Abstract
Alizarin red S (ARS) removal from wastewater using sheep wool as adsorbent was investigated. The influence of contact time, pH, adsorbent dosage, initial ARS concentration and temperature was studied. Optimum values were: pH = 2.0, contact time = 90 min, adsorbent dosage = 8.0 g/L. Removal of ARS under these conditions was 93.2%. Adsorption data at 25.0 °C and 90 min contact time were fitted to the Freundlich and Langmuir isotherms. R2 values were 0.9943 and 0.9662, respectively. Raising the temperature to 50.0 °C had no effect on ARS removal. Free wool and wool loaded with ARS were characterized by Fourier Transform Infrared Spectroscopy (FTIR). ARS loaded wool was used as adsorbent for removal of Cr(VI) from industrial wastewater. ARS adsorbed on wool underwent oxidation, accompanied by a simultaneous reduction of Cr(VI) to Cr(III). The results hold promise for wool as adsorbent of organic pollutants from wastewater, in addition to substantial self-regeneration through reduction of toxic Cr(VI) to Cr(III). Sequential batch reactor studies involving three cycles showed no significant decline in removal efficiencies of both chromium and ARS.
1. Introduction
Large amounts of dye-contaminated wastewaters are released yearly from leather, cosmetics, as well as the pharmaceutical, plastics and textile industries. Most of these dyes are skin irritants, mutagenic or carcinogenic [1,2]. Dye-polluted water decreases photosynthesis since light penetration is inhibited [3]. These dyes have complex aromatic structures, which give them thermal, optical and physicochemical stability, and thus, they could not be easily biodegraded by natural substances [4,5,6]. Alizarin red S (ARS) (Figure 1), or 1,2-dihydroxy-9,10-anthra-quinonesulfonic acid sodium salt, is a water soluble anthraquinone dye originally derived from the root of the madder plant [7]. It has been extensively employed since ancient times in dyeing textiles [8]. It is a strong oxidizing agent, and hence, must be stored away from moisture and heat [9].
Figure 1.
Alizarin red S (ARS).
A wide range of physical and chemical treatment technologies have been investigated for removing dyes from wastewater. They include coagulation, precipitation, adsorption, membrane filtration, electrochemical techniques and ozonation [10]. Among these, adsorption is the most widely employed, due to its high efficiency, non-toxicity, readily available adsorbents, low cost, ease of recovery and environmental sustainability [11,12,13]. Applicability largely depends on the cost and efficiency of adsorbent. Currently, various potential adsorbents are used for removal of specific organic compounds from wastewater. The adsorption of dyes transfers them from water effluent to solid phase, thereby decreasing effluent volume. Native mustard husk has been used in ARS removal from aqueous solutions, with thermodynamic studies indicating that the process is spontaneous and endothermic [14]. Pentaerythritol modified multi-walled carbon nanotubes (ox-MWCNT-PER) has been shown to be highly efficient in ARS removal [15]. Removal of ARS from wastewater using alumina as adsorbent has been investigated at optimum conditions [16]. Moreover, activated carbon is a good adsorbent for removal of ARS from wastewater, as indicated by favorable thermodynamic parameters [17].
Heavy metals, including chromium, are widely distributed in the environment as a result of numerous industrial applications. These include electroplating, chromate manufacture, wood preservation, galvanization, steel industry, paint, textile production, oxidative dyeing, cooling water towers, leather tanning, corrosion inhibitors and batteries [18]. As a result, heavy metals are found in many industrial wastewaters [19,20,21]. Chromium is a metallic element listed by the Environmental Protection Agency (EPA) as one of 129 priority pollutants [22,23]. It is found in the air, water and soil, and occurs in several oxidation states ranging from Cr(II) to Cr(VI), with the trivalent and hexavalent states being the most stable and common [1,2,3]. In natural waters, chromium is present in several forms, the most common of which are Cr(0), Cr(III), and Cr(VI). Cr(III) has very low solubility and is relatively stable, whereas Cr(VI) is environmentally mobile and highly toxic. Exposure to Cr(VI) causes various health problems, including skin and stomach irritation, dermatitis, liver damage, kidney circulation and nerve and tissue damage [24,25,26]. Numerous technologies have already been applied in the removal of Cr(VI) from aqueous solutions. These include adsorption, biosorption, ion-exchange, foam flotation, electrolysis, surface adsorption precipitation, reverse osmosis, sand filtration, chemical reduction/oxidation, electrochemical precipitation, membrane filtration and solvent extraction [27,28,29,30].
In previous studies by our group, sheep wool has been found to be efficient in Cr(VI) removal, with subsequent reduction to Cr(III) [31,32]. This study reports on the results of using sheep wool for the removal of ARS from wastewater and the possibility of regenerating the contaminated wool. The outcome of the research could be utilized in a sequential batch reactor for removal of the two pollutants from wastewaters.
2. Materials and Methods
2.1. Materials
All chemicals were of analytical grade and used without further purification. Solutions were prepared using distilled deionized water (DDW) and their concentrations determined spectrophotometrically. ARS was from BDH (Turkey). Potassium dichromate was from Riedel De-Haen (Germany). Acetone and 5-di-phenyl carbazide were from Sigma Aldrich (USA). Sheep wool (Sharjah animal market) was trimmed and riffled, then washed with water and detergent for two days.
2.2. Instrumentation
ARS and Cr(VI) concentrations were determined spectrophotometrically using Cary 50 (Varian, Australia). Total chromium was determined using Spectra AA220FS (Varian, Australia). Samples were shaken using Edmund Buhler shaker (KS-15/TH-15, Bodelshausen, Germany) at 25.0 ± 0.1 °C. pH was measured on a Thermo-Orion 210A + pH meter (USA) equipped with a combined glass electrode. IR spectra were obtained on a Spectrum One FTIR (Perkin Elmer, Waltham, MA, USA). ARS oxidation byproducts were detected using HPLC (Shimadzu, LC-2040C, Kyoto, Japan).
2.3. Methods
2.3.1. Reagent Preparation
A 1000 mg/L stock ARS solution was prepared at pH 2.0 and used to prepare standard solutions in the range 1–20 mg/L. Stock solutions containing 1000 mg/L Cr(VI) were prepared by dissolving potassium dichromate in DDW. Stock ligand solutions were prepared by mixing 0.20 mL of 0.05% of 1,5 diphenyl carbazide (in acetone) with 2 drops of 6.0 M sulfuric acid and 0.10 mL of sample. Standard Cr(VI) solutions were in the range 1–10 mg/L.
2.3.2. Determination of Cr ions Concentration
Total Cr concentration was determined by atomic absorption spectroscopy (AAS). The 1,5-diphenyl carbazide method could not be used in the determination of Cr(VI) alone, because Cr(VI) removal by wool-ARS complex was accompanied by formation of ARS oxidation by-products. ARS degradation by oxidation involves the cleavage of dye-chromophore components [33,34]. FTIR analysis of residual products from dye oxidation gave IR bands at 1717, 1623, 1387, 1105 and 1045 cm−1, attributed to >C=O (carbonyl), >C=C< (alkenes),-C-C-C (alkanes), SO42− and –C-O-C- groups, respectively. These by-products interfere with analysis of Cr(VI) by 1,5-diphenyl carbazide. Hence, only total Cr could be analyzed by AAS. The highest removal of Cr(VI) was 93%, as detected by AAS. However, previous studies by our group revealed that, at this short term contact, slight amounts of Cr(III) were released into solution [31]. This process causes incomplete removal of Cr(VI).
2.3.3. Adsorption Studies
Batch adsorption studies were carried out in flasks containing 50 mL of test solutions at the desired initial ARS concentrations. λmax for dye solutions was 261 nm. Batch adsorption studies were then carried out. Wool loaded with ARS was placed in flasks containing the desired initial Cr(VI) concentration and the contents shaken. Shaking conditions were: 1.5 h, 25.0 °C and 175 rpm. AAS was used to measure total Cr concentrations. Oxidation by-products of ARS by Cr(VI) were detected using HPLC under the following conditions: mobile phase 40:60% methanol:phosphate buffer, Pinnacle D8 C8 5 µm (RESTEK, Bellefonte, PA, USA), analytical wavelength 540 nm and 1.5 mL/min flow rate.
2.3.4. Regeneration In-Place Studies
Wool loaded with ARS was prepared by shaking wool with 100 mg/L ARS solution at optimum conditions. The resulting wool-ARS was washed with DDW and dried. The remaining ARS solution was analyzed spectrophotometrically. ARS removal was 88%. Wool-ARS was shaken with 50 mg/L Cr(VI) solution at optimum conditions for 4 days. Subsequently, the wool-ARS-Cr was collected, washed with DDW and dried. The remaining Cr(VI) solution was analyzed by AAS, giving a removal efficiency of 86.8%. The cycle was repeated three times.
3. Results and Discussion
3.1. Adsorption Studies
Removal of ARS was calculated using
where Co and Ce are initial and equilibrium ARS concentrations (mg/L), respectively.
% Removal = ((Co − Ce)/Co)∗100
3.1.1. Optimization Studies on ARS Removal by Wool
Effect of Contact Time
Figure 2 shows the effect of contact time on ARS removal. At all three pH values used, removal increases with increasing contact time until a maximum is reached at ca. 90 min, selected as the optimum time.
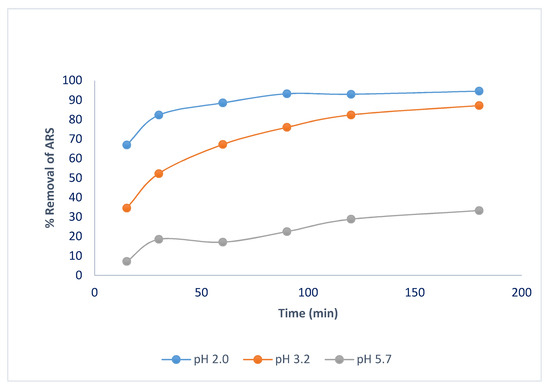
Figure 2.
Effect of contact time on ARS removal. Adsorbent dosage = 8.0 g/L, T = 25.0 °C, shaking speed = 175 rpm.
Effect of pH
ARS is employed as an indicator in acid-base titration, and changes color in the pH range 4.0 (yellow)-6.0 (red) [35]. At pH < 4.6, the acidic form predominates, whereas at pH > 6.0, it is mostly in the the basic form. Figure 3 shows the effect of pH on ARS removal. As pH increases, removal decreases from 90% at pH 2.0 reaching a minimum of <20% at pH > 5.0. Hence the acidic form ARS is the one more favorably adsorbed by wool. pH 2.0 was thus selected as optimum in subsequent measurements.
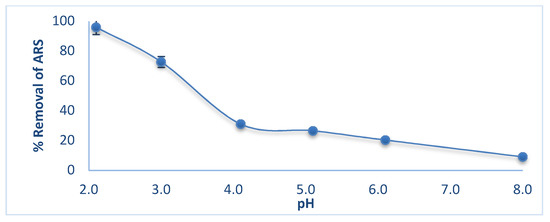
Figure 3.
Effect of pH on ARS removal by wool. Adsorbent dosage = 8.0 g/L, initial [ALS] = 100 mg/L, T = 25.0 °C, contact time = 90 min, shaking speed = 175 rpm.
Effect of Wool Dosage
Figure 4 shows the effect of wool dosage on ARS removal at optimum conditions. ARS removal increases with increasing wool dosage until a maximum 8.0 g/L. This dosage was selected as optimal and used in further experiments.
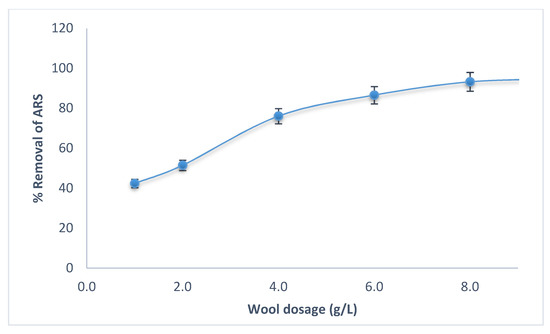
Figure 4.
Effect of adsorbent dosage on removal of ARS by wool at different wool dosages. Initial [ARS] = 100 mg/L, pH = 2.0, contact time = 90 min, T = 25.0 °C, shaking speed = 175 rpm.
Effect of Temperature
For initial ARS concentrations of 60 mg/L, 80 mg/L and 100 mg/L, increasing the temperature from 25.0 to 50.0 °C has little effect on ARS removal (Figure 5). The temperature of 25.0 °C was selected as optimal in adsorption isotherms.
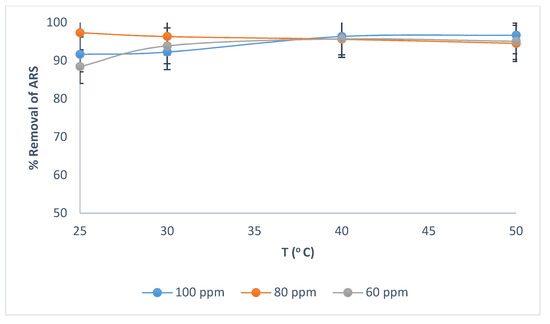
Figure 5.
Effect of temperature and initial ARS concentration on removal of ARS by wool. Adsorbent dosage = 8.0 g/L, contact time = 90 min, pH = 2.0, shaking speed = 175 rpm.
3.1.2. Adsorption Isotherms
The adsorption capacity (qe) for ARS removal was evaluated using
where qe is the equilibrium adsorption capacity (mg adsorbate/g adsorbent), V is the volume of solution (L) and m is the mass of adsorbent (g). At optimum adsorption parameters, qe was evaluated at several initial concentrations and the results fitted to the linearized forms of the Langmuir (Equation (3)) and Freundlich (Equation (4)) isotherms [36]. These are
where Ce is the equilibrium concentration (mg/L), qe is the amount adsorbed at equilibrium, in mg/g adsorbent, Q (mg/g) and b (L/mg) are the Langmuir constants, representing adsorption capacity and energy, respectively. Kf and n are the Freundlich constants. The poor fit to the Langmuir isotherm in Figure 6a indicates that adsorption does not follow this isotherm. This may be attributed to the nonequivalence adsorption sites on wool. Figure 6b shows the data fitted to the Freundlich isotherm. The good linearity of this plot clearly reveal that adsorption best fits the Freundlich isotherm, indicating that adsorption sites are not equivalent. The Freundlich constants, Kf and n, were 3.38 and 1.70, respectively.
qe = (Co − Ce) × V/m
Ce/qe = 1/Qb + Ce/Q
qe = Kf Ce1/n
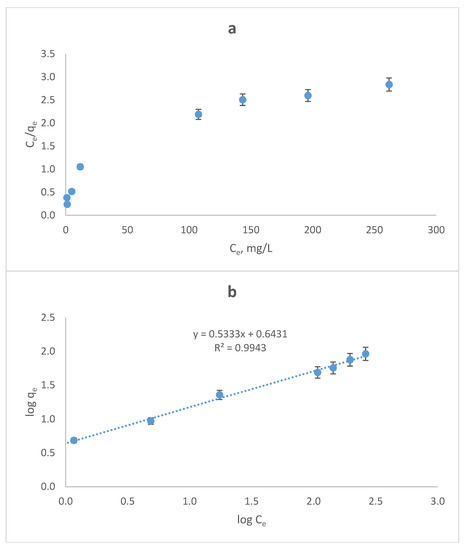
Figure 6.
(a) Langmuir and (b) Freundlich plots for adsorption of ARS by wool. Adsorbent dosage = 8.0 g/L, T = 25.0 °C, contact time = 90 min, pH = 2.0, shaking speed = 175 rpm.
3.1.3. Adsorption Kinetics
The kinetics of adsorption of ARS by wool was studies at pH 2 and were fitted to both Lagergren pseudo first order model (Equation (5)) and pseudo second order model (Equation (6)) [37,38].
Our data was found to best fit the pseudo second order model with R2 = 1 yielding a value for the second order rate constant (k2) of 0.134 g mg−1 min−1 at 25.0 °C.
3.2. Removal of ARS by Wool and by Wool Loaded with Cr(VI)
3.2.1. FTIR Spectra
An earlier publication by our group reported on Electron dispersive X-ray spectroscopy (EDS) and FTIR characterization of wool and wool loaded with Cr(VI) [31]. IR spectra of ARS were subsequently reported [33]. In this work, FTIR was used to monitor removal of ARS by wool, In Figure 7a, the peak at 3459 cm−1 is due to the –OH group, whereas peaks at 3094 cm−1 and 2926 cm−1 arise from =C-H groups in aromatic rings. Peaks at 2400 cm−1, 1666 cm−1, 1634 cm−1 and 1588 cm−1 are characteristic of anthraquinone molecules, whereas those at 1155 cm−1 and 728 cm−1 are for sulfonate groups. Figure 7b shows spectra of free wool with features described earlier [31]. Upon complexation with ARS, changes were observed (Figure 7c). Specifically, the broad peak at 4000–2800 cm−1 is sharpened, and a new band at 2400 cm−1 appears. Changes in the broad peak between 1600–800 cm−1 are also observed. These changes indicate that ARS is essentially taken up by wool. Adsorption of Cr(VI) by wool loaded with ARS results in further FTIR changes (Figure 7d), primarily in the 1200–800 cm−1 range. This observation indicates that ARS on wool undergoes oxidation by Cr(VI) to form aromatic byproducts.
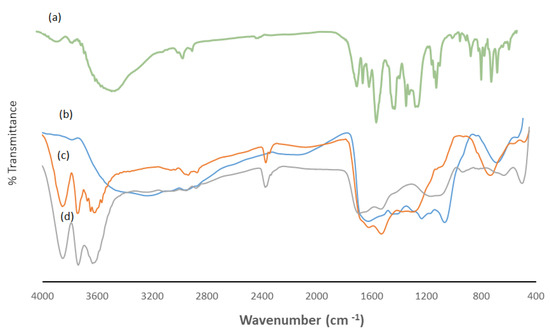
Figure 7.
FTIR spectra of (a) ARS, (b) free wool, (c) wool-ARS, (d) wool-ARS loaded with Cr(VI).
3.2.2. Oxidation Byproducts
Upon adsorption of Cr(VI) by wool loaded with ARS, oxidation by-products of ARS were formed (Figure 8), indicating that adsorbed Cr(VI) undergoes reduction to Cr (III). These by-products interfere with spectrophotometric determination of Cr(VI), with the result that concentrations of individual Cr species could not be determined. AAS, which gives total Cr, was hence used to follow the uptake of Cr(VI) by wool-ARS using short term contact time, so that Cr(III) has minimum interference [31]. The effect of contact time, pH, dosage and temperature were studied and optimized.
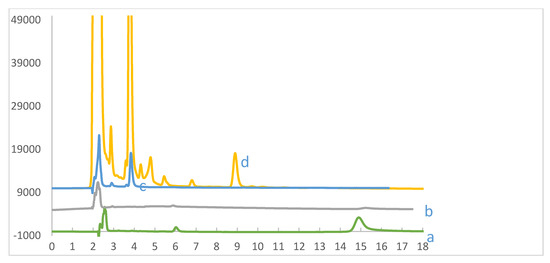
Figure 8.
HPLC chromatogram of aqueous solutions in equilibrium with ARS at pH 2.0. (a) ARS at pH 2 (green), (b) Wool loaded with ARS (grey), (c) ARS + Cr(VI) without wool (blue), (d) wool loaded with ARS after adsorption of Cr(VI) (yellow). Initial [ARS] = 1.0 mg/L, pH = 2.0; contact time 120 h, T = 25.0 °C, shaking speed = 175 rpm. Mobile phase is 40% methanol and 60% phosphate buffer, flow rate = 1.5 mL/min.
High performance liquid chromatography (HPLC) was used to analyse solutions in contact with wool, ARS or both, in presence or absence of Cr(VI). In Figure 8a (green), the ARS peak appears at 15 min retention time. Upon removal by wool, this peak disappears, due to ARS adsorption by wool (Figure 8b (grey)). This peak also disappears upon mixing Cr(VI) with ARS in solution without wool, a process that results in the appearance of a new peak at 4 min retention time, among other low intensity peaks that are not apparent in this chromatogram at this scale (Figure 8c (blue)). However, exposing wool loaded with ARS to Cr(VI) causes not only the disappearance of ARS peak at 15 min, but also the appearance of otherwise low intensity peaks in ARS-Cr(VI) without wool (Figure 8c) at retention times of 4.0, 4.4, 4.7, 5.5, 6.9 and 9.0 min (Figure 8d (yellow). These peaks indicate that the mechanism of ARS oxidation on wool is similar to that in aqueous solution. These peaks have been attributed to a variety of oxidation by-products [34,35]. Thus adsorption of Cr(VI) by wool loaded with ARS results in ARS oxidation with simultaneous release of oxidation by-products to solution. This process frees and regenerates wool from ARS for reuse.
3.2.3. Optimization Studies on Cr(VI) Removal by Wool Loaded with ARS
Effect of Contact Time
Figure 9 shows the effect of contact time on Cr(VI) removal by wool loaded with ARS. Equilibrium removal is reached after 40 min. This can be compared with the 90 min needed for removal of ARS alone (Figure 3). However, in a previous study, the optimum time for removal of Cr(VI) by free wool under short term conditions was 60 min [31]. Hence, adsorption is speeded by the presence of ARS on wool.
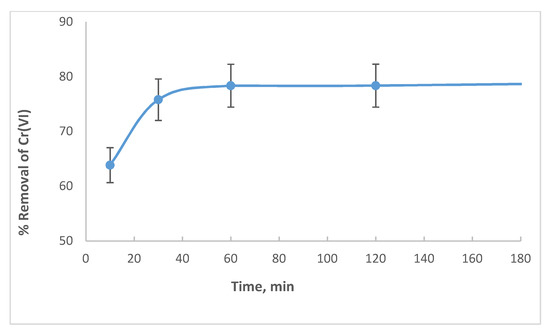
Figure 9.
Effect of contact time on Cr(VI) removal by wool loaded with ARS. Initial [Cr(VI)] concentration is 100 mg/L, adsorbent dosage = 8.0 g/L, pH = 6.0, T = 25.0 °C, shaking speed = 175 rpm.
Effect of pH
pH is an important factor that controls uptake of Cr(VI). Figure 10 reveals that removal of Cr(VI) by wool loaded with ARS reaches a maximum of 77.8% at pH 2.0, and remains almost constant at higher pH. In previous studies [39,40,41], the optimum pH for Cr(VI) removal by free wool was 2.0, indicating that reduction of Cr(VI) is catalyzed by hydrogen ions. In this study, removal of Cr(VI) by wool loaded with ARS is essentially independent of pH. pH 2.0 was selected as optimum for removal of Cr(VI) by wool loaded with ARS.
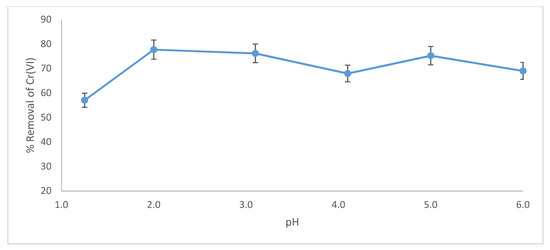
Figure 10.
Effect of pH on removal of Cr(VI) by wool loaded with ARS. Initial [Cr(VI)] concentration = 100 mg/L, adsorbent dosage = 8.0 g/L, contact time = 120 min., T = 25.0 °C, shaking speed = 175 rpm.
Effect of Adsorbent Dosage
The removal of Cr(VI) by wool loaded with ARS was studied at several adsorbent dosages (Figure 11). As the dosage increases, Cr(VI) removal increases until it reaches ca. 90.3%, at a dosage of 10.0 g/L. This is due to the increase of free active sites on wool and the increase in the number of sites loaded with ARS.
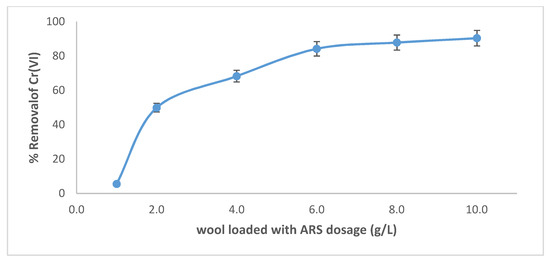
Figure 11.
Effect of adsorbent dosage on removal of Cr(VI) by wool loaded with ARS. Initial [Cr(VI)] concentration = 100 mg/L, pH = 2.0, T = 25.0 °C, contact time = 120 min, shaking speed = 175 rpm.
Effect of Temperature
Figure 12 shows the effect of temperature on removal of Cr(VI) by wool loaded with ARS. At constant temperature, removal varies with initial concentration in a semi-random trend. It increases as the initial concentration rises from 25 to 100 mg/L, then drops at 200 mg/L. The expectation is that removal ought to decrease with increasing initial concentration. The opposing trends within the two simultaneous processes that accompany removal may explain this apparent anomaly. The first is the adsorption of Cr(VI) by free sites on wool and the second is the oxidation of ARS by Cr(VI). In the first, removal decreases with increasing initial concentration, whereas in the second, the opposite effect is observed. Figure 12 also reveals that at constant initial Cr(VI) concentration, Cr(VI) removal decreases with increasing temperature. This observation indicates that the principal adsorption step is exothermic.
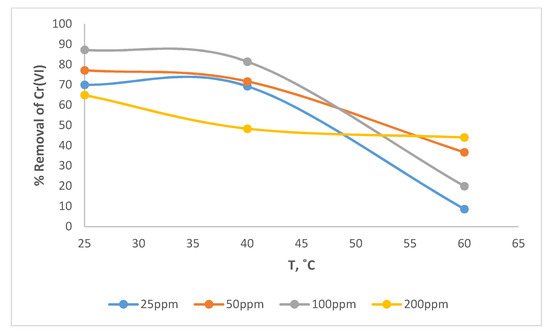
Figure 12.
Effect of temperature and Cr(VI) concentration on removal of Cr(VI) by wool loaded with ARS. Adsorbent dosage = 8.0 g/L, pH = 2.0, contact time = 120 min, shaking speed = 175 rpm.
3.3. Adsorption Mechanism
Based on the above results, Figure 13 shows a proposed two-step mechanism for removal of Cr(VI) by wool loaded with ARS (ARS-W). The second step is similar to that proposed in previous reports for removal of Cr(VI) by other natural adsorbents, but with ARS yielding oxidation products [39,41].
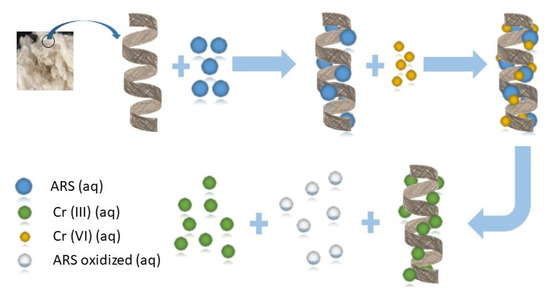
Figure 13.
Proposed mechanism for removal of Cr(VI) by wool loaded with ARS.
3.4. Isotherm Analysis
Adsorption data were fitted to both the Langmuir and Freundlich models. Figure 14 shows the dependence of adsorption capacity on Cr(VI) concentration after a 120 min contact, using wool loaded with ARS as adsorbent. The dependence does not resemble any known adsorption behavior, indicating that equilibrium was not achieved within this time. This is because removal by adsorption is followed by a time dependent redox process. Hence, the isotherm could not be fitted to any of the models that predicate equilibrium between adsorbate and adsorbent.
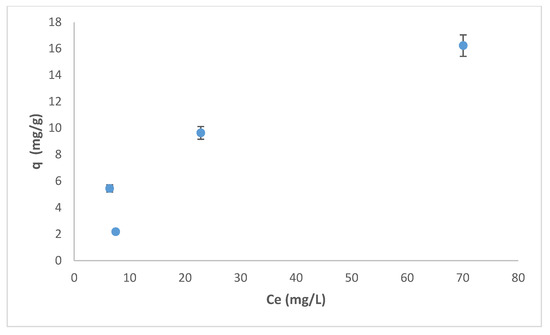
Figure 14.
Adsorption capacity (q) for the removal of Cr(VI) by wool loaded with ARS as function of Cr(VI) concentration. Contact time = 120 min, adsorbent dosage = 8.0 g/L, pH = 2.0, T = 25.0 °C, shaking speed = 175 rpm.
3.5. Regeneration Studies
Wool loaded with ARS has been used to investigate its regeneration by the simultaneous removal and reduction of Cr(VI) from industrial wastewater, followed by ARS oxidation. For this purpose, wool loaded with ARS was prepared by shaking wool with 100 mg/L ARS solution at optimum conditions. The resulting wool-ARS was washed with DDW and dried. Spectrophotometric analysis of the equilibrium solution gave ARS removal of 93.2%. Wool-ARS was then shaken with 50 mg/L Cr(VI) solution at optimum conditions for 4 days. Wool-ARS-Cr was washed with DDW and dried for further studies. AAS analysis of the equilibrium solution gave 86.8% Cr(VI) removal. The cycle was repeated four times. Figure 15 shows removal of ARS and Cr(VI) after each cycle. Removal of both ARS and Cr(VI) by wool varies in the range 60%–90%, even at the end of the fourth cycle. This finding suggests promising prospects for using a sequential batch reactor for the simultaneous removal of organic pollutants and Cr(VI), which is self-regenerated under field conditions.
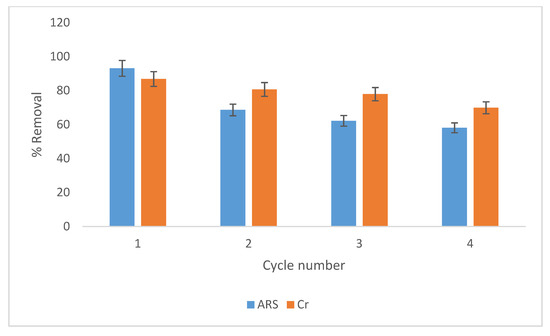
Figure 15.
Effect of different cycles on removal of Cr(VI) and ARS by wool. Adsorbent. dosage = 8.0 g/L, pH = 2.0, T = 25.0 °C, shaking speed = 175 rpm.
4. Conclusions
Removal efficiency of ARS by wool depends on contact time, adsorbent dosage, initial ARS concentration and pH. At 25.0 °C, The Freundlich adsorption isotherm gave a good fit to the adsorption data. ARS undergoes slow oxidization by Cr(VI) at pH 2.0. This oxidation and the ability of wool to adsorb Cr(VI) combine to form the basis of an effective method for sequential removal of ARS and Cr(VI), resulting in partial self-regeneration of wool from ARS. Wool loaded with ARS was efficient in removing Cr(VI) with the simultaneous appearance of oxidation ARS by-products. A sequential batch reactor, designed for four cycles, indicated no significant reduction in the ability of wool to remove ARS, followed by Cr(VI). A two-step mechanism for this removal has been proposed. The first involves fast adsorption of Cr(VI) on wool loaded with ARS, and the second involves an oxidation of ARS, followed by desorption of oxidation by-products into solution. Several of these by-products were detected by HPLC. The surface of wool before and after adsorption of ARS, followed by the adsorption of Cr(VI), which was characterized by FTIR. The results support the suggested mechanism. These findings form a basis for the design of batch sequential reactor for removal of ARS and Cr(VI), under field conditions and with zero liquid discharge.
Author Contributions
Z.A.S. and B.A.A. performed most of the experiments. This research was led by T.H.I., M.I.K. and F.H.J. were instrumental in the initiation, conceptualization and planning of this work. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the American University of Sharjah under grant number FRG17-R-17.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zazo, J.A.; Paull, J.S.; Jaffe, P.R. Influence of plants on the reduction of hexavalent chromium in wetland sediments. Environ. Pollut. 2008, 156, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wang, Y.; Li, M. A new carbon/ferrous sulfide/iron composite prepared by an in situ carbonization reduction method from hemp (Cannabis sativa L) stems and its Cr(VI) removal ability. ACS Sustain. Chem. Eng. 2014, 2, 1270–1279. [Google Scholar] [CrossRef]
- Sumathi, K.; Mahimairaja, S.; Naidu, R. Use of low-cost biological wastes and vermiculite for removal of chromium from tannery effluent. Bioresour. Technol. 2005, 96, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Zhai, X. Degradation of nitrobenzene by nano-TiO2 catalyzed ozonation. J. Mol. Catal. A Chem. 2007, 267, 41–48. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, X. Applications of conjugated polymer based composites in wastewater purification. RSC Adv. 2014, 4, 62160–62178. [Google Scholar] [CrossRef]
- Gholivand, M.; Yamini, Y.; Dayeni, M. Adsorptive removal of alizarin red-S and alizarin yellow GG from aqueous solutions using polypyrrole-coated magnetic nanoparticles. J. Environ. Chem. Eng. 2015, 3, 529–540. [Google Scholar] [CrossRef]
- Brown, D. Effect of colorants on the aquatic environment. Ecotox. Environ. Safe 1987, 13, 139–147. [Google Scholar] [CrossRef]
- Ding, F.; Sun, Y. Characterization of alizarin red S binding sites and structural changes on human serum albumin: A biophysical study. J. Hazard. Mater. 2011, 186, 352–359. [Google Scholar] [CrossRef]
- Ghaedi, M.; Hassanzadeh, A.; Nasiri, S.N. Multiwalled carbon nanotubes as adsorbents for the kinetic and equilibrium study of the removal of alizarin red S and morin. J. Chem. Eng. Data 2011, 5, 2511–2520. [Google Scholar] [CrossRef]
- Golder, A.K.; Hridaya, N.; Samanta, A.N. Electrocoagulation of methylene blue and eosin yellowish using mild steel electrodes. J. Hazard. Mater. 2005, 127, 134–140. [Google Scholar] [CrossRef]
- Tuzen, M.; Saygi, K.O.; Usta, C. Pseudomonas aeruginosa immobilized multiwalled carbon nanotubes as biosorbent for heavy metal ions. Bioresour. Technol. 2008, 99, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Duran, A.; Tuzen, M.; Soylak, M. Preconcentration of some trace elements via using multiwalled carbon nanotubes as solid phase extraction adsorbent. J. Hazard. Mater. 2009, 169, 466–471. [Google Scholar] [CrossRef]
- Chowdhury, T.; Zhang, L.; Zhang, J. Removal of arsenic(III) from aqueous solution using metal organic framework-graphene oxide nanocomposite. J. Nanomater. 2018, 8, 1062. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Mudhoo, A.; Chattopadhyaya, M. Kinetic, equilibrium thermodynamic studies and spectroscopic analysis of alizarin red S removal by mustard husk. J. Environ. Chem. Eng. 2013, 1, 1283–1291. [Google Scholar] [CrossRef]
- Yang, J.Y.; Jiang, X.Y.; Jiao, F.P. The oxygen-rich pentaerythritol modified multi-walled carbonnanotube as an efficient adsorbent for aqueous removal of alizarin yellow R and alizarin red S. Appl. Surf. Sci. 2018, 436, 198–206. [Google Scholar] [CrossRef]
- Rehman, R.; Mahmud, T.; Anwar, J. Removal of alizarin red S (dye) from aqueous media by using alumina as an adsorbent. J. Chem. Soc. Pak. 2011, 33, 228–232. [Google Scholar]
- Ghadia, M.; Najibi, A.; Hossaininian, H.; Shokrollahi, A.; Sylak, M. Kinetic and equilibrium study of alizarin red S removal by activated carbon. Toxicol. Environ. Chem. 2012, 94, 40–48. [Google Scholar] [CrossRef]
- Gautam, R.K.; Mudhoo, A.; Lofrano, G. Biomass-derived biosorbents for metal ions sequestration: Adsorbent modification and activation methods and adsorbent regeneration. J. Environ. Chem. Eng. 2014, 2, 239–259. [Google Scholar] [CrossRef]
- Saha, R.; Nandi, R.; Saha, B. Sources and toxicity of hexavalent chromium. J. Coord. Chem. 2011, 64, 1782–1806. [Google Scholar] [CrossRef]
- Espinosa, D.; Tenório, J. Thermal behavior of chromium electroplating sludge. Waste Manag. 2001, 21, 405–410. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K. Heavy metals toxicity and the environment. EXS 2012, 101, 133–164. [Google Scholar]
- Bartlett, L.; Vesilind, P.A. Chemistry and controversy: The regulation of environmental chromium. Environ. Eng. Policy 1998, 1, 81–86. [Google Scholar] [CrossRef]
- Fendorf, S.; Wielinga, B.W.; Hansel, C.M. Chromium transformations in natural environments: The role of biological and abiological processes in chromium(VI) reduction. Int. Geol. Rev. 2000, 4, 691–701. [Google Scholar] [CrossRef]
- Costa, M. Potential hazards of hexavalent chromate in our drinking water. Toxicol. Appl. Pharmacol. 2003, 188, 1–5. [Google Scholar] [CrossRef]
- Yang, J.; Yu, M.; Qiu, T. Adsorption thermodynamics and kinetics of Cr(VI) on KIP210 resin. J. Ind. Eng. Chem. 2014, 20, 480–486. [Google Scholar] [CrossRef]
- Yadav, S.; Srivastava, V.; Banerjee, S. Adsorption characteristics of modified sand for the removal of hexavalent chromium ions from aqueous solutions: Kinetic, thermodynamic and equilibrium studies. Catena 2013, 100, 120–127. [Google Scholar] [CrossRef]
- Li, Y.; Gao, B.; Wu, T. Hexavalent chromium removal from aqueous solution by adsorption on aluminum magnesium mixed hydroxide. Water Res. 2009, 43, 3067–3075. [Google Scholar] [CrossRef]
- Chang, Y.; Lim, J.; Yang, J.K. Removal of As(V) and Cr(VI) in aqueous solution by sand media simultaneously coated with Fe and Mn oxides. J. Ind. Eng. Chem. 2012, 18, 188–192. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Yakout, A.A.; Abdel-Aal, H. Speciation and selective biosorption of Cr(III) and Cr(VI) using nanosilica immobilized-fungi biosorbents. J. Environ. Eng. ASCE 2015, 141, 131–139. [Google Scholar] [CrossRef]
- Jumean, F.; Khamis, M.; Sara, Z. Concurrent removal and reduction of Cr(VI) by wool: Short and long term equilibration studies. Am. J. Anal. Chem. 2015, 6, 47–57. [Google Scholar] [CrossRef]
- Ray, P.; Sabri, M.; Ibrahim, H. Design and optimization of a batch sequential contactor for the removal of chromium(VI) from industrial wastewater using sheep wool as low cost adsorbent. Deswater 2018, 113, 109–113. [Google Scholar] [CrossRef]
- Ortiz, E.; Solis, H.; Noreña, L. Degradation of red anthraquinone dyes: Alizarin, alizarin S and alizarin complexone by ozonation. Int. J. Environ. Sci. Dev. 2017, 8, 255–259. [Google Scholar] [CrossRef]
- Asha, P.; Narayanamurthy, P.; Kavya, M.S. Kinetics and mechanism of oxidation of alizarin red-S using chloramine-T in acid medium. Int. J. Environ. Sci. Dev. 2016, 3, 2394–9333. [Google Scholar]
- Bishop, E. Indicators, 1st ed.; Pergamon: Oxford, UK, 1972; p. 250. [Google Scholar]
- Dada, A.; Olalekan, A.; Olatunya, A. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. J. Appl. Chem. 2012, 3, 38–45. [Google Scholar]
- Tan, L.A.; Hameed, B.H. Adsorption isotherms, kinetics, thermodynamics and desorption studies of basic dye on activated carbon derived from oil palm empty fruit bunch. J. Appl. Sci. 2010, 10, 2565–2571. [Google Scholar] [CrossRef]
- Hameed, B.H.; Mahmoud, D.K.; Ahmad, A.L. Equilibrium modeling and kinetic studies on the adsorption of basic dye by a low-cost adsorbent: Coconut (cocosnucifera) bunch waste. J. Hazard. Mater. 2008, 158, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Manassra, A.; Khamis, M.; Ihmied, T.; Eldakiky, M. Removal of chromium by continuous flow using wool packed columns. Electron. J. Environ. Agric. Food Chem. 2010, 9, 651–663. [Google Scholar]
- EPA Method. Chromium Hexavalent (Colorimetric). Educ. Publ. Awards (EPAA) 1992, 7196A, 1–6. [Google Scholar]
- Fiol, N.; Escudero, C.; Villaescusa, I. Chromium sorption and Cr(VI) reduction to Cr(III) by grape stalks and yohimbe Bark. Bioresour. Technol. 2008, 99, 5030–5036. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).