Abstract
The enthalpy of immersion for five activated carbons (with different surface chemistry) in cyclohexane and hexane was determined in order to observe the intensity of the solid–liquid interaction. The enthalpy of immersion was related to the properties of activated carbons, such as micropore volume, total basic groups content, and the EoWo product, that characterized each solid-liquid system. The values for the immersion enthalpy were between −21.2 and −91.7 J g−1 for cyclohexane and between −16.4 and −66.1 J g−1 for hexane. It showed greater interaction between the cyclohexane and the activated carbons and it was related to the properties of this adsorbate, such as molecular size and molecular arrangement. The difference in the enthalpy of immersion between the solvents per unit of micropore volume for the set of activated carbons was calculated obtaining a value of −487 J cm−3.
1. Introduction
The activities of daily life make most people spend much of their time in enclosed spaces, if the air quality is not in optimal, it can affect the health of those who remain in these places. Actually, the European Environment Agency (EEA) classifies this situation as one of the risk factors of high priority for general population health [1].
The pollutants have different origins: they are derived from combustion, agents and biological processes, gases and volatile organic compounds (VOCs). VOCs are a large and diverse class of chemical pollutants, so between 50 and 300 compounds can be found in indoor air environments [2].
With the persistent increase of volatile organic compounds and their harmful effects on human health and the environment, the development of effective techniques that allow the removal of these is of great importance. Control mechanisms have emerged, and they can generally be divided into methods of recovery and methods of destruction. Recovery methods include adsorption, condensation, absorption, and separation by membranes, while destruction techniques include incineration, catalytic oxidation and biological degradation. In comparison with destruction methods, which convert mainly VOCs into CO2 and H2O, recovery methods are more economical. In addition, incineration and most other methods of destruction, expend enormous amounts of energy to produce high temperatures that allow carrying out the reactions, and in turn, generate some toxic products, such as NOx, O3, OH radicals and secondary organic aerosols. Among the methods of recovery, adsorption is considered one of the most favorable methods due to its low cost and high efficiency, for which, carbonaceous materials have been widely used due to their versatility, selectivity, surface area, variety of porous structure, high capacity and rapid adsorption kinetics [3,4,5,6,7,8,9]. Characterizing activated carbons with the ability to adsorb VOCs allows knowing the interaction between these adsorbents and the adsorbent material [10].
It was proposed to study the interaction between the activated carbons with hexane and cyclohexane, since at the industrial level they have been widely used in a large number of household products, such as paints, varnishes, waxes, solvents, detergents or cleaning products [11]. In terms of their chemical structure, they were chosen because they are molecules with six carbon atoms that differ in their molecular size and arrangement, cyclohexane is a closed chain aliphatic compound while hexane is an open chain aliphatic compound.
The interaction activated carbon-VOCs can be studied by determining the isotherm of adsorption of the compound from the vapor phase on the solid and the enthalpy of immersion of the solid in the compound from the liquid phase [8]. Although the study of isotherms of adsorption is interesting, it is not discussed in this document, because it is intended to describe how the other technique, the immersion calorimetry, can generate information regarding the interaction between the solvent and the porous solid and relate the intensity of that interaction with the characteristics of the adsorbent and the adsorbate. The immersion enthalpy is a characterization parameter derived from the contact between a solid and a liquid, and this parameter varies, as said before, according to the textural and chemical characteristics of the solid and the properties of the liquid [12,13,14,15].
The thermal effects resulting from submerging a solid in a solvent, of non-polar type, can be related to the surface area of the solid through the models developed by Dubinin and Stoeckli [16]. The enthalpy of immersion for microporous solids, ΔHim, is related to the net heat of adsorption, according to the following expression:
The net heat of adsorption can be expressed as a function of the adsorption parameters, where θ is the degree of micropore filling, W/Wo, and for n, which is the term that is related to surface heterogeneity, the above equation is transformed:
where α is the coefficient of thermal expansion of the adsorbate, a represents the amount adsorbed to the relative pressure P/Po, ao corresponds to the adsorption limit value, Eo is the characteristic energy for the adsorption of the vapor.
When the net heat of adsorption is replaced in Equation (1), the expression is integrated, and it is considered that the microporous solid is activated carbon, with microporous volume Wo, which is immersed in a solvent with molar volume, Vm, the ratio for the enthalpy of immersion, ΔHim is obtained:
The determination of the immersion enthalpy of the activated carbon in solvents allows to quantify the liquid–solid interaction and it indicates for which solvent the interaction is greater. It leads to establishing a correlation with the energetic influence of the structure of the adsorbate.
Therefore, the enthalpy of immersion of five activated carbons with different textural and chemical properties in hexane and cyclohexane is determined in this work. Furthermore, the relationship between the immersion enthalpy with the micropore volume, the content of basic groups (that favor the solid–solvent interaction), the surface area and the EoWo product that is calculated from Equation (3) [10]. The change of the enthalpy in the solid–liquid interaction is calculated taking into account the structure of the solvents, and it is even more interesting because those are C6 hydrocarbon compounds but they differ in their size and the arrangement of the molecules and this affects the affinity with the solid, the interaction, the entrance to the pores and hence, the adsorption process. This is a solid–liquid interaction process where the intensity of this interaction between the adsorbent and the adsorbate is evaluated in energetic terms. This manuscript not only describes the interaction between two organic molecules (cyclohexane and hexane) and activated carbons modified in their chemical and textural properties but also how the system is affected according to the properties of its components: as for adsorbates, one is a closed chain aliphatic compound (cyclohexane), while the other is an open chain aliphatic compound (hexane). With respect to the adsorbents, they have different content of surface groups (lactonic, carboxylic and phenolic) that, in turn, modify the dispersive type interactions that may exist between this type of molecules and activated carbon. Further, the difference between the immersion enthalpy cyclohexane and hexane was calculated to indicate the contribution of the chemical structure of the two solvents in the solid–liquid interaction. In turn, a correlation between the immersion enthalpy, the adsorption energy and the microporous structure of the solid was established.
2. Materials and Methods
2.1. Preparation of Activated Carbons with Differences in Their Surface Chemistry
The starting sample was granular activated carbon prepared from coconut shell (CAG). A fraction of CAG was subjected to an oxidation process with a solution of nitric acid 6 M to obtain an oxidized activated carbon (CAN). Two fractions of CAN were exposed to thermal treatment at 723 K (5 h at a rate of 1.5 °C min−1, and then one hour at 723 K) and 1023 K (8 h at a rate of 1.5 °C min−1, and then one hour at 1023 K) under nitrogen atmosphere: CAN723 and CAN1023. A final sample was obtained by subjecting activated carbon GAC to a thermal treatment in N2 atmosphere, at 1173 K (10 h at a rate of 1.5 °C min−1, and then one hour at 1173 K), CAG1173 [17].
2.2. Nitrogen Adsorption Isotherms at 77 K
The textural parameters of the activated carbons: surface area and pore volume were evaluated by physical adsorption of nitrogen at 77 K in an Autosorb 3B, Quantachrome equipment. Micropore volume and apparent surface areas were determined by the Dubinin–Radushkevich and Brunauer–Emmett–Teller (BET) models, respectively [18].
2.3. Determination of the Content of Total Acidic and Basic Groups
The determination of the content of acidic and basic groups was performed according to the method proposed by Boehm [19] in which volumetric titrations were carried out. We weighed 0.5 g of the activated carbon and mixed with 50 mL of a solution of NaOH or HCl 0.1 M.
Subsequently, the mixtures were maintained at a constant temperature (298 K) and constant agitation for five days. Finally, a sample of 10 mL of each of the solutions in contact with the activated carbon was titrated with the corresponding titrant solutions previously standardized. The titrations were carried out using a CG 840B Shott potentiometer [19,20].
2.4. Determination of the Immersion Enthalpy in Cyclohexane and Hexane
For the calorimetric characterization, the immersion enthalpy of the activated carbons in cyclohexane and hexane was determined using a local microcalorimeter of heat conduction [21]. This calorimeter had thermopiles of semiconductor materials as thermal sensors and a stainless steel cell with capacity for 10 mL for the solvent.
We weighed 100 mg of the activated carbon and placed in a glass ampoule (with a capacity of approximately 2 mL and a fragile peak in the bottom to ensure its breaking at the time of the immersion of the activated carbon in the solvent) inside the calorimetric cell and the electric potential of the thermopiles was captured for approximately 40 min until the stable baseline was obtained. Later, the sample was immersed, the potential increased due to the wetting of the solid and it was registered until it returned to the baseline again. Finally, the electrical calibration was carried out [22,23,24,25,26].
3. Results
In Table 1, the textural and chemical characteristics of the activated carbons determined from the isotherms of N2 at 77 K and the volumetric titrations [27] were presented. The surface area was determined by the Brunauer–Emmett–Teller (BET) model. The total volume (VTotal) corresponded to the volume adsorbed at P/Po of 0.99 and the micropore volume (Wo) was evaluated by the Dubinin–Radushkevich (D–R) model. The pore size distribution (PSD) was also determined using the quenched solid density functional theory (QSDFT) for mixed slit pore/cylindrical pore geometry (hybrid slit-cylindrical equilibrium kernel), this PSD is shown in Figure 1. This pore shape was chosen because the determination was also made for the other geometries of the pore and this showed a lower percentage of error (between 0.753% and 0.902%).

Table 1.
Textural and chemical characteristics of activated carbons.
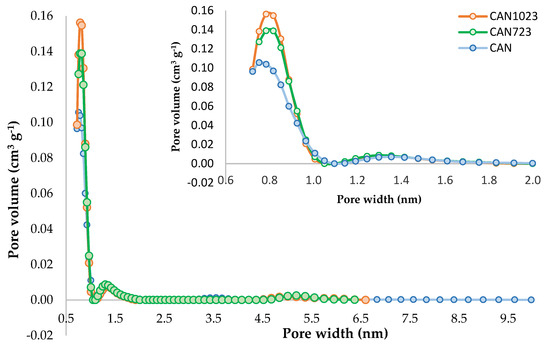
Figure 1.
Pore size distribution (PSD) for oxidized activated carbon (CAN), CAN723, and CAN1023 according to the quenched solid density functional theory (QSDFT) model.
In order to show the effect of the oxidation modification and the thermal treatment, the PSD of the three oxidized samples CAN, CAN723, and CAN1023 are shown in Figure 1. It was evidenced that the oxidized sample began to increase its pore volume proportionally with the increase in temperature, so that at 723 K, it increased approximately by 40%, and for CAN1023 increased again to approximately 60% with respect to the initial value of CAN, this could be due to the thermal stability of the oxygenated groups present on the surface, so that at a higher temperature there was greater removal of heteroatoms in the porous solid [28,29,30,31].
It was observed that the apparent surface area BET, presented variations with the chemical and thermal modifications: for the sample that is oxidized without thermal treatment, the surface area presents a slight detriment of 4% with respect to CAG, it was generated by the addition of surface groups derived from the interaction of the CAG with the nitric acid solution. On the other hand, the thermal modification of the samples for all cases (oxidized: CAN723, CAN1023, and non-oxidized CAG1173) presents a proportionality relationship between the increase in surface area and temperature, so that activated carbon subjected to higher temperature (1173 K) generated an increase of more than 18% [30]. The surface chemistry of the activated carbons is also an important factor in the solid–liquid interaction, so the total acidity and basicity of the solids were determined. Table 1 shows different contents of acidic and basic groups. It was observed that CAN and CAG1173 showed extreme values with basic group contents of 0.05 and 0.31 mol g−1, respectively [32].
Once the activated carbons were characterized, their immersion enthalpy in hexane and cyclohexane was determined to appreciate the difference in the value of the enthalpy due to the change in the surface characteristics of the solids and the change in the structure of the liquids that interacted with each solid. Then, the contribution to the enthalpy of immersion that was produced by the change in the structure of the solvent was calculated. Figure 2 and Figure 3 show the graphs of the change of the electric potential as a function of time resulting from the immersion of the activated carbons in cyclohexane and hexane.
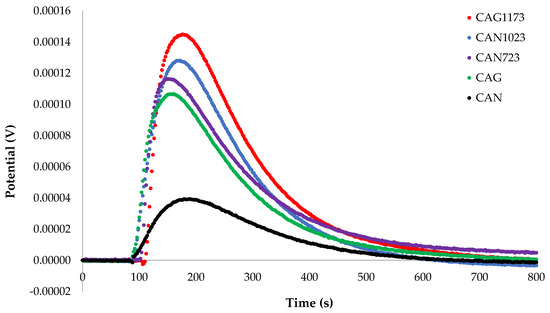
Figure 2.
Curves of electric potential as a function of the immersion time of activated carbons in cyclohexane.
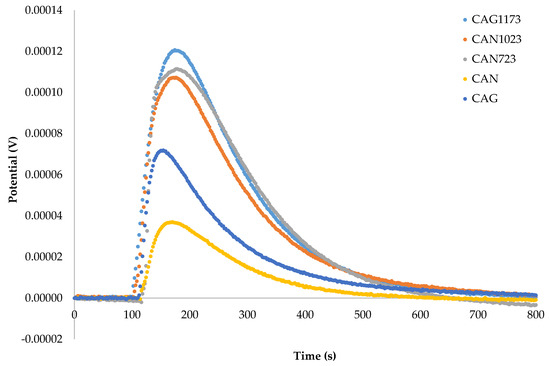
Figure 3.
Curves of electric potential as a function of the immersion time of activated carbons in hexane.
The enthalpy of immersion, ΔHim, was calculated from the area under the curve of the graphs that were generated by contacting the activated carbons with the solvents (Figure 2 and Figure 3): Cyclohexane (CAG: −55.34 J g−1; CAN: −21.23 J g−1; CAN723: −76.66 J g−1; CAN1023: −69.66 J g−1; CAG1173: −99.71), Hexane (CAG: −40.90 J g−1; CAN: −16.40 J g−1; CAN723: −57.60 J g−1; CAN1023: −53.40 J g−1; CAG1173: −66.10). It was observed that the lowest energy effect is shown for CAN and the highest energy effect corresponded to the activated carbon obtained by thermal treatment at 1173 K. Similar results are obtained for the immersion enthalpy of carbonaceous solids in a non-polar solvent, such as benzene [27].
It is known that the solid−liquid interaction, which is manifested in the immersion enthalpy, ΔHim, increases when the liquid molecules reach the micropores and this depends both on the size of the adsorbate and on the size distribution of the pores [33]. For this reason, the relationship between ΔHim of the activated carbons in the solvents and the micropore volume, Wo (obtained from the N2 adsorption isotherm), is shown in Figure 4.
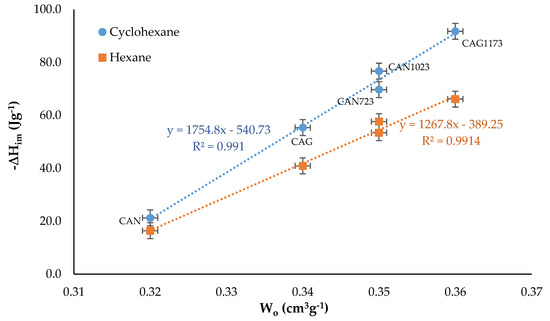
Figure 4.
Immersion enthalpy as a function of the micropore volume.
The result obtained when establishing the relationship was a directly proportional linear trend between the variables for the case of the immersion of the solids in the two solvents. However, since the values of the enthalpy of immersion in cyclohexane were greater than in hexane, the slope of the line presented for the immersion in this solvent was greater. It was also observed that for CAN the difference in the enthalpy of immersion was lower than for the other activated carbons, which indicates that as the volume of micropore decreased, the liquid−solid interaction became similar [34,35,36]. The linear trend described by the experimental data was in agreement with Equation (3), which represented the relationship between ΔHim and Wo for microporous activated carbons [16].
The difference between the values of the enthalpy of immersion in cyclohexane and hexane was calculated for each of the activated carbons to obtain the contribution that the structure of the solvent had in the solid−liquid interaction. Due to the ΔHim values that were obtained, it was observed that the interaction was greater between the activated carbons and the cyclohexane, therefore, one of the causes for the decrease in the value of the enthalpy of immersion was the change in the structure for two organic compounds with six carbons from cyclic shape to linear shape. In Figure 5 the difference in the immersion enthalpy was related to the micropore volume.
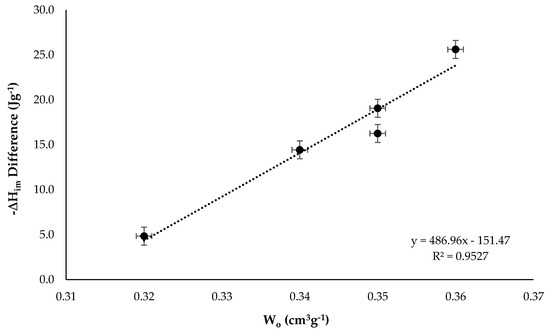
Figure 5.
Difference between the immersion enthalpy cyclohexane and hexane in function of the micropore volume.
The difference in ΔHim with respect to the micropore volume was also linear and it increased with increasing Wo, the highest value of 25.6 J g−1 was obtained for the activated carbon CAG1173 which was the one with the highest micropore volume value and the highest interaction, which indicated that the cyclohexane molecules due to their shape fitted better on the surface of the solid, presenting a higher value of the immersion enthalpy [37]. Figure 5 also allowed to calculate an average enthalpy change per unit volume for the set of activated carbons, it had a value of −487 J cm−3.
The other characteristic of the surface of an activated carbon that had a favorable influence on the energy interaction was the content of basic groups, since the thermal treatments carried out in this work increased the content of these groups due to the decomposition of the oxygenated groups that were removed from the surface and made it more hydrophobic, facilitating the interaction of non-polar solvents with the surface [32,38,39]. Figure 6 shows the relationship between ΔHim of activated carbons in cyclohexane and hexane and the content of total basic groups.
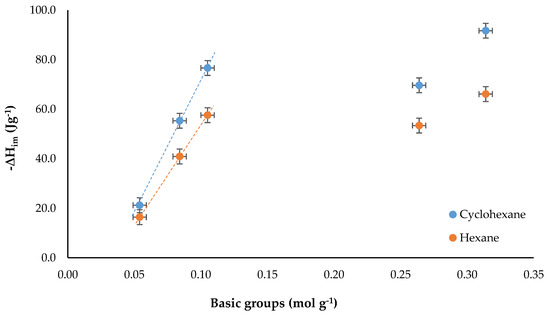
Figure 6.
Immersion enthalpy in function of the total basic groups content.
For both solvents it was observed that increasing the content of total basic groups increased the enthalpy of immersion, also, the experimental data were arranged in two trends. Likewise, the difference in the enthalpy of immersion in the two solvents was plotted according to the content of total basic groups, in order to observe more clearly the behavior of the set of solids. This result is presented in Figure 7.
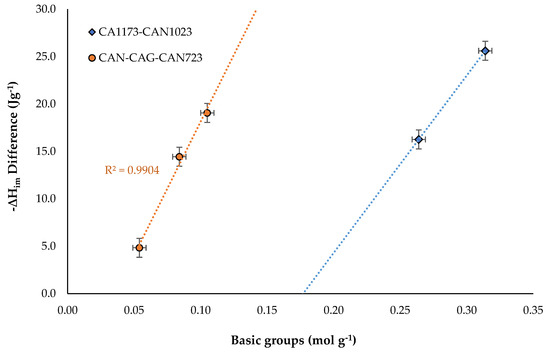
Figure 7.
Difference between the immersion enthalpy in cyclohexane and hexane in function of the total basic groups content.
Figure 7 corroborated the directly proportional behavior between the difference of ΔHim and the content of total basic groups of the surface of the solids, and that the set of activated carbons presented two different tendencies that were marked in the figure with an orange line for activated carbons with lower content of basic groups (showing a greater slope) and in blue for activated carbons with higher contents of basic groups (with lower slope). The difference in the enthalpy of immersion between the two solvents showed a total effect that included the interactions with the functional groups and with the surface. The relationship between ΔHim and the content of total acid groups was also observed, in which there was a tendency to decrease the enthalpy of immersion when increasing the content of acid groups, but the dispersion of the experimental values was high, and it did not allow obtaining clear information as in the case of the relationship with the total basic groups.
Figure 8 shows the relationship between ΔHim, and the surface area of the activated carbons because the interaction between the solvent and the surface of the solid increases if there is a larger contact surface.
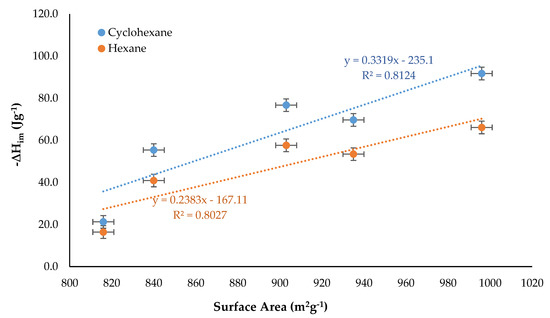
Figure 8.
Immersion enthalpy in function of the surface area of the activated carbons.
It was observed for the two solvents that there was a directly proportional relationship between the variables, however the experimental values showed a greater dispersion with respect to the trend line than when the values of the enthalpy of immersion were related to the micropore volume indicating that the latter best represented the interaction between the solid and the liquid that was manifested energetically in the system. Likewise, it can be seen that the interaction is greater among all activated carbons and cyclohexane and that from this ratio the average surface enthalpy for the cyclohexane can be calculated: 332 mJ m−2 and for hexane: 238 mJ m−2.
Finally, the product between the characteristic adsorption energy, Eo, and the micropore volume, Wo, obtained from Equation (3) was calculated. These two parameters were specific to the adsorption system and could be obtained from the immersion enthalpy of microporous solids in liquids in which the solids did not react. The EoWo product was related to the difference in the enthalpy of immersion between the cyclohexane and hexane for each activated carbon, then Figure 8 was obtained.
For the two solvents, directly proportional linear tendencies were obtained with a better adjustment for the case of the cyclohexane that had a cyclic structure, probably because this molecule could be located more easily on the surface of the activated carbon. It was also observed that the activated carbons with a lower content of oxygenated groups (which showed one of the highest values of difference in the enthalpy of immersion) presented a greater ordering.
Also, in Figure 9, CAN had points close to the two solvents showing less influence of the structure of the molecules of the liquids, which could be related to the lower microporosity and the presence of more oxygenated groups on the surface that interfered with the solid−liquid interaction.
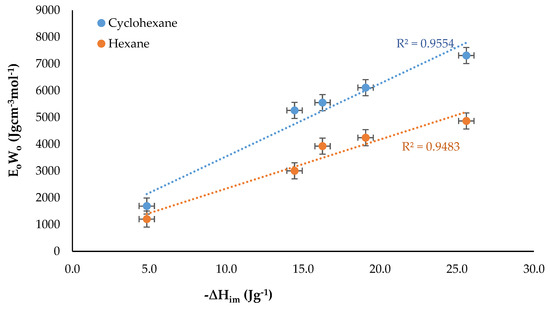
Figure 9.
EoWo obtained from the enthalpy of immersion of activated carbons into the solvents in function of the immersion enthalpy of the solvents.
4. Conclusions
The enthalpy of immersion of five activated carbons with different surface chemistry in cyclohexane showed values between −21.2 and −91.7 J g−1. For the same solids, the enthalpy of immersion in hexane, the values were between −16.4 and −66.1 J g−1.
The difference between the immersion enthalpy of the activated carbons in the two solvents was calculated, with values between −4.80 and −25.6 J g−1, it indicated the contribution of the chemical structure of the two solvents in the solid−liquid interaction.
It was established that the properties of activated carbons with the greatest influence on the solid−liquid interaction (evidenced by immersion enthalpy) were the micropore volume and the content of total basic groups. The average enthalpy per unit of micropore volume was calculated with a value of −487 J g−1.
For the activated carbons with the lower content of oxygenated groups, a linear relationship was established between the EoWo product and the difference between the immersion enthalpy of the solids in the two solvents.
Author Contributions
D.H.-M., L.G. and J.C.M.-P. conceived and designed the experiments; D.H.-M. performed the experiments; D.H.-M., L.G. and J.C.M.-P. analyzed the data; L.G. and J.C.M.-P. contributed reagents, materials and analysis tools; D.H.-M., L.G. and J.C.M.-P. wrote the paper.
Funding
Research programs for Associate Professors, Full Professors, and Emeritus Professors announced by the Faculty of Sciences of the University of the Andes, 20-12-2019-2020, 2019, according to the project “Enthalpy, free energy and adsorption energy of the activated carbon interaction and solutions of emerging organic compounds”.
Acknowledgments
The authors thank the Framework Agreement between the Universidad de los Andes and the Universidad Nacional de Colombia and the act of agreement established between the Chemistry Departments of the two universities. To the Colciencias Scholarship “Doctorados Nacionales 2016” Convocation 757. The authors also appreciate the grant for the funding of research programs for Associate Professors, Full Professors, and Emeritus Professors announced by the Faculty of Sciences of the University of the Andes, 20-12-2019-2020, 2019, according to the project “Enthalpy, free energy and adsorption energy of the activated carbon interaction and solutions of emerging organic compounds”.
Conflicts of Interest
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript or in the decision to publish the results.
References
- Saini, V.K.; Pires, J. Development of metal organic fromwork-199 immobilized zeolite foam for adsorption of common indoor VOCs. J. Environ. Sci. 2017, 55, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Gallego, E.; Roca, F.J.; Perales, J.F.; Guardino, X. Experimental evaluation of VOC removal efficiency of a coconut shell activated carbon filter for indoor air quality enhancement. Build Environ. 2013, 67, 14–25. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Creamer, A.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yu, Q.; Cui, Y.; Xie, F.; Li, W.; Li, Y.; Chen, M. Adsorption properties of activated carbon from reed with a high adsorption capacity. Ecol. Eng. 2017, 102, 443–450. [Google Scholar] [CrossRef]
- Yang, X.; Yi, H.; Tang, X.; Zhao, S.; Yang, Z.; Ma, Y.; Feng, T.; Cui, X. Behaviors and kinetics of toluene adsorption -desorption on activated carbons with varying pore structure. J. Environ. Sci. 2018, 67, 104–114. [Google Scholar] [CrossRef]
- Gil, R.R.; Ruiz, B.; Lozano, M.S.; Martín, M.J.; Fuente, E. VOCs removal by adsorption onto activated carbons from biocollagenic wastes of vegetable tanning. Chem. Eng. J. 2014, 245, 80–88. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Liu, J. Surface modification of coconut shell based activated carbon for the improvement of hydrophobic VOC removal. J. Hazard. Mater. 2011, 192, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, L.; Long, C.; Li, A. Enhanced adsorption and desorption of VOCs vapor on novel micro-mesoporous polymeric adsorbents. J. Colloid Interface Sci. 2014, 428, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.H. Recent developments in the physical adsorption of toxic organic vapours by activated carbons. Adsorpt. Sci. Technol. 2011, 29, 1–28. [Google Scholar] [CrossRef]
- Wang, G.; Dou, B.; Zhang, Z.; Wang, J.; Liu, H.; Hao, Z. Adsorption of benzene, cyclohexane and hexane on ordered mesoporous carbon. J. Environ. Sci. 2015, 30, 65–73. [Google Scholar] [CrossRef]
- Sarigiannis, D.A.; Karakitsios, S.P.; Gotti, A.; Liakos, I.; Katsoyiannis, L.A. Exposure to major volatile organic compounds and carbonyls in European indoor environments and associated health risk. Environ. Int. 2011, 37, 743–765. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Delgadillo, D.P.; Giraldo, L.; Moreno-Piraján, J.C. Preparation and calorimetry characterization of nitrogen-enriched activated carbons and their application in the removal of carbon dioxide. Eur. J. Chem. 2017, 8, 130–136. [Google Scholar] [CrossRef]
- Stoeckli, H.F.; Kraehenbuehl, F.; Ballerini, L.; De Bernardini, S. Recent developments in the Dubinin equation. Carbon 1989, 27, 125–128. [Google Scholar] [CrossRef]
- Bansal, R.C.; Donnet, J.B.; Stoeckli, H.F. Active Carbon; Marcel Dekker: New York, NY, USA, 1988. [Google Scholar]
- Moreno, J.C.; Giraldo, L. Determination of the immersion enthalpy of activated carbon by microcalorimetry of the heat conduction. Instrum. Sci. Technol. 2000, 28, 171–178. [Google Scholar] [CrossRef]
- Stoeckli, H.F. Dubinin’s theory and its contribution to adsorption science. Russ. Chem. B+ 2001, 50, 2265–2272. [Google Scholar] [CrossRef]
- Hernández-Monje, D.; Giraldo, L.; Moreno-Piraján, J.C. Study of Hexane Adsorption on Activated Carbons with Differences in Their Surface Chemistry. Molecules 2018, 23, 476. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F. Adsorption at the liquid–solid interface: Thermodynamics and methodology. In Adsorption by Powders and Porous Solids: Principles, Methodology and Applications; Rouquerol, J., Rouquerol, F., Llewellyn, P., Maurinm, G., Sing, K., Eds.; Academic Press: Kidlington, UK, 2014; pp. 105–158. [Google Scholar]
- Boehm, H.P. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Kim, Y.; Park, C. Titration Method for the Identification of Surface Functional Groups. In Materials Science and Engineering of Carbon Characterization; Inagaki, M., Kang, F., Eds.; Tsinghua University Press Limited: Hamamatsu, Japan; pp. 273–286.
- Vargas, D.P.; Giraldo, L.; Moreno-Piraján, J.C. Calorimetric study of the CO2 adsorption on carbon materials. J. Therm. Anal. Calorim. 2014, 117, 1299–1309. [Google Scholar] [CrossRef]
- Vargas, D.; Giraldo, L.; Moreno-Piraján, J.C. Accessible area and hydrophobicity of activated carbons obtained from the enthalpy characterization. Adsorption 2016, 22, 3–11. [Google Scholar] [CrossRef]
- Moreno, J.C.; Giraldo, L. Instrumentación calorimétrica aplicada a la determinación de entalpias de inmersión de sólidos porosos. In Sólidos Porosos Preparación, Caracterización y Aplicaciones; Moreno, J.C., Ed.; Uniandes: Bogotá, Colombia, 2007; pp. 281–297. [Google Scholar]
- Murillo, Y.S.; Giraldo, L.; Moreno-Piraján, J.C. Contribution enthalpic in the interaction of activated carbon with polar and apolar solvents. Arab. J. Chem. 2013, 6, 347–351. [Google Scholar] [CrossRef]
- Rodríguez, G.; Giraldo, L.; Moreno, J. Entalpías de inmersión de telas de carbón activado como parámetro de caracterización fisicoquímica. Rev. Colomb. Quím. 2009, 38, 32–36. [Google Scholar]
- Moreno-Piraján, J.C.; Giraldo, L. Study of Carbon Foams Synthesized by the Pyrolysis of Wastes Coconut Shells of African Palm at Different Conditions and use of Immersion Calorimetry as a Tool for Characterization. Orient. J. Chem. 2013, 29, 877–887. [Google Scholar] [CrossRef]
- Rodríguez, P.; Giraldo, L.; Moreno, J.C. Modified surface chemistry of activated carbons. Correlation with immersion enthalpy. J. Therm. Anal. Calorim. 2013, 114, 245–251. [Google Scholar] [CrossRef]
- Belhachemi, M.; Addoun, F. Effect of Heat Treatment on the Surface Properties of Activated Carbons. E- J. Chem. 2011, 8, 992–999. [Google Scholar] [CrossRef]
- Wang, R.; Lang, J.; Yan, X. Effect of surface area and heteroatom of porous carbon materials on electrochemical capacitance in aqueous and organic electrolytes. Sci. China Chem. 2014, 57, 1570–1578. [Google Scholar] [CrossRef]
- Yin, C.; Aroua, M.; Daud, W. Review of modifications of activated carbon for enhancing contaminant uptakes from aqueous solutions. Sep. Purif. Technol. 2007, 52, 403–411. [Google Scholar] [CrossRef]
- Mangun, C.L.; Benak, K.R.; Daley, M.A.; Economy, J. Oxidation of Activated Carbon Fibers: Effect on Pore Size, Surface Chemistry, and Adsorption Properties. Chem. Mater. 1999, 11, 3476–3483. [Google Scholar] [CrossRef]
- Jaramillo, J.; Álvarez, P.; Gómez-Serrano, V. Preparation and ozone-surface modification of activated carbon. Thermal stability of oxygen surface groups. Appl. Surf. Sci. 2010, 256, 5232–5236. [Google Scholar] [CrossRef]
- Silvestre-Albero, J.; Silvestre-Albero, A.; Rodríguez-Reinoso, F.; Thommes, M. Physical characterization of activated carbons with narrow microporosity by nitrogen (77.4 K), carbon dioxide (273 K) and argon (87.3 K) adsorption in combination with immersion calorimetry. Carbon 2012, 50, 3128–3133. [Google Scholar] [CrossRef]
- Gokce, Y.; Aktas, Z. Nitric acid modification of activated carbon produced from waste tea and adsorption of methylene blue and phenol. Appl. Surf. Sci. 2014, 313, 352–359. [Google Scholar] [CrossRef]
- Soudani, N.; Souissi-najar, S.; Ouederni, A. Influence of Nitric Acid Concentration on Characteristics of Olive Stone Based Activated Carbon. Chin. J. Chem. Eng. 2013, 21, 1425–1430. [Google Scholar] [CrossRef]
- Pereira, L.; Pereira, R.; Pereira, M.F.R.; van der Zee, F.P.; Cervantes, F.J.; Alves, M.M. Thermal modification of activated carbon surface chemistry improves its capacity as redox mediator for azo dye reduction. J. Hazard. Mater. 2010, 183, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Centeno, T.A.; Stoeckli, F. The assessment of surface areas in porous carbons by two model-independent techniques, the DR equation and DFT. Carbon 2010, 48, 2478–2486. [Google Scholar] [CrossRef]
- Montes-Morán, M.A.; Suárez, D.; Angel-Menéndez, J.; Fuente, E. The Basicity of Carbons. In Novel Carbon Adsorbents; Tascón, J.M.D., Ed.; Elsevier: London, UK, 2012; pp. 173–203. [Google Scholar]
- Kazmierczak-Razna, J.; Nowicki, P.; Wiśniewska, M.; Nosal-Wiercińska, A.; Pietrzak, R. Thermal and physicochemical properties of phosphorus-containing activated carbons obtained from biomass. J. Taiwan Inst. Chem. Eng. 2017, 80, 1006–1013. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).