Abstract
This article presents a regeneration method of a sodium hydroxide (NaOH) solution from a biogas upgrading unit through calcium carbonate (CaCO3) precipitation as a valuable by-product, as an alternative to the elevated energy consumption employed via the physical regeneration process. The purpose of this work was to study the main parameters that may affect NaOH regeneration using an aqueous sodium carbonate (Na2CO3) solution and calcium hydroxide (Ca(OH)2) as reactive agent for regeneration and carbonate slurry production, in order to outperform the regeneration efficiencies reported in earlier works. Moreover, Raman spectroscopy and Scanning Electron Microscopy (SEM) were employed to characterize the solid obtained. The studied parameters were reaction time, reaction temperature, and molar ratio between Ca(OH)2 and Na2CO3. In addition, the influence of small quantities of NaOH at the beginning of the precipitation process was studied. The results indicate that regeneration efficiencies between 53%–97% can be obtained varying the main parameters mentioned above, and also both Raman spectroscopy and SEM images reveal the formation of a carbonate phase in the obtained solid. These results confirmed the technical feasibility of this biogas upgrading process through CaCO3 production.
1. Introduction
Climate change is one of the major problems that has plagued humanity in recent times, consisting of a significant and lasting modification of local and global patterns of climate on the planet. The frequency and intensity of meteorological phenomena such as rainfall, hurricanes, storms, decreasing extent of ice, rising sea level and, above all, the increasing average temperature of the Earth’s atmosphere are the main evidences found by scientists that corroborate climate change [1,2]. According to the Intergovernmental Panel on Climate Change (IPCC) [1], the main origin is the anthropogenic emissions of so-called greenhouse gases (GHG), due to the use of fossil fuels such as coal, oil and natural gas for the production of electricity, transportation or industrial uses, CO2 being the most relevant among the greenhouse gases. For this reason, the use of renewables energies which reduce CO2 emissions could be found as one of the fields most investigated in the last decade [3,4,5,6,7,8,9,10,11].
One of the most promising renewable energy sources is biomass [12]. Biomethane is obtained by upgrading biogas produced from anaerobic digestion of different types of biomass. There are several ways to use this biomethane as an energy resource, which depend on technical, economic and legislative factors of each country [13]. Biomethane is an improved biogas from landfills, farms, sewage treatment plants, agriculture or other sources [14]. For use, biomethane must be submitted to a process called upgrading, which separates the undesired compounds (mainly CO2) and adapts its composition to the standards set by the legislation corresponding to that suitable for a fuel gas [15]. CO2 content in biogas as produced in anaerobic digestion varies between 35%–45% [16,17]. To remove CO2, very diverse techniques have been studied: Pressure Swing Adsorption (PSA) [18,19], Water Scrubbing (WS) [20,21], Organic Physical Scrubbing (OPS) [22,23], Chemical Absorption Scrubbing (CAS) [13,24], Membrane Separation (MS) [25,26] and Cryogenic Separation (CS) [27,28]. CAS is one of the most promising technique [29,30], both with amines (monoethanolamine (MEA) or Piperazine (PZ)) and caustic solvents (NaOH or potassium hydroxide (KOH)). Previous studies have reported high capture yields and selectivity to CO2, achieving similar capture efficiencies from 90% to 99% [31,32,33,34,35]. In the case of amine solvents, a high regeneration efficiency of the solvent can be obtained via physical regeneration, with an acceptable energy consumption [34]. However, compared with the previous solvents explained, when employing caustic solvents, an elevated energy consumption is necessary in order to regenerate the solvent physically, which makes these solvents less usable than the conventional MEA [35]. In this reaction, Na2CO3 or potassium carbonate (K2CO3) is obtained as a consequence of the absorption step.
An alternative path for CO2 utilization that avoids the energy penalty in the regeneration stage of the solvent would be the synthesis and separation of chemicals based on calcium (Ca+) by the precipitation processes into the solvent solution, as for example in Ca(OH)2 or residues with high Ca+ content. This alternative is very attractive from an economic point of view in order to drastically reduce costs of CO2 capture and valorize CO2 as a commercial by-product. An interesting by-product to be taken into account when alkaline hydroxides are used as solvents is CaCO3. CaCO3 can be produced through chemical reaction with Ca(OH)2 and precipitated as a solid [33], according to the next reaction:
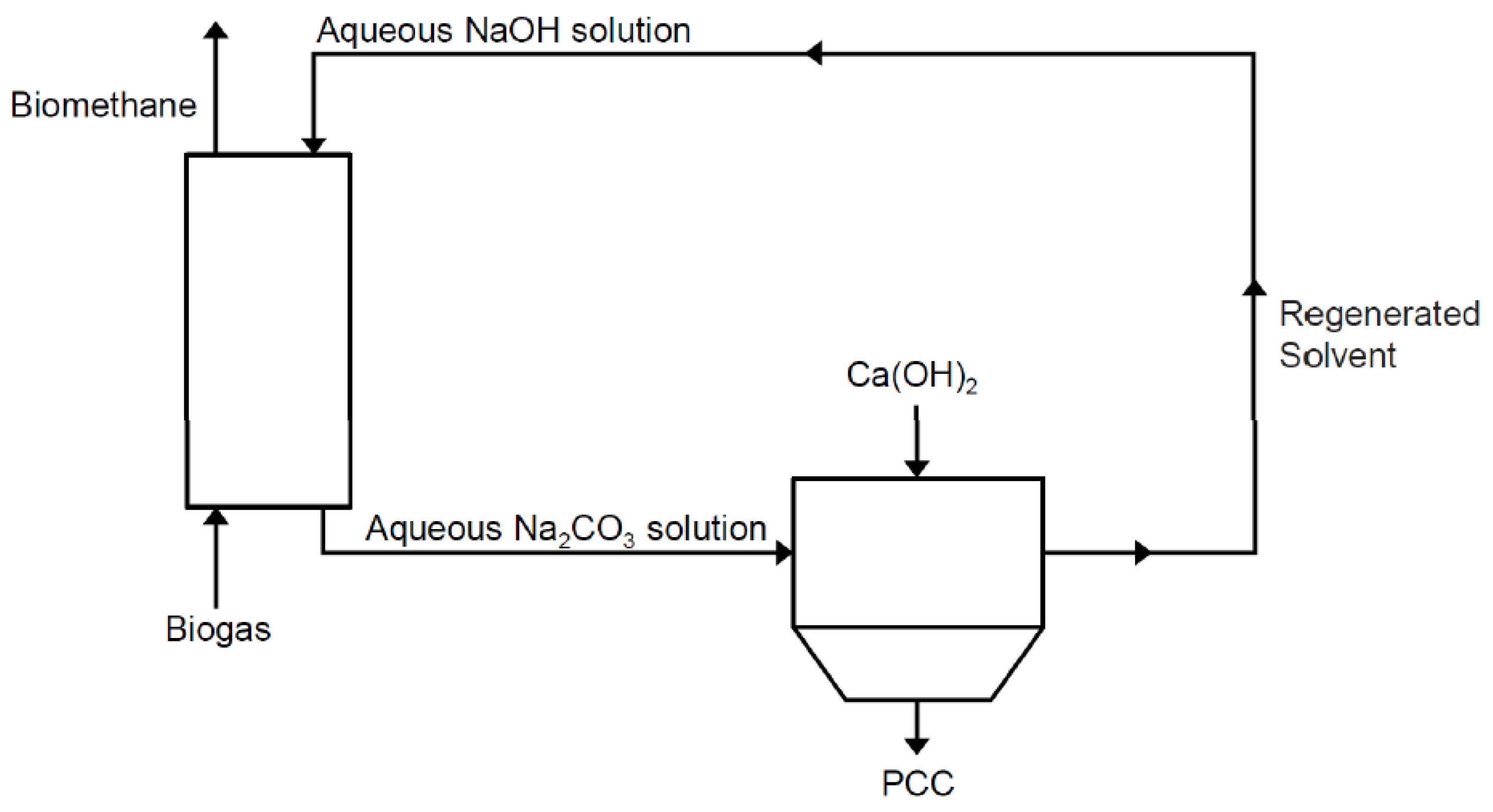
The type of CaCO3 obtained as a by-product is called Precipitated Calcium Carbonate (PCC). PCC is consumed in huge quantities and in variated applications for different industrial sectors, such as a filler for plastic materials, paper, foods, printing ink and medical necessities [36]. This synergy process between biogas upgrading, CO2 capture and PCC production is shown in Figure 1.
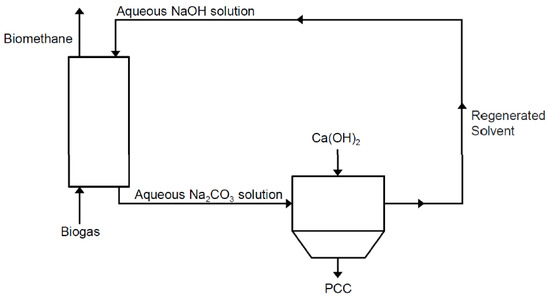
Figure 1.
Biogas upgrading through Precipitated Calcium Carbonate (PCC) production.
This process is much less energy intensive than physical regeneration previously studied by various authors [35,37,38], making the process economically attractive. Many researches focused on the carbonation of residues for storing CO2, as for instance steel slags [39], air pollution control residues [32,33], argon oxygen decarburization slags [23], incineration bottom ash [40] or basic oxygen furnace slags [41]. Baciocchi et al. [32,33] proposed an application of the process above explained using both NaOH and KOH as solvents, and Air Pollution Control residues as Ca+ sources, focusing on the amount of CO2 that could be definitely stored by this residues [32,42]. However, their results showed a non-valuable by-product from a commercial point of view and solvent regeneration efficiencies from 50% to 60% due to the employment of the residues. Therefore, the purpose of this work was to study the main parameters that may affect NaOH regeneration using an aqueous Na2CO3 solution and Ca(OH)2 as a reactive for regeneration and carbonate slurry production, in order to achieve better regeneration efficiencies than previous works and obtaining PCC as a valuable by-product. With this, the foundations for future works are laid, in which valuable by-products would be obtained that would allow to achieve a more economical and sustainable process for carbon capture and utilization.
2. Materials and Methods
2.1. Materials
Chemical compounds used in the experiments (Ca(OH)2, Na2CO3, CaCO3, NaOH) were provided by PanReac-AppliChem (Barcelona, Spain) (pure-grade or pharma-grade, 99% purity).
2.2. Regeneration Experiments
In general, the regeneration experiments were carried out following the methodology exposed below, which will be explained in greater depth later. First, the solutions of the reactants were prepared, at the same time that the instruments needed for the precipitation reaction were tuned. After these steps, the reaction was produced, which, once finished, was filtered and separated quickly for analyzing. The main parameters considered for results were NaOH regeneration efficiency and carbonate phase reached. The three most important variables studied in these experiments were the reaction time, the reaction temperature and the molar ratio between Ca(OH)2 and Na2CO3, which according to previous works may have a considerable effect on the regeneration efficiency of the process [32,33]. The matrix of experiments carried out can be found in Table 1. In order to study how each parameter affects by itself, a standard value was set for each of them, according to the bibliography for similar studies [31,32,33,35], and later they were varied one by one. The standard value for temperature reaction was set at 50 °C, molar ratio at 1.2 mol Ca/Na2CO3 and reaction time at 30 min. Furthermore, the influence of an initial addition of NaOH in the Na2CO3 solution was tested, in order to analyze the effect on NaOH regeneration.

Table 1.
Matrix of the experiments carried out.
Lab scale batch precipitation experiments were carried out in a 600 mL beaker placed in a water bath to control temperature tests. The value of the pH gives some clues of the compounds that may be present in the solution. Therefore, to check that the NaOH regeneration reaction had the desired effect, the pH was measured and checked to be in the range 12–14, which is characteristic for hydroxides solutions [31]. During each whole experiment time, the solutions were stirred by an electromagnetic magnet at a constant speed of 1000 rpm. For temperature and pH measuremsent, a thermometer and a pH-meter by Trison Instrument (BANDELIN electronic GmbH & Co. KG, Berlin, Germany) were employed. Measures were continually carried out and recorded in a data logger. Reproducibility checks were conducted resulting in an overall experimental error of ±2% for the regeneration efficiency calculations. The first steps of the procedures followed were to prepare both Na2CO3 and Ca(OH)2 solutions. In the case of Na2CO3, the aqueous solution was set at 20 g/100 mL according to the basis typical values expected after the absorption step [21,28], while the concentration of the Ca(OH)2 solution was stoichiometrically calculated for each test, as will be explained later. At the beginning of each experiment, a 200 mL distilled water slurry of Ca(OH)2 was poured into the beaker placed. After 15 min, 200 mL of Na2CO3 aqueous solution was added to start the reaction time. At the end of each experiment, the solution was vacuum filtered immediately and 50 mL sample was taken to determine the concentration of NaOH by inductively coupled plasma atomic emission spectroscopy. The solid obtained by filtration was dried at 105 °C to ensure a carbonate phase which was characterized by means of Scanning Electron Microscopy (SEM) and Raman spectroscopy.
Raman measurements of the powders samples were recorded using a Thermo DXR2 spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) equipped with a Leica DMLM microscope (Thermo Fisher Scientific, Waltham, MA, USA). The wavelength of applied excitation line was 532 nm ion laser and 50× objective of 8-mm optical was used to focus the depolarized laser beam on a sport of about 3 µm in diameter.
A JEOL JSM6400 (JEOL Ltd., Tokyo, Japan) operated at 20 KV equipped with energy dispersive X-ray spectroscopy (EDX) and a wavelength dispersive X-ray spectroscopy (WDS) systems was used for the microstructural/chemical characterization (SEM with EDS and WDS).
3. Results
This section reports the experimental results of the different tests carried out. The results of NaOH regeneration efficiency are presented, with reference to every parameter studied. NaOH regeneration efficiency is defined has follow:
As it has been set previously, NaOH regenerated was determined by inductively coupled plasma atomic emission spectroscopy, while the maximum NaOH to be regenerated can be easily stochiometrically calculated from the concentration of the Na2CO3 initial solution.
Then, some Raman spectroscopies of PCC are shown to demonstrate the carbonate phase reached, which are accompanied by some SEM images that contribute to verify the results predicted by Raman.
3.1. NaOH Regeneration
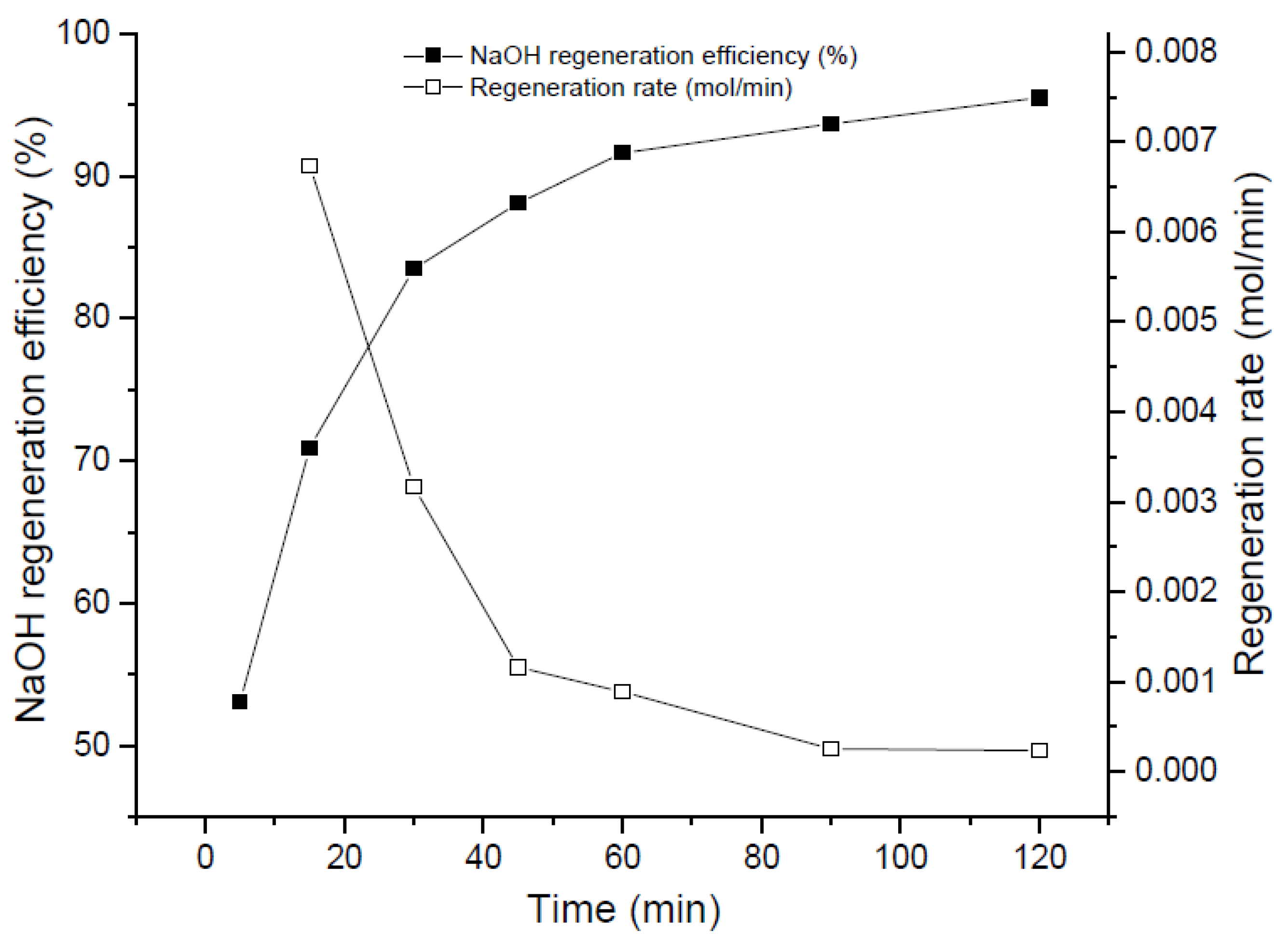
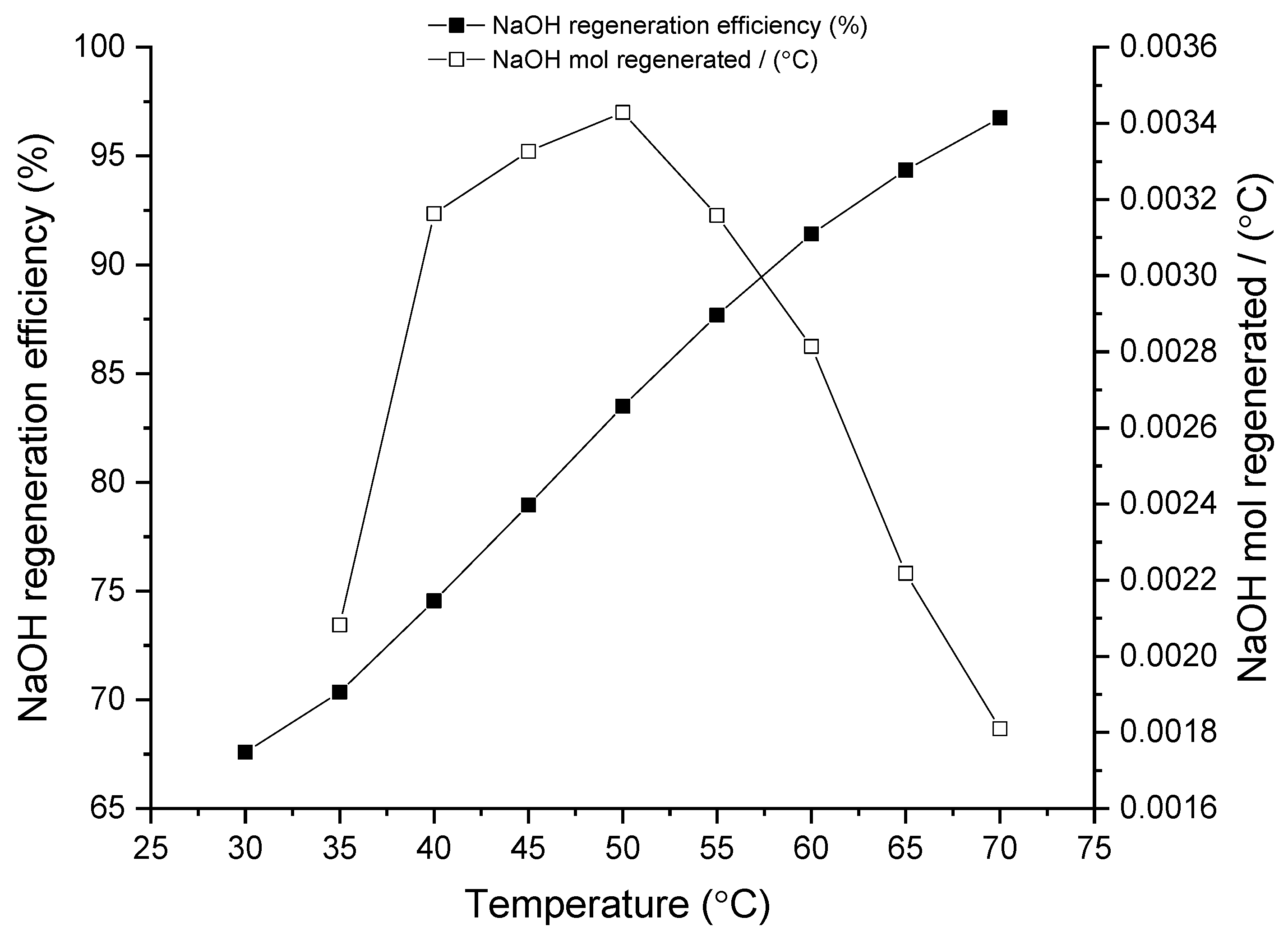
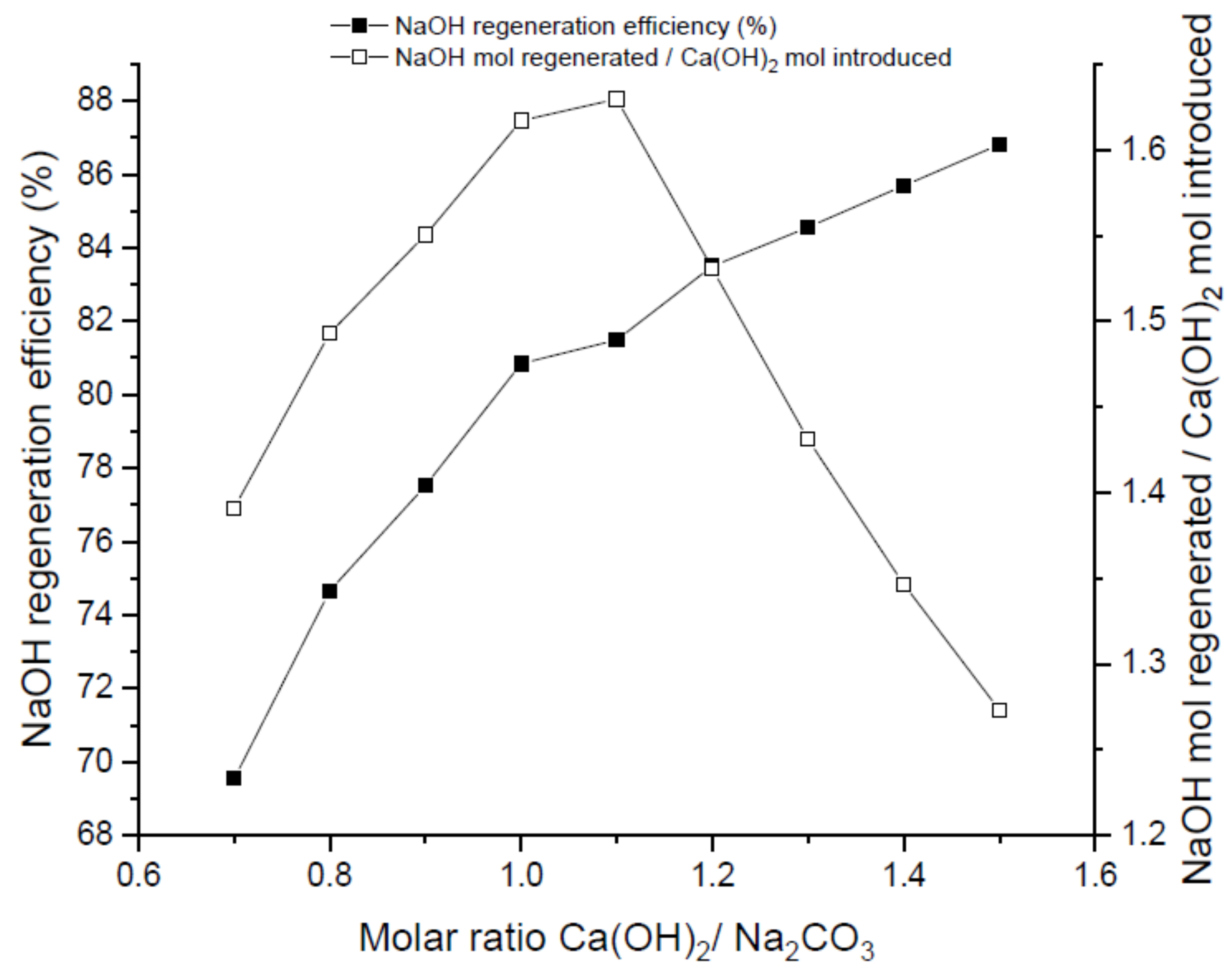
Figure 2, Figure 3 and Figure 4 show the regeneration efficiency curves of the filtered solutions resulting from regeneration experiments carried out with Ca(OH)2 at different reaction times, temperatures and molar ratios, respectively.
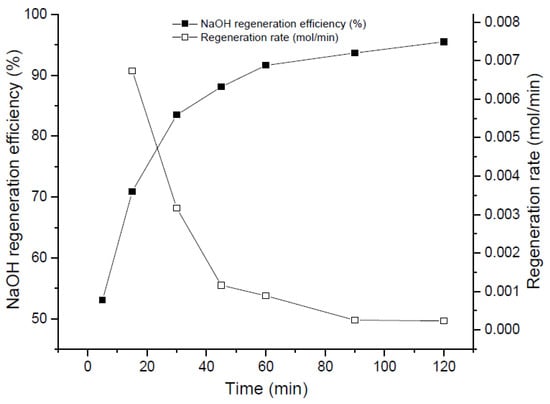
Figure 2.
Evolution of NaOH regeneration with time at fixed Temperature 50 °C and R = 1.2.
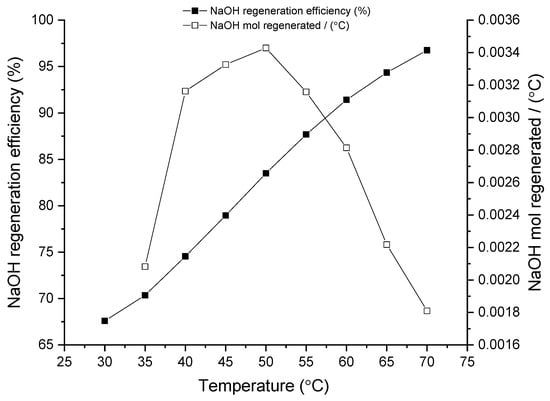
Figure 3.
Influence of temperature on NaOH regeneration. Experiments carried out at R = 1.2 and t = 30 min.
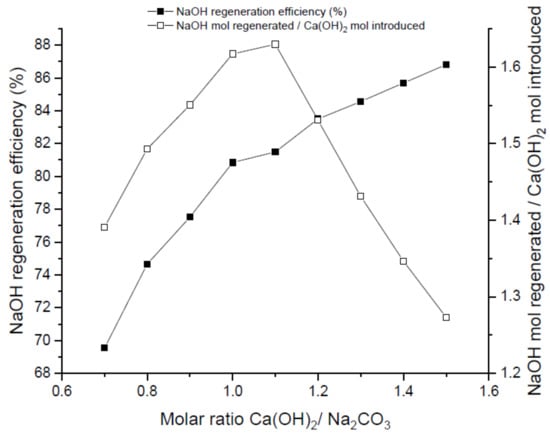
Figure 4.
Influence of molar ratio (R) in the regeneration experiments. Runs conducted at t = 30 min and 50 °C.
3.1.1. Reaction Time Effect
Figure 2 shows the effect of the reaction time in the regeneration phenomenon. As depicted in the plot, NaOH regeneration efficiency varies from 53% to 83% approximately from 5 min to 30 min of reaction time, and later, the slope of the curve changes drastically, passing through 91% regeneration efficiency at 60 min, until achieving a 95% of NaOH regeneration at 120 min. This means, in fact, that in a hypothetical real reactor, a duplication of its volume will be necessary to achieve an increase of 4% approximately (from 30 min to 60 min). As can be seen, from 60 min to 120 min, less than 0.001 mol NaOH is regenerated per minute. Thus, to operate at 120 min residence time is not worthy from a plant design point of view. The intersection of the two curves (regeneration rate and regeneration efficiency) indicates an interesting and very likely optimum operational point where a fair balance between both tendencies can be reached.
3.1.2. Temperature Influence
As for the temperature influence, Figure 3 shows the effect of different temperatures in the regeneration studies. Temperature effect reflects a linear trend showcasing a direct correlation between NaOH regeneration efficiency and process temperature. Indeed, in the best case scenario (at 70 °C) it can reach 97% of NaOH regeneration efficiency. Normalizing the regeneration capacity by the incremental temperature (empty symbols in the Figure) maximum is obtained at around 50 °C which somehow indicates that the increment in temperature has a stronger impact on the regeneration efficiency in the low-medium temperature range. This is an important result to be highlighted from an energy consumption perspective, as a temperature of 50 °C could be easily achieved through low-cost and/or renewable energy sources such as solar.
3.1.3. Molar Ratio Influence
Molar ratio inlet carbonate/precipitant agent is another important parameter to consider in the regeneration process. As can be seen in Figure 4, NaOH regeneration efficiency is favored by an increase of the molar ratio. Nevertheless, this increase of molar ratio promotes a higher quantity of Ca2+ ions that should be removed before recirculating the absorbent to the absorption tower in order to prevent accumulation of Ca(OH)2 which eventually may lead to fouling phenomenon in the tower. In parallel, as can be observed in Figure 4, NaOH mol regeneration per mol of Ca(OH)2 introduced decreases upon increasing the molar ratio above a threshold value of R = 1.1. This value set an optimum operational point beyond which no further benefits are envisaged from the process point of view.
3.1.4. Effect of NaOH Spark in the Regeneration Efficiency
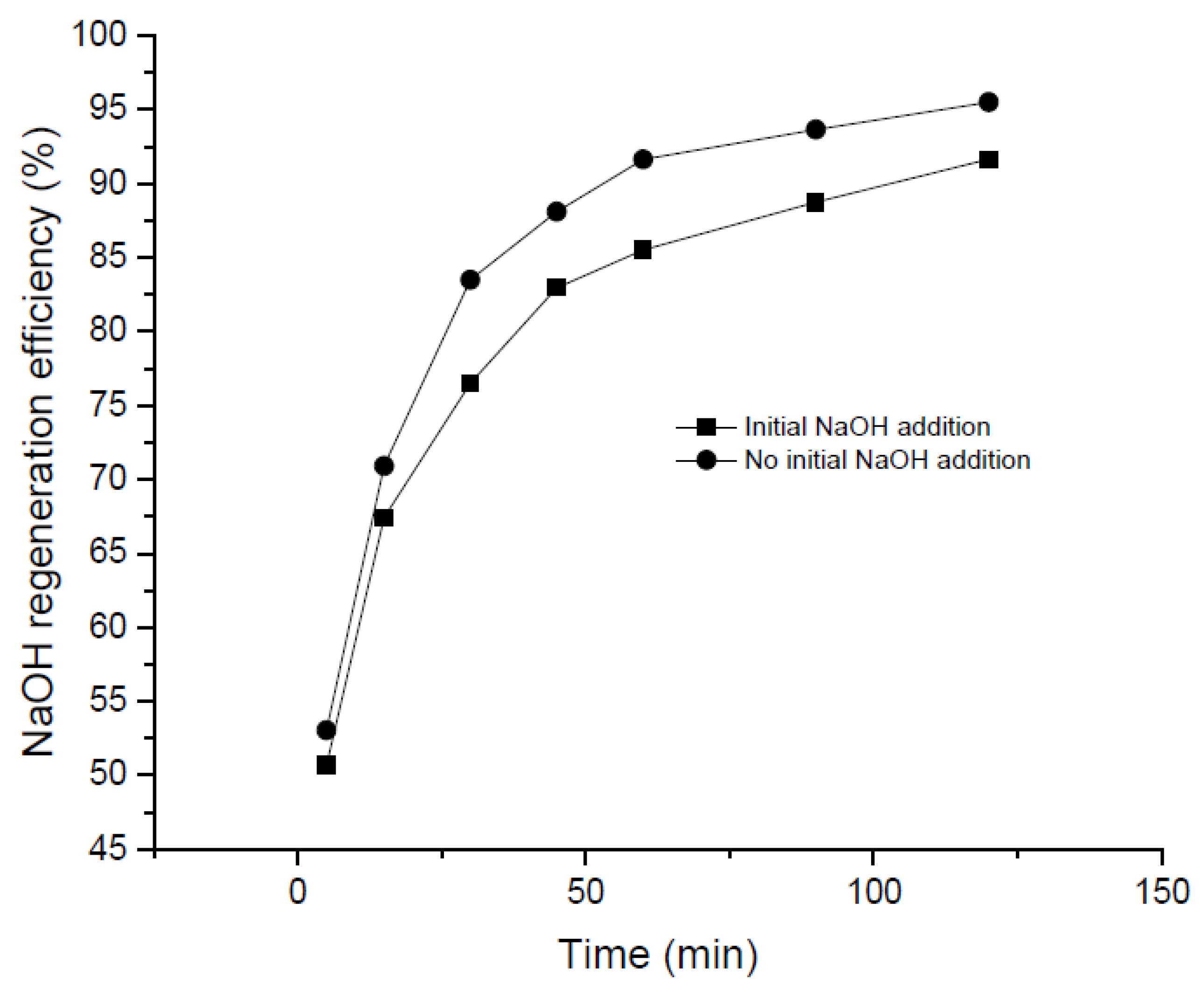
The addition of small quantities of NaOH at the beginning of the precipitation process may promote the recovery of NaOH (initially entering the precipitation reactor in the form of Na2CO3). Also, it should be taken into account that, in a real industrial plant, 100% conversion from NaOH to Na2CO3 would hardly be reached in the absorption stage. In this sense, small amounts of NaOH as “sparking species” were added to investigate its effect on the process. The impact exerted by the addition of an initial concentration of NaOH (1 M) in the Na2CO3 solution in terms of NaOH regeneration efficiency is reported in Figure 5. Analyzing this Figure, it may be noted that the NaOH regeneration was slower than the results obtained without an initial NaOH concentration. It seems that the presence of alkaline compounds do not benefit the regeneration process—a fact that can be related to the poorer solubility of Ca(OH)2 due to the ion common effect as previously observed elsewhere [32,36,43].
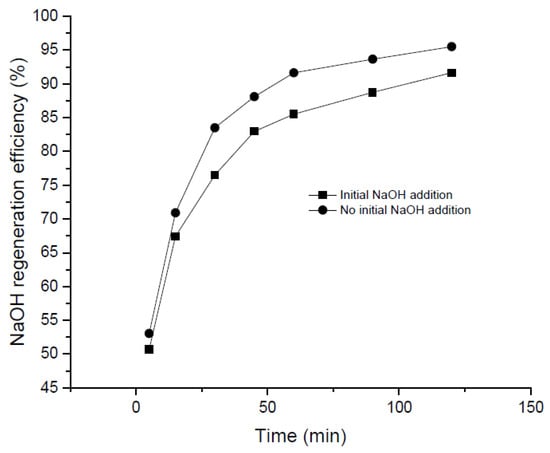
Figure 5.
Comparison curves of NaOH regeneration efficiency with and without initial addition of NaOH.
3.2. Physicochemical Characterization of the PCC
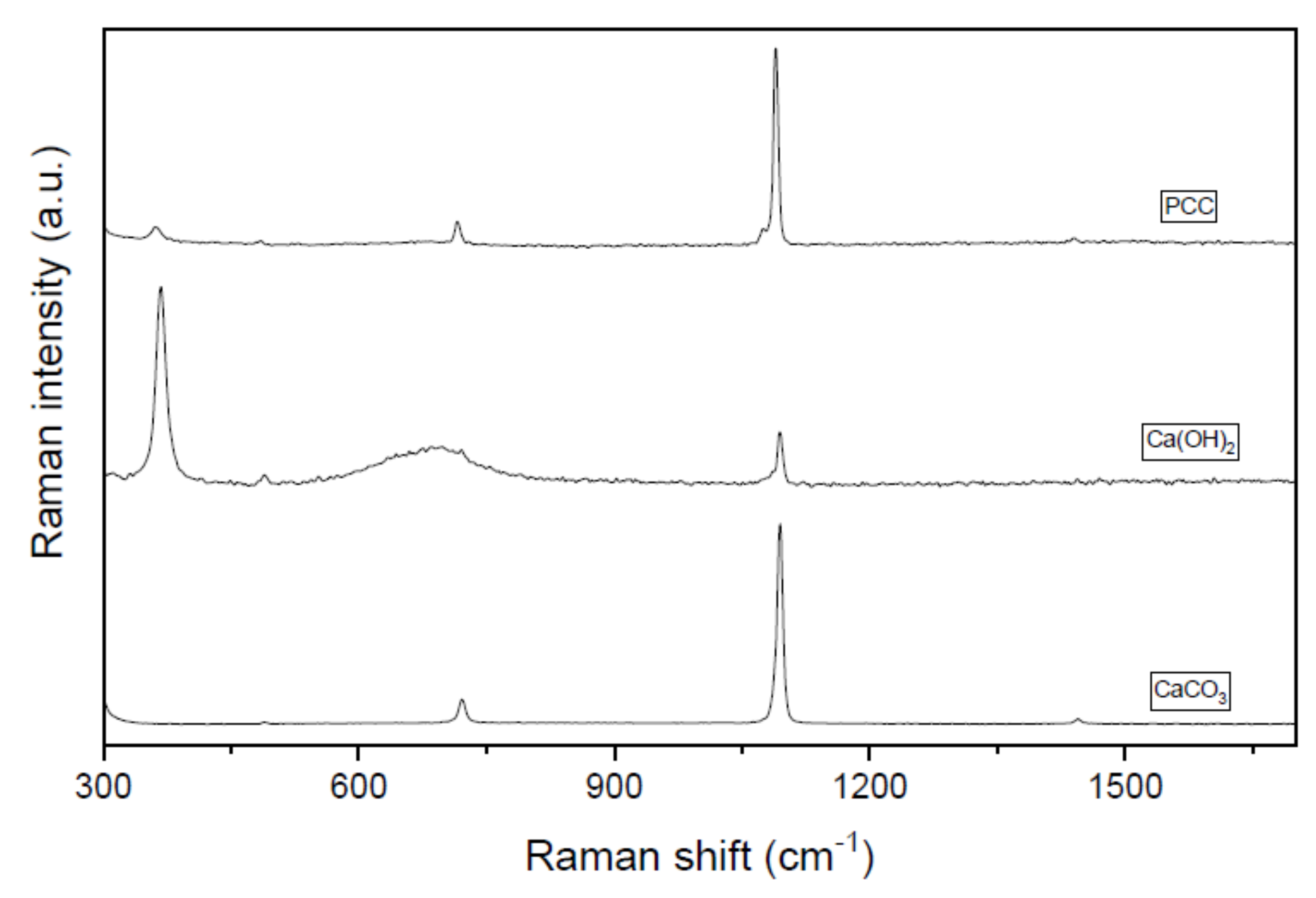
Aiming to determine the purity of the carbonates obtained during the recovery process, a combined Raman-SEM study was conducted on selected samples. Figure 6 shows the Raman spectra of the recovered carbonated after 30 min of reaction at 50 °C using a R = 1.2 in comparison with standards samples of pure CaCO3 and pure Ca(OH)2. CaCO3 typically presents a monoclinic structure belonging to the P21/c group [44]. The main characteristic band of CaCO3 polymorphs is a strong and narrow feature which appears at around 1100 cm−1. Also, another band ca. 700 cm−1 is typically ascribed to this type of structure [44]. As can be seen, these two peaks are presented in PCC spectra, confirming the successful precipitation process. In fact, the spectrum of our PCC sample resembles that of the CaCO3 standard as shown in Figure 6. Nevertheless, it must be highlighted that a certain amount of Ca(OH)2 remains present in our solid sample as intended by the Raman vibration mode at ca. 400 cm−1 which matches well with the most intense band on the Ca(OH)2 standard. In fact, these data correlate well with the regeneration efficiency data discussed above where 100% regeneration is never reached. In this sense, Raman experiments indicate that despite the fact that the regeneration process is highly effective, there is still some room for further improvements.
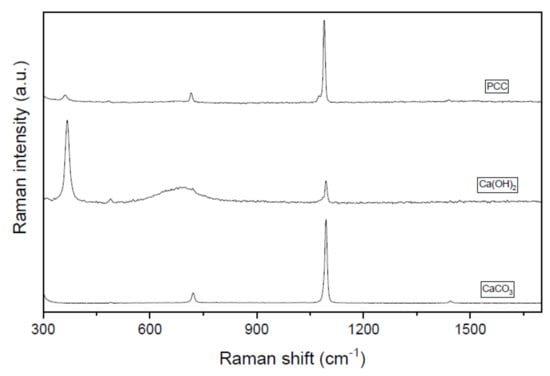
Figure 6.
Raman spectra of the PCC obtained (time = 30 min, T = 50 °C, R = 1.2) and the Ca(OH)2 and CaCO3 standards.
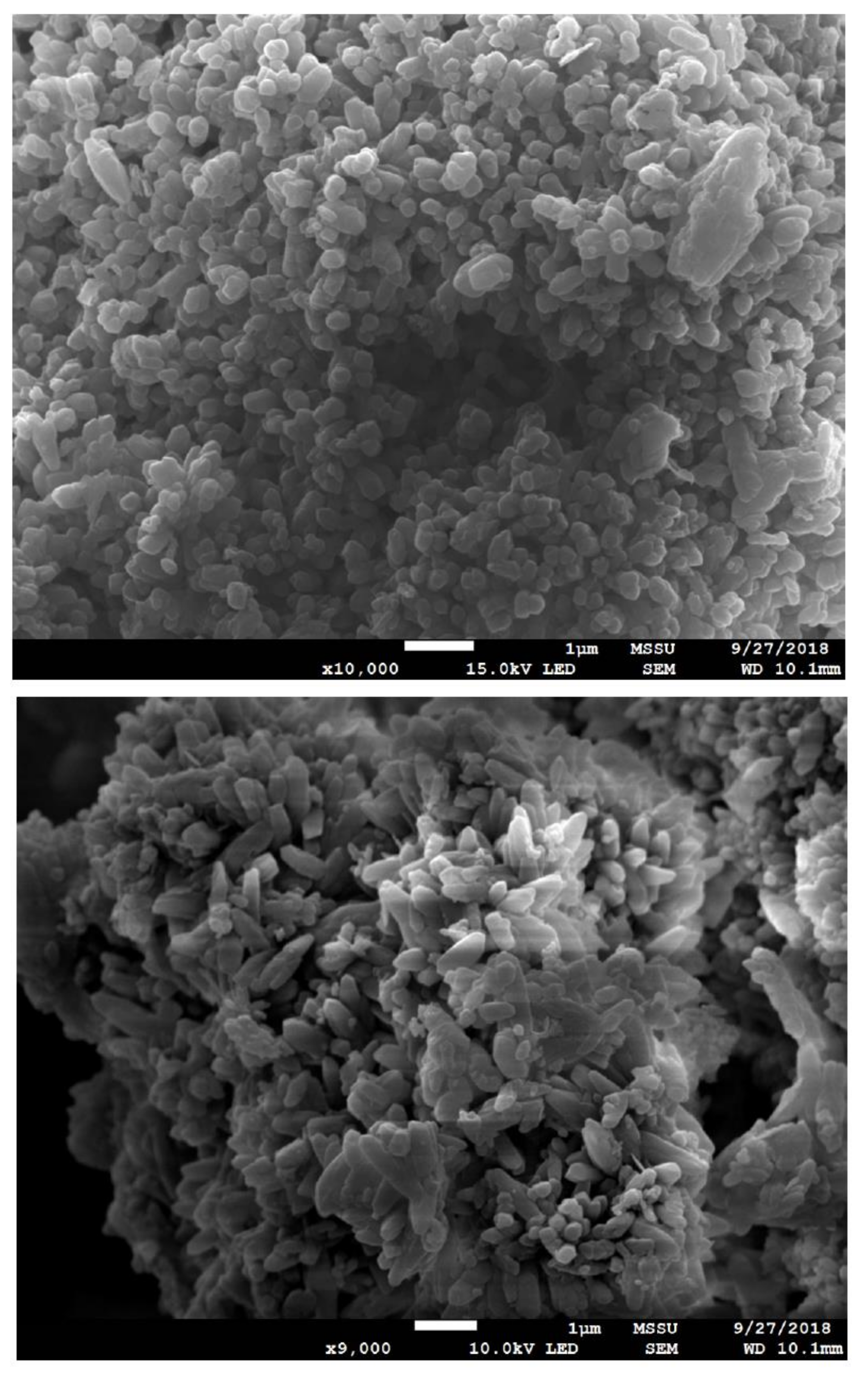
Scanning Electron Microscopy images are useful to gain further insights on the samples structures. Selected SEM images of different sections of the sample studied by Raman are presented in Figure 7. SEM images again confirmed the presence of CaCO3 with the typical morphology of calcite as previously observed by Altiner et al. [45]. In the case of SEM, it is hard to distinguish between CaCO3 and Ca(OH)2—especially when the amount of Ca(OH)2 is just a minor contribution in the overall sample composition. In general terms, our SEM study confirms that the successful carbonate precipitation is in good agreement with the regeneration efficiency studies and the Raman results.
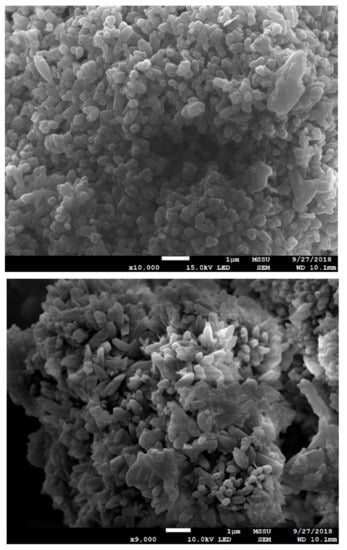
Figure 7.
SEM images of the PCC obtained (time = 30 min, T = 50 °C, R = 1.2).
4. Conclusions
The results obtained from this lab scale work have confirmed the technical feasibility of this biogas upgrading process through PCC production. In general, the majority of the tests have shown better regeneration efficiencies than previous studies identified in the first section of this work (53%–97% vs. 50%–60%) [32,33]. The multiple reaction parameters have a different impact on the overall process performance. For instance, it was identified that the ideal reaction time would be around 30–60 min for T = 50 °C and R = 1.2, leading to compact reactor units. As for the temperature effect, a maximum NaOH mol regenerated per grade is reached at 50 °C for t = 30 min and R = 1.2; this would be an advisable value for a real process since it could be reached easily through the employment of a renewable energy source. The molar ratio Ca(OH)2/Na2CO3 also influences the process, 1.1 being an ideal ratio to be implemented for realistic operations, for t = 30 min and T = 50 °C. This result has been chosen taking into account the maximum in the curve of NaOH mol regeneration per mol of Ca(OH)2. The presence of small quantities of NaOH do not benefit the regeneration process and in fact it produces a decrease in the regeneration efficiency due to the ion common effect; this would suggest an effort to get the maximum percentage of NaOH conversion to Na2CO3 in the absorption stage.
Raman and SEM studies confirm the large majority presence of CaCO3 on the recovered material. Interestingly, although the obtained solid is mainly composed by calcite type CaCO3, some traces of Ca(OH)2 are still present. This opens some room for further research to improve the regeneration process.
Author Contributions
Conceptualization, F.M.B.-M., L.F.V. and B.N.; Methodology, F.M.B.-M., M.R.-G., F.V. and T.R.R.; Data curation, F.M.B.-M. and T.R.R.; Writing—original draft preparation, F.M.B.-M., T.R.R., M.R.-G., B.N.; Writing—review and editing, F.M.B.-M., T.R.R., M.R.-G., B.N.; Supervision, T.R.R., M.R.-G., B.N.; Funding acquisition, B.N., L.F.V. and T.R.R.
Funding
This work was supported by University of Seville through its V PPIT-US. Financial support for this work was also provided by the EPSRC grant EP/R512904/1 as well as the Royal Society Research Grant RSGR1180353. This work was also partially sponsored by the CO2Chem UK through the EPSRC grant EP/P026435/1.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IPCC. Global Warming of 1.5 °C. Summary for Policymakers; Intergovernamental Panel on Climate Change: Incheon, Korea, 2018. [Google Scholar]
- International Energy Agency. World Energy Outlook Special Report: Energy and Climate Change; International Energy Agency: Paris, France, 2015. [Google Scholar]
- Decardi-Nelson, B.; Liu, S.; Liu, J. Improving Flexibility and Energy Efficiency of Post-Combustion CO2 Capture Plants Using Economic Model Predictive Control. Processes 2018, 6, 135. [Google Scholar] [CrossRef]
- Tollkötter, A.; Kockmann, N. Absorption and Chemisorption of Small Levitated Single Bubbles in Aqueous Solutions. Processes 2014, 2, 200–215. [Google Scholar] [CrossRef]
- Adams, T.A.; Hoseinzade, L.; Madabhushi, P.B.; Okeke, I.J. Comparison of CO2 Capture Approaches for Fossil-Based Power Generation: Review and Meta-Study. Processes 2017, 5, 44. [Google Scholar] [CrossRef]
- Taimoor, A.A.; Al-shahrani, S.; Muhammad, A. Ionic Liquid (1-Butyl-3-Metylimidazolium Methane Sulphonate) Corrosion and Energy Analysis for High Pressure CO2 Absorption Process. Processes 2018, 5, 45. [Google Scholar] [CrossRef]
- Li, J.; Ahmed, R.; Li, X. Thermodynamic Modeling of CO2-N2-O2-Brine-Carbonates in Conditions from Surface to High Temperature and Pressure. Energies 2018, 11, 2627. [Google Scholar] [CrossRef]
- Hu, J.; Galvita, V.; Poelman, H.; Marin, G. Advanced Chemical Looping Materials for CO2 Utilization: A Review. Materials 2018, 11, 1187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yan, Y.; Zhang, L.; Chen, Y.; Ju, S. CFD investigation of CO2 capture by methyldiethanolamine and 2-(1-piperazinyl)-ethylamine in membranes: Part B. Effect of membrane properties. J. Nat. Gas Sci. Eng. 2014, 19, 311–316. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, J.; Chen, F.; Li, H.; Zhang, W.; Qi, W. Progress in enhancement of CO2 absorption by nanofluids: A mini review of mechanisms and current status. Renew. Energy 2018, 118, 527–535. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, H.; Chang, H.; Pan, Z.; Luo, X. Machine learning predictive framework for CO2 thermodynamic properties in solution. J. CO2 Util. 2018, 26, 152–159. [Google Scholar] [CrossRef]
- Patel, D.; Kellici, S.; Saha, B. Green Process Engineering as the Key to Future Processes. Processes 2014, 10, 311–332. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Zhang, W.; Wang, J.; Soltaninan, M.R.; Olabie, A.G. Effectiveness of amino acid salt solutions in capturing CO2: A review. Renew. Sustain. Energy Rev. 2018, 98, 179–188. [Google Scholar] [CrossRef]
- Wheeler, P.; Holm-Nielsen, J.B.; Jaatinen, T.; Wellinger, A.; Lindberg, A.; Pettigrew, A. Biogas Upgrading and Utilisation; IEA Bioenergy: Paris, France, 1999; pp. 3–20. [Google Scholar]
- Petersson, A.; Wellinger, A. Biogas Upgrading Technologies—Developments and Innovations; IEA Bioenergy: Paris, France, 2009; Volume 20. [Google Scholar] [CrossRef]
- Zhou, K.; Chaemchuen, S.; Verpoort, F. Alternative materials in technologies for Biogas upgrading via CO2 capture. Renew. Sustain. Energy Rev. 2017, 79, 1414–1441. [Google Scholar] [CrossRef]
- Kadam, R.; Panwar, N.L. Recent advancement in biogas enrichment and its applications. Renew. Sustain. Energy Rev. 2017, 73, 892–903. [Google Scholar] [CrossRef]
- Alonso-Vicario, A.; Ochoa-Gómez, J.R.; Gil-Río, S.; Gómez-Jiménez-Aberasturi, O.; Ramírez-López, C.A.; Torrecilla-Soria, J.; Domínguez, A. Purification and upgrading of biogas by pressure swing adsorption on synthetic and natural zeolites. Microporous Mesoporous Mater. 2010, 134, 100–107. [Google Scholar] [CrossRef]
- Kim, Y.J.; Nam, Y.S.; Kang, Y.T. Study on a numerical model and PSA (pressure swing adsorption) process experiment for CH4/CO2 separation from biogas. Energy 2015, 91, 732–741. [Google Scholar] [CrossRef]
- Nie, H.; Jiang, H.; Chong, D.; Wu, Q.; Xu, C.; Zhou, H. Comparison of water scrubbing and propylene carbonate absorption for biogas upgrading process. Energy Fuels 2013, 27, 3239–3245. [Google Scholar] [CrossRef]
- Budzianowski, W.M.; Wylock, C.E.; Marciniak, P.A. Power requirements of biogas upgrading by water scrubbing and biomethane compression: Comparative analysis of various plant configurations. Energy Convers. Manag. 2017, 141, 2–19. [Google Scholar] [CrossRef]
- Niesner, J.; Jecha, D.; Stehlík, P. Biogas upgrading technologies: State of art review in European region. Chem. Eng. Trans. 2013, 35, 517–522. [Google Scholar] [CrossRef]
- Ozturk, B.; Demirciyeva, F. Comparison of biogas upgrading performances of different mixed matrix membranes. Chem. Eng. J. 2013, 222, 209–217. [Google Scholar] [CrossRef]
- Cousins, A.; Wardhaugh, L.T.; Feron, P.H.M. A survey of process flow sheet modifications for energy efficient CO2 capture from flue gases using chemical absorption. Int. J. Greenh. Gas Control Int. J. 2011, 5, 605–619. [Google Scholar] [CrossRef]
- Chen, X.Y.; Vinh-Thang, H.; Ramirez, A.A.; Rodrigue, D.; Kaliaguine, S. Membrane gas separation technologies for biogas upgrading. RSC Adv. 2015, 5, 24399–24448. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, Y.; Zhang, L.; Chen, Y.; Ran, J.; Pu, G.; Qin, C. Theoretical Study on CO2 Absorption from Biogas by Membrane Contactors: Effect of Operating Parameters. Ind. Eng. Chem. Res. 2014, 53, 14075–14083. [Google Scholar] [CrossRef]
- Tuinier, M.J.; Van Sint Annaland, M. Biogas purification using cryogenic packed-bed technology. Ind. Eng. Chem. Res. 2012, 51. [Google Scholar] [CrossRef]
- Chiesa, P.; Campanari, S.; Manzolini, G. CO2 cryogenic separation from combined cycles integrated with molten carbonate fuel cells. Int. J. Hydrogen Energy 2011, 36, 10355–10365. [Google Scholar] [CrossRef]
- Ryckebosch, E.; Drouillon, M.; Vervaeren, H. Techniques for transformation of biogas to biomethane. Biomass Bioenergy 2011, 35, 1633–1645. [Google Scholar] [CrossRef]
- Tippayawong, N.; Thanompongchart, P. Biogas quality upgrade by simultaneous removal of CO2 and H2S in a packed column reactor. Energy 2010, 35, 4531–4535. [Google Scholar] [CrossRef]
- Baciocchi, R.; Costa, G.; Gavasci, R.; Lombardi, L.; Zingaretti, D. Regeneration of a spent alkaline solution from a biogas upgrading unit by carbonation of APC residues. Chem. Eng. J. 2012, 179, 63–71. [Google Scholar] [CrossRef]
- Baciocchi, R.; Corti, A.; Costa, G.; Lombardi, L.; Zingaretti, D. Storage of carbon dioxide captured in a pilot-scale biogas upgrading plant by accelerated carbonation of industrial residues. Energy Procedia 2011, 4, 4985–4992. [Google Scholar] [CrossRef]
- Baciocchi, R.; Carnevale, E.; Costa, G.; Gavasci, R.; Lombardi, L.; Olivieri, T.; Zanchi, L.; Zingaretti, D. Performance of a biogas upgrading process based on alkali absorption with regeneration using air pollution control residues. Waste Manag. 2013, 33, 2694–2705. [Google Scholar] [CrossRef] [PubMed]
- Vega, F.; Cano, M.; Gallego, M.; Camino, S.; Camino, J.A.; Navarrete, B. Evaluation of MEA 5 M performance at different CO2 concentrations of flue gas tested at a CO2 capture lab-scale plant. Energy Procedia 2017, 114, 6222–6228. [Google Scholar] [CrossRef]
- Leonzio, G. Upgrading of biogas to bio-methane with chemical absorption process: Simulation and environmental impact. J. Clean. Prod. 2016, 131, 364–375. [Google Scholar] [CrossRef]
- Ahn, J.W.; Kim, J.H.; Park, H.S.; Kim, J.A.; Han, C.; Kim, H. Synthesis of single phase aragonite precipitated calcium carbonate in Ca(OH)2-Na2CO3-NaOH reaction system. Korean J. Chem. Eng. 2005, 22, 852–856. [Google Scholar] [CrossRef]
- Yeh, J.; Pennline, H.; Resnik, K. Study of CO2 absorption and desorption in a packed column. Energy Fuels 2001, 15, 272–278. [Google Scholar] [CrossRef]
- Rao, A.B.; Rubin, E.S. A Technical, Economic, and Environmental Assessment of Amine-Based CO2 Capture Technology for Power Plant Greenhouse Gas Control. Environ. Sci. Technol. 2002, 36, 4467–4475. [Google Scholar] [CrossRef] [PubMed]
- Librandi, P.; Costa, G.; De Souza, A.C.B.; Stendardo, S.; Luna, A.S.; Baciocchi, R. Carbonation of Steel Slag: Testing of the Wet Route in a Pilot-scale Reactor. Energy Procedia 2017, 114, 5381–5392. [Google Scholar] [CrossRef]
- Morone, M.; Costa, G.; Polettini, A.; Pomi, R.; Baciocchi, R. Valorization of steel slag by a combined carbonation and granulation treatment. Miner. Eng. 2014, 59, 82–90. [Google Scholar] [CrossRef]
- Santos, R.M.; Knops, P.C.M.; Rijnsburger, K.L.; Chiang, Y.W. CO2 Energy Reactor—Integrated Mineral Carbonation: Perspectives on Lab-Scale Investigation and Products Valorization. Front. Energy Res. 2016. [Google Scholar] [CrossRef]
- Baciocchi, R.; Carnevale, E.; Corti, A.; Costa, G.; Lombardi, L.; Olivieri, T.; Zanchi, L.; Zingaretti, D. Innovative process for biogas upgrading with CO2 storage: Results from pilot plant operation. Biomass Bioenergy 2013, 53, 128–137. [Google Scholar] [CrossRef]
- Konno, H.; Nanri, Y.; Kitamura, M. Crystallization of aragonite in the causticizing reaction. Powder Technol. 2002, 123, 33–39. [Google Scholar] [CrossRef]
- Dandeu, A.; Humbert, B.; Carteret, C.; Muhr, H.; Plasari, E.; Bossoutrot, J.M. Raman spectroscopy—A powerful tool for the quantitative determination of the composition of polymorph mixtures: Application to CaCO3 polymorph mixtures. Chem. Eng. Technol. 2006, 29, 221–225. [Google Scholar] [CrossRef]
- Altiner, M.; Yildirim, M. Production of precipitated calcium carbonate particles with different morphologies from dolomite ore in the presence of various hydroxide additives. Physicochem. Probl. Miner. Process 2017, 53, 413–426. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).