Abstract
Petrochemicals currently represent the predominant global source of energy and consumer products, including the starting materials used in the platform chemical, plastic polymer, and pharmaceutical industries. However, in recent years, the world’s approaches have shifted towards green chemistry and bio-based chemical production in an effort to reduce CO2 emissions and mitigate climate change. Over the past few decades, researchers have discovered that marine metabolites, primarily sourced from invertebrates, can be utilized to create sustainable and renewable chemicals. This review highlights the significance of advancing marine microorganism-based biotechnology and biochemistry in developing effective conversion systems to enhance the biological production of key platform chemicals, including those utilized as biomaterials and for energy. A background in marine metabolite biochemistry lays the groundwork for potential strategies to mitigate dependence on petroleum for consumer products. This is followed by a discussion of petroleum product replacement technologies, green chemistry alternatives, and CO2 mitigation efforts for the production of sustainable and renewable key platform chemicals.
1. Introduction
Petroleum-derived starting materials and platform chemicals offer valuable opportunities for innovation across multiple industries, ranging from pharmaceuticals to the food and beverage sector. By exploring their biochemical applications, we can harness their potential to develop more effective products and solutions that meet our evolving needs. Emphasizing sustainable practices in their use and production can further contribute to a healthier, more responsible future. Petroleum resources are finite and a major contributor to climate change; their depletion results in a surge in prices for energy, materials, and medicines worldwide. The dependency on such petrochemical-based products is one of the biggest challenges facing modern economies and the climate [1,2,3]. Therefore, the focus has shifted to “Green” starting materials sourced from bio-based, renewable sources that are non-toxic, carbon-neutral, biocompatible, and biodegradable [4,5]. Using materials derived from regenerative and sustainable terrestrial and marine sources may help ease environmental pollution and reduce dependence on fossil-based petroleum resources [6]. It has been found that, under favorable market conditions, producing chemicals from renewable resources could reach 113 million tons by 2050, accounting for 38% of the total organic chemical production [7].
Extensive research has revealed that marine metabolites (e.g., monoterpenes, carbohydrates, fatty acids, etc.) are promising candidates for use in various modern sectors. These include both biologically related medical sectors and non-biologically related industrial/biotechnological sectors. Given the unsustainable nature of petroleum-based resources, these marine metabolites could be the key to a more sustainable future [8].
Living systems comprise a complex network of metabolic reactions involving various enzymes and cells, enabling them to function as effective biocatalysts for chemical processes. Protein and cell biocatalysts offer selectivity, controlled reaction sequences, and the capacity to operate under environmentally friendly conditions, facilitating the efficient production of molecules and reducing costs while minimizing environmental impact. Biocatalysis enables the synthesis of chemical structures that may be difficult to achieve with traditional methods. Metabolic reactions produce fine chemicals, including pharmaceuticals and food additives, primarily from basic carbon sources such as glucose and carbon dioxide [9]. Although many of these compounds occur naturally, their commercial availability is often limited due to purification challenges and costs. Bio-based fine chemical synthesis addresses these limitations by sourcing essential components from low-cost materials [10]. The co-production of multiple chemicals from common carbon sources is economically advantageous. Conditions that promote rapid cell growth and efficient extraction methods from microorganisms enhance cost-effectiveness. Bio-production generally occurs at lower temperatures than traditional syntheses, contributing to cost reduction and environmental sustainability. Progress in engineering microorganisms has enabled the production of fuels, bulk chemicals, and valuable medications from inexpensive raw materials. Enzymes within cells can catalyze reactions in a single step, thereby optimizing the production of a wide array of molecules [11,12,13].
2. Petroleum-Derived Platform Chemicals and Biobased Production
In general, common platform chemicals (Figure 1) are derived from crude oil fractions (obtained from the refineries). They are used as precursors or basis materials for preparing chemical intermediates, building blocks, and polymers. A basic starting material has chemical moieties that act as linkages during polymerization or chemical reactions. The distillation of fossil fuel oil results in natural gas, aromatic compounds, naphtha, methane, ethane, propane, butane, heptane, cyclopentane, and cyclohexane. For example, ethylene has a carbon-carbon double bond that serves as a pi bond to form sigma-bond linkages with an adjacent ethylene molecule during the polymerization stage of polyethylene (PE) production. Building block compounds containing carbon-to-carbon double bonds (e.g., ethylene, isobutylene, acrylonitrile, vinyl chloride, styrene, methyl methacrylate, vinyl acetate, and isoprene) are typically used for addition polymerization [14]. Some platform chemicals, such as monocarboxylic acids (e.g., acetic and propionic acids) and monols (e.g., ethanol, propanol, n-/iso-butanol, and pentanols), are used as precursors to form polymers, followed by chemical reactions that convert them into building block compounds. Table 1 lists the world volume and price production of these platform chemicals.
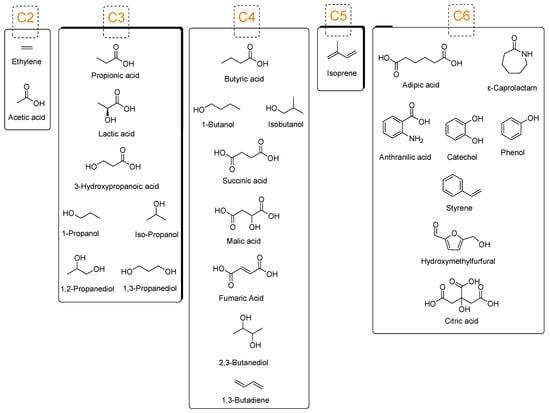
Figure 1.
Common platform chemicals derived from petroleum that are utilized for petrochemical production.

Table 1.
Global production of essential platform chemicals in the worldwide market by value and volume.
2.1. Two-Carbon Petroleum-Derived Chemicals
2.1.1. Ethylene
Ethylene (Et), the simplest alkene, is a hydrocarbon with the molecular formula C2H4. Ethylene possesses a carbon-carbon that is industrially separated from natural gas or petroleum through a heat treatment process at 800–900 °C (1470–1650 °F) [78,79,80]. Ethylene is widely utilized in the chemical and plastic polymer industries as a carbon-based material (Figure 2). Its global production was estimated at 120 million tons in 2008, over 150 million tons in 2016, and 305.9 million tons in 2022 [81,82]. A catalyzed hydration of ethylene produces ethanol; hydrogenation yields ethane, for instance. Ethylene is also used to make propan-1-ol by catalytic hydrogenation of propionaldehyde (using ethylene hydroformylation with carbon monoxide and hydrogen in the presence of a catalyst such as cobalt octacarbonyl or rhodium complex, propionaldehyde is produced by the oxo process) [83]. In addition to serving as a precursor to polymers, such as polyethylene, the most commonly used plastic or surfactants (e.g., ethylene oxide and ethylene glycol, polyethylene and polyethylene terephthalate (PET)) can be produced utilizing ethylene and ethylene glycol as building block compounds [84]. Ethylene, as a monomer to produce bioanalogs, such as bioplastics, is a potential solution for replacing petroleum-derived products [85].
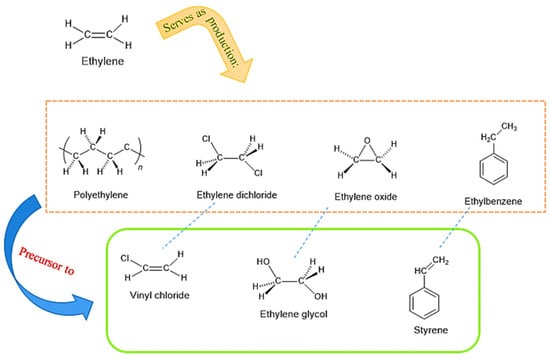
Figure 2.
Industrial uses of ethylene for producing polyethylene, ethylene dichloride, ethylene oxide, and ethylbenzene (from left to right; middle illustration). The compounds in the middle illustration are precursors to the compounds shown underneath. In oil refineries, 214 million metric tons of ethylene were produced in 2021 (Table 1). According to the U.S. National Renewable Energy Laboratory (NREL), the use of biomass (e.g., macro/microalgae) to produce bio-ethylene via the dehydration of bioethanol can reduce carbon dioxide (CO2) emissions by 70–80% compared to hydrocarbon processes in the chemical industry.
Figure 3 describes an alternative way for marine algae to produce ethylene (ethene) from acrylate [H2C=CH-COO–]. Acrylate is a precursor of ethylene [86,87] and can be cleaved from the secondary metabolite b-dimethylsul-phoniopropionate [DMSP (CH3)2S + CH2CH2COO–)] in an enzymatic reaction that also creates the volatile trace gas dimethyl sulfide (DMS [(CH3)2S]). The enzyme responsible for this reaction, DMSP lyase, has been found in several algal taxa, including benthic macroalgae and pelagic phytoplankton [88,89]. DMSP is an essential metabolite in many marine algae, accounting for between 48% and 100% of sulphur fluxes and 5% and 15% of carbon fluxes in marine microbial ecosystems [90]. Even though little research has been conducted on acrylate biosynthesis in marine organisms, it is noteworthy that DMSP-containing algal taxa have a high potential to produce it.
Additionally, DMSP is synthesized from methionine [91], the same amino acid that ultimately produces ethylene in higher plants. Among the various types of benthic algae, red and green macroalgae, also known as rhodophytes and chlorophytes, are the primary producers of DMSP and DMS [92]. These taxa are known to release ethylene. In a study, Broadgate et al. [93] showed that ten species of seaweed can produce ethylene. However, Ulva intestinalis, a chlorophyte, was found to have the highest ethene production rate, producing 62.93 pmol g−1 dry weight h−1. This species uses DMSP as its principal osmolyte, approximately 25 mmol kg−1 [94]. Additionally, experiments conducted by Watanabe and Kondo [86] showed that Codium latum (green alga), Porphyra tenera, P. aborescens (red algae), and several brown algae (phaeophytes) also produce ethylene. In another evaluation, only red and green algae showed the ability to synthesize ethylene. This production can be increased by adding the auxin hormone indole-3-acetic acid (IAA) and can be further enhanced by adding acrylate. It has been observed that P. perforata also produces ethylene [95], while the acellular macroscopic chlorophyte A. mediterranea uses ethylene for developmental differentiation [96].
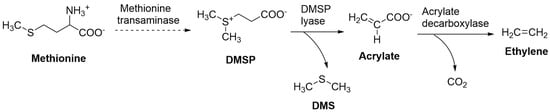
Figure 3.
Possible pathways for the production of ethylene by marine algae. DMS, DMSP, methionine transaminase [91], DMSP lyase [88], and acrylate decarboxylase [97].
2.1.2. Acetic Acid
Acetic acid, a carboxylic acid (an important C2 platform chemical), has the molecular formula C2H4O2, containing a carboxyl functional group attached to a methyl group. Among the products that are manufactured from this acid are vinyl acetate, acetic anhydride, and cellulose acetate, so vinyl acetate and acetic anhydride, known as building block compounds, form polymers such as polyvinyl acetate (PVA) and cellulose acetate. Acetic acid is produced by acetaldehyde oxidation, methanol carbonylation, and butane liquid-phase oxidation processes. The global acetic acid production was determined at 7 million tons in 2007 [98].
2.2. Three-Carbon Petroleum-Derived Chemicals
In industry, propionic acid (C3H6O2) is mainly produced through petrochemical routes, using ethylene as a starting material [99] and the oxidation of petrochemical raw materials such as propane or propionaldehyde [100]. Propionic acid is used extensively, including in the production of herbicides, cellulose fibers, perfumes, and pharmaceuticals [101].
2.2.1. Lactic Acid
Lactic acid (LA) (2-hydroxypropanoic acid) and 3-hydroxypropionic acid contain a hydroxyl group and a carboxyl group with molecular formula C3H6O3, and are multipurpose building blocks. They are well-known for manufacturing biodegradable polyesters, such as polylactide (PLA) and poly(3-hydroxypropionate). In 2007, world lactic acid production was estimated at 150,000 tons [98]. Currently, the production of 3-hydroxypropionic acid largely depends on petrochemical-synthetic pathways such as 1,3-propanediol, 3-hydroxypropionaldehyde oxidation, and acrylic acid hydration. Furthermore, lactic acid, a low molecular weight organic acid, has applications in various fields such as food, chemical, pharmaceutical, and medical. It can also be utilized to produce PLA, which is a safe, biocompatible, and biodegradable polymer that is a common material in the cosmetic industry. This alpha-hydroxyl acid also plays a crucial role in numerous biochemical pathways. Specifically, the use of lactic acid in the pharmaceutical and medical industries has become widespread for manufacturing purposes as follows:
- (a)
- Parenteral/I.V. solutions: Used to replenish body fluids and electrolytes [102] like Lactated Ringer’s and dialysis solutions;
- (b)
- Dialysis solutions: Sodium acetate is used as a dialysate fluid, but researchers recommend L(+) lactate for its fewer side effects [103];
- (c)
- Lactide glycolide copolymers: Favored for implantable drug delivery due to their biocompatibility and ability to dissolve in the body, making them suitable for drug-releasing matrices [104,105,106];
- (d)
- Ammonium lactate: Important for pharmaceutical uses, effective in moisturizing skin and treating severe dryness, also mitigates the drying effects of topical corticosteroids [107,108];
- (e)
- Mineral lactate formulations: Effective in treating anemia, hypertension, and osteoporosis, with key minerals including ferrous, calcium, manganese, magnesium, and zinc lactates [109];
- (f)
- Chiral synthesis: Central to pharmaceuticals, using natural chiral building blocks like lactic acid, with both (R) and (S) isomers available in high purities for cost-effective and versatile production [110].
Uchida and Murata [111] were the first to discover a method for producing lactic acid and ethanol through the fermentation of Ulva pinnatifida seaweed. They used cellulase for saccharification and a microbial consortium of Lactobacillus brevis, Debaryomyces hanseni var. hansenii, and Candida zeylanoides with yeast for cultivation. Adding this microbial consortium along with cellulase-induced lactic acid and ethanol fermentation in various kinds of seaweed. In a research conducted by Hwang et al. [112], they investigated using five different Lactobacilli strains for lactic acid production from hydrolysates of Enteromorpha prolifera. The results showed that the tested strains were more efficient in using E. prolifera hydrolysate for lactic acid production compared to corn stover hydrolysate. This could be due to differences in the composition of monosaccharides and lower furan content in macroalgae hydrolysate. This study supports the use of macroalgae carbohydrate hydrolysates as a competitive feedstock for biochemical production [112,113]. A similar study was performed by Mazumdar et al. [114], who tested an engineered E. coli strain containing the Streptococcus bovis/equinus L-lactate dehydrogenase to produce L-lactate from brown macroalgae Laminaria japonica hydrolysates as a carbon source [114]. Jang et al. [115] carried out both acid (H2SO4) and alkaline (NH4OH) hydrolysis of L. japonica to create a substrate for LA production in which the obtained mannitol was bioconverted into lactic acid by L. rhamnosus. In their following experiment, the researchers utilized a sulphuric acid hydrolysate of Gelidium amansii as the primary carbon source for lactic acid production by L. rhamnosus KY-3. They eliminated inhibitor components, including 5-HMF, furfural, and phenol. Monosugars glucose and galactose were converted into lactic acid. The resulting mixture contained a small quantity of acetic acid, formic acid, and ethanol [116].
2.2.2. 1-Propanol and Isopropanol
Alcohols such as 1-propanol and isopropanol can be utilized instead of methanol as the esterification reagent, and the esters formed exhibit decreased crystallization at low temperatures [117]. In the petroleum industry, 1-propanol is chemically synthesized from ethane, carbon monoxide, and hydrogen, whereas isopropanol is made from the hydration reactions between water and propene [118].
2.2.3. Propanediols
1,2-propanediol (PDO) and 1,3-propanediol are linear aliphatic glycols composed of two hydroxyl functional groups. These propanediols are base compounds for the production of polyester resins, antifreeze and de-icing agents, detergents, pharmaceuticals, cosmetics, and food products [36]. Specifically, monomer 1,3-PDO is broadly tapped for creating polymers extending from terephthalate to polyester polytrimethylene terephthalate (PTT). Both 1,2-PDO and 1,3-PDO are mostly gained chemosynthetically from propylene oxide and propenal, respectively [35,119]. 1,2-PD could be bioproduced from marine microorganisms. Merriman [37] achieved a promising outcome by optimizing the production of 1,2-PD through an algae fiber system using Thermoanaerobacterium thermosaccharolyticum bacteria. The extracts derived from the process contained various sugars, specifically C5 sugars such as xylose and arabinose, along with C6 sugars like glucose, galactose, and mannose. These sugars were subsequently bioconverted into 1,2-PD. Although Merriman’s study did not use real algal biomass, Merriman [37] recommends using U. lactuca, known for its high carbohydrate content, to potentially improve production processes. Additionally, research by Bikker et al. [120] has shown that 1,2-PD can be produced from U. lactuca biomass in combination with Clostridium beijerinckii NCIMB 8052. The study also noted that when sugars like glucose, rhamnose, and xylose were consumed at low concentrations, the main products formed were acetone, butanol, and ethanol (ABE).
2.3. Four-Carbon Petroleum-Derived Chemicals
2.3.1. Butyric Acid
Butyric acid, a four-carbon aliphatic fatty acid, is a starting material for the formation of cellulose acetate butyrate. It is yielded either by chemically oxidizing butane or butyraldehyde [121]. It has many applications in the food, perfume, and polymer industries (photographic films and eyeglass frames) [122].
2.3.2. Butanol
Butanol, a four-carbon alcohol, is a basic organic chemical with the molecular formula C4H10O. Current commercial production of butanol is based on petroleum-derived chemicals, which are produced by a two-step process involving the hydroformylation of propene to gain butyraldehyde and subsequent hydrogenation. It showed applications in industrial solvents and plays a role in producing important chemicals such as acetates, acrylate esters, amines, amino resins, butyl acrylates, glycol ethers, and methacrylates as intermediates. Butanol is an excellent solvent for manufacturing antibiotics, vitamins, and hormones, as well as a diluent for brake fluid formulations [123,124]. The European Medicines Agency (EMA) has declared that n-butanol is utilized as a preservative or stabilizing solvent in veterinary medicinal products. Additionally, n-butanol is used as a flavoring agent in various food products such as butter, cream, liquor, rum, and whiskey. It is also used as an extractant in food production to manufacture hormones, antibiotics, and vitamins. Additionally, n-butanol serves as a solvent for industrial and cleansing activities. The worldwide butanol market was valued at around USD 7 billion in 2020 and is projected to reach nearly 9 billion by 2026, with growth at a CAGR of 3.7% from 2021 to 2026 [125]. 1-butanol derivatives, butyl acrylate, and methacrylate esters can be applied for the production of latex surface coatings, enamels, and lacquers, while isobutanol ester derivatives, such as diisobutyl phthalate, are used as plasticizer agents [123,126].
In the past decade, numerous reports have been published on utilizing the macroalgae Ulva lactuca to produce n-butanol in a mixture of ABE [127,128,129]. Butanol can be made through bacterial fermentation with Clostridium strains such as Clostridium acetobutylicum or C. beijerinckii. This process is traditionally known as ABE fermentation. Macroalgae, specifically green and brown seaweeds, have also been researched for the production of acetone, butanol, ethanol, acetic, and butyric acids by Clostridium beijerinckii and C. saccharoperbutylacetonicum. Additionally, acetic and butyric acids can be produced by C. acetobutylicum (ca. 3–4 gABEglucose−1) [128,129,130]. Aqueous extracts of the brown algae Saccharina spp., which contain mannitol and laminarin, were subject to fermentation by C. acetobutylicum ATCC 824 to achieve butanol and ABE (yields: at 0.12 g g−1 and 0.16 g g−1, respectively, which are relatively low, but these can be improved to make industrial-scale acetone, butanol, and ethanol fermentations of brown seaweed economically feasible) [127].
2.3.3. Succinic Acid
Succinic acid (SA) is a four-carbon platform chemical generally produced commercially, primarily by hydrogenation of petroleum-based maleic acid or via the oxidation of butanediol [131], with applications in antifreeze liquids, coolants, solvents, pigments, polyesters, intermediates for the chemical industry (1,4-butanediol: BDO, derivatives), plasticizers, etc. [132]. SA serves as a building block for the synthesis of several industrial chemicals, including lacquers, sequestrants, buffers, and neutralizing agents [133]. Moreover, SA can be esterified to produce dimethyl succinate, a solvent marketed as environmentally friendly [134]. Water cooling systems for vehicles [135] are also used to improve the flotation of various ores and water repellency in the leather industry [136]. SA also promotes propionate production in the rumen and acts as a glycogenic material and a precursor for protein synthesis. This makes it a potential additive for animal feed for both ruminants and monogastric animals such as pigs, which could help reduce the usage of antibiotics like monocin and lasalocid in specific animal feeds. The crude succinate salt produced from carbohydrates could potentially find new markets as a product for animals [137]. Bioproduced SA has a broad spectrum of potential applications, ranging from pharmaceuticals and resins to the food industry, polyurethanes, cosmetics, de-icing solutions, solvents, and fine chemicals. As a platform chemical derived from renewable resources, it shows great promise. In the SA production, glucose and mannitol hydrolysates from the Laminaria japonica seaweed were used as carbon sources by engineered E. coli BS002 and recombinant E. coli KLPPP. (yielded: 17.4 and 22.4 g L−1, respectively) [138]. Palmaria palmata, a type of red algae, was subjected to pre-treatment and enzymatic hydrolysis, which resulted in a mixture of glucose and galactose sugars. The SA yield on galactose was almost three times higher than on glucose. In 2015, Alvarado-Morales and colleagues proposed an integrated biorefinery approach that aimed to produce SA and direct the by-products for food, added value products, and bioenergy production by converting extracted sugar from macroalgae Laminaria digitata to SA using Actinobacillus succinogenes 130Z (yield: 0.50 g (L h)−1) [139].
2.3.4. 2,3-Butanediol
2,3-Butanediol (2,3-BDO) is a crucial platform chemical widely used as an antifreeze agent. Its dehydration products have a broad range of applications, such as fuel additives, rubber production, food flavoring, and bacteriostatic additives [140]. Recently, researchers engineered an E. coli strain to efficiently produce 2,3-BDO and acetoin (A) using brown algae hydrolysate. The E. coli microaerobically utilized mannitol and glucose to synthesize 2,3-BDO + A fermentation products [141].
2.3.5. Malic Acid
Malic acid is an intermediate in the TCA cycle composed of two stereoisomers: the left-handed L-form and the right-handed D-form. Only L-malic acid is found in biological systems [142]. A variety of microorganisms have been observed to obtain L-malic acid [143]. Fungi, such as Aspergillus species and Schizophyllum commune, can produce a significant amount of L-malic acid from sugars through fermentation. Aspergillus flavus, for example, can produce 113 g L−1 of L-malic acid from 120 g L−1 of glucose. In another study, S. commune was used for the production of 43 g L−1 of L-malic acid from 50 g L−1 of glucose in an 8-L air-lift column fermentor in 110 h [144].
2.3.6. Fumaric Acid
The pharmaceutical industry uses fumaric acid to manufacture alexipharmic sodium dimercaptosuccinate and ferrous fumarate, which are used as optical bleaching agents. Additionally, fumaric acid esters, such as ethyl hydrogen fumarate, monoethyl fumarate, and dimethyl fumarate, are used to treat psoriasis patients who are not able to produce fumaric acid in their skin when exposed to light and multiple sclerosis (MS) [145,146,147]. Recently, it has been discovered that fumaric acid possesses antibacterial properties in Aloe vera L. [148]. Additionally, fumaric acid can be bioproduced through fermentation using Rhizopus species [145].
2.4. Five-Carbon Petroleum-Derived Chemicals
Isoprenes
Isoprene is a five-carbon building block for the synthesis of diverse polymers. The key source of isoprene is a by-product of ethylene generation by cracking naphtha. Isoprene yields are typically 2–5 wt% based on ethylene, although it may be increased by starting with a heavier raw material such as diesel. The first industrial synthesis of isoprene began with the dimerization of propylene to 2-methyl-1-pentene. This substance is then isomerized to 2-methyl-2-pentene, which is subsequently cracked with loss of methane to prepare isoprene; although the highly frequently used synthetic method is acid-catalyzed addition of formaldehyde to isobutene (Prins reaction) [131]. In living systems, isoprenoids represent the most remarkable and diverse group of natural products, with over 40,000 structurally distinct compounds critical to the survival of all classes of living organisms. These molecules are not just important; they are fundamental to essential biological functions, including respiration, electron transport, maintenance of membrane fluidity, hormone signaling, photosynthesis, and antioxidation, as well as the precise localization and regulation of protein activities [149,150]. Moreover, certain isoprenoids like carotenoids are strategically produced for commercial use as vital nutritional and medicinal additives, underscoring their importance in our health and well-being [151]. Isoprenoids are synthesized through a series of definitive condensations of five-carbon precursors: isopentenyl diphosphate (IPP) and its allyl isomer, dimethylallyl diphosphate (DMAPP). These precursors are generated via two established pathways: the mevalonate (MVA) pathway, predominant in most eukaryotes, and the 2-C-methyl-D-erythritol-1,4-phosphate (MEP) pathway, which operates in prokaryotes. The enzyme prenyltransferase is responsible for condensing IPP and DMAPP to produce key compounds known as prenyl pyrophosphates, including geranyl pyrophosphate (GPP), farnesyl pyrophosphate (FPP), geranylgeranyl pyrophosphate (GGPP), and various polyprenyl pyrophosphates. Prenyl pyrophosphates are decisively transformed into monoterpenes, sesquiterpenes, diterpenes, triterpenes, tetraterpenes, and polyprenyl side chains. The remarkable chemical diversity of isoprenoids is driven by specific terpene synthases and modifying enzymes, particularly cytochromes P450. Synthetic bioengineering strategies significantly enhance isoprenoid production in microorganisms such as E. coli and S. cerevisiae. These approaches lead to the successful synthesis of triterpenoids like amorpha-4,11-diene and artemisinic acid, which are vital precursors for the antimalarial drug artemisinin. Furthermore, they enable the efficient production of taxa-4(5),11(12)-diene, a key precursor for the potent antineoplastic agent Taxol. Additionally, synthetic bioengineering drives the synthesis of tetraterpenoids, including carotenoids like astaxanthin, showcasing its critical role in advancing these valuable compounds [152,153,154,155,156].
2.5. Six-Carbon Petroleum-Derived Chemicals
2.5.1. Adipic Acid
The adipic acid (1,6-hexanedioic acid) and its derivative, ɛ-caprolactam, are primarily utilized as building blocks for polyamides, including Nylon-6,6 and Nylon-6, respectively. The worldwide yearly preparation of adipic acid and ɛ-caprolactam is calculated at 2.2 and 4 million metric tons, respectively. Industrially, adipic acid is acquired by mixing the oxidation of cyclohexanone and cyclohexanol (ketone-alcohol oil); in contrast, ɛ-caprolactam is built by ɛ-aminocaproic acid cyclization [142]. The International Energy Agency (IEA) considers adipic acid the most significant dicarboxylic acid from an industrial perspective and has identified it as a suitable platform chemical for biobased production. Globally, adipic acid production will increase at a compound annual growth rate of 3–5%. It is being developed to produce adipic acid by microorganism fermentation using complex raw material streams and high concentrations of adipic acid at acidic pH, using a production host that is highly tolerant of complex raw material streams. Biomass must be produced and utilized efficiently in order to achieve a biobased economy. As a result, the raw materials used in a bioeconomy must be produced sustainably [157,158,159].
2.5.2. Anthranilic Acids, Catechols, and Phenols
Anthranilic acids (2-aminobenzoic acid), catechols (1,2-dihydroxybenzene), and phenols are a group of monoaromatic hydrocarbons inferred from the petrochemical industry. These monoaromatics have been produced globally as starting materials and are used extensively in different industries, including plastics, detergents, and pesticides. They also consist of significant constituents of numerous petroleum and fine chemical products [160,161].
2.5.3. Styrene
Styrene is a key monomer derived from benzene and ethylene, essential in the production of polymers and resins, with over 5.8 million metric tons consumed annually in the U.S. As a new drug delivery vehicle, polystyrene-integrated solid foams have also shown promise [162,163]. Styrene traditional synthesis requires more than three metric tons of steam per metric ton, making it the most energy-intensive of all commodity chemical production routes. It consumes nearly 200 trillion BTU of steam annually for domestic production alone, which is an exorbitant amount. Styrene can be biosynthesized using renewable resources like glucose instead of traditional methods. This innovative method harnesses the power of microorganisms to transform renewable resources directly and efficiently into styrene. By embracing this biotechnological approach, we pave the way for a sustainable and environmentally friendly solution that not only minimizes energy consumption but also reduces costs significantly. The use of these tiny biological powerhouses exemplifies how nature can contribute to a greener future, making the production process both eco-conscious and economically viable [84,164,165].
2.5.4. 5-Hydroxymethylfurfural
5-Hydroxymethylfurfural (5-HMF) is an aromatic heterocyclic furan substituted in the 2,5-position by hydroxide and aldehyde functional groups. Due to the 5-HMF’s unique chemical structure, there are large efforts to utilize 5-Hydroxymethylfurfurals as a starting point for the synthesis of chemicals. As its characteristics, 5-Hydroxymethylfurfural is an α,ω-bifunctional molecule with substituents at positions 2 and 5; thus, it could be either oxidized to a dicarboxylic acid or reduced to a diol. Both of them can be used to synthesize polymers. Also, it is a relatively unsaturated compound that can be turned into fuel molecules by hydrogenation [166]. Plastics, pharmaceuticals, food, and chemical industries also use 5-Hydroxymethylfurfural and its derivatives. By oxidizing 5-HMF, 2,5-furandicarboxylic acid (FDCA) is generated. In the same way that terephthalic acid is used to produce PET, FDCA can be used to produce polyethylene furanoate (PEF). By replacing polyethylene terephthalate with PEF, plastic bottles and packaging materials will have a significantly reduced carbon footprint. There is considerable promise in 5-hydroxymethylfurfural as a potential replacement for fossil-derived compounds. It is estimated that more than 175 valuable bio-based products are obtained from 5-Hydroxymethylfurfural [167,168,169]. In recent years, algal biomass has emerged as an ideal material for producing 5-hydroxy-methylfurfural. This is a compound commonly found in lignocellulosic acid hydrolysate, and it is mainly formed by the dehydration of hexose [170]. Gracilaria verrusca, with a solid-acid catalyst, released 5-Hydroxymethylfurfural, and red algae, Gelidium amansii, are also a source of it [71,171,172,173].
2.5.5. Citric Acid
Citric acid (CA) (C6H8O7) is one of the most essential biochemicals produced on an industrial scale. It has a broad range of applications across various industries, such as food and beverage, pharmaceutical, metal, and nutraceutical. It also has roles in cosmetics as a flavoring agent, sequestering agent, buffering agent, etc. [174]. In 2020, the global production of citric acid was estimated at 2.39 million tons. By 2026, the production is forecast to reach 2.91 million tons [175]. Additionally, in 2021, its worldwide sales were approximately $2.8 billion, and the market for citric acid is expected to grow further in the next decade [176]. According to statistical data, pharmaceuticals are responsible for 12% of the global production of this acid, whereas food consumes 70% [177]. For instance, the common medical use of citric acid is in formulations, including intramuscular and subcutaneous injections for parenteral administration, co-amorphous drugs, co-crystals, lyophilization (drug delivery), taste-masking, and effervescence. It is worth noting that the effectiveness of a treatment is heavily reliant on patient compliance. [178]. Following a worldwide increase in demand for biobased products instead of petroleum-derived ones, citric acid is a suitable candidate for replacement, as it is a bio-based monomer with three carboxylic and one hydroxyl group, providing hydrophilicity and crosslinking sites, as well as being non-toxic and cost-effective [179].
Aspergillus niger cultivation on carbohydrate-rich substrates is the primary method of producing citric acid. A study by Ramesh and Kalaiselvam (2011) demonstrated that the red macroalgae Gelidiella acerosa possess a high carbohydrate content (ca. 60% w/w) and can be used for citric acid production (obtained citric acid concentrations varied from 30 to 80 g at different pH) [180].
3. Challenges, Limitations, and Potentials of Petroleum-Derived Platform Chemicals from Marine Sources
This section assesses the commercial potential, scalability challenges, and optimal marine sources for key C2–C6 molecules, highlighting their relevance, technological maturity, and environmental benefits.
3.1. Commercial Potential
Lactic acid, succinic acid, butanol, 5-HMF, and ethylene are the most promising due to their large markets and bio-based production feasibility. Lactic acid, with a significant market (150,000 tons in 2007, likely higher in 2025) [28], SA with EUR 2.5 billion market, and butanol with USD 9 billion by 2026 support polymers, solvents, and biofuels, reducing reliance on energy-intensive petrochemical processes [127,139]. 5-HMF enables PEF, a sustainable PET alternative, while ethylene, 305.9 million tons in 2022, is critical for bioplastics [93,142]. Acetic acid, propionic acid, isoprene, and adipic acid hold potential but are less mature [180,181].
3.2. Scalability Challenges and Limitations
Scaling marine-derived production faces significant hurdles. These include low fermentation yields—such as 0.12–0.50 g g−1 for butanol, 20–40 g L−1 for lactic acid, 62.93 pmol g−1 dry weight h−1 for ethylene. Additionally, high energy costs associated with biomass pretreatment, the formation of by-products like acetic acid and ethanol, and seasonal variability in the composition of marine macro and microorganisms further complicate the process [84,127].
For instance, lactic acid production from Gelidium amansii can reach 30–40 g L−1, but this process is hindered by inhibitors such as 5-HMF. On the other hand, the production of succinic acid and butanol from Laminaria spp. suffers from low conversion efficiencies, with rates of 0.50 g L−1 h−1 and 0.12–0.16 g g−1, respectively [139]. Ethylene production relies on optimized DMSP lyase pathways, while isoprene faces challenges due to enzyme inefficiencies [28]. Techno-economic analyses (TEA) reveal that capital and pre-treatment costs are high. In contrast, life cycle assessments (LCAs) show lower greenhouse gas (GHG) emissions—ranging from 1.5 to 2 kg CO2 kg−1 produced, compared to 3–4 kg CO2 kg−1 from petrochemical processes. However, there are concerns about eutrophication risks [182]. Potential solutions include genetic engineering (such as optimizing Lactobacillus or E. coli), in situ product recovery (ISPR), and integrated biorefineries that enhance the value of co-products. These approaches offer significant industrial-scale potential by 2030 to 2035.
3.3. Sustainable Marine Sources
Macroalgae such as Ulva intestinalis, Gelidium amansii, Saccharina spp., and Laminaria japonica are outstanding choices due to their impressive carbohydrate content, which can reach up to 60% w/w. Their rapid growth and efficient use of land and freshwater make them superior candidates for sustainable applications [183]. Ulva spp. is highly effective for producing ethylene and lactic acid, thanks to its elevated DMSP and sugar content. In contrast, Gelidium and Laminaria play a vital role in supporting the production of 5-HMF, succinic acid, and citric acid [171,180]. Marine microorganisms, such as E. coli, Clostridium spp., and Lactobacillus rhamnosus, effectively utilize algal hydrolysates with fewer inhibitors when compared to terrestrial feedstocks like corn stover. This presents significant sustainability advantages, as confirmed by LCA [182].
In general, lactic acid, succinic acid, and 5-HMF are on the brink of commercial readiness, with a timeline of 5–7 years, thanks to their established fermentation pathways and significant market demand. In contrast, ethylene and butanol need yield improvements to reach similar levels of viability [93,139]. Besides, Acetic acid, propionic acid, isoprene, and adipic acid are currently lagging due to subpar yields and technological shortcomings. However, marine sources clearly outperform terrestrial feedstocks in key environmental metrics, significantly reducing greenhouse gas emissions and land use. To unlock the potential of a decarbonized, bio-based chemical industry, it is essential to overcome scalability challenges through advancements in synthetic biology, effective biorefinery integration, and robust policy incentives such as carbon credits. In the following section, we delve into effective biorefinery processes to tackle existing challenges and drive industrial-scale production forward.
4. Biorefinery
In today’s society, fossil fuel refineries provide most of our energy and consumer products. Our extreme dependence on fossil fuels, largely due to the intensive use of petrochemical derivatives (approximately 4% of oil is used in chemical and plastic production worldwide), releases about two-thirds of GHG into the atmosphere. GHG emissions, including carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O), are detrimentally affecting the Earth’s climate. It is concerning to note that carbon emissions have surged to their highest level in the last seven years. The latest figures show that energy-related carbon emissions have skyrocketed by approximately 2.0%, marking the fastest expansion rate in quite some time [184,185,186,187]. This implies an increment in emissions of approximately 0.6 gigatons, which is a cause for alarm. Moreover, due to the continuous rise in the price of fossil fuels, coupled with their uncertain availability and environmental impact, it is anticipated that the viability of oil exploitation will decline in the coming years. It is essential to promote alternative solutions that can help mitigate climate change and reduce the use of fossil fuels [184,185,186,187]. Globally, it is widely acknowledged that plant-based raw materials (i.e., biomass) can serve as feedstocks for industrial production, thereby replacing a significant portion of fossil resources. In addition to fossil fuels, it is the only source of C available on the planet. Thus, sustainable biomass production is of utmost importance, and expansion to include products from marine algae will be extremely valuable moving forward [188]. In a recent document issued by the IEA Bioenergy, the term “Biorefinery” was defined as a process for the sustainable conversion of biomass into a range of marketable products and energy [188].
The biorefinery concept encompasses various technologies that can extract biomass resources’ essential components (natural products), such as carbohydrates, proteins, and triglycerides. These components can then be transformed into value-added products, biofuels, platform chemicals, and power. Similar to today’s petroleum refineries, which produce a variety of fuels and products from petroleum, this concept is not only relevant but essential across numerous contexts. As we look to the future, we will take a decisive and progressive approach to transform significant sectors of the global economy and industry into a sustainable biobased community that champions environmental responsibility. We are committed to making this vision a reality, and we will succeed in driving this change forward. This will be built on the foundation of bioenergy, biofuels, and biobased products, with biorefineries serving as the backbone. Hence, biological and chemical processes will have to be established in order to replace oil with biomass in today’s production processes of commodities [189,190].
4.1. Biomass vs. Fossils as Source Raw Materials
Crude oil consists of a variety of organic hydrocarbon compounds, and the initial refining stage involves eliminating impurities and water. The next step is the distillation process, which separates crude oil into multiple fractions like diesel fuel, gasoline, kerosene, lubricating oils, and asphalts. Afterward, these fractions can undergo chemical transformations to produce a variety of industrial platform chemicals (Figure 1) and end products. Biomass composition differs from petroleum as it is not uniform and consists of a blend of carbon, hydrogen, and oxygen alongside minor components like nitrogen, sulfur, and mineral compounds. It is worth noting that biorefineries that use biomass feedstocks require a more diverse set of processing technologies. However, they are capable of producing a wider variety of products than petroleum refineries and can utilize a broader range of raw materials [191,192]. Naphtha, sourced from crude oil, serves as a raw material that is transformed into a limited number of platform chemicals. These chemicals are then utilized to produce a vast array of bulk chemicals. The critical property of naphtha feedstock is that it contains very little oxygen content, distinguishing it from biomass. As illustrated in Figure 4, a wide range of bulk chemicals can be made using only a handful of platform chemicals, namely, ethylene, propylene, C4-olefins (including butadiene, 1-butene, 2-butene, and isobutene), as well as the aromatics benzene, toluene, and xylene, commonly known as BTX. These platform chemicals containing hydrogen and carbon are utilized as solvents (such as toluene and benzene), as the starting materials for polymers (like ethylene, propylene, and butadiene), or are modified through the addition of elements such as nitrogen, oxygen, or chlorine [193,194,195,196].
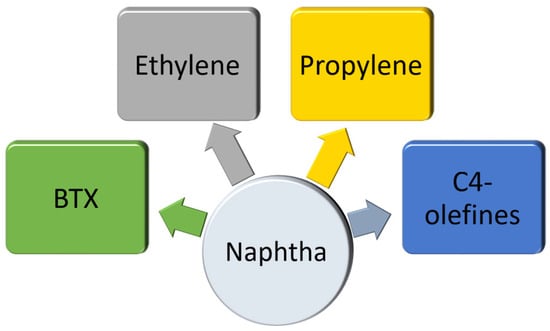
Figure 4.
Schematic of major platform chemicals produced from naphtha at petroleum refineries. Naphtha, as an intermediate hydrocarbon derived from refined crude oil and feedstock, is used for the production of hydrogen, ethylene, propylene, C4-olefins including butadiene, 1-butene, 2-butene, and isobutene; BTX, including aromatics benzene, toluene, and xylene.
It is forecasted that the biorefinery sector will create several platform chemicals that can be tapped to produce other commodities and bulk chemicals, thereby reducing environmental impact and cutting down on capital expenses (Figure 5 and Figure 6). The manufacture of many C1–C4 chemicals can be accomplished with renewable resources like starch, cellulose, or carbohydrates. In this case, Figure 6 presents some of the chemicals produced by microorganisms as a result of carbohydrate fermentation [197]. An important consideration in the production of biochemical products is the carbohydrate fraction of biomass feedstock, specifically the cellulose and hemicellulose found in lignocellulosic biomass. It is anticipated that this renewable carbon source will have a significant impact on this process. Biomass polysaccharides can be efficiently hydrolyzed into monosaccharides such as glucose, fructose, and xylose. These can be further turned into a variety of bio-platform molecules (bPMs) through fermentation or chemical synthesis. These bio-platform molecules serve as building blocks for numerous value-added chemicals and are similar to the petro-platform molecules found in present-day oil refineries. Also, bPMs have a strikingly higher oxygen content in comparison to platform molecules obtained from oil, such as ethylene and benzene. This will result in an intriguing shift in the chemistry field, where reduction chemistry is becoming more prevalent in comparison to environmentally damaging oxidation procedures. For instance, hydrogen gas is being used with a heterogeneous catalyst, resulting in a greener approach. One example of how one of these biobased platform chemicals can be used is Levulinic acid (C5H8O3), which is produced through the acid hydrolysis of C6 sugars. Due to its high reactivity, it can be transformed into plenty of chemical derivatives. This is possible because it possesses both a ketone carbonyl group and an acidic carboxyl group, allowing it to react as both a ketone and a fatty acid. To begin, cellulose undergoes hydrolysis to produce C6 sugars. Next, HMF is used to obtain levulinic acid efficiently at a 50% rate. Finally, the resulting levulinic acid is converted into either chemicals or fuel additives as desired (Figure 5 and Figure 6) [198,199,200,201,202,203,204,205,206].
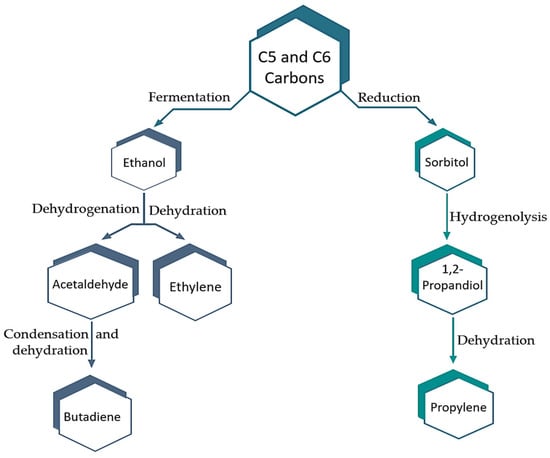
Figure 5.
Significant petrochemical platform chemicals are produced from lignocellulosic biomass feedstock [200].
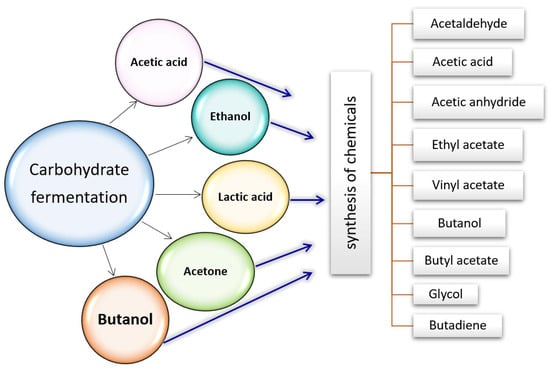
Figure 6.
Some chemicals are derived from carbohydrate fermentation by microorganisms. Ethanol is produced by fermentation of a sugar source (e.g., glucose (C6H12O6)), where one glucose molecule is degraded to two molecules of carbon dioxide and two molecules of ethanol. Ethanol is then converted to acetic acid through the loss of one carbon in the form of CO2 in the glucose–ethanol fermentation. Isomerization of glucose to fructose, the retro-aldol fragmentation of fructose to C3 intermediates, results in lactic acid production. In the side fermentation steps, acetone and butanol are obtained as well. Ultimately, each one of these starting materials is used for the production of other conventional industrial compounds. Lactic acid is a carboxylic acid widely found in nature. It can be converted into various compounds like acetaldehyde, acrylic acid, propanoic acid, 2,3-pentanedione, and dilactide due to its biofunctionality. Ethylene and acetaldehyde can be produced through the fermentation of ethanol. In India, over 20 companies are producing not only ethanol from sugar cane or molasses but also chemicals, such as acetic acid, acetic anhydride, and ethyl acetate, through ethanol fermentation. Vinyl acetate monomer (VAM) is a crucial component in the production of poly(vinyl acetate), which is widely used in the polymer industry for making resins and lattices. This makes VAM a valuable product in the market. To obtain esters such as ethyl acetate and butyl acetate, they are often synthesized from their products, namely ethanol, butanol, and acetic acid. n-butanol, on the other hand, is utilized as a solvent and intermediate for producing acrylates, ethers, and butyl acetate.
4.2. Algal Biorefinery
Marine algae biorefinery provides an eco-friendly way to utilize algae resources for a range of products, including various energy products, platform chemicals, and high-value products [207,208,209]. Indeed, after the extraction of biomolecules (such as bioactive compounds, proteins, gel polymeric materials, and pigments) from the cellulose-rich fraction, the remaining material can be processed into monomeric sugars for fermentation into various products. In recent decades, researchers have been working to reduce the world’s reliance on petrochemicals and petrol fuels. This has led to the development of many bioprocesses that focus on producing biofuels from both marine and freshwater algae. Some species, such as Botryococcus braunii, Nannochloropsis sp., and Schizochytrium sp., have been found to contain more than 700 kg of oil per ton of dry biomass [207,210,211].
Microalgae are considered a potentially important feedstock for a wide range of bio-based products, including biofuels, specialty chemicals, pharmaceuticals, cosmetics, and food products. Algal biomass is being studied worldwide as a sustainable source of simple sugars for bioethanol fermentation [212,213,214]. Carbohydrates obtained from both marine micro- and macroalgae can be used for the production of a variety of biochemicals and biomaterials (Figure 5 and Figure 6). The amount of carbohydrates present in microalgae varies widely from species to species [215]. Microalgae contain abundant amounts of two polysaccharides: cellulose and starch [208]. When broken down into their constituent sugars, glucose is the primary monosaccharide found in microalgae, comprising between 21% and 87% of the total carbohydrate content [216,217,218]. These polysaccharides offer a wide range of properties and potential applications, including biomedical and nutraceutical uses, as well as anti-adhesive, bioflocculant, and drag-reducing properties for ship engineering [219,220]. Compared to terrestrial plants, macroalgae have a major advantage for biorefinery purposes because they lack lignin. This simplifies carbohydrate extraction and saccharification, enabling them to be ideal for use in biotechnology processes ranging from biofuels to biochemicals, building blocks, and biomaterials. Additionally, marine plants such as algae have a high content of easily degradable carbohydrates (25–60% dw) and do not require arable land for growth, making them an attractive renewable feedstock. They grow in seawater, which means they do not compete with terrestrial food crops. Furthermore, the production yields of algae per unit area are higher because they are highly photosynthetic [221]. In summary, seaweeds have the ability to absorb CO2, have a rich carbohydrate content, and lack lignin, thus granting the potential for producing biofuels, biochemicals, and bioproducts [209].
Carbohydrate Bioconversion in Marine Algae
The conversion of carbohydrates from algae can play a significant role in the production of organic chemicals. Specific microbes can ferment sugars obtained from algae to produce commodity chemicals. In 2004, the US Department of Energy (DOE) recognized the potential of biorefinery carbohydrates and declared that a range of building block chemicals could be produced from them through chemical or biological conversion (Figure 5 and Figure 6) [222].
5. Monoterpenes
Monoterpenes (M-terpenes) belong to the terpenes family. Terpenes are a large class of natural products (NPs) produced by both terrestrial and marine organisms. This class of compounds is composed of isoprene units (C5H8) and encompasses innumerable molecules with a variety of structures [223]. Monoterpenes are composed of two linked isoprene units with the molecular formula C10H16 in a fully saturated form. They can be classified into three different subgroups: acyclic (geraniol, linalool, and myrcene), monocyclic (α-terpineol and terpinolene), and bicyclic (α-Pinene, thujone, camphor, and fenchone). These subgroups are further classified as follows: unsaturated hydrocarbons (limonene), alcohols (menthol), aldehydes and ketones (myrtenal, carvone), lactones (iridoids are monoterpene lactones, such as nepetalactone), and tropolones (γ-thujaplicin). Monoterpenes, which contain oxygen-containing functional groups, are also called monoterpenoids. The oxidation and cyclization of these compounds can occur in various ways. In many cases, these substances are found as secondary metabolites because they have such a low molecular weight [224]. In animals, a large percentage of monoterpenes are generally chiral and obtained through enzyme-controlled pathways; their chiral centers are denoted by the R* S* notations (even though chiral natural monoterpene cannot be structurally determined until their stereochemistry is known, the relative stereochemistry of those monocyclic and acyclic monoterpenes is well characterized) [225]. From the viewpoint of biogenesis, M-terpenes are produced by biosynthesis from units of isopentenyl pyrophosphate, which is synthesized from Acetyl-CoA through mevalonic acid in the HMG-CoA reductase pathway. Terpenes of this type are functionalized by molecular oxygen in many aerobic microorganisms. Model organisms such as Pseudomonas and Rhodococcus have been developed for the elucidation of pathways in aerobic bacteria. Microbial cultures have been reported to undergo numerous monoterpene transformations over the last decades, but the biochemical pathways have rarely been described. They have applications in pharmaceuticals, cosmetics, agriculture, food, and biochemistry [226,227,228,229]. Monoterpenes are widely investigated in terrestrial plants, but significantly fewer studies address the marine M-terpenes. Due to the lack of research on monoterpenes derived from marine organisms, structural variations, biosynthetic pathways, and their applications in various industries will be discussed in detail in the next section, and in particular in this review.
5.1. Marine Source Monoterpenes
Marine monoterpenes have shown unprecedented structures compared to their terrestrial counterparts in terms of halogenation and the arrangement of functional groups. These terpenes are produced by a broad range of marine organisms, such as fungi and bacteria [228,230,231,232]. Also, many invertebrates, such as mollusks [233], sponges [234,235], corals [231,236,237,238], ascidians [239,240], etc., have presented terpenes compounds in their secondary metabolites. In particular, over 101 marine monoterpenes have been reported thus far. As mentioned above, a large number of monoterpenes in marine organisms have undergone halogenation; terrestrial organisms do not appear to generate halogenated monoterpenes, and if they occur, they are rare relative to their marine counterparts. These halogen substituents indicate that marine and terrestrial M-terpenes differ significantly. The high chloride (1.99 × 107 µg L−1) and bromide (6.8 × 104 µg L−1) concentrations found in seawater can explain this, as terrestrial environments do not provide similar concentrations of halide ions. Seawater contains many times greater quantities of these ions than the terrestrial environment [225]. Another difference is that most monocyclic monoterpenes that are not halogenated are believed to form as a result of carbonium ion-induced cyclization; halonium ion-induced cyclizations, on the other hand, represent a novel ring-formation mechanism used by marine organisms. In an analogous biosynthetic event, plocamene monocyclics, which are unique carbon frameworks without terrestrial counterparts [225], might be generated. In terms of structural variation, it is estimated that more than 50% of the molecular weight of regular monoterpenoids (here called isoprene dimers) is halogenated. There is a surprising difference between marine sesquiterpenes and diterpenes, with monoterpenes in that only less than 30% of monoterpenes are oxygenated. It is possible to differentiate isoprene dimers (regular monoterpenoids) into four leading structural types (carbon frameworks), namely linear head-to-tail types (type A) and distinguished monocyclic types (types B, C, D). The acyclic compounds could be further subdivided into four groups: monoenes, dienes, trienes, and myrcenes; however, we could not find any evidence of terrestrial counterparts with monocyclic carbon skeletons (C) or (D).
Through biogenesis observations, it has been found that Pseudomonas sp., Aspergillus sp., and Penicillium sp. are responsible for producing these compounds, which are associated with their sponge host. As a result of investigations, 11% of the terpenes derived from marine fungal strains are monoterpenes. Additionally, it has been found that 20% of the fungal strains whose terpene compounds are produced from algae, and 9% are isolated from sponges (as symbionts) (Figure 7) [230].
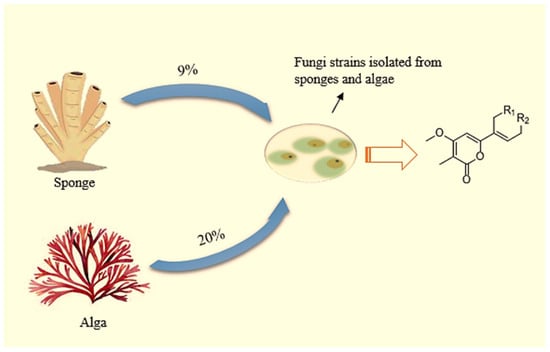
Figure 7.
A large percentage of monoterpenes are derived from microbial symbionts (e.g., fungi) isolated from sponges and algae.
5.2. Fungi
All monoterpene synthases initiate their carbocationic reaction by ionizing the substrate using divalent metal ions. After cyclizations, hydride shifts, or other rearrangements, the cationic intermediate undergoes a series of rearrangements. This reaction proceeds only until a proton loss or the addition of a nucleophile, and then it is terminated (Figure 8) [241]. Analogs of substrates, inhibitors, intermediates, and native enzymes are used to dissect this mechanism. The process converts approximately one-third of the substrate geranyl diphosphate (GDP) into acyclic products. This reaction occurs by ionizing the extended geranyl cation. Due to the (E)-geometry of the 2,3-double bond of the geranyl cation, the formation of cyclic products is hindered. Nevertheless, the cyclization of the geranyl cation into a six-membered ring is facilitated by the preliminary conversion of the geranyl cation into the tertiary linalyl cation. All cyclic monoterpenes are formed from the cisoid, anti-endo conformer of the linalyl cation, resulting in the cyclic α-terpinyl cation through the electrophilic attack of C1 on the C6–C7 double bond. Terpene synthase-mediated Wagner–Meerwein rearrangements are believed to form two other bicyclic monoterpene skeletons. In the case of terpene synthase, for instance, cyclizations, hydride shifts, or rearrangements could be performed in order to convert a single carbocation into a mixture of others [241].
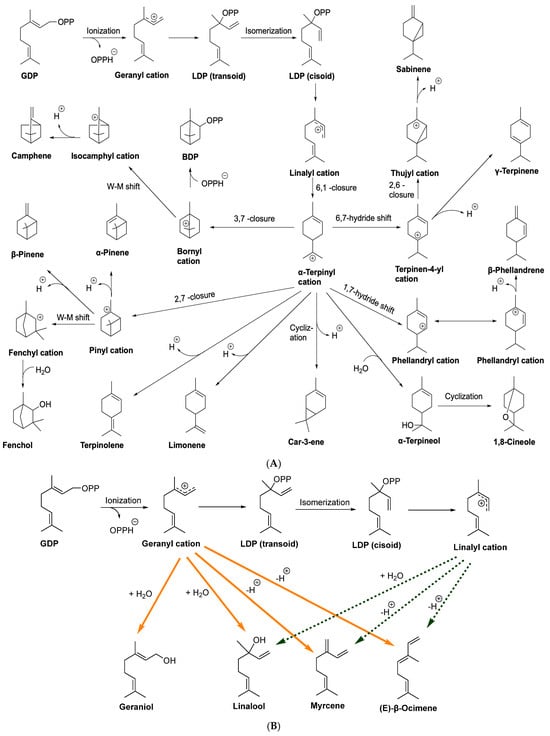
Figure 8.
The general summary of monoterpene synthase reactions to synthesize cyclic monoterpenes (A) and acyclic monoterpenes (B). GDP: geranyldiphosphate; LDP: linalyldiphosphate; BDP: bornyldiphosphate.
5.3. Algae
Cyclic and halogenated monoterpenes are among the most active compounds from this class (largely α-Pinene) [242,243,244,245,246,247,248]. A large portion of these compounds was found in marine algae, and they were synthesized mostly as a chemical defense against herbivores [249]. Macroalgae, which consist of red, Rhodophyta; brown, Phaeophyta; and green, Chlorophyta phyla, and microalgae, which consist of diatoms and cyanobacteria, provide a comprehensive list here are photosynthesizing organisms that sequester CO2 and biosynthesize isoprene and numerous monoterpenes. Marine monoterpenes are synthesized from terrestrial terpene precursors that are speculated to be associated with biological and low-temperature chemical formation mechanisms [250]. As a result, complex cyclic (ring-containing) or acyclic (linear) monoterpenes with unique ring structures (Figure 9) are commonly found in marine organisms that do not exist in terrestrial plants. Monoterpenes are found in marine macroalgae in abundant yields and have been widely observed to possess seasonal and geographical variations. In seawater, bromine and chlorine ions play an important role in the formation of halogenated monoterpenes, which are produced by bromoperoxidase abundant in many red algae [251]. Marine terpenoids differ from their terrestrial counterparts due to the halogen substituents. Investigations showed that red seaweed enzymes produce a variety of halogenated hydrocarbons and ketones during bromide ion addition. Nevertheless, the halogenated monoterpenes’ structures disclosed new patterns in which many compounds contained either only chlorine or a large proportion of chlorine in comparison with bromine [225]. Several of these compounds have been detected in red algae, such as Plocamium, Ochtodes, and Portieria [225,252]. Additionally, brown algae of the genus Dictyopteris contain monoterpenes but are much less concentrated than red algae [248]. Monoterpenes are biosynthesized via mevalonate (MVA) and/or methylerythritol phosphate (MEP) pathways (often involving haloperoxidase action). During the first step, building blocks such as all isopentenyl pyrophosphate (IPP), isoprenoids (considered as active isoprenes), and dimethylallyl pyrophosphate (DMAPP) are formed. In the cytosol, the MVA pathway begins with the condensation of three molecules of acetyl-CoA, followed by enzymatic conversions to produce isopentenyl pyrophosphate. Mevalonate kinase phosphorylates mevalonate as the main reaction in the formation of isopentenyl pyrophosphate (six different enzymes involved), while dimethylallyl pyrophosphate is generated from isopentenyl pyrophosphate by the seventh enzyme methylerythritol phosphate pathway (also referred to as deoxyxylulose-5-phosphate (DXP) pathway) initiates with the reaction between glyceraldehyde-3-phosphate (G3P) and pyruvate [253,254]. In addition to producing isopentenyl pyrophosphate, it can also produce dimethylallyl pyrophosphate because of the presence of the enzyme 4-hydroxy-3-methylbut-2-enyl diphosphate reductase (HDR) [254,255]. A monoterpene precursor, geranyl pyrophosphate (GPP), is formed when isopentenyl pyrophosphate is isomerized to dimethylallyl pyrophosphate [256]. The algae cells can follow each of these pathways. The methylerythritol phosphate pathway is present only in green algae, whereas several red algae can have either both methylerythritol phosphate pathways or only one. In regard to the biosynthesis of cyclic monoterpenes, as mentioned before, geranyl pyrophosphate is converted into the tertiary allylic isomer, linalyl pyrophosphate (LPP), through enzyme-catalyzed cyclization. A key step in this mechanism occurs when the divalent cation helps ionize the pyrophosphate group, allowing rotation around new single bonds and the generation of the highly reactive cyclic α-terpinyl cation (as an intermediate). As a result of several mechanisms such as oxidation, reduction, isomerization, or conjugation, the α-terpinyl cation intermediate will give rise to a wide range of monoterpene cyclic and bicyclic carbon skeletons [257,258]. In a study by Wise et al. (2002), neryl pyrophosphate (NPP), cis-isomer of geranyl pyrophosphate, and linalyl pyrophosphate were evaluated as alternative monoterpene substrates [258]. They found that by using neryl pyrophosphate and linalyl pyrophosphate as substrates, myrcene synthase formed cyclic structures that led to different product profiles. From neryl pyrophosphate, limonene was produced, while from linalyl pyrophosphate, myrcene, cis-ocimene, terpinene, and limonene were produced. Despite its capability to cyclize neryl pyrophosphate and LP, the enzyme is unable to cyclize the isomerization of geranyl pyrophosphate to the cyclization intermediate. It is worth noting that in macroalgae, the formation of acyclic monoterpenes with bromine or chlorine atoms or both is likely to be formed by haloperoxidase acting on either myrcene or ocimene [251,259]. Furthermore, as a result of electrophilic substitution with Cl, Br, and I, chloroperoxidase oxidizes and incorporates these molecules into the substrate [225]. In marine monoterpenes, the ochtodane ring (l-ethylidene-3,3-dimethylcyclohexane) may be created by bromonium ion-initiated cyclization from myrcene [260]. In this way, the C6–C7 olefin ring is closed first by bromination, and then an internal addition is made to the resulting cationic center. In a similar manner, 1,3-dimethyl-l-vinylcyclohexane ring and 2,4-dimethyl-1-vinylcyclohexane ring are precursors to ocimene. Multiple halogen substitutions in the monoterpenes were obtained from red algae, as discussed previously [225]. A mono- or dihalogenated myrcene derivative can be produced by turning geranyl pyrophosphate into myrcene using myrcene synthase. The myrcene derivative is then halogenated with haloperoxidases [261]. Halogenated cyclic compounds can be built from myrcene and ocimene as immediate precursors. Myrcene is a common precursor of halogenated monoterpenes in species from Portieria and Ochtodes, whereas ocimene is in macroalgae of the genus Plocamium (i.e., from Rhizophyllidaceae family, solely halogenated myrcene derivatives were isolated, whereas Plocamiaceae family just contains halogenated ocimene derivatives). Nevertheless, these halogenated substances are not only biologically synthesized by myrcene; enzyme cofactors play a crucial role in their production, and a certain set of enzyme cofactors is required to produce these compounds. The profound fact is that myrcene or ocimene can be directly yielded by HOPP loss from geranyl pyrophosphate [225].
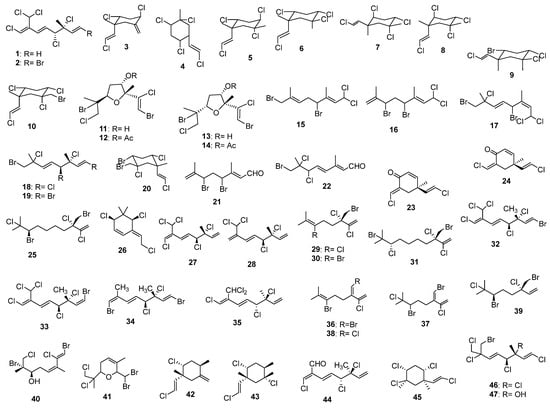
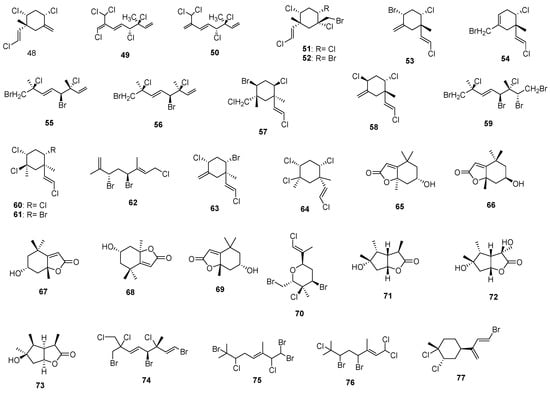
Figure 9.
Algal (a)cyclic monoterpenes 1–77.
5.4. Emission Rates of Isoprenes and Monoterpenes from Marine Photosynthetic Organisms
Isoprene, as mentioned earlier, is a crucial building block precursor for monoterpenes. It is the most common trace gas and is labeled as a biogenic volatile organic compound (BVOC) that has a role in protecting the ozone and atmospheric gases like methane and carbon monoxide [262]. Although isoprene is an important volatile organic compound, it also serves as a precursor to climate change via oxidative chemistry and secondary organic aerosol (SOA) formation [250,263]. Isoprene and monoterpenes emitted by macroalgae and microalgae, such as phytoplankton, are considered “oceanic emissions” due to their contribution as drivers of secondary organic aerosols. These are believed to affect the microphysical and radiational balance of the atmosphere via increasing CO2 and methane concentrations [250,262]. One study investigating marine aerosol production found that the red macroalga, Ochtodes secundiramea, produced the following monoterpene emissions in culture: myrcene, 32.6%; and (E)-10-bromomyrcene, 33.2% [250,258]. In another study, phytoplankton monoculture experiments revealed that marine gas-phase isoprene is produced at a greater scale than monoterpenes emissions. Specifically, during a phytoplankton bloom, the highest observed volatile organic compound emissions were measured at 375 ppt for isoprene but only 125 ppt for monoterpenes [250,264]. Similar laboratory monoculture studies have demonstrated that phytoplankton produce isoprene and monoterpenes, which have emission production rates that are dependent on speciation and environmental factors, such as water temperature, nutrients, and solar radiation [262,264]. Table 2 summarizes isoprene and monoterpene emissions from phytoplankton and other microalgae, which is important as a starting point for assessing their contributions to sustainable platform chemicals.
Many terrestrial monoterpenes are extracted via energy-intensive distillation methods that are nonrenewable and unsustainable in terms of resource consumption and by-product pollution, i.e., CO2. Marine photosynthetic organisms, specifically microalgae and macroalgae, offer an opportunity to utilize prevalent and existing emissions on an industrial scale. The production of marine photosynthetically produced isoprene and monoterpenes only requires CO2 and sunlight, unlike the cultivation of heterotrophic microsymbionts, which are often dependent on exogenous carbohydrate feedstocks [265]. Terrestrial-sourced monoterpenes, such as limonene and pinene extracted from citrus rinds and gum turpentine, can be substituted for marine-sourced monoterpenes (limonene and pinene from photosynthesizing microalgae, Table 2) that are naturally emitted into the environment. A large-scale transition away from the terrestrial production of isoprene and monoterpenes into an algal culture or capture of secondary organic aerosol-driving oceanic emissions will be crucial for combating climate change.

Table 2.
Isoprene and monoterpene emissions produced by macroalgae.
Table 2.
Isoprene and monoterpene emissions produced by macroalgae.
| Compound(s) | Species | Emission Rate | References |
|---|---|---|---|
| Limonene (cyclic monoterpene) | Nereocystis luetkeana, Alaria marginata (Brown algae) | ∼2.1 ppbV and 1.8 ppbV | [266] |
| Isoprene | Laminaria digitata, Ascophylum nodosum, Pelvetia canaliculata, Fucus vesiculosus, Fucus serratus, Halidrys siliquosa, Laminaria saccharina (Brown algae); Chondrus Crispis (Red alga); Asparagopsis armata (Red alga); Ulva intestinalis (Green alga) | 0.3–1.4 pmolesg−1 dry weighthr−1 3.5–5.3 pmolesg−1 dry weighthr−1 | [93,262,267] |
| Myrcene (E)-10-bromomyrcene (short-chained monoterpenes) | Ochtodes secundiramea (Red macroalga) | Myrcene- 32.6% (E)-10-bromomyrcene 33.2%* | [250,258] |
| Monoterpenes—N/A | Chaetoceros neogracilis, Chaetoceros debilis, Phaeodactylum tricornutum, Skeletonema costatum, Fragilariopsis kerguuellensis (Diatoms); Emiliania huxleyi (Coccolithophore); Trichodesmium sp., Synechococcus sp. (Cyanobacteria) | 0.3–68 nmol g [chlorophyll a]−1 day−1) | [250] |
| (–)-/(+)-pinene, myrcene, (+)-camphene, (–)-sabinene, (+)-3-carene, (–)-pinene, (–)-limonene, and p-ocimene (37% of total monoterpenes emitted) | Dunaliella tertiolecta (Green alga) | 226 nmol g [chlorophyll a]−1 day−1) | [250] |
| Isoprene | Phaeodactylum tricornutum, Chaetoceros neogracilis (Diatoms); Calcidiscus leptoporus, Emiliania huxleyi (Coccolithophores:); Dunaliella tertiolecta (Green alga | 2.8–28.5 pmol L−1-Chl a−1 (biomass-normalized concentration for C. neogracilis) | [250,262,268] |
| Isoprene | Prochlorococcus, Synechococcus (Cyanobacteria); Micromonas pusilla (Green alga); Pelagomonas calceolata (Flagellate); Emiliania huxleyi (Coccolithophore); Skeletonema costatum (Diatom) | 1–1.6 µmolesg−1 Chlday−1 (0.2–3.8 × 10−19 molescell−1 day−1) | [267] |
| Isoprene | Trichodesmium sp. (Cyanobacteria); Haptophytes, diatoms; Prochlorococcus sp. (Cyanobacteria) | 0–22 µmolesg−1 Chlday−1 | [269] |
| Isoprene | Emiliania huxleyi (Coccolithophor); Thalassiosira weissflogii, Thalassiosira pseudonana, Chaetoceros neogracile (Diatoms) | 0–67 µmolesg−1 Chlday−1 | [270] |
| Isoprene | Dunaliella tertiolecta, Phaeodactylum tricornutum, Thalassiosira pseudonana | 1 × 10−18–8.3 × 10−19 molescell−1 day−1 | [271,272] |
| Isoprene | Diatoms, Emiliania huxleyi, other coccolithophores, and other dinoflagellates | 0–6 × 109 moleculescm-2 sec−1 0.32 TgCyr−1 bottom-up; 11.6 TgCyr−1 top-down | [93,267,269,273,274,275,276,277,278,279,280,281] |
| α-Pinene | Diatoms | 0.013 bottom-up; 29.5 top-down | [281] |
Unit legend: parts per billion (ppb); by volume (V); nanomole (nmol) picomole (pmol); total ion current (TIC); %* as a percentage of TIC in the GC-MS trace; phytoplankton biomass—chlorophyll a (Chl a); biomass normalized concentration- picomol per liter of chlorophyll a (lpmol L−1 Chl a−1); “bottom-up” (sea to-air emission flux measurements); “top-down” (emission source estimate based on simul tion and observation data); tetragrams of carbon per year (TgCyr−1); N/A: not available.
6. Materials Processes of Marine Monoterpenes
6.1. Biotransformation of Marine Monoterpenes to Produce Biobased-Platform Chemicals
Terpenes such as monoterpenes have chiral centers and diverse functional groups, including olefin, hydroxyl, carboxylic, and carbonyl groups, making them versatile for conversion into various useful fine and bulk chemicals, including flavors, fragrances, solvents, pesticides, pharmaceuticals, and chiral intermediates that possess unique properties. Monoterpene conversion is a valuable process that can yield established industrial building blocks and fine products. Different catalysts and conditions can be employed to achieve the desired properties of the final product based on the terpene feedstock utilized, depending on the specific requirements. M-terpenes can be isomerized and transformed into various terpenoids and chemicals using either homogeneous catalysts (in the same phase) or heterogeneous catalysts (in a separate phase). These catalytic processes can also produce a range of different materials [282,283]. The use of monoterpenes as starting materials for the biotechnological production of natural chemicals has proven to be valuable across various industries. Therefore, the bioconversion of terpenes is of significant importance. Due to their structures, several monoterpenes have gained immense popularity in the industry, such as geraniol, nerol, citral, and limonene derivatives. They are biosynthesized or biotransformed by marine microorganisms. For example, the fungus Penicillium sp. has the ability to transform geraniol, nerol, a mixture of both called citrol, and a mixture of aldehydes known as citral, into 6-methyl-5-hepten-2-one. Citral is an aroma monoterpene that plays a key role in the perfumery industry. It is a cost-effective compound that is frequently used in the synthesis of menthol enantiomers. The other fascinating aroma compound is called (R)-(+)-limonene. Basidiomycete Pleurotus sapidus is capable of biotransforming this monoterpene, resulting in cis/trans-carveol and carvone production as the primary products (Figure 10).
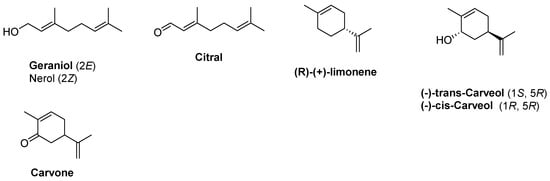
Figure 10.
Biosynthesized monoterpenes are used in various industries, such as the food and perfume industries, as additives used for product smell and taste. Geraniol and neral/nerol derivatives can be found in algae. Limonene is produced from neryl pyrophosphate in algal cells [258,284,285]. Lemon grass’s essential oil contains 75–85% citral, which is produced from geraniol by geraniol dehydrogenase cells [286,287]. Carvone is found in many plant essential oils, such as caraway, spearmint, and dill. However, microorganisms such as Bacillus sp. and their marine counterparts are able to biotransform α- and β-Pinene into carveol and carvone. Also, carvone could be obtained through nitrosochlorination of algal limonene to carvone [228,282].
6.1.1. Homogeneous Catalysts
In homogeneous catalysts, palladium (Pd) catalysts are employed for the oxidation (and isomerization) of monoterpenes like α- and β-Pinene and camphene, with hydrogen peroxide serving as the oxidant [288,289]. For instance, β-Pinene, which is a bicyclic monoterpene having an exocyclic alkene, can be transformed into pinocarveol, pinocarveol acetate, and myrtenyl acetate in different proportions based on the reaction conditions (Figure 11).
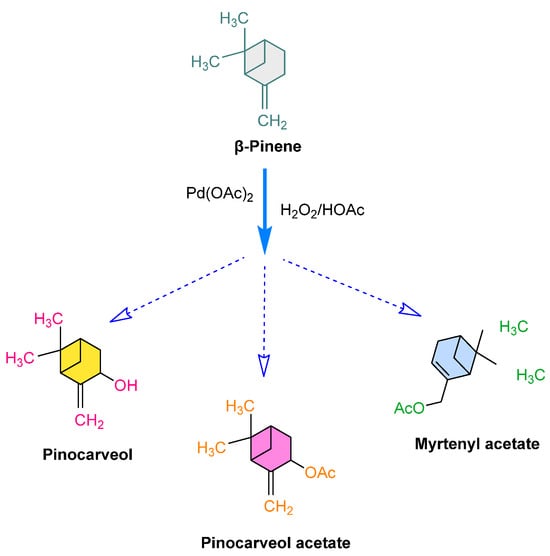
Figure 11.
β-Pinene oxidation in acetic acid solution to produce pinocarveol, pinocarveol acetate, and myrtenyl acetate in the presence of Pd(OAc)2, hydrogen peroxide [288].
Another example is camphene oxidation to camphene glycol acetate in acetic acid, which involves mechanisms such as hydroxypalladation or peroxypalladation with active Pd catalyst species (Figure 12) [283].
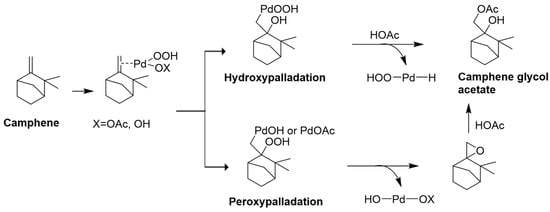
Figure 12.
Oxidation of camphene to camphene glycol acetate in acetic acid in the presence of an active palladium catalyst is done with two mechanisms: hydroxypalladation or peroxypalladation.
The process of oxidizing monoterpenes is often accomplished through the use of Re catalysts, particularly methyltrioxorhenium (MTO), which is a popular choice for olefin group epoxidation [290,291]. Typically, these reactions are carried out under low temperatures using H2O2 as an oxidant in the presence of a nitrogen base like pyridine (Figure 13). The nitrogen base not only enhances the reaction rate but also helps avoid ring-opening reactions and diol formation and accelerates the stability of the catalyst [292].
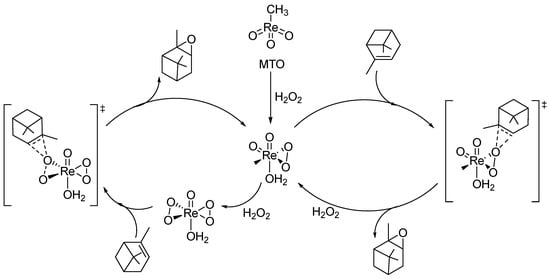
Figure 13.
The epoxidation of α-Pinene is effectively carried out using a methyltrioxorhenium (MTO) catalyst in conjunction with H2O2 as the oxidant. Both mono- and bisperoxo catalyst species play a role in this reaction, but the bisperoxo complex is definitely more abundant when excess H2O2 is present.
6.1.2. Heterogeneous Catalysts
Another significant process for the production of fine chemicals is the isomerization of olefins found in monoterpenes. Throughout this process, Rh catalysts have been specifically utilized. As an example, myrcene production involves utilizing the pyrolysis of β-Pinene or obtaining myrcene from alternate sources, which is subsequently transformed into geranyldiethylamine through the assistance of a lithium diethyl amide catalyst. In the following step, a Rh(I)–BINAP catalyst (Rh with chelates of either R- or S-2,2′-diphenylphosphino-1,1′-binaphthyl) is tapped to isomerize an alkene, transforming it from allylamine to the (1R,3R,4S) enamine. The enamine is then subjected to hydrolysis (acid) to produce citronellal, with subsequent ring closure catalyzed by ZnCl2 or ZnBr2 (Lewis acid). The remaining alkene is then reduced to yield (−)-menthol (Figure 14) [283].
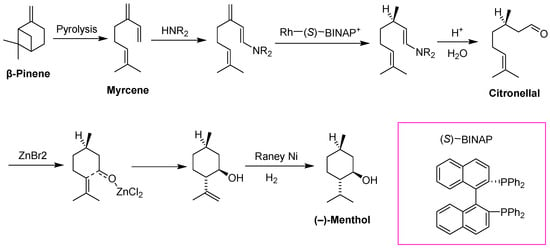
Figure 14.
(−)-menthol production begins with β-Pinene pyrolysis to myrcene production, according to the Takasago process.
A significant industrial process involves the production of aldehydes from monoterpenes. One standard method is to utilize rhodium-based catalysts to hydroformylate the monoterpenes, resulting in the formation of their corresponding aldehydes [293]. Recently, there has been interest in using the cycloaddition of CO2 to the epoxide of terpene oxide feedstocks, such as monoterpene oxide, as an alternative conversion pathway. This process results in the production of cyclic carbonates. Serving CO2 as a feedstock, CO2-based carbonates present a viable solution for repurposing an otherwise wasted carbon source. The properties of carbonates render them valuable in various applications, such as the polymer industry and as solvents [294,295]. An instance of this is when limonene dioxide is transformed into limonene dicarbonate using a tetrabutylammonium bromide (TBAB) catalyst. This process produces non-isocyanate polyurethanes that exhibit pH-dependent water solubility, enhanced thermal properties, and improved antimicrobial properties, among other features. As a result, poly(limonene carbonate) serves as a beneficial, eco-friendly base polymer [296,297]. There are various types of polymers that can be made from monoterpenes, specifically through cationic and radical polymerization methods. For example, Poly(β-Pinene) can be created through cationic polymerization of β-pinene using Lewis acid catalysts like EtAlCl2 or AlCl3, with the addition of cocatalysts or additives such as diphenyl ether to prevent undesired chain transfer reactions (Figure 15) [298,299,300,301,302]. Polymers derived from cyclic olefins are amorphous saturated hydrocarbons with main-chain cyclic units. These polymers have proven to be a popular superior alternative to conventional transparent polymers, such as poly(methyl methacrylate) (PMMA) and polycarbonate (PC), due to their high chemical resistance, low water absorption rate, good optical transparency in the near ultraviolet range, and are easy to fabricate (through appropriate additions or ring-opening polymerizations, cyclic monomers can be polymerized). For better sustainability, cyclic olefin polymers (COPs) are also biosynthesized using natural products from plant chemicals instead of petroleum-based industrial materials. One β-Pinene is an olefinic compound that is a well-known cationically polymerizable monomer composed of a fused four- and six-membered ring and a reactive exo-methylene moiety. In this case, the four-membered terpene polymerizes through its ring opening to produce a relatively stable tertiary carbocationic species and polymers with a six-membered alkene chain [303].
Figure 15.
Cationic polymerization of β-Pinene to cycloolefin polymer production [303].
Several terpenes, including limonene, α-Pinene, β-Pinene, and myrcene, have been extensively researched as potential starting materials for polymer syntheses. Specifically, refined limonene is utilized as a building block in certain terpene resins. The creation of tackifiers from terpenes can occur through cationic polymerization, utilizing potent Lewis acids like BF3 or AlCl3 alongside a co-catalyst. Phenol-containing terpene-based resins can be made through a Friedel–Craft alkylation reaction. Additionally, polyesters can be synthesized via polycondensation reaction and radical copolymerization from monoterpenes [304,305]. The reactions of α-pinene and limonene through dehydrogenation/dehydroisomerization are employed to produce p-Cymene, which is later oxidized to create bio-based p-Terephthalate using heterogeneous catalysts (Figure 16) [306].
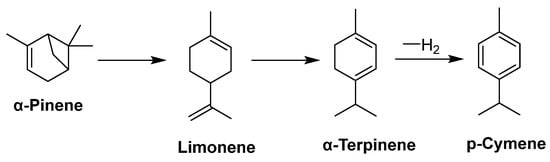
Figure 16.
α-Pinene dehydroisomerization into p-Cymene.
Also, the process of catalytic hydrogenation is commonly used to minimize the level of monoterpenes like citral and convert them into fragrant compounds such as geraniol and nerol. These compounds can further be subjected to hydrogenation to generate citronellol (Figure 17) [119,289,307].
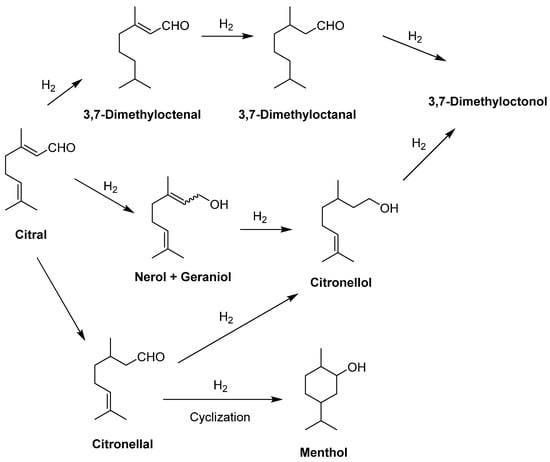
Figure 17.
Nonselective hydrogenation of citral results in citronellal, menthol, citronellol, 3,7-dimethyloctenal, 3,7-dimethyloctanal, 3,7-dimethyloctanol, nerol, and geraniol production.
The process of hydrogenating monoterpenes using molecular hydrogen is highly valuable, but its effectiveness relies heavily on the location and substitution of double bonds.
One crucial area of significant interest that has yet to gain more attention and has limited success so far is the replacement of petrochemical-based chemicals with biobased ones. Monoterpenes have the potential to produce fine aromatic chemicals as starting materials for producing petroleum-based platform chemicals or building blocks. It is estimated that approximately hundreds of thousands of tons of terpenes are produced. Considering that the current production volume of monoterpenes is in the range of hundreds of thousands to millions of tons, this can partially meet the needs of the industry [200]. Table 3 and Table 4 show the volume and market size of the industry’s favorite monoterpenes in the global and the USA markets.

Table 3.
The global production of monoterpenes in the worldwide market by value and volume.

Table 4.
The production of monoterpenes in the US market by value and volume [338].
6.2. Olefin Metathesis of Marine Monoterpenes and Potential for Petroleum Product Replacement
Olefin metathesis (OM) is the exchange of alkylidene moieties between substituted alkenes during the catalyzation process, first discovered in 1960–1970. Both cleavage and double bond formation are possible during olefin metathesis (Figure 18). The rearrangement of alkylidenes was initially believed to involve a bis(alkylidene)metal intermediate where both olefin ligands were coordinated with the metal atom. C-C double bonds are combined with metal-carbene complexes over two cycles of addition and cycloreversion as the next step, in olefin metathesis. Chemicals such as petrochemicals, polymers, oleochemicals, and specialty chemicals are obtainable through new routes made possible by Olefin metathesis. Interestingly, a growing number of natural products are also synthesized via the advanced ring-closing metathesis, which occurs similarly in olefin metathesis by biosynthesis of olefin derivatives (olefinic monomers and polyolefins) [341,342].
Figure 18.
General scheme of olefin metathesis.
Synthesized Polyolefins from Biobased Monomers
Polyolefins (POs) are synthetic polymers composed of olefinic monomers. Globally, they are produced and consumed in very large quantities. In recent years, the interest in polyolefins has sharply increased due to their wide range of properties and applications, recyclability, and low cost. They can be classified into ethylene-based polyolefins, propylene-based polyolefins, higher polyolefins, and polyolefin elastomers based on their monomeric unit and chain structure. There are many products that can arise from these polymers, including containers, grocery bags, home appliances, toys, automobile parts, adhesives, prosthetic implants, and engineering plastics. These materials are thermoplastic elastomers, thermosets, or thermoplastics and can be either amorphous or highly crystalline. This polymer acts as films, pipes/profiles, and fibers; therefore, they can be molded into various shapes and coated over other materials [343,344]. In light of the rapid rise in the mineral oil price, global dependency on oil-derived products, and rising concerns about diminishing supplies of these resources in the not-too-distant future, efforts are being made to replace nonrenewable raw materials in the petrochemical industry with renewable ones. In this context, a number of polyolefins synthesized from biobased monomers have been recommended, such as polyethylene (PE), polypropylene (PP), polyisobutylene, polyisoprene, and polybutadiene, in an effort to solve environmental concerns associated with petroleum-derived plastics. Besides, algae can fix CO2 by biological processes (as an advantage) in order to reduce ecosystem CO2 levels. Natural polyolefins can be substituted with petroleum-based polyolefins with regard to their properties and applications by using biobased monomers obtained from renewable feedstocks, such as algae. For instance, polyolefin/polysaccharide/biocomposites obtained from algae can be utilized in the automotive industry and industrial and biomedical applications such as tissue engineering and cell culture [345,346,347].
7. Marine Algal-Derived Fatty Acids
As the effects of global warming continue to worsen, there is a growing need to find alternatives to traditional fossil fuels. Biodiesel is one such alternative that shows great promise as a carbon-neutral fuel. Algal polyunsaturated fatty acids (PUFAs) are characterized by the presence of double bonds located three carbon atoms away from the terminal methyl group and have shown great use in the fuel industry. Two of these known fatty acids are eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Eicosapentaenoic acid contains 20 carbon atoms and five cis-double bonds, while docosahexaenoic acid has 22 carbon atoms and six cis-double bonds [348]. These fatty acids can make up to 22% of the algae’s weight [349]. For instance, a type of algae called Nannochloropsis salina produces triglycerides that contain fatty-acid chains ranging from 14 to 22 carbons in length. Apart from the health benefits, choosing algal oil can also have environmental advantages [348,350,351]. Algae grow much faster than terrestrial plants (up to 50 times faster), and they absorb CO2 from the environment while accumulating lipids. Algae can also be used as a feedstock for biodiesel, which is a type of transportation fuel [352]. These photosynthetic organisms have the potential to produce up to 547,830 US tons of biodiesel annually. This amount of production can satisfy 10% of the current biodiesel market while also producing 12,000 US tons of omega-3 fatty acids annually. This production level is estimated to meet 30% of the U.S. omega-3 fatty-acid market in 2024 [352]. As a result, developing a microalgal biorefinery that utilizes valuable components produced by the algal cell could reduce the cost of biofuels and satisfy bioproduct markets [352]. Besides, they are increasingly in demand in the global food and nutraceutical markets [349,353]. Research has demonstrated that they can bring down the incidence of cardiovascular issues [354], aid in the development of infants [355], lower high blood pressure [354,356], help to reduce inflammation [357], and boost immune system function [358].
8. Phlorotannins
Phlorotannins are a class of polyphenolic compounds that are produced in exceptional abundance by members of the Phaeophyceae class of macroalgae. The distinguishing characteristic of this chemical class is that they are comprised of the monomeric unit phloroglucinol. There are scattered reports of phloroglucinol derivatives found in terrestrial plants. However, their abundance and diversity from other marine algae groups or terrestrial sources are minimal compared to Phaeophytes. There are several subclasses of phlorotannins, which are defined by the manner in which phloroglucinol monomers are linked. These include ether linkages (Phlorethols and Fuhalols), phenyl linkages (Fucols), and dibenzodioxin linkages (Eckols and Carmalols). In nature, phlorotannins occur as complex mixtures ranging from molecular weights in the 126 MW to >600,000 MW range and are often comprised of diverse combinations of multiple linkage types. However, there are some chemotaxonomic effects that influence the relative abundance of different phlorotannin classes present in different genera—for example, the high abundance of Fucols in members of the genus Fucus [359]. For biorefinery applications, the consideration of the phlorotannin type and composition of the species selected for biomass processing may be a relevant factor regarding the ultimate feasibility and yield of the desired downstream commodity chemicals. A considerable amount of effort has been given to optimizing the extraction of phlorotannins and techniques for evaluating their respective yields from various Phaeophytes, which are optimally extracted with aqueous alcohol or acetone with yields ranging from ~100 mg to ~50,000 mg per 100 g dry weight (as measured by gallic acid equivalence—GAE) [360]. The high yields and reported antioxidant activity of phlorotannins have led to their development as food ingredients and nutraceuticals as replacements for additives that are currently synthetically derived from petroleum by-products [361]. Additionally, particular interest has been given to sourcing phlorotannins from dedicated aquaculture systems that are already in place to produce kelp species that are commonly consumed or used in the industrial production of alginates. (e.g., Saccharina latissima, Laminaria digitata, Macrocystis pyrifera) [362,363]. One emerging trend is the growing interest in developing large-scale macroalgae aquaculture and biorefining to capture carbon and produce industrial commodities, such as biofuels, pharmaceuticals, and platform chemicals, in a more sustainable production system than those currently in place [364,365]. As a result, this field is among the fastest-growing agricultural sectors and has garnered substantial international attention and investment. As macroalgae processing technologies advance and improvements are made to their feasibility at scale, they will progress towards cost-effective alternatives to petroleum products with a unique variety of biologically active compounds that show potential as sources for both replacements of synthetically derived additives and starting materials for important platform chemicals [366]. One such example is the conversion of abundant polymeric phlorotannins into phloroglucinol monomers that could be refined and converted into phenol to serve as the starting material for the blockbuster drug Aspirin (Figure 19).
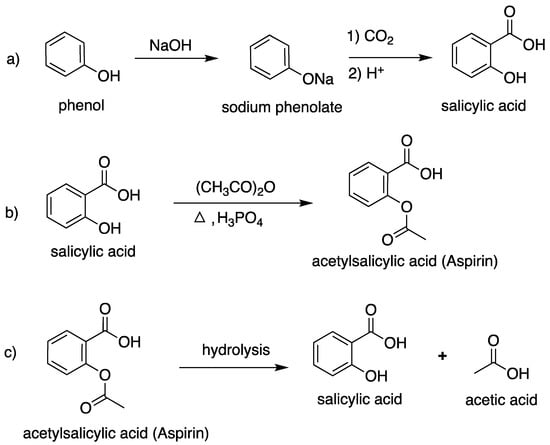
Figure 19.
Chemical pathways to synthetic NSAIDs were originally derived from nature. (a) phenol, a common petrochemical starting material, can be used to produce synthetic salicylic acid; (b) salicylic acid (isolated or synthesized) is acetylated at its benzene ring’s hydroxyl group, resulting in synthetic acetylsalicylic acid (Aspirin); (c) synthesized Aspirin is metabolized back to its precursor, salicylic acid, via hydrolysis.
9. Replacement of Petrochemicals Utilized in the Chemical Synthesis of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) and Other Pharmaceuticals with Natural Counterparts
Approximately 99% of pharmaceutical drugs, including nonsteroidal anti-inflammatory drugs, are chemically synthesized via petroleum-derived platform chemicals (petrochemicals) [367]. Aspirin, although a synthetic derivative of the natural product salicylic acid, isolated from the bark of willow trees (Salix spp.) and other species, is an example of a pharmaceutical that is produced commercially via synthesis rather than from natural extraction (Figure 19). The pain-relieving properties of naturally isolated salicylic acid have been documented for thousands of years, but a history of reported gastrointestinal issues has resulted in the synthesis of a modernized drug widely known today as aspirin [368]. This easily digestible variant (acetylsalicylic acid) was synthesized in 1897 via modification of the benzene ring’s hydroxyl group, which is ultimately metabolized back to salicylic acid by the body via hydrolysis (Figure 20) [369]. The first synthetic alternatives to naturally isolated salicylic acid were produced by organic chemists during the early 19th century, which utilized organic by-products, specifically phenol, from the synthetic dye and perfume industries [368].
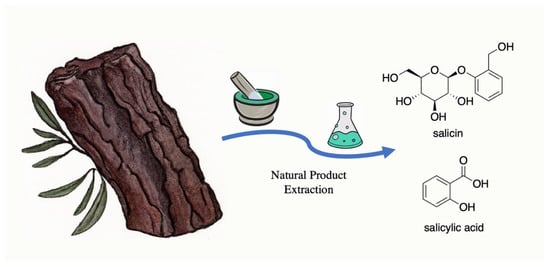
Figure 20.
The Willow tree (e.g., Salix alba) bark contains anti-inflammatory flavonoids (e.g., salicin) and the precursor/metabolized form of aspirin, salicylic acid.
Bayer and other pharmaceutical companies still use the synthetic chemistry route for producing acetylsalicylic acid to produce a centuries-old blockbuster drug that has been used by millions around the world. Nowadays, as mentioned, almost all therapeutics are synthesized from petroleum-derived platform chemicals due to financial and scalability incentives. This comes with an environmental cost regarding greenhouse gas emissions, resulting in carbon-positive pharmaceutical production.
Marine-derived polyphenolic compounds are potential solutions for combating industrially induced climate change by serving as natural, renewable, and sustainable starting materials. Phloroglucinol polymers (phlorotannins), flavonoid polymers (tannins), and other classes of marine polyphenols are largely common in marine vascular plants and brown macroalgae (Phaecophyceae class) [370]. The large-scale production of biological and renewable phenol is possible via the conversion of phloroglucinol polymers, mainly sourced from brown macroalgae. These “bio-phenols” can potentially replace petroleum-based starting materials, such as phenol, with toxic and nonbiodegradable properties that are detrimental to the environment [371]. The gradual transition to sustainable and renewable alternatives to petrochemical starting materials is crucial for mitigating CO2 and other greenhouse gas emissions, as well as fighting against the spread of toxic or nonbiodegradable microplastics.
10. Solutions and Perspectives
Advancements in microbial biotechnology and synthetic biology are paving the way for the scalable production of marine small molecules (e.g., monoterpenes), overcoming limitations of traditional extraction from macroorganisms [338,372]. By utilizing engineered bacteria, yeast, or microalgae, production can be more efficient than conventional methods. However, economic and technical challenges, such as low yields, toxicity, and high costs, must be addressed. Solutions like genetic engineering, integrated bioprocessing, and collaborative research are crucial for making industrial-scale production both economically viable and environmentally sustainable [373,374,375,376]. Marine biomass-based production undeniably presents a strong opportunity for sustainable alternatives to fossil and terrestrial methods, especially in significantly reducing greenhouse gas emissions and minimizing land-use impacts. However, the current high production costs and energy demands severely hinder economic competitiveness. To overcome these challenges, integrated biorefinery approaches, technological innovations, and robust policy incentives are essential. While microalgae systems may not yet be economically viable, they can produce high-value products [377,378]. In contrast, macroalgae systems demonstrate a greater cost-competitiveness when it comes to biofuels. Fortunately, there are TEA and LCA data that provide valuable insights into the superiority of marine biomass production—utilizing microalgae, macroalgae, and other aquatic organisms—compared to conventional commercial methods based on fossil fuels or terrestrial biomass [379,380,381]. These studies decisively evaluate the economic viability and environmental impacts of marine biomass systems, with a strong emphasis on biofuels, bioproducts, and high-value compounds such as monoterpenes.
The integration of synthetic biology, biorefinery systems, and advanced cultivation technologies will be realized within the next 3–5 years, paving the way for industrial-scale production of marine small molecules, such as monoterpenes, by 2030–2035 [379]. Pilot-scale facilities are set to deliver significant improvements and substantial cost reductions through co-product valorization and robust policy support.
Below is a concise summary of the commercial or pilot-plant scale production status of marine-derived molecules, specifically monoterpenes, fatty acids, and phlorotannins, discussed in this review:
- Commercial Scale: Omega-3 fatty acids (EPA, DHA)
- Pilot-Plant Scale: Phlorotannins are now being produced at the pilot scale, and excitingly, several commercial products are already available for consumers [382,383]. This marks a significant step forward in harnessing the benefits of these powerful compounds.
- Not Scaled yet: Marine monoterpenes remain at laboratory scale due to economic and technical challenges.
A promising strategy for scaling up marine monoterpene production to pilot or industrial levels involves leveraging synthetic biology and integrated biorefinery systems that utilize engineered marine microalgae and microbial cell factories, such as Yarrowia lipolytica, E. coli, Synechococcus, Nannochloropsis spp., and Rhodotorula toruloides [338]. By combining robust metabolic engineering with advanced cultivation systems and ISPR techniques, we will effectively address critical challenges like low yields, cytotoxicity, and high costs [338]. This approach will undoubtedly enable us to achieve cost-competitive production within the next 5–10 years approach will undoubtedly enable us to achieve cost-competitive production within the next 5–10 years.
- Metabolic Engineering for Higher Yields: The strategic overexpression of essential enzymes in MVA and MEP pathways, particularly limonene synthase, has proven successful, achieving production levels of up to 393.5 mgL−1 of limonene in R. toruloides [373]. With continued optimization, we are confident that we can reach yields of 1 to 5 gL−1, positioning us firmly within the realm of commercial viability. Furthermore, pilot-scale bioreactors designed to operate at 100 to 1000 L, utilizing these engineered strains, will be operational within the next 3 to 5 years. This advancement will enable the production of monoterpenes like limonene for high-value applications in fragrances and pharmaceuticals [373].
- Advanced Cultivation and ISPR: ISPR techniques, including small molecule in situ resin capture (SMIRC), reliably mitigate cytotoxicity by continuously removing volatile monoterpenes. The pilot-scale trials with Saccharomyces cerevisiae have consistently achieved stable production in 20-L bioreactors [338]. It firmly asserts that hybrid photobioreactor (PBR) systems utilizing ISPR are poised to scale up to 10,000-L pilot plants in the next 3–5 years [338]. This robust approach will not only significantly reduce energy costs but also drive industrial-scale production, particularly when integrated with renewable energy sources.
11. Conclusions
In summary, this review highlights the immense potential of marine metabolites as renewable and sustainable carbon-negative alternatives to petroleum-based chemicals. They offer diverse applications in pharmaceuticals, polymers, cosmetics, and other consumer products that can be made industrially by 2030–2035 through advancements in microbial engineering, optimized cultivation systems (e.g., macroalgae or microbial fermentation), and efficient extraction methods, such as ISPR and olefin metathesis with green ethylene. These innovations enable the production of high-value platform chemicals, such as styrene or propylene precursors, while addressing challenges like low yields, toxicity, high costs, and seasonal variability.
TEA and LCA underscore the environmental benefits of marine biomass over fossil- or terrestrial-based methods, including reduced GHG emissions and land-use impacts. However, high production costs and energy demands necessitate integrated biorefinery approaches, synthetic biology, and policy incentives to ensure economic competitiveness. Challenges such as catalyst decomposition in olefin metathesis and undesirable by-products in microbial fermentation require further research to enhance yield, purity, and environmental viability.
The structural diversity of marine monoterpenes, sourced from photosynthesizing organisms like macroalgae or naturally emitted BVOCs, supports their role as precursor molecules for chemical conversions into pharmaceutical intermediates and eco-friendly products. The shift toward green chemistry hinges on interdisciplinary collaboration, government legislation, and profit-driven incentives to globalize bio-based carbon-negative alternatives, ultimately decarbonizing industries reliant on fossil resources. By leveraging affordable substrates and biotransformation strategies, marine monoterpene production can meet commercial demands, revolutionizing the future of sustainable and renewable fine and specialty chemicals.
Author Contributions
Conceptualization: M.B., M.M.B. and M.T.H.; investigation: M.B.; writing—original draft preparation: M.B., M.M.B., S.C., A.H. and G.S.H.; drawing molecule structures: M.B., M.M.B., A.H. and G.S.H.; writing—review and editing: M.B., M.M.B., A.H. and M.T.H.; supervision: M.T.H. All authors have read and agreed to the published version of the manuscript.
Funding
Preparation of this review was supported in part by NIGMS R01GM145845-01 to M.T.H.
Data Availability Statement
No datasets were generated or analyzed during the current study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| PE | Polyethylene |
| TLA | Three-letter acronym |
| ACCO | 1-aminocyclopropane-1-carboxylic acid oxidase |
| CoA | Coenzyme A |
| TCA | Tricarboxylic cycle |
| MVA | Mevalonate |
| MEP | Methylerythritol phosphate |
| BVOC | Biogenic volatile organic compound |
| N/A | Not available |
| Et | Ethylene |
| PET | Polyethylene terephthalate |
| NREL | National Renewable Energy Laboratory |
| DMS | Dimethylsulphide |
| DMSP | Dimethylsulphoniopropionate |
| PVA | Polyvinyl acetate |
| LA | Lactic acid |
| PLA | Polylactide |
| TCA | Tricarboxylic acid cycle |
| PDO/PD | Propanediol |
| ABE | Acetone, butanol, and ethanol |
| EMA | European Medicines Agency |
| SA | Succinic acid |
| BDO | Butanediol |
| MS | Multiple sclerosis |
| DMAPP | Dimethylallyl diphosphate |
| IPP | Isopentenyl diphosphate |
| GPP | Geranyl pyrophosphate |
| FPP | Farnesyl pyrophosphate |
| GGPP | Geranylgeranyl pyrophosphate |
| IEA | International Energy Agency |
| 5-HMF | 5-Hydroxymethylfurfural |
| FDCA | 2,5-furandicaboxylic acid |
| PEF | Polyethylene furanoate |
| CA | Citric acid |
| BTX | Aromatics benzene, toluene, and xylene |
| bPMs | Bio-platform molecules |
| HMF | Hydroxymethylfuran |
| DOE | Department of Energy |
| VAM | Vinyl acetate monomer |
| M-terpenes | Monoterpenes |
| NPs | Natural products |
| HDR | 4-hydroxy-3-methylbut-2-enyl diphosphate reductase |
| DXP | Deoxyxylulose-5-phosphate |
| G3P | Glyceraldehyde-3-phosphate |
| LPP | Linalyl pyrophosphate |
| NPP | Neryl pyrophosphate |
| GDP | Geranyl diphosphate |
| LDP | Linalyldiphosphate |
| BDP | Bornyldiphosphate |
| la | Adriadysiolide |
| PKS | Polyketide synthetase |
| Pd | Palladium |
| MTO | Methyltrioxorhenium |
| TBAB | Tetrabutylammonium bromide |
| PMMA | Poly(methyl methacrylate) |
| PC | Polycarbonate |
| COPs | Cyclic olefin polymers |
| VOC | Volatile organic compound |
| OM | Olefin metathesis |
| POs | Polyolefins |
| PP | Polypropylene |
| SOA | Secondary organic aerosol |
| ppb | Parts per billion |
| V | Volume |
| nmol | Nanomole |
| pmol | Picomole |
| TIC | Total ion current |
| Chl a | Chlorophyll a |
| 1pmol/L/Chl a | Picomol per liter of chlorophyll a |
| TgC/yr | Tetragrams of carbon per year |
| PUFAs | Polyunsaturated fatty acids |
| EPA | Eicosapentaenoic acid |
| DHA | Docosahexaenoic acid |
| ISPR | In situ product recovery |
| SMIRC | Small molecule in situ resin capture |
| PBR | Hybrid photobioreactor |
| TEA | Techno-economic analysis |
| LCA | Life cycle assessment |
References
- Mani, R.; Bhattacharya, M. Properties of injection moulded blends of starch and modified biodegradable polyesters. Eur. Polym. J. 2001, 37, 515–526. [Google Scholar] [CrossRef]
- Dhurjati, P. Biorefineries-Industrial Processes and Products, Status Quo and Future Directions: Volumes 1 and 2 By Birgit Kamm, Patrick Gruber and Michael Kamm; Wiley Online Library: Hoboken, NJ, USA, 2008. [Google Scholar]
- Sharma, B.; Shrestha, A. Petroleum dependence in developing countries with an emphasis on Nepal and potential keys. Energy Strat. Rev. 2023, 45, 101053. [Google Scholar] [CrossRef]
- Iqbal, H.M.N.; Kyazze, G.; Tron, T.; Keshavarz, T. Laccase-Assisted Approach to Graft Multifunctional Materials of Interest: Keratin-EC Based Novel Composites and their Characterisation. Macromol. Mater. Eng. 2015, 300, 712–720. [Google Scholar] [CrossRef]
- Iqbal, H.M.N. Development of Bio-Composites with Novel Characteristics Through Enzymatic Grafting. Ph.D. Thesis, University of Westminster, London, UK, 2015. [Google Scholar]
- Huda, M.S.; Drzal, L.T.; Misra, M.; Mohanty, A.K.; Williams, K.; Mielewski, D.F. A Study on Biocomposites from Recycled Newspaper Fiber and Poly(lactic acid). Ind. Eng. Chem. Res. 2005, 44, 5593–5601. [Google Scholar] [CrossRef]
- Pachapur, V.L.; Sarma, S.J.; Brar, S.K.; Chaabouni, E. Platform chemicals: Significance and need. In Platform Chemical Biorefinery; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–20. [Google Scholar]
- Iqbal, H.M.N.; Kyazze, G.; Keshavarz, T. Advances in the Valorization of Lignocellulosic Materials by Biotechnology: An Overview. BioResources 2013, 8, 3157–3176. [Google Scholar] [CrossRef]
- Kissman, E.N.; Sosa, M.B.; Millar, D.C.; Koleski, E.J.; Thevasundaram, K.; Chang, M.C.Y. Expanding chemistry through in vitro and in vivo biocatalysis. Nature 2024, 631, 37–48. [Google Scholar] [CrossRef]
- Johnson, E.T.; Schmidt-Dannert, C. Light-energy conversion in engineered microorganisms. Trends Biotechnol. 2008, 26, 682–689. [Google Scholar] [CrossRef]
- Koeller, K.M.; Wong, C.-H. Enzymes for chemical synthesis. Nature 2001, 409, 232–240. [Google Scholar] [CrossRef]
- Keasling, J.D. Synthetic biology for synthetic chemistry. ACS Chem. Biol. 2008, 3, 64–76. [Google Scholar] [CrossRef]
- Hara, K.Y.; Araki, M.; Okai, N.; Wakai, S.; Hasunuma, T.; Kondo, A. Development of bio-based fine chemical production through synthetic bioengineering. Microb. Cell Factories 2014, 13, 173. [Google Scholar] [CrossRef]
- Odian, G. Principles of Polymerization; John Wiley & Sons. Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Hausinger, R.P.; Rifayee, S.B.; Thomas, M.G.; Chatterjee, S.; Hu, J.; Christov, C.Z. Biological formation of ethylene. RSC Chem. Biol. 2023, 4, 635–646. [Google Scholar] [CrossRef]
- Plettner, I.; Steinke, M.; Malin, G. Ethene (ethylene) production in the marine macroalga Ulva (Enteromorpha) intestinalis L.(Chlorophyta, Ulvophyceae): Effect of light-stress and co-production with dimethyl sulphide. Plant Cell Environ. 2005, 28, 1136–1145. [Google Scholar] [CrossRef]
- Market Size of Ethylene Worldwide in 2021, with a Forecast Until 2030. 2023. Available online: https://www.statista.com/statistics/1349781/ethylene-global-market-size/ (accessed on 24 March 2023).
- Production Capacity of Ethylene Worldwide from 2018 to 2023. 2023. Available online: https://www.statista.com/statistics/1067372/global-ethylene-production-capacity/#statisticContainer (accessed on 24 March 2023).
- Bloch, K.; Rittenberg, D. Sources of Acetic Acid in the Animal Body. J. Biol. Chem. 1944, 155, 243–254. [Google Scholar] [CrossRef]
- Yang, H.; Chen, T.; Wang, M.; Zhou, J.; Liebl, W.; Barja, F.; Chen, F. Molecular biology: Fantastic toolkits to improve knowledge and application of acetic acid bacteria. Biotechnol. Adv. 2022, 58, 107911. [Google Scholar] [CrossRef]
- Market Volume of Acetic Acid Worldwide from 2015 to 2022, with a Forecast for 2023 to 2030. 2023. Available online: https://www.statista.com/statistics/1245203/acetic-acid-market-volume-worldwide/ (accessed on 20 March 2023).
- Acetic Acid Market Size, Share & Industry Analysis, by Application (Vinyl Acetate Monomer (VAM), Purified Tereph-Thalic Acid (PTA), Ester Solvents, Acetic Anhydride, and Others), by End-Use Industry (Plastics & Polymers, Food & Beverages, Adhesives, Paints, and Coatings, Textile, and Others) and Regional Forecast, 2024–2032. 2021. Available online: https://www.fortunebusinessinsights.com/acetic-acid-market-103386 (accessed on 4 August 2025).
- Eş, I.; Khaneghah, A.M.; Hashemi, S.M.B.; Koubaa, M. Current advances in biological production of propionic acid. Biotechnol. Lett. 2017, 39, 635–645. [Google Scholar] [CrossRef]
- Market Volume of Propionic acid Worldwide from 2015 to 2022, with a Forecast for 2023 to 2030. 2023. Available online: https://www.statista.com/statistics/1245247/propionic-acid-market-volume-worldwide/ (accessed on 20 March 2023).
- Global Propionic Acid Market–Industry Trends and Forecast to 2029. 2022. Available online: https://www.databridgemarketresearch.com/reports/global-propionic-acid-market (accessed on 4 August 2022).
- Nguyen, N.K.; Dong, N.T.N.; Nguyen, H.T.; Le, P.H. Lactic acid bacteria: Promising supplements for enhancing the biological activities of kombucha. SpringerPlus 2015, 4, 91. [Google Scholar] [CrossRef]
- Lin, H.T.; Huang, M.Y.; Kao, T.Y.; Lu, W.J.; Lin, H.J.; Pan, C.L. Production of lactic acid from seaweed hydrolysates via lactic acid bacteria fermentation. Fermentation 2020, 6, 37. [Google Scholar] [CrossRef]
- Nagarajan, D.; Chen, C.-Y.; Ariyadasa, T.U.; Lee, D.-J.; Chang, J.-S. Macroalgal biomass as a potential resource for lactic acid fermentation. Chemosphere 2022, 309, 136694. [Google Scholar] [CrossRef]
- Sudhakar, M.; Dharani, G. Evaluation of seaweed for the production of lactic acid by fermentation using Lactobacillus plantarum. Bioresour. Technol. Rep. 2021, 17, 100890. [Google Scholar] [CrossRef]
- Lactic Acid Market Size, Share & Industry Analysis, by Raw Material (Sugarcane, Corn, Yeast Extract, and Others), by Form (Liquid and Dry), by Application (Polylactic Acid, Food & Beverages, Pharmaceutical, Cosmetics & Personal Care, and Others), and Regional Forecast, 2024–2032. 2022. Available online: https://www.fortunebusinessinsights.com/lactic-acid-market-102119 (accessed on 4 January 2022).
- Market Volume of Lactic Acid Worldwide from 2015 to 2022, with a Forecast for 2023 to 2030. 2023. Available online: https://www.statista.com/statistics/1310495/lactic-acid-market-volume-worldwide/ (accessed on 20 March 2023).
- Yang, H.; Zhang, C.; Lai, N.; Huang, B.; Fei, P.; Ding, D.; Hu, P.; Gu, Y.; Wu, H. Efficient isopropanol biosynthesis by engineered Escherichia coli using biologically produced acetate from syngas fermentation. Bioresour. Technol. 2020, 296, 122337. [Google Scholar] [CrossRef] [PubMed]
- Le, H.T.Q.; Lee, E.Y. Biological production of 2-propanol from propane using a metabolically engineered type I methanotrophic bacterium. Bioresour. Technol. 2022, 362, 127835. [Google Scholar] [CrossRef]
- Market Volume of Isopropyl Alcohol Worldwide from 2015 to 2022, with a Forecast for 2023 to 2030. 2023. Available online: https://www.statista.com/statistics/1245200/isopropyl-alcohol-market-volume-worldwide/ (accessed on 20 March 2023).
- Tao, Y.M.; Bu, C.Y.; Zou, L.H.; Hu, Y.L.; Zheng, Z.J.; Ouyang, J. A comprehensive review on microbial production of 1,2-propanediol: Micro-organisms, metabolic pathways, and metabolic engineering. Biotechnol. Biofuels 2021, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Saxena, R.K.; Anand, P.; Saran, S.; Isar, J.; Agarwal, L. Microbial production and applications of 1,2-propanediol. Indian J. Microbiol. 2010, 50, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Merriman, L.R. Algae to Propellants: Biosynthesis of 1,2-Propanediol from Algal Derived Sugars, in Chemical Engineering. Ph.D. Thesis, University Of Arkansas, Fayetteville, AR, USA, 2013. [Google Scholar]
- Saxena, R.; Anand, P.; Saran, S.; Isar, J. Microbial production of 1,3-propanediol: Recent developments and emerging opportunities. Biotechnol. Adv. 2009, 27, 895–913. [Google Scholar] [CrossRef]
- 1,3-Propanediol Market—By Source (Bio-Based, Petrochemical-Based), by Application (Polyurethane {Textile, Footwear, Automotive, Furniture}, Polytrimethylene Terephthalate, Personal Care & Detergents, Pharmaceutical, Food & Beverages, Heat Transfer Fluid, Inks and Coatings) & Global Forecasts to 2027. 2021. Available online: https://www.gminsights.com/industry-analysis/1-3-propanediol-market (accessed on 4 January 2021).
- Salvachúa, D.; Saboe, P.O.; Nelson, R.S.; Singer, C.; McNamara, I.; del Cerro, C.; Chou, Y.-C.; Mohagheghi, A.; Peterson, D.J.; Haugen, S.; et al. Process intensification for the biological production of the fuel precursor butyric acid from biomass. Cell Rep. Phys. Sci. 2021, 2, 100587. [Google Scholar] [CrossRef]
- Global Butyric Acid Market Size by Type (Synthetic, Renewable), by Application (Pharmaceutical, Biofuel, Food Additive & Flavoring, Animal Feed, Cosmetic, Plasticizer, Leather Tanning), Industry Analysis Report, Regional Outlook, Application Development Potential, Price Trend, Competitive Market Share & Forecast, 2021–2027. 2021. Available online: https://www.gminsights.com/industry-analysis/butyric-acid-market (accessed on 4 March 2021).
- Lan, E.I.; Liao, J.C. Microbial synthesis of n-butanol, isobutanol, and other higher alcohols from diverse resources. Bioresour. Technol. 2013, 135, 339–349. [Google Scholar] [CrossRef]
- Market Volume of n-Butanol Worldwide from 2015 to 2022, with a Forecast for 2023 to 2030. Available online: https://www.statista.com/statistics/1245211/n-butanol-market-volume-worldwide/ (accessed on 20 March 2023).
- Nghiem, N.P.; Kleff, S.; Schwegmann, S. Succinic Acid: Technology Development and Commercialization. Fermentation 2017, 3, 26. [Google Scholar] [CrossRef]
- Sahoo, K.K.; Datta, S.; Nayak, A.; Pranaw, K.; Dutta, D.; Goswami, G. Biological production of succinic acid: State of the art and future perspectives. Ind. Microbiol. Biotechnol. 2022, 427–461. [Google Scholar] [CrossRef]
- GVR Report Cover, Succinic Acid Market Size, Share & Trends Report. Succinic Acid Market Size, Share & Trends Analysis Report by Type (Petro-based, Bio-based), by End Use (Food & Beverages, Pharmaceuticals, Industrial), by Region (North America, Europe, APAC, CSA, MEA), and Segment Forecasts, 2022–2030. Available online: https://www.grandviewresearch.com/industry-analysis/succinic-acid-market (accessed on 4 January 2021).
- Goldberg, I.; Rokem, J.S.; Pines, O. Organic acids: Old metabolites, new themes. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2006, 81, 1601–1611. [Google Scholar] [CrossRef]
- Mondala, A.H. Direct fungal fermentation of lignocellulosic biomass into itaconic, fumaric, and malic acids: Current and future prospects. J. Ind. Microbiol. Biotechnol. 2015, 42, 487–506. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Zhang, J.; Chen, Y.; Xu, Q.; Song, P.; Li, Y.; Li, K.; Liu, H. Recent advances in microbial production of L-malic acid. Appl. Microbiol. Biotechnol. 2022, 106, 7973–7992. [Google Scholar] [CrossRef]
- Malic Acid Market Size, Share & Trends Analysis Report by Product (L-Malic Acid, D-Malic Acid, DL-Malic Acid), by End-Use (Food & Beverage, Pharmaceuticals, Personal Care & Cosmetics, Textiles), by Region, and Segment Forecasts, 2025–2030. 2022. Available online: https://www.grandviewresearch.com/industry-analysis/malic-acid-market (accessed on 4 January 2022).
- Fumaric Acid Market Outlook (2023 to 2033). 2023. Available online: https://www.factmr.com/report/526/fumaric-acid-market (accessed on 4 January 2023).
- Mori, Y.; Noda, S.; Shirai, T.; Kondo, A. Direct 1,3-butadiene biosynthesis in Escherichia coli via a tailored ferulic acid decarboxylase mutant. Nat. Commun. 2021, 12, 2195. [Google Scholar] [CrossRef] [PubMed]
- 1,3 Butadiene (BD) Market Share, Size, Trends, Industry Analysis Report, by Type (Extractive Distillation, and Oxidative Dehydrogenation); By Application; By Region; Segment Forecast, 2022–2030. 2022. Available online: https://www.polarismarketresearch.com/industry-analysis/1-3-butadiene-market (accessed on 4 August 2022).
- Morais, A.R.; Dworakowska, S.; Reis, A.; Gouveia, L.; Matos, C.T.; Bogdał, D.; Bogel-Łukasik, R. Chemical and biological-based isoprene production: Green metrics. Catal. Today 2015, 239, 38–43. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Monson, R.K. Isoprene research–60 years later, the biology is still enigmatic. Plant Cell Environ. 2017, 40, 1671–1678. [Google Scholar] [CrossRef]
- McGenity, T.J.; Crombie, A.T.; Murrell, J.C. Microbial cycling of isoprene, the most abundantly produced biological volatile organic compound on Earth. ISME J. 2018, 12, 931–941. [Google Scholar] [CrossRef]
- Isoprene Market Research Report Information by Grade (Polymer Grade And Chemical Grade), by Application (Styrene Isoprene Styrene, Isobutylene Isoprene, Polyisoprene, Block Co-Polymer, and Others), by End-Use Industry (Automotive, Medical, Construction, Personal Care, Sports & Leisure and Others) and by Region (North America, Europe, Asia-Pacific, and Rest of the World) –Market Forecast Till 2032. 2023. Available online: https://www.marketresearchfuture.com/reports/isoprene-industry-4799 (accessed on 4 August 2023).
- Deng, Y.; Ma, L.; Mao, Y. Biological production of adipic acid from renewable substrates: Current and future methods. Biochem. Eng. J. 2016, 105, 16–26. [Google Scholar] [CrossRef]
- Market Volume of Adipic Acid Worldwide in 2018 and 2023. 2023. Available online: https://www.statista.com/statistics/1113587/global-market-size-adipic-acid/ (accessed on 24 March 2023).
- Adipic Acid Market Size, Share & Trends Analysis Report by Application (Nylon 6, 6 Fiber, Nylon 6, 6 Resin, Polyurethane, Adipate Esters), by Region (North America, Europe, Asia Pacific), and Segment Forecasts, 2023–2030. 2022. Available online: https://www.grandviewresearch.com/industry-analysis/adipic-acid-market/toc (accessed on 24 March 2023).
- Aoki, K.; Uemori, T.; Shinke, R.; Nishira, H. Further Characterization of Bacterial Production of Anthranilic Acid from Aniline. Agric. Biol. Chem. 1985, 49, 1151–1158. [Google Scholar] [CrossRef]
- Anthranilic Acid Market- Size, Share, Industry Trends, and Forecasts (2025–2032). Available online: https://www.consegicbusinessintelligence.com/anthranilic-acid-market (accessed on 9 January 2025).
- Anthranilic Acid Market Size, Share, Growth, and Industry Analysis, by Types (Pharmaceutical Grade, Industrial Grade), Applications (Dye, Pharmaceutical, Others) and Regional Insights and Forecast to 2033. 2024. Available online: https://www.globalgrowthinsights.com/market-reports/anthranilic-acid-market-104384 (accessed on 23 May 2024).
- Ledesma, E.B.; Marsh, N.D.; Sandrowitz, A.K.; Wornat, M.J. An experimental study on the thermal decomposition of catechol. Proc. Combust. Inst. 2002, 29, 2299–2306. [Google Scholar] [CrossRef]
- Catechol Market Size, Share & Trends Analysis Report by End-Use, by Region, and Segment Forecasts, 2023 To 2030. 2023. Available online: https://www.grandviewresearch.com/industry-analysis/catechol-market-report (accessed on 27 January 2023).
- Roell, G.W.; Carr, R.R.; Campbell, T.; Shang, Z.; Henson, W.R.; Czajka, J.J.; Martín, H.G.; Zhang, F.; Foston, M.; Dantas, G.; et al. A concerted systems biology analysis of phenol metabolism in Rhodococcus opacus PD 630. Metab. Eng. 2019, 55, 120–130. [Google Scholar] [CrossRef]
- Phenol Industry Capacity and Capital Expenditure Forecasts with Details of Active and Planned Plants to 2028. 2024. Available online: https://www.globaldata.com/store/report/phenol-market-analysis/ (accessed on 4 June 2024).
- Market Volume of Phenols Worldwide from 2015 to 2022, with a Forecast for 2023 to 2030. 2023. Available online: https://www.statista.com/statistics/979265/global-phenol-market-volume/ (accessed on 11 July 2023).
- Lee, K.; Bang, H.B.; Lee, Y.H.; Jeong, K.J. Enhanced production of styrene by engineered Escherichia coli and in situ product recovery (ISPR) with an organic solvent. Microb. Cell Factories 2019, 18, 79. [Google Scholar] [CrossRef] [PubMed]
- Styrene Market Analysis: Plant Capacity, Production, Process, Technology, Operating Efficiency, Demand & Supply, End-Use, Foreign Trade, Sales, Channel, Regional Demand, Company Share, 2015–2035. 2022. Available online: https://www.chemanalyst.com/industry-report/styrene-market-650 (accessed on 4 December 2022).
- Jeong, G.-T.; Kim, S.-K.; Park, D.-H. Application of solid-acid catalyst and marine macro-algae Gracilaria verrucosa to production of fermentable sugars. Bioresour. Technol. 2015, 181, 1–6. [Google Scholar] [CrossRef]
- Hou, Q.; Qi, X.; Zhen, M.; Qian, H.; Nie, Y.; Bai, C.; Zhang, S.; Bai, X.; Ju, M. Biorefinery roadmap based on catalytic production and upgrading 5-hydroxymethylfurfural. Green Chem. 2020, 23, 119–231. [Google Scholar] [CrossRef]
- Global 5-hydroxymethylfurfural (5-HMF) Market Size by Source (Renewable Sources, Non-Renewable Sources), by Application (Food & Beverage, Pharmaceuticals), by End-User Industry (Food Industry, Healthcare Industry), by Formulation (Liquid Form, Powdered Form), by Distribution Channel (Online Retailing, Pharmacies), by Geographic Scope and Forecast. 2025. Available online: https://www.verifiedmarketreports.com/product/5-hydroxymethylfurfural-5-hmf-market/ (accessed on 4 April 2025).
- Citric Acid Market Size, Share & Trends Analysis Report by Form (Liquid, Powder), by Application (Pharmaceutical, Food & Beverages, Others), by Region, and Segment Forecasts, 2023–2030. 2023. Available online: https://www.grandviewresearch.com/industry-analysis/citric-acid-market (accessed on 4 January 2023).
- Citric Acid Market Analysis: Plant Capacity, Production, Operating Efficiency, Demand & Supply, End-User Industries, Type, Sales Channel, Redional Demand Comnany Share Trade 2015–203. 2023. Available online: https://www.chemanalyst.com/industry-report/citric-acid-market-695 (accessed on 4 September 2023).
- Angumeenal, A.; Venkappayya, D. An overview of citric acid production. LWT Food Sci. Technol. 2013, 50, 367–370. [Google Scholar] [CrossRef]
- Behera, B.C.; Mishra, R.; Mohapatra, S. Microbial citric acid: Production, properties, application, and future perspectives. Food Front. 2021, 2, 62–76. [Google Scholar] [CrossRef]
- Zimmermann, H.; Walzl, R. Ethylene. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Verlag, Germany, 2005. [Google Scholar]
- Chen, Y.; Kuo, M.J.; Lobo, R.; Ierapetritou, M. Ethylene production: Process design, techno-economic and life-cycle assessments. Green Chem. 2024, 26, 2903–2911. [Google Scholar] [CrossRef]
- Chadwick, S.S. Ullmann’s Encyclopedia of Industrial Chemistry. Ref. Serv. Rev. 1988, 16, 31–34. [Google Scholar] [CrossRef]
- Seddon, D. Petrochemical Economics: Technology Selection in a Carbon Constrained World; Imperial College Press: London, UK; World Scientific: London, UK, 2010; Volume 8. [Google Scholar]
- Ethylene Global Market Report 2023. 2023. Available online: https://www.researchandmarkets.com/reports/5733833/ethylene-global-market-report (accessed on 4 March 2023).
- Papa, A. Propanols. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Verlag, Germany, 2011. [Google Scholar]
- Jang, Y.S.; Kim, B.; Shin, J.H.; Choi, Y.J.; Choi, S.; Song, C.W.; Lee, J.; Park, H.G.; Lee, S.Y. Bio-based production of C2–C6 platform chemicals. Biotechnol. Bioeng. 2012, 109, 2437–2459. [Google Scholar] [CrossRef]
- De Bruycker, R.; Anthonykutty, J.M.; Linnekoski, J.; Harlin, A.; Lehtonen, J.; Van Geem, K.M.; Räsänen, J.; Marin, G.B. Assessing the potential of crude tall oil for the production of green-base chemicals: An experimental and kinetic modeling study. Ind. Eng. Chem. Res. 2014, 53, 18430–18442. [Google Scholar] [CrossRef]
- Watanabe, T.; Kondo, N. Ethylene evolution in marine algae and a proteinaceous inhibitor of ethylene biosynthesis from red alga. Plant Cell Physiol. 1976, 17, 1159–1166. [Google Scholar] [CrossRef]
- Abeles, F.B.; Morgan, P.W.; Saltveit, M.E., Jr. Ethylene in Plant Biology; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Steinke, M.; Kirst, G.O. Enzymatic cleavage of dimethylsulfoniopropionate (DMSP) in cell-free extracts of the marine macroalga Enteromorpha clathrata (Roth) Grev. (Ulvales, Chlorophyta). J. Exp. Mar. Biol. Ecol. 1996, 201, 73–85. [Google Scholar] [CrossRef]
- Steinke, M.; Wolfe, G.; Kirst, G. Partial characterisation of dimethylsulfoniopropionate (DMSP) lyase isozymes in 6 strains of Emiliania huxleyi. Mar. Ecol. Prog. Ser. 1998, 175, 215–225. [Google Scholar] [CrossRef]
- Simó, R.; Archer, S.D.; Pedrós-Alió, C.; Gilpin, L.; Stelfox-Widdicombe, C.E. Coupled dynamics of dimethylsulfoniopropionate and dimethylsulfide cycling and the microbial food web in surface waters of the North Atlantic. Limnol. Oceanogr. 2002, 47, 53–61. [Google Scholar] [CrossRef]
- Gage, D.A.; Rhodes, D.; Nolte, K.D.; Hicks, W.A.; Leustek, T.; Cooper, A.J.L.; Hanson, A.D. A new route for synthesis of dimethylsulphoniopropionate in marine algae. Nature 1997, 387, 891–894. [Google Scholar] [CrossRef]
- Blunden, G.; Smith, B.E.; Irons, M.W.; Yang, M.-H.; Roch, O.G.; Patel, A.V. Betaines and tertiary sulphonium compounds from 62 species of marine algae. Biochem. Syst. Ecol. 1992, 20, 373–388. [Google Scholar] [CrossRef]
- Broadgate, W.J.; Malin, G.; Küpper, F.C.; Thompson, A.; Liss, P.S. Isoprene and other non-methane hydrocarbons from seaweeds: A source of reactive hydrocarbons to the atmosphere. Mar. Chem. 2004, 88, 61–73. [Google Scholar] [CrossRef]
- Edwards, D.M.; Reed, R.H.; Chudek, J.A.; Foster, R.; Stewart, W.D.P. Organic solute accumulation in osmotically-stressed Enteromorpha intestinalis. Mar. Biol. 1987, 95, 583–592. [Google Scholar] [CrossRef]
- Zhang, W.; Yamane, H.; Chapman, D.J. The Phytohormone Profile of the Red Alga Porphyra perforata. Bot. Mar. 1993, 36, 257–266. [Google Scholar] [CrossRef]
- Driessche, T.V.; Vries, G.M.P.-D.; Guisset, J.-L. Tansley Review No. 91 Differentiation, growth and morphogenesis: Acetabularia as a model system. New Phytol. 1997, 135, 1–20. [Google Scholar] [CrossRef]
- Ghooprasert, P.; Spencer, M. Preparation and purification of an enzyme system for ethylene synthesis from acrylate. Physiol. Veg. 1975, 13, 579–589. [Google Scholar]
- Sauer, M.; Porro, D.; Mattanovich, D.; Branduardi, P. Microbial production of organic acids: Expanding the markets. Trends Biotechnol. 2008, 26, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Hohenschutz, H.; Dinkhauser, G.; Franz, D.; Buelow, H. Production of Propionic Acid. United States Patent 3835185, 1974. Available online: https://patents.google.com/patent/US3835185A/en (accessed on 4 August 2025).
- Ahmadi, N.; Khosravi-Darani, K.; Mortazavian, A.M. An overview of biotechnological production of propionic acid: From upstream to downstream processes. Electron. J. Biotechnol. 2017, 28, 67–75. [Google Scholar] [CrossRef]
- Boyaval, P.; Corre, C. Production of propionic acid. Le Lait 1995, 75, 453–461. [Google Scholar] [CrossRef]
- Patil, S.D.; Papadmitrakopoulos, F.; Burgess, D.J. Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis. J. Control. Release 2007, 117, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Narěbska, A.; Staniszewski, M. Separation of fermentation products by membrane techniques. I. Separation of lactic acid/lactates by diffusion dialysis. Sep. Sci. Technol. 1997, 32, 1669–1682. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Niederauer, G.G.; Agrawal, C.M. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly (lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Oliveira, J.E.; Medeiros, E.S.; Cardozo, L.; Voll, F.; Madureira, E.H.; Mattoso, L.H.; Assis, O.B. Development of poly (lactic acid) nanostructured membranes for the controlled delivery of progesterone to livestock animals. Mater. Sci. Eng. C 2013, 33, 844–849. [Google Scholar] [CrossRef]
- Ademola, J.; Frazier, C.; Kim, S.J.; Theaux, C.; Saudez, X. Clinical evaluation of 40% urea and 12% ammonium lactate in the treatment of xerosis. Am. J. Clin. Dermatol. 2002, 3, 217–222. [Google Scholar] [CrossRef]
- Pavicic, T.; Korting, H.C. Xerosis and callus formation as a key to the diabetic foot syndrome: Dermatologic view of the problem and its management. JDDG J. Der Dtsch. Dermatol. Ges. 2006, 4, 935–941. [Google Scholar] [CrossRef]
- Cadilla, R.; Larkin, A.; Stewart, E.; Trump, R.; Turnbull, P. Chemical Compounds. US Patent 20060142387 A1, 2004. Available online: https://patents.google.com/patent/WO2005011681A1/en (accessed on 4 August 2025).
- Datta, R.; Henry, M. Lactic acid: Recent advances in products, processes and technologies—A review. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2006, 81, 1119–1129. [Google Scholar] [CrossRef]
- Uchida, M.; Murata, M. Isolation of a lactic acid bacterium and yeast consortium from a fermented material of Ulva spp.(Chlorophyta). J. Appl. Microbiol. 2004, 97, 1297–1310. [Google Scholar] [CrossRef]
- Hwang, H.J.; Kim, S.M.; Chang, J.H.; Lee, S.B. Lactic acid production from seaweed hydrolysate of Enteromorpha prolifera (Chlorophyta). J. Appl. Phycol. 2012, 24, 935–940. [Google Scholar] [CrossRef]
- Hwang, H.J.; Lee, S.Y.; Kim, S.M.; Lee, S.B. Fermentation of seaweed sugars by Lactobacillus species and the potential of seaweed as a biomass feedstock. Biotechnol. Bioprocess Eng. 2011, 16, 1231–1239. [Google Scholar] [CrossRef]
- Mazumdar, S.; Bang, J.; Oh, M.-K. L-lactate production from seaweed hydrolysate of Laminaria japonica using metabolically engineered Escherichia coli. Appl. Biochem. Biotechnol. 2014, 172, 1938–1952. [Google Scholar] [CrossRef]
- Jang, S.-S.; Shirai, Y.; Uchida, M.; Wakisaka, M. Production of L (+)-lactic acid from mixed acid and alkali hydrolysate of brown seaweed. Food Sci. Technol. Res. 2011, 17, 155–160. [Google Scholar] [CrossRef]
- Jang, S.-S.; Shirai, Y.; Uchida, M.; Wakisaka, M. Potential use of Gelidium amansii acid hydrolysate for lactic acid production by Lactobacillus rhamnosus. Food Technol. Biotechnol. 2013, 51, 131–136. [Google Scholar]
- Hanai, T.; Atsumi, S.; Liao, J. Engineered synthetic pathway for isopropanol production in Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 7814–7818. [Google Scholar] [CrossRef]
- Jain, R.; Yan, Y. Dehydratase mediated 1-propanol production in metabolically engineered Escherichia coli. Microb. Cell Factories 2011, 10, 1–10. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Bikker, P.; van Krimpen, M.M.; van Wikselaar, P.; Houweling-Tan, B.; Scaccia, N.; van Hal, J.W.; Huijgen, W.J.; Cone, J.W.; López-Contreras, A.M. Biorefinery of the green seaweed Ulva lactuca to produce animal feed, chemicals and biofuels. J. Appl. Phycol. 2016, 28, 3511–3525. [Google Scholar] [CrossRef] [PubMed]
- Baroi, G.N.; Baumann, I.; Westermann, P.; Gavala, H.N. Butyric acid fermentation from pretreated and hydrolysed wheat straw by an adapted C lostridium tyrobutyricum strain. Microb. Biotechnol. 2015, 8, 874–882. [Google Scholar] [CrossRef]
- Zhang, A.; Yang, S.T. Engineering Propionibacterium acidipropionici for enhanced propionic acid tolerance and fermentation. Biotechnol. Bioeng. 2009, 104, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Park, J.H.; Jang, S.H.; Nielsen, L.K.; Kim, J.; Jung, K.S. Fermentative butanol production by Clostridia. Biotechnol. Bioeng. 2008, 101, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Mascal, M. Chemicals from biobutanol: Technologies and markets. Biofuels Bioprod. Biorefining 2012, 6, 483–493. [Google Scholar] [CrossRef]
- MarketsandMarkets. n-Butanol Market by Grade (Industrial, Pharmaceutical, Distribution Channel, Feedstock, Application Butyl Carboxylate, Direct Solvents, Specialty Chemicals, Rubber & Plasticizers), End-Use Industry and Region—Global Forecast to 2029. Available online: https://www.marketsandmarkets.com/Market-Reports/n-butanol-market-1089.html (accessed on 4 October 2024).
- García Ibarra, V.; Sendón, R.; García-Fonte, X.X.; Paseiro Losada, P.; Rodríguez Bernaldo de Quirós, A. Migration studies of butylated hydroxytoluene, tributyl acetylcitrate and dibutyl phthalate into food simulants. J. Sci. Food Agric. 2019, 99, 1586–1595. [Google Scholar] [CrossRef]
- Huesemann, M.H.; Kuo, L.-J.; Urquhart, L.; Gill, G.A.; Roesijadi, G. Acetone-butanol fermentation of marine macroalgae. Bioresour. Technol. 2012, 108, 305–309. [Google Scholar] [CrossRef]
- Potts, T.; Du, J.; Paul, M.; May, P.; Beitle, R.; Hestekin, J. The production of butanol from Jamaica bay macro algae. Environ. Prog. Sustain. Energy 2011, 31, 29–36. [Google Scholar] [CrossRef]
- Van der Wal, H.; Sperber, B.L.; Houweling-Tan, B.; Bakker, R.R.; Brandenburg, W.; López-Contreras, A.M. Production of acetone, butanol, and ethanol from biomass of the green seaweed Ulva lactuca. Bioresour. Technol. 2013, 128, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, N.; Blaschek, H.P. Clostridia and Process Engineering for Energy Generation. In Biomass to Biofuels: Strategies for Global Industries; Vertès, A.A.N.Q., Blaschek, H.P., Yukawa, H., Eds.; Wiley: Chichester, UK; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Takkellapati, S.; Li, T.; Gonzalez, M.A. An overview of biorefinery-derived platform chemicals from a cellulose and hemicellulose biorefinery. Clean Technol. Environ. Policy 2018, 20, 1615–1630. [Google Scholar] [CrossRef]
- Saxena, R.K.; Saran, S.; Isar, J.; Kaushik, R. Production and Applications of Succinic Acid. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 601–630. [Google Scholar]
- Corona-González, R.I.; Bories, A.; González-Álvarez, V.; Snell-Castro, R.; Toriz-González, G.; Pelayo-Ortiz, C. Succinic Acid Production with Actinobacillus succinogenes ZT-130 in the Presence of Succinic Acid. Curr. Microbiol. 2009, 60, 71–77. [Google Scholar] [CrossRef]
- Glassner, D.A.; Elankovan, P.; Beacom, D.R.; Berglund, K.A. Purification process for succinic acid produced by fermentation. Appl. Biochem. Biotechnol. 1995, 51–52, 73–82. [Google Scholar] [CrossRef]
- Zeikus, J.G.; Jain, M.K.; Elankovan, P. Biotechnology of succinic acid production and markets for derived industrial products. Appl. Microbiol. Biotechnol. 1999, 51, 545–552. [Google Scholar] [CrossRef]
- Özcan, Y.; Osmanoğlu, S.; İde, S. Crystal Structure of 2,2-Dimethyl Succinic Acid. Anal. Sci. 2003, 19, 1221–1222. [Google Scholar] [CrossRef][Green Version]
- Bergen, W.G.; Bates, D.B. Ionophores: Their Effect on Production Efficiency and Mode of Action. J. Anim. Sci. 1984, 58, 1465–1483. [Google Scholar] [CrossRef]
- Bai, B.; Zhou, J.-M.; Yang, M.-H.; Liu, Y.-L.; Xu, X.-H.; Xing, J.-M. Efficient production of succinic acid from macroalgae hydrolysate by metabolically engineered Escherichia coli. Bioresour. Technol. 2015, 185, 56–61. [Google Scholar] [CrossRef]
- Alvarado-Morales, M.; Gunnarsson, I.B.; Fotidis, I.A.; Vasilakou, E.; Lyberatos, G.; Angelidaki, I. Laminaria digitata as a potential carbon source for succinic acid and bioenergy production in a biorefinery perspective. Algal Res. 2015, 9, 126–132. [Google Scholar] [CrossRef]
- Celińska, E.; Grajek, W. Biotechnological production of 2, 3-butanediol—Current state and prospects. Biotechnol. Adv. 2009, 27, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, S.; Lee, J.; Oh, M.-K. Microbial production of 2,3 butanediol from seaweed hydrolysate using metabolically engineered Escherichia coli. Bioresour. Technol. 2013, 136, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, H.U.; Choi, S.; Yi, J.; Lee, S.Y. Microbial production of building block chemicals and polymers. Curr. Opin. Biotechnol. 2011, 22, 758–767. [Google Scholar] [CrossRef]
- Abbott, D.A.; Zelle, R.M.; Pronk, J.T.; Van Maris, A.J. Metabolic engineering of Saccharomyces cerevisiae for production of carboxylic acids: Current status and challenges. FEMS Yeast Res. 2009, 9, 1123–1136. [Google Scholar] [CrossRef]
- Kawagoe, M.; Hyakumura, K.; Suye, S.-I.; Miki, K.; Naoe, K. Application of bubble column fermentors to submerged culture of Schizophyllum commune for production of l-malic acid. J. Ferment. Bioeng. 1997, 84, 333–336. [Google Scholar] [CrossRef]
- Roa Engel, C.A.; Straathof, A.J.; Zijlmans, T.W.; van Gulik, W.M.; van der Wielen, L.A. Fumaric acid production by fermentation. Appl. Microbiol. Biotechnol. 2008, 78, 379–389. [Google Scholar] [CrossRef]
- Fox, R.J.; Gold, R. Dimethyl fumarate to treat multiple sclerosis. In Multiple Sclerosis Therapeutics; Cohen, J.A., Rudeck, R.A., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 387–392. [Google Scholar]
- Goldberg, I.; Rokem, J.S. Fumaric acid biosynthesis and accumulation. In Bioprocessing of Renewable Resources to Commodity Bioproducts; Bisaria, V.S., Kondo, A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 409–434. [Google Scholar]
- He, C.L.; Fu, B.D.; Shen, H.Q.; Jiang, X.L.; Wei, X.B. Fumaric acid, an antibacterial component of Aloe vera L. Afr. J. Biotechnol. 2011, 10, 2973–2977. [Google Scholar]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef]
- Wong, J.; Rios-Solis, L.; Keasling, J. Microbial production of isoprenoids. In Consequences of Microbial Interactions with Hydrocarbons, Oils, and Lipids: Production of Fuels and Chemicals; Springer: Berlin/Heidelberg, Germany, 2017; pp. 359–382. [Google Scholar]
- Chang, M.C.Y.; Keasling, J.D. Production of isoprenoid pharmaceuticals by engineered microbes. Nat. Chem. Biol. 2006, 2, 674–681. [Google Scholar] [CrossRef]
- Misawa, N. Pathway engineering for functional isoprenoids. Curr. Opin. Biotechnol. 2011, 22, 627–633. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, G.; Sun, Y.; Zheng, Y.; Jiang, X.; Liu, W.; Xian, M. Bio-isoprene production using exogenous MVA pathway and isoprene synthase in Escherichia coli. Bioresour. Technol. 2012, 104, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pfeifer, B.A. Heterologous production of plant-derived isoprenoid products in microbes and the application of metabolic engineering and synthetic biology. Curr. Opin. Plant Biol. 2014, 19, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Paddon, C.J.; Keasling, J.D. Semi-synthetic artemisinin: A model for the use of synthetic biology in pharmaceutical development. Nat. Rev. Microbiol. 2014, 12, 355–367. [Google Scholar] [CrossRef]
- Jongedijk, E.; Cankar, K.; Buchhaupt, M.; Schrader, J.; Bouwmeester, H.; Beekwilder, J. Biotechnological production of limonene in microorganisms. Appl. Microbiol. Biotechnol. 2016, 100, 2927–2938. [Google Scholar] [CrossRef] [PubMed]
- Diamond, G.M.; Murphy, V.; Boussie, T.R. Application of high throughput experimentation to the production of commodity chemicals from renewable feedstocks. In Modern Applications of High Throughput R&D in Heterogeneous Catalysis; Hagemeyer, A., Volpe, A.F., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2014; pp. 288–309. [Google Scholar]
- Wang, J.; Lin, M.; Xu, M.; Yang, S.T. Anaerobic fermentation for production of carboxylic acids as bulk chemicals from renewable biomass. In Anaerobes in Biotechnology; Hatti-Kaul, R., Mamo, G., Mattiasson, B., Eds.; Springer: Cham, Switzerland, 2016; pp. 323–361. [Google Scholar]
- Skoog, E.; Shin, J.H.; Saez-Jimenez, V.; Mapelli, V.; Olsson, L. Biobased adipic acid—The challenge of developing the production host. Biotechnol. Adv. 2018, 36, 2248–2263. [Google Scholar] [CrossRef]
- Schmidt, R.J. Industrial catalytic processes—Phenol production. Appl. Catal. A Gen. 2005, 280, 89–103. [Google Scholar] [CrossRef]
- E Balderas-Hernández, V.; Sabido-Ramos, A.; Silva, P.; Cabrera-Valladares, N.; Hernández-Chávez, G.; Báez-Viveros, J.L.; Martínez, A.; Bolívar, F.; Gosset, G. Metabolic engineering for improving anthranilate synthesis from glucose in Escherichia coli. Microb. Cell Factories 2009, 8, 19. [Google Scholar] [CrossRef]
- Pasquardini, L.; Lunelli, L.; Vanzetti, L.; Anderle, M.; Pederzolli, C. Immobilization of cationic rifampicin-loaded liposomes on polystyrene for drug-delivery applications. Colloids Surfaces B Biointerfaces 2008, 62, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, A.H.; Hamzah, M.Q.; Mezan, S.O.; Ameruddin, A.B.; Agam, M.A. Green Synthesis of Silver/Polystyrene Nano Composite (Ag/PS NCs) via Plant Extracts Beginning a New Era in Drug Delivery. Indian J. Sci. Technol. 2018, 11, 1–9. [Google Scholar] [CrossRef]
- McKenna, R.; Nielsen, D.R. Styrene biosynthesis from glucose by engineered E. coli. Metab. Eng. 2011, 13, 544–554. [Google Scholar] [CrossRef]
- Hayes, G.; Laurel, M.; MacKinnon, D.; Zhao, T.; Houck, H.A.; Becer, C.R. Polymers without Petrochemicals: Sustainable Routes to Conventional Monomers. Chem. Rev. 2022, 123, 2609–2734. [Google Scholar] [CrossRef]
- Wang, T.; Nolte, M.W.; Shanks, B.H. Catalytic dehydration of C6carbohydrates for the production of hydroxymethylfurfural (HMF) as a versatile platform chemical. Green Chem. 2013, 16, 548–572. [Google Scholar] [CrossRef]
- Patwardhan, P.R.; Brown, R.C.; Shanks, B.H. Product Distribution from the Fast Pyrolysis of Hemicellulose. ChemSusChem 2011, 4, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.K.; Abraham, A.; Mallapureddy, K.K.; Sukumaran, R.K. Lignocellulosic biorefinery wastes, or resources? In Waste Biorefinery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 267–297. [Google Scholar]
- Martins, F.C.; Alcantara, G.M.; Silva, A.F.S.; Melchert, W.R.; Rocha, F.R. The role of 5-hydroxymethylfurfural in food and recent advances in analytical methods. Food Chem. 2022, 395, 133539. [Google Scholar] [CrossRef]
- Yang, C.-F.; Huang, C.-R. Isolation of 5-hydroxymethylfurfural biotransforming bacteria to produce 2,5-furan dicarboxylic acid in algal acid hydrolysate. J. Biosci. Bioeng. 2018, 125, 407–412. [Google Scholar] [CrossRef]
- Park, J.-H.; Yoon, J.-J.; Park, H.-D.; Kim, Y.J.; Lim, D.J.; Kim, S.-H. Feasibility of biohydrogen production from Gelidium amansii. Int. J. Hydrogen Energy 2011, 36, 13997–14003. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chien, W.-C.; Chou, H.-K.; Yang, J.; Lin, H.-T.V. Sulfuric Acid Hydrolysis and Detoxification of Red Alga Pterocladiella capillacea for Bioethanol Fermentation with Thermotolerant Yeast Kluyveromyces marxianus. J. Microbiol. Biotechnol. 2014, 24, 1245–1253. [Google Scholar] [CrossRef]
- Mirzaei, H.M.; Karimi, B. Sulphanilic acid as a recyclable bifunctional organocatalyst in the selective conversion of lignocellulosic biomass to 5-HMF. Green Chem. 2015, 18, 2282–2286. [Google Scholar] [CrossRef]
- Nangare, S.; Vispute, Y.; Tade, R.; Dugam, S.; Patil, P. Pharmaceutical applications of citric acid. Futur. J. Pharm. Sci. 2021, 7, 1–23. [Google Scholar] [CrossRef]
- Research, E.M. Citric Acid Market Report and Forecast 2024–2029. 2024. Available online: https://www.expertmarketresearch.com/reports/citric-acid-market (accessed on 4 November 2024).
- Insights, F.M. Citric Acid Market Size, Growth, and Forecast for 2025 to 2035. 2025. Available online: https://www.futuremarketinsights.com/reports/citric-acid-market (accessed on 4 February 2024).
- Dhillon, G.S.; Brar, S.K.; Verma, M.; Tyagi, R.D. Recent Advances in Citric Acid Bio-production and Recovery. Food Bioprocess Technol. 2010, 4, 505–529. [Google Scholar] [CrossRef]
- Lambros, M.; Tran, T.; Fei, Q.; Nicolaou, M. Citric Acid: A Multifunctional Pharmaceutical Excipient. Pharmaceutics 2022, 14, 972. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Koo, J.M.; Kim, S.H.; Hwang, S.Y.; Im, S.S. Synthesis of super absorbent polymer using citric acid as a bio-based monomer. Polym. Degrad. Stab. 2017, 144, 128–136. [Google Scholar] [CrossRef]
- Ramesh, T.; Kalaiselvam, M. An Experimental Study on Citric Acid Production by Aspergillus niger Using Gelidiella acerosa as a Substrate. Indian J. Microbiol. 2011, 51, 289–293. [Google Scholar] [CrossRef]
- Espinosa-Ramírez, J.; Mondragón-Portocarrero, A.C.; Rodríguez, J.A.; Lorenzo, J.M.; Santos, E.M. Algae as a potential source of protein meat alternatives. Front. Nutr. 2023, 10, 1254300. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Akbar, S.; Okonkwo, V.; Smith, C.; Khan, S.; Shah, A.A.; Adnan, F.; Ijaz, U.Z.; Ahmed, S.; Badshah, M. Enrichment of the hydrogenotrophic methanogens for, in-situ biogas up-gradation by recirculation of gases and supply of hydrogen in methanogenic reactor. Bioresour. Technol. 2022, 345, 126219. [Google Scholar] [CrossRef]
- Kostas, E.T.; White, D.A.; Cook, D.J. Development of a bio-refinery process for the production of speciality chemical, biofuel and bioactive compounds from Laminaria digitata. Algal Res. 2017, 28, 211–219. [Google Scholar] [CrossRef]
- Popa, V.; Volf, I. Biomass as Renewable Raw Material to Obtain Bioproducts of High-Tech Value; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Arroyo, M.F.R.; Miguel, L.J. Low-carbon energy governance: Scenarios to accelerate the change in the energy matrix in ecuador. Energies 2020, 13, 4731. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Ravi, H.K. Toward petroleum-free with plant-based chemistry. Curr. Opin. Green Sustain. Chem. 2021, 28, 100450. [Google Scholar] [CrossRef]
- Solomon, S.; Qin, D.; Manning, M.; Chen, Z.; Marquis, M.; Averyt, K.; Tignor, M.; Miller, H. IPCC fourth assessment report (AR4). Clim. Chang. 2007, 374, 996. [Google Scholar]
- Cakmak, E.K.; Atasoy, M.; Owusu-Agyeman, I.; Khatami, K.; Cetecioglu, Z. Circular city concept for future biorefineries. In Clean Energy and Resource Recovery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 335–352. [Google Scholar]
- Ahorsu, R.; Medina, F.; Constantí, M. Significance and Challenges of Biomass as a Suitable Feedstock for Bioenergy and Biochemical Production: A Review. Energies 2018, 11, 3366. [Google Scholar] [CrossRef]
- Okolie, J.A.; Mukherjee, A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Next-generation biofuels and platform biochemicals from lignocellulosic biomass. Int. J. Energy Res. 2021, 45, 14145–14169. [Google Scholar] [CrossRef]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Kamm, B.; Schönicke, P.; Hille, C. Green biorefinery–industrial implementation. Food Chem. 2016, 197, 1341–1345. [Google Scholar] [CrossRef]
- Haveren, J.V.; Scott, E.L.; Sanders, J. Bulk chemicals from biomass. Biofuels Bioprod. Biorefining 2008, 2, 41–57. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of Biomass into Chemicals over Metal Catalysts. Chem. Rev. 2013, 114, 1827–1870. [Google Scholar] [CrossRef]
- Delidovich, I.; Hausoul, P.J.C.; Deng, L.; Pfützenreuter, R.; Rose, M.; Palkovits, R. Alternative Monomers Based on Lignocellulose and Their Use for Polymer Production. Chem. Rev. 2015, 116, 1540–1599. [Google Scholar] [CrossRef]
- Danner, H.; Braun, R. Biotechnology for the production of commodity chemicals from biomass. Chem. Soc. Rev. 1999, 28, 395–405. [Google Scholar] [CrossRef]
- Bozell, J.J.; Moens, L.; Elliott, D.; Wang, Y.; Neuenscwander, G.; Fitzpatrick, S.; Bilski, R.; Jarnefeld, J. Production of levulinic acid and use as a platform chemical for derived products. Resour. Conserv. Recycl. 2000, 28, 227–239. [Google Scholar] [CrossRef]
- Hayes, D.J.; Fitzpatrick, S.; Hayes, M.H.; Ross, J.R. The biofine process–production of levulinic acid, furfural, and formic acid from lignocellulosic feedstocks. In Biorefineries–Industrial Processes and Product; Kamm, B., Gruber, P.R., Kamm, M., Eds.; Wiley-VCH: Weinheim, Germany, 2006; pp. 139–164. [Google Scholar]
- Cherubini, F.; Strømman, A.H. Chemicals from lignocellulosic biomass: Opportunities, perspectives, and potential of biorefinery systems. Biofuels Bioprod. Biorefining 2011, 5, 548–561. [Google Scholar] [CrossRef]
- Van Putten, R.J.; Van Der Waal, J.C.; De Jong, E.D.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Farmer, T.J.; Mascal, M. Platform molecules. In Introduction to Chemicals From Biomass; James, C., Fabien, D., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 89–155. [Google Scholar]
- Kang, S.; Fu, J.; Zhang, G. From lignocellulosic biomass to levulinic acid: A review on acid-catalyzed hydrolysis. Renew. Sustain. Energy Rev. 2018, 94, 340–362. [Google Scholar] [CrossRef]
- Gerardy, R.; Debecker, D.P.; Estager, J.; Luis, P.; Monbaliu, J.C. Continuous flow upgrading of selected C2–C6 platform chemicals derived from biomass. Chem. Rev. 2020, 120, 7219–7347. [Google Scholar] [CrossRef]
- Vu, H.P.; Nguyen, L.N.; Vu, M.T.; Johir, M.A.H.; McLaughlan, R.; Nghiem, L.D. A comprehensive review on the framework to valorise lignocellulosic biomass as biorefinery feedstocks. Sci. Total Environ. 2020, 743, 140630. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.B.; Wu, T.Y. A review on solvent systems for furfural production from lignocellulosic biomass. Renew. Sustain. Energy Rev. 2021, 137, 110172. [Google Scholar] [CrossRef]
- Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H. Microalgae for the production of bulk chemicals and biofuels. Biofuels Bioprod. Biorefining Innov. A Sustain. Econ. 2010, 4, 287–295. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef]
- Cesário, M.T.; da Fonseca, M.M.R.; Marques, M.M.; de Almeida, M.C.M. Marine algal carbohydrates as carbon sources for the production of biochemicals and biomaterials. Biotechnol. Adv. 2018, 36, 798–817. [Google Scholar] [CrossRef] [PubMed]
- De Jong, E.; Jungmeier, G. Biorefinery concepts in comparison to petrochemical refineries. In Industrial Biorefineries & White Biotechnology; Pandey, A., Höfer, R., Larroche, C., Taherzadeh, M., Nampoothiri, K.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–33. [Google Scholar]
- Bwapwa, J.K.; Anandraj, A.; Trois, C. Possibilities for conversion of microalgae oil into aviation fuel: A review. Renew. Sustain. Energy Rev. 2017, 80, 1345–1354. [Google Scholar] [CrossRef]
- Kraan, S. Mass-cultivation of carbohydrate rich macroalgae, a possible solution for sustainable biofuel production. Mitig. Adapt. Strat. Glob. Chang. 2010, 18, 27–46. [Google Scholar] [CrossRef]
- Shukla, R.; Kumar, M.; Chakraborty, S.; Gupta, R.; Kumar, S.; Sahoo, D.; Kuhad, R.C. Process development for the production of bioethanol from waste algal biomass of Gracilaria verrucosa. Bioresour. Technol. 2016, 220, 584–589. [Google Scholar] [CrossRef]
- Varejão, J.M.; Nazaré, R. Ethanol production from macroalgae biomass. In Algal Biofuels; CRC Press: Boca Raton, FL, USA, 2017; pp. 189–200. [Google Scholar]
- Delattre, C.; Pierre, G.; Laroche, C.; Michaud, P. Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnol. Adv. 2016, 34, 1159–1179. [Google Scholar] [CrossRef]
- Brown, M.; Jeffrey, S.; Volkman, J.; Dunstan, G. Nutritional properties of microalgae for mariculture. Aquaculture 1997, 151, 315–331. [Google Scholar] [CrossRef]
- Renaud, S.M.; Thinh, L.-V.; Parry, D.L. The gross chemical composition and fatty acid composition of 18 species of tropical Australian microalgae for possible use in mariculture. Aquaculture 1999, 170, 147–159. [Google Scholar] [CrossRef]
- Knoshaug, E.; Laurens, L.; Kinchin, C.; Davis, R. Use of Cultivation Data from the Algae Testbed Public Private Partnership as Utilized in NREL’s Algae State of Technology Assessments; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2016.
- Yim, J.H.; Kim, S.J.; Ahn, S.H.; Lee, H.K. Characterization of a novel bioflocculant, p-KG03, from a marine dinoflagellate, Gyrodinium impudicum KG03. Bioresour. Technol. 2007, 98, 361–367. [Google Scholar] [CrossRef] [PubMed]
- de Jesus Raposo, M.F.; de Morais, A.M.; de Morais, R.M. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef]
- Jung, K.A.; Lim, S.-R.; Kim, Y.; Park, J.M. Potentials of macroalgae as feedstocks for biorefinery. Bioresour. Technol. 2013, 135, 182–190. [Google Scholar] [CrossRef]
- Werpy, T.; Holladay, J.; White, J. Top Value Added Chemicals From Biomass: Volume I. In Results of Screening for Potential Candidates from Sugars and Synthesis Gas; U.S. Department of Energy Office of Scientific and Technical Information: Oak Ridge, TN, USA, 2004. [Google Scholar]
- Zwenger, S.; Basu, C. Plant terpenoids: Applications and future potentials. Biotechnol. Mol. Biol. Rev. 2008, 3, 1. [Google Scholar]
- Zielińska-Błajet, M.; Feder-Kubis, J. Monoterpenes and Their Derivatives—Recent Development in Biological and Medical Applications. Int. J. Mol. Sci. 2020, 21, 7078. [Google Scholar] [CrossRef]
- Naylor, S.; Hanke, F.J.; Manes, L.V.; Crews, P. Chemical and biological aspects of marine monoterpenes. In Fortschritte der Chemie organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; Springer: Vienna, Austria, 1983; pp. 189–241. [Google Scholar]
- Davis, E.M.; Croteau, R. Cyclization enzymes in the biosynthesis of monoterpenes, sesquiterpenes, and diterpenes. In Biosynthesis: Aromatic Polyketides, Isoprenoids, Alkaloids; Springer: Berlin/Heidelberg, Germany, 2000; pp. 53–95. [Google Scholar]
- Breitmaier, E. Terpenes: Flavors, Fragrances, Pharmaca, Pheromones; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Marmulla, R.; Harder, J. Microbial monoterpene transformations—A review. Front. Microbiol. 2014, 5, 346. [Google Scholar] [CrossRef] [PubMed]
- Vil’, V.A.; Yaremenko, I.A.; Ilovaisky, A.I.; Terent’ev, A.O. Peroxides with Anthelmintic, Antiprotozoal, Fungicidal and Antiviral Bioactivity: Properties, Synthesis and Reactions. Molecules 2017, 22, 1881. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wu, Z.; Guo, H.; Liu, L.; Chen, S. A review of terpenes from marine-derived fungi: 2015–2019. Mar. Drugs 2020, 18, 321. [Google Scholar] [CrossRef]
- Ma, L.F.; Chen, M.J.; Liang, D.E.; Shi, L.M.; Ying, Y.M.; Shan, W.G.; Li, G.Q.; Zhan, Z.J. Streptomyces albogriseolus SY67903 produces eunicellin diterpenoids structurally similar to terpenes of the gorgonian Muricella sibogae, the bacterial source. J. Nat. Prod. 2020, 83, 1641–1645. [Google Scholar] [CrossRef]
- Gong, K.; Yong, D.; Fu, J.; Li, A.; Zhang, Y.; Li, R. Diterpenoids from Streptomyces: Structures, biosyntheses and bioactivities. ChemBioChem 2022, 23, e202200231. [Google Scholar] [CrossRef]
- Avila, C. Terpenoids in Marine Heterobranch Molluscs. Mar. Drugs 2020, 18, 162. [Google Scholar] [CrossRef]
- Chen, B.; Qiu, P.; Xu, B.; Zhao, Q.; Gu, Y.-C.; Fu, L.; Bi, S.; Lan, L.; Wang, C.-Y.; Guo, Y.-W. Cytotoxic and Antibacterial Isomalabaricane Terpenoids from the Sponge Rhabdastrella globostellata. J. Nat. Prod. 2022, 85, 1799–1807. [Google Scholar] [CrossRef]
- Majer, T.; Bhattarai, K.; Straetener, J.; Pohlmann, J.; Cahill, P.; Zimmermann, M.O.; Hübner, M.P.; Kaiser, M.; Svenson, J.; Schindler, M.; et al. Discovery of Ircinianin Lactones B and C—Two New Cyclic Sesterterpenes from the Marine Sponge Ircinia wistarii. Mar. Drugs 2022, 20, 532. [Google Scholar] [CrossRef] [PubMed]
- Elkhateeb, A.; El-Beih, A.A.; Gamal-Eldeen, A.M.; Alhammady, M.A.; Ohta, S.; Paré, P.W.; Hegazy, M.-E.F. New Terpenes from the Egyptian Soft Coral Sarcophyton ehrenbergi. Mar. Drugs 2014, 12, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Zhu, Z.D.; Chen, J.S.; Li, J.; Gu, Y.C.; Zhu, W.L.; Li, X.W.; Guo, Y.W. Xishacorenes A–C, diterpenes with bicyclo [3.3. 1] nonane nucleus from the Xisha soft coral Sinularia polydactyla. Org. Lett. 2017, 19, 4183–4186. [Google Scholar] [CrossRef]
- Bu, Q.; Yang, M.; Yan, X.Y.; Li, S.W.; Ge, Z.Y.; Zhang, L.; Yao, L.G.; Guo, Y.W.; Liang, L.F. Mililatensols A–C, new records of sarsolenane and capnosane diterpenes from soft coral Sarcophyton mililatensis. Mar. Drugs 2022, 20, 566. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Ibrahim, S.R.; Badr, J.M.; Youssef, D.T. Didemnaketals D and E, bioactive terpenoids from a Red Sea ascidian Didemnum species. Tetrahedron 2014, 70, 35–40. [Google Scholar] [CrossRef]
- Levert, A.; Foulon, V.; Fauchon, M.; Tapissier-Bontemps, N.; Banaigs, B.; Hellio, C. Antifouling Activity of Meroterpenes Isolated from the Ascidian Aplidium aff. densum. Mar. Biotechnol. 2020, 23, 51–61. [Google Scholar] [CrossRef]
- Degenhardt, J.; Köllner, T.G.; Gershenzon, J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 2009, 70, 1621–1637. [Google Scholar] [CrossRef]
- Zhai, G.; Chen, S.; Shen, H.; Guo, H.; Jiang, M.; Liu, L. Bioactive monoterpenes and polyketides from the ascidian-derived fungus Diaporthe sp. SYSU-MS4722. Mar. Drugs 2022, 20, 553. [Google Scholar] [CrossRef]
- Kladi, M.; Vagias, C.; Roussis, V. Volatile halogenated metabolites from marine red algae. Phytochem. Rev. 2004, 3, 337–366. [Google Scholar] [CrossRef]
- Cabrita, M.T.; Vale, C.; Rauter, A.P. Halogenated Compounds from Marine Algae. Mar. Drugs 2010, 8, 2301–2317. [Google Scholar] [CrossRef] [PubMed]
- El Gamal, A.A. Biological importance of marine algae. Saudi Pharm. J. 2010, 18, 1–25. [Google Scholar] [CrossRef]
- Peng, Y.; Hu, J.; Yang, B.; Lin, X.P.; Zhou, X.F.; Yang, X.W.; Liu, Y. Chemical composition of seaweeds. In Seaweed Sustainability; Tiwari, B.K., Troy, D.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 79–124. [Google Scholar]
- Ibrahim, M.; Salman, M.; Kamal, S.; Rehman, S.; Razzaq, A.; Akash, S.H. Algae-based biologically active compounds. In Algae Based Polymers, Blends, and Composites; Zia, K.M., Zuber, M., Ali, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 155–271. [Google Scholar]
- Zatelli, G.A.; Philippus, A.C.; Falkenberg, M. An overview of odoriferous marine seaweeds of the Dictyopteris genus: Insights into their chemical diversity, biological potential and ecological roles. Rev. Bras. Farm. 2018, 28, 243–260. [Google Scholar] [CrossRef]
- Paul, V.J.; McConnell, O.J.; Fenical, W. Cyclic monoterpenoid feeding deterrents from the red marine alga Ochtodes crockeri. J. Org. Chem. 1980, 45, 3401–3407. [Google Scholar] [CrossRef]
- Yassaa, N.; Peeken, I.; Zöllner, E.; Bluhm, K.; Arnold, S.; Spracklen, D.; Williams, J. Evidence for marine production of monoterpenes. Environ. Chem. 2008, 5, 391–401. [Google Scholar] [CrossRef]
- Butler, A.; Walker, J.V. Marine haloperoxidases. Chem. Rev. 1993, 93, 1937–1944. [Google Scholar] [CrossRef]
- Barahona, L.F.; Rorrer, G.L. Isolation of Halogenated Monoterpenes from Bioreactor-Cultured Microplantlets of the Macrophytic Red Algae Ochtodes s ecundiramea and Portieria h ornemannii. J. Nat. Prod. 2003, 66, 743–751. [Google Scholar] [CrossRef]
- Miziorko, H.M. Enzymes of the mevalonate pathway of isoprenoid biosynthesis. Arch. Biochem. Biophys. 2011, 505, 131–143. [Google Scholar] [CrossRef]
- Lohr, M.; Schwender, J.; Polle, J.E. Isoprenoid biosynthesis in eukaryotic phototrophs: A spotlight on algae. Plant Sci. 2012, 185–186, 9–22. [Google Scholar] [CrossRef]
- Lombard, J.; Moreira, D. Origins and Early Evolution of the Mevalonate Pathway of Isoprenoid Biosynthesis in the Three Domains of Life. Mol. Biol. Evol. 2010, 28, 87–99. [Google Scholar] [CrossRef]
- Polzin, J.; Rorrer, G.L. Selective production of the acyclic monoterpene β-myrcene by microplantlet suspension cultures of the macrophytic marine red alga Ochtodes secundiramea under nutrient perfusion cultivation with bromide-free medium. Algal Res. 2018, 36, 159–166. [Google Scholar] [CrossRef]
- Wise, M.L. Monoterpene biosynthesis in marine algae. Phycologia 2003, 42, 370–377. [Google Scholar] [CrossRef]
- Wise, M.L.; Rorrer, G.L.; Polzin, J.J.; Croteau, R. Biosynthesis of Marine Natural Products: Isolation and Characterization of a Myrcene Synthase from Cultured Tissues of the Marine Red Alga Ochtodes secundiramea. Arch. Biochem. Biophys. 2002, 400, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Hewson, W.D.; Hager, L.P. Bromoperoxidases and halogenated lipids in marine algae. J. Phycol. 1980, 16, 340–345. [Google Scholar] [CrossRef]
- Fenical, W. Natural Products Chemistry in the Marine Environment. Science 1982, 215, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Maliakal, S.; Cheney, D.P.; Rorrer, G.L. Halogenated monoterpene production in regenerated plantlet cultures of Ochtodes secundiramea (Rhodophyta, Cryptonemiales). J. Phycol. 2001, 37, 1010–1019. [Google Scholar] [CrossRef]
- Shaw, S.L.; Gantt, B.; Meskhidze, N.; Stetzer, O. Production and Emissions of Marine Isoprene and Monoterpenes: A Review. Adv. Meteorol. 2010, 2010, 408696. [Google Scholar] [CrossRef]
- Dayan, C.; Fredj, E.; Misztal, P.K.; Gabay, M.; Guenther, A.B.; Tas, E. Emission of biogenic volatile organic compounds from warm and oligotrophic seawater in the Eastern Mediterranean. Atmos. Chem. Phys. 2020, 20, 12741–12759. [Google Scholar] [CrossRef]
- Novak, G.A.; Bertram, T.H. Reactive VOC Production from Photochemical and Heterogeneous Reactions Occurring at the Air–Ocean Interface. Accounts Chem. Res. 2020, 53, 1014–1023. [Google Scholar] [CrossRef]
- Davies, F.K.; Jinkerson, R.E.; Posewitz, M.C. Toward a photosynthetic microbial platform for terpenoid engineering. Photosynth. Res. 2014, 123, 265–284. [Google Scholar] [CrossRef]
- Tokarek, T.W.; Brownsey, D.K.; Jordan, N.; Garner, N.M.; Ye, C.Z.; Osthoff, H.D. Emissions of C9–C16 hydrocarbons from kelp species on Vancouver Island: Alaria marginata (winged kelp) and Nereocystis luetkeana (bull kelp) as an atmospheric source of limonene. Atmos. Environ. X 2019, 2, 100007. [Google Scholar] [CrossRef]
- Shaw, S.L.; Chisholm, S.W.; Prinn, R.G. Isoprene production by Prochlorococcus, a marine cyanobacterium, and other phytoplankton. Mar. Chem. 2003, 80, 227–245. [Google Scholar] [CrossRef]
- Colomb, A.; Yassaa, N.; Williams, J.; Peeken, I.; Lochte, K. Screening volatile organic compounds (VOCs) emissions from five marine phytoplankton species by head space gas chromatography/mass spectrometry (HS-GC/MS). J. Environ. Monit. 2008, 10, 325–330. [Google Scholar] [CrossRef]
- Arnold, S.R.; Spracklen, D.V.; Williams, J.; Yassaa, N.; Sciare, J.; Bonsang, B.; Gros, V.; Peeken, I.; Lewis, A.C.; Alvain, S.; et al. Evaluation of the global oceanic isoprene source and its impacts on marine organic carbon aerosol. Atmos. Chem. Phys. 2009, 9, 1253–1262. [Google Scholar] [CrossRef]
- Gantt, B.; Meskhidze, N.; Kamykowski, D. A new physically-based quantification of marine isoprene and primary organic aerosol emissions. Atmos. Chem. Phys. 2009, 9, 4915–4927. [Google Scholar] [CrossRef]
- Alvarez, L.A.; Exton, D.A.; Timmis, K.N.; Suggett, D.J.; McGenity, T.J. Characterization of marine isoprene-degrading communities. Environ. Microbiol. 2009, 11, 3280–3291. [Google Scholar] [CrossRef]
- Evans, T. Biological Volatile Organic Compounds (BVOCs) emissions from the planktonic diatom Thalassiosira pseudonana. Ph.D. Thesis, Stony Brook University, Stony Brook, NY, USA, 2009. [Google Scholar]
- Bonsang, B.; Polle, C.; Lambert, G. Evidence for marine production of isoprene. Geophys. Res. Lett. 1992, 19, 1129–1132. [Google Scholar] [CrossRef]
- Baker, A.R.; Turner, S.M.; Broadgate, W.J.; Thompson, A.; McFiggans, G.B.; Vesperini, O.; Nightingale, P.D.; Liss, P.S.; Jickells, T.D. Distribution and sea-air fluxes of biogenic trace gases in the eastern Atlantic Ocean. Glob. Biogeochem. Cycles 2000, 14, 871–886. [Google Scholar] [CrossRef]
- Erickson III, D.J.; Hernandez, J.L. A global, high resolution, satellite-based model of air-sea isoprene flux. Geophys. Monogr. Series. 2002, 127, 333–341. [Google Scholar]
- Matsunaga, S.; Mochida, M.; Saito, T.; Kawamura, K. In situ measurement of isoprene in the marine air and surface seawater from the western North Pacific. Atmos. Environ. 2002, 36, 6051–6057. [Google Scholar] [CrossRef]
- Palmer, P.I.; Shaw, S.L. Quantifying global marine isoprene fluxes using MODIS chlorophyll observations. Geophys. Res. Lett. 2005, 32, 1–5. [Google Scholar] [CrossRef]
- Meskhidze, N.; Nenes, A. Phytoplankton and Cloudiness in the Southern Ocean. Science 2006, 314, 1419–1423. [Google Scholar] [CrossRef]
- Liakakou, E.; Vrekoussis, M.; Bonsang, B.; Donousis, C.; Kanakidou, M.; Mihalopoulos, N. Isoprene above the Eastern Mediterranean: Seasonal variation and contribution to the oxidation capacity of the atmosphere. Atmos. Environ. 2007, 41, 1002–1010. [Google Scholar] [CrossRef]
- Sinha, V.; Williams, J.; Meyerhöfer, M.; Riebesell, U.; Paulino, A.I.; Larsen, A. Air-sea fluxes of methanol, acetone, acetaldehyde, isoprene and DMS from a Norwegian fjord following a phytoplankton bloom in a mesocosm experiment. Atmos. Chem. Phys. 2007, 7, 739–755. [Google Scholar] [CrossRef]
- Luo, G.; Yu, F. A numerical evaluation of global oceanic emissions of α-pinene and isoprene. Atmos. Chem. Phys. 2010, 10, 2007–2015. [Google Scholar] [CrossRef]
- Rubulotta, G.; Quadrelli, E.A. Terpenes: A valuable family of compounds for the production of fine chemicals. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2019; pp. 215–229. [Google Scholar]
- Harman-Ware, A.E. Conversion of terpenes to chemicals and related products. In Chemical Catalysts for Biomass Upgrading; Crocker, M., Santillan-Jimenez, E., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 529–568. [Google Scholar]
- Güven, K.C.; Sezik, E.; Kaleağasıoğlu, F.; Erdugan, H.; Coban, B.; Karakaş, E. Vlatile Oils from Marine Macroalgae. In Natural Products; Ramawat, K., Merillom, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2883–2912. [Google Scholar]
- Cikoš, A.-M.; Jurin, M.; Čož-Rakovac, R.; Jokić, S.; Jerković, I. Update on Monoterpenes from Red Macroalgae: Isolation, Analysis, and Bioactivity. Mar. Drugs 2019, 17, 537. [Google Scholar] [CrossRef]
- Singh-Sangwan, N.; Sangwan, R.S.; Luthra, R.; Thakur, R.S. Geraniol dehydrogenase: A determinant of essential oil quality in lemongrass1. Planta Medica 1993, 59, 168–170. [Google Scholar] [CrossRef]
- Gupta, A.K.; Ganjewala, D. A study on biosynthesis of “citral” in lemongrass (C. flexuosus) cv. Suvarna. Acta Physiol. Plant. 2015, 37, 240. [Google Scholar] [CrossRef]
- Gusevskaya, E.; Robles-Dutenhefner, P.A.; Ferreira, V.M. Palladium-catalyzed oxidation of bicyclic monoterpenes by hydrogen peroxide. Appl. Catal. A Gen. 1998, 174, 177–186. [Google Scholar] [CrossRef]
- Monteiro, J.L.F.; Veloso, C.O. Catalytic Conversion of Terpenes into Fine Chemicals. Top. Catal. 2004, 27, 169–180. [Google Scholar] [CrossRef]
- Van Leeuwen, P.W. Homogeneous Catalysis: Understanding the Art; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Meier, M.A.; Weckhuysen, B.M.; Bruijnincx, P.C. (Eds.) Organometallics and Renewables; Springer: Berlin/Heidelberg, Germany, 2012; Volume 39. [Google Scholar]
- Adolfsson, H.; Copéret, C.; Chiang, J.P.; Yudin, A.K. Efficient Epoxidation of Alkenes with Aqueous Hydrogen Peroxide Catalyzed by Methyltrioxorhenium and 3-Cyanopyridine. J. Org. Chem. 2000, 65, 8651–8658. [Google Scholar] [CrossRef] [PubMed]
- Whiteker, G.T.; Cobley, C.J. Applications of rhodium-catalyzed hydroformylation in the pharmaceutical, agrochemical, and fragrance industries. In Organometallics as Catalysts in the Fine Chemical Industry; Beller, M., Blaser, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 35–46. [Google Scholar]
- North, M.; Pasquale, R.; Young, C. Synthesis of cyclic carbonates from epoxides and CO2. Green Chem. 2010, 12, 1514–1539. [Google Scholar] [CrossRef]
- Schäffner, B.; Schäffner, F.; Verevkin, S.P.; Börner, A. Organic Carbonates as Solvents in Synthesis and Catalysis. Chem. Rev. 2010, 110, 4554–4581. [Google Scholar] [CrossRef]
- Bähr, M.; Bitto, A.; Mülhaupt, R. Cyclic limonene dicarbonate as a new monomer for non-isocyanate oligo- and polyurethanes (NIPU) based upon terpenes. Green Chem. 2012, 14, 1447–1454. [Google Scholar] [CrossRef]
- Hauenstein, O.; Agarwal, S.; Greiner, A. Bio-based polycarbonate as synthetic toolbox. Nat. Commun. 2016, 7, 11862. [Google Scholar] [CrossRef]
- Kukhta, N.A.; Vasilenko, I.V.; Kostjuk, S.V. Room temperature cationic polymerization of β-pinene using modified AlCl3 catalyst: Toward sustainable plastics from renewable biomass resources. Green Chem. 2011, 13, 2362–2364. [Google Scholar] [CrossRef]
- Gandini, A.; Lacerda, T.M. From monomers to polymers from renewable resources: Recent advances. Prog. Polym. Sci. 2015, 48, 1–39. [Google Scholar] [CrossRef]
- Winnacker, M.; Rieger, B. Recent Progress in Sustainable Polymers Obtained from Cyclic Terpenes: Synthesis, Properties, and Application Potential. ChemSusChem 2015, 8, 2455–2471. [Google Scholar] [CrossRef]
- Llevot, A.; Dannecker, P.; von Czapiewski, M.; Over, L.C.; Söyler, Z.; Meier, M.A.R. Renewability is not Enough: Recent Advances in the Sustainable Synthesis of Biomass-Derived Monomers and Polymers. Chem. A Eur. J. 2016, 22, 11510–11521. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef]
- Satoh, K.; Nakahara, A.; Mukunoki, K.; Sugiyama, H.; Saito, H.; Kamigaito, M. Sustainable cycloolefin polymer from pine tree oil for optoelectronics material: Living cationic polymerization of β-pinene and catalytic hydrogenation of high-molecular-weight hydrogenated poly(β-pinene). Polym. Chem. 2013, 5, 3222–3230. [Google Scholar] [CrossRef]
- Firdaus, M. Terpenes as Renewable Resources for Organic and Macromolecular Chemistry. Ph.D. Thesis, Karlsruher Institut für Technologie (KIT), Karlsruhe, Germany, 2013. [Google Scholar]
- Firdaus, M.; Meier, M.A.R. Renewable polyamides and polyurethanes derived from limonene. Green Chem. 2012, 15, 370–380. [Google Scholar] [CrossRef]
- Neaţu, F.; Culică, G.; Florea, M.; Parvulescu, V.I.; Cavani, F. Synthesis of Terephthalic Acid by p-Cymene Oxidation using Oxygen: Toward a More Sustainable Production of Bio-Polyethylene Terephthalate. ChemSusChem 2016, 9, 3102–3112. [Google Scholar] [CrossRef]
- Buisman, G.J.H.; Lange, J.H.M. Arizona chemical: Refining and upgrading of bio-based and renewable feedstocks. In Industrial Biorenewables: A Practical Viewpoint; María, P.D.d., Ed.; Wiley: Hoboken, NJ, USA, 2016; pp. 21–62. [Google Scholar]
- Allenspach, M.; Steuer, C. α-Pinene: A never-ending story. Phytochem. 2021, 190, 112857. [Google Scholar] [CrossRef]
- Yang, J.; Nie, Q.; Ren, M.; Feng, H.; Jiang, X.; Zheng, Y.; Liu, M.; Zhang, H.; Xian, M. Metabolic engineering of Escherichia coli for the biosynthesis of alpha-pinene. Biotechnol. Biofuels 2013, 6, 1–10. [Google Scholar] [CrossRef]
- Alpha Pinene Market Size, Share, Growth, and Industry Analysis, by Type (≥95%, <95%), by Application (Aroma Chemicals, Adhesive & Tire Resins, Solvents & Cleaners, and Others), Regional Insights and Forecast from 2025 to 2033. 2021. Available online: https://www.businessresearchinsights.com/market-reports/alpha-pinene-market-101083 (accessed on 4 April 2021).
- Rozza, A.L.; de Mello Moraes, T.; Kushima, H.; Tanimoto, A.; Marques, M.O.; Bauab, T.M.; Hiruma-Lima, C.A.; Pellizzon, C.H. Gastroprotective mechanisms of Citrus lemon (Rutaceae) essential oil and its majority compounds limonene and β-pinene: Involvement of heat-shock protein-70, vasoactive intestinal peptide, glutathione, sulfhydryl compounds, nitric oxide and prostaglandin E2. Chem. Biol. Interact. 2011, 189, 82–89. [Google Scholar] [CrossRef]
- Global-Beta-Pinene-Research. 2021. Available online: https://www.marketresearch.com/QYResearch-Group-v3531/Global-Beta-Pinene-Research-14725965/ (accessed on 4 June 2021).
- Global Beta Pinene Industry Research Report, Competitive Landscape, Market Size, Regional Status and Prospect. 2022. Available online: https://www.marketresearchguru.com/global-beta-pinene-industry-research-report-competitive-landscape-market-21956714 (accessed on 1 November 2022).
- Viswanathan, S.; Neerackal, G.; Buyuksonmez, F. Removal of beta-pinene and limonene using compost biofilter. J. Air Waste Manag. Assoc. 2012, 63, 237–245. [Google Scholar] [CrossRef]
- Clark, G. Aroma chemical profile: Menthol: History, world consumption, synthetics, by-products, substitutes and derivatives. Perfum. Flavorist 2007, 32, 38–47. [Google Scholar]
- Dylong, D.; Hausoul, P.J.; Palkovits, R.; Eisenacher, M. Synthesis of (−)-menthol: Industrial synthesis routes and recent development. Flavour Fragr. J. 2022, 37, 195–209. [Google Scholar] [CrossRef]
- Kamatou, G.P.; Vermaak, I.; Viljoen, A.M.; Lawrence, B.M. Menthol: A simple monoterpene with remarkable biological properties. Phytochemistry 2013, 96, 15–25. [Google Scholar] [CrossRef]
- Batool, I.; Nisar, S.; Hamrouni, L.; Jilani, M.I. Extraction, production and analysis techniques for menthol: A review. Int. J. Chem. Biochem. Sci. 2018, 14, 71–76. [Google Scholar]
- Behr, A.; Johnen, L. Myrcene as a Natural Base Chemical in Sustainable Chemistry: A Critical Review. ChemSusChem 2009, 2, 1072–1095. [Google Scholar] [CrossRef]
- Surendran, S.; Qassadi, F.; Surendran, G.; Lilley, D.; Heinrich, M. Myrcene—What are the potential health benefits of this flavouring and aroma agent? Front. Nutr. 2021, 8, 699666. [Google Scholar] [CrossRef]
- Esmaeili, A.; Mirmhadi, H.R.; Rafiee, F. Biotransformation of menthol by Chlorella vulgaris and study of the pathways involved. Chem. Nat. Compd. 2014, 49, 1160–1161. [Google Scholar] [CrossRef]
- Global-Myrcene-Insights-Forecast. Available online: https://www.marketresearch.com/QYResearch-Group-v3531/Global-Myrcene-Insights-Forecast-30260294/ (accessed on 4 November 2021).
- de Santana, M.T.; de Oliveira, M.G.B.; Santana, M.F.; De Sousa, D.P.; Santana, D.G.; Camargo, E.A.; de Oliveira, A.P.; Almeida, J.R.G.d.S.; Quintans-Júnior, L.J. Citronellal, a monoterpene present in Java citronella oil, attenuates mechanical nociception response in mice. Pharm. Biol. 2013, 51, 1144–1149. [Google Scholar] [CrossRef]
- Citronella Oil Market by Type (Java and Ceylon), Application (Food & Beverages, Cosmetics, Arc Citronella l Market Overvie naceuticals), Nature (Conventional and Organic) and Region—Global Forecast to 2027. 2023. Available online: https://www.marketsandmarkets.com/Market-Reports/citronella-oil-market-4836977.html (accessed on 3 May 2023).
- Solanki, K.P.; Desai, M.A.; Parikh, J.K. Microwave intensified extraction: A holistic approach for extraction of citronella oil and phenolic compounds. Chem. Eng. Process. Process. Intensif. 2019, 146, 107694. [Google Scholar] [CrossRef]
- Zhao, W.; Nomura, K. Design of Efficient Molecular Catalysts for Synthesis of Cyclic Olefin Copolymers (COC) by Copolymerization of Ethylene and α-Olefins with Norbornene or Tetracyclododecene. Catalysts 2016, 6, 175. [Google Scholar] [CrossRef]
- Edwards, J.P.; Wolf, W.J.; Grubbs, R.H. The synthesis of cyclic polymers by olefin metathesis: Achievements and challenges. J. Polym. Sci. Part A Polym. Chem. 2018, 57, 228–242. [Google Scholar] [CrossRef]
- Cyclic Olefin Polymer Market by Type (Homopolymers, Copolymers), Process Type (Injection Molding, Extrusion), End-use Industry (Packaging, Automotive, Healthcare & Medical, Food & Beverages, Electrical & Electronics) and Region—Global Forecast to 2029. 2025. Available online: https://www.marketsandmarkets.com/Market-Reports/cyclic-olefin-polymer-market-220794705.html (accessed on 4 March 2025).
- Hassanien, M.F.R.; Assiri, A.M.A.; Alzohairy, A.M.; Oraby, H.F. Health-promoting value and food applications of black cumin essential oil: An overview. J. Food Sci. Technol. 2015, 52, 6136–6142. [Google Scholar] [CrossRef]
- Kurkcuoglu, M.; Tumen, G.; Baser, K.H.C. Essential Oil Constituents of Satureja boissieri from Turkey. Chem. Nat. Compd. 2001, 37, 329–331. [Google Scholar] [CrossRef]
- Dougnon, G.; Ito, M. Role of Ascaridole and p-Cymene in the Sleep-Promoting Effects of Dysphania ambrosioides Essential Oil via the GABAergic System in a ddY Mouse Inhalation Model. J. Nat. Prod. 2020, 84, 91–100. [Google Scholar] [CrossRef]
- Martín-Luengo, M.; Yates, M.; Domingo, M.M.; Casal, B.; Iglesias, M.; Esteban, M.; Ruiz-Hitzky, E. Synthesis of p-cymene from limonene, a renewable feedstock. Appl. Catal. B Environ. 2008, 81, 218–224. [Google Scholar] [CrossRef]
- Global p-Cymene Market Size By Application (Fragrance Industry, Pharmaceuticals), by End Use (Industrial, Commercial), by Source (Natural, Synthetic), by Purity Level (High Purity (99% and Above), Medium Purity (90%-99%)), by Distribution Channel (Direct Sales, Online Sales), by Geographic Scope and Forecast. 2025. Available online: https://www.verifiedmarketreports.com/product/p-cymene-market/ (accessed on 4 February 2023).
- Thielmann, J.; Muranyi, P. Review on the chemical composition of Litsea cubeba essential oils and the bioactivity of its major constituents citral and limonene. J. Essent. Oil Res. 2019, 31, 361–378. [Google Scholar] [CrossRef]
- Nhu-Trang, T.-T.; Casabianca, H.; Grenier-Loustalot, M.-F. Authenticity control of essential oils containing citronellal and citral by chiral and stable-isotope gas-chromatographic analysis. Anal. Bioanal. Chem. 2006, 386, 2141–2152. [Google Scholar] [CrossRef]
- Takabe, K.; Yamada, T.; Katagiri, T. A Facile Synthesis of Citral. Synth. Commun. 1983, 13, 297–301. [Google Scholar] [CrossRef]
- Citral Market Analysis and Forecast: 2025–2032. Available online: https://www.coherentmarketinsights.com/market-insight/citral-market-4436 (accessed on 4 April 2025).
- Wu, W.; Maravelias, C.T. Synthesis and techno-economic assessment of microbial-based processes for terpenes production. Biotechnol. Biofuels 2018, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Meskhidze, N.; Sabolis, A.; Reed, R.; Kamykowski, D. Quantifying environmental stress-induced emissions of algal isoprene and monoterpenes using laboratory measurements. Biogeosciences 2015, 12, 637–651. [Google Scholar] [CrossRef]
- Qi, C.; Zhao, H.; Li, W.; Li, X.; Xiang, H.; Zhang, G.; Liu, H.; Wang, Q.; Wang, Y.; Xian, M.; et al. Production of γ-terpinene by metabolically engineered Escherichia coli using glycerol as feedstock. RSC Adv. 2018, 8, 30851–30859. [Google Scholar] [CrossRef] [PubMed]
- Calderon, N.; Ofstead, E.A.; Ward, J.P.; Judy, W.A.; Scott, K.W. Olefin metathesis. I. Acyclic vinylenic hydrocarbons. J. Am. Chem. Soc. 1968, 90, 4133–4140. [Google Scholar] [CrossRef]
- Schuster, M.; Blechert, S. Olefin metathesis in organic chemistry. Angew. Chem. Int. Ed. Engl. 1997, 36, 2036–2056. [Google Scholar] [CrossRef]
- Sanchis, R.; Fenollar, O.; García, D.; Sánchez, L.; Balart, R. Improved adhesion of LDPE films to polyolefin foams for automotive industry using low-pressure plasma. Int. J. Adhes. Adhes. 2008, 28, 445–451. [Google Scholar] [CrossRef]
- Soares, J.B.; McKenna, T.F. Polyolefin Reaction Engineering, 1st ed.; Wiley Online Library: Hoboken, NJ, USA, 2012. [Google Scholar]
- Chen, G.-Q.; Patel, M.K. Plastics Derived from Biological Sources: Present and Future: A Technical and Environmental Review. Chem. Rev. 2012, 112, 2082–2099. [Google Scholar] [CrossRef] [PubMed]
- Nagakawa, Y.; Yunoki, S.; Saito, M. Liquid scintillation counting of solid-state plastic pellets to distinguish bio-based polyethylene. Polym. Test. 2014, 33, 13–15. [Google Scholar] [CrossRef]
- Zuber, M.; Zia, K.M.; Noreen, A.; Bukhari, S.A.; Aslam, N.; Sultan, N.; Jabeen, M.; Shi, B. Algae-Based Polyolefins. In Algae Based Polymers, Blends, and Composites; Zia, K.M., Zuber, M., Ali, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 499–529. [Google Scholar]
- Saini, R.K.; Keum, Y.-S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Adarme-Vega, T.C.; Lim, D.K.Y.; Timmins, M.; Vernen, F.; Li, Y.; Schenk, P.M. Microalgal biofactories: A promising approach towards sustainable omega-3 fatty acid production. Microb. Cell Factories 2012, 11, 96. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 Fatty Acids EPA and DHA: Health Benefits Throughout Life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- AlFadhly, N.K.Z.; Alhelfi, N.; Altemimi, A.B.; Verma, D.K.; Cacciola, F.; Narayanankutty, A. Trends and Technological Advancements in the Possible Food Applications of Spirulina and Their Health Benefits: A Review. Molecules 2022, 27, 5584. [Google Scholar] [CrossRef]
- Stokes, J.; Tu, R.; Peters, M.; Yadav, G.; Fabiano, L.A.; Seider, W.D. Omega-3 fatty acids from algae produced biodiesel. Algal Res. 2020, 51, 102047. [Google Scholar] [CrossRef]
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef]
- Balk, E.M.; Lichtenstein, A.H. Omega-3 Fatty Acids and Cardiovascular Disease: Summary of the 2016 Agency of Healthcare Research and Quality Evidence Review. Nutrients 2017, 9, 865. [Google Scholar] [CrossRef]
- Uauy, R.; Hoffman, D.R.; Birch, E.E.; Birch, D.G.; Jameson, D.M.; Tyson, J. Safety and efficacy of omega-3 fatty acids in the nutrition of very low birth weight infants: Soy oil and marine oil supplementation of formula. J. Pediatr. 1994, 124, 612–620. [Google Scholar] [CrossRef]
- Dokholyan, R.S.; Albert, C.M.; Appel, L.J.; Cook, N.R.; Whelton, P.; Hennekens, C.H. A trial of omega-3 fatty acids for prevention of hypertension. Am. J. Cardiol. 2004, 93, 1041–1043. [Google Scholar] [CrossRef]
- Layé, S.; Nadjar, A.; Joffre, C.; Bazinet, R.P. Anti-Inflammatory Effects of Omega-3 Fatty Acids in the Brain: Physiological Mechanisms and Relevance to Pharmacology. Pharmacol. Rev. 2018, 70, 12–38. [Google Scholar] [CrossRef]
- Labrousse, V.; Leyrolle, Q.; Amadieu, C.; Aubert, A.; Sere, A.; Coutureau, E.; Grégoire, S.; Bretillon, L.; Pallet, V.; Gressens, P.; et al. Dietary omega-3 deficiency exacerbates inflammation and reveals spatial memory deficits in mice exposed to lipopolysaccharide during gestation. Brain Behav. Immun. 2018, 73, 427–440. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Fucaceae: A Source of Bioactive Phlorotannins. Int. J. Mol. Sci. 2017, 18, 1327. [Google Scholar] [CrossRef]
- Meng, W.; Mu, T.; Sun, H.; Garcia-Vaquero, M. Phlorotannins: A review of extraction methods, structural characteristics, bioactivities, bioavailability, and future trends. Algal Res. 2021, 60, 102484. [Google Scholar] [CrossRef]
- Cassani, L.; Gomez-Zavaglia, A.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Seaweed-based natural ingredients: Stability of phlorotannins during extraction, storage, passage through the gastrointestinal tract and potential incorporation into functional foods. Food Res. Int. 2020, 137, 109676. [Google Scholar] [CrossRef]
- Purcell-Meyerink, D.; Packer, M.A.; Wheeler, T.T.; Hayes, M. Aquaculture Production of the Brown Seaweeds Laminaria digitata and Macrocystis pyrifera: Applications in Food and Pharmaceuticals. Molecules 2021, 26, 1306. [Google Scholar] [CrossRef]
- Wekre, M.E.; Holmelid, B.; Underhaug, J.; Pedersen, B.; Kopplin, G.; Jordheim, M. Characterization of high value products in the side-stream of Laminaria hyperborea alginate production—Targeting the phenolic content. Algal Res. 2023, 72, 103109. [Google Scholar] [CrossRef]
- Filote, C.; Santos, S.C.R.; Popa, V.I.; Botelho, C.M.S.; Volf, I. Biorefinery of marine macroalgae into high-tech bioproducts: A review. Environ. Chem. Lett. 2020, 19, 969–1000. [Google Scholar] [CrossRef]
- Johnston, K.G.; Abomohra, A.; French, C.E.; Zaky, A.S. Recent Advances in Seaweed Biorefineries and Assessment of Their Potential for Carbon Capture and Storage. Sustainability 2023, 15, 13193. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Jayashree, S.; Kumar, G.; Sharmili, S.A.; Gopal, M.; Dharmaraj, S.; Chen, W.-H.; Kothari, R.; Manasa, I.; Park, J.H.; et al. Recent developments in biorefining of macroalgae metabolites and their industrial applications—A circular economy approach. Bioresour. Technol. 2022, 359, 127235. [Google Scholar] [CrossRef]
- Hess, J.; Bednarz, D.; Bae, J.; Pierce, J. Petroleum and health care: Evaluating and managing health care’s vulnerability to petroleum supply shifts. Am. J. Public Health 2011, 101, 1568–1579. [Google Scholar] [CrossRef]
- Desborough, M.J.; Keeling, D.M. The aspirin story–from willow to wonder drug. Br. J. Haematol. 2017, 177, 674–683. [Google Scholar] [CrossRef]
- Rinsema, T.J. One hundred years of aspirin. Med. Hist. 1999, 43, 502–507. [Google Scholar] [CrossRef]
- Arnold, T.M.; Targett, N.M. Marine Tannins: The Importance of a Mechanistic Framework for Predicting Ecological Roles. J. Chem. Ecol. 2002, 28, 1919–1934. [Google Scholar] [CrossRef]
- Shirmohammadli, Y.; Efhamisisi, D.; Pizzi, A. Tannins as a sustainable raw material for green chemistry: A review. Ind. Crop. Prod. 2018, 126, 316–332. [Google Scholar] [CrossRef]
- Fordjour, E.; Mensah, E.O.; Hao, Y.; Yang, Y.; Liu, X.; Li, Y.; Liu, C.-L.; Bai, Z. Toward improved terpenoids biosynthesis: Strategies to enhance the capabilities of cell factories. Bioresour. Bioprocess. 2022, 9, 1–33. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, M.; Ren, Y.; Jin, G.; Tao, Y.; Lyu, L.; Zhao, Z.K.; Yang, X. Engineering Rhodosporidium toruloides for limonene production. Biotechnol. Biofuels 2021, 14, 1–11. [Google Scholar] [CrossRef]
- Martínez, H.; Santos, M.; Pedraza, L.; Testera, A.M. Advanced Technologies for Large Scale Supply of Marine Drugs. Mar. Drugs 2025, 23, 69. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Vonshak, A. Scaling up microalgal cultures to commercial scale. Eur. J. Phycol. 2017, 52, 407–418. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, X. Biosynthesis of monoterpenoid and sesquiterpenoid as natural flavors and fragrances. Biotechnol. Adv. 2023, 65, 108151. [Google Scholar] [CrossRef]
- Thomassen, G.; Vila, U.E.; Van Dael, M.; Lemmens, B.; Van Passel, S. A techno-economic assessment of an algal-based biorefinery. Clean Technol. Environ. Policy 2016, 18, 1849–1862. [Google Scholar] [CrossRef]
- Slegers, P.M.; Olivieri, G.; Breitmayer, E.; Sijtsma, L.; Eppink, M.H.M.; Wijffels, R.H.; Reith, J.H. Design of Value Chains for Microalgal Biorefinery at Industrial Scale: Process Integration and Techno-Economic Analysis. Front. Bioeng. Biotechnol. 2020, 8, 550758. [Google Scholar] [CrossRef]
- Osman, A.I.; Fang, B.; Zhang, Y.; Liu, Y.; Yu, J.; Farghali, M.; Rashwan, A.K.; Chen, Z.; Chen, L.; Ihara, I.; et al. Life cycle assessment and techno-economic analysis of sustainable bioenergy production: A review. Environ. Chem. Lett. 2024, 22, 1115–1154. [Google Scholar] [CrossRef]
- Kulas, D.G.; Thies, M.C.; Shonnard, D.R. Techno-Economic Analysis and Life Cycle Assessment of Waste Lignin Fractionation and Valorization Using the ALPHA Process. ACS Sustain. Chem. Eng. 2021, 9, 5388–5395. [Google Scholar] [CrossRef]
- Bhagwat, S.S.; Li, Y.; Cortés-Peña, Y.R.; Brace, E.C.; Martin, T.A.; Zhao, H.; Guest, J.S. Sustainable Production of Acrylic Acid via 3-Hydroxypropionic Acid from Lignocellulosic Biomass. ACS Sustain. Chem. Eng. 2021, 9, 16659–16669. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Kim, E.-A.; Son, K.-T.; Jeon, Y.-J. Bioactive properties and potentials cosmeceutical applications of phlorotannins isolated from brown seaweeds: A review. J. Photochem. Photobiol. B Biol. 2016, 162, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Shevyrin, V.A.; Kovaleva, E.G.; Flisyuk, E.V.; Shikov, A.N. Optimization of Extraction of Phlorotannins from the Arctic Fucus vesiculosus Using Natural Deep Eutectic Solvents and Their HPLC Profiling with Tandem High-Resolution Mass Spectrometry. Mar. Drugs 2023, 21, 263. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).