Abstract
Heavy oil, due to its complex hydrocarbon structure and resistance to degradation, poses significant environmental challenges. There is a lack of knowledge about the biodegradability of heavy oil in the natural environment under aerobic and anaerobic conditions. In this study, we used microbial communities of water and soil samples to investigate the biodegradation of heavy oil. Gas chromatography (GC) analysis was used to measure residual oil. Under aerobic conditions, soil-derived microorganisms demonstrated significantly higher degradation efficiency—achieving up to 80.3% removal—compared to water-derived samples, which showed a maximum degradation of 52.1%. Anaerobic conditions, on the other hand, clearly slowed down degradation; the maximum degradation rates in water and soil samples were 43.7% and 11.1%, respectively. Although no clear linear relationship was found, the correlation between initial microbial populations and degradation performance revealed that higher counts of heterotrophic and oil-degrading bacteria generally enhanced biodegradation. Under anaerobic conditions, especially, persistent hydrocarbon peaks in both environments suggest the presence of recalcitrant heavy oil fractions such as polycyclic aromatic hydrocarbons. In conclusion, this study emphasizes the crucial roles microbial sources and oxygen availability play in maximizing bioremediation techniques for environments contaminated with heavy oil.
1. Introduction
Petroleum hydrocarbons have been integral to human civilization for millennia. Historical records indicate that as early as 2500 B.C., these compounds were employed in ancient Egypt for embalming mummies and in Babylon, often regarded as the world’s first city, as adhesives in brick construction [1]. Since the 20th century, the widespread adoption of automobiles has driven an exponential increase in the use of petroleum hydrocarbons, particularly as fuels for internal combustion engines and as energy sources for thermal power generation [2]. Beyond energy applications, petroleum derivatives serve as lubricants to minimize mechanical friction, as raw materials for asphalt in road construction, and as key ingredients in cosmetic formulations such as conditioners and hair treatments. Currently, more than 6000 commercial products are derived from petroleum hydrocarbons, underscoring their essential role in modern society [3].
However, the extensive utilization of petroleum hydrocarbons has also led to significant environmental concerns. Oil spills from maritime tanker accidents and soil contamination from industrial wastewater discharge are among the most prominent examples of petroleum-related pollution [4]. Notable incidents include the 1989 Exxon Valdez oil spill in Alaska, which resulted in widespread ecological damage; the 1997 Diamond Grace spill in Tokyo Bay, which released approximately 1600 kiloliters(kL) of crude oil; and the Nakhodka spill in the Sea of Japan later that year, which discharged approximately 6200 kL of heavy oil. These events severely impacted marine and coastal ecosystems [5,6,7].
Large-scale contamination events have encouraged bioremediation as a practical and environmentally friendly method of restoration. This approach uses the inherent ability of pollution-degrading microorganisms to degrade hazardous molecules. Particularly in marine and terrestrial environments, bioremediation presents a reasonably affordable and environmentally friendly substitute for conventional physical and chemical remediation methods [8].
Usually, two main techniques define bioremediation methods: biostimulation and bioaugmentation. By adding nutrients and oxygen, biostimulation increases the activity of native hydrocarbon-degrading microorganisms, so fostering microbial degradation at sites already home to degraders. Currently, biostimulation is the most often used method in field applications [9], especially for biodegradable pollutants and widely distributed ones.
On the other hand, bioaugmentation is the introduction into contaminated surroundings of non-native but extremely effective microbial strains. First isolated and grown under laboratory conditions, these exogenous microorganisms are then used to improve degradation in places lacking enough natural degraders. This method is particularly helpful for persistent pollutants or in settings with low microbial variety [10].
1.1. Challenges in Heavy Oil Biodegradation
Though bioremediation seems to give hope, the effective cleanup of heavy oil contamination presents unique challenges. In comparison to the lighter crude oils, heavy oils are distinguished by strong viscosity, a high molecular weight, and a complicated chemical composition that entails a greater quantity of recalcitrant compounds, such as asphaltenes, resins, and high molecular weight polycyclic aromatic hydrocarbons (PAHs) [11]. Such structural complexities make heavy oils even intrinsically more difficult to break down by microorganisms; these may need specialized enzyme equipment and may necessitate longer incubation times [12]. Additionally, factors in nature, like nutrient limitation, less than optimal temperatures, and, most importantly, the presence or absence of oxygen, severely affect the rate and amount of heavy oil breakage, and this is why remediation of entire ecosystems across natural environments is a tremendous challenge. The realization that there exist those very challenges is the key to coming up with remediation strategies that are precise and effective in addressing this plentiful resource.
1.2. Advancements and Emerging Strategies in Bioremediation
Developments in molecular biology and environmental microbiology in the last decade are transforming what we know about bioremediation and its use. With the introduction of the so-called omics technologies (genomics, transcriptomics, proteomics, and metabolomics), a detailed characterization of microbial communities and their metabolic pathways involved in hydrocarbon degradation is made possible, which will lead to the establishment of novel oil-degrading microorganisms and enzymes with superior function [13]. Moreover, it is becoming more and more interesting to engineer microbial consortia, mixtures of multiple microbial species that can synergistically attack complex pollutants, to circumvent the problem of single strains [14]. Such engineered systems, as well as bioremediative interactions with other physical or chemical treatments (e.g., the biosurfactant production to enhance bioavailability), mark some novel steps towards further training the process of petroleum hydrocarbon remediation in challenging settings by enhancing its performance and potential applicability [15].
The biodegradation of petroleum hydrocarbons in natural environments is influenced by a range of factors, including the abundance and activity of degrading microorganisms, ambient temperature, pH, oxygen availability, and other site-specific parameters. Given the complexity of these interactions, a comprehensive evaluation of the environmental biodegradability of heavy oils is essential. Understanding the conditions under which heavy oils are more likely to degrade—or conversely, to persist—will be critical for predicting their environmental impact and for developing effective, targeted remediation strategies [15].
In this study, we focus on engine oil, a common type of lubricating oil and a representative heavy oil compound. Lubricating oil is produced on a global scale, and a significant portion of it is released into the environment during use, posing a major pollution challenge. The primary objective of this study is to assess the biodegradability of lubricating oil under various environmental conditions. We employed water and soil samples as microbial sources and cultured them in media containing lubricating oil as the sole carbon source. Additionally, we investigated the effects of different microbial sources (aquatic vs. terrestrial), oxygen availability (aerobic vs. anaerobic conditions), and initial microbial population levels on the rate and extent of oil degradation.
2. Materials and Methods
2.1. Sample Collection
Environmental water and soil samples were collected from various locations to serve as microbial sources for the aerobic heavy oil degradation experiments. Water samples were obtained in approximately 45 mL volumes using centrifuge tubes from rivers in Muroran and Noboribetsu, Hokkaido, Japan, as well as from factory effluent and well water sources within industrial facilities. Soil samples were collected in similar volumes from areas near a dry-cleaning establishment and from within a sugar manufacturing plant. In addition to these, two sludge samples collected from the Ranto wastewater treatment plant (Muroran, Japan) were also included and categorized as soil samples for the purposes of this study. In total, a diverse set of samples representing different environmental origins and microbial compositions was assembled.
2.2. Preparation of Microbial Suspensions
The collected environmental water samples were used directly in the biodegradation experiments. Until use, the samples were stored in a laboratory refrigerator at 4 °C to preserve microbial viability. In contrast, the soil samples required pretreatment to prevent the adsorption of heavy oil onto soil particles, which could interfere with the evaluation of microbial degradation activity. Therefore, microbial suspensions were extracted from the soil samples and used in the experiments. These suspensions were prepared one day before the start of the biodegradation tests.
Soil samples were similarly stored at 4 °C until processing. For each sample, 2 g of soil were placed into a 30 mL glass vial, and 20 mL of a sodium tripolyphosphate solution (5 mg/L) was added as a dispersing agent to facilitate microbial extraction. The mixture was shaken at 30 °C and 120 rpm for 24 h using a reciprocal shaker. After shaking, the vial was left undisturbed for 3 min to allow the sedimentation of larger particles. The resulting supernatant was carefully collected and filtered through Advantech No. 1 filter paper (pore size: 6 μm). A total of 15 mL of the filtrate was used as the microbial suspension for subsequent biodegradation experiments.
2.3. Biodegradation Experiment
2.3.1. Aerobic Degradation Experiment
For the aerobic degradation experiment, a 300 mL Erlenmeyer flask was used for each test. A total of 135 mL of sterilized Mineral Salt Medium (MSM; composition detailed in Table 1) was added to each flask. Sterilization was performed by autoclaving at 121 °C for 20 min. Following sterilization, heavy oil was added aseptically within a clean bench to achieve a final concentration of 1000 mg/L. The mixture was then emulsified using an ultrasonic cleaner and vortex mixer to ensure homogeneous dispersion of the heavy oil.

Table 1.
Sample collection locations.
Subsequently, 15 mL of the test sample or prepared microbial suspension was added to each flask, bringing the total working volume to 150 mL. The flasks were sealed with silicone stoppers and incubated under dark conditions at 30 °C with rotary shaking at 120 rpm to maintain aerobic conditions and promote microbial activity. Sampling was conducted at four time points: 0, 1, 2, and 3 weeks after inoculation. At each point, 10 mL of the culture medium was collected, and the residual heavy oil was extracted using hexane. The concentration of heavy oil in each sample was then quantified by gas chromatography [16]. To ensure accurate measurements, the culture medium was thoroughly mixed prior to sampling to achieve uniform emulsification of the heavy oil. As a negative control, an identical setup was prepared using autoclaved deionized water (sterilized at 121 °C for 20 min) in place of microbial suspension. This control system allowed for the assessment of abiotic loss or degradation of heavy oil.
2.3.2. Anaerobic Biodegradation Test of Heavy Oil
In a triangular flask, MSM medium (excluding Ca components) was added, autoclaved (121 °C, 20 min), and then placed in a clean bench. Heavy oil was added to the medium to achieve a final concentration of 1000 mg/L, and the MSM medium’s Ca components were added. Sodium resazurin was also added to a final concentration of 1 mg/L to visually confirm the anaerobic state. The heavy oil components in the medium were emulsified using an ultrasonic cleaner and vortex mixer to homogenize the oil [17].
Subsequently, 9 mL of the prepared medium was dispensed into 30 mL vials, and 1 mL of microbial suspension was added to each vial to prepare a total of 10 mL for each sample. A total of 8 vials (4 measurement points × 2) were prepared for each sample. The vials were then sealed with butyl rubber stoppers and aluminum caps, and the headspace was replaced with nitrogen gas to maintain anaerobic conditions. These vials were then incubated in the dark at 30 °C, shaking at 120 rpm.
Two vials of each sample were taken at the beginning of incubation and at one, two, and three weeks, and they were extracted using hexane. Gas chromatography was used to measure the remaining mass of heavy oil over time. Furthermore, the same procedure was used to create a microorganism-free control system by substituting autoclaved deionized water for microbial suspension. At each stage following the extraction of hexane, the amount of heavy oil that remained was measured.
2.4. Hexane Extraction
The remaining heavy oil in the culture medium was quantified using a liquid-liquid extraction technique based on hexane [18]. At each designated sampling point, 10 mL of the culture medium was transferred to a 50 mL centrifuge tube. To this were added 1 mL of 1N hydrochloric acid (Wako, special grade, 35.0–37.0%), 5 mL of hexane (Kanto Chemical, Tokyo, Japan, special grade, 96.0%), and 5 mL of acetone (Kanto Chemical, special grade, 99.5%). The mixture was vigorously shaken at 120 rpm for 3 min to assist in extracting the heavy oil into the organic phase. To separate the aqueous phase from the hexane layer, the sample was centrifuged for 10 min at 2500 rpm. The upper hexane layer that had been extracted was oil-containing and transferred into a sterile 50 mL centrifuge tube. The original tube was filled with an additional 5 mL of hexane, and the extraction process was repeated under the same conditions to raise the recovery efficiency once more. The recovered hexane fractions were mixed, then the solution was passed through nitrogen gas to evaporate under a nitrogen stream until the volume was reduced to approximately 1 mL. Following the transfer of this concentrated extract to a 2.0 mL GC vial, nitrogen gas completely evaporated the residual solvent. After that, the vial was filled with 75 μL of fresh hexane and 25 μL of a naphthalene solution—an internal standard for gas chromatographic analysis. Following preparation, gas chromatography (GC) was used to quantify the remaining heavy oil [19].
2.5. Gas Chromatography Analysis
A gas chromatograph (GC-2014, Shimadzu Corporation, Kyoto, Japan) with a flame ionization detector (FID) and a chromatopack data system (C-R24, Shimadzu Corporation, Kyoto, Japan) was used to perform a quantitative analysis of the extracted heavy oil. Naphthalene was used as the internal standard method to ensure precise quantification. A capillary column with dimensions of 0.25 mm inner diameter, 30 m length, and 0.25 μm film thickness was used for the analytical separation. The sensitivity and specificity of the FID detector in detecting hydrocarbons led to its selection.
The operating conditions for the gas chromatographic analysis of the test heavy oil are summarized in Table 2.

Table 2.
Optimal Conditions for GC Measurement.
3. Results
3.1. Heavy Oil Biodegradation Analysis by Gas Chromatography (GC)
The results of the heavy oil degradation experiment, as analyzed by gas chromatography (GC), are shown in Figure 1. Ten distinct peaks were observed (labeled peaks ① to ⑩). In the blank control system, which lacked microbial inoculation, no decrease in the peaks was detected over the experimental period, indicating no degradation occurred. The heavy oil peaks were compared with naphthalene, highlighted by a red circle in Figure 1.

Figure 1.
Gas Chromatography Results of the Control System. The peak of the internal standard substance, naphthalene, is marked with a red circle.
3.2. Heavy Oil Degradation in 3 Weeks Under Aerobic Conditions
After 3 weeks, heavy oil biodegradation was analyzed by gas chromatography to observe the change in peaks. As shown in Table 3, in the heavy oil degradation experiments using water samples as microbial sources, various major peaks of heavy oil components tend to remain undegraded. This suggests that the microorganisms in the water samples may not be as effective in degrading certain components of the heavy oil.

Table 3.
The Presence (×) or Absence (○) of Major Heavy Oil Peaks After 3 Weeks of aerobic conditions.
On the other hand, in the heavy oil degradation experiments using soil samples as microbial sources, most of the major heavy oil components were degraded, indicating that the microorganisms present in the soil samples were more efficient in breaking down the oil. However, a common observation in both the water and soil samples is that components corresponding to Peaks ⑦, ⑨, and ⑩ tend to remain even after the degradation process. This suggests that these specific components of heavy oil are more resistant to microbial degradation and are therefore likely to persist in the environment following contamination. Next, the total concentration of the major peak components for each sample was calculated, and the overall heavy oil concentration changes were summarized in Figure 2. In this figure, the remaining percentage of heavy oil was calculated using Equation (1), and its temporal changes are shown to illustrate the degradation progress over time.
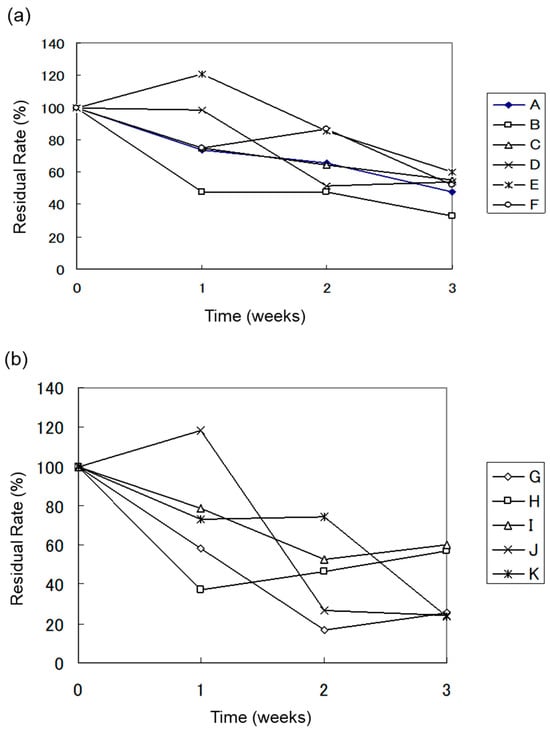
Figure 2.
(a) Aerobic Biodegradation Test Results of Heavy Oil (Water Sample), (b) Aerobic Biodegradation Test Results of Heavy Oil (Soil Sample).
The remaining rate (%) of heavy oil is calculated as follows:
- Cf: Concentration of heavy oil at each measurement time
- Ci: Initial concentration of heavy oil at the start of cultivation.
3.2.1. Biodegradation of Heavy Oil by Water Samples
Figure 2a,b show the biodegradation of heavy oil by water and soil samples under aerobic conditions over three weeks, respectively. The degradation percentage for each sample is summarized in Table 4. The residual rate (%) degradation percentage was calculated using Equations (1) and (2).

Table 4.
Biodegradation Rate After 3 Weeks in Aerobic Biodegradation Experiment of Heavy Oil.
3.2.2. The Effect of Initial Microbial Counts on the Biodegradation of Heavy Oil
The total bacterial count and heavy oil-degrading bacteria count in the samples used for the heavy oil degradation experiment are classified into general heterotrophic bacteria and heavy oil-degrading bacteria (those detected in media where heavy oil served as the sole carbon and energy source), as shown in Figure 3. In water samples, the total heterotrophic bacteria count ranged from 2.0 × 103 to 2.4 × 106 CFU/mL. The heavy oil-degrading bacteria ranged from 0 to 4.3 × 104 CFU/mL.
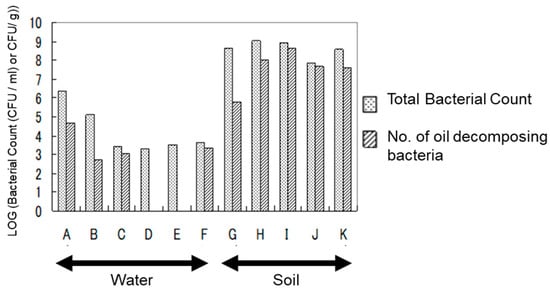
Figure 3.
Calculation of initial bacterial CFU.
In the soil environment, the total heterotrophic bacteria count ranged from 7.2 × 107 to 1.1 × 109 CFU/g. The heavy oil-degrading bacteria ranged from 6.3 × 105 to 4.3 × 108 CFU/g. This result suggests that microorganisms capable of degrading heavy oil and using it as a carbon source are widely distributed in general environments, with a particularly high abundance in soil.
Correlation Between Biodegradation Rate of Heavy Oil and Microbial Count in Water Samples Under Aerobic Conditions
In Figure 4a,b, the graphs are designed to determine if there is a relationship between the concentration of oil-degrading bacteria and the rate of biodegradation in water samples under aerobic conditions. Figure 4a shows the correlation between the heavy oil biodegradation rate and the total microbial count in water samples under aerobic conditions, while Figure 4b demonstrates the correlation between the heavy oil-degrading bacteria count and the biodegradation rate of heavy oil. There was no direct correlation between the concentration of oil-degrading bacteria and the rate of biodegradation in water samples. However, higher biodegradation rates were observed when the initial microbial count was high.
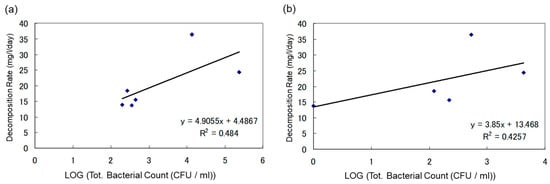
Figure 4.
(a) The Correlation Between Biodegradation Rate of Heavy Oil and Total Microbial Count in Water Samples Under Aerobic Conditions; (b) Correlation Between Biodegradation Rate of Heavy Oil and Heavy Oil-Degrading Bacteria Count in Water Samples Under Aerobic Conditions.
Correlation Between Biodegradation Rate of Heavy Oil and Microbial Count in Soil Samples Under Aerobic Conditions
In Figure 5a,b, the graphs are designed to determine if there is a relationship between the concentration of oil-degrading bacteria and the rate of biodegradation in soil samples under aerobic conditions. Figure 5a shows the correlation between total microbial count in soil samples under aerobic conditions and heavy oil biodegradation rate, while Figure 5b demonstrates the correlation between the biodegradation rate of heavy oil and the heavy oil-degrading bacteria count. There was no direct correlation between the concentration of oil-degrading bacteria and the rate of biodegradation in soil samples. However, higher biodegradation rates tended to occur when the initial microbial count was high.
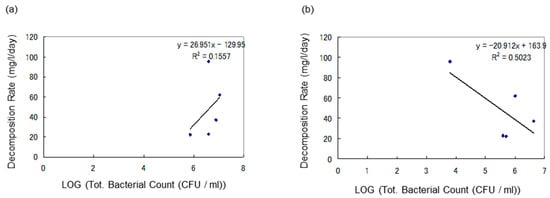
Figure 5.
(a) Correlation Between the Biodegradation Rate of Heavy Oil and the Total Bacteria Count in Soil Samples Under Aerobic Conditions, (b) Correlation Between Biodegradation Rate of Heavy Oil and Oil-Degrading Bacteria Count in Soil Samples Under Aerobic Conditions.
3.3. Heavy Oil Degradation Under Anaerobic Conditions
Anaerobic biodegradation tests of heavy oil were conducted, and residual heavy oil was analyzed using gas chromatography (GC). First, the GC results of the control samples for each week are shown (Figure 6). During the experimental period, no significant changes in the intensity of major peaks were observed, and there was no notable reduction in heavy oil components. Therefore, it was confirmed that any reduction in heavy oil components during the anaerobic biodegradation experiments was attributable to microbial degradation by oil-degrading bacteria. The heavy oil peaks were compared with naphthalene, highlighted by a red circle in Figure 1.
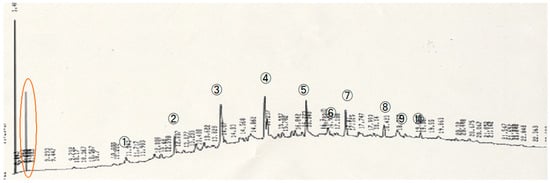
Figure 6.
Gas chromatography results of the control system under anaerobic conditions. The peak of the internal standard substance, naphthalene, is indicated with a red circle.
Analysis of Major Heavy Oil Peaks. Based on the GC analysis results, the concentrations of the major heavy oil peaks (① to ⑩) were calculated, and their changes over time were summarized in Figure 7 for each test system. After 3 weeks, the presence and absence of the major peaks were observed and summarized in Table 5.
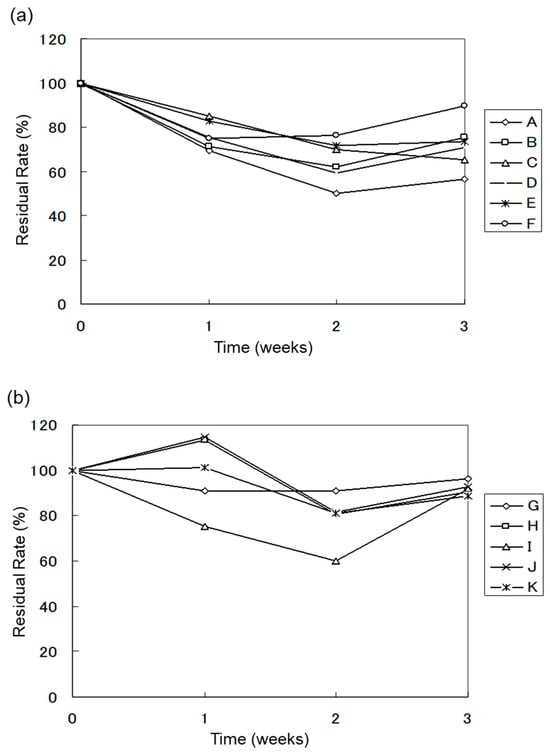
Figure 7.
(a) Anaerobic biodegradation test results of heavy oil (water sample); (b) Anaerobic biodegradation test results for heavy oil (soil samples).

Table 5.
Presence (×) or Absence (○) of Major Heavy Oil Peaks After 3 Weeks of Anaerobic Incubation.
3.3.1. Heavy Oil Degradation in 3 Weeks by Water and Soil Samples Under Anaerobic Conditions
Regardless of whether the sample is water or soil, under anaerobic conditions, heavy oil proves to be resistant to biodegradation by microorganisms, with many components of the heavy oil remaining intact. The data in Figure 7a,b highlight the limited biodegradation of heavy oil under anaerobic conditions. Over the course of the experiment, the residual rate remained relatively high, indicating that the biodegradation of heavy oil components was significantly hindered under anaerobic conditions. As shown in Figure 7a, the degradation experiment using water samples as the microbial source demonstrated a heavy oil degradation rate ranging from 10.2% to 43.7%. In contrast, the degradation experiment using soil samples as the microbial source exhibited a much lower heavy oil degradation rate, ranging from 3.7% to 11.1% (Figure 7b).
3.3.2. Effect of Initial Bacterial Count on the Biodegradation of Heavy Oil
To explore the impact of the initial bacterial count on the biodegradation of heavy oil, the degradation rate (mg/L/day) was calculated, and its correlation with both the initial total bacterial count and the initial heavy oil-degrading bacterial count was examined. The results of this analysis are presented in Figure 8 and Figure 9.
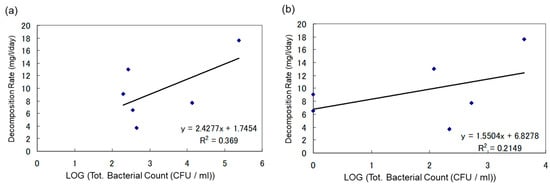
Figure 8.
(a) Correlation Between Degradation Rate of Heavy Oil and Total Bacterial Count in Water Sample Under Anaerobic Conditions; (b) Correlation Between Degradation Rate of Heavy Oil and Heavy Oil-Degrading Bacterial Count in Water Sample Under Anaerobic Conditions.
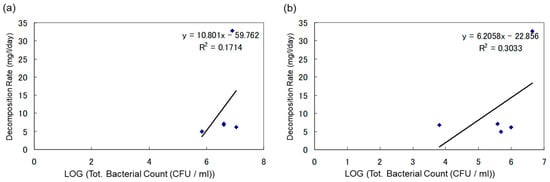
Figure 9.
(a) Correlation Between Degradation Rate of Heavy Oil and Total Bacterial Count in Soil Sample Under Anaerobic Conditions. (b) Correlation Between Degradation Rate of Heavy Oil and Heavy Oil-Degrading Bacterial Count in Soil Sample Under Anaerobic Conditions.
These figures allow assessment of the correlation between bacterial concentrations and biodegradation rates under anaerobic conditions, providing insight into the role of microbial populations in the breakdown of heavy oil in the tested environments.
Correlation Between Biodegradation Rate of Heavy Oil and Microbial Count in Water Samples Under Anaerobic Conditions
In Figure 8, the vertical axis represents the degradation rate of heavy oil (mg/L/day), and the horizontal axis represents the logarithmic values of the initial total bacterial count (CFU/mL) and the initial heavy oil-degrading bacterial count (CFU/mL).
The graphs are designed to determine if there is a relationship between the concentration of oil-degrading bacteria and the rate of biodegradation in soil samples under anaerobic conditions. Figure 8a indicates the correlation between total microbial count in water samples and heavy oil degradation rate, while Figure 8b shows the correlation between degradation rate of heavy oil and heavy oil degrading bacterial count in water samples. There was no direct correlation between the concentration of oil-degrading bacteria and the rate of biodegradation in soil samples. However, high biodegradation was observed when the initial microbial count was high.
Correlation Between Biodegradation Rate of Heavy Oil and Microbial Count in Soil Samples Under Anaerobic Conditions
In Figure 9, the vertical axis represents the degradation rate of heavy oil (mg/L/day), and the horizontal axis represents the logarithmic values of the initial total bacterial count (CFU/mL) and the initial heavy oil-degrading bacterial count (CFU/mL). Figure 9a demonstrates the correlation between total microbial count in soil samples and heavy oil degradation rate, while Figure 9b indicates the correlation between degradation rate of heavy oil and degrading bacterial count in soil samples. This study could not find a statistically significant correlation between CFU/mL and heavy oil decomposition rate.
4. Discussion
Significant energy resources, such as heavy oil reservoirs, may be recoverable. The enormous heavy oil deposits embedded in the Earth’s crust account for almost 70% of the world’s petroleum reserves [20]. Heavy oil deposits are seen as a promising substitute for conventional crude oil in the petroleum portfolios of the 21st century and to meet the world’s energy demands [21]. Environmental researchers are concerned about the massive amounts of oily residue produced by the world’s heavy oil extraction operations.
4.1. Heavy Oil Biodegradation Under Aerobic and Anaerobic Conditions
Our research demonstrated a marked difference in heavy oil biodegradation rates between aerobic and anaerobic conditions, as well as between soil and water samples.
4.1.1. Heavy Oil Biodegradation Under Aerobic Conditions
This study aimed to investigate the biodegradability of heavy oil in the environment and determine the microorganisms causing its breakdown. Biodegradation experiments were carried out using samples of soil and water. Experiments on the biodegradation of heavy oil under aerobic conditions used water and soil samples. The results revealed some degree of degradation in the water samples; however, the most important heavy oil components remained undegraded. On the other hand, the degradation process proved more successful on the soil sample since there were fewer residual heavy oil components. This suggests that in the soil environment, microorganisms are more efficient in breaking down heavy oil than in water surroundings [22].
In this study, heavy oil biodegradation under aerobic and anaerobic conditions shows how environmental conditions and microbial sources affect the degradation efficiency of petroleum hydrocarbons. Under aerobic conditions, soil-derived microbial communities acquired the best biodegradation efficiency after three weeks, that is, 80.3%. On the other hand, under the same conditions, the biodegradability rate of water-derived samples was 52.1%. These findings imply that more effective oil-degrading microbial populations found in soil environments are probably the outcome of enhanced microbial diversity, improved enzymatic capacity, and more adaptation to hydrocarbon exposure [23].
Many elements help to explain the remarkable performance of soil microorganisms in aerobic conditions. At first, soil often has a varied and complex microbial community comprising both general and specialized hydrocarbon-degrading bacteria [24]. Moreover, the presence of micro-niches, nutrients, and organic matter in soil could help some microorganisms survive and thrive. On the other hand, aqueous settings are marked by decreased nutrient availability and smaller microbial diversity, which might limit the metabolic activity and spread of hydrocarbon-degrading microorganisms. The noted difference in biodegradation rates between soil and water samples emphasizes the importance of microbial origin in bioremediation techniques. The total heterotrophic bacteria count in the water samples used for this study ranged from 2.0 × 103 to 2.4 × 106 CFU/mL; the count of oil-degrading bacteria ranged from 0 to 4.3 × 104 CFU/mL. By contrast, oil-degrading bacteria ranged from 6.3 × 105 to 4.3 × 108 CFU/g, and soil samples included a higher number of heterotrophic bacteria, ranging from 7.2 × 107 to 1.1 × 109 CFU/g. This implies that soil settings are more likely to feature oil-degrading bacteria [25].
Despite the substantial degradation that was accomplished in aerobic conditions, specific heavy oil components remained undegraded in both soil and water systems. The persistence of specific hydrocarbon peaks, particularly those that correspond to heavier or more structurally complex compounds, was consistently demonstrated in gas chromatography analysis. It is probable that these recalcitrant fractions are made up of high molecular weight polycyclic aromatic hydrocarbons or branched alkanes, which are inherently more difficult to degrade. The necessity of protracted treatment periods or the incorporation of more specialised microbial consortia in future remediation approaches is suggested by the persistence of such components [26].
4.1.2. Heavy Oil Biodegradation Under Anaerobic Conditions
Anaerobic conditions greatly slowed the breakdown of heavy oil. In anaerobic conditions, the soil samples showed a rate of biodegradation (11.1%), whereas the water samples showed high degradation, 43.7%, compared to the soil. The sharp contrast with the aerobic results highlights the crucial role that oxygen plays in promoting the microbial breakdown of complex hydrocarbons. While anaerobic microbial processes can break down some petroleum components, they are typically slower and limited to particular metabolic pathways, like sulfate reduction or methanogenesis, which may not adequately target the wide range of compounds found in heavy oil [27]. Due to the low degradation efficiency under anaerobic conditions, natural attenuation in oxygen-deprived environments is likely to be slow and incomplete. As a result, it might be essential to improve bioremediation results in these environments by boosting oxygen availability using methods like bioventing or biosparging. Alternatively, engineered microbial consortia or electron acceptor amendments might be taken into consideration to more successfully stimulate anaerobic degradation pathways [27].
4.2. Effect of Initial Microbial Count on Biodegradation Performance
The trend that we have witnessed in our research is that the greater the biodegradation rate, the greater the number of heterotrophic bacteria or oil-degrading bacteria [28]. Nonetheless, such a correlation as between the biodegradability of the heavy oil and the initial number of bacteria in the samples did not have a strict positive correlation. However, the biodegradation of heavy oil in an oxygenated environment is also visibly and markedly impacted by the types of oil-vaporising bacteria within the samples and the quantity of bacteria present.
4.3. Broader Implications and Future Directions
The results of this research have meaningful ramifications for solving the environmental predicaments of heavy oil extraction and spills, as well as coming up with sustainable technologies of remediation.
4.3.1. Environmental Concerns
The large quantity of oily flotsam created by the heavy oil mining activities, as well as the challenge to decompose, manage, and dispose of oily sludge, presents eminent fears to the environment [29,30]. The stacked deposition of this waste product is hazardous to the environment as it poses a risk to the nearby water, land, and air pollution [31]. Our findings underline the importance of having an efficient and clean environment remediation technique to make oily sludge safe to dispose of. It is this strongly expressed optimal biodegradation in soil systems within the context of aerobic conditions that reflects a probable route towards in situ or ex situ bioremediation of polluted land sites that can provide a sustainable alternative to expensive and largely problematic external remediation strategies such as land excavation or soil replacement [32,33]. This idea of reducing, recycling, repurposing, and disposing of the waste eventually, given its focus on waste management, finds a very strong parallel with the principles of bioremediation as a more ecologically sound and cost-effective process [34].
4.3.2. Technological Synergy and Symbiosis Prospects
The difference in the level of biodegradation observed between soil and water samples, as well as under different oxygen conditions, reveals the potential of technological synergy. In anaerobic or oxygen-limited environments (or in deep-sea sediments), the combination of bioremediation with a method to increase the amount of available oxygen, e.g., bioventing or biosparging, may offer considerable improvements in terms of degradation rate [28]. Additionally, the fact that recalcitrant heavy oil constituents still exist, as highlighted by studies, can encourage the creation of engineered microbial consortia or amendments of specific electron acceptors that act as the stimulus of anaerobic bioremediation in a more effective way [28]. The development of bespoke bioremediation agents, e.g., skilled in the degradation of specific types of oil, might be achieved by research on the isolation of oil-degrading microbial strains, especially those living in adapted soils. Interactions in the microbial community of one organism benefiting another, such as symbiotic associations offering specific metabolic pathways to break down complex hydrocarbons, are promising areas of future investigation. These synergies might be discovered and utilised, which could facilitate a complete and faster breakdown of even the most recalcitrant parts of heavy oils.
4.3.3. Scalability Perspectives of Real-World Industrial-Manufacturing Scales
Although this research will offer honest information at a laboratory level, industrial- and manufacturing-scale application of bioremediation technologies is an area of great concern by the industry itself. Bioremediation could be used at a large scale because its cost-effectiveness [35] and energy efficiency [35] at a smaller scale are good indicators of its application on a large scale. As an example, the bioreactors could be designed to replicate the ideal conditions that have been observed in the soil (e.g., aeration, nutrient availability, and regulated microbial numbers) and thus, over time, effectively treat the oily sludge that may be produced by an industrial process ex situ. The in situ remediation of contaminated sites, biostimulation (optimizing, e.g., nutrient availability) or bioaugmentation (a highly effective strain of microbes is introduced) can be a very promising strategy. Percentages of microbial counts and their relationship to the efficiency of degradation serve as a basis for establishing predictive models of remediation results on a greater scale. Their work should be further developed to optimize parameters, i.e., nutrient delivery, oxygen transfer rates, and inoculum size, to a stage at which the industrial application can be applied, and it can be ensured that the tremendous efficiency found in controlled experiments can be transferred into feasible, economically effective, and environmentally friendly solutions in dealing with heavy oil pollution worldwide [36].
5. Conclusions
Heavy oil is a significant environmental concern and requires sustainable biodegradation methods. This study found that the biodegradability of heavy oil was higher in the soil environment under aerobic conditions as compared to water. Under anaerobic conditions, there was a significant decrease in heavy oil degradation, which shows that heavy oil biodegradation is influenced by microbial source and oxygen availability. These findings suggest that future studies should focus on enhancing microbial activity and hydrocarbon degradation in aquatic systems. The study identified the effect of initial microbial count on degradation, suggesting that bioremediation using microbial consortia is an effective method for addressing heavy oil contamination. Additionally, maintaining aerobic conditions through aeration to carry out biostimulation could enhance the cleanup of anaerobic heavy oil contamination.
In spite of the promise of bioremediation, there remains a series of technological limitations that exist in these processes, such as incomplete biodegradation of the base fraction of heavy oil residues and the inefficiency of oxygen transfer in highly dissimilar, large-volume bioremediation sites. The prospect of overcoming these difficult challenges lies in the further development of optimization of microbial consortium, novel biostimulating methods, and the combination of bioremediation and other remediation technologies. Finally, further developments in the field have a lot wider application prospects because such solutions are environmentally friendly and cost-effective to reduce the world’s pollution of heavy oil in multiple industrial and natural environments.
Author Contributions
Conceptualization, Y.-C.C.; methodology, Y.-C.C.; software, Y.-C.C.; validation, formal analysis, S.A.; investigation, Y.-C.C.; resources, Y.-C.C.; data curation, Y.-C.C.; writing—original draft preparation, S.A. and I.; writing—review and editing, S.A. and I.; visualization, I.; supervision, Y.-C.C.; project administration, Y.-C.C.; funding acquisition, Y.-C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by JSPS KAKENHI (Grant Number 24K11471). Additionally, this research was supported by the Ogasawara Foundation for the Promotion of Science and Engineering (Japan) and the Iwatani Foundation for the Promotion of Science and Engineering (Japan).
Data Availability Statement
Data will be provided on demand.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GC | Gas Chromatography |
| FID | Flame Ionization Detector |
| MSM | Minimal Salt Medium |
| mg | Milligram |
| mL | Milliliter |
| CFU | Colony Forming Unit |
References
- Maurer, J.; Möhring, T.; Rullkötter, J.; Nissenbaum, A. Plant lipids and fossil hydrocarbons in embalming material of Roman Period mummies from the Dakhleh Oasis, Western Desert, Egypt. J. Archaeol. Sci. 2002, 29, 751–762. [Google Scholar] [CrossRef]
- Paykani, A.; Chehrmonavari, H.; Tsolakis, A.; Alger, T.; Northrop, W.F.; Reitz, R.D. Synthesis gas as a fuel for internal combustion engines in transportation. Prog. Energy Combust. Sci. 2022, 90, 100995. [Google Scholar] [CrossRef]
- Robinson, P.R. Natural Gas and Petroleum Products. In Petroleum Science and Technology: Downstream; Springer Nature: Cham, Switzerland, 2024; pp. 193–241. [Google Scholar]
- Sayed, F.A.; Eid, M.H.; El-Sherbeeny, A.M.; Abdel-Gawad, G.I.; Mohamed, E.A.; Abukhadra, M.R. Environmental and health risk assessment of polycyclic aromatic hydrocarbons and toxic elements in the red sea using Monte Carlo simulation. Sci. Rep. 2025, 15, 4122. [Google Scholar] [CrossRef]
- Peterson, C.H. The “Exxon Valdez” oil spill in Alaska: Acute, indirect and chronic effects on the ecosystem. Adv. Mar. Biol. 2001, 39, 1–103. [Google Scholar]
- Maki, H.; Hirayama, N.; Hiwatari, T.; Kohata, K.; Uchiyama, H.; Watanabe, M.; Yamasaki, F.; Furuki, M. Crude oil bioremediation field experiment in the Sea of Japan. Mar. Pollut. Bull. 2003, 47, 74–77. [Google Scholar] [CrossRef]
- Tazaki, K.; Fukuyama, A.; Tazaki, F.; Shintaku, Y.; Nakamura, K.; Takehara, T.; Katsura, Y.; Shimada, K. Twenty Years after the Nakhodka Oil Spill Accident in the Sea of Japan, How Has Contamination Changed? Minerals 2018, 8, 178. [Google Scholar] [CrossRef]
- Okeke, E.S.; Okoye, C.O.; Ezeorba, T.P.C.; Mao, G.; Chen, Y.; Xu, H.; Song, C.; Feng, W.; Wu, X. Emerging bio-dispersant and bioremediation technologies as environmentally friendly management responses toward marine oil spill: A comprehensive review. J. Environ. Manag. 2022, 322, 116123. [Google Scholar] [CrossRef]
- Anani, O.A.; Jeevanandam, J.; Adetunji, C.O.; Inobeme, A.; Oloke, J.K.; Yerima, M.B.; Thangadurai, D.; Islam, S.; Oyawoye, O.M.; Olaniyan, O.T. Application of biosurfactant as a noninvasive stimulant to enhance the degradation activities of indigenous hydrocarbon degraders in the soil. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 69–87. [Google Scholar]
- Mayans, B.; Antón-Herrero, R.; García-Delgado, C.; Delgado-Moreno, L.; Guirado, M.; Pérez-Esteban, J.; Escolástico, C.; Eymar, E. Bioremediation of petroleum hydrocarbons polluted soil by spent mushroom substrates: Microbiological structure and functionality. J. Hazard. Mater. 2024, 473, 134650. [Google Scholar] [CrossRef]
- Shahsavari, E.; Schwarz, A.; Aburto-Medina, A.; Ball, A.S. Biological degradation of polycyclic aromatic compounds (PAHs) in soil: A current perspective. Curr. Pollut. Rep. 2019, 5, 84–92. [Google Scholar] [CrossRef]
- Thapa, S.; Li, H.; OHair, J.; Bhatti, S.; Chen, F.C.; Nasr, K.A.; Johnson, T.; Zhou, S. Biochemical characteristics of microbial enzymes and their significance from industrial perspectives. Mol. Biotechnol. 2019, 61, 579–601. [Google Scholar] [CrossRef]
- Amini, F.; Giyahchi, M.; Moghimi, H. Bioremediation of Petroleum Contamination by Microorganisms: Role of Microbial Communities and Applications. Microb. Bioremediation Multiomics Technol. Sustain. Dev. Recent. Trends 2024, 13, 136. [Google Scholar]
- Petsas, A.S.; Vagi, M.C. Trends in the bioremediation of pharmaceuticals and other organic contaminants using native or genetically modified microbial strains: A review. Curr. Pharm. Biotechnol. 2019, 20, 787–824. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, B.A.; Aragaw, T.A.; Genet, M.B. Bioremediation of petroleum hydrocarbon contaminated soil: A review on principles, degradation mechanisms, and advancements. Front. Environ. Sci. 2024, 12, 1354422. [Google Scholar] [CrossRef]
- Nunal, S.N.; Santander-De Leon, S.M.S.; Hongyi, W.; Regal, A.A.; Yoshikawa, T.; Okunishi, S.; Maeda, H. Hydrocarbon degradation and bacterial community responses during remediation of sediment artificially contaminated with heavy oil. Biocontrol Sci. 2017, 22, 187–203. [Google Scholar] [CrossRef]
- Lavania, M.; Cheema, S.; Lal, B. Potential of viscosity reducing thermophillic anaerobic bacterial consortium TERIB# 90 in upgrading heavy oil. Fuel 2015, 144, 349–357. [Google Scholar][Green Version]
- Li, X.; Du, Y.; Wu, G.; Li, Z.; Li, H.; Sui, H. Solvent extraction for heavy crude oil removal from contaminated soils. Chemosphere 2012, 88, 245–249. [Google Scholar] [CrossRef]
- Adebusoye, S.A.; Ilori, M.O.; Amund, O.O.; Teniola, O.D.; Olatope, S.O. Microbial degradation of petroleum hydrocarbons in a polluted tropical stream. World J. Microbiol. Biotechnol. 2007, 23, 1149–1159. [Google Scholar] [CrossRef]
- Wen, Z.; Wang, J.; Wang, Z.; He, Z.; Song, C.; Liu, X.; Zhang, N.; Ji, T. Analysis of the world deepwater oil and gas exploration situation. Pet. Explor. Dev. 2023, 50, 1060–1076. [Google Scholar] [CrossRef]
- Rajendran, S.; Al-Samydai, A.; Palani, G.; Trilaksana, H.; Sathish, T.; Giri, J.; Saravanan, R.; Lalvani, J.I.J.; Nasri, F. Replacement of Petroleum Based Products With Plant-Based Materials, Green and Sustainable Energy—A Review. Eng. Rep. 2025, 7, e70108. [Google Scholar] [CrossRef]
- Chuah, L.F.; Chew, K.W.; Bokhari, A.; Mubashir, M.; Show, P.L. Biodegradation of crude oil in seawater by using a consortium of symbiotic bacteria. Environ. Res. 2022, 213, 113721. [Google Scholar] [CrossRef]
- Chaudhary, D.K.; Bajagain, R.; Jeong, S.W.; Kim, J. Insights into the biodegradation of diesel oil and changes in bacterial communities in diesel-contaminated soil as a consequence of various soil amendments. Chemosphere 2021, 285, 131416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, S.; Guo, P.; Guo, S. Characteristics of organic carbon metabolism and bioremediation of petroleum-contaminated soil by a mesophilic aerobic biopile system. Chemosphere 2021, 264, 128521. [Google Scholar] [CrossRef] [PubMed]
- Nejidat, A.; Meshulam, M.; Diaz-Reck, D.; Ronen, Z. Emergence of hydrocarbon-degrading bacteria in crude oil-contaminated soil in a hyperarid ecosystem: Effect of phosphate addition and augmentation with nitrogen-fixing cyanobacteria on oil bioremediation. Int. Biodeterior. Biodegrad. 2023, 178, 105556. [Google Scholar] [CrossRef]
- Yamini, V.; Rajeswari, V.D. Metabolic capacity to alter polycyclic aromatic hydrocarbons and its microbe-mediated remediation. Chemosphere 2023, 329, 138707. [Google Scholar] [CrossRef] [PubMed]
- Wartell, B.; Boufadel, M.; Rodriguez-Freire, L. An effort to understand and improve the anaerobic biodegradation of petroleum hydrocarbons: A literature review. Int. Biodeterior. Biodegrad. 2021, 157, 105156. [Google Scholar] [CrossRef]
- Koolivand, A.; Abtahi, H.; Parhamfar, M.; Saeedi, R.; Coulon, F.; Kumar, V.; Villaseñor, J.; Sartaj, M.; Najarian, N.; Shahsavari, M.; et al. The effect of petroleum hydrocarbons concentration on competition between oil-degrading bacteria and indigenous compost microorganisms in petroleum sludge bioremediation. Environ. Technol. Innov. 2022, 26, 102319. [Google Scholar] [CrossRef]
- Duan, Y.; Gao, N.; Sipra, A.T.; Tong, K.; Quan, C. Characterization of heavy metals and oil components in the products of oily sludge after hydrothermal treatment. J. Hazard. Mater. 2022, 424, 127293. [Google Scholar] [CrossRef]
- Hasan, A.M.; Kamal, R.S.; Farag, R.K.; Abdel-Raouf, M.E. Petroleum sludge formation and its treatment methodologies: A review. Environ. Sci. Pollut. Res. 2024, 31, 8369–8386. [Google Scholar] [CrossRef]
- Wang, X.; Jin, W.; Li, Y.; Liu, S.; Xu, J.; Liu, J.; Li, H.; Long, T. Treatment advances of hazardous solid wastes from oil and gas drilling and production processes. Chem. Eng. J. 2024, 497, 154182. [Google Scholar] [CrossRef]
- Xiaojie, Z.; Mukherjee, K.; Manna, S.; Das, M.K.; Kim, J.K.; Sinha, T.K. Efficient management of oil waste: Chemical and physicochemical approaches. In Advances in Oil-Water Separation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 439–467. [Google Scholar]
- Kwon, J.H.; Ji, M.K.; Kumar, R.; Islam, M.M.; Khan, M.A.; Park, Y.K.; Yadav, K.K.; Vaziri, R.; Hwang, J.H.; Lee, W.H.; et al. Recent advancement in enhanced soil flushing for remediation of petroleum hydrocarbon-contaminated soil: A state-of-the-art review. Rev. Environ. Sci. Bio/Technol. 2023, 22, 679–714. [Google Scholar] [CrossRef]
- Ogunbiyi, O.; Al-Rewaily, R.; Saththasivam, J.; Lawler, J.; Liu, Z. Oil spill management to prevent desalination plant shutdown from the perspectives of offshore cleanup, seawater intake and onshore pretreatment. Desalination 2023, 564, 116780. [Google Scholar] [CrossRef]
- Baloyi, J.; Mafunda, A. Novel Methods and Environment-Friendly Techniques for the Remediation of Environmental Petroleum Pollutants. In Environmental Hydrocarbon Pollution and Zero Waste Approach Towards a Sustainable Waste Management; Springer Nature: Cham, Switzerland, 2025; pp. 143–174. [Google Scholar]
- Kumar, V.; Shahi, S.K.; Singh, S. Bioremediation: An eco-sustainable approach for restoration of contaminated sites. In Microbial Bioprospecting for Sustainable Development; Springer Nature: Singapore, 2018; pp. 115–136. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).