Abstract
The aim of the present study was to investigate how different drying techniques (lyophilization, convective drying, and osmotic dehydration) affect the phytochemical profile, biological activities, color parameters, and antimicrobial potential of sweet potato peel from four varieties (white, pink, orange, and purple). Lyophilized orange peel showed the highest carotenoid content (21.31 mg β-carotene/100 g), while osmotic dehydration resulted in the highest retention of anthocyanins in purple peel (229.58 mg cyanidin-3-glucoside/100 g). Among phenolic compounds, the most abundant were caffeic and cinnamic acids, reaching up to 434.57 mg/100 g and 430.91 mg/100 g, respectively, in white peel. Antioxidant activity was strongest in purple peel, particularly in lyophilized samples. Convective drying enhanced anti-inflammatory activity in orange peel (68.25% inhibition), and all samples demonstrated significant α-glucosidase inhibition, with values up to 96.93%. Antimicrobial effects were observed only in purple peel extracts, which showed strong antifungal activity, especially against Saccharomyces cerevisiae (inhibition zone >50 mm). These results confirm that sweet potato peel holds considerable potential as a functional ingredient and that its bioactive value can be significantly influenced by the drying method applied.
1. Introduction
The principles of a circular economy emphasize the potential of waste and pollution as valuable resources, containing added-value molecules that can be repurposed to create new ingredients and materials. In the agricultural sector, food production generates an estimated 140 billion tons of biomass waste globally each year. This biomass is rich in valuable substances as a source of dietary fibers, aromas, natural colorants, and antioxidants, which can further be exploited in the pharmaceutical, cosmetics, and food industries [1,2].
Ipomoea batatas Lam. belongs to the plant family of Convolvulaceae. Its cultivation is widespread across the globe and is consumed in everyday use in countless countries. Based on their extremely nutritional and functional components, they are deemed as a vital staple in human food regimes; however, its processing for food purposes produces a large amount of waste that cannot be disregarded and currently holds little commercial value. Additionally, sweet potatoes are essential for the manufacture and processing of starch and a handful of various snacks [3]. Moreover, sweet potato peel contains different classes of polyphenols, carotenoids, and vitamins, making it a suitable raw material for isolating bioactive components that can be incorporated into functional foods or other products, exerting additional positive effects on human health [2]. Therefore, the sweet potato can be a typical example for a functional food concept.
Sweet potatoes are renowned for their high moisture content, exceeding 70%, which contributes to their perishable nature [4]. Effective storage of fresh vegetables and vegetable by-products typically requires low-temperature conditions, often associated with significant costs. Consequently, drying methods present a viable alternative for preserving the nutritional value of plant materials while enhancing their storage stability, minimizing packaging needs, and reducing transport weight [5]. Various drying techniques exist for fruits and vegetables, enabling extended storage and reducing postharvest losses [6]. Among these methods, freeze-drying, or lyophilization, is particularly valued for producing high-quality food products. This technique involves the sublimation of ice, removing water at low pressure, but it demands considerable energy compared to convective drying methods. A more recent and increasingly popular dehydration technique is osmotic dehydration, which leverages hypertonic solutions to facilitate water diffusion from the food matrix, effectively reducing moisture content by up to 50% without the energy costs associated with evaporation [7]. The potential applications of vegetable drying processes are extensive, with many techniques suitable for industrial implementation. Therefore, optimizing these methods for the dehydration of sweet potato peels is crucial for enhancing their functionality as food additives.
In terms of antimicrobial potential, sweet potatoes also show significant performance. Studies have indicated the presence of antibacterial and antifungal properties in sweet potato extracts. Specific phytonutrients and compounds present in sweet potatoes can contribute to the inhibition of the growth of certain bacteria and fungi, making it a potential tool for maintaining food hygiene and supporting general health [8]. The antimicrobial activity of different parts of the sweet potato varies depending on the bacterial strains, the type of sweet potato, the characteristics of the extract and the applied in vitro methods. The main antibacterial components in sweet potato extract are hydrophilic, anthocyanin pigments [9]. Although research on the antimicrobial activity of sweet potato tubers and peels is limited, the antimicrobial activity of the leaves has been established in numerous studies. For example, freeze-dried leaf powder of three sweet potato species has been found to effectively inhibit certain foodborne pathogens, including E. coli, Bacillus cereus, S. aureus. Also, dietary fiber from sweet potato tubers also shows inhibitory effects on the growth of foodborne bacteria. Enzymatically treated, these fibers from different varieties of sweet potato tubers also act on two probiotic Bifidobacterium species [10]. On the other hand, the antimicrobial potential of the sweet potato peel has not been sufficiently investigated, which opens the possibility for research on this part of the tuber.
The present study aimed to comprehensively evaluate the influence of different drying techniques, i.e., lyophilization, convective drying, and osmotic dehydration, on the phytochemical composition, antioxidant and anti-inflammatory activities, color attributes, and antimicrobial potential of sweet potato peel across four distinct varieties (white, pink, orange, and purple). Special attention was given to how each dehydration method affects the retention and stability of key bioactive compounds, such as carotenoids, phenolic acids, and anthocyanins, in order to better understand the potential of sweet potato peel as a valuable source of functional ingredients for food, nutraceutical, and cosmetic formulations.
2. Material and Methods
2.1. Sweet Potatoes and Processing Steps
In 2022, various SP varieties were sourced from a family farm in Kać, Novi Sad, Republic of Serbia (45.2993319° N, 19.9462154° E), which specializes in SP cultivation. The study focused on four distinct varieties, primarily differentiated by their flesh colors: white, pink, orange, and purple. The tubers were washed and manually peeled to a depth of approx. 1 mm. Collected peels (Figure 1) were dried using three different techniques: lyophilization, convective drying, and osmotic dehydration.

Figure 1.
Whole sweet potatoes (A) and fresh peels (B) of: (a) white; (b) pink; (c) orange; (d) purple.
Sweet potato peels were subjected to three drying methods:
- Lyophilization: Peels were initially freeze-dried at −40 °C for 2 h in a Martin Crist Alpha 2–4 freeze-drier (Osterode, Germany). The main drying phase took place at 0.01 bar, ranging from −40 to 20 °C over a period of 48 h. This was followed by a final drying step lasting 4 h at 0.005 mbar, with temperatures between 20 and 30 °C. Afterward, all samples were ground using a laboratory mill and passed through 72 and then 71 mesh sieves for homogenization, and they were stored in the refrigerator until further use.
- Convective Drying: Peels were dried in a single layer on a wire mesh tray within a drying chamber maintained at a temperature of 70 ± 1 °C until the samples reached a constant weight.
- Osmotic Dehydration: The osmotic pre-treatment was carried out using an 80% sugar beet molasses solution at room temperature for 5 h. Then, peels were rinsed with distilled water, gently blotted with tissue paper, and then stored in the freezer at −40 °C for 48 h to prepare them for the lyophilization step.
All samples were milled and stored in airtight containers at –20 °C until extraction.
2.2. Color Properties of Sweet Potato Peels
The color was measured in triplicate using a Minolta Chroma Meter CR-400 colorimeter (Konica Minolta Sensing Inc., Tokyo, Japan), which has a contact surface diameter of 8 mm. Prior to taking measurements, the device was calibrated with a white color standard. The results are expressed using the CIELab color system, where the coordinates are defined as follows: L* represents lightness (with 0 being black and 100 being white), a* indicates the red–green axis (with positive values representing red and negative values indicating green), and b* denotes the yellow–blue axis (with positive values indicating yellow and negative values indicating blue) [7].
2.3. Extraction Procedure of Sweet Potato Peels
The extraction of sweet potato peel samples was conducted based on the method described by Šeregelj et al. [2], with slight modifications. Namely, dried samples were extracted using an acetone/ethanol solvent mixture (36:64, v/v) in a solid-to-solvent ratio of 1:20 (w/v). The plant material underwent vortex homogenization for 1 min, followed by a 20-min treatment in an ultrasonic bath (RK 52 H, 1.8 L, SA GR, Lab Logistics Group GmbH, Meckenheim, Germany). Subsequently, centrifugation (LACE-24, COLO Lab Experts Slovenia, Gramma Libero, Belgrade, Serbia) was carried out at 4000 rpm for 5 min. The supernatant was then separated and stored until further use.
The choice of acetone/ethanol (36:64, v/v) as the extraction solvent was based on its proven efficiency in solubilizing a wide spectrum of bioactive compounds, particularly phenolics and carotenoids, which are abundant in sweet potato peels. This binary solvent system combines the polarity of ethanol with the strong solubilizing capacity of acetone, enhancing the recovery of both polar and semi-polar phytochemicals. Additionally, both solvents are widely used in food and nutraceutical research due to their compatibility with downstream analytical and application processes. The previous literature on root and peel extractions has reported high extraction yields and compound stability using similar solvent mixtures, validating its selection for this study.
2.4. Phytochemical Composition of Sweet Potato Peels
2.4.1. Spectrophotometric Analysis
Total carotenoids (TCar) in orange sweet potato peel were analyzed spectrophotometrically by the method of Nagata and Yamashita [11] adapted for a 96-well microplate. The obtained results were expressed as mg of β-carotene per 100 g dry weight (dw).
The pH single method, adapted to a 96-well microplate, was used for analyzing anthocyanins in purple sweet potato peel [12]. Total anthocyanins were expressed as cyanidin-3-glucoside equivalents per 100 g dry weight.
2.4.2. HPLC Analysis
Sweet potato peel extracts were analyzed by a chromatographic system Shimadzu Prominence (Shimadzu, Kyoto, Japan), as described in the work of Vučetić et al. [13]. Chromatograms were recorded using 280 and 330 nm wavelengths for phenolic acids. Separation was performed on a Luna C-18 RP column, 5 mm, 250 mm × 4.6 mm with a C18 guard column, 4 mm × 30 mm (both from Phenomenex, Torrance, CA, USA) and analyzing by Diode Array Detector SPD-M20A (Shimadzu, Kyoto, Japan). Two mobile phases, A (acetonitrile) and B (1% formic acid), were used at a flow rate of 1 mL/min with the following gradient profile: 0–10 min from 10 to 25% B, 10–20 min linear rise up to 60% B and from 20 to 30 min linear rise up to 70% B, followed by 10 min reverse to initial 10% B with additional 5 min of equilibration time. The results are reported as mg per 100 g dry weight.
2.5. Biological Potential of Sweet Potato Peels
2.5.1. Antioxidant Activity
The DPPH radical scavenging assay was conducted spectrophotometrically following the method outlined by Borjan et al. [14]. The values of DPPH radical scavenging activity were calculated using the following equation:
where Acontrol represents the absorbance of the blank and Asample is the absorbance of the juice sample. The results were reported as μmol Trolox equivalents (TE) per 100 g of dry weight.
DPPH = [(Acontrol − Asample)/Acontrol] × 100,
The reducing power (RP) was determined using the Oyaizu method, adapted for a 96-well microplate format [12]. Absorbance readings were taken at 700 nm, and the results were expressed as μmol Trolox equivalents (TE) per 100 g of dry weight.
The ABTS radical scavenging activity was assessed using a modified method based on Borjan et al. [14]. The absorbance of 250 μL of activated ABTS+● (with MnO2) was measured both before and 35 min after the addition of 2 μL of juice, incubated at 25 °C. The absorbance was recorded at 414 nm, with water serving as the blank. The results were expressed in μmol Trolox equivalents (TE) per 100 g of dry weight.
2.5.2. Pharmacological Activity
The anti-inflammatory activity (AIA) was assessed using a protein denaturation bioassay with egg albumin (sourced from fresh hen’s eggs), following the method described by Šovljanski et al. [7]. In short, 2 mL of the extract was incubated with 0.2 mL of egg albumin and phosphate-buffered saline (pH 6.4) at 37 °C for 15 min, followed by heating at 70 °C for 5 min. After cooling, the absorbance was recorded at 660 nm.
To evaluate in vitro antihyperglycemic activity (AHgA), the α-glucosidase inhibitory potential was tested using the method described by Ranitović et al. [15]. L-14-nitrophenyl α-D-glucopyranoside (2 mmol, 100 μL) and the samples (concentration 250 mg/mL, 20 μL), both dissolved in 10 mmol/L potassium phosphate buffer (pH 7.0), were combined in a well with the enzyme solution (56.66 mU/mL, 100 μL) to initiate the reaction. After a 10-min incubation at 37 °C, the absorbance was recorded at 405 nm.
2.5.3. Antimicrobial Potential
In order to examine the antimicrobial potential of the prepared extracts, eight test microorganisms were used, that is, two representatives each of Gram-negative and Gram-positive bacteria, as well as yeasts and molds. The following strains of the American Collection of Cultures of Microorganisms (ATCC) were used, which are deposited in the culture collection of the Department of Microbiology of the Faculty of Technology, Novi Sad: Pseudomonas aeruginosa ATCC 27853, Salmonella Typhimurium ATCC 13311, Bacillus cereus ATCC 11778, Staphylococcus aureus ATCC 25923, Saccharomyces cerevisiae ATCC 9763, Candida albicans ATCC 10231, Aspergillus brasiliensis ATCC 16404, and Penicillium aurantiogriseum ATCC16025. Cultures of test microorganisms were prepared by refreshing glycerol suspensions stored at −80 °C using the depleted technique on solid nutrient medium Mueller–Hinton agar (HiMedia, Mumbai, India) and Sabouroud Maltose Agar (HiMedia, Mumbai, India) for bacteria, i.e., yeasts and molds, respectively. Bacterial representatives were incubated for 24 h at 37 °C, while S. cerevisiae and C. albicans were incubated for 48 h at 30 and 37 °C, respectively. Mold representatives were incubated at a temperature of 25 °C for 5 days.
The disk-diffusion method was used to evaluate the antimicrobial potential of prepared sweet potato peel extracts. Within the selected method, the nutrient medium was inoculated with a suspension of microorganisms (approximately 6 log units CFU/mL, determined by the McFarland standard), and then 15 μL of the prepared extract was applied to three sterile disks in each petri plate. Mueller–Hinton agar (MHA, HiMedia, Mumbai, India) and Sabouraud Maltose agar (SMA, HiMedia, Mumbai, India) were used as appropriate nutrient media for bacteria, i.e., yeasts and molds, respectively. Sterile distilled water was used as a negative control, while positive controls included the commercially available antibiotic clavulanic acid (Sigma-Aldrich, St. Louis, MO, USA) and the antifungal actidione (3 mg/mL; Acros Organic, Fair Lawn, NJ, USA). The inhibition zone was measured with the HiAntibiotic Zone ScaleTM (HiMedia, Mumbai, India), while, in relation to the results of the tested extracts, the microorganisms were characterized as being either sensitive (inhibition zone diameter greater than 26 mm), intermediate (inhibition zone diameter between 22 and 26 mm), or resistant (inhibition zone diameter less than 26 mm).
Determination of the minimum inhibitory concentration (MIC values) was done for microorganisms that showed sensitivity to the applied extracts in the previous experimental step. Using the microdilution method, the MIC value was determined by testing a series of seven successive dilutions of extracts starting from the initial concentration (100 mg/mL). Dilutions of the extracts were prepared in sterile, distilled water, at a ratio of 1:1. The presence/absence of viable cells of sensitive microorganisms (growth/absence) was determined by seeding an aliquot from each well of the microtiter plates on the appropriate medium using the depletion method. The MIC was defined as the lowest extract concentration that inhibited visible microbial growth, with the next lower concentration permitting unrestricted culture development.
In order to examine the pharmacodynamic potential of the inhibitory effect of sweet potato extract, a kinetic study was performed, which included the determination of the number of sensitive microorganisms during a time-defined contact with the effective extract. Namely, the incubation of the inoculated Sabouraud Maltose Broth (SMB, HiMedia, Mumbai, India) was carried out for 48 h at the optimal temperature for the growth of sensitive microorganisms (molds at 25 °C, yeasts at 30 °C), while the determination of the concentration of microorganisms was done by the depletion method on a compatible solid substrate at the following contact time points: 0, 3, 6, 12, 18, 24, 36 and 48 h. The inoculated SMB medium without the addition of the extract served as a control.
2.6. Statistical Analysis
All analyses were performed in triplicate and the results are presented as mean values ± standard deviations. Prior to applying the one-way analysis of variance (ANOVA), the normality of data distribution and homogeneity of variances were evaluated using the Shapiro–Wilk and Levene’s tests, respectively. Only after confirming that the assumptions of parametric analysis were met, ANOVA followed by Tukey’s HSD post hoc test was applied to determine significant differences among groups at p < 0.05. Statistical analyses were carried out using the Origin 8.0 SRO software package and Microsoft Office Excel 2010.
3. Results and Discussion
3.1. Color Properties of Sweet Potato Peels
The color properties of sweet potato peels serve as important indicators of nutritional quality and health benefits across different varieties [16]. The final samples prepared for color testing are presented in Figure 2.

Figure 2.
Dried and powdered sweet potato peel samples obtained by (a) lyophilization; (b) osmotic dehydration; (c) convective drying.
The Lab* color values in sweet potato peels varied significantly with both variety and drying condition (Table 1). Lyophilization and convective drying yielded higher L* values across all varieties, indicating greater lightness compared to osmotic dehydration. This is likely due to minimal pigment degradation under low-temperature lyophilization and the limited Maillard browning at the relatively mild temperature of convective drying. In contrast, osmotic dehydration led to a notable reduction in L* values, especially in the white, pink, and orange varieties, likely due to solute infusion and pigment leaching or complexation. The a* values (redness) remained low in white peels under lyophilization and convective drying, signifying limited thermal-induced pigment transformation. However, osmotic dehydration caused a pronounced increase in a* values, particularly in the white (6.02), pink (6.55), and orange varieties, which may be attributed to sugar interactions and anthocyanin stabilization in the osmotic medium. The b* values (yellowness) increased in all varieties during osmotic dehydration, reflecting possible carotenoid retention or transformation due to limited oxidative degradation and pigment diffusion into the sugar matrix. Lyophilization and convective drying, on the other hand, maintained moderate b* values, likely due to the preservation of thermolabile pigments in the absence of direct solute interaction.

Table 1.
Color Properties of Different Sweet Potato Peels.
Compared to the previous work that investigated different sweet potatoes’ flesh drying using the same three drying methods [7], it can be noticed that the flesh is consistently lighter and more yellow (higher L* and b* values). At the same time, peels indicate stronger red tones (higher a*). Regarding the drying method, osmotic dehydration leads to darker peels and flesh with more reddish tones.
3.2. Spectrophotometric Analysis for Investigating Pigments in Orange and Purple Sweet Potatoes
As pigmentation is influenced by bioactive compounds such as anthocyanins in purple sweet potatoes and carotenoids in orange varieties, it plays a significant role in determining their antioxidant capacity and health-promoting effects. These pigments distinguish sweet potato varieties visually and correlate with the concentration of key compounds that contribute to their overall functional properties. Using fast spectrophotometric methods, total carotenoids were determined in orange sweet potato peels, while total anthocyanin content was investigated in purple sweet potato peels, with the results being presented in Table 2. No carotenoids or anthocyanins were found in white and pink sweet potato peels.

Table 2.
Total carotenoid and anthocyanin content in orange and purple sweet potatoes.
Orange and purple sweet potatoes are particularly interesting because of their characteristic color, belonging to carotenoids and anthocyanins, respectively. The differential effectiveness of drying methods in preserving specific compounds can be attributed to the distinct physicochemical properties of the bioactives and the conditions imposed by each technique [17]. For example, carotenoids are highly susceptible to oxidative degradation and isomerization under heat and light. Lyophilization, which operates at low temperatures and under vacuum, minimizes exposure to oxygen and heat, thereby preserving carotenoid integrity. Conversely, anthocyanins are water-soluble flavonoid pigments that are more stable in mildly acidic environments and can benefit from the solute infusion and reduced thermal exposure of osmotic dehydration. This method limits direct heat exposure and stabilizes anthocyanins through interactions with sugars in the hypertonic solution, reducing pigment leaching and degradation. The observed results align with these mechanisms, supporting a compound-specific optimization of drying approaches.
Based on the results, lyophilization appeared to be most suitable for carotenoid retention (21.31 mg/100 g), while osmotic dehydration was superior for preserving anthocyanins (229.58 mg/100 g). In the study by [18] orange sweet potato skin was convectively dried in an Autumnz Food Dehydrator at 60 °C, resulting in a total carotenoid yield of 11.7 mg/100 g dw, which is in accordance with the obtained results. Still, lyophilization and osmotic dehydration have better potential for carotenoid isolation from orange sweet potato peels, which is a step up from the classical drying method. Meanwhile, anthocyanins are considered one of the most integral constituent groups found in purple sweet potatoes, belonging mostly to cyanidins and peonidins, often acylated with ferulic, caffeic, and p-hydroxybenzoic acids [19]. As a drying method, osmotic dehydration and convective drying proved to be much better at anthocyanin retention than lyophilization. Even though lyophilization is considered highly effective for preserving sensitive compounds, when optimized for specific compounds, osmotic dehydration can offer superior results, based on its protective properties of solute uptake and controlled thermal exposure [20].
3.3. Qualitative and Quantitative HPLC Analysis of Phenolic Compounds
Phenolic compounds are important phytochemicals responsible for numerous health-promoting bioactivities, like antioxidant, anti-inflammatory, antiproliferative activity, and anti-diabetic [21]. Therefore, their isolation and identification from food waste, such as sweet potato peels, is important for the utilization of their unexploited potential (Table 3).

Table 3.
HPLC analysis of identified phenolic compounds in different sweet potato peels.
The following phenolic compounds were found in all four sweet potato peel varieties, in at least one of the tested drying methods: caffeic acid, gallic acid, cinnamic acid, and rosmarinic acid. Sinapic acid was present in all samples but purple sweet potato, while p-hydroxybenzoic acid was only absent from orange sweet potato peel. Additionally, syringic acid was found in pink (55.72 mg/100 g dw) and purple sweet potato peels (50.57 mg/100 g dw). In contrast, ferulic acid was only present in two orange sweet potato peel samples, where a higher amount was found in the lyophilized sample (39.12 mg/100 g dw), and catechin was only isolated from pink sweet potato peel after osmotic dehydration (338.80 mg/100 g dw). Gentisic acid exhibited a non-distinctive pattern between different samples. Whereas, in orange sweet potato peel, it was found in lyophilized and osmotically dehydrated samples (79.34 and 33.65 mg/100 g dw, respectively), while in purple sweet potato peel only the convectively dried sample contained gentisic acid (16.59 mg per 100 g dw). Cinnamic and caffeic acids were the most prevalent bioactive compounds found in all samples, with their highest levels belonging to the lyophilized white sweet potato peel, with 434.57 and 430.91 mg/100 g dw, respectively. Overall, the efficiency of phenolics’ preservation during the drying process was highly dependent on the type of sweet potato peel. This indicates the need for specific optimization of the drying method for targeted compounds and collection of a vast database capable of employing advanced statistical methods for predicting the optimal drying method correlated to the plant’s characteristics and desired bioactive constituents [7].
In the research of Makori et al. [22], four sweet potato varieties were separated into different edible parts and lyophilized. From detected phenolic acids, the most dominant were di-, mono-, and tri-O caffeoylquinic acids or caffeic acid, varying between cultivars and particular parts investigated. Caffeic acid found in different potato skins ranged from 5 to 34 mg/100 g dw, and the most prevalent compound was 4-O-caffeoylquinic acid at 124 mg/100 g dw, present in Yuzu cultivar, orange sweet potato. Meanwhile, in the research of Althwab et al. [23], sweet potato peel was separated, air-dried, and the following phenolic compounds were identified: chlorogenic, eryptochlorogenic, caffeic, vanillic, coumaric acid, and catechin. Other research has reported the presence of around 30 phenolic compounds and their derivatives found in flesh, leaves, and peels of purple sweet potatoes, with hydroxybenzoic, chlorogenic, caffeic, ferulic, and p-coumaric acids as primary phenolic acids [19]. Overall, although the literature consistently acknowledges phenolic acids as important constituents of sweet potato peels, cultivar differences, drying method, geographic origin, and agronomic conditions can lead to substantial variability in both the profile and concentration of these compounds, making direct comparisons across studies challenging.
3.4. Biological Activities of Different Sweet Potato Peels
The biological potential of food agro-waste is crucial for the valorization and future application of any by-products. It is reliant on the present residual phytochemicals and their preservation. Therefore, three antioxidant assays were employed to assess the antioxidant potential of four sweet potato peel varieties. Also, using different enzymes and quick spectrophotometric methods, anti-inflammatory and reducing power were determined. All results are presented in Table 4.

Table 4.
Biological activities of different sweet potato peels.
Among the three tested antioxidant assays, ABTS demonstrated the highest scavenging activity for white, pink, and orange sweet potato peels, varying from 890.41 to 2480.89 µM TE/100 g. Meanwhile, purple sweet potato peel exhibited the highest antioxidant activity, especially after lyophilization, with DPPH, ABTS, and reducing power reaching 6287.45, 5338.49, and 2325.85 µM TE/100 g, respectively. This is consistent with the previous results of high anthocyanin retention in lyophilized samples. Anthocyanins, as potent antioxidant agents, are also the reason for the purple cultivars’ amplified activity in all three antioxidant assays and have already been reported as important antioxidants in purple sweet potatoes [24,25]. Also, while reducing power was the lowest in a white lyophilized sample of sweet potatoes (788.02 µM TE/100 g), it was the highest in a purple lyophilized sample (2325.85 µM TE/100 g), proving the results to be more dependent on the type of sweet potato than the drying method used. Comparatively, the ferric reducing antioxidant potential of lyophilized sweet potato peels from different cultivars varied between 28 and 47 mg TE/100 g [22], showcasing even stronger potential.
Interestingly, these particular results highlight convective drying as the superior drying method for the preservation of sweet potato peels’ bioactivities, which was also the case in the previous study investigating drying effect on sweet potatoes’ flesh [7]. In association with the same study, sweet potato peels exhibited higher overall antioxidant activity than flesh, particularly in the pink variety. This can be attributed to the peels’ higher concentration of polyphenols, flavonoids, and anthocyanins, which are known to accumulate in outer tissues as part of plants’ defense mechanisms against environmental stressors such as UV radiation, pathogens, and mechanical injury. Notably, lyophilized pink sweet potato peels showed the lowest measured activities: 297.73 µM TE/100 g (DPPH), 890.41 µM TE/100 g (ABTS), and 451.67 µM TE/100 g, respectively. This is also supported by the work of Gabilondo et al. [25], where DPPH scavenging activity was found to be up to 10 times lower in the flesh of the sweet potato compared to the peel. The only exceptions were the results of the ABTS and the reducing power of purple sweet potato, where the peel’s activity was lower than the same plant’s activity found in the flesh. This is most likely due to the higher concentration of anthocyanins present in sweet potatoes’ flesh.
Orange sweet potato peels had the strongest anti-inflammatory activity, regardless of the drying method. This could be related to the presence of carotenoids and their anti-inflammatory activity. Carotenoids (β-carotene, β-cryptoxanthin, α-carotene, lutein) extracted from orange sweet potato peels in the study of Boukhers et al. [16] exhibited significant anti-inflammatory effects by impeding nitric oxide production and pro-inflammatory cytokines in cell and animal models. Antihyperglycemic activity was high in all tested samples, which could be connected to the presence of cinnamic acid, a known antidiabetic agent, found in all four sweet potato varieties [26]. Moreover, the purple sweet potato peels showed the lowest antihyperglycemic activity, which could be attributed to their lack of ferulic and sinapic acids, phenolic acids also characterized by their antidiabetic properties [27]. The difference in antihyperglycemic activity between different parts of sweet potatoes is very noticeable, and while, in the flesh itself, activity ranged from 1.49 to 61.56%, the peels showed consistently higher activity, from 68.53% to 96.93%, adding significant value for the valorization of this food waste [7].
3.5. Antimicrobial Potential of Different Sweet Potato Peels
Research on the antimicrobial potential of natural resources, especially agricultural waste such as fruit and vegetable residues, is becoming increasingly important in the search for new and effective antimicrobial agents. In the context of the global fight against antimicrobial resistance, fruit and vegetable waste represents underutilized sources of bioactive compounds that can guide the development of alternative antimicrobial treatments. Among the interesting by-products of agriculture is the skin of the sweet potato (sweet potato) due to its phenolic profile and antioxidant activity [28]. Sweet potatoes, widely cultivated for their nutritional properties, generate significant amounts of peel as a by-product in their production and preparation processes, representing a potentially natural source of compounds with antimicrobial properties. Table 5 shows the results of the assessment of the antimicrobial potential of the acetone–ethanol extracts of the peel of four sweet potato varieties dried in different ways. No antimicrobial potential for white, orange and pink I. batatas peels was found.

Table 5.
Assessment of antimicrobial potential using the disk-diffusion method.
The obtained results for purple peel samples indicate an insufficient concentration of bioactive compounds in the prepared extracts, which would enable the inhibition of the proliferative activity of prokaryote cells. The lack of inhibitory activity in the case of the tested bacteria indicates the absence of antibacterial potential in the purple sweet potato peel. Briefly, in the presence of the prepared extracts, there is unhindered growth and development of all tested representatives of bacteria—Pseudomonas aeruginosa, Salmonella Typhimurium, Bacillus cereus and Staphylococcus aureus. Although research on the antimicrobial activity of sweet potato peel extracts is scarce, the work of Naz et al. [29] investigated the ethanolic extract of the peel of the white variety of sweet potato and found the existence of low antibacterial activity (inhibition zone up to 18 mm) for S. aureus. A similar value of the zone of inhibition for S. aureus was obtained in the research of Singh et al. [30]; however, in this work, the sweet potato peel variety was not emphasized, nor was the extract agent used, so further comparison of these studies is limited. The absence of a bactericidal effect in the tested extracts is also confirmed by the presence of zones for all tested bacteria in the positive control (clavulanic acid), where inhibition zones of 13.3 ± 0.94, 25.0 ± 1.0, 18.7 ± 0.6 and 28.0 ± 0 mm were observed for P. aeruginosa, S. Typhimurium, B. cereus and S. aureus, respectively, while the negative control (distilled water) had the same performance as the tested peel extracts.
On the other hand, there is a noticeable difference in the effect on representatives of yeasts and molds. Namely, although extracts of white, orange and pink varieties of sweet potatoes peel did not act on these groups of microorganisms, extracts of purple varieties of sweet potatoes peel had an inhibitory effect on all tested eukaryotic representatives. The largest zone of inhibition (about 55 mm) was observed for the yeast Saccharomyces cerevisiae, while the smallest diameter of inhibition (about 13 mm) was defined for the yeast Candida albicans. On the other hand, an approximately equal fungicidal effect was observed for Aspergillus brasiliensis and Penicillium aurantiogriseum, with defined zones averaging around 23 mm. Compared to the controls, the examined commercial fungicide, actidione, had almost twice the value of the zone of inhibition for S. cerevisiae (26.33 ± 0.6 mm), while in the case of C. albicans it had a relatively higher value (27.0 ± 1.0 mm). In the case of the tested molds, the inhibitory effect of the control was at the same level as the tested extracts and was 22.0 ± 0.0 mm and 24.3 ± 0.6 mm for A. brasiliensis and P. aurantiogriseum, respectively. According to the obtained results, S. cerevisiae, A. brasiliensis and P. aurantiogriseum can be classified as sensitive microorganisms to the action of the extract of the purple variety of sweet potato peel prepared in the acetone–ethanol solvent. Also, regardless of the drying method, approximate values of the zone of inhibition were obtained for the same microorganism. This indicates uniformity in the composition of bioactive compounds that are carriers of antimicrobial potential in the bark of sweet potato, and the possibility of applying any of the examined methods for drying.
Furthermore, for sensitive microorganisms, the minimal concentrations of the extract with which the biocidal effect is achieved were experimentally defined (Figure 3). This graph shows the minimum inhibitory concentration (MIC) depending on three different drying methods—lyophilization, osmotic dehydration and convective drying—and represents their effectiveness in terms of inhibiting the growth of three sensitive microorganisms: S. cerevisiae, A. brasiliensis and P. aurantiogriseum.
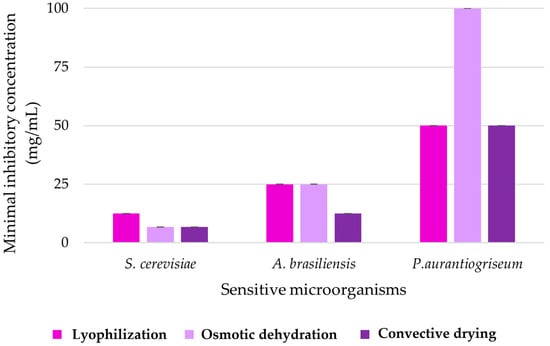
Figure 3.
Values of the minimum inhibitory concentration of the peel extract of the purple variety of sweet potato.
For the inhibitory effect of purple sweet potato peel extract, which was subjected to osmotic dehydration and conventional drying during preparation, the defined MIC values are 6.75 mg/mL, while for the freeze-dried peel extract twice the concentration was required for the same biocidal effect on S. cerevisiae. In contrast, in the case of the fungicidal effect on A. brasiliensis, samples dried by lyophilization and osmotic dehydration were effective in inhibition at a concentration of 25 mg/mL, while a concentration of 12.5 mg/mL of the sample dried by convective drying was equally effective for this mold. The highest MIC values were observed for P. aurantiogriseum, which requires an initial co-concentration value (100 mg/mL) in order to achieve the fungicidal effect of the sample obtained by osmotic dehydration, i.e., half of that value for the samples obtained by the other two methods of drying. This result additionally indicates the highest sensitivity of yeast representatives to the tested extracts, but also the specific antimicrobial potential among mold representatives. This may indicate that the drying methods may have a variable effect on the antimicrobial properties of the extracts, and that some of these methods may be better at preserving or potentially improving the antimicrobial properties of the products containing one of the tested extracts.
Defining pharmacodynamically the potential inhibitory effect of the extract of the peel of the purple variety of sweet potato was made possible through the conducted kinetic study (Table 6). This research step includes defining the concentration of microorganisms during the contact time with the tested extract and forming a kinetic model of its inhibitory action.

Table 6.
Time-kill kinetics study for purple peel sample during contact with sensitive microorganisms.
Based on the mentioned table, it is possible to analyze and compare the effectiveness of different methods of drying (lyophilization, osmotic dehydration, convective drying) of purple sweet potato peel when it comes to suppressing the growth of sensitive microorganisms over time. Also, the graphs show the curves of the control sample (i.e., the growth curve of microorganisms without contact with the tested extracts). Incubation of microbial suspensions without contact with extracts shows a constant increase in the concentration of microorganisms, indicating that without the addition of an inhibitory extract, there is no reduction in the number of microorganisms in the nutrient medium. For all three microorganisms, lyophilization and osmotic dehydration are more effective in reducing the number of microorganisms compared to convective drying, especially in the initial measurement points (0–12 h). After 12 h of contact, the concentration of microorganisms in the samples treated with extracts of lyophilized or osmotically dehydrated purple sweet potato skin was drastically reduced to a complete inhibition of microorganisms, which is not the case with convective drying, where the decrease in the concentration of microorganisms is milder. Specificity at the microorganism level can be defined through the moment of achieving the biocidal effect. Namely, for S. cerevisiae, the lyophilized and osmotically dehydrated sample lead to the elimination of microorganisms already after 12 h. A similar trend is observed for A. brasiliensis, with the fact that convective drying shows slightly better results in the initial hours for this mold. In the case of P. aurantiogriseum, convective drying appears to be more efficient than the previous two microorganisms, although it is still less efficient than lyophilization and osmotic dehydration as drying methods for purple sweet potato peel. The general conclusion is that lyophilization and osmotic dehydration, as methods of drying, make it possible to obtain extracts of the purple sweet potato variety that show a more effective antimicrobial effect compared to convective drying. Comparing these three microorganisms, it is evident that the tested extract has a faster and more effective antimicrobial effect on S. cerevisiae and A. brasiliensis compared to P. aurantiogriseum, which further confirms that the Penicillium species is the most resistant among sensitive microorganisms in this research. These differences may be the result of different defense mechanisms available to these microorganisms or different modalities of how the extract interacts with each of them. Further studies should focus on understanding the mechanisms by which the extract acts on these microorganisms in order to define and improve its antimicrobial use.
Analyzing all the data obtained from the study of the antimicrobial potential of sweet potato peel extracts, it can be concluded that the white, pink and orange varieties do not contain a sufficient amount of antimicrobial compounds that enable the inhibition of the growth of a wide range of tested microorganisms. On the other hand, the purple variety of sweet potato in its peel contains a favorable chemical composition that allowed defining the significant antimicrobial potential of this sample on the representative of the yeast S. cerevisiae, but also on both representatives of the mold: A. brasiliensis and P. aurantiogriseum. Also, the inhibitory activity of purple sweet potato peel extract on C. albicans was observed, but relatively low compared to the previously mentioned yeast species.
Consequently, it is possible to divide the research into several related units. Lyophilization and osmotic dehydration proved to be effective methods in inhibiting the growth of microorganisms, with a tendency to rapidly decrease cell viability in the initial hours of incubation. Convective drying also reduced the number of microorganisms, but not as effectively as the first two methods. This may indicate that the drying conditions and methods profile of the chemical composition and concentration of antimicrobial components in the extract. Furthermore, the results indicate variable effectiveness of drying methods against different microorganisms. For example, S. cerevisiae and A. brasiliensis showed greater sensitivity to the tested effects than P. aurantiogriseum. This may be related to the different resistance or sensitivity mechanisms inherent in each species, as well as to the different structures and components found in the sweet potato peel that could be responsible for the antimicrobial effect. In all investigated cases, contact with purple sweet potato extracts led to a decrease in the number of mentioned microorganisms over time, with the most pronounced inhibition of growth during the application of lyophilization and osmotic dehydration in the preparation steps of the purple sweet potato variety extract. This suggests that drying processes have a significant effect on the antimicrobial activity of the extract, which could be useful for applications in food preservation as well as in the production of natural antimicrobial agents. Given that some samples (especially those lyophilized and osmotically dehydrated) showed significant antimicrobial activity, there is potential for further research and development of these extracts as natural preservatives or antimicrobial agents. In general, the results of this research show promising antimicrobial properties of purple sweet potato peel extract and emphasize the importance of choosing the appropriate method of processing the peel in order to maximize these effects. While this study focused on controlled drying techniques—lyophilization, convective drying, and osmotic dehydration—to ensure reproducibility and optimize compound preservation, it is important to acknowledge that traditional methods, such as sun or air drying, are commonly used in low-resource settings. Although these methods are more cost-effective, they often suffer from greater variability due to environmental factors and longer processing times, which can affect the stability of thermolabile phytochemicals. Future studies should consider incorporating such conventional methods for comparative evaluation to better assess the trade-offs between bioactivity retention and economic feasibility.
3.6. Application Outlook: Valorization of Sweet Potato Peels
This study provides preliminary insight into how drying methods influence the antimicrobial potential of sweet potato peels, likely through effects on bioactive compound preservation and transformation. Future research should build on these findings by elucidating the specific chemical constituents responsible for antimicrobial activity and the mechanisms through which drying-induced changes modulate their bioefficacy. The findings of this study highlight not only the bioactive potential of dried sweet potato peels but also their suitability for integration into functional food, nutraceutical, or biopreservative applications. For example, peel extracts rich in phenolics and anthocyanins—especially those retained via lyophilization or osmotic dehydration—could serve as natural antioxidants or colorants in clean-label formulations. Future work should explore formulation into edible coatings, antioxidant-enriched flours, or dietary supplements. Additionally, their antimicrobial activity supports their exploration as natural preservatives in food systems. Scaling such applications will require techno-economic analysis and product-specific optimization.
4. Conclusions
This study underscores the importance of drying method selection in optimizing the antioxidant and antimicrobial properties of sweet potato peel extracts. Beyond compositional profiling, the valorization of this agro-industrial waste into functional ingredients offers a sustainable route for circular economy models in food systems. These results form a foundation for pilot-scale applications and product development in food and nutraceutical sectors. Color properties showcase residual pigments preserved by a carefully chosen drying method, especially in orange and purple sweet potatoes, due to rich carotenoid and anthocyanidin contents. Proper drying method appears to vary significantly based on the type of sweet potato, as well as the targeted preservation of bioactive compounds. For example, retention of carotenoids is higher after lyophilization, in comparison to anthocyanins’ retention being higher after osmotic dehydration. Furthermore, the antioxidant activity of white and pink sweet potatoes was the strongest after osmotic dehydration, while for orange and purple sweet potatoes, lyophilization has proved to be a superior drying method. Interestingly, convective drying was more beneficial for the anti-inflammatory and antihyperglycemic activities of the tested samples. Also, the selected drying method not only influences the amount of isolated phenolic compounds, but it also affects their profile. The results show that sweet potato peel is a promising by-product for applications in the food, pharmaceutical, and cosmetic industries, while demonstrating the importance of its preservation using an appropriate drying method. Future studies should aim to further optimize drying methods and fully elucidate the correlations between the preservation methods used and the content of isolated bioactive compounds.
Author Contributions
Conceptualization, V.T. and O.Š.; methodology, V.T., O.Š. and B.L.; validation, V.T., O.Š., B.L. and V.F.; formal analysis, A.V., T.C. and A.R. investigation, A.V., T.C. and A.R.; data curation, A.V. and T.C.; writing—original draft preparation, G.Ć., V.T. and O.Š.; writing—review and editing, G.Ć., V.T. and O.Š.; visualization, G.Ć. and V.F.; supervision, G.Ć. and V.T.; project administration, G.Ć. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science, Technological Development and Innovation, Republic of Serbia, grant No. 451-03-136/2025-03/200134 and 451-03-137/2025-03/200134.
Data Availability Statement
The original findings and contributions of this study are detailed within the article. For additional information, please contact the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mirabella, N.; Castellani, V.; Sala, S. Current Options for the Valorization of Food Manufacturing Waste: A Review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- Tripathi, N.; Hills, C.D.; Singh, R.S.; Atkinson, C.J. Biomass waste utilisation in low-carbon products: Harnessing a major potential resource. npj Clim. Atmos. Sci. 2019, 2, 35. [Google Scholar] [CrossRef]
- Šeregelj, V.; Ćetković, G.; Čanadanović-Brunet, J.; Tumbas Šaponjac, V.; Vulić, J.; Stajčić, S. Encapsulation and Degradation Kinetics of Bioactive Compounds from Sweet Potato Peel During Storage. Food Technol. Biotechnol. 2020, 58, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, F.; Ma, R.; Yang, T.; Liu, C.; Shen, W.; Jin, W.; Tian, Y. Advances in Valorization of Sweet Potato Peels: A Comprehensive Review on the Nutritional Compositions, Phytochemical Profiles, Nutraceutical Properties, and Potential Industrial Applications. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13400. [Google Scholar] [CrossRef]
- Sagar, V.R.; Suresh Kumar, P. Recent Advances in Drying and Dehydration of Fruits and Vegetables: A Review. J. Food Sci. Technol. 2010, 47, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Toydemir, G.; Boyacioglu, D.; Beekwilder, J.; Hall, R.D.; Capanoglu, E. A Review on the Effect of Drying on Antioxidant Potential of Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2016, 56, S110–S129. [Google Scholar] [CrossRef]
- Šovljanski, O.; Lončar, B.; Pezo, L.; Saveljić, A.; Tomić, A.; Brunet, S.; Filipović, V.; Filipović, J.; Čanadanović-Brunet, J.; Ćetković, G.; et al. Unlocking the Potential of the ANN Optimization in Sweet Potato Varieties Drying Processes. Foods 2024, 13, 134. [Google Scholar] [CrossRef]
- Pochapski, M.T.; Fosquiera, E.C.; Esmerino, L.A.; dos Santos, E.B.; Farago, P.V.; Santos, F.A.; Groppo, F.C. Phytochemical Screening, Antioxidant, and Antimicrobial Activities of the Crude Leaves’ Extract from Ipomoea Batatas (L.) Lam. Pharmacogn. Mag. 2011, 7, 165–170. [Google Scholar] [CrossRef]
- Wang, S.; Nie, S.; Zhu, F. Chemical Constituents and Health Effects of Sweet Potato. Food Res. Int. 2016, 89, 90–116. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Kido, M.; Kurata, R.; Kobayashi, T. Antibacterial Activity of Sweetpotato (Ipomoea batatas L.) Fiber on Food Hygienic Bacteria. Kagoshima Women’s Jr. Coll. 2011, 27, 5–17. [Google Scholar]
- Nagata, M.; Yamashita, I. Simple Method for Simultaneous Determination of Chlorophyll and Carotenoids in Tomato Fruit. Nippon. Shokuhin Kogyo Gakkaishi 1992, 39, 925–928. [Google Scholar] [CrossRef]
- Šeregelj, V.; Tumbas Šaponjac, V.; Pezo, L.; Kojić, J.; Cvetković, B.; Ilic, N. Analysis of Antioxidant Potential of Fruit and Vegetable Juices Available in Serbian Markets. Food Sci. Technol. Int. 2024, 30, 472–484. [Google Scholar] [CrossRef]
- Vučetić, A.; Šovljanski, O.; Pezo, L.; Gligorijević, N.; Kostić, S.; Vulić, J.; Čanadanović-Brunet, J. A Comprehensive Antioxidant and Nutritional Profiling of Brassicaceae Microgreens. Antioxidants 2025, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Borjan, D.; Šeregelj, V.; Andrejč, D.C.; Pezo, L.; Šaponjac, V.T.; Knez, Ž.; Vulić, J.; Marevci, M.K. Green Techniques for Preparation of Red Beetroot Extracts with Enhanced Biological Potential. Antioxidants 2022, 11, 805. [Google Scholar] [CrossRef]
- Ranitović, A.; Šovljanski, O.; Aćimović, M.; Pezo, L.; Tomić, A.; Travičić, V.; Saveljić, A.; Cvetković, D.; Ćetković, G.; Vulić, J.; et al. Biological Potential of Alternative Kombucha Beverages Fermented on Essential Oil Distillation By-Products. Fermentation 2022, 8, 625. [Google Scholar] [CrossRef]
- Boukhers, I.; Morel, S.; Kongolo, J.; Domingo, R.; Servent, A.; Ollier, L.; Kodja, H.; Petit, T.; Poucheret, P. Immunomodulatory and Antioxidant Properties of Ipomoea Batatas Flour and Extracts Obtained by Green Extraction. Curr. Issues Mol. Biol. 2023, 45, 6967–6985. [Google Scholar] [CrossRef]
- Belwal, T.; Cravotto, C.; Prieto, M.; Venskutonis, P.R.; Daglia, M.; Devkota, H.P.; Baldi, A.; Ezzat, S.M.; Gómez-Gómez, L.; Salama, M.M.; et al. Effects of Different Drying Techniques on the Quality and Bioactive Compounds of Plant-Based Products: A Critical Review on Current Trends. Dry. Technol. 2022, 40, 1539. [Google Scholar] [CrossRef]
- Ooi, S.F.; Sukri, S.A.M.; Zakaria, N.N.A.; Harith, Z.T. Carotenoids, Phenolics and Antioxidant Properties of Different Sweet Potatoes (Ipomoea Batatas) Varieties. IOP Conf. Ser. Earth Environ. Sci. 2021, 756, 012077. [Google Scholar] [CrossRef]
- Rosell, M.d.L.Á.; Quizhpe, J.; Ayuso, P.; Peñalver, R.; Nieto, G. Proximate Composition, Health Benefits, and Food Applications in Bakery Products of Purple-Fleshed Sweet Potato (Ipomoea Batatas L.) and Its By-Products: A Comprehensive Review. Antioxidants 2024, 13, 954. [Google Scholar] [CrossRef]
- Ahmed, I.; Qazi, I.M.; Jamal, S. Developments in Osmotic Dehydration Technique for the Preservation of Fruits and Vegetables. Innov. Food Sci. Emerg. Technol. 2016, 34, 29–43. [Google Scholar] [CrossRef]
- Šovljanski, O.; Cvetanović Kljakić, A.; Saveljić, A.; Tomić, A. Bioactivity and Bioavailability of Phenols from Plants. In Natural Products: Phytochemistry, Botany, Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2025; pp. 1–44. ISBN 978-3-642-36202-6. [Google Scholar]
- Makori, S.; Mu, T.; Sun, H.-N. Total Polyphenol Content, Antioxidant Activity, and Individual Phenolic Composition of Different Edible Parts of 4 Sweet Potato Cultivars. Nat. Prod. Commun. 2020, 15, 1934578X20936931. [Google Scholar] [CrossRef]
- Althawab, S.; Mousa, H.; Elzahar, K.; Mostafa, A. Protective Effect of Sweet Potato Peel against Oxidative Stress in Hyperlipidemic Albino Rats. Food Nutr. Sci. 2019, 10, 503–516. [Google Scholar] [CrossRef]
- Jiao, Y.; Jiang, Y.; Zhai, W.; Yang, Z. Studies on Antioxidant Capacity of Anthocyanin Extract from Purple Sweet Potato (Ipomoea Batatas L.). Afr. J. Biotechnol. 2012, 11, 7046–7054. [Google Scholar] [CrossRef]
- Gabilondo, J.; Corbino, G.; Chludil, H.; Malec, L. Bioactive Compounds of Two Orange-Fleshed Sweet Potato Cultivars (Ipomoea Batatas (L.) Lam.) in Fresh, Stored and Processed Roots. Appl. Food Res. 2022, 2, 100061. [Google Scholar] [CrossRef]
- Savych, A. Cinnamic acid and its derivatives in the herbal mixtures and their antidiabetic activity. Farmacia 2021, 69, 595–601. [Google Scholar] [CrossRef]
- Vinayagam, R.; Jayachandran, M.; Xu, B. Antidiabetic Effects of Simple Phenolic Acids: A Comprehensive Review. Phytother. Res. 2016, 30, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Basílio, L.S.P.; Nunes, A.; Minatel, I.O.; Diamante, M.S.; Di Lázaro, C.B.; Silva, A.C.A.F.e.; Vargas, P.F.; Vianello, F.; Maraschin, M.; Lima, G.P.P. The Phytochemical Profile and Antioxidant Activity of Thermally Processed Colorful Sweet Potatoes. Horticulturae 2024, 10, 18. [Google Scholar] [CrossRef]
- Naz, S.; Naqvi, S.A.R.; Khan, Z.A.; Mansha, A.; Ahmad, M.; Zahoor, A.F.; Hussain, Z. Antioxidant, Antimicrobial and Antiproliferative Activities of Peel and Pulp Extracts of Red and White Varieties of Ipomoea Batatas (L) Lam. Trop. J. Pharm. Res. 2017, 16, 2221–2229. [Google Scholar] [CrossRef]
- Singh, R.; Gupta, M.; Singhal, P.; Goyal, S.; Upadhyay, S. In Vitro Antimicrobial Activities of Vegetables (Potato, Cucumber, Sweet Potato and Ginger) Peel Wastes for Ecofriendly Microbial Management. Int. J. Bot. 2021, 6, 134–137. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).