Abstract
Citrus peel, a significant by-product of fruit processing, represents a rich source of carotenoids with strong antioxidant and health-promoting properties. The present study evaluated two green extraction techniques, cloud point extraction (CPE) and supramolecular solvent (SUPRAS)-based extraction, for carotenoids recovered from citron, orange, and tangerine peels. Whereas SUPRAS methods rely on a supramolecular solvent made of water, ethanol, and octanoic acid, CPE methods use surfactants and water, and both show a high potential to extract lipophilic components. CPE demonstrated superior efficiency in extracting total carotenoids and enhancing antioxidant activity, with orange peel extracts showing the highest concentrations. CPE and SUPRAS extracts were subsequently encapsulated using freeze-drying with chickpea protein isolate, achieving high encapsulation efficiencies (82.40–88.97%). The use of encapsulation technology is an effective strategy to protect carotenoids from environmental stressors. Color, morphological, and FTIR analyses confirmed the successful encapsulation and retention of carotenoids. Environmental impact was assessed using the EcoScale tool, revealing excellent sustainability for CPE (92 points) and satisfactory performance for SUPRAS-based extraction (70 points). The use of Generally Recognized As Safe (GRAS) solvents and plant-derived encapsulation materials makes this method highly suitable for clean-label product development across the food, cosmetic, and nutraceutical industries. In summary, the results point to a practical and sustainable approach to citrus waste valorization into valuable, health-promoting ingredients—supporting both circular economy goals and eco-friendly innovation.
1. Introduction
Citrus fruits, including citron (Citrus medica), orange (Citrus sinensis), and tangerine (Citrus reticulata), are consumed and relished worldwide for their fresh flavor, juiciness, and high nutritional value, offering numerous health benefits. Approximately 50–60% of citrus fruits are consumed fresh, while the remaining 40–50% undergo industrial processing to produce a wide range of value-added products, such as juices, jams, canned goods, flavoring agents, and cosmetics [1]. Thus processing generates substantial amounts of waste, comprising 55–70% of the total fresh fruit weight. Of this waste, peel accounts for 7–40% of the fresh fruit weight and 55–75% of the total dry fruit waste, while pulp and seeds contribute 20–55% and 0–5%, respectively, depending on the citrus species [2,3]. Furthermore, a significant portion of harvested citrus fruits fail to meet the quality standards for fresh consumption or industrial use, leading to their disposal and increasing waste accumulation. This practice creates significant environmental challenges, emphasizing the need for effective valorization strategies to reduce waste and maximize resource utilization [4].
The citrus peel is both a significant waste fraction and a valuable source of bioactive compounds and pigments such as carotenoids. As the demand for natural food additives continues to rise, the valorization of citrus by-products has gained increasing attention, particularly for the extraction of natural pigments and functional ingredients for use in the food, pharmaceutical, and cosmetic industries. Carotenoids, such as α-carotene, β-carotene, lutein, zeaxanthin, and β-cryptoxanthin, are among the most valuable compounds in citrus peel, contributing to their vibrant pigmentation and overall well-being through their antioxidant properties and pro-vitamin A activity [5]. In addition to their use as natural colorants, carotenoids have demonstrated anti-inflammatory, cardioprotective, and anticancer properties, making them highly desirable for functional products [6]. However, the application of carotenoids in functional products is often limited by their low bioavailability, instability under light and heat, and susceptibility to oxidation [7].
Preserving the bioactivity and safety of carotenoids during the extraction process is a critical challenge for industrial applications, as it directly impacts their functional properties and potential health benefits [8]. One promising extraction strategy is the use of green solvents, which offer mild process conditions that help maintain the structural integrity and stability of carotenoids [5,8,9]. Green solvents serve as sustainable alternatives to conventional organic solvents, which are often associated with toxicity and environmental concerns. A wide range of green solvents has been explored for carotenoid extraction, including supercritical fluids [10], vegetable oils [9], ionic liquids [11], and deep eutectic solvents (DESs) [12]. Surfactants, which can self-assemble into micelles, have also been investigated as green solvents for carotenoid recovery. Their amphiphilic nature enables the efficient solubilization of hydrophobic bioactives in aqueous environments, improving extraction yields while avoiding the use of hazardous organic solvents [13,14,15,16]. Additionally, supramolecular solvents, which are nanostructured liquids formed by the self-assembly of amphiphilic compounds in water or other green solvents, have emerged as a novel and efficient alternative for carotenoid extraction. These solvents provide tunable polarity, high extraction efficiency, and enhanced selectivity for carotenoids, making them an attractive option for a sustainable extraction approach [17,18]. The novelty of this study is not limited to a comparison of these two green extraction methods, but it also concerns their application to a biological matrix. To the best of our knowledge, no previous study has directly compared these two techniques in this context.
Once carotenoids have been extracted, they need to be preserved in various formulations to prevent degradation and maintain their biological properties. The use of encapsulation technology has emerged as an effective strategy to protect carotenoids from environmental stressors during their incorporation into functional (food) products as well as during storage [19]. Among the various encapsulation techniques, freeze-drying has gained prominence due to its ability to preserve heat-sensitive compounds by removing water under low-temperature and vacuum conditions. Encapsulation efficiency is strongly influenced by the choice of carrier. Plant-derived proteins have gained attention as sustainable encapsulation materials due to their biodegradability, emulsifying properties, and ability to form protective matrices around sensitive bioactive compounds [20]. Accordingly, the present study aimed to compare the efficiency of two green solvents and extraction techniques, cloud point extraction (CPE) using the non-ionic surfactant Tween 80 and supramolecular solvent (SUPRAS)-based extraction with a supramolecular solvent composed of water, ethanol, and octanoic acid, to extract carotenoids and antioxidants from citrus peels (citron, orange, and tangerine). Both approaches are in line with green chemistry principles and the Sustainable Development Goals (SDG 12: Responsible Consumption and Production; SDG 3: Good Health and Well-being; SDG 9: Industry, Innovation, and Infrastructure; and SDG 13: Climate Action). In addition, for the first time, CPE and SUPRAS carotenoid-rich extract was further encapsulated by the freeze-drying technique using chickpea protein isolate as a carrier. Finally, an assessment of the environmental impact, the cost of the extraction and encapsulation process, and the practicability of its use on an industrial scale was conducted. The results obtained in this study show citrus peel’s potential in future applications as a functional ingredient or food additive. This can be attributed to the following factors:
- The solvents used are food-grade and biocompatible, eliminating the need for removal and allowing for direct incorporation into bioactive formulations. This simplifies production, reduces energy consumption, and avoids solvent recovery steps. The solvents are composed of edible, Generally Recognized As Safe (GRAS) components, which are already approved by the Food and Drug Administration (FDA) and the European Commission as food colorants or dyes.
- The solvents may be used to create formulations suitable for various industries, including food, pharmaceuticals, and cosmetics, as carriers of natural carotenoids.
- Finally, chickpea protein isolate (CPI) can improve the stability and bioavailability of natural carotenoids in humans. Rich in essential amino acids and possessing excellent emulsifying properties, chickpea protein isolate acts as a protective carrier, preventing carotenoid degradation and enhancing carotenoid absorption. Additionally, its plant-based and sustainable nature makes it an ideal ingredient for functional foods, catering to the growing demand for clean-label and protein-enriched products.
2. Materials and Methods
2.1. Raw Samples
The citron was purchased from a local supermarket in Bar, Montenegro, while the orange and tangerine were obtained from a market in Novi Sad, Serbia. Citrus fruits were washed with distilled water, water-drained, and then peeled. The separated peel samples were frozen at −40 °C for 2 h in a Martin Crist Alpha 2–4 (Osterode, Germany) freeze-drier. The main drying process was performed at a pressure of 0.01 mbar and temperatures from −40 to 20 °C for 48 h. The final drying lasted 4 h at a pressure of 0.005 mbar and temperatures from 20 to 30 °C. The collected freeze-dried samples were stored at −20 °C until further use. The freeze-dried peel of each citrus sample was ground using a laboratory grinder (B800E high-speed grinder, Gorenje, Velenje, Slovenia), and the particle size was determined on a sieve set (CISA Cedaceria Industrial, Barcelona, Spain). The resulting particle sizes were 70.5 μm, 73 μm, and 71.5 μm for citron, orange, and tangerine peels, respectively. Ground samples were stored at −20 °C in an LGUEX 1500 freezer (Lab Logistics Group GmbH, Meckenheim, Germany) until extraction.
2.2. Cloud Point Extraction (CPE)
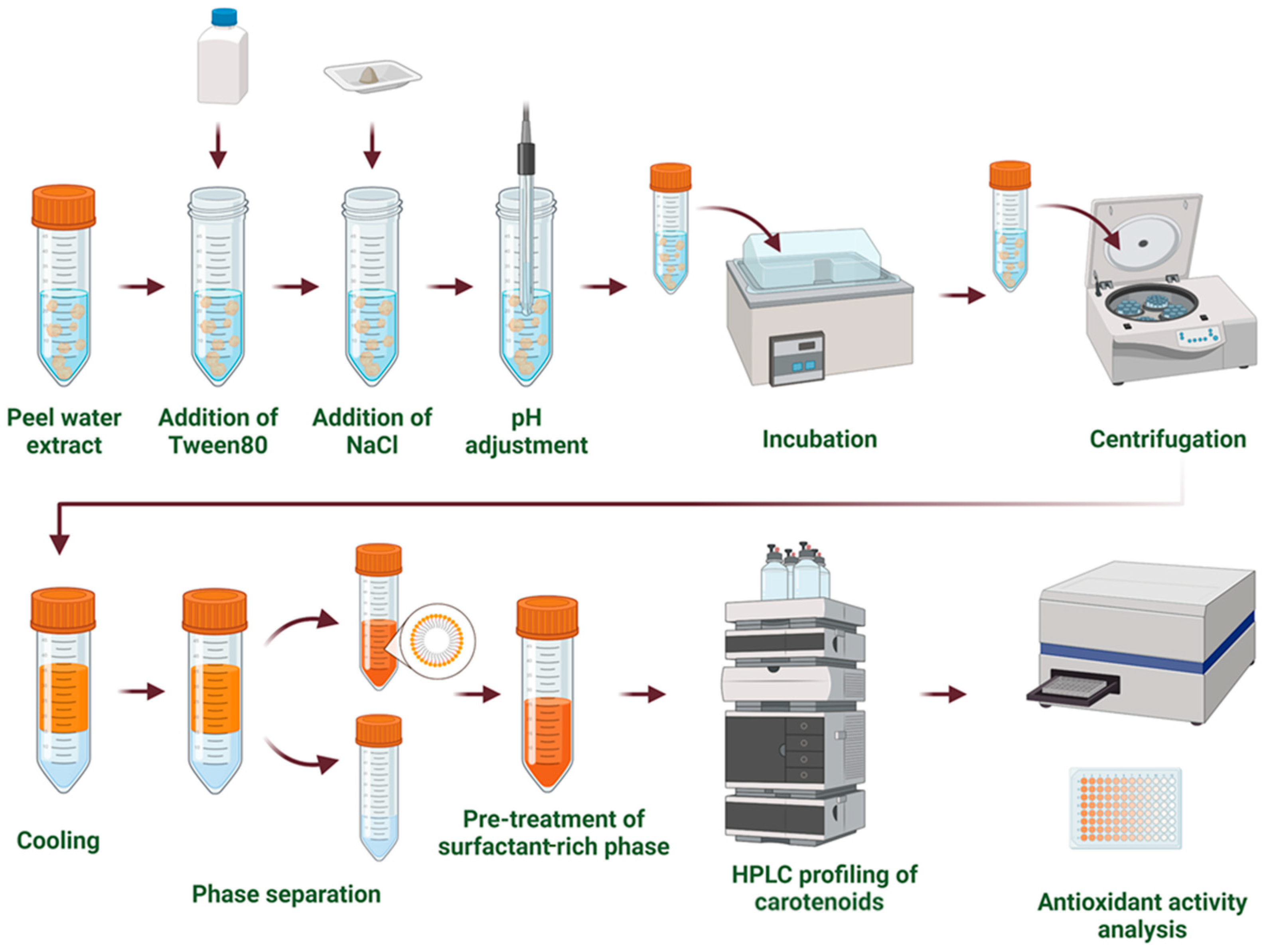
CPE was performed following the method described by Travičić et al. [14], under optimal conditions (Figure 1). Briefly, a peel sample, water, and Tween 80 were added to a 100 mL conical flask at a solid-to-liquid ratio of 1:70 (w/v) and a surfactant concentration of 10% (w/v). The pH was adjusted to 7.3, and the mixture was thoroughly stirred for 20 min on a magnetic stirrer (Heldolph, Schwabach, Germany) at 45 °C. After stirring, the sample was centrifuged (model: EBA 21, Hettich Zentrifugen, Tuttlingen, Germany) at 2470× g for 10 min to separate the supernatant. The supernatant was mixed with NaCl at a concentration of 18% (w/v) and incubated in a temperature-controlled water bath (model: VIMS elektrik, Loznica, Serbia) at 55 °C for 43 min. The bottom water phase was removed using a pipette, and the remaining surfactant phase was used to estimate total carotenoids (TCs) and antioxidant activity (AA).
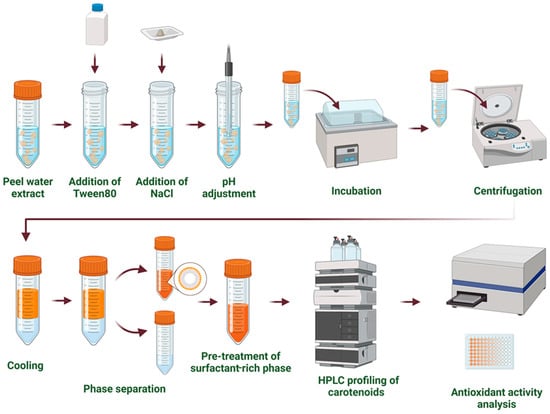
Figure 1.
Cloud point extraction (CPE) approach.
2.3. Supramolecular Solvent (SUPRAS)-Based Extraction
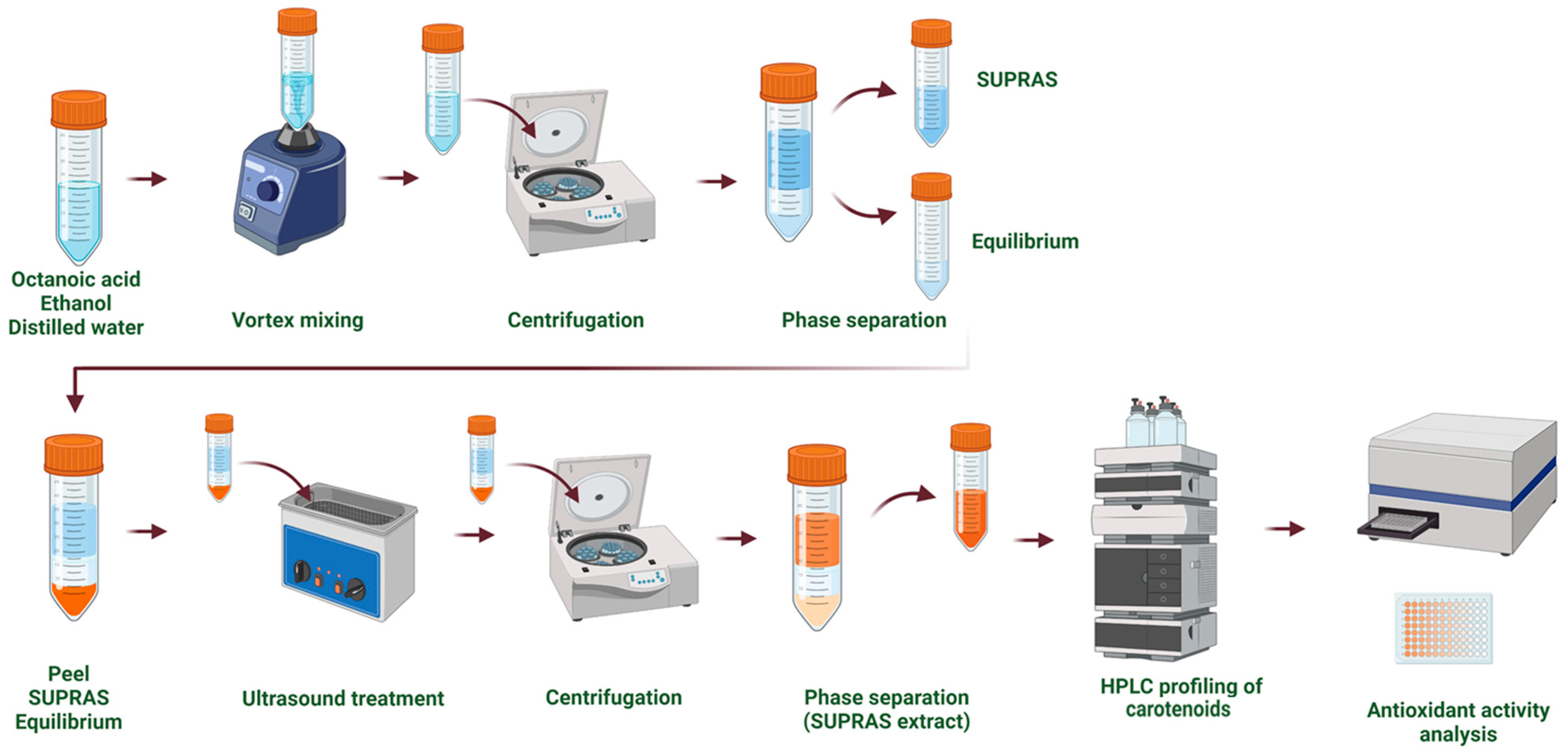
The SUPRAS extracts were obtained following the method outlined by Vučetić et al. [21] (Figure 2). Supramolecular solvents were prepared by combining ethanol (36% v/v), octanoic acid (5% v/v), and distilled acidified water (pH 3, 59% v/v). The resulting SUPRAS solvent consisted of two distinct phases, an upper SUPRAS phase and a lower equilibrium phase, which were separated and stored at 4 °C until further use. The SUPRAS extraction procedure involved the following steps: vortex mixing for 1 min (Heldolph, Schwabach, Germany), ultrasound treatment for 15 min in an ultrasonic bath (Elmasonic, Singen, Germany), and stirring at 300 rpm for 15 min using a laboratory shaker (Heidolph Unimax 1010, Schwabach, Germany) at room temperature. After extraction, the mixtures were centrifuged at 2470× g (Gramma Libero LACE 24, Belgrade, Serbia) for 20 min to separate the extract from the residual solid material.
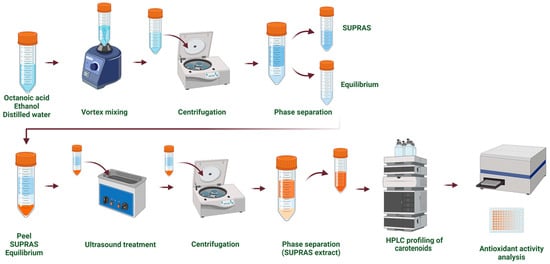
Figure 2.
Supramolecular solvent (SUPRAS)-based extraction approach.
2.4. Conventional Water Extraction (CWE)
Citron peel samples (1 g) were extracted in 70 mL distilled water for 3 h on a magnetic stirrer at 40 °C. The liquid extract was separated by centrifugation at 2470× g for 10 min to separate the extract from the solid material.
2.5. Characterization of Citrus Peel Extracts
2.5.1. HPLC Carotenoid Profiling
Carotenoid profiling was carried out using reverse-phase high-performance liquid chromatography, according to Šeregelj et al. [22]. For the analysis, 5 mL of each citron peel extract was transferred into a 25 mL screw-capped tube, wrapped in aluminum foil to protect it from light, and subjected to saponification under a nitrogen atmosphere. The reaction was performed at 70 °C for 45 min in a thermostatically controlled water bath (Haake E8, Berlin, Germany). The saponification mixture included 2.5 mL of ethanolic pyrogallol (60 g/L) as an antioxidant, 1 mL of 95% ethanol, 1 mL of sodium chloride solution (10 g/L), and 1 mL of potassium hydroxide (600 g/L). The samples were vortexed every 10 min during incubation. Following saponification, the samples were cooled in an ice bath and supplemented with 7.5 mL of sodium chloride solution (10 g/L). Carotenoids were then extracted using 15 mL of a hexane–ethyl acetate mixture (9:1, v/v). The organic phase was separated, evaporated under vacuum (model: R-210, Buchi, Flawil, Switzerland), and further dried under a nitrogen stream. The dry residue was reconstituted in 1 mL of methanol–tetrahydrofuran (95:5, v/v) and filtered through a 0.45 μm PTFE membrane before HPLC analysis.
HPLC was performed following the method described by Cvanić et al. [15] using a PerkinElmer Quasar C18 column (250 × 4.6 mm, 5 μm; PerkinElmer, Buckinghamshire, UK). The mobile phase consisted of methanol–tetrahydrofuran (95:5, v/v) stabilized with 0.1% butylated hydroxytoluene (BHT), delivered at a flow rate of 1 mL/min. Detection was conducted at 445 nm using a photodiode array detector (Waters 996 SPD-M20A, Shimadzu, Kyoto, Japan), with the wavelength range set from 200 to 600 nm. Carotenoid compounds were identified based on retention times and quantified by comparing the peak areas to those of external standards (calibration curves with r2 > 0.99). The results were expressed as milligrams per 100 g of dry sample (mg/100 g dw).
2.5.2. In Vitro Antioxidant Activity
Antioxidant activity was spectrophotometrically analyzed using the following methods: 2,2-diphenyl-1-picrylhydrazyl (DPPH), reducing power (RP), and 2,2′-azino-bis-3-ethyl benzo-thiazoline-6-sulphonic acid (ABTS), as described by Šovljanski et al. [23]. Antioxidant activity was expressed as millimoles of Trolox equivalent (TE) per 100 g of dried sample.
2.6. Encapsulation of CPE and SUPRAS Citrus Peel Extracts
The wall material used for encapsulating citrus peel extracts was a protein isolate from defatted chickpea seeds, prepared via alkaline extraction following the method described by Perović et al. [24]. For the freeze-drying encapsulation technique, 5 g of chickpea seed protein isolate was dissolved in 15 mL distilled water at 60 °C and kept under stirring until the temperature reached 30 °C. Six formulations were prepared by the addition of 3 mL of each CPE and SUPRAS citrus peel extract; formulations were homogenized at 11,000 rpm for 3 min at room temperature and freeze-dried.
2.7. Characterization of Citrus Peel Encapsulates
2.7.1. Encapsulation Efficiency (EE)
Encapsulation efficiency (EE) was determined following the method by Barbosa et al. [25], with minor modifications. For surface carotenoids, 0.25 g of the encapsulate was extracted with 5 mL acetone using a vortex for 20 s, followed by centrifugation at 4000 rpm for 10 min, and the supernatant was collected. To obtain the total carotenoid content, 0.25 g of the encapsulate was vortexed with water for 1 min to rupture the capsules and then extracted with 5 mL of methanol–dichlormetan (50:50 v/v). The layer with carotenoids was separated, and extraction was repeated using the same volumes of solvent to collect the total carotenoids. The determination of the carotenoid levels for these purposes was carried out by a spectrophotometric method [26].
2.7.2. Color Properties
Color measurements were performed using a Minolta reflectance colorimeter (Minolta ChromaMeter CR-300, Minolta, Osaka, Japan) based on the CIELab color system (L*, a*, and b*). Chroma (C*) was manually calculated using the formula √(a2 + b2).
2.7.3. Morphological Properties
The surface and microstructure of dried encapsulates and chickpea protein isolate were recorded by a Hitachi TM3030 Instrument scanning electron microscope (SEM) (Hitachi Ltd., Chiyoda, Tokyo, Japan).
2.7.4. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
The Fourier transform infrared (FTIR) spectra of prepared encapsulates were recorded by an FT/IR 6600 FTIR spectrometer (Jasco, Tokyo, Japan). The spectra were acquired over the range of 400–4000 cm−1 at a measuring resolution of 4 cm−1.
2.8. Green Metrics
The environmental impact was evaluated using the EcoScale method developed by Van Aken et al. [27]. This tool assigns penalty points (PPs) based on various factors, which are then deducted from a baseline score of 100, representing a completely environmentally friendly process. The EcoScale takes into account aspects such as solvent toxicity, energy requirements, extraction yield, and cost efficiency. In this study, the different solvents employed for carotenoid extraction were assessed using this system. The resulting scores were categorized as excellent (above 75), satisfactory (above 50), or inadequate (below 50).
2.9. Statistical Analysis
All analyses were performed in triplicate, and the results are presented as mean values ± standard deviations. Statistically significant differences between treatment means were determined using Tukey’s Honest Significant Difference (HSD) post hoc test following a one-way or factorial ANOVA (n = 3 repetitions). Statistical analyses were carried out using the Origin 8.0 SRO software package and Microsoft Office Excel 2010.
3. Results and Discussion
3.1. Characterization of Citrus Peel Extracts
3.1.1. Efficiency of Carotenoid Recovery
For hydrophobic compounds such as carotenoids, water in its pure state is not considered a suitable solvent due to its high polarity [28]. However, modern extraction strategies have focused on modifying water’s unique properties through the use of amphiphilic compounds or structuring agents to improve its affinity for lipophilic molecules. In the present study, water was used as a control, and carotenoids were not recovered under these conditions. As an answer to the need for advanced extraction systems, two alternative methods, such as CPE and SUPRAS-based extraction, were examined for their ability to extract carotenoids from citrus peels. These techniques exploit amphiphilic systems to create microenvironments capable of solubilizing both polar and non-polar regions of carotenoid molecules.
The results presented in Table 1 reveal the effective recovery of diverse carotenoids using both green extraction techniques. Among the techniques, CPE demonstrated superior efficiency compared to SUPRAS-based extraction across all three citrus peel types. Specifically, the total carotenoid yields obtained via CPE were 23.99 mg/100 g dw for citron, 45.57 mg/100 g dw for orange, and 34.08 mg/100 g dw for tangerine peel. In contrast, SUPRAS-based extraction showed lower efficiencies, with 52%, 58%, and 68% less total carotenoid recovery for citron, orange, and tangerine peels, respectively. This enhanced performance of CPE can be attributed to the formation of a surfactant-rich phase, which provides a hydrophobic microenvironment more suitable for solubilizing non-polar compounds like β-carotene and β-cryptoxanthin. Moreover, the moderate thermal conditions of CPE may aid in breaking cell wall structures and loosening chromoplasts, thus enhancing pigment release. Conversely, SUPRAS-based extraction relies primarily on hydrogen bonding and van der Waals interactions, which may be less efficient for extracting carotenoids that are deeply embedded within complex plant matrices [29]. Interestingly, specific carotenoids such as zeaxanthin were only detected in the SUPRAS extracts of citron peel, indicating selective solubilization based on the supramolecular structure of the solvent. In contrast, β-cryptoxanthin, detected in all CPE extracts, remained largely undetected in most SUPRAS samples. These observations confirm that solvent–analyte molecular interactions significantly influence both extraction efficiency and carotenoid selectivity [30].

Table 1.
Carotenoid profiling of citrus peel extracts.
Another key observation was the compositional variability in carotenoid profiles across citrus species. Orange peel showed the highest carotenoid concentration, consistent with literature reports that highlight Citrus sinensis as a rich source of β-cryptoxanthin and β-carotene, influenced by cultivar, maturity stage, and growth conditions. For example, Viñas-Ospino et al. [8] achieved substantial carotenoid yields from orange peel using ultrasound-assisted extraction with hydrophobic deep eutectic solvents (HDESs), while Murador et al. [31] employed ionic liquid-assisted UAE to extract carotenoids efficiently. However, while these emerging techniques show promise, HDES systems often require solvent tailoring and present viscosity-related processing challenges, and some ionic liquids remain limited in food-related applications due to toxicity concerns.
Xu et al. [32] previously reported that tangerine juice contained higher carotenoid concentrations than orange juice, but these differences likely arise from fruit part (juice vs. peel), postharvest handling, and method-specific factors. Carotenoids in juice are more bioavailable and accessible than those embedded in the waxy cuticle and fibrous peel matrix, which demands stronger extraction strategies [33,34].
When comparing the results of this study to those obtained using other technologies, CPE demonstrates notable advantages. Murador et al. [30] reported total carotenoid yields of only 32.08 ± 2.05 µg/g dw from orange peel using UAE with ionic liquids. Al-idee et al. [35] observed 21.18 mg/100 g dw from orange peel with acetone under optimized conditions, while UAE with olive oil yielded as little as 1.85 mg/100 g dw [36]. For tangerine peel, Saini et al. [37] reported the maximum lutein levels of 2.97 mg/100 g dw, well below the lutein content retrieved using both CPE and SUPRAS-based extraction in the present study.
Recent advances in enzyme-assisted extraction (EAE) and pressurized liquid extraction (PLE) have also shown potential. For instance, Manzoor et al. [35] reported that EAE enhanced carotenoid recovery from marigold petals, but this technique typically requires longer processing times, costlier enzymes, and fine process control. Pressurized liquid extraction (PLE), although efficient, involves elevated temperatures and pressures that may degrade heat-sensitive compounds or require specialized equipment. Compared to these, the CPE method employed here offers a simpler, scalable alternative that uses GRAS components, operates under mild conditions, and avoids the use of hazardous organic solvents.
3.1.2. In Vitro Antioxidant Activity
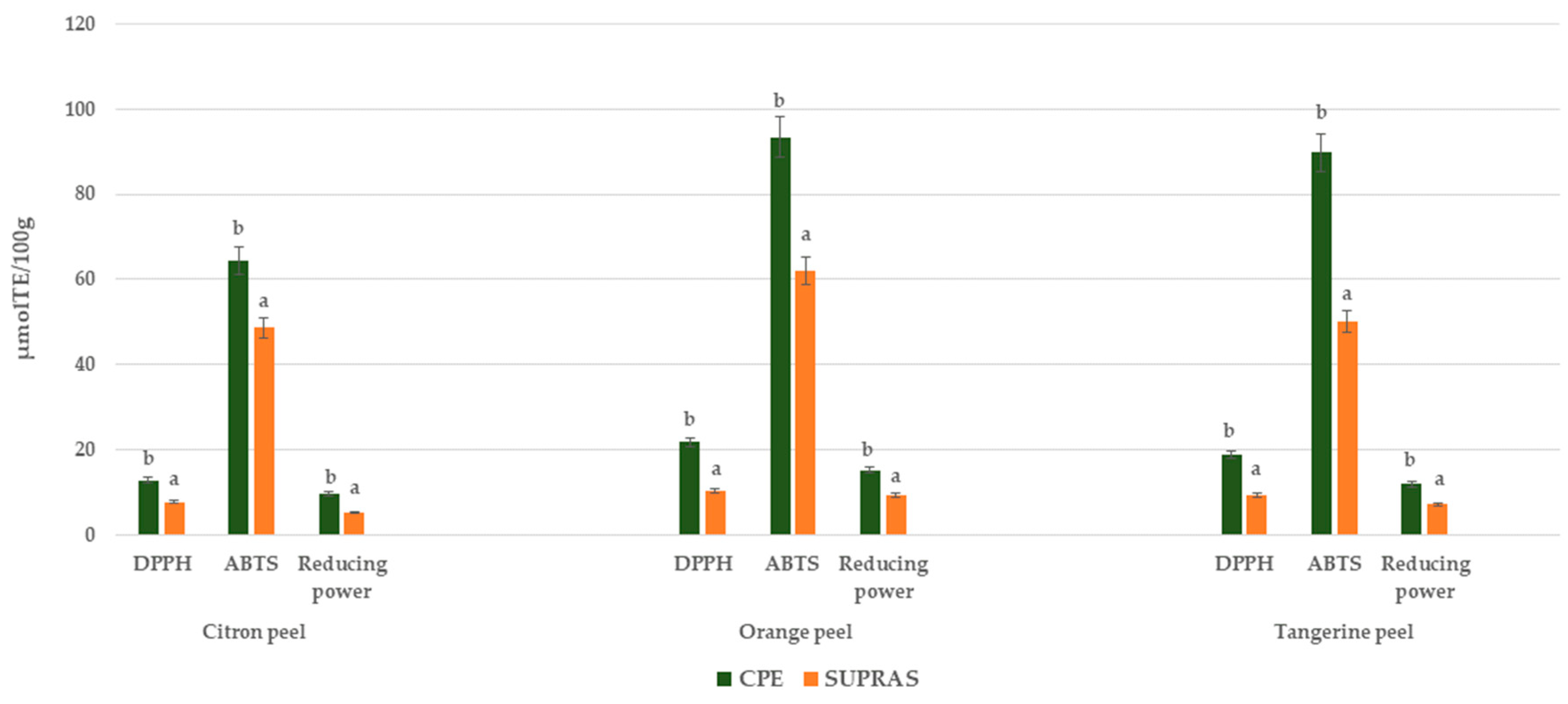
It is well known that antioxidant capacity depends on the chemical nature and mechanism of action of individual antioxidants; therefore, no single method can comprehensively assess the total antioxidant potential of a complex extract. Three in vitro antioxidant methods, namely DPPH, ABTS, and reducing power, were employed in this study, and the results are presented in Figure 3. In all three assays, CPE citrus peel samples exhibited greater antioxidant activity compared to SUPRAS extracts. The observed variation in antioxidant activity can be attributed to their distinct mechanisms of action. As outlined by Anticona et al. [38], the DPPH assay mainly evaluates the hydrogen atom-donating ability of antioxidants, reducing the stable DPPH• radical to a non-radical form. The higher activity level observed for CPE samples suggests that this method more effectively extracts hydrogen-donating compounds from citrus peels. Similarly, in the ABTS assay, which is suitable for detecting both hydrophilic and lipophilic antioxidant compounds, CPE extracts consistently showed superior radical scavenging activity. This indicates a broader and more efficient recovery of antioxidant constituents using the CPE method. The reducing power assay further supported these findings, with CPE samples again outperforming those from SUPRAS-based extraction, suggesting the stronger electron-donating capabilities of the extracted compounds.
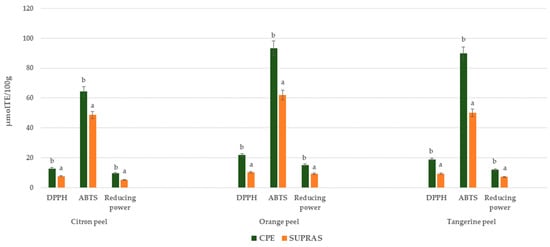
Figure 3.
In vitro antioxidant activity (DPPH, ABTS, and reducing power) for CPE and SUPRAS citrus peel extracts. Different letters above the bars indicate statistically significant differences (p ≤ 0.05) between extraction methods (CPE vs. SUPRAS-based extraction) for each antioxidant assay.
3.2. Characterization of Citrus Peel Encapsulates
3.2.1. Encapsulation Efficiency and Color Properties
Figure 4 illustrates the visual appearance of encapsulated CPE and SUPRAS citrus peel extracts. Encapsulation efficiency (EE) and color are valuable parameters that provide insight into the success of the encapsulation process, and the stability of the retained carotenoids in the resulting powders is shown in Table 2. The EE of carotenoids from citrus peel extracts, using chickpea protein isolate (CPI) as the wall material and freeze-drying as the encapsulation technique, ranged from 82.40% to 88.97%.
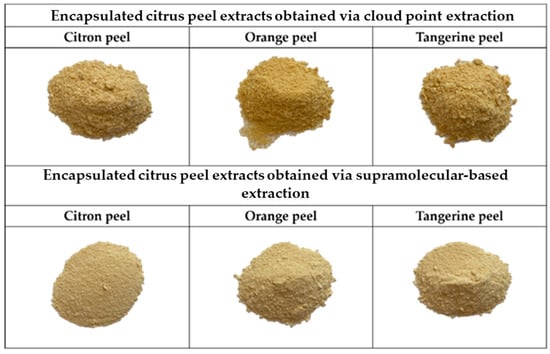
Figure 4.
Appearances of encapsulated CPE and SUPRAS citrus peel extracts.

Table 2.
Encapsulation efficiency and color properties of citrus peel encapsulates.
High EE values may be attributed to CPI’s amphiphilic nature, which facilitates strong interactions with both hydrophilic and hydrophobic bioactive compounds, enhancing entrapment within the freeze-dried matrix. This aligns with the findings by Karaca et al. [39], who highlighted the excellent emulsifying and film-forming properties of chickpea proteins. For conventional wall materials such as maltodextrin, gum arabic, whey protein, and inulin, EE values are typically in the range of 65–85%, depending on the extract type and processing conditions [7,40]. The superior performance of CPI observed in this study supports its application as a functional, plant-based alternative for encapsulating lipophilic bioactives such as carotenoids. In addition to its EE, CPI contributes to the clean-label profile of the final product and aligns with current trends in sustainable, allergen-free, and vegan product development. To ensure the practical applicability of the encapsulates, future research should include stability studies to assess their physicochemical and functional stability under various storage conditions. It is expected that the encapsulated powders will exhibit a favorable shelf-life, based on the chickpea protein isolate’s properties. Additionally, the antioxidant-rich nature of citrus waste extracts may further enhance oxidative stability by mitigating the degradation of sensitive bioactive compounds.
Regarding the color properties as valuable indicators of encapsulation performance and pigment content, the CIELab scalar coordinates (L*, a*, b*) and Chroma (C*) values were measured, and the results are presented in Table 2. The color characteristics of citrus peel encapsulates varied significantly depending on the extraction method used to obtain the initial bioactive-rich extracts. Encapsulates formulated with SUPRAS extracts exhibited consistently higher L* values (83.25–85.61), indicating a lighter appearance compared to those formulated from CPE extracts (75.28–78.01). This trend was also reflected in the Chroma (C*) values. This difference may be attributed to the lower carotenoid concentration in the SUPRAS extracts, resulting in reduced visual intensity in the final powders. Most samples were located in the region of negative a* and positive b* values, indicating a tendency toward greenish-yellow hues. However, the CPE orange peel sample showed a slightly positive a* value (0.15), reflecting a shift toward red tones. The b* values ranged from 10.14 to 25.63, with the highest recorded for the CPE orange peel encapsulate, indicating the most intense yellow coloration. This yellowing is associated with the higher carotenoid content in the encapsulated CPE orange peel extract.
3.2.2. Morphological Properties
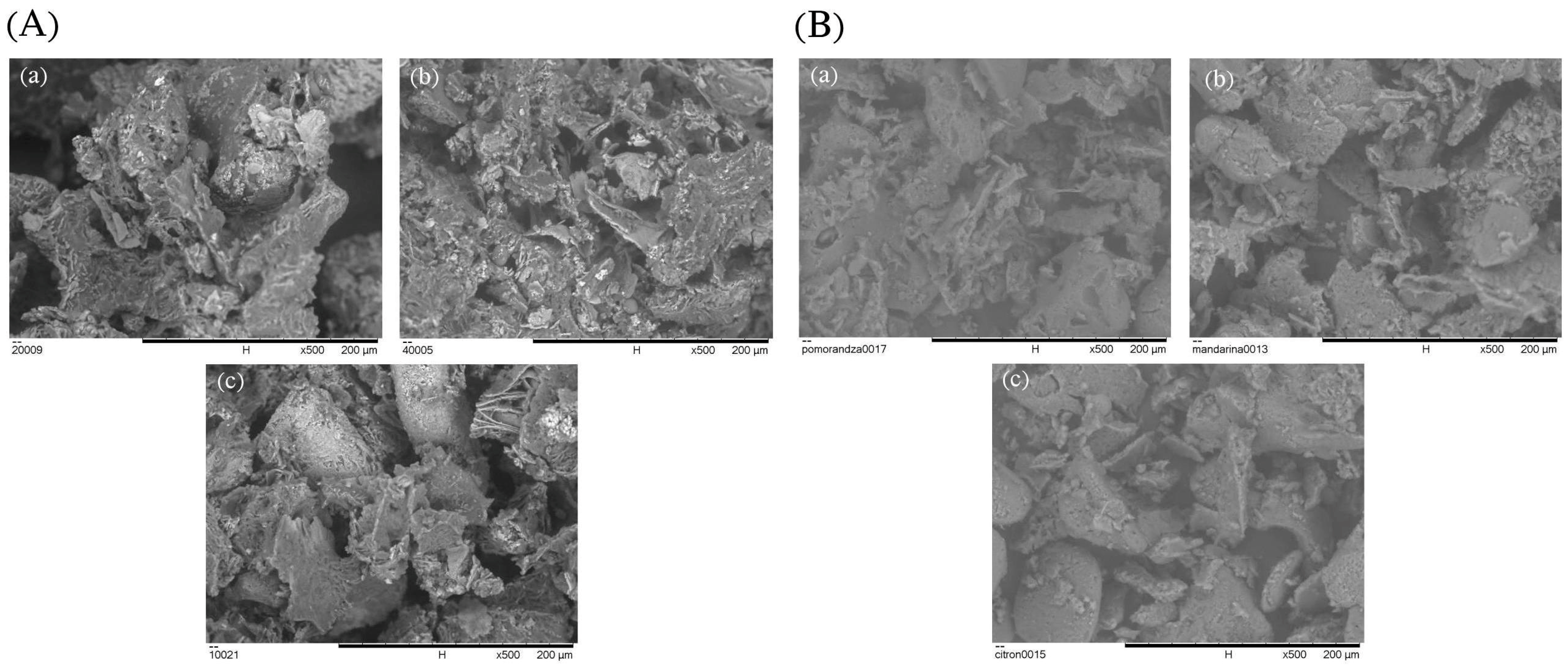
The surface morphology of the citrus peel encapsulates was examined through SEM imaging (Figure 5). All samples exhibited agglomerates of irregular and heterogeneous size, which can be attributed to the complex composition of plant-based extracts that interfere with the formation of uniform particles with well-defined morphology [41]. Such structures are also typical for freeze-dried products due to the low processing temperatures and the absence of mechanical forces during sublimation, which results in wrinkled, porous, and non-spherical particles [7,42]. Notably, morphological differences were observed between the encapsulates derived from CPE and SUPRAS extracts. CPE-based encapsulates showed larger and less organized particles, which may be attributed to the presence of complex phytochemicals in the extract that affect interactions with the wall material during freeze-drying. These components leading to more irregular structural features [43,44]. In contrast, the encapsulates obtained from SUPRAS extracts displayed smaller particles with sponge-like, porous structures and irregularly distributed surface patterns. These features likely reflect the microstructure of the amphiphilic supramolecular system used during extraction, which may influence the spatial arrangement of the encapsulated material and water content during drying [45]. Additionally, the SEM analysis of the chickpea protein isolate used as the encapsulating matrix revealed a broken-glass and flake-like morphology (Figure 6), which is a common structural outcome of protein-based systems subjected to freeze-drying [46].
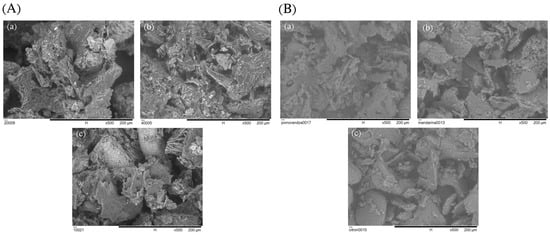
Figure 5.
Surface morphology (SEM images) of encapsulated citrus peel powders formulated using extracts obtained by (A) CPE and (B) SUPRAS methods: (a) orange, (b) tangerine, and (c) citron peels.

Figure 6.
SEM image of freeze-dried chickpea protein isolate used as encapsulating wall material.
3.2.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
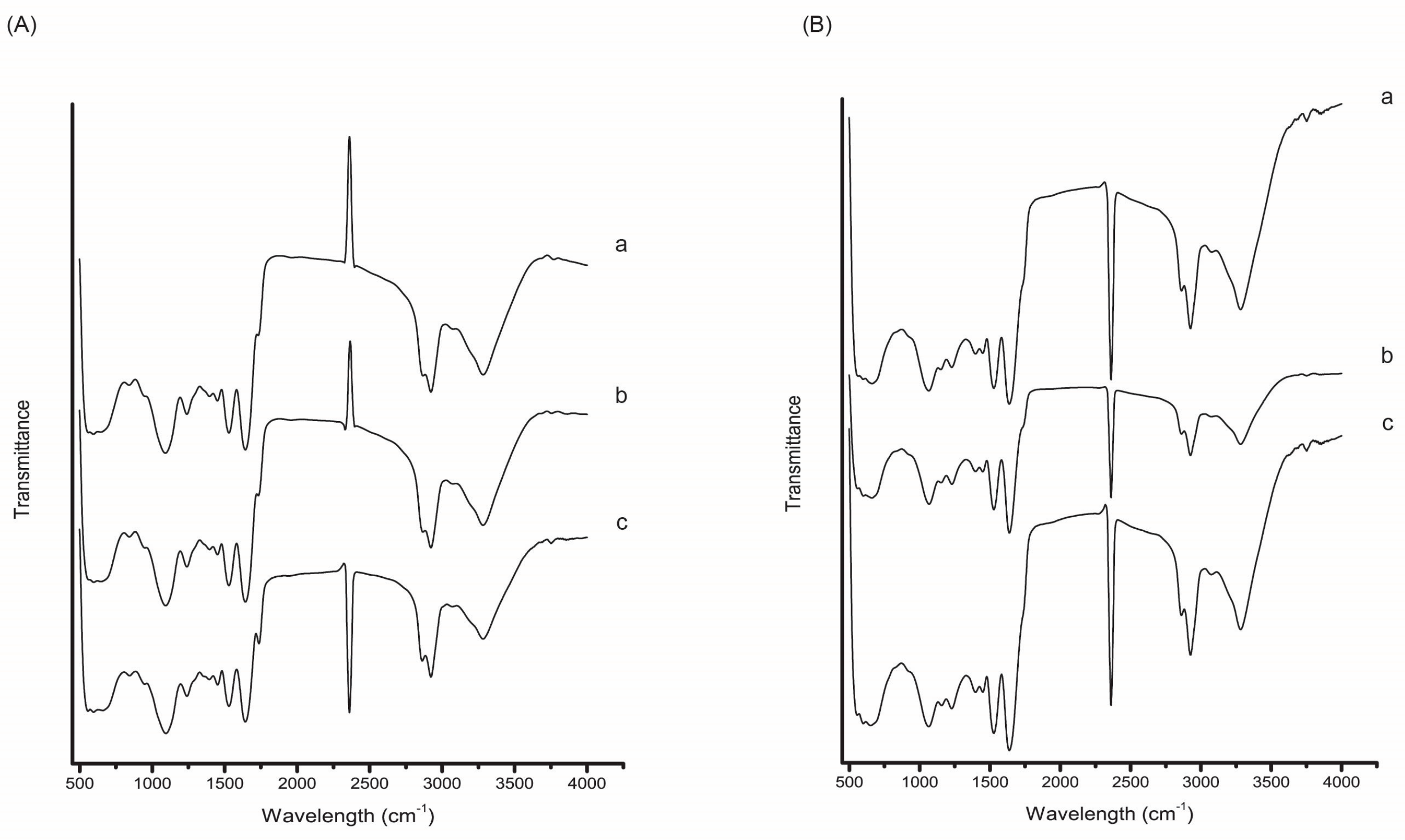
FTIR was employed to characterize the chemical structure of the citrus peel encapsulates and to verify the presence of carotenoids and interactions between the extract and wall material. The FTIR spectra of encapsulated orange, tangerine, and citron peel extracts are presented in Figure 7. Characteristic bands associated with carotenoid compounds were observed in all encapsulated samples, including broad O–H stretching vibrations around 3394 cm−1, C–H stretching near 2922 cm−1, and a weak C=O stretching band at approximately 1737 cm−1, in line with previously reported spectra of carotenoid-rich plant materials [47,48]. The presence of bands near 1400 cm−1 was also noted, corresponding to C–H bending. In addition to extract-specific signals, prominent bands corresponding to the chickpea protein isolate (used as the encapsulating matrix) were detected, such as amide I (~1650 cm−1) and amide II (~1540 cm−1) peaks, indicative of protein secondary structures. Differences between the encapsulates obtained from CPE and SUPRAS extracts were the most evident in the spectral regions of 2900–3000 cm−1 and 1500–1650 cm−1, which are commonly associated with carotenoid-related functional groups. Higher intensities in these regions were observed in the encapsulates prepared from CPE extracts, suggesting more efficient carotenoid retention compared to the SUPRAS-based encapsulates.
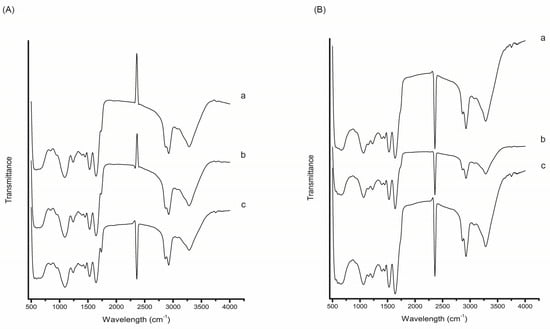
Figure 7.
FTIR spectra of citrus peel encapsulates obtained from (A) CPE and (B) SUPRAS extracts of (a) orange, (b) tangerine, and (c) citron peels.
3.3. EcoScale
Green metrics were utilized to assess the environmental impact and practical feasibility of the solvents and processing conditions used in the CPE and SUPRAS protocols for carotenoid extraction. The outcomes were obtained using the EcoScale tool, which quantifies the greenness of a procedure by assigning penalty points (PPs) to aspects that deviate from the ideal green chemistry principles. As described by Chemat et al. [49], a perfect green process is characterized by zero waste generation, minimal energy consumption, and limited or no use of hazardous chemicals. The EcoScale framework considers a score of 100 as indicative of a perfectly green process. The reliability of this tool has been validated in previous studies [8,50].
The CPE method achieved a score of 93 on the EcoScale, placing it in the category of excellent green techniques. This result is due to several favorable aspects of the procedure, including the use of water and Tween 80 (both GRAS and considered environmentally friendly). The method involves minimal safety risks, uses inexpensive reagents, and requires only mild conditions with short heating time. Notably, it avoids the use of flammable or corrosive substances, which makes it especially suitable for applications in the food and cosmetics industries where safety and sustainability are key priorities. On the other hand, the SUPRAS method scored 68, which falls within the acceptable range but indicates a lower level of greenness. This reduced score primarily results from the use of ethanol, octanoic acid, and hydrochloric acid. These substances are associated with flammability, corrosiveness, or irritant properties, which raise both safety and environmental issues. The differences in EcoScale scores between CPE and SUPRAS-based extraction are primarily due to the penalty points assigned to SUPRAS-based extraction for cost and safety. Both methods received similar scores for extraction yield. However, CPE incurred the same penalty points for temperature/time conditions, whereas SUPRAS-based extraction received points due to its technical setup.
4. Conclusions
The present study demonstrated that citrus peels, typically discarded as waste, can be effectively transformed into valuable sources of carotenoids using environmentally friendly extraction methods. Among the two green techniques evaluated, CPE significantly outperformed SUPRAS-based extraction in terms of carotenoid yield and antioxidant activity. CPE also achieved a high EcoScale score (93), confirming its superior environmental and safety profile. Further, it presents promising pathways for commercial and industrial scalability, particularly in the functional food, nutraceutical, and beverage sectors. Both extract types were successfully encapsulated using chickpea protein isolate (CPI) and freeze-drying, achieving high encapsulation efficiency (82.40–88.97%), color stability, and favorable morphological characteristics. The choice of chickpea protein isolate as an encapsulating matrix supports plant-based product trends, enhancing marketability, as well as the utilization of green technology extraction methods. Its emulsifying properties and compatibility with clean-label formulations make it a strong candidate for natural ingredient delivery systems. In general, this study provides a practical, scalable, and sustainable approach to the valorization of citrus processing waste, contributing to circular economy goals and the development of green product innovations. Future work will include the assessment of long-term storage stability, the bioaccessibility of encapsulated carotenoids, and application in real food systems.
Author Contributions
Conceptualization, V.T. and G.Ć.; methodology, T.C. and A.V.; software, L.P.; validation, T.C., A.V., M.P. and M.K.; formal analysis, T.C., A.V. and M.K.; investigation, V.T. and M.P.; data curation, V.T., G.Ć. and L.P.; writing—original draft preparation, V.T.; writing—review and editing, V.T., M.P. and L.P.; visualization, V.T. and G.Ć.; supervision, G.Ć.; project administration, V.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science, Technological Development and Innovation, Republic of Serbia, grant no. 451-03-136/2025-03/200134 and 451-03-137/2025-03/200134, and Provincial Secretariat for Higher Education and Scientific Research of Autonomous Province Vojvodina (Serbia) grant no. 003063971 2024 09418 003 000 000 001 04 002.
Data Availability Statement
The original findings and contributions of this study are detailed within the article. For additional information, please contact the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kato-Noguchi, H.; Kato, M. Pesticidal Activity of Citrus Fruits for the Development of Sustainable Fruit-Processing Waste Management and Agricultural Production. Plants 2025, 14, 754. [Google Scholar] [CrossRef] [PubMed]
- Mahato, N.; Sinha, M.; Sharma, K.; Koteswararao, R.; Cho, M.H. Modern Extraction and Purification Techniques for Obtaining High Purity Food-Grade Bioactive Compounds and Value-Added Co-Products from Citrus Wastes. Foods 2019, 8, 523. [Google Scholar] [CrossRef] [PubMed]
- Zema, D.A.; Calabrò, P.S.; Folino, A.; Tamburino, V.; Zappia, G.; Zimbone, S.M. Valorisation of Citrus Processing Waste: A Review. Waste Manag. 2018, 80, 252–273. [Google Scholar] [CrossRef]
- Ellouze, I. Citrus Bio-Wastes: A Source of Bioactive, Functional Products and Non-Food Uses. In Mediterranean Fruits Bio-Wastes: Chemistry, Functionality and Technological Applications; Ramadan, M.F., Farag, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 221–260. ISBN 978-3-030-84436-3. [Google Scholar]
- Andrade, M.A.; Barbosa, C.H.; Shah, M.A.; Ahmad, N.; Vilarinho, F.; Khwaldia, K.; Silva, A.S.; Ramos, F. Citrus By-Products: Valuable Source of Bioactive Compounds for Food Applications. Antioxidants 2023, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Hoe, B.C.; Priyangaa, A.; Nagarajan, J.; Ooi, C.W.; Ramanan, R.N.; Nagendra Prasad, K. Chapter 8—Carotenoids. In Nutraceutical and Functional Food Components, 2nd ed.; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 313–362. ISBN 978-0-323-85052-0. [Google Scholar]
- Šeregelj, V.; Ćetković, G.; Čanadanović-Brunet, J.; Šaponjac, V.T.; Vulić, J.; Lević, S.; Nedović, V.; Brandolini, A.; Hidalgo, A. Encapsulation of Carrot Waste Extract by Freeze and Spray Drying Techniques: An Optimization Study. LWT 2021, 138, 110696. [Google Scholar] [CrossRef]
- Viñas-Ospino, A.; Rita Jesus, A.; Paiva, A.; Esteve, M.J.; Frígola, A.; Blesa, J.; López-Malo, D. Comparison of Green Solvents for the Revalorization of Orange By-Products: Carotenoid Extraction and in Vitro Antioxidant Activity. Food Chem. 2024, 442, 138530. [Google Scholar] [CrossRef]
- Teramukai, K.; Kakui, S.; Beppu, F.; Hosokawa, M.; Miyashita, K. Effective Extraction of Carotenoids from Brown Seaweeds and Vegetable Leaves with Edible Oils. IFSET 2020, 60, 102302. [Google Scholar] [CrossRef]
- de Andrade Lima, M.; Kestekoglou, I.; Charalampopoulos, D.; Chatzifragkou, A. Supercritical Fluid Extraction of Carotenoids from Vegetable Waste Matrices. Molecules 2019, 24, 466. [Google Scholar] [CrossRef]
- Silva, T.P.; Alves, L.; Salgado, F.; Roseiro, J.C.; Łukasik, R.M.; Paixão, S.M. Ionic Liquids toward Enhanced Carotenoid Extraction from Bacterial Biomass. Molecules 2024, 29, 4132. [Google Scholar] [CrossRef]
- Sportiello, L.; Marchesi, E.; Tolve, R.; Favati, F. Green Extraction of Carotenoids from Pumpkin By-Products Using Natural Hydrophobic Deep Eutectic Solvents: Preliminary Insights. Molecules 2025, 30, 548. [Google Scholar] [CrossRef]
- Giovanoudis, I.; Athanasiadis, V.; Chatzimitakos, T.; Gortzi, O.; Nanos, G.D.; Lalas, S.I. Development of a Cloud Point Extraction Technique Based on Lecithin for the Recovery of Carotenoids from Liquid Tomato Wastewater. Waste 2023, 1, 105–114. [Google Scholar] [CrossRef]
- Travičić, V.; Cvanić, T.; Vidovic, S.; Pezo, L.; Hidalgo, A.; Šovljanski, O.; Ćetković, G. Sustainable Recovery of Polyphenols and Carotenoids from Horned Melon Peel via Cloud Point Extraction. Foods 2024, 13, 2863. [Google Scholar] [CrossRef] [PubMed]
- Cvanić, T.; Sulejmanovic, M.; Perovic, M.; Vulić, J.; Pezo, L.; Ćetković, G.; Travičić, V. Novel Green Strategy to Recover Bioactive Compounds with Different Polarities from Horned Melon Peel. Foods 2024, 13, 2880. [Google Scholar] [CrossRef]
- Vieira, F.A.; Ventura, S.P.M. Efficient Extraction of Carotenoids from Sargassum Muticum Using Aqueous Solutions of Tween 20. Mar. Drugs 2019, 17, 310. [Google Scholar] [CrossRef]
- Torres-Valenzuela, L.S.; Ayala-Aponte, A.; Rodriguez, L.; Rodriguez, A.; Navia-Porras, D.P. Supramolecular Solvent Extraction of Bioactive Compounds from Tamarillo (Solanum Betaceum) Byproducts. Discov. Food 2025, 5, 34. [Google Scholar] [CrossRef]
- Ueda, K.M.; Keiser, G.M.; Leal, F.C.; Farias, F.O.; Igarashi-Mafra, L.; Mafra, M.R. A New Single-Step Approach Based on Supramolecular Solvents (SUPRAS) to Extract Bioactive Compounds with Different Polarities from Eugenia Pyriformis Cambess (Uvaia) Pulp. Plant Foods Hum. Nutr. 2024, 79, 242–249. [Google Scholar] [CrossRef]
- Sereti, F.; Alexandri, M.; Papapostolou, H.; Papadaki, A.; Kopsahelis, N. Recent Progress in Carotenoid Encapsulation: Effects on Storage Stability, Bioaccessibility and Bioavailability for Advanced Innovative Food Applications. Food Res. Int. 2025, 203, 115861. [Google Scholar] [CrossRef]
- Ma, D.; Yang, B.; Zhao, J.; Yuan, D.; Li, Q. Advances in Protein-Based Microcapsules and Their Applications: A Review. Int. J. Biol. Macromol. 2024, 263, 129742. [Google Scholar] [CrossRef]
- Vučetić, A.; Pezo, L.; Šovljanski, O.; Vulić, J.; Travičić, V.; Ćetković, G.; Čanadanović-Brunet, J. Supramolecular Solvent-Based Extraction of Microgreens: Taguchi Design Coupled-ANN Multi-Objective Optimization. Processes 2024, 12, 1451. [Google Scholar] [CrossRef]
- Šeregelj, V.; Estivi, L.; Brandolini, A.; Ćetković, G.; Tumbas Šaponjac, V.; Hidalgo, A. Kinetics of Carotenoids Degradation during the Storage of Encapsulated Carrot Waste Extracts. Molecules 2022, 27, 8759. [Google Scholar] [CrossRef]
- Šovljanski, O.; Lončar, B.; Pezo, L.; Saveljić, A.; Tomić, A.; Brunet, S.; Filipović, V.; Filipović, J.; Čanadanović-Brunet, J.; Ćetković, G.; et al. Unlocking the Potential of the ANN Optimization in Sweet Potato Varieties Drying Processes. Foods 2024, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Perović, M.N.; Antov, M.G. The Influence of Enzymatic Pretreatment of Chickpea on Properties of Protein Nanoparticles Prepared by Heat Treatment. LWT 2022, 163, 113545. [Google Scholar] [CrossRef]
- Barbosa, M.I.M.J.; Borsarelli, C.D.; Mercadante, A.Z. Light Stability of Spray-Dried Bixin Encapsulated with Different Edible Polysaccharide Preparations. Food Res. Int. 2005, 38, 989–994. [Google Scholar] [CrossRef]
- Nagata, M.; Yamashita, I. Simple Method for Simultaneous Determination of Chlorophyll and Carotenoids in Tomato Fruit. J. Food Sci. Technol. 1992, 39, 925–928. [Google Scholar] [CrossRef]
- Aken, K.V.; Strekowski, L.; Patiny, L. EcoScale, a Semi-Quantitative Tool to Select an Organic Preparation Based on Economical and Ecological Parameters. Beilstein J. Org. Chem. 2006, 2, 3. [Google Scholar] [CrossRef]
- Kultys, E.; Kurek, M.A. Green Extraction of Carotenoids from Fruit and Vegetable Byproducts: A Review. Molecules 2022, 27, 518. [Google Scholar] [CrossRef]
- Zuin, V.G.; Ramin, L.Z. Green and Sustainable Separation of Natural Products from Agro-Industrial Waste: Challenges, Potentialities, and Perspectives on Emerging Approaches. Top. Curr. Chem. 2018, 376, 3. [Google Scholar] [CrossRef]
- Kurek, M.A.; Aktaş, H.; Pokorski, P.; Pogorzelska-Nowicka, E.; Custodio-Mendoza, J.A. A Comprehensive Review of Analytical Approaches for Carotenoids Assessment in Plant-Based Foods: Advances, Applications, and Future Directions. Appl. Sci. 2025, 15, 3506. [Google Scholar] [CrossRef]
- Murador, D.C.; Braga, A.R.C.; Martins, P.L.G.; Mercadante, A.Z.; de Rosso, V.V. Ionic Liquid Associated with Ultrasonic-Assisted Extraction: A New Approach to Obtain Carotenoids from Orange Peel. Food Res. Int. 2019, 126, 108653. [Google Scholar] [CrossRef]
- Xu, G.; Liu, D.; Chen, J.; Ye, X.; Ma, Y.; Shi, J. Juice Components and Antioxidant Capacity of Citrus Varieties Cultivated in China. Food Chem. 2008, 106, 545–551. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from Fruits and Vegetables: Chemistry, Analysis, Occurrence, Bioavailability and Biological Activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Marín, F.R.; Soler-Rivas, C.; Benavente-García, O.; Castillo, J.; Pérez-Alvarez, J.A. By-Products from Different Citrus Processes as a Source of Customized Functional Fibres. Food Chem. 2007, 100, 736–741. [Google Scholar] [CrossRef]
- Manzoor, S.; Rashid, R.; Prasad Panda, B.; Sharma, V.; Azhar, M. Green Extraction of Lutein from Marigold Flower Petals, Process Optimization and Its Potential to Improve the Oxidative Stability of Sunflower Oil. Ultrason. Sonochem. 2022, 85, 105994. [Google Scholar] [CrossRef]
- Savic Gajic, I.M.; Savic, I.M.; Gajic, D.G.; Dosic, A. Ultrasound-Assisted Extraction of Carotenoids from Orange Peel Using Olive Oil and Its Encapsulation in Ca-Alginate Beads. Biomolecules 2021, 11, 225. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.; Panesar, P.S.; Bera, M.B. Valuation of Citrus Reticulata (Kinnow) Peel for the Extraction of Lutein Using Ultrasonication Technique. Biomass Conv. Bioref. 2021, 11, 2157–2165. [Google Scholar] [CrossRef]
- Anticona, M.; Blesa, J.; Lopez-Malo, D.; Frigola, A.; Esteve, M.J. Effects of Ultrasound-Assisted Extraction on Physicochemical Properties, Bioactive Compounds, and Antioxidant Capacity for the Valorization of Hybrid Mandarin Peels. Food Biosci. 2021, 42, 101185. [Google Scholar] [CrossRef]
- Karaca, A.C.; Low, N.; Nickerson, M. Emulsifying Properties of Chickpea, Faba Bean, Lentil and Pea Proteins Produced by Isoelectric Precipitation and Salt Extraction. Food Res. Int. 2011, 44, 2742–2750. [Google Scholar] [CrossRef]
- Eun, J.-B.; Maruf, A.; Das, P.R.; Nam, S.-H. A Review of Encapsulation of Carotenoids Using Spray Drying and Freeze Drying. Crit. Rev. Food Sci. Nutr. 2020, 60, 3547–3572. [Google Scholar] [CrossRef]
- da Fonseca Machado, A.P.; Alves Rezende, C.; Alexandre Rodrigues, R.; Fernández Barbero, G.; de Tarso Vieira e Rosa, P.; Martínez, J. Encapsulation of Anthocyanin-Rich Extract from Blackberry Residues by Spray-Drying, Freeze-Drying and Supercritical Antisolvent. Powder Technol. 2018, 340, 553–562. [Google Scholar] [CrossRef]
- Chen, C.; Chi, Y.-J.; Xu, W. Comparisons on the Functional Properties and Antioxidant Activity of Spray-Dried and Freeze-Dried Egg White Protein Hydrolysate. Food Bioprocess Technol. 2012, 5, 2342–2352. [Google Scholar] [CrossRef]
- Guo, N.; Jiang, Y.-W.; Kou, P.; Liu, Z.-M.; Efferth, T.; Li, Y.-Y.; Fu, Y.-J. Application of Integrative Cloud Point Extraction and Concentration for the Analysis of Polyphenols and Alkaloids in Mulberry Leaves. J. Pharm. Biomed. Anal. 2019, 167, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.-C.; Shi, M.-Z.; Yu, Y.-L.; Cao, J. Simultaneous Extraction and Enrichment of Alkaloids from Lotus Leaf by In-Situ Cloud Point-Reinforced Ionic Liquid Assisted Mechanochemical Extraction Technology. Ind. Crops Prod. 2022, 183, 114968. [Google Scholar] [CrossRef]
- Algar, L.; Sicilia, M.D.; Rubio, S. Tailoring Supramolecular Solvents with Phosphoryl Groups for Highly Efficient Extraction of Chlorophenols in Natural Waters. Anal. Chim. Acta 2024, 1309, 342688. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, K.; Golding, J.B.; Vuong, Q.; Pristijono, P.; Stathopoulos, C.E.; Scarlett, C.J.; Bowyer, M. Encapsulation of Citrus By-Product Extracts by Spray-Drying and Freeze-Drying Using Combinations of Maltodextrin with Soybean Protein and ι-Carrageenan. Foods 2018, 7, 115. [Google Scholar] [CrossRef]
- de Lima Petito, N.; da Silva Dias, D.; Costa, V.G.; Falcão, D.Q.; de Lima Araujo, K.G. Increasing Solubility of Red Bell Pepper Carotenoids by Complexation with 2-Hydroxypropyl-β-Cyclodextrin. Food Chem. 2016, 208, 124–131. [Google Scholar] [CrossRef]
- Saha, N.; Samanta, A.K.; Chaudhuri, S.; Dutta, D. Characterization and Antioxidant Potential of a Carotenoid from a Newly Isolated Yeast. Food Sci. Biotechnol. 2015, 24, 117–124. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green Extraction of Natural Products. Origins, Current Status, and Future Challenges. TrAC 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Pressurized Aqueous Solutions of Deep Eutectic Solvent (DES): A Green Emergent Extraction of Anthocyanins from a Brazilian Berry Processing by-Product. Food Chem. X 2022, 13, 100236. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).