5-Fluorouracil Encapsulation in PLA Films: The Role of Chitosan Particles in Modulating Drug Release and Film Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of PLA and 5-FU-PLA Films
2.2.2. Preparation Protocol of PLA Films Loaded with Chitosan Particles
2.3. Characterisation of PLA, PLA + 5-FU and PLA + CH + 5-FU Structures
2.3.1. Film Thickness
2.3.2. Mechanical Properties
2.3.3. Water-Vapour Transmission Rate (WVTR) and Permeability (P)
2.3.4. Morphology
2.3.5. Water Contact Angle and Surface Energy Measurement
2.3.6. Phase State and Thermal Stability
2.3.7. Drug Release Test
2.3.8. Mathematical Modelling
3. Results and Discussion
3.1. Tensile Properties Test
3.2. Barrier Properties and Permeability
3.3. Morphology
3.4. Water Contact Angle and Surface Free Energy
3.5. Phase State and Thermal Behaviour
3.6. Drug Release Profiles and Optimal Model Fitting of the Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-FU | 5-fluorouracil |
| CH | Chitosan particles |
| P | Permeability |
| PLA | Poly(lactic acid); film based on poly(lactic acid) |
| PLA + 5-FU | film based on poly(lactic acid) and loaded with 5-fluorouracil |
| PLA + CH + 5-FU | PLA film containing drug-loaded chitosan particles |
| WVTR | Water Vapour Transmission Rate |
| YM | Young’s Modulus |
References
- Zhang, L.; Lv, J.; Yin, Y.; Ling, G.; Zhang, P. Rapidly Separable Microneedle Patch for the Controlled and Sustained Release of 5-Fluorouracil. Int. J. Pharm. 2023, 635, 122730. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.; Kashaw, S.K.; Sau, S.; Kushwah, V.; Jain, S.; Agrawal, R.K.; Iyer, A.K. PH Responsive 5-Fluorouracil Loaded Biocompatible Nanogels for Topical Chemotherapy of Aggressive Melanoma. Colloids Surf. B Biointerfaces 2019, 174, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, Y.-C. Topical Delivery of 5-Fluorouracil-Loaded Carboxymethyl Chitosan Nanoparticles Using Microneedles for Keloid Treatment. Drug Deliv. Transl. Res. 2020, 11, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Zahra, F.T.; Adil, M.; Amin, S.S.; Mohtashim, M.; Bansal, R.; Khan, H.Q. Efficacy of Topical 5% 5-Fluorouracil with Needling versus 5% 5-Fluorouracil Alone in Stable Vitiligo: A Randomised Controlled Study. J. Cutan. Aesthetic Surg. 2020, 13, 197–203. [Google Scholar] [CrossRef]
- Raviraj, V.; Pham, B.T.T.; Kim, B.J.; Pham, N.T.H.; Kok, L.F.; Painter, N.; Delic, N.C.; Jones, S.K.; Hawkett, B.S.; Lyons, J.G. Non-Invasive Transdermal Delivery of Chemotherapeutic Molecules in Vivo Using Superparamagnetic Iron Oxide Nanoparticles. Cancer Nanotechnol. 2021, 12, 6. [Google Scholar] [CrossRef]
- Santos, L.F.; Correia, I.J.; Silva, A.S.; Mano, J.F. Biomaterials for Drug Delivery Patches. Eur. J. Pharm. Sci. 2018, 118, 49–66. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic Acid (PLA) Controlled Delivery Carriers for Biomedical Applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Hijazi, N.; Le Moigne, N.; Rodier, E.; Sauceau, M.; Vincent, T.; Benezet, J.-C.; Fages, J. Biocomposite Films Based on Poly (Lactic Acid) and Chitosan Nanoparticles: Elaboration, Microstructural and Thermal Characterisation. Polym. Eng. Sci. 2018, 59, E350–E360. [Google Scholar] [CrossRef]
- Garavand, F.; Rouhi, M.; Jafarzadeh, S.; Khodaei, D.; Cacciotti, I.; Zargar, M.; Razavi, S.H. Tuning the Physicochemical, Structural, and Antimicrobial Attributes of Whey-Based Poly (L-Lactic Acid) (PLLA) Films by Chitosan Nanoparticles. Front. Nutr. 2022, 9, 880520. [Google Scholar] [CrossRef]
- Tığlı Aydın, R.S.; Pulat, M. 5-Fluorouracil Encapsulated Chitosan Nanoparticles for PH-Stimulated Drug Delivery: Evaluation of Controlled Release Kinetics. J. Nanomater. 2012, 2012, 313961. [Google Scholar] [CrossRef]
- Khan, T.A.; Azad, A.K.; Fuloria, S.; Nawaz, A.; Subramaniyan, V.; Akhlaq, M.; Safdar, M.; Sathasivam, K.V.; Sekar, M.; Porwal, O.; et al. Chitosan-Coated 5-Fluorouracil Incorporated Emulsions as Transdermal Drug Delivery Matrices. Polymers 2021, 13, 3345. [Google Scholar] [CrossRef] [PubMed]
- Sabitha, M.; Sanoj Rejinold, N.; Nair, A.; Lakshmanan, V.-K.; Nair, S.V.; Jayakumar, R. Development and Evaluation of 5-Fluorouracil Loaded Chitin Nanogels for Treatment of Skin Cancer. Carbohydr. Polym. 2013, 91, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Saha, M.; Mahata, L.C.; China, A.; Chatterjee, N. Krishna Das Saha Quercetin and 5-Fu Loaded Chitosan Nanoparticles Trigger Cell-Cycle Arrest and Induce Apoptosis in HCT116 Cells via Modulation of the P53/P21 Axis. ACS Omega 2023, 8, 36893–36905. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, Y.; Zhou, Y.; Guo, D.; Fan, Y.; Guo, F.; Zheng, Y.; Chen, W. Preparation of 5-Fluorouracil-Loaded Chitosan Nanoparticles and Study of the Sustained Release In Vitro and In Vivo. Asian J. Pharm. Sci. 2017, 12, 418–423. [Google Scholar] [CrossRef]
- Entezar-Almahdi, E.; Mohammadi-Samani, S.; Tayebi, L.; Farjadian, F. Recent Advances in Designing 5-Fluorouracil Delivery Systems: A Stepping Stone in the Safe Treatment of Colorectal Cancer. Int. J. Nanomed. 2020, 15, 5445–5458. [Google Scholar] [CrossRef]
- Milenkova, S.; Tashkov, S.; Zahariev, N.; Pilicheva, B.; Marudova, M. 5-Fluorouracil Encapsulated Chitosan Microspheres. J. Chem. Technol. Metall. 2024, 59, 887–896. [Google Scholar] [CrossRef]
- ASTM D882-91; Standard Test Methods for Tensile Properties of Thin Plastic Sheeting. American Society for Testing and Materials: Philadelphia, PA, USA, 2017. Available online: https://www.astm.org/d0882-00.html (accessed on 26 May 2025).
- Owens, D.K.; Wendt, R.C. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Elsawy, M.A.; Saad, G.R.; Sayed, A.M. Mechanical, Thermal, and Dielectric Properties of Poly (Lactic Acid)/Chitosan Nanocomposites. Polym. Eng. Sci. 2016, 56, 987–994. [Google Scholar] [CrossRef]
- Pünnel, L.C.; Lunter, D.J. Film-Forming Systems for Dermal Drug Delivery. Pharmaceutics 2021, 13, 932. [Google Scholar] [CrossRef]
- Chedik, L.; Baybekov, S.; Cosnier, F.; Marcou, G.; Varnek, A.; Champmartin, C. An Update of Skin Permeability Data Based on a Systematic Review of Recent Research. Sci. Data 2024, 11, 224. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Yue, L.; Huang, Y.; Wang, B.; Liu, P. Uncovering Key Mechanisms and Intervention Therapies in Aging Skin. Cytokine Growth Factor Rev. 2024, 79, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Guivier, M.; Chevigny, C.; Domenek, S.; Casalinho, J.; Perré, P.; Almeida, G. Water Vapour Transport Properties of Bio-Based Multilayer Materials Determined by Original and Complementary Methods. Sci. Rep. 2024, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Soltani, Z.; Tavakolipour, H.; Tabari, M. The Influence of Chitosan and Titanium Dioxide Nanoparticles Incorporated with Polylactic Acid on Prolonging Rye Bread Shelf Life. J. Food Meas. Characterisation 2022, 17, 1806–1816. [Google Scholar] [CrossRef]
- Singh, P.P.; Sharma, B.R.; Sidhu, K.S. Thermodynamics of Chloroform and Methanol Mixtures. Can. J. Chem. 1979, 57, 387–393. [Google Scholar] [CrossRef]
- Tümer, E.H.; Erbil, H.Y.; Akdoǧan, N. Wetting of Superhydrophobic Polylactic Acid Micropillared Patterns. Langmuir 2022, 38, 10052–10064. [Google Scholar] [CrossRef]
- Capra, P.; Musitelli, G.; Perugini, P. Wetting and Adhesion Evaluation of Cosmetic Ingredients and Products: Correlation of In Vitro-In Vivo Contact Angle Measurements. Int. J. Cosmet. Sci. 2017, 39, 393–401. [Google Scholar] [CrossRef]
- Choi, M.-J.; Woo, M.R.; Choi, H.-G.; Jin, S.G. Effects of Polymers on the Drug Solubility and Dissolution Enhancement of Poorly Water-Soluble Rivaroxaban. Int. J. Mol. Sci. 2022, 23, 9491. [Google Scholar] [CrossRef]
- Han, A.S.; Kim, J.; Park, J.W.; Jin, S.G. Novel Acyclovir-Loaded Film-Forming Gel with Enhanced Mechanical Properties and Skin Permeability. J. Drug Deliv. Sci. Technol. 2022, 70, 103213. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Castro, K.C.; de Costa, J.M.; Campos, M.G.N. Drug-Loaded Polymeric Nanoparticles: A Review. Int. J. Polym. Mater. Polym. Biomater. 2020, 71, 1–13. [Google Scholar] [CrossRef]
- Gautam, M.K.; Besan, M.; Pandit, D.; Mandal, S.; Chadha, R. Cocrystal of 5-Fluorouracil: Characterization and Evaluation of Biopharmaceutical Parameters. AAPS PharmSciTech 2019, 20, 149. [Google Scholar] [CrossRef] [PubMed]
- Samy, M.; Abdallah, H.M.; Awad, H.M.; Ayoub, H. Preparation, Characterisation and in Vitro Biological Activity of 5-Fluorouracil Loaded onto Poly (D, L-Lactic-Co-Glycolic Acid) Nanoparticles. Polym. Bull. 2022, 80, 6197–6219. [Google Scholar] [CrossRef]
- Banerjee, P.; Mukherjee, D.; Maiti, T.K.; Sarkar, N. Unveiling the Self-Assembling Behaviour of 5-Fluorouracil and Its N, N′-Dimethyl Derivative: A Spectroscopic and Microscopic Approach. Langmuir 2017, 33, 10978–10988. [Google Scholar] [CrossRef] [PubMed]
- Flores-León, J.R.; Rodríguez-Félix, D.E.; Quiroz-Castillo, J.M.; Burrola-Núñez, H.; Castillo-Ortega, M.M.; Encinas-Encinas, J.C.; Alvarado-Ibarra, J.; Santacruz-Ortega, H.; Valenzuela-García, J.L.; Herrera-Franco, P.J. Effect of Degradation on the Physicochemical and Mechanical Properties of Extruded Films of Poly (Lactic Acid) and Chitosan. ACS Omega 2024, 9, 9526–9535. [Google Scholar] [CrossRef]
- Jones, E.C.L.; Bimbo, L.M. Crystallisation Behaviour of Pharmaceutical Compounds Confined within Mesoporous Silicon. Pharmaceutics 2020, 12, 214. [Google Scholar] [CrossRef]
- Kelle, D.; Speth, K.R.; Martínez-Negro, M.; Mailänder, V.; Landfester, K. Banu Iyisan Effect of Protein Corona on Drug Release Behavior of PLGA Nanoparticles. Eur. J. Pharm. Biopharm. 2024, 207, 114611. [Google Scholar] [CrossRef]
- Kim, K.-J.; Hwang, M.-J.; Yun, Y.-H.; Yoon, S.-D. Synthesis and Drug Release Behaviour of Functional Montelukast Imprinted Inulin-Based Biomaterials as Asthma Treatment. J. Ind. Eng. Chem. 2022, 109, 221–229. [Google Scholar] [CrossRef]

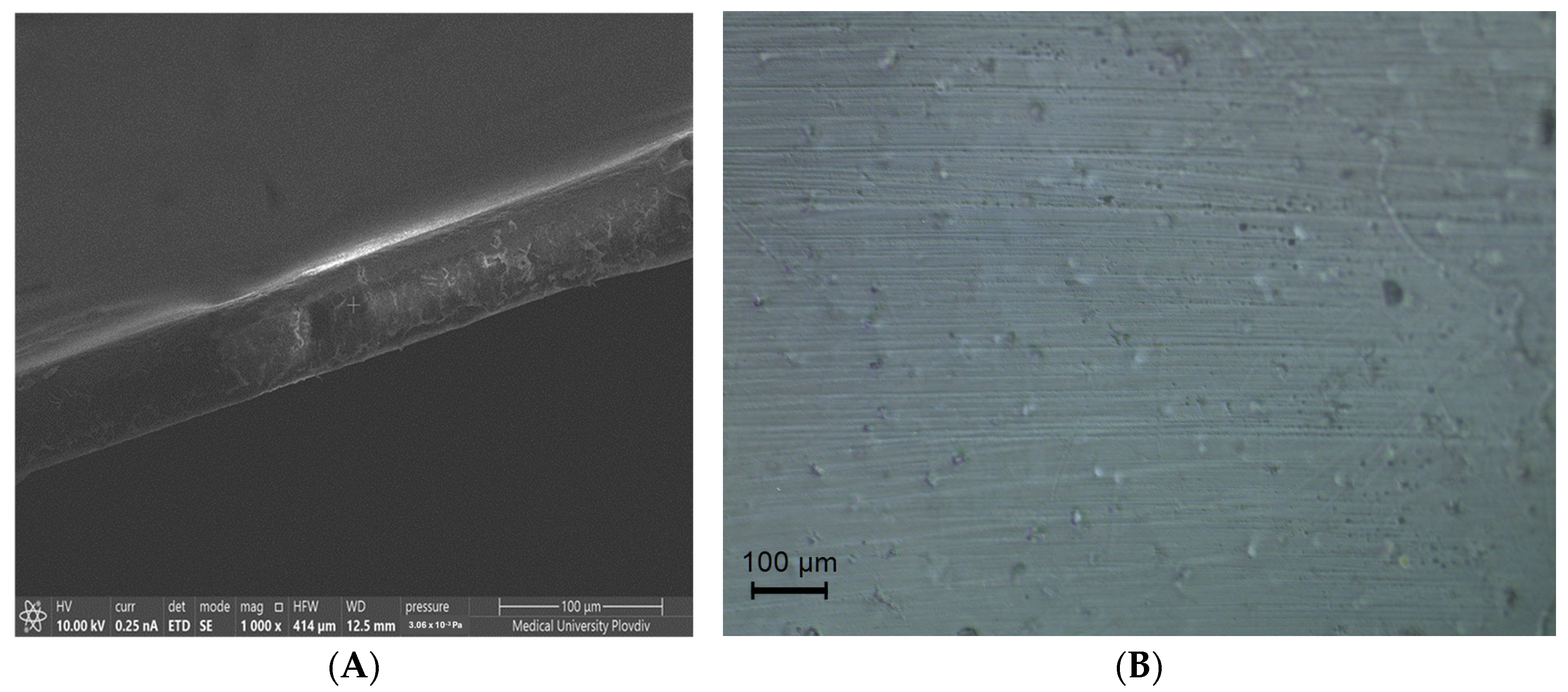
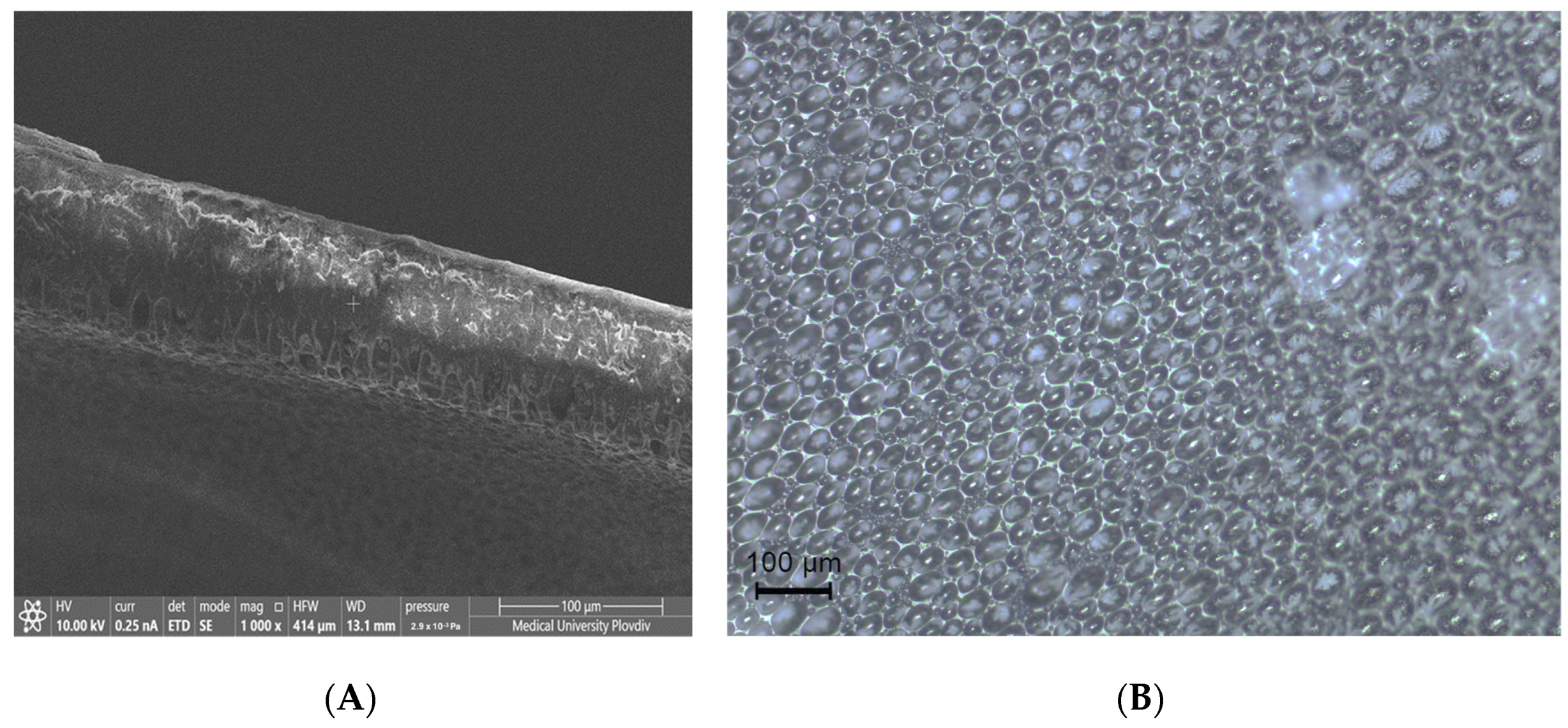
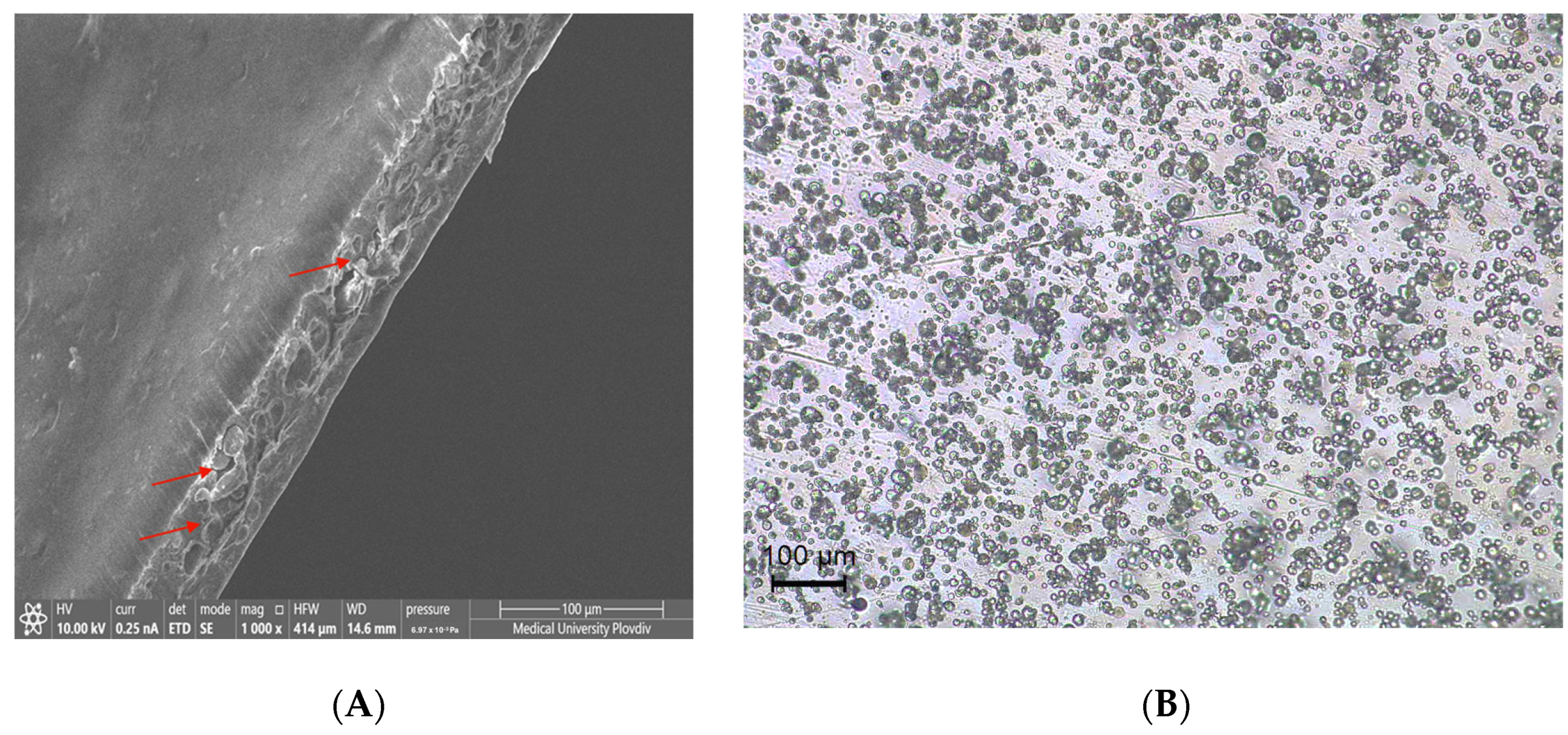
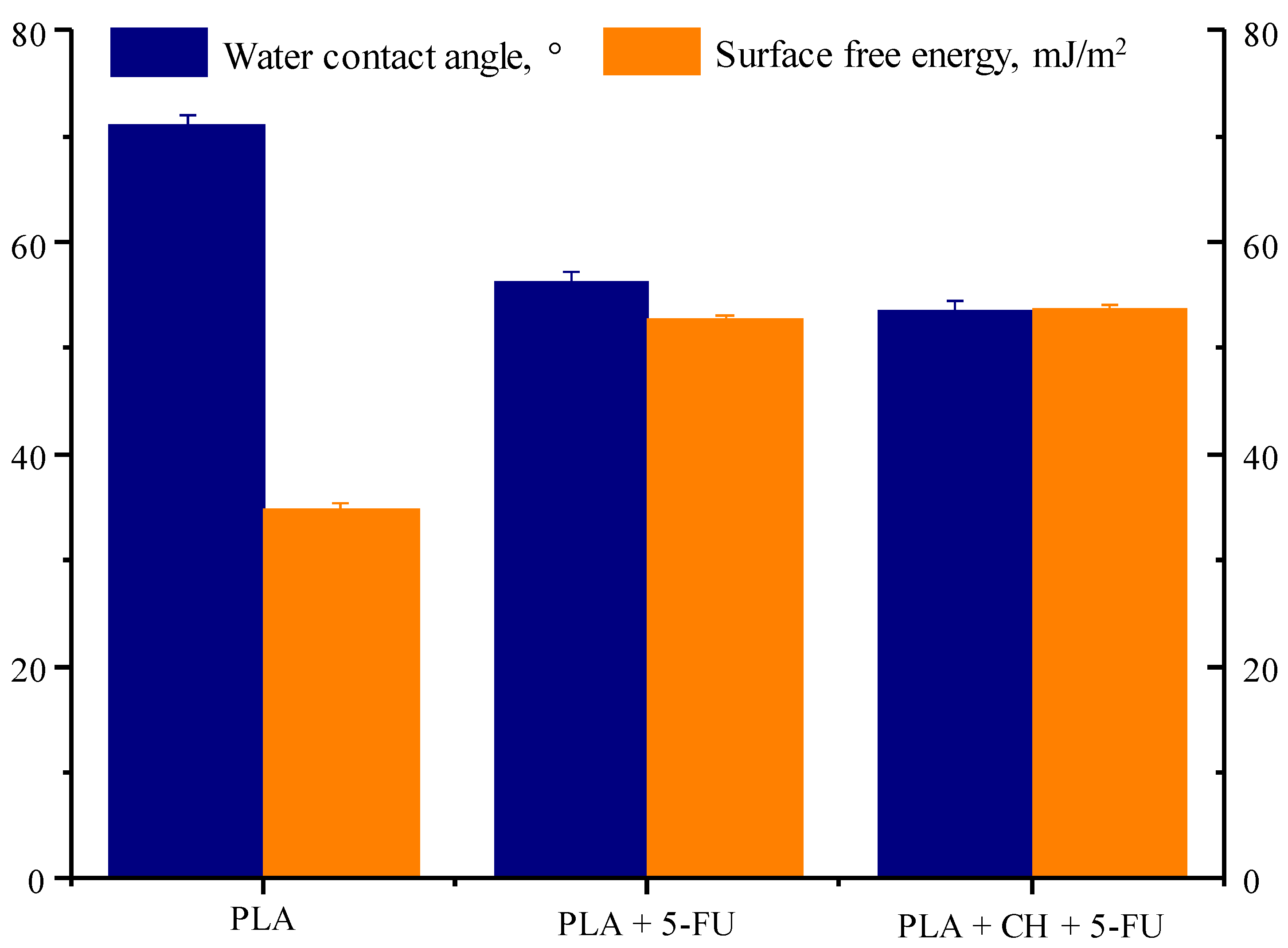

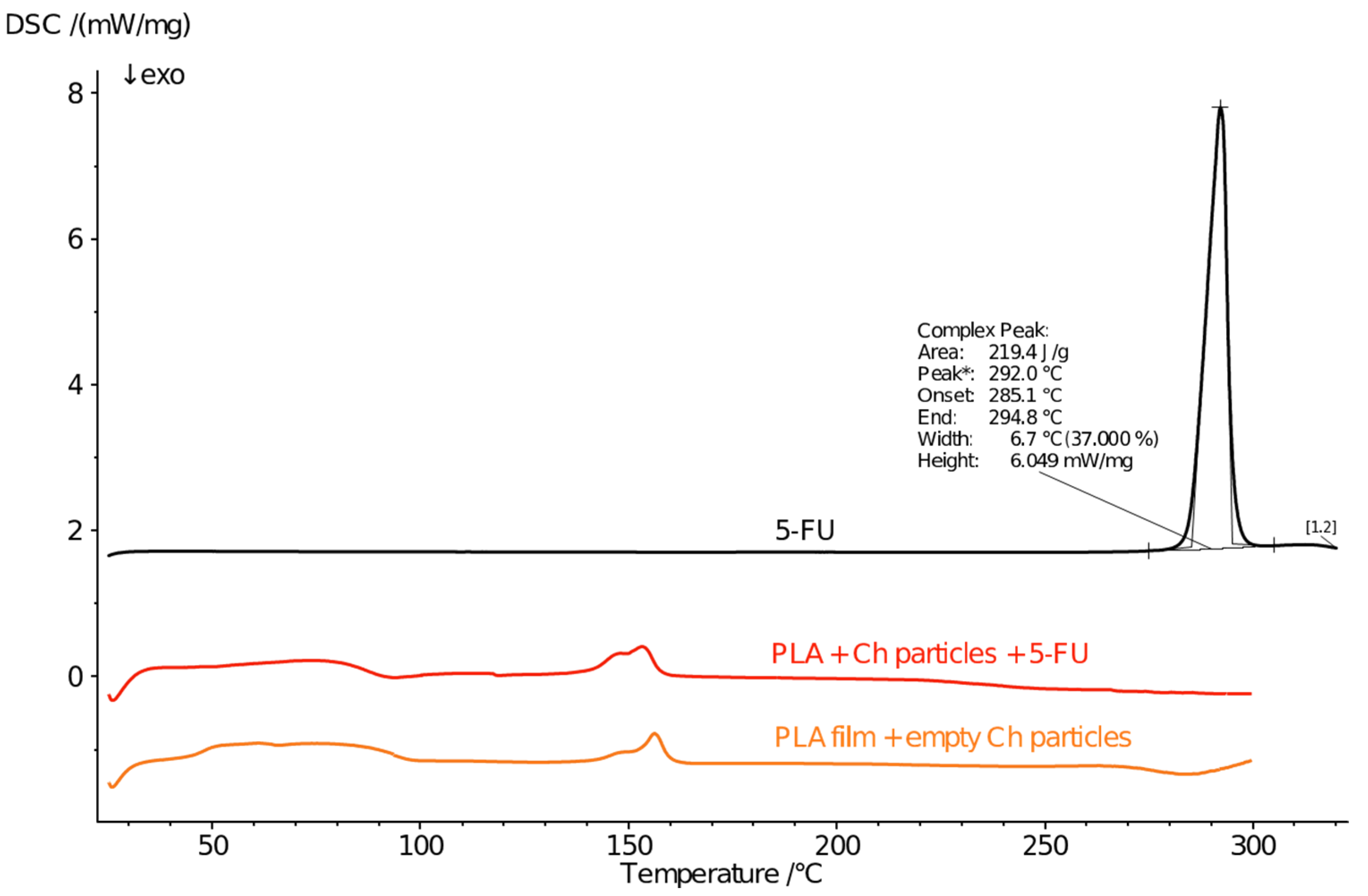
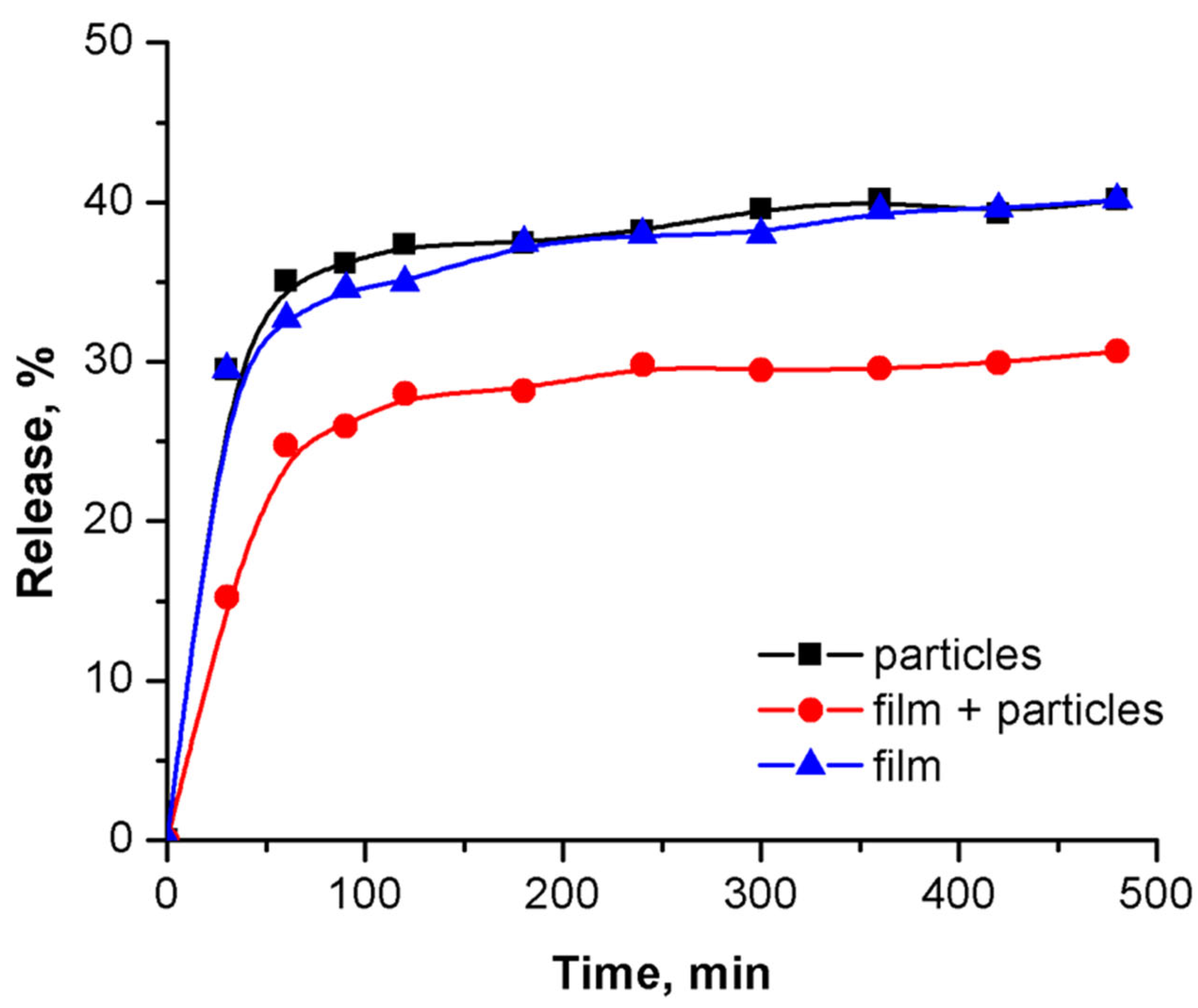
| PLA | PLA + 5-FU | PLA + CH + 5-FU | |
|---|---|---|---|
| Film thickness, µm | 62.9 ± 8.4 | 68.5 ± 5.8 | 62.6 ± 3.7 |
| Strain at break, % | 393 ± 28 | 7 ± 1 | 14 ± 2 |
| Stress at break, MPa | 30 ± 2 | 30 ± 6 | 24 ± 2 |
| Young’s Modulus, MPa | 292 ± 31 | 630 ± 100 | 514 ± 98 |
| PLA | PLA + 5-FU | PLA + CH + 5-FU | |
|---|---|---|---|
| WVTR, g/m2·24 h | 189 ± 7 | 241 ± 6 | 174 ± 9 |
| P × 10−13, g·mm/m2·24 h·kPa | 2.44 ± 0.09 | 1.56 ± 0.15 | 2.23 ± 0.12 |
| K | n | R2 | |
|---|---|---|---|
| CH + 5-FU | 23.4618 ± 1.4454 | 0.0897 ± 0.0115 | 0.9915 |
| PLA + 5-FU | 21.2348 ± 0.6289 | 0.1045 ± 0.0055 | 0.9981 |
| PLA + CH + 5-FU | 11.7193 ± 2.1062 | 0.1621 ± 0.0332 | 0.9468 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milenkova, S.; Marudova, M. 5-Fluorouracil Encapsulation in PLA Films: The Role of Chitosan Particles in Modulating Drug Release and Film Properties. Processes 2025, 13, 1961. https://doi.org/10.3390/pr13071961
Milenkova S, Marudova M. 5-Fluorouracil Encapsulation in PLA Films: The Role of Chitosan Particles in Modulating Drug Release and Film Properties. Processes. 2025; 13(7):1961. https://doi.org/10.3390/pr13071961
Chicago/Turabian StyleMilenkova, Sofia, and Maria Marudova. 2025. "5-Fluorouracil Encapsulation in PLA Films: The Role of Chitosan Particles in Modulating Drug Release and Film Properties" Processes 13, no. 7: 1961. https://doi.org/10.3390/pr13071961
APA StyleMilenkova, S., & Marudova, M. (2025). 5-Fluorouracil Encapsulation in PLA Films: The Role of Chitosan Particles in Modulating Drug Release and Film Properties. Processes, 13(7), 1961. https://doi.org/10.3390/pr13071961









