1. Introduction
Common flax (
Linum usitatissimum L.), a member of the Linaceae family, is a valuable annual crop of considerable economic importance. It grows primarily in temperate climates and is cultivated across Europe, Asia, and North Africa. Its cultivation history in the Middle East dates back to approximately 9000 BCE, making it one of the oldest domesticated crops in the world [
1,
2,
3]. Flax is mainly grown in two botanical varieties: oilseed flax (
Linum usitatissimum L. var.
brevimulticaulis Vav.), traditionally used in human nutrition, and fiber flax (
Linum usitatissimum L. var.
elongatum Vav.), used primarily in the textile industry [
2,
4]. Owing to its versatility, flax serves as a valuable raw material in the food, chemical, pharmaceutical, paper, and textile industries [
5].
In terms of composition, flaxseeds are a rich source of protein, energy, omega-3 fatty acids (mainly α-linolenic acid), lignans, polyphenols, dietary fiber, and various vitamins and minerals, all of which contribute to their high health-promoting potential. Polyphenols in flaxseed are primarily concentrated in the seed coat, where they occur as esters bound to 3-hydroxy-3-methylglutaric acid (HMG) residues and other compounds. These polyphenols may be released through the hydrolysis of ester bonds, facilitating their absorption and utilization in the human body [
6]. Among the polyphenolic compounds, phenolic acids—particularly caffeic, ferulic, and
p-coumaric acids—are notable for their strong antioxidant properties. The total phenolic acid content in flaxseed ranges from 8 to 10 g/kg dry matter, with approximately 5 g/kg present in esterified forms [
7,
8]. The flavonoid content in flaxseed ranges from 35 to 71 mg per 100 g and may reach 0.3–0.71 g/kg depending on the variety and cultivation conditions [
7,
9].
Flax is also rich in flavonoids, including C- and O-glycoflavones and basic cinnamic acids, which are synthesized during the early stages of plant development [
10]. Moreover, it contains lignins—complex phenolic polymers that serve a key structural role in plants and exhibit antioxidant and anti-inflammatory properties.
Numerous studies confirm the beneficial effects of flaxseed on cardiovascular health [
11,
12,
13,
14], as well as its antidiabetic, hepatoprotective, and anticancer potential [
15,
16,
17]. Some studies also indicate antipyretic and analgesic properties, as well as the ability to reduce inflammatory markers, such as CRP and TNF-α, and to improve kidney function in patients with systemic lupus erythematosus [
6,
18,
19,
20]. The health benefits of flaxseed consumption may be modulated by genetic factors, environmental conditions during cultivation, and processing techniques used in the food industry [
21].
One of the main products derived from oilseed flax processing is linseed oil, used primarily in food production and as a biofuel. In Poland, linseed oil is predominantly obtained via cold pressing—a method that is gaining popularity due to increasing consumer awareness of its superior nutritional value compared to refined oils [
4,
22]. Cold pressing is an oil extraction technique in which the temperature does not exceed 50 °C. This process is usually carried out using continuously operating screw presses and, less commonly, with hydraulic presses. The seed moisture content must be adjusted to suit the equipment used. To improve the extraction efficiency, seeds may be crushed and preheated prior to pressing [
5].
Flaxseed cake, also known as linseed press cake or meal, is a by-product of oil extraction. While traditionally used as a nutritious component in animal feed, its potential in food production is increasingly recognized [
23]. Flaxseed cake is particularly high in dietary fiber, which contributes to cholesterol reduction and limits glucose absorption. Additionally, cold-pressed flaxseed cake is rich in polyphenolic compounds, with reported concentrations ranging from 3.62 to 4.00 mg/g dry matter, which translates into strong free radical scavenging activity [
24]. It may also enhance the sensory qualities of food products [
25]. However, its nutritional composition—including protein, carbohydrate, and fiber content—depends on the flax variety and the processing methods applied.
Cookies are among the fifteen most purchased FMCG (Fast-Moving Consumer Goods) product categories in Poland. In 2015, Polish consumers spent approximately 2.6 billion PLN on nearly 145 million kilograms of cookies. Brand-name products accounted for over 68 million kilograms of this total, with private-label products reaching around 64 million kilograms; the remaining categories represented less than 13.2 million kilograms in total [
26].
Muffins, often compared to cupcakes—an iconic American dessert—originate from a tradition of simple, rustic baking. They are generally easier to prepare and have a more modest appearance than elaborately decorated cupcakes [
27]. The typical muffin preparation process involves mixing dry ingredients (e.g., flour and baking powder) separately from wet ingredients (e.g., eggs and butter), then briefly combining them to produce a slightly lumpy batter consistency [
28].
In recent years, there has been a growing interest in small, convenient “on-the-go” packaging formats that allow for quick consumption during everyday activities such as commuting or work breaks. At the same time, consumers increasingly expect cookies to be not only tasty but also nutritious and beneficial to health, encouraging manufacturers to seek new solutions to enhance their nutritional and functional values [
26].
One promising approach involves the use of natural technological ingredients that improve the sensory and nutritional quality of baked goods. The enrichment of cookies with plant-based raw materials, including flaxseed flour, has shown potential for increasing the levels of bioactive compounds and improving nutritional profiles. This is supported by several studies [
29,
30,
31,
32].
Kaur et al. [
30] demonstrated that the addition of flaxseed flour to cookies significantly alters their chemical composition, increases total polyphenol content (TPC), enhances antioxidant activity, and positively affects texture. Similarly, Karakoç et al. [
31] examined the substitution of wheat flour with oilseeds (pistachios, sesame, and flaxseed) in cookie recipes and found that both the type and quantity of added seeds significantly influenced the technological and sensory properties of the final products. Man et al. [
32], focusing on roasted flaxseed flour (RFSF), found that cookies enriched with RFSF exhibited improved nutritional and functional qualities. Both TPC and antioxidant activity (DPPH) increased proportionally with the amount of RFSF used. These findings align with the current market trends in which consumers seek convenient, tasty, and health-promoting food options.
Utilizing oil-pressing by-products not only reduces waste but also supports the development of functional foods. Research findings suggest that combining wheat flour with flax-based ingredients creates opportunities for developing nutritionally valuable bakery products. Such enriched cookies may offer an attractive alternative for health-conscious consumers. It is also important to consider the potential limitations resulting from the seasonality and variability of flax crop yields. In addition, fluctuations in the economic profitability of flax cultivation—especially in comparison to other oilseed crops such as rapeseed—may influence its availability. Nevertheless, considering that the current use of flaxseed cake in human nutrition is minimal, the risk of raw material shortage appears to be low. Its broader application could not only enhance the nutritional value of bakery products but also contribute to the effective utilization of oil extraction by-products.
It is also important to mention one of the potential limitations of using flaxseed cake in food applications, related to the presence of natural precursors that are converted to cyanide, known as cyanogenic glycosides (CGs) [
33,
34]. Although the oil-pressing process significantly reduces the enzymatic activity responsible for hydrolyzing CGs into hydrogen cyanide (HCN), residual amounts of these compounds may still be present in the cake. In a study by Yamashita et al. (2006), an effective method was proposed to remove these compounds through enzymatic hydrolysis using freshly ground flaxseed as a source of active β-glucosidases, followed by steam treatment to evaporate the released HCN [
33]. This approach allowed for the reduction of hydrogen cyanide content to below detectable levels without compromising essential nutritional components, such as protein, fat, dietary fiber, and lignans.
The aim of this study was to investigate the impact of incorporating flaxseed cake into wheat muffins on the levels of selected nutritional (ash, protein, fat, carbohydrates, dietary fiber) and bioactive compounds, specifically total polyphenols, phenolic acids, flavonols, and flavonoids, in the final products. Additionally, the antioxidant potential of the products was assessed using two free radical scavenging assays (DPPH and ABTS). The study also examined how different levels of flaxseed cake affected selected quality parameters of the final muffins, including color, texture, and volume.
3. Results and Discussion
3.1. Characteristics of Flax Cake
It is widely known that flax has long been regarded as a functional raw material with health-promoting properties, commonly used in food products. It is characterized by a high content of bioactive compounds, particularly polyphenols—mainly flavonoids such as C- and O-glycosylated flavones, which are the predominant flavonoids present in flaxseeds. Among the most important phenolic acids are
trans-ferulic acid and
trans-cinnamic acid, while compounds occurring in smaller amounts include caffeic,
p-coumaric, chlorogenic, gallic, sinapic, protocatechuic, and
p-hydroxybenzoic acids. Flax is also a rich source of lignans, with the primary compound being secoisolariciresinol diglucoside (SDG) [
9,
48].
Polyphenols, including flavonoids and phenolic acids such as caffeic, ferulic, and
p-coumaric acid, exhibit strong antioxidant, anti-inflammatory, and anticancer properties. These compounds protect cells against oxidative stress and reduce the risk of chronic diseases [
6,
49]. Phenolic acids are known to support cardiovascular health and aid in cancer prevention, while flavonoids also display antiviral and antiallergic effects [
8,
50].
Considering that flax exhibits numerous health-promoting properties and that the oil extracted from it is also known for its beneficial effects, it is reasonable to assume that the residual flaxseed cake obtained after oil-pressing may likewise have a positive impact on human health [
51,
52,
53,
54]. Therefore, the content of bioactive compounds from the polyphenol group was determined, and the antioxidant potential of the flaxseed cake was assessed.
It was found that the polyphenol content in flaxseed cake ranged from 6.63 to 14.76 mg catechin/g dry matter (DM). The higher value—14.76 mg catechin/g DM—was determined using the Folin–Ciocalteu reagent method (
Table 2), which is known to react not only with polyphenols but also with other food components, such as glycoalkaloids, vitamin C, and amino acids [
43,
44]. For this reason, alternative methods for polyphenol determination are often applied, such as the method by Mazza et al. [
41] with modifications by Oomah et al. [
42]. Using this approach, the polyphenol content in the tested flaxseed cake was approximately 6.63 mg catechin/g DM (
Table 2). It was confirmed that the polyphenol content measured without the use of the Folin–Ciocalteu reagent was lower than that determined using it. A high level of flavonoids and a concentration of phenolic acids at the level of 1.70 mg ferulic acid/g DM (
Table 2) were detected in the flaxseed cake. In the study by Čukelj et al. [
55], ferulic acid was identified as the dominant phenolic acid in flaxseed (81.4% of total phenolic acids), followed by p-coumaric acid (11.8%) and caffeic acid (6.8%). Among all the polyphenol groups, flavonols were present in the smallest amounts in the flaxseed cake (
Table 2). A sixfold lower content of flavonols compared to phenolic acids was recorded in flaxseed cake (
Table 2). Due to the high concentration of polyphenols, particularly flavonoids, the flaxseed cake exhibited strong radical scavenging activity, as confirmed by both ABTS and DPPH assays. A strong positive correlation was observed between the antioxidant activity and polyphenol content, confirming the relationship between the presence of these compounds and their free radical neutralizing capacity.
Flaxseed is widely recognized as a raw material of high nutritional and functional value. It is a rich source of protein, polyunsaturated fatty acids, lignans, dietary fiber, and various bioactive compounds with antioxidant, anti-inflammatory, and hypocholesterolemic properties. The nutritional composition of flaxseed cake, expressed per 100 g of dry matter (DM), confirmed that it is a valuable source of protein (32.64 g/100 g DM), carbohydrates (24.25 g/100 g DM), and dietary fiber (28.53 g/100 g DM), as well as a moderate source of fat (9.88 g/100 g DM) and minerals (4.70 g/100 g DM) (
Table 3). The total energy value was estimated at approximately 334.9 ± 8.6 kcal/100 g DM, indicating that flaxseed cake can also serve as a valuable energy source in food formulations.
Dietary fiber in flaxseed consists of a mixture of insoluble and soluble fractions, each exhibiting distinct physiological effects. The insoluble fraction supports gastrointestinal health by stimulating intestinal peristalsis and preventing constipation and diverticulosis. The soluble fraction, on the other hand, has been associated with lowering cholesterol levels, reducing postprandial glycemia, and offering potential anticancer benefits.
Due to its high content of protein and dietary fiber—exceeding the levels found in many other plant-based raw materials—flaxseed can be considered a valuable functional ingredient in food technology. The particularly high amount of insoluble fiber (19.74 ± 0.32 g/100 g DM) (
Table 3) makes it an attractive additive in products designed to support digestive health, as well as in the development of functional foods, including gluten-free formulations.
3.2. The Effect of Flaxseed Cake Addition Levels on the Polyphenol Content and Antioxidant Potential of Wheat Muffins
The proven high health-promoting potential of flaxseed cake, estimated based on its polyphenol content and insoluble dietary fiber and protein (
Table 2 and
Table 3), highlights the possibility of using this by-product as an enriching ingredient in various food products, such as wheat muffins.
The polyphenol content in muffins enriched with flaxseed cake was determined using two independent methods: one of them with the Folin–Ciocalteu reagent [
40] and the other without the above-mentioned reagent, following the method by Mazza et al. [
41] with modifications by Oomah et al. [
42].
It was found that muffins containing 10% to 40% flaxseed cake had a higher polyphenol content (measured without the Folin–Ciocalteu reagent) compared to the control sample (
Table 4). Even the smallest addition of 10% flaxseed cake into muffins led to a 45% increase in polyphenol content compared to the control. At 20% addition into muffins, the increase reached 160%, while 30% and 40% additions resulted in increases of 193% and 260%, respectively, in comparison to the control (
Table 4).
Similarly, the polyphenol content determined using the Folin–Ciocalteu reagent also increased proportionally with the amount of flaxseed cake added to the muffins. In samples containing 10% to 40% flaxseed cake, the polyphenol content increased from 2-fold up to 9.5-fold relative to the control (
Table 4). This significant increase highlights the strong influence of flaxseed cake on the enrichment of bioactive compounds in the final products.
Kaur et al. [
30] conducted a study on the effect of flaxseed flour addition on cookie properties and reported significant changes in polyphenol content. The use of flaxseed flour at levels ranging from 10% to 30% contributed to up to a 3-fold increase in total polyphenol content (TPC) in cookies. Similar results were obtained by Hao and Beta [
56], who studied Chinese steamed bread (CSB) enriched with barley, flaxseed, and extracts from barley and flaxseed hulls. They observed an increase in antioxidant activity and total polyphenol content in enriched samples compared to the control.
In a related study, Man et al. [
32] analyzed the effect of flaxseed flour addition to biscuits and found that a 40% addition led to a 25% increase in polyphenol content relative to the control. Even the lowest 10% addition resulted in an 11% increase [
32].
In the study by Plustea et al. [
57] on the enrichment of cookies with lupin, an increase in polyphenol content was observed in cookies containing lupin sprouts, ranging from a 4-fold to 9.5-fold increase compared to the control samples.
Natukunda et al. [
58] investigated the effect of tamarind seed powder on the bioactive properties of cookies. They demonstrated that cookies enriched with 10% tamarind seed powder contained 42% more polyphenols than the control [
58].
Ertaş and Asian [
59] analyzed the impact of raw and roasted hemp flour on the total polyphenol content in cookies. Their findings indicated that the polyphenol content increased in correlation with the proportion of both types of hemp flour. The most pronounced increase—up to 4 times higher than the control—was recorded in samples containing 20% roasted hemp flour.
In the study by Roppolo et al., the fortification of cookies with blackberry powder obtained through ultrasound-assisted hot-air drying (US-HAD) was investigated. The authors reported a significant increase in total phenolic content (TPC), reaching 13.88 mg GAE/g in cookies enriched with US-HAD powder [
60].
In the case of flavonoids, it was observed that the highest addition of flaxseed cake (40%) to wheat muffins resulted in more than a threefold increase in their content compared to the control muffins (
Table 5). The incorporation of 20% and 30% flaxseed cake led to increases in flavonoid content of approximately 120% and 130%, respectively, relative to the control (
Table 5). However, the increase in flavonoid content at these two levels was not statistically different (
p < 0.05), indicating that the effect was similar regardless of whether 20% or 30% of flaxseed cake was added (
Table 5). The smallest increase in flavonoid content—approximately 40%—was recorded in the sample containing 10% flaxseed cake compared to the control (
Table 5).
Natukunda et al. [
58] evaluated the effect of tamarind seed powder on the bioactive properties of cookies. Cookies enriched with 10% tamarind seed powder showed a 2.5-fold increase in flavonoid content compared to the control.
Regarding phenolic acid content in the analyzed muffin samples, it was found that the amount of phenolic acids increased progressively with higher levels of flaxseed cake. In samples containing 10% and 20% flaxseed cake, the phenolic acid content increased approximately sixfold, while in samples with 40% addition, the increase reached up to 40 times that of the control sample. These findings suggest that higher levels of flaxseed cake in muffins contribute significantly to the enhancement of phenolic acid content, which may positively influence the health-promoting properties of the final products.
In the case of flavonols, their levels in the analyzed products were minimal, which can be attributed to the inherently low flavonol content in the flaxseed cake itself. The addition of flaxseed cake in amounts ranging from 10% to 30% did not cause any significant changes in the flavonol content compared to the control sample. Only the 40% addition resulted in a slight, yet fivefold, increase in these bioactive compounds in the enriched muffins relative to the control.
In summary, it should be noted that the baking process (at temperatures around 180–200 °C) may lead to significant losses—up to 60%—of certain phenolic compounds [
61]. These losses can be caused by various factors, including thermal, enzymatic, and oxidative degradation, as well as isomerization/epimerization processes, particularly of catechins [
62], and decarboxylation of phenolic acids [
61,
63,
64].
Furthermore, a reduction in the phenolic content may also result from the formation of complexes with polysaccharides present in wheat flour used for muffin preparation [
62,
65]. Similarly, in a study by Xu and Chang [
66], baking at high temperatures (180 °C) reduced the total flavonoid content due to the breakdown of thermolabile flavonoid compounds and the degradation of complex polyphenols into simpler phenolic and non-phenolic compounds.
Despite the thermal degradation of polyphenol fractions during baking, the inclusion of flaxseed cake—an abundant source of bioactive compounds—ensures a considerable phenolic content in the final muffin products.
The antioxidant activity of the muffins was assessed using ABTS•+ and DPPH• radical assays, with results expressed in millimoles of Trolox equivalents per kilogram of dry matter (mM Trolox/kg DM).
In the ABTS assay, the control sample (without flaxseed cake) exhibited moderate antioxidant activity (14.63 mM Trolox/kg DM), which may be attributed to the presence of natural bioactive compounds in the flour and oil used in the formulation. The addition of just 10% flaxseed cake increased this value to 21.83 mM Trolox/kg DM, and further increments in flaxseed cake content resulted in a continued rise in the antioxidant capacity. The highest ABTS-based activity was recorded at 40% addition, reaching 45.00 mM Trolox/kg DM—more than three times the value of the control (
Figure 3).
In contrast, the DPPH method showed no measurable antioxidant activity in the control sample or in the sample with 10% flaxseed cake. A noticeable increase was first observed at the 20% addition level (9.07 mM Trolox/kg DM), followed by further increases at 30% (13.43 mM Trolox/kg DM) and 40% (18.02 mM Trolox/kg DM). This trend suggests a higher effectiveness in neutralizing DPPH radicals in muffins with greater amounts of flaxseed cake (
Figure 3).
It is important to emphasize that the enhanced antioxidant activity observed in the muffins is not solely attributed to the natural compounds present in flaxseed cake, such as lignans (e.g., secoisolariciresinol diglucoside-SDG), tocopherols, and phytosterols, but also to Maillard reaction products formed during the baking process, as well as the thermally transformed phenolic compounds with increased antioxidant potential [
62,
67,
68,
69].
Kaur et al. [
30] analyzed the effect of flaxseed flour addition on the antioxidant properties of cookies and reported a significant increase in antioxidant activity. Similarly, Man et al. [
32] demonstrated that the incorporation of roasted flaxseed flour (RFSF) into biscuits led to a progressive enhancement in DPPH-based antioxidant activity, proportional to the level of RFSF used in the formulation. The highest activity was recorded in sample B40, reaching 32.03% RSA, while the lowest value (13.67% RSA) was observed in the control sample. The antioxidant activity of the biscuits with 40% RFSF was approximately 2.5 times higher than that of the control. This increase can be explained by the fact that flaxseed is a powerful antioxidant source, particularly rich in lignans such as secoisolariciresinol diglucoside (SDG), which are also found in flaxseed oil [
32].
In the study by Natukunda et al. [
58], a 10% addition of tamarind seed powder (TSP) to cookies resulted in a fivefold increase in antioxidant activity. Similarly, Ertaş and Asian [
59] evaluated the antioxidant potential of cookies enriched with raw and roasted hemp flour. Their findings showed that both types of flour significantly enhanced the antioxidant activity compared to the control. The highest activity (40.88%) was observed in cookies with a higher proportion of roasted hemp flour, while cookies with raw hemp flour achieved a slightly lower value (38.21%).
In the study by Roppolo et al., where cookies with 10% blackberry powder obtained through ultrasound-assisted hot-air drying were analyzed, the antioxidant activity reached approximately 80–85 mmol TEAC/kg (ABTS) and 88–92 mmol TEAC/kg (DPPH). These values were higher than those obtained in our study at the same level of enrichment [
60].
3.3. The Effect of Flaxseed Cake Addition Level on the Nutritional Composition and Dietary Fiber Content of Wheat Muffins
The inclusion of flaxseed cake in the formulation had a clear impact on the nutritional composition of the analyzed muffin samples, resulting in significant changes across all evaluated nutrients. With increasing levels of flaxseed cake, a progressive rise in protein content was observed. In samples with the highest addition (M40L), the protein content was over 46% higher compared to the control sample (MK), indicating the high nutritional value of flaxseed cake as a source of plant-based protein (
Table 6).
An opposite trend was noted for available carbohydrates—their content systematically decreased as the flaxseed cake proportion increased. The carbohydrate level dropped from 70.43 g (MK) to 54.74 g (M40L), representing a reduction of approximately 22% (
Table 6). This decrease can be attributed to the “dilution” of starch by fiber- and protein-rich compounds present in the flaxseed cake.
A notable increase in fat content was also observed—flaxseed cake addition resulted in nearly a 31% rise in fat content (M40L vs. MK), which is explained by the residual oil retained in the flaxseed cake after cold pressing (
Table 6). This phenomenon was directly correlated with the increase in energy value of the flaxseed-enriched muffins (r = 0.96;
p = 0.009). This is understandable, considering that fat provides more than twice as much energy per gram as protein or carbohydrates.
The ash content (indicating the presence of mineral compounds) also increased with the addition of flaxseed cake, reaching its highest level in the M40L sample (3.6 g/100 g DM) (
Table 6). This is particularly important from the perspective of enriching the product with essential micronutrients.
As a result of the described changes, the energy value of the product increased moderately—total caloric content in the M40L sample was approximately 2% higher than in the control (MK) (
Table 6). Although this increase may seem minor, it is noteworthy that it was achieved alongside a significant improvement in the product’s nutritional profile.
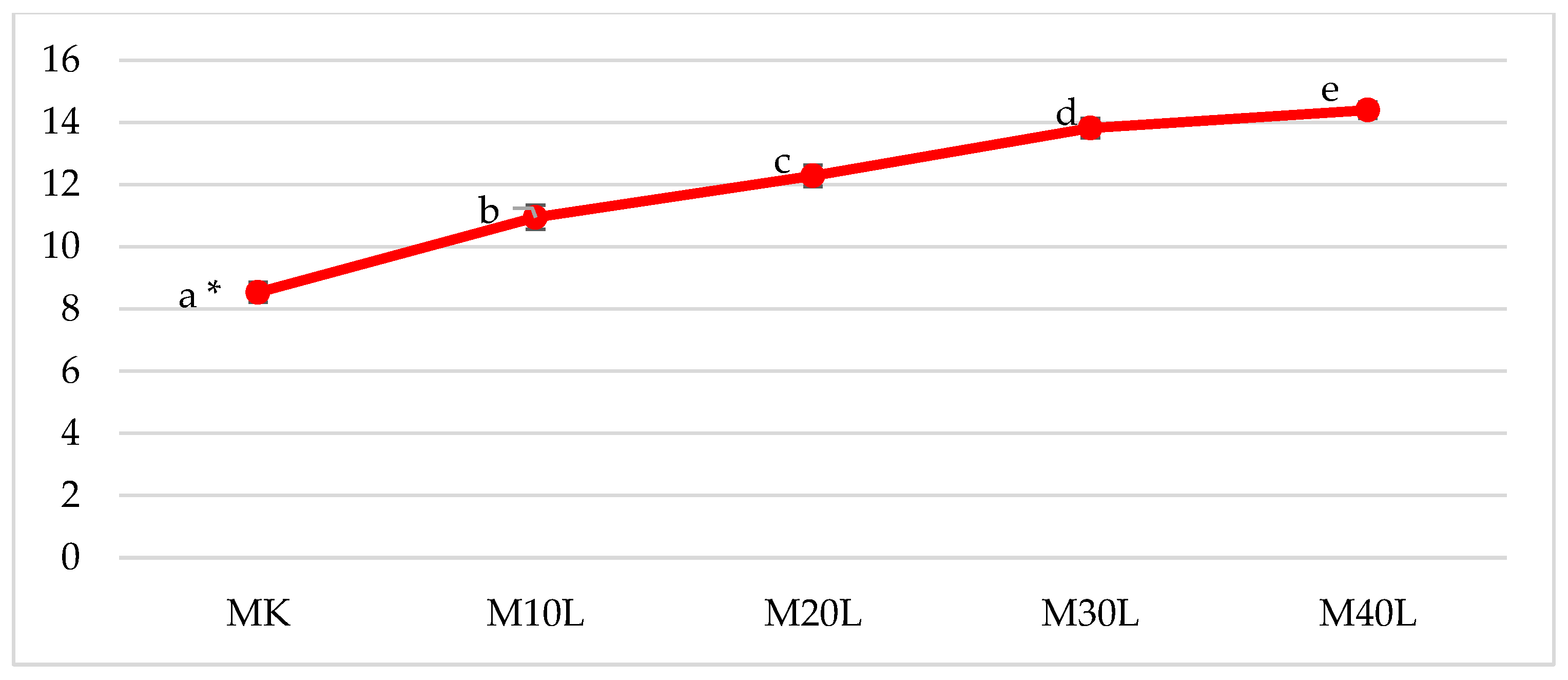
As the proportion of flaxseed cake in the muffin formulations increased, the theoretical satiety index (SI) of the final products also rose (
Figure 4). This index was calculated based on the protein and total dietary fiber content—two of the most satiating food components. The increase in SI was directly related to the higher levels of these components, which formed the basis of the calculation. Muffins enriched with 30% and 40% flaxseed cake exhibited 59% and 69% higher satiety indices, respectively, compared to the control sample. These findings are consistent with the observations of Holt et al. [
36], who also noted that increasing the protein and fiber content in food products leads to a greater sense of satiety. Therefore, the muffins obtained in this experiment can be considered to have the potential to prolong the feeling of fullness when compared to traditional muffins.
Similar results in nutritional composition were reported by Čukelj et al. [
55], where biscuits enriched with flaxseed (e.g., W–F—wholemeal wheat with flaxseed, R–W–F—rye and wholemeal wheat with flaxseed, O–W–F—oat and wholemeal wheat with flaxseed, etc.) had higher fat and protein contents than control samples (WW—white wheat, W—wholemeal wheat), while showing a simultaneous reduction in carbohydrate content. Notably, despite the increase in fat, the overall energy value of these products rose only slightly—by an average of 1.5–3%—similar to the muffins described in the present study.
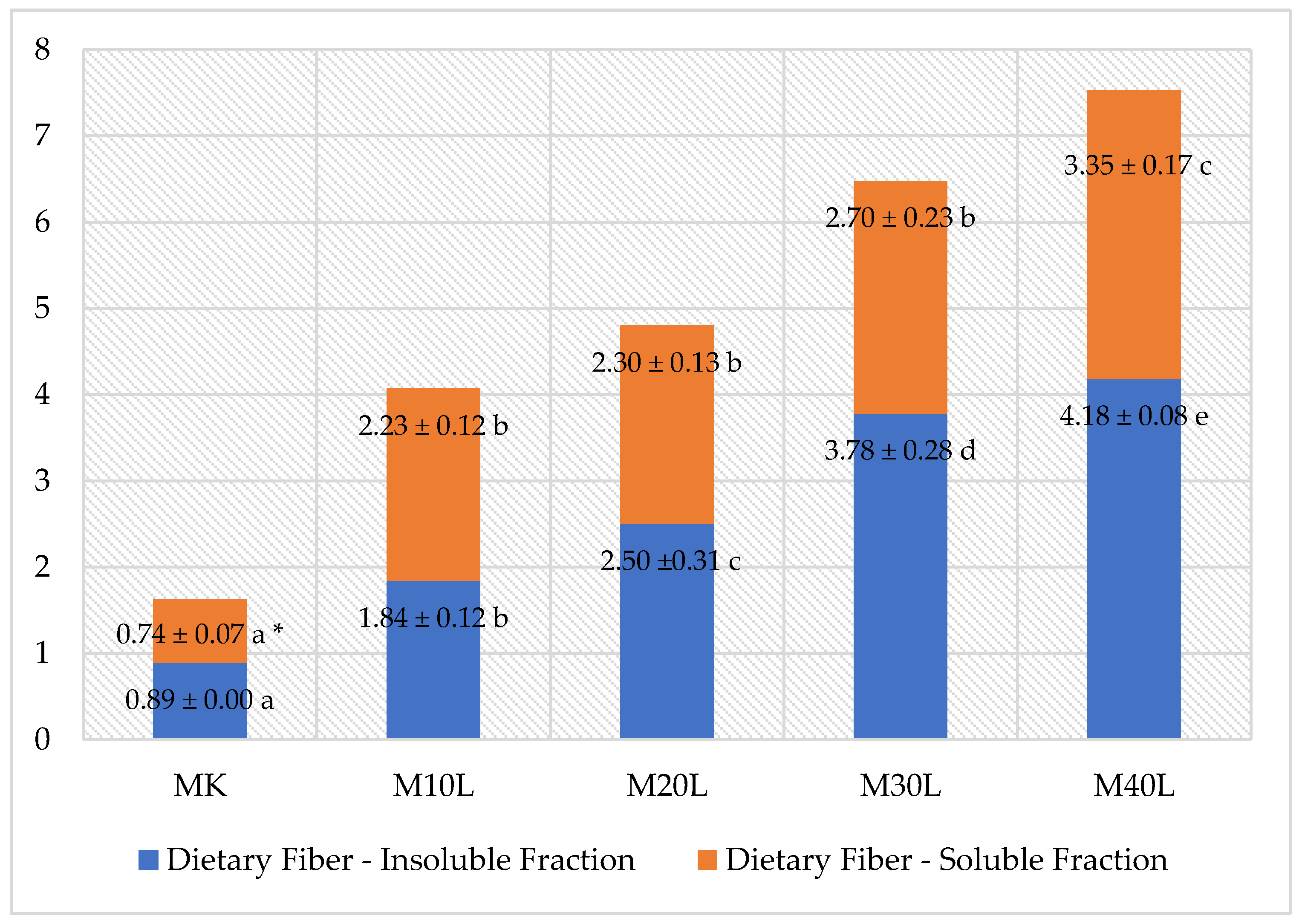
The enrichment of muffins with flaxseed cake significantly increased the total dietary fiber (TDF) content in the tested samples (
p < 0.05). TDF content rose nearly fourfold (by 361%)—from 1.63% in the control sample (MK) to 7.52% in the sample with the highest flaxseed cake addition (M40L) (
Figure 5). Insoluble dietary fiber (IDF) was the dominant fraction in all analyzed muffins, increasing proportionally with the flaxseed cake level and reaching its maximum at the highest addition (
Figure 5). This nearly fourfold increase in IDF is of particular importance for gastrointestinal health, as insoluble fiber promotes proper intestinal peristalsis and helps prevent constipation.
Although soluble dietary fiber (SDF) also showed an increasing trend with higher flaxseed cake content, significant changes were not observed at lower addition levels (approximately 2.2–2.7% for M10L–M30L). Only at the highest inclusion level (M40L) was a more than 3.5-fold increase in SDF observed compared to the control (MK −0.74%) (
Figure 5). At lower addition levels, the quantity of flaxseed cake was likely insufficient to significantly increase the proportion of SDF in the overall muffin mass.
The increase in soluble fiber may be functionally relevant, as it supports glycemic regulation and helps reduce LDL cholesterol levels—thus potentially enhancing the health-promoting properties of the muffins. A strong positive correlation was observed between the flaxseed cake addition level and TDF (r = 0.99; p < 0.001), IDF (r = 0.99; p < 0.001), and SDF (r = 0.97; p = 0.006), confirming that the amount of flaxseed cake used was the primary factor determining the fiber content in the final product.
These findings are consistent with the literature data, including studies by Ganorkar & Jain [
52] and Kajla et al. [
37], who also demonstrated that the addition of flax-derived raw materials—both seeds and cake—significantly increases dietary fiber content in baked goods.
3.4. Color of Wheat Muffins Enriched with Flaxseed Cake
Color is one of the key factors influencing consumer acceptance—it attracts attention and shapes the initial perception of product quality. In this study, the color parameters of muffins (L*, a*, b*) were analyzed, along with the total color difference ΔE00 compared to the control sample (MK).
The control sample (MK) was characterized by the highest lightness (L* = 79.48), intense yellow coloration (b* = 35.36), and the lowest a* value (6.62), indicating a typical bright muffin appearance. As the level of flaxseed cake increased (M10L–M40L), the L* value systematically and significantly decreased (
p < 0.05), reaching its lowest point in M40L (L* = 54.49) (
Table 7). Simultaneously, a reduction in b* values was observed, corresponding to a decline in yellow intensity, resulting in a more muted and less saturated color.
The a* value (redness) increased in M10L and M20L samples and then stabilized, indicating a moderate influence of flaxseed cake addition on this color component (
Table 7). To better visualize the extent of color changes, the total color difference ΔE
00 was calculated, accounting for human visual perception and reflecting perceptible color variations between the enriched muffins and the control. The ΔE
00 values exceeded the threshold of 5, which is considered the minimum level of color difference perceivable by the naked eye. This confirms that the incorporation of flaxseed cake significantly altered the appearance of the baked products. The greater the addition, the more noticeable the darkening and the decrease in yellow color saturation.
These results are consistent with the findings of Karakoç et al. [
31], Rrustemi et al. [
70], and Ertaş & Asian [
59], where the inclusion of plant-based ingredients led to reductions in L* and b* values and increased ΔE in baked products. This suggests that ingredients rich in phenolic compounds, natural pigments, and oils can significantly influence the color properties of bakery goods.
Similar trends were reported by Karakoç et al. [
31], who replaced wheat flour with various oilseeds and observed decreases in L* and b* values in cookies enriched with flaxseed, which the authors attributed to the presence of natural pigments and fats affecting final product coloration. Rrustemi et al. [
70] found that the replacement of wheat flour with defatted pumpkin and flaxseed cake produced a comparable effect—the muffins became darker and less vivid as the level of substitution increased. Likewise, Ertaş & Asian [
59], studying the addition of raw and roasted hemp flour, reported decreased L* and b* values and increased a* values, which they attributed to the growing presence of phenolic substances and pigments introduced via plant-based ingredients.
The similarities between these findings and those of the present study confirm that plant-based additives have a predictable impact on the color parameters of baked products, regardless of their botanical origin.
3.5. Volume of Wheat Muffins with Flaxseed Cake Addition
The fiber contained in flaxseed cake has the ability to absorb water, which affects the structure of the batter by limiting its ability to aerate and form pores. As a result, at higher proportions of flaxseed cake (e.g., 30% or 40%), the batter becomes denser, which leads to a decrease in muffin volume. Muffins with 10% flaxseed cake had the highest volume—69.85 mL—and the best volume index—139.71 mL/100 g flour, indicating proper texture. Increasing the addition level to 20% resulted in a slight reduction in volume to 65.79 mL and a volume index of 131.57 mL/100 g. In contrast, the addition of 40% flaxseed cake caused a significant decrease in both volume (59.00 mL) and volume index (118.00 mL/100 g), leading to a denser and less porous muffin structure (
Table 8). Therefore, increasing fiber content in the muffins contributed to a reduction in their volume, with muffins containing 10% flaxseed cake being the lightest and airiest, while those with 40% were the most compact.
3.6. Texture of Wheat Muffins with Flaxseed Cake Addition
The addition of 10% flaxseed cake to the muffins did not result in a significant increase in hardness compared to the control sample (
Table 9). Although the value was slightly higher than that of the control, the difference was minimal, likely due to the relatively low fiber content, which does not yet significantly affect the batter structure. Increasing the flaxseed cake content to 20–40% led to a noticeable reduction in hardness, from 7.97 N to 7.30 N (
Table 9). These results suggest that as the proportion of flaxseed cake in the muffin formulation increases, the product becomes softer in texture. This softening effect is likely associated with the presence of soluble dietary fiber, which has a high water-binding capacity, thereby increasing the moisture content of the crumb and improving its softness.
4. Conclusions
Flaxseed cake demonstrates both high nutritional value and serves as a source of numerous health-promoting compounds, including polyphenols, flavonoids, and phenolic acids. This composition contributes to its very high antioxidant activity, as confirmed by both ABTS and DPPH assays.
It was found that the incorporation of flaxseed cake into wheat muffins led to a significant increase in polyphenol and flavonoid content (up to threefold), as well as phenolic acids (up to 40-fold), indicating the potential for using flaxseed cake to enrich food products with bioactive compounds and enhance the antioxidant potential of the final product.
In addition, the enrichment of muffins with flaxseed cake positively influenced their nutritional profile—particularly visible in the increased levels of protein, fat, and dietary fiber, accompanied by a reduction in available carbohydrates. The nearly fourfold increase in total dietary fiber (TDF), with insoluble dietary fiber (IDF) as the dominant fraction, suggests that these products may offer not only greater satiety but also health benefits for the digestive system.
An increasing proportion of flaxseed cake in the muffins also resulted in noticeable changes in product appearance (darker color), a reduction in volume, and decreased hardness—indicating a softer texture.
The results indicate that a moderate addition of flaxseed cake (10–20%) is optimal for balancing the enhanced bioactive compound content with desirable quality attributes, making it a promising strategy for the development of health-oriented bakery products that are also appealing to consumers.
Furthermore, it is recommended that future research explore the economic aspects of flaxseed by-product utilization. Cost–benefit and techno-economic analyses would be essential to determine the feasibility and scalability of incorporating flaxseed cake into food production on an industrial level. Such evaluations could support broader implementation and commercialization of this functional ingredient.