N-Nitrosamines in Meat Products: Formation, Detection and Regulatory Challenges
Abstract
1. Introduction
2. Formation and Occurrence of NAs in Meat Products
3. Sources of Nitrites and Amines in Meat
4. Analytical Methods of Determination
5. Health Risks Associated with NAs
6. Legislation
7. Strategies to Reduce NA Formation
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| APCI | atmospheric pressure chemical ionization |
| CI-MS | chemical ionisation-mass spectrometry |
| CO2 | carbon dioxide |
| DMA | N-Methylmethanamine |
| EFSA | European Food Safety Authority |
| ESI | electrospray ionization |
| EU | European Union |
| FID | flame ionization detector |
| GC | gas chromatography |
| GCxGC | two-dimensional gas chromatography |
| GLC | gas-liquid chromatography |
| HPLC | high-performance liquid chromatography |
| HS-SPME | Headspace solid-phase microextraction |
| LC | liquid chromatography |
| LOD | limit of detection |
| LOQ | limit of quantification |
| ML | maximum level |
| MS | mass spectrometry |
| MS/MS | tandem mass spectrometry |
| N2O3 | nitrous anhydride |
| NaNO2 | sodium nitrite |
| NAs | N-nitrosamines |
| NCD | nitrogen-phosphorus detector |
| NDBA | N-nitrosodibutylamine |
| NDEA | N-nitrosodiethylamine |
| NDMA | N-nitrosodimethylamine |
| NDPA | N-nitrosodipropylamine |
| NDPhA | N-nitrosodiphenylamine |
| NMA | N-nitrosomethylaniline |
| NMEA | N-nitrosomethylethylamine |
| NMOR | N-nitrosomorpholine |
| NMTCA | N-nitroso-2-methylthiazolidine-4-carboxylic acid |
| NPIP | N-nitrosopiperidine |
| NPRO | N-nitrosoproline |
| NPYR | N-nitrosopyrrolidine |
| NSAR | N-nitrososarcosine |
| NVNA | non-volatile N-nitrosamine |
| UV-DAD | ultraviolet-diode array detector |
| VNA | volatile N-nitrosamine |
| WHO | World Health Organization |
References
- Wood, J.D. Meat Composition and Nutritional Value. Lawrie’s Meat Science, 8th ed.; Woodhead Publishing: New Delhi, India, 2017; pp. 635–659. [Google Scholar] [CrossRef]
- Ačkar, Đ.; Rot, T. Antimikrobno Djelovanje Aditiva u Mesnoj Industriji. MESO Prvi Hrvat. Časopis O Mesu 2019, 21, 26–30. [Google Scholar]
- Katalenić, M. Aditivi i Hrana. Medicus 2008, 17, 57–64. [Google Scholar]
- Lu, J.; Li, M.; Huang, Y.; Xie, J.; Shen, M.; Xie, M. A Comprehensive Review of Advanced Glycosylation End Products and N-Nitrosamines in Thermally Processed Meat Products. Food Control 2022, 131, 108449. [Google Scholar] [CrossRef]
- Moradi, S.; Shariatifar, N.; Akbari-adergani, B.; Molaee Aghaee, E.; Arbameri, M. Analysis and Health Risk Assessment of Nitrosamines in Meat Products Collected from Markets, Iran: With the Approach of Chemometric. J. Environ. Health Sci. Eng. 2021, 19, 1361–1371. [Google Scholar] [CrossRef]
- Mastanjević, K.; Kovačević, D.; Doničić, M.; Habschied, K. Levels of nitrite and nitrate content in traditional dry sausage “homemade kulen”. Meso Prvi Hrvatski Časopis o Mesu 2024, 5, 419–427. [Google Scholar] [CrossRef]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K.; Stewart, B.W.; et al. Carcinogenicity of Consumption of Red and Processed Meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- Li, L.; Wang, P.; Xu, X.; Zhou, G. Influence of Various Cooking Methods on the Concentrations of Volatile N-Nitrosamines and Biogenic Amines in Dry-Cured Sausages. J. Food Sci. 2012, 77, C560–C565. [Google Scholar] [CrossRef]
- Majou, D.; Christieans, S. Mechanisms of the Bactericidal Effects of Nitrate and Nitrite in Cured Meats. Meat Sci. 2018, 145, 273–284. [Google Scholar] [CrossRef]
- Deveci, G.; Tek, N.A. N-Nitrosamines: A Potential Hazard in Processed Meat Products. J. Sci. Food Agric. 2024, 104, 2551–2560. [Google Scholar] [CrossRef]
- Niklas, A.A.; Herrmann, S.S.; Pedersen, M.; Jakobsen, M.; Duedahl-Olesen, L. The Occurrence of Volatile and Non-Volatile N-nitrosamines in Cured Meat Products from the Danish Market. Food Chem. 2022, 378, 132046. [Google Scholar] [CrossRef]
- Park, J.E.; Seo, J.E.; Lee, J.Y.; Kwon, H. Distribution of Seven N-Nitrosamines in Food. Toxicol. Res. 2015, 31, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Özbay, S.; Şireli, U.T. The Effect of Ascorbic Acid, Storage Period and Packaging Material on the Formation of Volatile N-Nitrosamine in Sausages. J. Food Sci. Technol. 2022, 59, 1823–1830. [Google Scholar] [CrossRef]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.C.; Nebbia, C.S.; Nielsen, E.; et al. Risk Assessment of N-Nitrosamines in Food. EFSA J. 2023, 21, e07884. [Google Scholar] [CrossRef]
- De Mey, E.; De Maere, H.; Paelinck, H.; Fraeye, I. Volatile N-Nitrosamines in Meat Products: Potential Precursors, Influence of Processing, and Mitigation Strategies. Crit. Rev. Food Sci. Nutr. 2017, 57, 2909–2923. [Google Scholar] [CrossRef]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambré, C.; et al. Re-Evaluation of Sodium Nitrate (E 251) and Potassium Nitrate (E 252) as Food Additives. EFSA J. 2017, 15, e04787. [Google Scholar] [CrossRef]
- Sorour, M.A.; Mehanni, A.-H.E.; Mahmoud, E.-S.A.; El-Hawashy, R.M. Nitrate, Nitrite and N-Nitrosamine in Meat Products. J. Sohag Agriscience (JSAS) 2023, 2023, 121–135. [Google Scholar] [CrossRef]
- Pegg, R.B.; Honikel, K.O. Handbook of Fermented Meat and Poultry; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Shen, Q.; Zeng, X.; Kong, L.; Sun, X.; Shi, J.; Wu, Z.; Guo, Y.; Pan, D. Research Progress of Nitrite Metabolism in Fermented Meat Products. Foods 2023, 12, 1485. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, P.; Patarata, L.; Lorenzo, J.M.; Fraqueza, M.J. Nitrate Is Nitrate: The Status Quo of Using Nitrate through Vegetable Extracts in Meat Products. Foods 2021, 10, 3019. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Geng, Y.; Yao, J.; Ji, J.; Chen, F.; Xiao, J.; Hu, X.; Ma, L. N-Nitrosamines in Processed Meats: Exposure, Formation and Mitigation Strategies. J. Agric. Food Res. 2023, 13, 100645. [Google Scholar] [CrossRef]
- Shakil, M.H.; Trisha, A.T.; Rahman, M.; Talukdar, S.; Kobun, R.; Huda, N.; Zzaman, W. Nitrites in Cured Meats, Health Risk Issues, Alternatives to Nitrites: A Review. Foods 2022, 11, 3355. [Google Scholar] [CrossRef]
- Yurchenko, S.; Mölder, U. The Occurrence of Volatile N-Nitrosamines in Estonian Meat Products. Food Chem. 2007, 100, 1713–1721. [Google Scholar] [CrossRef]
- Gilani, P.S.; Fesahat, M.; Shariatifar, N. Nitrosamine in Meat and Meat Products: A Review. J. Food Saf. Hyg. 2023, 9, 217–226. [Google Scholar] [CrossRef]
- Honikel, K.O. The Use and Control of Nitrate and Nitrite for the Processing of Meat Products. Meat Sci. 2008, 78, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, G.I.; Bashammakh, A.S.; Alsibaai, A.A.; Alwael, H.; El-Shahawi, M.S. A Critical Overview on the Chemistry, Clean-up and Recent Advances in Analysis of Biogenic Amines in Foodstuffs. TrAC-Trends Anal. Chem. 2016, 78, 84–94. [Google Scholar] [CrossRef]
- Herrmann, S.S.; Granby, K.; Duedahl-Olesen, L. Formation and Mitigation of N-Nitrosamines in Nitrite Preserved Cooked Sausages. Food Chem. 2015, 174, 516–526. [Google Scholar] [CrossRef]
- Sallan, S.; Kaban, G.; Şişik Oğraş, Ş.; Çelik, M.; Kaya, M. Nitrosamine Formation in a Semi-Dry Fermented Sausage: Effects of Nitrite, Ascorbate and Starter Culture and Role of Cooking. Meat Sci. 2020, 159, 107917. [Google Scholar] [CrossRef] [PubMed]
- Drabik-Markiewicz, G.; Van den Maagdenberg, K.; De Mey, E.; Deprez, S.; Kowalska, T.; Paelinck, H. Role of Proline and Hydroxyproline in N-Nitrosamine Formation during Heating in Cured Meat. Meat Sci. 2009, 81, 479–486. [Google Scholar] [CrossRef]
- Lee, S.J.; Shin, J.H.; Sung, N.J.; Kim, J.G.; Hotchkiss, J.H. Effect of Cooking on the Formation of N-Nitrosodimethylamine in Korean Dried Seafood Products. Food Addit. Contam. 2003, 20, 31–36. [Google Scholar] [CrossRef]
- Drabik-Markiewicz, G.; Dejaegher, B.; De Mey, E.; Impens, S.; Kowalska, T.; Paelinck, H.; Vander Heyden, Y. Evaluation of the Influence of Proline, Hydroxyproline or Pyrrolidine in the Presence of Sodium Nitrite on N-Nitrosamine Formation When Heating Cured Meat. Anal. Chim. Acta 2010, 657, 123–130. [Google Scholar] [CrossRef]
- Bara, V.; Bara, C.; Bara, L. Nitrosamines Occurrence in Some Food Products; University of Oradea, Faculty of Environmental Protection: Oradea, Romania, 2011; pp. 27–34. [Google Scholar]
- Huang, M.C.; Chen, H.C.; Fu, S.C.; Ding, W.H. Determination of Volatile N-Nitrosamines in Meat Products by Microwave-Assisted Extraction Coupled with Dispersive Micro Solid-Phase Extraction and Gas Chromatography–Chemical Ionisation Mass Spectrometry. Food Chem. 2013, 138, 227–233. [Google Scholar] [CrossRef]
- Massey, R.C.; Key, P.E.; Jones, R.A.; Logan, G.L. Volatile, Non-Volatile and Total n-Nitroso Compounds in Bacon. Food Addit. Contam. 1991, 8, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Mavelle, T.; Bouchikhi, B.; Debry, G. The Occurrence of Volatile N-Nitrosamines in French Foodstuffs. Food Chem. 1991, 42, 321–338. [Google Scholar] [CrossRef]
- Canas, B.J.; Havery, D.C.; Joe, F.L., Jr.; Fazio, T. Current Trends in Levels of Volatile N-Nitrosamines in Fried Bacon and Fried-Out Bacon Fat. J. Assoc. Off. Anal. Chem. 1986, 69, 1020–1021. [Google Scholar] [CrossRef] [PubMed]
- Gloria1997; Gloria, M.B.A.; Barbour, J.F.; Scanian, R.A. N-Nitrosodimethylamine in Brazilian, U.S. Domestic, and U.S. Imported Beers. J. Agric. Food Chem. 1997, 45, 814–816. [Google Scholar] [CrossRef]
- Herrmann, S.S.; Duedahl-Olesen, L.; Granby, K. Simultaneous Determination of Volatile and Non-Volatile Nitrosamines in Processed Meat Products by Liquid Chromatography Tandem Mass Spectrometry Using Atmospheric Pressure Chemical Ionisation and Electrospray Ionisation. J. Chromatogr. A 2014, 1330, 20–29. [Google Scholar] [CrossRef]
- Herrmann, S.S.; Duedahl-Olesen, L.; Christensen, T.; Olesen, P.T.; Granby, K. Dietary Exposure to Volatile and Non-Volatile N-Nitrosamines from Processed Meat Products in Denmark. Food Chem. Toxicol. 2015, 80, 137–143. [Google Scholar] [CrossRef]
- Howe, G.R.; Harrison, L.; Howe, G.R. A short diet history for assessing dietary exposure to N-nitrosamines in epidemiologic studies. Am. J. Epidemiol. 1986, 124, 595–602. [Google Scholar] [CrossRef]
- Miller, B.J.; Billedeau, S.M.; Miller, D.W. Formation of N-Nitrosamines in Microwaved versus Skillet-Fried Bacon Containing Nitrite. Food Chem. Toxicol. 1989, 27, 295–299. [Google Scholar] [CrossRef]
- Österdahl, B.G.; Alriksson, E. Volatile Nitrosamines in Microwave-Cooked Bacon. Food Addit. Contam. 1990, 7, 51–54. [Google Scholar] [CrossRef]
- Österdahl, B.G. Volatile Nitrosamines in Foods on the Swedish Market and Estimation of Their Daily Intake. Food Addit. Contam. 1988, 5, 587–595. [Google Scholar] [CrossRef]
- Tricker, A.R.; Pfundstein, B.; Theobald, E.; Preussmann, R.; Spiegelhalder, B. Mean daily intake of volatile N-nitrosamines from foods and beverages in West Germany in 1989–1990. Food Chem. Toxicol. 1991, 29, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, A.J.; Hotchkiss, J.H.; Bisogni, C.A. N-Nitrosamine Ingestion from Consumer-Cooked Bacon. J. Food Sci. 1986, 51, 754–756. [Google Scholar] [CrossRef]
- Stuff, J.E.; Goh, E.T.; Barrera, S.L.; Bondy, M.L.; Forman, M.R. Construction of an N-Nitroso Database for Assessing Dietary Intake. J. Food Compos. Anal. 2009, 22, S42–S47. [Google Scholar] [CrossRef]
- Ozel, M.Z.; Gogus, F.; Yagci, S.; Hamilton, J.F.; Lewis, A.C. Determination of Volatile Nitrosamines in Various Meat Products Using Comprehensive Gas Chromatography–Nitrogen Chemiluminescence Detection. Food Chem. Toxicol. 2010, 48, 3268–3273. [Google Scholar] [CrossRef] [PubMed]
- Scheeren, M.B.; Sabik, H.; Gariépy, C.; Terra, N.N.; Arul, J. Determination of N-Nitrosamines in Processed Meats by Liquid Extraction Combined with Gas Chromatography-Methanol Chemical Ionisation/Mass Spectrometry. Food Addit. Contam. Part A 2015, 32, 1436–1447. [Google Scholar] [CrossRef]
- Campillo, N.; Viñas, P.; Martínez-Castillo, N.; Hernández-Córdoba, M. Determination of Volatile Nitrosamines in Meat Products by Microwave-Assisted Extraction and Dispersive Liquid–Liquid Microextraction Coupled to Gas Chromatography–Mass Spectrometry. J. Chromatogr. A 2011, 1218, 1815–1821. [Google Scholar] [CrossRef]
- De Mey, E.; De Klerck, K.; De Maere, H.; Dewulf, L.; Derdelinckx, G.; Peeters, M.C.; Fraeye, I.; Vander Heyden, Y.; Paelinck, H. The Occurrence of N-Nitrosamines, Residual Nitrite and Biogenic Amines in Commercial Dry Fermented Sausages and Evaluation of Their Occasional Relation. Meat Sci. 2014, 96, 821–828. [Google Scholar] [CrossRef]
- Herrmann, S.S.; Duedahl-Olesen, L.; Granby, K. Occurrence of Volatile and Non-Volatile N-Nitrosamines in Processed Meat Products and the Role of Heat Treatment. Food Control 2015, 48, 163–169. [Google Scholar] [CrossRef]
- Reinik, M.; Tamme, T.; Roasto, M.; Juhkam, K.; Jurtšenko, S.; Tenńo, T.; Kiis, A. Nitrites, Nitrates and N-Nitrosoamines in Estonian Cured Meat Products: Intake by Estonian Children and Adolescents. Food Addit. Contam. 2005, 22, 1098–1105. [Google Scholar] [CrossRef]
- Reinik, M.; Health Board, E. Nitrates, Nitrites, N-Nitrosamines and Polycyclic Aromatic Hydrocarbons in Food: Analytical Methods, Occurrence and Dietary Intake; Dissertation in colloid and enviromental chemistry, Tartu University Press: Tartu, Estonia, 2007; pp. 1–127. [Google Scholar]
- Özbay, S.; Şireli, U.T. Volatile N-Nitrosamines in Processed Meat Products and Salami from Turkey. Food Addit. Contam. Part B Surveill. 2021, 14, 110–114. [Google Scholar] [CrossRef]
- Gavinelli, M.; Fanelli, R.; Bonfanti, M.; Davoli, E.; Airoldi, L. Volatile Nitrosamines in Foods and Beverages: Preliminary Survey of the Italian Market. Bull. Environ. Contam. Toxicol. 1988, 40, 41–46. [Google Scholar] [CrossRef]
- Domańska-Blicharz, K.; Rachubik, J.; Kowalski, B. Occurrence of Volatile N-Nitrosamines in Polish Tinned Foods. Bull. Vet. Inst. Puławy 2005, 49, 319–322. [Google Scholar]
- Hospital, X.F.; Fernández, M.; Morales, P.; Alba, C.; Haza, A.I.; Hierro, E. Volatile N-Nitrosamines in Spanish Commercial Meat Products and in Fermented Sausages Prepared with Different Ingoing Amounts of Nitrate and Nitrite. Heliyon 2024, 10, e37487. [Google Scholar] [CrossRef]
- Mulvey, L.; Everis, L.; Leeks, D.; Hughes, H.; Wood, A. Alternatives to Nitrates and Nitrites in Organic Meat Products. 2010, pp. 1–84. Available online: https://www.researchgate.net/profile/Frank_T_Edelmann/post/Looking_for_nitrite_replacements_in_the_preservation_of_processed_meats/attachment/6008594dfb1e350001e73516/AS%3A982089893695488%401611159885034/download/OF0389_9454_FRP.pdf (accessed on 14 May 2025).
- Bedale, W.; Sindelar, J.J.; Milkowski, A.L. Dietary Nitrate and Nitrite: Benefits, Risks, and Evolving Perceptions. Meat Sci. 2016, 120, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Fraqueza, M.J.; Laranjo, M.; Elias, M.; Patarata, L. Microbiological Hazards Associated with Salt and Nitrite Reduction in Cured Meat Products: Control Strategies Based on Antimicrobial Effect of Natural Ingredients and Protective Microbiota. Curr. Opin. Food Sci. 2021, 38, 32–39. [Google Scholar] [CrossRef]
- Skibsted, L.H. Nitric Oxide and Quality and Safety of Muscle Based Foods. Nitric Oxide 2011, 24, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Garriga, M.; Aymerich, T. The Microbiology of Fermentation and Ripening; Wiley: Hoboken, NJ, USA, 2015; pp. 107–115. [Google Scholar]
- Patarata, L.; Martins, S.; Silva, J.A.; Fraqueza, M.J. Red Wine and Garlic as a Possible Alternative to Minimize the Use of Nitrite for Controlling Clostridium Sporogenes and Salmonella in a Cured Sausage: Safety and Sensory Implications. Foods 2020, 9, 206. [Google Scholar] [CrossRef] [PubMed]
- Sebranek, J.G.; Bacus, J.N. Cured Meat Products without Direct Addition of Nitrate or Nitrite: What Are the Issues? Meat Sci. 2007, 77, 136–147. [Google Scholar] [CrossRef]
- Abdullah, A.T.M.; Khan, T.A.; Sharif, M.; Mazumdar, R.M.; Rahman, M.M. Determination of Dietary Exposure and Extraction Efficiency of Nitrosamine from Cooked Meat. Curr. Res. Food Sci. 2022, 5, 491–497. [Google Scholar] [CrossRef]
- Domańska-Blicharz, K.; Michalski, M.; Kowalski, B. Effect of different storage conditions on nitrates and nitrites in polish edible offals processed meat products. Influence on n-nitrosamine content. Bull.-Vet. Inst. Pulawy 2004, 48, 63–68. [Google Scholar]
- Motaghifar, A.; Akbari-Adergani, B.; Rokney, N.; Mottalebi, A. Evaluating Red Meat Putrefaction in Long Term Storage in Freezing Condition Based on Co-Variation of Major Biogenic Amines and Total Volatile Nitrogen. Food Sci. Technol. 2021, 41, 123–128. [Google Scholar] [CrossRef]
- Jaguey-Hernández, Y.; Aguilar-Arteaga, K.; Ojeda-Ramirez, D.; Añorve-Morga, J.; González-Olivares, L.G.; Castañeda-Ovando, A. Biogenic Amines Levels in Food Processing: Efforts for Their Control in Foodstuffs. Food Res. Int. 2021, 144, 110341. [Google Scholar] [CrossRef] [PubMed]
- Schirone, M.; Esposito, L.; D’onofrio, F.; Visciano, P.; Martuscelli, M.; Mastrocola, D.; Paparella, A. Biogenic Amines in Meat and Meat Products: A Review of the Science and Future Perspectives. Foods 2022, 11, 788. [Google Scholar] [CrossRef]
- Komarova, N.V.; Velikanov, A.A. Determination of Volatile N-Nitrosamines in Food by High-Performance Liquid Chromatography with Fluorescence Detection. J. Anal. Chem. 2001, 56, 359–363. [Google Scholar] [CrossRef]
- Wang, L.H.; Hsia, H.C.; Wang, C.C. Simultaneous Determination of Five Volatile and Non-Volatile N-Nitrosamines in Biological Fluids and Cosmetic Products by Liquid Chromatography with Photodiode Array Detection. J. Liq. Chromatogr. Relat. Technol. 2006, 29, 1737–1751. [Google Scholar] [CrossRef]
- Avasilcai, L.; Nichifor, M.; Bireescu, G.; Cuciureanu, R. Evaluation of the Intake of Nitrate, Nitrite, Nitrosodiethylamine and Nitrosodimethylamine by Food Consumption. EQA-Int. J. Environ. Qual. 2014, 15, 33–39. [Google Scholar] [CrossRef]
- Li, W.; Chen, N.; Zhao, Y.; Guo, W.; Muhammd, N.; Zhu, Y.; Huang, Z. Online Coupling of Tandem Liquid-Phase Extraction with HPLC-UV for the Determination of Trace N-Nitrosamines in Food Products. Anal. Methods 2018, 10, 1733–1739. [Google Scholar] [CrossRef]
- Iammarino, M.; Mangiacotti, M.; Chiaravalle, A.E. Anion Exchange Polymeric Sorbent Coupled to High-Performance Liquid Chromatography with UV Diode Array Detection for the Determination of Ten N-Nitrosamines in Meat Products: A Validated Approach. Int. J. Food Sci. Technol. 2020, 55, 1097–1109. [Google Scholar] [CrossRef]
- Lehotay, S.J.; Sapozhnikova, Y.; Han, L.; Johnston, J.J. Analysis of Nitrosamines in Cooked Bacon by QuEChERS Sample Preparation and Gas Chromatography-Tandem Mass Spectrometry with Backflushing. J. Agric. Food Chem. 2015, 63, 10341–10351. [Google Scholar] [CrossRef]
- Chienthavorn, O.; Subprasert, P.; Insuan, W. Nitrosamines Extraction from Frankfurter Sausages by Using Superheated Water. Sep. Sci. Technol. 2014, 49, 838–846. [Google Scholar] [CrossRef]
- Amelin, V.G.; Lavrukhin, D.K. Combination of Microwave Heating Extraction and Dispersive Liquid-Liquid Microextraction for the Determination of Nitrosoamines in Foods Using Gas-Liquid Chromatography with a Mass-Spectrometric Detector. J. Anal. Chem. 2016, 71, 359–364. [Google Scholar] [CrossRef]
- Wang, Z.; Zhai, M.; Xia, X.; Yang, M.; Han, T.; Huang, M. A Simple Method for Monitoring Eight N-Nitrosamines in Beef Jerkys by Gas Chromatography-Tandem Mass Spectrometry with One-Step Treatment Coupled to Active Carbon Solid-Phase Extraction. Food Anal. Methods 2018, 11, 933–938. [Google Scholar] [CrossRef]
- Roasa, J.; Liu, H.; Shao, S. An Optimised HS-SPME-GC-MS Method for the Detection of Volatile Nitrosamines in Meat Samples. Food Addit. Contam. Part A 2019, 36, 396–404. [Google Scholar] [CrossRef]
- Sun, C.; Wang, R.; Wang, T.; Li, Q. Primary Evaluation of Nine Volatile N-Nitrosamines in Raw Red Meat from Tianjin, China, by HS-SPME-GC–MS. Food Chem. 2020, 310, 125945. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Bergkvist, L.; Wolk, A. Processed Meat Consumption, Dietary Nitrosamines and Stomach Cancer Risk in a Cohort of Swedish Women. Int. J. Cancer 2006, 119, 915–919. [Google Scholar] [CrossRef]
- Li, Y.; Hecht, S.S. Metabolic Activation and DNA Interactions of Carcinogenic N-Nitrosamines to Which Humans Are Commonly Exposed. Int. J. Mol. Sci. 2022, 23, 4559. [Google Scholar] [CrossRef]
- Santarelli, R.L.; Pierre, F.; Corpet, D.E. Processed Meat and Colorectal Cancer: A Review of Epidemiologic and Experimental Evidence. Nutr. Cancer 2008, 60, 131–144. [Google Scholar] [CrossRef]
- Larsson, S.C.; Wolk, A. Red and Processed Meat Consumption and Risk of Pancreatic Cancer: Meta-Analysis of Prospective Studies. Br. J. Cancer 2012, 106, 603–607. [Google Scholar] [CrossRef]
- Rohrmann, S.; Overvad, K.; Bueno-de-Mesquita, H.B.; Jakobsen, M.U.; Egeberg, R.; Tjønneland, A.; Nailler, L.; Boutron-Ruault, M.C.; Clavel-Chapelon, F.; Krogh, V.; et al. Meat Consumption and Mortality-Results from the European Prospective Investigation into Cancer and Nutrition. BMC Med. 2013, 11, 1–12. [Google Scholar] [CrossRef]
- Suvorov, D.V.; Shur, P.Z.; Fokin, V.A.; Lir, D.N.; Nurislamova, T.V.; Subbotina, D.Y. Health risk assessment taking into account N-nitrosamines’ concentrations in food products. Health Risk Anal. 2024, 2024, 37–44. [Google Scholar] [CrossRef]
- Seyyedsalehi, M.S.; Mohebbi, E.; Tourang, F.; Sasanfar, B.; Boffetta, P.; Zendehdel, K. Association of Dietary Nitrate, Nitrite, and N-Nitroso Compounds Intake and Gastrointestinal Cancers: A Systematic Review and Meta-Analysis. Toxics 2023, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Akyüz, M.; Ata, Ş.; Dinç, E. A Chemometric Optimization of Method for Determination of Nitrosamines in Gastric Juices by GC-MS. J. Pharm. Biomed. Anal. 2016, 117, 26–36. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Agents Classified by the IARC Monographs, Volumes 1–137; International Agency for Research on Cancer: Lyon, France, 2025. [Google Scholar]
- COMMISSION REGULATION (EU) 2023/2108 of 6 October 2023 Amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as Regards Food Additives Nitrites (E 249-250) and Nitrates (E 251-252). 2023. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02008R1333-20241216&qid=1747426974735 (accessed on 14 May 2025).
- Cintya, H.; Silalahi, J.; De Lux Putra, E.; Siburian, R. Public Health R. Analysis of Nitrosamines in Processed Meat Products in Medan City by Liquid Chromatography-Mass Spectrometry. Open Access Maced. J. Med. Sci. 2019, 7, 1382. [Google Scholar] [CrossRef]
- Sun, F.; Kong, B.; Chen, Q.; Han, Q.; Diao, X. N-Nitrosoamine Inhibition and Quality Preservation of Harbin Dry Sausages by Inoculated with Lactobacillus Pentosus, Lactobacillus Curvatus and Lactobacillus Sake. Food Control 2017, 73, 1514–1521. [Google Scholar] [CrossRef]
- Combet, E.; Paterson, S.; Iijima, K.; Winter, J.; Mullen, W.; Crozier, A.; Preston, T.; McColl, K.E.L. Fat Transforms Ascorbic Acid from Inhibiting to Promoting Acid-Catalysed N-Nitrosation. Gut 2007, 56, 1678–1684. [Google Scholar] [CrossRef]
- Li, L.; Shao, J.; Zhu, X.; Zhou, G.; Xu, X. Effect of Plant Polyphenols and Ascorbic Acid on Lipid Oxidation, Residual Nitrite and N-Nitrosamines Formation in Dry-Cured Sausage. Int. J. Food Sci. Technol. 2013, 48, 1157–1164. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Zhuang, H.; Chen, X.; Li, L.; Qiao, W.; Zhang, J. Effects of Plant Polyphenols and α-Tocopherol on Lipid Oxidation, Residual Nitrites, Biogenic Amines, and N-Nitrosamines Formation during Ripening and Storage of Dry-Cured Bacon. LWT-Food Sci. Technol. 2015, 60, 199–206. [Google Scholar] [CrossRef]
- Bors, W.; Foo, L.Y.; Hertkorn, N.; Michel, C.; Stettmaier, K. Chemical Studies of Proanthocyanidins and Hydrolyzable Tannins. Antioxid. Redox Signal. 2004, 3, 995–1008. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Lin, S.; Wang, Z.; Wang, C.; Wang, E.; Zhang, Y. Supercritical Fluid Extraction of Flavonoids from Maydis Stigma and Its Nitrite-Scavenging Ability. Food Bioprod. Process. 2011, 89, 333–339. [Google Scholar] [CrossRef]
- Ferreira, P.G.; Lima, M.A.S.S.; Bernedo-Navarro, R.A.; Conceição, R.A.; Linhares, E.; Sawaya, A.C.H.F.; Yano, T.; Salgado, I. Stimulation of Acidic Reduction of Nitrite to Nitric Oxide by Soybean Phenolics: Possible Relevance to Gastrointestinal Host Defense. J. Agric. Food Chem. 2011, 59, 5609–5616. [Google Scholar] [CrossRef]
- Deda, M.S.; Bloukas, J.G.; Fista, G.A. Effect of Tomato Paste and Nitrite Level on Processing and Quality Characteristics of Frankfurters. Meat Sci. 2007, 76, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Sallan, S.; Kaban, G.; Kaya, M. Nitrosamines in Sucuk: Effects of Black Pepper, Sodium Ascorbate and Cooking Level. Food Chem. 2019, 288, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Vivar-Vera, M.d.L.A.; Pérez-Silva, A.; Ruiz-López, I.I.; Hernández-Cázares, A.S.; Solano-Barrera, S.; Ruiz-Espinosa, H.; Bernardino-Nicanor, A.; González-Cruz, L. Chemical, Physical and Sensory Properties of Vienna Sausages Formulated with a Starfruit Dietary Fiber Concentrate. J. Food Sci. Technol. 2018, 55, 3303–3313. [Google Scholar] [CrossRef] [PubMed]
- Santhosha, S.G.; Jamuna, P.; Prabhavathi, S.N. Bioactive Components of Garlic and Their Physiological Role in Health Maintenance: A Review. Food Biosci. 2013, 3, 59–74. [Google Scholar] [CrossRef]
- Alirezalu, K.; Pateiro, M.; Yaghoubi, M.; Alirezalu, A.; Peighambardoust, S.H.; Lorenzo, J.M. Phytochemical Constituents, Advanced Extraction Technologies and Techno-Functional Properties of Selected Mediterranean Plants for Use in Meat Products. A Comprehensive Review. Trends Food Sci. Technol. 2020, 100, 292–306. [Google Scholar] [CrossRef]
- Choi, S.Y.; Chung, M.J.; Lee, S.J.; Shin, J.H.; Sung, N.J. N-Nitrosamine Inhibition by Strawberry, Garlic, Kale, and the Effects of Nitrite-Scavenging and N-Nitrosamine Formation by Functional Compounds in Strawberry and Garlic. Food Control 2007, 18, 485–491. [Google Scholar] [CrossRef]
- Akansel, B.; Yılmaz Oral, Z.F.; Sallan, S.; Kaban, G.; Kaya, M. Effect of Black Garlic on Microbiological Properties, Lipid Oxidation, Residual Nitrite, Nitrosamine Formation and Sensory Characteristics in a Semi-Dry Fermented Sausage. Foods 2023, 12, 1545. [Google Scholar] [CrossRef]
- Tiwari, M.K.; Jena, N.R.; Mishra, P.C. Mechanisms of Scavenging Superoxide, Hydroxyl, Nitrogen Dioxide and Methoxy Radicals by Allicin: Catalytic Role of Superoxide Dismutase in Scavenging Superoxide Radical. J. Chem. Sci. 2018, 130, 105. [Google Scholar] [CrossRef]
- Chung, M.J.; Lee, S.H.; Sung, N.J. Inhibitory Effect of Whole Strawberries, Garlic Juice or Kale Juice on Endogenous Formation of N-Nitrosodimethylamine in Humans. Cancer Lett. 2002, 182, 1–10. [Google Scholar] [CrossRef]
- Sharafan, M.; Jafernik, K.; Ekiert, H.; Kubica, P.; Kocjan, R.; Blicharska, E.; Szopa, A. Illicium verum (Star Anise) and Trans-Anethole as Valuable Raw Materials for Medicinal and Cosmetic Applications. Molecules 2022, 27, 650. [Google Scholar] [CrossRef]
- Chung, H.J. Evaluation of the Biological Activity of Extracts from Star-Anise (Illicium verum). J. Food Sci. Nutr. 2009, 14, 195–200. [Google Scholar] [CrossRef][Green Version]
- Freire, R.S.; Morais, S.M.; Catunda, F.E.A.; Pinheiro, D.C.S.N. Synthesis and Antioxidant, Anti-Inflammatory and Gastroprotector Activities of Anethole and Related Compounds. Bioorg Med. Chem. 2005, 13, 4353–4358. [Google Scholar] [CrossRef] [PubMed]
- Mehrnia, M.; Torun, M.; Şolpan, D. Degradation of N-Nitrosodiethylamine in Aqueous Solution by Gamma-Irradiation. Environ. Technol. 2021, 42, 4201–4207. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Xu, X.; Zhou, G.; Zhao, G.; Li, C.; Zhang, Y.; Chen, L.; Qi, J. Irradiated Chinese Rugao Ham: Changes in Volatile N-Nitrosamine, Biogenic Amine and Residual Nitrite during Ripening and Post-Ripening. Meat Sci. 2009, 81, 451–455. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, J.H.; Jo, C.; Lee, J.W.; Yook, H.S.; Byun, M.W. Effects of Gamma Irradiation on Residual Nitrite, Residual Ascorbate, Color, and N-Nitrosamines of Cooked Sausage during Storage. Food Control 2004, 15, 197–203. [Google Scholar] [CrossRef]
- Byun, M.W.; Ahn, H.J.; Kim, J.H.; Lee, J.W.; Yook, H.S.; Han, S.B. Determination of Volatile N-Nitrosamines in Irradiated Fermented Sausage by Gas Chromatography Coupled to a Thermal Energy Analyzer. J. Chromatogr. A 2004, 1054, 403–407. [Google Scholar] [CrossRef]
- Homme, C.L.; Sharp, J.O. Differential Microbial Transformation of Nitrosamines by an Inducible Propane Monooxygenase. Environ. Sci. Technol. 2013, 47, 7388–7395. [Google Scholar] [CrossRef]
| Meat Products | Concentration (µg/kg) | Literature | |||||
|---|---|---|---|---|---|---|---|
| N 1 | NDMA | NDEA | NPYR | NPIP | NDBA | ||
| Bacon, uncooked | 23 | - | - | - | - | - | [32] |
| Chinese pork sausage | 1 | 3.2 | 1.1 | 0.7 | 1.1 | 1.4 | [33] |
| Chinese cured pork | 1 | 2.4 | ND | ND | 1.4 | 0.6 | [33] |
| Bacon | 1 | 1.4 | 0.4 | 0.3 | 0.4 | 1.7 | [33] |
| Bacon smoked | 23 | 0.3–2.7 | ND–1.0 | ND–7.9 | ND–0.1 | ND–0.2 | [34,35] |
| Bacon fried | 365 | ND 2–5.0 | ND–0.7 | ND–200.0 | ND–1.2 | ND–0.3 | [23,32,36,37,38,39,40,41,42,43,44] |
| Bacon fat fried | 65 | 4.0–20.2 | – | 15.8–32.0 | ND–1.5 | – | [36,41,45] |
| Bacon, cooked in microwave | 24 | 0.3–4.5 | - | 0.1–21.3 | - | - | [42,46] |
| Beef sausage | 6 | 0.3 | 0.3 | 0.6 | 1.1 | 0.4 | [47] |
| Pork, smoked | 1 | <0.8 | <0.6 | 0.7–0.8 | 1.5–2.4 | 0.3–06 | [48] |
| Black pudding | 4 | 3.5 | - | 2.1 | 2.0 | 3.4 | [49] |
| Chorizo sausages | 10 | ND–109.4 | ND–0.4 | ND–0.9 | ND–0.001 | - | [46,50] |
| Frankfurter sausage | 24 | 0.2–2.2 | - | ND–0.5 | ND–1.9 | - | [23,46,49,51] |
| Hot dog | 1 | 2.1 | ND | ND | 0.2 | 0.4 | [33] |
| Ham, cooked | 108 | 0.2–4.9 | ND–2.7 | ND–5.3 | ND–1.8 | ND–4.6 | [23,46,48,49,51,52] |
| Ham | 21 | 0.6 | ND | ND | 0.2 | 0.2 | [33] |
| Ham, smoked | 42 | 0.1–3.1 | ND–1.5 | ND–19.5 | ND–4.5 | ND–2.6 | [23,35,38,49,53] |
| Ham, Turkey | 4 | 3.8 | - | 2.8 | 1.2 | - | [49] |
| Chicken salami | 4 | 2.4 | ND–2.3 | 2.3–3.5 | 2.4–2.6 | 1.0–4.0 | [54] |
| Beef salami | 22 | 0.1–3.6 | ND–3.6 | ND–4.6 | 0.5–4.4 | 0.3–4.2 | [54] |
| Turkey salami | 6 | 0.2–3.2 | 0.3–3.9 | ND–4.0 | 0.3–6.0 | 1.6–2.0 | [54] |
| Liver pâté | 13 | 0.3–2.9 | ND–1.9 | ND–1.4 | 0.1–0.9 | ND–0.3 | [35,49] |
| Mutton (or lamb), uncooked | 9 | - | - | - | - | - | [23] |
| Mutton (or lamb) fried | 10 | 1.0 | 0.6 | 2.6 | - | 0.3 | [23] |
| Canned pork | 22 | 1.1–3.3 | ND–62.8 | ND–2.4 | 1.0–1.6 | ND–55.6 | [23,49,55] |
| Tinned meat | 30 | 0.3–2.7 | - | - | 0.5–1.1 | - | [56] |
| Cooked pork, seasoned | 17 | 0.8–2.0 | ND–0.5 | ND–11.3 | ND–1.6 | ND–0.2 | [23,38,51] |
| Pork fat-free, fried | 10 | 0.4–0.6 | 0.2–0.3 | 2.4–3.6 | 0.3–0.5 | 0.2–0.4 | [23] |
| Pork fat-fried | 10 | 3.2–4.9 | 0.5–0.8 | 14.1–24.4 | 1.0–1.6 | 0.4–0.7 | [23] |
| Pork, barbecue | 9 | ND–1.2 | ND–0.3 | ND–6.5 | ND–1.5 | ND–0.2 | [23,40] |
| Pork, pickled | 91 | 0.8 | - | 0.4 | 0.3 | 0.2 | [23] |
| Pork, smoked | 55 | 0.1–1.3 | ND–1.6 | ND–7.5 | ND–2.9 | ND–0.7 | [23,35,38] |
| Pork, uncooked | 8 | - | - | - | - | - | [23] |
| Pork sausages | 13 | ND–3.3 | - | ND–0.4 | ND–0.2 | - | [49,51] |
| Poultry, canned | 13 | 0.93–0.94 | ND–3.0 | ND–2.0 | ND–1.1 | 0.5–68.4 | [23,55] |
| Poultry, fried | 23 | 1.2–1.3 | 0.7–0.9 | 15.2–20.7 | 1.1–1.1 | 0.3–0.4 | [23] |
| Poultry, cooked or grilled | 20 | 0.9–5.0 | ND–20.0 | ND–8.4 | ND–1.7 | ND–0.2 | [23,32,44] |
| Poultry, grilled, seasoned | 10 | 1.4 | 0.6 | 14.6 | 2.0 | 0.3 | [23] |
| Poultry, smoked | 14 | 0.7–2.1 | ND–0.3 | 0.4–22.1 | 0.3–5.3 | 0.2–6.3 | [23,53] |
| Poultry, uncooked | 10 | - | - | - | - | - | [23,32] |
| Poultry, other products | 4 | ND–0.5 | ND–0.8 | ND–0.4 | ND–2.6 | ND–2.8 | [35,38,49] |
| Salami | 148 | ND–5.0 | ND–4.6 | ND–2.7 | ND–1.2 | ND–1.7 | [23,35,38,47,49,51] |
| Sausage, cooked | 54 | ND–1.5 | ND–3.0 | ND–2.3 | ND–1.8 | ND–0.3 | [23,32,35,44,47,48,51] |
| Sausage, fried, grilled | 71 | 0.2–3.6 | 0.1–2.6 | ND–6.66 | ND–2.6 | ND–3.3 | [23,35,52,53] |
| Sausage, smoked | 174 | 0.1–1.4 | 0.1–2.6 | ND–3.1 | ND–2.3 | ND–0.6 | [23,35,52,53] |
| Analytical Method | Analyzed NAs | LOD (µg/kg) | Type of Sample | Reference |
|---|---|---|---|---|
| GCxGC/NCD | NDMA, NDEA, NDBA, NPIP, NPYR, NDPA | 1.61–3.86 | meat product | [47] |
| GC/MS | NDMA, NMEA, NDEA, NDBA, NPIP, NPYR, NMOR, NDPA, NDPhA | 0.003–0.014 | meat product | [49] |
| GC/CI-MS | NDMA, NMEA, NDEA, NDBA, NPIP, NPYR, NDPA | 0.01–0.12 | meat product | [33] |
| GC/FID, GC/MS | NDEA, NPIP, NPYR, NMOR | 0.47–1.48 | meat product | [76] |
| LC-(APCI/ESI) MS/MS | NDMA, NMEA, NDEA, NDBA, NPIP, NPYR, NMOR, NDPA, NMA, NSAR, NPRO, NTCA, NMTCA | 0.2–1.0 | meat product | [38] |
| GC-CI/MS | NDMA, NMEA, NDEA, NDBA, NPIP, NPYR, NMOR, NDPA, NDPhA | 0.15–0.37 | meat product | [48] |
| GC-MS/MS | NDMA, NDEA, NDBA, NPIP, NPYR | 0.1 | meat product | [75] |
| GLC/MS | NDMA, NMEA, NDEA, NDBA, NPIP, NPYR, NDPA | 0.1–0.5 | meat product | [77] |
| GC-MS/MS | NDMA, NMEA, NDEA, NDBA, NPIP, NPYR, NMOR, NDPA | 0.05–0.10 | beef meat | [78] |
| HS-SPME-GC-MS | NDMA *, NMEA, NDEA, NDBA, NPIP, NPYR, NMOR, NDPA, NDPhA ** | 0.16–3.6 * 56 ** 16 | meat product | [79] |
| HS-SPME-GC–MS | NDPA, NDEA, NMEA, NDPA, NDBA, NPIP, NPYR, NMOR, NDPhA | 1.45–3.15 | raw meat | [80] |
| HPLC/UV-DAD | NMEA, NDEA, NDBA, NPIP, NPYR, NMOR, NDPA, NDPheA, NMA, NDBzA | 20.1–111.6 | meat product | [74] |
| LC-(APCI/ESI) MS/MS | NDMA, NPRO, NTGA, NMEA, NPYR, NMTGA, NDEA, NPIP, NDPA, NDBA | 0.1–4.2 ng/g (LOQ) | cured meat products | [11] |
| Abbreviation | Name | CAS | Structure | IARC |
|---|---|---|---|---|
| NDMA | N-nitrosodimethylamine | 62-75-9 | 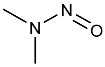 | 2A |
| NDEA | N-nitrosodiethylamine | 55-18-5 | 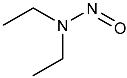 | 2A |
| NMU | N-Methyl-N-nitrosourea | 684-93-5 | 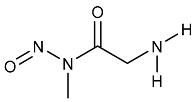 | 2A |
| MNNG | N-Methyl-N′-nitro-N-nitrosoguanidine | 70-25-7 | 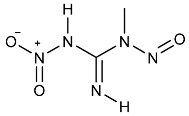 | 2A |
| ENU | N-Ethyl-N-nitrosourea | 759-73-9 | 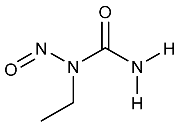 | 2A |
| NPYR | N-nitrosopyrrolidine | 930-55-2 | 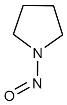 | 2B |
| NPIP | N-nitrosopiperidine | 100-75-4 |  | 2B |
| NDBA | N-nitrosodibutylamine | 924-16-3 |  | 2B |
| NDPA | N-nitrosodipropylamine | 621-64-7 | 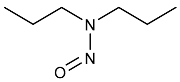 | 2B |
| NMEA | N-Nitrosomethylethylamine | 10595-95-6 | 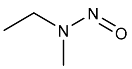 | 2B |
| NDELA | N-Nitrosodiethanolamine | 1116-54-7 |  | 2B |
| NSAR | N-Nitrososarcosine | 13256-22-9 | 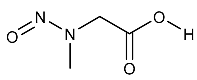 | 2B |
| NMVA | N-Nitrosomethylvinylamine | 4549-40-0 | 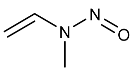 | 2B |
| NMOR | N-Nitrosomorpholine | 59-89-2 |  | 2B |
| MNPN | 3-(N-Nitrosomethylamino)propionitrile | 60153-49-3 | 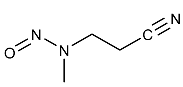 | 2B |
| NMU | N-Methyl-N-nitrosourethane | 615-53-2 | 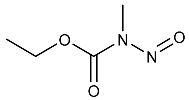 | 2B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rot, T.; Kovačević, D.; Habschied, K.; Mastanjević, K. N-Nitrosamines in Meat Products: Formation, Detection and Regulatory Challenges. Processes 2025, 13, 1555. https://doi.org/10.3390/pr13051555
Rot T, Kovačević D, Habschied K, Mastanjević K. N-Nitrosamines in Meat Products: Formation, Detection and Regulatory Challenges. Processes. 2025; 13(5):1555. https://doi.org/10.3390/pr13051555
Chicago/Turabian StyleRot, Tomislav, Dragan Kovačević, Kristina Habschied, and Krešimir Mastanjević. 2025. "N-Nitrosamines in Meat Products: Formation, Detection and Regulatory Challenges" Processes 13, no. 5: 1555. https://doi.org/10.3390/pr13051555
APA StyleRot, T., Kovačević, D., Habschied, K., & Mastanjević, K. (2025). N-Nitrosamines in Meat Products: Formation, Detection and Regulatory Challenges. Processes, 13(5), 1555. https://doi.org/10.3390/pr13051555







