Glutamate and Its Role in the Metabolism of Plants and Animals
Abstract
1. Introduction
2. Glutamate as a Chemical—Structure and Features
2.1. Physical and Chemical Properties of Glutamate
2.2. Glutamates
3. Glutamate Content and Bioavailability
Intestinal Microbiota as a Source of Glutamate
4. Glutamate—Mechanism of Action
5. Glutamate Receptors
6. Glutamate Transporters
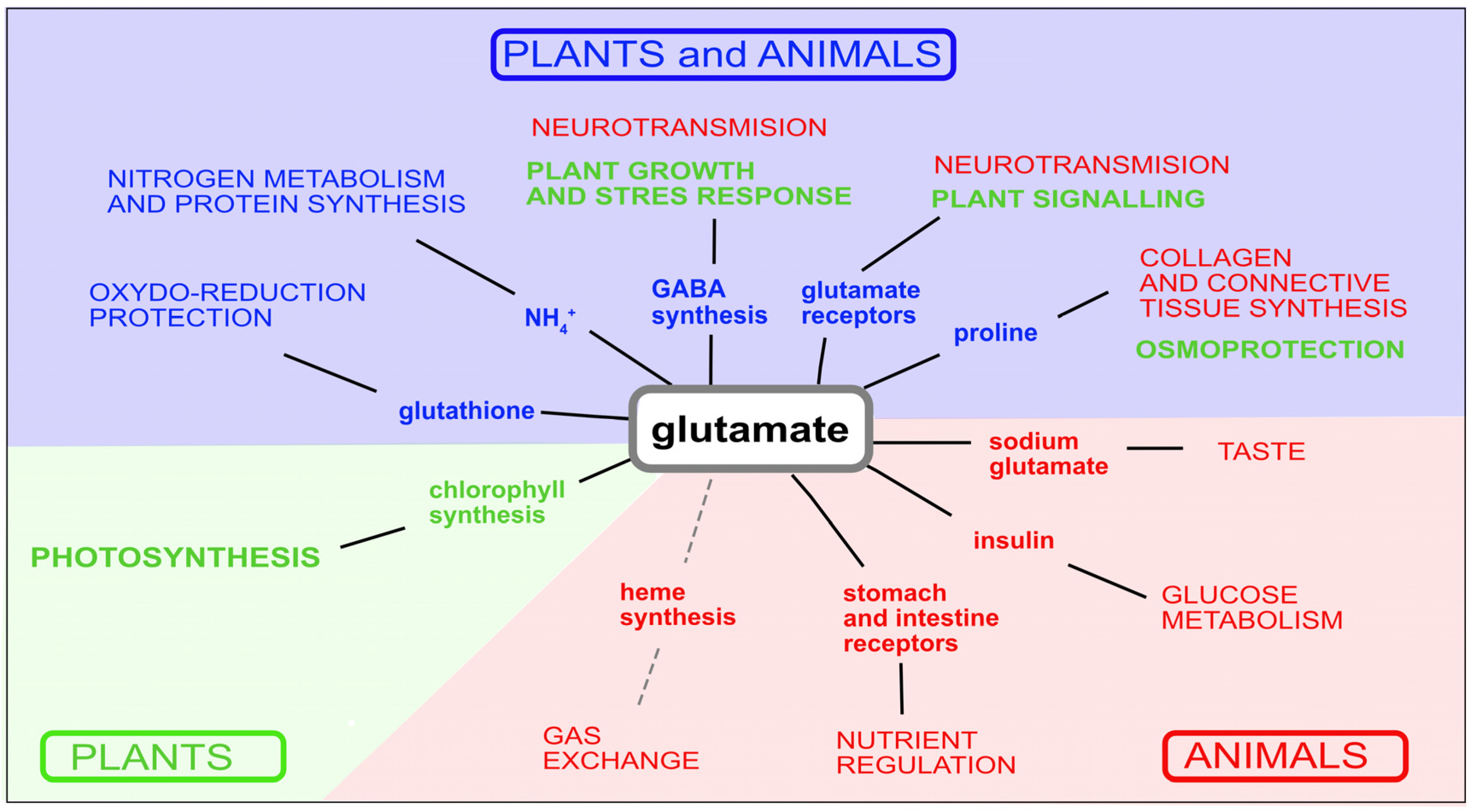
7. Glutamate Metabolism in Plants and Animals
7.1. Glutamate Synthesis and Catabolism in Plants and Animals
7.1.1. Glutamate Synthesis
7.1.2. Glutamate Catabolism
7.2. Role of Glutamate in Amino Acid and Protein Synthesis
7.3. GABA Synthesis
7.4. Chlorophyll and Heme Biosynthesis
7.5. Glutamate Is a Precursor of Proline (Osmoprotection)
7.6. Glutamate Is a Precursor of Glutathione (Oxidoreduction Protection)
8. Glutamate-Related Signalling
8.1. Animal Signalling
8.1.1. Neuronal Transmission
8.1.2. Extra-Neural Transmission
8.2. Plant Signalling
9. Glutamate in Food and Medicine Design
9.1. Glutamate in Food
9.2. Glutamate and Glutamine in Clinical Studies
10. Unresolved Questions and Practical Applications
10.1. Unresolved Questions for Future Research
- Glutamate metabolic pathways: Cross-kingdom comparisons of glutamate metabolism reveal conserved metabolic pathways that are common to plants and animals. The deep biochemical pathways of glutamate synthesis and catabolism are also shared by the occurrence of α-ketoglutarate, GABA, proline, and glutathione;
- Glutamate receptors: Plant GLR receptors are somewhat similar to animal iGLU receptors. However, the specific roles and signalling mechanisms of GLR proteins in plant metabolism, stress responses, and long-distance signalling remain unclear;
- Glutamate transporters: Although there is diversity among plant transporters and animal VGLUTs, they share the use of the proton motive force. Further study is required to identify other similarities and differences;
- Glutamate non-neuronal roles in animals: While the neurotransmitter function of glutamate is well established, its metabolic roles in non-neuronal tissues (e.g., ENS and brain–gut microbiota) require further study;
- Integrated approaches needed: More integrative studies combining biochemistry, molecular biology, physiology, and ethology are needed.
10.2. Practical Applications
- Searching for pharmaceuticals: Preclinical and comparative medical studies investigating the role of glutamate metabolism and signalling pathways in the development and treatment of many important social diseases are necessary. This is especially important for diseases related to the nervous system and nutrition-associated disorders, which may be related to glutamate metabolism and signalling;
- Searching for a good diet: A good, balanced diet should take into account the latest knowledge about glutamate metabolism and sensing, especially with regard to its content in food and its function in the ENS. Generally, plant proteins are richer in glutamate (40%) than animal proteins (11–22%). This could help to determine the potential impact of consumed foods on nervous system function and mental health. We should also consider the existence of the enterocyte barrier in the intestine and the BBB in the CNS, as well as brain–gut axis communication;
- Intestine microbiome and glutamate/glutamine cycle: This provides a solid physiological and biochemical foundation for the development of neurodietetics.
11. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MSG | Monosodium Glutamate |
| FDA | U.S. Food and Drug Administration |
| JECFA | Joint Expert Committee on Food Additives |
| FAO | Food and Agriculture Organisation |
| WHO | World Health Organisation |
| EFSA | European Food Safety Association |
| GRAS | Generally Recognized As Safe |
| FASEB | Federation of American Societies for Experimental Biology |
| VGLUTs | Vesicular glutamate transporters |
| EAATs | Excitatory amino acid transporters |
| NMDA | N-methyl-D-aspartate |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic |
| KA | Kainite Receptor |
| iGluRs | Ionotropic Glutamate Receptors |
| mGluRs | Metabotropic Glutamate Receptors |
| CCK | Cholecystokinin |
| GLP | Glucagon-Like Peptide |
| T1R1 | Taste Receptor type 1 member 1 |
| GS | Glutamine synthetase |
| GOGAT | Glutamate synthase |
| GAD | Glutamate decarboxylase |
| GABA | γ-aminobutyric acid |
| ROS | Reactive Oxygen Species |
| ALA | 5-aminolevulinic Acid |
| CNS | Central Nervous System |
| CRS | Chinese Restaurant Syndrome |
| MDD | Major depressive disorder |
| ENS | Enteric Nervous System |
| GLR | Glutamate Receptor-Like |
| ADHD | Attention-Deficit Hyperactivity Disorder |
| BBB | Blood-Brain Barrier |
| GDH | Glutamate Dehydrogenase |
| GAD | Glutamate Decarboxylase |
| A | Aminotransferases |
References
- Jinap, S.; Hajeb, P. Glutamate. Its Applications in Food and Contribution to Health. Appetite 2010, 55, 1–10. [Google Scholar] [PubMed]
- Loï, C.; Cynober, L. Glutamate: A Safe Nutrient, Not Just a Simple Additive. Ann. Nutr. Metab. 2022, 78, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Cynober, L. Metabolism of Dietary Glutamate in Adults. Ann. Nutr. Metab. 2018, 73 (Suppl. S5), 5–14. [Google Scholar] [CrossRef] [PubMed]
- Young, V.R.; Ajami, A.M. Glutamate: An Amino Acid of Particular Distinction. J. Nutr. 2000, 130, 892S–900S. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Mathé, G.; Couvreur, P.; Tew, K., II. Glutamine and Glutamate. Biomed. Pharmacother. 2002, 56, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Lima, M.; Procópio, J.; Pithon-Curi, T.; Bazotte, R.; Curi, R. Glutamine and Glutamate as Vital Metabolites. Braz. J. Med. Biol. Res. 2003, 36, 153–163. [Google Scholar] [CrossRef]
- Brosnan, J.T. Glutamate, at the Interface between Amino Acid and Carbohydrate Metabolism. J. Nutr. 2000, 130, 988S–990S. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Dai, Z.; Li, D.; Wang, J.; Wu, Z. Amino Acid Nutrition in Animals: Protein Synthesis and Beyond. Annu. Rev. Anim. Biosci. 2014, 2, 387–417. [Google Scholar] [CrossRef] [PubMed]
- Hudson, D.; Guevara, D.; Yaish, M.W.; Hannam, C.; Long, N.; Clarke, J.D.; Bi, Y.-M.; Rothstein, S.J. Gnc and Cga1 Modulate Chlorophyll Biosynthesis and Glutamate Synthase (Glu1/Fd-Gogat) Expression in Arabidopsis. PLoS ONE 2011, 6, e26765. [Google Scholar] [CrossRef]
- Tanaka, R.; Kobayashi, K.; Masuda, T. Tetrapyrrole Metabolism in Arabidopsis thaliana. Arab. Book 2011, 9, e0145. [Google Scholar] [CrossRef]
- Heuer, B. Osmoregulatory Role of Proline in Plants Exposed to Environmental Stresses. Handb. Plant Crop Stress 1999, 2, 675–695. [Google Scholar]
- Delauney, A.J.; Verma, D.P.S. Proline Biosynthesis and Osmoregulation in Plants. Plant J. 1993, 4, 215–223. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. Glutamate: A Truly Functional Amino Acid. Amino Acids 2013, 45, 413–418. [Google Scholar] [CrossRef]
- Moldovan, O.-L.; Rusu, A.; Tanase, C.; Vari, C.-E. Glutamate-a Multifaceted Molecule: Endogenous Neurotransmitter, Controversial Food Additive, Design Compound for Anti-Cancer Drugs. A Critical Appraisal. Food Chem. Toxicol. 2021, 153, 112290. [Google Scholar] [CrossRef]
- Zhou, Y.; Danbolt, N.C. Glutamate as a Neurotransmitter in the Healthy Brain. J. Neural Transm. 2014, 121, 799–817. [Google Scholar]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Glutamate (accessed on 12 May 2025).
- Bionumbers Amino Acids Size. Available online: https://bionumbers.hms.harvard.edu/bionumber.aspx?&id=106983 (accessed on 12 May 2025).
- Nelson, D.L.; Lehninger, A.L.; Cox, M.M. Lehninger Principles of Biochemistry; Macmillan: New York, NY, USA, 2008. [Google Scholar]
- Amino-Acids. 2025. Available online: https://aminoacidsguide.com/Glu.html (accessed on 12 June 2025).
- EFSA Efsa. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/4910 (accessed on 10 May 2025).
- Zanfirescu, A.; Ungurianu, A.; Tsatsakis, A.M.; Nițulescu, G.M.; Kouretas, D.; Veskoukis, A.; Tsoukalas, D.; Engin, A.B.; Aschner, M.; Margină, D. A Review of the Alleged Health Hazards of Monosodium Glutamate. Compr. Rev. Food. Sci. Food Saf. 2019, 18, 1111–1134. [Google Scholar] [CrossRef] [PubMed]
- COT Statement 2006/02. Available online: https://cot.food.gov.uk/sites/default/files/cot/cotstatementadditives.pdf (accessed on 10 May 2025).
- Tomé, D. The Roles of Dietary Glutamate in the Intestine. Ann. Nutr. Metab. 2018, 73 (Suppl. S5), 15–20. [Google Scholar] [CrossRef]
- Giacometti, T. Free and Bound Glutamate in Natural Products. In Glutamic Acid: Advances in Biochemistry and Physiology; Filer, L.J., Jr., Garattini, S., Kare, M.R., Reynolds, W.A., Wurtmaned, R.J., Eds.; Raven Press: New York, NY, USA, 1979; pp. 25–34. [Google Scholar]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Do-menico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Re-evaluation of glutamic acid (E 620), sodium glutamate (E 621), potassium glutamate (E 622), calcium glutamate (E 623), ammonium glutamate (E 624) and magnesium glutamate (E 625) as food additives. EFSA J. 2017, 15, e04910. [Google Scholar]
- Walker, R.; Lupien, J.R. The Safety Evaluation of Monosodium Glutamate. J. Nutr. 2000, 130, 1049S–1052S. [Google Scholar] [CrossRef]
- FDA2. Answ. Quest Monosodium Glutamate (MSG). 19 November 2012. Available online: https://examine.com/articles/is-msg-bad-for-your-health/?srsltid=AfmBOopNNoZRbYGyQQrGFEQoNlyCNYjd_27LDHFmB2XSRBw5iR_crj7z&utm_source=chatgpt.com (accessed on 10 May 2025).
- Bai, W.; Zhou, Y.-G. Homeostasis of the Intraparenchymal-Blood Glutamate Concentration Gradient: Maintenance, Imbalance, and Regulation. Front. Mol. Neurosci. 2017, 10, 400. [Google Scholar] [CrossRef]
- Bai, W.; Zhu, W.-L.; Ning, Y.-L.; Li, P.; Zhao, Y.; Yang, N.; Chen, X.; Jiang, Y.-L.; Yang, W.-Q.; Jiang, D.-P. Dramatic Increases in Blood Glutamate Concentrations Are Closely Related to Traumatic Brain Injury-Induced Acute Lung Injury. Sci. Rep. 2017, 7, 5380. [Google Scholar] [CrossRef] [PubMed]
- Kostyra, E. Substancje Wzmacniające Smak i ich Rola w Żywności Wygodnej. Postępy Tech. Przetwórstwa Spożywczego 2008, 2, 92–97. [Google Scholar]
- Ninomiya, K. Natural Occurrence. Food Rev. Int. 1998, 14, 177–211. [Google Scholar] [CrossRef]
- FDA1 Fda 1. Questions and Answers on Monosodium glutamate (MSG); 19 November 2012. Available online: https://www.fda.gov/food/food-additives-petitions/questions-and-answers-monosodium-glutamate-msg (accessed on 10 May 2025).
- Forde, B.G.; Lea, P.J. Glutamate in Plants: Metabolism, Regulation, and Signalling. J. Exp. Bot. 2007, 58, 2339–2358. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, D. Intestinal Hormones and Regulation of Satiety: The Case for Cck, Glp-1, Pyy, and Apo a-Iv. J. Parenter. Enter. Nutr. 2008, 32, 567–568. [Google Scholar] [CrossRef] [PubMed]
- Sukkar, S.G.; Vaccaro, A.; Ravera, G.B.; Borrini, C.; Gradaschi, R.; Massa Sacchi-Nemours, A.; Cordera, R.; Andraghetti, G. Appetite Control and Gastrointestinal Hormonal Behavior (Cck, Glp-1, Pyy 1–36) Following Low Doses of a Whey Protein-Rich Nutraceutic. Mediterr. J. Nutr. Metab. 2013, 6, 259–266. [Google Scholar] [CrossRef]
- Baj, A.; Moro, E.; Bistoletti, M.; Orlandi, V.; Crema, F.; Giaroni, C. Glutamatergic Signaling Along the Microbiota-Gut-Brain Axis. Int. J. Mol. Sci. 2019, 20, 1482. [Google Scholar]
- Mazzoli, R.; Pessione, E. The Neuro-Endocrinological Role of Microbial Glutamate and Gaba Signaling. Front. Microbiol. 2016, 7, 1934. [Google Scholar] [CrossRef] [PubMed]
- Pokusaeva, K.; Johnson, C.; Luk, B.; Uribe, G.; Fu, Y.; Oezguen, N.; Matsunami, R.; Lugo, M.; Major, A.; Mori-Akiyama, Y. Gaba-Producing Bifidobacterium Dentium Modulates Visceral Sensitivity in the Intestine. Neurogastroenterol. Motil. 2017, 29, e12904. [Google Scholar] [CrossRef]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota-Gut-Brain Axis and Its Therapeutic Applications in Neurodegenerative Diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar]
- Gruenbaum, B.F.; Merchant, K.S.; Zlotnik, A.; Boyko, M. Gut Microbiome Modulation of Glutamate Dynamics: Implications for Brain Health and Neurotoxicity. Nutrients 2024, 16, 4405. [Google Scholar] [CrossRef] [PubMed]
- Rousseaux, C.G. A Review of Glutamate Receptors I: Current Understanding of Their Biology. J. Toxicol. Pathol. 2008, 21, 25–51. [Google Scholar]
- Willard, S.S.; Koochekpour, S. Glutamate, Glutamate Receptors, and Downstream Signaling Pathways. Int. J. Biol. Sci. 2013, 9, 948. [Google Scholar] [CrossRef] [PubMed]
- Nisar, S.; Bhat, A.A.; Masoodi, T.; Hashem, S.; Akhtar, S.; Ali, T.A.; Amjad, S.; Chawla, S.; Bagga, P.; Frenneaux, M.P.; et al. Genetics of Glutamate and Its Receptors in Autism Spectrum Disorder. Mol. Psychiatry 2022, 27, 2380–2392. [Google Scholar]
- Crupi, R.; Impellizzeri, D.; Cuzzocrea, S. Role of Metabotropic Glutamate Receptors in Neurological Disorders. Front. Mol. Neurosci. 2019, 12, 20. [Google Scholar] [CrossRef]
- Chaudhari, N.; Pereira, E.; Roper, S.D. Taste Receptors for Umami: The Case for Multiple Receptors. Am. J. Clin. Nutr. 2009, 90, 738S–742S. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Staszewski, L.; Xu, H.; Durick, K.; Zoller, M.; Adler, E. Human Receptors for Sweet and Umami Taste. Proc. Natl. Acad. Sci. USA 2002, 99, 4692–4696. [Google Scholar] [CrossRef]
- Davenport, R. Glutamate Receptors in Plants. Ann. Bot. 2002, 90, 549–557. [Google Scholar] [CrossRef]
- Shigeri, Y.; Seal, R.P.; Shimamoto, K. Molecular Pharmacology of Glutamate Transporters, Eaats and Vgluts. Brain Res. Rev. 2004, 45, 250–265. [Google Scholar] [CrossRef]
- McCutcheon, S.L.; Bown, A.W. Evidence for a Specific Glutamate/H+ Cotransport in Isolated Mesophyll Cells. Plant Physiol. 1987, 83, 691–697. [Google Scholar] [CrossRef]
- Snedden, W.A.; Chung, I.; Pauls, R.H.; Bown, A.W. Proton/L-Glutamate Symport and the Regulation of Intracellular Ph in Isolated Mesophyll Cells. Plant Physiol. 1992, 99, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Stolarz, M.; Krol, E.; Dziubinska, H. Glutamatergic Elements in an Excitability and Circumnutation Mechanism. Plant Signal. Behav. 2010, 5, 1108–1111. [Google Scholar] [CrossRef] [PubMed]
- Balcar, V.J. Molecular Pharmacology of the Na+-Dependent Transport of Acidic Amino Acids in the Mammalian Central Nervous System. Biol. Pharm. Bull. 2002, 25, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Danbolt, N.C.; Furness, D.N.; Zhou, Y. Neuronal Vs Glial Glutamate Uptake: Resolving the Conundrum. Neurochem. Int. 2016, 98, 29–45. [Google Scholar] [PubMed][Green Version]
- Kolen, B.; Borghans, B.; Kortzak, D.; Lugo, V.; Hannack, C.; Guzman, R.E.; Ullah, G.; Fahlke, C. Vesicular Glutamate Transporters Are H+-Anion Exchangers That Operate at Variable Stoichiometry. Nat. Commun. 2023, 14, 2723. [Google Scholar] [CrossRef]
- Vandenberg, R.J.; Ryan, R.M. Mechanisms of Glutamate Transport. Physiol. Rev. 2013, 93, 1621–1657. [Google Scholar] [CrossRef]
- Liao, H.-S.; Chung, Y.-H.; Hsieh, M.-H. Glutamate: A Multifunctional Amino Acid in Plants. Plant Sci. 2022, 318, 111238. [Google Scholar]
- Coruzzi, G.M. Primary N-Assimilation into Amino Acids in Arabidopsis. Arab. Book 2003, 2, e0010. [Google Scholar] [CrossRef]
- Lea, P.J.; Miflin, B.J. Glutamate Synthase and the Synthesis of Glutamate in Plants. Plant Physiol. Biochem. 2003, 41, 555–564. [Google Scholar] [CrossRef]
- Bak, L.K.; Schousboe, A.; Waagepetersen, H.S. The Glutamate/Gaba-Glutamine Cycle: Aspects of Transport, Neurotransmitter Homeostasis and Ammonia Transfer. J. Neurochem. 2006, 98, 641–653. [Google Scholar] [CrossRef]
- McKenna, M.C. The Glutamate-Glutamine Cycle Is Not Stoichiometric: Fates of Glutamate in Brain. J. Neurosci. Res. 2007, 85, 3347–3358. [Google Scholar] [CrossRef]
- Hertz, L.; Rothman, D.L. Glutamine-Glutamate Cycle Flux Is Similar in Cultured Astrocytes and Brain and Both Glutamate Production and Oxidation Are Mainly Catalyzed by Aspartate Aminotransferase. Biology 2017, 6, 17. [Google Scholar] [CrossRef]
- Shen, J. Modeling the Glutamate—Glutamine Neurotransmitter Cycle. Front. Neuroenergetics 2013, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Burrin, D.G.; Stoll, B. Metabolic Fate and Function of Dietary Glutamate in the Gut1234. Am. J. Clin. Nutr. 2009, 90, 850S–856S. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P. Gaba Shunt in Durum Wheat. Front. Plant Sci. 2018, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Tarkowski, Ł.P.; Signorelli, S.; Höfte, M. Γ-Aminobutyric Acid and Related Amino Acids in Plant Immune Responses: Emerging Mechanisms of Action. Plant Cell Environ. 2020, 43, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Shelp, B.J.; Aghdam, M.S.; Flaherty, E.J. Γ-Aminobutyrate (GABA) Regulated Plant Defense: Mechanisms and Opportunities. Plants 2021, 10, 1939. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Maemura, K.; Kanbara, K.; Tamayama, T.; Hayasaki, H. Gaba and Gaba Receptors in the Central Nervous System and Other Organs. Int. Rev. Cytol. 2002, 213, 1–47. [Google Scholar] [PubMed]
- McCormick, D.A. Gaba as an Inhibitory Neurotransmitter in Human Cerebral Cortex. J. Neurophysiol. 1989, 62, 1018–1027. [Google Scholar] [CrossRef]
- Medlock, A.E.; Dailey, H.A. New Avenues of Heme Synthesis Regulation. Int. J. Mol. Sci. 2022, 23, 7467. [Google Scholar] [CrossRef]
- Burch, J.S.; Marcero, J.R.; Maschek, J.A.; Cox, J.E.; Jackson, L.K.; Medlock, A.E.; Phillips, J.D.; Dailey Jr, H.A. Glutamine Via α-Ketoglutarate Dehydrogenase Provides Succinyl-Coa for Heme Synthesis During Erythropoiesis. Blood 2018, 132, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Schubert, F.; Gallinat, J.; Seifert, F.; Rinneberg, H. Glutamate Concentrations in Human Brain Using Single Voxel Proton Magnetic Resonance Spectroscopy at 3 Tesla. Neuroimage 2004, 21, 1762–1771. [Google Scholar] [CrossRef]
- Nedergaard, M.; Takano, T.; Hansen, A.J. Beyond the Role of Glutamate as a Neurotransmitter. Nat. Rev. Neurosci. 2002, 3, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Filpa, V.; Moro, E.; Protasoni, M.; Crema, F.; Frigo, G.; Giaroni, C. Role of Glutamatergic Neurotransmission in the Enteric Nervous System and Brain-Gut Axis in Health and Disease. Neuropharmacology 2016, 111, 14–33. [Google Scholar] [PubMed]
- Kondoh, T.; Mallick, H.N.; Torii, K. Activation of the Gut-Brain Axis by Dietary Glutamate and Physiologic Significance in Energy Homeostasis. Am. J. Clin. Nutr. 2009, 90, 832S–837S. [Google Scholar] [CrossRef] [PubMed]
- Torii, K.; Uneyama, H.; Nakamura, E. Physiological Roles of Dietary Glutamate Signaling Via Gut–Brain Axis Due to Efficient Digestion and Absorption. J. Gastroenterol. 2013, 48, 442–451. [Google Scholar] [CrossRef]
- Kitamura, A.; Tsurugizawa, T.; Torii, K. Biological Significance of Glutamate Signaling During Digestion of Food through the Gut-Brain Axis. Digestion 2011, 83 (Suppl. S1), 37–43. [Google Scholar] [CrossRef]
- Delgado, T.C. Glutamate and Gaba in Appetite Regulation. Front. Endocrinol. 2013, 4, 103. [Google Scholar] [CrossRef] [PubMed]
- Dennison, K.L.; Spalding, E.P. Glutamate-Gated Calcium Fluxes in Arabidopsis. Plant Physiol. 2000, 124, 1511–1514. [Google Scholar] [CrossRef]
- Krol, E.; Dziubinska, H.; Trebacz, K.; Koselski, M.; Stolarz, M. The Influence of Glutamic and Aminoacetic Acids on the Excitability of the Liverwort Conocephalum Conicum. J. Plant Physiol. 2007, 164, 773–784. [Google Scholar] [CrossRef]
- Stolarz, M.; Krol, E.; Dziubinska, H.; Kurenda, A. Glutamate Induces Series of Action Potentials and a Decrease in Circumnutation Rate in Helianthus annuus. Physiol. Plant 2010, 138, 329–338. [Google Scholar] [CrossRef]
- Li, Z.-G.; Ye, X.-Y.; Qiu, X.-M. Glutamate Signaling Enhances the Heat Tolerance of Maize Seedlings by Plant Glutamate Receptor-Like Channels-Mediated Calcium Signaling. Protoplasma 2019, 256, 1165–1169. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.T.; Drewnowski, A.; Friedman, M.I. Is There a Relationship between Dietary Msg Obesity in Animals or Humans? Amino Acids 2014, 46, 2075–2087. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.S.; Branigan, D.; Li, M. Deciphering the Msg Controversy. Int. J. Clin. Exp. Med. 2009, 2, 329. [Google Scholar] [PubMed]
- Bayram, H.M.; Akgöz, H.F.; Kızıldemir, Ö.; Öztürkcan, S.A. Monosodium Glutamate: Review on Preclinical and Clinical Reports. Biointerface Res. Appl. Chem. 2023, 13, 149. [Google Scholar]
- Airaodion, A.I.; Ogbuagu, E.O.; Osemwowa, E.U.; Ogbuagu, U.; Esonu, C.E. Toxicological Effect of Monosodium Glutamate in Seasonings on Human Health. Glob. J. Nutri. Food Sci. 2019, 1, 522. [Google Scholar] [CrossRef]
- Baek, J.H.; Park, H.; Kang, H.; Kim, R.; Kang, J.S.; Kim, H.J. The Role of Glutamine Homeostasis in Emotional and Cognitive Functions. Int. J. Mol. Sci. 2024, 25, 1302. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, Y.P.; Ayed, C.; Li, B.; Liu, Y. An on-Line Study About Consumers’ Perception and Purchasing Behavior toward Umami Seasonings in China. Food Control 2020, 110, 107037. [Google Scholar] [CrossRef]
- Wang, S.; Adhikari, K. Consumer Perceptions and Other Influencing Factors About Monosodium Glutamate in the United States. J. Sens. Stud. 2018, 33, e12437. [Google Scholar] [CrossRef]
- Mariano, A.B. Assessment on Consumers’ Awareness and Perception Towards Monosodium Glutamate (Msg) and Its Effect to Their Buying Behavior. Int. J. Multidiscip. Res. 2025, 7, 1–30. [Google Scholar] [CrossRef]
- Ahangari, H.; Bahramian, B.; Khezerlou, A.; Tavassoli, M.; Kiani-Salmi, N.; Tarhriz, V.; Ehsani, A. Association between Monosodium Glutamate Consumption with Changes in Gut Microbiota and Related Metabolic Dysbiosis—A Systematic Review. Food Sci. Nutr. 2024, 12, 5285–5295. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Masuzawa, Y.; Kohmura, M.; Takumi, A.; Takeyama, H.; Miyazaki, S.; Gojobori, T.; Mineta, K. Glutamate-Sensing Genes Are Conserved among Populations Compared to Glutamate Metabolism Genes. Ann. Nutr. Metab. 2023, 79, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Zeng, Q.; Liu, P.; Wei, Y.; Guo, R.; Zhu, Y.; He, R.-R. Association of Glutamate Receptor Gene Polymorphisms with Attention-Deficit Hyperactivity Disorder Susceptibility: A Systematic Review and Meta-Analysis. Front. Genet. 2024, 15, 1348387. [Google Scholar] [CrossRef]
- Esterlis, I.; Holmes, S.E.; Sharma, P.; Krystal, J.H.; DeLorenzo, C. Metabotropic Glutamatergic Receptor 5 and Stress Disorders: Knowledge Gained from Receptor Imaging Studies. Biol. Psychiatry 2018, 84, 95–105. [Google Scholar]
- Carey, C.; Singh, N.; Dunn, J.T.; Sementa, T.; Mendez, M.A.; Velthuis, H.; Pereira, A.C.; Pretzsch, C.M.; Horder, J.; Hader, S.; et al. From Bench to Bedside: The Mglur5 System in People with and without Autism Spectrum Disorder and Animal Model Systems. Transl. Psychiatry 2022, 12, 395. [Google Scholar] [PubMed]
- Sarawagi, A.; Soni, N.D.; Patel, A.B. Glutamate and Gaba Homeostasis and Neurometabolism in Major Depressive Disorder. Front. Psychiatry 2021, 12, 637863. [Google Scholar]
- FDA3. 2025. Available online: https://www.hfpappexternal.fda.gov/scripts/fdcc/index.cfm?set=FoodSubstances&id=GLUTAMINE (accessed on 10 May 2025).
- Clinicaltrials. 2025. Available online: https://Clinicaltrials.Gov. (accessed on 5 May 2025).
- FDA4. 2004. Available online: https://Www.Accessdata.Fda.Gov/Drugsatfda_Docs/Label/2004/21677_Nutrestore_Lbl.Pdf (accessed on 10 May 2025).
- FDA5. Available online: https://Www.Fda.Gov/Drugs/Resources-Information-Approved-Drugs/Fda-Approved-L-Glutamine-Powder-Treatment-Sickle-Cell-Disease (accessed on 10 May 2025).
- Vagaggini, C.; D’Ursi, P.; Poggialini, F.; Fossa, P.; Francesconi, V.; Trombetti, G.; Orro, A.; Dreassi, E.; Schenone, S.; Tonelli, M. Deciphering the Landscape of Allosteric Glutaminase 1 Inhibitors as Anticancer Agents. Bioorg. Chem. 2025, 161, 108523. [Google Scholar] [CrossRef]
- Anderson, G.H.; Fabek, H.; Akilen, R.; Chatterjee, D.; Kubant, R. Acute Effects of Monosodium Glutamate Addition to Whey Protein on Appetite, Food Intake, Blood Glucose, Insulin and Gut Hormones in Healthy Young Men. Appetite 2018, 120, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Kahe, K.; Laferrère, B.; Castellanos, F.X.; Zhang, Y.; Mozaffarian, D. Monosodium Glutamate: A Hidden Risk Factor for Obesity? Obes. Rev. 2025, 26, e13903. [Google Scholar] [CrossRef]
- Insawang, T.; Selmi, C.; Cha’on, U.; Pethlert, S.; Yongvanit, P.; Areejitranusorn, P.; Boonsiri, P.; Khampitak, T.; Tangrassameeprasert, R.; Pinitsoontorn, C.; et al. Monosodium Glutamate (Msg) Intake Is Associated with the Prevalence of Metabolic Syndrome in a Rural Thai Population. Nutr. Metab. 2012, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zhao, L.; Daviglus, M.L.; Dyer, A.R.; Van Horn, L.; Garside, D.; Zhu, L.; Guo, D.; Wu, Y.; Zhou, B. Association of Monosodium Glutamate Intake with Overweight in Chinese Adults: The Intermap Study. Obesity 2008, 16, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Thu Hien, V.T.; Thi Lam, N.; Cong Khan, N.; Wakita, A.; Yamamoto, S. Monosodium Glutamate Is Not Associated with Overweight in Vietnamese Adults. Public Health Nutr. 2013, 16, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Shimada, A.; Castrillon, E.; Baad-Hansen, L.; Ghafouri, B.; Gerdle, B.; Wåhlén, K.; Ernberg, M.; Cairns, B.; Svensson, P. Increased Pain and Muscle Glutamate Concentration after Single Ingestion of Monosodium Glutamate by Myofascial Temporomandibular Disorders Patients. Eur. J. Pain 2016, 20, 1502–1512. [Google Scholar] [CrossRef]
- Vellisca, M.Y.; Latorre, J.I. Monosodium Glutamate and Aspartame in Perceived Pain in Fibromyalgia. Rheumatol. Int. 2014, 34, 1011–1013. [Google Scholar] [CrossRef] [PubMed]
- Kouzuki, M.; Taniguchi, M.; Suzuki, T.; Nagano, M.; Nakamura, S.; Katsumata, Y.; Matsumoto, H.; Urakami, K. Effect of Monosodium L-Glutamate (Umami Substance) on Cognitive Function in People with Dementia. Eur. J. Clin. Nutr. 2019, 73, 266–275. [Google Scholar] [CrossRef]



| Glutamate form |  | ||||||
| Net charge | +1 | 0 | −1 | −2 | |||
| Ion name | mono-cation | isoelectric | mono-anion | di-anion | |||
| pH | under 2 | 2–4 | 4–9 (physiological condition 7.4) | above 9 | |||
| pKa value | pK1 ~ 2.19 | isoelectric point 3.22 | pKR ~ 4.25 | pK2 ~ 9.67 | |||
| Dissociation | lack of dissociation | dissociation of H+ from the COOH group at carbon α | dissociation of H+ from the COOH group at side chain (R) | dissociation of H+ from the NH3+ group | |||
| Source | Free Glutamate Content (mg/100g) | Reference |
|---|---|---|
| plants | ||
| tomatoes (fresh) | 140–246 | [1,24] |
| peas | 106–200 | [1,30] |
| corn | 106–130 | [1,30] |
| spinach | 39–48 | [1] |
| carrots | 33 | [30] |
| green peppers | 32 | [30] |
| potatoes | 10–180 | [1,24,30] |
| plant-derived products | ||
| fruits | 5–18 | [1] |
| soy sauce (depending on the country) | 412–1264 | [1] |
| fermented beans (depending on the region) region) | 136–1700 | [1] |
| red algae (Porphyra) dried form | 1378 | [1] |
| meat | ||
| beef | 10–33 | [1,31] |
| pork | 9–23 | [1,31] |
| chicken | 22–44 | [1,31] |
| duck | 69 | [30] |
| mackerel | 36 | [30] |
| salmon | 20 | [30] |
| animal-derived products | ||
| milk | 2–22 | [1,30,31] |
| cheese (Ementaler-Parmesan) | 308–1200 | [1,24,30] |
| seafood | ||
| scallop | 140 | [1] |
| snow crab | 19 | [1] |
| Alaskan king crab | 72 | [1] |
| white shrimp | 20 | [1] |
| fish sauce (depending on the country) | 727–1383 | [1] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stolarz, M.; Hanaka, A. Glutamate and Its Role in the Metabolism of Plants and Animals. Processes 2025, 13, 2084. https://doi.org/10.3390/pr13072084
Stolarz M, Hanaka A. Glutamate and Its Role in the Metabolism of Plants and Animals. Processes. 2025; 13(7):2084. https://doi.org/10.3390/pr13072084
Chicago/Turabian StyleStolarz, Maria, and Agnieszka Hanaka. 2025. "Glutamate and Its Role in the Metabolism of Plants and Animals" Processes 13, no. 7: 2084. https://doi.org/10.3390/pr13072084
APA StyleStolarz, M., & Hanaka, A. (2025). Glutamate and Its Role in the Metabolism of Plants and Animals. Processes, 13(7), 2084. https://doi.org/10.3390/pr13072084







