Synthesis of 2,3-Dihydrobenzofuran Chalcogenides Under Visible Light: A Sustainable Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. General Procedure
2.2. Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cordeiro, P.; Rebelo, A.; Menezes, V.; Reis, J.S.; Nascimento, V. Fontes Naturais e Sintéticas de derivados de 2,3-Dihidrobenzofuranos: Uma Abordagem Recente. Rev. Virtual Quím. 2023, 15, 374–401. [Google Scholar] [CrossRef]
- Chen, Z.; Pitchakuntla, M.; Jia, Y. Synthetic approaches to natural products containing 2,3-dihydrobenzofuran skeleton. Nat. Prod. Rep. 2019, 36, 666–690. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.-W.; Chai, H.-B.; Keller, W.J.; Kinghorn, D.A. Lignans and Other Constituents of the Fruits of Euterpe oleracea (Açai) with Antioxidant and Cytoprotective Activities. J. Agric. Food Chem. 2008, 56, 7759–7764. [Google Scholar] [CrossRef] [PubMed]
- Dapkekar, A.B.; Sreenivasulu, C.; Kishore, D.R.; Satyanarayana, G. Recent Advances Towards the Synthesis of Dihydrobenzofurans and Dihydroisobenzofurans. Asian J. Org. Chem. 2022, 11, e202200012. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, F.-M.; Zhang, C.-S.; Liu, S.-Z.; Tian, J.-M.; Wang, S.-H.; Zhang, X.-M.; Tu, Y.-Q. Enantioselective synthesis of cis-hydrobenzofurans bearing all-carbon quaternary stereocenters and application to total synthesis of (−)-morphine. Nature 2019, 10, 2507. [Google Scholar] [CrossRef]
- Zarin, M.K.Z.; Dehaen, W.; Salehi, P.; Asl, A.A.B. Synthesis and Modification of Morphine and Codeine, Leading to Diverse Libraries with Improved Pain Relief Properties. Pharmaceutics 2023, 15, 1779. [Google Scholar] [CrossRef]
- Da Fonseca, A.C.C.; De Queiroz, L.N.; Felisberto, J.S.; Ramos, Y.J.; Marques, A.M.; Wermelinger, G.F.; Pontes, B.; Moreira, D.L.; Robbs, B.K. Cytotoxic effect of pure compounds from Piper rivinoides Kunth against oral squamous cell carcinoma. Nat. Prod. Res. 2021, 35, 6163–6167. [Google Scholar] [CrossRef]
- Sun, D.; Zhao, Q.; Li, C. Total Synthesis of (+)-Decursivine. Org. Lett. 2011, 13, 5302–5305. [Google Scholar] [CrossRef]
- Ashraf, R.; Zahoor, A.F.; Ali, K.G.; Nazeer, U.; Saif, M.J.; Mansha, A.; Chaudhry, A.R.; Irfan, A. Development of novel transition metal-catalyzed synthetic approaches for the synthesis of a dihydrobenzofuran nucleus: A review. RSC Adv. 2024, 14, 14539–14581. [Google Scholar] [CrossRef]
- Chen, W.; Yang, X.-D.; Li, Y.; Yang, L.-J.; Wang, X.-Q.; Zhang, G.-L.; Zhang, H.-B. Design, synthesis and cytotoxic activities of novel hybrid compounds between dihydrobenzofuran and imidazole. Org. Biomol. Chem. 2011, 9, 4250–4255. [Google Scholar] [CrossRef]
- Wang, D.-H.; Yu, J.-Q. Highly Convergent Total Synthesis of (+)-Lithospermic Acid via a Late-Stage Intermolecular C−H Olefination. J. Am. Chem. Soc. 2011, 133, 5767–5769. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-A.; Yue, L.; Zhu, J.; Ren, H.; Zhang, H.; Hu, D.-Y.; Han, G.; Feng, J.; Nan, Z.-D. Total synthesis of Tasimelteon. Tetrahedron Lett. 2019, 60, 1986–1988. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Srinivasan, V.; Poeggeler, B.; Hardeland, R.; Cardinali, D.P. Drug Insight: The use of melatonergic agonists for the treatment of insomnia—Focus on ramelteon. Nat. Clin. Pract. Neurol. 2007, 3, 221–228. [Google Scholar] [CrossRef]
- Scheide, M.R.; Schneider, A.R.; Jardim, G.A.M.; Martins, G.M.; Durigon, D.C.; Saba, S.; Rafique, J.; Braga, A.L. Electrochemical synthesis of selenyl-dihydrofurans via anodic selenofunctionalization of allyl-naphthol/phenol derivatives and their anti-Alzheimer activity. Org. Biomol. Chem. 2020, 18, 4916–4921. [Google Scholar] [CrossRef]
- Azevedo, A.R.; Cordeiro, P.S.; Strelow, D.N.; Andrade, K.N.; Neto, M.R.S.; Fiorot, R.G.; Brüning, C.A.; Braga, A.L.; Lião, L.M.; Bortolatto, C.F.; et al. Green Approach for the Synthesis of Chalcogenyl-2,3-dihydrobenzofuran Derivatives Through Allyl-phenols/Naphthols and Their Potential as MAO-B Inhibitors. Chem. Asian J. 2023, 18, e202300586. [Google Scholar] [CrossRef]
- Hellwig, P.S.; Barcellos, A.M.; Furst, C.G.; Alberto, E.E.; Perin, G. Oxyselenocyclization of 2-Allylphenols for the Synthesis of 2,3-Dihydrobenzofuran Selenides. ChemistrySelect 2021, 6, 13884–13889. [Google Scholar] [CrossRef]
- Bartz, R.H.; Souz, P.S.; Iarocz, L.E.B.; Hellwig, P.S.; Jacob, R.G.; Silva, M.S.; Lenardão, E.J.; Perin, G. Greening the Synthesis of 2,3-Dihydrobenzofuran Selenides: I2/TBHP-Promoted Selenocyclization of 2-Allylphenols. Eur. J. Org. Chem. 2025, 28, e202401243. [Google Scholar] [CrossRef]
- Zhou, C.F.; Zhang, Y.-Q.; Ling, Y.; Ming, L.; Xi, X.; Liu, G.-Q.; Zhang, Y. Time-economical synthesis of selenofunctionalized heterocycles via I2O5-mediated selenylative heterocyclization. Org. Biomol. Chem. 2022, 20, 420–426. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Magolda, R.L.; Sipio, W.J.; Barnette, W.E.; Lysenko, Z.; Joullie, M.M. Phenylselenoetherification. A highly efficient cyclization process for the synthesis of oxygen- and sulfur-heterocycles. J. Am. Chem. Soc. 1980, 102, 3784–3793. [Google Scholar] [CrossRef]
- Kostić, M.; Verdía, P.; Fernández-Stefanuto, V.; Puchta, R.; Tojo, E. A mild and efficient procedure for alkenols oxyselenocyclization by using ionic liquids. J. Phys. Org. Chem. 2019, 32, e3928. [Google Scholar] [CrossRef]
- Okuma, K.; Seto, J.-I. Synthesis of Indoles, 3,1-Benzoxazines, and Quinolines from 2-Alkenylanilides and Active Seleniums. Phosphorus Sulfur Silicon Relat. Elem. 2010, 185, 1014–1020. [Google Scholar] [CrossRef]
- Chen, R.; Zheng, T.; Jaing, X.; Yeung, Y.-Y. Cyclopropenium Sulfide as Lewis Base Catalyst for Chemoselective and Regioselective Electrophilic Selenylation of Phenols. ACS Catal. 2024, 14, 9198–9206. [Google Scholar] [CrossRef]
- Tiecco, M.; Testaferri, L.; Tingoli, M.; Bartoli, D.; Balducci, R. Ring-closure reactions initiated by the peroxydisulfate ion oxidation of diphenyl diselenide. J. Org. Chem. 1990, 55, 429–434. [Google Scholar] [CrossRef]
- Yoon, T.P.; Ischay, M.A.; Du, J. Visible light photocatalysis as a greener approach to photochemical synthesis. Nat. Chem. 2010, 2, 527–532. [Google Scholar] [CrossRef]
- Vergaelen, M.; Verbraeken, B.; Van Guyse, J.F.R.; Podevyn, A.; Tigrine, A.; De la Rosa, V.R.; Monnery, B.D.; Hoogenboom, R. Ethyl acetate as solvent for the synthesis of poly(2-ethyl-2- oxazoline). Green Chem. 2020, 22, 1747–1753. [Google Scholar] [CrossRef]
- Peglow, T.J.; Nobre, P.C.; Thomaz, J.P.S.S.C.; Vieira, M.M.; Junior, H.C.S.; Dalberto, B.T.; Schneider, P.H.; Rodembusch, F.S.; Nascimento, V. Synthesis and Photophysical Evaluation of Dialkynyl-N-(het)arylpyrroles: Promising Key Compounds in Fluorescence Chemistry. Asian J. Org. Chem. 2024, 13, e202300655. [Google Scholar] [CrossRef]
- Peglow, T.J.; Martins, C.C.; Da Motta, K.P.; Luchese, C.; Wilhelm, E.A.; Stieler, R.; Schneider, P.H. Synthesis and biological evaluation of 5-chalcogenyl-benzo[h]quinolines via photocyclization of arylethynylpyridine derivatives. New J. Chem. 2022, 46, 23030–23038. [Google Scholar] [CrossRef]
- Li, Y.-N.; Chen, F.; Zhang, X.-G.; Tu, H.-Y. Iodine-Mediated Regioselective Radical Cyclization of o-Vinylaryl Isocyanides with Disulfides/Diselenides Leading to 2-Chalcogenated Quinolines. Adv. Synth. Catal. 2023, 365, 3814–3818. [Google Scholar] [CrossRef]
- Meng, Z.; Shi, M.; Wei, Y. Iodine radical mediated cascade [3 + 2] carbocyclization of ene-vinylidenecyclopropanes with thiols and selenols via photoredox catalysis. Org. Chem. Front. 2024, 11, 1395–1403. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, G.; Jiang, L.; Dong, Y.; Zhan, X.; Sun, F.; Du, Y. Construction of C-S and C-Se Bonds Mediated by Hypervalent Iodine Reagents Under Metal-Free Conditions. Curr. Org. Chem. 2022, 26, 1935–1953. [Google Scholar] [CrossRef]
- Tian, S.-Y.; Ai, J.-J.; Han, J.-H.; Rao, W.; Shen, S.-S.; Sheng, D.; Wang, S.-Y. Photoinduced Construction of Thieno [3,4-c]quinolin-4(5H)-ones/Selenopheno [3,4-c]quinolin-4(5H)-ones Using Diphenyl Disulfide or Diphenyl Diselenide as Sulfur or Selenium Sources. J. Org. Chem. 2023, 88, 828–837. [Google Scholar] [CrossRef]
- Jiang, S.; Leng, Y.; Wang, P.; Sui, K.; Ma, N.; Wu, Y. Visible-Light-Induced Regioselective Selenohydroxylation of Enamine Amides with Diaryl Diselenides. Eur. J. Org. Chem. 2024, 27, e202400600. [Google Scholar] [CrossRef]
- Yang, H.; Li, W.; Wang, Y.; Zhu, H.; Le, Z.; Xie, Z. Photo-induced organoselenium-catalyzed synthesis of 2-substituted quinazoline derivatives. J. Mol. Struct. 2024, 1297, 136940. [Google Scholar] [CrossRef]
- Chillal, A.S.; Bhawale, R.T.; Kshirsagar, U.A. Photoinduced Regioselective Chalcogenation and Thiocyanation of 4H-Pyrido [1,2-a] pyrimidin-4-ones Under Benign Conditions. Eur. J. Org. Chem. 2023, 26, e202300665. [Google Scholar] [CrossRef]
- Dalberto, B.T.; Vieira, M.M.; Padilha, N.B.; Stieler, R.; Schneider, P.H. Visible-Light-Mediated Cyclization of 1,3-Diones and Chalcogenoalkynes: An Eco-Friendly Protocol for the Regioselective Formation of Polysubstituted Chalcogenofurans. ChemCatChem 2024, 16, e202400869. [Google Scholar] [CrossRef]
- Rodrigues, I.; Barcellos, A.M.; Belladona, A.L.; Roehrs, J.A.; Cargnelutti, R.; Alves, D.; Perin, G.; Schumacher, R.F. Oxone®-mediated direct arylselenylation of imidazo [2,1-b]thiazoles, imidazo [1,2-a]pyridines and 1H-pyrazoles. Tetrahedron 2018, 74, 4242–4246. [Google Scholar] [CrossRef]
- Bian, M.; Hua, J.; Ma, T.; Xu, J.; Cai, C.; Yang, Z.; Liu, C.; He, W.; Fang, Z.; Guo, K. Continuous-flow electrosynthesis of selenium-substituted iminoisobenzofuran via oxidative cyclization of olefinic amides and diselenides. Org. Biomol. Chem. 2021, 19, 3207–3212. [Google Scholar] [CrossRef]
- Beukeaw, D.; Noikham, M.; Yotphan, S. Iodine/persulfate-promoted site-selective direct thiolation of quinolones and uracils. Tetrahedron 2019, 75, 130537. [Google Scholar] [CrossRef]
- Kumar, P.; Bhalla, A. Reaction Pattern and Mechanistic Aspects of Iodine and Iodine-Based Reagents in Selenylation of Aliphatic, Aromatic, and (Hetero)Cyclic Systems. Top Curr. Chem. 2024, 382, 12. [Google Scholar] [CrossRef]
- Kosso, A.R.O.; Kabri, Y.; Broggi, J.; Redon, S.; Vanelle, P. Sequential Regioselective Diorganochalcogenations of Imidazo [1,2-a]pyrimidines Using I2/H3PO4 in Dimethylsulfoxide. J. Org. Chem. 2020, 85, 3071–3081. [Google Scholar] [CrossRef]
- Win, K.M.N.; Sonawane, A.D.; Koketsu, M. Synthesis of selenated tetracyclic indoloazulenes via iodine and diorganyl diselenides. Org. Biomol. Chem. 2021, 19, 3199–3206. [Google Scholar] [CrossRef] [PubMed]
- Palomba, M.; Angeli, A.; Galdini, R.; Hughineata, A.J.; Pering, G.; Lenardão, E.J.; Marini, F.; Santi, C.; Supuran, C.T.; Bagnoli, L. Iodine/Oxone® oxidative system for the synthesis of selenylindoles bearing a benzenesulfonamide moiety as carbonic anhydrase I, II, IX, and XII inhibitors. Org. Biomol. Chem. 2024, 22, 6532–6542. [Google Scholar] [CrossRef] [PubMed]
- Dhurey, A.; Mandal, S.; Pramanik, A. I2/DMSO-Promoted Synthesis of Diaryl Sulfide- and Selenide-Embedded Arylhydrazones. J. Org. Chem. 2023, 88, 5377–5390. [Google Scholar] [CrossRef] [PubMed]
- Thedy, M.E.C.; Gularte, M.M.; Azeredo, J.B.; Braga, A.L. I2/DMSO Mediated Direct Selenylation of Uracils with Diorganoyl Diselenides—A Simple Protocol to Access 5-Selanyl-Uracils. ChemistrySelect 2024, 9, e202402057. [Google Scholar] [CrossRef]
- Jiang, H.; Schen, H.; Li, C.; Jin, Z.; Shang, Y.; Chen, Y.; Yi, M.; Du, J.; Gui, Q.-W. Synthesis of Seleno Oxindoles via Iodine-Induced Radical Cyclization of N-Arylacrylamides with Diorganyl Diselenides. Synthesis 2022, 54, 2669–2676. [Google Scholar] [CrossRef]
- Yi, R.; Liu, S.; Gao, H.; Liang, Z.; Xu, X.; Li, N. Iodine-promoted direct thiolation (selenylation) of imidazole with disulfides (diselenide): A convenient and metal-free protocol for the synthesis of 2-arylthio(seleno)imidazole. Tetrahedron 2020, 76, 130951. [Google Scholar] [CrossRef]
- Liu, M.; Li, Y.; Yu, L.; Xu, Q.; Jiang, X. Visible light-promoted, iodine-catalyzed selenoalkoxylation of olefins with diselenides and alcohols in the presence of hydrogen peroxide/air oxidant: An efficient access to α-alkoxyl selenides. Sci. China Chem. 2018, 61, 294–299. [Google Scholar] [CrossRef]
- Du, H.-A.; Tang, R.-Y.; Deng, C.-L.; Liu, Y.; Li, J.-H.; Zhang, X.-G. Iron-Facilitated Iodine-Mediated Electrophilic Annulation of N,N-Dimethyl-2-alkynylanilines with Disulfides or Diselenides. Adv. Synth. Catal 2011, 353, 2739–2748. [Google Scholar] [CrossRef]
- Tran, V.H.; Nguyen, A.T.; Kim, H.-K. Tin(II) Chloride-Catalyzed Direct Esterification and Amidation of tert-Butyl Esters Using α,α-Dichlorodiphenylmethane Under Mild Conditions. J. Org. Chem. 2023, 88, 13291–13302. [Google Scholar] [CrossRef]
- Masuyama, Y.; Hayashi, M.; Suzuki, N. SnCl2-Catalyzed Propargylic Substitution of Propargylic Alcohols with Carbon and Nitrogen Nucleophiles. Eur. J. Org. Chem. 2013, 2013, 2914–2921. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Agrawal, N.; Mali, S.M.; Jadhav, S.V.; Gopi, H.N. Tin(ii) chloride assisted synthesis of N-protected γ-amino β-keto esters through semipinacol rearrangement. Org. Biomol. Chem. 2010, 8, 4855–4860. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, P.; Perumal, P.T. SnCl2·2H2O—An Alternative to Lewis Acidic Ionic Liquids. Chem. Lett. 2006, 35, 632–633. [Google Scholar] [CrossRef]
- Akiyama, T.; Onuma, Y. Tin(ii) chloride mediated allylation of aldimines generated in situ with allylstannane in water. J. Chem. Soc. 2002, 1, 1157–1158. [Google Scholar] [CrossRef]
- Aichhorn, S.; Himmelsbach, M.; Schöfberger, W. Synthesis of quinoxalines or quinolin-8-amines from N-propargyl aniline derivatives employing tin and indium chlorides. Org. Biomol. Chem. 2015, 13, 9373–9380. [Google Scholar] [CrossRef]
- Marjani, A.P.; Khalafy, J.; Salami, F.; Mohammadlou, M. Tin(II) Chloride Catalyzed Synthesis of New Pyrazolo [5,4-b]quinolines under Solvent-Free Conditions. Synthesis 2015, 47, 1656–1660. [Google Scholar] [CrossRef]
- Menezes, F.D.L.; Guimarães, M.D.O.; Silva, M.J. Highly Selective SnCl2-Catalyzed Solketal Synthesis at Room Temperature. Ind. Eng. Chem. Res. 2013, 52, 16709–16713. [Google Scholar] [CrossRef]
- Schendera, E.; Unkel, L.-N.; Quyen, P.P.H.; Salkewitz, G.; Hoffmann, F.; Villinger, A.; Brasholz, M. Visible-Light-Mediated Aerobic Tandem Dehydrogenative Povarov/Aromatization Reaction: Synthesis of Isocryptolepines. Chem. Eur. J. 2020, 26, 269–274. [Google Scholar] [CrossRef]
- Maejima, S.; Yamaguchi, E.; Itoh, A. Intermolecular Tandem Addition/Esterification Reaction of Alkenes with Malonates Leading to γ-Lactones Mediated by Molecular Iodine under Visible Light Irradiation. Adv. Synth. Catal. 2017, 359, 3883–3887. [Google Scholar] [CrossRef]
- Maejima, S.; Yamaguchi, E.; Itoh, A. trans-Diastereoselective Syntheses of γ-Lactones by Visible Light-Iodine-Mediated Carboesterification of Alkenes. ACS Omega 2019, 4, 4856–4870. [Google Scholar] [CrossRef]
- Maejima, S.; Yamaguchi, E.; Itoh, A. Three-Component Iminolactonization Reaction via Bifunctionalization of Olefins Using Molecular Iodine and Visible Light. J. Org. Chem. 2020, 85, 10709–10718. [Google Scholar] [CrossRef]



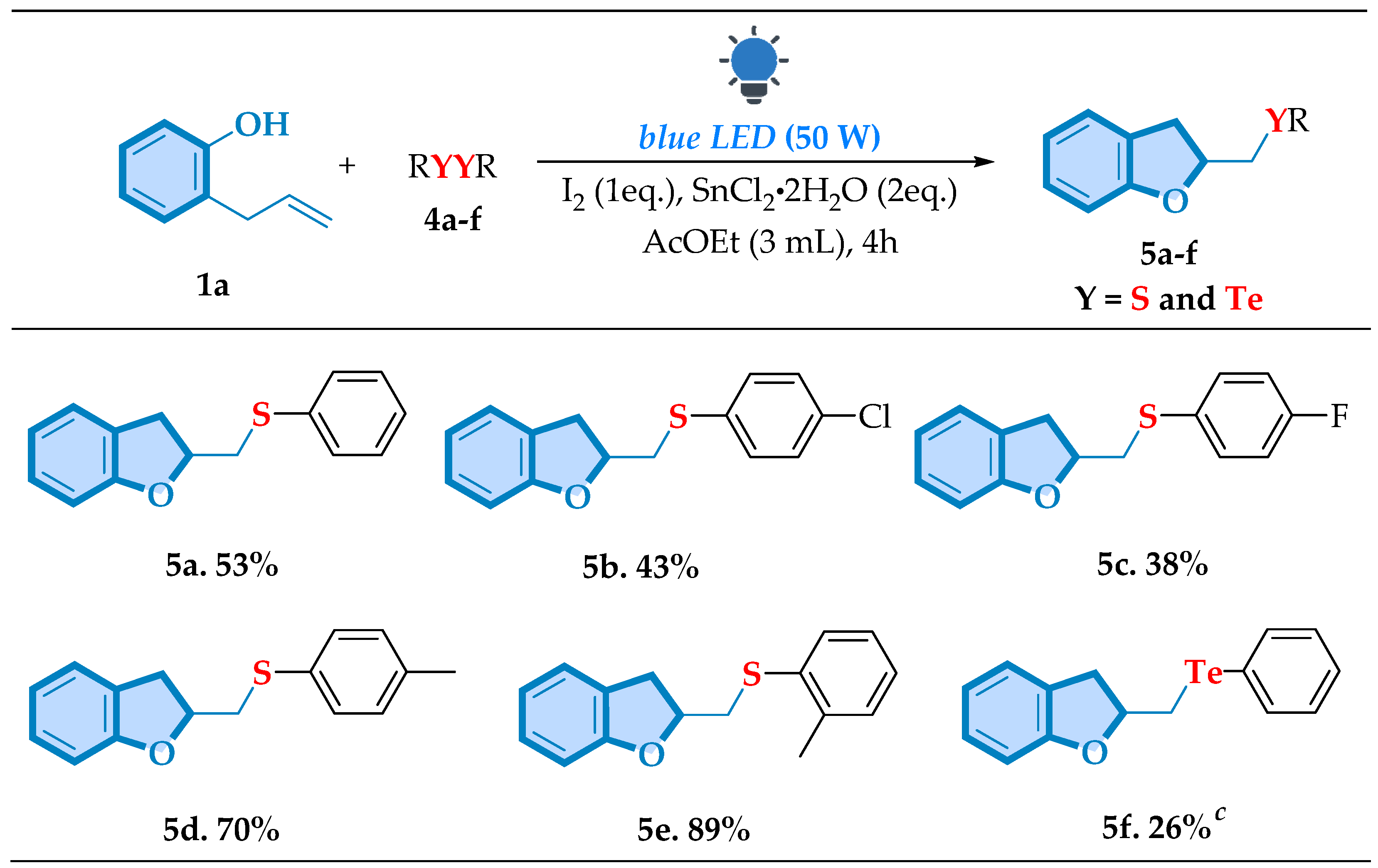
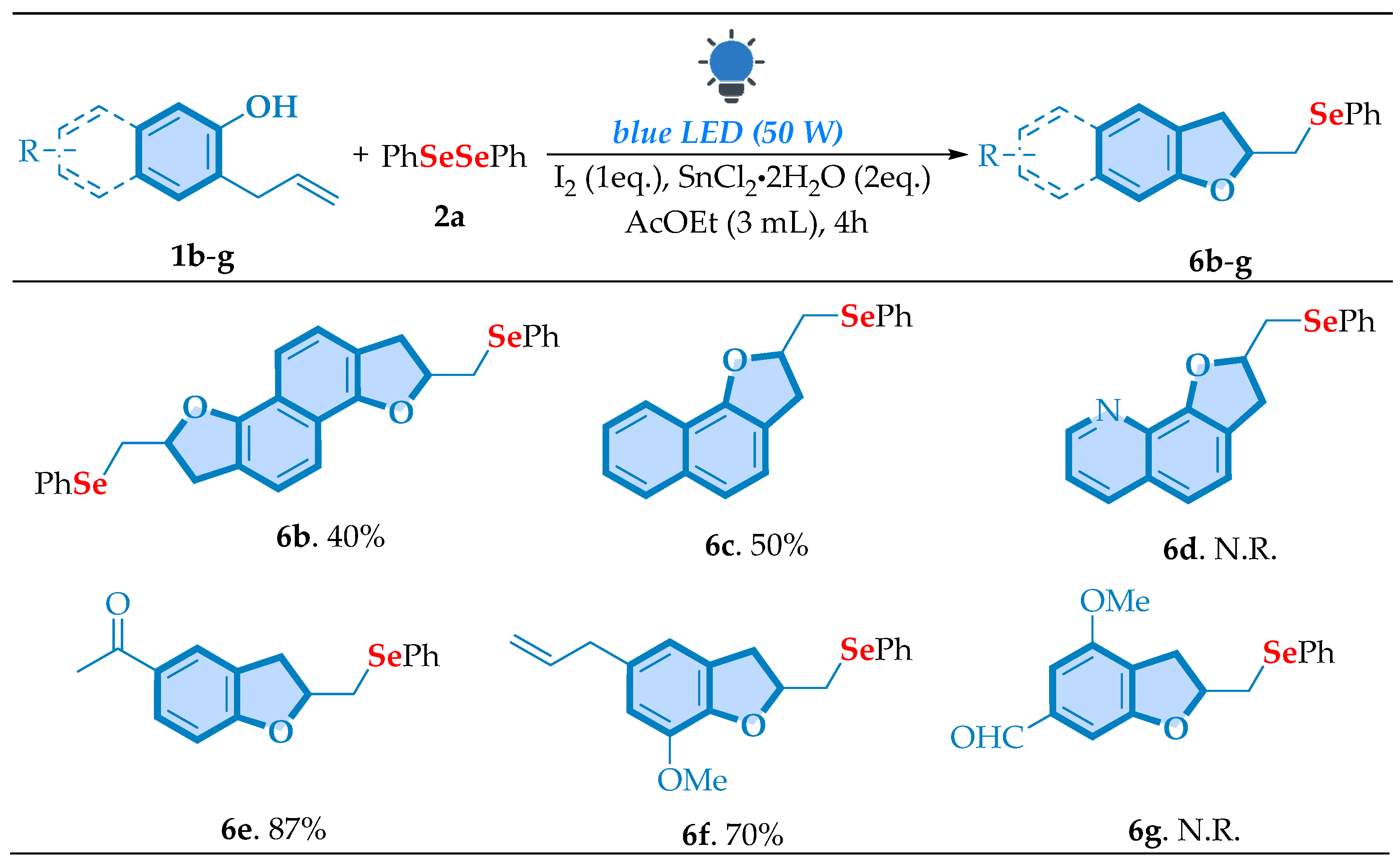
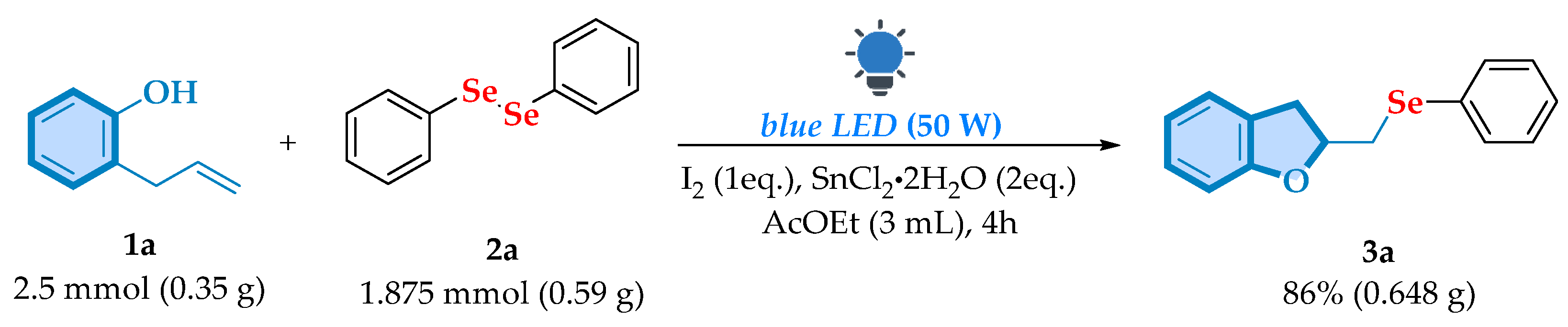


 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | I2 (Equiv.) | Visible Light | Photocatalyst | Additive (Equiv.) | Solvent | Time (h) | Yield (%) b |
| 1 | - | Blue LED | Eosin Y (5 mol%) | - | DCM | 24 | 41 |
| 2 | - | Blue LED | Eosin Y (5 mol%) | InCl3 (2 equiv.) | DCM | 24 | 56 |
| 3 | - | Blue LED | Eosin Y (5 mol%) | SnCl2·2H2O (1 equiv.) | DCM | 24 | 58 |
| 4 | - | Blue LED | Eosin Y (5 mol%) | SnCl2·2H2O (2 equiv.) | DCM | 24 | 63 |
| 5 | - | Blue LED | Eosin Y (5 mol%) | SnCl2·2H2O (4 equiv.) | DCM | 24 | 50 |
| 6 | - | Blue LED | Eosin Y (5 mol%) | SnCl4 (2 equiv.) | DCM | 24 | NR |
| 7 | 1 | Blue LED | Eosin Y (5 mol%) | SnCl2·2H2O (2 equiv.) | DCM | 4 | 72 |
| 8 | 1 | Blue LED | - | SnCl2·2H2O (2 equiv.) | DCM | 4 | 83 |
| 9 c | 1 | Blue LED | - | SnCl2·2H2O (2 equiv.) | DCM | 4 | 98 |
| 10 c | 2 | Blue LED | - | SnCl2·2H2O (2 equiv.) | DCM | 4 | 95 |
| 11 c | 0.1 | Blue LED | - | SnCl2·2H2O (2 equiv.) | DCM | 4 | traces |
| 12 c | 1 | Green LED | - | SnCl2·2H2O (2 equiv.) | DCM | 4 | NR |
| 13 c | 1 | White LED | - | SnCl2·2H2O (2 equiv.) | DCM | 4 | traces |
| 14 c | 1 | Blue LED | - | SnCl2·2H2O (2 equiv.) | MeCN | 4 | 55 |
| 15 c | 1 | Blue LED | - | SnCl2·2H2O (2 equiv.) | acetone | 4 | 45 |
| 16 c | 1 | Blue LED | - | SnCl2·2H2O (2 equiv.) | Toluene | 4 | 40 |
| 17 c | 1 | Blue LED | - | SnCl2·2H2O (2 equiv.) | Hexane | 4 | traces |
| 18 c | 1 | Blue LED | - | SnCl2·2H2O (2 equiv.) | AcOEt | 4 | 95 |
| 19 c | 1 | Blue LED | - | SnCl2·2H2O (2 equiv.) | THF | 4 | 55 |
| 20 c | 1 | Blue LED | - | SnCl2·2H2O (2 equiv.) | EtOH | 4 | NR |
| 21 c,d | 1 | Blue LED | - | SnCl2·2H2O (2 equiv.) | AcOEt | 4 | 56 |
| 22 c,e | 1 | - | - | SnCl2·2H2O (2 equiv.) | AcOEt | 4 | 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, L.S.; Silva, M.C.; Nobre, P.C.; Peglow, T.J.; Nascimento, V. Synthesis of 2,3-Dihydrobenzofuran Chalcogenides Under Visible Light: A Sustainable Approach. Processes 2025, 13, 1038. https://doi.org/10.3390/pr13041038
Gomes LS, Silva MC, Nobre PC, Peglow TJ, Nascimento V. Synthesis of 2,3-Dihydrobenzofuran Chalcogenides Under Visible Light: A Sustainable Approach. Processes. 2025; 13(4):1038. https://doi.org/10.3390/pr13041038
Chicago/Turabian StyleGomes, Luana S., Millena C. Silva, Patrick C. Nobre, Thiago J. Peglow, and Vanessa Nascimento. 2025. "Synthesis of 2,3-Dihydrobenzofuran Chalcogenides Under Visible Light: A Sustainable Approach" Processes 13, no. 4: 1038. https://doi.org/10.3390/pr13041038
APA StyleGomes, L. S., Silva, M. C., Nobre, P. C., Peglow, T. J., & Nascimento, V. (2025). Synthesis of 2,3-Dihydrobenzofuran Chalcogenides Under Visible Light: A Sustainable Approach. Processes, 13(4), 1038. https://doi.org/10.3390/pr13041038







