Abstract
This review comprehensively examines the advancements and challenges in anaerobic digestion (AD) for biogas production, emphasising technological, microbial, and policy perspectives. It highlights the AD significant potential for valorising diverse organic substrates, including manure, food waste, and microalgae, thereby contributing to renewable energy generation and greenhouse gas mitigation. Key operational factors influencing biogas yield include substrate composition, temperature (preferably mesophilic conditions), pH (6.5–7.5), and the substrate-to-inoculum ratio (SIR), all of which significantly affect microbial activity and process stability. Co-digestion strategies and pretreatments are examined for their roles in enhancing biodegradability and methane yield, respectively. Microbial community dynamics, particularly responses to feedstock heterogeneity and operational parameters, are integral to process optimisation. Advances in metagenomics have provided insights into microbial resilience and adaptation to conditions such as high ammonium levels. This review also discusses various modelling approaches, including kinetic models and machine learning techniques, for predicting and optimising biogas production. Additionally, policy frameworks within regions such as the European Union and Brazil, along with economic incentives and regulatory hurdles, are also considered crucial for scaling up deployment. Challenges such as digestate management and high capital costs persist, underscoring the need for integrated strategies to enhance the sustainability and viability of AD-based biogas projects.
1. Introduction
Anaerobic digestion (AD) is a biological process that transforms organic matter into biogas (primarily methane (CH4) and carbon dioxide (CO2)) through the metabolic activity of diverse microbial consortia under oxygen-free conditions. This process comprises four sequential stages: hydrolysis, acidogenesis, acetogenesis, and methanogenesis, each catalysed by specific microbial groups as hydrolytic bacteria (e.g., Clostridium, Bacteroides), fermentative bacteria (Lactobacillus), syntrophic acetogens (Syntrophomonas), and methanogenic archaea (Methanosaeta, Methanosarcina) [1], as illustrated in Figure 1.
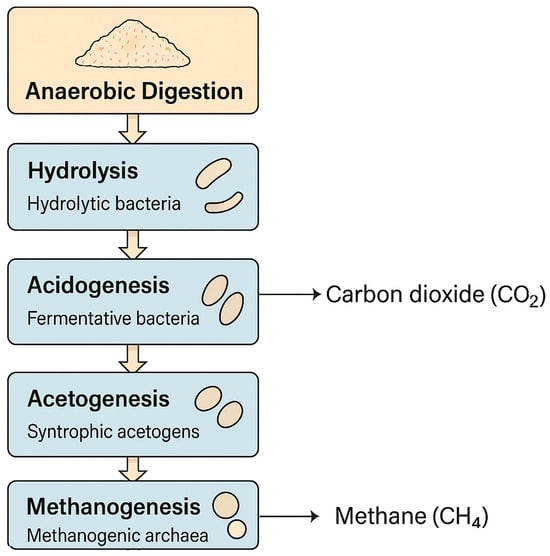
Figure 1.
Anaerobic digestion process for biogas production.
AD has gained prominence as a sustainable technology for waste valorisation and renewable energy generation, particularly in the context of circular bioeconomy and climate mitigation strategies [2]. Its versatility allows the treatment of various feedstocks, including agricultural residues, food waste, and microalgae, while producing biogas that can be upgraded to biomethane for industrial and transportation applications [3]. Despite its potential, AD systems face challenges related to substrate complexity, process instability, and economic feasibility. These issues have prompted the development of advanced pretreatment methods, co-digestion strategies, and predictive modelling approaches [4,5].
AD is a promising technology for managing organic waste and generating bioenergy [6,7,8]. However, conventional AD processes frequently encounter operational challenges, including structural complexity, diverse substrate characteristics, low productivity, inefficient biodegradability, and poor stability [9]. These limitations can hinder biogas and biomethane production. To overcome these shortcomings and enhance AD performance, the scientific community has increasingly emphasised various process intensification strategies [9]. This review aims to provide a comprehensive overview of recent advances and persistent challenges in AD, integrating technological, microbial, and policy perspectives to support its broader implementation.
2. Strategies to Optimise Anaerobic Co-Digestion for Biomethane Production
Pretreatment of substrates has considerably influenced AD efficiency. Examples include the thermo-alkali pretreatment of sewage sludge, which resulted in a 36% (Figure 1) increase in biogas production (using 2.29 M NaOH at 88.50 °C). Similarly, thermal-alkali pretreatment of dairy cattle manure achieved a 23.6% (Figure 2) improvement in methane production (with 10% NaOH at 100 °C for 5 min). Bio-physicochemical pretreatment of oily wastewater increased biogas production by 280% (with Bacillus sp., sonication, and acidification), as depicted in Figure 2 [9]. Pretreatments with N-methylmorpholine-N-oxide on rice straw, wheat straw, and soft spruce improved methane yield by 400–1200%. Conversely, Thermal pretreatment of food waste also resulted in a 23.68% increase in methane yield [10].
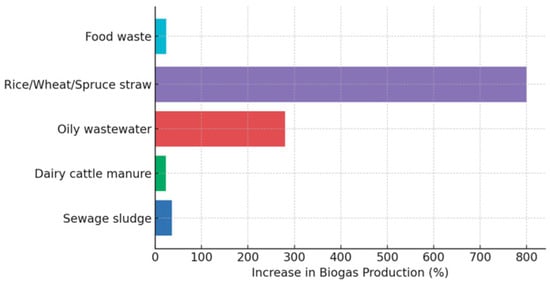
Figure 2.
Comparative efficiency of pretreatment methods [9,10].
Figure 2 illustrates how different substrates respond to pretreatment in terms of biogas production improvement. The core insight is that lignocellulosic materials such as rice, wheat, and spruce straw, exhibit the highest potential for enhancement, achieving increases significantly greater than other feedstocks. This suggests that pretreatment is particularly effective for fibrous, structurally complex substrates where hydrolysis is a limiting step. In contrast, food waste, sewage sludge, and dairy manure show minimal gains, indicating that these substrates are already highly degradable and less dependent on pretreatment. Oily wastewater demonstrates a moderate improvement, reflecting its high energy content but also the need for strategies to overcome inhibitory compounds. Overall, the figure highlights that pretreatment benefits are substrate-specific and most impactful for recalcitrant biomass. A comparison of most of the treatments presented in Figure 2, in terms of ml CH4/gVS, is provided in Table 1.

Table 1.
Methane yield of different pretreatments.
However, physical pretreatments have been shown to affect cellulose crystallinity, degree of polymerisation, particle size, surface area, and pore size of the starting material. Thermochemical pretreatment reduces particle size and increases volatile solids (VS) reduction and chemical oxygen demand (COD) solubility [9,11]. From a process stability perspective, hydrolysis rates are high in physical techniques (especially microwave) and combined approaches, while biological methods exhibit slower kinetics [9]. Chemical techniques, however, show variable results depending on the operating conditions. Regarding the generation of inhibitory compounds, physical and combined methods tend to form toxic compounds (phenolic derivatives), particularly under extreme thermal conditions. Chemical techniques (acid/alkali) also present this drawback, unlike ozonolysis. Conversely, biological methods do not generate inhibitors, which constitutes a significant advantage [9] (Figure 3).
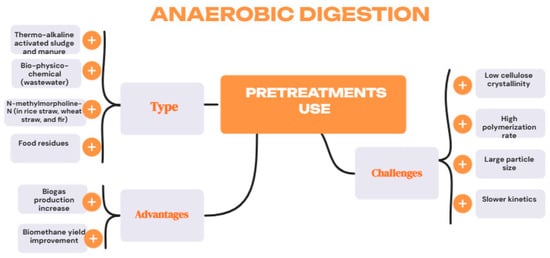
Figure 3.
Pretreatment in anaerobic digestion. Types, advantages and challenges.
In terms of technical and economic aspects, biological techniques stand out due to their low energy requirements, which favour their implementation in sustainable systems. Conversely, physical, chemical, and combined methods involve high energy costs and are limited to large-scale applications. In terms of operational cost, biological (particularly fungal) methods are generally cost-effective, whereas physical and chemical methods incur high costs associated with equipment, reagents, and waste treatment. Enzymatic pretreatment, although effective, can be expensive due to the high cost of commercial enzymes [9].
One of the most effective approaches to boost AD yield is anaerobic co-digestion (An-CoD) [12,13]. This process involves the simultaneous biotreatment of a mixture of at least two different substrates or feedstocks under anaerobic conditions. An-CoD offers multiple advantages over monodigestion of individual feedstocks [14,15]. For example, it improves substrate biodegradation, increases biogas and biomethane production, and optimises cost-effectiveness. The flexibility of An-CoD allows for adjusting the carbon-to-nitrogen (C/N) ratio [10], improving pH buffering capacity, and balancing essential macronutrients such as carbon, sulphur, phosphorus, and nitrogen. Additionally, it facilitates the handling of substrates containing toxic or inhibitory components, by diluting them to levels less detrimental to the microbial community. Productivity in co-digestion has been reported to show significant improvements, ranging from 25% to 400% compared to monodigestion. Examples of successful combinations include sewage sludge co-digested with agricultural waste, municipal solid waste, cattle manure, and chicken manure [9]. Co-digestion has also demonstrated the ability to enhance the biodegradation of micropollutant organic compounds through increased co-metabolic heterotrophism [16].
In the case of a mixture of litter, fruit and vegetable wastes (40:60), a maximum methane yield was achieved by saving 2% extra water [17]. Co-digestion of horse manure and food waste in three-stage reactors resulted in an increased methane yield of 11–23% [9,18]. Co-digestion of brewery waste (wastewater and sludge) with brewery spent grains (12.5%) obtained the highest methane yield which was 20.66 times higher than the control [19], Table 2 shows the description of the An-CoD systems discussed above.

Table 2.
Systems description of different co-digestions [9,17,18,19].
An-CoD has established itself as an effective strategy to improve the efficiency and stability of organic waste digestion processes. One of its key benefits is the management of inhibitors and the improvement of the system’s buffering capacity, thereby facilitating the dilution of toxic compounds [20,21]. The use of food waste as an acidifier has been shown to improve lignocellulosic biomass hydrolysis by 28% and to reduce cellulose crystallinity by 6.7%. Further, the addition of grass clippings can help stabilise pH levels [22].
Regarding the C/N ratio (Figure 4), co-digestion allows more efficient adjustments than mono-digestion. For example, a C/N ratio of 25 in the co-digestion of rice straw with Hydrilla verticillata maximised biogas production. In contrast, a C/N ratio of 30, using food waste and rice straw, increased methane yield by 94.41%. vs. removal is also significantly improved with optimised mixtures: up to 73% for primary sludge and plant residues (70:30), and 72.87% with cow manure, flax, and wheat straw (50:25:25), yielding methane yields of 351 mL CH4/gVS [22].
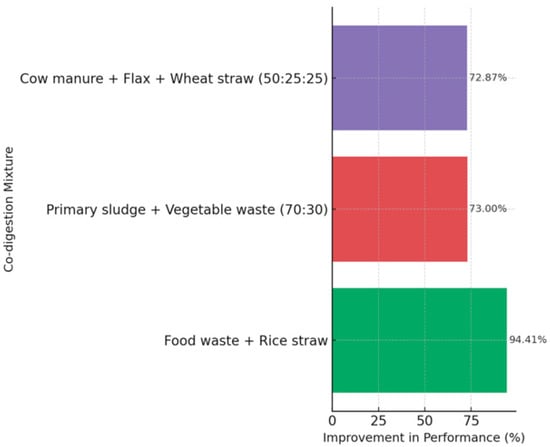
Figure 4.
Effect of C/N Ratio and Co-Digestion Composition on Biogas Performance [22].
While all combinations significantly enhance process efficiency, the mixture of food waste with rice straw stands out with the highest improvement, suggesting a strong synergistic effect, likely due to its balanced nutrient profile and high biodegradability. In contrast, mixtures involving cow manure with lignocellulosic residues and primary sludge with vegetable waste show moderate gains, indicating that structural complexity and fibre content may limit microbial accessibility. Overall, the results highlight that substrate selection is critical, and pairing easily degradable organic matter with complementary fibrous material can substantially optimise biogas yield.
Standard operating parameters include mesophilic temperatures (37 °C) [17], substrate-to-inoculant ratios of 0.3–0.9 [23,24], and total solids levels adjusted to avoid volatile fatty acid (VFA) accumulation. At the microbiological level, co-digestion stimulates synergies between raw materials and improves microbial enzymatic activity [22].
From a technical and economic point of view, co-digestion is considered a cost-effective technology, applicable on a large scale, and requiring lower equipment requirements than high-density AD [25]. Economic analyses depend on the local context (feedstock, quality control, and technology) [14]. Bioenergy recovery can reach 0.348 kWh/kg TVS and 1556 MJ/kg TVS in heat, avoiding 0.114 kg CO2-eq/kg TVS, thereby highlighting its potential as a strategy for climate mitigation and energy valorisation of waste [19].
Reactor configuration and operating conditions are also determining factors for optimising biogas and biomethane production. Several modifications have been suggested, such as the use of two-stage or multi-stage reactor systems, where hydrolysis and acidogenesis are separated from acetogenesis and methanogenesis, allowing pH and temperature conditions to be optimised independently [26]. Temperature phased AD reactors, which involve a thermophilic stage followed by a mesophilic stage, have also proven effective. Optimal reactor design, along with control of factors such as pH, agitation, temperature, hydraulic retention time (HRT), OLR and C/N ratio, is essential for the efficient operation of biodigesters [27]. Temperature, in particular, is a crucial parameter, with mesophilic conditions (approximately 30–40 °C) and thermophilic conditions (approximately 40–60 °C) being the most common. Although thermophilic digestion is more efficient due to its higher reaction rate, it is also more susceptible to process instability, as evidenced by VFA accumulation and elevated ammonia levels.
Bioelectrochemical systems, including microbial electrochemical cells (MEC), represent a significant advancement and have emerged as promising technologies to enhance the efficiency of AD. These systems enhance the conversion of organic matter to methane, stabilise the process, and improve effluent quality [21,28]. Table 3 presents the methane yield increase and other characteristics for various systems.

Table 3.
Bioelectrochemical systems description [21,28].
At the microbial level, EAD promotes DIET between species and stimulates the growth of electroactive microorganisms such as Pseudomonas sp. and Acinetobacter sp., favouring methanogenesis. However, technical challenges remain. The low technological maturity of EAD requires a greater understanding of the microbe-electrode interface [21]. Additionally, the cost of electrodes is a key constraint to scaling up [18]. Despite this, the process shows high potential, with an energy conversion ratio of 12.8 and a global warming potential of only 39.6 g CO2-eq/MJ achieved in animal manure EAD [21]. The theoretical resource of EAD applied to organic solid waste in China is equivalent to 82.6% of the national natural gas consumption, underscoring its potential as a sustainable energy solution [21].
On the other hand, biogas purification technologies represent an essential component for biomethane production [29], although they do not directly intensify AD. Their primary objective is to increase the methane concentration in biogas to levels of 95–99%, which enables maximising its calorific value and viability in industrial, residential, and transportation applications [9]. The presence of impurities such as carbon dioxide (CO2), hydrogen sulphide (H2S), ammonia (NH3) and siloxanes negatively affects the energy and operational value of biogas. CO2 reduces the calorific value and makes transportation more expensive (Figure 5). At the same time, H2S is highly corrosive and must be kept below 1000 ppm for boilers, between 200 and 1000 ppm for internal combustion engines, and at 5 mg/m3 for use in gas networks or vehicles. NH3, also toxic and corrosive, should be limited to concentrations between 3 and 20 mg/m3. Siloxanes, as they form abrasive deposits on equipment, require extremely low levels, below 0.1 ppm in turbines and 5 to 10 mg/m3 in other applications.
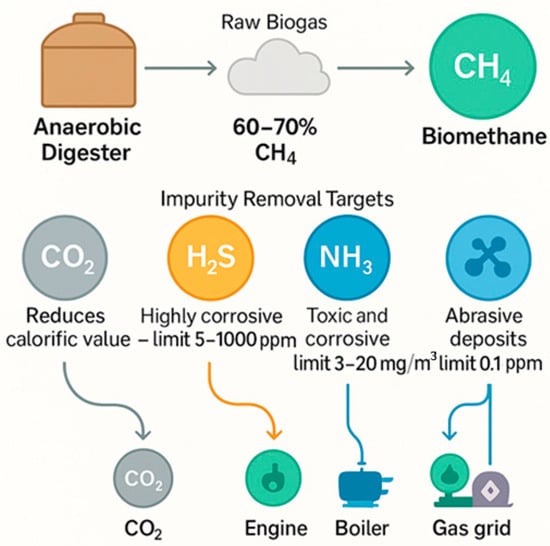
Figure 5.
Critical limits of Biogas Impurities and Their Impact on Biogas Upgrading Efficiency.
Among the purification strategies, chemical absorption by scrubbing with organic solvents allows reaching a CH4 purity of 99%, with losses of less than 0.1% and a low energy consumption of 0.05–0.25 kWh/Nm3. However, this option involves significant investment, maintenance and thermal demand costs. Membrane separation is presented as a viable option in terms of energy efficiency and costs, although its performance decreases in gas-liquid configurations. In the case of adsorption, solid porous materials such as activated carbon, silica gels, and metal-organic frameworks are used, which combine organic and inorganic components and offer high impurity capture capacity.
On the other hand, biological technologies have been explored as sustainable alternatives. In situ chemoautotrophic methanation, which involves injecting hydrogen into the digester to convert CO2 to CH4, can achieve methane concentrations of 80 to 100%. However, this approach can generate a pH increase above 8.5, inhibiting methanogenic activity. The ex situ version is carried out in a separate reactor, where hydrogen is produced through electrolysis, resulting in high energy consumption. Despite this, when renewable energy is used, an emission reduction of up to 71.4% can be achieved by using cattle manure as a substrate [26]. Additionally, biotrickling filters have been effectively used for H2S removal, achieving removal rates of between 80% and 100%. Other studies have optimised the production of biogas and biomethane from biomass such as Laminaria digitata and food waste, using two-stage AD with hydrothermal pretreatment (Table 4).

Table 4.
Two-stage AD with hydrothermal pretreatment systems description [30,31].
This approach also improved COD solubilisation and carbohydrate monomer release. Although the use of sulfuric acid or its combination with enzymolysis raised carbon conversion to 55.2%, these conditions showed lower energy conversion efficiencies (39.5–40.7%) with respect to untreated biomass or biomass treated with mild hydrothermal pretreatment alone [30,31].
Together, these technologies are crucial to ensuring the quality of biomethane, enhancing its economic and environmental performance, and positioning it as a genuine alternative to natural gas in a sustainable energy system. Ultimately, the viability of these optimisation strategies depends on a thorough analysis of their technical, economic, and environmental feasibility. While An-CoD has proven to be economically viable in several case studies, with a positive net return value and a favourable payback period, the high cost of some enzymatic pretreatments and the difficulty of synthesising, storing and transporting additives such as Zero-valent iron particles (ZVI) may limit its large-scale application. Therefore, life cycle analysis (LCA) and techno-economic analysis (TEA) are crucial to ensure that innovations not only improve biomethane production but also contribute to a sustainable circular bioeconomy approach [7,21].
Techno-economic considerations are paramount in the selection and application of biogas purification technologies, with investment and maintenance costs representing a primary determinant. Traditional physical and chemical technologies exhibit economic disadvantages, primarily due to their high energy demands and reliance on expensive chemical inputs, which substantially escalate operating costs. In contrast, biological technologies emerge as more economically viable alternatives, requiring less energy and obviating the need for expensive reagents, thereby reducing overall process costs.
An economic analysis, conducted within the Italian context, estimated the price of purchased electricity at 0.15 USD/kWh, whereas the cost of natural gas reached 0.24 USD/Nm3. Revenues from biomethane sales exhibit considerable fluctuations, with values ranging from 0.4 to 1.2 USD/Nm3, contingent upon the type and level of available government subsidies [32]. These variations in public policies directly influence the commercial viability of biomethane and its subsequent adoption within the energy market.
From an environmental perspective, the analysis also considered the potential for carbon dioxide emissions reduction. Estimated savings of 0.202 kg CO2/kWh were achieved when biomethane replaced natural gas, with even greater savings of 0.325 kg CO2/kWh observed when compared to electricity generated by the Italian grid The most significant environmental benefit was observed when 64% of the biogas underwent processing through the purification unit (F = 64%), representing the optimal scenario for maximising CO2 savings [32]. These results underscore the critical importance of integrating technical efficiency, economic feasibility, and environmental benefits into the design of biomethane production systems.
In summary (Figure 6), while AD presents a promising technology for biogas and biomethane production from organic waste, it confronts challenges such as substrate complexity, low productivity, inefficient biodegradability, and process instability [3]. To overcome these limitations, various process intensification strategies have been developed. Pretreatments (physical, chemical, thermal, biological, or combined) aim to enhance the accessibility of organic matter to microorganisms [7]. However, physical and chemical pretreatments often entail high energy requirements and costs [25]. Furthermore, some can generate toxic by-products that inhibit the process [22]. Although biological pretreatments are generally more cost-effective and prevent the formation of toxic by-products, their efficiency may be slower, and their industrial scalability remains limited by the cost of enzymes [10].
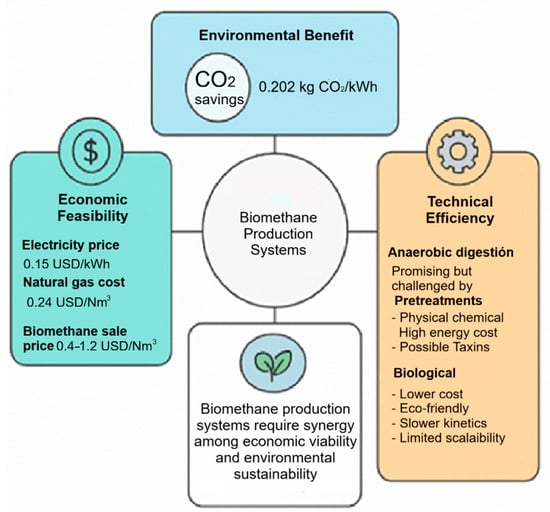
Figure 6.
Integrated evaluation of Biogas and Biomethane Production: Economic, Environmental, and Technical Perspectives.
An-CoD offers several advantages, including a favourable C/N ratio balance, dilution of inhibitory compounds, improved nutrient balance (encompassing macronutrients and trace elements), enhanced process stability, and optimised OLR [14]. An-CoD can lead to a significant increase in biogas and biomethane production, with reported yields up to 400% higher than those observed in monodigestion [33]. Moreover, it represents an economical strategy capable of valorising multiple waste streams [15,34]. However, inappropriate substrate selection may generate antagonistic rather than synergistic effects [22], and in some cases, may increase the COD of the effluent or necessitate additional pretreatments [3].
Finally, process intensification through the use of additives is a rapidly growing strategy [28]. Conductive and adsorbent materials, such as biochar, activated carbon or magnetite, can mitigate the accumulation of toxic compounds (ammonia, VFA) and promote DIET [18]. This enhancement improves process stability, accelerates methanogenesis, and increases methane production [28]. Similarly, materials with ion exchange capacity, such as zeolites and bentonites, can immobilise biomass, allowing reactors to operate with lower HRT and higher OLR [9]. Nanomaterials (e.g., metallic nanoparticles of iron, cobalt, nickel, and titanium dioxide), owing to their unique characteristics—high surface area and reactivity—stimulate microbial activity and accelerate cell growth, thereby enhancing substrate degradation and biomethane production [27]. However, the primary drawbacks of these additives are their high cost and the potential risk of toxicity if added in excess [18]. Further research is therefore essential to fully understand the mechanisms of DIET and to validate the techno-economic feasibility of these technologies on a large scale [35].
3. Functional Additives
In addition to the strategic selection of substrates, process intensification through additives has also gained attention. These additives can be classified into several categories, each with a distinct mechanism of action. One category involves the addition of macro- and micronutrients (trace metals), with supplementation using metals such as Ca2+, Fe2+, Ni2+, Zn2+, Mg2+, Cu2+, Cd2+, and W6+ at specific concentrations, which has been shown to improve biogas performance. These metals are crucial components of metalloenzymes that drive the biochemical activities of bacteria in the AD process. For example, nickel (Ni2+) can accelerate the conversion of acetate to methane, and the addition of ZVI has resulted in a substantial increase in total biomethane generation. However, it is vital to avoid excessive addition of heavy metals, as this could reduce the biochemical activity of methanogens and potentially lead to a collapse of the AD process [9].
During hydrolysis and acidogenesis, metals such as Fe and Zn can act as conductive materials, facilitating DIET and thereby enhancing the degradation of complex compounds and the production of VFA. In the subsequent acetogenesis and methanogenesis phases, Ni and Co are essential components of cofactors (e.g., coenzyme F430, cobalamins) for key enzymes involved in methanogenesis, whilst Fe contributes to iron–sulphur proteins and cytochromes [36]. Additionally, Mg and Ca play a stabilising role in cellular activity and can mitigate ammonia inhibition. The effectiveness of these additives depends on their bioaccessibility; the labile fraction of metals determines their functional impact, and chelating agents can increase metal bioavailability, reducing the required dosage [37].
Another category involves the use of conductive and absorbent materials. The accumulation of toxic and inhibitory compounds, such as ammonia or VFA, can significantly reduce biogas yield. The application of conductive carbon-based or adsorbent materials can mitigate this inhibition and thus improve production. These materials facilitate DIET, a syntrophic mechanism that expedites substrate degradation [35,38]. DIET promotes methanogen enrichment and accelerates the initiation of methanogenesis. Examples of these materials include activated carbon (in powdered or granular form), biochar, and carbon cloth. However, the high price of carbon-based nanomaterials currently prevents their widespread adoption. Therefore LCA and TEA are needed to fully understand this technology [18,27].
Biochar exerts multifaceted effects across the biochemical stages of AD. During hydrolysis and acidogenesis, its high surface area and porosity enhance microbial colonisation and aggregation, whilst stimulating the activity of hydrolytic enzymes such as proteases and amylases, thereby accelerating the breakdown of complex organic matter. In acetogenesis and methanogenesis, biochar acts as an electron conductor, facilitating DIET between syntrophic bacteria and methanogenic archaea, which expedites the conversion of VFA into methane. This conductive property promotes thermodynamically favourable methanogenic pathways, including acetoclastic and hydrogenotrophic routes, and enriches key microbial taxa such as Methanosarcina, Methanosaeta, and Syntrophomonas. Additionally, biochar improves the bioavailability of trace elements (Fe, Co, Ni), enhancing enzymatic activity and metabolic efficiency.
From a stability perspective, biochar buffers pH fluctuations and mitigates ammonia and VFA accumulation, reducing inhibition and shortening recovery times after acidification or stress events. Its adsorptive capacity further removes toxic compounds, thereby improving cell viability. Synergistic effects are observed when biochar is combined with optimal organic loading and effluent recirculation, maximising methane yield and system resilience. Moreover, biochar properties—such as pyrolysis temperature and surface functional groups—can be tailored to modulate conductivity and adsorption, enabling targeted performance improvements according to substrate type and process stage.
A third category involves the use of materials with ion exchange capacity, where materials such as zeolites, bentonites [39], clay and manganese oxides can function as support matrices to immobilise biomass in the biodigester. This immobilisation allows for operating the reactor with a lower HRT and higher OLR, thereby improving the AD process. Zeolites can also be modified to increase their ion exchange capacity and supply micronutrients [9].
The combined application of bentonite and hematite introduces complementary benefits across the biochemical stages of AD. During hydrolysis and acidogenesis, bentonite’s high ion-exchange and adsorption capacity buffers VFA accumulation and stabilises pH, creating a favourable environment for microbial activity and preventing early inhibition. Simultaneously, hematite (Fe2O3) releases Fe3+ ions that stimulate iron-reducing bacteria and facilitate the conversion of metabolic intermediates, enhancing electron transfer within syntrophic communities. In acetogenesis and methanogenesis, hematite acts as a conductive material, promoting DIET between syntrophic bacteria and methanogenic archaea, accelerating VFA conversion and methane production.
Bentonite serves as a support matrix, improving hematite dispersion and stability, thus maximising its conductive effect and interaction with biomass. This composite material enriches hydrolytic and acidogenic bacteria as well as hydrogenotrophic methanogens, optimising microbial structure for efficient digestion. Synergistic effects include improved resilience to acidification, shortened lag phases, and methane yields up to 3.3–12.3 times higher than control systems. Furthermore, the bentonite–hematite combination enhances microbial diversity and accelerates system recovery under stress conditions, positioning it as a promising additive strategy for process stability and performance optimisation.
It has been suggested that the preferable approach for the addition of bentonite/calcium to the AD system is to mix fats, oils, and grease (FOG) with bentonite/calcium before their incorporation into AD, which generates an effect on ammonia inhibition in thermophilic anaerobic reactors degrading animal wastes [40]. Table 5 details this information.

Table 5.
Additives used in AD [9,27,38].
Finally, the use of nanomaterials has demonstrated benefits in promoting substrate degradation and improving AD yield. Their unique characteristics, including a high surface area, numerous active sites, and high reactivity, stimulate microbial activity and accelerate cell growth. Nanomaterials, including metallic nanoparticles of iron, copper, cobalt, silver, and nickel, can enhance DIET and interspecies hydrogen transfer. Iron nanoparticle supplementation has been reported to increase biomethane and biogas production, accompanied by low levels of H2S.
Nanoparticles promote faster substrate degradation and reduce bioconversion time [9]. In particular, iron nanoparticles have been shown to improve biogas production with a low H2S content. The use of TiO2 and Fe2O2 has doubled biogas production and significantly reduced hydrogen sulphide, with daily H2S removal rates between 53–62% using concentrations of Fe2O3 (20–100 mg/L) and TiO2 (100–500 mg/L). ZVI, magnetite and their mixture have also been used to increase methane production (Figure 7) [18].
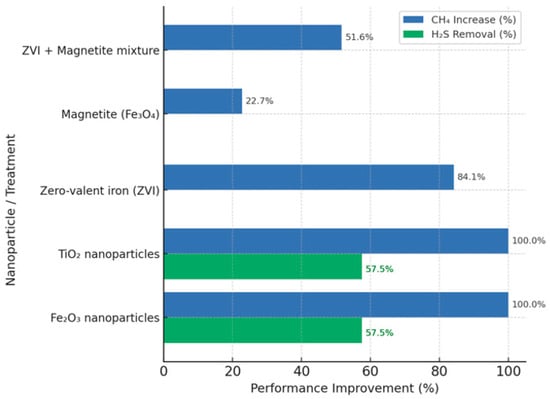
Figure 7.
Effect of metal nanoparticles on biogas production and H2S removal efficiency [18,41,42].
The superior behaviour of TiO2 and Fe2O3 as shown in Figure 7, can be attributed to their high surface reactivity and conductive properties. These attributes facilitate DIET and accelerate syntrophic interactions, which are critical for methanogenesis. In contrast, ZVI and magnetite exhibit moderate gains, indicating that conductivity alone is insufficient without optimised surface chemistry. The observed trend suggests that both electron mobility and catalytic activity govern process efficiency, thereby reinforcing the role of advanced nanomaterials as multifunctional agents for boosting biogas production whilst stabilising digester conditions.
In terms of process stability, nanomaterials exhibit properties such as a high surface area, an abundance of active sites, high reactivity, and specificity, which favour the control of the oxide-reduction potential of ZVI. In addition to removing H2S, it stimulates the enzymes involved in the acidification and methanogenesis stages of the process. Regarding the microbial impact, bioavailable metal components enhance enzymatic activity, accelerate cell growth, favour DIET, and optimise inter-species hydrogen transfer, reinforcing the efficiency of the overall process.
Metallic and composite nanoparticles exert significant effects across the biochemical stages of AD. During hydrolysis and acidogenesis, nanoparticles such as Fe3O4, TiO2, and NiFe2O4 enhance the degradation of complex organic matter and accelerate VFA production by improving enzymatic activity and the solubilisation of recalcitrant compounds, thereby increasing substrate availability for subsequent stages [41,42]. In acetogenesis and methanogenesis, conductive nanoparticles (Fe, Ni, Co) facilitate DIET between syntrophic bacteria and methanogenic archaea, expediting VFA conversion to methane. These particles also release essential trace ions (Fe2+, Ni2+) that act as cofactors for key enzymes, stimulating microbial metabolism and coenzyme synthesis, whilst enriching functional communities such as Methanosarcina and Geobacter.
From a stability perspective, nanoparticles buffer pH, reduce VFA accumulation, and mitigate ammonia or sulphide inhibition. Certain nanoparticles, such as Fe3O4, promote extracellular polymeric substance secretion, improving microbial aggregation and resilience under stress. Synergistic effects arise when nanoparticles are combined with other additives (e.g., K+, activated carbon) or applied under optimised conditions, significantly boosting methane yield and system robustness. Multi-component nanoparticles (e.g., Fe2O3–TiO2, NiO–TiO2) outperform single-metal particles in enhancing hydrolysis and methanogenesis, though excessive dosages can inhibit microbial activity [43]. Particle size and concentration are critical determinants of efficacy, with optimal dosing maximising benefits whilst avoiding toxicity.
In light of the recent advancements aimed at enhancing the efficiency, applicability, environmental impact, and economic viability of AD, the subsequent section will introduce the technological innovations developed to upgrade this process. A simplified scheme of additives to AD is illustrated in Figure 8.
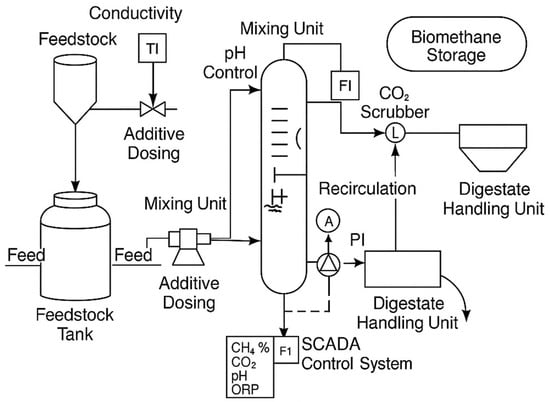
Figure 8.
AD process with additives [9,27].
4. Innovations in Biogas Upgrading Technologies
The valorisation of biogas, a key product of AD, has gained increasing importance in the pursuit of renewable energy sources and greenhouse gas mitigation [44,45]. This is driven by the global energy transition, which positions biomass, and biogas in particular, as crucial elements in reducing dependence on fossil fuels and lowering carbon footprints. Unrefined biogas primarily comprises methane (40–75%) and carbon dioxide (25–55%), along with other impurities such as hydrogen sulphide, water vapour, ammonia, and siloxanes, all of which limit its calorific value and potential applications [44,46]. To overcome these limitations and transform it into biomethane, a higher-quality natural gas substitute, various upgrading technologies have been developed and are constantly evolving [45,47]. These innovations focus on both CO2 removal and conversion, as well as optimisation of purification processes.
Innovations in biogas upgrading technologies, spurred by the growing demand for renewable energy and the urgent need to mitigate carbon dioxide emissions, have shifted towards more comprehensive solutions. These transcend mere component separation, now encompassing the crucial pretreatment phase for impurity removal. In this context, Le Pera et al. [48] highlighted combined pretreatment technologies for cleaning raw biogas from large-scale digesters in Italy, underscoring the necessity of removing trace compounds, such as H2S, to protect upgrading equipment and ensure high biomethane purity for industrial use.
Biological approaches, including in situ and ex situ methanation, as well as microbial electrosynthesis (MES), present an attractive option for transforming CO2 into methane or other valuable by-products. These methods operate under more moderate conditions and with a lower carbon and energy footprint, thereby minimising the reliance on costly chemicals. Continuing this focus on CO2 valorisation via biological routes, Struk, Kushkevych, and Vítezóva [49] reviewed the concepts and configurations of photobioreactors that utilise microorganisms for biogas upgrading through photosynthetic CO2 conversion. Similarly, Mhadmhan et al. [50] investigated direct catalytic methanation of CO2 to produce high-quality biomethane (targeting approximately 95% CH4) using NiMg/Carbon nanotubes-SiO2 fibre catalysts, optimising their performance under various temperature, pressure, and space velocity conditions.
However, these pathways still face substantial challenges, including the optimisation of hydrogen mass transfer, the achievement of high methane purity in specific processes, and scalability to an industrial level. In parallel, physicochemical methods have seen improvements, such as the evolution of membranes and cryogenic techniques, along with the exploration of new sorbents and solvents [51], all of which contribute to higher efficiencies and lower costs. In the quest for more efficient solutions, Moya et al. [52] focused on designing biogas upgrading processes using ionic liquids. This approach achieved a drastic reduction in energy requirements (from 0.785 to 0.211 kWh/Nm3) and highlighted the autothermal potential of these processes, where the primary energy contribution is electrical energy consumption. However, these innovative technologies often remain susceptible to biogas impurities, inherent methane losses, and, in many cases, entail significant upfront investment and operating costs. Ongoing research is therefore focused on addressing these limitations, pursuing hybrid configurations and integrating them with by-product valorisation to achieve technically, economically, and environmentally sustainable biogas upgrading on a large scale, thereby transforming waste into a high-value energy resource.
One of the most promising areas of innovation is biological biogas upgrading, which aims to convert CO2 to methane rather than merely removing it [53]. Within this category, biological methanation, both in situ and ex situ, has attracted considerable attention [54]. In situ chemoautotrophic methanisation involves the direct injection of hydrogen into the anaerobic digester, where indigenous methanogens, such as Methanosarcina sp. and Methanobacterium sp., utilise endogenous CO2 to produce methane. This process can raise the methane content to 80–100% [55,56]. However, it faces challenges related to increased pH, which can inhibit methanogenesis, and a low hydrogen mass transfer rate. These issues are typically addressed by co-digestion with acidic waste or pH control [57].
Ex situ biological methanation technologies, on the other hand, process raw biogas in a separate anaerobic reactor where CO2 is biologically converted to methane by reacting with hydrogen obtained from water electrolysis [56]. These technologies have demonstrated methane yields exceeding 97% when using sludge as feedstock [56]. Furthermore, MES and MEC have emerged as innovative and sustainable approaches [58]. Single chamber microbial systems utilise electrogenic and methanogenic microorganisms to convert CO2 into methane, thereby improving the methane purity of biogas to up to 96% and potentially generating other value-added products, such as acetic acid, which increases the overall value of biogas [59,60]. Technologies employing microalgae are also innovative (Table 6), performing photosynthetic biofixation of CO2, reducing its content in biogas to 2–6%, and producing active biomass that can be reused for methane production or high-value bioproducts, concurrently treating wastewater [53].

Table 6.
Summary of biogas upgrading technologies innovation trends.
Beyond biological solutions, physicochemical methods have also yielded notable innovations. Membrane separation technology, in particular, has rapidly gained prominence, surpassing adsorption and absorption, largely driven by advances in materials science [46]. Polymer membranes are especially suitable for biogas upgrading, and multi-stage configurations can achieve high methane recovery rates (99%) and purity levels of 95–99% [62]. Continued progress in materials engineering and science is anticipated to further improve its competitiveness [63,64]. Cryogenic separation is another short- to medium-term trend, with innovations enabling the pressureless conversion of biogas into liquefied biomethane and solid CO2 [65]. Overall, these innovations are crucial given the cost variability in biogas production [66].
Improvements to conventional adsorption technologies include the development of new packing materials for water scrubbing columns, which can reduce the required operating pressure. Additionally, adsorbents with linear isotherm behaviour can decrease the energy demand for regeneration [67,68]. The use of flash tanks under vacuum for water regeneration in high-pressure water washing has resulted in significant improvements: biomethane purity increased by 10%, CO2 removal by 14%, and methane recovery by 19% [68]. Furthermore, the implementation of anisotropic packing in high-pressure water washing absorption columns, when adapted to reduce the gas flow rate, enables the achievement of biomethane purities higher than 97% [68].
Hybrid and integrated approaches that combine different technologies to maximise efficiency and sustainability are also being explored [53]. For example, systems that integrate concentrated photovoltaic thermal collectors with biogas upgrading units have been proposed to provide thermal and electrical energy, reducing fossil fuel consumption and increasing biomethane production [69]. The application of nanotechnology, using nanoparticles and nanocatalysts as additives in AD, has been demonstrated to enhance substrate degradation, increase biogas and biomethane production, and reduce pollutants such as H2S and CO2 [45,56]. Biochar materials and biomass ash are also used as additives to enhance AD performance, which indirectly affects the quality of the resulting biogas [45,61]. A simplified process scheme of AD with pretreatment and bioelectrochemical systems is illustrated in Figure 9.
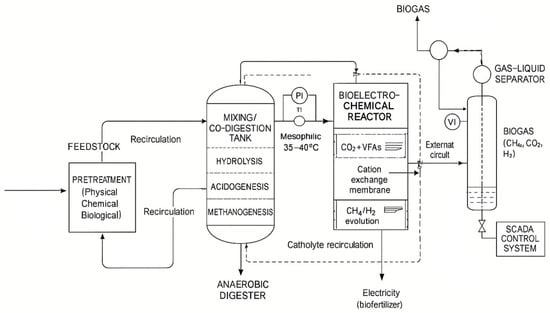
Figure 9.
AD process with pretreatment and bioelectrochemical systems [9,28].
In addition to the direct improvement of biogas, innovations include the valorisation of captured CO2 into other valuable products. A novel technology that enhances the mass transfer efficiency of CO2 removal through microchannel devices enables the use of CO2-rich water byproducts for the production of calcium nanocarbonate or as a “liquid CO2 fertiliser” for agricultural greenhouses, gradually releasing CO2 to support plant growth [70]. This represents a shift towards materials-oriented technologies, verified at the pilot scale, that convert a byproduct into valuable resources. In summary, innovations in biogas upgrading technology are moving towards more sustainable and cost-effective solutions, driven by the combination of biological processes, the development of new materials, the optimisation of physicochemical systems, and the integration of technologies to achieve higher biomethane efficiency and purity [71].
Therefore, innovations in biogas upgrading technologies, driven by the growing demand for renewable energy and the urgency to mitigate carbon dioxide emissions, have moved towards more comprehensive solutions that transcend mere component separation. Biological approaches, such as in situ and ex situ methanisation, as well as MES, offer the advantage of transforming CO2 into methane or valuable by-products, operating under more moderate conditions and with a lower carbon and energy footprint, thereby minimising the need for costly chemicals. However, these pathways must still overcome substantial challenges, including optimisation of hydrogen mass transfer, low methane purity in specific processes, and industrial-scale scalability.
In parallel, improvements in physicochemical methods, such as the evolution of membranes or cryogenic techniques, along with the exploration of new sorbents and solvents, point to higher efficiencies and lower costs. However, these innovative technologies often grapple with susceptibility to biogas impurities, inherent methane losses and, in many cases, significant upfront investment and operating costs. Ongoing research is focused on addressing these limitations, pursuing hybrid configurations and integration with by-product valorisation to achieve biogas upgrading that is technically, economically and environmentally sustainable on a large scale, transforming waste into a high-value energy resource.
5. Operating Parameters and Predictive Models
AD is solidifying its position as a fundamental strategy for bioenergy production and sustainable waste management, thereby driving intensive research into its operating parameters and the development of predictive models to optimise the process. Studies focus on understanding the inherent complexities of this biological system, characterised by its non-linear interactions and the synergistic activity of diverse microbial populations [72,73]. These models not only facilitate the prediction of reactor behaviour but are also crucial for design, scale-up, and performance control, thereby reducing reliance on costly and time-consuming laboratory experiments [74,75].
One of the most robust and detailed approaches is the Anaerobic Digestion Model No. 1 (ADM1), developed by the International Water Association (IWA) [76,77,78,79]. This structured model thoroughly describes the biochemical and physicochemical phases, including the disintegration and hydrolysis of components such as carbohydrates, lipids, and proteins, as well as acidogenesis, acetogenesis, methanogenesis, ionic interactions, and gas-liquid transfer [72,76]. The equation of this model is expressed in Equation (1) [80].
where is the flow (m3·d−1) of inlet () or liquid (), Sstream,i is the concentration of liquid components (nominally kgCOD·m–3), is volume (m3), is the component index, is the process index, is the kinetic rate of process j, and is the rate coefficients for component i on process j (nominally kgCOD·m–3). A detailed list of parameters can be seen in D.J. Batstone et al. [80]. However, its industrial-scale application presents challenges due to the large number of required parameters and the need for comprehensive and continuous substrate characterisation, which is often infeasible in large-scale plants [5,78,81].
To address the limitations of ADM1, several extensions and simplifications have been proposed. For example, the extension of Anaerobic Digestion Model No. 1 (ADM1_ME) was developed to describe biomethanation dynamics, incorporating syngas flow and adapting the mass transfer coefficient for different reactor configurations, such as bubble column and continuous stirred-tank reactors [82,83]. This extended model equations are presented in Equations (2)–(4).
is the concentration of the component j in the liquid phase, is the inlet flow rates of liquid, is the liquid volume, is the inlet concentration of the component j in the liquid phase, Yk is the yield of biomass k, is the stoichiometric coefficients, is the growth rate of biomass k, is the mass transfer rate of component i, is the concentration of biomass k in the liquid phase, is the inlet concentration of biomass k in the liquid phase, is the decay rate of biomass k, is the concentration of component i in gas phase, is the flow rates of gas, is the inlet concentration of component i in gas phase, is the gas volumes, and is the outlet gas flow rate [82].
Other modifications of ADM1 have allowed for considering specific effects, such as inhibition by free ammonia [84], VFA production [85,86], or the metabolism of different components [76]. Simplified versions, such as ADM1-R3, strike a balance between accuracy and practicality by reducing the number of processes and parameters, thereby facilitating their implementation in large-scale agricultural plants where substrate variability and data availability may be limited [77,78].
Conventional kinetic models are fundamental tools for analysing the biomethanation potential (BMP) and the biodegradability of substrates [75,84,87,88,89]. Models such as the (modified) Gompertz (Equation (5)), First Order (Equation (6)), Logistic (Equation (7)), or Richards models (Equation (8)) are widely used to fit experimental data of cumulative methane production over time, providing key parameters such as maximum production rate, maximum potential production, and lag phase [74,85,88].
is the cumulative gas volume (ml CH4/gVS), is the maximum specific methane production potential gas volume (ml CH4/gVS), is the maximum specific methane production rate (ml CH4/gVS.d), is the lag phase (d), is the digestion time (d), is exp(1) = 2.7183, is the rate constant for methane (1/d), is the coefficient of curve’s shape, and is the dimensionless kinetic parameter [74,85].
These models have been applied to a wide range of substrates, including food waste, peanut shells, molasses, and microalgae biomass [86,90]. While effective in describing behaviour under predetermined conditions, their main limitation is their inability to predict performance under operating conditions different from those studied, as they do not directly capture the influence of the physicochemical parameters of the process or substrate [91]. Recently, the use of fractional calculus to generalise first-order models has been explored, seeking to overcome some of these limitations [88].
Temperature is a critical operational parameter that directly influences microbial activity and, consequently, biogas production [92,93]. Mesophilic conditions (30–40 °C) are generally preferred and considered optimal for many AD processes [85,88,93]. Accurate temperature control is crucial for process stability, and neglecting thermal inertia can result in an overestimation of biogas production [93]. The pH is another determining factor, as methanogenic bacteria are susceptible to its values, with an optimal range of 6.5–7.5 [4]. A pH outside this range can inhibit microbial activity, thereby reducing biogas production [73,86,92].
The SIR and solids concentration (TS/VS) are crucial for ensuring an adequate microbial population and organic matter availability [4,74,85,91,92]. Optimal SIR reduces process times and increases productivity [87]. However, very low TS/VS may not be sufficient for microbial activity, while excessively high concentrations may hinder mixing and mass transfer. Substrate composition, particularly the C/N ratio, is crucial for maintaining the nutritional balance of microorganisms [87,94]. An inadequate C/N can lead to ammonia inhibition or accumulation of VFA, negatively affecting methane production. Other components, such as carbohydrates, proteins, and lipids, also influence biogas and methane yield [78].
Co-digestion is an effective strategy to overcome the limitations of mono-digestion, such as nutrient imbalance or low biodegradability, thereby resulting in higher biomethane yields [74,92]. Pretreatments (chemical, thermal, and ultrasonic) also significantly improve yields by breaking down the biomass structure, making it more accessible to microorganisms and increasing biomethane production, while reducing the dormancy phase [73,75,95]. Optimisation of these pretreatments is crucial to maximise yields. HRT is another influential factor in process stability and efficiency [5,96]. Agitation conditions, pressure, and the addition of supplements such as biochar or yeast can also be optimised to improve reactor performance [73,91,96].
Machine Learning (ML) and Artificial Intelligence (AI) models have gained ground as powerful tools for predicting and optimising AD performance, especially in complex, large-scale systems where mechanistic models can be computationally intensive or require difficult-to-obtain data [81,97,98]. Algorithms such as Artificial Neural Networks (ANN) [4,99], Long Short-Term Memory (LSTM) [5,72,77], Extreme Gradient Boosting (XGBoost), Random Forest, and Support Vector Machine (SVM) [81,85,92], as well as fuzzy logic [97], have demonstrated their ability to model complex non-linear relationships and predict biogas and methane production with high accuracy [85,92,100]. Their advantage lies in their ability to predict behaviour without the need for detailed prior knowledge of the system’s kinetics or stoichiometry, based on the identification of patterns in the input and output data [5,100].
ML models are beneficial for identifying the importance of input characteristics [77], allowing plant operators to focus on the most influential parameters for optimisation. Hybrid approaches, combining ML with techniques such as Response Surface Methodology (RSM) and Genetic Algorithms (GA), have been developed for parameter optimisation and biogas yield prediction [99]. These hybrid methods have proven to be highly effective, for example, in the co-digestion of food waste and manure [92]. The incorporation of HRT as a “search window” in LSTM models, acting as a memory of the reactor, has also significantly improved predictive accuracy [5], Table 7 presents a summary of information on predictive models.

Table 7.
Summary of developed models.
Mathematical modelling of AD varies significantly by application scale, reflecting differences in data requirements and operational complexity. At the laboratory scale, BMP tests under idealised conditions commonly employ empirical kinetic models (such as Gompertz, First-Order, and Logistic), which provide cost-effective tools for estimating parameters like maximum methane release and lag phase. Mechanistic models, notably ADM1, offer detailed dynamic simulations but face limitations in industrial settings due to extensive substrate characterisation requirements. Simplified versions (e.g., ADM1-R3) mitigate these constraints and enable large-scale process optimisation, including inhibition modelling. In contrast, data-driven approaches using ML, such as XGBoost, Random Forest, LSTM, and Temporal Fusion Transformer, excel at capturing nonlinear, time-dependent dynamics in full-scale digesters, achieving predictive accuracies up to R2 = 0.999 with long-term operational data.
However, ML models are less suitable for early design stages due to their reliance on extensive historical datasets. Direct extrapolation from BMP to continuous large-scale systems remains unreliable because of mixing and mass transfer effects; dynamic models incorporating degradation kinetics reduce scaling errors and improve prediction accuracy. Hybrid frameworks that integrate BMP-derived constraints with ML architectures further enhance robustness and forecasting precision for industrial applications.
In summary (Figure 10), the literature reveals an evolution towards a more integrated approach to AD modelling. While mechanistic models, such as ADM1, provide a deep understanding of the underlying processes, their complexity and data requirements limit their direct industrial-scale application. In contrast, conventional kinetic models offer a useful simplification for analysing BMP and latency phases, but their predictive scope is limited to the conditions studied. ML and AI models complement these tools by offering powerful predictive capability for large-scale and complex systems, often without the need for deep mechanistic knowledge, and allowing the identification of the most influential operating parameters. This combination of empirical, mechanistic, and data analysis-based approaches is essential to advance the optimisation of the AD process and sustainably maximise biomethane production.
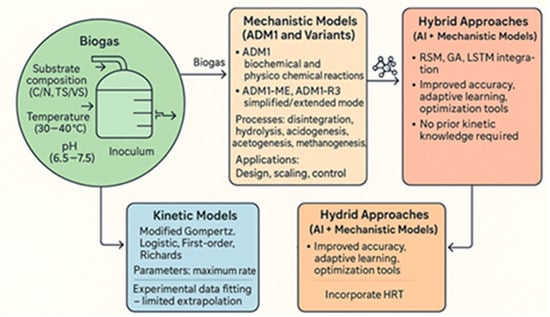
Figure 10.
Evolution and integration of modelling approaches in anaerobic digestion systems.
6. Microbiology and Microbial Community Dynamics
AD is a complex biological process, driven by an intricate network of microbial communities that transform organic matter into biogas, mainly methane and carbon dioxide, and a stabilised digestate [101,102]. The efficiency of the AD process critically depends on microbial activity and the interactions between diverse populations, including hydrolytic and fermentative bacteria, syntrophic bacteria, and methanogenic archaea [103,104]. A thorough understanding of the dynamics of these communities is therefore crucial to optimise biomethane production and manage digester stability [105].
The AD process comprises four successive biochemical phases: hydrolysis, acidogenesis, acetogenesis, and methanogenesis, each catalysed by specific microbial groups [106,107]. Hydrolysis, the initial phase, involves the degradation of insoluble organic polymers such as polysaccharides, proteins and lipids are degraded into soluble monomers and oligomers through the action of enzymes secreted by hydrolytic bacteria of genera such as Clostridium, Bacillus and Bacteroides [108,109]. This phase is often considered the rate-limiting step in AD, as it determines the availability of substrate for subsequent phases.
Immediately following hydrolysis, acidogenesis—a typically rapid reaction in the anaerobic conversion of complex organic matter—occurs. During this stage, fermentative bacteria, such as Clostridium sp., Bacillus sp. and Lactobacillus sp., utilise the sugars, glycerol, and amino acids derived from hydrolysis to produce VFAs such as acetic, propionic and butyric acids, as well as alcohols, H2, and CO2 [1,109]. Accumulation of VFAs in high concentrations can cause instability in the operational pH of the digester, thereby negatively affecting the process [107,108].
Acetogenesis involves the oxidation of VFAs, medium- and long-chain fatty acids, to acetic acid, H2, and CO2 by proton-reducing acetogenic bacteria, such as those from the Clostridiaceae and Syntrophomonadaceae families [108]. An increase in the abundance of syntrophic acetate oxidisers and syntrophic fatty acid oxidisers, including Clostridium ultunense, Syntrophomonas sp. and Syntrophus sp., has been correlated with enhanced methane production. These microorganisms oxidise fatty acids to acetate and H2, which are subsequently utilised by methanogenic archaea [110].
Finally (Figure 11), methanogenesis is the process of methane production from acetate, alcohols, and CO2 reduction, facilitated by the action of methanogenic archaea. This step is fundamental for closing the cycle in AD and is carried out by acetoclastic, hydrogenotrophic, and methylotrophic archaea [106,107]. Methanogenic archaea are particularly sensitive to process conditions, such as pH and temperature, which underscores the importance of a stable environment for their activity [104].
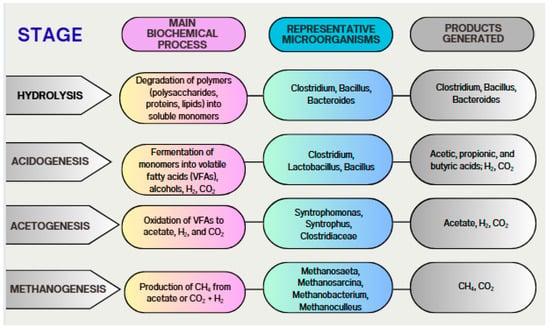
Figure 11.
Biochemical stages and representative microorganisms in anaerobic digestion.
The diversity and dynamics of microbial communities in anaerobic digesters are influenced by a multitude of operational factors, such as substrate type, temperature, pH, OLR, and HRT [111,112]. For example, an increase in temperature can significantly decrease the diversity of archaeal and bacterial communities [105]. Heterogeneity in organic waste induces changes in the digester microbiota, which, in turn, affects the intricate symbiotic networks and the stability of biomethanation. Moreover, the composition of the anaerobic microbial flora is highly dependent on the microorganisms arriving with the freshly fed substrates, which also serve as a source of inoculum [108].
Among the predominant bacterial phyla in AD, Firmicutes, Bacteroidetes, and Proteobacteria are ubiquitous and play crucial roles in lignocellulose degradation and VFA production [1]. Firmicutes is well known for its ability to degrade proteins, lipids, and polysaccharides, in addition to producing VFAs, especially butyrate, and acetic acid, a fundamental substrate for acetoclastic methanogenesis [104,109]. Bacteroidetes are important hydrolysers of complex carbohydrates and proteins [113]. Other phyla, such as Chloroflexi, Actinobacteria, Thermotogae and Synergistetes also contribute significantly to the process [109,112].
As for archaea, Methanosaeta and Methanosarcina are dominant genera crucial for methane production [114,115]. Methanosaeta is an obligate acetoclastic methanogen that strictly utilises acetate for methanogenesis, being the most abundant in sludge samples from anaerobic digesters [116]. On the other hand, Methanosarcina is more adaptive, being able to utilise acetate, hydrogen/carbon dioxide, and other C1 substrates, which allows it to proliferate under different VFA concentration conditions, including acidic conditions and high acetate concentrations [117]. Other important hydrogenotrophic methanogens include Methanobacterium, Methanoculleus and Methanospirillum, which have a higher affinity for dehydrogen ions for uptake during digestion [118,119].
The efficiency of AD is intrinsically linked to interspecific interactions and syntrophy among microorganisms [119]. Interspecific electron transfer, whether molecularly mediated or DIET, is critical for acetogenesis and methanogenesis [40,101]. DIET, which involves direct electron transfer through conductive filamentous structures such as electric pili or cytochromes, is an area of increasing interest because of its potential for diffusionless electron transfer, accelerating methane production and alleviating stress conditions such as high OLR and low pH [113].
Heterogeneity in substrate composition is a key determinant of microbial dynamics [108,109,111]. For instance, co-digestion of polysaccharide- or lipid-rich residues results in noticeable changes in acidogen and acetogen populations [108]. The addition of FOG can both balance nutrients and regulate microbial composition, and thereby stimulate metabolic activities and enhancing biogas production [120,121]. In polysaccharide waste digestion, a positive association is observed between propionate-oxidising acetogens (Anaerolinea, Bellilinea, Levilinea, Longilinea) and acetoclastic methanogens (Methanosaeta, Methanosarcina), which in turn accelerates methanogenic activity [108].
Operational conditions also modulate the communities. Temperature, for example, is a crucial factor affecting hydrolysis and methanogenesis, differentiating communities into mesophilic and thermophilic systems [105,112]. The dominant bacterial communities in mesophilic digesters typically include Firmicutes, Bacteroidetes, Cloacimonetes, and Chloroflexi, whereas in thermophilic systems, Thermotogae and Firmicutes predominate [112]. Furthermore, the fluctuation in abundance of genera such as Methanosarcina and Methanosaeta is also related to temperature, showing that Methanosarcina is more resilient to variations than Methanosaeta [112,115].
To improve the performance and stability of AD, various strategies have been explored, including bioaugmentation and the addition of additives [122,123]. Bioaugmentation with lignocellulolytic microbiotas, such as those from cow rumen or termite gut, holds promise for improving lignocellulose biodegradation by enriching the degrading communities and supplementing the native inoculum with enhanced methanogenic activity [103,107]. The addition of biochar, for instance, can mitigate inhibitors, promote process stability and increase biomethane production by providing a specific surface area for microbial colonisation and improving DIET [35].
Micronutrients such as Co, Ni, Fe, Mg and Ca are essential for the metabolic machinery of AD microbes, maintaining enzymatic activity and biochemical processes [35,40]. Their optimal supplementation has been shown to improve biomethane production by increasing the expression of specific genes, reducing VS, and promoting DIET, as well as increasing the abundance of Methanobrevibacter and Methanosarcina [40]. Similarly, microaeration has also shown benefits by increasing hydrolase activity, VFA production and oxidation/oxidation and hydrogen sulphide mitigation, which indirectly enhances methanogenesis and the abundance of genera such as Clostridium, Methanosarcina, and Methanobacterium [40]. A summary of microbiology topics is shown in Figure 12.
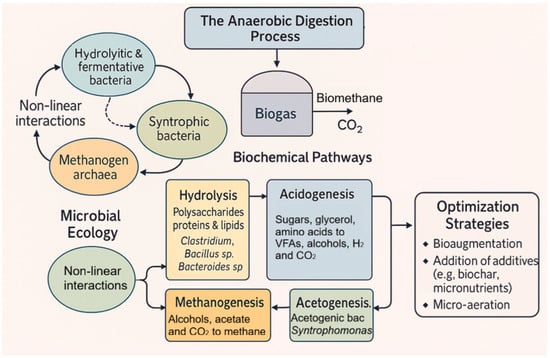
Figure 12.
Microbial ecology and biochemical pathways in anaerobic digestion systems.
The advancement of microbial characterisation techniques has been fundamental to a deeper understanding of AD. High-throughput ‘omics’ approaches, such as 16S rRNA gene amplicon sequencing, metagenomics, metatranscriptomics, and metaproteomics, have revolutionised the ability to identify microbial ecology and dynamics in anaerobic digesters [105]. These tools make it possible to predict the functional capabilities of complex communities, directly assess microbial function under specific growth conditions, and generate multidimensional data on how microorganisms respond to changes in AD conditions [1,105]. Metagenomics, in particular, is an efficient method for determining the complex structure of the system’s microbiota and for performing analyses of metabolic mechanisms, including identifying previously unknown species with significant functional potential [105].
Multi-omics approaches allow detailed insight into the diversity and composition of microbial communities. A prominent example of this application is the work of Wang et al. [124], which focuses on deciphering the microbial “fingerprints”, i.e., diversity, composition and function, of methanogenesis in hybrid anaerobic electro-biological digestion systems. The authors emphasise the importance of understanding the microbe-electrode interface and the synergistic and interactive communication of microbiota to enhance system performance. They highlight the need to explore an open hybrid anaerobic electro-biological digestion systems database that includes performance data and microbial community analysis to provide better guidance for future applications. In addition, they emphasise the use of cutting-edge multi-omics technologies, such as epicPCR and single-cell sequencing, to unveil the metabolic capacity of species, as well as the combination of microsensors with microscale microscopy to explore in situ biofilm dynamics and proton and electron transfer mechanisms [124].
The application of multi-omics approaches, including metatranscriptomics and metabolomics, has elucidated the molecular mechanisms governing microbial community function in AD, beyond the influence of temperature and ammonia nitrogen. At the molecular level, ammonia inhibition has been shown to disrupt specific metabolic pathways, such as acetoclastic methanogenesis, by suppressing the expression of key enzymes (e.g., methyl-CoM reductase in Methanothrix) and inducing VFA accumulation. Nevertheless, microbial communities exhibit self-regulation through the selection and activation of more tolerant taxa, such as Methanosarcina, which restore functionality via overexpression of genes and proteins associated with alternative methanogenic pathways [125]. Furthermore, the inhibition of propionate oxidation under ammonia stress correlates with suppression of the methylmalonyl-CoA pathway in syntrophic bacteria such as Pelotomaculum, highlighting the critical role of enzymatic regulation and syntrophic cooperation in maintaining process stability [126].
Environmental and operational factors, including OLR, HRT, pH, substrate composition, the presence of toxic compounds (e.g., triclocarban), and the addition of conductive materials, also modulate microbial structure and function. For instance, organic loading and retention time influence microbial network modularity and the emergence of functional biomarkers, whereas substrate composition and inhibitors can alter gene expression patterns and antibiotic resistance. Mechanisms such as DIET, mediated by conductive pili and multi-heme cytochromes, have been identified as key regulators of metabolic efficiency and resilience under high organic loading or low-pH conditions [101].
Ultimately, community robustness and functionality depend on functional redundancy, alpha diversity, and adaptive capacity through trophic network reorganisation and activation of alternative metabolic routes, ensuring methane production and system stability under fluctuating environmental and operational conditions [127]. These findings underscore the importance of integrating molecular-level insights with process optimisation strategies to enhance AD performance and its biotechnological applications.
On the other hand, applied voltages modelled microbial populations in microbial electrolyte cell-assisted AD systems. They reveal that a voltage of 0.8 V optimally enriches fermentative and electroactive acidogenic bacteria, which improves hydrolysis-acidification efficiency and DIET. Furthermore, they found a positive correlation between Methanosarcin and Methanoculleus with these functional bacteria. Specifically, Methanosarcina is more abundant at low voltages, while Methanoculleus thrives at medium to high voltages, driven by hydrogen production. Thus, the applied voltage not only alters the composition of the microbial community but also regulates the interactions between key groups, directly influencing their metabolic pathways to optimise methane production [128].
In summary, the microbiology of AD is a dynamic and essential field for the development of sustainable waste management and energy production technologies. The interconnection between microbial communities, their metabolic pathways and operational parameters is the key to process stability and efficiency. The application of ‘omics’ approaches and strategies, such as bioaugmentation or the addition of specific additives, promises to improve the performance of large-scale anaerobic digesters further, transforming waste biomass into a valuable renewable energy source [35,105].
7. Policies, Barriers and Market Prospects
AD is presented as a key technology for transitioning towards a circular bioeconomy and decarbonisation, as it transforms waste organic matter into biomethane, a versatile energy vector, and valuable by-products, such as biofertilisers [129,130]. This process not only contributes to waste management but also offers a pathway to reduce greenhouse gas emissions and dependence on fossil fuels, aligning with global goals such as the Paris Agreement [131,132]. For this technology to reach its full potential, it is crucial to understand the policies that drive it, the barriers that limit its adoption, and the market prospects that define its economic and environmental viability.
At the policy level, the European Union, through directives such as the Renewable Energy Directive (RED-II), has established a framework for promoting the use of energy from renewable sources, including biomethane [2,133]. Specifically in Italy, initiatives such as the National Recovery and Resilience Plan and the Common Agricultural Policy aim to promote the development of AD and composting plants, recognising their dual benefits in waste management and energy production [134]. These policies are complemented by various economic incentives, such as feed-in tariffs for biomethane injected into the gas grid [135], tax exemptions or reductions, such as the sales tax agreement in Brazil [136], and investment subsidies that are especially important for smaller installations [137]. The trading of biomethane emissions savings in the European Union Emissions Trading Scheme is also considered a key factor in the financial viability of smaller-scale processes [2].
China has established a comprehensive policy framework to promote AD and biogas production, aligning with environmental sustainability and circular economy objectives. This framework is underpinned by national laws such as the Renewable Energy Law, Agriculture Law, Energy Conservation Law, Livestock Law, and Circular Economy Law, which explicitly support the biogas sector and AD technology [138]. Financial incentives have played a pivotal role, with substantial subsidies provided by central and local governments between 2003 and 2012 for household digesters and large-scale plants. Since 2015, national subsidies have shifted towards industrial-scale facilities and demonstration projects, while local subsidies for domestic digesters remain region-specific [139]. Market-driven instruments, including feed-in tariffs, carbon reduction credits, and tax exemptions for biogas equipment, have further stimulated investment and technological development [140]. China also maintains internationally recognised technical standards for biogas projects, encompassing digestate management and organic fertiliser regulations [141].
Despite these advances, challenges persist in policy implementation and coordination, which remain less effective compared to European frameworks, particularly regarding commercialisation strategies [142]. Moreover, the subsidy system is criticised for its inflexibility, with recommendations to shift from input-based (construction) to output-based (production) incentives [141]. Recent regulations aim to improve agricultural utilisation of digestate; however, its market penetration continues to be limited [142].
The United States has developed a robust policy framework for AD and biogas production, primarily driven by federal and state-level incentives aimed at reducing greenhouse gas emissions and promoting renewable fuels. At the federal level, the Renewable Fuel Standard recognises biogas from AD systems as a cellulosic biofuel, enabling producers to generate Renewable Identification Numbers that can be traded, thereby incentivising biogas utilisation in the transport sector [143]. The Inflation Reduction Act in 2022 further strengthens this framework by offering Investment Tax Credits and expanding the 45Q tax credit for carbon capture and storage, benefiting AD projects that integrate emission-reduction technologies. Specifically, the 45Q credit provides economic incentives for CO2 capture and sequestration during AD, promoting systems with negative carbon emissions [143].
At the state level, California leads with its Low Carbon Fuel Standard, which encourages the production of low- or negative-carbon fuels, including biogas and biomethane, while supporting carbon capture and storage integration. Complementary initiatives such as the Bioenergy Action Plan and the state’s emissions trading market reinforce AD development [144]. Other states implement Renewable Portfolio Standards, indirectly incentivising biogas generation, alongside targeted programmes such as annual permits for waste conversion facilities in Iowa and greenhouse gas reduction schemes in Maryland [144]. Collectively, these policies position AD as a strategic technology for decarbonisation and renewable energy expansion in the United States.
India has established a multi-layered policy structure to promote AD for biogas production, with a strong emphasis on rural energy access, waste management, and sustainable development. The National Biogas and Manure Management Programme remains the cornerstone initiative, designed to deploy family-sized digesters in rural areas through a top-down, supply-driven approach, supported by government-set targets and state-level implementation [145]. While the programme provides subsidies, technical assistance, and training, it faces persistent challenges related to user awareness, affordability, and maintenance, prompting calls for a more community-oriented, bottom-up strategy [146]. Recent initiatives complement this framework, notably the Sustainable Alternative Towards Affordable Transportation scheme, which aims to install 5000 compressed biogas plants by last year, targeting an annual production of 15 million metric tonnes of compressed biogas [147].
Similarly, the Galvanising Organic Bio-Agro Resources Dhan programme focuses on converting cattle dung and organic waste into biogas, aligning with the Swachh Bharat (Clean India) Mission [147]. Additional schemes, such as Waste-to-Energy and the New National Biogas and Organic Manure Programme, promote biogas generation from municipal, agricultural, and industrial waste streams, offering incentives for decentralised and zone-based plants [148]. Collectively, these policies underscore India’s commitment to leveraging AD as a tool for energy security, waste valorisation, and climate mitigation.
Regions with market-oriented and performance-linked policies (e.g., EU, US) demonstrate higher technological maturity because their frameworks incentivise efficiency and innovation rather than merely infrastructure development. These policies foster large-scale plants equipped with advanced pretreatment technologies, digital monitoring systems, and integration of carbon capture and storage, enabling both operational optimisation and alignment with decarbonisation goals. In contrast, subsidy-dependent or socially driven frameworks (China, India) prioritise coverage and socio-economic objectives, resulting in widespread adoption of basic digesters but limited technological sophistication. The reliance on input-based subsidies and fragmented coordination in these regions discourages investment in high-efficiency systems, hybrid configurations, and upgrading technologies, thereby constraining their contribution to global climate targets. This divergence highlights that policy design not only determines adoption rates but also dictates the depth of technological innovation, scalability, and the ability of AD systems to integrate into advanced energy markets.
However, deployment of AD faces significant barriers, with high capital costs being a primary concern [134]. In particular, microalgae cultivation, harvesting, and processing systems, along with wastewater treatment and sludge drying units, account for a substantial portion of the initial investment in polygeneration plants [2]. Yet, several technological and management strategies can mitigate these challenges. From a design perspective, high-solids digestion and compact reactor configurations increase volumetric productivity, reducing infrastructure size and associated costs. Simplified and modular systems, such as single-stage reactors or decentralised plants, further lower investment requirements. Performance-enhancing techniques—including low-cost adsorbents, conductive materials, nanoparticles, and microaeration—enable smaller reactor footprints while maintaining efficiency. Energy optimisation through heat recovery and integration of renewable sources also contributes to cost reduction. Economically, economies of scale, co-digestion partnerships, and shared infrastructure improve financial viability, while feed-in tariffs, subsidies, and advanced process control systems maximise operational efficiency and minimise unnecessary expenditure [149].
Another major challenge lies in the logistics and transportation of organic waste, particularly in rural areas where collection infrastructure is limited, resulting in high transportation costs and rapid material degradation [134]. Combining decentralised biogas plants with regional collection centres, optimised transport routes, and community education initiatives offers an effective solution to logistical constraints in rural areas. This integrated approach reduces feedstock transport costs, enhances local participation, and facilitates the implementation of AD systems, while promoting circular economy principles at the community level [134].
The management of the resulting digestate also presents challenges, given its large quantity, high water content, and the associated costs of storage and transportation [129]. Advanced digestate valorisation technologies enable the transformation of this by-product into valuable resources for agriculture, industry, and energy systems [150]. Key approaches include nutrient recovery for fertiliser production, conversion into biochar for soil enhancement and carbon sequestration, and the synthesis of bioplastics and biofertilisers. Emerging applications also explore the development of advanced materials and energy carriers, positioning digestate as a feedstock for high-value products rather than a disposal challenge. Integrating these technologies into AD systems is essential to achieve efficient resource recovery and close material loops, thereby supporting a circular bioeconomy and improving the overall sustainability of biogas production.
Denmark has established a highly integrated biogas value chain that exemplifies circular bioeconomy principles by coupling renewable energy generation with nutrient recycling. This model is based on the co-digestion of livestock manure, agricultural residues, and organic waste to produce biogas, which is subsequently upgraded to biomethane and injected into the national gas grid. A distinctive feature of the Danish approach is the systematic valorisation of digestate as an organic fertiliser, achieved through solid–liquid separation and nutrient optimisation processes [151]. This integration reduces greenhouse gas emissions, mitigates ammonia volatilisation, and enhances soil fertility, thereby creating a closed-loop system that benefits both energy and agricultural sectors. Policy instruments such as feed-in tariffs and CO2 reduction obligations have been instrumental in supporting this industrial chain, ensuring economic viability and scalability within Denmark’s renewable energy framework.
Germany has made significant progress in phosphorus recovery from AD by-products, driven by strict regulations mandating the recycling of at least 50% of phosphorus from sewage sludge. One prominent technology is magnetic separation of vivianite, an iron phosphate mineral formed during digestion when sufficient iron is present. Pilot studies report recovery efficiencies exceeding 80% in three passes, producing a reusable phosphorus concentrate and meeting legal requirements. The process performance depends on phosphorus bound to vivianite, which can be enhanced through controlled iron dosing [152]. Additionally, struvite precipitation (magnesium ammonium phosphate) is widely applied to recover phosphorus from the liquid fraction of digestate under optimised pH and Mg:P ratios [153]. Emerging materials, such as magnetic biochar, further improve vivianite formation and separation, increasing overall recovery efficiency. These technologies transform residual phosphorus into high-value fertiliser products, supporting circular economy principles and securing a sustainable nutrient supply [154].
From a regulatory and technical perspective, the lack of a comprehensive and harmonised legislative framework, as well as the bureaucracy in environmental licencing processes, hinders the development of the sector in countries such as Brazil [131,132]. In addition, the fragmentation of data on the types of raw materials available, as observed in the United Kingdom, complicates decision-making and the formulation of coherent policies [155]. At the operational level, optimisation of co-digestion, including reactor design and feedstock mixing ratios, still requires further research to maximise biogas production and overcome process stability limitations [136,137]. The variability in digestate quality and the difficulty in establishing new markets for this by-product also represent an impediment to its valorisation [129].
Despite these challenges, the market prospects for biomethane are promising, driven by its ability to decarbonise various sectors such as agriculture, heavy transport and heating [2]. It is estimated that biomethane could satisfy a significant part of the global natural gas demand, contributing to a considerable reduction in greenhouse gas emissions [156]. Economic feasibility is a key factor, and studies show that, on an industrial scale, biomethane production can achieve a negative marginal abatement cost, indicating a net environmental and economic benefit. The cost-effectiveness of the process is strongly influenced by the operating expenses and the plant capacity factor [2].
The integration of AD into polygeneration systems that co-produce energy (biomethane), food (Spirulina), and biofertiliser (digestate) is an environmentally and economically beneficial approach. This product diversification improves financial viability and maximises emission savings [2]. It has been observed that biomethane production costs can be competitive with those of natural gas, especially in scenarios with high natural gas prices or when supported by government incentives [136]. The global potential for CO2 removal and biomethane production from waste biomass is substantial, with figures suggesting a significant capacity to offset greenhouse gas emissions [130].
In conclusion, AD is a crucial technology in the energy transition and circular bioeconomy, with significant potential for decarbonisation and waste valorisation [157]. Although significant barriers related to capital costs, logistics and digestate management persist, as well as the need for clearer regulatory frameworks, continued support through policies and incentives is vital to overcome these obstacles [158]. Continued research and development are essential to improve process efficiency, optimise the use of various feedstocks, and ensure the competitiveness of biomethane in the global energy market [155].
8. Conclusions
AD stands as a cornerstone technology for renewable energy generation and circular bioeconomy, offering a dual benefit of waste valorisation and greenhouse gas mitigation. The study highlights significant progress in process intensification strategies, including pretreatment methods, An-CoD, and functional additives such as biochar, conductive materials, and nanomaterials. These approaches collectively enhance substrate biodegradability, improve process stability, and accelerate methanogenesis through mechanisms like DIET and nutrient balancing. Co-digestion emerges as a particularly effective strategy, enabling synergistic interactions between substrates, optimising C/N ratios, and mitigating inhibitory compounds, with reported methane yield improvements up to 400%.
Technological innovations extend beyond digestion to biogas upgrading, where advanced physicochemical and biological methods such as membrane separation, catalytic methanation, MEC, and microalgae-assisted CO2 fixation are driving higher methane purity and energy efficiency. Hybrid and integrated systems that combine upgrading with CO2 valorisation and by-product recovery reinforce the sustainability of biomethane production.
The study underscores the critical role of microbial community dynamics, revealing how multi-omics approaches and bioaugmentation strategies can optimise hydrolysis, acidogenesis, and methanogenesis. Functional redundancy and syntrophic cooperation, particularly through DIET, are pivotal for resilience under stress conditions such as high organic loading or ammonia inhibition.
Predictive modelling is another key advancement, with mechanistic models (ADM1 and its variants) providing detailed process insights, while empirical kinetic models remain essential for BMP analysis. Emerging ML and AI frameworks (including LSTM, XGBoost, and hybrid approaches) offer robust predictive capabilities for large-scale systems, reducing reliance on costly experimental trials and enabling real-time optimisation.
Despite these technological strides, economic and regulatory barriers persist. High capital costs, logistical challenges in feedstock collection, and digestate management remain major constraints. Policy frameworks and market incentives such as feed-in tariffs, carbon credits, and tax exemptions are vital to foster adoption, with performance-linked schemes proving more effective than input-based subsidies. Regional disparities in policy design influence technological maturity, scalability, and integration into advanced energy markets.
Looking forward, the study advocates for integrated strategies combining process optimisation, microbial engineering, advanced modelling, and supportive policy instruments. LCA and TEA are essential to ensure that innovations deliver not only technical gains but also environmental and economic sustainability. Ultimately, AD is positioned as a transformative technology for achieving global decarbonisation targets, provided that research, innovation, and policy converge to overcome existing barriers and unlock its full potential.
Author Contributions
Conceptualisation, J.N.-P.; methodology, J.C.D.-Q.; formal analysis, J.C.D.-Q.; investigation, J.C.D.-Q.; resources, R.E.-V., J.L.B.-G., P.B. and M.L.-F.; data curation, M.L.-F. and J.L.B.-G.; writing—original draft preparation, J.C.D.-Q.; writing—review and editing, J.C.D.-Q., R.E.-V., P.B. and M.L.-F.; supervision, J.N.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
During the preparation of this manuscript, the authors used Consensus for the purposes of finding relevant literature and Grammarly for English revision.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AD | Anaerobic digestion |
| ADM1 | Anaerobic Digestion Model No. 1 |
| ADM1_ME | Extension of Anaerobic Digestion Model No. 1 |
| AI | Artificial Intelligence |
| ANN | Artificial Neural Networks |
| An-CoD | Anaerobic co-digestion |
| BMP | Biomethanation potential |
| COD | Chemical oxygen demand |
| C/N | Carbon-to-nitrogen |
| DIET | Direct interspecies electron transfer |
| EAD | Electro-anaerobic digestion |
| FOG | Fats, oils and grease |
| GA | Genetic algorithm |
| HRT | Hydraulic retention time |
| IWA | International Water Association |
| LCA | Life cycle analysis |
| LSTM | Long Short-Term Memory |
| MEC | Microbial electrochemical cells |
| MES | Microbial electrosynthesis |
| ML | Machine Learning |
| OLR | Organic loading rate |
| RED-II | Renewable Energy Directive |
| RSM | Response surface methodology |
| SIR | substrate-to-inoculum ratio |
| SVM | Support Vector Machine |
| TEA | Techno-economic analysis |
| TS/VS | Solids concentration |
| VFA | Volatile fatty acid |
| VS | Volatile solids |
| XGBoost | Extreme Gradient Boosting |
| ZVI | Zero-valent iron particles |
References
- Harirchi, S.; Wainaina, S.; Sar, T.; Nojoumi, S.A.; Parchami, M.; Parchami, M.; Varjani, S.; Khanal, S.K.; Wong, J.; Awasthi, M.K.; et al. Microbiological Insights into Anaerobic Digestion for Biogas, Hydrogen or Volatile Fatty Acids (VFAs): A Review. Bioengineered 2022, 13, 6521–6557. [Google Scholar] [CrossRef]
- Bose, A.; O’Shea, R.; Lin, R.; Long, A.; Rajendran, K.; Wall, D.; De, S.; Murphy, J.D. The Marginal Abatement Cost of Co-Producing Biomethane, Food and Biofertiliser in a Circular Economy System. Renew. Sustain. Energy Rev. 2022, 169, 112946. [Google Scholar] [CrossRef]
- Ganesh Saratale, R.; Kumar, G.; Banu, R.; Xia, A.; Periyasamy, S.; Dattatraya Saratale, G. A Critical Review on Anaerobic Digestion of Microalgae and Macroalgae and Co-Digestion of Biomass for Enhanced Methane Generation. Bioresour. Technol. 2018, 262, 319–332. [Google Scholar] [CrossRef]
- Cordoba, V.E.; Mussi, J.; De Paula, M.; Acosta, G.G. Prediction of Biomethane Production of Cheese Whey by Using Artificial Neural Networks. IEEE Lat. Am. Trans. 2023, 21, 1032–1039. [Google Scholar] [CrossRef]
- Salamattalab, M.M.; Hasani Zonoozi, M.; Molavi-Arabshahi, M. Innovative Approach for Predicting Biogas Production from Large-Scale Anaerobic Digester Using Long-Short Term Memory (LSTM) Coupled with Genetic Algorithm (GA). Waste Manag. 2024, 175, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.J.; Ferreira, A.F.; Fernandes, E.C. Biogas and Biomethane Production Potential via Anaerobic Digestion of Manure: A Case Study of Portugal. Renew. Sustain. Energy Rev. 2023, 188, 113846. [Google Scholar] [CrossRef]
- Shah, S.V.; Yadav Lamba, B.; Tiwari, A.K.; Chen, W.H. Sustainable Biogas Production via Anaerobic Digestion with Focus on CSTR Technology: A Review. J. Taiwan Inst. Chem. Eng. 2024, 162, 105575. [Google Scholar] [CrossRef]
- Hu, Y.; Bao, Y.; Meng, J.; Li, D.; Yuan, X.; Dong, Z. Biomethane Production Efficiency and Degradation Mechanism of Tar-Rich Coal in Anaerobic Digestion. Fuel 2024, 375, 132560. [Google Scholar] [CrossRef]
- Aworanti, O.A.; Ajani, A.O.; Agbede, O.O.; Agarry, S.E.; Ogunkunle, O.; Laseinde, O.T.; Kalam, M.A.; Fattah, I.M.R. Enhancing and Upgrading Biogas and Biomethane Production in Anaerobic Digestion: A Comprehensive Review. Front. Energy Res. 2023, 11, 1170133. [Google Scholar] [CrossRef]
- Neri, A.; Bernardi, B.; Zimbalatti, G.; Benalia, S. An Overview of Anaerobic Digestion of Agricultural By-Products and Food Waste for Biomethane Production. Energies 2023, 16, 6851. [Google Scholar] [CrossRef]
- Salman, C.A.; Schwede, S.; Thorin, E.; Yan, J. Enhancing Biomethane Production by Integrating Pyrolysis and Anaerobic Digestion Processes. Appl. Energy 2017, 204, 1074–1083. [Google Scholar] [CrossRef]
- de la Cruz-Azuara, J.E.; Ruiz-Marin, A.; Canedo-Lopez, Y.; Aguilar-Ucan, C.A.; Ceron-Breton, R.M.; Ceron-Breton, J.G.; Anguebes-Franseschi, F. Biomethane Production from the Two-Stage Anaerobic Co-Digestion of Cow Manure: Residual Edible Oil with Two Qualities of Waste-Activated Sludge. Energies 2024, 17, 2848. [Google Scholar] [CrossRef]
- Philipp, M.; Ackermann, H.; Barbana, N.; Pluschke, J.; Geißen, S.U. Possibilities for Anaerobic Digestion of Slaughter Waste and Flotates for Biomethane Production. Water 2023, 15, 1818. [Google Scholar] [CrossRef]
- Noor, R.S.; Ahmed, A.; Abbas, I.; Hussain, F.; Umair, M.; Noor, R.; Sun, Y. Enhanced Biomethane Production by 2-Stage Anaerobic Co-Digestion of Animal Manure with Pretreated Organic Waste. Biomass Convers. Biorefinery 2023, 13, 2833–2847. [Google Scholar] [CrossRef]
- Ripoll, V.; Agabo-García, C.; Perez, M.; Solera, R. Improvement of Biomethane Potential of Sewage Sludge Anaerobic Co-Digestion by Addition of “Sherry-Wine” Distillery Wastewater. J. Clean. Prod. 2020, 251, 119667. [Google Scholar] [CrossRef]
- Carneiro, R.B.; Gomes, G.M.; Camargo, F.P.; Zaiat, M.; Santos-Neto, Á.J. Anaerobic Co-Metabolic Biodegradation of Pharmaceuticals and Personal Care Products Driven by Glycerol Fermentation. Chemosphere 2024, 357, 142006. [Google Scholar] [CrossRef]
- D’Silva, T.C.; Isha, A.; Verma, S.; Shirsath, G.; Chandra, R.; Vijay, V.K.; Subbarao, P.M.V.; Kovács, K.L. Anaerobic Co-Digestion of Dry Fallen Leaves, Fruit/Vegetable Wastes and Cow Dung without an Active Inoculum—A Biomethane Potential Study. Bioresour. Technol. Rep. 2022, 19, 101189. [Google Scholar] [CrossRef]
- Dhull, P.; Lohchab, R.K.; Kumar, S.; Kumari, M.; Shaloo; Bhankhar, A.K. Anaerobic Digestion: Advance Techniques for Enhanced Biomethane/Biogas Production as a Source of Renewable Energy. Bioenergy Res. 2023, 17, 1228–1249. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Tena, M.; Sillero, L.; Magrini, F.E.; Sophiatti, I.V.M.; Gaio, J.; Paesi, S.; Forster-Carneiro, T.; Solera, R.; Perez, M. Application of Anaerobic Co-Digestion of Brewery by-Products for Biomethane and Bioenergy Production in a Biorefinery Concept. Bioenergy Res. 2023, 16, 2560–2573. [Google Scholar] [CrossRef]
- Pan, S.Y.; Tsai, C.Y.; Liu, C.W.; Wang, S.W.; Kim, H.; Fan, C. Anaerobic Co-Digestion of Agricultural Wastes toward Circular Bioeconomy. iScience 2021, 24, 102704. [Google Scholar] [CrossRef]
- Wang, C.; Feng, D.; Xia, A.; Nizami, A.S.; Huang, Y.; Zhu, X.; Zhu, X.; Liao, Q.; Murphy, J.D. A Comparative Life Cycle Assessment of Electro-Anaerobic Digestion to Evaluate Biomethane Generation from Organic Solid Waste. Renew. Sustain. Energy Rev. 2024, 196, 114347. [Google Scholar] [CrossRef]
- Ibro, M.K.; Ancha, V.R.; Lemma, D.B. Impacts of Anaerobic Co-Digestion on Different Influencing Parameters: A Critical Review. Sustainability 2022, 14, 9387. [Google Scholar] [CrossRef]
- Castel, L.; Cazaudehore, G.; Beigbeder, J.B.; Guyoneaud, R.; Peyrelasse, C. Pilot-Scale Study of CO2 Enrichment Effects on Anaerobic Digestion Performance. Chem. Eng. Sci. 2025, 304, 121070. [Google Scholar] [CrossRef]
- Quintana-Najera, J.; Blacker, A.J.; Fletcher, L.A.; Ross, A.B. Influence of Augmentation of Biochar during Anaerobic Co-Digestion of Chlorella Vulgaris and Cellulose. Bioresour. Technol. 2022, 343, 126086. [Google Scholar] [CrossRef] [PubMed]
- Rawoof, S.A.A.; Kumar, P.S.; Vo, D.V.N.; Subramanian, S. Sequential Production of Hydrogen and Methane by Anaerobic Digestion of Organic Wastes: A Review. Environ. Chem. Lett. 2021, 19, 1043–1063. [Google Scholar] [CrossRef]
- Ferrari, G.; Holl, E.; Steinbrenner, J.; Pezzuolo, A.; Lemmer, A. Environmental Assessment of a Two-Stage High Pressure Anaerobic Digestion Process and Biological Upgrading as Alternative Processes for Biomethane Production. Bioresour. Technol. 2022, 360, 127612. [Google Scholar] [CrossRef]
- Saravanakumar, A.; Sudha, M.R.; Chen, W.H.; Pradeshwaran, V.; Ashokkumar, V.; Selvarajoo, A. Biomethane Production as a Green Energy Source from Anaerobic Digestion of Municipal Solid Waste: A State-of-the-Art Review. Biocatal. Agric. Biotechnol. 2023, 53, 102866. [Google Scholar] [CrossRef]
- Kaur, G.; Basak, N.; Kumar, S. State-of-the-Art Techniques to Enhance Biomethane/Biogas Production in Thermophilic Anaerobic Digestion. Process Saf. Environ. Prot. 2024, 186, 104–117. [Google Scholar] [CrossRef]
- Roy, U.K.; Radu, T.; Wagner, J.L. Carbon-Negative Biomethane Fuel Production: Integrating Anaerobic Digestion with Algae-Assisted Biogas Purification and Hydrothermal Carbonisation of Digestate. Biomass Bioenergy 2021, 148, 106029. [Google Scholar] [CrossRef]
- Ding, L.; Cheng, J.; Lin, R.; Deng, C.; Zhou, J.; Murphy, J.D. Improving Biohydrogen and Biomethane Co-Production via Two-Stage Dark Fermentation and Anaerobic Digestion of the Pretreated Seaweed Laminaria Digitata. J. Clean. Prod. 2020, 251, 119666. [Google Scholar] [CrossRef]
- Ding, L.; Cheng, J.; Qiao, D.; Li, H.; Zhang, Z. Continuous Co-Generation of Biohydrogen and Biomethane through Two-Stage Anaerobic Digestion of Hydrothermally Pretreated Food Waste. Energy Convers. Manag. 2022, 268, 116000. [Google Scholar] [CrossRef]
- Caposciutti, G.; Baccioli, A.; Ferrari, L.; Desideri, U. Biogas from Anaerobic Digestion: Power Generation or Biomethane Production? Energies 2020, 13, 743. [Google Scholar] [CrossRef]
- Jha, B.; Isha, A.; Trivedi, A.; Chandra, R.; Subbarao, P.M.V. Anaerobic Co-Digestion of Rice Straw and de-Oiled Rice Bran for Biomethane Production. Energy Rep. 2021, 7, 704–710. [Google Scholar] [CrossRef]
- Kusmayadi, A.; Huang, C.Y.; Kit Leong, Y.; Lu, P.H.; Yen, H.W.; Lee, D.J.; Chang, J.S. Integration of Microalgae Cultivation and Anaerobic Co-Digestion with Dairy Wastewater to Enhance Bioenergy and Biochemicals Production. Bioresour. Technol. 2023, 376, 128858. [Google Scholar] [CrossRef]
- Saif, I.; Thakur, N.; Zhang, P.; Zhang, L.; Xing, X.; Yue, J.; Song, Z.; Nan, L.; Yujun, S.; Usman, M.; et al. Biochar Assisted Anaerobic Digestion for Biomethane Production: Microbial Symbiosis and Electron Transfer. J. Environ. Chem. Eng. 2022, 10, 107960. [Google Scholar] [CrossRef]
- Ghiotto, G.; De Bernardini, N.; Orellana, E.; Fiorito, G.; Cenci, L.; Kougias, P.; Campanaro, S.; Treu, L. Impact of Trace Metal Supplementation on Anaerobic Biological Methanation under Hydrogen and Carbon Dioxide Starvation. NPJ Biofilms Microbiomes 2025, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Bardi, M.; Vinardell, S.; Astals, S.; Koch, K. Opportunities and Challenges of Micronutrients Supplementation and Its Bioavailability in Anaerobic Digestion: A Critical Review. Renew. Sustain. Energy Rev. 2023, 186, 113689. [Google Scholar] [CrossRef]
- Świechowski, K.; Rasaq, W.A.; Syguła, E. Anaerobic Digestion of Brewer’s Spent Grain with Biochars—Biomethane Production and Digestate Quality Effects. Front. Energy Res. 2023, 11, 1141684. [Google Scholar] [CrossRef]
- Salama, E.S.; Saha, S.; Kurade, M.B.; Dev, S.; Chang, S.W.; Jeon, B.H. Recent Trends in Anaerobic Co-Digestion: Fat, Oil, and Grease (FOG) for Enhanced Biomethanation. Prog. Energy Combust. Sci. 2019, 70, 22–42. [Google Scholar] [CrossRef]
- Thakur, N.; Sharma, M.; Alghamdi, H.; Zheng, Y.; Xue, W.; Jeon, B.H.; Salama, E.S.; Li, X. A Recent Trend in Anaerobic Digestion (AD): Enhancement of Microbiome and Digestibility of Feedstocks via Abiotic Stress Factors for Biomethanation. Chem. Eng. J. 2023, 472, 145047. [Google Scholar] [CrossRef]
- Li, S.; Cao, Y.; Zhao, Z.; Zhang, Y. Regulating Secretion of Extracellular Polymeric Substances through Dosing Magnetite and Zerovalent Iron Nanoparticles To Affect Anaerobic Digestion Mode. ACS Sustain. Chem. Eng. 2019, 7, 9655–9662. [Google Scholar] [CrossRef]
- Ghofrani-Isfahani, P.; Baniamerian, H.; Tsapekos, P.; Alvarado-Morales, M.; Kasama, T.; Shahrokhi, M.; Vossoughi, M.; Angelidaki, I. Effect of Metal Oxide Based TiO2 Nanoparticles on Anaerobic Digestion Process of Lignocellulosic Substrate. Energy 2020, 191, 116580. [Google Scholar] [CrossRef]
- Baniamerian, H.; Ghofrani-Isfahani, P.; Tsapekos, P.; Alvarado-Morales, M.; Shahrokhi, M.; Angelidaki, I. Multicomponent Nanoparticles as Means to Improve Anaerobic Digestion Performance. Chemosphere 2021, 283, 131277. [Google Scholar] [CrossRef]
- Carranza-Abaid, A.; Wanderley, R.R.; Knuutila, H.K.; Jakobsen, J.P. Analysis and Selection of Optimal Solvent-Based Technologies for Biogas Upgrading. Fuel 2021, 303, 121327. [Google Scholar] [CrossRef]
- Francisco López, A.; Lago Rodríguez, T.; Faraji Abdolmaleki, S.; Galera Martínez, M.; Bello Bugallo, P.M. From Biogas to Biomethane: An In-Depth Review of Upgrading Technologies That Enhance Sustainability and Reduce Greenhouse Gas Emissions. Appl. Sci. 2024, 14, 2342. [Google Scholar] [CrossRef]
- Comesaña-Gándara, B.; García-Depraect, O.; Santos-Beneit, F.; Bordel, S.; Lebrero, R.; Muñoz, R. Recent Trends and Advances in Biogas Upgrading and Methanotrophs-Based Valorization. Chem. Eng. J. Adv. 2022, 11, 100325. [Google Scholar] [CrossRef]
- Adnan, A.I.; Ong, M.Y.; Nomanbhay, S.; Chew, K.W.; Show, P.L. Technologies for Biogas Upgrading to Biomethane: A Review. Bioengineering 2019, 6, 92. [Google Scholar] [CrossRef]
- Le Pera, A.; Sellaro, M.; Pellegrino, C.; Limonti, C.; Siciliano, A. Combined Pre-Treatment Technologies for Cleaning Biogas before Its Upgrading to Biomethane: An Italian Full-Scale Anaerobic Digester Case Study. Appl. Sci. 2024, 14, 2053. [Google Scholar] [CrossRef]
- Struk, M.; Kushkevych, I.; Vítězová, M. Biogas Upgrading Methods: Recent Advancements and Emerging Technologies. Rev. Environ. Sci. Biotechnol. 2020, 19, 651–671. [Google Scholar] [CrossRef]
- Mhadmhan, S.; Ngamcharussrivichai, C.; Hinchiranan, N.; Kuchonthara, P.; Li, Y.; Wang, S.; Reubroycharoen, P. Direct Biogas Upgrading via CO2 Methanation to High-Quality Biomethane over NiMg/CNT-SiO2 Fiber Catalysts. Fuel 2022, 310, 122289. [Google Scholar] [CrossRef]
- Martín-Hernández, E.; Guerras, L.S.; Martín, M. Optimal Technology Selection for the Biogas Upgrading to Biomethane. J. Clean. Prod. 2020, 267, 122032. [Google Scholar] [CrossRef]
- Moya, C.; Santiago, R.; Hospital-Benito, D.; Lemus, J.; Palomar, J. Design of Biogas Upgrading Processes Based on Ionic Liquids. Chem. Eng. J. 2022, 428, 132103. [Google Scholar] [CrossRef]
- Assunção, L.R.C.; Mendes, P.A.S.; Matos, P.; Borschiver, S. Technology Roadmap of Renewable Natural Gas: Identifying Trends for Research and Development to Improve Biogas Upgrading Technology Management. Appl. Energy 2021, 292, 116849. [Google Scholar] [CrossRef]
- Iglesias, R.; Muñoz, R.; Polanco, M.; Díaz, I.; Susmozas, A.; Moreno, A.D.; Guirado, M.; Carreras, N.; Ballesteros, M. Biogas from Anaerobic Digestion as an Energy Vector: Current Upgrading Development. Energies 2021, 14, 2742. [Google Scholar] [CrossRef]
- Fu, S.; Angelidaki, I.; Zhang, Y. In Situ Biogas Upgrading by CO2-to-CH4 Bioconversion. Trends Biotechnol. 2021, 39, 336–347. [Google Scholar] [CrossRef]
- Sher, F.; Smječanin, N.; Hrnjić, H.; Karadža, A.; Omanović, R.; Šehović, E.; Sulejmanović, J. Emerging Technologies for Biogas Production: A Critical Review on Recent Progress, Challenges and Future Perspectives. Process Saf. Environ. Prot. 2024, 188, 834–859. [Google Scholar] [CrossRef]
- Pothakos, V.; De Vuyst, L.; Zhang, S.J.; De Bruyn, F.; Verce, M.; Torres, J.; Callanan, M.; Moccand, C.; Weckx, S. Temporal Shotgun Metagenomics of an Ecuadorian Coffee Fermentation Process Highlights the Predominance of Lactic Acid Bacteria. Curr. Res. Biotechnol. 2020, 2, 1–15. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, Y.; Dhar, B.R. A Critical Review of Microbial Electrolysis Cells Coupled with Anaerobic Digester for Enhanced Biomethane Recovery from High-Strength Feedstocks. Crit. Rev. Environ. Sci. Technol. 2022, 52, 50–89. [Google Scholar] [CrossRef]
- Chu, X.; Ren, Y.; Qu, G.; Ren, N.; Xie, R.; Cheng, M.; Chen, X.; Wang, Z.; Yuan, Y. Current Status of Research on Microbial Electrocatalytic CH4 Production for Biogas Upgrading and Challenges. J. Environ. Chem. Eng. 2024, 12, 112088. [Google Scholar] [CrossRef]
- Chung, T.H.; Dhillon, S.K.; Shin, C.; Pant, D.; Dhar, B.R. Microbial Electrosynthesis Technology for CO2 Mitigation, Biomethane Production, and Ex-Situ Biogas Upgrading. Biotechnol. Adv. 2024, 77, 108474. [Google Scholar] [CrossRef]
- Khan, M.U.; Lee, J.T.E.; Bashir, M.A.; Dissanayake, P.D.; Ok, Y.S.; Tong, Y.W.; Shariati, M.A.; Wu, S.; Ahring, B.K. Current Status of Biogas Upgrading for Direct Biomethane Use: A Review. Renew. Sustain. Energy Rev. 2021, 149, 111343. [Google Scholar] [CrossRef]
- Baena-Moreno, F.M.; le Saché, E.; Pastor-Pérez, L.; Reina, T.R. Membrane-Based Technologies for Biogas Upgrading: A Review. Environ. Chem. Lett. 2020, 18, 1649–1658. [Google Scholar] [CrossRef]
- Fajrina, N.; Yusof, N.; Ismail, A.F.; Aziz, F.; Bilad, M.R.; Alkahtani, M. A Crucial Review on the Challenges and Recent Gas Membrane Development for Biogas Upgrading. J. Environ. Chem. Eng. 2023, 11, 110235. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Kumar, J.; Vu, M.T.; Mohammed, J.A.H.; Pathak, N.; Commault, A.S.; Sutherland, D.; Zdarta, J.; Tyagi, V.K.; Nghiem, L.D. Biomethane Production from Anaerobic Co-Digestion at Wastewater Treatment Plants: A Critical Review on Development and Innovations in Biogas Upgrading Techniques. Sci. Total Environ. 2021, 765, 142753. [Google Scholar] [CrossRef]
- Naquash, A.; Qyyum, M.A.; Haider, J.; Lim, H.; Lee, M. Renewable LNG Production: Biogas Upgrading through CO2 Solidification Integrated with Single-Loop Mixed Refrigerant Biomethane Liquefaction Process. Energy Convers. Manag. 2021, 243, 114363. [Google Scholar] [CrossRef]
- Di Profio, P.; Ciulla, M.; Di Giacomo, S.; Barbacane, N.; Wolicki, R.D.; Fontana, A.; Moffa, S.; Pilato, S.; Siani, G. Emerging Green Strategies for Biogas Upgrading through CO2 Capture: From Unconventional Organic Solvents to Clathrate and Semi-Clathrate Hydrates. J. Mol. Liq. 2023, 391, 123196. [Google Scholar] [CrossRef]
- Golmakani, A.; Ali Nabavi, S.; Wadi, B.; Manovic, V. Advances, Challenges, and Perspectives of Biogas Cleaning, Upgrading, and Utilisation. Fuel 2022, 317, 123085. [Google Scholar] [CrossRef]
- Wantz, E.; Lemonnier, M.; Benizri, D.; Dietrich, N.; Hébrard, G. Innovative High-Pressure Water Scrubber for Biogas Upgrading at Farm-Scale Using Vacuum for Water Regeneration. Appl. Energy 2023, 350, 121781. [Google Scholar] [CrossRef]
- Su, B.; Wang, H.; Zhang, X.; He, H.; Zheng, J. Using Photovoltaic Thermal Technology to Enhance Biomethane Generation via Biogas Upgrading in Anaerobic Digestion. Energy Convers. Manag. 2021, 235, 113965. [Google Scholar] [CrossRef]
- Guo, J.; He, P.; Wu, H.; Xi, Y.; Li, C.; Zhang, H.; Zhou, J.; Liao, J.; Lü, F. Novel Material-Oriented Valorization of Biogas Can Achieve More Carbon Reduction than Traditional Utilization by Bioelectricity or Biomethane. Bioresour. Technol. 2024, 395, 130333. [Google Scholar] [CrossRef] [PubMed]
- Kazimierowicz, J.; Dębowski, M.; Zieliński, M. Innovative Method for Biomethane Production Based on a Closed Cycle of Biogas Upgrading and Organic Substrate Pretreatment—Technical, Economic, and Technological Fundamentals. Energies 2025, 18, 1033. [Google Scholar] [CrossRef]
- Jeong, K.; Abbas, A.; Shin, J.; Son, M.; Kim, Y.M.; Cho, K.H. Prediction of Biogas Production in Anaerobic Co-Digestion of Organic Wastes Using Deep Learning Models. Water Res. 2021, 205, 117697. [Google Scholar] [CrossRef]
- Upadhyay, A.; Kovalev, A.A.; Zhuravleva, E.A.; Kovalev, D.A.; Litti, Y.V.; Masakapalli, S.K.; Pareek, N.; Vivekanand, V. A Review of Basic Bioinformatic Techniques for Microbial Community Analysis in an Anaerobic Digester. Fermentation 2023, 9, 62. [Google Scholar] [CrossRef]
- Matobole, K.; Seodigeng, T.; Banza, M.; Rutto, H. Modeling of the Biomethane Production from Ultrasonic Pretreated Fruit and Vegetable Waste via Anaerobic Digestion. J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng. 2024, 59, 513–522. [Google Scholar] [CrossRef]
- Olatunji, K.O.; Olugbemide, A.D.; Akerejola, R.F.; Madyira, D.M. Kinetic Modelling of the Biomethane Production Potential of Acidic Pretreated Groundnut Shells. Biomass Convers. Biorefinery 2025, 15, 17139–17153. [Google Scholar] [CrossRef]
- Bertacchi, S.; Ruusunen, M.; Sorsa, A.; Sirviö, A.; Branduardi, P. Mathematical Analysis and Update of Adm1 Model for Biomethane Production by Anaerobic Digestion. Fermentation 2021, 7, 237. [Google Scholar] [CrossRef]
- Meola, A.; Weinrich, S. Hybrid Modelling of Dynamic Anaerobic Digestion Process in Full-Scale with LSTM NN and BMP Measurements. In Proceedings of the European Symposium on Artificial Neural Networks, Computational Intelligence and Machine Learning, Bruges, Belgium, 4–6 October 2023; pp. 543–548. [Google Scholar] [CrossRef]
- Tisocco, S.; Weinrich, S.; Lyons, G.; Wills, M.; Zhan, X.; Crosson, P. Application of a Simplified ADM1 for Full-Scale Anaerobic Co-Digestion of Cattle Slurry and Grass Silage: Assessment of Input Variability. Front. Environ. Sci. Eng. 2023, 18, 1–15. [Google Scholar] [CrossRef]
- Youssef, B.; Mohamed, S.; Abderrahim, F. Towards Enhanced Prediction of Biomethane Production Using Random Forest and Artificial Neural Network Models. In Proceedings of the 2023 IEEE International Conference on Agrosystem Engineering, Technology & Applications (AGRETA), Shah Alam, Malaysia, 9 September 2023; pp. 23–27. [Google Scholar] [CrossRef]
- Batstone, D.J.; Keller, J.; Angelidaki, I.; Kalyuzhnyi, S.V.; Pavalostathis, S.G.; Rozzi, A.; Sanders, W.T.M.; Siegrist, H.; Vavilin, V.A. The IWA Anaerobic Digestion Model No 1 (ADM1). Water Sci. Technol. 2002, 45, 65–73. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, D.; Wen, Z.; Fei, F.; Caicedo, L.; Yuan, K.; Shang, R. Interpretable Machine Learning for Predicting Biomethane Production in Industrial-Scale Anaerobic Co-Digestion. Sci. Total Environ. 2020, 712, 134574. [Google Scholar] [CrossRef]
- Acosta-Pavas, J.C.; Robles-Rodríguez, C.E.; Morchain, J.; Dumas, C.; Cockx, A.; Aceves-Lara, C.A. Dynamic Modeling of Biological Methanation for Different Reactor Configurations: An Extension of the Anaerobic Digestion Model No. 1. Fuel 2023, 344, 128106. [Google Scholar] [CrossRef]
- Arotingo Guandinango, H.P.; del Espín Valladares, R.C.; Núñez Pérez, J.; Lara Fiallos, M.V.; Pereda Reyes, I.; Pais-Chanfrau, J.M. Modelisation of the Biomethane Accumulation in Anaerobic Co-Digestion of Whey and Sugarcane Molasse Mixtures. Fermentation 2023, 9, 834. [Google Scholar] [CrossRef]
- Ripoll, V.; Solera, R.; Perez, M. Validation of a New Kinetic Model for Biomethane Production from Anaerobic Co-Digestion of Sewage Sludge and Sherry-Wine Distillery Wastewater. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Fidan, B.; Bodur, F.G.; Öztep, G.; Güngören-Madenoğlu, T.; Kabay, N.; Baba, A. Comparison of Conventional and Machine Learning Models for Kinetic Modelling of Biomethane Production from Pretreated Tomato Plant Residues. Ind. Crops Prod. 2025, 223, 120235. [Google Scholar] [CrossRef]
- Olatunji, K.O.; Mootswi, K.D.; Olatunji, O.O.; Zwane, M.I.; van Rensburg, N.J.; Madyira, D.M. Anaerobic Co-Digestion of Food Waste and Groundnut Shells: Synergistic Impact Assessment and Kinetic Modeling. Waste Biomass Valorization 2025, 16, 3745–3760. [Google Scholar] [CrossRef]
- Mouftahi, M.; Tlili, N.; Hidouri, N.; Bartocci, P.; Fantozzi, F. Bioenergy Recovery from Southern Tunisia’s Organic Wastes: Analysis and Kinetic Modeling Study of Biomethane Production. Biomass Convers. Biorefinery 2023, 13, 6345–6361. [Google Scholar] [CrossRef]
- Da Silva, C.; Peces, M.; Jaques, A.; Muñoz, J.J.; Dosta, J.; Astals, S. Fractional Calculus as a Generalized Kinetic Model for Biochemical Methane Potential Tests. Bioresour. Technol. 2024, 396, 130412. [Google Scholar] [CrossRef]
- van der Berg, D.J.; Teke, G.M.; Görgens, J.F.; van Rensburg, E. Predicting Commercial-Scale Anaerobic Digestion Using Biomethane Potential. Renew. Energy 2024, 235, 121304. [Google Scholar] [CrossRef]
- Ahire, P.D.; Upadhyay, A.; Talwar, P.; Khatri, H.; Singh, R.; Lindenberger, C.; Pareek, N.; Vivekanand, V. Tool Development for Estimation of Biomethane Potential of Different Food Waste for a Sustainable Bioeconomy. Biomass Bioenergy 2024, 182, 107107. [Google Scholar] [CrossRef]
- Khashaba, N.H.; Ettouney, R.S.; Abdelaal, M.M.; Ashour, F.H.; El-Rifai, M.A. Artificial Neural Network Modeling of Biochar Enhanced Anaerobic Sewage Sludge Digestion. J. Environ. Chem. Eng. 2022, 10, 107988. [Google Scholar] [CrossRef]
- Ahmad, A.; Yadav, A.K.; Singh, A.; Singh, D.K. A Comprehensive Machine Learning-Coupled Response Surface Methodology Approach for Predictive Modeling and Optimization of Biogas Potential in Anaerobic Co-Digestion of Organic Waste. Biomass Bioenergy 2024, 180, 106995. [Google Scholar] [CrossRef]
- Calise, F.; Cappiello, F.L.; Cimmino, L.; Dentice d’Accadia, M.; Vicidomini, M. Dynamic Analysis and Investigation of the Thermal Transient Effects in a CSTR Reactor Producing Biogas. Energy 2023, 263, 126010. [Google Scholar] [CrossRef]
- Henrotin, A.; Hantson, A.L.; Dewasme, L. Dynamic Modeling and Parameter Estimation of Biomethane Production from Microalgae Co-Digestion. Bioprocess. Biosyst. Eng. 2023, 46, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Yadav, A.K.; Pal, A.; Hasan, S. A Novel Intensified Anaerobic Co-Digestion Process for High-Quality Biomethane Production Using Ultrasound Energy: Optimization and Robustness Tests with ML-RSM-TLBO Algorithm. Energy Convers. Manag. 2025, 325, 119408. [Google Scholar] [CrossRef]
- Meola, A.; Winkler, M.; Weinrich, S. Metaheuristic Optimization of Data Preparation and Machine Learning Hyperparameters for Prediction of Dynamic Methane Production. Bioresour. Technol. 2023, 372, 128604. [Google Scholar] [CrossRef]
- Ferreira, C.M.; Akisue, R.A.; de Sousa Júnior, R. Mathematical Modeling and Computational Simulation Applied to the Study of Glycerol and/or Molasses Anaerobic Co-Digestion Processes. Processes 2023, 11, 2121. [Google Scholar] [CrossRef]
- Long, F.; Wang, L.; Cai, W.; Lesnik, K.; Liu, H. Predicting the Performance of Anaerobic Digestion Using Machine Learning Algorithms and Genomic Data. Water Res. 2021, 199, 117182. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, H.; Li, X. Low-Temperature Hydrothermal Modification with Fe/C Catalysts for Enhancing Corn Stover Anaerobic Digestion Performance and Modeling Development for Predicting Biomethane Yield. Catalysts 2025, 15, 362. [Google Scholar] [CrossRef]
- Sappl, J.; Harders, M.; Rauch, W. Machine Learning for Quantile Regression of Biogas Production Rates in Anaerobic Digesters. Sci. Total Environ. 2023, 872, 161923. [Google Scholar] [CrossRef]
- Akram, J.; Song, C.; El Mashad, H.M.; Chen, C.; Zhang, R.; Liu, G. Advances in Microbial Community, Mechanisms and Stimulation Effects of Direct Interspecies Electron Transfer in Anaerobic Digestion. Biotechnol. Adv. 2024, 76, 108398. [Google Scholar] [CrossRef]
- Ng, H.J.; Goh, K.M.; Yahya, A.; Abdul-Wahab, M.F. Microbial Community Dynamics and Functional Potentials in the Conversion of Oil Palm Wastes into Biomethane. 3 Biotech. 2024, 14, 91. [Google Scholar] [CrossRef]
- Basak, B.; Kumar, R.; Tanpure, R.S.; Mishra, A.; Tripathy, S.K.; Chakrabortty, S.; Roh, H.S.; Yadav, K.K.; Chung, W.; Jeon, B.H. Roles of Engineered Lignocellulolytic Microbiota in Bioaugmenting Lignocellulose Biomethanation. Renew. Sustain. Energy Rev. 2025, 207, 114913. [Google Scholar] [CrossRef]
- Bonugli-Santos, R.C.; Marteres, T.J.; Luiz, F.N.; Somer, J.G.; Mari, Â.G.; Passarini, M.R.Z. Prolonged Acetogenic Phase and Biological Succession during Anaerobic Digestion Using Swine Manure. Folia Microbiol. 2022, 67, 733–745. [Google Scholar] [CrossRef]
- Kabaivanova, L.; Nacheva, L.; Petrova, P. Microbial Community Structure in Anaerobic Digestion for Green Energy Production: A Mini Review. Open Access J. Microbiol. Biotechnol. 2024, 9, 1–4. [Google Scholar] [CrossRef]
- Bedoya, K.; Hoyos, O.; Zurek, E.; Cabarcas, F.; Alzate, J.F. Annual Microbial Community Dynamics in a Full-Scale Anaerobic Sludge Digester from a Wastewater Treatment Plant in Colombia. Sci. Total Environ. 2020, 726, 138479. [Google Scholar] [CrossRef]
- Faisal, S.; Thakur, N.; Jalalah, M.; Harraz, F.A.; Al-Assiri, M.S.; Saif, I.; Ali, G.; Zheng, Y.; Salama, E.S. Facilitated Lignocellulosic Biomass Digestibility in Anaerobic Digestion for Biomethane Production: Microbial Communities’ Structure and Interactions. J. Chem. Technol. Biotechnol. 2021, 96, 1798–1817. [Google Scholar] [CrossRef]
- Saha, S.; Basak, B.; Hwang, J.-H.; Salama, E.-S.; Chatterjee, P.K.; Jeon, B.-H. Microbial Symbiosis: A Network towards Biomethanation. Trends Microbiol. 2020, 28, 968–984. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, V.K.; Bhatia, A.; Kubota, K.; Rajpal, A.; Ahmed, B.; Khan, A.A.; Kazmi, A.A.; Kumar, M. Microbial Community Dynamics in Anaerobic Digesters Treating Organic Fraction of Municipal Solid Waste. Environ. Technol. Innov. 2021, 21, 101303. [Google Scholar] [CrossRef]
- Ruiz Hernández, V.C.; Legaría Solano, J.P.; Sahagún Castellanos, J.; De la O Olan, M. Variabilidad Genética En Algunas Especies Cultivadas y Silvestres de Amaranto. Rev. Mex. Ciencias Agrícolas 2018, 9, 405–416. [Google Scholar] [CrossRef]
- Blasco, L.; Kahala, M.; Ervasti, S.; Tampio, E. Dynamics of Microbial Community in Response to Co-Feedstock Composition in Anaerobic Digestion. Bioresour. Technol. 2022, 364, 128039. [Google Scholar] [CrossRef]
- de Jonge, N.; Davidsson, Å.; la Cour Jansen, J.; Nielsen, J.L. Characterisation of Microbial Communities for Improved Management of Anaerobic Digestion of Food Waste. Waste Manag. 2020, 117, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Xie, J.; Wang, Y.; Wei, W.; Li, L.; Li, L.; Li, W.; Lv, S.; He, J.; Zhang, L. Electro-Assisted Anaerobic Digestion of Waste Activated Sludge for Biomethane Production Enhanced by Hyperosmosis of Sodium Chloride: Characteristics and Microbial Mechanisms. Process Saf. Environ. Prot. 2024, 187, 1390–1402. [Google Scholar] [CrossRef]
- Ketsub, N.; Whatmore, P.; Abbasabadi, M.; Doherty, W.O.S.; Kaparaju, P.; O’Hara, I.M.; Zhang, Z. Effects of Pretreatment Methods on Biomethane Production Kinetics and Microbial Community by Solid State Anaerobic Digestion of Sugarcane Trash. Bioresour. Technol. 2022, 352, 127112. [Google Scholar] [CrossRef]
- Wei, Y.; Li, Z.; Ran, W.; Yuan, H.; Li, X. Performance and Microbial Community Dynamics in Anaerobic Co-Digestion of Chicken Manure and Corn Stover with Different Modification Methods and Trace Element Supplementation Strategy. Bioresour. Technol. 2021, 325, 124713. [Google Scholar] [CrossRef]
- Pasalari, H.; Gharibi, H.; Darvishali, S.; Farzadkia, M. The Effects of Different Pretreatment Technologies on Microbial Community in Anaerobic Digestion Process: A Systematic Review. J. Environ. Health Sci. Eng. 2024, 22, 439–453. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, L.; Zuo, X.; Yan, B.; Li, X.; Yuan, H. Depolymerization of Corn Stover by Urea-Hydrothermal Pretreatment for Efficient Biomethane Production and Microbial Community Analysis of Anaerobic Digestion. J. Clean. Prod. 2022, 380, 134978. [Google Scholar] [CrossRef]
- Pan, S.; Liu, Q.; Wen, C.; Li, Z.; Du, L.; Wei, Y. Producing Biogas from Rice Straw: Kinetic Analysis and Microbial Community Dynamics. Bioenergy Res. 2021, 14, 1338–1348. [Google Scholar] [CrossRef]
- Rabii, A.; Aldin, S.; Dahman, Y.; Elbeshbishy, E. A Review on Anaerobic Co-Digestion with a Focus on the Microbial Populations and the Effect of Multi-Stage Digester Configuration. Energies 2019, 12, 1106. [Google Scholar] [CrossRef]
- Agabo-García, C.; Solera, R.; Pérez, M. First Approaches to Valorizate Fat, Oil and Grease (FOG) as Anaerobic Co-Substrate with Slaughterhouse Wastewater: Biomethane Potential, Settling Capacity and Microbial Dynamics. Chemosphere 2020, 259, 127474. [Google Scholar] [CrossRef]
- Usman, M.; Salama, E.S.; Arif, M.; Jeon, B.H.; Li, X. Determination of the Inhibitory Concentration Level of Fat, Oil, and Grease (FOG) towards Bacterial and Archaeal Communities in Anaerobic Digestion. Renew. Sustain. Energy Rev. 2020, 131, 110032. [Google Scholar] [CrossRef]
- Bucci, L.; Ghiotto, G.; Zampieri, G.; Raga, R.; Favaro, L.; Treu, L.; Campanaro, S. Adaptation of Anaerobic Digestion Microbial Communities to High Ammonium Levels: Insights from Strain-Resolved Metagenomics. Environ. Sci. Technol. 2024, 58, 580–590. [Google Scholar] [CrossRef]
- Ozsefil, I.C.; Miraloglu, I.H.; Ozbayram, E.G.; Ince, B.; Ince, O. Bioaugmentation of Anaerobic Digesters with the Enriched Lignin-Degrading Microbial Consortia through a Metagenomic Approach. Chemosphere 2024, 355, 141831. [Google Scholar] [CrossRef]
- Wang, B.; Liu, W.; Liang, B.; Jiang, J.; Wang, A. Microbial Fingerprints of Methanation in a Hybrid Electric-Biological Anaerobic Digestion. Water Res. 2022, 226, 119270. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, W.; Dong, Q.; Wu, D.; Yang, P.; Peng, Y.; Li, L.; Peng, X. Integrated Multi-Omics Analyses Reveal the Key Microbial Phylotypes Affecting Anaerobic Digestion Performance under Ammonia Stress. Water Res. 2022, 213, 118152. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Kim, N.-K.; Lee, S.-H.; Trinh, H.P.; Park, H.-D. Gene Abundance and Microbial Syntrophy as Key Drivers of Anaerobic Digestion Revealed through 16S RRNA Gene and Metagenomic Analysis. Chemosphere 2024, 370, 144028. [Google Scholar] [CrossRef]
- Wu, Z.-L.; Shi, W.-J.; Zhang, L.; Xia, Z.; Gou, M.; Sun, Z.-Y.; Tang, Y.-Q. Investigating the Robustness of Microbial Communities in Municipal Sludge Anaerobic Digestion under Organic Loading Rate Disturbance. J. Environ. Manag. 2024, 372, 123326. [Google Scholar] [CrossRef]
- Zhao, Q.; Yuan, H.; Li, X. Effect of Applied Voltages on Corn Stover Biomethanation and Microbial Community Characteristics in a Microbial Electrolytic Cell-Assisted Anaerobic Digestion System. Processes 2025, 13, 1271. [Google Scholar] [CrossRef]
- Farghali, M.; Osman, A.I.; Umetsu, K.; Rooney, D.W. Integration of Biogas Systems into a Carbon Zero and Hydrogen Economy: A Review; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; Volume 20, ISBN 0123456789. [Google Scholar]
- Feng, Y.; Rosa, L. Global Biomethane and Carbon Dioxide Removal Potential through Anaerobic Digestion of Waste Biomass. Environ. Res. Lett. 2024, 19, 024024. [Google Scholar] [CrossRef]
- Costa, J.M.; Aguiar, A.B.S.; Montanari, A.F.P.; Damasceno, B.G.; Duran, K.A.; Jerônimo, K.A.; Silva, M.M.; Silva, T.C.T.; Rodriguez, R.P. Environmental Aspects and Perspectives of the Brazilian Market for Biogas and Biomethane from Anaerobic Digestion: A Review. Bioenergy Res. 2024, 17, 59–72. [Google Scholar] [CrossRef]
- Meisterl, K.; Sastre, S.; Puig-Ventosa, I.; Chifari, R.; Martínez Sánchez, L.; Chochois, L.; Fiorentino, G.; Zucaro, A. Circular Bioeconomy in the Metropolitan Area of Barcelona: Policy Recommendations to Optimize Biowaste Management. Sustainability 2024, 16, 1208. [Google Scholar] [CrossRef]
- Lombardi, L.; Francini, G. Techno-Economic and Environmental Assessment of the Main Biogas Upgrading Technologies. Renew. Energy 2020, 156, 440–458. [Google Scholar] [CrossRef]
- Bux, C.; Cangialosi, F.; Amicarelli, V. Biomethane and Compost Production by Anaerobic Digestion of Organic Waste: Suggestions for Rural Communities in Southern Italy. Sustainability 2023, 15, 15644. [Google Scholar] [CrossRef]
- Murano, R.; Maisano, N.; Selvaggi, R.; Pappalardo, G.; Pecorino, B. Critical Issues and Opportunities for Producing Biomethane in Italy. Energies 2021, 14, 2431. [Google Scholar] [CrossRef]
- Hosseini, T.; Culley, S.A.; Zecchin, A.; Maier, H.R.; Ashman, P.J. Impact of Co-Digestion and Degree of Centralization on the Yield and Viability of Biomethane Production: A Case Study in Regional Australia. Energy Convers. Manag. X 2024, 22, 100585. [Google Scholar] [CrossRef]
- Pappalardo, G.; Selvaggi, R.; Pecorino, B. Biomethane Production Potential in Southern Italy: An Empirical Approach. Renew. Sustain. Energy Rev. 2022, 158, 112190. [Google Scholar] [CrossRef]
- Alengebawy, A.; Ran, Y.; Osman, A.; Jin, K.; Samer, M.; Ai, P. Anaerobic Digestion of Agricultural Waste for Biogas Production and Sustainable Bioenergy Recovery: A Review. Environ. Chem. Lett. 2024, 22, 2641–2668. [Google Scholar] [CrossRef]
- Chung, C.-C.; Liu, L.; Zhang, Y.; Wang, Y.; Wei, Z. Evolution of Biogas Policies in China (2001–2019): Dynamics of Policy Instruments towards Energy Transitions. J. Clean. Prod. 2022, 379, 134642. [Google Scholar] [CrossRef]
- Huang, X. The Promotion of Anaerobic Digestion Technology Upgrades in Waste Stream Treatment Plants for Circular Economy in the Context of “Dual Carbon”: Global Status, Development Trend, and Future Challenges. Water 2024, 16, 3718. [Google Scholar] [CrossRef]
- Zheng, L.; Chen, J.; Zhao, M.; Cheng, S.; Wang, L.; Mang, H.-P.; Li, Z. What Could China Give to and Take from Other Countries in Terms of the Development of the Biogas Industry? Sustainability 2020, 12, 1490. [Google Scholar] [CrossRef]
- Xue, S.; Song, J.; Wang, X.; Shang, Z.; Sheng, C.; Li, C.; Zhu, Y.; Liu, J. A Systematic Comparison of Biogas Development and Related Policies between China and Europe and Corresponding Insights. Renew. Sustain. Energy Rev. 2020, 117, 109474. [Google Scholar] [CrossRef]
- Leonhardt, B.; Tyson, R.; Taw, E.; Went, M.; Sanchez, D. Policy Analysis of CO2 Capture and Sequestration with Anaerobic Digestion for Transportation Fuel Production. Environ. Sci. Technol. 2023, 57, 11401–11409. [Google Scholar] [CrossRef]
- Ankathi, S.; Chaudhari, U.; Handler, R.; Shonnard, D. Sustainability of Biogas Production from Anaerobic Digestion of Food Waste and Animal Manure. Appl. Microbiol. 2024, 4, 418–438. [Google Scholar] [CrossRef]
- Ghosh, A.; Sarkar, J.; Das, B. A Critical Analysis on Anaerobic Digestion of OFMSW in India. Waste Valoris. Recycl. 2019, 2, 237–245. [Google Scholar] [CrossRef]
- Mittal, S.; Ahlgren, E.; Shukla, P. Barriers to Biogas Dissemination in India: A Review. Energy Policy 2018, 112, 361–370. [Google Scholar] [CrossRef]
- Meena, P.; Pal, A.; Gautam, S. Zone-Wise Biogas Potential in India: Fundamentals, Challenges, and Policy Considerations. Environ. Sci. Pollut. Res. 2023, 31, 1841–1862. [Google Scholar] [CrossRef]
- Gross, T.; Breitenmoser, L.; Kumar, S.; Ehrensperger, A.; Wintgens, T.; Hugi, C. Anaerobic Digestion of Biowaste in Indian Municipalities: Effects on Energy, Fertilizers, Water and the Local Environment. Resour. Conserv. Recycl. 2021, 170, 105569. [Google Scholar] [CrossRef]
- Ellacuriaga, M.; Cascallana, J.G.; González, R.; Gómez, X. High-Solid Anaerobic Digestion: Reviewing Strategies for Increasing Reactor Performance. Environments 2021, 8, 80. [Google Scholar] [CrossRef]
- Zielińska, M.; Bułkowska, K. Sustainable Management and Advanced Nutrient Recovery from Biogas Energy Sector Effluents. Energies 2024, 17, 3705. [Google Scholar] [CrossRef]
- Nghia, N.K.; Thy, C.T.A.; Xa, L.T.; Luan, D.T. Recycling of Biogas Wastewater to Organic Fertilizers and Influence of Organic Fertilizers on Maize, Mung Bean Growth and Yield under the Field Conditions. Appl. Environ. Biotechnol. 2022, 7, 16–27. [Google Scholar] [CrossRef]
- Wijdeveld, W.; Prot, T.; Sudintas, G.; Kuntke, P.; Korving, L.; Loosdrecht, M. Pilot-Scale Magnetic Recovery of Vivianite from Digested Sewage Sludge. Water Res. 2022, 212, 118131. [Google Scholar] [CrossRef]
- Lorick, D.; Macura, B.; Ahlström, M.; Grimvall, A.; Harder, R. Effectiveness of Struvite Precipitation and Ammonia Stripping for Recovery of Phosphorus and Nitrogen from Anaerobic Digestate: A Systematic Review. Environ. Evid. 2020, 9, 27. [Google Scholar] [CrossRef]
- Wu, Z.-Z.; Zheng, Q.; Zhang, Y.; Pang, Y.; Huang, T.; Peng, D. Phosphorus Recovery from Waste-Activated Sludge through Vivianite Crystallization Enhanced by Magnetic Biochar. J. Clean. Prod. 2023, 392, 136294. [Google Scholar] [CrossRef]
- Bywater, A.; Heaven, S.; Zhang, Y.; Banks, C.J. Potential for Biomethanisation of CO2 from Anaerobic Digestion of Organic Wastes in the United Kingdom. Processes 2022, 10, 1202. [Google Scholar] [CrossRef]
- Sun, H.; Wang, E.; Li, X.; Cui, X.; Guo, J.; Dong, R. Potential Biomethane Production from Crop Residues in China: Contributions to Carbon Neutrality. Renew. Sustain. Energy Rev. 2021, 148, 111360. [Google Scholar] [CrossRef]
- Wu, B.; Lin, R.; Bose, A.; Huerta, J.D.; Kang, X.; Deng, C.; Murphy, J.D. Economic and Environmental Viability of Biofuel Production from Organic Wastes: A Pathway towards Competitive Carbon Neutrality. Energy 2023, 285, 129322. [Google Scholar] [CrossRef]
- Pavicic, J.; Mavar, K.N.; Brkic, V.; Simon, K. Development, Advantages and Challenges in Europe. Energies 2022, 15, 2940. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).