Abstract
The synthesis of dimethyl carbonate (DMC) from methanol and CO2 has also received widespread attention, and K2CO3 is usually used as a catalyst in the synthesis of DMC. In this work, the role of K2CO3 in synthesizing dimethyl carbonate (DMC) from methanol and CO2 was revisited. Interestingly, NMR results indicated that K2CO3 can react with methanol to form carbonate CH3OCOO−, an essential intermediate in the synthesis of DMC, which can be transformed into DMC in the presence of CH3I. In other words, K2CO3 can act as not only a catalyst but also a reactant to synthesize DMC from methanol and CO2.
1. Introduction
Global warming is a serious environmental issue primarily due to the dramatic increase in anthropogenic CO2 emissions, which has triggered disastrous effects on the ecosystems of the Earth. Developing effective methodologies to reduce CO2 emissions plays a critical role in curbing global warming [1]. Carbon capture and utilization (CCU) technology is recognized as a promising strategy to efficiently control and reduce carbon emissions, in which CO2 is trapped from carbon sources and then transformed into valuable chemicals [2,3]. To date, many strategies have been developed for transforming CO2 into high-value chemicals, such as carboxylic acids, carbamates, and organic carbonates [4,5]. The synthesis of dimethyl carbonate (DMC) from methanol and CO2 has also gained widespread attention due to its simple synthesis procedures and high atom economy [6].
DMC is regarded as a green organic compound due to its low toxicity and good biodegradability [7]. Because of its unique properties, DMC has been utilized in a wide range of fields. It can be used as a gasoline additive and an ideal electrolyte solvent in lithium-ion batteries [8]. Moreover, the applications of DMC can also be found in paints, pesticides, pharmaceutical products, polycarbonate synthesis, and methylation processes [9]. Although the conversion of CO2 and methanol (CH3OH) to DMC is a promising and green reaction, this reaction suffers from the inherent drawbacks, including thermodynamic limitations and the activation of the relatively inert C=O bond of CO2, which results in a low yield of DMC [10,11,12,13]. Therefore, developing efficient catalysts for this reaction is of particular importance. Various catalysts have been designed and used for the synthesis of DMC from CO2 and CH3OH, such as alkali metal carbonate salts, organometallic complexes, ionic liquids, metal organic frameworks, nanomaterials based on metal oxides, and heteropoly acid compounds [14,15].
Alkali carbonate salts are cheap catalysts that are usually used in organic synthesis, and the applications of alkali carbonate catalysts in the synthesis of DMC from CO2 and CH3OH have also been widely investigated [16]. Among them, K2CO3 is found to be the most efficient catalyst [17,18]. It should be noted that CH3I is used as a promoter during the synthesis of DMC when K2CO3 is used as the catalyst. The catalytic mechanism of K2CO3 for the formation of DMC is also proposed in the literature [19], which includes the following steps. The acid–base reaction occurs between K2CO3 and CH3OH with the formation of CH3O− anion and bicarbonate anion , and CH3O− anion can attack CO2 to form the carbonate anion CH3OCOO−. The nucleophilic group CH3OCOO− will react with CH3I to form DMC and give I− anion. The I− anion interacts with to form HI, and thus is regenerated. Then, CH3I is recycled through the reaction between HI and CH3OH [20].
Herein, the role of K2CO3 in the synthesis of DMC was revisited. Surprisingly, the results disclosed that anion can directly react with -OH of CH3OH to form CH3OCOO− carbonate in the absence of CO2. As mentioned above, CH3OCOO− carbonate is an intermediate in the formation of DMC. In other words, K2CO3 is not only a catalyst but also a reagent in the synthesis of DMC, which may be a reason for the deactivation of K2CO3 in the formation of DMC from CO2 and CH3OH.
2. Materials and Methods
2.1. Materials and Characterizations
K213CO3 (99%) and CD3OD (99.8%) were obtained from Innochem (Beijing, China).
1H NMR (400 MHz) and 13C NMR (100.6 MHz) spectra were obtained on a Bruker spectrometer. The NMR spectra were recorded at 25 °C, and a 5 mm PABBO probe was used. In the 1H NMR experiment, the relaxation delay was 1.0 s, the acquisition time was 4.09 s, and the number of scans was 16. In the 13C NMR experiment, the number of scans was 1024, the relaxation delay was 2.0 s, and the acquisition time was 1.36 s. For the HSQC experiment, the relaxation delay was 2.0 s, and the acquisition time was 0.10 s. For the HMBC experiment, the relaxation delay was 1.5 s, and the acquisition time was 0.38 s.
2.2. Synthesis of K213CO3-CD3OD Mixture
K213CO3 was added into CD3OD at room temperature, and the mass fraction of K213CO3 was 3 wt%. The mixture was stirred at room temperature for 24 h.
2.3. NMR Data
K213CO3-CD3OD: 13C NMR (100.6 MHz, CD3OD-d4) δ = 161.3, 170.0 ppm.
3. Results and Discussion
NMR spectra were used to investigate interactions between K2CO3 and CH3OH. Due to the low solubility of K2CO3 in CH3OH, the NMR spectra of K213CO3 in methanol-d4 were recorded to obtain the strong signal of carbonate anion in 13C NMR spectra. The mixture of K213CO3-CD3OD (3 wt%) was stirred 24 h at room temperature. After stirring, solid particles of K2CO3 can still be observed at the bottom of the mixture, suggesting K2CO3 was not completely dissolved in methanol, and the methanol phase became a suspension. The suspension was used to obtain NMR spectra.
As can be seen in Figure 1a, the carbon signals of and CD3OD appear at 170.0 [21] and 49.0 ppm, respectively. Besides these two peaks, a new peak was found at 161.3 ppm, which can be attributed to the carbonyl carbon of methanol-based carbonate [22,23].

Figure 1.
(a) The 13C NMR spectra of K213CO3-CD3OD; (b) The 1H NMR spectra of CD3OD and K213CO3-CD3OD; (c) The 1H-13C HSQC spectra of K213CO3-CD3OD.
The 1H NMR spectra of CD3OD with and without K213CO3 were also investigated and shown in Figure 1b. After mixing CD3OD with K213CO3, the residual -OH peak and CHD2- peak in deuterated methanol shifted downfield from 4.84 and 3.31 ppm to 5.03 and 3.34 ppm, respectively. Interestingly, a new peak can be observed at 3.38 ppm after the addition of K213CO3 into CD3OD. This peak can be attributed to the signal of CH3- group of CH3OH, which is supported by the HSQC spectra (Figure 1c). The appearance of CH3- signal revealed that can act as a catalyst for the hydrogen–deuterium exchange reaction between -OH and the deuterated methyl group [24,25]. Moreover, the hydrogen signals of CH3OCOO− and CHD2OCOO− were detected using 1H-13C HMBC spectra (Figure 2). As can be seen in Figure 2, hydrogen signals of CH3OCOO− and CHD2OCOO− were correlated with the carbonyl carbon of methanol-based carbonate, suggesting again the carbonate carbon from K2CO3 was attached to the O atom of methanol.
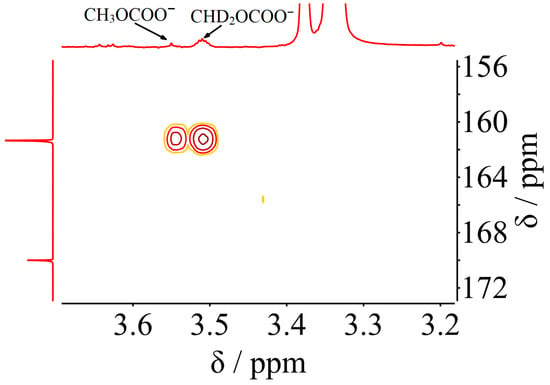
Figure 2.
The 1H-13C HMBC spectra of K213CO3-CD3OD.
Based on the above NMR results, the possible reaction steps between CH3OH and are shown as follows.


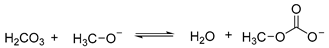





Using Equations (2) and (3), the following Equation (5) can be obtained.
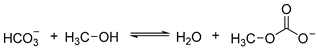
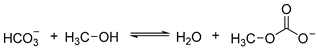
The overall reaction between CH3OH and can be presented by Equation (6) below.
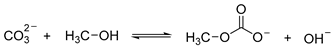

As revealed by Equation (6), can react with CH3OH to form CH3OCOO− carbonate, which can react with CH3I to form DMC. Therefore, it is reasonable to conclude that K2CO3 also acts as a reactant in the synthesis of DMC.
Based on the above results, the possible reaction mechanism for the synthesis of DMC from CO2 and methanol with K2CO3 as a reactant is shown in Scheme 1. When CO2 is introduced into the reaction system, CO2 can react with OH− to form . The formed can react with CH3OH to form CH3OCOO− (Equation (5)). In other words, both CO2 and can be transformed to CH3OCOO−, and then DMC is formed after the reaction between CH3OCOO− and CH3I. In addition, it is worth noting that the role of K2CO3 as a reactant may be a reason for the deactivation of K2CO3 during the synthesis of DMC [20], which is detrimental to the stability and reusability of K2CO3. As reported by Fujita and co-authors, the amounts of K2CO3 recycled after the synthesis of DMC were not high [20]. The effect of reaction parameters, such as temperature, pressure, and concentration, on the DMC yield had been studied by Hou and co-authors [26].
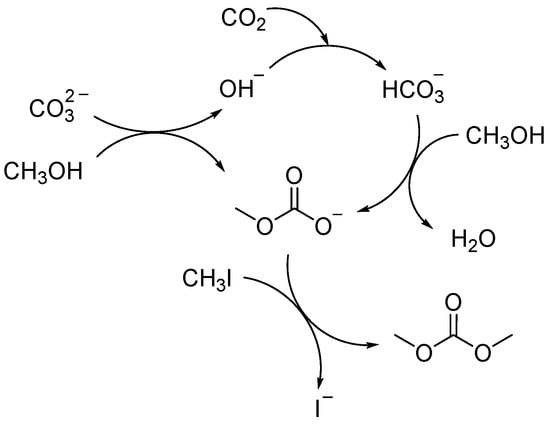
Scheme 1.
The possible reaction mechanism of the synthesis of DMC from CO2 and CH3OH with K2CO3 as a reactant.
4. Conclusions
In summary, the reaction between K2CO3 and CH3OH was revealed. K2CO3 could react with CH3OH to form carbonate CH3OCOO−, which was an important intermediate and can react with CH3I to form DMC. The results demonstrated that K2CO3 can function as not only a catalyst but also a reactant during the synthesis of DMC from CO2 and CH3OH. The insights of this work may be useful to understand the role of K2CO3 in the DMC synthesis and offer valuable information for the design of efficient catalysts for synthesizing DMC.
Author Contributions
Conceptualization, D.Y. and X.D.; methodology, D.Y., C.W. and X.D.; investigation, Y.Z., M.C., D.Y. and C.W.; data curation, Y.Z. and M.C.; writing—original draft preparation, D.Y., C.W. and X.D.; writing—review and editing, D.Y. and X.D.; supervision, D.Y.; funding acquisition, C.W. and D.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the National Natural Science Foundation of China (No. 22102158 and 21503196) and Fundamental Research Funds for the Central Universities (No. 265QZ2022003 and 2652019111).
Data Availability Statement
The data used in this work are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, H.; Zheng, Y.; Li, J.; Li, L.; Wang, X. AI for Nanomaterials Development in Clean Energy and Carbon Capture, Utilization and Storage (CCUS). ACS Nano 2023, 17, 9763–9792. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Catanescu, C.O.; Bird, R.E.; Satagopan, S.; Baum, Z.J.; Lotti Diaz, L.M.; Zhou, Q.A. Trends in Research and Development for CO2 Capture and Sequestration. ACS Omega 2023, 8, 11643–11664. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Guo, X.; Zhou, A.; Chen, Z.A.; Jin, D.; Fan, M.; Ma, T. Fundamentals and Recent Progress in Magnetic Field Assisted CO2 Capture and Conversion. Small 2024, 20, 2305533. [Google Scholar] [CrossRef] [PubMed]
- Kumar De, S.; Won, D.-I.; Kim, J.; Kim, D.H. Integrated CO2 capture and electrochemical upgradation: The underpinning mechanism and techno-chemical analysis. Chem. Soc. Rev. 2023, 52, 5744–5802. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, S.; Li, L.; Feng, J.; Li, K.; Huang, Z.; Lin, H. Integrated CO2 capture and utilization: A review of the synergistic effects of dual function materials. Catal. Sci. Technol. 2024, 14, 790–819. [Google Scholar] [CrossRef]
- Fu, L.; Ren, Z.; Si, W.; Ma, Q.; Huang, W.; Liao, K.; Huang, Z.; Wang, Y.; Li, J.; Xu, P. Research progress on CO2 capture and utilization technology. J. CO2 Util. 2022, 66, 102260. [Google Scholar] [CrossRef]
- Li, L.; Liu, W.; Chen, R.; Shang, S.; Zhang, X.; Wang, H.; Zhang, H.; Ye, B.; Xie, Y. Atom-Economical Synthesis of Dimethyl Carbonate from CO2: Engineering Reactive Frustrated Lewis Pairs on Ceria with Vacancy Clusters. Angew. Chem. Int. Ed. 2022, 61, e202214490. [Google Scholar] [CrossRef]
- Raza, A.; Ikram, M.; Guo, S.; Baiker, A.; Li, G. Green Synthesis of Dimethyl Carbonate from CO2 and Methanol: New Strategies and Industrial Perspective. Adv. Sustain. Syst. 2022, 6, 2200087. [Google Scholar] [CrossRef]
- Kumar, P.; Srivastava, V.C.; Štangar, U.L.; Mušič, B.; Mishra, I.M.; Meng, Y. Recent progress in dimethyl carbonate synthesis using different feedstock and techniques in the presence of heterogeneous catalysts. Catal. Rev. 2021, 63, 363–421. [Google Scholar] [CrossRef]
- Signorile, M.; Salusso, D.; Crocellà, V.; Paganini, M.C.; Bordiga, S.; Bonino, F.; Ferri, D. Surface species in direct liquid phase synthesis of dimethyl carbonate from methanol and CO2: An MCR-ALS augmented ATR-IR study. Phys. Chem. Chem. Phys. 2023, 25, 8392–8402. [Google Scholar] [CrossRef]
- Wang, S.; Chen, C.; Yang, P.; Guo, Y.; Wang, B.; Niu, H.; Zhang, Q.; Wang, J. Facile One-Pot Preparation of Organotin-Oxometalate Polymers for Efficient Synthesis of Dimethyl Carbonate from CO2. Ind. Eng. Chem. Res. 2024, 63, 10151–10161. [Google Scholar] [CrossRef]
- Ohno, H.; Ikhlayel, M.; Tamura, M.; Nakao, K.; Suzuki, K.; Morita, K.; Kato, Y.; Tomishige, K.; Fukushima, Y. Direct dimethyl carbonate synthesis from CO2 and methanol catalyzed by CeO2 and assisted by 2-cyanopyridine: A cradle-to-gate greenhouse gas emission study. Green Chem. 2021, 23, 457–469. [Google Scholar] [CrossRef]
- Faria, D.J.; Moreira dos Santos, L.; Bernard, F.L.; Selbacch Pinto, I.; Carmona da Motta Resende, M.A.; Einloft, S. Dehydrating agent effect on the synthesis of dimethyl carbonate (DMC) directly from methanol and carbon dioxide. RSC Adv. 2020, 10, 34895–34902. [Google Scholar] [CrossRef]
- Shi, D.; Heyte, S.; Capron, M.; Paul, S. Catalytic processes for the direct synthesis of dimethyl carbonate from CO2 and methanol: A review. Green Chem. 2022, 24, 1067–1089. [Google Scholar] [CrossRef]
- Tamboli, A.H.; Chaugule, A.A.; Kim, H. Catalytic developments in the direct dimethyl carbonate synthesis from carbon dioxide and methanol. Chem. Eng. J. 2017, 323, 530–544. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, Y.; Williams, B.L.; Xiao, M.; Wang, S.; Han, D.; Sun, L.; Meng, Y. Catalytic materials for direct synthesis of dimethyl carbonate (DMC) from CO2. J. Clean. Prod. 2021, 279, 123344. [Google Scholar] [CrossRef]
- Shao, W.; Zhang, X.; Xie, Y. Engineering active sites and recognizing mechanisms for CO2 fixation to dimethyl carbonate. Trends Chem. 2023, 5, 312–323. [Google Scholar] [CrossRef]
- Wang, D.; Shi, F.; Wang, L. A Review of Catalysts for Synthesis of Dimethyl Carbonate. Catalysts 2024, 14, 259. [Google Scholar] [CrossRef]
- Sakakura, T.; Choi, J.-C.; Yasuda, H. Transformation of Carbon Dioxide. Chem. Rev. 2007, 107, 2365–2387. [Google Scholar] [CrossRef]
- Fujita, S.-I.; Bhanage, B.M.; Ikushima, Y.; Arai, M. Synthesis of dimethyl carbonate from carbon dioxide and methanol in the presence of methyl iodide and base catalysts under mild conditions: Effect of reaction conditions and reaction mechanism. Green Chem. 2001, 3, 87–91. [Google Scholar] [CrossRef]
- Li, Q.; Bao, Z.; Akhmedov, N.G.; Li, B.A.; Duan, Y.; Xing, M.; Wang, J.; Morsi, B.I.; Li, B. Unraveling the Role of Glycine in K2CO3 Solvent for CO2 Removal. Ind. Eng. Chem. Res. 2022, 61, 12545–12554. [Google Scholar] [CrossRef]
- Xie, Y.; Parnas, R.; Liang, B.; Liu, Y.; Tao, C.; Lu, H. Synthesis and characterization of switchable ionic compound based on DBU, CH3OH, and CO2. Chin. J. Chem. Eng. 2015, 23, 1728–1732. [Google Scholar] [CrossRef]
- Gu, Q.; Fang, J.; Xu, Z.; Ni, W.; Kong, K.; Hou, Z. CO2 promoted synthesis of unsymmetrical organic carbonate using switchable agents based on DBU and alcohols. New J. Chem. 2018, 42, 13054–13064. [Google Scholar] [CrossRef]
- Gu, J.-G.; Wang, C.-X.; Hu, G.-Q.; Shen, K.; Zhang, H.-H. K2CO3/18-Crown-6-Catalyzed Selective H/D Exchange of Heteroarenes with Bromide as a Removable Directing Group. Org. Lett. 2023, 25, 3055–3059. [Google Scholar] [CrossRef] [PubMed]
- Bew, S.P.; Hiatt-Gipson, G.D.; Lovell, J.A.; Poullain, C. Mild Reaction Conditions for the Terminal Deuteration of Alkynes. Org. Lett. 2012, 14, 456–459. [Google Scholar] [CrossRef]
- Hou, Z.; Han, B.; Liu, Z.; Jiang, T.; Yang, G. Synthesis of dimethyl carbonate using CO2 and methanol: Enhancing the conversion by controlling the phase behavior. Green Chem. 2002, 4, 467–471. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).