Abstract
The amount of microplastics (MPs) present in marine ecosystems are a growing concern, with potential impacts on human health because they are associated with an increase in the ecotoxicity of certain foods, such as fish. As a result, there has been a growing interest in developing effective methods for the analysis of MPs in marine waters. Traditional methods for MP analysis involve visual inspection and manual sorting, which can be time-consuming and subject to human error. However, novel methods have been developed that offer more efficient and accurate analyses. One such method is based on spectroscopy, such as Fourier transform infrared spectroscopy (FTIR). Another method involves the use of fluorescent dyes, which can selectively bind to microplastics and allow for their detection under UV light. Additionally, machine learning approaches have been developed to analyze large volumes of water samples for MP detection and classification. These methods involve the use of specialized algorithms that can identify and classify MPs based on their size, shape, and texture. Overall, these novel methods offer more efficient and accurate analyses of MPs in marine waters, which is essential for understanding the extent and impacts of MP pollution and for developing effective mitigation strategies. However, there is still a need for continued research and development to optimize these methods and improve their sensitivity and accuracy.
1. Introduction
Microplastics (MPs), defined as plastic waste with dimensions less than 5 mm [1,2], are recognized as an emerging environmental pollutant and have garnered considerable attention due to their possible negative effects on living organisms. MPs are categorized as main or secondary according to their sources. Marine litter, particularly MPs and nano-plastics (NPs), is widely disseminated and is recognized as a growing threat to the environment and human health. It is well recognized that maritime habitats are among the most damaged, and coastal zones are among the most polluted. Carpenter and Smith published the first paper warning about the presence of plastic pellets on the surface of the North Atlantic Ocean in 1972 [3]. Yet, it has only been in the last ten years that there has been a widespread increase in concern, both in the scholarly community and in society, about the impact of plastic-based pollution on the marine environment [4].
The proper approach for the identification of MPs should be selected based on the quantity of samples and the microplastic size range of interest; adopting a good identification method for microplastics is critical for analyzing microplastic contamination. Plastics are primarily classified based on physical qualities such as size, shape, and color [5]. The study of Coyle, Hardiman, and O’Driscoll presented polymers which (i) float as: Low-density polyethylene (LDPE), Polyethylene (PE), High-density polyethylene (HDPE), Polypropylene (PP), and Polystyrene (Expanded) (PS), and (ii) sink as: Polystyrene (PS), Polystyrene Acrylonitrile (PSA), Acrylonitrile butadiene styrene (ABS), Polyamide (Nylon) (PA), Polymethyl methacrylate (Acrylic) (PMMA), Polyvinyl Chloride (PVC), Polylactic acid (PLA), Polycarbonate (PC), Polyethylene terephthalate (PET), Polyoxymethylene (POM), Polyester (Poly), and Cellulose acetate. The specific gravity of seawater is 1.025 while for the floating plastic polymers it is under 1.05, and for those that sink, the specific gravity ranges from 1.04 (PS) to 1.44 (POM) [6]. Size is normally determined by a particle’s longest dimension; size categories can be used if appropriate. Researchers prefer to utilize five basic categories when describing microplastic forms, while the nomenclature used differs between study organizations as presented in Table 1 [7].

Table 1.
Codifications used to classify microplastics based on morphology [7].
The annual production of plastics worldwide with an estimation for the next three decades [8] is presented in Figure 1. Therefore, it is expected that research into the traces that plastic leaves in the environment, both water and the environment of the human population, will be of special interest.
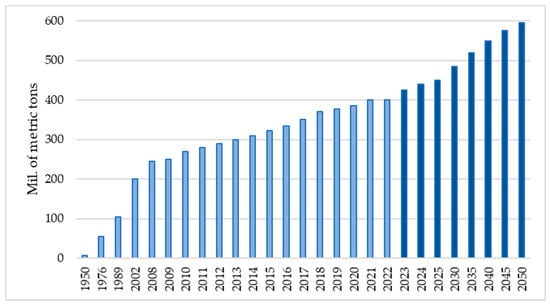
Figure 1.
Production forecast of plastics worldwide from 1950 to 2050 in millions of metric tons [6] where the dark blue bars present the estimation based on the interpolation method.
Humans are exposed to MPs due to their increasing presence in the environment and are exposed to them through the consumption of branches, inhalation, and skin contact [9]. Previous research indicates that exposed persons may experience (i) immune system disorder, (ii) oxidative stress, (iii) neurotoxicity, (iv) cytotoxicity, and (v) the transfer of MPs to other tissues as a result of exposure to MPs [10,11]. Of great concern is that MPs have also been detected in human breast milk and the projection of daily consumption is up to 5 g/week from water, food, and consumer products [12]. Therefore, the detection of MPs is an important factor in order to detect the sources of human and animal exposure, and to qualitatively and quantitatively assess which methods to use in their detection, as well as to develop guidelines for their minimization [6]. That is why we studied which scientific fields deal with microplastics. A comprehensive systematic review of the available papers in the last decade (since January 2013 until December 2022), investigating the topic “microplastics” found that the number of studies grows exponentially starting with only 35 papers in 2013, while ten years later (in 2022) almost 120 times more papers were published on the topic, 4108 of them [13]. However, the study of microplastics has spread into various scientific fields, from sciences that primarily deal with the study of the environment, through to chemical and engineering disciplines to public health and green sustainable science, as presented in Figure 2. The interest of the scientific community in the methods used in the detection of microplastics that end up in the human food chain is presented with an overview of studies investigating the potential of three vibrational spectroscopic methods, also categorized as the green one (minimized sample preparation, fast, and not time-consuming). Fourier-transform infrared spectroscopy (FTIR), Near-infrared spectroscopy (NIR), and Raman spectroscopy are non-destructive analyses that provide detailed information at the molecular level (chemical structure, molecular interactions, etc.), and they are based on the interaction of light with chemical bonds within the sample. Their applicability is evident in the number of published papers on the topics “microplastics” + “FTIR”, “Raman”, or “NIR”. As can be seen (Figure 3), since the year 2000 the number of publications increases, but the increase has been extremely exponential in the last 10 years [13].
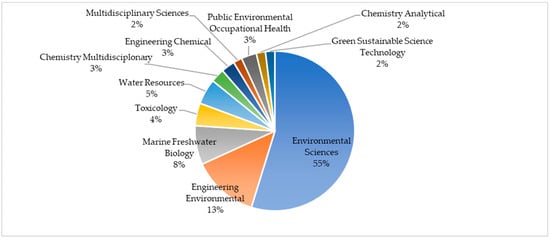
Figure 2.
Proportion of published papers in the last 10 years (2013–2022) investigating microplastics in Web of Science categories (first 10 categories) [13].
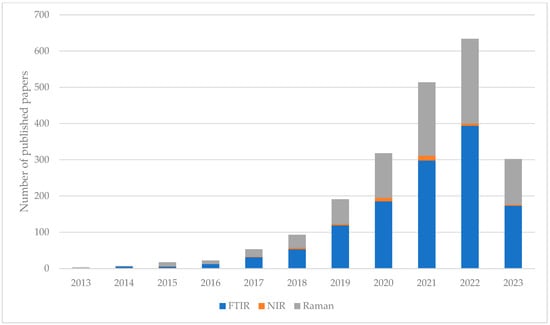
Figure 3.
Overview of published papers (since 2013 till June 2023) investigating three spectroscopic methods in the detection of microplastics, FTIR, NIR, and Raman, based on the Web of Science core collection.
Microplastic transmission along the sea level and exposure to humans begins with the fragmentation of plastic waste. The next step is marine organisms swallowing them which, if consumed (seafood consumes all of the microplastics or dismisses fish as a vehicle of plastic additives and harmful pollution), they end up in the human food chain, so people are exposed [14]. However, it has not yet been clarified whether microplastics affect humans, and if they do, how extremely problematic it is that plastic often contains additives or other additional substances, and they can be the cause of potentially harmful effects when being exposed to them. Therefore, it is necessary to carefully study the entire theme related to the microplastics in food and water [15]. The European Food Security Administration (EFSA) emphasizes that there is not enough information on the impact of microplastics in food and from the environment to the human body. It estimates the necessity of evening the research numbers and further developing and standardizing the methods they use for the analysis of microplastics and assessments of the potential risks they may represent. This is a necessity to facilitate comparisons with the results of different research institutions and to obtain clearer insight into the situation [16]. Therefore, the aim of this work is to contribute to the understanding of the sources of microplastics in food and to provide an overview of their detection methods (with an emphasis on the FTIR and NIR methods) as well as Europe’s vision regarding microplastics.
2. Analytical Methods for Analysis of Microplastics
It is crucial to have a reliable analytical approach to detect, measure, and identify MPs of varied materials, sizes, and shapes from environmental, agricultural, and food matrices to improve the risk assessments of MPs to people [17,18]. For microplastic investigation, spectroscopic methods, particularly vibrational spectroscopy (Raman and infrared), are often utilized, as for food analyses as well [19,20], coupled with chemometric tools [21,22,23].
2.1. FTIR Microscopic Analysis of Microplastics
Microplastic particles have been found in various bottled water brands. The consequences of microplastics on human health are unknown; however, the presence of microplastics in food and drinks, potentially carrying priority organic pollutants (POPs), is a serious issue. Bottled water analysis can reveal the existence, identity, size, and quantity of microplastics. Infrared (IR) spectroscopy is the principal analytical technique for polymer identification. Fourier-Transform Infrared spectrophotometers (FTIR) measure the light absorption in the wavelength range of 2.5–25 µm (wavenumber range: 4000 to 400 cm−1), what is called the mid-infrared range. The application of IR microscopy allows for the detection and identification of microplastics as small as a few millimeters. IR microscopy, either using point mode or IR imaging, has been shown to be an excellent analytical technique for the detection and identification of microplastics present in bottled water, and can be applied to a much larger range of samples containing microplastics using appropriate sample collection and clean-up. All brands of bottled water contained microplastics in the size range of 20–200 microns with some fibers more than 2 mm in length. The bottled waters contained considerably less fibers and particles than were present in the tap water sample. The types of microplastics present varied quite considerably and the vast majority were not plastic materials used in the manufacture of plastic drinks bottles. The origin of the microplastics needs to be determined within the individual manufacturer’s sites to eliminate the problem, or extra filtration steps could be introduced to remove the microplastics. In addition, it needs to be determined whether the microplastics present form a health risk to consumers [24,25,26,27,28,29,30]. An overview of the functional groups of untreated microplastics, which float, is presented in Table 2.

Table 2.
The functional group from untreated microplastics (PE, LDPE, HDPE, PP, and PS) identified with FTIR.
On the contrary, the study by Gerdes and coworkers [38] finds that for low to medium particle counts, FTIR microscopy is a reliable approach (150–1000 particles per sample). Increased particle counts promote clogging and uneven distributions, which impede accurate quantification because choked particles escape detection. As a result, at high particle concentrations, MP numbers are likely to be underestimated. This technology of MP detection through FTIR microscopy offers some advantages over other spectrometric methods, but it also has certain limitations: Raman microscopy detects particles up to 1 μm in size but requires time-consuming sample preparation. This sample preparation is necessary to avoid blanks and matrix fluorescence. Furthermore, every microscopic approach encounters problems with heterogenic particle dispersion on the filter material and blockages caused by excessive particle numbers. The problem with high particle counts worsens as the measurable particle size decreases. This may be explained by the fact that particles smaller than 20 μm are one to two orders of magnitude more frequent than particles larger than 20 μm, resulting in a greater number of particles that must be studied [39].
There are spectrometric methods such as pyrolysis GC-MS or thermal extraction desorption (TED). GC-MS can be entirely automated, resulting in a greater sample throughput. The detection and quantification limits are quite high (LOD and LOQ). Moreover, the matrix’s pyrolysis products can interfere with the measurement, and MP particle size distributions cannot be obtained. This FTIR technology provides a simple and reliable method that may be used in any laboratory without limitation. Sample preparation and measurements take a reasonable amount of time for research purposes (one day for sample preparation, four-to-eight hours for measurements) and have no severe constraints. Depending on the analytical question, this approach can be used with a wide range of environmental materials and particle sizes as small as 20 μm [26,40].
2.2. Near-Infrared (NIR) Spectroscopy
NIR spectroscopy is widely used in food and beverage analyses [41,42] but the near-infrared light region is also used in plastic differentiation [43,44]. NIR spectroscopy does not only analyze the surface but it penetrates deeper into the sample (e.g., material containing microplastics) and is fast without the need for sample preparation, is cost-effective, and environmentally friendly (no requirements for chemicals or gases) [22]. As with any sample, so with plastics, when NIR light hits the sample (in the range of 800–2500 nm), the molecules absorb the electromagnetic radiation and produce molecular overtones and combined vibrations. In plastic materials, differences can be recognized in the characteristic bands of carbon bonded with oxygen or hydrogen (C–O and C–H) and nitrogen bonded with hydrogen (N–H), which are observed in plastic materials [45].
Studies dealing with the identification of microplastics by NIR spectroscopy have shown successful predictions of microplastics in soil samples [46] where untreated soil samples were directly measured with a spectroradiometer (in the 350–1000 nm range). Paul et al. [47] showed in their research the high efficiency of NIR spectroscopy in combination with chemometrics (using the software Unscrambler X) in examining the presence of microplastics in soil samples and for determining the size, quantity, and age of the plastic. Their research showed that PE, PP, PS, PVC, and Polyethylene terephthalate (PET) were effectively detected by NIR in real and artificial soil samples at 1 wt.%. They used the Model Support Vector Machine (SVM) [43]. The application of NIR spectroscopy in the identification of types of marine microplastics was examined in the study by Pakhomova et al. [48] where a MicroNIR spectrophotometer was used. The advantages of using a MicroNIR device is that it can be used on-site for imaging. In order to verify the success of the measurement method, a spectrum library of the most frequently found microplastics from marine waste was created and with the help of software (MicroNIR) the recorded spectra of samples were compared, whereby the accuracy of the identification of microplastics in environmental samples was 96%. However, it should be pointed out here that the MicroNIR spectrophotometer identified microplastics > 1 mm in size, and for the purpose of verification, the samples were identified with an ATR-FTIR device, in order to confirm accuracy. In investigating microplastics in the coastal environments of the Arabian Gulf [49], the FTIR/FT-NIR spectrometer was used with 89% effective identification of the type of polymers (LDPPE, PP, PET, and PP) from sediments and seawater. The quantification of ternary microplastic mixtures containing PP, PE, and PS through an ultra-compact near-infrared spectrometer coupled with chemometric tools were investigated [50], as well as with a Miniaturized Near-Infrared (MicroNIR) spectrometer. Prediction of the previously mentioned polymers is high with R2 > 0.9, and the chemical nature of the examined samples was checked by Raman spectroscopy (before and after fragmentation). The potential of portable NIR devices has certainly shown its application potential in the detection of microplastics [48,50], although their usual range is 900–1700 nm.
2.3. Pyrolysis Gas Chromatography–Mass Spectrometry (Py-GC-MS)
The pyrolysis gas chromatography–mass spectrometry (Py-GC-MS) technique has a lot of potential in environmental analysis. This method is primarily utilized for the chemical identification of macromolecules that, because of their large size, cannot be described by liquid or gas chromatography. These macromolecules are broken down into smaller molecules by pyrolysis (controlled heat breakdown), which may then be separated by gas chromatography and identified by mass spectrometry. This approach has typically been employed in environmental samples to characterize organic matter and human food compounds, pollutants, lignins, and so on. It is capable to identifying the many types of chemical units that comprise macromolecules. Furthermore, this approach has lately seen a significant increase in the chemical characterization of microplastics found in environmental samples. This has prompted its application in this form of matrix [51,52,53,54,55,56,57,58].
2.4. Comparison of Microscopic and Spectroscopic Identification Methods
Because the detected and recognized MPs have shrunk in size, quantitative analysis with the naked eye or a microscope is limited. Little microplastics should be identified using the spectroscopic approach [58]. If only a few samples are being evaluated, FTIR or Raman detection of plastic-like particles is indicated. This approach is reasonably accurate for determining the quantity and type of polymer of microplastics. When evaluating many samples, it is best to utilize a mix of microscopy and spectroscopic approaches. To define sample-based criteria for the identification of main and typical microplastics in sample groups based on matrix, season, and location, a screening study employing FTIR or Raman should be performed first. The stereomicroscope should then be used to count microplastics based on these criteria [59]. NIR spectroscopy in combination with chemometric tools and microplastic databases can be extremely useful, especially if MicroNIR devices have been used for on-site measurements [48,50].
Because the detected and recognized microplastics have shrunk in size, quantitative analysis with the naked eye or a microscope is limited. More precise standardized methodologies and analytical techniques in the field of microplastics study must yet be investigated.
3. Recent Techniques for Detecting Microplastics
Despite the fact that the number of publications and interest in MP research has expanded quickly, it is still difficult to compare the collected results due to the use of diverse techniques in MP assay. There is an urgent need for a uniform approach to MP quantification processes in order to create comparable assessments [60]. This short review is an attempt which sums up the most notable recent technologies of analyzing MPs through various examples, magnifying both the pros and cons of each technique and giving a concise conclusion at the end of the article.
3.1. Photocatalysis
Photocatalysis is a well-established ecologically beneficial process that makes use of free and infinite sun energy. This method has seen significant usage in water filtration recently because of its excellent ability to degrade antibiotics, insecticides, and dyes. The mechanism of photocatalytic degradation is believed to be the interaction between ROSs (e.g., hydroxyl (OH), superoxide (O2)) generated on the surface of semi-conductors and the organic substrate, which breaks the chemical bonds of organic pollutants and causes their complete mineralization toward CO2 and H2O. However, organic materials can be immediately oxidized into CO2 and H2O by photo-excited holes (h + VB) created by an electron transfer from the valence band to the conduction band [61].
To maximize the benefits of photocatalysis, future research should concentrate on: (1) developing practical photocatalytic materials; (2) describing the mechanisms of MPs degradation; (3) quantitatively assessing the ecological risks posed by the degradation intermediates; and (4) further refining recycling techniques [62]. Scientists should pay more attention to both photocatalytic and microbial technologies because they have the potential to provide long-term water security and ecological stability [63].
3.2. Catalytic Advanced Oxidation Process (AOP)
AOPs are well-known for their ability to remove organic pollutants by producing ROSs with high standard reduction potentials, such as sulfate radical (SO4, E0 = 3.1 V against NHE) and hydroxyl radical (•OH, E0 = 2.7 V vs. NHE). This approach has efficiently decomposed or mineralized a wide range of contaminants, including colors, antibiotics, and POPs (persistent organic pollutants), because of their great oxidation capabilities [61,61,62,63,64,65,66].
Aging caused by AOPs is becoming more common [67]. AOPs are effective methods for degrading organic pollutants by generating many reactive oxygen species (ROS) to attack their internal structure [61], which are important in determining the characteristics of aged MPs and explaining the formation pathway of secondary MPs. At the moment, (heat-activated) persulfate, Fenton/photo-Fenton, O3/H2O2, UV/H2O2, UV/Cl2, and other agents are mostly employed to age MPs. The reactive species generated during photo-oxidation inducing the breakage of C–C bonds in the main chain has been proven to be the indirect photoaging process of polyolefin MPs such as PE, PS, and PP [68].
3.3. Biodegradation (Enzymatic Catalysis)
Biodegradation is a crucial technique for removing chemical pollutants produced by bacteria in addition to abiotic breakdown. Microorganisms create the corrosive chemicals in the chemical process of biodegradation. The polymer backbone may be broken by microbes or extracellular enzymes during the biodegradation process, changing the surface properties or mechanical strength as well as the average molecular weight of the polymer. On the other hand, commercial plastics have a strong biodegradability resistance. Due to their long molecular chains and absence of functional groups, polyolefins are more resistant to biodegradation [68].
3.4. Membrane-Based Filtration
Due to their significant benefits of high separation efficiency and small plant size, filtration techniques such as microfiltration (MF), ultrafiltration (UF), reverse osmosis (RO), dynamic membranes (DM), and MBRs have been demonstrated to be practical for producing high-quality water from a primary or secondary effluent [69,70,71]. Numerous studies have been conducted on dynamic membrane technology as a possible rival for cutting-edge water treatment methods. Recently, membrane technologies have been enhanced by integrating them with other techniques, leading to an overall increase in the efficiency of MPs removal. MBR, a heterogeneous reaction system made up of a biological reactor and a membrane system, has been created for better wastewater treatment [72].
3.5. Adsorption
Adsorption has also been used to absorb the pollutants in water, such as heavy metals and organic toxins [59]. Adsorption performance is determined by ion exchange, 𝜋–𝜋 contacts, hydrophobic interactions, and hydrogen–bond interactions between adsorbents and contaminants. This approach has recently been used to reduce MPs in the water treatment process. Yuan et al. used 3D reduced graphene oxide to test the adsorption efficiency of PS MPs (3DRGO) [73]. MPs eliminated throughout the multi-stage wastewater treatment operations generally persist in the sludge. This MP-rich sludge is frequently handled further, either as landfill or as agriculture fertilizer. Despite the fact that a range of procedures are used to eliminate dangerous things before they are used in agriculture, the presence of MPs is neglected [74]. Due to a lack of rational treatment to recycle or entirely eliminate the separated and collected MPs debris and particles, the aforementioned strategies based on simple physical separation cannot permanently address the pollution of environmental MPs, regardless of how many advancements have been made.
3.6. Coagulation
Coagulation is often utilized in modern water facilities during advanced treatment to generate high-quality drinking water. The coagulants employed in this technique, such as ferric sulfate or aluminum sulfate, cause the aggregation of suspended particulate materials into flocs [75], which then sediment and may be easily removed from water. The hydrogen bonding and/or electrostatic interactions between the coagulants and suspended particles are critical for achieving high separation efficiency during this procedure [33]. In addition to the use of additives, the surface qualities of MPs have a major impact on the coagulation efficiency of MPs removal [76] Changes in the surface chemistry and roughness of MPs, for example, during weathering processes in the natural environment, might influence the MPs affinity for coagulants and flocculants. Lapointe et al. investigated the efficacy of coagulation in removing pristine and weathered MPs [77]. In conclusion, increasing the effectiveness of MPs removal during the coagulation process requires the development of novel, environmentally friendly flocs stabilizing chemicals as well as additional oxidation processes (such as UV and ozone) to improve the interaction between flocs and MPs [61].
Figure 4 shows a schematic review of all the mentioned methods with their pros and cons. The comparison clearly states that improvements in futuristic methods are needed and that there is still room for improvements of the existing ones [61].
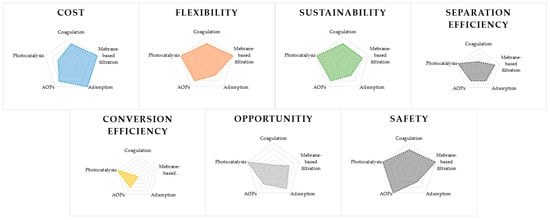
Figure 4.
Comparison of the general performance indicators for existing MPs removal techniques [61].
3.7. Novel Method for Assessing Microplastics through Mixing
The MP ratio test is designed to test whether particulate material (the test particle) is harmful when co-occurring in a mixture with naturally occurring particles (the reference particle) over a range of suspended solids concentrations. Namely, if the harmfulness of the particle being tested is greater than the reference particle, then reducing its share in the mixture with the reference particles should reduce the total toxicity. However, the additive effect assumption is necessary (additive effect of microplastics, above the critical mortality threshold, is positively responding to %MP). The above enables that for each test concentration of suspended solids in which there are different ratios of reference particles and particles being tested, a dose–response relationship can be established for each mixture in the concentration range [11,45]. Suspended solids (e.g., clay and cellulose) in the same size range as microplastics are prevalent in the environment. As a result, it must be determined if the addition of MPs to these background amounts of particulate material poses a risk [38]. This approach offers a unique method for disentangling the influence of MPs from that of other particulates by the repeated dilution of microplastic and reference particles in mixtures. It illustrates the method’s applicability by immobilizing Daphnia magna in polyethylene terephthalate (test microplastic; median particle diameter ~5 μm) and kaolin clay (reference material; ~3 μm). This methodology offers the potential to standardize the ecotoxicological testing of particulates, including MPs [20] and to estimate the critical levels for the suspended microplastic that can be considered as hazardous.
3.8. Separation of False Positive Microplastics
In this further innovative example [78], a novel method for quantitative and qualitative microplastics analysis was devised employing a two-phase (ethyl acetate–water) system in conjunction with confocal Raman spectroscopy. Microplastics can be effectively extracted based on the hydrophobic–lipophilic interaction by the addition of hydrophobic organic solvents. A certain amount of water and organic solvent is added to the sample being analyzed, the ratio of which is 0.2~0.3. MPs may be separated from false positive MPs in beach sand and marine sediment thanks to the hydrophobic–lipophilic interaction (HLI) of the two-phase system. The results show that the polypropylene (PP), polyethylene terephthalate (PET), polyvinyl chloride (PVC), polyamide 66 (PA 66), polycarbonate (PC), and polyethylene (PE) microplastics have recovery rates of greater than 92.98%. Additionally, the suggested method may identify antibiotics that are lipophilic and hydrophobic and are adsorbent on microplastics, such as sulfamethoxazole (SMX), erythromycin (EM), madimycin (MD), and josamycin (JOS). These antibiotics are eliminated via the dissolving precipitating process [77]. This novel study technique opens up new possibilities for detecting microplastics and hazardous chemicals in the marine environment.
3.9. Scanner-Based Combination for Analyzing Microplastic Particles
Another software-targeted novel approach described in a study [79] aims at combining a low-cost approach for assessing the form and size spectrum of all MP particles in the sample with the standard National Oceanic and Atmospheric Administration (NOAA) methodology [80] for MP extraction from seawater (four stations in Sevastopol bay, the Black Sea). For image acquisition, a standard flatbed scanner with a slide adaptor was used, and MP dispersive parameters (particle abundance, shape, and size spectrum) were measured using the ImageJ software. Their pilot study was aimed at combining the previously mentioned MP extraction protocol from seawater (NOAA) with a method for analyzing the spectrum of shapes and sizes (cheap and simple method) for all MP particles that compose the observed sample. Their conclusions emphasized that for the particle analysis, the Feret’s diameter and circularity (or roundness) appeared to be the most effective shape descriptors. The entire silhouette area of MP particles was proven to generate a reliable estimate of the overall mass of MP particles. In terms of abundance and mass, the first valid estimations of MP concentrations in Black Sea coastal waters (Sevastopol Bay) items amounted to 0.6 to 7 μg m3 and 6 to 750 μg m3, respectively. MP inflow to bay waters and movement along the bay appear to be governed by a complicated mix of variables including rainfall, wind regimes, currents, and Black River discharge. The authors showed the potential of the conducted pilot study and the transfer of image analysis in the MPs analysis; however, many questions related to the methods used to quantify MP pollution were also opened. For example, (i) the protocol does not include procedures for the wet peroxidation of organic substances and density separation of plastics or (ii) complexity of the procedure (complicated and time-consuming) and non-standardized differentiation of MPs.
So, this method has its advantages and disadvantages, and further refinements of the method are necessary. However, despite this, these data are among the few that are an indicator of pollution in that area. Using a simple image analysis, a study by Lorenzo-Navarro et al. [81] was conducted, which applied the acquisition of an image with a flatbed scanner that, with the use of the so-called computer vision for the analysis of obtained images and machine learning, aimed to develop the classifiers of different types of MP particles. Here, the samples of pure MPs were to a good extent equivalent to the extraction of MPs from a natural seawater sample. However, the idea is still far from potentially “standard” because the mathematical tools for analysis, recognition, and classification of MP are extremely complex and demanding.
3.10. Scanner-Based Combination for Analyzing Microplastic Particles
The first application of quantitative 1H NMR spectroscopy (qNMR) that presents a novel method for the qualitative and quantitative analysis of MPs in solution is presented by Peez et al. [82]. This method, such as previously mentioned, was suggested due to the evident great need for research in the field of quantitative analysis of MP particles that are independent of size. In their model samples, polyethylene (PE) granules (<300 µm), polyethylene terephthalate (PET) fibers (≈500 µm), and polystyrene (PS) beads (0.5 to 1 mm) each underwent qualitative and quantitative analysis as prototypical MP particles using the calibration curve method. The remaining proton signal of the deuterated solvent was employed as an internal standard. The method’s linearity is characterized with the R-squared value over 0.994 for all polymer types, and its accuracy is between 99.4 and 99.9%. The limit of detection (LOD) is 19–21 g/mL and the limit of quantification (LOQ) is 74–85 mg/mL, indicating that the LOD and LOQ are seen in an ecologically relevant size. In this paper, it was shown that (i) the content of the tested MP particles in model samples can be determined with high precision by means of the calibration curve method, (ii) particle sizes (upper and/or lower) do not exist because the MP particles are dissolved and can be determined qualitatively and quantitatively, which certainly supports the claim that size-independent qualitative and quantitative identification of microplastic particles in model samples is attainable using qNMR [82]. Despite all the above, there are still limitations for real samples (from the environment) and additional research is necessary that will study (i) the digestion of biological matrices and the recovery rates of MP contents in the sample and (ii) the separation of MPs from other ingredients in the sample. One of the possible solutions is the combination of non-destructive spectroscopic methods (FTIR, NIR, or Raman) in the analysis of MP particle size distribution with qNMR to determine the number of MPs in the observed sample.
3.11. A Cheap, Green, and Fast Analytical Procedure for MP Extraction
Another attempt to produce a both efficient and cheap validation technique for MP assessment was made by the use of a new extraction method [83]. Since sediments are known to be a primary sink for numerous organic and inorganic pollutants, the goal of this research was to design and test a quick and low-cost approach for assessing MP contamination in intertidal sediments from the Gulf of Biscay (Pays de la Loire region, France). MPs extracted by the protocol they suggested: (i) from dried sediments, (ii) centrifuged using milliQ water, (iii) filtered through nitrate cellulose (12 µm), and (iv) directly identified on the membrane filters after a supernatant filtration stage, using FTIR spectroscopy in reflection mode. This method examined sediments collected at three sites during two seasons, and for the first time, the number of repeats required to achieve a satisfactory representativeness was investigated. The optimal number of repetitions was determined to be 10 replicates, each of 25 g. The average MP concentration in sediments was 67 (±76) MPs/kg dw (N = 60), with no significant differences at either 28 locations or seasons.
The findings underlined the limit of significant MP accumulation (>1 mm) in intertidal mudflats. MP concentrations in seawater, sediments, and bivalves from the same area on the French Atlantic coast were discussed, indicating a potential preferential filtering of tiny MPs (20–50 μm) by the bivalves, which needs be validated by laboratory tests. This study emphasizes the pervasiveness of MPs in the marine environment once more and the potential of new methods which need more confirmations [84].
3.12. Machine Learning Approach in Microplastic Analysis
A key drawback of traditional meta-analysis techniques is their lack of spatial resolution. Among the broader scientific community, however, machine learning-aided meta-analysis has the potential to address this issue. In comparison to conventional statistical meta-analysis approaches, machine learning techniques provide greater flexibility and are largely free from a priori assumptions by drawing conclusions from the raw data and turning them into useable models. The term learning itself implies (i) acquiring new knowledge or (ii) improving or (iii) updating skills, while machine learning (ML) unifies problem solving in a way that helps machines ‘discover’ their ‘own’ algorithms [85]. Approaches to machine learning can be applied to structured and unstructured data such as large language models, computer vision, speech recognition, fields with extremely large datasets, such as medicine or spectral data of chemical compounds, where it is too expensive to develop algorithms for performing individual tasks that would lead to some conclusions. The terms “Machine learning” and “data mining” often overlap and use the same methods; however, the focus on discovering (previously) unknown properties in data is characteristic of data mining, while the focus on predictions based on known properties learned from data is characteristic of machine learning [86]. The attempts to create international microplastic databases are still in their infancy, and predictive machine learning algorithms need vast datasets or big data to gain improved accuracy [84]. One outstanding step toward making global marine microplastic data accessible for machine learning is the creation of the first-of-its-kind marine microplastic database by Nyadjro et al., 2020 [87]. Thus, there is a lack of comprehensive databases on marine microplastics that would allow (i) focusing on one specific type of marine litter, microplastics, to establish NCEI as the primary location for data management, (ii) collating and providing a comprehensive repository for the information needed to study marine debris, and (iii) free data through: the Global Ocean Currents Database; The World Ocean Database, and the Surface Sea Database. For the implementation of activities related to marine debris in NOAA NCEI, the following is required: (i) the development of a database (identification, procurement, and uploading), (ii) program for marine debris and NCEI (the so-called Marine Debris Exchange House), (iii) user engagement and information services, (iv) develop microplastic sensor measurement, and (v) provide ArcGIS visualization (facilitate retrieval of ongoing microplastics sensor data) [87]. In accordance with the above, an overview of the research that supports the aforementioned needs and goals follows.
3.12.1. Computer-Assisted Analysis of Microplastics
As already mentioned, in recent years, the prevalent strategy has been to employ spectral library searches to automatically identify sample spectra [88]. Subsequent research, however, has revealed that this technique is fairly limiting in some settings, which has led to advancements for making library searches more resilient while also paving the way for the introduction of more complex machine learning algorithms. A novel study [84] proposes a model-based machine learning strategy based on random decision forests that was used to analyze large FPA-FTIR data sets of environmental samples. The model is applicable to complicated matrices and can discriminate between more than 20 distinct polymer kinds. The model’s performance under these difficult conditions is demonstrated using eight distinct data sets. In addition, Monte Carlo cross validation was used to calculate error rates such as sensitivity, specificity, and precision [88].
The data are processed in four phases once the FTIR picture is imported and calibrated using the ENVI import function:
- Detection of the filter substrate;
- Classification of the remaining pixels;
- Postprocessing of the classification and;
- Particle detection and characterization.
They have come to the conclusions that the Bayreuth Microplastics Finder (BMF) technique incorporates a dual control or four-eye principle, which we advocate owing to sample preparation and data collecting issues [88]:
- Even though their concentrations are often relatively low, MPs may agglomerate which increases the possibility of particles partially overlapping. Additionally, because the present machine learning model does not enable the detection of mixed spectra, the RDF model cannot accurately classify overlapping areas. Yet, by utilizing the particle editor, it is rather simple to leverage the underlying visual picture to accurately create particle shapes. The researcher can thereby correct any potential bias.
- As a result of their rigidity, fibers may not lay flat on top of the filter surface, and any protruding portions may not fall within the detector’s focus plane. A single fiber may therefore be seen as a collection of unconnected segments (see Figure 1 for an example). Once more, this problem may be fixed by connecting the parts using the visual picture and inserting class pixels in the middle. Primpke et al. [89] claim that placing a BaF2 window over the sample guarantees that the fibers are positioned within the microscope’s focus plane. If there are a lot of microfibers to study, this can be a different strategy.
- If MPs are thicker over a specific threshold, total absorption (TA) might become another significant issue in transmission measurements. If enough information on peak locations is retained, the TA effect could still make it possible to identify polymers for spectra that are less badly damaged. The random decision forest (RDF) model that is currently being used has been particularly trained to enable the categorization of such spectra. However, there are certain particles that can only be partially identified, necessitating human user interaction once more. In these cases, the visual picture in conjunction with the particle editor is used.
3.12.2. Quantum Cascade Laser Imaging Approach in Analyzing MPs
The deterioration significantly affects the microplastics’ infrared spectra and can make identification difficult. The detection of weathered microplastics is a challenging topic, and this work offers a fresh solution. In order to capture the infrared spectra of diverse polymeric particles, a quantum cascade laser (LDIR) was employed (81,291 individual particles). Two supervised machine learning (ML) models, Subspace k-Nearest Neighbor (Sub-kNN) and Boosted Decision Tree (BDT), were trained to recognize the spectrum characteristics of labeled particles and then used to identify unlabeled samples. Using 10-fold cross validation, the models’ identification accuracy was 89.7% and 77.1%, respectively. The Sub-kNN or BDT models were able to identify almost 90% of the samples. After that, samples that the supervised ML model was unable to classify were clustered using a non-supervised ML model called Density-based Spatial Clustering of Applications with Noise (DBSCAN). This made it possible to find more microplastic subgroups. The identification process may then be sped up by manually labeling a subset of each group’s spectra (for example, the centroids of each cluster), which also enables the addition of fresh labeled samples to the original supervised ML [90].
3.12.3. Training and Evaluating Machine Learning Algorithms MPs Classification
Another investigation of machine learning classification techniques which were used on the FTIR spectra of marine MPs gathered for this study is furthered. The performance of the predictions for 13 classes of polymers was assessed using a comparison of effective classification models. To prevent bias during the training and selection phases, a pipeline approach was used in conjunction with a strict methodology. The use of an oversampling approach to correct for dataset imbalance also contributed. The minimization function aims and performance metric employed were the log-loss. For a quick and practical automated characterization of microplastics, our examination of a Support Vector Machine Classifier shows a favorable link between simplicity and performance [91].
3.12.4. Deep Learning Approach for Automatic MPs Analysis
In order to automatically count and categorize microplastic particles in the range of 1–5 mm from photographs acquired with a digital camera or a mobile phone with a resolution of 16 million pixels or higher, a deep learning network architecture is proposed in this research [92]. The suggested architecture includes a first stage for segmenting the image’s particles that is carried out using the U-Net neural network. Following the separation of the various particles, a second stage using the VGG16 neural network divides them into three categories: fragments, pellets, and lines. These three categories were chosen because they are the most prevalent within the size range that is being considered. Images from two digital cameras and one smartphone were used in the experimental evaluation. The samples utilized in the studies were taken in August 2018 at the Beach del Poris beach on Tenerife Island, Spain. In the trials on segmenting particles, a Jaccard Index value of 0.8 is attained, and the microplastic particles are classified with an accuracy of 98.11%. The suggested architecture outperforms a comparable, previously published system using conventional computer vision methods in terms of speed. As investigated by Han and coworkers [93], a deep learning model based on the mask region conventional neural network (Mask R–CNN) was developed and used for the classification, localization, and segmentation of MPs (1–5 mm). In doing so, a MP deep learning dataset was constructed and used for training and validation of the Mask R–CNN model (using only optical cameras and available image processing software). However, in both studies the classification, localization, segmentation, and computer performance of the developed models were evaluated. When precisely developing any model, it is important on which data set the learning and validation of the model for the classification, localization, and segmentation of real MPs with different morphologies and at different scales is carried out. The study using Mask R-CNN included a microplastic dataset including 3000 images, and was tested on 250 images with the precision over 93% [93]. The study by Lorenzo-Navaro et al. [81] used for training and testing 49 images of mixed microplastic samples and 20% of training samples vas used as validation with an average accuracy over 98%. However, such approach also pointed out limitations as (i) in monitoring microplastics, this is their visual identification/screening process which was a labor-intensive task that needs to be conducted by trained individuals and (ii) before classifying and counting MP particles, sample cleaning must be carried out to remove non-plastic material [45,93].
All of the above point to the need for establishing Europe’s attitude and vision towards plastic in our environment and pollution.
4. Europe’s Program on Solving the Pollution Problem
An important factor in the world’s vision against pollution in marine (and other) environments can be traced with Europe’s most leading project holder, Horizon Europe, which is the EU’s key funding program for research and innovation. Scientific communities in the form of various institutes, universities, and faculties work together with companies on an advanced problem. Table 3 shows Horizon 2020 projects that have contributed to the execution of the ZPAP flagships [94].

Table 3.
Horizon 2020 initiatives that have aided in the implementation of the ZPAP flagships [94].
As seen in Table 2, 70.4% of overall projects are either fully or partially marine-pollution oriented. This approach of companies being involved in scientific research provides a fertile ground for all present and upcoming challenges on MPs water pollution. Moreover, Table 4 presents a systematic overview of Europe’s vision for a new plastics economy, on which all future scientific research should be based. The vision [61] contains all the key elements for plastic recycling combined with innovative solutions, which are a direct consequence of all current and future methods for MPs analyses. The consumers (end users of food which potentially contains microplastics) were not left out in the consideration of Europe, and their attitude related to microplastics (Table 5) was also systematized, through the eyes of the EU.

Table 4.
The vision for Europe’s new plastics economy [95].

Table 5.
Europe’s current attitude to plastics economy [94].
If the leading institutions of each country adhere to the stated principles and plans [61], there will certainly be less microplastic pollution, and rivers, lakes, and seas will be cleaner, and thus the food that comes to us from them.
5. Conclusions
From what is presented, it is evident that the traditional methods of detecting microplastics are becoming obsolete, slowly giving way to spectroscopic methods, where the potential of NIRs and FTIRs is recognized when coupled with additional data. Standard methods of detecting microplastics are often time-consuming, labor-intensive, and have limited accuracy in identifying and quantifying microplastics in water samples.
Recent studies have shown that machine learning algorithms have been successfully employed in identifying microplastics in water samples with high accuracy rates. With the advancement of technology and the continued development of sophisticated algorithms, it is expected that machine learning will become even more effective in detecting and quantifying microplastics in the environment. Although machine learning has shown potential in the analysis of microplastics, it is important to note that its effectiveness depends on the quality and diversity of the training data and algorithms used. The potential of machine learning algorithms in the recognition of microplastics and their quantification is manifested by high accuracy rates, such as 93% accuracy, whereas a machine learning tool used is the mask region conventional neural network (Mask R-CNN). In addition, machine learning, along with other analytical techniques, can contribute to enhancing MP detection and analysis. A combination of traditional methods, spectroscopic techniques, and machine learning algorithms can offer a comprehensive and robust approach to understanding MP pollution.
The potential impact of microplastics on marine ecosystems and human health cannot be overstated. It is crucial to continue research efforts in this area and to explore innovative approaches to detect and mitigate the effects of microplastics in the environment. Machine learning is a powerful tool that has the potential to transform our understanding of microplastic pollution and to contribute to more effective strategies for its prevention and mitigation. Therefore, it is safe to say that machine learning is indeed the future of detecting microplastics in the environment.
Author Contributions
Conceptualization, V.M., Ž.C., M.I., P.A. and J.G.K.; methodology, V.M. and J.G.K.; software, V.M.; validation, V.M., Ž.C., M.I., P.A. and J.G.K.; formal analysis, V.M., M.I. and J.G.K.; investigation, V.M., Ž.C., M.I., P.A. and J.G.K.; data curation, V.M., Ž.C., M.I., P.A. and J.G.K.; writing—original draft preparation, V.M., Ž.C., M.I., P.A. and J.G.K.; writing—review and editing, V.M., Ž.C., M.I., P.A. and J.G.K.; visualization, V.M., Ž.C., M.I., P.A. and J.G.K.; supervision, J.G.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ivleva, N.P.; Wiesheu, A.C.; Niessner, R. Microplastic in Aquatic Ecosystems Angew. Chem. Int. Ed. 2017, 56, 1720. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of Microplastic on Shorelines Woldwide: Sources and Sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, E.J.; Anderson, S.J.; Harvey, G.R.; Miklas, H.P.; Peck, B.B. Polystyrene Spherules in Coastal Waters. Science 1972, 178, 749–750. [Google Scholar] [CrossRef]
- Llorca, M.; Álvarez-Muñoz, D.; Ábalos, M.; Rodríguez-Mozaz, S.; Santos, L.H.; León, V.M.; Campillo, J.A.; Martínez-Gómez, C.; Abad, E.; Farré, M. Microplastics in Mediterranean coastal area: Toxicity and impact for the environment and human health. Trends Environ. Anal. Chem. 2020, 27, e00090. [Google Scholar] [CrossRef]
- Gajdoš, J.; Galić, K.; Kurtanjek, Ž.; Ciković, N. Gas permeability and DSC characteristics of polymers used in food packaging. Polym. Test. 2000, 20, 49–57. [Google Scholar] [CrossRef]
- Coyle, R.; Hardiman, G.; Driscoll, K.O. Microplastics in the marine environment: A review of their sources, distribution processes, uptake and exchange in ecosystems. CSEE 2020, 2, 100010. [Google Scholar] [CrossRef]
- Lusher, A.L.; Welden, N.A.; Sobral, P.; Cole, M. Sampling, isolating and identifying microplastics ingested by fish and invertebrates. Anal. Methods 2016, 9, 1346–1360. [Google Scholar] [CrossRef]
- PlasticsEurope (PEMRG); Conversio; nova-institute, Statista 2022. Available online: https://plasticseurope.org/wp-content/uploads/2022/10/PE-PLASTICS-THE-FACTS_V7-Tue_19-10-1.pdf. (accessed on 6 June 2023).
- Bhuyan, S. Effects of Microplastics on Fish and in Human Health. Front. Environ. Sci. 2022, 10, 827289. [Google Scholar] [CrossRef]
- Lee, Y.; Cho, J.; Sohn, J.; Kim, C. Health Effects of Microplastic Exposures: Current Issues and Perspectives in South Korea. Yons. Med. J. 2023, 64, 301–308. [Google Scholar] [CrossRef]
- Ghosh, S.; Sinha, J.K.; Ghosh, S.; Vashisth, K.; Han, S.; Bhaskar, R. Microplastics as an Emerging Threat to the Global Environment and Human Health. Sustainability 2023, 15, 10821. [Google Scholar] [CrossRef]
- Ordoñez, M. Microplastics and Health Risks: What Do We Really Know? Available online: https://www.webmd.com/a-to-z-guides/news/20221028/microplastics-health-risks-what-do-we-really-know (accessed on 25 July 2023).
- Web of Science (2023) Topic: Microplastics. Available online: https://www.webofscience.com/wos/woscc/summary/4e417e27-5582-44d1-9eba-7d3d38586c01-95f2c4f5/relevance/1 (accessed on 5 July 2023).
- Dimensions, © 2023 Digital Science & Research Solutions, Inc. Available online: https://app.dimensions.ai/discover/publication (accessed on 5 June 2023).
- Mercogliano, R.; Avio, C.G.; Regoli, F.; Anastasio, A.; Colavita, G.; Santonicola, S. Occurrence of Microplastics in Commercial Seafood under the Perspective of the Human Food Chain. A Review. J. Agric. Food Chem. 2020, 68, 5296–5301. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Piniero, M.A. Microplastics: Focus on Food and Health; Publications Office of the European Union: Luxembourg, 2018. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (Contam). Presence of Microplastics and Nanoplastics in Food, with Particular Focus on Seafood. EFSA J. 2016, 14, e04501. [Google Scholar] [CrossRef]
- Chen, G.; Fu, Z.; Yang, H.; Wang, J. An overview of analytical methods for detecting microplastics in the atmosphere. TrAC Trends Anal. Chem. 2020, 130, 115981. [Google Scholar] [CrossRef]
- Filipec, S.V.; Valinger, D.; Mikac, L.; Ivanda, M.; Kljusurić, J.G.; Janči, T. Influence of Sample Matrix on Determination of Histamine in Fish by Surface Enhanced Raman Spectroscopy Coupled with Chemometric Modelling. Foods 2021, 10, 1767. [Google Scholar] [CrossRef] [PubMed]
- Janči, T.; Valinger, D.; Kljusurić, J.G.; Mikac, L.; Filipec, S.V.; Ivanda, M. Determination of histamine in fish by Surface Enhanced Raman Spectroscopy using silver colloid SERS substrates. Food Chem. 2017, 224, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Ćurko, J.; Kljusurić, J.G.; Matošić, M.; Crnek, V.; López-Vázquez, C.M.; Garcia, H.A.; Brdjanović, D.; Valinger, D. Use of near-infrared spectroscopy on predicting wastewater constituents to facilitate the operation of a membrane bioreactor. Chemosphere 2021, 272, 129899. [Google Scholar] [CrossRef]
- Valinger, D.; Longin, L.; Grbeš, F.; Benković, M.; Jurina, T.; Kljusurić, J.G.; Tušek, A.J. Detection of honey adulteration—The potential of UV-VIS and NIR spectroscopy coupled with multivariate analysis. LWT-Food Sci. Technol. 2021, 145, 111316. [Google Scholar] [CrossRef]
- Grgić, F.; Jurina, T.; Valinger, D.; Kljusurić, J.G.; Tušek, A.J.; Benković, M. Near-Infrared Spectroscopy Coupled with Chemometrics and Artificial Neural Network Modeling for Prediction of Emulsion Droplet Diameters. Micromachines 2022, 13, 1876. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Methods for sampling and detection of microplastics in water and sediment: A critical review. TrAC Trends Anal. Chem. 2019, 110, 150–159. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, C.G. A comparative study on the distribution behavior of microplastics through FT-IR analysis on different land uses in agricultural soils. Environ. Res. 2015, 93, 202–209. [Google Scholar] [CrossRef]
- Huppertsberg, S.; Knepper, T. Validation of an FT-IR microscopy method for the determination of microplastic particles in surface waters. Methodsx 2020, 7, 100874. [Google Scholar] [CrossRef] [PubMed]
- Tagg, A.S.; Sapp, M.; Harrison, J.P.; Ojeda, J.J. Identification and Quantification of Microplastics in Wastewater Using Focal Plane Array-Based Reflectance Micro-FT-IR Imaging. Anal. Chem. 2015, 87, 6032–6040. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.; Woods, S.; Lowry, S.; Sukumaran, S. Tracking Microplastics in the Environment via FT-IR Microscopy. Spectroscopy 2017, 32, 17–23. [Google Scholar]
- Soo-Ah, C.; Won-Bo, C.; Su-Bin, K.; Jae-Hak, C.; Hyo-Jin, K. Identification of Microplastics in Sea Salts by Raman Microscopy and FT-IR Microscopy. JAST 2019, 32, 243–251. [Google Scholar] [CrossRef]
- Hupperstsberg, S.; Knepper, T.P. Instrumental analysis of microplastics—Benefits and challenges. Anal. Bioanal. Chem. 2018, 410, 6343–6352. [Google Scholar] [CrossRef] [PubMed]
- Tofa, T.S.; Kunjali, K.L.; Paul, S.; Dutta, J. Visible light photocatalytic degradation of microplastic residues with zinc oxide nanorods. Environ. Chem. Lett. 2019, 17, 1341–1346. [Google Scholar] [CrossRef]
- Amelia, D.; Karamah, E.F.; Mahardika, M.; Syafri, E.; Rangappa, S.M.; Siengchin, S.; Asrofi, M. Effect of advanced oxidation process for chemical structure changes of polyethylene microplastics. Mater. Today: Proc. 2020, 52, 2501–2504. [Google Scholar] [CrossRef]
- Mohan, S.; Prabakaran, A. Infrared and laser raman spectra of polyethylene and its normal coordinate analysis. Asian J. Chem. 1989, 1, 162–165. [Google Scholar]
- Krimm, S.; Liang, C.Y.; Sutherland, G.B.B.M. Infrared Spectra of High Polymers. II. Polyethylene. J. Chem. Phys. 1956, 25, 549–562. [Google Scholar] [CrossRef]
- Gulmine, J.; Janissek, P.; Heise, H.; Akcelrud, L. Polyethylene characterization by FTIR. Polym. Test. 2002, 21, 557–563. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, L.; Sutton, D.; Wang, X.; Lin, T. Needleless Melt-Electrospinning of Polypropylene Nanofibres. J. Nanomater. 2012, 2012, 382639. [Google Scholar] [CrossRef]
- Fang, J.; Xuan, Y.; Li, Q. Preparation of polystyrene spheres in different particle sizes and assembly of the PS colloidal crystals. Sci. China Technol. Sci. 2010, 53, 3088–3093. [Google Scholar] [CrossRef]
- Gerdes, Z.; Hermann, M.; Ogonowski, M.; Gorokhova, E. A novel method for assessing microplastic effect in suspension through mixing test and reference materials. Sci. Rep. 2019, 9, 10695. [Google Scholar] [CrossRef]
- Eo, S.; Hong, S.H.; Song, Y.K.; Lee, J.; Lee, J.; Shim, W.J. Abundance, composition, and distribution of microplastics larger than 20 μm in sand beaches of South Korea. Environ. Pollut. 2018, 238, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Monnot, M.; Sun, Y.; Asia, L.; Wong-Wah_Chung, P.; Doumeng, P.; Moulin, P. Microplastics in different water samples (seawater, freshwater, and wastewater): Methodology approach for characterization using micro-FTIR spectroscopy. Water Res. 2023, 232, 119711. [Google Scholar] [CrossRef] [PubMed]
- Tušek, A.J.; Jurina, T.; Čulo, I.; Valinger, D.; Kljusurić, J.G.; Benković, M. Application of NIRs coupled with PLS and ANN modelling to predict average droplet size in oil-in-water emulsions prepared with different microfluidic devices. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 270, 120860. [Google Scholar] [CrossRef]
- Balbino, S.; Vincek, D.; Trtanj, I.; Egređija, D.; Gajdoš-Kljusurić, J.; Kraljić, K.; Obranović, M.; Škevin, D. Assessment of Pumpkin Seed Oil Adulteration Supported by Multivariate Analysis: Comparison of GC-MS, Colourimetry and NIR Spectroscopy Data. Foods 2022, 11, 835. [Google Scholar] [CrossRef]
- Rani, M.; Ducoli, S.; Federici, S.; Depero, L.E. Influx of Near-Infrared Technology in Microplastic Community: A Bibliometric Analysis. Microplastics 2023, 2, 107–121. [Google Scholar] [CrossRef]
- Tirkey, A.; Upadhyay, L.S.B. Microplastics: An overview on separation, identification and characterization of microplastics. Mar. Pollut. Bull. 2021, 170, 112604. [Google Scholar] [CrossRef]
- Crawford, C.B.; Quinn, B. Microplastic Pollution; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Corradini, F.; Bartholomeus, H.; Lwanga, E.H.; Gertsen, H.; Geissen, V. Science of the Total Environment Predicting soil microplastic concentration using vis-NIR spectroscopy. Sci. Total. Environ. 2019, 650, 922–932. [Google Scholar] [CrossRef]
- Paul, A.; Wander, L.; Becker, R.; Goedecke, C.; Braun, U. High-throughput NIR spectroscopic (NIRS) detection of microplastics in soil. Environ. Sci. Pollut. Res. 2019, 26, 7364–7374. [Google Scholar] [CrossRef] [PubMed]
- Pakhomova, S.; Zhdanov, I.; Van Bavel, B. Polymer Type Identification of Marine Plastic Litter Using a Miniature Near-Infrared Spectrometer (MicroNIR). Appl. Sci. 2020, 10, 8707. [Google Scholar] [CrossRef]
- Abayomi, O.A.; Range, P.; Al-Ghouti, M.A.; Obbard, J.P.; Almeer, S.H.; Ben-Hamadou, R. Microplastics in coastal environments of the Arabian Gulf. Mar. Pollut. Bull. 2017, 124, 181–188. [Google Scholar] [CrossRef]
- Marchesi, C.; Rani, M.; Federici, S.; Alessandri, I.; Vassalini, I.; Ducoli, S.; Borgese, L.; Zacco, A.; Núñez-Delgado, A.; Bontempi, E.; et al. Quantification of ternary microplastic mixtures through an ultra-compact near-infrared spectrometer coupled with chemometric tools. Environ. Res. 2023, 216, 114632. [Google Scholar] [CrossRef]
- Picó, Y.; Barceló, D. Pyrolysis gas chromatography-mass spectrometry in environmental analysis: Focus on organic matter and microplastics. TrAC Trends Anal. Chem. 2020, 130, 115964. [Google Scholar] [CrossRef]
- Fischer, M.; Scholz-Böttcher, B.M. Simultaneous Trace Identification and Quantification of Common Types of Microplastics in Environmental Samples by Pyrolysis-Gas Chromatography–Mass Spectrometry. Environ. Sci. Technol. 2017, 51, 5052–5060. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Scholz-Böttcher, B.M. Microplastics analysis in environmental samples—Recent pyrolysis-gas chromatography-mass spectrometry method improvements to increase the reliability of mass-related data. Anal. Methods 2019, 11, 2489–2497. [Google Scholar] [CrossRef]
- Dierkes, G.; Lauschke, T.; Becher, S.; Schumacher, H.; Földi, C.; Ternes, T. Quantification of microplastics in environmental samples via pressurized liquid extraction and pyrolysis-gas chromatography. Anal. Bioanal. Chem. 2019, 411, 6959–6968. [Google Scholar] [CrossRef]
- Primpke, S.; Fischer, M.; Lorenz, C.; Gerdts, G.; Scholz-Böttcher, B.M. Comparison of pyrolysis gas chromatography/mass spectrometry and hyperspectral FTIR imaging spectroscopy for the analysis of microplastics. Anal. Bioanal. Chem. 2020, 412, 8283–8298. [Google Scholar] [CrossRef]
- Dibke, C.; Fischer, M.; Scholz-Böttcher, B.M. Microplastic Mass Concentrations and Distribution in German Bight Waters by Pyrolysis–Gas Chromatography–Mass Spectrometry/Thermochemolysis Reveal Potential Impact of Marine Coatings: Do Ships Leave Skid Marks? Environ. Sci. Technol. 2021, 55, 2285–2295. [Google Scholar] [CrossRef]
- Albignac, M.; Ghiglione, J.F.; Labrune, C.; ter Halle, A. Determination of the microplastic content in Mediterranean benthic macrofauna by pyrolysis-gas chromatography-tandem mass spectrometry. Mar. Pollut. Bull. 2022, 181, 113882. [Google Scholar] [CrossRef] [PubMed]
- Hermabessiere, L.; Rochman, C.M. Microwave-Assisted Extraction for Quantification of Microplastics Using Pyrolysis–Gas Chromatography/Mass Spectrometry. Environ. Toxicol. Chem. 2021, 40, 2733–2741. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Rani, M.; Lee, J.; Shim, W.J. A comparison of microscopic and spectroscopic identification methods for analysis of microplastics in environmental samples. Mar. Pollut. Bull. 2015, 93, 202–209. [Google Scholar] [CrossRef]
- Yuan, Z.; Nag, R.; Cummins, E. Human health concerns regarding microplastics in the aquatic environment—From marine to food systems. Sci. Total. Environ. 2022, 823, 153730. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Sherrell, P.C.; Chen, J.; Wang, H.; Zhang, W.; Yang, J. How to Build a Microplastics-Free Environment: Strategies for Microplastics Degradation and Plastics Recycling. Adv. Sci. 2022, 9, 2103764. [Google Scholar] [CrossRef]
- He, J.; Han, L.; Wang, F.; Ma, C.; Cai, Y.; Ma, W.; Xu, E.G.; Xing, B.; Yang, Z. Photocatalytic strategy to mitigate microplastic pollution in aquatic environments: Promising catalysts, efficiencies, mechanisms, and ecological risks. Crit. Rev. Environ. Sci. Technol. 2023, 53, 504–526. [Google Scholar] [CrossRef]
- Ebrahimbabaie, P.; Yousefi, K.; Pichtel, J. Photocatalytic and biological technologies for elimination of microplastics in water: Current status. Sci. Total. Environ. 2022, 806, 150603. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Ren, W.; Nie, G.; Li, X.; Duan, X.; Zhang, Y.; Wang, S. Fast and Long-Lasting Iron(III) Reduction by Boron Toward Green and Accelerated Fenton Chemistry. Angew. Chem. Int. Ed. 2020, 59, 16517–16526. [Google Scholar] [CrossRef]
- Giannakis, S.; Lin, K.-Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Oturan, N.; Raffy, S.; Cretin, M.; Esmilaire, R.; van Hullebusch, E.; Esposito, G.; Oturan, M.A. Sub-stoichiometric titanium oxide (Ti4O7) as a suitable ceramic anode for electrooxidation of organic pollutants: A case study of kinetics, mineralization and toxicity assessment of amoxicillin. Water Res. 2016, 106, 171–182. [Google Scholar] [CrossRef]
- Li, S.; Hu, J. Transformation products formation of ciprofloxacin in UVA/LED and UVA/LED/TiO2 systems: Impact of natural organic matter characteristics. Water Res. 2018, 132, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lim, F.Y.; Hu, J. Characteristics and behaviors of microplastics undergoing photoaging and Advanced Oxidation Processes (AOPs) initiated aging. Water Res. 2023, 232, 119628. [Google Scholar] [CrossRef]
- Cvetnić, T.S.; Šalić, A.; Benković, M.; Jurina, T.; Valinger, D.; Kljusurić, J.G.; Zelić, B.; Tušek, A.J. A Systematic Review of Enzymatic Kinetics in Microreactors. Catalysts 2023, 13, 708. [Google Scholar] [CrossRef]
- Poerio, T.; Piacentini, E.; Mazzei, R. Membrane Processes for Microplastic Removal. Molecules 2019, 24, 4148. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ding, L.; Wang, H. MXene-Based Membranes for Separation Applications. Small Sci. 2021, 1, 2100013. [Google Scholar] [CrossRef]
- Gurung, K.; Ncibi, M.C.; Fontmorin, J.M.; Särkkä, H.; Sillanpää, M. Incorporating Submerged MBR in Conventional Activated Sludge Process for Municipal Wastewater Treatment: A Feasibility and Performance Assessment. J. Membr. Sci. Technol. 2016, 6, 1000158. [Google Scholar] [CrossRef]
- Yuan, F.; Yue, L.; Zhao, H.; Wu, H. Study on the adsorption of polystyrene microplastics by three-dimensional reduced graphene oxide. Water Sci. Technol. 2020, 81, 2163–2175. [Google Scholar] [CrossRef]
- Mahon, A.M.; O’connell, B.; Healy, M.G.; O’connor, I.; Officer, R.; Nash, R.; Morrison, L. Microplastics in Sewage Sludge: Effects of Treatment. Environ. Sci. Technol. 2017, 51, 810–818. [Google Scholar] [CrossRef]
- Iyare, P.U.; Ouki, S.K.; Bond, T. Microplastics removal in wastewater treatment plants: A critical review. Environ. Sci. Water Res. Technol. 2020, 6, 2664–2675. [Google Scholar] [CrossRef]
- Perren, W.; Wojtasik, A.; Cai, Q. Removal of Microbeads from Wastewater Using Electrocoagulation. ACS Omega 2018, 3, 3357–3364. [Google Scholar] [CrossRef]
- Lapointe, M.; Farner, J.M.; Hernandez, L.M.; Tufenkji, N. Understanding and Improving Microplastic Removal during Water Treatment: Impact of Coagulation and Flocculation. Environ. Sci. Technol. 2020, 54, 8719–8727. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, W.-Y.; Liao, Y.; Sun, R.; Hu, J.; Lu, Z.; Chang, M.; Yang, J.; Dai, Z.; Zhou, C.; et al. Separation of false-positive microplastics and analysis of microplastics via a two-phase system combined with confocal Raman spectroscopy. J. Hazard. Mater. 2022, 440, 129803. [Google Scholar] [CrossRef] [PubMed]
- Mukhanov, V.S.; Litvinyuk, D.A.; Sakhon, E.G.; Bagaev, A.V.; Veerasingam, S.; Venkatachalapathy, R. A new method for analyzing microplastic particle size distribution in marine environmental samples. Ecol. Montenegrin. 2019, 23, 77–86. [Google Scholar] [CrossRef]
- Noaa Draft Standardocean Mapping Protocol Prepared by The Interagency Working Group on Ocean and Coastal Mapping for The National Ocean Mapping, Exploration, and Characterization Council. Available online: https://iocm.noaa.gov/standards/Standard_Ocean_Mapping_Protocol_draft_Feb2023.pdf (accessed on 25 July 2023).
- Lorenzo-Navarro, J.; Castrillón-Santana, M.; Gómez, M.; Herrera, A.; Marín-Reyes, P.A. Automatic Counting and Classification of Microplastic Particles. In Proceedings of the ICPRAM 2018-Proceedings of the 7th International Conference on Pattern Recognition Applications and Methods, Funchal, Portugal, 16–18 January 2018; pp. 646–652. [Google Scholar] [CrossRef]
- Peez, N.; Janiska, M.-C.; Imhof, W. The first application of quantitative 1H NMR spectroscopy as a simple and fast method of identification and quantification of microplastic particles (PE, PET, and PS). Anal. Bioanal. Chem. 2019, 411, 823–833. [Google Scholar] [CrossRef]
- Phuong, N.N.; Poirier, L.; Lagarde, F.; Kamari, A.; Zalouk-Vergnoux, A. Microplastic abundance and characteristics in French Atlantic coastal sediments using a new extraction method. Environ. Pollut. 2018, 243, 228–237. [Google Scholar] [CrossRef]
- Kannankai, M.P.; Babu, A.J.; Radhakrishnan, A.; Alex, R.K.; Borah, A.; Devipriya, S.P. Machine learning aided meta-analysis of microplastic polymer composition in global marine environment. J. Hazard. Mater. 2022, 440, 129801. [Google Scholar] [CrossRef]
- Dineva, K.; Atanasova, T. Systematic look at machine learning algorithms—Advantages, disadvantages and practical applications. Int. Multidiscip. Sci. GeoConference SGEM 2020, 20, 317–324. [Google Scholar] [CrossRef]
- Knights, V.; Kolak, M.; Markovikj, G.; Kljusurić, J.G. Modeling and Optimization with Artificial Intelligence in Nutrition. Appl. Sci. 2023, 13, 7835. [Google Scholar] [CrossRef]
- Nyajdro, E.D.; Wang, Z.; Boyer, T.; Cross, S.L.; Cebrian, J. NOAA NCEI Global Marine Microplastics Database Initiative. In Proceedings of the AGU Fall Meeting Abstracts, Online, 1–17 December 2020. [Google Scholar]
- Hufnagl, B.; Stibi, M.; Martirosyan, H.; Wilczek, U.; Möller, J.N.; Löder, M.G.J.; Laforsch, C.; Lohninger, H. Computer-Assisted Analysis of Microplastics in Environmental Samples Based on μFTIR Imaging in Combination with Machine. Environ. Sci. Technol. Lett. 2022, 9, 90–95. [Google Scholar] [CrossRef]
- Primpke, S.; Dias, P.A.; Gerdts, G. Automated identification and quantification of microfibres and microplastics. Anal. Methods 2019, 11, 2138–2147. [Google Scholar] [CrossRef]
- Tian, X.; Beén, F.; Bäuerlein, P.S. Quantum cascade laser imaging (LDIR) and machine learning for the identification of environmentally exposed microplastics and polymers. Environ. Res. 2022, 212, 113569. [Google Scholar] [CrossRef] [PubMed]
- Back, H.d.M.; Junior, E.C.V.; Alarcon, O.E.; Pottmaier, D. Training and evaluating machine learning algorithms for ocean microplastics classification through vibrational spectroscopy. Chemosphere 2022, 287, 131903. [Google Scholar] [CrossRef]
- Lorenzo-Navarro, J.; Castrillón-Santana, M.; Sánchez-Nielsen, E.; Zarco, B.; Herrera, A.; Martínez, I.; Gómez, M. Deep learning approach for automatic microplastics counting and classification. Sci. Total. Environ. 2021, 765, 142728. [Google Scholar] [CrossRef] [PubMed]
- Han, X.-L.; Jiang, N.-J.; Hata, T.; Choi, J.; Du, Y.-J.; Wang, Y.-J. Deep learning based approach for automated characterization of large marine microplastic particles. Mar. Environ. Res. 2023, 183, 105829. [Google Scholar] [CrossRef] [PubMed]
- European Research Executive Agency. Horizon Projects Supporting the Zero Pollution Action Plan, European Commission, Directorate-General for Research and Innovation 2022. Available online: https://rea.ec.europa.eu/publications/horizon-projects-supporting-zero-pollution-action-plan_en (accessed on 5 July 2023).
- EC 2018 A European Strategy for Plastics in a Circular Economy. Available online: https://ec.europa.eu/environment/circular-economy/pdf/plastics-strategy.pdf (accessed on 5 July 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).