Abstract
The increasingly intense consumption of plastics and, above all, their improper disposal in the environment are causing serious environmental concerns. Great efforts have been made for the development of new methods aimed at facilitating and speeding up the identification and sorting of different materials in the plastic recycling process. In this field, new strategies based on fluorescent tagging have been developed. This work concerns the synthesis and characterization of new fluorescent copolymers of polyethylene (PE) and polystyrene (PS), which are among the most produced and consumed plastic materials. The synthesized copolymers are potentially suitable for use as fluorescent markers of PE and PS. Ethylene-co-N-pentenyl carbazole (P(E-co-PK)) and styrene-co-4-(N-carbazolyl)methyl styrene (P(S-co-SK)) copolymers were prepared by Ziegler–Natta and free radical polymerization, respectively. If excited at 300 nm, both P(E-co-PK)s and P(S-co-SK)s give fluorescence emissions resulting in them being optically active. Moreover, due to the low amount of fluorescent units, they show chemico-physical properties such as those of their corresponding homopolymers (PE and PS). P(E-co-PK)s and P(S-co-SK)s have been also tested as fluorescent markers of PE and PS. The experimental results demonstrate that from PE/P(E-co-PK) and PS/P(S-co-SK) blends prepared using only 1% by weight of fluorescent copolymer, distinguishable fluorescent emissions can be still detected.
1. Introduction
Due to their incredible versatility and wide functionality as well as their low costs, the demand for and consumption of plastic materials are constantly growing worldwide. It is expected that global plastics production, estimated to be 390.7 million metric tons in 2021, will continue to grow in the coming decades [1,2,3]. Unfortunately, the continuous increase of plastic products placed on the market is also giving rise to serious concerns due to the indiscriminate and thoughtless disposal of plastic waste in the environment. In the last decade, plastic waste mismanagement has become such an urgent concern that the 2015 European action plan for the circular economy has valued plastics as one of five priority areas [4]. A “new plastics economy”, which has among its key elements the expansion and improvement of the non-biodegradable plastic recycling processes, has been promoted [5]. In this context, many researchers from both academia and industry have devoted their studies to new strategies leading to the improvement of collection, sorting and recycling technologies [6]. To improve both the cheapness and speed of plastic mechanical recycling processes, automated sorting systems based on the intrinsic chemico-physical properties of polymers have been developed. For this purpose, infrared (IR) and near-infrared (NIR) spectroscopy allowing for recognition of the chemical functional groups present in a specific material have been largely utilized [7,8,9,10]. However, spectroscopic methods for recognizing plastics can fail due to possible degradation of the material over time affecting its identification. On the other hand, the use of tracer additives giving to a specific plastic material a unique identification feature has been proposed too [11,12,13,14,15,16,17,18,19,20]. Processes based on intelligent luminescent labels for rapid automatic sorting packaging have been recently developed [21]. Fluorescent markers to tag different plastics allowing for their identification and consequent sorting during the mechanical recycling process have been tested [22]. A fluorescent tracer can be applied to the plastic as a coating or by adding it in the compounding step [23]. Both methods necessarily involve the use of selected tracers that are stable during the entirety of the plastic products lifetime and that do not decompose during the recycling processes of plastic materials usually involving high temperatures [23]. The fluorescent coating can be accidentally removed from the plastic material it covers, and moreover, it is not suitable for materials such as polyethylene or polypropylene due to their low adhesiveness. Hence, mixing a fluorescent tracer into a specific plastic material is probably the best way to tag it.
Recently, some of us reported the synthesis of new copolymers of propylene and the fluorescent comonomer N-pentenyl-carbazole (P(P-co-PK) contained in low amounts (see Scheme 1) [24]. Such copolymer samples can be readily prepared by copolymerization of the two comonomers (N-pentenyl-carbazole and propylene) using Ziegler–Natta (Z-N) catalytic systems and they can show chemico-physical properties very similar to isotactic polypropylene (i-PP) as well as the distinctive fluorescence of the carbazole group. We also demonstrated that P(P-co-PK)/commercial i-PP homogeneous blend films containing 1% by weight of P(P-co-PK) retain the same optical feature of P(P-co-PK).
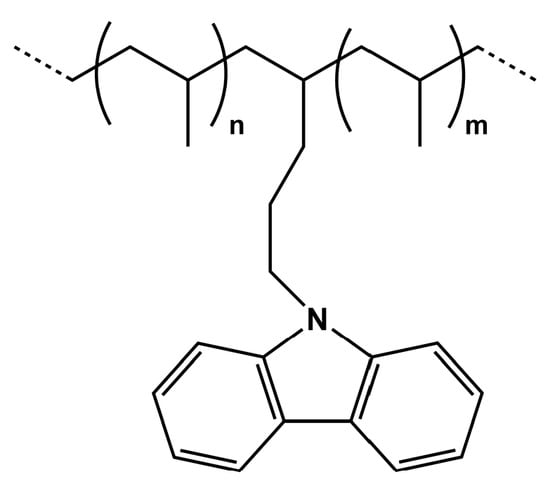
Scheme 1.
Propylene-co-N-pentenyl-carbazole copolymer structure.
Therefore, we suggested that P(P-co-PK)s, due to their high compatibility with i-PP and similar stability, can be used for the fluorescent tagging of i-PP allowing for its detection in the recycling processes of plastics [24]. Starting from these foregoing results, we hypothesized that this approach could be used to obtain fluorescent copolymers of other common plastic materials such as polyethylene (PE) and polystyrene (PS), thus making it suitable to be used for the fluorescent tagging of their homologous homopolymers.
In this paper, we report the synthesis and the microstructural, chemico-physical, and optical characterization of new fluorescent copolymers such as poly(ethylene-co-N-pentenyl carbazole) (P(E-co-PK)) and poly(styrene-co-4-(N-carbazolyl)methyl styrene) (P(S-co-SK)). P(E-co-PK) samples were obtained by Z-N insertion copolymerization, whereas P(S-co-SK)s were prepared by free radical copolymerization. For all of the copolymerization tests, the used amount of the fluorescent comonomer was low enough so that the copolymer samples exhibited chemico-physical properties very close to their homologous homopolymer ones, but high enough to exhibit fluorescent properties, too. Finally, the first results concerning the effectiveness of P(E-co-PK)s and P(S-co-SK)s as fluorescent labels of their commercial homologous homopolymers are also reported.
2. Materials and Methods
2.1. Materials
All of the manipulations involving air- and moisture-sensitive compounds were carried out under a dried and purified nitrogen atmosphere by using Schlenk or glovebox techniques. The glassware and vials used in the ethylene copolymerization were dried in an oven at 120 °C overnight and exposed to a vacuum–nitrogen cycle three times. The reagents and solvents were purchased from Sigma-Aldrich s.r.l. (Milano, Italy). Toluene was purified by refluxing it for 48 h over sodium and distilling it before use; methylaluminoxane (MAO) was used as 10% wt. toluene solution. The catalytic precursor rac-dimethylsilylbis(1-indenyl) zirconium dichloride was purchased from Strem Chemicals, Inc. (Newburyport, MA, USA) and was used as received.
2.2. Measurements
The NMR spectra of the monomers, N-pentenyl-carbazole (PK) and 4-(N-carbazolyl)methyl styrene (SK),were acquired by using a Bruker Advance 400 spectrometer (Bruker, Germany) (1H, 400 MHz; 13C, 100 MHz) operating at 298 K. The samples were prepared by dissolving 5 mg of the monomer in 0.5 mL of deuterated chloroform (CDCl3). The used internal chemical shift reference was tetramethylsilane (TMS). The NMR spectra of P(E-co-PK)s were acquired by using an ASCEND 600 spectrometer (Bruker, Germany) (1H, 600 MHz; 13C, 150 MHz) operating at 373 K. The samples were prepared by dissolving 20 mg of P(E-co-PK) in 0.5 mL of TCDE (tetrachlorodideuteroethane). The used internal chemical shift reference was hexamethyldisiloxane (HMDS). The NMR spectra of P(S-co-SK)s were recorded on an ASCEND 600 spectrometer (1H, 600 MHz; 13C, 150 MHz) operating at 298 K. The samples for the NMR analysis were prepared by dissolving them in 0.5 mL of CDCl3 (deuterated chloroform) and 20 mg of P(S-co-SK). The used internal chemical shift reference was tetramethylsilane (TMS).
Molecular masses (Mn and Mw) and dispersities (Mw/Mn) of P(E-co-PK)s were obtained by an integrated HT-SEC system at 135 °C. o-dichlorobenzene (ODCB) was used as the mobile phase, with a flow rate of 0.8 mL/min. The measurements were acquired with a GPCV 2000 system from Waters (USA) equipped with a differential refractometer (DRI) as the concentration detector; column set: three Shodex HT (806M-805-804) from Showa Denko (Japan). SEC calibration: a relative calibration—Log(M) = ƒ(V): M = molecular weight, V = elution volume— built with polystyrene (PS) standards was used. The polynomial relative calibration curve (3rd order) was constructed with seventeen narrow MWD PS standards with peak molecular weights (Mp) ranging from 5,480,000 g/mol and 162 g/mol (PS monomer).
Molecular masses (Mn and Mw) and dispersities (Mw/Mn) of P(S-co-SK)s were measured by an Agilent GPC/SEC instrument (Agilent, Santa Clara, CA, USA), using THF as the eluent (1.0 mL/min.) at 25 °C. Polystyrene standard samples with narrow molecular weight distributions were used as the reference.
Thermogravimetric analysis (TGA) measurements were carried out by using a TGA Q500 apparatus manufactured by TA Instruments (Waters/TA instruments, New Castle, DE, USA) in flowing N2 (100 cm3/min). Five mg of the polymer samples was placed into platinum crucibles and heated at 10 °C/min from 20° to 800 °C.
Thermal analysis was carried out by using a TA-DSC Q20 apparatus manufactured by Waters/TA instruments (Waters, New Castle, DE, USA) in flowing N2. Five to seven mg of polymer samples was placed into aluminum crucibles and heated/cooled runs were carried out in the range 0–250 °C at 10 °C/min.
Wide-angle X-ray diffraction (WAXD) measurements were carried out on the polymer powder samples using a Bruker D8 Advance automatic diffractometer (Bruker, Germany), in reflection, by Ni-filtered Cu-Ka average radiation (1.5418 Å).
UV-vis measurement was carried out using a Varian Cary 50 spectrophotometer (Agilent, Santa Clara, CA, United States) and photoluminescence recorded by a Varian Cary Eclipse spectrophotometer (Agilent, Santa Clara, CA, USA). Polymer thin (100 nm) films, prepared on a quartz substrate, were obtained by spin coating toluene or chloroform polymer solutions (1 mg/mL) in air at 500 rpm for 60 s.
The morphologies of blend film samples were investigated by scanning electron microscopy (SEM) using a CAMBRIDGE S360 microscope (Zeiss, Germany). The cross-sections of the film samples were obtained in liquid nitrogen by brittle fractures. Prior to SEM observation, the films were sputter-coated with a thin layer of gold.
2.3. Synthesis of the Monomers N-Pentenyl-Carbazole and 4-(N-Carbazolyl)methyl Styrene
The monomers, N-pentenyl-carbazole and 4-(N-carbazolyl)methyl styrene, were synthesized as already reported in [24,25], respectively.
N-pentenyl-carbazole: 9.36 g (0.056 mol) of carbazole and 25.0 g (0.168 mol) of 5-bromo-1-pentene were introduced in a round bottom flask of 100 mL. Thirty mL of a sixteen M NaOH solution and benzyl triethyl ammonium bromide (TBEA) (4.57 g, 0.0168 mol) was added to the mixture and stirred overnight at 60 °C. Then, the mixture was poured into water and extracted with ethyl acetate thrice. The ethyl acetate solution was dried over anhydrous MgSO4 for 2 h and then filtered. The crude product was obtained by evaporation of the solvent (ethyl acetate). It was then purified on silica gel column chromatography (hexane/ ethyl acetate (8:2)) to give a white solid with an 85% yield. N-pentenyl-carbazole was dried over phosphorus pentoxide (P2O5) under vacuum and stored in a glass ampoule under nitrogen.
N-pentenyl-carbazole characterization: 1H NMR (400 MHz, CDCl3) δ: 2.01 (2H, m, CH2), 2.19 (2H, m, CH2), 4.34 (2H, t, NCH2), 5.07 (2H, dd, CH2=CH), 5.88 (1H, m, CH), 7.29 (2H, d, Ph), 7.45 (2H, t, Ph), 7.50 (2H, t, Ph), 8.15, (2H, d, Ph); 13C NMR (100 MHz, CDCl3) δ: 140.33, 137.45, 125.55, 122.81, 120.32, 118.14, 115.48, 108.61, 42.31, 31.11, 27.88.
4-(N-carbazolyl)methyl styrene: Both 11.7 g (0.070 mol) of carbazole and 29.6 mL (0.21 mol) of 4-vinylbenzyl chloride were dissolved in toluene in a round bottom flask of 100 mL. Thirty-five mL of a sixteen M NaOH solution and benzyl triethyl ammonium bromide (TBEA) (5.72 g, 0.021 mol) was added to the toluene solution. The mixture was stirred overnight at 60 °C. Then, the mixture was poured into water and extracted with chloroform thrice. The chloroform solution was dried over anhydrous MgSO4 for 2 h and then filtered. The crude product was obtained by evaporation of the solvent (chloroform). It was then purified on silica gel column chromatography. A mixture of hexane/dichloromethane (2:1)) was used as an eluent to give a white solid with a 70% yield. 4-(N-carbazolyl) methyl styrene was dried over phosphorus pentoxide (P2O5) under vacuum and stored in a glass ampoule under nitrogen.
4-(N-carbazolyl)methyl styrene characterization: 1H NMR (400 MHz, CDCl3) δ: 5.21 (1H, d, CH2=CH), 5.53 (2H, s, NCH2), 5.67 (1H, d, CH2=CH), 6.63–6.70 (1H, m, CH), 7.11 (2H, d, Ar), 7.25–7.47 (6H, m, Ar), 8.15, (2H, d, Ar); 13C NMR (100 MHz, CDCl3) δ: 140.80, 137.09, 136.91, 136.48, 126.82, 126.06, 123.23, 120.60, 119.43, 114.14, 109.08, 46.56.
2.4. Ethylene-N-Pentenyl-Carbazole Copolymerizations
A 50 mL round bottom flask, containing a magnetic stirrer, was charged with N-pentenyl-carbazole (run 1: 0.32 mmol, 75 mg; run 2: 0.64 mmol, 150 mg) and 12 mL of dry toluene under N2 atmosphere at room temperature (20 °C). One mL of a toluene solution of MAO (10% by weight) was added. The N2 atmosphere in the reaction flask was then replaced by constant ethylene over-pressure. After 5 min, a dry toluene solution of rac-dimethylsilylbis(1-indenyl) zirconium dichloride (0.011 mmol (5.0 mg)) was injected to start polymerization. The reaction mixture was stirred for 15 min, and then the polymerization was quenched with a few milliliters of ethanol. After coagulation in an excess of acidified ethanol, the polymer was recovered by filtration, washed several times with fresh ethanol, and dried at room temperature in vacuum. Finally, the copolymer samples were extracted before with hexane to eliminate traces of unreacted N-pentenyl-carbazole monomer, and then with toluene to eliminate possible catalytic residues by using a Kumagawa extractor. The polymers were almost insoluble in hexane and soluble in toluene. The toluene soluble fraction was used for all of the analyses and tests.
2.5. Styrene4-(N-Carbazolyl)methyl Styrene Copolymerizations
In a 50 mL round bottom flask equipped with a magnetic stir bar, 4-(N-carbazolyl)methyl styrene (run 3: 0.88 mmol, 250 mg; run 4: 1.76 mmol, 500 mg) and styrene (44 mmol (5.0 mL)) were dissolved in 5 mL of dry toluene. Benzoyl peroxide (BPO) (0.41 mmol (100 mg)) was added to the reaction mixture and the flask was heated at 80 °C for 2 h. The ethanol coagulated polymer was recovered by filtration, washed with fresh ethanol several times, and dried at room temperature in vacuum. Finally, the copolymer samples were also extracted before with hexane and then with toluene by using a Kumagawa extractor. The toluene soluble fraction was used for all of the analyses and tests.
2.6. Polymerization of Ethylene
Under N2 atmosphere, in a 50 mL round bottom flask containing a magnetic stir bar, 1 mL of MAO solution (toluene, 10% in weight) was added to 12 mL of dry toluene at room temperature (20 °C). The N2 atmosphere in the reaction flask was replaced by constant ethylene over-pressure. After 5 min, a dry toluene solution of rac-dimethylsilylbis(1-indenyl) zirconium dichloride (7.0 mg (0.0156 mmol)) was injected to start polymerization, The reaction mixture was stirred for 15 min, and then the polymerization was quenched by introducing a few milliliters of ethanol. After coagulation in an excess of acidified ethanol, the polymer was recovered by filtration, washed several times with fresh ethanol, and dried at room temperature in vacuum. Finally, the copolymer samples were also extracted before with hexane and then with toluene by using a Kumagawa extractor. The toluene soluble fraction was used for all of the analyses and tests.
2.7. Polymerization of Styrene
In a 50 mL round bottom flask equipped with a magnetic stir bar, 5.0 mL (44 mmol) of styrene was dissolved in 5 mL of dry toluene. Benzoyl peroxide (BPO) (0.41 mmol (100 mg)) was added to the reaction mixture and the flask was heated at 80 °C for 2 h. Then, the polymer was coagulated in an excess of ethanol, recovered by filtration, washed several times with fresh ethanol, and dried under vacuum at room temperature. Finally, the copolymer samples were also extracted before with hexane and then with toluene by using a Kumagawa extractor. The toluene soluble fraction was used for all of the analyses and tests.
2.8. Preparation of PE/P(E-co-PK) and PS/P(S-co-SK) Polymer Blends
PE/P(E-co-PK) and PS/P(S-co-SK) polymer blend samples were prepared by dissolving 500 mg of PE or PS and 5 mg of P(E-co-PK) or P(S-co-SK) in 10 mL of toluene at 100 °C under stirring. The homogeneous polymer mixture was then poured into an excess of ethanol, the solid was recovered by filtration, and then dried under vacuum at 40 °C overnight. Polymer blend films were obtained by the solution casting method from their solution (5 mg/mL).
3. Results and Discussion
3.1. (P(E-co-PK)s and (P(S-co-SK)s Synthesis and Microstructural Characterization
Ethylene-co-N-pentenyl carbazole copolymer samples (P(E-co-PK)s) were synthesized by insertion Ziegler–Natta polymerization [26] using the catalytic system rac-dimethylsilylbis(1-indenyl) zirconium dichloride/MAO (see Scheme 2, path a). Styrene-co-4-(N-carbazolyl)methyl styrene) copolymer samples (P(S-co-SK)s) were instead prepared by free radical polymerization using benzoyl peroxide (BPO) as an initiator (see Scheme 2, path b).
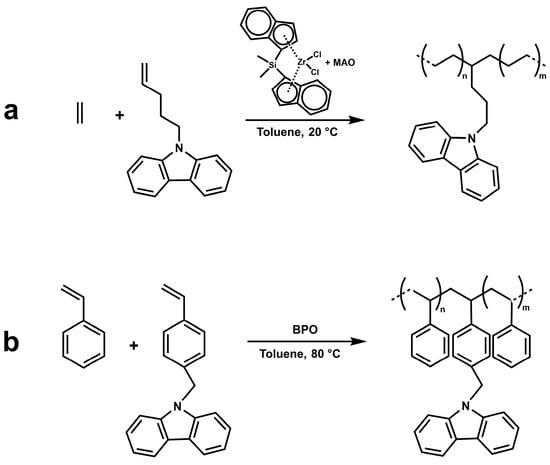
Scheme 2.
Copolymer synthesis.
Since the main aim of this study was to achieve fluorescent copolymer samples with chemico-physical properties such as those of the corresponding commercial non-fluorescent homopolymers, all of the copolymerization tests were conducted so that the percent of fluorescent comonomer units inserted into the copolymer chains never exceeds 4%. The copolymerization results are summarized in Table 1.

Table 1.
Copolymerization results.
The copolymer microstructures were evaluated by 13C NMR analysis. All of the assignments have been made by distortionless enhancement by polarization transfer 90 and 135 (DEPT-90 andDEPT-135) 13C NMR experiments, [27,28] based on the data reported in the literature for carbons in similar environments, [25,29,30,31,32,33,34] and by using Grant and Paul additivity rules [35].
In Figure 1 and Figure 2, the 13C NMR spectra of P(E-co-PK) (run 1) and P(S-co-SK) (run 3) samples are shown. Both copolymers have microstructures presenting long non fluorescent comonomer homosequences (PE and PS, respectively) interspersed with isolated units of fluorescent comonomer (PK and SK, respectively). For the sake of clarity, the experimental carbon chemical shifts of the signals related to copolymer microstructures are listed in Tables T1 and T2 of S.I.
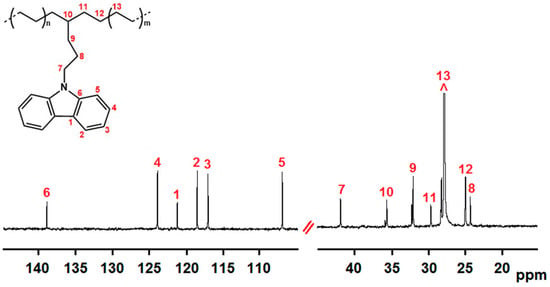
Figure 1.
13C NMR spectrum of P(E-co-PK) (run 1). (TCDE solvent, HMDS scale, 100 °C).
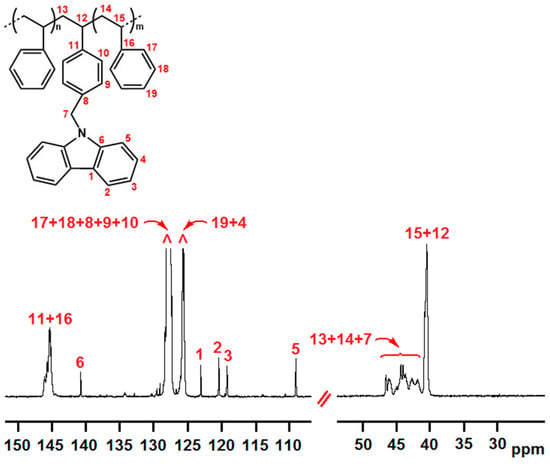
Figure 2.
13C NMR spectrum of P(S-co-SK) (run 3). (CDCl3 solvent, TMS scale, 25 °C).
Two-dimensional DOSY NMR experiments were also conducted to demonstrate the successful copolymerization of all of our samples. In Figure 3 and Figure 4, the DOSY maps with the 1H NMR spectra projected on top of run 1 and 3 are depicted. Both show that 1H NMR signals of non-fluorescent comonomer homosequences and fluorescent comonomer isolated units of any copolymer sample present the same diffusion coefficient (2.02 × 10−11 m2/s for P(E-co-PK) and 3.45 × 10−11 m2/s for P(S-co-SK)), according to an efficient copolymerization of the two monomers. These data are consistent with GPC analysis showing monomodal curves for all of the copolymer samples (see Figures S1–S4 in S.I.).
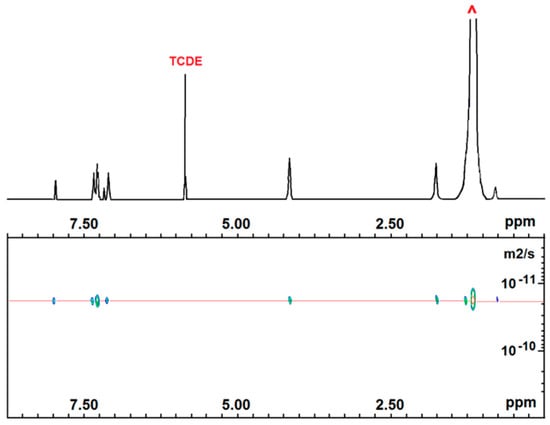
Figure 3.
Six hundred MHz 2D DOSY NMR spectrum of P(E-co-PK) (run 1). (TCDE solvent, HMDS scale, 100 °C).
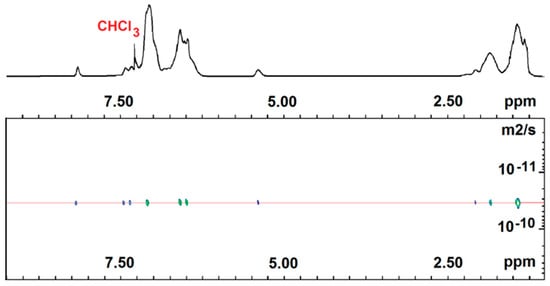
Figure 4.
Six hundred MHz 2D DOSY NMR spectrum of P(S-co-SK) (run 3). (CDCl3 solvent, TMS scale, 25 °C).
3.2. WAXD, Thermal, and Optical Characterization
In Figure 5, the X-ray diffraction spectra of runs 1 and 3 are reported. The run 1 (P(E-co-PK)) pattern shows two sharp high-intensity diffraction peaks at 2θ values of 21.6° and 23.6° as well as several lower intensity peaks as typically observed in semicrystalline PE samples [36]. Similar to the PS WAXD pattern, [37] run 3 (P(S-co-SK)) reveals at 2θ = 19.5° an intense and broad peak, to be assigned to the amorphous phase, along with a typical so-called “polymerization peak” at 2θ = 10.1°.
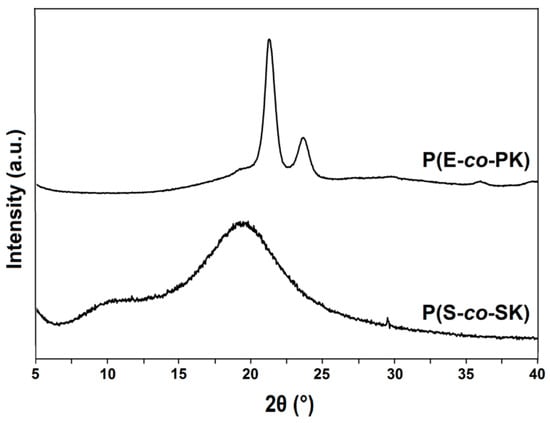
Figure 5.
WAXD patterns of runs 1 and 3 of Table 1.
The results of the thermal analysis showed that all of the copolymer samples (P(E-co-PK)s and P(S-co-SK)s) have high decomposition temperatures (see Table 1 and Figures S5–S8 in S.I.), comparable with those of the corresponding homopolymers (PE and PS, respectively). The DSC analysis results of P(E-co-PK) (run 1) and P(S-co-SK) (run 3) are shown in Figure 6. As expected, the P(E-co-PK) thermogram presents an endothermic peak due to PE block melting, while in the P(S-co-SK) thermogram, only a glass transition phenomenon is detected.
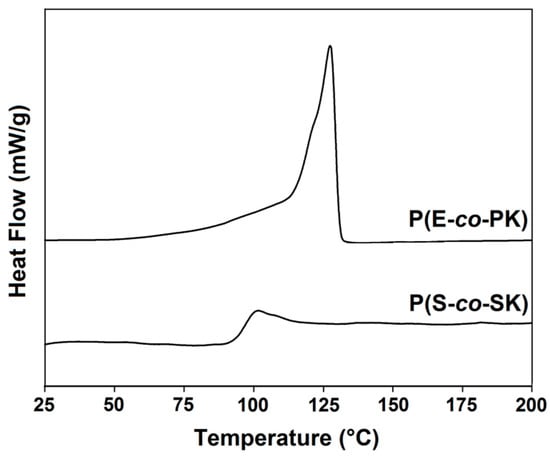
Figure 6.
Second DSC heating thermograms of runs 1 and 3 of Table 1.
UV-vis absorbance spectra on the films of all of the prepared copolymer samples were carried out. As an example, the run 1 and 3 spectra are reported in Figure 7. Although P(E-co-PK) and P(S-co-SK) copolymers contain a very low amount of PK and SK units, their UV-vis spectra clearly show two peak maxima at ~334 and ~349 nm, which are distinctive of carbazole groups [38,39].
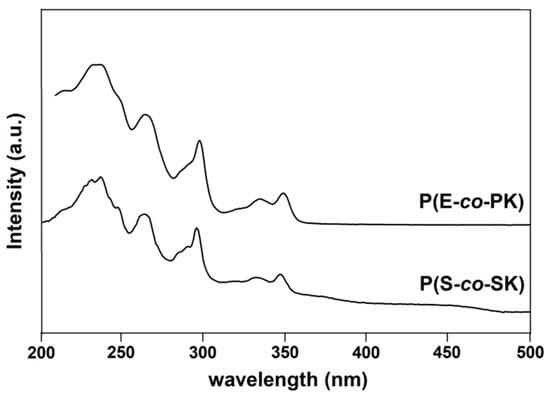
Figure 7.
UV-vis absorption spectra of runs 1 and 3 of Table 1.
The film emission spectra of run 1 and 3 are shown in Figure 8. All of the samples present high energy bands at 351 and 366 nm (Λecc = 300 nm) due to the carbazole emission [28,29,30,33,34,40,41]. As expected, due to the absence of two close carbazole-containing units in the copolymer chains, no excimer emission has been detected.
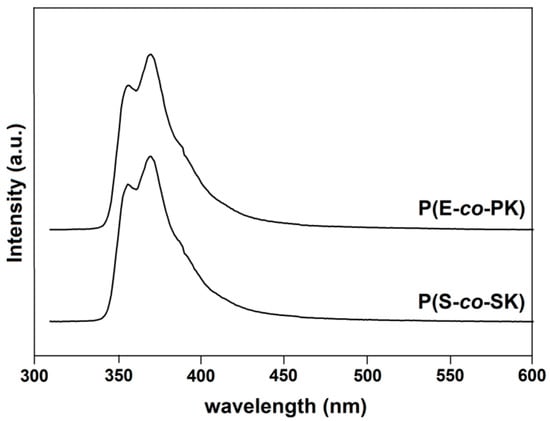
Figure 8.
Emission spectra of runs 1 and 3 of Table 1 (Λecc = 300 nm).
3.3. Fluorescence Study of P(E-co-PK)/PE and P(S-co-SK)/PS Polymer Blends
All of the synthesized copolymers, P(E-co-PK)s and P(S-co-SK)s, contain an amount of fluorescent comonomer units low enough to allow them to exhibit very similar chemico-physical properties to their corresponding homopolymers, PE and PS, but, on the other hand, they are high enough to make them fluorescence traceable. Preliminary studies were conducted to verify the effectiveness of our copolymers as fluorescent markers of their commercial homologous homopolymers. Thus, common polymer samples of linear semicrystalline PE and amorphous PS were synthesized (see experimental part and S.I. for their synthesis and characterization) to be used to prepare polymer blends. In detail, the PE and PS samples were mixed with 1% by weight of P(E-co-PK) (run 1) and P(S-co-SK) (run 3), respectively. P(E-co-PK) and PE were dissolved in toluene, while P(S-co-SK) and PS were dissolved in chloroform. Then, both mixtures were coagulated in ethanol. Finally, polymer blend films were prepared by solution casting. The SEM images of the cross section of the PE/P(E-co-PK) and PS/P(S-co-PS) blend films are presented in Figure 9. Both have a homogeneous morphology that is completely similar to the corresponding homopolymers, PE and PS, respectively (for the comparison see Figures S9 and S10 in S.I.).
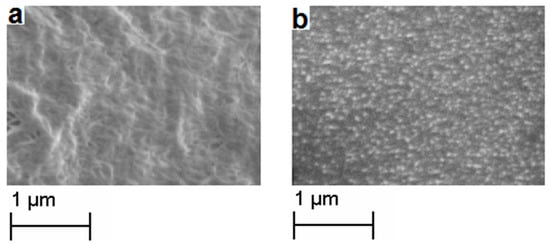
Figure 9.
SEM images of the (a) PE/P(E-co-PK) and (b) PS/P(S-co-PS) blend films.
In Figure 10, the fluorescence spectra of the PE/P(E-co-PK) and PS/P(S-co-PS) blend film samples, carried out at room temperature using an excitation wavelength of 300 nm, are depicted. In both spectra, the bands at 351 and 366 nm detected in the P(E-co-PK) and P(S-co-PS) copolymer samples (see Figure 8) attributable to the emission of the carbazole units are still present. Although these results are preliminary, they indicate that our copolymers are suitable for use as fluorescent markers of commercial polymers.
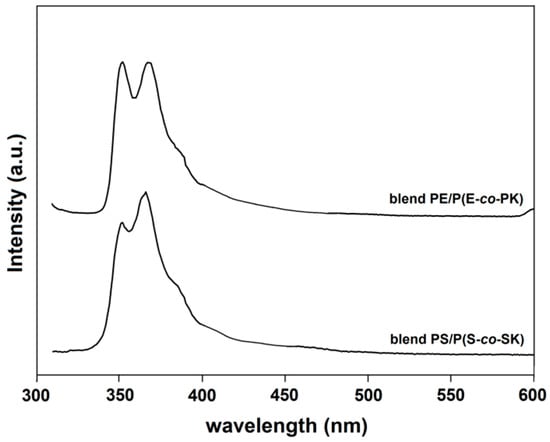
Figure 10.
Emission spectra of the PE/P(E-co-PK) (top) and PS/P(S-co-SK) (button) polymer blends containing 1% by weight of P(E-co-PK) and P(S-co-SK), respectively, (Λecc = 300 nm).
4. Conclusions
P(E-co-PK) and P(S-co-PS) copolymers containing a low amount of fluorescent units (PK and SK) were prepared to obtain fluorescent PEs and PSs, respectively. P(E-co-PK)s were synthesized by Ziegler–Natta polymerization using the homogeneous catalytic system rac-dimethylsilylbis(1-indenyl) zirconium dichloride/MAO, while P(S-co-SK)s were prepared by free radical polymerization using BPO as an initiator.
NMR analysis allowed us to identify all of the copolymer microstructures presenting isolated fluorescent units interspersed in long non-fluorescent comonomer homosequences. Due to the low amount of fluorescent units, never higher than 4%, both P(E-co-PK)s and P(S-co-PS)s have chemico-physical properties very similar to their corresponding non-fluorescent homopolymers (PE and PS, respectively). In particular, they have the same thermal stability. Contextually, P(E-co-PK)s and P(S-co-PS)s are optically active, thus showing the typical fluorescence of the carbazole group.
Thus, P(E-co-PK) and P(S-co-PS) copolymers were tested to verify if they could be suitable as fluorescent markers of their corresponding non-fluorescent homopolymers (PE and PS, respectively). P(E-co-PK)/PE and P(S-co-SK)/PS polymer blends containing 1% by weight of P(E-co-PK) and P(S-co-SK), respectively, were prepared and analyzed by fluorescence spectroscopy. Both of the P(E-co-PK)/PE and P(S-co-SK)/PS polymer blends results were homogeneous and fluorescence traceable.
Taking into account that the mechanical plastic recycling processes usually involve high-temperature treatments and that thermally stable fluorescent markers are therefore required, our fluorescent copolymers should be particularly suitable for the application of methods based on plastic fluorescent tagging.
Supplementary Materials
The following supporting information can be down loaded at: https://www.mdpi.com/article/10.3390/pr11020515/s1, Figure S1: GPC trace of run 1 (P(E-co-PK sample); Figure S2: GPC trace of run 2 (P(E-co-PK sample); Figure S3: GPC trace of run 3 (P(S-co-SK sample); Figure S4: GPC trace of run 4 (P(S-co-SK sample); Figure S5: TGA trace of runs 1 (P(E-co-PK sample); Figure S6: TGA trace of runs 2 (P(E-co-PK sample); Figure S7: TGA trace of runs 3 (P(S-co-SK sample); Figure S8: TGA trace of runs 4 (P(S-co-SK sample); Figure S9: SEM images of PE/P(E-co-PK) blend and PE films; Figure S10: SEM images of PS/P(S-co-SK) blend and PS films; Figure S11: 1H NMR spectrum of PS sample; Figure S12: 13C NMR spectrum of PS sample; Figure S13: TGA trace of PS sample; Figure S14: DSC curve of PS sample; Figure S15: X ray diffraction pattern of PS sample; Figure S16: 13C NMR spectrum of PE sample; Figure S17: TGA trace of PE sample; Figure S18: DSC curve of PE sample; Figure S19: X ray diffraction pattern of PE sample; Table T1: 13C NMR Assignments of P(E-co-PK); Table T2: 13C NMR Assignments of P(S-co-SK).
Author Contributions
Conceptualization, S.P., V.V. and F.G.; investigation, F.T., M.M., C.C. and R.F.; methodology, A.C.B., R.F. and F.T.; validation, all authors; writing—original draft, S.P., L.I. and V.V.; writing—review and editing, F.G., M.M. and C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study received financial support from the University of Salerno (FARB). We thank Mariagrazia Napoli (University of Salerno), Patrizia Oliva (University of Salerno), and Ivano Immediata (University of Salerno) for their technical assistance.
Data Availability Statement
All data are reported in the manuscript and/or in the Supporting Information.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Global Plastics Market Research Review 2021–2022: An Analysis of Developments by Key Plastics Manufacturers. Available online: https://www.globenewswire.com/en/news-release/2022/04/26/2428757/28124/en/Global-Plastics-Market-Research-Review-2021-2022-An-Analysis-of-Developments-by-Key-Plastics-Manufacturers.html (accessed on 26 April 2022).
- Colnik, M.; Kotnik, P.; Knez, Ž.; Škerget, M. Chemical Recycling of Polyolefins Waste Materials Using Supercritical Water. Polymers 2022, 14, 4415. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R. Chapter 2—Production, use, and fate of synthetic polymers. In Plastic Waste and Recycling; Letcher, T.M., Ed.; Academic Press: Cambridge, UK, 2020; pp. 13–32. [Google Scholar] [CrossRef]
- European Commission. Closing the Loop—An EU Action Plan for the Circular Economy; Communication from the Commission (COM(2015) 614 Final); European Commission: Brussels, Belgium, 2015.
- European Commission. A European Strategy for Plastics in a Circular Economy; Communication from the Commission to the European parliament (COM (2018) 28 Final); European Commission: Brussels, Belgium, 2018.
- Johnson, H.; Chambers, L.C.; Holloway, J.O.; Bousgas, A.; Akhtar-Khavari, A.; Blinco, J.; Barner-Kowollik, C. Using precision polymer chemistry for plastics traceability and governance. Polym. Chem. 2022, 13, 6082–6090. [Google Scholar] [CrossRef]
- Henriksen, M.L.; Karlsen, C.B.; Klarskov, P.; Hinge, M. Plastic classification via in-line hyperspectral camera analysis and unsupervised machine learning. Vib. Spectrosc. 2022, 118, 103329. [Google Scholar] [CrossRef]
- Schmidt, F.; Christiansen, N.; Lovrincic, R. The Laboratory at Hand: Plastic Sorting Made Easy. Photonics Views 2020, 17, 56–59. [Google Scholar] [CrossRef]
- Beigbeder, J.; Perrin, D.; Mascaro, J.F.; Lopez-Cuesta, J.M. Study of the physico-chemical properties of recycled polymers from waste electrical and electronic equipment (WEEE) sorted by high resolution near infrared devices. Resour. Conserv. Recycl. 2013, 78, 105–114. [Google Scholar] [CrossRef]
- Wu, X.; Li, J.; Yao, L.; Xu, Z. Auto-sorting commonly recovered plastics from waste household appliances and electronics using near-infrared spectroscopy. J. Clean. Prod. 2020, 246, 118732. [Google Scholar] [CrossRef]
- Brunner, S.; Fomin, P.; Kargel, C. Automated sorting of polymer flakes: Fluorescence labeling and development of a measurement system prototype. Waste Manag. 2015, 38, 49–60. [Google Scholar] [CrossRef]
- Serranti, A.; Gargiulo, G.; Bonifazi, G. Hyperspectral imaging for process and quality control in recycling plants of polyolefin flakes. J. Near Infrared Spectrosc. 2012, 20, 573–581. [Google Scholar] [CrossRef]
- Serranti, S.; Fraunholcz, N.; Di Maio, F.; Bonifazi, G. Recycling-oriented characterization of polyolefin packaging waste. Waste Manag. 2013, 33, 574–584. [Google Scholar] [CrossRef]
- Maris, E.; Aoussat, A.; Naffrechoux, E.; Froelich, D. Polymer tracer detection systems with {UV} fluorescence spectrometry to improve product recyclability. Miner. Eng. 2012, 29, 77–88. [Google Scholar] [CrossRef]
- Bezati, F.; Froelich, D.; Massardier, V.; Maris, E. Addition of X-ray fluorescent tracers into polymers, new technology for automatic sorting of plastics: Proposal for selecting some relevant tracers. Resour. Conserv. Recycl. 2011, 55, 1214–1221. [Google Scholar] [CrossRef]
- Langhals, H.; Zgela, D.; Schlücker, T. High performance recycling of polymers by means of their fluorescence lifetimes. Green Sustainable Chem. 2014, 4, 144–150. [Google Scholar] [CrossRef]
- Langhals, H.; Schmid, T.; Herman, M.; Zwiener, M.; Hofer, A. Binary fluorescence labeling for the recovery of polymeric materials for recycling. Int. J. Environ. Eng. Sci. Technol. Res. 2013, 1, 124–132. [Google Scholar] [CrossRef]
- Massardier, V.; Louizi, M.; Maris, E.; Froelich, D. High shear dispersion of tracers in polyolefins for improving their detection. Polímeros 2015, 25, 466–476. [Google Scholar] [CrossRef]
- Ahmad, S.R. A new technology for automatic identification and sorting of plastics for recycling. Environ. Technol. 2004, 25, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Hui, D.; Singh, R.; Ahuja, I.P.S.; Feo, L.; Fraternali, F. Recycling of plastic solid waste: A state of art review and future applications. Compos. Part B 2017, 115, 409–422. [Google Scholar] [CrossRef]
- Harris, P.G.; Fern, G.R.; Silver, J. Method and Apparatus for Identifying Articles with a Luminescent Marker for Recycling. U.S. Patent No. 11,318,500, 3 May 2022. [Google Scholar]
- Arenas, A.; Beltrán, F.R.; Alcázar, V.; de la Orden, M.U.; MartínezUrreaga, J. Fluorescence labeling of high density polyethylene for identification and separation of selected containers in plastics waste streams. Comparison of thermal and photochemical stability of different fluorescent tracers. Mater. Today Commun. 2017, 12, 125–132. [Google Scholar] [CrossRef]
- Pilon, L.; Stewart, A.; Bahia, R.; Hintschich, S.; Willner, C.; Eder, H. Removable Identification Technology to Differentiate Food Contact PET in Mixed Waste Streams: Interim Report. Polymark 2015, 1–23. Available online: https://www.semanticscholar.org/paper/Removable-Identification-Technology-to-Food-Contact-Pilon-Stewart/8735a984209e3f5ec506da9148cbe44a64c19e1f (accessed on 26 April 2022).
- Pragliola, S.; Grisi, F.; Vitale, V.; Sacco, O.; Venditto, V.; Izzo, L.; Grimaldi, A.; Baranzini, N. New fluorescence labeling isotactic polypropylenes as a tracer: A proof of concept. Polym. Chem. 2022, 13, 2685–2693. [Google Scholar] [CrossRef]
- Botta, A.; Pragliola, S.; Capacchione, C.; Rubino, A.; Liguori, R.; De Girolamo Del Mauro, A.; Venditto, V. Synthesis of poly(4-(N-carbazolyl)methyl styrene)s: Tailoring optical properties through stereoregularity. Eur. Polym. J. 2017, 88, 246–256. [Google Scholar] [CrossRef]
- Venditto, V.; Guerra, G.; Corradini, P.; Fusco, R. Mechanism of monomer insertion for heterogeneous isospecific Ziegler Natta catalytic models. Eur. Polym. J. 1991, 27, 45–54. [Google Scholar] [CrossRef]
- Doddrell, D.M.; Pegg, D.T.; Bendall, M.R. Distortionless enhancement of NMR signals by polarization transfer. J. Magn. Res. 1982, 48, 323–327. [Google Scholar] [CrossRef]
- Aubert, P.; Sledz, J.; Schue, F.; Brevard, C. Etude structurale du polypentadiene-1,3 par spectrometrie de RMN du 13C. J. Polym. Sci. Polym. Chem. Ed. 1981, 19, 955–972. [Google Scholar] [CrossRef]
- Botta, A.; Pragliola, S.; Venditto, V.; Rubino, A.; Aprano, S.; Del Mauro De Girolamo, A.; Maglione, M.G.; Minarini, C. Synthesis, characterization, and use as emissive layer of white organic light emitting diodes of the highly isotactic poly(N-pentenyl-carbazole). Polym. Compos. 2015, 36, 1110–1117. [Google Scholar] [CrossRef]
- Cho, Y.S.; Kim, S.W.; Ihn, C.S.; Lee, J.-S. Anionic Polymerisation of 4-(9-Carbazolyl)Methylstyrene. Polymer 2001, 42, 7611–7616. [Google Scholar] [CrossRef]
- Ates, M.; Uludag, N.; Sarac, A.S. Synthesis of 2-(9H-carbazole-9-yl)ethyl methacrylate: Electrochemical impedance spectroscopic study of poly(2-(9H-carbazole-9-yl)ethyl methacrylate) on carbon fiber. J. Appl. Polym. Sci. 2011, 121, 3475–3482. [Google Scholar] [CrossRef]
- Lessard, B.H.; Ling, E.J.Y.; Maric, M. Fluorescent, Thermoresponsive Oligo(ethylene glycol) Methacrylate/9-(4-Vinylbenzyl)-9H-carbazole Copolymers Designed with Multiple LCSTs via Nitroxide Mediated Controlled Radical Polymerization. Macromolecules 2012, 45, 1879–1891. [Google Scholar] [CrossRef]
- Liguori, R.; Botta, A.; Rubino, A.; Pragliola, S.; Venditto, V. Stereoregular polymers with pendant carbazolyl groups: Synthesis, properties and optoelectronic applications. Synth. Met. 2018, 246, 185–194. [Google Scholar] [CrossRef]
- Pragliola, S.; De Vita, R.; Longo, P. Aqueous emulsion polymerization of styrene and substituted styrenes using titanocene compounds. Polymer 2013, 54, 1583–1587. [Google Scholar] [CrossRef]
- Grant, D.M.; Paul, E.G. Carbon-13 Magnetic Resonance. II. Chemical Shift Data for the Alkanes. J. Am. Chem. Soc. 1964, 86, 2984–2990. [Google Scholar] [CrossRef]
- Caminiti, R.; Pandolfi, L.; Ballirano, P. Structure of Polyethylene from X-Ray Powder Diffraction: Influence of the Amorphous Fraction on Data Analysis. J. Macromol. Sci.-Phys. B 2000, 39, 481–492. [Google Scholar] [CrossRef]
- Ayyagari, C.; Bedrov, D.; Smith, G.D. Structure of Atactic Polystyrene: A Molecular Dynamics Simulation Study. Macromolecules 2000, 33, 6194–6199. [Google Scholar] [CrossRef]
- Berlman, I.B. Handbook of Fluorescence Spectra of Aromatic Molecules Academic; Elsevier: New York, NY, USA, 1971. [Google Scholar]
- Johnson, P.C.; Offen, H.W. Excimer Fluorescence of Poly(N-vinylcarbazole). J. Chem. Phys. 1971, 55, 2945–2949. [Google Scholar] [CrossRef]
- Botta, A.; Costabile, C.; Venditto, V.; Pragliola, S.; Liguori, R.; Rubino, A.; Alberga, D.; Savarese, M.; Adamo, C. Optoeletronic properties of poly(N-alkenyl-carbazole)s driven by polymer stereoregularity. J. Pol. Sci. Pol. Chem. 2018, 59, 242–251. [Google Scholar] [CrossRef]
- Izzo, L.; Lisa, P.; Sacco, O.; Pragliola, S. Synthesis of Di-Block Copolymers Poly (Propylene oxide)-block-Poly (9-(2,3-epoxypropyl) Carbazole) via Sequential Addition of Monomers in One Pot. Polymers 2021, 13, 763. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).