Abstract
In this study, the essential oil (EO) from the peel of the Greek citrus hybrid Citrus sinensis cv New Hall - Citrus aurantium was studied in terms of its antimicrobial properties as well as its effect on Saccharomyces cerevisiae. According to the analysis of the EO, 48 compounds are contained in it, with the main compounds being limonene, β-pinene, myrcene, α-pinene, valencene, and α-terpineol. As regards its antimicrobial properties, the EO was evaluated against nine human pathogenic microorganisms, six bacteria, and three fungi. Taking the results into account, it was apparent that Gram-negative bacteria were the most susceptible to the addition of the EO, followed by the Gram-positive bacteria, and finally the examined yeasts. The minimum inhibitory concentrations were found to be lower compared to other studies. Finally, the effect of the EO on the biochemical behavior of the yeast Saccharomyces cerevisiae LMBF Y-16 was investigated. As the concentration of the EO increased, the more the exponential phase of the microbial growth decreased; furthermore, the biomass yield on the glucose consumed significantly decreased with the addition of the oil on the medium. The addition of the EO in small concentrations (e.g., 0.3 mL/L) did not present a remarkable negative effect on both the final biomass concentration and maximum ethanol quantity produced. In contrast, utilization of the extract in higher concentrations (e.g., 1.2 mL/L) noticeably inhibited microbial growth as the highest biomass concentration achieved, maximum ethanol production, and yield of ethanol produced per glucose consumed drastically declined. Concerning the composition of cellular lipids, the addition of the EO induced an increment in the concentration of cellular palmitic, stearic, and linoleic acids, with a concomitant decrease in the cellular palmitoleic acid and oleic acids.
1. Introduction
Rutaceae is a well-known family of flowering plants that consists of more than 400 genera with 3500 species. The various species typically have four or five distinct flower sections that emit a potent aroma. They come in a variety of shapes and sizes, from little trees to shrubs and herbs. Rutoideae, Dictyolomatoideae, Flindersioideae, Seathelioideae, Toddalioideae, Aurantioideae, and Rhabdondreoideae are the seven subfamilies that make up the Rutaceae family [1]. The most important genus, from an economical point of view, in the subfamily of Aurantioideae is Citrus, which includes the sweet orange (C. sinensis), sour orange (C. aurantium), lemon (C. limon), grapefruit (C. paradisi), and lime (C. aurantifolia). Since many of the described species are hybrids, the taxonomy and systematics of the genus are complicated, and the exact number of natural species is unknown [2,3]. Brazil, China, and the USA are among the countries with the largest citrus production, worldwide, while considerable amounts are also produced in the Mediterranean region [4]. In Greece, Newhall navel oranges are broadly cultivated and used in the food industry.
One product with wide usage from the citrus species is its essential oil (EO). EOs are generally recognized as safe (GRAS) and, as such, they have been used extensively as natural food preservatives, both for traditionally and industrially produced food products. Moreover, the use of EOs is highly accepted by consumers, due to the pleasant odor and taste that they bestow on the food products [5]. The Citrus species are a rich source of EO of high value that can be obtained using proper procedures [6]. As indicated by Verzera et al., the species of the genus Citrus produce three different kinds of essential oils depending on the part of the plant in which they are produced (fruit pericarp, leaves, flowers) [7,8,9]. The composition of Citrus EO varies due to many factors, such as the harvest year [10], harvest date [11], cultivar [12], extraction system [13], geographic origin, ripeness of the fruit, and the extraction method. For instance, increasing temperatures and decreasing rainfalls over the years can impact the composition of the EO [10]; while the advancements in the extraction methods over the years have resulted in EO with various compositions [13]. According to the literature, EOs are complex mixtures of volatile constituents with limonene as the most abundant compound, ranging from 32% to 98% in the oil, including monoterpenes, sesquiterpenes, and their oxygenated derivatives [9,14,15]. Moreover, EOs contain bioactive metabolites such as limonene, linalool, and citral; therefore, EOs often exhibit antimicrobial properties [4,16].
Microbes are double-edged swords for the food industry. Various types of foods are produced with the use of microorganisms, such as cheese, yogurt, bread, cocoa, wine, beverages, etc. [17]. However, microorganisms are also responsible for food spoilage and foodborne diseases. As such, not only is much emphasis placed on the study of microorganisms to produce valuable products, but also on the study of ways to eliminate or decrease the viability of microorganisms. A good example is the species Saccharomyces cerevisiae. It is the yeast responsible for alcoholic fermentation in wine. Moreover, it has also been studied in relation to its potential to produce ethanol in various fermentation configurations [18,19,20]. These reports currently attract noticeable industrial interest due to the constant increase in conventional fuel prices, with ethanol having the potential to replace these conventional fuels, at least partially, in various engines and heating systems. Production of bioethanol on industrial-scale operations is mainly based on trials performed by wild-type or genetically modified S. cerevisiae strains. Amongst other issues that are imposed during various fermentative processes in industrial biotechnology, one major point is related to the maintenance of aseptic conditions during the performed processes. During the alcohol fermentation process, a variety of bacteria can potentially contaminate the facilities, affecting the overall efficiency of performed processes. Therefore, it would be of interest to identify potential compounds, the addition of which into the fermentation medium could reduce the problem related to the contamination by microorganisms not performing the alcoholic fermentation. As such, acid treatment and antibiotics are employed in an effort to decontaminate the facilities or control the growth of the yeast; however, both methods exhibit certain limitations [21]. Therefore, EOs that exhibit antimicrobial activity could potentially be used for such reasons.
The present investigation aims to examine the effect of EO from Citrus sinensis cv New Hall - Citrus aurantium upon the biochemical behavior (e.g., biomass production, substrate uptake, metabolites production, composition in the fatty acids of cellular lipids) of S. cerevisiae yeast. EO was also examined for its chemical composition, as well as for its antimicrobial activity, against nine human pathogenic microorganisms, six bacteria, and three fungi.
2. Materials and Methods
2.1. Plant Material
Fresh fruits were collected at the stage of maturity from healthy trees that were cultivated under the same pedoclimatic and cultural conditions as grown in Poros Horticulture Institute, Poros Island, Greece. The orange trees were cultivated according to the protocol of the Institute. This tree plant station consists of 800 acres, of which 300 acres are used for Citrus species cultivation. It is a horticultural station where varieties of national tree catalogs are maintained.
The physical and chemical criteria were considered to make sure the oranges were at their maturity stage. Fruit limbs and branches were green in color, their epicarps were orange, and they weighed between 150 and 200 g. Additionally, the total soluble solids (TSSs) (°Brix) were between 10 and 12, the titratable acidity (TA) (reported as % citric acid) was >0.8, and the TSS/TA ratio was above 12.
Randomly, 5 trees and 16 fruits from each tree were chosen. The fruits, after their collection, were transferred to the lab. They were washed with tap water and carefully dried with a paper towel.
2.2. Extraction of Essential Oil
The fruits were cut, and the pericarp (albedo and flavedo) was peeled off carefully and collected. Orange peels weighing 1 Kg were cold-pressed, and enough water was added. The EO was isolated from the aqueous peel oil emulsion by centrifugation (10 min at 15,000 rpm) [22]. After recording the yield of oil, it was dried with anhydrous sodium sulfate and stored at 4 °C until further analysis. Analyses were carried out within a week from the EO extraction.
2.3. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
The EO was analyzed using a Hewlett Packard Gas Chromatograph 7820A Series II Plus linked to Hewlett Packard 5977Β mass spectrometer system. The stationary phase consisted of a 30 m length, 0.25 mm id, and 0.5 µm film thickness HP5-MS capillary column. The GC oven temperature was regulated between 60 °C and 280 °C at a rate of 3 °C/min. Helium was used as a carrier gas, at a flow rate of 2.2 mL/min, split ratio 1:10, injector temperature 220 °C, and ionization voltage 70 eV. The identification of the compounds was conducted using NIST98 and Wiley mass spectral databases as well as comparison with existing literature [23,24].
2.4. Antimicrobial Activity of the EO
The in vitro antibacterial study of the studied EO was carried out by the agar dilution technique. Experiments were carried out against the Gram-positive Staphylococcus aureus (ATCC 25923) and Staphylococcus epidermidis (ATCC 12228), and the Gram-negative Escherichia coli (ATCC 25922), Enterobacter cloacae (ATCC 13047), Klebsiella pneumoniae (ATCC 13883) and Pseudomonas aeruginosa (ATCC 227853), as well as the human pathogen fungi Candida albicans (ATCC 10231), C. tropicalis (ATCC 13801), and C. glabrata (ATCC 28838). Before the assay, stock solutions were prepared (at concentrations of 10 and 1 mg/mL). Then, the stock solutions were serially diluted in broth medium (100 μL of Müller–Hinton broth or Sabouraud broth), in 96 well plates, and 1 μL of the microbial suspension (prepared in sterile distilled water) was inoculated in each well and the plates were incubated at 37 °C. The Minimum Inhibitory Concentrations (MIC) were calculated as the lowest concentrations preventing visible growth. Besides the EO, the standard antibiotics netilmicin and amoxicillin (for bacteria) and 5-fluocytosine and amphotericin B (for fungi) were tested. The experiments were repeated three times and the results were expressed as average values [25].
2.5. Saccharomyces cerevisiae Culture Conditions
Saccharomyces cerevisiae, isolated from naturally fermented grapes, was used. The strain was isolated from the Laboratory of Food Microbiology and Biotechnology (Department of Food Science and Human Nutrition, Agricultural University of Athens) and obtained the reference number LMBF Y-16. The strain was maintained in Yeast Extract Peptone Dextrose (YPD) slants, with sub-cultures every 3 months for the viability to be maintained. All cultures were performed in submerged-flask experiments. Culture conditions were employed from a previous study [26].
2.6. Determinations and Analyses
Cells were harvested by centrifugation (Heraeus Sepatech Suprafuge-22 apparatus) at 7000× g for 20 min. Distilled water was added to the cell pellet and, after vortexing for 1 min, the sample was centrifuged at 7000× g for 20 min. Cell concentration (X, g/L) was determined from the dry weight (T = 95 ± 5 °C/24 h). The concentration of the Dissolved Oxygen (DO) in the growth medium was determined with a selective electrode (oxi200 Sensodirect, Lovinbod). After stopping the shaking, the electrode was inserted into the flask and, under shaking, measurements were taken after 10 min. All trials were conducted under fully aerobic conditions [DO > 20% (v/v)]. pH measurement was conducted in a Jenway 3020 pH meter. The concentration of glucose (Glc) was carried out with the dinitrosalicylic acid (DNS) method [27]. Ethanol (Eth) concentration in the culture medium was determined with the aid of HPLC [26]. For the extraction of the oil, a mixture of chloroform/methanol 2:1 (v/v) was used on the dry biomass. The amount of oil was determined gravimetrically. Lipids were converted to methyl-esters and analyzed, as previously described [26].
In all the kinetic experiments performed, each experimental point presented in the figures and tables is the mean value of two measurements deriving from two independent experiments (SE in most of the experimental points was ≤18%).
2.7. Statistical Analysis
For conducting all analyses, three batches of EO were prepared, and each batch was analyzed three times, resulting in a total of 9 measurements. The Shapiro–Wilk test was used to examine the normal distribution of the results. The Mann–Whitney U test and the Kruskal–Wallis test were used to assess statistically significant differences between the samples. All statistical analyses were carried out using the SPSS (version 26) (SPSS Inc., Chicago, IL, USA) software.
3. Results and Discussion
3.1. Composition of the EO
The cold-pressed EO from the fresh peels (pericarps) of Citrus sinensis cv New Hall - Citrus aurantium gave a white-yellow oil with a distinct citrus odor. The yield was calculated to be 0.39% w/v. The obtained EO was analyzed with the use of GC-MS in order to examine the compounds that comprise the EO. Results are given in Table 1. As can be seen, 48 compounds were identified in the EO. Limonene proved to be the major component (76.25%), which is in accordance with previous reports [28,29]. Moreover, β-pinene, myrcene, α-pinene, valencene, and α-terpineol were also present in high amounts (4.14%, 1.12%, 1.07%, 1.15%, and 1.29%, respectively). The results are similar to that of previous studies [9,30].

Table 1.
Chemical constituents of EO from the pericarp of Citrus cinensis cv New Hall - Citrus aurantium.
3.2. Antibacterial Activity of the EO
The next step was to study the antimicrobial activity of the EO against nine human pathogenic microbes, two Gram-positive and four Gram-negative bacteria, as well as three fungi strains. According to the results presented in Table 2, the EO showed stronger activity against the Gram-positive bacteria S. aureus and S. epidermidis (MIC values 520 and 460 μg/mL, respectively) and moderate activity against the rest of the Gram-negative bacteria (MIC values 740–900 μg/mL). The EO exerted weaker activity against all Candida strains (MIC values 940–1100 μg/mL). Therefore, it can be concluded that the Gram-positive bacteria examined were found to be less resistant to the presence of the EO than the Gram-negative bacteria, while the tested fungi appeared more resistant than the bacteria. This is an asset for the examined EO, since, in many cases, EO from citrus species exhibit higher antibacterial activity towards Gram-negative species in comparison to Gram-positive species, since the latter have a thick cell membrane that bestows them with an enhanced resistance [31]. Moreover, the Gram-negative species are surrounded by an outer membrane that restricts the diffusion of hydrophobic compounds (such as limonene), thus, rendering them more resistant [32]. Additionally, the lower efficiency of the EO compared to the antibacterial and antifungal compounds can be attributed to the high percentage of limonene in the EO, since limonene is highly volatile and has low solubility in water, thus, resulting in lower antimicrobial activity of the EO [31]. In regard to the aforementioned MIC values, they are lower compared to previous reports. For instance, Pedrosa et al. reported MIC values > 2000 µg/mL for Candida tropicalis when EO and mild heating were applied [33]. Likewise, the MIC values of orange EO for S. aureus, B. subtilis, K. pneumoniae, P. aeruginosa, and E. coli were reported to be >12,800 μg/mL [34].

Table 2.
Antimicrobial activity of the EO (MIC μg/mL).
3.3. Effect of the EO on Saccharomyces cerevisiae Growth
The next step was to determine the effect of EO addition on the growth of S. cerevisiae. To this end, two concentrations of EO were studied (i.e., 0.3 and 1.5 mL/L). The yeasts grown in the absence of EO were considered as the control. Naturally, despite aerobiosis, the microorganism proceeded with glucose fermentation that resulted in the accumulation of ethanol quantities into the culture medium [Crabtree positive yeast—see, i.e., [35,36,37,38]]. As such, the biomass and ethanol produced, as well as the consumption of glucose, were examined. Results of the study are provided in Figure 1A–C and Table 3. From the results, it was apparent that the addition of the lowest examined concentration of EO did not affect the production of ethanol to a high extent. In the control experiment, ethanol production was found to be 11.9 g/L, while at the lowest examined concentration the value was found to be 11.8 g/L (p > 0.05). Likewise, the ethanol conversion yields on glucose consumed (YEth/Glc) were found to be 0.47 and 0.46 g/g, respectively (p > 0.05). After glucose depletion from the culture medium, ethanol was typically consumed and further biomass was generated [37,38]. It is the so-called “ethanol makes, accumulate consume” phenomenon, in which, despite the presence of oxygen in the culture medium, the microorganism rapidly assimilated glucose by performing alcoholic fermentation in the early growth steps; after the glucose depletion from the growth medium, the ethanol was subjected to oxidation in order for the further production of biomass to be performed (“diauxic” growth conditions).
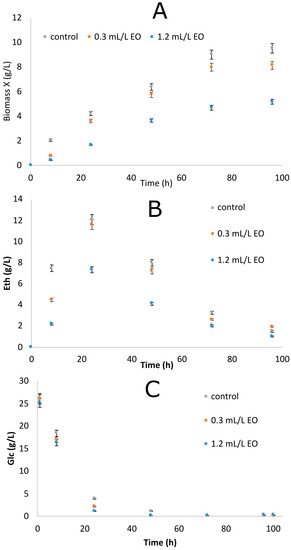
Figure 1.
Changes in biomass (X, g/L) (A), ethanol (Eth, g/L) (B), and glucose (Glc, g/L) (C) during growth of Saccharomyces cerevisiae in the absence (control) and presence of EO from the Citrus sinensis cv Citrus aurantium plant.

Table 3.
Βiomass concentration (X, g/L), ethanol concentration (Eth, g/L), and remaining glucose quantity (Glcr, g/L) at two fermentation points; the point when the maximum concentration of biomass (24) and maximum concentration of ethanol (72) was achieved. The conversion yield of ethanol produced per glucose consumed (YEth/Glc, g/g) is also presented at the point in which maximum ethanol concentration was achieved.
Both the maximum and lowest biomass concentrations achieved for the control were 9.7 and 8.1 g/L, respectively, suggesting that a small inhibitory effect of the added oil towards biomass synthesis was exerted when the EO was added into the medium. When higher quantities of EO were added into the medium (e.g., 1.2 mL/L), remarkably, lower quantities (p < 0.05) of ethanol (in terms of both absolute values in g/L and values of product synthesized per g of sugar consumed) and biomass were observed (see Table 3 and Figure 1), indicating the significant negative effect of C. sinensis cv C. aurantium EO upon microbial growth when added in high amounts into the culture medium. In contrast, despite the addition of the EO, sugar was consumed by the microorganisms at comparable rates (Figure 1C); the glucose consumption rate for all cases was nearly 1.2 g/L/h, with glucose being almost completely assimilated within 24 h after inoculation.
S. cerevisiae rapidly consumed glucose and produced non-negligible quantities of ethanol in the culture medium. Although the maximum quantity of ethanol produced in the present work (11.9 g/L) was not very high—the highest final concentrations of ethanol reported in the literature are between 60 and 110 g/L [39,40,41,42]—it was noted that even if the fermentations were not optimized, the conversion yield of ethanol produced per glucose consumed YEth/Glc (0.46–0.47 g/g in control and test culture, respectively, for p > 0.05) was very close to the theoretical maximum of about 94% of the maximum theoretically achievable value. Therefore, the present S. cerevisiae strain can be considered as a promising bio-ethanol producer [43].
In the technology of modern bioethanol production (also in wine-making or beer-making facilities), there is increasing concern in relation to the preservation of biodiversity and the employment of “new”, “novel”, wild-type Saccharomyces (or potentially non-Saccharomyces) strains (and not genetically engineered strains) as microbial candidates amenable to produce ethanol in high concentrations and conversion yields [36,41,44,45,46]. Despite the fact that in several instances, and for various strains, the application of alcoholic fermentation under aerobic conditions (due to the “Crabtree” effect) can result in lower alcoholic production compared to the anaerobic trials [46], this seems not to be the case for the wild-type LMBF Y-16 strain that presented exceptionally high conversion yields of ethanol produced per unit of glucose consumed in the current investigation. In general, the production of ethanol (in terms of both absolute values in g/L and relative values in g per g of sugar consumed) can be influenced by the aeration imposed and the addition of natural compounds (such as the EO of Citrus sinensis cv Citrus aurantium plant) into the growth medium. Furthermore, it can be influenced by parameters such as the initial concentration of sugar and the resultant oxidative stress, osmotic stress, and substrate inhibition when high concentrations are imposed; the initial C/N ratio, with a decrease in ethanol production at imposed high nitrogen-limited conditions; the recalcitrance of compounds found due to the hydrolysis of lignocellulosic materials, etc. [36,41,44,45,46].
3.4. Effect of the EO on the Lipids of Saccharomyces cerevisiae
The total cellular lipids of S. cerevisiae were extracted in order to investigate whether the addition of the essential oil influenced both the quantity and the composition of cellular lipids of the strain. The addition of the EO induced significant (p < 0.05) differentiations concerning the composition of cellular lipids for S. cerevisiae yeast (Table 4). The composition in fatty acids did not present significant modifications as a function of the fermentation time. However, the addition of the EO resulted in noticeable modifications in the cellular fatty acid composition of the investigated microorganism; the addition of EO into the medium, even in small concentrations, clearly increased the quantity of cellular C16:0, concentration, decreasing simultaneously alongside palmitoleic acid (Δ9C16:1). Furthermore, cellular Δ9C18:1 concentration slightly decreased while Δ9,12C18:2 simultaneously increased when the amount of essential oil increased into the medium. Finally, the concentration of cellular C18:0 was small, but it presented the tendency to increase with the EO addition increment. From the above data, it may be assumed that the addition of the EO of C. sinensis cv New Hall - C. aurantium had a negative effect on the Δ9 desaturase of cellular C16:0 and a positive effect on the Δ12 desaturase of cellular Δ9C18:1. Similar physiological behavior has been observed by various S. cerevisiae strains; the cellular fatty acid contents of C12:0 and C14:0 notably increased at conditions that did not favor remarkable microbial growth (high initial ethanol concentrations into the medium) [47]. In contrast to these results, in genetically engineered ethanol-tolerant S. cerevisiae strains, an increment of added ethanol into the culture medium (from 0 to 5%) noticeably increased (p < 0.05) the concentration of cellular Δ9C18:1 (from 25 to 50%, w/w, of total lipids), while, inversely, the concentrations of saturated fatty acids (C18:0 and, principally, C16:0) noticeably decreased (p < 0.05) [48]. On the other hand, the addition of various natural compounds (such as phenol-containing compounds of olive-mill wastewaters, hydroglycerolic extracts of onion peels, Origanum vulgare L. essential oil, cyclopropenic fatty acids, etc.) into the growth medium of several types of yeast strains (i.e., Cryptococcus, curvatus, Rhodosporidium toruloides, Yarrowia lipolytica, etc.) resulted in a non-systematic effect upon the fatty acid composition of the cellular lipids produced by the yeast strains. In several cases, the addition of natural compounds may result in the increase in the saturation content of the cellular fatty acids [49,50,51]. In other cases, this addition may not have serious effects on the fatty acid composition of the cellular lipids produced; it may even increase the unsaturation content of the yeast cellular lipids [51,52].

Table 4.
Fatty acid composition (%, w/w) of cellular lipids produced by Saccharomyces cerevisiae in the absence and presence of EO.
4. Conclusions
The interest in using plant-derived antimicrobial compounds is growing unabated. EOs are a highly promising source of such compounds. In this study, it was demonstrated that EO from Citrus sinensis cv New Hall - Citrus aurantium is a highly promising EO that can be used for antimicrobial purposes. It exhibits lower MIC values for common pathogenic microbes in comparison to other EOs from other species of oranges. Moreover, it was demonstrated that a concentration of 0.3 mL/L of the EO scarcely exhibits any effect on the normal growth of S. cerevisiae and its ethanol production. However, increased concentrations significantly reduced its growth. Nonetheless, the use of EOs is a promising strategy to replace existing methods for the decontamination of bio-ethanol production facilities.
Author Contributions
Conceptualization, S.I.L., S.P. and I.C.; methodology, S.I.L., C.G., S.P., P.D. and I.C.; software, E.B., V.A. and T.C.; validation, S.I.L., E.B., V.A., T.C., C.G., S.P. and I.C.; formal analysis, V.A., T.C., C.G., E.B. and I.C.; investigation, S.I.L., C.G., E.B., P.D. and I.C.; resources, S.I.L. and I.C.; data curation, V.A., T.C., C.G. and I.C.; writing—original draft preparation, T.C., C.G. and I.C.; writing—review and editing, S.I.L., O.G., S.P., E.B., V.A., T.C., C.G. and I.C.; visualization, T.C.; supervision, S.I.L. and I.C.; project administration, S.I.L. and I.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Appelhans, M.S.; Bayly, M.J.; Heslewood, M.M.; Groppo, M.; Verboom, G.A.; Forster, P.I.; Kallunki, J.A.; Duretto, M.F. A new subfamily classification of the Citrus family (Rutaceae) based on six nuclear and plastid markers. Taxon 2021, 70, 1035–1061. [Google Scholar] [CrossRef]
- Nicolosi, E.; Deng, Z.N.; Gentile, A.; La Malfa, S.; Continella, G.; Tribulato, E. Citrus phylogeny and genetic origin of important species as investigated by molecular markers. Theor. Appl. Genet. 2000, 100, 1155–1166. [Google Scholar] [CrossRef]
- Schwartz, T.; Nylinder, S.; Ramadugu, C.; Antonelli, A.; Pfeil, B.E. The Origin of Oranges: A Multi-locus Phylogeny of Rutaceae Subfamily Aurantioideae. Syst. Bot. 2016, 40, 1053–1062. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, K.; Deng, W.; Zhong, B.; Yang, W.; Chun, J. Chemical composition and antimicrobial activity of Gannan navel orange (Citrus sinensis Osbeck cv. Newhall) peel essential oils. Food Sci. Nutr. 2018, 6, 1431–1437. [Google Scholar] [CrossRef]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef]
- Razola-Díaz, M.d.C.; Guerra-Hernández, E.J.; García-Villanova, B.; Verardo, V. Recent developments in extraction and encapsulation techniques of orange essential oil. Food Chem. 2021, 354, 129575. [Google Scholar] [CrossRef] [PubMed]
- Verzera, A.; Trozzi, A.; Gazea, F.; Cicciarello, G.; Cotroneo, A. Effects of rootstock on the composition of bergamot (Citrus bergamia Risso et Poiteau) essential oil. J. Agric. Food Chem. 2003, 51, 206–210. [Google Scholar] [CrossRef]
- Bora, H.; Kamle, M.; Mahato, D.K.; Tiwari, P.; Kumar, P. Citrus essential oils (CEOs) and their applications in food: An overview. Plants 2020, 9, 357. [Google Scholar] [CrossRef]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Blázquez, M.A.; Granell, A. Volatile Compounds in Citrus Essential Oils: A Comprehensive Review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef]
- Gioffrè, G.; Ursino, D.; Labate, M.L.C.; Giuffrè, A.M. The peel essential oil composition of bergamot fruit (Citrus bergamia, Risso) of Reggio Calabria (Italy): A review. Emir. J. Food Agric. 2020, 32, 835. [Google Scholar] [CrossRef]
- Rowshan, V.; Najafian, S. Changes of Peel Essential Oil Composition of Citrus aurantium L. During Fruit Maturation in Iran. J. Essent. Oil-Bearing Plants 2015, 18, 1006–1012. [Google Scholar] [CrossRef]
- Maria, G.A.; Riccardo, N. Citrus bergamia, Risso: The peel, the juice and the seed oil of the bergamot fruit of Reggio Calabria (South Italy). Emir. J. Food Agric. 2020, 32, 522–532. [Google Scholar] [CrossRef]
- Ferhat, M.A.; Boukhatem, M.N.; Hazzit, M.; Meklati, B.Y.; Chemat, F. Cold Pressing, Hydrodistillation and Microwave Dry Distillation of Citrus Essential Oil from Algeria: A Comparative Study. Electron. J. Biol. 2016, 1, 30–41. [Google Scholar]
- Moufida, S.; Marzouk, B. Biochemical characterization of blood orange, sweet orange, lemon, bergamot and bitter orange. Phytochemistry 2003, 62, 1283–1289. [Google Scholar] [CrossRef]
- Tranchida, P.Q.; Bonaccorsi, I.; Dugo, P.; Mondello, L.; Dugo, G. Analysis of Citrus essential oils: State of the art and future perspectives. A review. Flavour Fragr. J. 2012, 27, 98–123. [Google Scholar] [CrossRef]
- Kademi, H.I.; Garba, U. Citrus peel essential oils: A review on composition and antimicrobial activities. Int. J. Food Saf. Nutr. Public Health Technol. 2017, 9, 38–44. [Google Scholar]
- Tamang, J.P.; Shin, D.-H.; Jung, S.-J.; Chae, S.-W. Functional Properties of Microorganisms in Fermented Foods. Front. Microbiol. 2016, 7, 578. [Google Scholar] [CrossRef]
- Benjamin, B.; Bakare, D.V.; Effiong, T.E. Saccharomyces cerevisiae Bio-Ethanol Production as an Alternative Source of Sustainable Energy Ethanol Production using Saccharomyces cerevisiae. Int. J. Res. Appl. Sci. Biotechnol. 2020, 7, 190–194. [Google Scholar] [CrossRef]
- Kasavi, C.; Finore, I.; Lama, L.; Nicolaus, B.; Oliver, S.G.; Toksoy Oner, E.; Kirdar, B. Evaluation of industrial Saccharomyces cerevisiae strains for ethanol production from biomass. Biomass Bioenergy 2012, 45, 230–238. [Google Scholar] [CrossRef]
- Akhtar, N.; Karnwal, A.; Upadhyay, A.K.; Paul, S.; Mannan, M.A.U. Saccharomyces cerevisiae bio-ethanol production, a sustainable energy alternative. Asian J. Microbiol. Biotechnol. Environ. Sci. 2018, 20, S200–S204. [Google Scholar]
- Ceccato-Antonini, S.R.; Shirahigue, L.D.; Varano, A.; da Silva, B.N.; Brianti, C.S.; de Azevedo, F.A. Citrus essential oil: Would it be feasible as antimicrobial in the bioethanol industry? Biotechnol. Lett. 2023, 45, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.; Jin, R.; Sun, J.; Ma, B.; Bao, X. Evaluation of mechanical-pressed essential oil from Nanfeng mandarin (Citrus reticulata Blanco cv. Kinokuni) as a food preservative based on antimicrobial and antioxidant activities. LWT 2018, 95, 346–353. [Google Scholar] [CrossRef]
- Drive, G.; Suite, A.; Stream, C.; Spectrometry, C.M.; Adams, R.P. Identification of Essential Oil Components by Gas Review: Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2005; Volume 8, ISBN 9781932633214. [Google Scholar]
- Pyrgioti, E.; Graikou, K.; Cheilari, A.; Chinou, I. Assessment of Antioxidant and Antimicrobial Properties of Selected Greek Propolis Samples (North East Aegean Region Islands). Molecules 2022, 27, 8198. [Google Scholar] [CrossRef] [PubMed]
- Widelski, J.; Graikou, K.; Ganos, C.; Skalicka-Wozniak, K.; Chinou, I. Volatiles from Selected Apiaceae Species Cultivated in Poland—Antimicrobial Activities. Processes 2021, 9, 695. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Gortzi, O.; Margeli, E.; Chinou, I.; Galiotou-Panayotou, M.; Lalas, S. Effect of Citrus essential oil addition upon growth and cellular lipids of Yarrowia lipolytica yeast. Eur. J. Lipid Sci. Technol. 2008, 110, 997–1006. [Google Scholar] [CrossRef]
- Wood, I.P.; Elliston, A.; Ryden, P.; Bancroft, I.; Roberts, I.N.; Waldron, K.W. Rapid quantification of reducing sugars in biomass hydrolysates: Improving the speed and precision of the dinitrosalicylic acid assay. Biomass Bioenergy 2012, 44, 117–121. [Google Scholar] [CrossRef]
- Gaff, M.; Esteban-Decloux, M.; Giampaoli, P. Bitter orange peel essential oil: A review of the different factors and chemical reactions influencing its composition. Flavour Fragr. J. 2020, 35, 247–269. [Google Scholar] [CrossRef]
- Farahmandfar, R.; Tirgarian, B.; Dehghan, B.; Nemati, A. Comparison of different drying methods on bitter orange (Citrus aurantium L.) peel waste: Changes in physical (density and color) and essential oil (yield, composition, antioxidant and antibacterial) properties of powders. J. Food Meas. Charact. 2020, 14, 862–875. [Google Scholar] [CrossRef]
- Ngan, T.T.K.; Nguyen, O.B.; Muoi, N.V.; Truc, T.T.; My, V.T.N. Chemical composition and antibacterial activity of orange (citrus sinensis) essential oils obtained by hydrodistillation and solvent free microwave extraction. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 991. [Google Scholar]
- Lin, X.; Cao, S.; Sun, J.; Lu, D.; Zhong, B.; Chun, J. The chemical compositions, and antibacterial and antioxidant activities of four types of citrus essential oils. Molecules 2021, 26, 3412. [Google Scholar] [CrossRef] [PubMed]
- Ben Hsouna, A.; Ben Halima, N.; Smaoui, S.; Hamdi, N. Citrus lemon essential oil: Chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 2017, 16, 146. [Google Scholar] [CrossRef]
- de Souza Pedrosa, G.T.; de Carvalho, R.J.; Berdejo, D.; de Souza, E.L.; Pagán, R.; Magnani, M. Control of Autochthonous Spoilage Lactic Acid Bacteria in Apple and Orange Juices by Sensorially Accepted Doses of Citrus Spp. Essential Oils Combined with Mild Heat Treatments. J. Food Sci. 2019, 84, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 2006, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Sarris, D.; Papanikolaou, S. Biotechnological production of ethanol: Biochemistry, processes and technologies. Eng. Life Sci. 2016, 16, 307–329. [Google Scholar] [CrossRef]
- Terpou, A.; Dimopoulou, M.; Belka, A.; Kallithraka, S.; Nychas, G.-J.E.; Papanikolaou, S. Effect of Myclobutanil Pesticide on the Physiological Behavior of Two Newly Isolated Saccharomyces cerevisiae Strains during Very-High-Gravity Alcoholic Fermentation. Microorganisms 2019, 7, 666. [Google Scholar] [CrossRef]
- Aggelis, G.; Athanassopoulos, N.; Paliogianni, A.; Komaitis, M. Effect of a Teucrium polium L. extract on the growth and fatty acid composition of Saccharomyces cerevisiae and Yarrowia lipolytica. Int. J. Gen. Mol. Microbiol. 1998, 73, 195–198. [Google Scholar] [CrossRef]
- Parapouli, M.; Vasileiadis, A.; Afendra, A.S.; Hatziloukas, E. Saccharomyces cerevisiae and its industrial applications. AIMS Microbiol. 2020, 6, 1–31. [Google Scholar] [CrossRef]
- Roukas, T. Ethanol production from non-sterilized beet molasses by free and immobilized Saccharomyces cerevisiae cells using fed-batch culture. J. Food Eng. 1996, 27, 87–96. [Google Scholar] [CrossRef]
- Roukas, T. Kinetics of ethanol production from carob pods extract by immobilized Saccharomyces cerevisiae cells. Appl. Biochem. Biotechnol. 1994, 44, 49–64. [Google Scholar] [CrossRef]
- Çaylak, B.; Vardar Sukan, F. Comparison of different production processes for bioethanol. Turk. J. Chem. 1998, 22, 351–359. [Google Scholar]
- Wang, R.; Ji, Y.; Melikoglu, M.; Koutinas, A.; Webb, C. Optimization of innovative ethanol production from wheat by response surface methodology. Process Saf. Environ. Prot. 2007, 85, 404–412. [Google Scholar] [CrossRef]
- Lin, Y.; Tanaka, S. Ethanol fermentation from biomass resources: Current state and prospects. Appl. Microbiol. Biotechnol. 2006, 69, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Sarris, D.; Matsakas, L.; Aggelis, G.; Koutinas, A.A.; Papanikolaou, S. Aerated vs non-aerated conversions of molasses and olive mill wastewaters blends into bioethanol by Saccharomyces cerevisiae under non-aseptic conditions. Ind. Crops Prod. 2014, 56, 83–93. [Google Scholar] [CrossRef]
- Roukas, T.; Kotzekidou, P. From food industry wastes to second generation bioethanol: A review. Rev. Environ. Sci. Biotechnol. 2022, 21, 299–329. [Google Scholar] [CrossRef]
- Basa, K.; Papanikolaou, S.; Dimopoulou, M.; Terpou, A.; Kallithraka, S.; Nychas, G.J.E. Trials of Commercial-and Wild-Type Saccharomyces cerevisiae Strains under Aerobic and Microaerophilic/Anaerobic Conditions: Ethanol Production and Must Fermentation from Grapes of Santorini (Greece) Native Varieties. Fermentation 2022, 8, 249. [Google Scholar] [CrossRef]
- del Castillo Agudo, L. Lipid content of Saccharomyces cerevisiae strains with different degrees of ethanol tolerance. Appl. Microbiol. Biotechnol. 1992, 37, 647–651. [Google Scholar] [CrossRef]
- You, K.M.; Rosenfield, C.L.; Knipple, D.C. Ethanol tolerance in the yeast Saccharomyces cerevisiae is dependent on cellular oleic acid content. Appl. Environ. Microbiol. 2003, 69, 1499–1503. [Google Scholar] [CrossRef]
- Moreton, R.S. Modification of fatty acid composition of lipid accumulating yeasts with cyclopropene fatty acid desaturase inhibitors. Appl. Microbiol. Biotechnol. 1985, 22, 42–45. [Google Scholar] [CrossRef]
- Chatzifragkou, A.; Petrou, I.; Gardeli, C.; Komaitis, M.; Papanikolaou, S. Effect of Origanum vulgare L. essential oil on growth and lipid profile of Yarrowia lipolytica cultivated on glycerol-based media. J. Am. Oil Chem. Soc. 2011, 88, 1955–1964. [Google Scholar] [CrossRef]
- Xenopoulos, E.; Giannikakis, I.; Chatzifragkou, A.; Koutinas, A.; Papanikolaou, S. Lipid Production by Yeasts Growing on Commercial Xylose in Submerged Cultures with Process Water Being Partially Replaced by Olive Mill Wastewaters. Processes 2020, 8, 819. [Google Scholar] [CrossRef]
- Filippousi, R.; Diamantopoulou, P.; Stavropoulou, M.; Makris, D.P.; Papanikolaou, S. Lipid production by Rhodosporidium toruloides from biodiesel-derived glycerol in shake flasks and bioreactor: Impact of initial C/N molar ratio and added onion-peel extract. Process Biochem. 2022, 123, 52–62. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).