Abstract
The increasing occurrence of toxic cyanobacteria in water sources, driven by climate change and eutrophication, is of great concern worldwide today. Cyanobacterial blooms can negatively affect water bodies and generate harmful secondary metabolites, namely microcystins (MCs), which significantly impair water quality. Various adsorbents used for MC removal from water sources were assessed in this investigation. Activated carbon constitutes the most widely used adsorbent for treating contaminated waters due to its high affinity for adsorbing MCs. Alternative adsorbents have also been proposed and reported to provide higher efficiency, but the studies carried out so far in this regard are still insufficient. The mechanisms implicated in MC adsorption upon different adsorbents should be further detailed for a better optimization of the adsorption process. Certainly, adsorbent characteristics, water pH and temperature are the main factors influencing the adsorption of MCs. In this context, optimization studies must be performed considering the effectiveness, economic aspects associated with each adsorbent. This review provides guidelines for more practical field applications of the adsorption in the treatment of waters actually contaminated with MCs.
1. Introduction
Cyanobacteria are among the most primitive and widespread life forms on Earth. Over thousands of years, harmful cyanobacterial blooms (CyanoHABs) have naturally occurred from various species of cyanobacteria derived from freshwater and marine waters. Several species of cyanobacteria are known to produce a wide variety of metabolites. Among the most commonly produced metabolites, which have proved to be harmful and resistant, are microcystins (MC), which are considered a risk to ecosystem sustainability and human health [1].
These structurally and biochemically diverse metabolites, often referred to as MCs, include terpenoids, lactones, and alkaloids that function as metal chelators and protease inhibitors [2]. MCs are well known as a toxic secondary metabolite released into the water by approximately 40 species of cyanobacteria [3]. Cyanobacteria generate toxins as a response to different environmental stressors such as eutrophication, global warming, nutrient inputs resulting from human activities as well as interspecies chemical competition between species [4,5].
Being a global environmental problem, MC pollution has been attracting increasing attention [6,7]. Numerous existing approaches have been adopted to remove CyanoHABs and their deleterious MCs from water sources. Indeed, some technologies, like coagulation–flocculation, air floatation, sedimentation, filtration, and disinfection, show high removal efficiencies of cyanobacteria cells [8,9,10,11]. These conventional methods are, however, ineffective for removing soluble MCs released into water during cell aging and/or as a result of cell deterioration caused by external stressors or by the treatment itself [12,13]. Effective treatment options that have successfully been used to remove extracellular MCs include adsorption [4], nanofiltration [14], ozonation [15], chlorination, and photolysis with ultraviolet radiation at 185 and 254 nm [16].
According to the literature, adsorption has been widely studied, and is considered one of the most attractive treatment alternatives for removing pollutants from water [17] including dissolved extracellular MCs from water sources. Adsorption exhibits significant advantages over conventional methods, including technical, economic, and environmental considerations [18], and it can be applied on an as-needed basis, since CyanoHAB problems are transient and intermittent in nature [19,20]. Activated carbon (AC) is among the commonly used adsorbents for effective MC removal, due to its porous nature and large specific surface area [18]. Several studies have shown that AC adsorption is extensively applied for removal of residual organic pollutants, including dissolved MCs from water sources, due to its high mesoporosity [21].
To date, more research has been conducted on various adsorbents for removing MCs from water. However, most of these studies have focused on AC and the remaining studies on other adsorbents have been conducted only on a laboratory scale. Recently, some generalizations have been proposed, in particular regarding factors influencing the adsorption process of MCs and the possible associated mechanisms; however, more studies are still needed in order to further optimize the adsorption process on each kind of adsorbent for an improved efficiency.
The challenge of sustainable water management is to develop environmentally friendly, economically viable and energy-efficient processes for the treatment and conservation of water resources. As a result, successful approaches will provide high pollutant removal and nutrient retrieval efficiencies while simultaneously reducing the carbon footprint, reducing waste, and safeguarding human health and the environment [22]. In this context, the interest in sustainable and environmentally friendly water treatment processes using several types of adsorbents to remove organic matter and MCs has increased in recent years.
This review provides an overview of the origins, characteristics, and effects of MCs, an evaluation of various adsorbents used for the removal MCs and the major factors affecting their adsorption, and a discussion of the possible mechanisms involved in the adsorption of MCs. The main challenges and limitations of the practical application of adsorption processes in removing MCs from water bodies will also be highlighted.
2. Origin and Characteristics of MCs and Their Impact on Water Sources
Eutrophication of freshwater resources and the presence of CyanoHABs are still expanding geographically because of anthropogenic activity and global climate change [23,24]. In marine and freshwater, the incidence and severity of blooms have significantly increased [25,26,27]. The most common bloom-forming cyanobacteria in freshwater bodies is Microcystis aeruginosa [28,29,30]. CyanoHABs can thrive under a wide variety of environmental conditions, and are especially prolific and competitive. Their formation, occurrence and frequency are controlled by many factors, such as climatic conditions and anthropogenic activities [31].
One characteristic of cyanobacteria that is becoming increasingly widely recognized is their capacity to produce toxic secondary metabolites, called cyanotoxins, or bioactive compounds that affect animal and human physiology, such as lipopolysaccharides, peptides and alkaloids [32]. Genomic studies suggest that some cyanobacteria are capable of generating over a hundred distinct bioactive molecules, with variable levels of toxicity [33,34]. Cyanotoxins are mainly produced in the exponential and stationary developmental stages, and are usually stored in cyanobacterial cells. They are commonly liberated into the surrounding waters by natural lysis of the cells after their death [32,35] or through certain water processing that leads to cell damage and toxin release [35,36].
Numerous investigations have identified the presence of cyanotoxins in surface waters and at the inlets of drinking water treatment plants [37,38,39,40,41]. According to a comprehensive national survey conducted in French surface waters consisting of 26 sampling sites over 24 months, concentrations of up to 1000 µg/L of cyanotoxins were reported [42,43]. Liu et al. [44] reported maximum dissolved cyanotoxin concentrations of 35 µg/L during the 2004 Wuxi drinking water crisis in Lake Taihu, China. McQuaid et al. [41] found significantly higher cyanotoxin values during water blooms in China, with total cyanotoxin concentrations of up to 127 µg/L of MC-LR being found. Otherwise, minimum concentrations below 1 µg/L of dissolved cyanotoxins were measured in Lake Taihu and Lake Dianchi in China, even when the concentrations of suspended Microcystis cells exceeded 109 cells/L [45]. Hence, the World Health Organization (WHO) has set an interim guideline of 1 µg/L for cyanotoxins in drinking water [46].
Cyanotoxins are classified, according to their side effects on animal cells or organs, as neurotoxins (homoanatoxin-a, anatoxin-a, anatoxin-a (S), hematotoxins-a and saxitoxins), hapatotoxins (cylindrospermopsin, MCs and nodularin), and cytotoxins (debromoaplysiatoxin, lipopolysaharide endotoxin, aplysiatoxin and lingbyatoxin). Cyanotoxins can exhibit additional effects on skin, have carcinogenic potential, and can be gastrointestinal irritants [2,47,48,49,50,51].
Microcystins (MCs) are the prevalent and dominant class of cyanotoxins identified in freshwater [31]. MCs are mainly produced by Microcystis; however, a variety of other genera have been found to produce MCs, including Planktothrix, Anabaena taxa, and Nostoc and Gloeotrichia [52,53], sometimes at relatively high concentrations [54]. The main route of exposure to MCs for humans is through drinking water, although other pathways like food, recreational waters, and dietary supplements may be important for some crops and individuals. Indeed, they are considered to be potential tumor promoters, leading to diarrhea, nausea, vomiting and even death [31,55,56,57]. MCs are also recognized as very potent acute liver toxins, with an LD50 in mice of approximately 50–500 µg/kg via intraperitoneal injection (Carmichael, 1997). In addition, they can cause several problems in drinking water treatment plants, like increased turbidity of water [58] and plugging of filters, thus reducing filter run-times and consequently increasing backwash frequencies [49]. MCs are diverse in terms of their chemical structure, and consist of more than 279 congeners, varying in their toxicity by up to one order of magnitude, as reported by Bouaïcha et al. [59]. The molecular weight of MCs ranges from 985 to 1024 Da. Their molecules are characterized by the presence of two carboxylic groups and one amino group in their molecules, enabling them to bear a negative charge at a pH between 3 and 12 [60]. Most MC congeners have the same general structure of cyclo-(D-alanine1-X2-D-MeAsp3-Z4-Adda5-D-glutamate6-Mdha7) [61,62,63] (Figure 1).
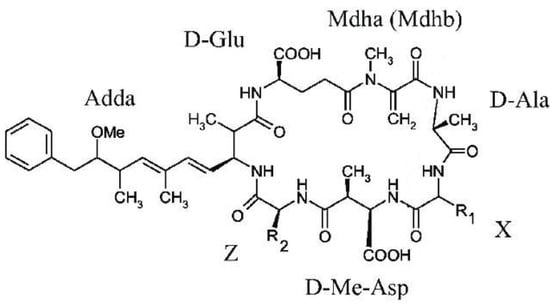
Figure 1.
General chemical structure of microcystins.
They are designated by the one-letter abbreviations to indicate the two variable amino acids present. Other minor variations are known in the chemical structure, including the lack of the methyl group and in the ADDA part of the molecule [64,65]. Therefore, the most common toxic MCs, which are produced especially by M. aeruginosa, are of particular concern in environmental research, including MC-LR, MC-RR, MC-LA, and MC-YR (Figure 2) [66]. However, other congeners may be found, such as MC-LF and MC-LW. Among the four mentioned MCs, MC-LR has been reported to exhibit the highest chemical stability, the largest molecule size, the highest solubility in water, and the most complex structure, and is thus considered to be the most toxic congener [67]. In the water intakes of six drinking water treatment plants located in southeast of Quebec (Canada), MC-LR was found to be the dominant toxin present, with a maximum concentration of 3.5 µg/L in untreated water and 0.04 µg/L in treated water [68].
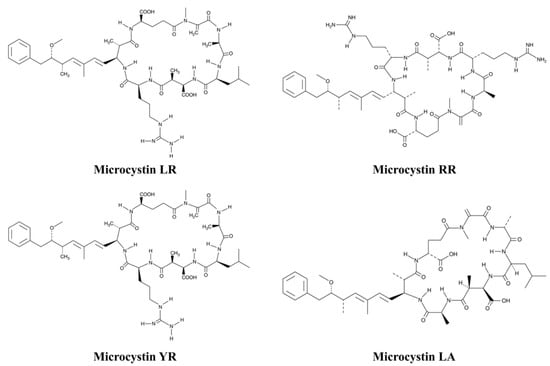
Figure 2.
Chemical structure of the common toxic microcystins’ congeners.
3. Removal of MCs by Adsorption from Water Sources
3.1. Assessment of Various Adsorbents for MC Removal
Adsorption is considered one of the most effective technologies for removing organic pollutants, including MCs [69,70,71]. A wide variety of adsorbents (e.g., AC, zeolites, and clays) have been used, showing highly efficient adsorption capacities towards MCs [72,73]. MCs are large molecules, with a volume of 2.63 nm [74], an area of 1.8 nm [75], and a molecular length of about 1.9 nm [76]; thus, only adsorbents with adequate surface area, porosity, or valence bonds can be effectively and selectively used to remove MCs.
Numerous studies have been carried out using the conditions found in water treatment plants in order to evaluate the effectiveness of adsorbents for the removal of MCs. Most of the studies related to MC adsorption have been conducted on the MC-LR congener, in particular [56,77,78]. MC-LR is a large molecule (MW = 994 Da) that is a complex aggregate of amino acids. Hence, it is hydrophobic in an aqueous solution. This is critically relevant to addressing the physical and surface chemical properties of the chosen adsorbent for MC removal [79].
As shown in Table 1, the most common adsorbent in drinking water treatment plants is AC, due to its high affinity for MCs. AC has been shown to be an effective adsorbent for the removal of a wide variety of organic and inorganic pollutants due to its exceptionally high surface area (50 to 1500 m2/g). It is also characterized by well-developed internal microscopy and a large spectrum of surface functional groups [80]. The use of AC provides several inherent advantages, including lower carbon usage rates for many applications and the ease of regenerating spent carbon. Among other advantages, it also possesses the advantage of being a less expensive material, only requiring capital expenditure for feeding and contact [81]. However, it has been reported that, after saturation, AC could be itself constitute a waste material that needs to be managed, which may limit its large-scale application in many areas. In addition, some instability due to the effect of temperature on some surface functional groups can also be observed [82]. Indeed, in solution, AC forms agglomerates with various pore sizes, increasing the surface area of the hydrophobic interior, enhancing the adsorption of organic materials such as MCs. Two types of AC have been used: powdered activated carbon (PAC) and granular activated carbon (GAC). The former is typically used as a transient treatment option to address transient contaminants, and is added to water intermittently in the form of powder or slurry as needed, while the latter is applied in fixed beds to reduce naturally occurring organics, taste and odor compounds, and synthetic organic compounds in industrial waters, and is typically used in continuous-flow column reactors [3,39]. GAC has received more consideration than PAC due to the fact that GAC is used in columns or beds, which provide higher adsorption efficiencies and more control over the process. The higher cost of GAC is frequently compensated by higher yields, especially with the continuous removal of organics. Initiation and propagation of biological activity on GAC may be beneficial in breaking out additional organic molecules [39].

Table 1.
An overview of adsorbent performance in removing MCs.
Depending on the nature of the feedstock and the preparation method, the properties of the AC and its adsorption capacity towards MCs vary significantly. According to the literature, it has been confirmed that MCs are preferentially adsorbed into mesopores (2 to 50 nm) rather than macro- and micropores due to their size (1.33 to 2.94 nm) [31]. Many of the studies shown in Table 1 reported that coal- and wood-based coagulants are the most effective for MC adsorption due to their large mesopore volume. For instance, Cook and Newcombe [83] investigated the performance of wood and charcoal PACs in the adsorption of MC-RR, MC-LR, MC-YR, and MC-LA for 3 days at pH 6.0–8.5. The homogeneous surface diffusion model was used to predict the kinetics of MC-LR and MC-LA adsorption. The results showed differences in the adsorption of each of the studied congeners, with removal efficiencies for both adsorbents in the following order: MC-RR > MC-YR > MC-LR > MC-LA. Equilibrium and batch kinetic tests show that MC-LR was more readily adsorbed than MC-LA, and the percentage of removal was found to be independent of the initial concentration of each MC congener. According to the authors, the number of negatively charged carboxyl, D-glutamate and -methylaspartate groups and positively charged basic amino groups is controlled by the net charge of the molecule. Similarly, Ho et al. [56] also reported that MC congeners exhibited different adsorption behaviors in contact with PAC in the order MC-RR > MC-YR > MC-LR > MC-LA, as reported by Cook and Newcombe [83]. Further studies have shown that wood AC presents higher MC adsorption due to the mesoporous nature of the carbon material [84,85,86]. Donati et al. [87] similarly evaluated MC-LR removal using two coconut shell-based PACs, three charcoal-based PACs, two wood-based PACs, and one peat moss-based PAC. Although all PACs gave typical isotherms of high-affinity monolayer adsorption, the level of MC-LR adsorption varied greatly between the PACs and showed a distinct trend. The results demonstrated that wood-based PAC had the largest volume of micropores and mesopores, followed by coal, coconut shell- and peat moss-based PACs. The findings indicated that the most effective adsorption was achieved by wood-based PACs, with a maximum adsorption capacity of 280 µg/mg followed by charcoal-based PACs with adsorption capacities of 116, 75 and 70 µg/mg MC-LR per mg carbon. The lowest maximum adsorption capacities were given by coconut shell-based PACs (20 and 40 µg/mg), followed by peat moss-based PACs (20 µg/mg).
Next to AC, biochar (BC) is increasingly being recognized as a potential technical and ecological adsorbent for organic and inorganic pollutants from contaminated waters. BC is a pyrogenic black carbon derived from the thermal conversion of feedstocks in O-limited conditions [88,89,90]. Feedstocks used for BC come from a wide range of sources, and are readily available in large quantities and at low cost [91]. BC has a relatively large surface area, a porous structure, high negative charge, and is resistant to degradation [92,93], and it is believed to be a favorable adsorbent for contaminant management in the aquatic environment [94,95,96]. It is also characterized by high electrical conductivity and stability, even at lower production temperatures [97]. BC production requires less energy compared to AC, which requires higher temperature and an activation process [98]. Furthermore, BC can be reused several times after regeneration, and may become an ideal resource for environmental technologies in water treatment due to its economic and environmental benefits [99].
Several BCs derived from diverse feedstock have been evaluated with respect to the removal of MCs from contaminated waters [100,101,102], and some possible mechanisms responsible for MC adsorption have been proposed [100,101]. Nevertheless, few studies have focused on the ability of BC to adsorb MCs in drinking water and wastewater. Among the numerous structural congeners of MCs, only MC-LR has been investigated in adsorption studies on BC. For instance, Zheng et al. [103] evaluated the ability of iron-activated BCs (FA-BCs) to remove MC-LR. Their findings demonstrated that the surface area and adsorption capacity of the FA–BC ratio improved when the iron impregnation ratio was increased from 86 m2/g and 0.76 mg/g to 835 m2/g and 9.00 mg/g.
It has been reported that BC properties are strongly affected by the pyrolysis conditions, with temperature being the dominant factor. Under conditions of higher pyrolysis temperatures, BC provides higher surface area and aromaticity, resulting in a higher capacity for MC adsorption due to the electron donor–acceptor interaction [104]. Li et al. [100] showed that manure-based BC produced at 600 °C exhibited better MC-LR adsorption capacity, owing to a higher mesopore volume. In the same study, the manure-based BC at a pyrolysis temperature of 600 °C showed increased adsorption capacity for MC-LR due to the fact that the ash rendered polar groups and organic matter readily available for adsorption. Li et al. [101] demonstrated that BC produced at 300 °C exhibited very good adsorption capacity, although it possessed a low surface area. Wei and Lu [105] also investigated the performance of BC at different pyrolysis temperatures (300, 400, 500, 600 and 700 °C) for the removal of MC-LR. BC pyrolyzed at 700 °C was found to have a specific surface area of 360.9 m2/g and a maximum adsorption capacity of 10.96 µg/g, which was twice as high as that observed for BC pyrolyzed at 300 °C. For all adsorbents, the adsorption kinetics was observed to show a good fit with the pseudo-second order kinetic model (R2: 0.94–0.99), and the adsorption equilibrium was well depicted with the Freundlich model (R2 > 0.9). Similarly, Liu et al. [106] studied the ability of BC to sanitize water from different MC congeners under different preparation conditions using a spent mushroom substrate—produced under CO2 (BCs) or N2 gas (BNs)—under pyrolysis temperatures of 300 and 600 °C. The results showed considerable variation in the distribution coefficients Kd (0.98–19.2 L/kg) between MCs (MC-YR > MC-RR > MC-LR) and BCs (BC600 > BN600 > BC300 > BN300), depending on the combined effects of hydrophobicity, electrostatic attraction, H-bonding, cation bridging, and the number of adsorption sites on the BCs. The adsorption of MCs was a pH-dependent and endothermic physisorption process that followed pseudo-second-order kinetics (R2 = 0.99) and the linear isotherm model (R2 > 0.88) [106].
Even with increases in the application of BC in water and wastewater treatment [107], many deficiencies still exist in the use of BC as a convenient adsorbent for MCs removal. Most of the existing data concentrate on MC-LR adsorption, whereas few are available for other MC congeners. In this context, a comparative study on the adsorption of different microcystin congeners by BCs is necessary, as they usually exist as a mixture of various congeners in the environment [108]; therefore, it is imperative to study the co-adsorption of various MCs on BCs. In addition, an understanding of the underlying mechanisms associated with the specific properties of microcystins needs to be established.
Furthermore, mesoporous carbon (e.g., from silica) has also been shown to possess important adsorption capacity towards MC-LR [109]. Mesoporous carbon is a new material considered to possess interesting properties, including high specific surface area and pore volume, tunable pore sizes and geometries, and good chemical and mechanical stability [110]. Park et al. [109] investigated various types of mesoporous carbon: two types of commercial mesoporous carbon (MC1 and MC2), and MC3 synthesized at 900 °C. The results showed that MC1 was the most suitable type for MC-LR removal, with a maximum adsorption capacity of 35,670.49 mg/g. The MC kinetic process was found to fit the pseudo second-order model (R2 = 0.98), while the equilibrium isotherm models demonstrated the best fit for the Langmuir model for the equilibrium data of MC1 and MC2, suggesting that single-layer adsorption had occurred. Nevertheless, only a small number of investigations have been performed on mesoporous carbon as an adsorbent for MC-LR [111,112,113]. It has been reported that mesoporous carbon can serve as an additional adsorbent for MC-LR removal, because it is primarily composed of mesopores (2–50 nm) and has a unique interconnected channel structure, high stability, and large pore volume. Teng et al. [111,112] studied the adsorption of MC-LR on mesoporous carbon, and demonstrated an adsorption capacity 10 times greater for MC-LR than that of PAC. Moreover, Zhang and Jiang [114] further noted that the use of mesoporous carbon decreased the MC-LR concentration to <1 mg/L, due to its possessing numerous accessible mesopores, despite using lower doses of mesoporous carbon than what was used for PAC. Huang et al. [113] functionalized mesoporous carbon to increase its adsorption capacity for both MC-LR and MC-RR. The results showed that functionalized mesoporous carbon achieved its maximum adsorption capacity of 2360 and 2868 µg/g at pH 7, according to the Langmuir model for MC-LR and MC-RR, respectively. Park et al. [109] showed that mesoporous carbon contained a mesoporosity in the range of 81–91.3%, suggesting that the mesoporous structure had been properly constructed. The mesoporous carbon was effective for the removal of MC-LR to below the WHO guideline level after a 10 min reaction with a carbon dosage of 20 mg/L.
3.2. Factors Influencing the Adsorption of MCs
3.2.1. Characteristics of Adsorbent
To understand the adsorption process, and to select a suitable adsorbent for a target cyanotoxin, an analysis of the physico-chemical properties of the adsorbent is necessary.
The adsorption efficiency of an adsorbent towards a particular organic compound is determined not only through its concentration in solution, but also particularly through its specific surface area, pore size distribution (internal pore structure), and surface chemistry [121,122,123,124]. The affinity of the adsorbent for adsorbing MCs is also controlled by the size and the conformation of the molecule [83,87]. Donati et al. [87] and Pendleton et al. [85] have suggested that the size and conformation of MC molecules, as well as adsorbent pore volume, seem to be the dominant parameters controlling MC adsorption, with minimal influence of electrostatic interactions due to the hydrophobicity of the molecule and the low number of ionizable functional groups.
It has been stated that adsorbents with a specific surface area of approximately 670 m2/g and above are efficient for the adsorption of MCs [125]. Nevertheless, the effectiveness of MC adsorption is strongly related to the pore size distribution of the targeted adsorbent, rather than the absolute value of its surface area [126]. For instance, AC has a large surface area, generally between 400 and 1500 m2/g, giving it a large number of available adsorption sites [31]. It has been reported that AC with a surface area that is mainly distributed as secondary micropores with a diameter between 0.8 and 2 nm can be used for the adsorption of MCs, and smaller mesopores can contribute to further improving the adsorption efficiency [13,121]. In contrast, other studies have shown that AC possesses a limited ability to adsorb the relatively large MC-LR due to its microporous structure (pore size < 2 nm) [109]. Similarly, prior studies have reported that both micropores and macropores (>50 nm) are inefficient for the removal of MC-LR, while mesopores contribute considerably in the adsorption process [31,87,127]. Pelekani and Snoeyink [127] confirmed that an important property influencing the adsorption process is the pore size distribution (PSD) of the adsorbent, which determines the fraction of the total pore volume that is accessible for the adsorbate. An adsorbent with a sufficiently large PSD can adsorb both large and small organic matter, including MCs, with an adsorption efficiency that depends on direct competition for the available adsorption sites [128,129]. Several studies have stated that mesoporous adsorbents (pore size 2–50 nm) are more efficient for adsorbing MCs than macropore-dominated adsorbents [56,85], as mesopores more readily accommodate MCs that are around 1–3 nm in size [85,87]. Wood-based AC, with a relatively high mesopore distribution of about 0.8 cm3/g, has been reported to have a greater removal extent and faster removal rate towards MCs than charcoal, coconut, and peat-based AC, which have mesopore volumes of less than 0.2 cm3/g [56,85,87].
It is important to ensure that the target adsorbent PSD is adequate for the size of the target pollutants, particularly when they are present as a mixture of substances of different molecular weights. Alternatively, both large and small organic matter, including MCs, can be effectively adsorbed on AC exhibiting a large PSD. In this case, adsorption efficiency depends on the existence of direct concurrence for adsorption sites [128,129]. Thus, the adsorption capacity depends on the accessibility of the organic molecules to the internal surface of the adsorbent, which is a function of their size [78]. Newcombe et al. [130] claimed that the adsorption strength increases with decreasing pore size owing to the increased number of contact points between the adsorbate and the adsorbent surface.
The surface chemistry of the adsorbent is another important parameter affecting the adsorption rate of MCs. The surface of the adsorbent may have an acidic character as a result of functional groups, namely carboxyls, phenols, lactones, and acid anhydrides [131,132,133], dissociating to produce a negative surface charge [134]. Similarly, several studies have reported that various adsorbents possess a fundamental character that can be ascribed to delocalized π-electrons within the basal planes, nitrogen functionalities, and surface oxygen groups, such as pyrones [131], chromenes [135], diketones, and quinones [136], which accept protons from solution and are responsible for the positive charge of the surface.
Therefore, according to the source of the carbon and the activation method, the porosity and the surface activity vary. Hence, the appropriate selection of adsorbent must reflect the nature of the MCs to be removed, taking into consideration the chemical properties of each adsorbent used.
3.2.2. pH
The pH of the water influences adsorption processes significantly, since it determines the adsorbent surface charge and the speciation and degree of ionization of the adsorbate, and directly affects electrostatic interactions [137]. Several studies on the effect of pH on the adsorption of MC-LR have demonstrated that the adsorption increases with decreasing pH value (from 8 to 2.5). For instance, Zhu et al. [78] noted that high pH values, which resulted in an electrostatic repulsion between the negatively charged MC molecules and AC, were responsible for the low adsorption capacity of PACs. Nevertheless, the electrostatic repulsion disappeared at low pH levels, and the MC molecules became electrically neutral, thus enhancing their adsorption onto PACs. According to [79], the weak acidic functional groups of MCs became more available for adsorption under acidic conditions. A further study performed by Hnatukova et al. [137] showed that the adsorption efficiency of M. aeruginosa on two GACs with pHPZC of 5.5 and 6.7 was approximately two times at pH 5 that at pH 8.5 [135]. Lanaras et al. [76] reported that at low pH, the MC molecules clustered together, and the size of the molecules decreased, thus increasing the available adsorption surface area of the targeted adsorbent. Meanwhile, AC contains more acidic functional groups under acidic conditions. The adsorption capacity of AC increased due to the formation of hydrogen bonds between MC molecules and the surface of AC [76]. On the other hand, increasing the pH leads to acidic oxygen functional groups on the GAC dissociating, thus providing a negative charge, whereas the negative charge increased in MC molecules as a result of the dissociation of functional groups, such as -COOH- and -S-, leading to strong electrostatic repulsive interactions [135]. The same study studied the efficiency of two commercially adsorbents (Filtrasorb 200 and Norit 1240) in removing M. aeruginosa and organic matter including MCs. The two carbons studied exhibited positive and negative charges as a function of pH. The Filtrasorb 400 carbon had a pHpzc of approximately 6.7, while the N1240 was found to be a more acidic carbon, with a pHpzc of approximately 5.5. Consequently, both carbons exhibited a net positive charge under applied acidic conditions of pH 5 and a net negative charge at a basic pH of 8.5. Bjelopavlic et al. [132] also noted that solution pH influenced the characteristics (protonation/deprotonation) of surface functional groups, thus determining the surface charge of adsorbent surface and therefore the electrostatic interaction between the adsorbent and the MC molecules. Therefore, with the function of pH, positively or negatively charged functional groups predominate in MC molecules [136].
3.2.3. Temperature
The adsorption of MCs has also been shown to be influenced by the temperature of the adsorption medium [133,138]. It has been noted that the solution temperature has a strong effect on the kinetic energy, on the solubility of the adsorbed molecules, and on the solution viscosity, which may affect the adsorption of organic molecules [133,138]. Zhu et al. [78] demonstrated that the adsorption of MCs (MC-LR and MC-RR) at 15 °C was higher than that at 25 or 35 °C on wood carbon. In the same study, both Gibbs and van’t Hoff equations were adopted to assess the effects of temperature on the adsorption equilibrium of MCs on wood carbon. On the basis of the results, it was deduced that the adsorption process of MC-RR and LC-LR was dominated by physical adsorption. A negative Gibbs free energy was found, suggesting that the adsorption process of MCs occurs spontaneously. The same study reported that variations in adsorption enthalpy were negative, suggesting that the adsorption process is exothermic in nature. Therefore, using a lower temperature would promote the adsorption of MCs. The same conclusion has also been reported by Moreno-Castilla [133] and Sebben and Pendleton [139]. Park et al. [109] also investigated the adsorption efficiency of MCs on mesoporous carbon using thermodynamic models. The results indicated that MC-LR adsorption was exothermic nature on mesoporous carbon, as shown by the negative value of enthalpy variation ( = −9.63 to 72.23 kJ/mol). The negative value of the change in Gibb’s free energy (= −13.73 to 17.41 kJ/mol) indicated that MC-LR adsorption on mesoporous carbon was a spontaneous reaction that demonstrated a higher affinity for the adsorption of MC-LR on mesoporous carbon at lower temperatures. The highest capacity of adsorption was achieved at 25 °C, although the adsorption of MC-LR on mesoporous carbon was not greatly affected when the temperature varied within the range of 25–45 °C.
Nevertheless, both positive and negative effects of temperature changes on adsorption capacity can occur, mainly depending on the type of adsorbate and adsorbent [140,141]. Conversely, other studies have indicated an increase in the amount of MCs adsorbed on AC with increasing temperature; thus, the process is endothermic [142]. On the other hand, the negative value of the adsorption entropy supported a decrease in the randomness of the MC molecules on the surface of PAC, which is attributed to structural changes in the adsorbate and the adsorbent. Consequently, it was concluded that the adsorption capacities of the MCs decreased with increasing temperature. The enhanced adsorption efficiency at higher temperatures can be explained by the fact that with increasing temperature, molecules interact with each other more easily. This behavior leads to the formation of larger associations that are able to bind to active centers on the surface of adsorbent [138,143,144]. Indeed, a switch from monolayer to multilayer adsorption may occur at higher temperatures using higher concentrations of a given substance [145]. Additional studies have also indicated that some organic compounds must first break the bonds of other molecules in the adsorbate before being adsorbed onto the target adsorbent. This bond breaking process between the treated water and the adsorbate may require energy input, such as an increase in the temperature of the solution [146,147].
Several investigations have been conducted to evaluate the effect of different temperatures on MC adsorption, concluding that the process can be either exothermic or endothermic. These investigations, though, seldom explain the possible reasons for the thermodynamic behavior of the substances studied. At times, the outcomes are incongruent, and further research on this issue is needed. The Langmuir and Freundlich isotherm models remain the two most commonly used models regarding adsorption in solution. The Langmuir isotherm model is mainly adapted to solution adsorption data when a plateau is observed in the adsorbed amount with a decreasing equilibrium concentration of the solution. This suggests that maximum sorption corresponds to a monolayer adsorption of molecules on a solid surface possessing a finite number of sorption sites [148]. Meanwhile, the Freundlich isotherm model is often suited to solution adsorption when no apparent plateau can be observed in equilibrium solutions at relatively higher concentrations. It is an empirical model and indicates whether the adsorbent presents a heterogeneous surface [149]. These models have been widely used to address the effects of temperature on the adsorption process. According to Rabe et al. [140], temperature affects not only the adsorption equilibrium, but also the adsorption kinetics. Therefore, a thorough analysis of the adsorption thermodynamics can shed light on the molecular interactions between the adsorbate and the adsorbent at the phase interface.
3.3. Possible Adsorption Mechanisms
The interaction of MCs with different types of adsorbents is associated with different mechanisms, which are interrelated with the properties of the adsorbent, the characteristics of the MC molecules, and the solution characteristics [60,133].
The mechanisms most frequently involved, and which have been reported to predominate in the adsorption of MCs, are associated with the non-specific dispersion forces [123,132,133], such as van der Waals forces, which are universal short-range attractive forces that act between all kinds of particles. Other types of interactions like hydrogen bonds and interactions between hydrophobic parts of the carbon skeleton (i.e., graphitic surfaces lacking any functionality), which are mainly due to the aversion of the hydrophobic species to water and not to their attraction to the surface, have also been reported [133]. Such interactions are highly prevalent, and are vera important, since they occur between all types of particles. Strong but close interactions may arise via electrostatic forces and hydrogen bonds between polar or polarizable molecules of the adsorbate and the predominantly oxygen-containing groups located in the pore structure of the adsorbent or on the edges of the graphene carbon layers [150]. They are linked to particular sites in the structures of the adsorbent and the adsorbate, and are thus considered specific.
Different types of interactions that could be involved in the adsorption of MCs have been described in the literature (Figure 3) [122,132,133,151]. It has been reported that the interaction between MCs and AC are restricted to electrostatic and hydrophobic interactions [56,60,83]. Although similar in molecular size, the MC congeners interact with the adsorbent in a manner corresponding to their relative hydrophobicity, with the more hydrophobic forms (e.g., MC-LF) being more rapidly adsorbed, and to a greater extent than the less hydrophobic forms (e.g., MC-LY). Meanwhile, electrostatic interactions occur between the MCs’ ionic functional groups and the charged functional groups on the adsorbent (for instance, the oxygen-containing groups) [152]. Campinas and Rosa [60] reported that electrostatic interactions between MCs and ACs are affected by ionic strength; an increase in ionic strength leads to an increase in adsorption due to the reduction in electrostatic repulsion. Several studies have reported that the ionic strength effect depends on adsorbate variables, including surface concentration and molecular size [153,154,155]. Indeed, adsorption carried out by attractive electrostatic interactions can be expected to decrease with increasing ionic strength in the presence of low concentrations. Randtke and Snoeyink [154] reported that an increase in salt concentration can increase the adsorption capacity of GAC significantly due to the ionic strength effect, which depends on the molecular size of the adsorbate. Similarly, an additional study performed by O’Connor et al. [156] stated the influence and the dominance of electrostatic interactions during the adsorption of amino acid onto mesoporous silica. The results showed a pH change for solutions with a final pH > 5, which was somewhat lower than that at a final pH of 4.5–5. This may indicate a shift in the adsorption mechanism with less ion exchange and more electrostatic binding as the silica surface becomes more deprotonated.
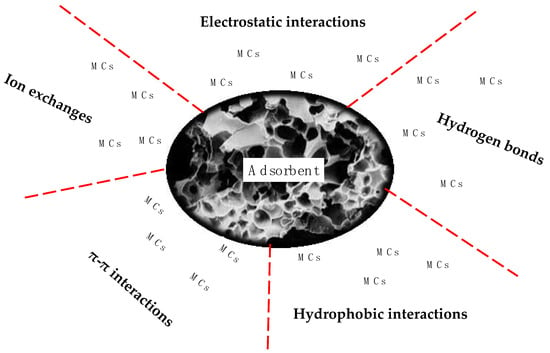
Figure 3.
Potential mechanisms for MC adsorption.
Hydrophobic and π–π interactions have been recognized as an additional mechanism controlling the adsorption of MCs onto adsorbents [20,135,157,158]. Hydrophobic interactions are a result of the tendency of molecules to adsorb onto a carbon surface instead of remaining dissolved in water [130]. Attractive π–π interactions are formed between delocalized π-electrons from the polyaromatic basal planes of the adsorbent and π-electrons from the aromatic structures of the MC molecules [135,158]. Substantive electrolytes also play an important role in MC adsorption, and this function is apparently different for different MC congeners. According to Campinas and Rosa [60], adsorption of MC-LR on PAC from water containing CaCl2 showed higher adsorption efficiency than from water containing KCl. The same study confirmed that the increase in electrolyte concentration screened electrostatic interactions, resulting in improved adsorption kinetics, especially under neutral to acidic pH conditions, for the following negatively charged MC-LY, MC-LW and MC-LF.
Several studies have shown that general hydrogen bonding is also prevalent in the adsorption of very low molecular weight cyanobacterial components, including MCs [125,159,160]. Hydrogen bonds are typically formed between the protonated functional groups of the adsorption participants, as has been reported for the N heteroatoms of the guanidyl group in arginine molecules and the hydroxyl groups of AC [139,158]. Similar trends were observed for the adsorption of the abundant amino acids in MCs using two types of AC (Filrasorb (FTL) and Picabiol (PIC)). The results showed that the dominant adsorption mechanism was electrostatic interactions for both FTL and PIC. These interactions were weaker due to the lower negative charge of FTL caused by the smaller amount of acidic functional groups; therefore, the overall adsorption efficiency was lower [20].
Therefore, the contribution of the above interactions to the adsorption of MCs on the target adsorbent remains questionable, since each adsorbent has its own characteristics, and each solution has its own specific characteristics. Therefore, analyzing the relative importance of molecular size and surface concentration for the effect of ionic strength is equally important. I would also be of interest to consider model compounds with different molecular sizes in order to understand whether the behavior of MCs can be predicted on the basis of surface concentration and molecular size.
4. Real World Application of Adsorption for the Removal of MCs: Performances and Limitations
The control of cyanobacteria in raw water supplies, and thereby MCs in the drinking water system, can be dealt with at several different points, from source to tap. Preventing cyanobacterial growth by preventing natural eutrophication of the water is the most important long-term management objective for cyanobacterial control. However, the final point at which cyanobacteria and MCs can be controlled is within the water treatment system. Once the MCs are dissolved in water, adsorption remains the most widely used method for removing MCs, as it requires an operational process that is less complicated than conventional treatment techniques, and may offer higher removal efficiency. Several adsorbents have been studied to sanitize water of MCs, including AC [56,77,86] and BC [100,118,126].
To date, AC remains the most widely used adsorbent in drinking water treatment plants. It is used, when needed, to remove the extracellular MCs released in the water at the final step of the water treatment process [119,161]. The effectiveness of AC for the removal of MCs varies depending on the origin of the carbon, contact time, and the initial concentration of the MCs, although effective removal of MCs generally requires a high dosage of AC, sometimes ranging from 20 to 30 mg/L [31,56,119,161]. Nevertheless, the effectiveness of AC can be affected by the properties of the water, i.e., natural organic matter content, pH, the presence of anions, and temperature [78,84,162,163]. Depending on the structural properties of AC and its interaction with the target MC molecule, its performance can sometimes be limited. To further improve MC removal efficiency, several treatment methods have been used in combined systems. By combining coagulation–flocculation, adsorption, and ultrafiltration, drinking water quality has been effectively improved in terms of MC removal [4]. Within laboratory-scale experiments, PAC added simultaneously during the coagulation processes at 5 mg/L made it possible to achieve a removal efficiency of up to 34% for MCs and over 50% for neurotoxins. At the pilot scale, a PAC dosage of 20 mg/L followed by a pre-ozonation treatment reduced M. aeruginosa bloom-derived MCs by more than 90%. Further studies have confirmed that PAC doses greater than 20 mg/L are often required to achieve nearly complete removal of MCs [164,165,166]. A further study concluded that the combination of these methods with adsorption may improve DOC and MC removal, while also resulting in membrane fouling [167]. However, the main drawback of these multi-step processes is the potential release of intracellular MCs during the treatment, leading to the use of increased doses of adsorbent and thus increased process costs [168]. During the real application of adsorption for the removal of MCs, the presence of natural organic matter in contaminated water should also be taken into consideration, as organic substances can compete with MC molecules for the available adsorption sites on the adsorbent surface, inducing a reduction in adsorption efficiency [162]. Most of the studies performed so far have been focused on the application of adsorption to remove MCs in less complex media. These studies lack a systematic approach for evaluating the removal efficiency of MCs in real conditions with more complex waters. Additional research is still needed to improve our knowledge of the effectiveness of adsorption for removal of MCs from real water sources, both in the laboratory and at field scale. A rapid evaluation of treatment efficiency in drinking water treatment plans is essential for ensuring the safety of the drinking water supply. In the case of adsorption columns, operating periods are strongly influenced by natural organic matter, and thus the systems must be regularly monitored to confirm their performance [169]. Restrictions on the use of AC have led to increased interest in developing alternative carbon adsorbents to sanitize water from MCs, including graphene oxide, carbon nanotubes, synthetic polymers and biomaterials [115,117,170,171], demonstrating not only better adsorption capacity, but also rapid adsorption kinetics [86,115].
Compared to the large literature on AC, research on other adsorbent materials is rather limited, and consists primarily of laboratory-scale studies. Therefore, knowledge concerning the extent to which environmental factors and characteristics of MCs affect their adsorption behavior is still limited. Nevertheless, some of these alternative carbon-based adsorbents demonstrate high potential for the effective removal of MCs.
5. Conclusions and Outlook
Climate change and related environmental factors are leading to widespread blooms of toxic cyanobacteria in fresh water, making it more challenging to protect water supplies. MCs are frequently detected in various water supplies, and can result in great risk to various ecosystems and impair human health. Adsorption has been reported as a suitable technology for eliminating various MC congeners. Nevertheless, the adsorption process is influenced by various factors, such as surface area, porosity, surface chemistry of the adsorbent, water characteristics and pH, and type of MCs. Adsorbents with enhanced mesoporosity have been reported to be more favorable for adsorbing MCs. The water pH and ionic strength are the main properties controlling the adsorption efficiency. Adsorption temperature represents an additional consideration that typically impacts adsorption as a physical process, although for MC adsorption, insufficient studies are available to clearly demonstrate the effects of temperature. Electrostatic interactions, hydrogen bonds, the hydrophobic effect and π–π interactions have been recognized as mechanisms implicated in MC adsorption. The combination and scope of these interactions are highly correlated with the pH and ionic strength of the contaminated water. However, additional research is still needed to more deeply understand the primary mechanism involved in the MC adsorption process. AC has been reported to be the most widely used adsorbent for removing MCs from contaminated water. Other studies have demonstrated, however, that alternative adsorbents provide higher efficiency on removing MCs than AC. The difficulties associated with regeneration and spent AC disposal are reasons to move in the direction of using environmentally friendly adsorbents based on biomass waste (e.g., BC) and modified sorbents (e.g., graphene oxide, functionalized mesoporous carbon, silica, and coated magnetic nanoparticles). However, the cost of synthesizing modified adsorbents is higher than that of AC, and most research to date has been limited to the laboratory scale. To this end, optimization studies must be performed considering the economic and environmental aspects associated with the treatment technology. Therefore, more investigations must be performed in the field in order to determine more practical applications using continuous flow column experiments involving real water with a multicomponent composition.
Author Contributions
Conceptualization, W.E.B. and M.L. (Manfred Lübken); methodology, W.E.B. and G.E.; writing—original draft preparation, W.E.B.; writing—review and editing, W.E.B., G.E. and M.L. (Manfred Lübken); visualization, A.Y., M.D. and A.O.; supervision, M.L. (Manfred Lübken), M.L. (Mohammed Loudiki) and A.Y.; funding acquisition, M.L. (Manfred Lübken). All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported by the German Academic Exchange Service (DAAD, No. 57552339). The APC was funded by the Open Access Publication Funds of the Ruhr-Universität Bochum.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ibelings, B.W.; Chorus, I. Accumulation of Cyanobacterial Toxins in Freshwater “Seafood” and Its Consequences for Public Health: A Review. Environ. Pollut. 2007, 150, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.W.; Lazorchak, J.M.; Howard, M.D.A.; Johnson, M.V.V.; Morton, S.L.; Perkins, D.A.K.; Reavie, E.D.; Scott, G.I.; Smith, S.A.; Steevens, J.A. Are Harmful Algal Blooms Becoming the Greatest Inland Water Quality Threat to Public Health and Aquatic Ecosystems? Environ. Toxicol. Chem. 2016, 35, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Westrick, J.A.; Szlag, D.C.; Southwell, B.J.; Sinclair, J. A Review of Cyanobacteria and Cyanotoxins Removal/Inactivation in Drinking Water Treatment. Anal. Bioanal. Chem. 2010, 397, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Kajjumba, G.W.; Ejjada, M.; Masrura, S.U.; Marti, E.J.; Khan, E.; Jones-lepp, T.L. Recent Advancements in the Removal of Cyanotoxins from Water Using Conventional and Modified Adsorbents—A Contemporary Review. Water 2020, 12, 2756. [Google Scholar] [CrossRef]
- Lehman, P.W.; Kurobe, T.; Lesmeister, S.; Baxa, D.; Tung, A.; Teh, S.J. Impacts of the 2014 Severe Drought on the Microcystis Bloom in San Francisco Estuary. Harmful Algae 2017, 63, 94–108. [Google Scholar] [CrossRef]
- Henao, E.; Rzymski, P.; Waters, M.N. A Review on the Study of Cyanotoxins in Paleolimnological Research: Current Knowledge and Future Needs. Toxins 2019, 12, 6. [Google Scholar] [CrossRef]
- Hu, C.; Rzymski, P. Programmed Cell Death-like and Accompanying Release of Microcystin in Freshwater Bloom-Forming Cyanobacterium Microcystis: From Identification to Ecological Relevance. Toxins 2019, 11, 706. [Google Scholar] [CrossRef]
- Serrà, A.; Philippe, L.; Gómez, E. Removal of Cyanobacteria and Cyanotoxins in Waters. Toxins 2021, 13, 636. [Google Scholar] [CrossRef]
- Gitis, V.; Hankins, N. Water Treatment Chemicals: Trends and Challenges. J. Water Process Eng. 2018, 25, 34–38. [Google Scholar] [CrossRef]
- El Bouaidi, W.; Essalhi, S.; Douma, M.; Tazart, Z.; Ounas, A.; Enaime, G.; Yaacoubi, A.; Loudiki, M. Evaluation of the Potentiality of Vicia Faba and Opuntia Ficus Indica as Eco-Friendly Coagulants to Mitigate Microcystis Aeruginosa Blooms. Desalin. Water Treat. 2020, 196, 198–213. [Google Scholar] [CrossRef]
- El Bouaidi, W.; Libralato, G.; Douma, M.; Ounas, A.; Yaacoubi, A.; Lofrano, G.; Albarano, L.; Guida, M.; Loudiki, M. A Review of Plant-Based Coagulants for Turbidity and Cyanobacteria Blooms Removal. Environ. Sci. Pollut. Res. 2022, 29, 42601–42615. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.W.K.; House, J.; Velzeboer, R.M.A.; Drikas, M.; Burch, M.D.; Steffensen, D.A. The Effect of Ferric Chloride Flocculation on Cyanobacterial Cells. Water Res. 1998, 32, 808–814. [Google Scholar] [CrossRef]
- Newcombe, G.; Nicholson, B. Water Treatment Options for Dissolved Cyanotoxins. J. Water Supply Res. Technol. 2004, 53, 227–239. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Yin, Q.; Yu, S.; Gao, N.; Wang, X.; Chen, J. Different Drinking Water Production Schemes to Treat Authentic Algae-Laden Source Water: Removal of 2-Methylisoborneol in Different Forms. Chem. Eng. J. 2022, 440, 135962. [Google Scholar] [CrossRef]
- Jasim, S.Y.; Saththasivam, J. Advanced Oxidation Processes to Remove Cyanotoxins in Water. Desalination 2017, 406, 83–87. [Google Scholar] [CrossRef]
- Chintalapati, P.; Mohseni, M. Degradation of Cyanotoxin Microcystin-LR in Synthetic and Natural Waters by Chemical-Free UV/VUV Radiation. J. Hazard. Mater. 2020, 381, 120921. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Najafi, F.; Neshat, A. Poly (Amidoamine-Co-Acrylic Acid) Copolymer: Synthesis, Characterization and Dye Removal Ability. Ind. Crops Prod. 2013, 42, 119–125. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Sadeghi, U.; Maleki, A.; Hayati, B.; Najafi, F. Synthesis of Cationic Polymeric Adsorbent and Dye Removal Isotherm, Kinetic and Thermodynamic. J. Ind. Eng. Chem. 2014, 20, 2745–2753. [Google Scholar] [CrossRef]
- Fu, H.; Liu, H.; Mao, J.; Chu, W.; Li, Q.; Alvarez, P.J.J.; Qu, X.; Zhu, D. Photochemistry of Dissolved Black Carbon Released from Biochar: Reactive Oxygen Species Generation and Phototransformation. Environ. Sci. Technol. 2016, 50, 1218–1226. [Google Scholar] [CrossRef]
- Cermakova, L.; Fialova, K.; Kopecka, I.; Baresova, M.; Pivokonsky, M. Investigating Adsorption of Model Low-MW AOM Components onto Different Types of Activated Carbon–Influence of Temperature and PH Value. Environ. Technol. 2022, 43, 1152–1162. [Google Scholar] [CrossRef]
- Wang, S.; Jiao, Y.; Rao, Z. Selective Removal of Common Cyanotoxins: A Review. Environ. Sci. Pollut. Res. 2021, 28, 28865–28875. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.H.; Guo, W.; Chen, Z.; Zhang, T.C. Green Technologies for Sustainable Water Management: Introduction and Overview. Green Technol. Sustain. Water Manag. 2016, 2016, 1–34. [Google Scholar]
- Márquez-Pacheco, H.; Hansen, A.M.; Falcón-Rojas, A. Phosphorous Control in a Eutrophied Reservoir. Environ. Sci. Pollut. Res. 2013, 20, 8446–8456. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Gardner, W.S.; Mccarthy, M.J.; Peierls, B.L.; Wilhelm, S.W. Algal Blooms: Noteworthy Nitrogen Algal Blooms: Proactive Strategy Ocean Acidification Foils Chemical Signals. Science 2014, 346, 2014–2016. [Google Scholar]
- Douma, M.; Manaut, N.; Oudra, B.; Loudiki, M. First Report of Cyanobacterial Diversity and Microcystins in a Microcystis Strain from Sidi Boughaba, a Moroccan Coastal Lagoon. African J. Aquat. Sci. 2016, 41, 445–452. [Google Scholar] [CrossRef]
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E.; et al. Eutrophication and Harmful Algal Blooms: A Scientific Consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Huisman, J. Climate: Blooms like It Hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef]
- Loudiki, M.; Oudra, B.; Sabour, B.; Sbiyyaa, B.; Vasconcelos, V. Taxonomy and Geographic Distribution of Potential Toxic Cyanobacterial Strains in Morocco. Ann. Limnol. 2002, 38, 101–108. [Google Scholar] [CrossRef]
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A Review of the Global Ecology, Genomics, and Biogeography of the Toxic Cyanobacterium, Microcystis Spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef]
- Douma, M.; Ouahid, Y.; Loudiki, M.; Del Campo, F.F.; Oudra, B. The First Detection of Potentially Toxic Microcystis Strains in Two Middle Atlas Mountains Natural Lakes (Morocco). Environ. Monit. Assess. 2017, 189, 39. [Google Scholar] [CrossRef]
- He, X.; Liu, Y.L.; Conklin, A.; Westrick, J.; Weavers, L.K.; Dionysiou, D.D.; Lenhart, J.J.; Mouser, P.J.; Szlag, D.; Walker, H.W. Toxic Cyanobacteria and Drinking Water: Impacts, Detection, and Treatment. Harmful Algae 2016, 54, 174–193. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Sun, P.; Zhang, J.; Esquivel-Elizondo, S.; Wu, Y. Microorganisms-Based Methods for Harmful Algal Blooms Control: A Review. Bioresour. Technol. 2018, 248, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Méjean, A.; Paci, G.; Gautier, V.; Ploux, O. Biosynthesis of Anatoxin-a and Analogues (Anatoxins) in Cyanobacteria. Toxicon 2014, 91, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Calteau, A.; Fewer, D.P.; Latifi, A.; Coursin, T.; Laurent, T.; Jokela, J.; Kerfeld, C.A.; Sivonen, K.; Piel, J.; Gugger, M. Phylum-Wide Comparative Genomics Unravel the Diversity of Secondary Metabolism in Cyanobacteria. BMC Genom. 2014, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, J.; Bornmann, K.; Schmidt, W. Relevance of Intra-and Extracellular Cyanotoxins for Drinking Water Treatment. Acta Hydrochim. Hydrobiol. 2002, 30, 7–15. [Google Scholar] [CrossRef]
- Qian, H.; Yao, Q.; Tai, C.; Deng, Z.; Gan, J.; Ou, H. Identification and Characterization of Acetyltransferase-type Toxin-antitoxin Locus in Klebsiella Pneumoniae. Mol. Microbiol. 2018, 108, 336–349. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Azevedo, S.M.F.O.; An, J.S.; Molica, R.J.R.; Jochimsen, E.M.; Lau, S.; Rinehart, K.L.; Shaw, G.R.; Eaglesham, G.K. Human Fatalities Form Cyanobacteria: Chemical and Biological Evidence for Cyanotoxins. Environ. Health Perspect. 2001, 109, 663–668. [Google Scholar] [CrossRef]
- Lahti, K.; Rapala, J.; Kivimäki, A.L.; Kukkonen, J.; Niemelä, M.; Sivonen, K. Occurrence of Microcystins in Raw Water Sources and Treated Drinking Water of Finnish Waterworks. Water Sci. Technol. 2001, 43, 225–228. [Google Scholar] [CrossRef]
- Svrcek, C.; Smith, D.W. Cyanobacteria Toxins and the Current State of Knowledge on Water Treatment Options: A Review. J. Environ. Eng. Sci. 2004, 3, 155–185. [Google Scholar] [CrossRef]
- Merel, S.; Clément, M.; Thomas, O. State of the Art on Cyanotoxins in Water and Their Behaviour towards Chlorine. Toxicon 2010, 55, 677–691. [Google Scholar] [CrossRef]
- McQuaid, N.; Zamyadi, A.; Prévost, M.; Bird, D.F.; Dorner, S. Use of in Vivo Phycocyanin Fluorescence to Monitor Potential Microcystin-Producing Cyanobacterial Biovolume in a Drinking Water Source. J. Environ. Monit. 2011, 13, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Froment-Vedrine, M.; Lasfargues, G.; Coutureau, F.; Villa, A. L’Agence Française de Sécurité Sanitaire de l’environnement et Du Travail (Afsset). Administration 2005, 209, 19. [Google Scholar]
- de Gaulle, G. De l’Agence Française de Sécurité Sanitaire Des Aliments Relatif à l’évaluation Qualitative Du Risque Sanitaire Pour l’homme Lié à La Présence Dans l’eau Destinée à La Consommation Humaine et Dans Divers Effluents Aqueux de Virus Influenza Hautement Patho. Available online: https://www.anses.fr/fr/system/files/EAUX2005sa0332.pdf (accessed on 31 May 2022).
- Liu, Y.; Chen, W.; Li, D.; Huang, Z.; Shen, Y.; Liu, Y. Cyanobacteria-/Cyanotoxin-Contaminations and Eutrophication Status before Wuxi Drinking Water Crisis in Lake Taihu, China. J. Environ. Sci. 2011, 23, 575–581. [Google Scholar] [CrossRef]
- Zhang, W.; Fang, T.; Xu, X. Study on Photodegradation of Cyanobacterial Toxin in Blooms of Dianchi Lake. Zhongguo Huanjing Kexue 2001, 21, 1–3. [Google Scholar]
- Tran, C.D.; Duri, S.; Delneri, A.; Franko, M. Chitosan-Cellulose Composite Materials: Preparation, Characterization and Application for Removal of Microcystin. J. Hazard. Mater. 2013, 252, 355–366. [Google Scholar] [CrossRef]
- Falconer, I.R.; Humpage, A.R. Health Risk Assessment of Cyanobacterial (Blue-Green Algal) Toxins in Drinking Water. Int. J. Environ. Res. Public Health 2005, 2, 43–50. [Google Scholar] [CrossRef]
- Graham, J.L.; Loftin, K.A.; Meyer, M.T.; Ziegler, A.C. Cyanotoxin Mixtures and Taste-and-Odor Compounds in Cyanobacterial Blooms from the Midwestern United States. Environ. Sci. Technol. 2010, 44, 7361–7368. [Google Scholar] [CrossRef]
- Ho, L.; Dreyfus, J.; Boyer, J.; Lowe, T.; Bustamante, H.; Duker, P.; Meli, T.; Newcombe, G. Fate of Cyanobacteria and Their Metabolites during Water Treatment Sludge Management Processes. Sci. Total Environ. 2012, 424, 232–238. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Madamwar, D.; Incharoensakdi, A. Bloom Dynamics of Cyanobacteria and Their Toxins: Environmental Health Impacts and Mitigation Strategies. Front. Microbiol. 2015, 6, 1–22. [Google Scholar] [CrossRef]
- Tokodi, N.; Drobac, D.; Lazić, G.; Petrović, T.; Marinović, Z.; Lujić, J.; Malešević, T.P.; Meriluoto, J.; Svirčev, Z. Screening of Cyanobacterial Cultures Originating from Different Environments for Cyanotoxicity and Cyanotoxins. Toxicon 2018, 154, 1–6. [Google Scholar] [CrossRef]
- Carey, C.C.; Haney, J.F.; Cottingham, K.L. First Report of Microcystin-LR in the Cyanobacterium Gloeotrichia Echinulata. Environ. Toxicol. An Int. J. 2007, 22, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Beattie, K.A.; Ressler, J.; Wiegand, C.; Krause, E.; Codd, G.A.; Steinberg, C.E.W.; Pflugmacher, S. Comparative Effects and Metabolism of Two Microcystins and Nodularin in the Brine Shrimp Artemia Salina. Aquat. Toxicol. 2003, 62, 219–226. [Google Scholar] [CrossRef]
- Faassen, E.J.; Lürling, M. Occurrence of the Microcystins MC-LW and MC-LF in Dutch Surface Waters and Their Contribution to Total Microcystin Toxicity. Mar. Drugs 2013, 11, 2643–2654. [Google Scholar] [CrossRef] [PubMed]
- Nishiwaki-Matsushima, R.; Ohta, T.; Nishiwaki, S.; Suganuma, M.; Kohyama, K.; Ishikawa, T.; Carmichael, W.W.; Fujiki, H. Liver Tumor Promotion by the Cyanobacterial Cyclic Peptide Toxin Microcystin-LR. J. Cancer Res. Clin. Oncol. 1992, 118, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Lambling, P.; Bustamante, H.; Duker, P.; Newcombe, G. Application of Powdered Activated Carbon for the Adsorption of Cylindrospermopsin and Microcystin Toxins from Drinking Water Supplies. Water Res. 2011, 45, 2954–2964. [Google Scholar] [CrossRef] [PubMed]
- Ilieva, V.; Kondeva-Burdina, M.; Georgieva, T.; Pavlova, V. Toxicity of Cyanobacteria. Organotropy of Cyanotoxins and Toxicodynamics of Cyanotoxins by Species. Pharmacia 2019, 66, 91. [Google Scholar] [CrossRef]
- Scheffer, M.; Hosper, S.H.; Meijer, M.L.; Moss, B.; Jeppesen, E. Alternative Equilibria in Shallow Lakes. Trends Ecol. Evol. 1993, 8, 275–279. [Google Scholar] [CrossRef]
- Bouaïcha, N.; Miles, C.O.; Beach, D.G.; Labidi, Z.; Djabri, A.; Benayache, N.Y.; Nguyen-Quang, T. Structural Diversity, Characterization and Toxicology of Microcystins. Toxins 2019, 11, 714. [Google Scholar] [CrossRef]
- Campinas, M.; Rosa, M.J. The Ionic Strength Effect on Microcystin and Natural Organic Matter Surrogate Adsorption onto PAC. J. Colloid Interface Sci. 2006, 299, 520–529. [Google Scholar] [CrossRef]
- Botes, D.P.; Tuinman, A.A.; Wessels, P.L.; Viljoen, C.C.; Kruger, H.; Williams, D.H.; Santikarn, S.; Smith, R.J.; Hammond, S.J. The Structure of Cyanoginosin-LA, a Cyclic Heptapeptide Toxin from the Cyanobacterium Microcystis Aeruginosa. J. Chem. Soc. Perkin Trans. 1 1984, 2311–2318. [Google Scholar] [CrossRef]
- Rinehart, K.L.; Namikoshi, M.; Choi, B.W. Structure and Biosynthesis of Toxins from Blue-Green Algae (Cyanobacteria). J. Appl. Phycol. 1994, 6, 159–176. [Google Scholar] [CrossRef]
- Sivonen, K.; Niemelä, S.I.; Niemi, R.M.; Lepistö, L.; Luoma, T.H.; Räsänen, L.A. Toxic Cyanobacteria (Blue-Green Algae) in Finnish Fresh and Coastal Waters. Hydrobiologia 1990, 190, 267–275. [Google Scholar] [CrossRef]
- Namikoshi, M.; Rinehart, K.L.; Dahlem, A.M.; Beasley, V.R.; Carmichael, W.W. Total Synthesis of Adda, the Unique C20 Amino Acid of Cyanobacterial Hepatotoxins. Tetrahedron Lett. 1989, 30, 4349–4352. [Google Scholar] [CrossRef]
- Carmichael, W.W. Cyanobacteria Secondary Metabolites—The Cyanotoxins. J. Appl. Bacteriol. 1992, 72, 445–459. [Google Scholar] [CrossRef]
- Xian, Q.; Chen, H.; Liu, H.; Zou, H.; Yin, D. Isolation and Identification of Antialgal Compounds from the Leaves of Vallisneria Spiralis L. by Activity-Guided Fractionation. Environ. Sci. Pollut. Res. 2006, 13, 233–237. [Google Scholar] [CrossRef]
- He, X.; Chen, J.; Wu, D.; Wang, J.; Xin, M.; Liu, L.; Sun, P.; Wang, B. Occurrence, Distribution, Source, and Influencing Factors of Lipophilic Marine Algal Toxins in Laizhou Bay, Bohai Sea, China. Mar. Pollut. Bull. 2020, 150, 110789. [Google Scholar] [CrossRef]
- Robert, C.; Tremblay, H.; DeBlois, C. Cyanobactéries et Cyanotoxines Au Québec: Suivi à Six Stations de Production d’eau Potable (2001–2003); Service des Eaux Municipales, Direction Générale des Politiques, Développement Durable, Environnement et Parcs: Québec, QC, Canada, 2005. [Google Scholar]
- Jeon, J.; Soon, A.; Park, J.; Hong, S.; Cho, K.; Deok Yu, B. Adsorption and Surface Diffusion of Pt Atoms on Hydroxylated MgO (001) Surfaces. J. Phys. Soc. Japan 2013, 82, 34603. [Google Scholar] [CrossRef]
- Lobanga, K.P.; Haarhoff, J.; Van Staden, S.J. Treatability of South African Surface Waters by Activated Carbon. Water Sa 2013, 39, 379–384. [Google Scholar] [CrossRef][Green Version]
- Jamil, S.; Loganathan, P.; Listowski, A.; Kandasamy, J.; Khourshed, C.; Vigneswaran, S. Simultaneous Removal of Natural Organic Matter and Micro-Organic Pollutants from Reverse Osmosis Concentrate Using Granular Activated Carbon. Water Res. 2019, 155, 106–114. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Arulkumar, M.; Palvannan, T. Utilization of Agro-Industrial Waste Jatropha Curcas Pods as an Activated Carbon for the Adsorption of Reactive Dye Remazol Brilliant Blue R (RBBR). J. Clean. Prod. 2012, 22, 67–75. [Google Scholar] [CrossRef]
- Ampiaw, R.E.; Yaqub, M.; Lee, W. Adsorption of Microcystin onto Activated Carbon: A Review. Membr. Water Treat. 2019, 10, 405–415. [Google Scholar]
- Dawson, C. Analysis of an Upwind-Mixed Finite Element Method for Nonlinear Contaminant Transport Equations. SIAM J. Numer. Anal. 1998, 35, 1709–1724. [Google Scholar] [CrossRef]
- De Figueiredo, D.R.; Azeiteiro, U.M.; Esteves, S.M.; Gonçalves, F.J.M.; Pereira, M.J. Microcystin-Producing Blooms - A Serious Global Public Health Issue. Ecotoxicol. Environ. Saf. 2004, 59, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Lanaras, T.; Cook, C.M.; Eriksson, J.E.; Meriluoto, J.A.O.; Hotokka, M. Computer Modelling of the 3-Dimensional Structures of the Cyanobacterial Hepatotoxins Microcystin-LR and Nodularin. Toxicon 1991, 29, 901–906. [Google Scholar] [CrossRef]
- Şengül, A.B.; Ersan, G.; Tüfekçi, N. Removal of Intra- and Extracellular Microcystin by Submerged Ultrafiltration (UF) Membrane Combined with Coagulation/Flocculation and Powdered Activated Carbon (PAC) Adsorption. J. Hazard. Mater. 2018, 343, 29–35. [Google Scholar] [CrossRef]
- Zhu, S.; Yin, D.; Gao, N.; Zhou, S.; Wang, Z.; Zhang, Z. Adsorption of Two Microcystins onto Activated Carbon: Equilibrium, Kinetic, and Influential Factors. Desalin. Water Treat. 2016, 57, 23666–23674. [Google Scholar] [CrossRef]
- Huang, W.J.; Cheng, B.L.; Cheng, Y.L. Adsorption of Microcystin-LR by Three Types of Activated Carbon. J. Hazard. Mater. 2007, 141, 115–122. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Gómez-Serrano, V.; Álvarez, P.M.; Alvim-Ferraz, M.C.M.; Dias, J.M. Activated Carbon Modifications to Enhance Its Water Treatment Applications. An Overview. J. Hazard. Mater. 2011, 187, 1–23. [Google Scholar] [CrossRef]
- 81. Najm, I.N.; Snoeyink, V.L.; Lykins, B.W., Jr.; Adams, J.Q. Using Powdered Activated Carbon: A Critical Review. J. Am. Water Works Assoc. 1991, 83, 65–76. [Google Scholar] [CrossRef]
- Van Pelt, A.H.; Simakova, O.A.; Schimming, S.M.; Ewbank, J.L.; Foo, G.S.; Pidko, E.A.; Hensen, E.J.M.; Sievers, C. Stability of Functionalized Activated Carbon in Hot Liquid Water. Carbon N. Y. 2014, 77, 143–154. [Google Scholar] [CrossRef]
- Cook, D.; Newcombe, G. Removal of Microcystin Variants with Powdered Activated Carbon. Water Sci. Technol. Water Supply 2002, 2, 201–207. [Google Scholar] [CrossRef]
- Lee, J.; Walker, H.W. Effect of Process Variables and Natural Organic Matter on Removal of Microcystin-LR by PAC−UF. Environ. Sci. Technol. 2006, 40, 7336–7342. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, P.; Schumann, R.; Wong, S.H. Microcystin-LR Adsorption by Activated Carbon. J. Colloid Interface Sci. 2001, 240, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Gong, A.; He, H.; Zhou, J.; Wei, Y.; Lv, L. Adsorption of Microcystins by Carbon Nanotubes. Chemosphere 2006, 62, 142–148. [Google Scholar] [CrossRef]
- Donati, C.; Drikas, M.; Hayes, R.; Newcombe, G. Microcystin-LR Adsorption by Powdered Activated Carbon. Water Res. 1994, 28, 1735–1742. [Google Scholar] [CrossRef]
- Sizmur, T.; Fresno, T.; Akgül, G.; Frost, H.; Moreno-Jiménez, E. Biochar Modification to Enhance Sorption of Inorganics from Water. Bioresour. Technol. 2017, 246, 34–47. [Google Scholar] [CrossRef]
- Enaime, G.; Lübken, M. Agricultural Waste-Based Biochar for Agronomic Applications. Appl. Sci. 2021, 11, 8914. [Google Scholar] [CrossRef]
- Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Wichern, M.; Lübken, M. Hydrothermal Carbonization of the Filter Bed Remained after Filtration of Olive Mill Wastewater on Olive Stones for Biofuel Application. Biomass Convers. Biorefinery 2020, 12, 1237–1247. [Google Scholar] [CrossRef]
- Tan, R.R.; Bandyopadhyay, S.; Foo, D.C.Y. Graphical Pinch Analysis for Planning Biochar-Based Carbon Management Networks. Process Integr. Optim. Sustain. 2018, 2, 159–168. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a Sorbent for Contaminant Management in Soil and Water: A Review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U., Jr. Organic and Inorganic Contaminants Removal from Water with Biochar, a Renewable, Low Cost and Sustainable Adsorbent–a Critical Review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Inyang, M.; Dickenson, E. The Potential Role of Biochar in the Removal of Organic and Microbial Contaminants from Potable and Reuse Water: A Review. Chemosphere 2015, 134, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Kaetzl, K.; Lübken, M.; Nettmann, E.; Krimmler, S.; Wichern, M. Slow Sand Filtration of Raw Wastewater Using Biochar as an Alternative Filtration Media. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kaetzl, K.; Lübken, M.; Gehring, T.; Wichern, M. Efficient Low-Cost Anaerobic Treatment of Wastewater Using Biochar and Woodchip Filters. Water 2018, 10, 818. [Google Scholar] [CrossRef]
- Huggins, T.M.; Haeger, A.; Biffinger, J.C.; Ren, Z.J. Granular Biochar Compared with Activated Carbon for Wastewater Treatment and Resource Recovery. Water Res. 2016, 94, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhang, N.; Xing, C.; Cui, Q.; Sun, Q. The Adsorption, Regeneration and Engineering Applications of Biochar for Removal Organic Pollutants: A Review. Chemosphere 2019, 223, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Yao, C.; Liu, Y.; Zhang, C.; Jia, L.; Li, D.; Fu, Z.; Sun, D.; Robert Kirk, S.; Yin, D. Bamboo-Derived Porous Biochar for Efficient Adsorption Removal of Dibenzothiophene from Model Fuel. Fuel 2018, 211, 121–129. [Google Scholar] [CrossRef]
- Li, J.; Cao, L.; Yuan, Y.; Wang, R.; Wen, Y.; Man, J. Comparative Study for Microcystin-LR Sorption onto Biochars Produced from Various Plant- and Animal-Wastes at Different Pyrolysis Temperatures: Influencing Mechanisms of Biochar Properties. Bioresour. Technol. 2018, 247, 794–803. [Google Scholar] [CrossRef]
- Li, L.; Qiu, Y.; Huang, J.; Li, F.; Sheng, G.D. Mechanisms and Factors Influencing Adsorption of Microcystin-LR on Biochars. Water. Air. Soil Pollut. 2014, 225, 2220. [Google Scholar] [CrossRef]
- Liu, Y.; Lonappan, L.; Brar, S.K.; Yang, S. Impact of Biochar Amendment in Agricultural Soils on the Sorption, Desorption, and Degradation of Pesticides: A Review. Sci. Total Environ. 2018, 645, 60–70. [Google Scholar] [CrossRef]
- Zheng, H.; Feng, N.; Yang, T.; Shi, M.; Wang, X.; Zhang, Q.; Zhao, J.; Li, F.; Sun, K.; Xing, B. Individual and Combined Applications of Biochar and Pyroligneous Acid Mitigate Dissemination of Antibiotic Resistance Genes in Agricultural Soil. Sci. Total Environ. 2021, 796, 148962. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sung, J.-K.; Yang, J.E.; Ok, Y.S. Effects of Pyrolysis Temperature on Soybean Stover-and Peanut Shell-Derived Biochar Properties and TCE Adsorption in Water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Lu, J. Adsorption of Microcystin-LR by Rice Straw Biochars with Different Pyrolysis Temperatures. Environ. Technol. Innov. 2021, 23, 101609. [Google Scholar] [CrossRef]
- Liu, B.L.; Fu, M.M.; Xiang, L.; Feng, N.X.; Zhao, H.M.; Li, Y.W.; Cai, Q.Y.; Li, H.; Mo, C.H.; Wong, M.H. Adsorption of Microcystin Contaminants by Biochars Derived from Contrasting Pyrolytic Conditions: Characteristics, Affecting Factors, and Mechanisms. Sci. Total Environ. 2021, 763, 143028. [Google Scholar] [CrossRef]
- Kaetzl, K.; Lübken, M.; Uzun, G.; Gehring, T.; Nettmann, E.; Stenchly, K.; Wichern, M. On-Farm Wastewater Treatment Using Biochar from Local Agroresidues Reduces Pathogens from Irrigation Water for Safer Food Production in Developing Countries. Sci. Total Environ. 2019, 682, 601–610. [Google Scholar] [CrossRef]
- Díez-Quijada, L.; Prieto, A.I.; Guzmán-Guillén, R.; Jos, A.; Cameán, A.M. Occurrence and Toxicity of Microcystin Congeners Other than MC-LR and MC-RR: A Review. Food Chem. Toxicol. 2019, 125, 106–132. [Google Scholar] [CrossRef]
- Park, J.A.; Jung, S.M.; Yi, I.G.; Choi, J.W.; Kim, S.B.; Lee, S.H. Adsorption of Microcystin-LR on Mesoporous Carbons and Its Potential Use in Drinking Water Source. Chemosphere 2017, 177, 15–23. [Google Scholar] [CrossRef]
- Libbrecht, W.; Verberckmoes, A.; Thybaut, J.W.; Van Der Voort, P.; De Clercq, J. Soft Templated Mesoporous Carbons: Tuning the Porosity for the Adsorption of Large Organic Pollutants. Carbon N. Y. 2017, 116, 528–546. [Google Scholar] [CrossRef]
- Teng, W.; Wu, Z.; Fan, J.; Chen, H.; Feng, D.; Lv, Y.; Wang, J.; Asiri, A.M.; Zhao, D. Ordered Mesoporous Carbons and Their Corresponding Column for Highly Efficient Removal of Microcystin-LR. Energy Environ. Sci. 2013, 6, 2765–2776. [Google Scholar] [CrossRef]
- Teng, W.; Wu, Z.; Fan, J.; Zhang, W.X.; Zhao, D. Amino-Functionalized Ordered Mesoporous Carbon for the Separation of Toxic Microcystin-LR. J. Mater. Chem. A 2015, 3, 19168–19176. [Google Scholar] [CrossRef]
- Huang, W.; Chen, J.; He, F.; Tang, J.; Li, D.; Zhu, Y.; Zhang, Y. Effective Phosphate Adsorption by Zr/Al-Pillared Montmorillonite: Insight into Equilibrium, Kinetics and Thermodynamics. Appl. Clay Sci. 2015, 104, 252–260. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, L. Fabrication of Novel Rattle-Type Magnetic Mesoporous Carbon Microspheres for Removal of Microcystins. J. Mater. Chem. 2011, 21, 10653–10657. [Google Scholar] [CrossRef]
- Pavagadhi, S.; Tang, A.L.L.; Sathishkumar, M.; Loh, K.P.; Balasubramanian, R. Removal of Microcystin-LR and Microcystin-RR by Graphene Oxide: Adsorption and Kinetic Experiments. Water Res. 2013, 47, 4621–4629. [Google Scholar] [CrossRef] [PubMed]
- Park, J.A.; Kang, J.K.; Jung, S.M.; Choi, J.W.; Lee, S.H.; Yargeau, V.; Kim, S.B. Investigating Microcystin-LR Adsorption Mechanisms on Mesoporous Carbon, Mesoporous Silica, and Their Amino-Functionalized Form: Surface Chemistry, Pore Structures, and Molecular Characteristics. Chemosphere 2020, 247, 125811. [Google Scholar] [CrossRef] [PubMed]
- Krupadam, R.J.; Patel, G.P.; Balasubramanian, R. Removal of Cyanotoxins from Surface Water Resources Using Reusable Molecularly Imprinted Polymer Adsorbents. Environ. Sci. Pollut. Res. 2012, 19, 1841–1851. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Kan, E. Thermally Enhanced Adsorption and Persulfate Oxidation-Driven Regeneration on FeCl3-Activated Biochar for Removal of Microcystin-LR in Water. Chemosphere 2022, 286, 131950. [Google Scholar] [CrossRef] [PubMed]
- Campinas, M.; Rosa, M.J. Removal of Microcystins by PAC/UF. Sep. Purif. Technol. 2010, 71, 114–120. [Google Scholar] [CrossRef]
- Zeng, S.; Kan, E. Adsorption and Regeneration on Iron-Activated Biochar for Removal of Microcystin-LR. Chemosphere 2021, 273, 129649. [Google Scholar] [CrossRef]
- Dastgheib, S.A.; Karanfil, T. Adsorption of Oxygen by Heat-Treated Granular and Fibrous Activated Carbons. J. Colloid Interface Sci. 2004, 274, 1–8. [Google Scholar] [CrossRef]
- Newcombe, G.; Dixon, D. Adsorption of Industrial Pollutants. Interface Sci. Drink. Water Treat. Theory Appl. 2006, 9, 155. [Google Scholar]
- Selmi, T.; Enaime, G.; Kesraoui, A.; Baçaoui, A.; Seffen, M. Dye Removal by Activated Carbon Produced from Agave Americana Fibers: Stochastic Isotherm and Fractal Kinetic Studies. Environ. Sci. Pollut. Res. 2021, 28, 46580–46591. [Google Scholar] [CrossRef] [PubMed]
- Enaime, G.; Ennaciri, K.; Ounas, A.; Baçaoui, A.; Seffen, M.; Selmi, T.; Yaacoubi, A. Preparation and Characterization of Activated Carbons from Olive Wastes by Physical and Chemical Activation: Application to Indigo Carmine Adsorption. J. Mater. Environ. Sci. 2017, 8, 4125–4137. [Google Scholar]
- Jović, B.; Kordić, B.; Miškov, V.; Tričković, J.; Kovačević, M.; Petrović, S. Amides as a Model System of Low Molar Mass Algal Organic Matter. Influence on the Adsorption of p-Nitrophenol on Activated Carbon. Arab. J. Chem. 2020, 13, 59–66. [Google Scholar] [CrossRef]
- Li, X.; Pei, H.; Hu, W.; Meng, P.; Sun, F.; Ma, G.; Xu, X.; Li, Y. The Fate of Microcystis Aeruginosa Cells during the Ferric Chloride Coagulation and Flocs Storage Processes. Environ. Technol. 2015, 36, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Pantelić, D.; Svirčev, Z.; Simeunović, J.; Vidović, M.; Trajković, I. Cyanotoxins: Characteristics, Production and Degradation Routes in Drinking Water Treatment with Reference to the Situation in Serbia. Chemosphere 2013, 91, 421–441. [Google Scholar] [CrossRef] [PubMed]
- Pelekani, C.; Snoeyink, V.L. Competitive Adsorption in Natural Water: Role of Activated Carbon Pore Size. Water Res. 1999, 33, 1209–1219. [Google Scholar] [CrossRef]
- Ebie, K.; Li, F.; Azuma, Y.; Yuasa, A.; Hagishita, T. Pore Distribution Effect of Activated Carbon in Adsorbing Organic Micropollutants from Natural Water. Water Res. 2001, 35, 167–179. [Google Scholar] [CrossRef]
- Newcombe, G.; Drikas, M.; Hayes, R. Influence of Characterised Natural Organic Material on Activated Carbon Adsorption: II. Effect on Pore Volume Distribution and Adsorption of 2-Methylisoborneol. Water Res. 1997, 31, 1065–1073. [Google Scholar] [CrossRef]
- Barton, S.S.; Evans, M.J.B.; Halliop, E.; MacDonald, J.A.F. Acidic and Basic Sites on the Surface of Porous Carbon. Carbon N. Y. 1997, 35, 1361–1366. [Google Scholar] [CrossRef]
- Bjelopavlic, M.; Newcombe, G.; Hayes, R. Adsorption of NOM onto Activated Carbon: Effect of Surface Charge, Ionic Strength, and Pore Volume Distribution. J. Colloid Interface Sci. 1999, 210, 271–280. [Google Scholar] [CrossRef]
- Moreno-Castilla, C. Adsorption of Organic Molecules from Aqueous Solutions on Carbon Materials. Carbon N. Y. 2004, 42, 83–94. [Google Scholar] [CrossRef]
- Álvarez-Merino, M.A.; Fontecha-Cámara, M.A.; López-Ramón, M.V.; Moreno-Castilla, C. Temperature Dependence of the Point of Zero Charge of Oxidized and Non-Oxidized Activated Carbons. Carbon N. Y. 2008, 46, 778–787. [Google Scholar] [CrossRef]
- Hnatukova, P.; Kopecka, I.; Pivokonsky, M. Adsorption of Cellular Peptides of Microcystis Aeruginosa and Two Herbicides onto Activated Carbon: Effect of Surface Charge and Interactions. Water Res. 2011, 45, 3359–3368. [Google Scholar] [CrossRef] [PubMed]
- Safarikova, J.; Baresova, M.; Pivokonsky, M.; Kopecka, I. Influence of Peptides and Proteins Produced by Cyanobacterium Microcystis Aeruginosa on the Coagulation of Turbid Waters. Sep. Purif. Technol. 2013, 118, 49–57. [Google Scholar] [CrossRef]
- Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Lübken, M. Biochar for Wastewater Treatment—Conversion Technologies and Applications. Appl. Sci. 2020, 10, 3492. [Google Scholar] [CrossRef]
- Schreiber, B.; Brinkmann, T.; Schmalz, V.; Worch, E. Adsorption of Dissolved Organic Matter onto Activated Carbon—The Influence of Temperature, Absorption Wavelength, and Molecular Size. Water Res. 2005, 39, 3449–3456. [Google Scholar] [CrossRef]
- Sebben, D.; Pendleton, P. Amino Acid + Silica Adsorption Thermodynamics: Effects of Temperature. J. Chem. Thermodyn. 2015, 87, 96–102. [Google Scholar] [CrossRef]
- Rabe, M.; Verdes, D.; Seeger, S. Understanding Protein Adsorption Phenomena at Solid Surfaces. Adv. Colloid Interface Sci. 2011, 162, 87–106. [Google Scholar] [CrossRef]
- Wang, L.; Liu, L.; Zheng, B. Eutrophication Development and Its Key Regulating Factors in a Water-Supply Reservoir in North China. J. Environ. Sci. 2013, 25, 962–970. [Google Scholar] [CrossRef]
- Başar, C.A. Applicability of the Various Adsorption Models of Three Dyes Adsorption onto Activated Carbon Prepared Waste Apricot. J. Hazard. Mater. 2006, 135, 232–241. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, G.; Jia, X. Re-Moval of Microcystin-LR from Drinking Water Using a Bam-Boo-Based Charcoal Adsorbent Modified with Chitosan. J. Environ. Sci. 2011, 23, 1983–1988. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, M.; Yang, H.; Jo, J.; Han, D.; Jeon, Y.-J.; Cho, S. Enrichment and Purification of Marine Polyphenol Phlorotannins Using Macroporous Adsorption Resins. Food Chem. 2014, 162, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Scheufele, F.B.; Módenes, A.N.; Borba, C.E.; Ribeiro, C.; Espinoza-Quiñones, F.R.; Bergamasco, R.; Pereira, N.C. Monolayer–Multilayer Adsorption Phenomenological Model: Kinetics, Equilibrium and Thermodynamics. Chem. Eng. J. 2016, 284, 1328–1341. [Google Scholar] [CrossRef]
- Li, X.M.; Yang, Q.; Huang, K.; Zeng, G.M.; Liao, D.X.; Liu, J.J.; Long, W.F. Screening and Characterization of a Bioflocculant Produced by Aeromonas Sp. Biomed. Environ. Sci. 2007, 20, 274. [Google Scholar]
- Anastopoulos, I.; Kyzas, G.Z. Are the Thermodynamic Parameters Correctly Estimated in Liquid-Phase Adsorption Phenomena? J. Mol. Liq. 2016, 218, 174–185. [Google Scholar] [CrossRef]
- Taran, F.; Nazemi, A.H.; Sadraddini, A.A.; Dinpazhuh, Y. Investigation of Conservative and Non-Conservative Solute Transport in Soil and Comparison of Three Adsorption Models Using HYDRUS-2D. J. Mater. Environ. Sci. 2016, 7, 2082–2093. [Google Scholar]
- Moafi, H.F.; Ansari, R.; Ostovar, F. Ag2O/Sawdust Nanocomposite as an Efficient Adsorbent for Removal of Hexavalent Chromium Ions from Aqueous Solutions. J. Mater. Environ. Sci. 2016, 7, 2051–2068. [Google Scholar]
- Andreu, A.; Stoeckli, H.F.; Bradley, R.H. Specific and Non-Specific Interactions on Non-Porous Carbon Black Surfaces. Carbon N. Y. 2007, 45, 1854–1864. [Google Scholar] [CrossRef]
- Yoon, J.-Y.; Kim, J.-H.; Kim, W.-S. The Relationship of Interaction Forces in the Protein Adsorption onto Polymeric Microspheres. Colloids Surfaces A Physicochem. Eng. Asp. 1999, 153, 413–419. [Google Scholar] [CrossRef]
- Crittenden, B.; Patton, A.; Jouin, C.; Perera, S.; Tennison, S.; Echevarria, J.A.B. Carbon Monoliths: A Comparison with Granular Materials. Adsorption 2005, 11, 537–541. [Google Scholar] [CrossRef]
- Kilduff, J.E.; Karanfil, T.; Chin, Y.-P.; Weber, W.J. Adsorption of Natural Organic Polyelectrolytes by Activated Carbon: A Size-Exclusion Chromatography Study. Environ. Sci. Technol. 1996, 30, 1336–1343. [Google Scholar] [CrossRef]
- Randtke, S.J.; Snoeyink, V.L. Evaluating GAC Adsorptive Capacity. J. Am. Water Work. Assoc. 1983, 75, 406–413. [Google Scholar] [CrossRef]
- Li, Y.-H.; Di, Z.; Ding, J.; Wu, D.; Luan, Z.; Zhu, Y. Adsorption Thermodynamic, Kinetic and Desorption Studies of Pb2+ on Carbon Nanotubes. Water Res. 2005, 39, 605–609. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.J.; Hokura, A.; Kisler, J.M.; Shimazu, S.; Stevens, G.W.; Komatsu, Y. Amino Acid Adsorption onto Mesoporous Silica Molecular Sieves. Sep. Purif. Technol. 2006, 48, 197–201. [Google Scholar] [CrossRef]
- Kopecka, I.; Pivokonsky, M.; Pivokonska, L.; Hnatukova, P.; Safarikova, J. Adsorption of Peptides Produced by Cyanobacterium Microcystis Aeruginosa onto Granular Activated Carbon. Carbon N. Y. 2014, 69, 595–608. [Google Scholar] [CrossRef]
- Cermakova, L.; Kopecka, I.; Pivokonsky, M.; Pivokonska, L.; Janda, V. Removal of Cyanobacterial Amino Acids in Water Treatment by Activated Carbon Adsorption. Sep. Purif. Technol. 2017, 173, 330–338. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chu, W.; Li, H.; Boyd, S.A.; Teppen, B.J.; Mao, J.; Lehmann, J.; Zhang, W. Quantification and Characterization of Dissolved Organic Carbon from Biochars. Geoderma 2019, 335, 161–169. [Google Scholar] [CrossRef]
- Chae, S.; Noeiaghaei, T.; Oh, Y.; Kim, I.S.; Park, J.S. Effective Removal of Emerging Dissolved Cyanotoxins from Water Using Hybrid Photocatalytic Composites. Water Res. 2019, 149, 421–431. [Google Scholar] [CrossRef]
- Cook, D.; Newcombe, G. Comparison and Modeling of the Adsorption of Two Microcystin Analogues onto Powdered Activated Carbon. Environ. Technol. 2008, 29, 525–534. [Google Scholar] [CrossRef]
- Campinas, M.; Viegas, R.M.C.; Rosa, M.J. Modelling and Understanding the Competitive Adsorption of Microcystins and Tannic Acid. Water Res. 2013, 47, 5690–5699. [Google Scholar] [CrossRef]
- Stoquart, C.; Vázquez Rodríguez, G.A.; Servais, P.; Sauvé, S.; Barbeau, B. Micropollutant Removal Potential by Aged Powdered Activated Carbon. J. Environ. Eng. 2016, 142, 4016058. [Google Scholar] [CrossRef]
- Bruchet, A.; Bernazeau, F.; Baudin, I.; Pieronne, P. Algal Toxins in Surface Waters: Analysis and Treatment. Water Supply 1998, 16, 619–623. [Google Scholar]
- Hart, J.; Fawell, J.K.; Croll, B. The Fate of Both Intra- and Extracellular Toxins during Drinking Water Treatment. Water Supply 1998, 16, 611–616. [Google Scholar]
- Mouchet, P.; Bonnelye, V. Solving Algae Problems: French Expertise and World-Wide Applications. J. Water Supply Res. Technol. 1998, 47, 125–141. [Google Scholar] [CrossRef]
- Pivokonsky, M.; Kopecka, I.; Cermakova, L.; Fialova, K.; Novotna, K.; Cajthaml, T.; Henderson, R.K.; Pivokonska, L. Current Knowledge in the Field of Algal Organic Matter Adsorption onto Activated Carbon in Drinking Water Treatment. Sci. Total Environ. 2021, 799, 149455. [Google Scholar] [CrossRef]
- Dixon, M.B.; Richard, Y.; Ho, L.; Chow, C.W.K.; O’Neill, B.K.; Newcombe, G. Integrated Membrane Systems Incorporating Coagulation, Activated Carbon and Ultrafiltration for the Removal of Toxic Cyanobacterial Metabolites from Anabaena Circinalis. Water Sci. Technol. 2011, 63, 1405–1411. [Google Scholar] [CrossRef]
- Kelly, N.E.; Javed, A.; Shimoda, Y.; Zastepa, A.; Watson, S.; Mugalingam, S.; Arhonditsis, G.B. A Bayesian Risk Assessment Framework for Microcystin Violations of Drinking Water and Recreational Standards in the Bay of Quinte, Lake Ontario, Canada. Water Res. 2019, 162, 288–301. [Google Scholar] [CrossRef]
- Bialczyk, J.; Natkański, P.; Kuśtrowski, P.; Czaja-Prokop, U.; Bober, B.; Kaminski, A. Removal of Cyanobacterial Anatoxin-a from Water by Natural Clay Adsorbents. Appl. Clay Sci. 2017, 148, 17–24. [Google Scholar] [CrossRef]
- Melegari, S.P.; Matias, W.G. Preliminary Assessment of the Performance of Oyster Shells and Chitin Materials as Adsorbents in the Removal of Saxitoxin in Aqueous Solutions. Chem. Cent. J. 2012, 6, 1–8. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).