A CFD Investigation on the Aerosol Drug Delivery in the Mouth–Throat Airway Using a Pressurized Metered-Dose Inhaler Device
Abstract
1. Introduction
2. CFD Model Development
2.1. Geometry and Material
2.2. Mesh Independency Study
2.3. Governing Equations
2.4. Boundary Conditions
2.5. Numerical Controls and Computational Power
3. Results and Discussion
3.1. CFD Model Validation
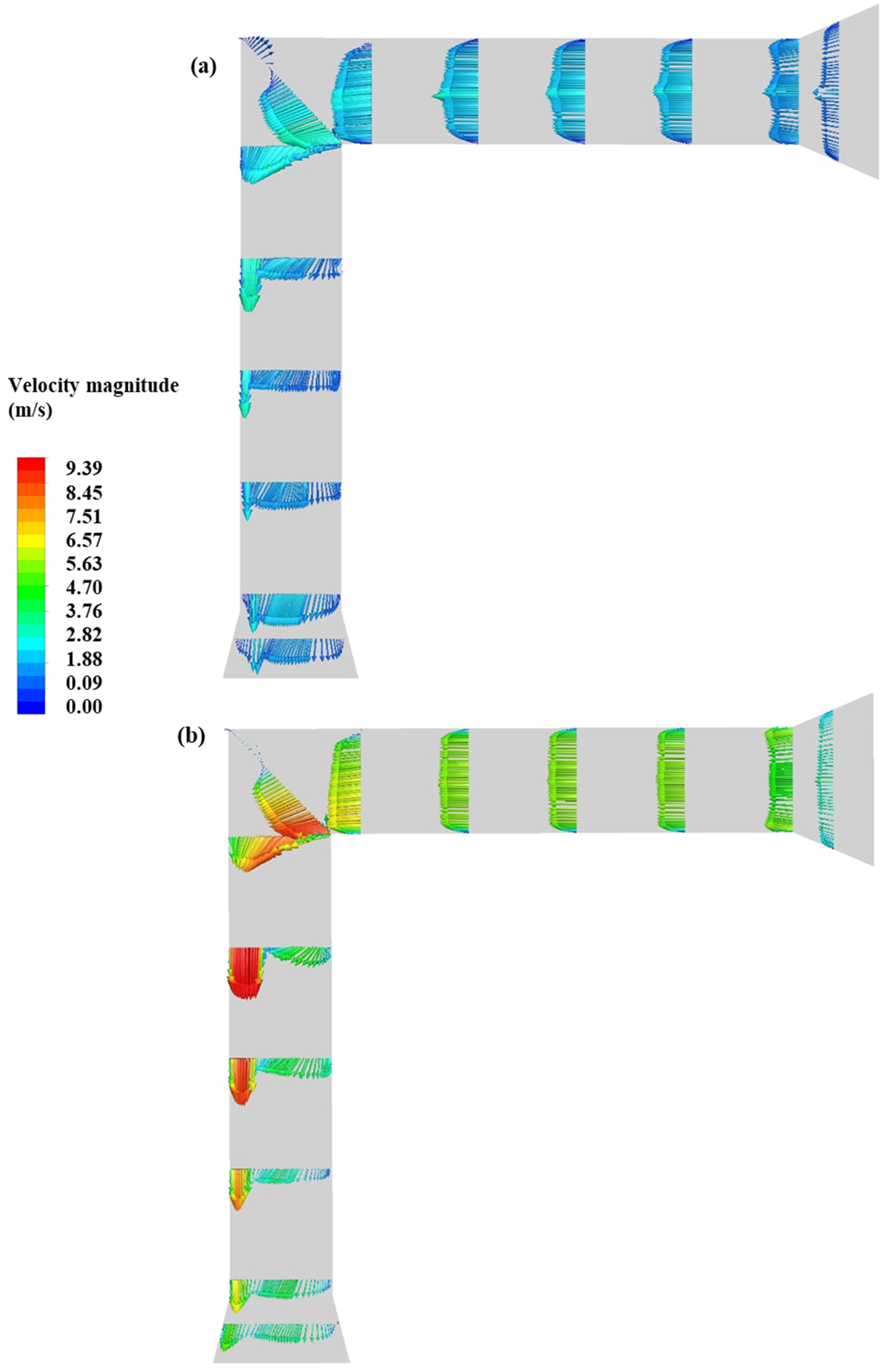
3.2. Impact of Flow Rate
3.3. Impact of Cone Angle
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Abbreviations | |
| COPD | chronic obstructive pulmonary disease |
| pMDI | pressurized metered-dose inhaler |
| CFD | computational fluid dynamics |
| APSD | aerodynamic particle size distribution |
| IP | induction port |
| USP | United States pharmacopeia |
| TKE | turbulent kinetic energy |
| 3D | 3- dimensional |
| BDP | beclomethasone dipropionate particles |
| RMSE | root-mean-square error |
| SIMPLE | semi-implicit method for pressure-linked equations |
| MMAD | mass median aerodynamic diameter |
| Notations | |
| particle diameter, (µm) | |
| mean diameter, (µm) | |
| turbulent kinetic energy, (m2/s2) | |
| constant drag coefficient, (-) | |
| particle velocity, (m/s) | |
| Re | Reynolds number, (-) |
| spread parameter, (-) | |
| fraction of total mass, (-) | |
| X | dynamic shape factor, (-) |
| coefficient of proportionality, (-) | |
| aerodynamic diameter, (µm) | |
| time-averaged velocity, (m/s) | |
| static pressure, (Pa) | |
| Greek letters | |
| standard density, (kg/m3) | |
| fluid specific mass, (kg) | |
| particle density, (kg/m3) | |
| kinematic viscosity, (m2/s) | |
| turbulent viscosity, (m2/s) | |
| viscosity of gas, (m2/s) | |
| gas density, (kg/m3) | |
| turbulent dissipation rate, (s−1) | |
| , , , β, | constant coefficient of SST model, (-) |
| mass flow rate, (kg/s) |
References
- Oliveira, R.F.; Teixeira, S.F.; Silva, L.F.; Teixeira, J.C.; Antunes, H. Development of new spacer device geometry: A CFD study (part I). Comput. Methods. Biomech. Biomed. Eng. 2012, 15, 825–833. [Google Scholar] [CrossRef] [PubMed]
- McDonald, K.J.; Martin, G.P. Transition to CFC-free metered dose inhalers into the new millennium. Int. J. Pharm. 2000, 201, 89–107. [Google Scholar] [CrossRef]
- Rau, J.L. The inhalation of drugs: Advantages and problems. Respir. Care 2005, 50, 367–382. [Google Scholar] [PubMed]
- Cochrane, M.G.; Bala, M.V.; Downs, K.E.; Mauskop, J.; Ben-Joseph, R.H. Inhaled corticosteroids for asthma therapy: Patient compliance, devices, and inhalation technique. Chest 2000, 117, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Kofman, C.; Berlinski, A.; Zaragoza, S.; Teper, A. Aerosol therapy for pediatric outpatients. J. Respir. Care. Pract. 2004, 117, 26–28. [Google Scholar]
- Dutta, R.; Spence, B.; Wei, X.; Dhapare, S.; Hindle, M.; Longest, P.W. CFD guided optimization of nose-to-lung aerosol delivery in adults: Effects of inhalation waveforms and synchronized aerosol delivery. Pharm. Res. 2020, 37, 199. [Google Scholar] [CrossRef]
- Ahookhosh, K.; Saidi, M.; Mohammadpourfard, M.; Aminfar, H.; Hamishehkar, H.; Farnoud, A.; Schmid, O. Flow structure and particle deposition analyses for optimization of a pressurized metered dose inhaler (pMDI) in a model of tracheobronchial airway. Eur. J. Pharm. Sci. 2021, 164, 105911. [Google Scholar] [CrossRef]
- Clarke, A.R. Medical aerosol inhalers: Past, present, and future. Aerosol Sci. Technol. 1995, 22, 374–391. [Google Scholar] [CrossRef]
- Longest, P.W.; Hindle, M. CFD simulations of enhanced condensational growth (ECG) applied to respiratory drug delivery with comparisons to in vitro data. J. Aerosol Sci. 2010, 41, 805–820. [Google Scholar] [CrossRef]
- Martin, A.R.; Finlay, W.H. The effect of humidity on the size of particles delivered from metered-dose inhalers. Aerosol Sci. Technol. 2005, 39, 283–289. [Google Scholar] [CrossRef]
- Mitchell, J.P.; Nagel, M.W.; Wiersema, K.J.; Doyle, C.C.; Migounov, V.A. The effect of humidification on the size distribution of aerosols delivered to the mechanically ventilated patient. In Proceedings of the 14th International Society for Aerosols in Medicine Congress, Baltimore, MD, USA, 14–18 June 2003. [Google Scholar]
- Hindle, M.; Longest, P.W. Evaluation of enhanced condensational growth (ECG) for controlled respiratory drug delivery in a mouth-throat and upper tracheobronchial model. Pharm. Res. 2010, 27, 1800–1811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Finlay, W.H.; Matida, E.A. Particle deposition measurements and numerical simulation in a highly idealized mouth–throat. J. Aerosol Sci. 2004, 35, 789–803. [Google Scholar] [CrossRef]
- Dhand, R. Inhalation therapy with metered-dose inhalers and dry powder inhalers in mechanically ventilated patients. Respir. Care. 2005, 50, 1331–1345. [Google Scholar] [PubMed]
- Zhu, B.; Traini, D.; Young, P. Aerosol particle generation from solution-based pressurized metered dose inhalers: A technical overview of parameters that influence respiratory deposition. Pharm. Dev. Technol. 2015, 20, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.; Heimbecher, S.; Hart, J.L.; Sequeira, J. Influence of the metering chamber volume and actuator design on the aerodynamic particle size of a metered dose inhaler. Drug Dev. Ind. Pharm. 2003, 29, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Ruzycki, C.A.; Javaheri, E.; Finlay, W.H. The use of computational fluid dynamics in inhaler design. Expert Opin. Drug Deliv. 2013, 10, 307–323. [Google Scholar] [CrossRef]
- Vinchurkar, S.; Longest, P.W.; Peart, J. CFD simulations of the Andersen cascade impactor: Model development and effects of aerosol charge. J. Aerosol Sci. 2009, 40, 807–822. [Google Scholar] [CrossRef]
- Oliveira, R.F.; Ferreira, A.C.; Teixeira, S.F.; Teixeira, J.C.; Marques, H.C. pMDI spray plume analysis: A CFD study. In Proceedings of the World Congress on Engineering, London, UK, 3–5 July 2013. [Google Scholar]
- Buchmann, N.A.; Duke, D.J.; Shakiba, S.A.; Mitchell, D.M.; Stewart, P.J.; Traini, D.; Honnery, D.A. Novel high-speed imaging technique to predict the macroscopic spray characteristics of solution based pressurized metered dose inhalers. Pharm. Res. 2014, 31, 2963–2974. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Zhou, Y.; Su, W.C. Deposition of particles in human mouth–throat replicas and a USP induction port. J. Aerosol Med. Pulm. Drug Deliv. 2015, 28, 147–155. [Google Scholar] [CrossRef]
- Bass, K.; Longest, P.W. Recommendations for simulating microparticle deposition at conditions similar to the upper airways with two-equation turbulence models. J. Aerosol Sci. 2018, 119, 31–50. [Google Scholar] [CrossRef]
- Koullapis, P.; Kassinos, S.C.; Muela, J.; Perez-Segarra, C.; Rigola, J.; Lehmkuhl, O.; Nicolaou, L. Regional aerosol deposition in the human airways: The SimInhale benchmark case and a critical assessment of in silico methods. Eur. J. Pharm. Sci. 2018, 113, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Duke, D.J.; Scott, H.N.; Kusangaya, A.J.; Kastengren, A.L.; Matusik, K.; Young, P.; Honnery, D. Drug distribution transients in solution and suspension-based pressurized metered dose inhaler sprays. Int. J. Pharm. 2019, 566, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Ganderton, D.; Lewis, D.; Davies, R. The formulation and evaluation of a CFC-free budesonide pressurized metered dose inhaler. Respir. Med. 2003, 97, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Smyth, H.; Brace, G.; Barbour, T.; Gallion, J.; Grove, J.; Hickey, A.J. Spray pattern analysis for metered dose inhalers: Effect of actuator design. Pharm. Res. 2006, 23, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Fadl, A.; Wang, J.; Zhang, Z.; Cheng, Y.S. Effects of MDI spray angle on aerosol penetration efficiency through an oral airway cast. J. Aerosol Sci. 2007, 38, 853–864. [Google Scholar] [CrossRef]
- Gavtash, B.; Versteeg, H.; Hargrave, G.; Myatt, B.; Lewis, D.; Church, T.; Brambilla, G. Multi-physics theoretical approach to predict pMDI spray characteristics. Drug Deliv. Lungs. 2017, 27, 73–76. [Google Scholar]
- Yousefi, M.; Inthavong, K.; Tu, J. Effect of pressurized metered dose inhaler spray characteristics and particle size distribution on drug delivery efficiency. J. Aerosol Med. Pulm. Drug Deliv. 2017, 30, 359–372. [Google Scholar] [CrossRef]
- Chen, Y.; Young, P.M.; Murphy, S.; Fletcher, D.F.; Long, E.; Lewis, D.; Traini, D. High-speed laser image analysis of plume angles for pressurised metered dose inhalers: The effect of nozzle geometry. AAPS PharmSciTech 2017, 18, 782–789. [Google Scholar] [CrossRef]
- Lewis, D.A.; O’Shea, H.; Church, T.K.; Brambilla, G.; Traini, D.; Young, P.M. Exploring the impact of sample flowrate on in vitro measurements of metered dose inhaler performance. Int. J. Pharm. 2016, 514, 420–427. [Google Scholar] [CrossRef][Green Version]
- ANSYS® Inc. ANSYS Fluent Theory Guide; Release. R1, v193; ANSYS® Inc.: Canonsburg, PA, USA, 2019. [Google Scholar]
- Newman, S.P. Principles of metered-dose inhaler design. Respir. Care 2005, 50, 1177–1190. [Google Scholar]
- Mortazavi, H.; Pakzad, L. The hydrodynamics and mixing performance in a moving baffle oscillatory baffled reactor through computational fluid dynamics (CFD). Processes 2020, 8, 1236. [Google Scholar] [CrossRef]
- Moskal, A.; Gradoń, L. Temporary and spatial deposition of aerosol particles in the upper human airways during breathing cycle. J. Aerosol Sci. 2002, 33, 1525–1539. [Google Scholar] [CrossRef]
- Longest, P.W.; Holbrook, L.T. In silico models of aerosol delivery to the respiratory tract—Development and applications. Adv. Drug Deliv. Rev. 2012, 64, 296–311. [Google Scholar] [CrossRef] [PubMed]
- Crowe, C.T.; Troutt, T.R.; Chung, J. Numerical models for two-phase turbulent flows. Annu. Rev. Fluid Mech. 1996, 28, 11–43. [Google Scholar] [CrossRef]
- Anderson, T.B.; Jackson, R. Fluid mechanical description of fluidized beds. Equations of motion. Ind. Eng. Chem. Fundam. 1967, 6, 527–539. [Google Scholar] [CrossRef]
- Wilcox, D.C. Turbulence Modeling for CFD; DCW industries: Palm Drive La Canada Flintridge, CA, USA, 1998; Volume 2, pp. 103–217. [Google Scholar]
- Versteeg, H.K.; Malalasekera, W. An Introduction to Computational Fluid Dynamics: The Finite Volume Method; Pearson education: London, UK, 2007. [Google Scholar]
- Paul, M.M.; Pakzad, L. Bubble size distribution and gas holdup in bubble columns employing non-Newtonian liquids: A CFD Study. Can. J. Chem. Eng. 2022, 1–17. [Google Scholar] [CrossRef]
- Li, Z.; Kleinstreuer, C.; Zhang, Z. Simulation of airflow fields an microparticle deposition in realistic human lung airway models. part i: Air flow patterns. Eur. J. Mech. B/Fluids 2007, 26, 632–649. [Google Scholar] [CrossRef]
- Clift, R.; Grace, J.R.; Weber, M.E. Bubbles, Drops, and Particles; Academic Press Inc.: New York, NY, USA, 1978. [Google Scholar]
- Buckley, R.; Loyalka, S. Cunningham correction factor and accommodation coefficient: Interpretation of Millikan’s data. J. Aerosol Sci. 1989, 20, 347–349. [Google Scholar] [CrossRef]
- Cunningham, E. On the velocity of steady fall of spherical particles through fluid medium. Proceedings of the Royal Society of London. Math. Phys. 1910, 83, 357–365. [Google Scholar]
- Inthavong, K.; Ye, Y.; Ding, S.; Tu, J. Comparative study of the effects of acute asthma in relation to a recovered airway tree on airflow patterns. In Proceedings of the 13th International Conference on Biomedical Engineering, Singapore, 3–6 December 2008; pp. 1555–1558. [Google Scholar]
- Alatrash, A.; Matida, E. Characterization of medication velocity and size distribution from pressurized metered-dose inhalers by phase Doppler anemometry. J. Aerosol Med. Pulm. Drug Deliv. 2016, 29, 501–513. [Google Scholar] [CrossRef]
- Dunham, R.Q. Rosin-Rammler Distributions in ANSYS Fluent (No. LA-UR-12-24026); Los Alamos National Lab (LANL): Los Alamos, NM, USA, 2012. [Google Scholar]
- Sarkar, S.; Peri, S.P.; Chaudhuri, B. Investigation of multiphase multicomponent aerosol flow dictating pMDI-spacer interactions. Int. J. Pharm. 2017, 529, 264–274. [Google Scholar] [CrossRef]
- Finlay, W.H.; Darquenne, C. Particle size distributions. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 178–180. [Google Scholar] [CrossRef]
- Li, W.I.; Perzl, M.; Heyder, J.; Langer, R.; Brain, J.D.; Englmeier, K.H.; Edwards, D.A. Aerodynamics and aerosol particle deaggregation phenomena in model oral-pharyngeal cavities. J. Aerosol Sci. 1996, 27, 1269–1286. [Google Scholar] [CrossRef]
- Nave, R.; Mueller, H. From inhaler to lung: Clinical implications of the formulations of ciclesonide and other inhaled corticosteroids. Int. J. Gen. Med. 2013, 6, 99. [Google Scholar]
- Feddah, M.R.; Brown, K.F.; Gipps, E.M.; Davies, N.M. In-vitro characterization of metered dose inhaler versus dry powder inhaler glucocorticoid products: Influence of inspiratory flow rates. J. Pharm. Pharm. Sci. 2007, 3, 318–324. [Google Scholar]
- Cheng, Y.S.; Zhou, Y.; Chen, B.T. Particle deposition in a cast of human oral airways. Aerosol Sci. Technol. 1999, 31, 286–300. [Google Scholar] [CrossRef]
- Longest, P.W.; Hindle, M.; Choudhuri, S.D.; Xi, J. Comparison of ambient and spray aerosol deposition in a standard induction port and more realistic mouth–throat geometry. J. Aerosol Sci. 2008, 39, 572–591. [Google Scholar] [CrossRef]
- Tang, P.; Kwok, P.C.L.; Tong, Z.; Yang, R.; Raper, J.A.; Chan, H.K. Does the United States Pharmacopeia throat introduce de-agglomeration of carrier-free powder from inhalers? Pharm. Res. 2012, 29, 1797–1807. [Google Scholar] [CrossRef]
- Gjellerup, C.; Frederiksen, S.O. CFD and PIV investigation of the flow inside the USP throat and in a replica of the human upper airways. Joint Dissertation (MSc), Technical University of Denmark, Lyngby, Denmark, 2007. [Google Scholar]











| Flow Rate (L/min) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 18.0 | 28.3 | 45.0 | 60.0 | 80.0 | |||||
| Spray Cone Angle (°) | |||||||||
| 2 | 4 | 8 | 10 | 17 | 20 | ||||
| Parameter | Cell Size (mm) | Number of Cells |
|---|---|---|
| Mesh A | 2.00 | 114,825 |
| Mesh B | 1.98 | 222,150 |
| Mesh C | 1.68 | 414,589 |
| Mesh D | 0.82 | 812,448 |
| Distribution model | Rosin–Rammler |
| Minimum diameter (µm) | 0.45 |
| Maximum diameter (µm) | 9 |
| Mean diameter (µm) | 1.828 |
| Spread parameter (-) | 1.3676 |
| Initial velocity (m/s) | 100 |
| Spray cone angle (°) | 17 |
| Flow rates (L/min) | 18.0 | 28.3 | 45.0 | 60.0 | 80.0 |
| Particle deposition in IP (%) | 12.4 | 9.5 | 8.1 | 7.5 | 7.1 |
| MMAD (µm) | 1.8 ± 0.1 | 1.6 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dastoorian, F.; Pakzad, L.; Kozinski, J.; Behzadfar, E. A CFD Investigation on the Aerosol Drug Delivery in the Mouth–Throat Airway Using a Pressurized Metered-Dose Inhaler Device. Processes 2022, 10, 1230. https://doi.org/10.3390/pr10071230
Dastoorian F, Pakzad L, Kozinski J, Behzadfar E. A CFD Investigation on the Aerosol Drug Delivery in the Mouth–Throat Airway Using a Pressurized Metered-Dose Inhaler Device. Processes. 2022; 10(7):1230. https://doi.org/10.3390/pr10071230
Chicago/Turabian StyleDastoorian, Farnia, Leila Pakzad, Janusz Kozinski, and Ehsan Behzadfar. 2022. "A CFD Investigation on the Aerosol Drug Delivery in the Mouth–Throat Airway Using a Pressurized Metered-Dose Inhaler Device" Processes 10, no. 7: 1230. https://doi.org/10.3390/pr10071230
APA StyleDastoorian, F., Pakzad, L., Kozinski, J., & Behzadfar, E. (2022). A CFD Investigation on the Aerosol Drug Delivery in the Mouth–Throat Airway Using a Pressurized Metered-Dose Inhaler Device. Processes, 10(7), 1230. https://doi.org/10.3390/pr10071230






