Abstract
The filamentous fungus Penicillium canescens, isolated from oil-polluted soil, was evaluated for its ability to dissipate high-molecular-weight polycyclic aromatic hydrocarbons (PAH). The study was conducted in a microcosm containing 180 g of historical PAH-contaminated soil under non-sterile conditions with two incubation temperatures (14 °C and 18 °C) on a 12-h cycle. The experiment was conducted over 8 months, with four experimental conditions created by varying the volumes of the bulking agent and vegetable oil (olive oil) and the time of addition of these compounds. The PAH dissipation performance of the fungal augmentation treatment was compared with that achieved with a biostimulated soil (bulking agent and vegetable oil) and with the untreated soil as control. The greatest PAH dissipation was obtained with P. canescens bioaugmentation (35.71% ± 1.73), with 13 of the 16 US EPA PAH significantly dissipated, at rates above 18%, and particularly high-molecular-weight PAH, composed of more than three fused aromatic rings. Nematode toxicity tests indicated a significant decrease in the toxicity of soil bioaugmented by this fungus. Fulvic and humic contents were significantly increased by this treatment. All these results suggest that bioaugmentation with P. canescens can be used to restore soils with long-term PAH contamination.
1. Introduction
Polycyclic aromatic hydrocarbons (PAH), produced mainly by anthropogenic activities that use fossil energy sources, such as coal and oil, are widespread pollutants in the environment. They represent a real danger to human health and biotic communities in terrestrial and aquatic ecosystems. Indeed, some PAH are known to be toxic, mutagenic, carcinogenic, and teratogenic []. Historical industrial activities have resulted in numerous PAH-polluted soils [] requiring the development of clean-up techniques. PAHs can be divided into two categories: low molecular weight PAH (LMW PAH) composed of less than four aromatic rings and high molecular weight PAH (HMW PAH) composed of four or more rings. In historical contaminated soils, HMW PAH can be strongly bound to soil organic matter (SOM) and form nonextractable residues (NER) []. NER strongly sequestered by SOM cannot be degraded by natural soil attenuation and is hazardous when mobilised.
Different techniques are available to clean up PAH-polluted soils, i.e., physical (thermal treatment) chemical (oxidation or solvent extraction). These techniques are often very expensive leading to a soil structure deterioration. However, in recent years, soft chemical oxidation methods, i.e., Fenton-like oxidation, which are more respectful of the soil integrity, have also been developed []. Among biological ones, bioremediation by fungi is one of the most cost-effective and soil-friendly []. Fungi, representing the dominant living biomass in soils, have an important advantage over bacteria with their mycelial network able to prospect a larger surface of soil. Moreover, the colonization of soil by fungal mycelium results in enmeshment and aggregation of soil particles and improvement of soil structure, sometimes facilitating contaminant bioavailability []. Compared with bacteria, filamentous fungi show some other advantages in the transport or translocation of essential substances, including nutrients and water, and the pollutant itself, over significant distances [,]. Fungal mycelia can also act as “highways” in facilitating the transport of pollutant-degrading bacteria over distance in soil which can enhance bioremediation [].
Concerning organic pollutants biodegradation, Basidiomycota and Ascomycota have been extensively investigated for their ability to degrade PAH. White rot fungi belonging to Basidiomycota produce extracellular enzymes, such as lignin peroxidases, manganese peroxidases, and laccases capable of degrading PAH []. However, the conditions (lignin and/or moisture) necessary for the secretion of enzymes are rarely present in PAH-polluted soils. Moreover, these fungi are weak competitors against resident microflora [], in contrast to some Ascomycota fungi. Ascomycota fungi include a high diversity of fungal species, common inhabitants of extremely polluted areas [,,]. Their occurrence and their contribution to pollutant degradation has been highlighted in numerous recent studies [,,]. These Ascomycota fungi possess some advantages in comparison to white-rot fungi, i.e., their fast growth at neutral pH and their intracellular metabolism of xenobiotics, mostly mediated by Phase I and Phase II detoxification metabolism, mainly by cytochrome P450s, as described in eukaryotic organisms [].
In soils, the hydrophobic nature of PAH often limits the efficiency of its dissipation using biological agents, because of their low bioavailability, especially for HMW PAH. Indeed, because of the interaction of HMW PAH with soil organic matter, the sorption and desorption of PAH greatly affect the mycoremediation efficiency achieved with polluted soils, especially in aged historical soils. The use of vegetable oils of various botanical origins (peanut, sunflower, olive, rapeseed, and soybean, among others) in the treatment of PAH-contaminated soils has been documented in recent bibliographic reviews [,]. To enhance the desorption of PAH strongly bound to soil organic matter, especially in an aged contaminated soil, an original and cheap emulsifying solution was designed for use as a biodegradable surfactant in the present study.
The objective of this work was to study the ability of a telluric saprotrophic Ascomycota fungus, Penicillium canescens to dissipate the 16 PAH identified as priority pollutants by the US Environmental Protection Agency (EPA) in industrial aged PAH-contaminated soil microcosms on a laboratory scale. This fungus was previously isolated from a brownfield aged PAH-contaminated soil sampled in the North of France []. This fungus was selected as it showed a high capacity to degrade benzo(a)pyrene (BaP) in mineral medium. Thus, the aim of this study was not to explore the metabolism of the selected Ascomycota fungus in details but to compare it effectiveness in presence of an original cheap emulsifying solution designed for purpose as a biodegradable surfactant. Three comparative treatments for microcosms were followed: P. canescens bioaugmentation, biostimulation of endogenous microflora, and natural attenuation. To consider the potential risk of generating PAH metabolites more toxic than the initial compound [], the remaining toxicity was assessed after 8 months of incubation using an ecotoxicological test with Caenorhabditis elegans (Nematoda, Rhabditidae, Secernentea). Finally, a gravimetric analysis of humic and fulvic acids (HFA) was undertaken to evaluate possible SOM changes after treatment with P. canescens.
2. Materials and Methods
2.1. Chemicals and Materials
All chemical products were reagent grade of the highest purity available and were obtained from Sigma-Aldrich (St. Louis, MO, USA). PAH-polluted soil (125.63 ± 5.6 mg PAH kg−1 soil) was kindly provided by EACM (France) from an old petrochemical factory in the north of France. No additional data were provided to us for confidentiality reasons. The standard mineral salt medium (MM) included (g L−1) KCl (0.5), NaH2PO4 ⋅ 2H2O (1.2), MgSO4 (0.7), NO3NH4 (2), and CuSO4 ⋅ 5H2O (0.01). For fungus preparation inoculum, the mineral salt medium (FMM) consisted of KCl (0.5), NaH2PO4 ⋅ 2H2O (0.6), Na2PO4 ⋅ 2H2O (1.04), MgSO4 (0.5), NO3NH4 (7.8), and CuSO4 ⋅ 5H2O (0.05). The emulsifying solution (ES) was prepared by mixing olive oil (either 2.34 or 4.68 g) and L-α-Lecithin (either 0.36 or 0.72 g) in MM with an Ultra-Turrax apparatus (15,000 rpm) for 5 min. A malt yeast extract agar (MYEA) medium was used for routine growth.
2.2. Microorganism and Inoculum Preparation
Penicillium canescens, previously isolated in our laboratory with high capacity for dissipating benzo(a)pyrene (BaP) in mineral liquid medium [], was selected for the microcosm study. A mixture composed of 50% wood chips and 50% cardboard chips was used as the fungal carrier and bulking agent. The bulking agent mixture (200 g) was carefully mixed with malt (66 g) in 2-L Erlenmeyer flasks. Bulking agent hydration was achieved by FMM to obtain 70% water holding capacity (WHC). 2-Liter Erlenmeyer flasks with hydrated carriers were sterilised twice (at 136 °C for 10 min) at 3-day intervals. Ten mycelium agar plugs (7 mm in diameter) from a 7-day-old P. canescens pre-culture on MYEA were added to the flasks. The cultures were incubated at room temperature (18 ± 1 °C) for 15 days.
2.3. Microcosm Setup
All treatments are summarised in Table 1. The microcosm experiments were conducted in quadruplicate in 520-mL transparent microboxes containing either 160 or 180 g of dry-mass equivalent 2-mm-sieved PAH-contaminated non-sterile soil. Four treatment strategies were evaluated: (NA) natural attenuation, (BS) biostimulation with only the endogenous microflora, (BA) bioaugmentation inoculated with P. canescens, and a control (C) corresponding to air-dried untreated soil for evaluating abiotic PAH losses. For NA, only distilled water was added to the soil to maintain WHC at 95%. For the BA and BS treatments, two experimental parameters concerning fungal inoculum quantity and the time of ES addition were tested. For the BS1 treatment, 180 g of soil were mixed with 20 g of uninoculated bulking agent, and the whole was simultaneously hydrated with ES. For the BS3 treatment, 180 g of soil were mixed with ES four days before the addition of the bulking agent. BS2 and BS4 only differ from BS1 and BS3, respectively, in the bulking agent quantity and the ES volume necessary to obtain 95% of WHC. The same strategy was used for the BA treatments, with the only difference being the prior inoculation of the bulking agent by P. canescens. Microcosms were incubated for 8 months at temperature cycles (14 °C/18 °C) of 12 h under static conditions in the dark. Filters in the microboxes lids allowed air renewal in each microcosm. WHC was adjusted to 95% each month with distilled water by gravimetric control.

Table 1.
Experimental design and treatments.
2.4. PAH Extraction and Analysis
After 8 months incubation, soils were air dried (20 ± 1 °C) for one week, homogenised, and passed through a 2-mm sieve to remove bulking agent. For each quadruplicate, the residual levels of PAH were analysed in soil samples. PAH levels were determined via gas chromatography and a mass spectrometry detector (GC/MS) by the AGROLAB laboratory (Dijon, France) according to French standard method (NF ISO 18287 August 2006/X31-170).
2.5. PAH Bioavailability
1-Butanol (BuOH) was selected as the extraction solvent for measuring PAH bioavailability in soil (adapted from Umeh et al. [,]). After 8 months of incubation, 3 mL of BuOH was added to 1 g of PAH-polluted soil in a 22-mL glass centrifuge tube for each quadruplicate. The soil–BuOH mixture was vortexed for 1 min and centrifuged (4500× g for 15 min). The PAH concentration of the BuOH supernatant was determined by the same method described in Section 2.4.
2.6. Ecotoxicological Tests
Soil ecotoxicity was evaluated with bioassays conducted with Caenorhabditis elegans (Nematoda, Rhabditidae, and Secernentea). Different ecotoxicity variables (growth inhibition, fertility, and reproduction) were assessed for the best PAH dissipation treatment in comparison to the control C (by ELISOL environment, Congénies, France) according to NF ISO 10872, July 2010.
2.7. Fulvic and Humic Acids Extraction
A classical extraction of humic and fulvic acids (HFA) was undertaken, as recommended by the International Humic Substances Society. As a first step, 5 g of PAH-polluted dried soil (in quadruplicate) were mixed with 20 mL of chloroform in a 50-mL centrifuge tube. The chloroform–soil mixture was vortexed for 1 min and centrifuged (4500× g for 15 min). The supernatant containing PAH was removed. The soil washing operation conducted with chloroform was repeated until no ultraviolet (UV) fluorescence was detected in the chloroformic phase at 360 nm. After 2 days of drying at 60 °C, PAH-free soil was mixed with 20 mL of 0.1 M NaOH solvent. The NaOH-soil mixture was vortexed for 5 min, left to stand for 1 h, and centrifuged (4500× g for 20 min). Brown–yellow supernatant was collected and stored at 4 °C. This experimental step was repeated until the colour extract was negligible. All supernatant fractions were pooled, dialysed, lyophilised, and weighed. The elementary composition of the humic substances was determined using an elemental analyser (ThermoFisher Scientific, Illkirch, France).
2.8. Statistical Tests
The PAH dissipation results achieved are expressed by the mean value ± standard error of the quadruplicate for each treatment. After verification of the data normality using the Shapiro–Wilk test (p ≥ 0.05), the Mann–Whitney U test was used to compare the results of the treatments with those of the control (p ≤ 0.05). The differences between the treatments and C were determined to be significant (s) or non-significant (ns). Principal component analysis (PCA) was performed on the following four PAH variables: dissipation, water solubility, the log of the octanol/water coefficient (Log Kow) and the initial concentration. Observations were made for the 16 EPA PAH. PCA was performed using the Addinsoft XLSTAT statistical and data analysis tool (Paris, France; https://www.xlstat.com, accessed on 31 January 2022).
3. Results and Discussion
3.1. PAH Dissipation in Soil Microcosms: Bioaugmentation versus Biostimulation
PAH dissipation was assessed after 8 months of incubation in an historical polluted soil with a total PAH contamination of approximately 123.63 mg kg−1 of soil. After testing the normality (Shapiro–Wilk test), the Mann–Whitney U test was used to compare all BA (bioaugmentation with P. canescens) and BS (biostimulation) treatments with the control C. The results confirmed that PAH dissipation for the 16 EPA PAH were statistically significant (p = 0.05) in all cases. As shown in Figure 1, the treatments can be classified according to the percentage of total PAH dissipated in the following order: BA4, BS4, BS3, BA2, BS2, BA3, BA1, BS1, and NA. The best PAH decrease was achieved by BA4 (35.71% ± 1.73), which is the only treatment whose results were significantly different from those of all other treatments.

Figure 1.
Average PAH dissipation rates of treatments after 8 months of incubation. Error bars represent standard deviations. Letters designate groups with no statistically significant differences. Treatments are identified in Table 1.
Table 2 details the 16 EPA PAH dissipation rates for NA, BS4, and BA4. The BA4 treatment dissipated 13 of the 16 PAH at rates greater than 18%, while NA and BS4 dissipated only 7 and 9 of the PAH, respectively (>10%). Moreover, for BA4, the final concentration of each PAH dissipated was significantly different from the initial PAH concentration in the soil, except for NAPH, ACE and DBahA. These 3 PAH were present at very low concentrations in the soil. This result underlines that this fungus P. canescens is able to dissipate not only LMW PAH but also HMW ones. This a very interesting result for industrial scale-up.

Table 2.
Concentration of PAH in initial soil and PAH dissipation in soil microcosms after 8 months of incubation (mean value of quadruplicates). Treatments are identified in Table 1.
BA4 is a fungal-bioaugmented microcosm assay (BA) inoculated with a twofold ES added 4 days before the addition of a twofold bulking agent inoculated with P. canescens. These three parameters, i.e., fungal inoculation with the selected fungus, the quantity and nature of the bulking agent, and the timing of the ES addition were very important to the dissipation rates achieved. In fact, the variation of any one of these parameters led to a decrease in the PAH dissipation rate, as observed for other treatments. Indeed, for the same experimental conditions, the substitution of P. canescens (BA4) by endogenous microflora (BS4) led to a significant reduction of 11.8% in PAH dissipation. The experiments were conducted in non-sterile soils, so it is not possible to conclude whether this result is directly linked to P. canescens or to it interactions with endogenous fungi and bacteria. However, the significant reduction in PAH dissipation between BA4 and BS4 is consistent with the positive involvement of P. canescens. This hypothesis is reinforced by the following results. First, as shown in Table 1, a single-dose input of bulking agent inoculated with P. canescens (BA3) resulted in a significant 20.5% reduction in PAH dissipation, compared to BA4. Moreover, BbF, BkF, BaP, BghiP, and IcdP are very stable chemical PAH, with five or six aromatic rings, which are insoluble in water and classified as persistent hydrophobic contaminants. Their dissipation at a rate higher than 18% in BA4 most likely indicates that P. canescens has a high capacity to degrade hydrophobic aromatic molecules. Such a significantly lower reduction of five- and six-ringed PAH is very rarely obtained in similar studies conducted in aged soils [].
The selected P. canescens strain was isolated in a previous study from a former bus depot in northern France and was selected as the most efficient strain in BaP degradation (44.2%) in a mineral medium []. The literature review underlines the capabilities of P. canescens to degrade other pollutants such as biphenyls [], fluorene [], dibenzofuran [] and hexadecane [] in mineral medium. It was described by Say et al. [] as biosorbent for heavy metals (lead, cadmium, mercury, and arsenic ions) from aqueous solutions and was therefore suggested as a potential strain for the clean-up of the environment, especially the aqueous systems. Additionally, in recent reviews, Leitao [] and Zehra et al. [] emphasised that other Penicillium species have greater economic pollutant transformation potential than other imperfect fungal strains because Penicillium species can degrade various types of xenobiotics with low cosubstrate requirements. Therefore, this species could be of interest for developing economically feasible processes for pollutant transformation. However, few studies on this subject have been conducted in natural complex environments, such as soil and industrial effluents, with a real scale-up in in situ and ex situ bioreactor landfills applications. The present study, conducted in historical polluted soil, underlines the potential of P. canescens for both 16 EPA PAH dissipation and establishment in non-sterile soil with endogenous microflora, a real novelty result.
Finally, we deliberately chose to alternate the temperature between 14 °C to 18 °C ever 12 h to mimic the nychthemeral cycle in field conditions, unlike microcosm studies commonly performed at constant temperatures (approximately 25 °C). Temperature is an important environmental parameter, which influences, among other things, fungal enzymatic activities, including those involved in PAH dissipation. A decrease in nocturnal temperatures might favour the growth of endogenous psychrophilic organisms’ growth, which are not necessarily competent in PAH dissipation. This nychthemeral cycle is very rarely considered in pollutant degradation studies conducted at laboratory scales. The next step would be upscaling from this laboratory research to a pilot study and then to a full-scale test of remediation of industrial historical polluted soils using Penicillium strains. This would be achieved in further research conducted with design offices in bioremediation.
A second group of four treatments, statistically non-significant with respect to NA, can be distinguished, with total PAH dissipation percentages inferior to 20%: BS2, with 18.31% ± 4.18; BA3, with 13.73% ± 10.35; BA1, with 11.46% ± 2.73; and BS1, with 10.04% ± 2.52. These treatments did not present all of the conditions necessary for significant dissipation. Either the endogenous microflora development in biostimulation treatments could be competitive to P. canescens but not very efficient in PAH dissipation. Or the quantity of the emulsifying solution was insufficient, as explained below.
3.2. PAH Dissipation in Soil Microcosms: Importance of the Emulsifying Solution
Three combined parameters, i.e., the inoculated strain, the emulsifying solution composition and quantity added seemed to be very important in PAH dissipation, as shown in Figure 1 and Table 2. Indeed, after BA4 treatment with 35.71% ± 1.73 total PAH dissipation, a third group of statistically significant treatments with NA can be distinguished, with a total PAH dissipation of approximately 20–23%: BS4 with 22.49% ± 2.56, BS3 with 20.55% ± 4 and BA2 with 20.55% ± 5.95. The first two treatments, BS4 and BS3, are biostimulation experiments, differing only in the quantities of ES and bulking agent added: a single dose for BS3 and a double dose for BS4. In both cases, ES was added 4 days before the addition of the non-inoculated bulking agent. The BA2 treatment is a bioaugmentation one, with a double dose of ES and bulking agent concomitantly added with P. canescens.
We deliberately formulated ES with olive oil and lecithin for the following reasons. ES could act as an extra carbon and energy source available in soil, thus favouring microflora proliferation. The strategy including nutrient supplementation, especially with carbon one to enhance the growth and activity of microorganisms, is frequently described as a bioremediation approach in both biostimulation and bioaugmentation experiments, especially in soils in which carbon starvation frequently occur. However, in environments in which nutrients can be limiting factors, the application of carbon-rich nutrients to soil enhances also the growth of other soil microbiota. This could result in the exponential growth of unwanted microorganisms, thereby creating competition for the bioaugmented fungi. This might be counterproductive if the most competitive microbial populations are not the best pollutant-degrading ones [] leading to bioremediation failures. The average PAH dissipation rate (approximately 20–23%) observed in BS4, BS3, and BA2 could thus be explained in comparison with the BA4 treatment, with 35.71% ± 1.73 total PAH dissipation. Moreover, the ES application in the biostimulation treatments led to more contrasting results with no significant effects on PAH with five or more rings. Indeed, the BS4 treatment dissipated only 9 of the 16 PAH and preferentially dissipated two-, three-, and four-rings PAH, in comparison with the very wide distribution of the 13 of 16 dissipated PAH for BA4 (Table 2). On the other hand, ES, composed of olive oil and lecithin, was formulated with the hope of enhancing biodegradation. The hydrophobic nature of vegetable oil, which is largely composed of triglycerides (93–99%), with smaller amounts of phospholipids, fatty acids, and neutral lipids, could influence the desorption, solubility, and extraction of PAH present in soils, i.e., through pure physical chemical processes that encourage the partitioning of PAH in oils [,]. These PAH resist to biodegradation in particular due to the adsorption of aged compounds to organic matter and clay, which restrains the pollutant bioavailability for microorganisms. So, the role of vegetable oil and lecithin could act as enhancing PAH mass transfer and therefore biodegradability. Oils have previously been used as surfactants and carbon sources in PAH dissipation experiments. On average, 86% of 16 PAH was dissipated in contaminated soil using white rot fungi (Pleurotus ostreatus) in the presence of fish oil [].
The aim of the present study conducted in non-sterile soil, a complex environment, was not to determine which of the mechanisms described previously was involved but to find the optimal conditions permitting the best dissipation rate of PAH even those which are the most recalcitrant. What is important to note is that BA4 is a fungal–bioaugmented microcosm inoculated with twofold ES added four days before the addition of a twofold bulking agent inoculated with P. canescens. These three parameters, i.e., fungal inoculation with the selected fungus P. canescens, the quantity and nature of bulking agent, and the timing of the addition (four days before) were, in this case, necessary to obtain such a high dissipation rate.
3.3. Multivariable Analysis Based on Four PAH Parameters: Dissipation, Initial Concentration in Soil, Water Solubility, and Log Kow
A multivariate study was conducted to understand the factors influencing the dissipation of the 16 PAH for the BA4 and NA treatments (Figure 2). Four PAH parameters—dissipation %, PAH initial concentration, water solubility, and Log Kow—were studied using principal component analysis (PCA). For better understanding, the Table 3 detailed the physical and chemical properties of PAH.
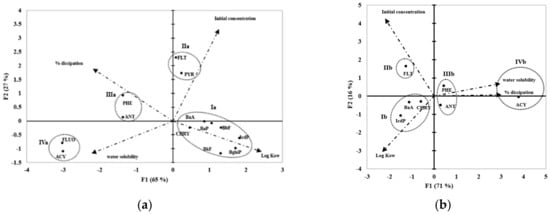
Figure 2.
Principal component analysis (PCA) showing the two first axes (axes F1 and F2 of total variance) for the PAH distribution. (a): BA4 treatment; (b): NA treatment. The PCA was performed on four PAH parameters: % dissipation, PAH initial concentration, water solubility, and Log Kow. For PAH abbreviations, see Table 3. Only PAH types with significant dissipation are shown.

Table 3.
Selected physical and chemical properties of the 16 US EPA PAH.
The inertia percentages recorded for the first two axes were 65% and 27% for BA4 (Figure 2a) and 71% and 16% for NA (Figure 2b). The Euclidian distance computed from the variables led to the classification of four PAH groups, labelled I to IV. Groups Ia (BA4) and Ib (NA) contained PAH with a high Log Kow. Group II included FLT and PYR for BA4 and only FLT for NA. These two PAH are present in high initial concentrations in the studied soil. PHE and ANT, i.e., HAP with three aromatic rings, form group III in both cases. Finally, group IV contained relatively water-soluble PAH. IVa was composed of ACY and FLUO, while IVb was made up solely of ACY.
As expected, a correlation between % dissipation and water solubility was clearly observed for BA4 and NA, as ACY, PHE, and ANT (with a water solubility from 0.07 to 3.9 mg L−1) were highly dissipated. However, let us recall that ACY and ANT are present at very low initial concentrations.
BA4 led to a FLUO dissipation not observed in NA, which was most likely due to the presence of P. canescens. Other differences can also be noted. In group Ib, only three PAH with high Log Kow were significantly dissipated: BaA, CHRY, and IcdP. In BA4, group Ia exhibited dissipation for four additional high-Log Kow PAH: BbF, BkF, BaP, and Bghip. This result would suggest that the presence of P. canescens in BA4 provides better high-Log Kow i.e., better HMW PAH dissipation than with endogenous microflora alone. Such a dissipation of high-Log Kow PAH in microcosms by filamentous fungi has been reported with other fungi [,,]. However, PAH dissipation was approximately 3.6 greater for BA4 in comparison with NA. This difference underlined again the increased PAH mass transfer from soil to the formulated ES enhancing PAH bioavailability in BA4 (as explained above). Figure 2a clearly shows that the dissipation rate of PAH depends not only on their intrinsic physicochemical properties but also on their initial concentrations, as observed for Group IIa. Indeed, PYR, with its Log Kow of 4.88, was dissipated 1.4-fold more than CHRY, which has a Log Kow of the same rank i.e., 5.16.
3.4. Nematode Toxicity Tests
In this study, untreated soil (control C) and the best treatment (BA4) were tested with a standardised toxicity test, using the nematode, Caenorhabditis elegans, as the test organism. This organism was chosen as a well-known experimental model with high sensitivity to different contaminants and representing important functional levels in soil and aquatic ecosystems. It has been shown in several studies to have high potential to be extensively integrated within Environmental Risk Assessment (ERA) routines [,]. The growth, fertility, and reproduction of C. elegans in the soils were compared with the exposure in standard soil LUFA (Table 4). A significant difference was observed for the growth of C. elegans, (29% and 16% inhibition for C and BA4, respectively). C. elegans showed 100% fertility in the reference LUFA soil, as well as in both treatments. Based on the nematode fertility (the presence of eggs in the adults), only 37% of the nematodes exposed to C contained eggs (63% nematode reproduction inhibition), whereas 100% of the nematodes were gravid in BA4 soil. All these results clearly indicate a toxicity decrease in BA4. In such a practical study comparing soil biotreatments, the use of a terrestrial ecotoxicology test seems to be very relevant. Indeed, the purpose of this study was not to characterize the PAH degradation metabolites produced during the best treatment but to find the optimal conditions of PAH dissipation and also to prove its effectiveness not only in terms of pollution abatement but also in reducing the soil toxicity. This is why terrestrial ecotoxicity tests are complementary realized to relevant treatments (based in this study on three parameters, i.e., fungal inoculation with the selected fungus, the quantity and nature of the bulking agent, and the timing of the ES addition).

Table 4.
Inhibition of growth, fertility, and reproduction in untreated (C) and BA4-treated soil in comparison to the standard soil LUFA (mean value of triplicates).
The use of free-living nematodes (principally C. elegans) for evaluating soil toxicity is just the beginning especially for organic pollutants. The advantages of C. elegans are a short lifespan, simple and inexpensive cultivation (300–350 eggs per nematode), and a rapid response to environmental change []. C. elegans can be used to rapidly assess chemical toxicity, with soil pollutants inhibiting C. elegans growth and reproduction []. The use of soil nematodes for soil health assessment may be more widely adopted complementary to chemical measurements of the concentration of pollutants. Our important results suggest a fungal bioremediation process to recover soil health features. From a global environmental perspective, soil health protects against erosion, desertification, and pollution, which threaten not only landscape sustainability but human health as well. These aspects should be more often considered into a global approach to the remediation of historically contaminated soils.
3.5. PAH Bioavailability
In order to investigate the toxicity decrease in BA4 compared to C, bioavailable PAH were assessed. We selected BuOH as a solvent for extracting bioavailable PAH, as recommended by Semple’s research team [,] for risk-based PAH-polluted soils management. As Figure 3 shows, the bioavailable PAH level in BA4 was dramatically reduced, by 41% compared to C. After 8 months of incubation with P. canescens, the bioavailable PAH in BA4 only represented 29.8% of the initial total PAH, compared to 45% in C. This interesting result could partly explain the toxicity decrease versus the nematode, C. elegans, as the test organism, observed for BA4. The toxicity abatement could also be due to the biodegradation in BA4 of other toxic chemical molecules initially present in the soil, as the studied soil comes from a former petrochemical plant (not taken into consideration).

Figure 3.
Comparison of the rate of bioavailable PAH between the fungal biotreated (BA4) soil after 8 months of incubation and untreated soil (C).
Another important result was that not all of the bioavailable PAH were degraded in BA4. Similar research on biostimulation or fungal bioaugmentation has resulted in the maximum PAH dissipation after an incubation period of only 2 months [,]. In our study, we measured PAH dissipation after 8 months of incubation (final measurement), so it is likely that PAH biodegradation reached a steady state in BA4. This PAH dissipation plateau may be due to either energetic substrate depletion being necessary for fungal biodegradation or, over time, endogenous microflora development being more competitive but perhaps less efficient for PAH dissipation.
3.6. Organic Matter Change
During the laboratory experiments carried out for the present study, a difference in colour was observed with the naked eye for BA4. The soil was a darker brown colour than the C soil. A basic SOM study was undertaken. Table 5 shows a significant increase in the HFA content, by a factor of 2.4 between C and BA4, with an HFA carbon content for BA4 five times higher than for C. These findings suggest that biotreatment with P. canescens significantly increases the SOM content. SOM production is an extremely complex process involving primarily the biodegradation and chemomodification of organic matter of plant and animal origin by soil microorganisms and pedofauna []. Several hypotheses could explain the SOM increase in BA4. First, saprotrophic fungi possess numerous enzymatic systems (e.g., lignin peroxidases, peroxidases, laccases, and P450, among others) capable of biodegrading and modifying OM. The molecules obtained from these enzymatic mechanisms serve as precursors for SOM formation []. In BA4, P. canescens and endogenous microorganisms most likely used either the bulking agent cellulose and lignin (15% loss of carrier mass, data not shown) or olive oil (from ES) to produce SOM molecular precursors []. The second hypothesis is based on the fact that some fungi can produce humic acids [,] or humic-like substances []. More work is required to investigate the SOM increase in BA4. Nevertheless, this result is very encouraging for fungal remediation of polluted soils, as the neo-formed SOM could be used not only as a substrate to feed soil fauna but also as a carbon sink.

Table 5.
Comparison of humic and fulvic acids and carbon masses between untreated (C) and bioaugmented (BA4) soils after 8 months of incubation (mean value of quadruplicates ± standard error).
3.7. Limits and Future Research Perspectives
In order to develop a low-cost mycoremediation technique that respects soil integrity, we tested the PAH dissipation efficiency of Penicillium canescens in a non-sterile soil microcosm. Our study originality was based on olive oil use both as a PAH desorbing agent and also as a non-conventional carbon and energy source for P. canescens. The main result obtained is the ability of P. canescens bioaugmentation treatment (BA4) to dissipate PAH compared to the biostimulation treatment (BS4). The studied P. canescens strain, previously selected as the most efficient strain in BaP degradation in a mineral medium [], was also efficient for dissipating the 16 EPA PAH in non-sterile historical polluted soil, a real novelty result. One of the factors that probably contributed to this result is the competitive nature of this fungus, well known to be a producer of antibiotics such as canescin [] and antifungal extrolites []. However, its ability to compete against endogenous microflora could be the cause of the plateau reached for PAH dissipation. Indeed, a too high competitiveness can be counterproductive by limiting the installation of microorganisms’ consortia able to biodegrade PAH metabolites produced by the inoculated fungus. So, the complex interactions between the fungus population inoculated and endogenous communities (bacteria, pedofauna) should be further investigated in order to optimize the structure and the degradative functions of the consortium for obtaining best degradation rates. Soil metagenomic methods during PAH bioremediation treatment could provide insight on the structure of complex microbial population [,].
The aim of the present study was not to determine which mechanisms involved in remediation obtained. However, concerning the original ES formulated with olive oil and lecithin, three mechanisms described in the literature guided our experiment design: (1) the ES could act as a nutrient supplementation, (2) it could also influence the desorption, solubility, and extraction of PAH present in soils, especially for HMW PAH, with very low water solubility and high Log Kow, (3) finally, it could also induce an increase of the intracellular H2O2 concentration, due to the normal lipid metabolic pathway, permitting fortuitously PAH oxidation by fungal cells, a new metabolic engineering strategy we proposed with Fusarium solani in in vitro cultures []. The mechanisms involved in xenobiotic biotransformation among these potential mechanisms required further research focus for improving mycoremediation in soils.
Finally, we are also conscious of the extrapolation limits of these results obtained in 200 g soil microcosm to field level. The next step would be upscaling from this laboratory research to a pilot study (with soil masses of the order of a ton) and then to a full-scale test (in bioreactor landfills with industrial historical polluted soils using Penicillium strains) via research conducted with environmental consulting firms in soil remediation.
4. Conclusions
For more than two decades, fungal remediation techniques (biostimulation and/or bioaugmentation) have demonstrated their effectiveness in reducing PAH soil pollution while preserving soil integrity. Many European countries have legal requirements for reducing PAH concentrations in soils to below specified thresholds to prevent soils from being considered as waste. For example, in France, the threshold is 50 mg PAH kg−1 of soil. In this study, the best PAH abatement rate obtained in BA4 was approximately 81 mg PAH kg−1 of soil. According to the French legal standard, this soil would be considered waste and could therefore be treated by a thermal process requiring heating to over 800 °C and producing sterile ash. Our study clearly shows that low-cost (half the cost thermal desorption) fungal technology offers an alternative to use natural components and fungal biodiversity to obtain a soil with low ecotoxicity. It is desirable and even recommendable to encourage researchers promoting a change in the law concerning risk assessment in the field of contaminated soils. It would be desirable to couple more often ecotoxicological approaches based on sentinel and relevant organisms with clean-up thresholds. Moreover, this fungal technology remediation contributes to enrich with sequestered organic-matter carbon with a minimum of energy consumption (a low-energy technology). In the context of climate change and environment sustainability, the preservation of soil health must become imperative. Only this change of paradigm will enable us to promote bioremediation methods in the future to protect the soil health for One health.
Author Contributions
Conceptualization, E.V. and C.R.; methodology, E.V. and C.R.; validation, E.V.; formal analysis, E.V.; investigation, E.V.; resources, E.V. and C.R.; data curation, E.V.; writing—original draft preparation, E.V. and C.R.; writing—review and editing, E.V. and C.R.; visualization, E.V. and C.R.; funding acquisition, E.V. and C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Region of Hauts-de-France with Start-AiRR Program. The APC was funded by UCEIV Laboratory—ULCO.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
PAH-polluted soil was kindly provided by EACM (France).
Conflicts of Interest
The authors declare no conflict of interest.
References
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures. IARC Monogr. Eval. Carcinog. Risks. Hum. 2010, 92, 1–853. [Google Scholar]
- Panagos, P.; Van Liedekerke, M.; Yigini, Y.; Montanarella, L. Contaminated Sites in Europe: Review of the Current Situation Based on Data Collected through a European Network. J. Environ. Public Health 2013, 2013, 158764. [Google Scholar] [CrossRef] [PubMed]
- Umeh, A.; Duan, L.; Naidu, R.; Semple, K. Time-Dependent Remobilisation of Non-Extractable Benzo[a]Pyrene Residues in Contrasting Soils: Effects of Aging, Spiked Concentration, and Soil Properties. Environ. Sci. Technol. 2018, 52, 12295–12305. [Google Scholar] [CrossRef] [PubMed]
- Mazarji, M.; Minkina, T.; Sushkova, S.; Mandzhieva, S.; Fedorenko, A.; Bauer, T.; Soldatov, A.; Barakhov, A.; Dudnikova, T. Biochar-Assisted Fenton-like Oxidation of Benzo[a]Pyrene-Contaminated Soil. Environ. Geochem. Health 2022, 44, 195–206. [Google Scholar] [CrossRef]
- Medaura, M.C.; Guivernau, M.; Moreno-Ventas, X.; Prenafeta-Boldú, F.X.; Viñas, M. Bioaugmentation of Native Fungi, an Efficient Strategy for the Bioremediation of an Aged Industrially Polluted Soil with Heavy Hydrocarbons. Front. Microbiol. 2021, 12, 713. [Google Scholar] [CrossRef]
- Harms, H.; Schlosser, D.; Wick, L.Y. Untapped Potential: Exploiting Fungi in Bioremediation of Hazardous Chemicals. Nat. Rev. Microbiol. 2011, 9, 177–192. [Google Scholar] [CrossRef]
- Furuno, S.; Foss, S.; Wild, E.; Jones, K.C.; Semple, K.T.; Harms, H.; Wick, L.Y. Mycelia Promote Active Transport and Spatial Dispersion of Polycyclic Aromatic Hydrocarbons. Environ. Sci. Technol. 2012, 46, 5463–5470. [Google Scholar] [CrossRef]
- Wick, L.Y.; Remer, R.; Würz, B.; Reichenbach, J.; Braun, S.; Schäfer, F.; Harms, H. Effect of Fungal Hyphae on the Access of Bacteria to Phenanthrene in Soil. Environ. Sci. Technol. 2007, 41, 500–505. [Google Scholar] [CrossRef]
- Cerniglia, C.E.; Sutherland, J.B. Degradation of Polycyclic Aromatic Hydrocarbons by Fungi. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 2079–2110. [Google Scholar]
- Marco-Urrea, E.; García-Romera, I.; Aranda, E. Potential of Non-Ligninolytic Fungi in Bioremediation of Chlorinated and Polycyclic Aromatic Hydrocarbons. New Biotechnol. 2015, 32, 620–628. [Google Scholar] [CrossRef]
- Aranda, E. Promising Approaches towards Biotransformation of Polycyclic Aromatic Hydrocarbons with Ascomycota Fungi. Curr. Opin. Biotechnol. 2016, 38, 1–8. [Google Scholar] [CrossRef]
- Zafra, G.; Absalón, Á.E.; Cuevas, M.D.C.; Cortés-Espinosa, D.V. Isolation and Selection of a Highly Tolerant Microbial Consortium with Potential for PAH Biodegradation from Heavy Crude Oil-Contaminated Soils. Water Air Soil Pollut. 2014, 225, 1826. [Google Scholar] [CrossRef]
- Covino, S.; D’Annibale, A.; Stazi, S.R.; Cajthaml, T.; Čvančarová, M.; Stella, T.; Petruccioli, M. Assessment of Degradation Potential of Aliphatic Hydrocarbons by Autochthonous Filamentous Fungi from a Historically Polluted Clay Soil. Sci. Total Environ. 2015, 505, 545–554. [Google Scholar] [CrossRef]
- Godoy, P.; Reina, R.; Calderón, A.; Wittich, R.-M.; García-Romera, I.; Aranda, E. Exploring the Potential of Fungi Isolated from PAH-Polluted Soil as a Source of Xenobiotics-Degrading Fungi. Environ. Sci. Pollut. Res. Int. 2016, 23, 20985–20996. [Google Scholar] [CrossRef]
- Yap, C.L.; Gan, S.; Ng, H.K. Application of Vegetable Oils in the Treatment of Polycyclic Aromatic Hydrocarbons-Contaminated Soils. J. Hazard. Mater. 2010, 177, 28–41. [Google Scholar] [CrossRef]
- Rathankumar, A.K.; Saikia, K.; Kumar, P.S.; Varjani, S.; Kalita, S.; Bharadwaj, N.; George, J.; Vaidyanathan, V.K. Surfactant-Aided Mycoremediation of Soil Contaminated with Polycyclic Aromatic Hydrocarbon (PAHs): Progress, Limitation, and Countermeasures. J. Chem. Technol. Biotechnol. 2021, 97, 391–408. [Google Scholar] [CrossRef]
- Fayeulle, A.; Veignie, E.; Schroll, R.; Munch, J.C.; Rafin, C. PAH Biodegradation by Telluric Saprotrophic Fungi Isolated from Aged PAH-Contaminated Soils in Mineral Medium and Historically Contaminated Soil Microcosms. J. Soils Sediments 2019, 19, 3056–3067. [Google Scholar] [CrossRef]
- Ghosal, D.; Ghosh, S.; Dutta, T.K.; Ahn, Y. Current State of Knowledge in Microbial Degradation of Polycyclic Aromatic Hydrocarbons (PAHs): A Review. Front. Microbiol. 2016, 7, 1369. [Google Scholar] [CrossRef]
- Umeh, A.C.; Duan, L.; Naidu, R.; Semple, K.T. Comparison of Single- and Sequential-Solvent Extractions of Total Extractable Benzo[a]Pyrene Fractions in Contrasting Soils. Anal. Chem. 2018, 90, 11703–11709. [Google Scholar] [CrossRef]
- Umeh, A.C.; Duan, L.; Naidu, R.; Semple, K.T. Extremely Small Amounts of B[a]P Residues Remobilised in Long-Term Contaminated Soils: A Strong Case for Greater Focus on Readily Available and Not Total-Extractable Fractions in Risk Assessment. J. Hazard. Mater. 2019, 368, 72–80. [Google Scholar] [CrossRef]
- Schauer, F.; Borriss, R. Biocatalysis and Biotransformation. In Advances in Fungal Biotechnology for Industry, Agriculture, and Medicine; Tkacz, J.S., Lange, L., Eds.; Springer: Boston, MA, USA, 2004; pp. 237–306. [Google Scholar] [CrossRef]
- Garon, D.; Sage, L.; Seigle-Murandi, F. Effects of Fungal Bioaugmentation and Cyclodextrin Amendment on Fluorene Degradation in Soil Slurry. Biodegradation 2004, 15, 1–8. [Google Scholar] [CrossRef]
- Hammer, E.; Schoefer, L.; Mikolasch, A.; Hundt, K.; Schauer, F. Formation of Glucoside Conjugates during Biotransformation of Dibenzofuran by Penicillium canescens SBUG-M 1139. Appl. Microbiol. Biotechnol. 2001, 57, 390–394. [Google Scholar] [CrossRef]
- Poyntner, C.; Prem, M.; Mann, O.; Blasi, B.; Sterflinger, K. Selective Screening: Isolation of Fungal Strains from Contaminated Soils in Austria. Die Bodenkult. J. Land Manag. Food Environ. 2017, 68, 157–169. [Google Scholar] [CrossRef][Green Version]
- Say, R.; Yilmaz, N.; Denizli, A. Removal of Heavy Metal Ions Using the Fungus Penicillium canescens. Adsorpt. Sci. Technol. 2003, 21, 643–650. [Google Scholar] [CrossRef]
- Leitão, A.L. Potential of Penicillium Species in the Bioremediation Field. Int. J. Environ. Res. Public. Health 2009, 6, 1393–1417. [Google Scholar] [CrossRef]
- Zehra, A.; Dubey, M.K.; Meena, M.; Aamir, M.; Patel, C.B.; Upadhyay, R.S. Chapter 14—Role of Penicillium Species in Bioremediation Processes. In New and Future Developments in Microbial Biotechnology and Bioengineering; Gupta, V.K., Rodriguez-Couto, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 247–260. [Google Scholar] [CrossRef]
- Lladó, S.; Covino, S.; Solanas, A.M.; Viñas, M.; Petruccioli, M.; D’annibale, A. Comparative Assessment of Bioremediation Approaches to Highly Recalcitrant PAH Degradation in a Real Industrial Polluted Soil. J. Hazard. Mater. 2013, 248–249, 407–414. [Google Scholar] [CrossRef]
- Bogan, B.W.; Sullivan, W.R. Physicochemical Soil Parameters Affecting Sequestration and Mycobacterial Biodegradation of Polycyclic Aromatic Hydrocarbons in Soil. Chemosphere 2003, 52, 1717–1726. [Google Scholar] [CrossRef]
- Eggen, T. Application of Fungal Substrate from Commercial Mushroom Production—Pleurotus ostreatus—for Bioremediation of Creosote Contaminated Soil. Int. Biodeterior. Biodegrad. 1999, 44, 117–126. [Google Scholar] [CrossRef]
- Potin, O.; Rafin, C.; Veignie, E. Bioremediation of an Aged Polycyclic Aromatic Hydrocarbons (PAHs)-Contaminated Soil by Filamentous Fungi Isolated from the Soil. Int. Biodeterior. Biodegrad. 2004, 54, 45–52. [Google Scholar] [CrossRef]
- Queirós, L.; Pereira, J.L.; Gonçalves, F.J.M.; Pacheco, M.; Aschner, M.; Pereira, P. Caenorhabditis elegans as a Tool for Environmental Risk Assessment—Emerging and Promising Applications for a “Nobelized Worm”. Crit. Rev. Toxicol. 2019, 49, 411–429. [Google Scholar] [CrossRef]
- Hägerbäumer, A.; Höss, S.; Heininger, P.; Traunspurger, W. Experimental Studies with Nematodes in Ecotoxicology: An Overview. J. Nematol. 2015, 47, 11–27. [Google Scholar]
- Lu, Q.; Liu, T.; Wang, N.; Dou, Z.; Wang, K.; Zuo, Y. A Review of Soil Nematodes as Biological Indicators for the Assessment of Soil Health. Front. Agric. Sci. Eng. 2020, 7, 275–281. [Google Scholar] [CrossRef]
- Höss, S.; Jänsch, S.; Moser, T.; Junker, T.; Römbke, J. Assessing the Toxicity of Contaminated Soils Using the Nematode Caenorhabditis elegans as Test Organism. Ecotoxicol. Environ. Saf. 2009, 72, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- Winquist, E.; Björklöf, K.; Schultz, E.; Räsänen, M.; Salonen, K.; Anasonye, F.; Cajthaml, T.; Steffen, K.T.; Jørgensen, K.S.; Tuomela, M. Bioremediation of PAH-Contaminated Soil with Fungi—From Laboratory to Field Scale. Int. Biodeterior. Biodegrad. 2014, 86, 238–247. [Google Scholar] [CrossRef]
- Hayes, M.H.; Swift, R. Vindication of Humic Substances as a Key Component of Organic Matter in Soil and Water. Adv. Agron. 2020, 163, 1–37. [Google Scholar] [CrossRef]
- Grinhut, T.; Hadar, Y.; Chen, Y. Degradation and Transformation of Humic Substances by Saprotrophic Fungi: Processes and Mechanisms. Fungal Biol. Rev. 2007, 21, 179–189. [Google Scholar] [CrossRef]
- Khatami, S.; Deng, Y.; Tien, M.; Hatcher, P.G. Lignin Contribution to Aliphatic Constituents of Humic Acids through Fungal Degradation. J. Environ. Qual. 2019, 48, 1565–1570. [Google Scholar] [CrossRef]
- Kumada, K.; Hurst, H.M. Green Humic Acid and Its Possible Origin as a Fungal Metabolite. Nature 1967, 214, 631–633. [Google Scholar] [CrossRef]
- Schnitzer, M.; Neyroud, J.A. Further Investigations on the Chemistry of Fungal “Humic Acids”. Soil Biol. Biochem. 1975, 7, 365–371. [Google Scholar] [CrossRef]
- Jia, C.; Li, X.; Zhang, L.; Francis, D.; Tai, P.; Gong, Z.; Liu, W. Extracellular Polymeric Substances from a Fungus Are More Effective than Those from a Bacterium in Polycyclic Aromatic Hydrocarbon Biodegradation. Water Air Soil Pollut. 2017, 228, 1627–1631. [Google Scholar] [CrossRef]
- Brian, P.W.; Hemming, H.G.; Moffatt, J.S.; Unwin, C.H. Canescin, an Antibiotic Produced by Penicillium Canescens. Trans. Brit. Mycol. Soc. 1953, 36, 243–247. [Google Scholar] [CrossRef]
- Nicoletti, R.; Lopez-Gresa, M.P.; Manzo, E.; Carella, A.; Ciavatta, M.L. Production and Fungitoxic Activity of Sch 642305, a Secondary Metabolite of Penicillium Canescens. Mycopathologia 2007, 163, 295–301. [Google Scholar] [CrossRef]
- Gorovtsov, A.; Demin, K.; Sushkova, S.; Minkina, T.; Grigoryeva, T.; Dudnikova, T.; Barbashev, A.; Semenkov, I.; Romanova, V.; Laikov, A.; et al. The Effect of Combined Pollution by PAHs and Heavy Metals on the Topsoil Microbial Communities of Spolic Technosols of the Lake Atamanskoe, Southern Russia. Environ. Geochem. Health 2021. online ahead of print. [Google Scholar] [CrossRef]
- Delsarte, I.; Rafin, C.; Mrad, F.; Veignie, E. Lipid Metabolism and Benzo[a]Pyrene Degradation by Fusarium Solani: An Unexplored Potential. Environ. Sci. Pollut. Res. Int. 2018, 25, 12177–12182. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).