Abstract
Healthcare facilities require flexible layouts that can adapt quickly in the face of various disruptions. COVID-19 confirmed this need for both healthcare and manufacturing systems. Starting with the transfer of decision support systems from manufacturing, this paper generalizes layout re-design activities for complex systems by presenting a simulation framework. Through a real case study concerning the proliferation of nosocomial cross-infection in an intensive care unit (ICU), the model developed in systems dynamics, based on a zero order immediate logic, allows reproducing the evolution of the different agencies (e.g., physicians, nurses, ancillary workers, patients), as well as of the cyber-technical side of the ICU, in its general but also local aspects. The entire global workflow is theoretically founded on lean principles, with the goal of balancing the need for minimal patient throughput time and maximum efficiency by optimizing the resources used during the process. The proposed framework might be transferred to other wards with minimal adjustments; hence, it has the potential to represent the initial step for a modular depiction of an entire healthcare facility.
1. Introduction
Hospital leadership has to cope daily with the unpredictability of the demand for care, the need to find the trade-off between effective therapies at minimum cost, and limited material and human resources. The usual improvement strategies involve both investment in infrastructure and increasing operational costs. The latter are related to the management of the newly added location and also to the new human resources allocated. Relative economic and financial sustainability is difficult to achieve because it runs counter to budget constraints imposed by national institutes providing healthcare [1,2]. Typical factors, such as the need to increase healthcare services [3] to meet an increasing rate of demand, the need to reduce operating costs, the growing trend toward the specialization of care processes, and the patient-centered philosophy [4], are key to designing improvement strategies to maximize the efficiency and effectiveness of clinical performance. Consequently, patient management based on the rational use of resources would be less costly.
Traditionally, the underlying philosophy of lean principles aims to better manage existing resources by reducing waste in any productive activity, including clinical activities. For example, lean has already proven successful in the manufacturing sector. Typical results include reduced operating costs, reduced cycle time, and increased customer satisfaction. Problems such as the budget deficit, hospital infections, the need to reduce care risks and the number of deaths, the limited supply of services, the charges of endemic inefficiency, the public interests, the lack of safety and quality, and the low level of personnel motivation affect all kinds of health organizations all over the world. The novelty of lean management techniques is the ability to improve quality (providing better care in a timely manner), to make the job less stressful and more rewarding, and at the same time to increase efficiency and productivity [5].
In this paper, a system dynamics (SD)-based simulation framework is proposed to reproduce the systemic complexity of a typical hospital ward, and subsequently has been tested and validated in a use case within the Intensive Care Unit (ICU) of one of the largest hospitals in Italy. The framework, designed according to lean principles, is designed to provide mitigation solutions which are easy to implement so to cope with the overwhelming complexity of healthcare systems.
The ICU department from which the actual comparison data were extracted complains of an endemic spread of nosocomial cross-infection. The problem is actually widespread [6].
To the best of our knowledge, there is no research in the literature that has developed simulation models based on lean principles in SD that address the problem of nosocomial cross-infection. Given the compelling relevance of this issue, starting from an initial configuration model, two alternative scenarios have been developed which have proven to increase the throughput of the clinical activities and to reduce the clinical treatment costs as well as the infection prevalence.
The paper is structured as follows: Section 2 briefly describes the literature related to the present research; Section 3 details the methods used to derive the simulation framework; Section 4 highlights the results obtained through the application of the simulation model; Section 5 concludes and outlines future research.
2. Related Literature
Over the past two decades, educators and healthcare professionals have sought to implement lean tools in healthcare [7,8,9]. Successful lean implementation depends on educating clinicians about continuous improvements in lean tools and making sure that they are part of the improvement team. Based on the revealed enablers and barriers, a comprehensive lean implementation framework was created and then used in conjunction with engineering tools in a large hospital. The results of the implementation showed a 60% reduction in cycle time, an 80% reduction in operational costs, and many other benefits [10]. The most meaningful and, at the same time, the most counterintuitive idea is founded on the principle that it is possible to do more with less stress to the system [11].
Hospital wards are recognized as complex systems [12,13]. A complex system can be defined as a set of connected components whose interactions, which are marked by circular and nonlinear causal relationships, are the main driver that generates the dynamic behavior of the system itself [14]. The continuous evolution of events (i.e., dynamic complexity) is often characterized by different objectives and interests that, in different circumstances, may conflict with each other. In such situations, it is difficult to understand how, where, and when to operate, since most of the interventions could generate unexplained consequences [15].
The characteristics and behavior of dynamic healthcare systems are typically generated by a large number of factors, such as the degree of connection of the relationships, the degree of nonlinearity of the relationships, the degree of system adaptability, the self-regenerative behaviors, and the complexity of the feedback structures, which show the system dependence on the previous states. It follows that in order to represent this complexity opportunely, there is need of an approach of the theory of the systems [16]. The process of modeling and simulation is the instrument that is able to implement the “inductive” path allowing to face the complexity of the system without trivializing it.
Simulation is a particular kind of modeling of reality that allows situations or environments to be recreated for the purpose of practice, learning, evaluation, testing, or to gain understanding of human systems or actions [17].
Hospital facilities design is among the oldest applications of simulation in healthcare [18]. More recently, several simulation models have been proposed to address facilities’ problems. For example, Koizumi, Kuno, and Smith used a queuing model to analyze congestion processing in patient flows [19].
An SD model was proposed to evaluate the performance of the testing center in a medical diagnostic laboratory. The aim of the research was to improve the service for patients, with the goal of reducing the average waiting time and its variability, providing a valuable contribution to the design of the testing center [20].
The use of simulation has been widely used even by the World Health Organization, for example, in finding the best strategies to mitigate the effects of COVID-19 [21].
Generally, increasing stakeholder engagement has a positive impact on projects, and simulation has proven to be an effective engagement tool. This suggests the value of introducing the use of facilitated workshops in hospitals [22]. A notable gap in stakeholder engagement is the model coding phase, where a conceptual model is transformed into a simulation model [23]. Considering the latter fact, Abdelghany and Eltawil reviewed different integrated simulation approaches used in healthcare, e.g., discrete event simulation (DES), agent-based simulation (ABS), and SD [24]. Among these, the major advantage of using SD is that it allows for the consideration of systemic feedbacks; hence, it is particularly suitable for representing systems’ complex reinforcing dynamics. Simulation-based decision aids can often serve to produce visually clear information. This is a significant advantage given that medical and nursing staff are particularly prone to information overload [25,26]. Improving stakeholder health literacy can achieve positive feedback effects on a hospital’s ability to manage critical issues [27]. For example, often physicians must decide whether to deny a needy patient access to a full ICU or create a vacancy by prematurely discharging a current occupant. In the medical literature, the influencing factors are identified, the whole patient discharge process has been described, and the consequences for the patient’s health have been analyzed. In 2020, Azcarate, Esparza, and Mallor provided a review of medical and mathematical literature on patient discharge decisions and proposed a simulation framework capable of modeling realistically the patient discharge process [28].
In healthcare organizations, everything is affected by everything else. Therefore, there is a need to take into account both systemic complexity from a top-down point of view and detail complexity from a bottom-up point of view. The solution proposed by Brailsford, Desai, and Viana involves the construction of a hybrid model that exploits the peculiarities of the DES in order to detail the single parts of the hospital and, at the same time, using the features of the SD to build a glue substructure to represent both the environmental dynamics and the connecting relationships between the different subsystems [29]. More specifically, the proposed model evaluated hospital performance by considering the dynamic behavior of the Chlamydia trachomatis pathogen.
Such hybrid approach between the DES and SD represents a notable first step towards the formulation of integrated modeling systems in healthcare.
Chahal, K. et al. [30], after identifying the reasons why there is a need for hybrid simulation, defined a standard procedure for creating hybrid DES/SD simulation models in healthcare. Specifically, the proposed procedure is divided into three phases: the first one allows to identify the problem in order to understand if it is necessary to use a hybrid approach; the second phase is characterized by the separate development of DES and SD models with the purpose of identifying the interaction points between the models in order to formulate their mutual relationship; the third phase allows to investigate the interactions between the models according to the spatiotemporal coupling.
In the wake of a growing trend toward the hybridization of simulation approaches, the search for the perfect hybrid has undergone further developments with [31], which made a comparison highlighting the main pros and cons between SD and DES in healthcare settings.
The year 2016 seems to have been a fruitful year for the effort toward hybrid models: Zulkepli and Eldabi conceptualized modeling by the use of a hybrid simulation, its advantages, and issues. That research work was focused on patient pathways within large healthcare systems and suggested when and how to implement hybrid solutions [32]. In [33], the hybrid approach has been used to generate cardiac ill population samples, thereby making hybrid models more and more important in healthcare; ref. [34] provided a step-by-step simulation tutorial in real-world healthcare settings, with particular concern in privacy and security.
As the various studies and numerous applications point out, the well-established simulation philosophies that contrast the high-level holistic view of dynamically connected systems (typical of SD modelers) with the detailed “microscopic” view of individual systems (typical of DES modelers) show some clear points of contact that are progressively strengthened by the processes of “hybridization” and by the simultaneous evolution of simulation software. This evolution underlies an increasingly consolidated trend of simulation software vendors to develop DES platforms with continuous features and SD platforms with discrete features. The authors of [35] reviewed and summarized publications related to various simulation tools in healthcare. Moreover, the paper finalized five main types of simulations, arguing that future studies should focus on developing a framework that supports a problem-based selection of simulation types.
The literature on hybrid models suggests that the integration of SD and DES is an effective and efficient solution as a decision support system. However, the framework implemented in the present work should not be intended as such a traditional approach. The discrete process is realized within the SD simulation environment itself. A previous attempt of such an approach can be traced back to [36,37,38,39,40]. The idea, by now consolidated for the authors, is to follow the states’ evolution of the system, e.g., being time-driven, that is, recording the values of the state’s variable states at regular intervals of time. During these intervals, the events that follow are the results of dynamic relationships based on feedback control. With this paradigm, however, we are only able to control the transition from one state to the next, at each finite delta T, losing the information of the transition during one state to the next. Therefore, the state-to-state evolutionary path can be used to build differential equations in an infinitesimal dt; hence, the system’s evolution changes accordingly to the finite interval of the simulation.
Recently, the combined approach of SD and DES has been used to plan resources relative to the COVID-19 pandemic [41], proving to be a useful and effective decisional support tool.
3. Materials and Methods
3.1. Types of Variables Used in SD
The main analogical formalism on which the SD is based is that of the bathtub. That is to say that the evolution of the states of a system can be modeled similarly to containers of finite capacity that can be filled and emptied, operating on the flow rates in input and output. A model in SD is constituted, therefore, by state variables (i.e., indicating the situation of the system in every instant) and by flow variables (i.e., concurring to modify the state of the system). Such formalism is known as the stock and flow (or level and rate) diagram, and constitutes the archetypal structure of whichever model of simulation in SD. The stocks (levels) are fundamental in order to generate the behavior in a system; the flows (rates) cause the change of the stocks. Such analogical formalism corresponds to a logical–mathematical formalism expressed in terms of differential equations.
There are three variable types used in SD: levels, which represent the system states changing over time; auxiliaries, which combine and reformulate information as it passes through the system; constants, which represent information unchanged by the simulation but that can be changed by the user through input controls.
Levels are variables with memory whose value is determined by the flow in and the flow out. At the time t, the level value is represented by the expression below, where dt is the time step of the simulation run. Levels conserve the matter that flows in and out of them.
Levelt+dt = Levelt + inflowst − outflowst
Such a definition is used to calculate the initial value of the level variable: the value at start-up. In addition, a level contains a flow definition regarding how the flows accumulate in each time step. The flows are also controlled by other variables (one per flow) that work as flow rates. In the framework proposed below, we used a custom formalism. The level variables have been indicated with the caption in [variable_name]. In our framework, we used a custom formalism. The level variables have been indicated with the caption in [variable_name] while the flow variables are indicated with the ev [variable_name] one.
In general, there are three types of flows available in SD: continuous, discrete, and logical. If one or more flows regarding a level is continuous, the level data type must be either real or complex due to the nature of continuous flows. Otherwise, if the flow is discrete, the level can be an integer. For logical flows, both the flow rate and the level must be logical. The flows are controlled by flow rates, and when creating level-and-flow structures, it is important that the controlling flow rates and the levels match each other’s definitions (including units, data types, and dimensions). Specifically, to the proposed framework, the discrete formalism is in use. That happens since each patient outflow is implemented by associating a stochastically generated care path, hence it is discrete.
While continuous flows represent quantities being transported between levels, discrete flows represent transactions of given quantities at given times (i.e., the so-called tokens). While continuous flows are integrated over the time step (and is thus dependent of the length of the time step), discrete flows represent a quantity that is accumulated by the level of each time step (and is thus independent of the length of the time step). The expressions for continuous flows and discrete flows are shown below (as they appear in the flow expression for the connected level).
Continuous flow = dt · Rate
Discrete flow = Rate
A flow is defined by two parts: the flow rate variable definition and the equation flow specified for the level variables connected to that flow. To create a discrete flow, the flow rate must use a “zero order integration setting”. The difference between “Zero Order” and “Zero Order Immediate” settings is shown in the expressions below, where L is the level and F the controlling flow rate:
Zero Order: Lt + dt = Lt + Ft
Zero Order Immediate: Lt + dt = Lt + Ft + dt
The time at which the flow rate F is calculated and at which the flow is added to the level L is different.
In Zero Order, the integration is performed at the end of the time step. In Zero Order Immediate, the integration occurs at the beginning of the time step. As a consequence, the variable will only be evaluated upon the entry of the time step and stay constant for the remainder. The flow rate’s value, calculated in the current time step, is then immediately accumulated by the level, hence the name “immediate”.
When using immediate discrete flows, the value of the flow rate will remain constant over the time step. When using a zero order immediate flow, this allows us to create a structure immediately assigning a value to a level when the condition occurs.
3.2. The Simulation Framework
The proposed simulation framework consists of five main parts:
- The “patients” module: The patients follow a predetermined three-stepped ICU pathway made of acceptance, therapeutic care, and transfer to the inpatient ward. The latter, being outside the ICU, is represented simply by removing the patients at the end of the pathway;
- The “chain of events” module: This is the simulation module subsuming routine care procedures as they take place in the ICU. The care procedures have been identified in a previous stage from the medical literature;
- The “resource management system” module: The hospital’s internal management system that decides and redistributes the allocation of resources for the ICU, e.g., the available beds and the employed personnel (both nursing and medical);
- The “hourglass” module: The purpose of this module is to align and measure the care activities with ongoing simulation time;
- The “variables management” module: This module serves both input settings and performance parameter evaluation of the ICU. The variables management module operates also as a front-end interface to both set control variables and observe ICU behavior through some specific key performance indicators (KPIs).
In the following the five parts of the framework are thoroughly discussed one by one:
3.2.1. Patients
This part of the framework models the states that patients undergo during their stay in the ICU. It is represented by a sequential structure of twelve variables: six level variables (patient; patients_in; patients_in_therapy; patients_exiting_ICU; patients_in_ post_therapy; patients_discharged) and six flow variables (ev_patient_in; ev_bed_free; ev_exit_ICU; ev_post_therapy; ev_discharge) (Figure 1).
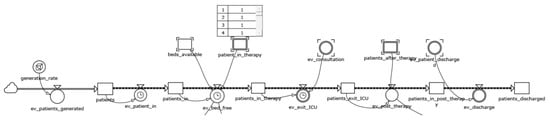
Figure 1.
The Patient module.
The first level variable (patients) takes into account patients incoming in the ICU. Their number is established at a pace set by a patients_generation_rate adjusted accordingly to real data. Excessive patients_generation_rate might result in a possible congestion condition of the department in case of the unavailability of beds. The patients_in_therapy level variable indicates the amount of patients that are in the ICU at each simulation time; therefore, it is upper-bounded by beds capacity. The patients_exiting_ICU variable accounts for the level of patients ending the intensive care and then destined to post-therapy care, consisting in washing and cleaning. Such state is represented by patients_in_post_therapy. Patients_discharged indicates the total number of patients who left the department.
The ev_patient_in represents the flow that transfer tokens from patients to patient_in. The flow is active only if there is an integer number of patients to be admitted in the ICU.
The ev_bed_free flow variable allocates the previously generated patients into patients_in_therapy level. It acts as a control valve permitting the flow only when there are simultaneously both incoming patients_in and beds_available.
The flow between patients_in_therapy and patients_exiting_ICU states is controlled by ev_exit_ICU. The flow is positive when both the conditions <patients in the previous level> and <completed current therapeutic activities> are met.
The ev_post_therapy variable is the flow allocating patients to post-treatment procedures. The ev_discharge variable controls patient flow from patients_in_post_therapy to patient_discharged and is triggered when the previous level is full or when there are patients who have completed all care in the post-treatment setting.
3.2.2. Chain of Events
The level variables patients_in and patients_in_post_therapy constitute the blocks that process patient operations as a result of a circular programming activity. These variables have been linked with the chain of events—the module that defines how all the states vary within the model. These structures have the duty of scanning the sequence of operations performed by physicians and nurses in the ward until the moment of discharge. The model adopts an array logic: each patient care cycle follows a scheduled pathway in which each next step can be activated only if the resources (bed, medical, nurse, equipment) are available (Figure 2).
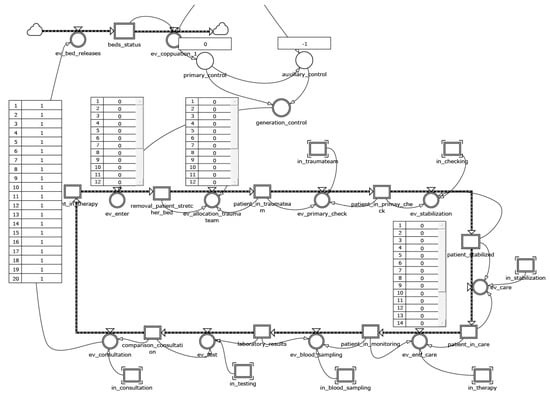
Figure 2.
The chain of events module.
3.2.3. Resource Management System
The resources management system is realized by using two level variables linked to some flow variables determining the transition from the state seize to the release one of the resources (Figure 3). Each of these flows are triggered if the resource’s availability is different from zero and there are entities that require it. Each resource is seized for a time interval proportional to its usage and equal to a value that begets accordingly to a real data fitting distribution. At the expiry of the time of use, the resource is released. Such mechanism is replicated both for physicians and nurses.
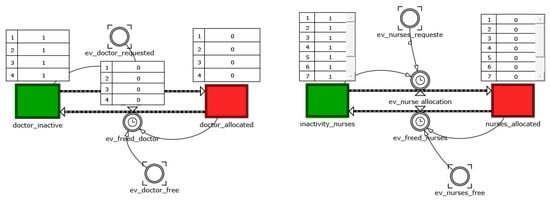
Figure 3.
Resource management system.
3.2.4. Hourglass
This module reproduces the timing of any intensive care procedure, and also allows for the triggering of state evolution, not only when resources become available, but also in the event that the time associated with the activities runs out. For example, the timing of patient stabilization is triggered by the ev_stabilization flow variable (Figure 4).
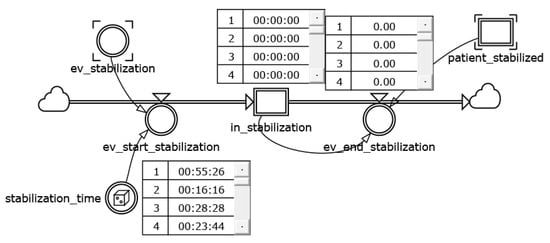
Figure 4.
A detail of hourglass consumption of time mechanism.
The reached state of stabilization recalls the value from the variable stabilization_time that is copied in the level variable in_stabilization. At the same time, the ev_end_stabilization drains the stabilization time until its exhaustion for every corresponding increment of simulation time.
The in_stabilization state activates the evolution from the state patient_stabilized to the patient_in_care one in the chain of events (Figure 2).
Table 1 accounts for the main time-related parameters provided by the hourglass module.

Table 1.
Time-related parameters.
3.2.5. Variables Management
As previously mentioned, this module allows front-end management of the entire model. That is a required feature, since the model needs to be validated and subsequently tuned in search for optimality. The identified parameters are:
- Average Length of Stay (ALOS), the average length of stay calculated as the ratio of the amount of actual hours of hospitalization to the number of patients leaving the ICU:
- ICU Throughput, which is defined as the reciprocal of the ALOS:
- Average Daily Admissions (ADA), the average of patients_in per day [patients]
- Average Daily Dismissions (ADD), the average daily of patients_discharged per day [patients]
- Average Daily Census (ADC), which quantifies the daily ICU saturation (measured in patients). It can be calculated as:
- Bed Turnover Rate (BTR), which is the average duration, in days, that elapses between the discharge of one patient and the admission of the next inpatient to the same bed over any period of time:
- Patient Turnover (PT), which measures the number of times the ICU renews the inpatients.
Although several software solutions were available, it was chosen to implement the framework in Powersim, which allows, in our opinion, to schematize complex systems relatively easily with simple building blocks. It must be said, however, that such simplicity of the atomic building blocks makes it rather difficult to build realistic models of the different behaviors. It becomes necessary in the fact of the use of an elevated number of variables of level and flow, and, moreover, connected in various ways. Powersim uses the same variable of level and flow in order to realize parts of the framework that supply the useful data of output to the analysis.
Finally, it is possible to obtain the results for the scenario analysis independently from the integration step in which the states of the system are updated. This allows the generalization of the output analysis. As a result, there is no dependence of the results on whether Powersim was used.
3.3. Data Analisys and Model Validation
Data analysis was preliminary to both the construction of the simulation model, which uses stochastic functions built on the real data, and the model validation phase.
The data used were collected based on hospital reports recorded for approximately 3 years. In these reports, the access times to the ICU facilities, the types of pathologies, and, for each patient, the “exit” events from the ICU sections both towards other hospital departments and towards the exit were recorded.
In order to analyze the data, inference analyses were carried out to identify the mathematical functions that best approximate the successions of the data.
These functions make it possible to construct the phenomenon of arrivals and, for the different pathologies, the treatment services associated with the departments of the hospital structure.
The simulation model was validated by using real data collected in four ICUs (burns, neurosurgery, liver, and postoperative) of a large hospital located in South Italy. The reports analyzed referenced recorded admissions/discharges. This made it possible to identify the month in which the ICUs was most loaded, i.e., October. In addition, shared the demand rates by time slot and relative to an ordinary day of the same month, standardized operation times from intensive care protocols, and finally, the statistical distribution of length of stay based on a sample of 1563 registered admissions were collected and used to calibrate the model.
The demand rate for a typical October day was identified as a function of the hourly curve of admissions divided according to three time slots in order to obtain three Poisson generations with an appropriately summarized mean (Table 2).

Table 2.
Poisson distribution with associated average values.
Further processing identified the statistical distribution of ALOS. The latter shows an exponential hourly trend of an average 242.85 h (Figure 5).
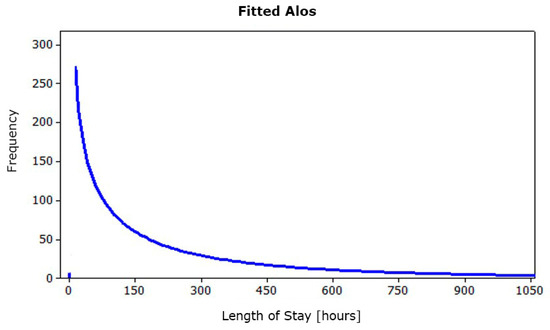
Figure 5.
Fitted ALOS.
Using Kelton’s formula [42], the number of runs that allow to obtain an acceptable error with a probability of 99% is about 2870; therefore, it can be considered not affected by random input data.
Subsequently, the simulation runs have been carried out over a two-month period monitoring the output parameter inferred from the level variable patients_discharged. This result, compared with the admissions/discharge records obtained during the same time slot, totally confirms the simulation model’s validity: the simulated value (117 patients) is totally comparable to the theoretical one (122 patients) identified in the real data analysis stage.
4. Results
The obtained scenario of the validation phase represents the baseline of the analyzed ICU (Figure 6). The department consists of 20 beds equally equipped to the highest level.
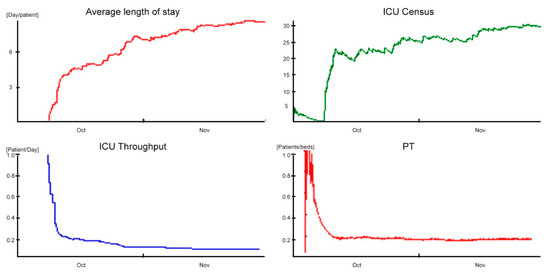
Figure 6.
Outcomes in baseline scenario.
The one-month ALOS in simulation time stabilizes around a value of 8.5 days/patient (approximately 204 h), with a standard deviation of 7.5 days/patient (180 h).
Comparing the simulation data with those reported in the study [43] (e.g., the ALOS equal to 7.5 patient-days and the standard deviation equal to 2.9), it shows a discrepancy of one day at the ALOS and standard deviation, respectively. In addition, the ADM equal to 2.23 patient-days and the ADD equal to 1.9 patient-days are summarized by the ICU Throughput performance indicator, which shows a decreasing trend in transient settling around a controlled value of 0.12 patient-days. The time differences and indicators just described clearly demonstrate that the ICU accepts incoming patients faster than it discharges them, consequently generating congestion problems.
These phenomena are further justified by the low daily PT of 0.10 patients/bed and the high bed utilization (98%). The saturation sustained by the ICU was identified thanks to the ADC indicator, and influenced by the ADA and ALOS variables, is around a value of 18.9. This coefficient was used as a term of comparison between the performances in the different scenarios. Finally, with regard to staff utilization, in the time slot characterized by the highest demand for care (1:00 p.m. to 7:00 p.m.), medical and nursing staff utilization amounted to 78% and 75%, respectively, compared to ward congestion.
The simulation model fully confirms the critical problems of the department highlighted by both experts in the field and scientific publications. These problems, resulting from the combination of conflicting factors, such as the demand for intensive care and the limited availability of life support stations, can generate phenomena of congestion defined as “bed blocking” [44].
The causes of the phenomenon are substantially two:
- The delayed discharge, which occurs when patients, although clinically ready to be discharged from the ICU, cannot be transferred to inpatient wards due to bed unavailability;
- The so-called nosocomial cross infection. Nosocomial or hospital infection means any disease with an infectious origin, with microbial or viral origin, clinically recognizable, lacking or incubating at the time of admission, which is acquired by patients during the hospital stay. This hazard strongly impacts hospital units from both an organizational and economic point of view. In particular, critically ill patients develop about 25% of all nosocomial infections that we can recognize in the hospital and about 90% of hospital epidemic events concern ICUs.
Both factors trigger a chain mechanism that deteriorates the ICU’s level of service, increasing the value of ALOS, decreasing the throughput of clinical activities, and, inevitably, increasing overall costs.
To reduce the aforementioned inefficiencies, the simulation model, validated on real data, has been used to build alternative scenarios with the aim of improving the system’s performances.
Below are the two, which were found to be the most effective, imagined following lean principles: the two solutions reschedule the activities and redesign in a viable way the layout, trying at the same time to minimize costs and reorganizational efforts.
4.1. Scenario 1: Insertion of a Post-Hospitalization Buffer
The first lean solution aims to achieve a ward configuration that facilitates the removal of stabilized patients through the creation of an area operating as a buffer between the ICU and the ordinary hospitalization wards. This area is designed to facilitate, on the one hand, the discharging process and therefore the bed’s releasing, and, on the other hand, the identification of the final ordinary hospitalization location. According to the literature data, the maximum allowed length of stay in an ICU after that the patient can be discharged is equal to 7.5 days (180 h).
An improvement that is immediately effective might be realized by introducing a buffer that allows the time limit to be contained in the 7.5 days. In particular, there is a reduction of 12% of ALOS, whose value is equal to 7.5. This decrease has a positive impact both for the ADM, with an increase of 70%, to a value of 3.80 patients/day both for the ADD, with an increase of 68% to a value equal to 3.2 patients/day; consequently, the ICU Throughput increases to 8% with 0.13 patients/day. According to the obtained results, the saturation indicated by the ADC amounts to 28.5 and shows a higher value than the obtained one in the baseline scenario.
The new configuration’s positive effects are further highlighted by the daily PT indicator, which is equal to a value of 0.14 patients/bed underlining an increase of 40% if compared to the baseline (Figure 7).
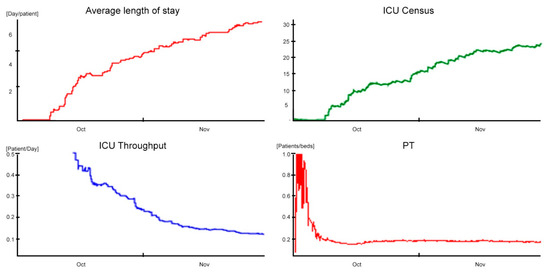
Figure 7.
Outcomes in post-hospitalization buffer scenario.
4.2. Scenario 2: The “Cell Design” Infection’s Management
An additional configuration was designed to limit the effect of the bed blocking phenomena and, consequently, the extension of the recovery period. It is known to correlate with the risk of nosocomial cross-infection, which produces an increase in costs, human and economic, for people and hospital as well.
Pathogens need a vector to infect patients. The transmission vehicles most frequently involved in nosocomial infections are the hands of healthcare workers and air conditioning. It is not uncommon for antibiotic-resistant bacteria to find optimal conditions to reproduce on air conditioning filters. Consequently, in under-crowded ward conditions and a prolonged length of stay, the inpatients are exposed to a higher chance of being infected.
Taking for granted the accuracy in the execution of care practices, preventive hand cleaning and sterilization of instruments, the use of disposable equipment, and the timely detection of bacteria, a scenario has been identified in which the ICU is configured to limit the phenomena of cross-infection. The layout in this case consists of a distribution of beds that follows a cell logic: a “noninfected” cell consisting of twelve beds and an “infected” cell consisting of the remaining eight beds appropriately isolated. Figure 8 and Figure 9 show, respectively, the patient and the chain of events modules for the present scenario.
Figure 8.
The patient module for the cell design scenario.
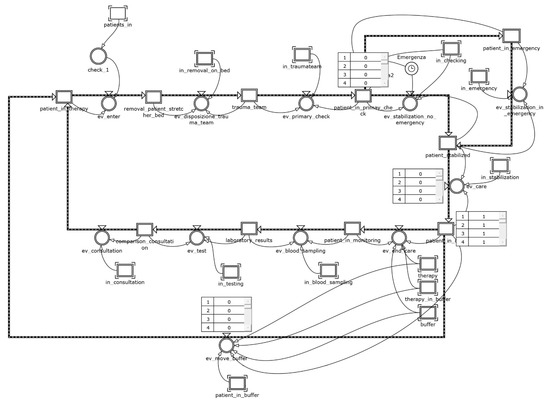
Figure 9.
The chain of events module for the cell design scenario.
The incidence referred to the inpatient stay of nosocomial cross-infected patients was obtained from data provided by the Italian Group for the Evaluation of Interventions in Intensive Care [45], which conducted a continuous monitoring study on 133 ICUs (87.2% multifunctional, 4.5% neuro-surgical, 8.3% other) distributed on the national territory. Ten percent of patients develop at least one during hospitalization; specifically, 3% had infections on admission and during hospitalization; the remaining 7%, although not infected on admission, developed infections during recovery in the ICU. The latter fraction of patients represents the major cause of the spread of nosocomial infection in crowded conditions and is difficult to contain. Under this assumption, the condition in which patients develop infection during their inpatient stay is the most severe. With this idea in mind, it is possible to classify patients in descending order of nosocomial harm: infected patients receive greater nosocomial harm than uninfected patients; in addition, those who develop infection in the inpatient stay receive greater nosocomial harm than those who present on admission. The result of this classification gives us the incidence and mean range of ALOS by class (Table 3).

Table 3.
Range of length of stay per class of infection. Severity of infection decreases with the class.
From this, it follows that the proposed layout for the cell design scenario can efficiently manage the flow of patients. In particular, the timely isolation of the infected during admission or in the first hours of stay prevents the uncontrolled spread of nosocomial cross-infection, reducing the overall ALOS and the saturation of the department. As a result, not only does it decrease average hospitalization time, but it tends to safeguard long-term residents more. The results of the model are confirmed in research studies [46,47]. The implementation of preventive procedures for prompt detection of nosocomial cross-infection sentinel germs, together with the isolation of infected patients, allows to move from the baseline scenario with an exponential hospitalization with an average of 9–10 days (totally in accordance with the data) to a final scenario in which uninfected patients have an average hospitalization of 3 days and infected patients have an average hospitalization of 34 days. In detail, the patient module is realized using a 2 × 1 array, whose first row represents the infected cells and the second one the not-infected ones. A submodule was prepared to share a time slot generation according to a percentage of 10% on the first cell and of 90% on the second one; then, for the two cells, a treatment time of 34 and 3 days, respectively, was set. With this refinement, the results show further improvements for the ALOS (6.32 days/patient), with a reduction of 16% compared to the posthospitalization buffer scenario (Figure 10).
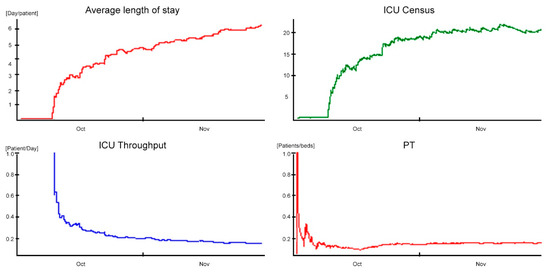
Figure 10.
Outcomes in cell design scenario.
ADM and ADD do not seem affected by such improvements, remaining quite unchanged (3.33 patients/day and 3 patients/day, respectively); the ICU throughput increases to 23% (0.16 patients/day). ADC indicator amounts to 21.04 and shows a lower value of saturation than the one obtained in the posthospitalization buffer scenario.
The improvements in the cell design scenario are even more evident when looking at the PT indicator, which reaches an average value equal to 0.14 patients/bed, and it shows an increase of 70% in comparison with the baseline.
Table 4 summarizes the performances in the different scenarios.

Table 4.
KPIs for the three scenarios.
5. Conclusions
This research paper explored the use of SD in the evaluation and management of discrete systems by proposing a framework suitable for hospital departments. In particular, the framework modifies the classical model by introducing the zero order immediate approach. Moreover, both the macroscopic aspects (traditionally associated to the SD) and the microsimulative ones (usually faced with discrete-event techniques) have been integrated in the proposed framework. Concerning the model tailored to the case study and implemented on arrays pertaining to the care pathway, it faithfully reproduces the behavior of the real twin department, offering management decision support. The use of lean principles has been shown to produce improvement scenarios with immediate benefits at a low implementation cost, such as the dynamic reconfiguration through cell-based design for the management of patients who develop nosocomial cross-infection.
Unfortunately, SD offers few building blocks for the detailed description of systems; consequently, it is only possible to account for complex internal relationships at the price of elaborate micromodeling structures (e.g., arrays). The worst consequence is that you get models that are not immediately readable, especially for those not accustomed to such an approach. We believe that these shortcomings are abundantly counterbalanced by the advantageous possibility of implementing different feedback logics—thus, inherently systemic and possibly nonlinear—in the modeling.
The model has been implemented in Powersim, a choice that, in our opinion, allows a certain freedom of maneuver in the design of the models. This freedom of expression is paid for by an excessively large number of level and flow variables. However, the results of the analysis are well generalizable given the independence of the integration step at which the system is updated. Independence from update status also implies independence of results from the particular software employed.
The framework, per se, with minimal adjustments, can produce models capable of describing other hospital departments. It follows that the repeated application of such modular framework allows, in principle, to model an entire healthcare infrastructure. With more redesign effort instead, you can certainly transfer it to other application domains.
Finally, the proposed tool allows the classification of patients, and associated medical procedures, through a cell design. Although it was designed for the management of nosocomial cross infection, without any imaginative effort, it can replicate for other infectious diseases, such as COVID-19 or the next pandemic threat.
The impact on COVID-19 of this model can be identified in the ability to manage the critical infrastructure/components of a hospital system, to withstand the pressure of an increasing number of infected patients who require their use. In Scenario 2, the “Cell Design” infection’s management analyzes what the benefit of a cell layout is, with the realization of an isolated ward for the quarantines imposed by infectious viruses. The benefit is measured in terms of improvements on the crossing time of patients affected by cross-infection compared to the case of a classic layout with patients sharing the same space. The layout in cells can reduce, due to isolation, the cross-infection, decreasing the transmissibility of the disease and increasing the healing time.
Therefore, the use of the framework is configured as a decision support to re-engineer the layout of critical hospital departments, thus improving the effectiveness of care.
Author Contributions
Conceptualization, E.R. and A.F.; methodology, E.R.; validation, C.R.; formal analysis, C.R.; resources, A.C.C.; data curation, A.C.C. and A.F.; writing—original draft preparation, A.C.C.; writing—review and editing, A.F. and E.R.; visualization, A.F.; supervision, C.R.; project administration, C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the data collection.
Data Availability Statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
Acknowledgments
The authors would like to thank the physicians and nurses whose valuable contributions made this research possible.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rocha, A.; Costa, A.S.; Figueira, J.R.; Ferreira, D.C.; Marques, R.C. Quality assessment of the Portuguese public hospitals: A multiple criteria approach. Omega 2021, 105, 102505. [Google Scholar] [CrossRef]
- Shander, A.; Fleisher, L.A.; Barie, P.S.; Bigatello, L.M.; Sladen, R.N.; Watson, C.B. Clinical and economic burden of postoperative pulmonary complications: Patient safety summit on definition, risk-reducing interventions, and preventive strategies. Crit. Care Med. 2011, 39, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- Askari, M.; Klaver, N.S.; van Gestel, T.J.; van de Klundert, J. Intention to use Medical Apps Among Older Adults in the Netherlands: Cross-Sectional Study. J. Med. Internet Res. 2020, 22, e18080. [Google Scholar] [CrossRef] [PubMed]
- Schiza, E.C.; Neokleous, K.C.; Petkov, N.; Schizas, C.N. A patient centered electronic health: EHealth system development. Technol. Health Care 2015, 23, 509–522. [Google Scholar] [CrossRef]
- Marsilio, M.; Pisarra, M.; Rubio, K.; Shortell, S. Lean adoption, implementation, and outcomes in public hospitals: Benchmarking the US and Italy health systems. BMC Health Serv. Res. 2022, 22, 122. [Google Scholar] [CrossRef]
- Klevens, R.M.; Edwards, J.R.; Richards, C.L., Jr.; Horan, T.C.; Gaynes, R.P.; Pollock, D.A.; Cardo, D.M. Estimating health care-associated infections and deaths in U.S. Hospitals, 2002. Public Health Rep. 2007, 122, 160–166. [Google Scholar] [CrossRef]
- Wickramasinghe, N.; Al-Hakim, L.; Gonzalez, C.M.; Tan, J. Lean Thinking for Healthcare; Springer: New York, NY, USA, 2014; ISBN 9781461480365. [Google Scholar]
- Souza, D.L.; Korzenowski, A.L.; Alvarado, M.M.; Sperafico, J.H.; Ackermann, A.E.F.; Mareth, T.; Scavarda, A.J. A systematic review on lean applications’ in emergency departments. Healthcare 2021, 9, 763. [Google Scholar] [CrossRef]
- Gabow, P.A.; Goodman, P.L. The lean prescription: Powerful medicine for our ailing healthcare system. In The Lean Prescription: Powerful Medicine for Our Ailing Healthcare System; CRC Press, Taylor & Francis: Boca Raton, FL, USA, 2014; pp. 1–155. ISBN 9781482246391. [Google Scholar]
- Abdallah, A.A. Healthcare Engineering: A Lean Management Approach. J. Healthc. Eng. 2020, 2020, 8875902. [Google Scholar] [CrossRef]
- Browning, T.R.; de Treville, S. A lean view of lean. J. Oper. Manag. 2021, 67, 640–652. [Google Scholar] [CrossRef]
- Benson, H. Chaos and complexity: Applications for healthcare quality and patient safety. J. Healthc. Qual. 2005, 27, 4–10. [Google Scholar] [CrossRef]
- Zimmerman, B. How Complexity Science Is Transforming Healthcare. In The SAGE Handbook of Complexity and Management; SAGE: London, UK, 2011; ISBN 9781446201084. [Google Scholar]
- Sugihara, G.; May, R.; Ye, H.; Hsieh, C.H.; Deyle, E.; Fogarty, M.; Munch, S. Detecting causality in complex ecosystems. Science 2012, 338, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Cinaroglu, S. Complexity in healthcare management: Why does Drucker describe healthcare organizations as a double-headed monster? Int. J. Healthc. Manag. 2016, 9, 11–17. [Google Scholar] [CrossRef]
- Engelseth, P.; White, B.E.; Mundal, I.; Eines, T.F.; Kritchanchai, D. Systems modelling to support the complex nature of healthcare services. Health Technol. 2021, 11, 193–209. [Google Scholar] [CrossRef]
- Zeigler, B.P.; Muzy, A.; Kofman, E. Theory of Modeling and Simulation: Discrete Event & Iterative System Computational Foundations; Academic Press: London, UK, 2018; ISBN 9780128134078. [Google Scholar]
- Kennedy, O.G. The use of computer simulation in health care facility design. In Proceedings of the Winter Simulation Conference, San Francisco, CA, USA, 17–19 January 1973; pp. 172–198. [Google Scholar]
- Koizumi, N.; Kuno, E.; Smith, T.E. Modeling patient flows using a queuing network with blocking. Health Care Manag. Sci. 2005, 8, 49–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohleder, T.R.; Bischak, D.P.; Baskin, L.B. Modeling patient service centers with simulation and system dynamics. Health Care Manag. Sci. 2007, 10, 1–12. [Google Scholar] [CrossRef]
- Currie, C.S.M.; Fowler, J.W.; Kotiadis, K.; Monks, T.; Onggo, B.S.; Robertson, D.A.; Tako, A.A. How simulation modelling can help reduce the impact of COVID-19. J. Simul. 2020, 14, 83–97. [Google Scholar] [CrossRef] [Green Version]
- Ozkaynak, M.; Sircar, C.M.; Frye, O.; Valdez, R.S. A systematic review of design workshops for health information technologies. Informatics 2021, 8, 34. [Google Scholar] [CrossRef]
- Naumann, R.B.; Guynn, I.; Clare, H.M.; Lich, K.H. Insights from system dynamics applications in addiction research: A scoping review. Drug Alcohol Depend. 2022, 231, 109237. [Google Scholar] [CrossRef]
- Abdelghany, M.; Eltawil, A.B. Linking approaches for multi-methods simulation in healthcare systems planning and management. Int. J. Ind. Syst. Eng. 2017, 26, 275–290. [Google Scholar] [CrossRef]
- Patriarca, R.; Falegnami, A.; Bilotta, F. Embracing simplexity: The role of artificial intelligence in peri-procedural medical safety. Expert Rev. Med. Devices 2019, 16, 77–79. [Google Scholar] [CrossRef]
- Abdullah, S.S.; Rostamzadeh, N.; Sedig, K.; Garg, A.X.; McArthur, E. Visual analytics for dimension reduction and cluster analysis of high dimensional electronic health records. Informatics 2020, 7, 17. [Google Scholar] [CrossRef]
- Ola, O.; Sedig, K. Health literacy for the general public: Making a case for non-trivial visualizations. Informatics 2017, 4, 33. [Google Scholar] [CrossRef] [Green Version]
- Azcarate, C.; Esparza, L.; Mallor, F. The problem of the last bed: Contextualization and a new simulation framework for analyzing physician decisions. Omega 2020, 96, 102120. [Google Scholar] [CrossRef]
- Brailsford, S.C.; Desai, S.M.; Viana, J. Towards the holy grail: Combining system dynamics and discrete-event simulation in healthcare. In Proceedings of the Winter Simulation Conference, Baltimore, ML, USA, 5–8 December 2010; pp. 2293–2303. [Google Scholar]
- Chahal, K.; Eldabi, T.; Young, T. A conceptual framework for hybrid system dynamics and discrete event simulation for healthcare. J. Enterp. Inf. Manag. 2013, 26, 50–74. [Google Scholar] [CrossRef]
- Gönül-Sezer, E.D.; Ocak, Z. Comparison of System Dynamics and Discrete Event Simulation Approaches. In Simulation and Modeling Methodologies, Technologies and Applications; Springer: London, UK, 2016; Volume 442, ISBN 9783319312941. [Google Scholar]
- Zulkepli, J.; Eldabi, T. Towards a framework for conceptual model hybridization in healthcare. In Proceedings of the Winter Simulation Conference, Washington, DC, USA, 11–14 December 2016; Volume 2016, pp. 1597–1608. [Google Scholar]
- Mielczarek, B.; Zabawa, J. Modeling healthcare demand using a hybrid simulation approach. In Proceedings of the Winter Simulation Conference, Washington, DC, USA, 11–14 December 2016; pp. 1535–1546. [Google Scholar]
- Alvarado, M.; Lawley, M.; Li, Y. Healthcare simulation tutorial: Methods, challenges, and opportunities. In Proceedings of the Winter Simulation Conference, Washington, DC, USA, 11–14 December 2016; pp. 236–247. [Google Scholar]
- Nawawi, M.K.M.; Zulkepli, J.; Khalid, R.; Abidin, N.Z. Modeling in Healthcare: Selection of Simulation Types. Univers. J. Public Health 2021, 9, 401–409. [Google Scholar] [CrossRef]
- Romano, E.; Assante, D. A Management of Intensive Care Unit based on the System Dynamics Model. In Proceedings of the 5th International Conference on Applied Economics, Business and Development, Chania, Greece, 27–29 August 2013. [Google Scholar]
- Romano, E.; Santillo, L.C.; Zoppoli, P. Transformation of a production/assembly washing machine lines into a lean manufacturing system. WSEAS Trans. Syst. Control 2009, 4, 65–76. [Google Scholar]
- Murino, T.; Naviglio, G.; Romano, E. Optimal size of kanban board in a single stage multi product system. WSEAS Trans. Syst. Control 2010, 5, 464–473. [Google Scholar]
- Romano, E.; Guizzi, G.; Chiocca, D. A decision support tool, implemented in a system dynamics model, to improve the effectiveness in the hospital emergency department. Int. J. Procure. Manag. 2015, 8, 141–168. [Google Scholar] [CrossRef] [Green Version]
- Romano, E.; Iuliano, D. A simulation/optimisation approach to support the resource allocation in service firms. Int. J. Procure. Manag. 2018, 11, 53–75. [Google Scholar] [CrossRef]
- Garcia-Vicuña, D.; Esparza, L.; Mallor, F. Hospital preparedness during epidemics using simulation: The case of COVID-19. Cent. Eur. J. Oper. Res. 2022, 30, 213–249. [Google Scholar] [CrossRef]
- Kelton, W.D. Simulation with Arena; Zupick, N.B., Ed.; McGraw-Hill Education: New York, NY, USA, 2015; ISBN 0073401315. [Google Scholar]
- Bertolini, G.; Rossi, C.; Brazzi, L.; Radrizzani, D.; Rossi, G.; Arrighi, E.; Simini, B. The relationship between labour cost per patient and the size of intensive care units: A multicentre prospective study. Intensive Care Med. 2003, 29, 2307–2311. [Google Scholar] [CrossRef] [PubMed]
- Zulkepli, J.; Eldabi, T.; Mustafee, N. Hybrid simulation for modelling large systems: An example of integrated care model. In Proceedings of the 2012 Winter Simulation Conference (WSC), Berlin, Germany, 9–12 December 2012; pp. 1–12. [Google Scholar]
- Boffelli, S.; Rossi, C.; Anghileri, A.; Giardino, M.; Carnevale, L.; Messina, M.; Neri, M.; Langer, M.; Bertolini, G.; Marco, A.; et al. Continuous quality improvement in intensive care medicine. The GiViTI Margherita Project-Report 2005. Minerva Anestesiol. 2006, 72, 419–432. [Google Scholar] [PubMed]
- Kharaba, A.; Algethamy, H.; Hussein, M.; Al-Hameed, F.M.; Alghamdi, A.; Hamdan, A.; Fatani, J.; Elhazmi, A.; Alkhalaf, H.; Barghash, B.; et al. Incidence, outcomes, and predictors of Acinetobacter infection in Saudi Arabian critical care units. J. Crit. Care 2021, 66, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Leroy, O.; Gangneux, J.-P.; Montravers, P.; Mira, J.-P.; Gouin, F.; Sollet, J.-P.; Carlet, J.; Reynes, J.; Rosenheim, M.; Regnier, B.; et al. Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: A multicenter, prospective, observational study in France (2005–2006). Crit. Care Med. 2009, 37, 1612–1618. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).