Abstract
Background: Pediatric non-alcoholic fatty liver disease (NAFLD) is a major public health concern. Aminotransferase (ALT) is frequently used for screening and monitoring, but few studies have reported typical patterns of ALT elevation in children. Methods: TARGET-NASH is a real-world longitudinal observational cohort of patients with NAFLD receiving care across the United States. Analyses included children enrolled between 1 August 2016, and 12 October 2020, with at least one ALT measurement after enrollment. Peak ALT was based on the first and last available record and categorized into clinical cut points: <70 IU/L, >70–<250 IU/L, and >250 IU/L. A chi-squared test was used to compare differences in proportions, and a Kruskal–Wallis test was used to compare the medians and distributions of continuous responses. Results: Analyses included 660 children with a median age of 13 years. Of the 660, a total of 187 had undergone a biopsy and were more likely to be Hispanic or Latino (67% vs. 57%, p = 0.02) and to have cirrhosis (10% vs. 1%, p < 0.001). The highest ALT scores ranged from 28 U/L to 929 U/L; however, these scores varied across time. The prevalence of cirrhosis or any liver fibrosis stage was most common among children with a peak ALT > 70 U/L. Conclusions: Large variability was seen in ALT among children, including many values > 250 U/L. Higher levels of ALT were associated with increased prevalence of comorbidities and more advanced stages of NAFLD. These findings support an increased need for therapeutics and disease severity assessment in children with peak ALT > 70 U/L.
1. Introduction
Pediatric non-alcoholic fatty liver disease (NAFLD) is a major public health problem that has raised concern worldwide. In recent years, NAFLD has developed into one of the most common chronic diseases affecting children as young as two years of age, and the prevalence is continuing to increase [1,2,3]. The rate of pediatric patients with NAFLD has more than doubled in recent decades and has persisted across gender and racial/ethnic subgroups [4]. A meta-analysis of studies examining the prevalence of NAFLD worldwide showed a pooled prevalence of 7.6% in the general population and approximately 36% among children with obesity. With the increased prevalence of obesity among pediatric patients worldwide, the rate of NAFLD could continue to rise in the coming years [1,5,6].
While the risk factors associated with NAFLD in pediatric patients are similar to adults, the presence of either metabolic syndrome (MetS) or central adiposity places children at an increased risk for severe steatosis, inflammation, and advanced fibrosis [7,8]. In children, the long-term implications and trajectory of the disease are still unknown; however, some studies suggest that NAFLD presenting in childhood may be more severe compared to disease that initiates in adulthood [9]. For example, at diagnosis, 15% of the children have advanced liver fibrosis compared to 11.5% of adults [10,11], a crucial histologic feature that predicts mortality in adult patients with NAFLD.
Alanine aminotransferase (ALT) is commonly used both to screen and monitor the severity of NAFLD in clinical practice and clinical trials due in part to its availability and cost-effectiveness [10]. Despite the common utilization of ALT, there is a paucity of data describing both the range and clinical characteristics associated with ALT elevation among children with NAFLD in “real-world” practice. While the median or mean ALT for a cohort is commonly reported, it is less well documented what percentage of children with NAFLD have very high ALT levels. Further, ALT is commonly used as an inclusion criterion in NAFLD clinical trials, and ALT > 250 U/L is frequently an exclusion criterion due to insufficient data on children with levels > 250 U/L. This study describes the distribution of peak ALT levels as well as the distribution of clinical characteristics by both history of a biopsy and peak serum ALT category (≤70, >70 to ≤250, and >250 U/L) among children enrolled in the real-world data cohort TARGET-NASH.
2. Methods
2.1. Cohort
TARGET-NASH is a real-world longitudinal observational cohort of pediatric and adult patients with NAFLD receiving care in both academic and community Hepatology, Gastroenterology, and Endocrinology practices across the United States. Upon enrollment, patients who consent to participate provide access to their medical records for three years prior to the date of enrollment and are then followed prospectively for up to five years. Patient narratives, laboratory values, pathology reports, and imaging data are extracted from the medical record and uploaded into a secured database at six-month intervals from the date of enrollment. Demographics, comorbidities, concomitant medications, interventions for NAFLD, and liver disease progression are represented in the database as well as adverse outcomes, including cardiovascular and neoplastic complications. A detailed description of the TARGET-NASH study design has been previously published [12,13]. Approvals from central and/or local institutional review boards were obtained prior to subject recruitment and enrollment.
This analysis included children enrolled in TARGET-NASH between 1 August 2016 and 12 October 2020 receiving care across 12 academic specialty hepatology and gastroenterology pediatric institutions in the US with at least one ALT measurement from the date of enrollment. For peak ALT and follow-up derivations, any ALTs collected more than 3.5 years prior to the date of enrollment are excluded.
2.2. Liver Disease Case Definition
All patients had a diagnosis of NAFLD at enrollment by their treating physician based on either liver biopsy, imaging, or a pragmatic case definition and were being managed in usual clinical practice. Keeping in line with the real-world data approach, the NAFLD definition used reflects what occurs in common practice rather than within guideline recommendations. A categorization of cirrhosis was based on findings from liver biopsies or imaging with a clinical designation of cirrhosis.
2.3. Patient Characteristics
Demographics including age, gender, ethnicity, any medication use, as well as any history of comorbid disease were obtained at the time of enrollment. Body mass index (BMI) z-score and weight percentile were calculated based on the most recent height and weight values closest to enrollment using the Centers for Disease Control 2000 growth charts. Weight category was derived from BMI percentiles, with classification as underweight (BMI < 5 percentile), normal weight (BMI 5 to <85 percentile), overweight (BMI 85 to <95 percentile), and obese (BMI ≥ 95 percentile).
Laboratory measures including aspartate aminotransferase (AST), ALT, albumin, total bilirubin, alkaline phosphatase (ALP), hemoglobin, and platelet count were ascertained from the values closest to enrollment. Liver histology was obtained from the most recent radiographic and biopsy reports.
2.4. Alanine Aminotransferase (ALT)
Peak ALT was ascertained over the longitudinal follow-up period from the child’s first medical record on file to the last available record on file. ALT was categorized into a three-level variable according to the following a priori clinical cut points: ≤70 IU/L, >70–≤ 250 IU/L, and >250 IU/L. These cutoffs are based on inclusion criteria commonly used in clinical trials of children with NAFLD. These inclusion criteria usually require an ALT value higher than three times the upper limit of normal and lower than ten times the upper limit normal. For patients with a minimum of five ALT measurements at least 1 month apart, ALT trajectories across each of the clinical cut points (≤70 IU/L, >70–≤250 IU/L, >250 IU/L) were generated. ALTs collected within 3 months of the onset of cholestasis, cholelithiasis, pancreatitis, sepsis, bariatric surgery, and other conditions indicative of gallbladder disease were excluded from the ALT trajectory analysis as the ALT level may be indicative of these conditions and not the underlying liver disease. A secondary review was performed on records obtained from three patients with the highest ALT values (929, 819, 715). All three patients had clinical data and liver biopsies performed indicating NASH without evidence of other etiologies and were therefore included in the study.
2.5. Statistical Analysis
The mean and standard deviations were calculated across continuous measures of patient characteristics, while counts and percentages were provided for categorical responses.
A chi-squared test was used to compare the differences in proportions across categories of patient characteristics. For cell counts less than 5, a Fisher’s exact test was used. A Kruskal–Wallis test was used to compare the medians and distributions of continuous responses, and a Fisher’s exact test was used for group comparisons. A p-value of <0.05 was considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
3. Results
3.1. Study Population
The analysis included 660 children diagnosed with NAFLD and enrolled in TARGET-NASH between 1 August 2016 and 12 October 2020 who had at least one ALT measurement. The median age of children in the cohort was 13 years, and 67% were white, 60% were Hispanic or Latino, and 70% were male. The median BMI z-score was 2.36 (range 0–4.5), and 93% of children were obese. The prevalence of NAFLD cirrhosis was 3.6%, and 18.6% of children were diagnosed with Type 2 diabetes (Table 1). Of the 660 patients, 101 patients (15.3%) had a history of anxiety or depression, 89 patients (13.5%) had a history of pediatric ADHD, and 71 patients (10.8%) had a history of psoriasis or other autoimmune skin diseases.

Table 1.
Demographics and Characteristics of Pediatric Patients Enrolled in TARGET-NASH.
3.2. Patient Characteristics by Receipt of Biopsy
Of the 660 children with NAFLD, a total of 187 had a biopsy reported. Patients who had or had not received a biopsy did not differ significantly regarding age, gender, and race. However, patients who received a biopsy were more likely to be Hispanic or Latino (67% vs. 57%, p = 0.02) or have cirrhosis (10% vs. 1%, p < 0.001) and diabetes (27% vs. 15%, p < 0.001). Children with a reported biopsy were found to have a higher peak ALT and AST than those without a biopsy (ALT: 169.0 U/L vs. 88.0 U/L, p < 0.001; AST: 91.0 U/L vs. 52.0 U/L, p < 0.001). Laboratory values for albumin, hemoglobin, and ALP were also found to be significantly different between those who did or did not have a biopsy (Table 1).
Biopsy recipients used either metformin (26% vs. 13%, p < 0.001) or vitamin E (36% vs. 6%, p < 0.001) more frequently and had a longer follow-up time between the first ALT measurement and the last ALT measurement available (median = 34.9 months vs. 26.2 months, p < 0.001) (Table 1).
3.3. Patient Characteristics by Peak ALT Category
The prevalence of cirrhosis was more common among children with a peak ALT > 70 U/L compared to children with a peak ALT ≤ 70 U/L. Children with a peak ALT between 71 and 250 U/L were 2.8 times as likely to have cirrhosis (compared to ALT ≤ 70 U/L), and children with a peak ALT > 250 U/L were eight times more likely to have cirrhosis compared to children with a peak ALT ≤ 70 U/L. The prevalence of Type 2 diabetes was 2.2 times as likely among children with an ALT > 250 U/L compared to children with a peak ALT between 71 and 250 and children with a peak ALT ≤ 70 U/L (ALT ≤ 70 U/L, 17%; ALT between 71–250, 16%; ALT > 250 U/L, 36%; p < 0.001) (Table 2).

Table 2.
Demographic and Patient Characteristics by Peak ALT Categories.
The distribution of age, race, ethnicity, and BMI z-score were similar between peak ALT categories; however, the male-to-female ratio was significantly higher among children with a peak ALT > 70 U/L. Among children with an ALT value > 70 U/L, children were nearly twice as likely to be of Hispanic or Latino ethnicity (Hispanic/Latino: 60%, Non-Hispanic: 40%). Median AST and ALP increased across peak ALT categories, and there was a significant difference across peak ALT categories in median hemoglobin and platelets (p = 0.002 and p = 0.008, respectively). Other than AST, hemoglobin, and platelets, the distribution of biochemical values were not significantly different by peak ALT level. The proportion of children that were prescribed either metformin or vitamin E was higher among children with a peak ALT > 250 U/L than among children with a peak ALT between 71–250 U/L or a peak ALT ≤ 70 U/L (metformin: p = 0.001 and vitamin E: <0.001, respectively) (Table 2). The median time (in months) from the first ALT to the last ALT was greatest for patients with a peak ALT > 250 (ALT ≤ 70 U/L, 28.0; ALT > 70–≤250 U/L, 28.3; ALT > 250 U/L, 31.1; p = 0.152).
3.4. Liver Histology
Of the 187 children who had a liver biopsy, reports obtained from each institution showed that the prevalence of grade 3 steatosis was more than 3 times as likely among children with a peak ALT > 70 U/L compared to children with peak ALT ≤70 U/L (ALT ≤ 70 U/L, 17%; ALT 70–<250 U/L, 51%; ALT ≥ 250 U/L, 50%; p = 0.113). The proportion of children with evidence of liver fibrosis stage, assessed by Brunt or NAS scoring, was greater among children in the highest peak ALT category compared to the lowest peak category (ALT ≤ 70 U/L, 58%; ALT > 70–≤250 U/L, 66%; ALT > 250 U/L, 75%; p = 0.372). Lobular inflammation grade 2 (>4 foci under 20× field) and hepatocyte ballooning grade 2 (many ballooned cells) were only present among children with ALT >70 U/L. Moreover, the proportion of children with a NAS score of 5 or greater with a peak ALT > 70 U/L (50%) was 4.5 times that of children with a peak ALT < 70 U/L (11%). Of those receiving a biopsy, 85% had steatohepatitis, and 15% had advanced fibrosis (fibrosis stage 3 or greater). Nearly half of all the children who had a biopsy had a NAS total score ≥ 5 (48%) (Appendix A Table A2).
3.5. Peak ALT by Patient Characteristics
There were no statistically significant differences in median peak ALT levels between males and females, body mass index percentiles, or age and race categories (Figure 1). Children who identified as being Hispanic or Latino had a peak ALT significantly higher than those who did not (106.0 U/L vs. 98.5 U/L, p = 0.018). Peak ALT was also significantly higher in children with any history of metformin (130.0 U/L vs. 100.0 U/L, p = 0.003) or Vitamin E use (162.0 U/L vs. 98.0 U/L, p < 0.001). The median peak ALT value among children with Type 2 diabetes was higher than children without a history of Type 2 diabetes (137.0 U/L vs. 99.0 U/L, p = 0.004). (Figure 1; Table 3).
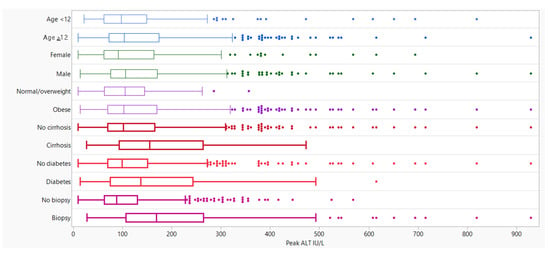
Figure 1.
Median and Quartile Range (q1 and q3) of Peak ALT by Patient Characteristics. Box plots with a bolded line indicate the significance differences in mean peak ALT across response values at a level of 0.05 (refer to Table 3).

Table 3.
Peak ALT values by patient characteristics.
Children who underwent a liver biopsy (n = 187) had a median peak ALT value that was nearly double that of children who did not have a biopsy (n = 473) (169.0 vs. 88.0; p < 0.001) (Figure 1; Table 1). Additionally, children with steatohepatitis or the presence of fibrosis had a higher median peak ALT value than children without these histological features (Appendix A Table A1).
3.6. ALT over Time
Data from pediatric patients with at least five ALT measurements were utilized to investigate the ALT fluctuation across time (Figure 2). Generally, most children who were in the ≤70 IU/L ALT category at their initial measurement (Time 1) stayed at that level over time, with a small percentage moving to the ALT > 250 IU/L category (<1%). Overall, the increasing trend of the percentage of children in the ≤70 IU/L ALT category and the transitions from higher ALT categories to the ≤70 IU/L ALT suggest an improvement in ALT over time. Forty-four percent of children had an ALT between 71 and 250 IU/L at their initial time point and improved slightly over time with some moving into the ALT ≤ 70 IU/L category, while a smaller number of children worsened to an ALT > 250 IU/L at later time points. There was a small number of patients (5%) with ALT > 250 IU/L at their initial time point, and these patients generally remained in the higher ALT category, with only three instances of improving to ≤70 IU/L at some point (Figure 2). Appendix A Table A3 is a numerical representation of the transitions between ALT categories across each of the five time points. The majority of children started with an ALT ≤ 70 IU/L (51%, n = 170) followed by 71–250 IU/L (44%, n = 148) and >250 IU/L (5%, n = 17), and by time point five, these numbers had changed to 54% (n = 181), 41% (n = 137), and 5% (n = 17), respectively (Appendix A Table A3).
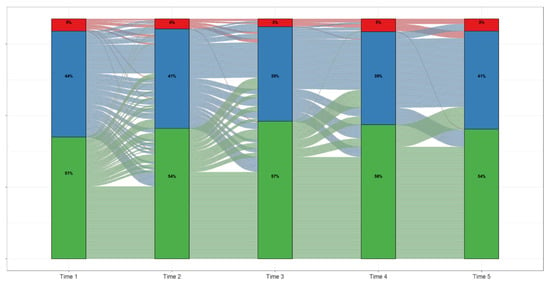
Figure 2.
ALT Trajectories Among Pediatric Patients Enrolled in TARGET-NASH.
4. Discussion
This study describes the distribution of clinical and metabolic characteristics among a large cohort of children with NAFLD stratified by receipt of biopsy (biopsy, no biopsy) and peak ALT level (≤70 U/L, >70–≤250 U/L, and >250 U/L). The prevalence of NAFLD, a disease associated with a high risk for premature morbidity and mortality [14], has substantially increased with the increasing rates of obesity in children [1]. Despite the controversial role of ALT in predicting NAFLD severity in children [15,16], ALT is frequently used in clinical practice to monitor disease activity. Overall, there was substantial variability in ALT values among children in this study population. Contrary to trends in adult patients with NAFLD, there were a considerable number of children with NAFLD with ALT values higher than 250 U/L. In this cohort, approximately 12% of patients had a peak ALT > 250 U/L with values ranging between 252 and 929 U/L.
The variations shown in ALT trajectory across categories indicate that the disease state is not static. Patients with higher ALT values were more likely to remain in the higher ALT category and were associated with comorbidities such as Type 2 diabetes and cirrhosis. Conversely, children who initially had ALT < 70 U/L tended to stay in this category and have a less severe disease. While no trends can define the situation for an individual patient, these data suggest a decreased monitoring frequency in children with lower ALT and the importance of further assessment in children with high ALT.
Children who underwent a liver biopsy were more likely to be Hispanic and have higher ALT levels compared to those who did not. This may reflect clinical concern for more significant liver disease driving the decision to perform the biopsy. Hispanic children have the highest risk of NAFLD and appear to have a higher likelihood of advanced liver fibrosis [17,18]. While liver biopsies are the gold standard for assessing disease severity, it is an invasive procedure that has been associated with morbidity, and in some cases, mortality [19,20]. ALT has been shown to have moderate specificity and sensitivity in detecting NAFL, while also being non-invasive and inexpensive [21], with mean ALT values measured over time proving to be a valid biomarker of histologic changes in children [22]. In this study, patients with peak ALT values higher than 70 presented a higher prevalence of cirrhosis, NAS score, and higher grades of fibrosis, inflammation and ballooning. Hispanics were the most prevalent among the highest ALT category. In addition to liver disease severity, the prevalence of Type 2 diabetes was also greater in patients with ALT values higher than 70. Type 2 diabetes has been associated with NAFLD in adult populations, and this can be translated to pediatric populations, as ALT is used as a surrogate for liver fat accumulation. A rise in ALT may reflect not only a greater degree of insulin resistance but also a higher possibility of developing Type 2 diabetes.
The degree of ALT elevation was also associated with a higher prevalence of prescription medication use. The percentage of children that had documented use of metformin, as well as a history of Type 2 diabetes, was higher among children with an elevated ALT (>70 U/L). Vitamin E was more frequently prescribed in children with ALT > 70 U/L. At present, medications are not recommended in the treatment of pediatric patients with NAFLD under the guidance of the NASHPGHAN guidelines [10]. However, like the patients within this study, Vitamin E has been used by clinicians for treating biopsy-proven NASH based on promising results within the adult population [23,24].
This real-world observational study conducted in academic and community sites included a large and diverse multicenter population of children that were diagnosed and managed for NAFLD in usual clinical practice. Previous studies examining pediatric NAFLD have occurred mainly in randomized controlled trials [23,25], thus limiting the sample to a stringent population with very specific inclusion/exclusion criteria and an inability to look at the progression over time in a real-world population [26]. The wide inclusion criteria for this study allowed the analysis of those patients with normal to mildly elevated values of ALT, a population often overlooked.
There were limitations to this study. Not all children in the cohort had a liver biopsy; however, most of them had either an MRI or US of the liver. Both methods are widely used to assess the presence of NAFLD in a clinical setting and in large population-based studies [27,28]. Second, the available biopsies were analyzed by the pathologist in charge of each institution in a routine clinical setting, which might have increased the variability in pathology interpretation. Another limitation was the high percentage of children identified as Hispanics or Latinos, which most likely reflects the distribution of the disease. In this study, even though children of Hispanic or Latino origin are at the highest risk for developing NAFLD [18], the percentage of children who were Hispanic or Latino in each category of peak ALT was similar. This may be due to the diversity of the population of Hispanics or Latinos in this study, misclassification due to the self-reported nature of ethnicity, or the way ALT was categorized using a priori values. Despite these limitations, the findings add to the scientific literature by describing the trends in ALT related to NAFLD during childhood.
In conclusion, pediatric patients with NAFLD enrolled in TARGET-NASH showed large variability in ALT. Peak ALT scores ranged from 10 U/L to 929 U/L, likely influenced both by phase of disease and severity. There were a surprisingly high number of pediatric patients with a peak ALT ≥ 250 within this diverse real-world cohort. Future studies following ALT trajectories over longer time periods and with clinical outcome data would be beneficial for understanding NAFLD among pediatric patients.
Author Contributions
Conceptualization, E.C.-L., H.L.M., C.S., P.M., T.M., S.P., M.N.K., B.M., A.R.M., B.R., S.A.X. and M.B.V.; Data curation, C.S. and B.M.; Methodology, H.L.M., C.S., J.B., P.M., T.M., S.P., M.N.K., B.M., A.R.M., B.R., S.A.X. and M.B.V.; Writing—review & editing, E.C.-L., H.L.M., C.S., J.B., P.M., T.M., S.P., M.N.K., B.M., A.R.M., B.R., S.A.X. and M.B.V. All authors have read and agreed to the published version of the manuscript.
Funding
Target RWE is the sponsor of the TARGET-NASH study and is solely responsible for data collection and analysis and the decision to publish, as well as preparation of the manuscript with co-authors. TARGET-NASH is a collaboration among academic and community investigators and the pharmaceutical industry.
Institutional Review Board Statement
The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received. The study conformed to the US Federal Policy for the Protection of Human Subjects. Ethics approval was obtained from the Western Institutional Review Board (Approval Code—WCG 20161381), approval date is 22 June 2016.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study by each of the participating sites in TARGET-NASH.
Data Availability Statement
Due to privacy issues, data is not publicly available.
Acknowledgments
Target RWE is responsible for designing and conducting the TARGET NASH study and the collection, management, and analysis of the data. A complete list of participating sites and the associated TARGET-NASH investigators can be found in Appendix A Table A4. TARGET-NASH Investigators are responsible for the recruitment of patients and acquisition of data for the TARGET-NASH study. We would like to thank Stephanie Watkins, for her contribution to this study.
Conflicts of Interest
E.C.L., J.B., P.M., T.M., S.P., M.N.K., B.R. and S.K. have no conflict to report. H.L.M., C.S., B.M., A.R.M. are TARGET employees. MV has research funding or is an advisor to Allergan, Boehringer-Ingelheim, Immuron, Intercept, Resonance Health, and Shire.
Appendix A

Table A1.
Peak ALT by characteristics of liver histology 1.
Table A1.
Peak ALT by characteristics of liver histology 1.
| Number of Patients (n = 660) | Mean (SD) | Median (Min–Max) | p-Value 1 | |
|---|---|---|---|---|
| Liver Biopsy | <0.001 | |||
| Yes | 187 | 211.2 (150.7) | 169.0 (28–929) | |
| No | 473 | 110.5 (77.21) | 88.0 (10–568) | |
| NAS Total Score | 0.668 | |||
| 0 | 1 | 72.0 (-) | 72.0 (72–72) | |
| 1 | 5 | 131.2 (100.3) | 87.0 (28–280) | |
| 2 | 10 | 184.3 (196.1) | 117.5 (43–694) | |
| 3 | 20 | 199.7 (147.5) | 147.5 (65–651) | |
| 4 | 48 | 206.3 (151.4) | 157.0 (61–819) | |
| 5 | 54 | 232.1 (130.1) | 196.5 (54–608) | |
| 6 | 23 | 235.3 (132.1) | 234.0 (95–715) | |
| Steatohepatitis on Brunt and/or NAS 2,3 | 0.002 | |||
| Yes | 153 | 227.0 (156.3) | 175.0 (31–929) | |
| No | 27 | 128.7 (75.74) | 109.0 (28–286) | |
| Fibrosis 3,4 | ||||
| Yes | 127 | 230.4 (163.6) | 183.0 (31–929) | 0.011 |
| No | 60 | 170.7 (109.5) | 130.5 (28–552) | |
| Advanced Fibrosis 3,5 | 0.756 | |||
| Yes | 28 | 219.4 (144.0) | 175.0 (31–715) | |
| No | 159 | 209.8 (152.3) | 169.0 (28–929) |
1p-value is for the comparison of mean peak ALT; 2 Participant has either steatohepatitis on Brunt assessment or NAS Score ≥ 4; 3 Summaries are provided for participants with biopsy; 4 Fibrosis is determined by fibrosis stage ≥ 1 on either Brunt or NAS; 5 Advanced fibrosis is determined by fibrosis stage ≥ 3 on either Brunt or NAS.

Table A2.
Characteristics of Liver histology.
Table A2.
Characteristics of Liver histology.
| Peak ALT | All Participants (n = 660) | p-Values 4 | |||
|---|---|---|---|---|---|
| Summary | ≤70 IU/L | 71 to 250 IU/L | >250 IU/L | ||
| (n = 164) | (n = 415) | (n = 81) | |||
| Liver Histology | |||||
| Participant with Biopsy, n (%) | 12 (7.3%) | 123 (29.6%) | 52 (64.2%) | 187 (28.3%) | -- |
| Steatosis–Brunt and/or NAS | |||||
| Steatohepatitis on Brunt and/or NAS 1 | <0.001 | ||||
| No | 6 (50.0%) | 18 (15.3%) | 3 (6.0%) | 27 (15.0%) | |
| Yes | 6 (50.0%) | 100 (84.7%) | 47 (94.0%) | 153 (85.0%) | |
| Not Available | 0 | 5 | 2 | 7 | |
| Fibrosis Stage–Brunt or NAS | |||||
| Fibrosis, n (%) 2 | 0.372 | ||||
| No | 5 (41.7%) | 42 (34.1%) | 13 (25.0%) | 60 (32.1%) | |
| Yes | 7 (58.3%) | 81 (65.9%) | 39 (75.0%) | 127 (67.9%) | |
| Not Available | 0 | 0 | 0 | 0 | |
| Advanced Fibrosis, n (%) 3 | 0.848 | ||||
| No | 11 (91.7%) | 104 (84.6%) | 44 (84.6%) | 159 (85.0%) | |
| Yes | 1 (8.3%) | 19 (15.4%) | 8 (15.4%) | 28 (15.0%) | |
| Not Available | 0 | 0 | 0 | 0 | |
| Brunt Scoring | |||||
| Steatohepatitis status, n (%) | 0.033 | ||||
| Not Steatohepatitis | 3 (37.5%) | 11 (11.7%) | 2 (4.9%) | 16 (11.2%) | |
| Steatohepatitis | 5 (62.5%) | 83 (88.3%) | 39 (95.1%) | 127 (88.8%) | |
| Not Available | 2 | 21 | 7 | 30 | |
| Inflammation Grade, n (%) | 0.585 | ||||
| 0 | 2 (20.0%) | 6 (6.5%) | 4 (9.5%) | 12 (8.3%) | |
| 1 | 7 (70.0%) | 69 (74.2%) | 31 (73.8%) | 107 (73.8%) | |
| 2 | 0 (0.0%) | 13 (14.0%) | 5 (11.9%) | 18 (12.4%) | |
| 3 | 1 (10.0%) | 5 (5.4%) | 2 (4.8%) | 8 (5.5%) | |
| Not Available | 0 | 22 | 6 | 28 | |
| Fibrosis Stage, n (%) | 0.589 | ||||
| 0 | 5 (50.0%) | 37 (32.2%) | 12 (25.0%) | 54 (31.2%) | |
| 1 | 3 (30.0%) | 40 (34.8%) | 14 (29.2%) | 57 (32.9%) | |
| 2 | 1 (10.0%) | 19 (16.5%) | 14 (29.2%) | 34 (19.7%) | |
| 3 | 1 (10.0%) | 16 (13.9%) | 8 (16.7%) | 25 (14.5%) | |
| 4 | 0 (0.0%) | 3 (2.6%) | 0 (0.0%) | 3 (1.7%) | |
| Not Available | 0 | 0 | 0 | 0 | |
| NAS Scoring | |||||
| Steatosis Grade, n (%) | 0.113 | ||||
| 0 | 0 (0.0%) | 2 (1.8%) | 0 (0.0%) | 2 (1.2%) | |
| 1 | 6 (50.0%) | 17 (15.5%) | 10 (20.0%) | 33 (19.2%) | |
| 2 | 4 (33.3%) | 35 (31.8%) | 15 (30.0%) | 54 (31.4%) | |
| 3 | 2 (16.7%) | 56 (50.9%) | 25 (50.0%) | 83 (48.3%) | |
| Not Available | 0 | 6 | 1 | 7 | |
| Lobular Inflammation Grade, n (%) | 0.051 | ||||
| 0 | 4 (40.0%) | 10 (9.8%) | 4 (8.7%) | 18 (11.4%) | |
| 1 | 6 (60.0%) | 65 (63.7%) | 28 (60.9%) | 99 (62.7%) | |
| 2 | 0 (0.0%) | 27 (26.5%) | 14 (30.4%) | 41 (25.9%) | |
| Not Available | 2 | 14 | 5 | 21 | |
| Hepatocyte Ballooning Grade, n (%) | 0.856 | ||||
| 0 | 4 (44.4%) | 31 (30.4%) | 12 (26.7%) | 47 (30.1%) | |
| 1 | 5 (55.6%) | 59 (57.8%) | 28 (62.2%) | 92 (59.0%) | |
| 2 | 0 (0.0%) | 12 (11.8%) | 5 (11.1%) | 17 (10.9%) | |
| Not Available | 3 | 14 | 6 | 23 | |
| NAS Total Score, n (%) | 0.043 | ||||
| 0 | 0 (0.0%) | 1 (0.9%) | 0 (0.0%) | 1 (0.6%) | |
| 1 | 1 (11.1%) | 3 (2.8%) | 1 (2.2%) | 5 (3.1%) | |
| 2 | 4 (44.4%) | 4 (3.7%) | 2 (4.3%) | 10 (6.2%) | |
| 3 | 1 (11.1%) | 14 (13.1%) | 5 (10.9%) | 20 (12.3%) | |
| 4 | 2 (22.2%) | 32 (29.9%) | 14 (30.4%) | 48 (29.6%) | |
| 5 | 1 (11.1%) | 36 (33.6%) | 17 (37.0%) | 54 (33.3%) | |
| 6 | 0 (0.0%) | 16 (15.0%) | 7 (15.2%) | 23 (14.2%) | |
| 7 | 0 (0.0%) | 1 (0.9%) | 0 (0.0%) | 1 (0.6%) | |
| Not Available | 3 | 9 | 5 | 17 | |
| NAS Total Score Grouped, n (%) | 0.009 | ||||
| 0–3 | 6 (66.7%) | 22 (20.8%) | 8 (17.4%) | 36 (22.4%) | |
| 4–6 | 3 (33.3%) | 84 (79.2%) | 38 (82.6%) | 125 (77.6%) | |
| Not Available | 3 | 10 | 5 | 18 | |
| NAS Total Score | 0.001 | ||||
| Median (n) | 2.0 (9) | 4.0 (107) | 5.0 (46) | 4.0 (162) | |
| Min–Max | 1–5 | 0–7 | 1–6 | 0–7 | |
| Fibrosis Stage, n (%) | 0.321 | ||||
| 0 | 0 (0.0%) | 5 (55.6%) | 0 (0.0%) | 5 (35.7%) | |
| 1 | 1 (50.0%) | 2 (22.2%) | 2 (66.7%) | 5 (35.7%) | |
| 2 | 1 (50.0%) | 2 (22.2%) | 1 (33.3%) | 4 (28.6%) | |
| Not Available | 10 | 107 | 48 | 165 | |
1 Participant has either steatohepatitis on Brunt assessment or NAS Score ≥ 4. 2 Fibrosis is determined by fibrosis stage ≥ 1 on either Brunt or NAS. 3 Advanced fibrosis is determined by fibrosis stage ≥ 3 on either Brunt or NAS. 4 p-value is from an exact test.

Table A3.
ALT Transition Percentages in Pediatric Participants.
Table A3.
ALT Transition Percentages in Pediatric Participants.
| Timepoint 2 | ||||
| Timepoint 1 | ALT ≤ 70 IU/L | ALT 71 to 250 IU/L | ALT > 250 IU/L | Participant Count |
| ALT ≤ 70 IU/L | 79.4% | 20.0% | 0.6% | 170 (50.7%) |
| ALT 71–250 IU/L | 31.1% | 65.5% | 3.4% | 148 (44.2%) |
| ALT > 250 IU/L | 5.9% | 47.05% | 47.05% | 17 (5.1%) |
| Participant Count | 182 (54.3%) | 139 (41.4%) | 14 (4.1%) | |
| Timepoint 3 | ||||
| Timepoint 2 | ALT ≤ 70 IU/L | ALT 71 to 250 U/L | ALT > 250 IU/L | Participant Count |
| ALT ≤ 70 IU/L | 82.4% | 17.6% | 0% | 182 (54.3%) |
| ALT 71–250 IU/L | 29.5% | 66.2% | 4.3% | 139 (41.4%) |
| ALT > 250 IU/L | 7.1% | 57.1% | 39.4% | 14 (4.1%) |
| Participant Count | 192 (57.3%) | 132 (39.4%) | 11 (3.2%) | |
| Timepoint 4 | ||||
| Timepoint 3 | ALT ≤ 70 IU/L | ALT 71 to 250 U/L | ALT > 250 IU/L | Participant Count |
| ALT ≤ 70 IU/L | 80.2% | 19.3% | 0.5% | 192 (57.3%) |
| ALT 71–250 IU/L | 25.0% | 69.7% | 5.3% | 132 (39.4%) |
| ALT >250 IU/L | 0% | 0.1% | 90.9% | 11 (3.2%) |
| Participant Count | 187 (55.8%) | 130 (38.8%) | 18 (5.4%) | |
| Timepoint 5 | ||||
| Timepoint 4 | ALT ≤ 70 IU/L | ALT 71 to 250 U/L | ALT > 250 IU/L | Participant Count |
| ALT ≤ 70 IU/L | 83.4% | 16.6% | 0% | 187 (55.8%) |
| ALT 71–250 IU/L | 18.5% | 73.9% | 7.7% | 130 (38.8%) |
| ALT > 250 IU/L | 5.6% | 55.6% | 38.9% | 18 (5.4%) |
| Participant Count | 181 (54.0%) | 137 (40.9%) | 17 (5.1%) | |

Table A4.
List of Participating Sites and Principal Investigators.
Table A4.
List of Participating Sites and Principal Investigators.
| The Co-Authors would Like to Thank the Members of the TARGET-NASH Consortium: |
|---|
| Manal Abdelmalek (Duke/Durham, NC, USA) |
| Humberto Aguilar (Louisiana Research Center/Shreveport, LA, USA) |
| Aijaz Ahmed (Stanford University/Palo Alto, CA, USA) |
| Alina Allen (Mayo Clinic/Rochester, MN, USA) |
| Sarah Barlow (UT Southwestern Children’s Health/Dallas, TX, USA) |
| Sid Barritt (University of North Carolina Chapel Hill/Chapel Hill, NC, USA) |
| David Bernstein (Northwell Health/Manhasset, NY, USA) |
| Kaylan Bhamidimarri (University of Miami/Miami, FL, USA) |
| Liana Billings (Northshore University Health System/Skokie, IL, USA) |
| Kyle Brown (University of Iowa Hospitals and Clinics/Iowa City, IA, USA) |
| Robert Brown (Weill Cornell Medical College/New York, NY, USA) |
| Karen Corbin (Advent Health Diabetes Institute/Orlando, FL, USA) |
| Kenneth Cusi (University of Florida Health Division of Endocrinology/Gainsville, FL, USA) |
| Andrew deLemos (Center for Liver Disease and Transplant at CMC/Charlotte, NC, USA) |
| Karan Emerick (Connecticut Children’s Hospital/Hartford, CT, USA) |
| Roberto Firpi-Morell (University of Florida Hepatology Research/Gainsville, FL, USA) |
| Maged Adel Ghali (University of Florida–Jacksonville/Jacksonville, FL, USA) |
| Zachary Henry (University of Virginia/Charlottesville, VA, USA) |
| Whitney Jackson (University of Colorado/Aurora, CO, USA) |
| Sujit Janardhan (Rush University Medical Center/Chicago, IL, USA) |
| Mohammad Kabbany (Cleveland Clinic Children’s/Cleveland, OH, USA) |
| Nyingi Kemmer (Tampa General Medical Group/Tampa, FL, USA) |
| David Koch (MUSC/Charleston, SC, USA) |
| Justin Kupec (West Virginia University/Morgantown, WV, USA) |
| Charles Landis (Harborview Medical Center/Seattle, WA, USA) |
| Mary Katherine Lawrence (Carteret Medical Center/Morehead City, NC, USA) |
| Cynthia Levy (Schiff Center for Liver Disease–University of Miami/Miami, FL, USA) |
| Steven Lidofsky (University of Vermont Medical Center/Burlington, VT, USA) |
| Anna Lok (University of Michigan/Ann Arbor, MI, USA) |
| Velimir Luketic (Virginia Commonwealth University/Richmond, VA, USA) |
| Enrique Martinez (Atlanta Gastro/Atlanta, GA, USA) |
| Craig McClain (University of Louisville/Louisville, KY, USA) |
| Patrick McKiernan (UPMC Children’s Hospital of Pittsburgh/Pittsburgh, PA, USA) |
| Ellen Mitchell (St. Christopher’s Hospital for Children/Philadelphia, PA, USA) |
| Mazen Noureddin (California Liver Research Institute/Pasadena, CA, USA) |
| Sirish Palle (University of Oklahoma/Oklahoma City, OK, USA) |
| Yen Pham (Baylor College of Medicine/Houston, TX, USA) |
| David Pound (Indianapolis Gastroenterology Research Foundation/Indianapolis, IN, USA) |
| Rajender Reddy (University of Pennsylvania/Philadelphia, PA, USA) |
| Fredric Regenstein (Tulane/New Orleans, LA, USA) |
| Mary Rinella (Northwestern University/Chicago, IL, USA) |
| Fedja Rochling (University of Nebraska Medical Center/Omaha, NE, USA) |
| Bryan Rudolph (Children’s Hospital at Montefiore/Bronx, NY, USA) |
| Vinod Rustgi (Rutgers, Robert Wood Johnson Medical School/New Brunswick, NJ, USA) |
| Adnan Said (University of Wisconsin Madison/Madison, WI, USA) |
| Niharika Samala (Indiana University/Indianapolis, IN, USA) |
| Souvik Sarkar (University of California Davis/Sacramento, CA, USA) |
| Kenneth Sherman (University of Cincinnati Health LLC/Cincinnati, OH, USA) |
| Mitchell Shiffman (Bon Secours Liver Institute of Virginia–MIH/Richmond, VA, USA) |
| Coleman Smith (Georgetown University, Medstar/Washington DC, USA) |
| Jawahar Taunk (Advance Gastroenterology Associates, LLC/Palm Harbor, FL, USA) |
| Brent Tetri (St. Louis University/St. Louis, MO, USA) |
| Paul Thuluvath (Mercy Medical Center/Baltimore, MD, USA) |
| Huy Trinh (Silicon Valley Research Institute/San Jose, CA, USA) |
| Elizabeth Verna (Columbia University Medical Center/New York, NY, USA) |
| Miriam Vos (Emory/Atlanta, GA, USA) |
| L. Michael Weiss (Gastro Florida/Clearwater, FL, USA) |
| Mark Wong (Banner University Medical Center/Phoenix, AZ, USA) |
| Kathleen Wyne (Ohio State University/Columbus, OH, USA) |
| Stavra Xanthakos (Cincinnati Children’s Hospital/Cincinnati, OH, USA) |
References
- Anderson, E.L.; Howe, L.; Jones, H.; Higgins, J.; Lawlor, D.; Fraser, A. The Prevalence of Non-Alcoholic Fatty Liver Disease in Children and Adolescents: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0140908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwimmer, J.B.; Ugalde-Nicalo, P.; Welsh, J.A.; Angeles, J.E.; Cordero, M.; Harlow, K.E. Effect of a Low Free Sugar Diet vs Usual Diet on Nonalcoholic Fatty Liver Disease in Adolescent Boys: A Randomized Clinical Trial. JAMA 2019, 321, 256–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suri, A.; Song, E.; van Nispen, J.; Voigt, M.; Armstrong, A.; Murali, V.; Jain, A. Advances in the Epidemiology, Diagnosis, and Management of Pediatric Fatty Liver Disease. Clin. Ther. 2021, 43, 438–454. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Karpen, S.; Vos, M.B. Increasing Prevalence of Nonalcoholic Fatty Liver Disease Among United States Adolescents, 1988–1994 to 2007–2010. J. Pediatr. 2013, 162, 496–500.e1. [Google Scholar] [CrossRef] [Green Version]
- Lobstein, T.; Jackson-Leach, R.; Moodie, M.L.; Hall, K.D.; Gortmaker, S.L.; Swinburn, B.A.; James, W.P.T.; Wang, Y.F.; McPherson, K. Child and adolescent obesity: Part of a bigger picture. Lancet 2015, 385, 2510–2520. [Google Scholar] [CrossRef] [Green Version]
- Van Dommelen, P.; Schönbeck, Y.; Van Buuren, S.; HiraSing, R.A. Trends in a life threatening condition: Morbid obesity in Dutch, Turkish and Moroccan children in The Netherlands. PLoS ONE 2014, 9, e94299. [Google Scholar] [CrossRef]
- Nobili, V.; Bedogni, G.; Canani, R.B.; Brambilla, P.; Cianfarani, S.; Pietrobelli, A.; Agostoni, C. The potential role of fatty liver in paediatric metabolic syndrome: A distinct phenotype with high metabolic risk? Pediatr. Obes. 2012, 7, e75–e80. [Google Scholar] [CrossRef]
- Patton, H.M.; Yates, K.; Unalp-Arida, A.; Behling, C.A.; Huang, T.T.-K.; Rosenthal, P.; Sanyal, A.J.; Schwimmer, J.; Lavine, J.E. Association Between Metabolic Syndrome and Liver Histology Among Children With Nonalcoholic Fatty Liver Disease. Am. J. Gastroenterol. 2010, 105, 2093–2102. [Google Scholar] [CrossRef] [Green Version]
- Holterman, A.L.; Guzman, G.; Fantuzzi, G.; Wang, H.; Aigner, K.; Browne, A.; Holterman, M. Nonalcoholic fatty liver disease in severely obese adolescent and adult patients. Obesity 2013, 21, 591–597. [Google Scholar] [CrossRef] [Green Version]
- Vos, M.B.; Abrams, S.H.; Barlow, S.E.; Caprio, S.; Daniels, S.R.; Kohli, R.; Mouzaki, M.; Sathya, P.; Schwimmer, J.B.; Sundaram, S.S.; et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J. Pediatr. Gastroenterol. Nutr. 2017, 64, 319–334. [Google Scholar] [CrossRef] [Green Version]
- Angulo, P.; Kleiner, D.E.; Dam-Larsen, S.; Adams, L.A.; Björnsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Keach, J.C.; Lafferty, H.D.; Stahler, A.; et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2015, 149, 389–397.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barritt, A.; Gitlin, N.; Klein, S.; Lok, A.S.; Loomba, R.; Malahias, L.; Powell, M.; Vos, M.B.; Weiss, L.M.; Cusi, K.; et al. Design and rationale for a real-world observational cohort of patients with nonalcoholic fatty liver disease: The target-nash study. Contemp. Clin. Trials 2017, 61, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Barritt, A.S.; Watkins, S.; Gitlin, N.; Klein, S.; Lok, A.S.; Loomba, R.; Schoen, C.; Reddy, K.R.; Trinh, H.N.; Mospan, A.R.; et al. Patient Determinants for Histologic Diagnosis of NAFLD in the Real World: A target-nash Study. Hepatol. Commun. 2021, 5, 938–946. [Google Scholar] [CrossRef]
- Newton, K.P.; Hou, J.; Crimmins, N.A.; LaVine, J.E.; Barlow, S.E.; Xanthakos, S.A.; Africa, J.; Behling, C.; Donithan, M.; Clark, J.M.; et al. Prevalence of Prediabetes and Type 2 Diabetes in Children with Nonalcoholic Fatty Liver Disease. JAMA Pediatr. 2016, 170, e161971. [Google Scholar] [CrossRef] [PubMed]
- Schwimmer, J.B.; Dunn, W.; Norman, G.; Pardee, P.E.; Middleton, M.S.; Kerkar, N.; Sirlin, C. SAFETY Study: Alanine Aminotransferase Cutoff Values Are Set Too High for Reliable Detection of Pediatric Chronic Liver Disease. Gastroenterology 2010, 138, 1357–1364.e2. [Google Scholar] [CrossRef] [Green Version]
- Molleston, J.P.; Schwimmer, J.; Yates, K.P.; Murray, K.F.; Cummings, O.W.; Lavine, J.E.; Brunt, E.M.; Scheimann, A.O.; Unalp-Arida, A. Histological Abnormalities in Children with Nonalcoholic Fatty Liver Disease and Normal or Mildly Elevated Alanine Aminotransferase Levels. J. Pediatr. 2014, 164, 707–713.e3. [Google Scholar] [CrossRef] [Green Version]
- Goldner, D.; Lavine, J.E. Nonalcoholic Fatty Liver Disease in Children: Unique Considerations and Challenges. Gastroenterology 2020, 158, 1967–1983.e1. [Google Scholar] [CrossRef]
- Marzuillo, P.; Del Giudice, E.M.; Santoro, N. Pediatric fatty liver disease: Role of ethnicity and genetics. World J. Gastroenterol. 2014, 20, 7347–7355. [Google Scholar] [CrossRef]
- Harwood, J.; Bishop, P.; Liu, H.; Nowicki, M. Safety of Blind Percutaneous Liver Biopsy in Obese Children A Retrospective Analysis. J. Clin. Gastroenterol. 2010, 44, e253–e255. [Google Scholar] [CrossRef]
- Matos, H.; Noruegas, M.J.; Gonçalves, I.; Sanches, C. Effectiveness and safety of ultrasound-guided percutaneous liver biopsy in children. Pediatr. Radiol. 2012, 42, 1322–1325. [Google Scholar] [CrossRef]
- Draijer, L.G.; Feddouli, S.; Bohte, A.E.; Slootweg, O.V.B.; Rijcken, T.H.P.; Benninga, M.A.; Stoker, J.; Koot, B.G.P. Comparison of diagnostic accuracy of screening tests ALT and ultrasound for pediatric non-alcoholic fatty liver disease. Eur. J. Pediatr. 2019, 178, 863–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arsik, I.; Frediani, J.K.; Frezza, D.; Chen, W.; Ayer, T.; Keskinocak, P.; Jin, R.; Konomi, J.V.; Barlow, S.E.; Xanthakos, S.A.; et al. Alanine Aminotransferase as a Monitoring Biomarker in Children with Nonalcoholic Fatty Liver Disease: A Secondary Analysis Using TONIC Trial Data. Children 2018, 5, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavine, J.E.; Schwimmer, J.B.; Van Natta, M.L.; Molleston, J.P.; Murray, K.F.; Rosenthal, P. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: The TONIC randomized controlled trial. JAMA 2011, 305, 1659–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudolph, B. Diagnostic and Treatment Dilemmas in Pediatric NAFLD. Clin. Liver Dis. 2021, 18, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, A.E.; Charatcharoenwitthaya, P.; Treeprasertsuk, S.; Benson, J.T.; Enders, F.; Angulo, P. The natural history of non-alcoholic fatty liver disease in children: A follow-up study for up to 20 years. Gut 2009, 58, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Mouzaki, M.; Trout, A.T.; Arce-Clachar, A.C.; Bramlage, K.; Kuhnell, P.; Dillman, J.R.; Xanthakos, S. Assessment of Nonalcoholic Fatty Liver Disease Progression in Children Using Magnetic Resonance Imaging. J. Pediatr. 2018, 201, 86–92. [Google Scholar] [CrossRef]
- Pacifico, L.; Celestre, M.; Anania, C.; Paolantonio, P.; Chiesa, C.; Laghi, A. MRI and ultrasound for hepatic fat quantification:relationships to clinical and metabolic characteristics of pediatric nonalcoholic fatty liver disease. Acta Paediatr. 2007, 96, 542–547. [Google Scholar] [CrossRef]
- Harrison, S.A.; Rinella, M.E.; Abdelmalek, M.F.; Trotter, J.F.; Paredes, A.H.; Arnold, H.L.; Kugelmas, M.; Bashir, M.R.; Jaros, M.J.; Ling, L.; et al. NGM282 for treatment of non-alcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2018, 391, 1174–1185. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).