Abstract
Background: Laron Syndrome (LS) is a rare hereditary form of dwarfism occurring, with few exceptions, in Jewish, Muslim, and Asian populations or their descendants spread over all continents. It is caused by deletions or mutations in the GH-Receptor gene, resulting in high serum levels of a structurally and biologically normal, but inactive GH and low-to-undetectable IGF-I. Aim: To summarize the disabilities and handicaps observed in patients with LS, from infancy through adult age. Results: Diagnosing, treating and following a cohort of 76 patients with LS (in many cases from infancy into adult age) enabled our department to study not only their growth and social achievements, but also the difficulties these patients encounter in life. The longstanding IGF-I deficiency caused somatic and biochemical changes which led to disabilities starting in infancy and becoming more severe with advancing age. The most serious symptoms LS patients have are dwarfism, progressive obesity, diabetes, fatty liver, cardiovascular disease, and neurological and orthopedic problems, leading to difficulties in vocational training, occupation, and social life, all lowering the Quality of Life (QoL) of these patients. Conclusions: Early initiation of IGF-I replacement treatment in patients with Laron Syndrome prevents and reverses some of the symptoms associated with longstanding IGF-I deficiency.
1. Introduction
Laron Syndrome (LS—OMIM #262500, MedDRA #10082851, SCID #38196001, ICD-10 E34.3, GARD 6859, UMLS C0271568, MeSH D046150, Orpha 633) or GH insensitivity is a rare disease of growth failure falling in the category of “Orphan Diseases”. It constitutes the best characterized entity of congenital IGF-I deficiency [1]. LS was first described in 1966 [2] and 1968 [3] in Yemenite Jews. Following these reports, more diagnoses were established in children originating from the Mediterranean, the Middle East, Asia, or South and Central America [4,5]. Despite their resemblance with genetic isolated growth hormone (GH) deficiency [6], these patients had high serum GH and low-to-undetectable insulin-like growth factor I (IGF-I) which did not respond to exogenous GH administration [7,8] demonstrating GH insensitivity [9,10]. By analyzing liver membranes from two LS patients, it was found that the resistance to GH is due to the inability of the GH molecule to bind to its receptor [11]. Chromatography and PCR methods revealed that the underlying pathology is caused by deletions or mutations in the GH-Receptor gene [12,13]. The great majority of the defects were found to be in the extracellular domain of the GH-Receptor gene [14]. The analysis of our cohort, all comprising consanguineous families, led to the conclusion that LS is genetically transmitted by a fully penetrant autosomal recessive mechanism [15]. It is noteworthy that only patients who are homozygous or double heterozygotes for GH-R defects express the full characteristics of the syndrome. The resemblance of the bone X-rays of our patients with the skeleton of an 18,000-year-old female with dwarfism on the Island of Flores led us to hypothesize that the founder gene originated in Indonesia [16]. For over 50 years we have followed a large cohort of LS patients [17], many of whom we have followed from early childhood into adult age. This enabled us to study not only the clinical and biochemical consequences of IGF-I deficiency and IGF-I replacement in LS patients but also their adjustment to society at various age levels.
The aim of this report is to describe the disabilities and handicaps observed in our large cohort of patients with Laron Syndrome.
2. Subject and Methods
The data were extracted from the Medical Records of the Endocrinology and Diabetes Research Unit of the Schneider Children’s Medical Center. Sixty-nine patients and their families lived in Israel and seven were referred from other countries.
Only patients with a proven and documented diagnosis of Laron Syndrome, i.e., high serum GH, low serum IGF-I, and lack of response upon GH administration with or without genetic analysis were included in the study.
The study was approved by the Rabin Medical Center Ethics Committee (RMC: 619-22).
3. Results
Below, we have listed the main disabilities and symptoms observed in LS patients studied by us.
3.1. Growth and Development
Dwarfism is present at birth (42–46 cm) and progresses until closure of the long bone epiphysis, leading to an adult height of 116–142 cm in males and 108–136 cm in females [18] (Figure 1). The upper/lower body ratio reveals short lower limbs compared to the trunk. The head circumference (i.e., brain volume) is smaller than normal [19], and there is underdevelopment of the facial bones [20,21] which leads to a protruding forehead, saddle nose, sunset look, and sparse hair resulting in a characteristic abnormal facies from infancy [3,6] (Figure 2). The hands and feet are small (acromicria) [22,23], resulting in difficulties finding appropriate clothing. Some infants are obliged to wear doll shoes [24] due to an inability to find small enough shoes. These unusual features, together with the dwarfism, draw attention, causing stress to parents and antagonism in surrounding people. Due to the abnormal structure of the cranium, LS patients have a retarded growth of the ocular globe [25,26] causing myopia and the need for spectacles. The retinal vascularization is reduced [27] and with advancing age, a minority of patients develop glaucoma. Some are born with strabismus or cataracts; all these issues impair vision. LS patients also develop a neurosensory hearing defect [28]. The development, size, morphology, and composition of the teeth are defective [29] and orthodontic treatment is required. Due to the immaturity of the carbohydrate system, infants and young children suffer from hypoglycemia and seizures [30]. This feature is reversed with the increase in the degree of the adipose tissue during puberty.
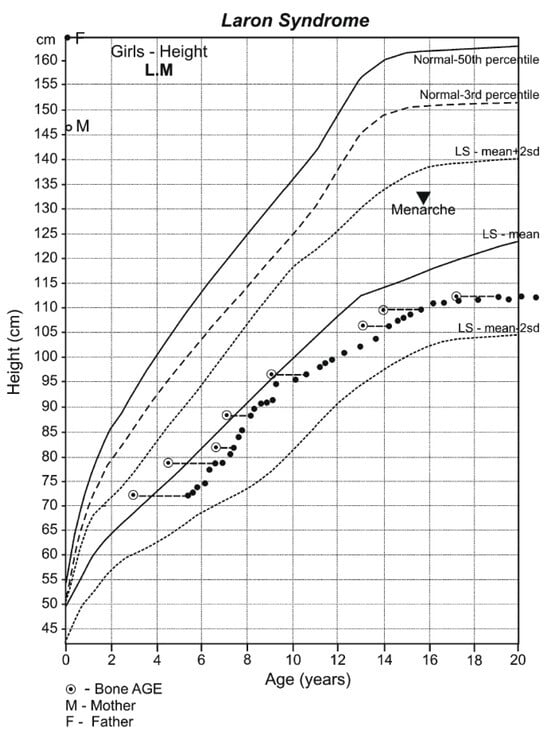
Figure 1.
Growth pattern of an untreated girl with Laron Syndrome. Drawn on the Laron Syndrome-specific growth chart (Laron Z et al. Arch Dis Child. 1993, 68: 768-70) [31]; reproduced with permission from Laron Z, Kopchivk JJ. Laron Syndrome—from Man to Mouse. Heidelberg, Springer—Verlag 2011 [32].
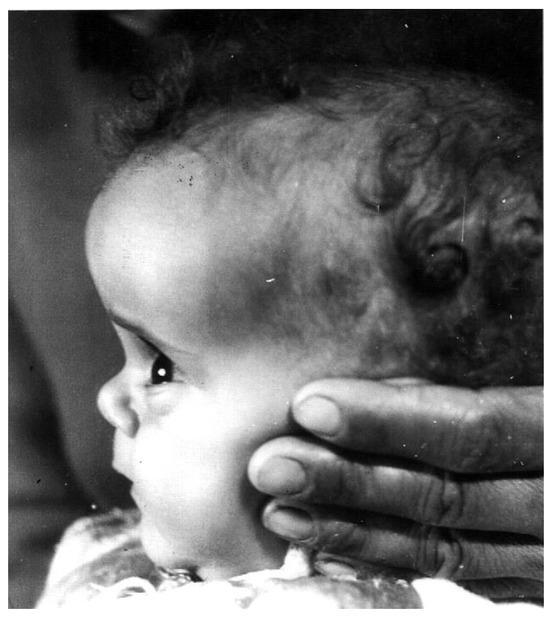
Figure 2.
Characteristic features of a 1-year-old girl with Laron Syndrome. Note the protruding forehead, saddle nose, and sparse hair [3,6]. Reproduced with permission from: Laron Z, Kopchivk JJ. Laron Syndrome—from Man to Mouse. Heidelberg, Springer: Verlag 2011 [33].
3.2. School Age
To adjust to the dwarfism, low benches and tables are needed. Deficiencies in protein metabolism, such as an abnormal amino acid pattern [34] and low procollagen levels [35], lead to underdevelopment of the muscular system and weakness [36,37] which impedes mobility, participation in competitive sport activities, and carrying heavy school bags. Children with LS also find it difficult to manage the steps when getting on and off regular school buses. Due to their abnormal appearance, obesity, and size, LS children are often bullied by their classmates.
3.3. Sexual Development and Puberty
Penis size, testicular volume, and female genitalia are small at all ages [38,39] but do not impair sexual activity and reproduction later in life [17,40]. Puberty is delayed in both sexes [41] which differentiates patients with LS from their peers and causes feelings of inferiority.
3.4. Adult Life
The progressive obesity [33] (Figure 3), independent of food intake [42], impedes a normal life routine and causes clinical and biochemical complications, such as insulin resistance, glucose intolerance, diabetes, hyperlipidemia, fatty liver, and cardiovascular disease, all characteristics of the “Metabolic Syndrome” [43].
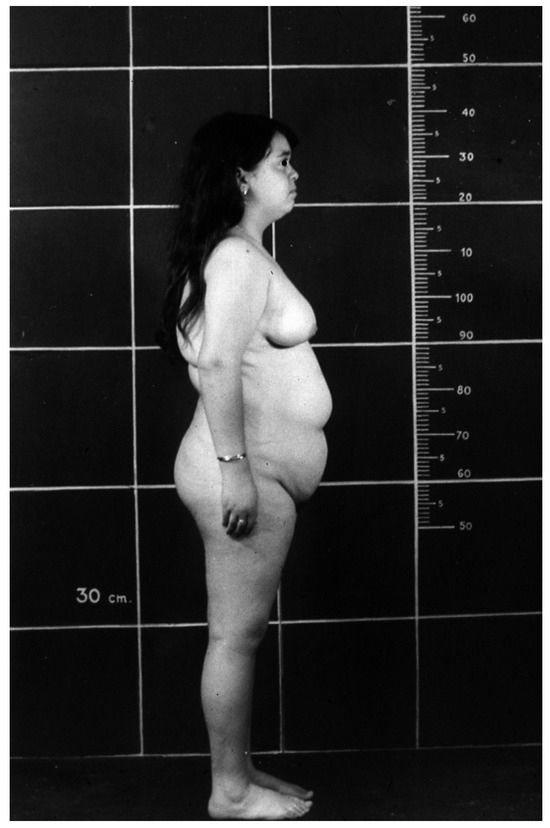
Figure 3.
Obesity in a 16-year-old girl with Laron Syndrome. Reproduced with permission from Laron Z, Kopchivk JJ. Laron Syndrome—from Man to Mouse. Heidelberg, Springer: Verlag 2011 [33].
3.5. Limitation in Mobility
Two of our patients and 25% of the Ecuadorian patients have congenital dislocation of the hip or Perthes Disease [44]. If not treated, together with the weakness of the muscular system and marked obesity, serious walking impairment may occur. A reduced left heart output and reduced lung function also impair exercise capacity [45].
3.6. Neurological Abnormalities
Using X-rays, CT, and MRI examinations of the skull and brain, we found a series of structural pathologies in the central nervous system (CNS) leading to neurologic deficits [46]. Among these are underdeveloped sinuses, degenerative changes of the white matter with occasional cerebellar atrophy, and progressive development of spinal stenosis [47]. An unusual brain lesion was found in one of our patients [48]. A similar lesion was reported in an LS patient from Mexico [49]. Both were adult female patients who died suddenly. Few patients had focal epilepsy [50] and due to the reduced dimensions of the larynx and obesity, LS patients often suffer from sleep apnea [51,52].
3.7. Psycho-Social Aspects
Psychological studies of LS children and adults revealed that some of the children present a low score on the Wechsler and Bender tests [53,54]. The low stature and academic limitations of some patients cause difficulties with regard to vocational training and finding occupations [17]. A minority of the patients we have followed achieved academic degrees, with one achieving a PhD degree. Some of the patients with healthy partners are married and have children. Others have difficulties finding partners.
The many symptoms, social limitations, and difficulties these patients face at any age are reflected in the emotional states, disappointment, and even depression reported by most LS patients. Raising a child with Laron Syndrome and being a patient with many limitations causes great psychological problems in the family and in the patient themselves, with a negative impact on Quality of Life [55] (Figure 4). The fact that patients with LS are protected from developing malignancies [56,57] was not found to affect their QoL.
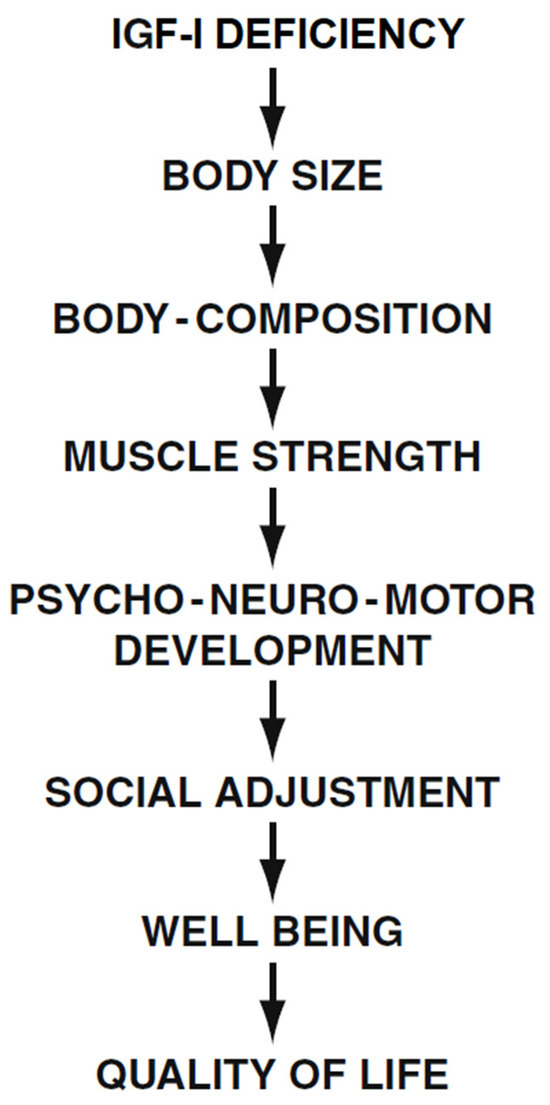
Figure 4.
Effect of long-term IGF-I deficiency on social adjustment and Quality of Life (QoL) in patients with Laron Syndrome. Reproduced with permission from Laron Z, Kopchivk JJ. Laron Syndrome—from Man to Mouse. Heidelberg, Springer: Verlag 2011 [32].
3.8. Treatment
The only treatment for LS is replacement of the genetic deficiency of IGF-I. IGF-I is a peptide which needs to be injected daily, preferably once a day [58,59]. Administering it twice or in greater doses causes unnecessary and unpleasant adverse effects [60]. IGF-I treatment accelerates linear growth [52,58,61,62] and growth of the head circumference (i.e., brain size) [19]. Even when the treatment is continuous, most children do not reach normal height. Whereas short-term treatment reduces adiposity, long-term administration of IGF-I stimulates the development of obesity [63]. As IGF-I treatment is currently approved only for children, to stimulate growth, the symptoms associated with late complications are not prevented. In a short clinical trial of IGF-I treatment of adult patients with LS, we found that IGF-I had beneficial effects such as lowering blood insulin, cholesterol [64] and serum Lp (a) [65], and that it improved left heart ventricular function [66].
4. Discussion
Laron Syndrome is a rare disease but a unique model for understanding the role of IGF-I in the process of growth and body composition [1,67]. After its discovery in 1966 [2], more and more patients were referred to our clinic and reports from other countries were published in the scientific literature. Long-term follow up was reported only by two clinics with many patients (i.e., in Ecuador [68] and Israel [17]). As our patients lived in a small country with easy access to a multidisciplinary medical team and provision of complete insurance for all medical expenses, we had a better opportunity to follow our patients than our Ecuadorian colleagues, whose patients are dispersed in locations far from the clinic, meaning that some of their examinations had to be performed in the USA. Comparing the findings between the two cohorts, it is evident that the patients in Ecuador had all the complications, disabilities, and symptoms found in our LS patients [69,70], including morphological changes in the brain structure [71]. It is noteworthy that the patients in Ecuador showed a high incidence of cardiac disease (27%), stroke, alcoholism, and a high incidence of accidents (20%) [69]. It thus seems that independent of the local conditions, the disease led to a reduced QoL.
Future Perspectives
All of the disabilities and handicaps of patients with LS are inherent to the genetic IGF-I deficiency. Height can be improved by early initiation of IGF-I replacement treatment. Even if normal height cannot be achieved, social adjustment is improved. The recently introduced weight reduction treatments using GLP-1 receptor agonists or gastric sleeve operation started by a few of our patients reduced obesity and reduced some of its biochemical complications. The marked loss of weight also had positive psychological effects. Unfortunately, both IGF-I and GLP-1-R antagonists are available to only a small number of LS patients due to regulatory and economic factors in some countries. The same is true for pregestational diagnosis and implantation of only healthy embryos in an attempt to prevent new patients with LS in families at risk. Gene therapy may also be a possible future treatment [72].
5. Conclusions
Patients with Laron Syndrome due to defects in the GH-R gene and resulting lifelong IGF-I deficiency suffer from many disabilities starting in infancy, comprising biochemical and clinical changes in all body systems. These abnormalities cause many physical symptoms and psycho-social problems, which are only partially alleviated by IGF-I replacement treatment.
Funding
This research received no external funding.
Institutional Review Board Statement
Approved by the ethics committee of the Rabin Medical Center (619 and 22, 11 November 2022).
Informed Consent Statement
An informed consent form was signed by each patient or parent at refer.
Data Availability Statement
The patients or parents approved the use of academic data for academic purposes.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Laron, Z.; Werner, H. Laron syndrome—A historical perspective. Rev. Endocr. Metab. Disord. 2021, 22, 31–41. [Google Scholar] [CrossRef]
- Laron, Z.; Mannheimer, S. Measurement of human growth hormone. Description of the method and its clinical applications. Isr. J. Med. Sci. 1966, 2, 115–119. [Google Scholar] [PubMed]
- Laron, Z.; Pertzelan, A.; Karp, M. Pituitary dwarfism with high serum levels of growth hormone. Isr. J. Med. Sci. 1968, 4, 883–894. [Google Scholar] [PubMed]
- Rosenbloom, A.L.; Guevara-Aguirre, J.; Rosenfeld, R.G.; Fielder, P.J. The little women of Loja: Growth hormone receptor-deficiency in an inbred population of southern Ecuador. N. Engl. J. Med. 1990, 323, 1367–1374. [Google Scholar] [CrossRef]
- Rosenfeld, R.G.; Rosenbloom, A.L.; Guevara-Aguirre, J. Growth hormone (GH) insensitivity due to primary GH receptor deficiency. Endocr. Rev. 1994, 15, 369–390. [Google Scholar] [CrossRef] [PubMed]
- Laron, Z. Deficiencies of growth hormone and somatomedins in man. Spec. Top. Endocrinol. Metab. 1983, 5, 149–199. [Google Scholar]
- Daughaday, W.H.; Laron, Z.; Pertzelan, A.; Heins, J.N. Defective sulfation factor generation: A possible etiological link in dwarfism. Trans. Assoc. Am. Physicians 1969, 82, 129–138. [Google Scholar]
- Laron, Z.; Pertzelan, A.; Karp, M.; Kowadlo-Silbergeld, A.; Daughaday, W.H. Administration of growth hormone to patients with familial dwarfism with high plasma immunoreactive growth hormone. Measurement of sulfation factor, metabolic, and linear growth responses. J. Clin. Endocrinol. Metab. 1971, 33, 332–342. [Google Scholar] [CrossRef]
- Laron, Z.; Kowadlo-Silbergeld, A.; Eshet, R.; Pertzelan, A. Growth hormone resistance. Ann. Clin. Res. 1980, 12, 269–277. [Google Scholar]
- Laron, Z. Laron syndrome (primary growth hormone resistance or insensitivity): The personal experience 1958–2003. J. Clin. Endocrinol. Metab. 2004, 89, 1031–1044. [Google Scholar] [CrossRef]
- Eshet, R.; Laron, Z.; Pertzelan, A.; Dintzman, M. Defect of human growth hormone in the liver of two patients with Laron type dwarfism. Isr. J. Med. Sci. 1984, 20, 8–11. [Google Scholar] [PubMed]
- Godowski, P.J.; Leung, D.W.; Meacham, L.R.; Galgani, J.P.; Hellmiss, R.; Keret, R.; Rotwein, P.S.; Parks, J.S.; Laron, Z.; Wood, W.I. Characterization of the human growth hormone receptor gene and demonstration of a partial gene deletion in two patients with Laron type dwarfism. Proc. Natl. Acad. Sci. USA 1989, 86, 8083–8087. [Google Scholar] [CrossRef]
- Amselem, S.; Duquesnoy, P.; Attree, O.; Novelli, G.; Bousnina, S.; Postel-Vinay, M.C.; Goosens, M. Laron dwarfism and mutations of the growth hormonereceptor gene. N. Engl. J. Med. 1989, 321, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Shevah, O.; Laron, Z. Genetic Aspects. In Laron Syndrome—From Man to Mouse; Laron, Z., Kopchick, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 29–52. [Google Scholar]
- Adam, A.; Josefsberg, Z.; Pertzelan, A.; Zadik, Z.; Chemke, J.M.; Laron, Z. Occurrence of four types of growth hormone related dwarfism in Israeli communities. Eur. J. Pediatr. 1981, 137, 35–39. [Google Scholar] [CrossRef]
- Hershkovitz, I.; Kornreich, L.; Laron, Z. Comparative skeletal features between Homo floresiensis and patients with primary growth hormone insensitivity (Laron Syndrome). Am. J. Phys. Anthropol. 2007, 134, 198–208. [Google Scholar] [CrossRef]
- Laron, Z.; Kauli, R. Fifty seven years of follow-up of the Israeli cohort of Laron Syndrome patients-From discovery to treatment. Growth Horm. IGF Res. 2016, 28, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Laron, Z.; Kauli, R. Linear growth pattern of untreated Laron syndrome patients. In Laron Syndrome—From Man to Mouse; Laron, Z., Kopchick, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 63–89. [Google Scholar]
- Laron, Z.; Iluz, M.; Kauli, R. Head circumference in untreated and IGF-I treated patients with Laron syndrome: Comparison with untreated and hGH-treated children with isolated growth hormone deficiency. Growth Horm. IGF Res. 2012, 22, 49–52. [Google Scholar] [CrossRef]
- Scharf, A.; Laron, Z. Skull changes in pituitary dwarfism and the syndrome of familial dwarfism with high plasma immunoreactive growth hormone—A Roentgenologic study. Horm. Metab. Res. 1972, 4, 93–97. [Google Scholar] [CrossRef]
- Konfino, R.; Pertzelan, A.; Laron, Z. Cephalometric measurements of familial dwarfism and high plasma immunoreactive growth hormone. Am. J. Orthod. 1975, 68, 196–201. [Google Scholar] [CrossRef]
- Konen, O.; Silbergeld, A.; Lilos, P.; Kornreich, L.; Laron, Z. Hand size and growth in untreated and IGF-I treated patients with Laron syndrome. J. Pediatr. Endocrinol. Metab. 2009, 22, 235–239. [Google Scholar] [CrossRef]
- Silbergeld, A.; Lilos, P.; Laron, Z. Foot length before and during insulin-like growth factor-I treatment of children with Laron syndrome compared to human growth hormone treatment of children with isolated growth hormone deficiency. J. Pediatr. Endocrinol. Metab. 2007, 20, 1325–1328. [Google Scholar] [CrossRef]
- Shurka, E.; Laron, Z. Adjustment and rehabilitation problems of children and adolescents with growth retardation. I. Familial dwarfism with high plasma immunoreactive human growth hormone. Isr. J. Med. Sci. 1975, 11, 352–357. [Google Scholar]
- Bourla, D.H.; Laron, Z.; Snir, M.; Lilos, P.; Weinberger, D.; Axer-Siegel, R. Insulinlike growth factor I affects ocular development: A study of untreated and treated patients with Laron syndrome. Ophthalmology 2006, 113, 1197.e1–1197.e5. [Google Scholar] [CrossRef]
- Kornreich, L.; Konen, O.; Lilos, P.; Laron, Z. The globe and orbit in Laron syndrome. AJNR Am. J. Neuroradiol. 2011, 32, 1560–1562. [Google Scholar] [CrossRef]
- Hellström, A.; Carlsson, B.; Niklasson, A.; Segnestam, K.; Boguszewski, M.; de Lacerda, L.; Savage, M.; Svensson, E.; Smith, L.; Weinberger, D.; et al. IGF-I is critical for normal vascularization of the human retina. J. Clin. Endocrinol. Metab. 2002, 87, 3413–3416. [Google Scholar] [CrossRef]
- Attias, J.; Zarchi, O.; Nageris, B.I.; Laron, Z. Cochlear hearing loss in patients with Laron syndrome. Eur. Arch. Otorhinolaryngol. 2012, 269, 461–466. [Google Scholar] [CrossRef]
- Laron, Z. The Teeth in Patients with Laron Syndrome. In Laron Syndrome—From Man to Mouse; Laron, Z., Kopchick, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 213–217. [Google Scholar]
- Laron, Z. Laron-type dwarfism (hereditary somatomedin deficiency): A review. Adv. Int. Med. 1984, 51, 117–150. [Google Scholar]
- Laron, Z.; Lilos, P.; Kinger, B. Growth curves for Laron Syndrome. Arch. Dis. Child 1993, 68, 768–770. [Google Scholar]
- Laron, Z.; Kopchivk, J.J. Laron Syndrome—from Man to Mouse; Springer: Verlag: Heidelberg, 2011. [Google Scholar]
- Laron, Z.; Klinger, B. Body fat in Laron syndrome patients: Effect of insulin-like growth factor I treatment. Horm. Res. 1993, 40, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Barazani, C.; Werner, H.; Laron, Z. Changes in plasma amino acids metabolites, caused by long-term IGF-I deficiency, are reversed by IGF-I treatment—A pilot study. Growth Horm. IGF Res. 2020, 52, 101312. [Google Scholar] [CrossRef] [PubMed]
- Klinger, B.; Jensen, L.T.; Silbergeld, A.; Laron, Z. Insulin-like growth factor-I raises serum procollagen levels in children and adults with Laron syndrome. Clin. Endocrinol. 1996, 45, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Brat, O.; Ziv, I.; Klinger, B.; Avraham, M.; Laron, Z. Muscle force and endurance in untreated and human growth hormone or insulin-like growth factor-I-treated patients with growth hormone deficiency or Laron syndrome. Horm. Res. 1997, 47, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Epstein, Y.; Hadid, A.; Laron, Z.; Moran, D.S.; Vaisman, N. Muscle–Bone Relationship in Patients with Laron Syndrome. In Laron Syndrome—From Man to Mouse; Laron, Z., Kopchick, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 171–174. [Google Scholar]
- Laron, Z.; Klinger, B. Effect of insulin-like growth factor-I treatment on serum androgens and testicular and penile size in males with Laron syndrome (primary growth hormone resistance). Eur. J. Endocrinol. 1998, 138, 176–180. [Google Scholar] [CrossRef]
- Laron, Z. Development and biological function of the female gonads and genitalia in IGF-I deficiency—Laron syndrome as a model. Pediatr. Endocrinol. Rev. 2006, 3 (Suppl. S1), 188–191. [Google Scholar]
- Laron, Z.; Kauli, R. Sexual Development in Patients with Laron Syndrome. In Laron Syndrome—From Man to Mouse; Laron, Z., Kopchick, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 101–118. [Google Scholar]
- Laron, Z.; Sarel, R.; Pertzelan, A. Puberty in Laron type dwarfism. Eur. J. Pediatr. 1980, 134, 79–83. [Google Scholar] [CrossRef]
- Ginsberg, S.; Laron, Z.; Bed, M.A.; Vaisman, N. The obesity of patients with Laron Syndrome is not associated with excessive nutritional intake. Obes. Res. Clin. Pract. 2009, 3, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Laron, Z.; Kauli, R.; Silbergeld, A. Adult patients with Laron syndrome tend to develop the metabolic syndrome. Growth Horm. IGF Res. 2024, 78, 101605. [Google Scholar] [CrossRef] [PubMed]
- Laron, Z.; Kauli, R. Orthopedic Problems in Laron Syndrome. In Laron Syndrome—From Man to Mouse; Laron, Z., Kopchick, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 323–324. [Google Scholar]
- Ben-Dov, I.; Gaides, M.; Scheinowitz, M.; Wagner, R.; Laron, Z. Reduced exercise capacity in untreated adults with primary growth hormone resistance (Laron syndrome). Clin. Endocrinol. 2003, 59, 763–767. [Google Scholar] [CrossRef]
- Kornreich, L.; Horev, G.; Schwarz, M.; Karmazyn, B.; Laron, Z. Craniofacial and brain abnormalities in Laron syndrome (primary growth hormone insensitivity). Eur. J. Endocrinol. 2002, 146, 499–503. [Google Scholar] [CrossRef]
- Kornreich, L.; Horev, G.; Schwarz, M.; Karmazyn, B.; Laron, Z. Laron syndrome abnormalities: Spinal stenosis, os odontoideum, degenerative changes of the atlanto-odontoid joint, and small oropharynx. AJNR Am. J. Neuroradiol. 2002, 23, 625–631. [Google Scholar]
- Kornreich, L.; Laron, Z. An unusual brain lesion in a patient with Laron Syndrome. Endocrine 2024, 84, 1266–1267. [Google Scholar] [CrossRef]
- Castilla-Cortazar, I.; Femat-Roldán, G.; Rodríguez-Rivera, J.; Aguirre, G.A.; García-Magariño, M.; Martín-Estal, I.; Espinosa, L.; Díaz-Olachea, C. Mexican case report of a never-treated Laron syndrome patient evolving to metabolic syndrome, type 2 diabetes, and stroke. Clin. Case Rep. 2017, 5, 1852–1855. [Google Scholar] [CrossRef]
- Goldberg, L.; Laron, Z.; Kornreich, L.; Scheuerman, O.; Goldberg-Stern, H.; Kraus, D. Focal Epilepsy in Individuals with Laron Syndrome. Horm. Res. Paediatr. 2022, 95, 286–290. [Google Scholar] [CrossRef]
- Dagan, Y.; Abadi, J.; Lifschitz, A.; Laron, Z. Severe obstructive sleep apnoea syndrome in an adult patient with Laron syndrome. Growth Horm. IGF Res. 2001, 11, 247–249. [Google Scholar] [CrossRef]
- Laron, Z.; Kauli, R.; Rosenzweig, E. Sleep and Sleep Disorders in Patients with Laron Syndrome. In Laron Syndrome—From Man to Mouse; Laron, Z., Kopchick, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 317–320. [Google Scholar]
- Frankel, J.J.; Laron, Z. Psychological aspects of pituitary insufficiency in children and adolescents with special reference to growth hormone. Isr. J. Med. Sci. 1968, 4, 953–961. [Google Scholar]
- Laron, Z. Psychological Aspects in Patients with Laron Syndrome. In Laron Syndrome—From Man to Mouse; Laron, Z., Kopchick, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 325–333. [Google Scholar]
- Laron, Z. Adjustment and Rehabilitation Problems of Children, Adolescents, and Adults with Laron Syndrome. In Laron Syndrome—From Man to Mouse; Laron, Z., Kopchick, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 335–337. [Google Scholar]
- Shevah, O.; Laron, Z. Patients with congenital deficiency of IGF-I seem protected from the development of malignancies: A preliminary report. Growth Horm. IGF Res. 2007, 17, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Steuerman, R.; Shevah, O.; Laron, Z. Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. Eur. J. Endocrinol. 2011, 164, 485–489. [Google Scholar] [CrossRef]
- Laron, Z.; Anin, S.; Klipper-Aurbach, Y.; Klinger, B. Effects of insulin-like growth factor on linear growth, head circumference, and body fat in patients with Laron-type dwarfism. Lancet 1992, 339, 1259–1262. [Google Scholar] [CrossRef] [PubMed]
- Laron, Z. Emerging treatment options for patients with Laron syndrome. Expert. Opin. Orphan Drugs 2014, 2, 681–694. [Google Scholar] [CrossRef]
- Laron, Z. Insulin-like growth factor-I treatment of children with Laron syndrome (primary growth hormone insensitivity). Pediatr. Endocrinol. Rev. 2008, 5, 766–771. [Google Scholar]
- Klinger, B.; Laron, Z. Three year IGF-I treatment of children with Laron syndrome. J. Pediatr. Endocrinol. Metab. 1995, 8, 149–158. [Google Scholar] [CrossRef]
- Laron, Z. IGF-I Treatment of Patients with Laron Syndrome. In Laron Syndrome—From Man to Mouse; Laron, Z., Kopchick, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 343–380. [Google Scholar]
- Bisker-Kassif, O.; Kauli, R.; Lilos, P.; Laron, Z. Biphasic response of subscapular skinfold thickness to hGH or IGF-1 administration to patients with congenital IGHD, congenital MPHD and Laron syndrome. Obes. Res. Clin. Pract. 2014, 8, e55–e62. [Google Scholar] [CrossRef]
- Klinger, B.; Anin, Z.; Flexer, Z.; Laron, Z. IGF-1 treatment of adult patients with Laron Syndrome. In Lessons from Laron Syndrome (LS) 1966–1992; Laron, Z., Parks, J.S., Eds.; Karger: Basel, Switzerland, 1993; Volume 24, pp. 237–243. [Google Scholar]
- Laron, Z.; Werner, H. Administration of insulin like growth factor I (IGFI) lowers serum lipoprotein(a)-impact on atherosclerotic cardiovascular disease. Growth Horm. IGF Res. 2023, 71, 101548. [Google Scholar] [CrossRef]
- Scheinowitz, M.; Feinberg, M.S.; Laron, Z. IGF-I replacement therapy in children with congenital IGF-I deficiency (Laron syndrome) maintains heart dimension and function. Growth Horm. IGF Res. 2009, 19, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Rosenbloom, A.L. A half-century of studies of growth hormone insensitivity/Laron syndrome: A historical perspective. Growth Horm. IGF Res. 2016, 28, 46–50. [Google Scholar] [CrossRef]
- Rosenbloom, A.L.; Guevara-Aguirre, J.; Rosenfeld, R.G.; Francke, U. Growth hormone receptor deficiency in Ecuador. J. Clin. Endocrinol. Metab. 1999, 84, 4436–4443. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Aguirre, J.; Balasubramanian, P.; Guevara-Aguirre, M.; Wei, M.; Madia, F.; Cheng, C.W.; Hwang, D.; Martin-Montalvo, A.; Saavedra, J.; Ingles, S.; et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci. Transl. Med. 2011, 3, 70ra13. [Google Scholar] [CrossRef]
- Guevara-Aguirre, J.; Teran, E.; Lescano, D.; Guevara, A.; Guevara, C.; Longo, V.; Gavilanes, A.W.D. Growth hormone receptor deficiency in humans associates to obesity, increased body fat percentage, a healthy brain and a coordinated insulin sensitivity. Growth Horm. IGF Res. 2020, 51, 58–64. [Google Scholar] [CrossRef]
- Nashiro, K.; Guevara-Aguirre, J.; Braskie, M.N.; Hafzalla, G.W.; Velasco, R.; Balasubramanian, P.; Wei, M.; Thompson, P.M.; Mather, M.; Nelson, M.D.; et al. Brain Structure and Function Associated with Younger Adults in Growth Hormone Receptor-Deficient Humans. J. Neurosci. 2017, 37, 1696–1707. [Google Scholar] [CrossRef]
- Werner, H. Toward gene therapy of Laron syndrome. Gene Ther. 2022, 29, 319–321. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).