The Epidemiology of Nontuberculous Mycobacteria in Cystic Fibrosis
Abstract
Highlights
- NTM is a concern for patients with CF even in the era of CFTR modulators
- The prevalence of NTM in CF patients varies considerably among studies making difficult to have a global estimation of the NTM burden in CF
- Further multicenter prospective studies are needed to precisely assess the global burden of NTM in CF in the era of CFTR modulators
Abstract
1. Introduction
- Incidence;
- Prevalence;
- Predominant NTM species;
- Geographic distribution.
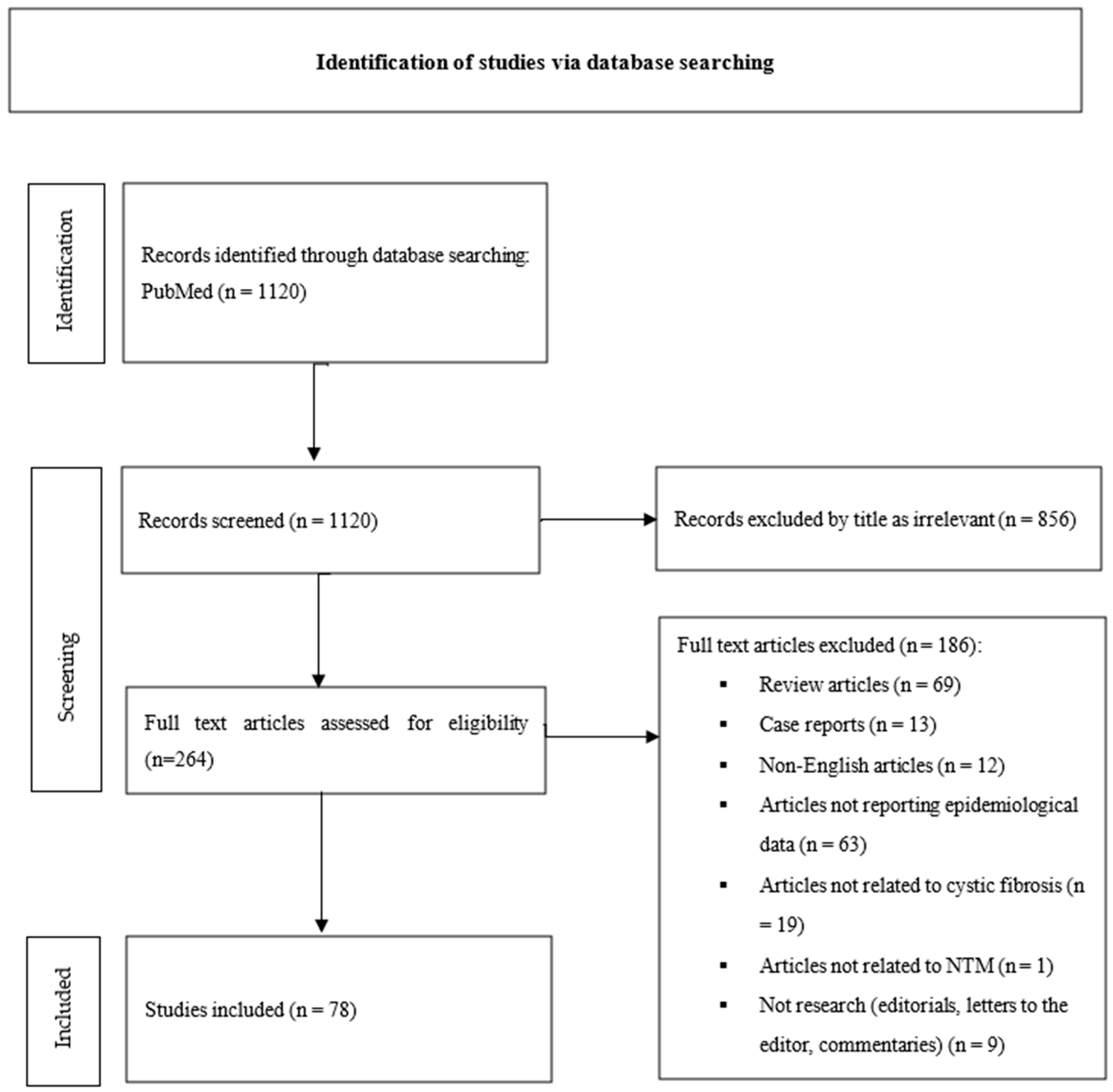
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
- Population: individuals diagnosed with cystic fibrosis.
- Topic: report of the prevalence and/or incidence of NTM infections.
- Study design: prospective or retrospective observational studies, case–control studies, or cross-sectional studies.
- Language: published in English.
- Review articles, meta-analyses, case reports, editorials, letters to the editor, and commentaries.
- Studies focusing exclusively on patients with other chronic pulmonary diseases (e.g., bronchiectasis only).
- Studies primarily addressing treatment rather than the epidemiological characteristics of NTM infections.
- Articles lacking sufficient data on the incidence and/or prevalence of NTM infections.
2.3. Study Selection
- Review articles (n = 69);
- Case reports (n = 13);
- Non-English articles (n = 12);
- Articles not reporting epidemiological data (n = 63);
- Articles not related to cystic fibrosis (n = 19);
- Articles not related to NTM (n = 1);
- Not research (editorials, letters to the editor, and commentaries) (n = 9).
2.4. Data Analysis
3. Results
4. Discussion
4.1. Prevalence of NTM Infections
4.2. Incidence
4.3. NTM Species
4.4. Lung Function
4.5. Nontuberculous Mycobacteria Pulmonary Disease (NTM-PD)
4.6. Co-Colonization with Other Microorganisms
4.7. Geographic Distribution
4.8. CFTR Modulators
4.9. Strengths and Limitations
5. Conclusions
- An increasing trend in the prevalence of nontuberculous mycobacteria infection among patients with cystic fibrosis, as reported in multiple recent studies. This highlighted rise may partially reflect improved surveillance and diagnostic awareness over time.
- Impaired lung function and microbial co-colonization appear to be significant risk factors for NTM infection.
- The introduction and widespread use of CFTR modulators may contribute to a decline in NTM infections, although current evidence remains insufficient to draw firm conclusions.
- Further multicenter prospective studies are needed in order to
- ○
- Clarify the possible effect of CFTR modulators on NTM prevalence in CF.
- ○
- Specify the other factors that affect the epidemiology of NTM prevalence in CF.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABPA | Allergic Bronchopulmonary Aspergillosis |
| ATS | American Thoracic Society |
| BCSA | Burkholderia cepacia Selective Agar |
| CF | Cystic Fibrosis |
| CFFPR | Cystic Fibrosis Foundation Patient Registry |
| CFFR | Cystic Fibrosis Foundation Registry |
| CFTR | Cystic Fibrosis Transmembrane Conductance Regulator |
| ECFSPR | European Cystic Fibrosis Society Patient Registry |
| ETI | Elexacaftor/Texacaftor/Ivacaftor |
| FEF25–75 | Forced Expiratory Flow 25–75 |
| FEV1 | Forced Expiratory Volume in the 1st second |
| FVC | Forced Vital Capacity |
| IDSA | Infectious Diseases Society of America |
| MABSC | Mycobacterium abscessus Complex |
| MAC | Mycobacterium avium complex |
| MGIT | Mycobacterial Growth Indicator Tube |
| NTM | Nontuberculous Mycobacteria |
| NTM-PD | Nontuberculous Mycobacteria Pulmonary Disease |
| PEG | Percutaneous Endoscopic Gastrostomy |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RGM | Rapidly Growing Mycobacteria |
| U.K. | United Kingdom |
| U.S.A. | United States of America |
References
- De Boeck, K. Cystic fibrosis in the year 2020: A disease with a new face. Acta Paediatr. 2020, 109, 893–899. [Google Scholar] [CrossRef]
- Rafeeq, M.M.; Murad, H.A.S. Cystic fibrosis: Current therapeutic targets and future approaches. J. Transl. Med. 2017, 15, 84. [Google Scholar] [CrossRef]
- Brown, S.D.; White, R. Keep them breathing: Cystic fibrosis pathophysiology, diagnosis, and treatment. J. Am. Acad. Physician Assist. 2017, 30, 23–27. [Google Scholar] [CrossRef]
- Elborn, J.S. Cystic fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Lu, M.; Saddi, V. Disease caused by non-tuberculous mycobacteria in children with cystic fibrosis. Paediatr. Respir. Rev. 2019, 29, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Floto, R.A.; Olivier, K.N. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax 2016, 71, i1–i22. [Google Scholar] [CrossRef] [PubMed]
- Diagnosis and Treatment of Disease Caused by Nontuberculous Mycobacteria. This official statement of the American Thoracic Society was approved by the Board of Directors, March 1997. Medical Section of the American Lung Association. Am. J. Respir. Crit. Care Med. 1997, 156, S1–S25. [Google Scholar]
- Griffith, D.E.; Aksamit, T.; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416, Erratum in Am. J. Respir. Crit. Care Med. 2007, 175, 744–745. [Google Scholar] [CrossRef] [PubMed]
- Kurz, S.G.; Zha, B.S. Summary for Clinicians: 2020 Clinical Practice Guideline Summary for the Treatment of Nontuberculous Mycobacterial Pulmonary Disease. Ann. Am. Thorac. Soc. 2020, 17, 1033–1039. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, 1230. [Google Scholar] [CrossRef]
- Arvind, B.; Medigeshi, G.R. Aetiological agents for pulmonary exacerbations in children with cystic fibrosis: An observational study from a tertiary care centre in northern India. Indian J. Med. Res. 2020, 151, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Al-Momani, H.; Perry, A. Nontuberculous mycobacteria in gastrostomy fed patients with cystic fibrosis. Sci. Rep. 2017, 7, 46546. [Google Scholar] [CrossRef]
- Kilby, J.M.; Gilligan, P.H. Nontuberculous mycobacteria in adult patients with cystic fibrosis. Chest 1992, 102, 70–75. [Google Scholar] [CrossRef]
- Aitken, M.L.; Burke, W. Nontuberculous mycobacterial disease in adult cystic fibrosis patients. Chest 1993, 103, 1096–1099. [Google Scholar] [CrossRef]
- Bange, F.C.; Brown, B.A. Lack of transmission of Mycobacterium abscessus among patients with cystic fibrosis attending a single clinic. Clin. Infect. Dis. 2001, 32, 1648–1650. [Google Scholar] [CrossRef]
- Leitritz, L.; Griese, M. Prospective study on nontuberculous mycobacteria in patients with and without cystic fibrosis. Med. Microbiol. Immunol. 2004, 193, 209–217. [Google Scholar] [CrossRef]
- Girón, R.M.; Domingo, D. Micobacterias ambientales en pacientes adultos con fibrosis quística [Nontuberculous mycobacteria in patients with cystic fibrosis]. Arch. Bronconeumol. 2005, 41, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Chalermskulrat, W.; Sood, N. Non-tuberculous mycobacteria in end stage cystic fibrosis: Implications for lung transplantation. Thorax 2006, 61, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Paschoal, I.A.; de Oliveira Villalba, W. Cystic fibrosis in adults. Lung 2007, 185, 81–87. [Google Scholar] [CrossRef]
- Coolen, N.; Morand, P. Reduced risk of nontuberculous mycobacteria in cystic fibrosis adults receiving long-term azithromycin. J. Cyst. Fibros. 2015, 14, 594–599. [Google Scholar] [CrossRef]
- Sherrard, L.J.; Tay, G.T. Tropical Australia is a potential reservoir of non-tuberculous mycobacteria in cystic fibrosis. Eur. Respir. J. 2017, 49, 1700046. [Google Scholar] [CrossRef]
- Fernández-Caso, B.; Vázquez, R. Prevalence and importance of non-tuberculous mycobacteria in adult patients with cystic fibrosis in a hospital in Madrid. Enferm. Infecc. Microbiol. Clin. (Engl. Ed.). 2020, 38, 323–326. [Google Scholar] [CrossRef]
- Richter, W.J.; Sun, Y. Vitamin D Deficiency Is Associated with Increased Nontuberculous Mycobacteria Risk in Cystic Fibrosis. Ann. Am. Thorac. Soc. 2021, 18, 913–916. [Google Scholar] [CrossRef]
- Wyrostkiewicz, D.; Opoka, L. Nontuberculous Mycobacterial Lung Disease in the Patients with Cystic Fibrosis—A Challenging Diagnostic Problem. Diagnostics 2022, 12, 1514. [Google Scholar] [CrossRef]
- Gross, J.E.; Caceres, S. Investigating Nontuberculous Mycobacteria Transmission at the Colorado Adult Cystic Fibrosis Program. Am. J. Respir. Crit. Care Med. 2022, 205, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Mianowski, L.; Doléans-Jordheim, A. One year of ETI reduces lung bacterial colonisation in adults with cystic fibrosis. Sci. Rep. 2024, 14, 29298. [Google Scholar] [CrossRef] [PubMed]
- Örlős, Z.; Lőrinczi, L.K. Epidemiology, microbiology and clinical impacts of non-tuberculous mycobacteria in adult patients with cystic fibrosis. Heliyon 2024, 11, e41324. [Google Scholar] [CrossRef]
- Gross, J.E.; Finklea, J.D. Genomic epidemiology of Mycobacterium abscessus at an adult cystic fibrosis programme reveals low potential for healthcare-associated transmission. ERJ Open Res. 2024, 10, 00165–02024. [Google Scholar] [CrossRef]
- Fauroux, B.; Delaisi, B. Mycobacterial lung disease in cystic fibrosis: A prospective study. Pediatr. Infect. Dis. J. 1997, 16, 354–358. [Google Scholar] [CrossRef]
- Esther, C.R., Jr.; Henry, M.M. Nontuberculous mycobacterial infection in young children with cystic fibrosis. Pediatr. Pulmonol. 2005, 40, 39–44. [Google Scholar] [CrossRef]
- Radhakrishnan, D.K.; Yau, Y. Non-tuberculous mycobacteria in children with cystic fibrosis: Isolation, prevalence, and predictors. Pediatr. Pulmonol. 2009, 44, 1100–1106. [Google Scholar] [CrossRef]
- Cândido, P.H.C.; Nunes, L.D.S.; Marques, E.A.; Folescu, T.W.; Coelho, F.S.; de Moura, V.C.N.; da Silva, M.G.; Gomes, K.M.; Lourenço, M.C.D.S.; Aguiar, F.S.; et al. Multidrug-resistant nontuberculous mycobacteria isolated from cystic fibrosis patients. J. Clin. Microbiol. 2014, 52, 2990–2997. [Google Scholar] [CrossRef]
- Satana, D.; Erkose-Genc, G. Prevalence and drug resistance of mycobacteria in Turkish cystic fibrosis patients. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Bouso, J.M.; Burns, J.J. Household proximity to water and nontuberculous mycobacteria in children with cystic fibrosis. Pediatr. Pulmonol. 2017, 52, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.I.; Kulkarni, H. Early detection of non-tuberculous mycobacteria in children with cystic fibrosis using induced sputum at annual review. Pediatr. Pulmonol. 2019, 54, 257–263. [Google Scholar] [CrossRef]
- Gardner, A.I.; McClenaghan, E. Epidemiology of Nontuberculous Mycobacteria Infection in Children and Young People with Cystic Fibrosis: Analysis of UK Cystic Fibrosis Registry. Clin. Infect. Dis. 2019, 68, 731–737, Erratum in Clin. Infect. Dis. 2021, 72, 910–911. [Google Scholar] [CrossRef]
- Yan, J.; Kevat, A. Investigating transmission of Mycobacterium abscessus amongst children in an Australian cystic fibrosis centre. J. Cyst. Fibros. 2020, 19, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.A.; Bokobza, I. Eradication success for non-tuberculous mycobacteria in children with cystic fibrosis. Eur. Respir. J. 2021, 57, 2003636. [Google Scholar] [CrossRef]
- Abidin, N.Z.; Gardner, A.I. Trends in nontuberculous mycobacteria infection in children and young people with cystic fibrosis. J. Cyst. Fibros. 2021, 20, 737–741. [Google Scholar] [CrossRef]
- Singh, J.; Hunt, S. The changing epidemiology of pulmonary infection in children and adolescents with cystic fibrosis: An 18-year experience. Sci. Rep. 2024, 14, 9056. [Google Scholar] [CrossRef]
- Mulherin, D.; Coffey, M.J. Skin reactivity to atypical mycobacteria in cystic fibrosis. Respir. Med. 1990, 84, 273–276. [Google Scholar] [CrossRef]
- Hjelte, L.; Petrini, B. Prospective study of mycobacterial infections in patients with cystic fibrosis. Thorax 1990, 45, 397–400. [Google Scholar] [CrossRef]
- Hjelt, K.; Højlyng, N. The role of Mycobacteria Other Than Tuberculosis (MOTT) in patients with cystic fibrosis. Scand. J. Infect. Dis. 1994, 26, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Torrens, J.K.; Dawkins, P. Non-tuberculous mycobacteria in cystic fibrosis. Thorax 1998, 53, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.; Maiz, L. Nontuberculous mycobacteria in patients with cystic fibrosis. Clin. Infect. Dis. 2001, 32, 1298–1303. [Google Scholar] [CrossRef]
- Sermet-Gaudelus, I.; Le Bourgeois, M. Mycobacterium abscessus and children with cystic fibrosis. Emerg. Infect. Dis. 2003, 9, 1587–1591. [Google Scholar] [CrossRef]
- Olivier, K.N.; Weber, D.J. Nontuberculous Mycobacteria in Cystic Fibrosis Study Group. Nontuberculous mycobacteria. I: Multicenter prevalence study in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003, 167, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Devine, M.; Moore, J.E. Detection of mycobacterial DNA from sputum of patients with cystic fibrosis. Ir. J. Med. Sci. 2004, 173, 96–98. [Google Scholar] [CrossRef]
- Mussaffi, H.; Rivlin, J. Nontuberculous mycobacteria in cystic fibrosis associated with allergic bronchopulmonary aspergillosis and steroid therapy. Eur. Respir. J. 2005, 25, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Pierre-Audigier, C.; Ferroni, A. Age-related prevalence and distribution of nontuberculous mycobacterial species among patients with cystic fibrosis. J. Clin. Microbiol. 2005, 43, 3467–3470. [Google Scholar] [CrossRef]
- Ferroni, A.; Vu-Thien, H.; Lanotte, P.; Le Bourgeois, M.; Sermet-Gaudelus, I.; Fauroux, B.; Marchand, S.; Varaigne, F.; Berche, P.; Gaillard, J.L.; et al. Value of the chlorhexidine decontamination method for recovery of nontuberculous mycobacteria from sputum samples of patients with cystic fibrosis. J. Clin. Microbiol. 2006, 44, 2237–2239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Valenza, G.; Tappe, D. Prevalence and antimicrobial susceptibility of microorganisms isolated from sputa of patients with cystic fibrosis. J. Cyst. Fibros. 2008, 7, 123–127. [Google Scholar] [CrossRef]
- Levy, I.; Grisaru-Soen, G. Multicenter cross-sectional study of nontuberculous mycobacterial infections among cystic fibrosis patients, Israel. Emerg. Infect. Dis. 2008, 14, 378–384. [Google Scholar] [CrossRef]
- Roux, A.L.; Catherinot, E. Jean-Louis Herrmann for the OMA Group. Multicenter study of prevalence of nontuberculous mycobacteria in patients with cystic fibrosis in France. J. Clin. Microbiol. 2009, 47, 4124–4128. [Google Scholar] [CrossRef]
- Seddon, P.; Fidler, K. Prevalence of nontuberculous mycobacteria in cystic fibrosis clinics, United Kingdom, 2009. Emerg. Infect. Dis. 2013, 19, 1128–1130. [Google Scholar] [CrossRef]
- Esther, C.R., Jr.; Esserman, D.A. Chronic Mycobacterium abscessus infection and lung function decline in cystic fibrosis. J. Cyst. Fibros. 2010, 9, 117–123. [Google Scholar] [CrossRef]
- Binder, A.M.; Adjemian, J. Epidemiology of nontuberculous mycobacterial infections and associated chronic macrolide use among persons with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2013, 188, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Qvist, T.; Johansen, I.S. Urine lipoarabinomannan point-of-care testing in patients affected by pulmonary nontuberculous mycobacteria—Experiences from the Danish Cystic Fibrosis cohort study. BMC Infect. Dis. 2014, 14, 655. [Google Scholar] [CrossRef]
- Martiniano, S.L.; Sontag, M.K. Clinical significance of a first positive nontuberculous mycobacteria culture in cystic fibrosis. Ann. Am. Thorac. Soc. 2014, 11, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Adjemian, J.; Olivier, K.N. Nontuberculous mycobacteria among patients with cystic fibrosis in the United States: Screening practices and environmental risk. Am. J. Respir. Crit. Care Med. 2014, 190, 581–586. [Google Scholar] [CrossRef]
- Raidt, L.; Idelevich, E.A. Increased Prevalence and Resistance of Important Pathogens Recovered from Respiratory Specimens of Cystic Fibrosis Patients During a Decade. Pediatr. Infect. Dis. J. 2015, 34, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, O.; Mussaffi, H. Increasing nontuberculous mycobacteria infection in cystic fibrosis. J. Cyst. Fibros. 2015, 14, 53–62. [Google Scholar] [CrossRef]
- Phelippeau, M.; Dubus, J.C. Prevalence of Mycobacterium lentiflavum in cystic fibrosis patients, France. BMC Pulm. Med. 2015, 15, 131. [Google Scholar] [CrossRef][Green Version]
- Qvist, T.; Gilljam, M. Scandinavian Cystic Fibrosis Study Consortium (SCFSC). Epidemiology of nontuberculous mycobacteria among patients with cystic fibrosis in Scandinavia. J. Cyst. Fibros. 2015, 14, 46–52. [Google Scholar] [CrossRef]
- Kopp, B.T.; Nicholson, L. Geographic variations in cystic fibrosis: An analysis of the U.S. CF Foundation Registry. Pediatr. Pulmonol. 2015, 50, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Campos-Herrero, M.I.; Chamizo, F.J. Nontuberculous mycobacteria in cystic fibrosis patients on the Island of Gran Canaria. A population study. J. Infect. Chemother. 2016, 22, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Eltringham, I.; Pickering, J. Comparison of Mycobacterial Growth Indicator Tube with Culture on RGM Selective Agar for Detection of Mycobacteria in Sputum Samples from Patients with Cystic Fibrosis. J. Clin. Microbiol. 2016, 54, 2047–2050. [Google Scholar] [CrossRef]
- Preece, C.L.; Perry, A. A novel culture medium for isolation of rapidly-growing mycobacteria from the sputum of patients with cystic fibrosis. J. Cyst. Fibros. 2016, 15, 186–191. [Google Scholar] [CrossRef]
- Salsgiver, E.L.; Fink, A.K. Changing Epidemiology of the Respiratory Bacteriology of Patients with Cystic Fibrosis. Chest 2016, 149, 390–400. [Google Scholar] [CrossRef]
- Viviani, L.; Harrison, M.J. Epidemiology of nontuberculous mycobacteria (NTM) amongst individuals with cystic fibrosis (CF). J. Cyst. Fibros. 2016, 15, 619–623. [Google Scholar] [CrossRef]
- Cavalli, Z.; Reynaud, Q. High incidence of non-tuberculous mycobacteria-positive cultures among adolescent with cystic fibrosis. J. Cyst. Fibros. 2017, 16, 579–584. [Google Scholar] [CrossRef]
- Plongla, R.; Preece, C.L. Evaluation of RGM Medium for Isolation of Nontuberculous Mycobacteria from Respiratory Samples from Patients with Cystic Fibrosis in the United States. J. Clin. Microbiol. 2017, 55, 1469–1477. [Google Scholar] [CrossRef]
- Aiello, T.B.; Levy, C.E. Prevalence and clinical outcomes of nontuberculous mycobacteria in a Brazilian cystic fibrosis reference center. Pathog. Dis. 2018, 76, fty051. [Google Scholar] [CrossRef]
- Scohy, A.; Gohy, S. Comparison of the RGM medium and the mycobacterial growth indicator tube automated system for isolation of non-tuberculous mycobacteria from sputum samples of cystic fibrosis patients in Belgium. J. Clin. Tuberc. Other Mycobact. Dis. 2018, 13, 1–4. [Google Scholar] [CrossRef]
- Eikani, M.S.; Nugent, M. Clinical course and significance of nontuberculous mycobacteria and its subtypes in cystic fibrosis. BMC Infect. Dis. 2018, 18, 311. [Google Scholar] [CrossRef]
- Adjemian, J.; Olivier, K.N. Epidemiology of Pulmonary Nontuberculous Mycobacterial Sputum Positivity in Patients with Cystic Fibrosis in the United States, 2010–2014. Ann. Am. Thorac. Soc. 2018, 15, 817–826, Erratum in Ann. Am. Thorac. Soc. 2018, 15, 1114–1115. [Google Scholar] [CrossRef]
- Hatziagorou, E.; Orenti, A. Changing epidemiology of the respiratory bacteriology of patients with cystic fibrosis-data from the European cystic fibrosis society patient registry. J. Cyst. Fibros. 2020, 19, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Low, D.; Wilson, D.A. Screening practices for nontuberculous mycobacteria at US cystic fibrosis centers. J. Cyst. Fibros. 2020, 19, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.; Belmonte, O. High Prevalence of Nontuberculous Mycobacteria in Cystic Fibrosis Patients in Tropical French Reunion Island. Pediatr. Infect. Dis. J. 2021, 40, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Ademhan Tural, D.; Emiralioglu, N. The frequency and related factors of non-tuberculosis mycobacteria infections among patients with cystic fibrosis. Pediatr. Int. 2021, 63, 1369–1375. [Google Scholar] [CrossRef]
- Foote, S.L.; Lipner, E.M. Environmental predictors of pulmonary nontuberculous mycobacteria (NTM) sputum positivity among persons with cystic fibrosis in the state of Florida. PLoS ONE 2021, 16, e0259964. [Google Scholar] [CrossRef]
- Lipner, E.M.; Crooks, J.L. Nontuberculous mycobacterial infection and environmental molybdenum in persons with cystic fibrosis: A case-control study in Colorado. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 289–294. [Google Scholar] [CrossRef]
- Zomer, D.; van Ingen, J.; Dutch CF Registry Steering Group. Epidemiology and management of nontuberculous mycobacterial disease in people with cystic fibrosis, the Netherlands. J. Cyst. Fibros. 2023, 22, 327–333. [Google Scholar] [CrossRef]
- Wetzstein, N.; Diricks, M. Molecular Epidemiology of Mycobacterium abscessus Isolates Recovered from German Cystic Fibrosis Patients. Microbiol. Spectr. 2022, 10, e0171422. [Google Scholar] [CrossRef]
- Ricotta, E.E.; Prevots, D.R. CFTR modulator use and risk of nontuberculous mycobacteria positivity in cystic fibrosis, 2011–2018. ERJ Open Res. 2022, 8, 00724-2021. [Google Scholar] [CrossRef]
- Mercaldo, R.A.; Marshall, J.E. Detecting clusters of high nontuberculous mycobacteria infection risk for persons with cystic fibrosis—An analysis of U.S. counties. Tuberculosis 2023, 138, 102296, Erratum in Tuberculosis 2023, 142, 102347. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.E.; Mercaldo, R.A. Incidence of nontuberculous mycobacteria infections among persons with cystic fibrosis in the United States (2010–2019). BMC Infect. Dis. 2023, 23, 489. [Google Scholar] [CrossRef] [PubMed]
- Steindor, M.; Hafkemeyer, S. Epidemiological trends in nontuberculous mycobacterial infection among people with cystic fibrosis in Germany. Int. J. Infect. Dis. 2023, 129, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, Q.; Bricca, R. Risk factors for nontuberculous mycobacterial isolation in patients with cystic fibrosis: A meta-analysis. Pediatr. Pulmonol. 2020, 55, 2653–2661. [Google Scholar] [CrossRef]
- Prieto, M.D.; Alam, M.E. Global burden of nontuberculous mycobacteria in the cystic fibrosis population: A systematic review and meta-analysis. ERJ Open Res. 2023, 9, 00336-2022. [Google Scholar] [CrossRef]
| Study | Year | Study Population | Study Design | Country | Prevalence (%, n/N) | MAC/ MABSC (%, n/N) | |
|---|---|---|---|---|---|---|---|
| 1 | Kilby JM et al., 1992 [13] | 1981–1990 | 87 | Retrospective observational | U.S.A. | Overall: 20.0 (17/87) | - |
| 2 | Aitken ML et al., 1993 [14] | 1990–1991 | 64 | Prospective cross-sectional | U.S.A. | Overall: 12.5 (8/64) | - |
| 3 | Bange FC et al., 2001 [15] | 1997–1999 | 214 | Retrospective | Germany | Overall: 7.0 (15/214) | - |
| 4 | Leitritz L. et al., 2004 [16] | 1999–2001 | 91 | Prospective observational | Germany | Overall: 11.0 (10/91) Point: 3.3 (-) | - |
| 5 | Girón RM et al., 2005 [17] | 1997–2001 | 28 | Prospective cohort | Spain | Overall: 25 (7/28) | - |
| 6 | Chalermskulrat W. et al., 2006 [18] | 1990–2003 | 146 | Retrospective cohort | U.S.A. | Prior transplantation: 19.7 (26/132) Post-transplantation: 13.7 (20/146) | 45 (13/29 isolates) /41 (12/29 isolates) |
| 7 | Paschoal IA. et al., 2007 [19] | 2003–2004 | 54 | Retrospective cross-sectional | Brazil | Overall: 11.0 (9/54) | - |
| 8 | Coolen N. et al., 2015 [20] | 2006–2010 | 347 | Case–control | France | Overall: 12.4 (41/347) | 48.7 (20/41) /58.5 (24/41) |
| 9 | Al-Momani H. et al., 2017 [12] | 2017 | 16 | Observational cross-sectional | United Kingdom | Overall: 43 (7/16) | - |
| 10 | Sherrard LJ. et al., 2017 [21] | 2001–2013 | 434 | Retrospective observational cohort | Australia | Overall: 14 (54/375) | - |
| 11 | Fernández-Caso B.et al., 2019 [22] | 92 | Retrospective observational | Spain | Overall: 30.4 (28/92) | - | |
| 12 | Richter WJ. et al., 2021 [23] | 2007–2018 | 254 | Retrospective cohort | U.S.A. | Overall: 19.7 (50/254) | 58 (29/50) /36 (18/50) |
| 13 | Wyrostkiewicz D. et al., 2022 [24] | 2010–2020 | 151 | Retrospective observational | Poland | Overall: 7 (11/151) | 55 (6/11)/9 (1/11) |
| 14 | Gross JE. et al., 2022 [25] | 2012–2018 | 507 | Retrospective cohort | U.S.A. | Overall: 32.5 (165/507) | - |
| 15 | Mianowski L. et al., 2024 [26] | 2020–2021 | 198 | Retrospective observational | France | 2Y prior ETI: 7.2 (14/194) 1Y after ETI: 1.7 (3/179) | - |
| 16 | Örlős Z. et al., 2024 [27] | 2020–2022 | 232 | Retrospective cohort | Hungary | Point: 16.8 (39/232) Annual: 5.7 (11/192) (2020) –12.9 (30/232) (2022) | 41 (16/39)/38.5 (15/39) |
| 17 | Gross JE et al., 2024 [28] | 2013–2018 | 294 | Retrospective observational | U.S.A. | Overall: 24.1 (71/294) | -/70.4 (50/71) |
| Study | Year | Study Population | Study Design | Country | Prevalence (%, n/N) | MAC/ MABSC (%) | |
|---|---|---|---|---|---|---|---|
| 1 | Fauroux B. et al., 1997 [29] | 1997 | 106 | Prospective | France | Overall: 6.6 (7/106) | - |
| 2 | Esther Jr. et al., 2005 [30] | 1993–2002 | 545 | Retrospective observational | U.S.A. | Overall: BAL cohort: 6.1 (7/114) Registry cohort: 3.9 (17/431) Annual: 2.0 (-) | - |
| 3 | Radhakrishnan DK.et al., 2009 [31] | 2004 | 98 | Prospective cohort | Canada | Point: 6.1 (6/98) | 66 (4/6)/33 (2/6) |
| 4 | Cândido PH et al., 2014 [32] | 2009–2012 | 129 | Prospective cohort | Brazil | Overall: 7.75 (10/129) | - |
| 5 | Satana D. et al., 2014 [33] | 2003–2008 | 130 | Retrospective observational | Turkey | Overall: 3.07 (4/130) | -/60.9 (14/23 isolates) |
| 6 | Bouso JM et al., 2017 [34] | 2012–2015 | 65 | Retrospective observational | U.S.A. | Overall: 32.2 (21/65) | 66.7(14/21)/23.8 (5/21) |
| 7 | Ahmed MI et al., 2019 [35] | 2012–2016 | 42 | Prospective observational cohort | United Kingdom | - | - |
| 8 | Gardner AI. et al., 2019 [36] | 2010–2015 | 5333 | Retrospective cohort | United Kingdom | Overall: 5.4 (288/5333) Annual: 1.3–3.8 (-) | - |
| 9 | Yan J. et al., 2020 [37] | 2013–2017 | 99 | Retrospective | Australia | Overall: 11 (99/328 total population) | -/6.7 (22/328 total population) |
| 10 | Arvind B. et al., 2020 [11] | 2013–2015 | 104 | Prospective observational | India | 0 (0/104) | - |
| 11 | Hughes DA et al., 2021 [38] | 2011–2018 | 567 | Retrospective cohort | United Kingdom | Overall: 10.4 (59/657) | - |
| 12 | Abidin NZ. et al., 2022 [39] | 2016–2018 | 4687 | Retrospective observational | United Kingdom | Overall: 6.5 (303/4687) Annual: 3.1–3.6 (-) | 30.4 (92/303) /58.1 (176/303) |
| 13 | Singh J. et al., 2024 [40] | 2002–2019 | 419 | Retrospective observational cohort | Australia | Overall: 0.72 (-/419) | - |
| Study | Year | Study Population | Study Design | Country | Prevalence (%, n/N) | MAC/ MABSC (%) | |
|---|---|---|---|---|---|---|---|
| 1 | Mulherin D. et al., 1990 [41] | 1989 | 43 | Prospective observational | United Kingdom | Overall: 2.3 (1/43) | - |
| 2 | Hjelte L. et al., 1990 [42] | 1990 | 54 | Prospective observational | Sweden | Overall: 9.3 (5/54) | - |
| 3 | Hjelt K. et al., 1994 [43] | 1987–1988 | 185 | Prospective cross-sectional | Denmark | Point: 1.6 (3/185) | - |
| 4 | Torrens JK et al., 1998 [44] | 1989–1994 | 372 | Retrospective case–control | United Kingdom | Overall: 3.8 (14/372) | - |
| 5 | Oliver A. et al., 2001 [45] | 2000 | 37 | Prospective observational | Spain | Overall: 16.1 (6/37) | - |
| 6 | Sermet-Gaudelus I. et al., 2003 [46] | 1996–1999 | 296 | Prospective cohort | France | Overall: 9.8 (29/296) | |
| 7 | Olivier KN. et al., 2003 [47] | 2002 | 986 | Prospective cross-sectional | U.S.A. | Overall: 13 (128/986) | 72 (92/138)/16 (18/128) |
| 8 | Devine M. et al., 2004 [48] | 2004 | 182 | Retrospective cross-sectional | United Kingdom | Overall: Pediatric 0.9 (1/116) Adult 3.0 (2/66) | |
| 9 | Mussaffi H. et al., 2005 [49] | 1997–2003 | 139 | Retrospective cohort | Israel | Overall: 8.6 (12/139) | - |
| 10 | Pierre-Audigier C. et al., 2005 [50] | 2000 | 386 | Prospective cross-sectional | France | Overall: 8.1 (31/385) | 21.2 (7/33)/39.4 (13/33) |
| 11 | Ferroni A. et al., 2006 [51] | 2004–2005 | 289 | Comparative | France | Overall: 11.0 (32/289) | 5 (3/60 isolates) /72 (43/60 isolates) |
| 12 | Valenza G. et al., 2008 [52] | 2006 | 60 | Cross-sectional | Germany | Point: 13.3 (8/60) | - |
| 13 | Levy Ι. et al., 2008 [53] | 2001–2003 | 186 | Retrospective cross-sectional | Israel | Overall: 22.6 (42/186) | 14.3 (6/42)/31 (13/42) |
| 14 | Roux AL. et al., 2009 [54] | 2004 | 1582 | Prospective cross-sectional | France | Overall: 6.6 (104/1582) | 22 (23/104)/48 (50/104) |
| 15 | Seddon P. et al., 2009 [55] | 2009 | 7122 | Cross-sectional | United Kingdom | Overall: 4.2 (300/7122) Pediatric 3.3(110/3317) Adult 5.0 (190/3805) | - |
| 16 | Esther CR Jr et al., 2010 [56] | 2000–2007 | 1216 | Retrospective observational | U.S.A. | Overall:13.7 (166/1216) Mean annual: 10.8 (-) | 59 (98/166)/41 (68/166) |
| 17 | Binder AM. et al., 2013 [57] | 2003–2011 | 5403 | Nested case–control | U.S.A. | Point: 4 (191/5403) | 64 (122/191)/36 (69/191) |
| 18 | Qvist T et al., 2014 [58] | 2012–2013 | 198 | Prospective cohort | Denmark | Overall: 12 (23/198) | - |
| 19 | Martiniano SL. et al., 2014 [59] | 2000–2010 | 650 | Retrospective cohort | U.S.A. | Overall: 14.8 (96/650) Pediatric 9.6 (48/499) Adult 31.7 (48/151) | Pediatric 75 (36/48)/21 (10/48) Adult 69 (33/48)/27 (13/48) |
| 20 | Adjemian J. et al., 2014 [60] | 2010–2011 | 10,527 | Retrospective observational | U.S.A. | Overall: 14 (1384/10,527) | - |
| 21 | Raidt L. et al., 2015 [61] | 2001–2011 | 94 | Retrospective observational | Germany | Annual: 0 (0/94) 2001 –7.4 (7/94) 2011 | - |
| 22 | Bar-On O. et al., 2015 [62] | 2002–2011 | 110 | Retrospective observational | Israel | Annual: 5 (4/79) 2003 –14.5 (16/110) 2011 | 24(-)/46(-) of all isolates |
| 23 | Phelippeau M. et al., 2015 [63] | 2010–2014 | 354 | Prospective observational | France | Overall: 7.1 (25/354) | 32 (8/25)/48 (12/25) |
| 24 | Qvist T. et al., 2015 [64] | 2000–2012 | 1411 | Retrospective | Scandinavia | Overall: 11 (157/1411) | 32 (51/157)/45 (70/157) |
| 25 | Kopp BT et al., 2015 [65] | 2007–2012 | 30,896 | Retrospective | U.S.A. | Overall: 8.1 (2512/30,896) | - |
| 26 | Campos-Herrero MI. et al., 2016 [66] | 2002–2012 | 44 | Retrospective observational | Spain | Overall: 40.9 (18/44) Mean annual: 14.1 (-) | -/37 (7/18) |
| 27 | Eltringham I. et al., 2016 [67] | 2015 | 187 | Prospective | United Kingdom | Overall: 15 (28/187) | -/90 (21/28) |
| 28 | Preece CL. et al., 2016 [68] | 2014 | 210 | Prospective observational | United Kingdom | Overall: 15.7 (33/210) | -/10 (21/210) |
| 29 | Salsgiver EL. et al., 2016 [69] | 2010–2012 | 13,169 | Retrospective observational | U.S.A. | Annual: 12(-) | 6.94/5.03 of all pts tested |
| 30 | Viviani L. et al., 2016 [70] | 2009 | 13,593 | Cross-sectional | Europe | Overall: 2.7 (374/13,593) | - |
| 31 | Cavalli Z. et al., 2017 [71] | 2009–2014 | 401 | Retrospective case–control | France | Overall: 12 (48/401) | 56.3 (27/48) /37.5 (18/48) |
| 32 | Plongla R. et al., 2017 [72] | 2015–2016 | 487 | Prospective | U.S.A. | Overall: 14.2 (69/487) | 3.3 (-)/5.5 (-) of all isolates |
| 33 | Aiello TB. et al., 2018 [73] | 2018 | 117 | Cross-sectional observational | Brazil | Overall: 6 (7/117) | 42.9 (3/7)/57.1 (4/7) |
| 34 | Scohy A. et al., 2018 [74] | 2016–2017 | 124 | Prospective | Belgium | Overall: 10.5 (13/124) | -/55 (11/20 isolates) |
| 35 | Eikani MS et al., 2018 [75] | 2003–2023 | 360 | Retrospective, case–control | U.S.A. | Overall: 8.3 (30/360) | - |
| 36 | Adjemian J. et al., 2018 [76] | 2010–2014 | 16,153 | Retrospective cohort | U.S.A. | Overall: 20 (3211/16,153) Annual: 11–13.4 (-) | 61 (1949/3211) /39 (1249/3211) |
| 37 | Hatziagorou E. et al., 2019 [77] | 2011–2016 | 42,958 | Observational | Europe | Overall: 3.3 (-) | - |
| 38 | Low D. et al., 2020 [78] | 2010–2014 | 17,177 | Retrospective cohort | U.S.A. | Annual: 10.9 (890/8172) 2010 –12.9 (1462/11,348) 2014 | 51.2 (749/1.462) /37.4 (547/1.462) |
| 39 | Ho D. et al., 2021 [79] | 2002–2015 | 171 | Retrospective observational | Reunion Island | Overall:26.4 (51/171) | 27.4 (14/51) /60.8 (31/51) |
| 40 | Ademhan D. et al., 2021 [80] | 2012–2020 | 485 | Retrospective cohort | Turkey | - | - |
| 41 | Foote SL. et al., 2021 [81] | 2010–2017 | 979 | Nested case–control | U.S.A. | - | - |
| 42 | Lipner EM et al., 2022 [82] | 2007–2019 | 193 | Nested case–control | U.S.A. | - | 76.2 (147/193) /42.3 (82/193) |
| 43 | Zomer D. et al., 2022 [83] | 2013–2019 | 1516 | Retrospective case–control | Netherlands | Annual: 1 (14/1353) 2013 –3.6 (55/1516) 2019 | 30.9 (42/136 isolates)/47.1 (64/136 isolates) |
| 44 | Wetzstein N. et al., 2022 [84] | 2015–2020 | 6295 | Retrospective observational | Germany | Overall: 1.9 (106/5462 in registry) (2015) –3.0 (177/5869) (2017) | 15.1 (16/106) 2015 –36.3 (65/179) 2020/46.6 (82/176) 2018 –62.3 (66/106) 2015 |
| 45 | Ricotta EE. et al., 2022 [85] | 2011–2018 | 17,403 | Retrospective cohort | U.S.A. | Overall: 23 (4002/17,403) | - |
| 46 | Mercaldo RA. et al., 2022 [86] | 2010–2019 | 25,305 | Retrospective | U.S.A. | - | - |
| 47 | Marshall JE. et al., 2023 [87] | 2010–2019 | 3771 | Retrospective cohort | U.S.A. | - | - |
| 48 | Steindor M. et al., 2023 [88] | 2016–2020 | 6295 | Retrospective observational | Germany | Annual: 7.53 (133/1767 tested) 2016 –8.76(185/2112 tested) 2020 | 2.15 (38/1767 tested) 2016 –3.08 (65/2112) 2020/ 3.63 (84/2317) 2019 –4.47 (79/1767) 2016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotiropoulou, A.; Loukou, I.; Vliora, C.; Douros, K.; Moustaki, M. The Epidemiology of Nontuberculous Mycobacteria in Cystic Fibrosis. Children 2025, 12, 1270. https://doi.org/10.3390/children12091270
Sotiropoulou A, Loukou I, Vliora C, Douros K, Moustaki M. The Epidemiology of Nontuberculous Mycobacteria in Cystic Fibrosis. Children. 2025; 12(9):1270. https://doi.org/10.3390/children12091270
Chicago/Turabian StyleSotiropoulou, Aikaterini, Ioanna Loukou, Christiana Vliora, Konstantinos Douros, and Maria Moustaki. 2025. "The Epidemiology of Nontuberculous Mycobacteria in Cystic Fibrosis" Children 12, no. 9: 1270. https://doi.org/10.3390/children12091270
APA StyleSotiropoulou, A., Loukou, I., Vliora, C., Douros, K., & Moustaki, M. (2025). The Epidemiology of Nontuberculous Mycobacteria in Cystic Fibrosis. Children, 12(9), 1270. https://doi.org/10.3390/children12091270





