Bilateral Symmetrical Brain MRI Findings in Acute Necrotising Encephalopathy Type 1
Abstract
1. Introduction
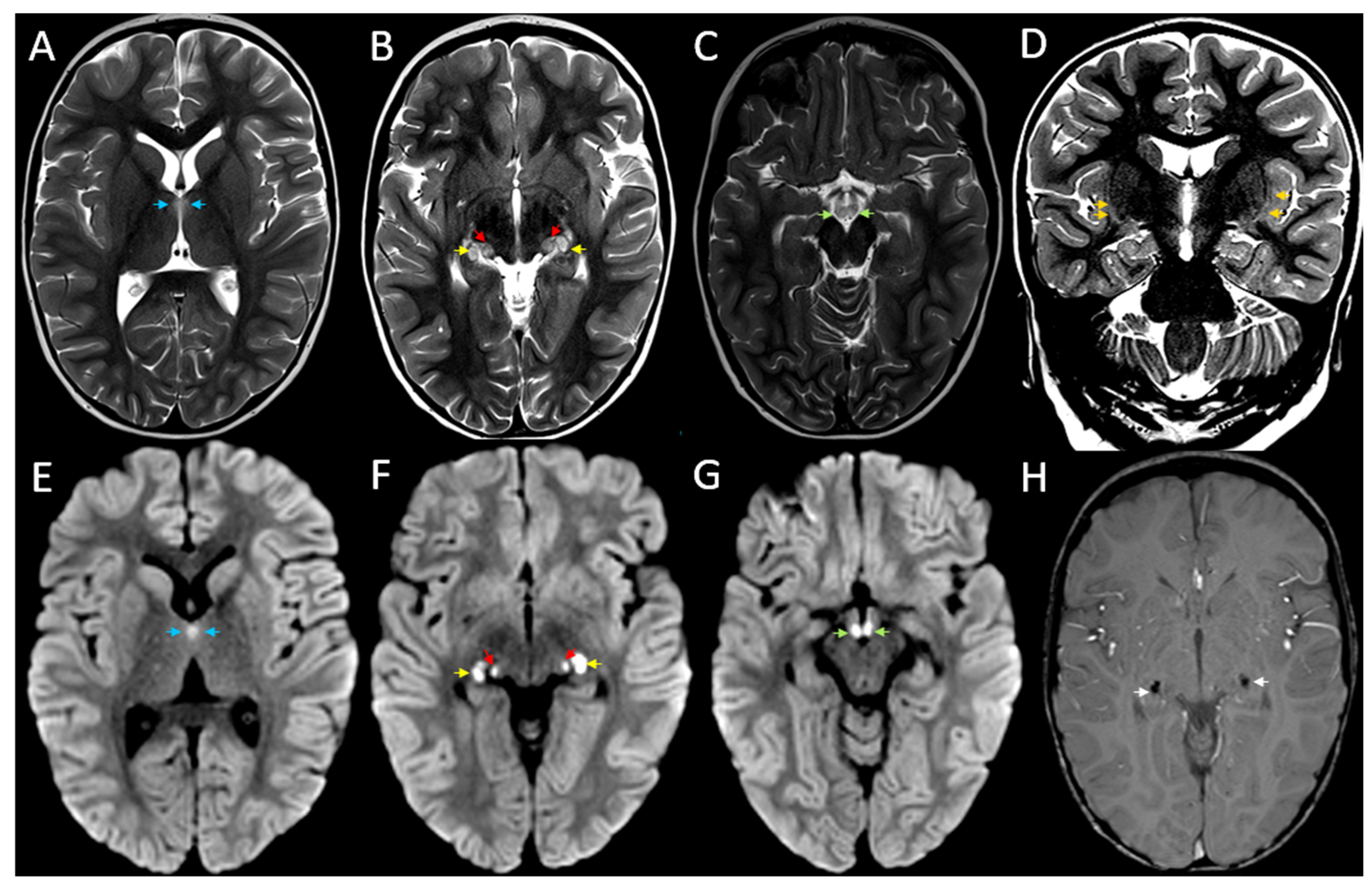
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mizuguchi, M. Acute necrotizing encephalopathy of childhood: A novel form of acute encephalopathy prevalent in Japan and Taiwan. Brain Dev. 1997, 19, 81–92. [Google Scholar] [CrossRef]
- Mizuguchi, M.; Abe, J.; Mikkaichi, K.; Noma, S.; Yoshida, K.; Yamanaka, T.; Kamoshita, S. Acute necrotising encephalopathy of childhood: A new syndrome presenting with multifocal, symmetric brain lesions. J. Neurol. Neurosurg. Psychiatry 1995, 58, 555–561. [Google Scholar] [CrossRef]
- Wong, A.M.; Simon, E.M.; Zimmerman, R.A.; Wang, H.S.; Toh, C.H.; Ng, S.H. Acute necrotizing encephalopathy of childhood: Correlation of MR findings and clinical outcome. AJNR Am. J. Neuroradiol. 2006, 27, 1919–1923. [Google Scholar]
- Jiang, J.; Wang, Y.E.; Palazzo, A.F.; Shen, Q. Roles of Nucleoporin RanBP2/Nup358 in Acute Necrotizing Encephalopathy Type 1 (ANE1) and Viral Infection. Int. J. Mol. Sci. 2022, 23, 3548. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.M.; Ahsan, N.; Ho, E.; Santoro, J.D. Genetic Acute Necrotizing Encephalopathy Associated with RANBP2: Clinical and Therapeutic Implications in Pediatrics. Mult. Scler. Relat. Disord. 2020, 43, 102194. [Google Scholar] [CrossRef] [PubMed]
- Desgraupes, S.; Etienne, L.; Arhel, N.J. RANBP2 evolution and human disease. FEBS Lett. 2023, 597, 2519–2533. [Google Scholar] [CrossRef]
- Neilson, D.E.; Adams, M.D.; Orr, C.M.; Schelling, D.K.; Eiben, R.M.; Kerr, D.S.; Anderson, J.; Bassuk, A.G.; Bye, A.M.; Childs, A.-M.; et al. Infection-triggered familial or recurrent cases of acute necrotizing encephalopathy caused by mutations in a component of the nuclear pore, RANBP2. Am. J. Hum. Genet. 2009, 84, 44–51. [Google Scholar] [CrossRef]
- Neilson, D.E. The interplay of infection and genetics in acute necrotizing encephalopathy. Curr. Opin. Pediatr. 2010, 22, 751–757. [Google Scholar] [CrossRef]
- Horváthy-Szőcs, A.; Liptai, Z.; Dobner, S.; Rudas, G.; Barsi, P. A Closer Investigation of the Synchronous Bilateral Pattern of MRI Lesions in Acute Necrotizing Encephalopathy Type 1. AJNR Am. J. Neuroradiol. 2021, 42, 2251–2253. [Google Scholar] [CrossRef]
- Lee, Y.J.; Hwang, S.K.; Kwon, S. Acute Necrotizing Encephalopathy in Children: A Long Way to Go. J. Korean Med. Sci. 2019, 34, e143. [Google Scholar] [CrossRef]
- Weitkamp, J.H.; Spring, M.D.; Brogan, T.; Moses, H.; Bloch, K.C.; Wright, P.F. Influenza A virus-associated acute necrotizing encephalopathy in the United States. Pediatr. Infect. Dis. J. 2004, 23, 259–263. [Google Scholar] [CrossRef]
- Okumura, A.; Mizuguchi, M.; Kidokoro, H.; Tanaka, M.; Abe, S.; Hosoya, M.; Aiba, H.; Maegaki, Y.; Yamamoto, H.; Tanabe, T.; et al. Outcome of acute necrotizing encephalopathy in relation to treatment with corticosteroids and gammaglobulin. Brain Dev. 2009, 31, 221–227. [Google Scholar] [CrossRef]
- Chatur, N.; Yea, C.; Ertl-Wagner, B.; Yeh, E.A. Outcomes in influenza and RANBP2 mutation-associated acute necrotizing encephalopathy of childhood. Dev. Med. Child Neurol. 2022, 64, 1008–1016. [Google Scholar] [CrossRef]
- Yamamoto, H.; Okumura, A.; Natsume, J.; Kojima, S.; Mizuguchi, M. A severity score for acute necrotizing encephalopathy. Brain Dev. 2015, 37, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.R.; Sedani, S.; Lim, M.; Wassmer, E.; Absoud, M. RANBP2 mutation and acute necrotizing encephalopathy: 2 cases and a literature review of the expanding clinico-radiological phenotype. Eur. J. Paediatr. Neurol. 2015, 19, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.R.; Speck-Martins, C.E.; Martins, B.J.A.F.; Izumi, A.P.; La Rocque-Ferreira, A. Variable Presentation and Reduced Penetrance in Autosomal Dominant Acute Necrotizing Encephalopathy Related to RANBP2 Variant. J. Pediatr. Genet. 2021, 12, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.M.; Zhang, S.M.; Yao, C.; Luo, M.-Q.; Ma, H.-J.; Lei, T.; Yuan, C.-H.; Wu, G.-F.; Hu, J.-S.; Cai, C.-Q.; et al. The Clinical and Imaging Characteristics Associated With Neurological Sequelae of Pediatric Patients With Acute Necrotizing Encephalopathy. Front. Pediatr. 2021, 9, 655074. [Google Scholar] [CrossRef]
- Wu, X.; Wu, W.; Pan, W.; Wu, L.; Liu, K.; Zhang, H.L. Acute necrotizing encephalopathy: An underrecognized clinicoradiologic disorder. Mediat. Inflamm. 2015, 2015, 792578. [Google Scholar] [CrossRef]
- Yoshida, T.; Tamura, T.; Nagai, Y.; Ueda, H.; Awaya, T.; Shibata, M.; Kato, T.; Heike, T. MRI gadolinium enhancement precedes neuroradiological findings in acute necrotizing encephalopathy. Brain Dev. 2013, 35, 921–924. [Google Scholar] [CrossRef]
- Sarigecili, E.; Ucar, H.K.; Havali, C.; Cansu, A.; Aydin, K. Acute necrotizing encephalopathy associated with RANBP2 mutation: Value of MRI findings for diagnosis and intervention. Acta Neurol. Belg. 2023, 123, 571–582. [Google Scholar] [CrossRef]
- Fischell, S.Z.; Fischell, J.; Kliot, T.; Tumulty, J.; Thompson, S.J.; Raees, M.Q. Case report: Acute necrotizing encephalopathy: A report of a favorable outcome and systematic meta-analysis of outcomes with different immunosuppressive therapies. Front. Neurol. 2023, 14, 1239746. [Google Scholar] [CrossRef]
- Li, K.; Zhang, T.; Liu, G.; Jin, P.; Wang, Y.; Wang, L.; Xu, M.; Liu, C.; Liu, Y.; Zhou, T.; et al. Plasma exchange therapy for acute necrotizing encephalopathy of childhood. Pediatr. Investig. 2021, 5, 99–105. [Google Scholar] [CrossRef]
- Kelly, E.; Harvey, J.; Brion, K.; Fletcher, J.; Slee, M. Relapsing necrotising encephalomyelopathy due to RANBP2 mutation. Pract. Neurol. 2019, 19, 360–363. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoppe, A.T.; Ghia, T.; Warne, R.; Shipman, P.; Lakshmanan, R. Bilateral Symmetrical Brain MRI Findings in Acute Necrotising Encephalopathy Type 1. Children 2025, 12, 974. https://doi.org/10.3390/children12080974
Hoppe AT, Ghia T, Warne R, Shipman P, Lakshmanan R. Bilateral Symmetrical Brain MRI Findings in Acute Necrotising Encephalopathy Type 1. Children. 2025; 12(8):974. https://doi.org/10.3390/children12080974
Chicago/Turabian StyleHoppe, Alexander T., Twinkle Ghia, Richard Warne, Peter Shipman, and Rahul Lakshmanan. 2025. "Bilateral Symmetrical Brain MRI Findings in Acute Necrotising Encephalopathy Type 1" Children 12, no. 8: 974. https://doi.org/10.3390/children12080974
APA StyleHoppe, A. T., Ghia, T., Warne, R., Shipman, P., & Lakshmanan, R. (2025). Bilateral Symmetrical Brain MRI Findings in Acute Necrotising Encephalopathy Type 1. Children, 12(8), 974. https://doi.org/10.3390/children12080974





