Highlights
What are the main findings?
- Individualized anesthetic planning with total intravenous anesthesia and blood conservation is essential in neuromuscular scoliosis surgery.
- Implementing Enhanced Recovery After Surgery principles facilitates faster recovery and reduces complications in this high-risk population.
What is the implication of the main finding?
- A multidisciplinary, evidence-based approach improves perioperative outcomes.
- Standardized protocols enhance safety and recovery in pediatric neuromuscular scoliosis surgery.
Abstract
Background/Objectives: Scoliosis surgery in pediatric patients, particularly those with neuromuscular disorders, is associated with increased perioperative risk due to respiratory insufficiency, cardiovascular comorbidities, and nutritional deficiencies. This review aims to summarize current evidence-based approaches to anesthetic management in this vulnerable population. Methods: A comprehensive literature review was conducted focusing on anesthetic strategies and multidisciplinary protocols used in the perioperative care of children with neuromuscular conditions undergoing scoliosis surgery. Emphasis was placed on intraoperative neurophysiological monitoring (IONM), blood conservation techniques, and Enhanced Recovery After Surgery (ERAS) principles. Results: Key management strategies include individualized preoperative risk assessment, use of total intravenous anesthesia (TIVA) to preserve IONM signal integrity, and the implementation of blood conservation methods such as antifibrinolytic therapy and intraoperative cell salvage. Additional perioperative considerations include maintaining normothermia, careful positioning, and multimodal analgesia. Postoperative care should incorporate structured respiratory support and early mobilization within the ERAS pathway to promote recovery and reduce complications. Conclusions: The perioperative care of pediatric patients with neuromuscular scoliosis undergoing spinal surgery requires a multidisciplinary and individualized anesthetic approach. Adherence to evidence-based protocols, including TIVA, blood management strategies, and ERAS principles, is essential for minimizing perioperative complications and improving outcomes in this high-risk group.
1. Introduction
The surgical correction of scoliosis in pediatric patients with neuromuscular disorders remains one of the most complex challenges in pediatric anesthesiology [,]. Neuromuscular scoliosis (NMS), defined as a structural spinal deformity secondary to an underlying neuromuscular condition (e.g., cerebral palsy [CP; see Figure 1 and Figure 2], spinal muscular atrophy [SMA], Duchenne muscular dystrophy [DMD]), is frequently encountered in clinical practice [,,]. NMS arises from progressive muscular weakness and impaired postural control, often leading to severe three-dimensional deformities and thoracic insufficiency [,,]. Epidemiologically, scoliosis is common across neuromuscular conditions, with prevalence increasing alongside functional severity and age. In CP, population-based pediatric cohorts show that at 10 years ≈1% (Gross Motor Function Classification System [GMFCS] I–II), ≈5% (GMFCS III), ≈10% (GMFCS IV), and ≈30% (GMFCS V) have scoliosis; by 20 years, ≈75% of those in GMFCS V reach a Cobb angle ≥ 40°, whereas none in GMFCS I do so [,]. In SMA, pediatric series report scoliosis in ≈90% of type I, 80–90% of type II, and ≈50% of type III patients [,]. In DMD, curves typically emerge after loss of ambulation. However, contemporary long-term corticosteroid therapy—by prolonging ambulation—has been associated with a lower incidence and delayed onset compared with earlier cohorts []. These differences underscore the perioperative burden and the importance of condition-specific anesthetic planning.
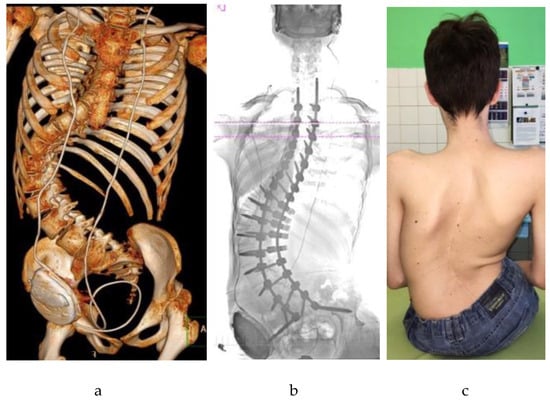
Figure 1.
An adolescent patient with neuromuscular scoliosis secondary to cerebral palsy and hydrocephalus, following posterior spinal fusion with pelvic fixation: (a) Three-dimensional computed tomography (CT) reconstruction showing a severe scoliotic deformity with rotational and translational components, corrected by posterior instrumentation; (b) Anteroposterior radiograph illustrating long-segment posterior spinal fusion extending to the pelvis; (c) Postoperative clinical image in the seated position showing a midline surgical scar and improved spinal alignment.
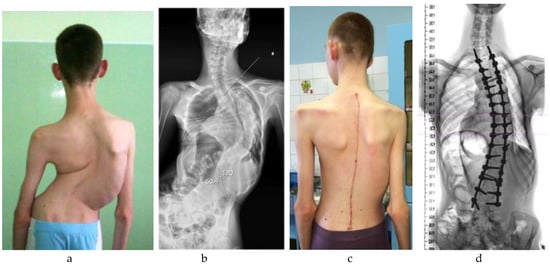
Figure 2.
An adolescent patient with neuromuscular scoliosis secondary to cerebral palsy. In contrast to the case presented in Figure 1, this patient retained ambulatory function and did not require pelvic fixation: (a) Preoperative clinical image demonstrating moderate coronal imbalance with visible scapular asymmetry; (b) Preoperative standing anteroposterior radiograph showing a structural thoracolumbar scoliosis without pelvic obliquity; (c) Postoperative clinical image showing improved trunk alignment and a well-healed midline incision; (d) Postoperative radiograph demonstrating correction of the scoliotic curve with segmental posterior instrumentation terminating above the pelvis.
Children with NMS frequently present with multisystem comorbidities—restrictive pulmonary disease with ineffective airway clearance and recurrent infections, swallowing dysfunction with gastroesophageal reflux, and malnutrition—which collectively elevate perioperative risk and mandate individualized anesthetic strategies []. Additional complexity arises from altered pharmacokinetics and an increased risk of respiratory compromise during long posterior fusions []. Furthermore, anesthetic maintenance must preserve the integrity of intraoperative neurophysiological monitoring (IONM), a topic expanded in the intraoperative section [].
This review synthesizes perioperative anesthetic principles specific to NMS with a pragmatic, clinician-focused emphasis on risk stratification, airway and respiratory management, IONM-compatible anesthesia, blood conservation, and postoperative care within multidisciplinary Enhanced Recovery After Surgery (ERAS) pathways []. A narrative review format was adopted because the pediatric evidence base spans heterogeneous sources—society guidelines and consensus statements (e.g., Scoliosis Research Society, Pediatric Orthopaedic Society of North America, and anesthesia societies), pediatric cohort studies, technique-oriented reports, and neurophysiology recommendations—with substantial variability in populations, interventions, and outcomes that preclude meaningful meta-analysis [,,,]. A narrative synthesis enables integration of guideline-level recommendations with pragmatic perioperative protocols most relevant to anesthesiologists managing NMS. Methods detail our search process and evidence weighting.
2. Materials and Methods
2.1. Design and Scope
This narrative review synthesizes current evidence on perioperative anesthetic management for pediatric spinal deformity surgery, focusing on NMS.
2.2. Data Sources and Search Strategy
The primary database was PubMed/MEDLINE, queried from January 2010 through 30 August 2024. Earlier landmark studies were included when foundational to current practice. Reference lists of key articles and webpages of relevant societies (e.g., Scoliosis Research Society [SRS], Pediatric Orthopaedic Society of North America [POSNA], and major anesthesia societies) were hand-searched. Search strings combined controlled vocabulary (Medical Subject Headings [MeSH]) and free-text keywords reflecting the perioperative pathway (e.g., neuromuscular scoliosis, pediatric/children, anesthesia/anesthetic management, airway, ventilation, total intravenous anesthesia, intraoperative neuromonitoring, tranexamic acid/blood conservation, ERAS). Terms on surgical planning—computed tomography (CT) for pedicle-screw or osteotomy planning, navigation/robotic assistance, and halo-gravity traction (HGT)—were added to capture evidence relevant to Section 4. The search was last updated on 30 August 2024.
2.3. Eligibility Criteria
Eligible studies included peer-reviewed, primarily English-language publications directly applicable to pediatric perioperative care in NMS: guidelines, consensus statements, clinical trials, case series, cohort studies, and systematic or narrative reviews. Two Slovak publications were retained for their original data on spinal decompression techniques. Excluded materials were non-peer-reviewed works, single-patient case reports, conference abstracts without full text, and adult-only series without pediatric relevance. Where pediatric data were limited, high-quality adolescent or mixed-population studies were included when perioperative pathways were compatible.
2.4. Study Selection and Yield
Titles and abstracts, followed by full texts, were independently screened by two authors, with disagreements resolved by consensus. Across all PubMed queries, 653 records were identified. After de-duplication across query modules, 574 unique records were screened at the title and abstract level; a total of 89 publications, including those identified through hand-searching of society webpages and citation lists, were retained for qualitative synthesis. This approach en-sures quantitative transparency while remaining consistent with the narrative design of the review.
2.5. Quality Considerations and Evidence Weighting
Given heterogeneity in study designs, populations (e.g., CP, SMA, DMD, MMC), interventions, and outcomes, no single formal risk-of-bias instrument was applied. Instead, a prespecified qualitative hierarchy was used:
- (i)
- Society guidance (SRS, POSNA, anesthesia societies) and multicenter/prospective pediatric cohorts were weighted more heavily than single-center descriptive series;
- (ii)
- Age- and diagnosis-specific applicability (pediatric NMS over adolescent idiopathic scoliosis (AIS)/adult data) was prioritized;
- (iii)
- Recency, sample size, and presence of comparators informed interpretation when recommendations conflicted.
Use of adolescent or mixed-population evidence is explicitly indicated where pediatric NMS data were lacking.
2.6. Data Extraction and Synthesis
From included sources, we abstracted study scope; population and diagnosis; anesthetic techniques (including IONM compatibility); ventilation strategies for restrictive disease; blood-conservation measures (including tranexamic acid protocols); perioperative pathways (prehabilitation, extubation or non-invasive ventilation (NIV) criteria, ERAS elements); and complication incidences. Findings were synthesized thematically by perioperative phase: preoperative assessment and optimization, intraoperative management, perioperative complications, and postoperative care.
2.7. Ethics
No original human or animal data were generated for this review; institutional ethics approval was not required.
3. Preoperative Assessment and Optimization
The therapeutic approach to spinal deformities in pediatric patients relies on accurate classification that considers age, underlying etiology, and the characteristics and extent of the spinal curvature. This assessment is essential for selecting a tailored treatment strategy. The primary goals of surgical intervention are spinal stabilization, prevention of curve progression, and maximal safe deformity correction, accompanied by pain reduction, improved functional capacity, and enhanced quality of life for patients and caregivers.
Preoperative preparation for scoliosis surgery in children is a complex, multiphase process aimed at creating optimal conditions for safe surgery under general anesthesia. Its main objectives are to conduct a thorough perioperative risk assessment, optimize the child’s clinical condition, formulate an individualized anesthetic plan, and define the scope of required intra- and postoperative monitoring. Given the physiological demands of spinal surgery on multiple organ systems, the extent of preoperative evaluation must be adapted to age, diagnosis, and comorbidities, with early involvement of a multidisciplinary team (anesthesiology, orthopedics, neurology, pulmonology, cardiology, rehabilitation, nutrition) and clear care pathways [].
3.1. Anesthetic Pre-Assessment
Evaluation should encompass the child’s overall medical condition and potential difficulties in airway management. Baseline laboratory tests typically comprise a complete blood count, coagulation profile, and basic metabolic panel; additional testing is guided by comorbidities and medications (e.g., antiepileptics, intrathecal baclofen). Depending on clinical findings, further specialist consultations—neurology, pulmonology, and cardiology—may be warranted [].
3.2. Pulmonary Evaluation
Assessment is tailored to age, clinical status, and cooperation. Recommended investigations include overnight oxygen saturation monitoring and/or capnography to screen for nocturnal hypoventilation, with post-sleep arterial or capillary blood gas analysis when feasible. Polysomnography provides details on sleep-related breathing disturbances. Cooperative children should undergo spirometry (forced vital capacity [FVC], forced expiratory volume in 1 s [FEV1]); in neuromuscular disorders, evaluation of cough strength and maximal inspiratory/expiratory pressures (MIP/MEP) is advised. In severe thoracic deformity or abnormal pulmonary function, chest CT can be considered to evaluate airway compression and parenchymal disease [,].
3.3. Respiratory Optimization
A proactive strategy is recommended, emphasizing early airway-clearance techniques (e.g., oscillatory devices, mechanical insufflation–exsufflation), respiratory physiotherapy, and timely initiation or optimization of NIV when indicated. These interventions reduce perioperative pulmonary complications and facilitate recovery; planned postoperative NIV and routine cough-assist should be considered in high-risk children [,,].
3.4. Cardiac Evaluation
Cardiac assessment is essential when cardiovascular involvement is suspected or confirmed. Electrocardiography and transthoracic echocardiography are recommended—particularly for disorders associated with cardiomyopathy or conduction abnormalities (e.g., DMD)—to guide hemodynamic targets, rhythm surveillance, and anesthetic planning [,].
3.5. Nutritional Optimization and Aspiration Risk
Malnutrition is common and independently associated with adverse outcomes in neuromuscular scoliosis. Early dietetic assessment, caloric/protein optimization, and correction of micronutrient deficiencies (e.g., iron, vitamin D) are recommended. In selected patients with severe dysphagia or reflux—especially in SMA and DMD—prehabilitation may include establishing a reliable enteral route (e.g., gastrostomy) weeks to months before surgery to stabilize weight and reduce aspiration risk. Aspiration mitigation involves reflux management when present, adherence to pediatric fasting guidance, individualized modified rapid-sequence induction in high-risk airways, and explicit airway-protection strategies at extubation (e.g., cuff-leak assessment, optimal positioning, early NIV) [,].
3.6. Psychological Preparation and Family Communication
Structured preoperative counseling reduces anxiety and improves adherence to postoperative respiratory therapy and mobilization. Discussions should set expectations for the ICU course, pain control, the potential need for NIV and cough-assist, and escalation pathways; they should also—sensitively—acknowledge the possibility of serious complications, including neurological injury and mortality, to support informed consent and shared decision-making [].
3.7. Blood Management Planning
Preoperative blood-conservation planning should include protocolized antifibrinolysis (tranexamic acid, TXA) [,,,], temperature management, and intraoperative cell salvage when available. Cross-matching should reflect the estimated blood volume and anticipated loss based on curve severity and planned fusion length. Given the potential for major hemorrhage in long posterior fusions, institutions should maintain explicit massive transfusion protocol (MTP) readiness with predefined activation criteria, component therapy guidance, and point-of-care coagulation testing where available.
Transfusion thresholds should be defined within institutional protocols and individualized to each child’s physiologic status and comorbidities, in coordination with postoperative monitoring plans.
Centers are encouraged to adopt a standardized TXA protocol consistent with local experience and safety monitoring. Evidence supports TXA effectiveness across pediatric scoliosis, with several cohorts showing greater blood-sparing at higher doses and randomized/meta-analytic data in AIS confirming benefit at lower doses. Intraoperative details on coagulation management and dosing strategies are discussed in Section 5.5.
3.8. Venous Thromboembolism (VTE) Risk and Prophylaxis
Although pediatric VTE after elective spine surgery is rare in mixed pediatric cohorts, NMS may carry a relatively higher risk owing to immobility, prolonged procedures, central venous access, and multisystem comorbidity. Routine mechanical prophylaxis is recommended for all patients, and selective pharmacologic prophylaxis based on individual risk and institutional protocol, consistent with contemporary pediatric consensus [].
3.9. Premedication and Anxiolysis
Premedication, an important perioperative planning component, should be tailored to the child‘s condition and comorbidities. Benzodiazepines are often avoided in children with neuromuscular scoliosis because of risks of respiratory depression, agitation, and postoperative cognitive effects; where anxiolysis is required, alternatives such as dexmedetomidine, clonidine, or low-dose ketamine may be considered, alongside non-pharmacologic strategies (child-life preparation, caregiver presence, distraction techniques) to minimize sedative burden [,].
4. Surgical Planning and Deformity Preparation
4.1. Preoperative Imaging and Navigation
Beyond pulmonary assessment, CT delineates pedicle morphology, vertebral rotation, and canal dimensions, thereby informing pedicle screw trajectories and osteotomy strategy in severe three-dimensional deformity and dysplastic pedicles. In experienced centers, CT-based navigation or robotic assistance can improve placement accuracy and reduce unplanned screw revision; the benefit must be balanced against radiation exposure and institutional resources. Where pediatric NMS-specific evidence is limited, evidence from adolescent cohorts is cautiously extrapolated due to shared posterior instrumentation steps [,,].
4.2. Halo-Gravity Traction (HGT) for Severe, Rigid Curves
In children with large, rigid deformities and compromised respiratory mechanics, preoperative HGT can progressively reduce curve magnitude, improve ventilatory mechanics, and facilitate safer definitive correction with less need for aggressive osteotomies. Contemporary pediatric studies report meaningful coronal/sagittal correction with an acceptable safety profile when traction is escalated gradually with regular neurological checks, diligent pin-site care, and pressure-injury prevention. Courses beyond approximately 3 months seldom add benefit and increase care burden. Reported complications (generally infrequent) include pin-site infection/loosening, transient neuropraxia or cranial-nerve symptoms, neck discomfort, and dysphagia; standardized protocols and multidisciplinary oversight mitigate these risks [,,,,,,].
4.3. Implications for Anesthesia and Team
Surgical planning that incorporates CT-guided trajectory/osteotomy maps and, when indicated, staged HGT should be tightly coordinated with anesthesia to: (i) anticipate airway management and positioning in traction; (ii) align hemodynamic goals with neuromonitoring requirements during gradual correction and definitive fusion; (iii) plan analgesia and skin/pressure protection during traction periods; and (iv) ensure clear intraoperative communication regarding traction adjustments and IONM milestones [,,,,,,,,,].
5. Anesthetic Techniques and Intraoperative Considerations
Scoliosis correction is among the most complex procedures in pediatric orthopedic practice, demanding meticulous anesthetic planning due to its technical demands and elevated perioperative risk. Key intraoperative considerations include optimal patient positioning and appropriate anesthetic techniques—particularly in the context of IONM—ventilatory management, blood conservation strategies, and early planning for postoperative intensive care [,,,].
Although NMS is often approached as a single entity, perioperative management should reflect condition-specific risks and priorities; the matrix below highlights high-impact differences relevant to anesthetic planning (Table 1) [,,].

Table 1.
High-impact anesthetic differences across NMS conditions (DMD/BMD = Duchenne/Becker; SMA = Spinal muscular atrophy; CP = Cerebral palsy; MMC = Myelomeningocele/spina bifida).
5.1. Intraoperative Respiratory and Positioning Management in NMS
Given the reduced chest wall compliance and restrictive mechanics in NMS—exacerbated by prone positioning—we favor pressure-controlled ventilation (PCV) or pressure-regulated volume control (PRVC; also termed pressure-controlled ventilation–volume guaranteed [PCV–VG]), targeting low tidal volumes (~6–7 mL·kg−1 of predicted body weight [PBW]) with individualized PEEP (~5–8 cmH2O) to maintain oxygenation and dynamic compliance without impeding venous return. Gentle recruitment after turning prone, avoidance of nitrous oxide, maintenance of EtCO2 ~35–40 mmHg and normoxia, and limitation of peak and plateau pressures help protect the lung and preserve IONM signal quality [,].
Several factors may increase intra- and postoperative morbidity in these patients, including systemic comorbidities, major blood loss, risk of neurological injury, and the physiologic effects of prone positioning. When placing the patient in the prone position, adequately supporting the thorax and abdomen is essential to prevent increased intra-abdominal and intrathoracic pressures, which can impair venous return and cardiopulmonary interactions (Figure 3). Compression of the inferior vena cava may reduce preload and promote epidural venous engorgement, increasing the risk of bleeding from these highly vascular structures. Improper positioning may also reduce chest wall excursion, diminish pulmonary compliance, and decrease functional residual capacity [].
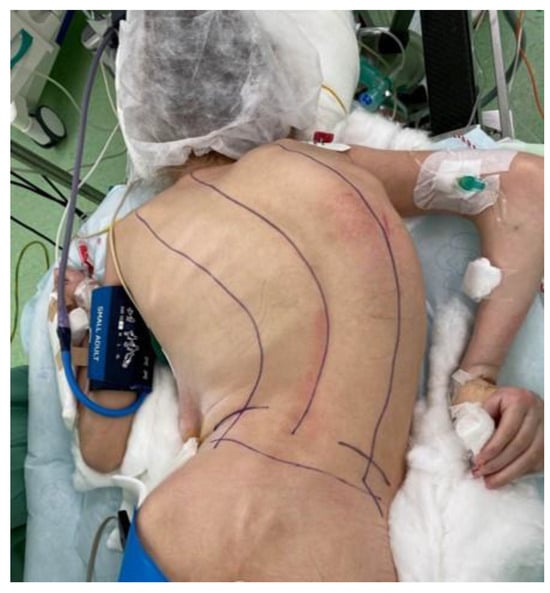
Figure 3.
Intraoperative prone positioning in a pediatric patient undergoing scoliosis correction. Proper support of the thorax and abdomen and alignment of the spine are essential to minimize intra-abdominal pressure and avoid positioning-related complications.
The surgical and anesthetic teams are responsible for ensuring safe and physiologically favorable positioning. Before surgical draping, proper alignment must be confirmed; all pressure points protected; and measures taken to prevent nerve injuries to the brachial plexus, ulnar nerve, dorsal foot nerves, and lateral femoral cutaneous nerve. The endotracheal tube must be secured to avoid dislodgement. The eyes should be protected from direct pressure, with commercial face pillows or mirrors recommended for facial observation. A bite block should be inserted to protect oral structures during motor evoked potential (MEP) stimulation. If the face is not readily visible, indirect visualization using simple tools (e.g., smartphone camera) may be helpful [].
Perioperative visual loss is a rare but potentially devastating complication of prone spinal surgery. Its estimated incidence is approximately 3.09 per 10,000 spine procedures [,]. Reported mechanisms include anterior and posterior ischemic optic neuropathy, central retinal artery occlusion, cortical blindness, and posterior reversible encephalopathy syndrome []. Although the precise pathophysiology in the prone position remains unclear, data from the American Society of Anesthesiologists Postoperative Visual Loss Registry identify prolonged anesthesia duration and considerable intraoperative blood loss as key contributing factors [].
5.2. IONM
IONM markedly reduces the risk of neurological injury by assessing the functional integrity of neural pathways []. It enables early detection of irritation or injury to critical neural structures and facilitates real-time surgical adjustments. Historically, the Stagnara wake-up test was used intraoperatively to assess motor function in the lower limbs; however, this technique has largely been replaced with modern electrophysiological modalities [,].
Combined somatosensory-evoked potentials (SSEPs) and MEPs have become the standard of care for spinal deformity surgery. Successful IONM requires close collaboration among the surgeon, anesthesiologist, and neurophysiologist, with anesthetic management carefully tailored to preserve signal integrity.
TIVA using propofol and short-acting opioids (e.g., remifentanil) is preferred for IONM. Target-controlled infusion (TCI) systems are commonly used to ensure stable anesthetic depth. Typical ranges used with IONM include propofol 100–200 μg·kg−1·min−1 (or TCI ≈ 2–4 μg·mL−1) combined with remifentanil 0.05–0.2 μg·kg−1·min−1; adjunct ketamine 0.25–0.5 mg·kg−1 bolus then 0.1–0.3 mg·kg−1·h−1 can reduce propofol requirements without degrading MEPs; if dexmedetomidine is used, keep low infusions ≈ 0.2–0.5 μg·kg−1·h−1 and monitor for possible MEP attenuation []. In contrast, inhalational agents, nitrous oxide, and benzodiazepines depress MEP amplitudes and prolong latencies, and are generally avoided. Dexmedetomidine may also suppress MEPs, although its effects on SSEPs remain less clearly defined [].
Non-depolarizing neuromuscular blockers may be administered during induction, but should be discontinued once IONM begins []. Physiological disturbances such as hypotension, hypovolemia, anemia, and hypothermia can impair monitoring quality and must be promptly addressed if changes in evoked potentials occur [,]. The structured multidisciplinary protocol for responding to IONM signal changes is presented in Table 2.

Table 2.
Multidisciplinary checklist for immediate interventions following IONM signal loss.
A well-coordinated intraoperative approach, encompassing optimized ventilation, maintenance of hemodynamic stability, effective thermoregulation, and management of anticipated blood loss, combined with precisely tailored anesthetic strategies and reliable neurophysiological monitoring, is key to ensuring surgical safety and preserving neurological function.
5.3. Perioperative Monitoring
The extent of perioperative vital sign monitoring during scoliosis surgery depends on the procedure’s type and duration, anticipated blood loss, and patient comorbidities. Standard monitoring during general anesthesia for spinal deformity surgery includes electrocardiography, heart rate, oxygen saturation (SpO2), invasive blood pressure, body temperature, ventilatory parameters, hourly urine output, and anesthesia depth. Near-infrared spectroscopy serves as a useful adjunct for continuous, noninvasive monitoring of regional cerebral oxygenation.
5.4. Perioperative Temperature Management
Intraoperative hypothermia is a major concern in pediatric scoliosis surgery, particularly in patients with neuromuscular disorders. Reported incidence in elective pediatric surgery ranges from 2.7% to 74%, depending on procedure type, age, and intraoperative conditions [,]. Children with neuromuscular conditions, severe spinal deformities, and low body mass index are especially vulnerable. Severe hypothermia, a 2.5 °C drop in core body temperature from baseline, can markedly impair IONM by reducing the quality of evoked potentials. Moreover, even mild hypothermia (1–2 °C decrease) has been shown to triple the risk of surgical site infections, prolong hospitalization by approximately 20%, and increase intraoperative blood loss [].
Preventive measures should include preoperative warming, active intraoperative warming, use of warmed intravenous fluids, and continuous temperature monitoring via esophageal or nasopharyngeal probes. Recommended warming modalities include forced-air warming blankets, underbody warming pads, and fluid warming systems. Strict maintenance of normothermia is essential to reduce perioperative complications and supports better recovery.
5.5. Blood Loss in Scoliosis Surgery
Surgical correction of spinal deformities in children is frequently associated with substantial blood loss, posing a serious and potentially life-threatening risk (Figure 4). Massive hemorrhage considerably increases perioperative morbidity and mortality in this vulnerable population []. Even in patients without preexisting coagulopathy, extensive procedures may lead to acquired consumptive and dilutional coagulopathy, with hypofibrinogenemia often representing the earliest and most critical abnormality. Efforts to maintain normovolemia using crystalloids and red blood cell (RBC) transfusions can further aggravate coagulopathy by diluting clotting factors, underscoring the need for prompt and accurate assessment of hemostasis [,].
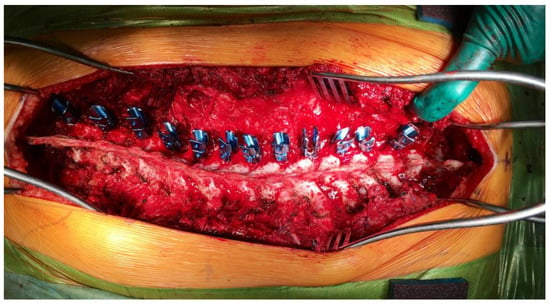
Figure 4.
Intraoperative image during posterior spinal fusion surgery for neuromuscular scoliosis, illustrating the placement of pedicle screws on the left side of the spine in full density. The image demonstrates the extensive surgical exposure and the vascular nature of the operative field, highlighting the potential for substantial intraoperative blood loss. The photo emphasizes the need for meticulous hemostasis, targeted antifibrinolytic therapy, and real-time coagulation monitoring to minimize transfusion requirements and perioperative complications.
Goal-directed coagulation management with viscoelastic testing (Rotational Thromboelastometry [ROTEM] or thromboelastography [TEG]) is recommended because it allows timely and targeted replacement therapy (e.g., fibrinogen concentrate or cryoprecipitate when indicated) and provides a more physiological alternative to fixed-ratio transfusion protocols []. Given the risks associated with allogeneic transfusions, clearly defined restrictive transfusion thresholds are essential; current pediatric guidelines support thresholds of approximately 7 g/dL for hemodynamically stable patients, individualized to physiology, comorbidity, and surgical complexity [].
Modern pediatric blood-sparing strategies integrate preoperative anemia optimization, blood-sparing anesthetic techniques, antifibrinolysis, intraoperative cell salvage, and institutional readiness for a massive transfusion protocol (MTP) in high-risk cases. Among these, antifibrinolytics—particularly TXA—are the most effective pharmacologic adjuncts in reducing blood loss during scoliosis surgery. Pediatric dosing regimens vary, including low-dose continuous infusions (e.g., 10–20 mg/kg loading dose followed by 0.25–2 mg/kg/h) and high-dose protocols (e.g., 50 mg/kg loading dose followed by 5 mg/kg/h), selected according to anticipated blood loss, comorbidity, and institutional experience [].
Comparative pediatric data demonstrate lower estimated blood loss and transfusion requirements with high-dose versus low-dose TXA in AIS []. Moreover, disease-specific evidence in NMS confirms the benefit of antifibrinolysis: in cerebral palsy, TXA significantly reduced intraoperative blood loss and cell-salvage transfusion and outperformed ε-aminocaproic acid (EACA), with no increase in adverse events; differences in total allogeneic transfusion were not significant []; in spinal muscular atrophy, a 20-year cohort showed a marked reduction in blood loss and transfusion volume with TXA, along with a trend toward fewer postoperative pulmonary complications [].
Collectively, these findings support the implementation of a standardized TXA protocol as part of a multimodal, goal-directed blood management strategy. In patients with severe, rigid neuromuscular curves or anticipated massive blood loss, many pediatric spine centers adopt higher dosing regimens (e.g., 50–100 mg/kg loading followed by 5–10 mg/kg/h infusion), consistent with institutional guidelines. While current evidence supports the efficacy and safety of TXA in this population, vigilant thromboembolic and neurologic monitoring remains essential [,,].
6. Perioperative Complications
The choice of surgical approach for spinal deformity correction—anterior, posterior, or combined—depends on deformity type and severity. Combined approaches are frequently employed for complex and rigid curvatures. These procedures are among the most challenging in spinal surgery and carry the risk of various complications. Reported complication rates for deformity correction and spinal fusion in adolescent idiopathic scoliosis range from 5% to 23% []. Higher complication rates are observed in congenital and neuromuscular scoliosis due to associated comorbidities and more complex deformities.
Complications can be classified as intraoperative or postoperative. The most serious involve neurological injuries that may occur during or after surgery. Local intraoperative complications include dural sac injury, bleeding, bone structural damage, and direct organ injury, and those related to improper positioning. Postoperative complications may also include gastrointestinal issues such as ileus and, rarely, pancreatitis.
6.1. Surgical Site and Neurological Complications
Postoperative complications include surgical site (SSI) infections, venous thromboembolism, gastrointestinal issues, and implant-related problems. SSI is among the most common complications of spinal surgery, with a reported incidence of approximately 2.7% within 90 days postoperatively []. Risk factors include obesity, male sex, prolonged surgery, and neuromuscular disease. Major blood loss requiring transfusion—previously considered routine—is now recognized as a preventable complication.
Anterior spinal artery syndrome (ASAS) represents a severe ischemic insult to the spinal cord and may cause permanent neurological deficits. It results from ischemia in the territory of the anterior spinal artery, which supplies approximately two-thirds of the cord. The mid-thoracic region (T3–T8) is particularly vulnerable due to its relatively poor vascularization, whereas the artery of Adamkiewicz—functionally the most important radicular vessel—enters between T9 and T12 in about 75% of individuals. Mechanical injury, compression, or traction on these arteries can result in profound neurological impairment manifested by motor deficits, sensory disturbances, radicular pain, and bowel or bladder dysfunction.
The prognosis of ASAS remains generally poor, with a mortality rate near 20%, and roughly half of survivors experiencing minimal neurological recovery []. Although complete prevention of spinal cord ischemia is impossible, its risk can be mitigated by maintaining adequate mean arterial pressure, avoiding rapid deformity correction, ensuring effective hemostasis, and optimizing oxygen delivery. IONM should be standard practice, combining SSEPs and MEPs where possible, although these modalities may not detect all ischemic events; postoperative deterioration can still occur despite normal intraoperative traces.
6.2. Gastrointestinal Complications
Superior mesenteric artery syndrome (SMAS) is a rare but serious gastrointestinal complication caused by duodenal compression between the abdominal aorta and the superior mesenteric artery. First described by Rokitansky in 1842 [], SMAS is associated with marked weight loss, anorexia nervosa, malabsorption, and hypercatabolic states. Risk factors include asthenic body habitus and previous spinal surgery. Symptoms typically manifest between postoperative days 6–12 as spinal “lengthening” narrows the aortomesenteric angle. Reported prevalence after scoliosis surgery ranges from 0.5% to 2.4% [,]. Clinical features include early satiety, nausea, vomiting, post-prandial pain, bloating, and, in severe cases, high intestinal obstruction.
6.3. Hemorrhagic Complications
Massive bleeding is defined as blood loss exceeding one total blood volume within 24 h, 50% within 3 h, or ≈10% every 10 min. Scoliosis correction is a prolonged procedure with potential for significant blood loss and acquired coagulopathy. Typical intraoperative blood loss in AIS is often reported at ≈300–1000 mL, varying with curve magnitude, osteotomies, and fusion length; losses are generally higher in NMS.
Blood loss correlates with comorbidities, Cobb angle, scoliosis type, number of fused levels, and surgical duration. Patients with neuromuscular disorders face the highest risk because of severe, rigid deformities and longer operative times. The greatest intraoperative blood loss has been observed in DMD, with substantial losses also observed in myelomeningocele, SMA, and CP []. When blood loss exceeds 50% of the estimated circulating volume, the risk of complications rises sharply.
Intraoperative blood management—including goal-directed coagulation therapy, viscoelastic monitoring, and standardized antifibrinolysis with TXA—is detailed in Section 5.5.
6.4. Pulmonary Complications
Pulmonary complications are a major cause of postoperative morbidity and mortality in pediatric scoliosis surgery. Their incidence is fivefold higher in children with non-idiopathic scoliosis than in idiopathic cases and greater after anterior than posterior approaches []. Common events include atelectasis, hemothorax, pneumothorax, and pleural effusion; less frequent are pneumonia, pulmonary edema, and upper airway obstruction. Children with neuromuscular disorders frequently require postoperative ventilatory support.
In a single-center cohort of 133 children, Al-Iede et al. [] reported postoperative pulmonary complications in approximately 18% of pediatric patients. Major risk factors included neuromuscular disease, Cobb angle > 90°, reduced forced vital capacity, and elevated serum bicarbonate. Preoperative polysomnography helps identify nocturnal hypoventilation, and preoperative NIV use is associated with significantly fewer postoperative pulmonary complications and an approximately 6-day shorter hospital stay.
6.5. Summary and Risk Stratification
The incidence and spectrum of perioperative complications vary according to etiology, deformity severity, and comorbidity (Table 3). NMS carries the highest overall risk, reflecting underlying respiratory compromise, poor nutrition, and prolonged, high-volume fusions. A structured institutional pathway that integrates standardized antifibrinolysis, goal-directed hemostasis, infection prevention, and early respiratory support is essential to reduce morbidity.

Table 3.
Major perioperative complications in pediatric scoliosis surgery.
7. Postoperative Care and Complication Management
The postoperative phase following pediatric scoliosis surgery is critical and requires close monitoring and multidisciplinary coordination to prevent complications and promote recovery. Effective pain control, respiratory support, hemodynamic stability, and early mobilization are cornerstones of postoperative management.
7.1. Pain Management
Optimal pain control is best achieved using multimodal analgesia, including scheduled administration of acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs), supplemented with opioids as needed. Opioid-sparing strategies are particularly important in patients at high risk of respiratory depression or prolonged ventilator weaning. Adjuvants such as gabapentinoids or dexmedetomidine infusions may reduce opioid consumption.
Historically, patient-controlled analgesia with opioids has been the mainstay of postoperative pain management after correction of adolescent idiopathic scoliosis []. However, concerns regarding adverse effects, current clinical practice increasingly favors a multimodal analgesic approach that allows early opioid tapering []. Epidural catheters delivering continuous local anesthetic infusions can substantially improve postoperative pain control in children undergoing scoliosis surgery [].
Regional anesthesia techniques, such as bilateral two-level erector spinae plane blocks (ESPBs) at T4 and T10 using 0.2% ropivacaine under ultrasound guidance, may also be considered []. This approach does not interfere with intraoperative neuromonitoring and has been shown to reduce intraoperative propofol and remifentanil requirements, as well as the need for postoperative systemic analgesics [].
Emerging evidence indicates that surgical-site infiltration with liposomal bupivacaine can extend early analgesia and reduce opioid requirements after posterior spinal fusion in adolescents []. While evidence in pediatric populations remains limited, its inclusion in multimodal regimens is increasingly explored within ERAS-based protocols [].
Pain management should therefore combine scheduled NSAIDs with supplemental opioids, local or regional anesthesia, and adjuvant agents, tailored to the child’s clinical condition and risk profile.
7.2. Respiratory and Hemodynamic Support
Respiratory support is essential, particularly in patients with restrictive lung disease or impaired cough due to neuromuscular weakness. NIV, chest physiotherapy (including assisted cough), and early mobilization should be implemented proactively. Oxygen supplementation and blood gas monitoring can guide respiratory support during the immediate postoperative period.
Extubation should be carefully planned once the child demonstrates adequate spontaneous ventilation, an effective cough, and stable gas exchange (pH ≥ 7.30 with PaCO2 close to the child’s pre-operative/baseline value and SpO2 ≥ 92–94% on FiO2 ≤ 0.4). In neuromuscular scoliosis or in patients with pre-existing nocturnal hypoventilation, planned post-extubation NIV (e.g., BiPAP) reduces respiratory failure and re-intubation rates [].
Hemodynamic monitoring should continue in the ICU, particularly for patients with substantial intraoperative blood loss, fluid shifts, or underlying cardiac disease. Regular assessment of hemoglobin levels, coagulation profiles, and fluid balance is essential for goal-directed therapy.
7.3. Nutrition and Monitoring
Early initiation of enteral nutrition is recommended to support wound healing and recovery. Nutritional status should be closely monitored, particularly in patients with pre-existing malnutrition or feeding difficulties. In severe neuromuscular disease, gastrostomy-assisted enteral feeding may enhance caloric intake and postoperative rehabilitation outcomes [].
7.4. Complication Surveillance
Common postoperative complications include respiratory failure, wound infection, ileus, nausea, vomiting, and neurological deficits. A high index of suspicion and early intervention are crucial. The emergence of new motor or sensory deficits should prompt immediate neuroimaging and neurosurgical consultation. Immediate decompression of spinal canal stenosis and careful spinal cord deliberation can help restore neurological function in cases of progressive or incomplete deficits [,].
7.5. Enhanced Recovery and Family Involvement
ERAS pathways for pediatric spinal surgery aim to standardize goal-directed perioperative care and have been associated with fewer complications and shorter hospital stay [,] (Table 4). For medically fragile neuromuscular phenotypes, key adaptations include:

Table 4.
Overview of key ERAS principles in pediatric scoliosis surgery (adapted from [,,,]).
- A respiratory pathway with planned postoperative NIV, cough-assist devices, early airway clearance, and nocturnal gas-exchange surveillance (e.g., SMA, advanced DMD).
- A cardiac-first plan in DMD/Becker Muscular Dystrophy (BMD), with continuous ECG monitoring or telemetry and cautious fluid targets.
- Agent selection aligned with disease biology and IONM requirements—notably the absolute avoidance of succinylcholine and volatile anesthetics in DMD/BMD, with TIVA as standard.
- Strict latex-free processes for myelomeningocele and other high-risk groups.
- Individualized VTE prophylaxis, prioritizing mechanical measures and aligning low-molecular-weight heparin use with neuraxial management decisions.
Structured family education using a teach-back approach—covering respiratory equipment use, analgesic plan, wound and urinary tract infection red flags, and escalation pathways—enhances adherence, safety, and confidence at discharge [,].
Integrating these measures within institutional ERAS pathways fosters a consistent, multidisciplinary approach to postoperative care and may reduce complication rates in this complex population [,,,].
8. Conclusions
The anesthetic treatment of scoliosis presents numerous challenges, primarily due to the condition’s pathophysiology and complexity. General anesthesia for scoliosis correction in children should be provided exclusively by experienced pediatric anesthesiologists in specialized centers equipped for intensive postoperative care and continuous hemodynamic and respiratory monitoring.
Preoperative management requires a meticulous multidisciplinary approach to evaluate the patient’s overall condition and optimize perioperative safety. Spinal deformity correction represents one of the most complex orthopedic procedures, demanding precise anesthetic planning because of its high-risk nature and technical demands. Key intraoperative priorities include selecting anesthetic techniques compatible with intraoperative neurophysiological monitoring, ensuring optimal patient positioning, maintaining protective ventilation strategies, effective blood-loss control, and comprehensive postoperative intensive care planning (Table 5).

Table 5.
Perioperative management in children undergoing scoliosis surgery (NMS-adapted).
According to current neuroanesthesia guidelines, TIVA delivered via TCI with continuous depth-of-anesthesia monitoring remains the technique of choice for optimizing transcranial motor evoked potentials during spine surgery. Blood-loss management requires vigilant monitoring, timely transfusion decision-making, and targeted hemostatic intervention to maintain hemodynamic stability and prevent coagulopathy.
The implementation of ERAS protocols—which integrate well-defined preoperative, intraoperative, and postoperative elements—has significantly improved perioperative outcomes in pediatric scoliosis surgery (Table 6). Such structured pathways shorten hospitalization, reduce complication rates by up to 63%, attenuate postoperative pain, and may contribute to lower healthcare costs [,].

Table 6.
ERAS in scoliosis surgery: core elements and NMS-specific adaptations.
Anesthesia for children with neuromuscular diseases, who frequently present with scoliosis, requires expertise and familiarity with the unique perioperative challenges of this vulnerable patient population [].
Author Contributions
Conceptualization, B.N.; methodology, B.N., S.U. and R.R.; investigation, B.N. and B.L.; resources, B.N., B.L. and Š.P.; writing—original draft preparation, B.N. and V.D.; writing—review and editing, B.N.; supervision, V.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable. No new data were created or analyzed in this study.
Acknowledgments
The authors express their sincere gratitude to their colleagues from the Department of Pediatric Anesthesiology and Intensive Medicine, Faculty of Medicine, Comenius University and National Institute of Children’s Diseases, Bratislava, Slovakia, and from the Department of Orthopedics, Faculty of Medicine, Comenius University and National Institute of Children’s Diseases, Bratislava, Slovakia, for their valuable insights and clinical experience that contributed to this review.
Conflicts of Interest
The authors declare no conflicts of Interest.
Abbreviations
| AIS | Adolescent Idiopathic Scoliosis |
| BMD | Becker Muscular Dystrophy |
| CP | Cerebral Palsy |
| CT | Computed Tomography |
| CVC | Central Venous Catheter |
| DMD | Duchenne Muscular Dystrophy |
| EBV | Estimated Blood Volume |
| ECG | Electrocardiography |
| ERAS | Enhanced Recovery After Surgery |
| ESPB | Erector Spinae Plane Block |
| FVC | Forced Vital Capacity |
| GMFCS | Gross Motor Function Classification System |
| IONM | Intraoperative Neurophysiological Monitoring |
| IV (PIV) | Peripheral Intravenous (catheter) |
| LMWH | Low-Molecular-Weight Heparin |
| MAP | Mean Arterial Pressure |
| MEP | Motor Evoked Potential |
| MMC | Myelomeningocele (Spina Bifida) |
| NDNMB | Non-Depolarizing Neuromuscular Blocker |
| NIV | Non-Invasive Ventilation |
| NMB | Neuromuscular Blocker |
| NMS | Neuromuscular Scoliosis |
| NSAID | Non-Steroidal Anti-Inflammatory Drug |
| OR | Operating Room |
| PBW | Predicted Body Weight |
| PEEP | Positive End-Expiratory Pressure |
| PICU | Pediatric Intensive Care Unit |
| SMA | Spinal Muscular Atrophy |
| SSEP | Somatosensory Evoked Potential |
| TCI | Target-Controlled Infusion |
| TIVA | Total Intravenous Anesthesia |
| TOF | Train-of-Four |
| TXA | Tranexamic Acid |
| UTI | Urinary Tract Infection |
| VTE | Venous Thromboembolism |
| VT | Tidal Volume |
References
- Wang, L.; Du, Y.; Huang, N.; Yin, N.; Du, J.; Yang, J.; Jiang, L.; Mao, Y. Clinical characteristics and anaesthetic management of severe scoliosis patients with spinal muscular atrophy: Case series. Ann. Med. Surg. 2024, 86, 643–649. [Google Scholar] [CrossRef]
- Murphy, R.F.; Mooney, J.F. Current concepts in neuromuscular scoliosis. Curr. Rev. Musculoskelet. Med. 2019, 12, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Cloake, T.; Gardner, A. The management of scoliosis in children with cerebral palsy: A review. J. Spine Surg. 2016, 2, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Dowling, J.J.; de Gonorazky, H.D.; Cohn, R.D.; Campbell, C. Treating pediatric neuromuscular disorders: The future is now. Am. J. Med. Genet. A 2018, 176, 804–841. [Google Scholar] [CrossRef] [PubMed]
- Rummey, C.; Flynn, J.M.; Corben, L.A.; Delatycki, M.B.; Wilmot, G.; Subramony, S.H.; Bushara, K.; Duquette, A.; Gomez, C.M.; Hoyle, J.C.; et al. Scoliosis in Friedreich’s ataxia: Longitudinal characterization in a large heterogeneous cohort. Ann. Clin. Transl. Neurol. 2021, 8, 1239–1250. [Google Scholar] [CrossRef]
- Hägglund, G.; Pettersson, K.; Czuba, T.; Persson-Bunke, M.; Rodby-Bousquet, E. Incidence of scoliosis in cerebral palsy: A population-based study of 962 young individuals. Acta Orthop. 2018, 89, 443–447. [Google Scholar] [CrossRef]
- Pettersson, K.; Wagner, P.; Rodby-Bousquet, E. Development of a risk score for scoliosis in children with cerebral palsy. Acta Orthop. 2020, 91, 203–208. [Google Scholar] [CrossRef]
- Ciftci, S.; Ulusaloglu, A.C.; Shrader, M.W.; Scavina, M.T.; Mackenzie, W.G.; Heinle, R.; Neal, K.M.; Stall, A.; Howard, J.J. Scoliosis development in spinal muscular atrophy: The influences of genetic severity, functional level, and disease-modifying treatments. J. Pediatr. Orthop. 2024; ahead of print. [Google Scholar] [CrossRef]
- Ruythooren, F.; Moens, P. Spinal muscular atrophy scoliosis in the era of background therapies—A review of the literature. J. Clin. Med. 2024, 13, 3467. [Google Scholar] [CrossRef]
- Archer, J.E.; Gardner, A.; Roper, H.P.; Chikermane, A.A.; Tatman, A.J. Duchenne muscular dystrophy: The management of scoliosis. J. Spine Surg. 2016, 2, 185–194. [Google Scholar] [CrossRef]
- Scoliosis Research Society (SRS). Informational & Position Statements (Quality & Safety Resource Library). Scoliosis Research Society. Available online: https://www.srs.org/Education/Quality-and-Safety/Informational--Position-Statements (accessed on 15 September 2025).
- Fletcher, N.D.; Ghag, R.; Hedequist, D.J.; Imrie, M.N.; Bennett, J.T.; Glotzbecker, M.P.; POSNA QSVI Spine Committee. Perioperative blood pressure management for patients undergoing spinal fusion for pediatric spinal deformity: Current concept review. J. Pediatr. Orthop. Soc. N. Am. 2023, 5, 602. [Google Scholar] [CrossRef]
- Welborn, M.C.; Redding, G.; Evers, P.; Nicol, L.; Bauer, D.F.; Iyer, R.R.; Poon, S.; Hwang, S. Pre-op considerations in neuromuscular scoliosis deformity surgery: Proceedings of the half-day course at the 58th annual meeting of the Scoliosis Research Society. Spine Deform. 2024, 12, 867–876. [Google Scholar] [CrossRef]
- Vitale, M.; Roye, B.; Bloom, Z.; Kunes, J.; Matsumoto, H.; Roye, D.; Farrington, D.; Flynn, J.; Halanski, M.; Hasler, C.; et al. Best practices for the orthopaedic care of children with spinal muscular atrophy: A consensus statement from the European Neuromuscular Centre Standard of Care Orthopaedic Working Group. J. Pediatr. Orthop. Soc. N. Am. 2022, 4, 296. [Google Scholar] [CrossRef]
- Hudec, J.; Prokopová, T.; Kosinová, M.; Gál, R. Anesthesia and perioperative management for surgical correction of neuromuscular scoliosis in children: A narrative review. J. Clin. Med. 2023, 12, 3651. [Google Scholar] [CrossRef] [PubMed]
- Sedra, F.; Shafafy, R.; Sadek, A.-R.; Aftab, S.; Montgomery, A.; Nadarajah, R. Perioperative Optimization of Patients with Neuromuscular Disorders Undergoing Scoliosis Corrective Surgery: A Multidisciplinary Team Approach. Glob. Spine J. 2021, 11, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Khirani, S.; Bersanini, C.; Aubertin, G.; Bachy, M.; Vialle, R.; Fauroux, B. Non-Invasive positive pressure ventilation to facilitate the post-operative respiratory outcome of spine surgery in neuromuscular children. Eur. Spine J. 2014, 23 (Suppl. S4), S406–S411. [Google Scholar] [CrossRef] [PubMed]
- Chatwin, M.; Wakeman, R.H. Mechanical Insufflation–Exsufflation: Considerations for Improving Clinical Practice. J. Clin. Med. 2023, 12, 2626. [Google Scholar] [CrossRef]
- Willis, L.D. 2022 Year in Review: Mechanical Insufflation–Exsufflation. Respir. Care 2023, 68, 275–283. [Google Scholar] [CrossRef]
- Buddhe, S.; Cripe, L.; Friedland-Little, J.; Kertesz, N.; Eghtesady, P.; Finder, J.; Hor, K.; Judge, D.P.; Kinnett, K.; McNally, E.M.; et al. Cardiac Management of the Patient with Duchenne Muscular Dystrophy. Pediatrics 2018, 142 (Suppl. S2), S72–S81. [Google Scholar] [CrossRef]
- OrphanAnesthesia. Duchenne Muscular Dystrophy—Anaesthesia Recommendations. Available online: https://www.orphananesthesia.eu (accessed on 15 September 2024).
- Naume, M.M.; Hoei-Hansen, C.E.; Born, A.P.; Brekke, G.; Høj, A.; Nielsen, M.R.; Borgwardt, L.; Vissing, J.; Dirks, J.; Rye, A.K.S.; et al. A Prospective Study on the Feasibility and Effect of an Optimized Perioperative Care Protocol in Pediatric Neuromuscular Scoliosis Surgery. J. Clin. Med. 2024, 13, 7848. [Google Scholar] [CrossRef]
- Mustafa, M.S.; Shafique, M.A.; Zaidi, S.D.E.Z.; Qamber, A.; Rangwala, B.S.; Ahmed, A.; Zaidi, S.M.F.; Rangwala, H.S.; Uddin, M.M.N.; Ali, M.; et al. Preoperative anxiety management in pediatric patients: A systematic review and meta-analysis of randomized controlled trials on the efficacy of distraction techniques. Front. Pediatr. 2024, 12, 1353508. [Google Scholar] [CrossRef]
- Johnson, D.J.; Johnson, C.C.; Goobie, S.M.; Nami, N.; Wetzler, J.A.; Sponseller, P.D.; Frank, S.M. High-dose Versus Low-dose Tranexamic Acid to Reduce Transfusion Requirements in Pediatric Scoliosis Surgery. J. Pediatr. Orthop. 2017, 37, e552–e557. [Google Scholar] [CrossRef]
- Shrestha, I.K.; Ruan, T.-Y.; Lin, L.; Tan, M.; Na, X.-Q.; Qu, Q.-C.; Chen, J.-C.; Si, Y.-Y.; Tao, J.-P. The efficacy and safety of high-dose tranexamic acid in adolescent idiopathic scoliosis: A meta-analysis. J. Orthop. Surg. Res. 2021, 16, 53. [Google Scholar] [CrossRef]
- Oetgen, M.E.; Litrenta, J. Perioperative blood management in pediatric spine surgery. J. Am. Acad. Orthop. Surg. 2017, 25, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Ahlers, C.G.; Lan, M.; Schoenecker, J.G.; Borst, A.J. Blood Loss and Transfusion in a Pediatric Scoliosis Surgery Cohort in the Antifibrinolytic Era. J. Pediatr. Hematol. Oncol. 2022, 44, e701–e706. [Google Scholar] [CrossRef] [PubMed]
- ICM-VTE Pediatric Delegates. Recommendations from the ICM-VTE: Pediatric. J. Bone Joint Surg. Am. 2022, 104 (Suppl. S1), 238–251. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Zhang, W.; Peng, Y.; Liang, A.; Du, K.; Huang, D. Use of computed tomographic reconstruction to establish the ideal entry point for pedicle screws in idiopathic scoliosis. Eur. Spine J. 2012, 21, 23–30. [Google Scholar] [CrossRef]
- Linden, G.S.; Ghessese, S.; Cook, D.; Hedequist, D.J. Pedicle Screw Placement in Adolescent Idiopathic Scoliosis: A Comparison between Robotics Coupled with Navigation versus the Freehand Technique. Sensors 2022, 22, 5204. [Google Scholar] [CrossRef]
- Baky, F.J.; Milbrandt, T.; Echternacht, S.; Stans, A.A.; Shaughnessy, W.J.; Larson, A.N. Intraoperative Computed Tomography-Guided Navigation for Pediatric Spine Patients Reduced Return to Operating Room for Screw Malposition Compared with Freehand/Fluoroscopic Techniques. Spine Deform. 2019, 7, 577–581. [Google Scholar] [CrossRef]
- LaValva, S.M.; Pahys, J.M.; Garg, S.; Bumpass, D.B.; Sucato, D.J.; Kelly, M.P.; Lenke, L.G.; Gupta, M.C.; Sponseller, P.D.; Boachie-Adjei, O.; et al. Preoperative Halo-Gravity Traction for Severe Pediatric Spinal Deformity: Can It Replace a Vertebral Column Resection? J. Pediatr. Orthop. Soc. N. Am. 2023, 5, e496. [Google Scholar] [CrossRef]
- Nemani, V.M.; Kim, H.J.; Bjerke-Kroll, B.T.; Yagi, M.; Sacramento-Dominguez, C.; Akoto, H.; Papadopoulos, E.C.; Sanchez-Perez-Grueso, F.; Pellise, F.; Nguyen, J.T.; et al. Preoperative halo-gravity traction for severe spinal deformities at an SRS-GOP site in West Africa: Protocols, complications, and results. Spine 2015, 40, 153–161. [Google Scholar] [CrossRef]
- Garabekyan, T.; Hosseinzadeh, P.; Iwinski, H.J.; Muchow, R.D.; Talwalkar, V.R.; Walker, J.; Milbrandt, T.A. The results of preoperative halo-gravity traction in children with severe spinal deformity. J. Pediatr. Orthop. B 2014, 23, 1–5. [Google Scholar] [CrossRef]
- Popescu, M.B.; Ulici, A.; Carp, M.; Haram, O.; Ionescu, N.S. The use and complications of halo-gravity traction in children with scoliosis. Children 2022, 9, 1701. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, Y.; Qi, L.; Wu, S.; Li, J.; Wang, Y.; Jiang, B. Does preoperative halo-gravity traction reduce the degree of deformity and improve pulmonary function in severe scoliosis patients with pulmonary insufficiency? A systematic review and meta-analysis. Front. Med. 2021, 8, 767238. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hai, Y.; Han, B.; Zhou, L.; Zhang, Y. Preoperative halo-gravity traction combined with one-stage posterior spinal fusion surgery following for severe rigid scoliosis with pulmonary dysfunction: A cohort study. BMC Surg. 2024, 24, 286. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, A.L.; Ramo, B.S.; Johnston, C.E. Halo gravity traction for severe pediatric spinal deformity: A clinical concepts review. Spine Deform. 2019, 7, 395–403. [Google Scholar] [CrossRef]
- Antolovich, G.C.; Cooper, M.S.; Johnson, M.B.; Lundine, K.; Yang, Y.; Frayman, K.; Vandeleur, M.; Sutherland, I.; Peachey, D.; Gadish, T.; et al. Perioperative Care of Children with Severe Neurological Impairment and Neuromuscular Scoliosis—A Practical Pathway to Optimize Perioperative Health and Guide Decision Making. J. Clin. Med. 2022, 11, 6769. [Google Scholar] [CrossRef]
- Children’s Mercy Kansas City. Neuromuscular Spinal Fusion Enhanced Recovery After Surgery (ERAS) Pathway: Synopsis. 2025. Available online: https://www.childrensmercy.org/siteassets/media-documents-for-depts-section/documents-for-health-care-providers/block-clinical-practice-guidelines/mobileview/neuromuscular-spinal-fusion-synopsis.pdf (accessed on 16 September 2025).
- Elmeshneb, M.A.; Hassanin, M.A.; Elnady, B.; Sleem, A.; Le, G.T.; Patel, M.S.; Quraishi, N.A. Surgical complications in neuromuscular scoliosis surgery: Systematic review and meta-analysis of the last ten years. Eur. Spine J. 2024, 33, 2666–2676. [Google Scholar] [CrossRef]
- Kwee, M.M.; Ho, Y.H.; Rozen, W.M. The prone position during surgery and its complications: A systematic review and evidence-based guidelines. Int. Surg. 2015, 100, 292–303. [Google Scholar] [CrossRef]
- Afrasinei, M.; Greaney, D. Face monitoring during prone position. Paediatr. Anaesth. 2022, 32, 486–487. [Google Scholar] [CrossRef]
- Shen, Y.; Drum, M.; Roth, S. The prevalence of perioperative visual loss in the United States: A 10-year study from 1996 to 2005 of spinal, orthopedic, cardiac, and general surgery. Anesth. Analg. 2009, 109, 1534–1545. [Google Scholar] [CrossRef]
- Nickels, T.J.; Manlapaz, M.R.; Farag, E. Perioperative visual loss after spine surgery. World J. Orthop. 2014, 5, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.A.; Roth, S.; Posner, K.L.; Cheney, F.W.; Caplan, R.A.; Newman, N.J.; Domino, K.B. The American Society of Anesthesiologists Postoperative Visual Loss Registry: Analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology 2006, 105, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, L.; Ghosh, S.; Dillon, D.; Palumbo, L.; Woodland, P.; Thalayasingam, P.; Lethbridge, M. Intraoperative neurophysiology monitoring in scoliosis surgery in children. Clin. Neurophysiol. Pract. 2019, 4, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Tsirikos, A.I.; Duckworth, A.D.; Henderson, L.E.; Michaelson, C. Multimodal intraoperative spinal cord monitoring during spinal deformity surgery: Efficacy, diagnostic characteristics, and algorithm development. Med. Princ. Pract. 2020, 29, 6–17. [Google Scholar] [CrossRef]
- Zuccaro, M.; Zuccaro, J.; Samdani, A.F.; Pahys, J.M.; Hwang, S.W. Intraoperative neuromonitoring alerts in a pediatric deformity center. Neurosurg. Focus 2017, 43, E8. [Google Scholar] [CrossRef]
- Walker, C.T.; Kim, H.J.; Park, P.; Lenke, L.G.; Weller, M.A.; Smith, J.S.; Nemergut, E.C.; Sciubba, D.M.; Wang, M.Y.; Shaffrey, C.; et al. Neuroanesthesia guidelines for optimizing transcranial motor evoked potential neuromonitoring during deformity and complex spinal surgery: A Delphi consensus study. Spine 2020, 45, 911–920. [Google Scholar] [CrossRef]
- Alkhatip, A.A.A.M.; Mills, K.E.; Hogue, O.; Sallam, A.; Hamza, M.K.; Farag, E.; Yassin, H.M.; Wagih, M.; Ahmed, A.M.I.; Helmy, M.H.; et al. The effects of dexmedetomidine on intraoperative neurophysiologic monitoring modalities during corrective scoliosis surgery in pediatric patients: A systematic review. Paediatr. Anaesth. 2024, 34, 112–120. [Google Scholar] [CrossRef]
- Nakahari, H.; Wilton, N.C.T.; Kojima, T. Anesthesia management of neonates and infants requiring intraoperative neurophysiological monitoring: A concise review. Paediatr. Anaesth. 2023, 33, 526–531. [Google Scholar] [CrossRef]
- Okamura, M.; Saito, W.; Miyagi, M.; Shirasawa, E.; Imura, T.; Nakazawa, T.; Mimura, Y.; Yokozeki, Y.; Kuroda, A.; Kawakubo, A.; et al. Incidence of unintentional intraoperative hypothermia in pediatric scoliosis surgery and associated preoperative risk factors. Spine Surg. Relat. Res. 2021, 5, 154–159. [Google Scholar] [CrossRef]
- Lai, L.L.; See, M.H.; Rampal, S.; Ng, K.S.; Chan, L. Significant factors influencing inadvertent hypothermia in pediatric anesthesia. J. Clin. Monit. Comput. 2019, 33, 1105–1112. [Google Scholar] [CrossRef]
- Vrbica, K.; Hudec, J.; Hrdy, O.; Galko, M.; Horalkova, H.; Demlova, R.; Kubelova, M.; Repko, M.; Gal, R. Effect of prophylactic fibrinogen concentrate in scoliosis surgery (EFISS): A study protocol of two-arm, randomised trial. BMJ Open 2023, 13, e071547. [Google Scholar] [CrossRef]
- Carson, J.L.; Stanworth, S.J.; Guyatt, G.; Valentine, S.; Dennis, J.; Bakhtary, S.; Cohn, C.S.; Dubon, A.; Grossman, B.J.; Gupta, G.K.; et al. Red blood cell transfusion: 2023 AABB international guidelines. JAMA 2023, 330, 1892–1902. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.S.; Yunus, S.N.; Ng, C.C.; Chan, C.Y.W.; Chiu, C.K.; Kwan, M.K. Tranexamic acid in pediatric scoliosis surgery: A prospective randomized trial comparing high-dose and low-dose tranexamic acid in adolescent idiopathic scoliosis undergoing posterior spinal fusion surgery. Spine 2021, 46, E1170–E1177. [Google Scholar] [CrossRef] [PubMed]
- Dhawale, A.A.; Shah, S.A.; Sponseller, P.D.; Bastrom, T.; Neiss, G.; Yorgova, P.; Newton, P.O.; Yaszay, B.; Abel, M.F.; Shufflebarger, H.; et al. Are antifibrinolytics helpful in decreasing blood loss and transfusions during spinal fusion surgery in children with cerebral palsy scoliosis? Spine (Phila Pa 1976) 2012, 37, E549–E555. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.-H.; Lin, S.-Y.; Wu, M.-H.; Tien, Y.-C.; Jong, Y.-J.; Liang, W.-C.; Lu, Y.-M.; Shih, C.-L.; Lu, C.-C. Intravenous tranexamic acid reduces blood loss and transfusion volume in scoliosis surgery for spinal muscular atrophy: Results of a 20-year retrospective analysis. Int. J. Environ. Res. Public Health 2021, 18, 9959. [Google Scholar] [CrossRef]
- Murphy, R.F.; Mooney, J.F., III. Complications following spine fusion for adolescent idiopathic scoliosis. Curr. Rev. Musculoskelet. Med. 2016, 9, 462–469. [Google Scholar] [CrossRef]
- Rudic, T.N.; Althoff, A.D.; Kamalapathy, P.; Bachmann, K.R. Surgical site infection after primary spinal fusion surgery for adolescent idiopathic scoliosis: An analysis of risk factors from a nationwide insurance database. Spine (Phila Pa 1976) 2023, 48, E101–E106. [Google Scholar] [CrossRef]
- Novy, J.; Carruzzo, A.; Maeder, P.; Bogousslavsky, J. Spinal cord ischemia: Clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch. Neurol. 2006, 63, 1113–1120. [Google Scholar] [CrossRef]
- Rokitansky, C. Handbuch der Pathologischen Anatomie; Braumüller & Seidel: Wien, Austria, 1842; Volume 3. [Google Scholar]
- Bernotavičius, G.; Saniukas, K.; Karmonaitė, I.; Zagorskis, R. Superior mesenteric artery syndrome. Acta Med. Litu. 2016, 23, 155–164. [Google Scholar] [CrossRef]
- Altiok, H.; Lubicky, J.P.; DeWald, C.J.; Herman, J.E. The superior mesenteric artery syndrome in patients with spinal deformity. Spine (Phila Pa 1976) 2005, 30, 2164–2170. [Google Scholar] [CrossRef]
- Shapiro, F.; Zurakowski, D.; Sethna, N.F. Tranexamic acid diminishes intraoperative blood loss and transfusion in spinal fusions for Duchenne muscular dystrophy scoliosis. Spine (Phila Pa 1976) 2007, 32, 2278–2283. [Google Scholar] [CrossRef] [PubMed]
- Al-Iede, M.M.; Al-Zayadneh, E.; Bridge, C.; Alqutawneh, B.; Waters, K. Risk factors for postoperative pulmonary complications in children with severely compromised pulmonary function secondary to severe scoliosis. Pediatr. Pulmonol. 2020, 55, 2782–2790. [Google Scholar] [CrossRef] [PubMed]
- Al-Mohrej, O.A.; Aldakhil, S.S.; Al-Rabiah, M.A.; Al-Rabiah, A.M. Surgical treatment of adolescent idiopathic scoliosis: Complications. Ann. Med. Surg. 2020, 52, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Reames, D.L.; Smith, J.S.; Fu, K.M.G.; Polly, D.W., Jr.; Ames, C.P.; Berven, S.H.; Perra, J.H.; Glassman, S.D.; McCarthy, R.E.; Knapp, R.D., Jr.; et al. Complications in the surgical treatment of 19,360 cases of pediatric scoliosis: A review of the Scoliosis Research Society Morbidity and Mortality database. Spine 2011, 36, 1484–1491. [Google Scholar] [CrossRef]
- Sharma, S.; Wu, C.; Andersen, T.; Wang, Y.; Hansen, E.S.; Bünger, C.E. Prevalence of complications in neuromuscular scoliosis surgery: A literature meta-analysis from the past 15 years. Eur. Spine J. 2013, 22, 1230–1249. [Google Scholar] [CrossRef]
- Yin, S.; Tao, H.; Du, H.; Feng, C.; Yang, Y.; Yang, W.; Duan, C. Postoperative pulmonary complications following posterior spinal instrumentation and fusion for congenital scoliosis. PLoS ONE 2018, 13, e0207657. [Google Scholar] [CrossRef]
- Gabel, B.C.; Schnell, E.C.; Dettori, J.R.; Jeyamohan, S.; Oskouian, R. Pulmonary complications following thoracic spinal surgery: A systematic review. Glob. Spine J. 2016, 6, 296–303. [Google Scholar] [CrossRef]
- Ma, L.; Yu, X.; Zhang, J.; Shen, J.; Zhao, Y.; Li, S.; Huang, Y. Risk factors of postoperative pulmonary complications after primary posterior fusion and hemivertebra resection in congenital scoliosis patients younger than 10 years old: A retrospective study. BMC Musculoskelet. Disord. 2022, 23, 89. [Google Scholar] [CrossRef]
- Monagle, P.; Cuello, C.A.; Augustine, C.; Bonduel, M.; Brandão, L.R.; Capman, T.; Chan, A.K.C.; Hanson, S.; Male, C.; Meerpohl, J.; et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Treatment of pediatric venous thromboembolism. Blood Adv. 2018, 2, 3292–3316. [Google Scholar] [CrossRef]
- Mulpuri, N.; Sanborn, R.M.; Pradhan, P.; Miller, P.E.; Canizares, M.F.; Shore, B.J. Pediatric orthopaedic venous thromboembo-lism: A systematic review investigating incidence, risk factors, and outcome. JBJS Open Access 2024, 9, e23.00107. [Google Scholar] [CrossRef]
- Seki, H.; Ideno, S.; Ishihara, T.; Watanabe, K.; Matsumoto, M.; Morisaki, H. Postoperative pain management in patients undergoing posterior spinal fusion for adolescent idiopathic scoliosis: A narrative review. Scoliosis Spinal Disord. 2018, 13, 17. [Google Scholar] [CrossRef]
- Chin, K.J.; Dinsmore, M.J.; Lewis, S.; Chan, V. Opioid-sparing multimodal analgesia with bilateral bi-level erector spinae plane blocks in scoliosis surgery: A case report of two patients. Eur. Spine J. 2020, 29, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hong, R.A.; Robbins, C.B.; Gibbons, K.M.; Holman, A.E.; Caird, M.S.; Farley, F.A.; Abbott, M.D.; Burke, M.C. Intrathecal morphine and oral analgesics provide safe and effective pain control after posterior spinal fusion for adolescent idiopathic scoliosis. Spine 2018, 43, E98–E104. [Google Scholar] [CrossRef] [PubMed]
- Domagalska, M.; Ciftsi, B.; Janusz, P.; Reysner, T.; Daroszewski, P.; Kowalski, G.; Wieczorowska-Tobis, K.; Kotwicki, T. Effectiveness of the bilateral and bilevel erector spinae plane block (ESPB) in pediatric idiopathic scoliosis surgery: A randomized, double-blinded, controlled trial. J. Pediatr. Orthop. 2024, 44, e634–e640. [Google Scholar] [CrossRef] [PubMed]
- Akesen, S.; Güler, S.B.; Akesen, B. Bilateral bi-level erector spinae plane blocks in scoliosis surgery: A retrospective comparative study. Acta Orthop. Traumatol. Turc. 2022, 56, 327–332. [Google Scholar] [CrossRef]
- Changoor, S.; Giakas, A.; Sacks, K.; Asma, A.; Lang, R.S.; Yorgova, P.; Rogers, K.; Gabos, P.G.; Shah, S.A. The role of liposomal bupivacaine in multimodal pain management following posterior spinal fusion for adolescent idiopathic scoliosis: Faster and farther with less opioids. Spine (Phila Pa 1976) 2024, 49, E11–E16. [Google Scholar] [CrossRef]
- Hey, G.; Mehkri, Y.; Mehkri, I.; Boatright, S.; Duncan, A.; Patel, K.; Gendreau, J.; Chandra, V. Enhanced recovery after surgery pathways in pediatric spinal surgery: A systematic review and meta-analysis. World Neurosurg. 2024, 190, 329–338. [Google Scholar] [CrossRef]
- Mooney, J.F., III. Perioperative enteric nutritional supplementation in pediatric patients with neuromuscular scoliosis. J. S. Orthop. Assoc. 2000, 9, 202–206. [Google Scholar] [PubMed]
- Chrenko, R. Open-door laminoplasty in cervical myelopathy using titanium miniplate system: Initial clinical experience. Miniinvaz. Chir. Endosk. 2015, 1, 15–21. [Google Scholar]
- Chrenko, R. Unilateral laminotomy and bilateral decompression of degenerative lumbar stenosis—Clinical outcome of 169 operated patients. Miniinvaz. Chir. Endosk. 2015, 4, 13–20. [Google Scholar]
- Gadiya, A.D.; Koch, J.E.J.; Patel, M.S.; Shafafy, M.; Grevitt, M.P.; Quraishi, N.A. Enhanced recovery after surgery (ERAS) in adolescent idiopathic scoliosis (AIS): A meta-analysis and systematic review. Spine Deform. 2021, 9, 893–904. [Google Scholar] [CrossRef]
- Pennington, Z.; Cottrill, E.; Lubelski, D.; Ehresman, J.; Lehner, K.; Groves, M.L.; Sponseller, P.; Sciubba, D.M. Clinical utility of enhanced recovery after surgery pathways in pediatric spinal deformity surgery: Systematic review of the literature. J. Neurosurg. Pediatr. 2021, 27, 225–238. [Google Scholar] [CrossRef]
- Rafeeqi, T.; Pearson, E.G. Enhanced recovery after surgery in children. Transl Gastroenterol Hepatol. 2021, 6, 46. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vattipalli, S.; Rath, G.P.; Athiraman, U. Anesthesia for Children with Neuromuscular Diseases. In Fundamentals of Pediatric Neuroanesthesia; Rath, G.P., Ed.; Springer: Singapore, 2021; pp. 579–594. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).