Abstract
Atopic dermatitis (AD) is a chronic and recurrent inflammatory skin condition characterized by itching, eczematous plaques, and dry skin. Despite ongoing research, its exact cause remains elusive. In this study, we aimed to explore the factors that influence the severity of AD in children and assess the relationship between serum vitamin D levels and the disease’s severity. We enrolled 96 AD patients in our investigation, evaluated their clinical condition using the Scoring Atopic Dermatitis (SCORAD) index, and compared them to a group of 90 healthy controls. Our analysis revealed that serum vitamin D levels and eosinophil counts significantly impacted the SCORAD index (p < 0.001). According to standardized regression coefficients, for each incremental unit in serum vitamin D levels, the SCORAD index exhibited a decrease of 0.449 units. Similarly, a one-unit increase in eosinophil count resulted in a 0.009 unit increase in the SCORAD index. It is worth noting that the influence of serum vitamin D levels on disease severity surpasses that of eosinophil counts and atopic conditions. In our patient cohort, we uncovered a negative correlation (r = −0.419, p < 0.001) between serum vitamin D levels and the SCORAD index. Our findings suggest that low serum vitamin D levels may have a more substantial impact on AD severity than atopic conditions and eosinophilia. Furthermore, we observed a negative association between the severity of AD and serum 25(OH)D3 levels.
1. Introduction
Atopic dermatitis (AD) is a chronic and recurrent inflammatory skin disorder characterized by itching, dry skin, and eczematous plaques [1,2]. The pathogenesis of AD is not completely known but is associated with environmental, genetic, biochemical, and immunological factors [3,4]. The incidence is higher in metropolitan families and provinces with high socioeconomic standing, and the prevalence is roughly 15% to 30% in children and 2% to 10% in adults [5,6]. The disease manifests itself predominantly in early childhood; 45% of patients develop symptoms before six months, and 60% of patients before their first birthday [7].
The disorder severity can be assessed with the Scoring Atopic Dermatitis (SCORAD) index [8]. The SCORAD index consists of three criteria: (A) extent (percentage of skin surface affected according to the rule of nine); (B) intensity (incorporation of erythema, edema/papules, the outcome of itching, oozing/crust appearance, lichenification, and dryness, six parameters evaluated on a scale of 0–3; 0: absent, 1: mild, 2: moderate, 3: severe); and (C) subjective symptoms (for the last three days, the severity of pruritus, the impact of sleep, and the overall condition of the skin on daily life are questioned, and the responses are evaluated on a scale of 1 to 10) [9]. The formula A/5 + 7B/2 + C is applied to all the obtained data, and the SCORAD score is calculated. If the index score falls between 0 and 24.9, it is categorized as mild; between 25 and 50 is moderate; and above 50 is severe atopic dermatitis [9].
In addition to the well-described function in calcium homeostasis and immunity, vitamin D has miscellaneous skin functions, such as keratinocyte proliferation, skin barrier function, and apoptosis [10]. In addition, vitamin D may affect autoimmune diseases, infections, malignancies, cardiovascular diseases, neuropsychiatric disorders, and allergic disease courses, including AD [11,12]. It is believed that the anti-inflammatory effect of vitamin D plays a role in its therapeutic efficacy in modulating disease progression [12] In recent years, the significance of vitamin D’s anti-inflammatory effect has been highlighted multiple times [12,13]. In a study conducted by Karampinis et al. low serum vitamin D levels have been shown to lead to higher rates of exacerbations in patients with psoriasis [14]. Reinholz et al. emphasized that lower serum 25-hydroxyvitamin D3 (25(OH)D3) conditions are associated with higher IgE sensitivity, food allergy, and asthma [15]. Another study reported that the SCORAD index score has a negative correlation with vitamin D [16]. However, other studies have emphasized that they have not found a relationship between AD and vitamin D [17,18]. The effect of vitamin D levels on the course of AD is controversial; therefore, we aimed to evaluate the factors affecting the severity of AD in children and to evaluate the relationship between serum vitamin D levels and the severity of AD.
2. Materials and Methods
Ninety-six patients diagnosed with AD and 90 healthy controls without chronic systemic or allergic diseases were enrolled in our case–control study, which was conducted from September 2022 to November 2022.
We collected clinical evaluation, SCORAD index data, and laboratory analysis results, including complete blood count, food, and aero allergen serum-specific immunoglobulin E (IgE), serum total IgE level, and serum 25(OH)D3 concentration, from patient records retrospectively. The patients were diagnosed with AD according to the Hanifin and Rajka criteria by a pediatric allergist. Atopic dermatitis severity was assessed using the SCORAD index. Based on this index, AD was categorized into mild AD (0–24.9), moderate AD (25–50), and severe AD (>50) [9].
We excluded the patients who used systemic glucocorticoid therapy the previous month and used vitamin D supplements the prior six months from the study. Also, we excluded patients who received local steroid treatment from the study as the current treatment might affect the eczema harshness and the SCORAD index. In addition, we excluded patients receiving multi-vitamin supplements and drugs that have the potential to affect serum 25(OH)D3 levels (like antacids or anticonvulsants or systemic steroid therapy), patients suffering from a systemic disorder or with clinical signs consistent with rickets-like limb deformities, obese individuals, patients with upper respiratory tract illness, chronic lung disease (asthma or others), an impaired immune system, neurological or metabolic diseases. The participants’ serum vitamin D levels were evaluated in the autumn. Participants living in the same region had similar levels of sunlight exposure. Sunlight is an important source of vitamin D [19]. The dietary characteristics of the patients were not evaluated. Those with malnutrition were excluded from the study.
2.1. Skin Prick Tests
For forearm skin prick tests, we evaluated patients who had not received antihistamines for seven days, applying serum histamine (10 mg/mL) and 0.9% saline as positive and negative references, respectively. We gauged responses at the fifteenth minute. We defined the positive reaction as ≥3 mm above the negative control [20].
We performed the prick tests with the same antigens (egg yolk, egg white, milk, peanut, hazelnut, walnut, wheat, fish, soy, Dermatophagoides farinea, Dermatophagoides pteronyssinus, Alternaria alternata, dog and cat dander, cockroaches–mix, and grass–mix, grain–mix, tree–mix, and weed–mix pollens) (Lofarma S.p.A, Milan, Italy) by using Stallerpoint (Allertech, Doral, FL, USA).
2.2. Total Eosinophil Count, Specific IgE in Serum, Total IgE, and Atopy
We obtained peripheral blood eosinophil counts from hemogram parameters and measured total IgE from serum (by nephelometric method with a machine from Siemens Healthcare Diagnostics Products, Marburg, Germany) and assayed causal or suspected food (milk, egg white, egg yolk, peanut, hazelnut, walnut, wheat, fish, soy) and aero (Dermatophagoides farinea, Dermatophagoides pteronyssinus, Alternaria, alternata, Aspergillus, cockroaches mix, cat and dog dander, and grass-mix and tree-mix pollens) allergen-specific IgE levels with the Immuno-CAP system (UniCAP; Uppsala, Sweden) (≥0.35 kIU/L was considered to be positive). The patient’s atopy condition was described by positivity to at least one allergen by skin prick test (SPT; wheal ≥3 mm larger than negative control) or specific IgE testing (≥0.35 kIU/L) [20]. Individuals with no positive results were classified as non-atopic (non-sensitized).
2.3. 25-Hydroxyvitamin D3 Levels
We took peripheral venous blood samples at 6–10 h fasting and measured serum 25-(OH)D3 levels used the liquid chromatography-tandem mass spectrometry procedure (Waters Quattro Premier XE TM, Waters Corp, Milford, MA, USA). Although the most active form in the blood is 1–25(OH) vitamin D, its half-life is quite short, about 3–4 h [21]. In contrast, the half-life of 25-(OH)D3 is on average about 3 weeks, and this form accumulates mainly in adipose tissue [21]. Digested and synthesized by the skin, vitamin D is rapidly converted to 25-(OH)D3, but only a fraction of the 25-(OH)D3 in serum is converted to the active metabolite 1,25-(OH) vitamin D. Serum levels of 1,25-(OH) vitamin D are very low or there is no correlation with vitamin D stores [21,22]. The concentration of 25(OH) vitamin D3 in serum is higher than 1–25(OH) vitamin D3 [22]. For these reasons, we used the 25(OH)D3 form of vitamin D to assess serum levels in our study. Serum 25-hydroxyvitamin D3 levels ranging from 10.1 to 24 ng/mL were considered mild to moderate vitamin D deficiency, levels between 24.1 and 80 ng/mL were considered optimal, and levels above 80 ng/mL were considered toxic [23]. We evaluated serum 25-hydroxyvitamin D3 levels between September and November 2022.
2.4. Statistical Analysis
We conducted the data analysis employing IBM SPSS Statistics Standard Concurrent User V 26 and MedCalc® Statistical Software version 19.6, both widely recognized statistical package applications. Descriptive statistics are reported as the count of instances (n), proportions (%), mean ± standard deviation, and median (with interquartile range). To assess data normality, we employed the Shapiro–Wilk test and verified the homogeneity of variances using the Levene test. When dealing with non-normally distributed continuous variables, we resorted to the Mann–Whitney U test for between-group comparisons. For assessing relationships between deficiency indices and continuous variables, we computed the Spearman correlation coefficient. When examining the associations between categorical variables, we employed both the Pearson chi-square test and Fisher’s exact test. To delve deeper into our analysis, we employed multiple linear regression analysis. This allowed us to investigate the impact of serum vitamin D deficiency, eosinophilia, and atopy on the SCORAD index. We rigorously assessed the suitability of the linear regression model, taking into account various diagnostic checks. These checks included the Shapiro–Wilk test to evaluate residual normality, Durbin–Watson statistics to assess autocorrelation, variance inflation factor statistics, and the tolerance test to gauge linearity assumptions. We affirm that all necessary assumptions were met for the two regression models we constructed. A significance level of p < 0.05 was deemed statistically significant. Formun Üstü
2.5. Ethics
We obtained the study approval from the ethics committee of Kartal Dr. Lütfi Kırdar Training and Research Hospital (Date: 26 October 2022, Number: 2022/514/236/12) and conducted the study in accordance with the Helsinki criteria.
3. Results
We enrolled a total of 186 participants in our study, comprising 90 individuals in the control group and 96 patients, all of whom were assessed during the autumn season. There were no notable disparities in terms of gender (p = 0.999) and age (p = 0.855) distribution between the two groups. However, the patient group exhibited significantly higher eosinophil and total IgE levels, while their vitamin D levels were notably lower (p = 0.018 and p = 0.001, respectively) (Table 1).

Table 1.
Comparison of variables by group.
In 56 of the patients (equivalent to 58.3%), there was an evident deficiency in vitamin D levels. Despite the data presented in Table 2, which indicate that females had a slightly higher median SCORAD index compared to males, this disparity did not reach statistical significance (p = 0.072). However, a notable discrepancy was observed in the SCORAD index concerning vitamin D status (p < 0.001). Specifically, the group afflicted by severe vitamin D deficiency exhibited a markedly elevated SCORAD index in contrast to the mild to moderate and optimal groups (p < 0.001). In contrast, there was no statistically noteworthy contrast between the mild to moderate and optimal groups. Furthermore, it is worth noting that the SCORAD scores in the sensitive cohort were significantly elevated compared to those in the non-sensitive group (p = 0.002). Similarly, within the subset of individuals with food allergies, the SCORAD index demonstrated a substantial increase in comparison to the group void of food allergies (p = 0.002). Nonetheless, among individuals with specific food allergies (such as milk, egg white, and egg yolk), the SCORAD index exhibited a statistically significant elevation in comparison to those lacking such allergies (respectively, p = 0.009, p = 0.002, p = 0.037) (refer to Table 2 for details). Nevertheless, we did not uncover any noteworthy distinctions in the SCORAD index among those with allergies to other food items (such as peanuts, hazelnuts, walnuts) (p > 0.05) or aeroallergens (such as mites, pollen, cat, dog epithelium) (p = 0.422, p = 0.204, p = 0.512, respectively).

Table 2.
Comparison of disease severity with vitamin D levels and atopy in the patient group (n = 96).
We observed a noteworthy (p = 0.001) positive association between the overall eosinophil count and the SCORAD index (r = 0.336). However, the correlation coefficients between age, gender, total IgE, and the SCORAD index did not reach statistical significance (p = 0.064, p = 0.194, respectively) (Table 3).

Table 3.
Correlations between SCORAD index and age, gender, total eosinophil count, and log total IgE.
The effect of serum vitamin D levels, eosinophil count, and atopy on disease severity was evaluated by multiple linear regression analysis (Table 4). Gender and age were not considered in the analysis as they were confounding factors. We determined the final factors affecting disease severity by the stepwise method. In the regression analysis, atopy was excluded from the model, and as a result, serum vitamin D levels and eosinophil counts were found to be effective on the SCORAD index (p < 0.001). According to Table 4, as serum vitamin D level increases by one unit, the SCORAD index decreases by 0.449 units, and as eosinophil value increases by one unit, the SCORAD index increases by 0.009 units. According to standardized regression coefficients (absolute values), the effect of serum vitamin D levels on disease severity is greater than that of eosinophil counts.

Table 4.
Evaluation of factors influencing the SCORAD index using multiple linear regression analysis.
As shown in Table 5, AD severity was identified as mild in 33 (34.3%), moderate in 52 (54.2%), and severe in 11 (11.5%) cases. Furthermore, we observed a significant disparity in serum vitamin D levels among the groups with different disease severities (p < 0.001). Serum vitamin D levels were statistically lower in the severe AD group compared to the mild and moderate groups. However, the difference in serum vitamin D levels between the mild and moderate groups did not reach statistical significance (Table 5).

Table 5.
Comparison of vitamin D levels regarding disease severity.
In all patients, we found a statistically significant (p < 0.001) moderately negative correlation (r = −0.419) between serum vitamin D levels and the SCORAD index (Figure 1). Moreover, within the atopic group, a statistically significant (p = 0.001) moderate (r = −0.460) negative association was observed between vitamin D and the SCORAD index. Additionally, in patients without atopy, there was a statistically significant (p = 0.031) weak negative correlation (r = −0.305) between vitamin D and the SCORAD index.
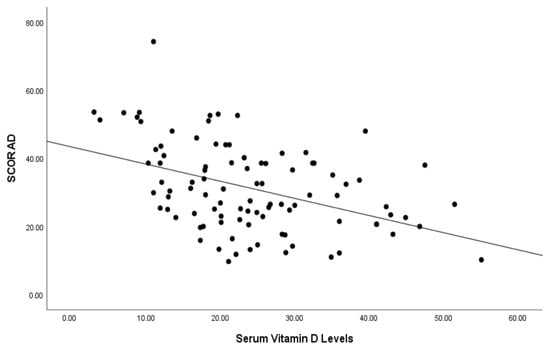
Figure 1.
Correlation of serum vitamin D levels (ng/mL) and SCORAD index of patients.
4. Discussion
Easily accessible and practical laboratory tests can serve as valuable tools for assessing the severity of AD [24]. In this context, we investigated the relationship between serum vitamin D levels and the severity of AD, as well as the impact of other factors. Our study’s findings underscore an inverse correlation between serum vitamin D levels and the severity of AD in children. This highlights the substantial influence of vitamin D deficiency on AD severity, surpassing the effects of atopy and eosinophilia. The well-established roles of vitamin D in immunomodulation and calcium homeostasis come into play here [10,25]. Vitamin D plays a pivotal role in modulating the production of cytokines, particularly those involved in the Th1/Th2 balance [26]. Th2 cytokines, such as interleukin-4 (IL-4), IL-5, and IL-13, are relevant to the pathogenesis of AD and may also have a regulatory impact on vitamin D production [26]. Also, vitamin D has antimicrobial properties, and its deficiency has an association with increased susceptibility to infections [25]. Skin colonization by Staphylococcus aureus has an association with the development and exacerbation of AD, and vitamin D may reduce bacterial colonization on the skin [25]. Recent studies have underlined vitamin D’s potential role in AD, and its supplementation can support AD treatment.
Most vitamin D is obtained through exposure of the skin to sunlight. Sunlight, especially ultraviolet B (UVB) radiation, has a significant immunomodulatory effect on the skin. Exposure to UVB radiation stimulates the production of vitamin D in the skin, which plays a critical role in immune system regulation [27]. It is observed that at higher geographic latitudes, where there is less sunlight exposure and reduced vitamin D production, there is a higher prevalence of AD [28]. Vitamin D can influence not only calcium regulation but also immunomodulation. Specifically, vitamin D can affect local calcium balance and regulate gene transcription by targeting vitamin D receptors that regulate immune system responses [29]. Additionally, vitamin D has been shown to inhibit the release of markers such as IL-6 and tumor necrosis factor-alpha (TNF-α), which promote inflammation and worsen AD symptoms [30]. Moreover, vitamin D is highlighted for its ability to enhance the production of antimicrobial peptides in the skin, strengthening the skin’s natural defense and thereby reducing the risk of infection or exacerbation [31]. Furthermore, it is believed that vitamin D may contribute to the maintenance of skin integrity by supporting the production of critical proteins like filaggrin and involucrin, which are essential for skin barrier function [10].
The relationship between vitamin D levels and the severity of AD remains a contentious topic in the existing literature. In our study, the mean serum 25(OH)D3 levels were notably lower in children with AD when compared to the control group. This observation aligns with a study by Wang et al., which involved 498 pediatric AD patients and 328 individuals in a control group; they also reported statistically lower average serum levels of 25(OH)D3 in the AD group [5]. Our findings are consistent with similar investigations that compared children with AD to healthy control groups, consistently finding lower mean serum 25(OH)D3 levels in the patient groups [32,33]. In contrast to our results, two separate studies among children did not reveal a statistical difference between the AD group and the control group in terms of serum 25(OH)D3 levels [2,34]. It is worth noting that in one of these studies, there were disparities in the evaluation seasons of the participants, especially considering that the average vitamin D values were significantly lower than those observed in our study [2]. However, a meta-analysis encompassing 11 studies demonstrated a significant difference between the patient and control groups [35]. This implies that the risk of vitamin D deficiency in pediatric AD might be considerably higher, consistent with the findings of a meta-analysis conducted by Kim and colleagues, which also indicated an elevated risk of vitamin D deficiency in pediatric AD [36].
Our findings suggest that individuals with severe AD exhibit lower levels of vitamin D compared to those with mild and moderate AD. Furthermore, we have identified a strong negative correlation between serum vitamin D levels and the severity of AD. These results are consistent with prior research, which consistently reported an inverse relationship between serum vitamin D levels and the severity of AD [5,16,34,37]. In a recent study conducted by Bulut et al., a negative correlation was observed between serum 25(OH)D3 levels and the severity of AD in 120 pediatric patients, which is in agreement with our findings [4]. Additionally, in line with the outcomes of our study, serum 25(OH)D3 levels were statistically lower in patients with severe AD compared to other groups [4]. Similar studies in the existing literature have reported a negative association between disease severity and serum 25(OH)D3 levels [2,5,16,34,37]. However, it is worth noting that some studies, in contrast to our findings, did not establish a correlation between disease severity and serum 25(OH)D3 levels [17,18,38]. Raj et al. also found a negative correlation between serum vitamin D levels and disease severity. They emphasized that vitamin D supplementation had the most beneficial impact in severe atopic dermatitis cases and the least in mild cases [34]. Considering these findings, assessing serum vitamin D levels could be advisable for severe AD patients or cases where disease severity does not improve as expected despite appropriate treatment. To gain further insights, large-scale, multicenter, prospective studies are necessary to clarify this relationship. Another aspect worthy of investigation is whether vitamin D deficiency contributes to increased skin lesions due to its detrimental effect on barrier function, or if the deficiency arises as a consequence of reduced vitamin production in diseased skin.
We observed a significant increase in the SCORAD index in the group without sensitivities compared to the non-sensitive group. Although the SCORAD index was notably higher in the food allergy group, we did not find a significant impact of inhaled allergen sensitivity on the SCORAD index. Similarly, Lee et al. reported elevated SCORAD indices in patients with food allergies [38]. In a study conducted within our nation, Akan et al. uncovered that serum 25(OH)D3 levels displayed an adverse connection with the seriousness of AD within the cohort characterized by allergic sensitivities. Conversely, no discernible correlation emerged within the group devoid of sensitivities [39]. In our investigation, we detected a statistically significant albeit slight inverse relationship between serum 25(OH)D3 levels and the SCORAD index among individuals lacking sensitivities. Epidemiological investigations have spotlighted the role of vitamin D in the development of sensitivities [40]. Vitamin D impedes the proliferation of T cells and contributes to the conversion of CD4+ T cells into regulatory T cells, which are acknowledged for their capacity to suppress pro-allergic mechanisms [41]. Consequently, we emphasize the importance of vigilantly monitoring serum 25(OH)D3 levels, particularly in AD patients with sensitivities.
In our study, we found that serum vitamin D deficiency had a greater impact on the severity of AD than atopy. Although the existing literature on this subject is limited, Baek et al. found a similar correlation between the SCORAD index and serum vitamin D levels when compared to atopy, which aligns with our findings [42]. In this study, Baek et al. found that low 25(OH)D3 levels increased food allergen sensitivity risk and were related to the severity of AD [42]. Eosinophils are closely linked with the effector component of immune response mediated by type 2 T helper cells, which plays a pivotal role in allergic reactions [43]. Specifically, 1,25(OH) vitamin D has been shown to extend the lifespan of eosinophils and enhance the expression of CXCR4, a receptor involved in chemotaxis and immune regulation [44]. Additionally, it has been observed that vitamin D can reduce eosinophil necrosis and limit the release of cytolytic peroxidase [45]. Nevertheless, the ongoing debate revolves around the association between serum vitamin D levels and the eosinophil count in the bloodstream. While a few studies have established a link between lower vitamin D levels in the blood and elevated eosinophilia, the majority of research has not reported a significant correlation [46,47,48]. In our investigation, we uncovered a statistically significant positive connection between the SCORAD index and the overall eosinophil count, whereas no notable correlation emerged regarding factors like age, gender, total IgE, and the SCORAD index. Correspondingly, Cheon’s study and the findings of Baek and colleagues corroborated our results by identifying affirmative correlations between the SCORAD index and the total eosinophil count [33,42]. Furthermore, in line with our findings, studies conducted by Su and team, as well as Baek and others, failed to establish a connection between total IgE levels and the SCORAD index [2,42]. When evaluating the impact of serum vitamin D levels and eosinophils on disease severity, we observed that serum vitamin D levels had a more pronounced effect on disease severity in comparison to eosinophilia. For every unit increase in serum vitamin D levels, we observed a reduction of 0.449 units in the SCORAD index. Conversely, for each unit increase in eosinophil count, we noted a mere 0.009 unit increase in the SCORAD index. To clarify this relationship, we are of the opinion that comprehensive, multi-center studies are essential.
Limitations and Strengths
One of the primary limitations of our study is the inability to assess factors that could influence the severity of atopic dermatitis, such as environmental pollutants or psychosomatic factors. The limitation of being a single center and having a small sample size can be considered as other limitations of our study. Comprehensive assessment of the impact of vitamin D deficiency, eosinophil count, and atopy on AD is the strength of the study. We established that serum 25(OH)D3 deficiency exhibited a stronger predictive value for the SCORAD index compared to eosinophilia and atopy in a regression model. This is another strength of the study.
5. Conclusions
Based on our analysis of the findings, it appears that reduced serum vitamin D levels could exert a more significant influence on the severity of AD when contrasted with atopy and eosinophilia. We identified an inverse relationship between AD severity and serum 25(OH)D3 levels. In essence, this implies that individuals with lower serum 25(OH)D3 levels may encounter more pronounced symptoms of the disease.
Author Contributions
Conceptualization, F.Ç. and M.T.K.; methodology, F.Ç. and M.T.K.; validation, F.Ç. and M.T.K.; formal analysis, F.Ç. and M.T.K.; investigation, F.Ç. and M.T.K.; resources, F.Ç. and M.T.K.; data curation, F.Ç. and M.T.K.; writing—original draft preparation, F.Ç. and M.T.K.; writing—review and editing, F.Ç. and M.T.K.; visualization, F.Ç. and M.T.K.; supervision, F.Ç. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of University of Health Sciences, Kartal Dr. Lütfi Kırdar Training and Research Hospital Istanbul, Türkiye (protocol code 2022/514/236/12 date of 26 October 2022).
Informed Consent Statement
Since the study was conducted retrospectively, no informed consent was obtained.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mesjasz, A.; Zawadzka, M.; Chałubiński, M.; Trzeciak, M. Is Atopic Dermatitis Only a Skin Disease? Int. J. Mol. Sci. 2023, 24, 837. [Google Scholar] [CrossRef] [PubMed]
- Su, O.; Bahalı, A.G.; Demir, A.D.; Ozkaya, D.B.; Uzuner, S.; Dizman, D.; Onsun, N. The relationship between severity of disease and vitamin D levels in children with atopic dermatitis. Postepy Dermatol. Alergol. 2017, 34, 224–227. [Google Scholar] [CrossRef]
- Lara-Corrales, I.; Huang, C.M.; Parkin, P.C.; Rubio-Gomez, G.A.; Posso-De Los Rios, C.J.; Maguire, J.; Pope, E. Vitamin D level and supplementation in pediatric atopic dermatitis: A randomized controlled trial. J. Cutan. Med. Surg. 2019, 23, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Bulut, M.; Ozceker, D.; Tamay, Z. The relationship between serum vitamin D levels and childhood atopic dermatitis. Ann. Med. Res. 2020, 27, 1722–1727. [Google Scholar] [CrossRef]
- Wang, S.S.; Hon, K.L.; Kong, A.P.; Pong, H.N.; Wong, G.W.; Leung, T.F. Vitamin D deficiency is associated with diagnosis and severity of childhood atopic dermatitis. Pediatr. Allergy Immunol. 2014, 25, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Bieber, T. Atopic dermatitis. Ann. Dermatol. 2010, 22, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Kowalska-Olędzka, E.; Czarnecka, M.; Baran, A. Epidemiology of atopic dermatitis in Europe. J. Drug Assess. 2019, 8, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Munawwarah, L.; Evalina, R.; Sofyani, S. Serum 25-hydroxyvitamin-D level and atopic dermatitis severity in children. Paediatr. Indones. 2017, 57, 234–238. [Google Scholar] [CrossRef][Green Version]
- Oranje, A.P.; Glazenburg, E.J.; Wolkerstorfer, A.; de Waard-van der Spek, F.B. Practical issues on interpretation of scoring atopic dermatitis: The SCORAD index, objective SCORAD and the three-item severity score. Br. J. Dermatol. 2007, 157, 645–648. [Google Scholar] [CrossRef]
- Umar, M.; Sastry, K.S.; Al Ali, F.; Al-Khulaifi, M.; Wang, E.; Chouchane, A.I. Vitamin D and the Pathophysiology of Inflammatory Skin Diseases. Skin Pharmacol. Physiol. 2018, 31, 74–86. [Google Scholar] [CrossRef]
- Bergler-Czop, B.; Brzezińska-Wcisło, L. Serum vitamin D level—The effect on the clinical course of psoriasis. Postepy Dermatol. Alergol. 2016, 33, 445–449. [Google Scholar] [CrossRef]
- Ao, T.; Kikuta, J.; Ishii, M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef] [PubMed]
- L Bishop, E.; Ismailova, A.; Dimeloe, S.; Hewison, M.; White, J.H. Vitamin D and Immune Regulation: Antibacterial, Antiviral, Anti-Inflammatory. JBMR Plus 2020, 5, e10405. [Google Scholar] [CrossRef]
- Karampinis, E.; Goudouras, G.; Ntavari, N.; Bogdanos, D.P.; Roussaki-Schulze, A.V.; Zafiriou, E. Serum vitamin D levels can be predictive of psoriasis flares up after COVID-19 vaccination: A retrospective case control study. Front. Med. 2023, 10, 1203426. [Google Scholar] [CrossRef]
- Reinholz, M.; Ruzicka, T.; Schauber, J. Vitamin D and its role in allergic disease. Clin. Exp. Allergy 2012, 42, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Peroni, D.G.; Piacentini, G.L.; Cametti, E.; Chinellato, I.; Boner, A.L. Correlation between serum 25-hydroxyvitamin D levels and severity of atopic dermatitis in children. Br. J. Dermatol. 2011, 164, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Robl, R.; Uber, M.; Abagge, K.T.; Lima, M.N.; Carvalho, V.O. Serum Vitamin D Levels Not Associated with Atopic Dermatitis Severity. Pediatr. Dermatol. 2016, 33, 283–288. [Google Scholar] [CrossRef]
- Chiu, Y.E.; Havens, P.L.; Siegel, D.H.; Ali, O.; Wang, T.; Holland, K.E.; Galbraith, S.S.; Lyon, V.B.; Drolet, B.A. Serum 25-hydroxyvitamin D concentration does not correlate with atopic dermatitis severity. J. Am. Acad. Dermatol. 2013, 69, 40–46. [Google Scholar] [CrossRef]
- Chalcraft, J.R.; Cardinal, L.M.; Wechsler, P.J.; Hollis, B.W.; Gerow, K.G.; Alexander, B.M.; Keith, J.F.; Larson-Meyer, D.E. Vitamin D Synthesis Following a Single Bout of Sun Exposure in Older and Younger Men and Women. Nutrients 2020, 12, 2237. [Google Scholar] [CrossRef]
- Ahn, J.Y.; Choi, B.S. Clinical Evaluation of Specific Immunoglobulin E in Sputum in Pediatric Patients. Pediatr. Allergy Immunol. Pulmonol. 2018, 31, 73–77. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D status: Measurement, interpretation, and clinical application. Ann. Epidemiol. 2009, 19, 73–78. [Google Scholar] [CrossRef]
- Grant, W.B.; Boucher, B.J.; Bhattoa, H.P.; Lahore, H. Why vitamin D clinical trials should be based on 25-hydroxyvitamin D concentrations. J. Steroid Biochem. Mol. Biol. 2018, 177, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Kennel, K.A.; Drake, M.T.; Hurley, D.L. Vitamin D deficiency in adults: When to test and how to treat. Mayo Clin. Proc. 2010, 85, 752–758. [Google Scholar] [CrossRef]
- Altaş, U.; Akgün Ünlü, D.; Güllüce, H.; Tunce, E.; Altaş, Z.M.; Söğütlü, Y.; Özkars, M.Y. Evaluation of the relationship between laboratory parameters and allergy tests in children with atopic dermatitis. Chron. Precis. Med. Res. 2023, 4, 168–171. [Google Scholar]
- Di Rosa, M.; Malaguarnera, M.; Nicoletti, F.; Malaguarnera, L. Vitamin D3: A helpful immuno-modulator. Immunology 2011, 134, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A.; Arkwright, P.D.; Brüggen, M.C.; Busse, W.; Gadina, M.; Guttman-Yassky, E.; Kabashima, K.; Mitamura, Y.; Vian, L.; Wu, J.; et al. Type 2 immunity in the skin and lungs. Allergy 2020, 75, 1582–1605. [Google Scholar] [CrossRef] [PubMed]
- Erem, A.S.; Razzaque, M.S. Vitamin D-independent benefits of safe sunlight exposure. J. Steroid Biochem. Mol. Biol. 2021, 213, 105957. [Google Scholar] [CrossRef]
- Fenner, J.; Silverberg, N.B. Oral supplements in atopic dermatitis. Clin. Dermatol. 2018, 36, 653–658. [Google Scholar] [CrossRef]
- Mutgi, K.; Koo, J. Update on the role of systemic vitamin D in atopic dermatitis. Pediatr. Dermatol. 2013, 30, 303–307. [Google Scholar] [CrossRef]
- Zhang, Y.; Leung, D.Y.; Richers, B.N.; Liu, Y.; Remigio, L.K.; Riches, D.W.; Goleva, E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J. Immunol. 2012, 188, 2127–2135. [Google Scholar] [CrossRef]
- Luger, T.; Amagai, M.; Dreno, B.; Dagnelie, M.A.; Liao, W.; Kabashima, K.; Schikowski, T.; Proksch, E.; Elias, P.M.; Simon, M.; et al. Atopic dermatitis: Role of the skin barrier, environment, microbiome, and therapeutic agents. J. Dermatol. Sci. 2021, 102, 142–157. [Google Scholar] [CrossRef] [PubMed]
- El Taieb, M.A.; Fayed, H.M.; Aly, S.S.; Ibrahim, A.K. Assessment of serum 25-hydroxyvitamin d levels in children with atopic dermatitis: Correlation with SCORAD index. Dermatitis 2013, 24, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Cheon, B.R.; Shin, J.E.; Kim, Y.J.; Shim, J.W.; Kim, D.S.; Jung, H.L.; Park, M.S.; Shim, J.Y. Relationship between serum 25-hydroxyvitamin D and interleukin-31 levels, and the severity of atopic dermatitis in children. Korean J. Pediatr. 2015, 58, 96–101. [Google Scholar] [CrossRef]
- Raj, K.A.P.; Handa, S.; Narang, T.; Sachdeva, N.; Mahajan, R. Correlation of serum vitamin D levels with severity of pediatric atopic dermatitis and the impact of vitamin D supplementation on treatment outcomes. J. Dermatolog. Treat. 2022, 33, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Hattangdi-Haridas, S.R.; Lanham-New, S.A.; Wong, W.H.S.; Ho, M.H.K.; Darling, A.L. Vitamin D Deficiency and Effects of Vitamin D Supplementation on Disease Severity in Patients with Atopic Dermatitis: A Systematic Review and Meta-Analysis in Adults and Children. Nutrients 2019, 11, 1854. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, S.-N.; Lee, Y.W.; Choe, Y.B.; Ahn, K.J. Vitamin D Status and Efficacy of Vitamin D Supplementation in Atopic Dermatitis: A Systematic Review and Meta-Analysis. Nutrients 2016, 8, 789. [Google Scholar] [CrossRef] [PubMed]
- Lubis, H.H.; Nababan, K.A.; Paramita, D.A. Correlation of low vitamin D status with atopic dermatitis severity in children. Bali Med. J. 2021, 10, 291–295. [Google Scholar] [CrossRef]
- Lee, S.A.; Hong, S.; Kim, H.J.; Lee, S.H.; Yum, H.Y. Correlation between serum vitamin d level and the severity of atopic dermatitis associated with food sensitization. Allergy Asthma Immunol. Res. 2013, 5, 207–210. [Google Scholar] [CrossRef]
- Akan, A.; Azkur, D.; Ginis, T.; Toyran, M.; Kaya, A.; Vezir, E.; Ozcan, C.; Ginis, Z.; Kocabas, C.N. Vitamin D level in children is correlated with severity of atopic dermatitis but only in patients with allergic sensitizations. Pediatr. Dermatol. 2013, 30, 359–363. [Google Scholar] [CrossRef]
- Liu, X.; Wang, G.; Hong, X.; Wang, D.; Tsai, H.J.; Zhang, S.; Arguelles, L.; Kumar, R.; Wang, H.; Liu, R.; et al. Gene-vitamin D interactions on food sensitization: A prospective birth cohort study. Allergy 2011, 66, 1442–1448. [Google Scholar] [CrossRef]
- Suaini, N.H.A.; Zhang, Y.; Vuillermin, P.J.; Allen, K.J.; Harrison, L.C. Immune Modulation by Vitamin D and Its Relevance to Food Allergy. Nutrients 2015, 7, 6088–6108. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Shin, Y.H.; Chung, I.H.; Kim, H.J.; Yoo, E.G.; Yoon, J.W.; Jee, H.M.; Chang, Y.E.; Han, M.Y. The link between serum vitamin D level, sensitization to food allergens, and the severity of atopic dermatitis in infancy. J. Pediatr. 2014, 165, 849–854.e1. [Google Scholar] [CrossRef] [PubMed]
- Spencer, L.A.; Weller, P.F. Eosinophils and Th2 immunity: Contemporary insights. Immunol. Cell Biol. 2010, 88, 250–256. [Google Scholar] [CrossRef]
- Skrobot, A.; Demkow, U.; Wachowska, M. Immunomodulatory Role of Vitamin D: A Review. Adv. Exp. Med. Biol. 2018, 1108, 13–23. [Google Scholar]
- Ethier, C.; Yu, Y.; Cameron, L.; Lacy, P.; Davoine, F. Calcitriol Reduces Eosinophil Necrosis Which Leads to the Diminished Release of Cytotoxic Granules. Int. Arch. Allergy Immunol. 2016, 171, 119–129. [Google Scholar] [CrossRef]
- Souto Filho, J.T.D.; de Andrade, A.S.; Ribeiro, F.M.; Alves, P.A.S.; Simonini, V.R.F. Impact of vitamin D deficiency on increased blood eosinophil counts. Hematol. Oncol. Stem Cell Ther. 2018, 11, 25–29. [Google Scholar] [CrossRef] [PubMed]
- De Groot, J.C.; van Roon, E.N.; Storm, H.; Veeger, N.J.; Zwinderman, A.H.; Hiemstra, P.S.; Bel, E.H.; ten Brinke, A. Vitamin D reduces eosinophilic airway inflammation in nonatopic asthma. J. Allergy Clin. Immunol. 2015, 135, 670–675.e3. [Google Scholar] [CrossRef] [PubMed]
- Dogru, M.; Kirmizibekmez, H.; Yesiltepe Mutlu, R.G.; Aktas, A.; Ozturkmen, S. Clinical effects of vitamin D in children with asthma. Int. Arch. Allergy Immunol. 2014, 164, 319–325. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).