Abstract
Lymphedema is a condition resulting from mutations in various genes essential for lymphatic development and function, which leads to obstruction of the lymphatic system. Secondary lymphedema is a progressive and incurable condition, most often manifesting after surgery for breast cancer. Although its causation appears complex, various lines of evidence indicate that genetic predisposition may play a role. Previous studies show that mutations in connexin 47 are associated with secondary lymphedema. We have tested the hypothesis that connexin 37 gene mutations in humans are associated with secondary lymphedema following breast cancer surgery. A total of 2211 breast cancer patients were screened and tested for reference single nucleotide polymorphisms (SNPs) of the GJA4 gene (gap junction protein alpha 4 gene). The results presented in this paper indicate that two SNPs in the 3’ UTR (the three prime untranslated region) of the GJA4 gene are associated with an increased risk of secondary lymphedema in patients undergoing breast cancer treatment. Our results provide evidence of a novel genetic biomarker for assessing the predisposition to secondary lymphedema in human breast cancer patients. Testing for the condition-associated alleles described here could assist and inform treatment and post-operative care plans of breast cancer patients, with potentially positive outcomes for the management of disease progression.
1. Introduction
Connexins are a large family of six-subunit transmembrane hemi-channels. A total of 21 connexin genes have been described in humans, and 20 in mice [1,2]. Individual hemi-channels (connexons) as part of a gap junction channel allow for the diffusion of ions and small molecules between the extracellular space and the cytosol, and gap junction channels facilitate the diffusion of ions, metabolites, and signalling molecules between cells [3,4].
Lymphedema is an incurable condition resulting from obstruction of the lymphatic system, characterised by localised fluid retention, swelling, and susceptibility to infection. The condition is sub-classified into two varieties: the so-called primary lymphedema is inherited, resulting from mutations in various genes essential for lymphatic development and function, whilst secondary lymphedema is generally a post-operative complication of surgery, usually affecting women undergoing treatment for breast cancer [5,6,7]. The estimates of the proportion of patients affected range from 2–80%, no doubt partly reflecting differences in measurement and diagnostic criteria [2]. Several other medical factors, such as the stage of cancer at the time of diagnosis, the pathological involvement of lymph nodes, the number of dissected lymph nodes during breast cancer surgery, the type and extent of surgery, and also the extent and method of radio- and chemotherapy are considered important in the development of secondary lymphedema in breast cancer patients. Additionally, patient age, body mass index, and degree of physical activity have all been suggested to influence the risk of developing secondary lymphedema [7,8].
Intriguingly, there is also some evidence for genetic predisposition to secondary lymphedema [9,10]. For example, mutations in hepatocyte growth factor/high affinity hepatocyte growth factor receptor/mesenchymal-epithelial transition (HGF/MET) have been reported in both primary and secondary lymphedema [9]. This protein is expressed in lymphatic endothelial cells and has functions in cell growth, mobility, differentiation, and intercellular junctions [9]. Another set of mutations associated with secondary lymphedema affect the connexin Cx47 [8]. Similar mutations are also associated with Pelizaues–Merzbacher-like disease (PMLD) [11], spastic paraplegia [12], and primary lymphedema [11,12]. It has been shown that Cx43 is abundantly expressed in the ventricular myocardium and in cardiac neural crest cells and plays an important role in human congenital heart disease [13].
Connexins adopt complex tertiary structures achieved through the coordination of six subunits, representing a “connexon”, which is capable of generating a gap junction by docking to another connexon on an adjacent cell [14]. This suggests a general model in which a genetic predisposition to form inappropriate cellular junctions may explain the development of some secondary lymphedemas.
Here, we demonstrate that polymorphisms in another connexin, Cx37, are differentially distributed in patients with and without secondary lymphedema, following surgery for breast cancer. Cx37 is a good candidate marker because it is expressed in the lymphatic system and endothelial cells [15]. Furthermore, single nucleotide polymorphisms (SNPs) in GJA4 (the gene that codes for Cx37) have previously been shown to be associated with myocardial infarction and atherosclerosis, suggesting (by analogy with the wide-ranging effects of mutations in HGF/MET and Cx47), that Cx37 could have a role in secondary lymphedema [16].
2. Experimental Section
2.1. Patients
From an initial screen of 2211 breast cancer patients (admitted to the Sayyed-Al-Shohada hospital in Isfahan, Iran, between 2009–2015) at least 6 months post chemotherapy, written consents were obtained and blood samples collected from 102 patients aged between 35 and 70. Patients were selected for this study if they had breast cancer “lower than stage IIIC” and “tumour size between 3 and 10 cm”. From the patients with the above characteristics, 51 patients with secondary lymphedema (case group) and 51 patients without secondary lymphedema (control group) were randomly selected and were further analysed. The staging system of the International Society of Lymphology (ISL) was used to characterize the severity of lymphedema, considering the “softness” or “firmness” of the limb, and all patients in the case group had moderate to severe lymphedema [17].
All 102 patients either had modified radical mastectomy (MRM) or breast conserving surgery (BCS). In the case group, an average of 4.7 and in control group an average of 2.2 lymph nodes were involved. Also, 69% of patients from the case group and 64% of patients from the control group had BCS, and 31% of patients from the case group and 46% of patients from the control group had MRM.
During the surgery, at least six axillary lymph nodes were removed, and the patients had chemotherapy and radiation therapy (supplementary material). The external beam radiation method was applied to all patients using a linear accelerator on an outpatient basis, 5 days a week, over 5 to 7 weeks, depending on each particular situation. The radiotherapy treatment included the breast and the regional axillary lymph nodes, and there was no clear correlation between the radiotherapy of regional lymph nodes and the occurrence of secondary lymphedema. DNA extraction from blood samples was performed using PrimePrep Genomic DNA isolation kit (GeNet Bio, Daejon, Korea) [18].
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the Tabriz University of Medical Sciences, Iran (IR.MUI.REC.1394.2.058 (1 August 2014)).
2.2. High-Resolution Melting Analysis
High-Resolution Melting (HRM) is an inexpensive, accurate, homogeneous, and post-PCR method, which enables researchers to analyse genetic variations such as SNPs, mutations, and methylations in PCR amplicons [19]. The primers for amplification of rs3543 and rs705193 were designed using Primer3web software (version 4.1.0, Howard Hughes Medical Institute, Ashburn, VA, USA; http://primer3.ut.ee/). The primer sequences and product sizes are shown in Table 1. PCR amplification and HRM analysis were performed in a reaction volume of 10 µL, with High-Resolution Master Mix (Solis BioDyne, Tartu, Estonia; https://www.sbd.ee/), 0.5 µL of each primer (10 pmol), and 30 ng DNA. HRM analysis was performed using a Corbett Rotor-Gene 6000 (Germantown, MD, USA).

Table 1.
Primer sequences and amplicon sizes for rs3543 and rs705193 used in High-Resolution Melting (HRM) analysis.
The polymerase chain reaction (PCR) procedure started with a pre-incubation at 95 °C for 15 min, followed by 40 cycles of denaturation (95 °C for 15 s), annealing (60 °C for 20 s), and extension (72 °C for 20 s). The melting analysis of the amplicons was carried out from 75 °C to 95 °C at 0.2 °C/s. The samples with different melting profiles were selected for direct sequencing by an ABI 3130 sequencer (Applied Biosystems, Waltham, MA, USA; http://www.thermofisher.com).
2.3. Statistical Analysis
SPSS version 22 (IBM SPSS,) was used for all statistical analyses. Parametric analyses were conducted on continuous data with normal distribution, otherwise non-parametric analyses were applied. The significance level of p < 0.05 was used in each analysis.
3. Results
3.1. Physiological Parameters
Clinical records of patients’ age, height, weight (at the time of sampling and after surgery and radiotherapy), and body surface area were collected; the statistical analyses showed no significant differences between lymphedema case and control groups for these parameters (Table 2). The effects of the physiological parameters on the presence of secondary lymphedema were further evaluated using binary logistic regression (Table 3). The regression revealed no significant effects (Cox & Snell R2 = 0.040, Nagelkerke R2 = 0.053, p > 0.05) of these parameters on the presence of secondary lymphedema.

Table 2.
Comparisons of physiological measurements and tumour measurements between the control and case groups.

Table 3.
Effects of age, height, weight, and body surface area on the presence of secondary lymphedema.
Age, height, and weight odds ratios were close to 1, indicating no effects (Table 2), while an increase in the body surface area did appear to correlate with an increased risk of secondary lymphedema, yet the effect was not statistically significant (p = 0.604).
3.2. Tumour Parameters
In the case group, 35 patients went through the MRM surgical procedure and 16 through the BCS procedure, while in the control group 28 patients underwent the MRM procedure and 23 the BCS procedure. There was no statistical significant difference between the two groups (Cramer’s V = 0.141, p = 0.154). The lymph nodes removed during surgery varied from patient to patient. The highest number of nodes removed in the control group was 20 and the lowest 6; 51% had 6–10 nodes removed. In comparison to the control group, the patients in the case group had a maximum of 28 lymph nodes removed (1 patient) and a minimum of 6; 66.7% had 6–10 nodes removed. However, the statistical analyses showed no significant differences in the number of lymph nodes removed between the two groups (Table 2).
From the lymph nodes removed, the number of nodes that were invaded by the tumour was assessed (Figure 1); over one-third of the control group had no lymph nodes affected (37.3%), while a similar number in the case group had up to two nodes affected (39.2%). Although the Moses test of Extreme Reaction (nonparametric tests algorithms) revealed no statistically significant difference in the range of the lymph nodes involved (p = 0.132), the Mann–Whitney test of distribution indicated a statistically highly significant difference between the control and the case groups (p = 0.002).
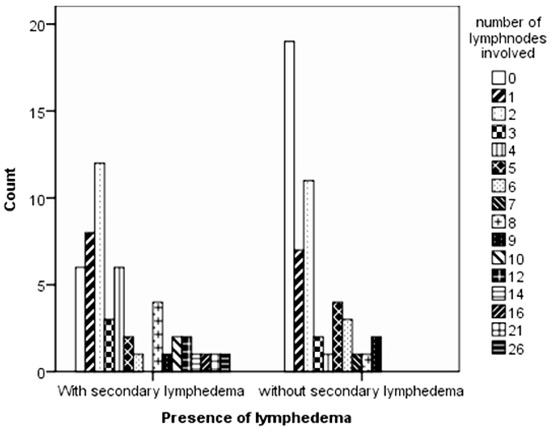
Figure 1.
Differences in the number of lymph nodes invaded by tumour cells between the control and case groups.
Binary logistic regression analyses (Table 4) revealed a moderate effects of the tumour parameters on the presence of lymphedema (Cox & Snell R2 = 0.231, Nagelkerke R2 = 0.309); the overall effect was statistically significant (p = 0.001). The multivariate model correctly predicted 72.1% (31 out of 45) of those with secondary lymphedema and 70.6% (36 out of 51) of those without secondary lymphedema; the overall accuracy was 71.3%.

Table 4.
Effects of clinical parameters and genotypes assessed on the secondary lymphedema.
3.3. Connexin 37 Genotypes
3.3.1. Melting Profiles
Figure 2 and Figure 3 present the melting profiles of rs3543 and rs705193 genotypes, respectively. Homozygous wild-type, mutant, and heterozygote samples are shown on a standard normalized melt curve in Figure 2 and Figure 3. The results for rs3543 show three different melting profiles of analysed amplicons and two melting profiles for rs705193.
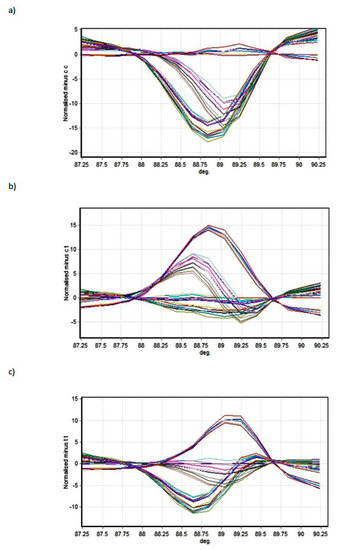
Figure 2.
High-Resolution Melting (HRM) detection of rs3543 genotype: (a) genotype CC, (b) genotype CT, and (c) genotype TT. Curve colors represent different samples.
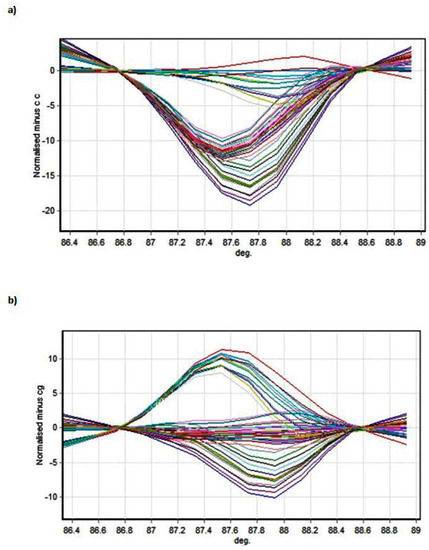
Figure 3.
HRM detection of rs705193 genotypes: (a) genotype CC and (b) genotype CG. Curve colors represent different samples.
3.3.2. Genotypes and Allele Frequencies
Categorical cross-tabulation analyses identified significant associations of allele type T (C→T mutation) with the presence of secondary lymphedema in rs3543 (Table 5). The CC genotype was more abundant in the control group (without lymphedema), while the genotypes CT and TT showed moderate and significant associations with the presence of secondary lymphedema in the case group, respectively. Cramer’s V revealed a medium association which was statistically highly significant (Cramer’s V = 0.385, p = 0.001).

Table 5.
Cross-tabulation analysis of rs3543 allele frequencies in the case and the control groups.
Similarly, in rs705193, the C to G mutation contributed significantly to the increased risk of secondary lymphedema (Table 6), while the genotype CG showed significant influence. Cramer’s V indicated a medium association which was highly significant (Cramer’s V = 0.356, p = 0.001).

Table 6.
Cross-tabulation analysis of rs705193 allele frequencies in the case and the control groups.
Interestingly, rs3543 and rs705193 were strongly associated with each other in both the case and the control groups (Cramer’s V 0.803 and 0.819 respectively, p = 0.003). The association was not influenced by the allele type TT in rs3543 (−1.96 < z < 1.96, Table 7).

Table 7.
Association of rs3543 and rs705193 in the case and the control groups.
It was evident that for rs3543, the allele type TT had a similar distribution in both the case and the control groups; CT had a small difference between the two groups, and CC had a significant difference between the groups. The absence of the rs3543’s CT and rs705193’s CC combination, together with the lack of the CC and CC combination, contributed to the secondary lymphedema.
4. Discussion
The results presented in this paper indicate that two SNPs in the 3’ UTR of the GJA4 gene are associated with an increased risk of secondary lymphedema in patients being treated for breast cancer. GJA4 was chosen because it encodes Cx37; other studies have already described that two genes (GJC2 encoding connexin 47 and MET gene) also involved in junction formation have mutations associated with the predisposition to secondary lymphedema [20,21]. The results thus provide strong support for the hypothesis that secondary lymphedema is caused at least partly by genetic factors that presumably lead to inappropriate formation of cellular junctions and, consequently, blockage of the lymphatic system. This has important implications for the diagnosis and treatment of lymphedema.
In comparison to the BCS procedure, although statistically not significant, MRM surgical procedure seemed to increase the odds of secondary lymphedema (odds ratio = 2.766, p = 0.075, Table 4). Tumour size, number of lymph nodes removed during the surgery, and number of lymph nodes being invaded by the tumour had little impact on the presence of lymphedema (odds ratio close to 1 and p > 0.05).
The Wald statistic did not indicate that the β coefficients for the genotypes were statistically significantly different from 0 (p > 0.05), however the odds ratios for rs3543 (CC) and in particular for rs705193 (CC) showed their odds in favour of without lymphedema (internal value without lymphedema 0, with lymphedema 1).
It is important to note that the SNPs detected are in a region annotated as a 3’UTR, meaning that a direct effect on the protein sequence is unlikely (albeit we have not shown directly that the protein sequence is actually unaffected by the variation, and there remains a possibility that the annotation of this region may be erroneous). Likely, therefore, the mutation associated with secondary lymphedema affects the post-transcriptional fate of the mRNA through effects on stability, as several microRNAs have already been shown to target other connexin family members [22].
Alternatively, there might be effects on transcription through long-range interactions. Finally, it is possible that the variation is functionally insignificant and rather an artefact of linkage or some other confounding variable. Though possible, we consider this latter unlikely in view of the fact that other secondary lymphedema-associated mutations also affect junction-forming proteins [23].
From the molecular pathological point of view, the results presented here suggest that a fruitful approach to secondary lymphedema may be to characterise the cell–cell junctions in healthy and pathological tissues, with the aim of determining, for example, whether the problem is fundamentally linked to junctions that are too tight or too loose [15,16]. Given that the 3’UTR of genes is often involved in RNA stability, we may speculate that the mutations result in loss of function, i.e., less RNA and therefore less protein, which would probably manifest as “too loose” junctions. Alternatively, if the mutations remove a microRNA target, the effect would be increased translation, possibly manifesting as “too tight” junctions. This fundamental and essential work is however beyond the scope of the present study.
The lymphatic drainage pathways of the breast (axillary, internal mammary, and supraclavicular nodal groups) are the regional areas most likely to be involved with metastatic breast cancer, and it has been shown that patients who undergo more extensive surgery, have many lymph nodes removed, or have radiation therapy to the axilla or groin after surgery are more likely to develop lymphedema [24].
The next step of our research, also to increase the strength of our results and conclusion, will be to increase the sample size and to collect similar samples from different geographical areas and other ethnic groups. It is important to notice the importance of ethnicity on the genetic variations and of the sample size, because too big or too small sample sizes have limitations that can compromise the conclusions drawn from studies.
5. Conclusions
The results in this study confirm that the number of lymph nodes being invaded by breast tumours had a statistically significant impact on the presence of lymphedema and that increased lymph node invasion correlated with an increased probability of secondary lymphedema.
Significantly, we have discovered a novel predictive biomarker for the predisposition to secondary lymphedema in breast cancer patients, following surgical intervention. Testing for the condition-associated allele should help inform the treatment and post-operative care of patients, with desirable outcomes for the management of breast cancer. Further study of genes involved in junction formation may reveal additional secondary lymphedema-associated polymorphisms, and hence extra biomarkers, offering an exciting new area of breast cancer research.
Supplementary Materials
The Physiological and tumour parameters (.xlsx) are available online at http://www.mdpi.com/2227-9059/6/1/23/s1.
Acknowledgments
Authors are immensely grateful to all patients who voluntarily participated in study. The production of this research paper would not have been possible without all of them. We would also like to show our gratitude to Mahdiyeh Behnam, the Medical Centre of Genome (Isfahan, Iran), for her contribution and help toward the laboratory experiments.
Author Contributions
M.H., S.M.M.A., F.M., and M.S. conceived and designed the experiments; M.H., E.M., and M.K. performed the experiments; M.H., M.A.B., B.M., M.K., and Q.X. analyzed the data; M.H., M.S. contributed reagents/materials/analysis tools; M.H., M.A.B., C.Y., Q.X., and J.M. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Pfenniger, A.; Wohlwend, A.; Kwak, B.R. Mutations in connexin genes and disease. Eur. J. Clin. Investig. 2011, 41, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, R.; Willecke, K. Connexin-caused genetic diseases and corresponding mouse models. Antioxid. Redox Signal. 2009, 11, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.J.; Xu, X.; Lo, C.W. Connexins and cell signalling in development and disease. Annu. Rev. Cell Dev. Biol. 2004, 20, 811–838. [Google Scholar] [CrossRef] [PubMed]
- Laird, D. The gap junction proteome and its relationship to disease. Trends Cell Biol. 2010, 20, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Poage, E.; Singer, M.; Armer, J.; Poundall, M.; Shellabarger, M.J. Demystifying lymphedema: Development of the lymphedema putting evidence into practice card. Clin. J. Oncol. Nurs. 2008, 12, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Dagenais, S.; Erickson, R.P.; Arlt, M.F.; Glynn, M.W.; Gorski, J.L.; Seaver, L.H.; Glover, T.W. Mutations in FOXC2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedema distichiasis syndrome. Am. J. Hum. Genet. 2000, 67, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.; Janda, M.; Cornish, B.; Battistutta, D.; Newman, B. Lymphedema after breast cancer: Incidence, risk factors, and effect on upper body function. J. Clin. Oncol. 2008, 26, 3536–3542. [Google Scholar] [CrossRef] [PubMed]
- Soran, A.; D’Angelo, G.; Begovic, M.; Ardic, F.; Harlak, A.; Samuel Wieand, H.; Vogel, V.G.; Johnson, R.R. Breast cancer related lymphedema—What are the significant predictors and how they affect the severity of lymphedema? Breast J. 2006, 12, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Finegold, D.N.; Schacht, V.; Kimak, M.A.; Lawrence, E.C.; Foeldi, E.; Karlsson, J.M.; Baty, C.J.; Ferrell, R.E. HGF and MET mutations in primary and secondary lymphedema. Lymphat. Res. Biol. 2008, 6, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Finegold, D.N.; Baty, C.J.; Knickelbein, K.Z.; Perschke, S.; Noon, S.E.; Campbell, D.; Karlsson, J.M.; Huang, D.; Kimak, M.A.; Lawrence, E.C.; et al. Connexin 47 mutations increase risk for secondary lymphedema following breast cancer treatment. Clin. Cancer Res. 2012, 18, 2382–2390. [Google Scholar] [CrossRef] [PubMed]
- Henneke, M.; Combes, P.; Diekmann, S.; Bertini, E.; Brockmann, K.; Burlina, A.P.; Kaiser, J.; Ohlenbusch, A.; Plecko, B.; Rodriguez, D.; et al. GJA12 mutations are a rare cause of Pelizaeus-Merzbacher-like disease. Neurology 2008, 70, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Orthmann-Murphy, J.; Salsano, E.; Abrams, C.; Bizzi, A.; Uziel, G.; Freidin, M.M.; Lamantea, E.; Zeviani, M.; Scherer, S.S.; Pareyson, D.; et al. Hereditary spastic paraplegia is a novel phenotype for GJA12/GJC2 mutations. Brain 2009, 132, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.Y.; Xie, L.J.; Linask, K.L.; Zhang, C.; Zhao, X.Q.; Yang, Y.; Zhou, G.M.; Wu, Y.J.; Marquez-Rosado, L.; McElhinney, D.B.; et al. Evaluating the role of connexin43 in congenital heart disease: Screening for mutations in patients with outflow tract anomalies and the analysis of knock-in mouse models. J. Cardiovasc. Dis. Res. 2011, 2, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Saez, J.; Berthoud, V.; Branes, M.; Martinez, A.D.; Beyer, E.C. Plasma membrane channels formed by connexins: Their regulation and functions. Physiol. Rev. 2003, 83, 1359–1400. [Google Scholar] [CrossRef] [PubMed]
- Pfenniger, A.; Derouette, J.; Verma, V.; Lin, X.; Foglia, B.; Coombs, W.; Roth, I.; Satta, N.; Dunoyer-Geindre, S.; Sorgen, P.; et al. Gap junction protein Cx37 interacts with endothelial nitric oxide synthase in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Izawa, H.; Ichihara, S.; Takatsu, F.; Ishihara, H.; Hirayama, H.; Sone, T.; Tanaka, M.; Yokota, M. Prediction of the risk of myocardial infarction from polymorphisms in candidate genes. N. Engl. J. Med. 2002, 347, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2013 Consensus Document of the International Society of Lymphology. Lymphology 2013, 46, 1–11. [Google Scholar]
- GeNet Bio. PrimePrep Genomic DNA Isolation Kit Manual from Blood and Tissue. Available online: www.genetbio.com/en/page9.html (accessed on 3 August 2014).
- Reed, G.H.; Kent, J.O.; Wittwer, C.T. High-resolution DNA melting analysis for simple and efficient molecular diagnostics. Pharmacogenomics 2007, 8, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Ferrell, R.; Baty, C.J.; Kimak, M.; Karlsson, J.M.; Lawrence, E.C.; Franke-Snyder, M.; Meriney, S.D.; Feingold, E.; Finegold, D.N. GJC2 missense mutations cause human lymphedema. Am. J. Hum. Genet. 2010, 86, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Ostergaard, P.; Simpson, M.; Brice, G.; Mansour, S.; Connell, F.C.; Onoufriadis, A.; Child, A.H.; Hwang, J.; Kalidas, K.; Mortimer, P.S.; et al. Rapid identification of mutations in GJC2 in primary lymphoedema using whole exome sequencing combined with linkage analysis with delineation of the phenotype. J. Med. Genet. 2011, 48, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Calderón, J.F.; Retamal, M.A. Regulation of Connexins Expression Levels by MicroRNAs, an Update. Front. Physiol. 2016, 7, 558. [Google Scholar] [CrossRef] [PubMed]
- Miaskowski, C.; Dodd, M.; Paul, S.M.; West, C.; Hamolsky, D.; Abrams, G.; Cooper, B.A.; Elboim, C.; Neuhaus, J.; Schmidt, B.L.; et al. Lymphatic and Angiogenic Candidate Genes Predict the Development of Secondary Lymphedema following Breast Cancer Surgery. PLoS ONE 2013, 8, e60164. [Google Scholar] [CrossRef] [PubMed]
- Sanghani, M.; Balk, E.M.; Cady, B. Impact of axillary lymph node dissection on breast cancer outcome in clinically node negative patients: A systematic review and meta-analysis. Cancer 2009, 115, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).