Role of the Th2-like Immune Response in Obesity: IL-4 as a Metabolic Regulator and IL-13 as an Effector of Muscle Energy Metabolism
Abstract
1. Introduction
2. Obesity: A Chronic Inflammatory… Disease?
3. The Immune System and Inflammation in Obesity
3.1. Innate Immune Response in Obesity
3.2. Adaptive Immune Response in Obesity
Th2 Cell Differentiation and Its Central Role in Type 2 Cytokine Regulation
4. IL-4 as a Metabolic Modulator
5. IL-5 Coordinates the Eosinophil Distribution
6. IL-13 in Metabolic Homeostasis and Energy Expenditure
7. Th2 Response in Obesity and Asthma
8. Emerging Drugs and Biologic Therapies Regulating Th2 Cytokine Activity in Obesity
8.1. IL-4-Based Therapeutic Approaches
8.2. IL-5-Based Therapeutic Approaches
9. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| WHO | World Health Organization |
| T2D | Type 2 Diabetes |
| CVD | Cardiovascular Diseases |
| MASLD | Metabolic-associated Steatotic Liver Disease |
| WAT | White adipose tissue |
| AT | Adipose Tissue |
| PWO | People With Obesity |
| ILC2s | Group 2 Innate Lymphoid Cells |
| IL | Interleukin |
| IFN-γ | Interferon-gamma |
| TNF-α | Tumor Necrosis Factor alpha |
| BMI | Body Mass Index |
| GATA3 | GATA Binding Protein 3 |
| IgG1/IgE | Immunoglobulin G1/E |
| HDL-C | High-Density Lipoprotein Cholesterol |
| SAT | Subcutaneous Adipose Tissue |
| BAT | Brown Adipose Tissue |
| VAT | Visceral Adipose Tissue |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| HGF | Hepatocyte Growth Factor |
| NFκB | Nuclear Factor Kappa-light-chain-enhancer of Activated B Cells |
| IK-Kβ | IκB Kinase beta |
| JNK | c-Jun N-terminal Kinase |
| NLRP3 | NOD-, LRR- and pyrin domain-containing protein 3 |
| IR | Insulin Resistance |
| EAT | Epicardial Adipose Tissue |
| PVAT | Perivascular Adipose Tissue |
| LDL | Low -Density Lipoprotein |
| HDL | High -dDensity Lipoprotein |
| LPS | Lipopolysaccharides |
| TLR | Toll-like receptor |
| DCs | Dendritic Cells |
| CD | Cluster of Differentiation (e.g., CD4, CD8, CD25) |
| CLS | Crown-like Structures |
| CCL2 | C-C Motif Chemokine Ligand 2 |
| LTB4 | Leukotriene B4 |
| SV | Stromal Vascular |
| PRRs | Pattern Recognition Receptors |
| CCR | C-C Chemokine Receptor |
| MMEs | Metabolic- Activated Macrophages |
| ABCA1 | ATP-Bbinding Cassette Transporter |
| TPH-1 | Tryptophan Hydrolase 1 |
| UCP-1 | Uncoupling Protein 1 |
| HFD | High Fat Diet |
| NETs | Neutrophil Extracellular Traps |
| CRAMP | Cathelicidin-Related Antimicrobial Peptide |
| DIO | Diet-Induced Obesity |
| SpDCs | Splenic Dendritic Cells |
| FAO | Fatty Acid Oxidation |
| Ag | Antigen |
| Tc1 | Type 1 Cytotoxic T cells |
| PD-1 | Programmed cell death 1 |
| TCR | T Cell Receptor |
| Teff2 | T-effector CD4+ cells |
| APCs | Antigen Presenting Cells |
| IL-4Rα | Interleukin 4 Receptor alpha |
| FA/FAs | Fatty Acid(s) |
| STAT | Signal Transducer and Activator of Transcription |
| PPARα | Peroxisome Proliferator-Activated Receptor alpha |
| Fgf21 | Fibroblast Growth Factor 21 |
| AKT | Protein Kinase B |
| HSL | Hormone- Sensitive Lipase |
| cAMP | Cycle adenosine monophosphate |
| PKA | Protein Kinase A |
| Tregs | Regulatory T Cells |
| Th1/Th2/Th17 | T helper 1/2/17 cells |
| AT-EOS | Adipose Tissue Eosinophils |
| TGF-β | Transforming Growth Factor beta |
| MIP-1α | Macrophage Inflammatory Protein-1 alpha |
| AAMs | Alternative Activated Macrophages |
| ERK | Extracellular signal-regulated kinase |
| ERR | Estrogen- Related Receptor |
| GDFIS | Growth Differentiation Factor 15 |
| GFRAL | GDNF Family Receptor alpha-like |
References
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 6 August 2025).
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, V.; Hogan, A.E.; Fallon, P.G.; Schwartz, C. Obesity-Mediated Immune Modulation: One Step Forward, (Th)2 Steps Back. Front. Immunol. 2022, 13, 932893. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.M.; Weschenfelder, J.; Sander, C.; Minkwitz, J.; Thormann, J.; Chittka, T.; Mergl, R.; Kirkby, K.C.; Faßhauer, M.; Stumvoll, M.; et al. Inflammatory Cytokines in General and Central Obesity and Modulating Effects of Physical Activity. PLoS ONE 2015, 10, e0121971. [Google Scholar] [CrossRef] [PubMed]
- Koyasu, S.; Moro, K. Type 2 innate immune responses and the natural helper cell. Immunology 2011, 132, 475. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.; Cummings, D.E.; Eckel, R.H.; Cohen, R.V.; Wilding, J.P.H.; Brown, W.A.; Stanford, F.C.; Batterham, R.L.; Farooqi, I.S.; Farpour-Lambert, N.J.; et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025, 13, 221–262. [Google Scholar] [CrossRef]
- Jin, X.; Qiu, T.; Li, L.; Yu, R.; Chen, X.; Li, C.; Proud, C.G.; Jiang, T. Pathophysiology of obesity and its associated diseases. Acta Pharm. Sin. B 2023, 13, 2403. [Google Scholar] [CrossRef]
- Busebee, B.; Ghusn, W.; Cifuentes, L.; Acosta, A. Obesity: A Review of Pathophysiology and Classification. Mayo Clin. Proc. 2023, 98, 1842–1857. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Catrysse, L.; van Loo, G. Inflammation and the Metabolic Syndrome: The Tissue-Specific Functions of NF-κB. Trends Cell Biol. 2017, 27, 417–429. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Wan, C.; Liu, Y.; Wang, Y.; Meng, C.; Zhang, Y.; Jiang, C. NLRP3 inflammasome mediates M1 macrophage polarization and IL-1β production in inflammatory root resorption. J. Clin. Periodontol. 2020, 47, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Stanek, A.; Brożyna-Tkaczyk, K.; Myśliński, W. The role of obesity-induced perivascular adipose tissue (Pvat) dysfunction in vascular homeostasis. Nutrients 2021, 13, 3843. [Google Scholar] [CrossRef]
- Boicean, A.; Ichim, C.; Sasu, S.M.; Todor, S.B. Key Insights into Gut Alterations in Metabolic Syndrome. J. Clin. Med. 2025, 14, 2678. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, L.; Yang, L.; Chu, H. The critical role of gut microbiota in obesity. Front. Endocrinol. 2022, 13, 1025706. [Google Scholar] [CrossRef]
- Den Besten, G.; Bleeker, A.; Gerding, A.; van Eunen, K.; Havinga, R.; van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.-J.; et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a pparg-dependent switch from lipogenesis to fat oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef]
- Blaszczak, A.M.; Jalilvand, A.; Hsueh, W.A. Adipocytes, Innate Immunity and Obesity: A Mini-Review. Front. Immunol. 2021, 12, 650768. [Google Scholar] [CrossRef] [PubMed]
- Savulescu-Fiedler, I.; Mihalcea, R.; Dragosloveanu, S.; Scheau, C.; Baz, R.O.; Caruntu, A.; Scheau, A.-E.; Caruntu, C.; Benea, S.N. The Interplay between Obesity and Inflammation. Life 2024, 14, 856. [Google Scholar] [CrossRef] [PubMed]
- Castoldi, A.; De Souza, C.N.; Câmara, N.O.S.; Moraes-Vieira, P.M. The macrophage switch in obesity development. Front. Immunol. 2016, 6, 637. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ren, Y.; Chang, K.; Wu, W.; Griffiths, H.R.; Lu, S.; Gao, D. Adipose tissue macrophages as potential targets for obesity and metabolic diseases. Front Immunol. 2023, 14, 1153915. [Google Scholar] [CrossRef]
- Ramkhelawon, B.; Hennessy, E.J.; Ménager, M.; Ray, T.D.; Sheedy, F.J.; Hutchison, S.; Wanschel, A.; Oldebeken, S.; Geoffrion, M.; Spiro, W.; et al. Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity. Nat. Med. 2014, 20, 377–384. [Google Scholar] [CrossRef]
- Mclaughlin, T.; Ackerman, S.E.; Shen, L.; Engleman, E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J. Clin. Investig. 2017, 127, 5–13. [Google Scholar] [CrossRef]
- Yabut, J.M.; Desjardins, E.M.; Chan, E.J.; Day, E.A.; Leroux, J.M.; Wang, B.; Crane, E.D.; Wong, W.; Morrison, K.M.; Crane, J.D.; et al. Genetic deletion of mast cell serotonin synthesis prevents the development of obesity and insulin resistance. Nat. Commun. 2020, 11, 463. [Google Scholar] [CrossRef]
- Altamura, S.; Lombardi, F.; Palumbo, P.; Cinque, B.; Ferri, C.; Del Pinto, R.; Pietropaoli, D. The Evolving Role of Neutrophils and Neutrophil Extracellular Traps (NETs) in Obesity and Related Diseases: Recent Insights and Advances. Int. J. Mol. Sci. 2024, 25, 13633. [Google Scholar] [CrossRef] [PubMed]
- Altamura, S.; Lombardi, F.; Palumbo, P.; Cinque, B.; Ferri, C.; Del Pinto, R.; Pietropaoli, D. Obesity-induced Endothelial Dysfunction is Prevented by Neutrophil Extracellular Trap Inhibition. Sci. Rep. 2018, 8, 4881. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chakarov, S. Eosinophils in obesity and obesity-associated disorders. Discov. Immunol. 2023, 2, kyad022. [Google Scholar] [CrossRef] [PubMed]
- Soedono, S.; Cho, K.W. Adipose tissue dendritic cells: Critical regulators of obesity-induced inflammation and insulin resistance. Int. J. Mol. Sci. 2021, 22, 8666. [Google Scholar] [CrossRef]
- Chen, I.; Awasthi, D.; Hsu, C.-L.; Song, M.; Chae, C.-S.; Dannenberg, A.J.; Cubillos-Ruiz, J.R. High-Fat Diet–Induced Obesity Alters Dendritic Cell Homeostasis by Enhancing Mitochondrial Fatty Acid Oxidation. J. Immunol. 2022, 209, 69–76. [Google Scholar] [CrossRef]
- Vijay, J.; Gauthier, M.-F.; Biswell, R.L.; Louiselle, D.A.; Johnston, J.J.; Cheung, W.A.; Belden, B.; Pramatarova, A.; Biertho, L.; Gibson, M.; et al. Single-cell analysis of human adipose tissue identifies depot- and disease-specific cell types. Nat. Metab. 2019, 2, 97–109. [Google Scholar] [CrossRef]
- Winer, D.A.; Winer, S.; Shen, L.; Wadia, P.P.; Yantha, J.; Paltser, G.; Tsui, H.; Wu, P.; Davidson, M.G.; Alonso, M.N.; et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 2011, 17, 610–617. [Google Scholar] [CrossRef]
- Frasca, D.; Romero, M.; Diaz, A.; Blomberg, B.B. Obesity accelerates age defects in B cells, and weight loss improves B cell function. Immun. Ageing 2023, 20, 35. [Google Scholar] [CrossRef]
- Wang, L.; Sun, P.; Wu, Y.; Wang, L. Metabolic tissue-resident CD8+ T cells: A key player in obesity-related diseases. Obes. Rev. 2021, 22, e13133. [Google Scholar] [CrossRef]
- Rocha, V.Z.; Folco, E.J.; Sukhova, G.; Shimizu, K.; Gotsman, I.; Vernon, A.H.; Libby, P. Interferon-γ, a Th1 cytokine, regulates fat inflammation: A role for adaptive immunity in obesity. Circ. Res. 2008, 103, 467–476. [Google Scholar] [CrossRef]
- Barbosa, P.; Pinho, A.; Lazaro, A.; Paula, D.; Campos, J.C.; Tralhao, J.G.; Pereira, M.J.; Paiva, A.; Laranjeira, P.; Carvalho, E.; et al. High percentage of immune Th1 and Tc1 cells infiltrating visceral adipose tissue in people with obesity. Obes. Res. Clin. Pract. 2024, 18, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Shi, X.; Zhang, B.; Liu, H.; Zhang, L.; Ding, W.; Zhao, Y. The imbalance of Th17/Th1/Tregs in patients with type 2 diabetes: Relationship with metabolic factors and complications. J. Mol. Med. 2011, 90, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan-Bogdan, M.; McDonnell, M.E.; Shin, H.; Rehman, Q.; Hasturk, H.; Apovian, C.M.; Nikolajczyk, B.S. Elevated Proinflammatory Cytokine Production by a Skewed T Cell Compartment Requires Monocytes and Promotes Inflammation in Type 2 Diabetes. J. Immunol. 2011, 186, 1162–1172. [Google Scholar] [CrossRef]
- Zi, C.; He, L.; Yao, H.; Ren, Y.; He, T.; Gao, Y. Changes of Th17 cells, regulatory T cells, Treg/Th17, IL-17 and IL-10 in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Endocrine 2022, 76, 263–272. [Google Scholar] [CrossRef]
- Gyllenhammer, L.E.; Lam, J.; Alderete, T.L.; Allayee, H.; Akbari, O.; Katkhouda, N.; Goran, M.I. Lower omental t-regulatory cell count is associated with higher fasting glucose and lower β-cell function in adults with obesity. Obesity 2016, 24, 1274–1282. [Google Scholar] [CrossRef]
- DiToro, D.; Winstead, C.J.; Pham, D.; Witte, S.; Andargachew, R.; Singer, J.R.; Wilson, C.G.; Zindl, C.L.; Luther, R.J.; Silberger, D.J.; et al. Differential IL-2 expression defines developmental fates of follicular versus nonfollicular helper T cells. Science 2018, 361, 6407. [Google Scholar] [CrossRef] [PubMed]
- Ruterbusch, M.; Pruner, K.B.; Shehata, L.; Pepper, M. In Vivo CD4+ T Cell Differentiation and Function: Revisiting the Th1/Th2 Paradigm. Annu. Rev. Immunol. 2020, 38, 705–725. [Google Scholar] [CrossRef]
- Bernstein, Z.J.; Shenoy, A.; Chen, A.; Heller, N.M.; Spangler, J.B. Engineering the IL-4/IL-13 axis for targeted immune modulation. Immunol. Rev. 2023, 320, 29–57. [Google Scholar] [CrossRef]
- Bao, K.; Reinhardt, R.L. The differential expression of IL-4 and IL-13 and its impact on type-2 immunity. Cytokine 2015, 75, 25–37. [Google Scholar] [CrossRef]
- Qiu, Y.; Nguyen, K.D.; Odegaard, J.I.; Cui, X.; Tian, X.; Locksley, R.M.; Palmiter, R.D.; Chawla, A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 2014, 157, 1292–1308. [Google Scholar] [CrossRef]
- Knudsen, N.H.; Stanya, K.J.; Hyde, A.L.; Chalom, M.M.; Alexander, R.K.; Liou, Y.-H.; Starost, K.A.; Gangl, M.R.; Jacobi, D.; Liu, S.; et al. Interleukin-13 drives metabolic conditioning of muscle to endurance exercise. Science 2020, 368, 6490. [Google Scholar] [CrossRef]
- Ricardo-Gonzalez, R.R.; Red Eagle, A.; Odegaard, J.I.; Jouihan, H.; Morel, C.R.; Heredia, J.E.; Mukundan, L.; Wu, D.; Locksley, R.M.; Chawla, A. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc. Natl. Acad. Sci. USA 2010, 107, 22617–22622. [Google Scholar] [CrossRef]
- Shiau, M.Y.; Lu, H.F.; Chang, Y.H.; Chiu, Y.C.; Shih, Y.L. Characterization of proteins regulated by interleukin-4 in 3T3-L1 adipocytes. SpringerPlus 2015, 4, 242. [Google Scholar] [CrossRef]
- Tsao, C.H.; Shiau, M.Y.; Chuang, P.H.; Chang, Y.H.; Hwang, J. Interleukin-4 regulates lipid metabolism by inhibiting adipogenesis and promoting lipolysis. J. Lipid Res. 2014, 55, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Shiau, M.-Y.; Chuang, P.-H.; Yang, C.-P.; Hsiao, C.-W.; Chang, S.-W.; Chang, K.-Y.; Liu, T.-M.; Chen, H.-W.; Chuang, C.-C.; Yuan, S.-Y.; et al. Mechanism of Interleukin-4 Reducing Lipid Deposit by Regulating Hormone-Sensitive Lipase. Sci. Rep. 2019, 9, 11974. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Yang, C.-P.; Wang, Y.-Y.; Hsiao, C.-W.; Chen, W.-Y.; Liao, S.-L.; Lo, Y.-L.; Chang, Y.-H.; Hong, C.-J.; Chen, C.-J. Interleukin-4 improves metabolic abnormalities in leptin-deficient and high-fat diet mice. Int. J. Mol. Sci. 2020, 21, 4451. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.D.; Li, T.; Ghannam, H.; Rau, C.M.; Masuda, M.Y.; Madura, J.A.; Jacobsen, E.A.; De Filippis, E. Linking adipose tissue eosinophils, IL-4, and leptin in human obesity and insulin resistance. JCI Insight 2024, 9, e170772. [Google Scholar] [CrossRef]
- Kim, H.J.; Jung, Y. The Emerging Role of Eosinophils as Multifunctional Leukocytes in Health and Disease. Immune Netw. 2020, 20, e24. [Google Scholar] [CrossRef]
- Molofsky, A.B.; Nussbaum, J.C.; Liang, H.-E.; Van Dyken, S.J.; Cheng, L.E.; Mohapatra, A.; Chawla, A.; Locksley, R.M. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J. Exp. Med. 2013, 210, 535–549. [Google Scholar] [CrossRef]
- Rozenberg, P.; Reichman, H.; Zab-Bar, I.; Itan, M.; Pasmanik-Chor, M.; Bouffi, C.; Qimron, U.; Bachelet, I.; Fulkerson, P.C.; Rothenberg, M.E.; et al. CD300f:IL-5 cross-talk inhibits adipose tissue eosinophil homing and subsequent IL-4 production. Sci. Rep. 2017, 7, 5922. [Google Scholar] [CrossRef]
- Luo, J.; Chen, W.; Liu, W.; Jiang, S.; Ye, Y.; Shrimanker, R.; Hynes, G.; Klenerman, P.; Pavord, I.D.; Xue, L.; et al. IL-5 antagonism reverses priming and activation of eosinophils in severe eosinophilic asthma. Mucosal Immunol. 2024, 17, 524–536. [Google Scholar] [CrossRef]
- Stanya, K.J.; Jacobi, D.; Liu, S.; Bhargava, P.; Dai, L.; Gangl, M.R.; Inouye, K.; Barlow, J.L.; Ji, Y.; Mizgerd, J.P.; et al. Direct control of hepatic glucose production by interleukin-13 in mice. J. Clin. Investig. 2012, 123, 261–271. [Google Scholar] [CrossRef] [PubMed]
- MacHado, M.V.; Yang, Y.; Diehl, A.M. The benefits of restraint: A pivotal role for IL-13 in hepatic glucose homeostasis. J. Clin. Investig. 2012, 123, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Duffen, J.; Zhang, M.; Masek-Hammerman, K.; Nunez, A.; Brennan, A.; Jones, J.E.; Morin, J.; Nocka, K.; Kasaian, M. Modulation of the IL-33/IL-13 Axis in Obesity by IL-13Rα2. J. Immunol. 2018, 200, 1347–1359. [Google Scholar] [CrossRef] [PubMed]
- Darkhal, P.; Gao, M.; Ma, Y.; Liu, D. Blocking high-fat diet-induced obesity, insulin resistance and fatty liver by overexpression of Il-13 gene in mice. Int. J. Obes. 2015, 39, 1292–1299. [Google Scholar] [CrossRef]
- Wunderlich, C.M.; Hövelmeyer, N.; Wunderlich, F.T. Mechanisms of chronic JAK-STAT3-SOCS3 signaling in obesity. JAKSTAT 2013, 2, e23878. [Google Scholar] [CrossRef]
- Kang, Y.E.; Kim, H.J.; Shong, M. Regulation of Systemic Glucose Homeostasis by T Helper Type 2 Cytokines. Diabetes Metab. J. 2019, 43, 549–559. [Google Scholar] [CrossRef]
- Webb, L.M.; Wojno, E.D.T. Exercising Immunity: Interleukin-13 Flexes Muscle. Immunity 2020, 52, 902–904. [Google Scholar] [CrossRef]
- Leiria, L.O.S.; Martins, M.A.; Saad, M.J.A. Obesity and asthma: Beyond TH2 inflammation. Metabolism 2015, 64, 172–181. [Google Scholar] [CrossRef]
- Ogulur, I.; Mitamura, Y.; Yazici, D.; Pat, Y.; Ardicli, S.; Li, M.; D’Avino, P.; Beha, C.; Babayev, H.; Zhao, B.; et al. Type 2 immunity in allergic diseases. Cell. Mol. Immunol. 2025, 22, 211–242. [Google Scholar] [CrossRef]
- Pinkerton, J.W.; Kim, R.Y.; Brown, A.C.; Rae, B.E.; Donovan, C.; Mayall, J.R.; Carroll, O.R.; Ali, M.K.; Scott, H.A.; Berthon, B.; et al. Relationship between type 2 cytokine and inflammasome responses in obesity-associated asthma. J. Allergy Clin. Immunol. 2022, 149, 1270–1280. [Google Scholar] [CrossRef]
- Phu, T.A.; Ng, M.; Vu, N.K.; Bouchareychas, L.; Raffai, R.L. IL-4 polarized human macrophage exosomes control cardiometabolic inflammation and diabetes in obesity. Mol. Ther. 2022, 30, 2274–2297. [Google Scholar] [CrossRef]
- Choi, E.W.; Lee, M.; Song, J.W.; Kim, K.; Lee, J.; Yang, J.; Lee, S.H.; Kim, I.Y.; Choi, J.-H.; Seong, J.K. Fas mutation reduces obesity by increasing IL-4 and IL-10 expression and promoting white adipose tissue browning. Sci. Rep. 2020, 10, 12001. [Google Scholar] [CrossRef]
- Chen, S.M.; Hsiao, C.W.; Chen, Y.J.; Hong, C.J.; Lin, J.C.; Yang, C.P.; Chang, Y.H. Interleukin-4 inhibits the hypothalamic appetite control by modulating the insulin-AKT and JAK-STAT signaling in leptin mutant mice. Environ. Toxicol. 2024, 39, 3980–3990. [Google Scholar] [CrossRef] [PubMed]
- Arndt, L.; Lindhorst, A.; Neugebauer, J.; Hoffmann, A.; Hobusch, C.; Alexaki, V.-I.; Ghosh, A.; Blüher, M.; Wolfrum, C.; Glaß, M.; et al. The Role of IL-13 and IL-4 in Adipose Tissue Fibrosis. Int. J. Mol. Sci. 2023, 24, 5672. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, M.; Patrawala, M.; Levy, J.M.; Shih, J.; Lee, F.E. Association of antieosinophil therapy with decreased body mass index in patients with severe asthma: A preliminary retrospective analysis. Ann. Allergy Asthma Immunol. 2019, 122, 649. [Google Scholar] [CrossRef] [PubMed]
- Ten Have, L.; Visser, E.; Meulmeester, F.L.; Bendien, S.A.; Braunstahl, G.-J.; Broeders, M.E.; Fieten, K.B.; Hashimoto, S.; van Huisstede, A.; Langeveld, B.; et al. Long-Term Weight Changes After Starting Anti–IL-5/5Ra Biologics in Severe Asthma: The Role of Oral Corticosteroids. J. Allergy Clin. Immunol. Pract. 2023, 11, 2748–2756.e3. [Google Scholar] [CrossRef]
- Beck, L.A.; Thaçi, D.; Hamilton, J.D.; Graham, N.M.; Bieber, T.; Rocklin, R.; Ming, J.E.; Ren, H.; Kao, R.; Simpson, E. Dupilumab Treatment in Adults with Moderate-to-Severe Atopic Dermatitis. N. Engl. J. Med. 2014, 371, 130–139. [Google Scholar] [CrossRef]
- Simpson, E.L.; Pink, A.E.; Blauvelt, A.; Gooderham, M.; Armstrong, A.W.; Worm, M.; Katoh, N.; Peris, K.; Puig, L.; Barbarot, S. Tralokinumab Efficacy Over 1 Year in Adults with Moderate-to-Severe Atopic Dermatitis: Pooled Data from Two Phase III Trials. Am. J. Clin. Dermatol. 2023, 24, 939–952. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Guttman-Yassky, E.; Thaçi, D.; Irvine, A.D.; Stein Gold, L.; Blauvelt, A.; Simpson, E.L.; Chu, C.-Y.; Liu, Z.; Gontijo Lima, R. Two Phase 3 Trials of Lebrikizumab for Moderate-to-Severe Atopic Dermatitis. N. Engl. J. Med. 2023, 388, 1080–1091. [Google Scholar] [CrossRef]
- Ortega, H.G.; Liu, M.C.; Pavord, I.D.; Brusselle, G.G.; FitzGerald, J.M.; Chetta, A.; Humbert, M.; Katz, L.E.; Keene, O.N.; Yancey, S.W.; et al. Mepolizumab Treatment in Patients with Severe Eosinophilic Asthma. N. Engl. J. Med. 2014, 371, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Cushen, B.; Menzies-Gow, A. Benralizumab: An updated treatment of eosinophilic asthma. Expert Rev. Respir. Med. 2020, 14, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Tomkinson, A.; Tepper, J.; Morton, M.; Bowden, A.; Stevens, L.; Harris, P.; Lindell, D.; Fitch, N.; Gundel, R.; Getz, E.B. Inhaled vs subcutaneous effects of a dual IL-4/IL-13 antagonist in a monkey model of asthma. Allergy Eur. J. Allergy Clin. Immunol. 2010, 65, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Kau, A.L.; Korenblat, P.E. Anti-Interleukin 4 and 13 for Asthma Treatment in the Era of Endotypes. Curr. Opin. Allergy Clin. Immunol. 2014, 14, 570–575. [Google Scholar] [CrossRef]
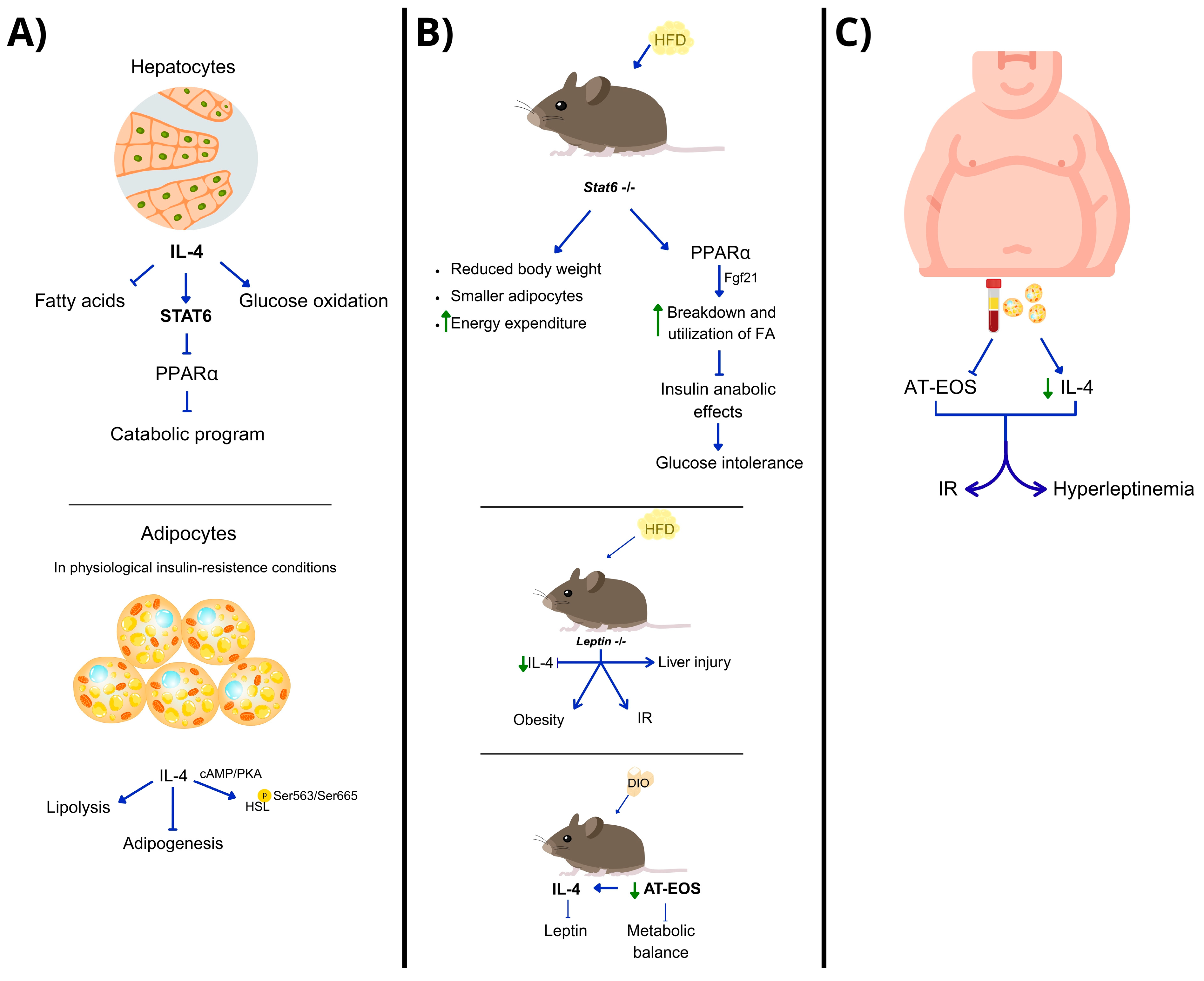
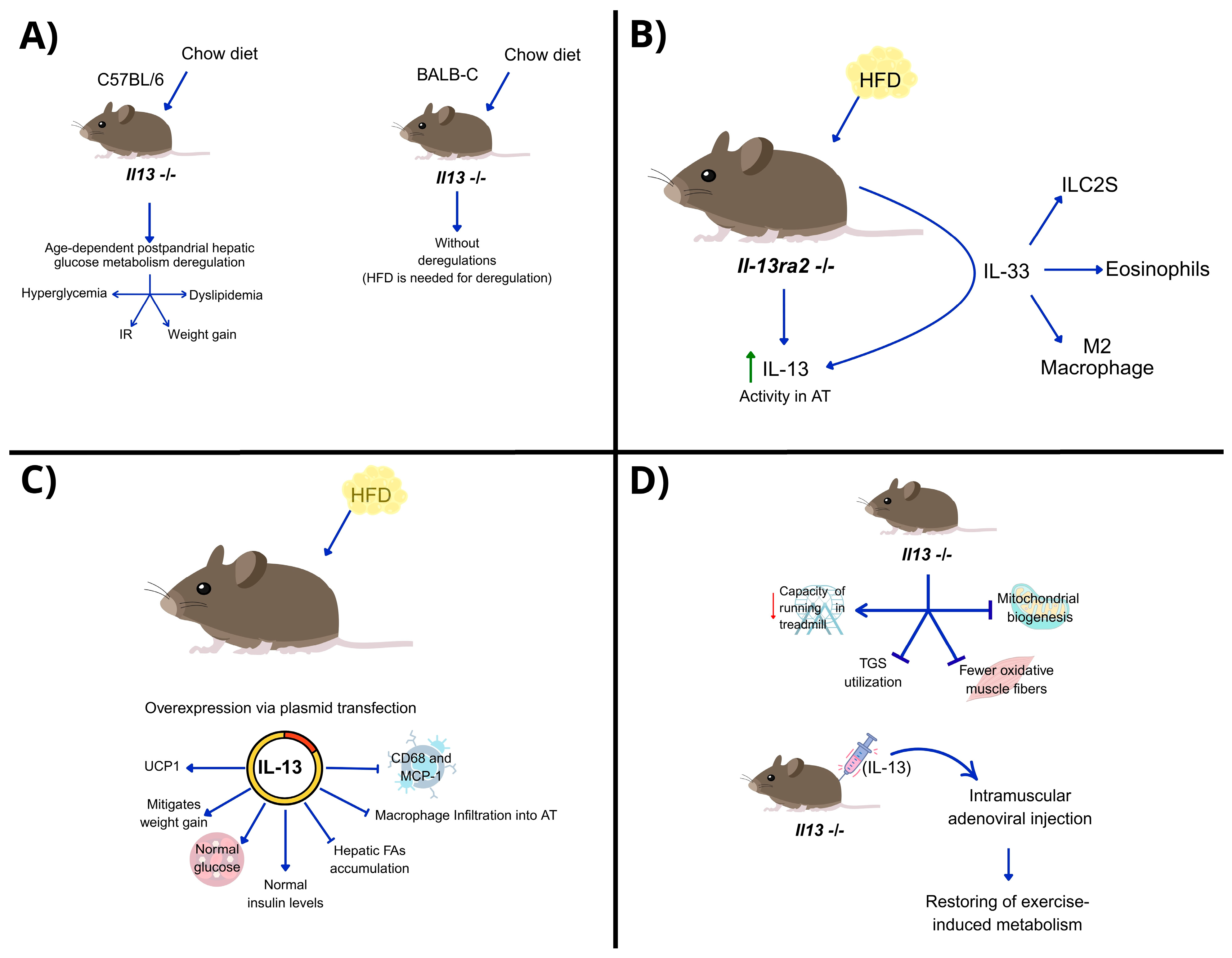
| Area | Pathways Involved | Therapeutic Implications | Ref. |
|---|---|---|---|
| Liver (hepatocytes) | IL-4 → STAT6 → PPARα trans-repression | IL-4 may regulate hepatic metabolism; STAT6 is key for balance between lipid and glucose metabolism. | [45] |
| White adipose tissue (WAT)/adipocytes | IL-4→ STAT6 → ↓ adipogenesis IL-4 → HSL activation via cAMP/PKA | IL-4 enhances lipid breakdown in WAT; potential in early intervention before IR develops. | [46,47,48] |
| HFD/DIO-leptin-deficient models and 3T3-L1 cell line | ↓ IL-4 → obesity, hyperglycemia, IR IL-4 deficiency → ↓ AT-EOS IL-4 supplementation restores STAT3/STAT6/AKT | IL-4 restores metabolic balance in obesity and leads to browning of white adipocytes. | [49] |
| Human adipocytes (ex vivo or in vitro) | IL-4 regulates leptin via eosinophils | IL-4 could regulate hyperleptinemia and improve outcomes in obesity. | [50] |
| Area | Pathways Involved | Therapeutic Implications | Ref. |
|---|---|---|---|
| Visceral Adipose Tissue (VAT) | IL-5 → eosinophil release and tissue distribution | IL-5 is essential for VAT eosinophil homeostasis, impacting metabolic regulation. | [51] |
| Eosinophil recruitment | IL-13 eotaxins IL-4 endothelial integrins | Redundant mechanisms support eosinophil recruitment; IL-5 is critical but not exclusive. | [51] |
| Innate lymphoid cells type 2 (ILC2s) | IL-33 → ILC2 activation → IL5/IL13 production | Targeting ILC2 activation via IL-33 may enhance VAT immune–metabolic function. | [52] |
| Regulation by CD300f receptor | CD300f → ERK/Akt signaling → IL-5 and IL-4 modulation | Modulating CD300f may influence local immune–metabolic function. | [53] |
| Fibrosis and asthma models | IL-5 → TGF-β pathway → profibrotic eosinophil activity | IL-5 may be a therapeutic target in fibrotic and inflammatory diseases. | [54] |
| Area | Pathway Involved | Therapeutic Implications | Ref. |
|---|---|---|---|
| Liver (hepatocytes) | IL-13 → STAT3 phosphorylation → ↓ gluconeogenesis | IL-13 regulates glucose metabolism via STAT3, especially under metabolic stress. | [56] |
| Adipose tissue (AT) | IL-13Ra2 → ↓ IL-13 activity IL-33 → ↑ IL-13 and anti-inflammatory immune cells | Blocking IL-13Ra2 may restore IL-13 function in obesity. IL-13 reduces adipose inflammation. | [57] |
| Skeletal muscle | IL-13 → ↑ UCp1, ERRα and ERRγ expression → ↑ thermogenesis and FA metabolism | IL-13 supports muscle metabolic reprogramming and exercise adaptation via non-STAT6 signaling. | [58,59] |
| Exercise-induced adaptation | Endurance exercise → ↑ILC2 → IL-13/STAT3 activation | IL-13 may mediate physiological adaptation to exercise and stress; potential therapeutic target for metabolic muscle disorders. | [44] |
| Cytokine | Model/Study Design | Main Findings | Ref. |
|---|---|---|---|
| IL-4 | In vitro (THP-1 macrophages, 3T3-L1 adipocytes) + in vivo (mice, HFD, WT, Ldlr−/−) | IL-4-polarized (M2-like) macrophage exosomes increased anti-inflammatory miRNAs, reduced miR-33, enhanced GLUT4, UCP1, OXPHOS, mitochondrial function, and lipophagy; improved glucose tolerance and reduced AT/liver inflammation. | [65] |
| IL-4 | In vivo (Fas-mutant MRL/lpr mice, HFD, cold exposure) | Fas mutation increased IL-4, IL-10, UCP1, and tyrosine hydroxylase; promoted M2 macrophages and WAT browning, protecting against HFD-induced obesity. | [66] |
| IL-4 | In vivo (Leptin145E/145E mice) | IL-4 reduced body weight, food intake, glucose; modulated hypothalamic neuropeptides (↓AgRP, NPY; ↑POMC); improved insulin–AKT and JAK–STAT signaling; increased UCP1 in WAT. | [67] |
| IL-4 | Ex vivo WAT organotypic culture + in vivo (mice) | IL-4 and IL-13 induced fibrosis-related gene/protein expression via IL-4R and macrophages; partially confirmed in vivo; role in human obesity unclear. | [68] |
| IL-5 | Clinical retrospective study (n = 51, severe asthma, anti-IL-5 therapy) | Average BMI decrease of ~1 point; greater reduction in obese subgroup (~1.7 points); confounding factors limit obesity-specific conclusions. | [69] |
| IL-5 | Clinical study (patients with severe asthma, anti-IL-5 therapy) | Similar BMI changes; no consistent differences between agents; effects on body composition unclear. | [70] |
| IL-5 | In vivo (Il5 Tg/Cd300f−/− mice, HFD) | Increased eosinophils and IL-4 in AT; enhanced M2 macrophages; less weight gain and better glucose tolerance; CD300f deletion amplified these effects. | [70] |
| Agent/Modality | Target/Pathway | Type | Approved Indications (Non-Metabolic) | Evidence in Obesity/Metabolic Disease | Clinical Trials in Obesity | Ref. |
|---|---|---|---|---|---|---|
| Dupilumab | IL-4Rα (blocks IL-4 and IL-13 signaling) | Antagonist mAb | Atopic dermatitis, eosinophilic asthma, CRSwNP, eosinophilic esophagitis, prurigo nodularis | No interventional obesity trials: metabolic effects not established | None identified | [71] |
| Tralokinumab | IL-13 | Antagonist mAb | Atopic dermatitis | No obesity trials; metabolic outcomes not primary endpoints | None identified | [72] |
| Lebrikizumab | IL-13 | Antagonist mAb | Atopic dermatitis (regional approvals) | No obesity trials reported | None identified | [73] |
| Mepolizumab | IL-5 | Antagonist mAb | Severe eosinophilic asthma; EGPA; HES | Retrospective asthma cohort: mean BMI −1.0 overall; −1.7 in obese subgroup; confounded by corticosteroid changes | None identified | [74] |
| Reslizumab | IL-5 | Antagonist mAb | Severe eosinophilic asthma | Heterogeneous BMI changes in asthma cohorts | None identified | [75] |
| Benralizumab | IL-5Rα (eosinophil depletion via ADCC) | Antagonist mAb | Severe eosinophilic asthma | No consistent metabolic signal reported | None identified | [75] |
| IL-4-polarized macrophage exosomes | IL-4-induced M2 programs (miR-21/99a/146b/378a↑, miR-33↓) | Extracellular vesicles (preclinical) | None | In vitro and mice: anti-inflammatory; ↑ browning; improved glucose tolerance | None (preclinical only) | [65] |
| Pitrakinra (Aerovant) | IL-4/IL-13 (antagonist peptide) | Investigational (discontinued) | None | No obesity data; asthma and eczema studies only | None identified | [76] |
| Pascolizumab/Anrukinzumab | IL-4 or IL-13 | Antagonist mAbs (discontinued) | None | No metabolic evidence | None identified | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méndez-García, L.A.; Solleiro-Villavicencio, H.; Bueno-Hernández, N.; Cérbulo-Vázquez, A.; Escobedo, G.; Esquivel-Velázquez, M.; Fonseca-Sánchez, M.A. Role of the Th2-like Immune Response in Obesity: IL-4 as a Metabolic Regulator and IL-13 as an Effector of Muscle Energy Metabolism. Biomedicines 2025, 13, 2208. https://doi.org/10.3390/biomedicines13092208
Méndez-García LA, Solleiro-Villavicencio H, Bueno-Hernández N, Cérbulo-Vázquez A, Escobedo G, Esquivel-Velázquez M, Fonseca-Sánchez MA. Role of the Th2-like Immune Response in Obesity: IL-4 as a Metabolic Regulator and IL-13 as an Effector of Muscle Energy Metabolism. Biomedicines. 2025; 13(9):2208. https://doi.org/10.3390/biomedicines13092208
Chicago/Turabian StyleMéndez-García, Lucía A., Helena Solleiro-Villavicencio, Nallely Bueno-Hernández, Arturo Cérbulo-Vázquez, Galileo Escobedo, Marcela Esquivel-Velázquez, and Miguel A. Fonseca-Sánchez. 2025. "Role of the Th2-like Immune Response in Obesity: IL-4 as a Metabolic Regulator and IL-13 as an Effector of Muscle Energy Metabolism" Biomedicines 13, no. 9: 2208. https://doi.org/10.3390/biomedicines13092208
APA StyleMéndez-García, L. A., Solleiro-Villavicencio, H., Bueno-Hernández, N., Cérbulo-Vázquez, A., Escobedo, G., Esquivel-Velázquez, M., & Fonseca-Sánchez, M. A. (2025). Role of the Th2-like Immune Response in Obesity: IL-4 as a Metabolic Regulator and IL-13 as an Effector of Muscle Energy Metabolism. Biomedicines, 13(9), 2208. https://doi.org/10.3390/biomedicines13092208







