Application of Human Epineural Patch (hEP) as a Novel Strategy for Nerve Protection and Enhancement of Regeneration After Nerve Crush Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
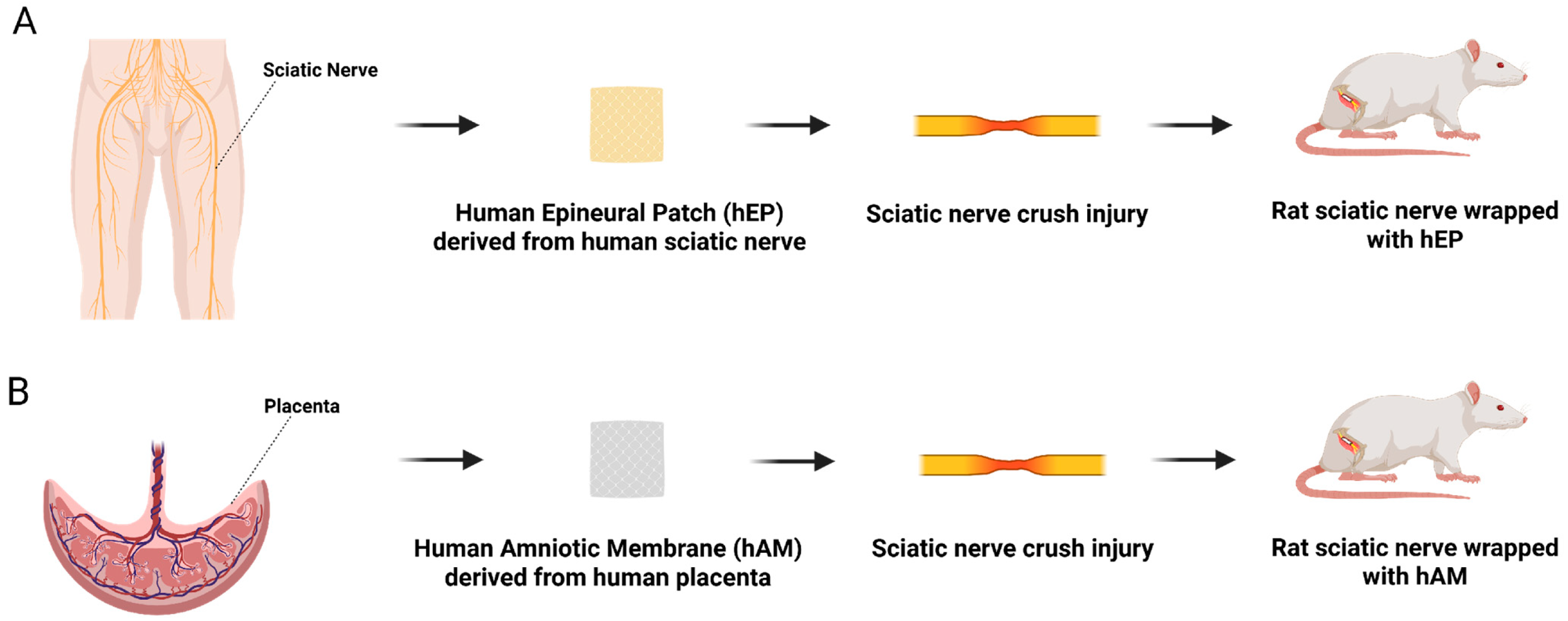
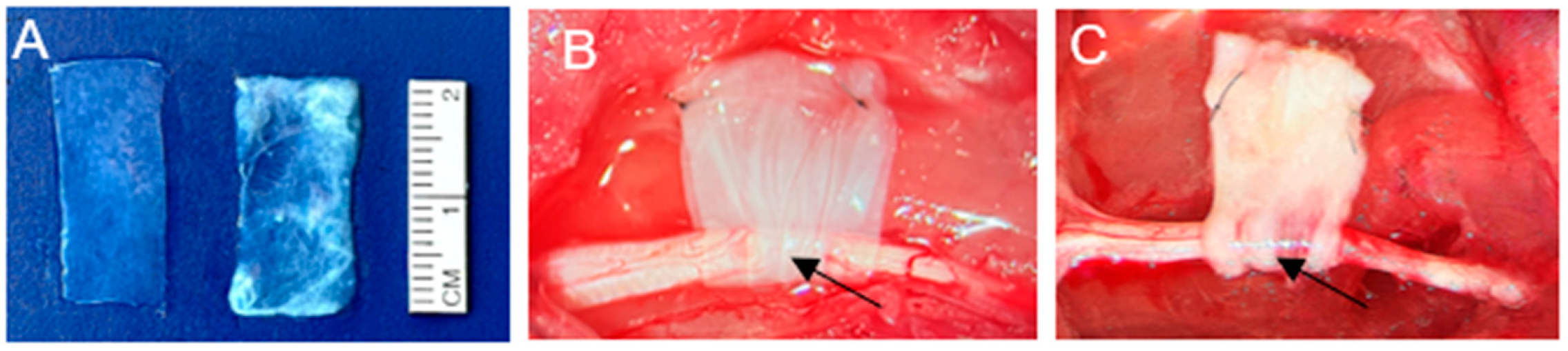
2.2. Human Epineural Patch Creation
2.3. Human Amniotic Membrane
2.4. Surgical Procedure
Postsurgical Supportive Treatment
2.5. Experimental Groups and Study Design
2.6. Functional Tests and Histomorphometric Analysis
2.6.1. Functional Motor Assessment by Toe-Spread Test
2.6.2. Functional Sensory Assessment with Pinprick Test
2.6.3. Macroscopic Assessment of the hEP and hAM at the Sciatic Nerve Injury Site
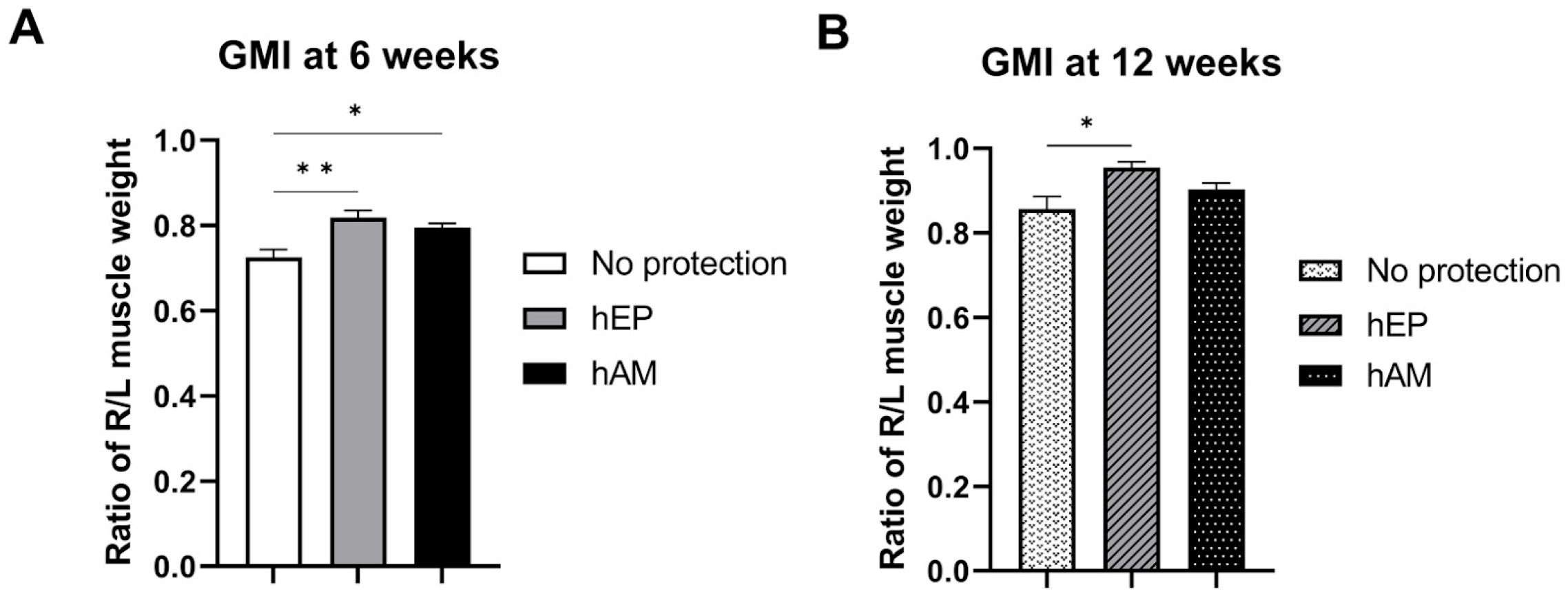
2.6.4. Assessment of Muscle Denervation Atrophy by Gastrocnemius Muscle Index
2.6.5. Histomorphometric Analysis
2.7. Assessment of Immune Responses
2.8. Statistical Analysis
3. Results Following Sciatic Nerve Crush Injury in a 6-Week Study
3.1. Toe-Spread Test
3.2. Pinprick Test
3.3. Gastrocnemius Muscle Index
3.4. Myelin Thickness
3.5. Fiber Diameter
3.6. Percentage of Myelinated Fibers
3.7. Axonal Density
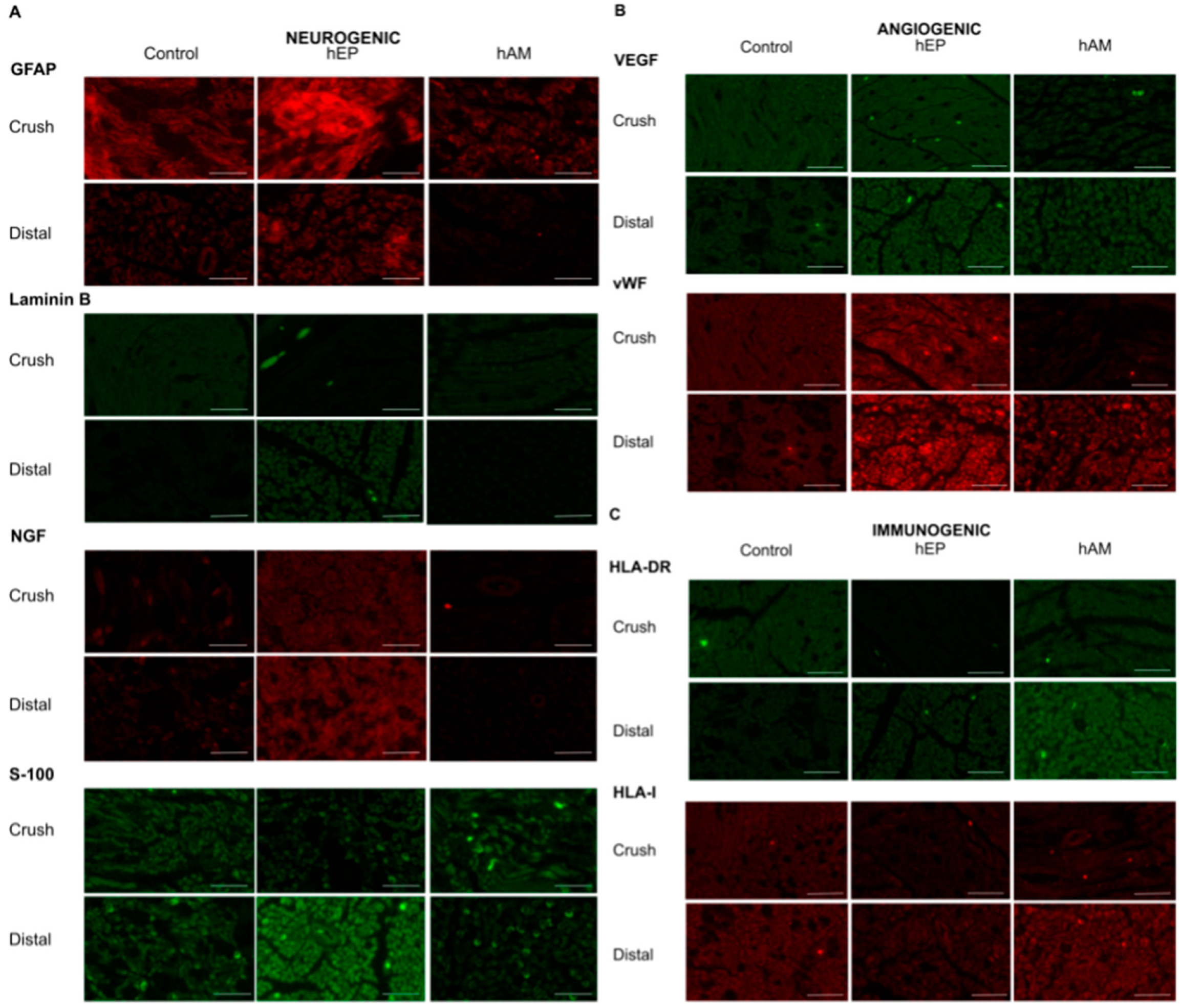
3.8. GFAP Expression
3.9. Laminin B Expression
3.10. NGF Expression
3.11. S-100 Expression
3.12. VEGF Expression
3.13. vWF Expression
3.14. HLA-DR Expression
3.15. HLA-I Expression
4. Results Following Sciatic Nerve Crush Injury in a 12-Week Study
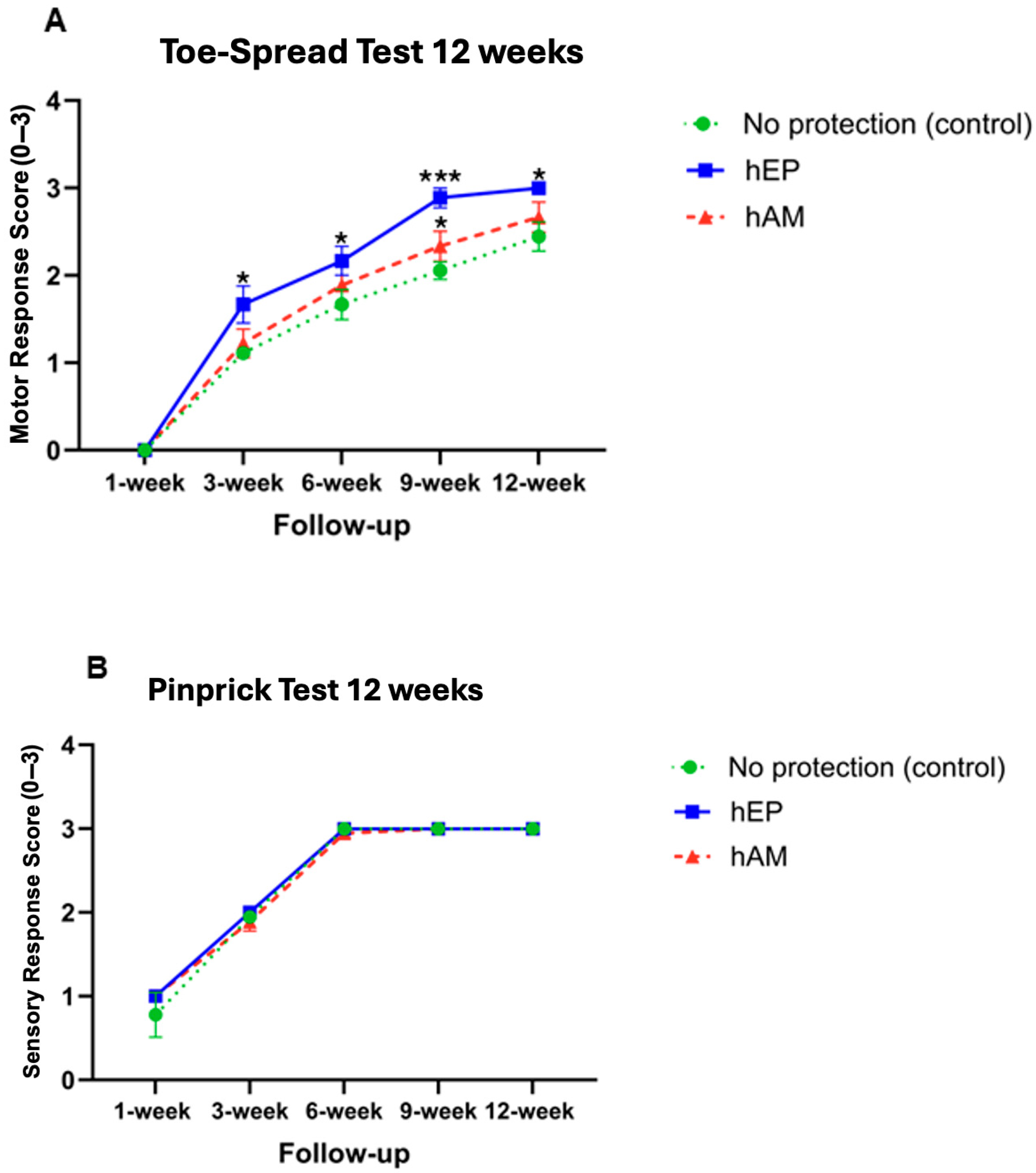
4.1. Toe-Spread Test
4.2. Pinprick Test
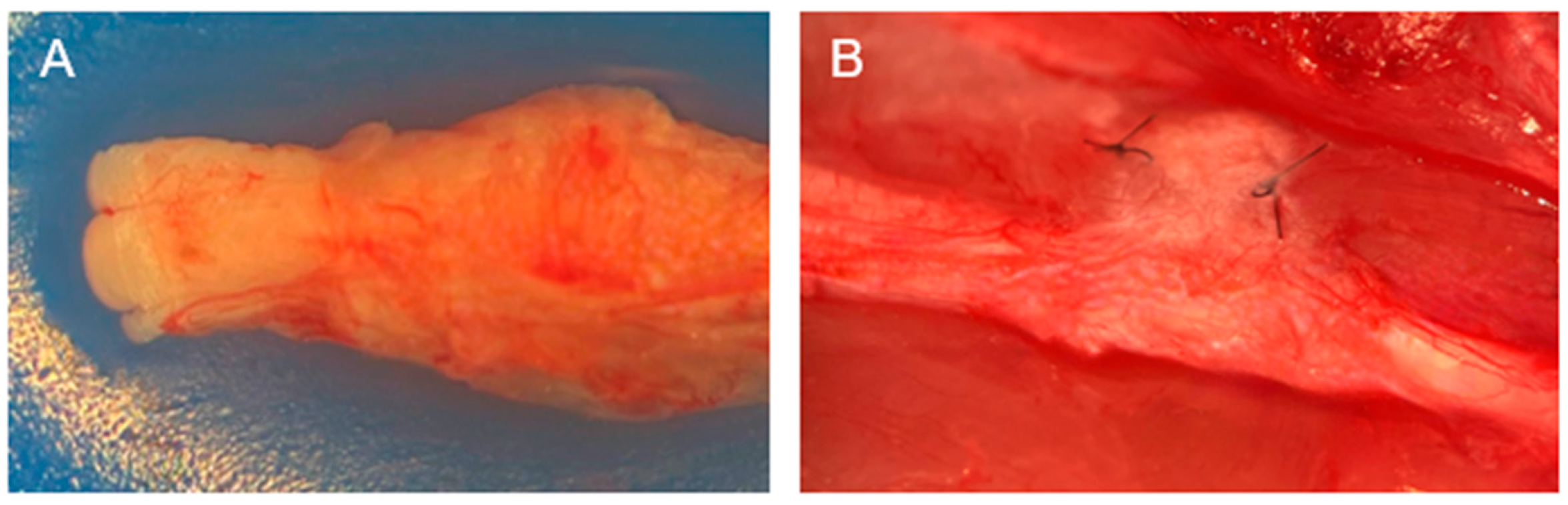
4.3. Gross Assessment
4.4. Gastrocnemius Muscle Index
4.5. Myelin Thickness
4.6. Fiber Diameter
4.7. Myelinated Fibers Percentage
4.8. Axonal Density
4.9. GFAP Expression
4.10. Laminin B Expression
4.11. NGF Expression
4.12. S-100 Expression
4.13. VEGF Expression
4.14. vWF Expression
4.15. HLA-DR Expression
4.16. HLA-I Expression
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAALAC | American Association for the Accreditation of Laboratory Animal Care |
| ACC | Animal Care Committee |
| CTR | Crush/Transection/Repair |
| GFAP | Glial Fibrillary Acidic Protein |
| GMI | Gastrocnemius Muscle Index |
| hAM | human Amniotic Membrane |
| hEP | human Epineural Patch |
| HLA-DR | Human Leukocyte Antigen—DR |
| HLA-I | Human Leukocyte Antigen—Class I |
| MTF | Musculoskeletal Transplant Foundation |
| NGF | Nerve Growth Factor |
| S-100 | S-100 Protein |
| SEM | Standard Error of the Mean |
| VEGF | Vascular Endothelial Growth Factor |
| vWF | von Willebrand Factor |
References
- Kretschmer, T.; Antoniadis, G.; Braun, V.; Rath, S.A.; Richter, H.P. Evaluation of iatrogenic lesions in 722 surgically treated cases of peripheral nerve trauma. J. Neurosurg. 2001, 94, 905–912. [Google Scholar] [CrossRef]
- Modrak, M.; Talukder, M.A.H.; Gurgenashvili, K.; Noble, M.; Elfar, J.C. Peripheral nerve injury and myelination: Potential therapeutic strategies. J. Neurosci. Res. 2020, 98, 780–795. [Google Scholar] [CrossRef] [PubMed]
- Lopes, B.; Sousa, P.; Alvites, R.; Branquinho, M.; Sousa, A.C.; Mendonça, C.; Atayde, L.M.; Luís, A.L.; Varejão, A.S.P.; Maurício, A.C. Peripheral Nerve Injury Treatments and Advances: One Health Perspective. Int. J. Mol. Sci. 2022, 23, 918. [Google Scholar] [CrossRef] [PubMed]
- Kuffler, D.P.; Foy, C. Restoration of Neurological Function Following Peripheral Nerve Trauma. Int. J. Mol. Sci. 2020, 21, 1808. [Google Scholar] [CrossRef]
- Siemionow, M.; Demir, Y.; Mukherjee, A.L. Repair of peripheral nerve defects with epineural sheath grafts. Ann. Plast. Surg. 2010, 65, 546–554. [Google Scholar] [CrossRef]
- Selim, O.A.; Lakhani, S.; Midha, S.; Mosahebi, A.; Kalaskar, D.M. Three-Dimensional Engineered Peripheral Nerve: Toward a New Era of Patient-Specific Nerve Repair Solutions. Tissue Eng. Part B Rev. 2022, 28, 295–335. [Google Scholar] [CrossRef]
- Wu, S.; Shen, W.; Ge, X.; Ao, F.; Zheng, Y.; Wang, Y.; Jia, X.; Mao, Y.; Luo, Y. Advances in Large Gap Peripheral Nerve Injury Repair and Regeneration with Bridging Nerve Guidance Conduits. Macromo. Biosci. 2023, 26, e2300078. [Google Scholar] [CrossRef] [PubMed]
- Siemionow, M.; Uygur, S.; Ozturk, C.; Siemionow, K. Techniques and materials for enhancement of peripheral nerve regeneration: A literature review. Microsurgery 2013, 33, 318–328. [Google Scholar] [CrossRef]
- Niknejad, H.; Peirovi, H.; Jorjani, M.; Ahmadiani, A.; Ghanavi, J.; Seifalian, A.M. Properties of the amniotic membrane for potential use in tissue engineering. Eur. Cells Mater. 2008, 15, 88–99. [Google Scholar] [CrossRef]
- Mamede, A.C.; Botelho, M.F. Amniotic Membrane: Origin, Characterization, and Medical Applications; Springer: Dordrecht, The Netherlands, 2015. [Google Scholar]
- Kim, S.S.; Sohn, S.K.; Lee, K.Y.; Lee, M.J.; Roh, M.S.; Kim, C.H. Use of human amniotic membrane wrap in reducing perineural adhesions in a rabbit model of ulnar nerve neurorrhaphy. J. Hand Surg. 2010, 35, 214–219. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Yang, J.; Fan, Z.H.; Wang, D.L.; Wang, Y.Y.; Zhang, T.; Yu, L.M.; Yu, C.Y. Fresh human amniotic membrane effectively promotes the repair of injured common peroneal nerve. Neural Regen. Res. 2019, 14, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Leal-Marin, S.; Kern, T.; Hofmann, N.; Pogozhykh, O.; Framme, C.; Börgel, M.; Figueiredo, C.; Glasmacher, B.; Gryshkov, O. Human Amniotic Membrane: A review on tissue engineering, application, and storage. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1198–1215. [Google Scholar] [CrossRef]
- Ramuta, T.Ž.; Kreft, M.E. Human Amniotic Membrane and Amniotic Membrane-Derived Cells: How Far Are We from Their Use in Regenerative and Reconstructive Urology? Cell Transplant. 2018, 27, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Lubiatowski, P.; Unsal, F.M.; Nair, D.; Ozer, K.; Siemionow, M. The epineural sleeve technique for nerve graft reconstruction enhances nerve recovery. Microsurgery 2008, 28, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Siemionow, M.; Duggan, W.; Brzezicki, G.; Klimczak, A.; Grykien, C.; Gatherwright, J.; Nair, D. Peripheral nerve defect repair with epineural tubes supported with bone marrow stromal cells: A preliminary report. Ann. Plast. Surg. 2011, 67, 73–84. [Google Scholar] [CrossRef]
- Siemionow, M.; Uygur, S.; Madajka, M. Application of Epineural Sheath as a Novel Approach for Fat Volume Maintenance. Ann. Plast. Surg. 2017, 79, 606–612. [Google Scholar] [CrossRef]
- Siemionow, M.; Bobkiewicz, A.; Cwykiel, J.; Uygur, S.; Francuzik, W. Epineural Sheath Jacket as a New Surgical Technique for Neuroma Prevention in the Rat Sciatic Nerve Model. Ann. Plast. Surg. 2017, 79, 377–384. [Google Scholar] [CrossRef]
- Klimczak, A.; Siemionow, M.; Futoma, K.; Jundzill, A.; Patrzalek, D. Assessment of immunologic, proangiogenic and neurogenic properties of human peripheral nerve epineurium for potential clinical application. Histol. Histopathol. 2017, 32, 1197–1205. [Google Scholar] [CrossRef]
- Siemionow, M.; Cwykiel, J.; Uygur, S.; Kwiecien, G.; Oztürk, C.; Szopinski, J.; Madajka, M. Application of epineural sheath conduit for restoration of 6-cm long nerve defects in a sheep median nerve model. Microsurgery 2019, 39, 332–339. [Google Scholar] [CrossRef]
- Siemionow, M.; Strojny, M.M.; Kozlowska, K.; Brodowska, S.; Grau-Kazmierczak, W.; Cwykiel, J. Application of Human Epineural Conduit Supported with Human Mesenchymal Stem Cells as a Novel Therapy for Enhancement of Nerve Gap Regeneration. Stem Cell Rev. Rep. 2022, 18, 642–659. [Google Scholar] [CrossRef]
- Kozłowska, K.; Różczka, K.; Strojny, M.M.; Brodowska, S.; Lopez, A.; Siemionow, M. Human Mesenchymal Stem Cells Enhance Nerve Regeneration in Nerve Gap Repair with Human Epineural Conduit of a Large—Unmatched Diameter. J. Surg. 2022, 10, 193–205. [Google Scholar] [CrossRef]
- Strojny, M.M.; Kozlowska, K.; Brodowska, S.; Różczka, K.; Siemionow, M. Assessment of Human Epineural Conduit of Different Size Diameters on Efficacy of Nerve Regeneration and Functional Outcomes. J. Reconstr. Microsurg. 2023, 39, 392–404. [Google Scholar] [CrossRef]
- Gudemez, E.; Ozer, K.; Cunningham, B.; Siemionow, K.; Browne, E.; Siemionow, M. Dehydroepiandrosterone as an enhancer of functional recovery following crush injury to rat sciatic nerve. Microsurgery 2002, 22, 234–241. [Google Scholar] [CrossRef]
- Siemionow, M.; Radecka, W.; Kozlowska, K.; Chambily, L.; Brodowska, S.; Kuc, D.; Filipek, G.; Budzynska, K. Protective Effect of the Human Epineural Patch Application after Sciatic Nerve Crush Injury Followed by Nerve Transection and End-to-End Repair. Arch. Immunol. Ther. Exp. 2025, 73. [Google Scholar] [CrossRef]
- Long, B.; Liang, S.Y.; Gottlieb, M. Crush injury and syndrome: A review for emergency clinicians. Am. J. Emerg. Med. 2023, 69, 180–187. [Google Scholar] [CrossRef]
- Bage, T.; Power, D.M. Iatrogenic peripheral nerve injury: A guide to management for the orthopaedic limb surgeon. EFORT Open Rev. 2021, 6, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.C.; Leung, Y.Y. Innovations in Peripheral Nerve Regeneration. Bioengineering 2024, 11, 444. [Google Scholar] [CrossRef] [PubMed]
- Czarnecki, P.; Huber, J.; Szymankiewicz-Szukała, A.; Górecki, M.; Romanowski, L. End-to-Side vs. Free Graft Nerve Reconstruction-Experimental Study on Rats. Int. J. Mol. Sci. 2023, 24, 10428. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Hong, J.; Piao, Y.; Shin, H.J.; Lee, S.J.; Rhyu, I.J.; Yi, M.H.; Kim, J.; Kim, D.W.; Beom, J. Extracorporeal shockwave therapy enhances peripheral nerve remyelination and gait function in a crush model. Adv. Clin. Exp. Med. 2020, 29, 819–824. [Google Scholar] [CrossRef]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Qasim, M.; Zafar, S.; Aziz, N.; Razzaq, A.; Hussain, R.; de Aguilar, J.G.; et al. Current Status of Therapeutic Approaches against Peripheral Nerve Injuries: A Detailed Story from Injury to Recovery. Int. J. Biol. Sci. 2020, 16, 116–134. [Google Scholar] [CrossRef]
- Siemionow, M.; Brzezicki, G. Chapter 8: Current techniques and concepts in peripheral nerve repair. Int. Rev. Neurobiol. 2009, 87, 141–172. [Google Scholar] [CrossRef]
- Thakker, A.; Sharma, S.C.; Hussain, N.M.; Devani, P.; Lahiri, A. Nerve wrapping for recurrent compression neuropathy: A systematic review. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 549–559. [Google Scholar] [CrossRef]
- Radecka, W.; Nogalska, W.; Siemionow, M. Peripheral Nerve Protection Strategies: Recent Advances and Potential Clinical Applications. J. Funct. Biomater. 2025, 16, 153. [Google Scholar] [CrossRef]
- Crosio, A.; Ronchi, G.; Fornasari, B.E.; Odella, S.; Raimondo, S.; Tos, P. Experimental Methods to Simulate and Evaluate Postsurgical Peripheral Nerve Scarring. J. Clin. Med. 2021, 10, 1613. [Google Scholar] [CrossRef] [PubMed]
- Kerns, J.M.; Walter, J.S.; Patetta, M.J.; Sood, A.; Hussain, A.K.; Chung, J.J.; Deshpande, A.; DesLaurier, J.T.; Dieter, R.A.; Siemionow, M.; et al. Histological Assessment of Wallerian Degeneration of the Rat Tibial Nerve Following Crush and Transection Injuries. J. Reconstr. Microsurg. 2021, 37, 391–404. [Google Scholar] [CrossRef]
- Wolfe, E.M.; Mathis, S.A.; de la Olivo Muñoz, N.; Ovadia, S.A.; Panthaki, Z.J. Comparison of human amniotic membrane and collagen nerve wraps around sciatic nerve reverse autografts in a rat model. Biomater. Biosyst. 2022, 6, 100048. [Google Scholar] [CrossRef] [PubMed]
- Mayrhofer-Schmid, M.; Klemm, T.T.; Aman, M.; Kneser, U.; Eberlin, K.R.; Harhaus, L.; Boecker, A.H. Shielding the Nerve: A Systematic Review of Nerve Wrapping to Prevent Adhesions in the Rat Sciatic Nerve Model. J. Pers. Med. 2023, 13, 1431. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, S.; Markal, N.; Siemionow, K.; Araneo, B.; Siemionow, M. Effect of subepineurial dehydroepiandrosterone treatment on healing of transected nerves repaired with the epineurial sleeve technique. Microsurgery 2003, 23, 49–55. [Google Scholar] [CrossRef]
- Tetik, C.; Ozer, K.; Ayhan, S.; Siemionow, K.; Browne, E.; Siemionow, M. Conventional versus epineural sleeve neurorrhaphy technique: Functional and histomorphometric analysis. Ann. Plast. Surg. 2002, 49, 397–403. [Google Scholar] [CrossRef]
- Fenelon, M.; Etchebarne, M.; Siadous, R.; Grémare, A.; Durand, M.; Sentilhes, L.; Torres, Y.; Catros, S.; Gindraux, F.; L’Heureux, N.; et al. Assessment of fresh and preserved amniotic membrane for guided bone regeneration in mice. J. Biomed. Mater. Res. A 2020, 108, 2044–2056. [Google Scholar] [CrossRef]
- Fénelon, M.; Catros, S.; Meyer, C.; Fricain, J.C.; Obert, L.; Auber, F.; Louvrier, A.; Gindraux, F. Applications of Human Amniotic Membrane for Tissue Engineering. Membranes 2021, 11, 387. [Google Scholar] [CrossRef] [PubMed]
- Law, E.J.; Taib, H.; Berahim, Z. Amniotic Membrane: An Approach to Periodontal Regeneration. Cureus 2022, 14, e27832. [Google Scholar] [CrossRef] [PubMed]
- Bolívar, S.; Udina, E. Preferential regeneration and collateral dynamics of motor and sensory neurons after nerve injury in mice. Exp. Neurol. 2022, 358, 114227. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Sun, C.; Zhao, H.; Lin, H.; Han, Q.; Wang, J.; Ma, H.; Chen, B.; Xiao, Z.; Dai, J. Improvement of sciatic nerve regeneration using laminin-binding human NGF-beta. PLoS ONE 2009, 4, e6180. [Google Scholar] [CrossRef]
- Bélanger, E.; Henry, F.P.; Vallée, R.; Randolph, M.A.; Kochevar, I.E.; Winograd, J.M.; Lin, C.P.; Côté, D. In vivo evaluation of demyelination and remyelination in a nerve crush injury model. Biomed. Opt. Express. 2011, 2, 2698–2708. [Google Scholar] [CrossRef]
- Knöferle, J.; Koch, J.C.; Ostendorf, T.; Michel, U.; Planchamp, V.; Vutova, P.; Tönges, L.; Stadelmann, C.; Brück, W.; Bähr, M.; et al. Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 6064–6069. [Google Scholar] [CrossRef]
- Agoston, D.V. How to Translate Time? The Temporal Aspect of Human and Rodent Biology. Front. Neurol. 2017, 17, 92. [Google Scholar] [CrossRef]

| Experimental Group Number | Repair Method | Number of Athymic Nude Rats per Group |
|---|---|---|
| 6 weeks study | ||
| 1 | no protective wrapping | n = 6 |
| 2 | hEP | n = 6 |
| 3 | hAM | n = 6 |
| 12 weeks study | ||
| 1 | no protective wrapping | n = 6 |
| 2 | hEP | n = 6 |
| 3 | hAM | n = 6 |
| 6 Weeks Study | ||||
|---|---|---|---|---|
| Parameter | Location | Control (Mean ± SEM) | hEP (Mean ± SEM) | hAM (Mean ± SEM) |
| Myelin Thickness | ||||
| (μm) | Proximal | 0.532 ± 0.016 | 0.571 ± 0.017 | 0.677 ± 0.018 **** |
| Crush | 0.372 ± 0.009 | 0.326 ± 0.008 | 0.460 ± 0.018 **** | |
| Distal | 0.334 ± 0.007 **** | 0.278 ± 0.008 | 0.320 ± 0.007 **** | |
| % of Myelinated Fibers | ||||
| (%) | Proximal | 84.711 ± 1.570 | 91.185 ± 0.628 *** | 89.454 ± 0.865 ** |
| Crush | 86.509 ± 0.621 | 84.102 ± 0.666 | 86.991 ± 0.864 * | |
| Distal | 84.637 ± 0.852 | 86.191 ± 0.588 ** | 82.970 ± 0.719 | |
| Fiber Diameter | ||||
| (μm) | Proximal | 11.770 ± 0.359 | 12.568 ± 0.213 | 12.006 ± 0.179 |
| Crush | 8.029 ± 0.077 * | 8.577 ± 0.104 **** | 9.720 ± 0.190 **** | |
| Distal | 8.196 ± 0.087 | 8.871 ± 0.117 **** | 8.246 ± 0.096 **** | |
| Axonal Density | ||||
| (axons/μm2) | Proximal | 28.722 ± 3.477 | 41.111 ± 2.021 ** | 39.000 ± 1.690 * |
| Crush | 47.167 ± 2.306 | 60.556 ± 4.027 ** | 47.611 ± 2.432 | |
| Distal | 44.389 ± 2.171 | 47.444 ± 2.173 | 48.222 ± 1.821 | |
| 6-Week Study | |||
|---|---|---|---|
| Control | hEP | hAM | |
| NEUROGENIC | |||
| GFAP | |||
| Crush | 2.333 ± 0.333 | 2.833 ± 0.167 | 1.583 ± 0.083 |
| Distal | 1.500 ± 0.500 | 1.833 ± 0.601 | 0.583 ± 0.083 |
| Laminin B | |||
| Crush | 1.167 ± 0.167 | 1.167 ± 0.333 | 1.167 ± 0.167 |
| Distal | 0.500 ± 0.000 | 1.500 ± 0.500 * | 0.333 ± 0.167 |
| NGF | |||
| Crush | 0.500 ± 0.289 | 1.250 ± 0.629 | 0.667 ± 0.167 |
| Distal | 0.333 ± 0.167 | 1.833 ± 0.601 | 0.500 ± 0.289 |
| S-100 | |||
| Crush | 1.667 ± 0.167 | 1.833 ± 0.333 | 1.833 ± 0.333 |
| Distal | 2.167 ± 0.167 | 2.500 ± 0.289 * | 1.167 ± 0.441 |
| ANGIOGENIC | |||
| VEGF | |||
| Crush | 1.167 ± 0.441 | 1.833 ± 0.167 | 1.167 ± 0.441 |
| Distal | 1.167 ± 0.167 | 1.833 ± 0.333 | 1.333 ± 0.167 |
| wVF | |||
| Crush | 1.167 ± 0.667 | 2.167 ± 0.167 * | 0.583 ± 0.083 |
| Distal | 1.167 ± 0.167 | 2.500 ± 0.500 | 1.833 ± 0.167 |
| IMMUNOGENIC | |||
| HLA-DR | |||
| Crush | 1.500 ± 0.289 | 0.833 ± 0.601 | 1.333 ± 0.441 |
| Distal | 1.000 ± 0.289 | 0.833 ± 0.167 | 1.500 ± 0.500 |
| HLA-I | |||
| Crush | 1.400 ± 0.430 | 0.900 ± 0.187 | 1.000 ± 0.274 |
| Distal | 1.700 ± 0.255 | 0.900 ± 0.292 | 1.500 ± 0.224 |
| 12 Weeks Study | ||||
|---|---|---|---|---|
| Parameter | Location | Control (Mean ± SEM) | hEP (Mean ± SEM) | hAM (Mean ± SEM) |
| Myelin Thickness | ||||
| (μm) | Proximal | 0.664 ± 0.030 | 0.641 ± 0.020 | 0.881 ± 0.037 **** |
| Crush | 0.502 ± 0.023 **** | 0.294 ± 0.008 | 0.351 ± 0.010 **** | |
| Distal | 0.421 ± 0.026 **** | 0.372 ± 0.012 *** | 0.274 ± 0.007 | |
| % of Myelinated Fibers | ||||
| (%) | Proximal | 89.582 ± 0.748 | 88.475 ± 1.607 | 92.128 ± 0.474 * |
| Crush | 88.713 ± 0.656 | 89.784 ± 0.548 | 87.881 ± 0.664 | |
| Distal | 88.479 ± 0.507 | 89.537 ± 0.431 * | 87.262 ± 0.665 | |
| Fiber Diameter | ||||
| (μm) | Proximal | 11.701 ± 0.166 | 14.142 ± 0.189 **** | 11.859 ± 0.176 |
| Crush | 10.322 ± 0.151 **** | 7.677 ± 0.088 | 8.748 ± 0.086 **** | |
| Distal | 8.409 ± 0.114 | 8.957 ± 0.093 *** | 8.574 ± 0.093 * | |
| Axonal Density | ||||
| (axons/μm2) | Proximal | 27.778 ± 2.535 | 40.222 ± 1.832 **** | 38.167 ± 1.172 ** |
| Crush | 45.944 ± 3.845 | 56.056 ± 3.785 | 59.222 ± 2.697 * | |
| Distal | 54.667 ± 2.974 | 59.333 ± 3.734 | 57.556 ± 3.781 | |
| 12-Week Study | |||
|---|---|---|---|
| Control | hEP | hAM | |
| NEUROGENIC | |||
| GFAP | |||
| Crush | 1.833 ± 0.601 * | 2.500 ± 0.289 | 0.750 ± 0.433 |
| Distal | 1.667 ± 0.601 | 1.333 ± 0.167 | 0.333 ± 0.167 |
| Laminin B | |||
| Crush | 2.333 ± 0.333 | 2.000 ± 0.289 * | 0.583 ± 0.220 ** |
| Distal | 1.167 ± 0.441 | 1.667 ± 0.333 | 0.750 ± 0.382 |
| NGF | |||
| Crush | 0.500 ± 0.289 | 1.750 ± 0.144 * | 0.667 ± 0.333 |
| Distal | 0.667 ± 0.167 | 0.917 ± 0.300 | 0.167 ± 0.167 |
| S-100 | |||
| Crush | 1.667 ± 0.441 | 2.167 ± 0.167 | 1.667 ± 0.167 |
| Distal | 1.333 ± 0.333 | 2.000 ± 0.289 * | 0.833 ± 0.167 |
| ANGIOGENIC | |||
| VEGF | |||
| Crush | 2.333 ± 0.667 | 2.667 ± 0.167 | 2.000 ± 0.500 |
| Distal | 1.500 ± 0.500 | 2.167 ± 0.601 | 1.833 ± 0.167 |
| wVF | |||
| Crush | 1.667 ± 0.601 | 2.167 ± 0.833 | 2.000 ± 0.500 |
| Distal | 0.833 ± 0.333 | 1.500 ± 0.289 | 0.917 ± 0.546 |
| IMMUNOGENIC | |||
| HLA-DR | |||
| Crush | 2.500 ± 0.289 * | 1.083 0.363 | 2.000 ± 0.577 |
| Distal | 1.333 ± 0.333 | 0.833 ± 0.167 | 2.333 ± 0.333 * |
| HLA-I | |||
| Crush | 2.000 ± 0.289 * | 0.917 ± 0.300 * | 2.333 ± 0.333 * |
| Distal | 0.917 ± 0.363 | 0.833 ± 0.167 | 0.667 ± 0.220 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozlowska, K.; Radecka, W.; Brodowska, S.; Chambily, L.; Kuc, D.; Lopez, A.; Siemionow, M. Application of Human Epineural Patch (hEP) as a Novel Strategy for Nerve Protection and Enhancement of Regeneration After Nerve Crush Injury. Biomedicines 2025, 13, 1633. https://doi.org/10.3390/biomedicines13071633
Kozlowska K, Radecka W, Brodowska S, Chambily L, Kuc D, Lopez A, Siemionow M. Application of Human Epineural Patch (hEP) as a Novel Strategy for Nerve Protection and Enhancement of Regeneration After Nerve Crush Injury. Biomedicines. 2025; 13(7):1633. https://doi.org/10.3390/biomedicines13071633
Chicago/Turabian StyleKozlowska, Katarzyna, Weronika Radecka, Sonia Brodowska, Lucile Chambily, Dominika Kuc, Amber Lopez, and Maria Siemionow. 2025. "Application of Human Epineural Patch (hEP) as a Novel Strategy for Nerve Protection and Enhancement of Regeneration After Nerve Crush Injury" Biomedicines 13, no. 7: 1633. https://doi.org/10.3390/biomedicines13071633
APA StyleKozlowska, K., Radecka, W., Brodowska, S., Chambily, L., Kuc, D., Lopez, A., & Siemionow, M. (2025). Application of Human Epineural Patch (hEP) as a Novel Strategy for Nerve Protection and Enhancement of Regeneration After Nerve Crush Injury. Biomedicines, 13(7), 1633. https://doi.org/10.3390/biomedicines13071633







