Abstract
Objectives: Knee osteoarthritis (KOA) rehabilitation aims to assess the impact of pain reduction on kinesiophobia and outpatient welfare, emphasizing the interconnectedness of biopsychosocial factors in the rehabilitative process. Methods: The study involved a sample of KOA patients undergoing outpatient physical therapy. Forty patients (n = 40), aged 40–88, with acute or chronic knee osteoarthritis (Kellegren-Lawrence staging score I–II–III) were collected in Patients undergoing physical therapy using quantum molecular resonance (QMR) technology. The analysis employed a cross-lagged panel model to examine the relationships between perceived pain, kinesiophobia, and quality of life during the rehabilitative plan. Results: Rehabilitation significantly reduced pain levels and kinesiophobia while improving the quality of life for outpatients. The analysis demonstrated that pain reduction had a substantial causal influence on kinesiophobia and life conditions, both immediately following treatment and during follow-up. Conclusions: The findings underscore the importance of considering biopsychosocial factors in KOA rehabilitative treatment, highlighting the dynamic interplay between pain perception, kinesiophobia, and quality of life throughout the rehabilitation process.
Keywords:
kinesiophobia; pain; biopsychosocial approach; rehabilitation; osteoarthitis; inflammation 1. Introduction
The biopsychosocial (BPS) approach to medicine was introduced in 1977 by Engel [1]. According to this model, healthcare and overall well-being depend not only on the physical or biochemical aspects of the body (the biomedical model) but also on psychological and social factors [2,3,4,5]. The likelihood of developing certain diseases or pathologies is, thus, shaped by a complex interplay of biological, psychological, and social factors—extending beyond mere physical health [3,6].
The BPS model emphasizes the dynamic relationships among biological, psychological, and social factors, which collectively influence health conditions and psychological well-being [6]. To effectively analyze these interactions, it is essential to determine the contribution of each factor and how they influence one another. These relationships are often conceptualized as dynamic processes, which are typically studied through statistical analyses, such as analysis of variance or correlation studies, rather than deterministic mathematical models [6,7]. Because of the complex, interactive nature of these variables, the BPS approach tends to focus on individual specificity, recognizing that each pathology has a unique history linked to a person’s development and life circumstances rather than categorizing patients into fixed groups [3,6].
This individualized perspective is reflected in the use of multivariate statistical models that account for personal differences, helping to clarify the contribution of various factors to disease etiology and progression. Such models are particularly beneficial in managing chronic conditions or long-term pathologies, like musculoskeletal diseases such as osteoarthritis, where extended rehabilitation is often necessary [8,9].
In particular, knee osteoarthritis (KOA) is a global health concern, especially prevalent among the elderly over 65 years of age [10]. This condition is characterized by moderate to severe pain and disability, which significantly impair quality of life and contribute to rising public health costs [11]. Beyond physical symptoms, psychological factors—such as anxiety, stress, and fear of pain (kinesiophobia)—also play a vital role in shaping patients’ experiences during rehabilitation [12,13].
Kinesiophobia, a component of the fear-avoidance model, is defined as an excessive, irrational fear of movement due to perceived vulnerability to injury or re-injury [12]. This fear can negatively influence the lifestyle of KOA outpatients by fostering beliefs that movement may cause further damage, leading to avoidance behaviors. Such avoidance reduces physical activity, which can result in muscle weakness, joint instability, and ultimately, a vicious cycle of worsening symptoms and decreased autonomy [14,15,16].
Research suggests that rehabilitation can positively impact KOA patients by reducing kinesiophobia, even if their pain levels remain relatively unchanged [17]. However, it remains unclear whether improvements are primarily due to psychological effects, such as decreased fear of movement, or physical benefits, like pain reduction and increased joint stability. Some studies indicate that psychological factors, including fear and anxiety, can diminish with rehabilitation, especially in overweight patients, thereby enhancing overall well-being [17,18,19,20].
Despite these insights, the existing literature on kinesiophobia and osteoarthritis often lacks a cross-lagged or longitudinal perspective. Rehabilitation in KOA is a dynamic process where biological, psychological, and social factors are interconnected, aligning with the BPS model [21].
Our hypothesis considers that kinesiophobia could act as a significant barrier to effective rehabilitation, leading patients to avoid activities that could alleviate symptoms or improve joint function; therefore, it is crucial to examine how these factors influence each other over time. To better understand these complex interactions, our study focused on analyzing how perceived pain (a biological factor), kinesiophobia (a psychological factor), and quality of life (a social and life condition factor) change during a specific rehabilitative treatment for KOA outpatients.
This fear of movement often creates a vicious cycle: avoidance behaviors lead to muscle weakness and joint deterioration, which in turn exacerbate pain and disability. Consequently, addressing kinesiophobia is essential for successful rehabilitation. Effective strategies typically involve educational approaches and therapeutic interventions, such as cognitive–behavioral therapy, personalized exercise programs, and integrated physical therapy. These methods aim to help patients understand that controlled, gradual movement is safe and beneficial, thereby reducing fear and promoting activity.
The primary goal of our study was to assess whether, during rehabilitation, reductions in perceived pain—achieved through physical therapy—also lead to decreases in kinesiophobia and improvements in quality of life. In other words, we aimed to explore the dynamic processes activated by rehabilitation: whether improvements in physical symptoms directly influence psychological factors and social well-being, or if reductions in fear and avoidance behaviors are primarily responsible for enhancing patients’ overall quality of life.
Understanding these interactions is vital for developing more effective, individualized treatment plans. By applying a BPS perspective, our research emphasizes the importance of considering biological, psychological, and social dimensions simultaneously. This comprehensive approach could help identify which factors are most influential at different stages of rehabilitation and how they interact over time, ultimately leading to better management of KOA and similar chronic conditions.
2. Materials and Methods
We conducted a longitudinal design model, which is suitable for research in rehabilitation per the TRENDS guidelines [22].
2.1. Participants
Forty patients (n = 40) from the Clinical Hospital “SS Annunziata” of Chieti (Italy), referred for rehabilitation, underwent physiotherapy with two experienced physiotherapists. Eligibility was determined during clinical consultation by an orthopedic expert in KOA and based on specified criteria: aged 40–88, with acute or chronic knee osteoarthritis and pain (visual analog scale > 3). The inclusion criteria were: both male and female subjects and radiographic evidence for Kellegren–Lawrence staging score (I–II–III). Patients with the following symptoms were excluded: favism, hemolytic anemia, severe hyperthyroidism, graves’ disease, thrombocytopenia < 50,000 and severe coagulopathy, severe cardiovascular instability, coagulation disorders, alcohol abuse, hemochromatosis, patients treated with dietary supplements, pregnancy and lactation, psychiatric disorders, less than three months after previous knee infiltration, septic arthritis and/or febrile conditions, and history of contraindications to current instrumental physiotherapy (previous cancer). Subjects with rheumatic and autoimmune diseases and a recent history of trauma and/or distortions of the knee (ligaments) were excluded from the study. Recruitment spanned six months, with informed consent obtained. No new medications or therapies were introduced during the study. This stringent selection process ensured patients met criteria and maintained stable treatment regimens, enhancing research reliability.
2.2. Treatment Rehabilitative Protocol
Physical therapy was performed with the Q-Physio model electro-medical device (code 4001006), serial number D06164121, by Telea Electronic Engineering Srl (Vicenza—Italy). Patients underwent the following treatment by this electrotherapy: three sessions per week, for a total of 6 sessions, each lasting 30 min.
Rehabilitative treatments were performed with the patient in the supine position in the semi-flexion knee, and four electrodes were used: on the supra-patellar area surface, between the medial femoral condyle and the medial tibial condyle, on the area between the lateral femoral condyle and the lateral tibial condyle, and on the popliteal angle surface. The floating electrode was placed between the couch and the patient’s gluteal region to maximize the contact area. The device generates alternating electric currents characterized by high-frequency (4–64 MHz) and low-intensity waves [23,24].
2.3. Outcome Measures
Pain and function were assessed using the visual analogue scale (VAS) [25,26], the knee injury and osteoarthritis outcome scale (KOOS) [27], and the Tampa scale of kinesiophobia (TSK) [28,29].
VAS measures pain intensity on a continuous horizontal 10 cm scale with two start and end points marked “no pain” and “worst pain ever”. The higher the distance forms the start point of the line (on the left), and the higher the perceived pain. Values can range from 0 (= no pain) to 10 (= maximum pain).
KOOS, a self-completed questionnaire, gauges knee-related issues across five subscales (frequency and intensity of pain during functional activities; symptoms like stiffness, swelling, presence of joint noise or locking ROM limitation; difficulty with activities of daily living; difficulty with recreational/sports activities; knee-related quality of life). The item score was measured on a Likert scale from 0 (= no difficulty) to 4 (= very difficult). The global score is then transformed to a percentage that can vary from 0 (= highest presence of knee problems) to 100 (= absence of knee problems).
TSK evaluates pain-related beliefs and fear of movement; TSK-13, in the most common version, rates items on a 4-point Likert scale ranging from 1 “strongly disagree” to 4 “strongly agree”. The global score varies from 13 to 52. Higher scores indicate greater fear. TSK-13 categorizes items into activity avoidance (e.g., “I’m afraid that I might injure myself if I exercise”) and somatic focus factors, providing insight into subjects’ perspectives on pain and movement (e.g., “Pain always means I have injured my body”).
2.4. Timelines
All patients were evaluated at T0 = before treatment; T1 = at the end of treatment; T2 = one month after the end of treatment (follow-up).
2.5. Statistical Analysis
We calculated the descriptive statistics (mean and standard deviation, SD) of measures taken in the three phases T0 (before treatment), T1 (after treatment), and T2 (follow-up).
Cross-lagged panel models (CLPMs) are statistical models analyzing longitudinal data with observations at multiple time points [30]. They estimate relationships between variables over time, distinguishing directional influences and stability. CLPMs use regression to estimate cross-lagged paths (β1 and β2), between variables, autoregressive paths (β3 and β4) indicating variable stability, and synchronous correlations (rX0Y0 and rX1Y1). Autoregressive coefficients closer to zero suggest high variance. In a basic CLPM with variables X0, X1, Y0, and Y1 variables, equations express the model’s mathematical structure. CLPMs enable an understanding of how variables influence each other over time, which is essential in longitudinal data analysis. The CLPM is mathematically expressed as a set of equations:
and the relative path-diagram is shown in Figure 1.
Y1 = β1X0 + β3Y0
X1 = β4X0 + β2Y0
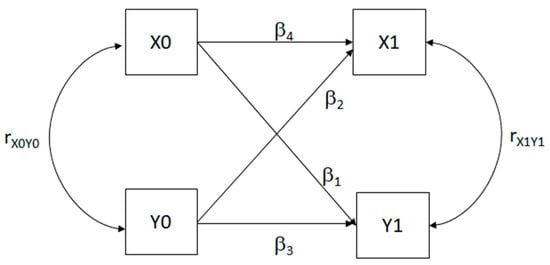
Figure 1.
Path diagram of a simple cross-lagged panel model (CLPM). X0 and Y0 = starting measures; X1 and Y1 = consequent measures. β1 and β2 = coefficient of crossed connections for causal predominance; β3 and β4 = autoregressive coefficients; rX0Y0 and rX1Y1 = synchronous correlations.
If β3 and β4 are significantly different, X and Y are stable. Significant β1 and β2, with differing values, suggest causal predominance. When β1 > β2, X0 influences Y1 more than Y0 influences X1; if β1 < β2, the reverse holds. Testing causal predominance via structural equation models in r compares CLPMs with free (β1 ≠ β2) versus equal (β1 = β2) cross-lagged coefficients. If no statistical difference occurs, no causal predominance exists. We tested CLPM using two-wave data (T0, T1, T2), comparing Model M1 with Models M2, M3, and M4. M1 is the model in which every parameter for cross-relations is set free. M2 is the model in which the crossed relation coefficients of the first wave of measurements from T0 to T1 are set equal (β1 ≠ β2). M3 is the model in which the crossed relation coefficients of the second wave of measurements from T1 to T2 are set equal each (β3 ≠ β4). M4 is the model in which all the crossed relation is set equal (β1 ≠ β2 and β3 ≠ β4).
Each model’s Akaike information criterion (AIC), Bayesian information criterion (BIC), χ2, df, p(χ2), and χ2 differences were reported, determining significance between Model M1 and others (M2, M3, and M4). Models with the lowest AIC and BIC show the best fit. All the analyses were made with JASP 0.18.1 software [31]. CPLM offers advantages over traditional methods of analysis because (a) it allows us to determine whether prior variables predict later variables and vice versa (directionality of relationship); (b) it includes autoregressive paths that control for prior variables (associations between variables are not merely due to individual differences); (c) it allows the analysis of reciprocal influences over time (dynamic interplay between variables).
3. Results
For the descriptive statistics, 64.7% of patients were female and 62.7% were unemployed or retired; 80.4% were married and 19.6% were single. The mean age was 64.6 years (SD = 11.1), and the mean BMI was 26.9 (SD = 6.2) (Figure 2, flow diagram).
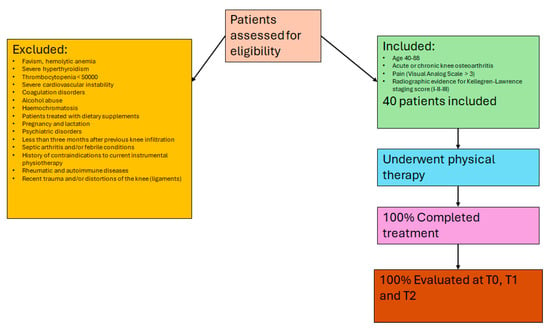
Figure 2.
Flow diagram showing patient recruitment, with inclusion and exclusion criteria, and completion rates for each phase of the study.
Table 1 shows the descriptive analysis for VAS, TSK, and KOOS taken in the three moments T0, T1, and T2. Table 1 demonstrates a consistent reduction in pain from T0 to T1 (first wave) and from T1 to T2 (second wave). Moreover, there is a continual improvement in knee functionality and quality of life, evidenced by the increasing KOOS scores, alongside a consistent decrease in kinesiophobia, particularly noticeable in the first wave. Consequently, rehabilitation proves effective in alleviating pain and kinesiophobia while enhancing outpatient quality of life (Supplementary materials Table S1. Shows Regression coefficients, covariances and variances of model M1 between VAS and TSK scores or between VAS and KOOS scores.)

Table 1.
Descriptive statistics (mean and SD) for VAS, KOOS, and TSK, taken in different time phases (T0, T1, and T2).
Figure 3 shows the path diagram of the CLPM that relates VAS measures to TSK measures. Significant coefficients and correlations are indicated with asterisks.
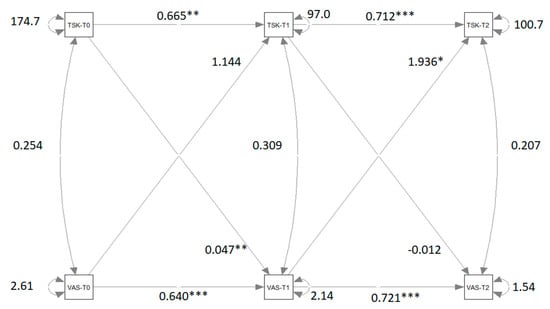
Figure 3.
Path diagram of the cross-lagged panel model that relates VAS measures to TSK measures. Significant β coefficients and correlations r are reported with asterisks. Note: VAS = visual analog Scale; TSK = Tampa Scale of Kinesiophobia; T0 = pre-treatment condition; T1 = post-treatment condition; T2 = follow-up. ** = p < 0.01; *** = p < 0.001. (*** Very significant; ** Moderate significant; * significant).
Table 2 reports the coefficient estimations for the four models M1, M2, M3, and M4 and ANOVA results of the comparison between the models.

Table 2.
Coefficient estimations for the four models M1, M2, M3, and M4 and ANOVA results of the comparison between the models for VAS and TSK measures. In M1, the coefficients of the two waves (from T0 to T1 and from T1 to T2) are set differently (β1 ≠ β2 and β3 ≠ β4); in M2 β1 = β2 and β3 ≠ β4; in M3 β1 ≠ β2 and β3 = β4; in M4 β1 = β2 and β3 = β4.
The differences between M2 and M1 and between M4 and M1 are not significant. The difference between M3 and M1 is significant. The significant difference between M3 and M1 indicates that the crossed-relation coefficients of the second wave are not equivalent and that there is a causal predominance of VAS to TSK. This causal predominance is not present in the first wave.
Figure 4 shows the path diagram of the CLPM that relates KOOS measures to TSK measures.
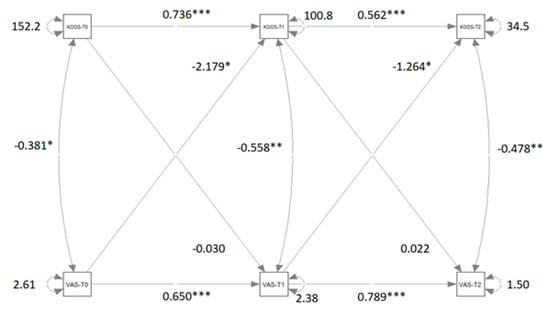
Figure 4.
Path diagram of the Cross-lagged panel model that relates VAS measures to KOOS measures. Significant β coefficients and correlations r are reported with asterisks. Note: VAS = visual analog scale; KOOS = knee injury and osteoarthritis outcome score; T0 = pre-treatment condition; T1 = post-treatment condition; T2 = follow-up. ** = significant at p < 0.01; *** = significant at p < 0.001. (*** Very significant; ** Moderate significant; * significant).
Table 3 reports the coefficient estimations for the four models M1, M2, M3, and M4 and ANOVA results of the comparison between the models. The disparities between M2 and M1, as well as between M4 and M1, are significant. Although the difference between M3 and M1 is not statistically significant, it approaches significance closely. The cross-lagged coefficients of both the first and second waves are not statistically equivalent, indicating a causal predominance of VAS over KOOS. This causal predominance in both waves is notably underscored by the significant difference between M4 and M1.

Table 3.
Coefficient estimations for the four models M1, M2, M3, and M4 and ANOVA results of the comparison between the models for VAS and KOOS measures. In M1 the coefficients of the two waves (from T0 to T1 and from T1 to T2) are set differently (β1 ≠ β2 and β3 ≠ β4); in M2 β1 = β2 and β3 ≠ β4; in M3 β1 ≠ β2 and β3 = β4; in M4 β1 = β2 and β3 = β4.
4. Discussion
Pain is a physical sensation that can profoundly impact human well-being, constituting a multidimensional phenomenon as it affects both the social and psychological aspects of outpatients’ lives [32]. Pain undeniably has a substantial effect on the well-being of individuals suffering from articulation diseases like arthritis or osteoarthritis [13,33]. According to certain studies [12,13], the fear of pain can be regarded as an alternative or concomitant cause of the reduction in outpatients’ well-being. The primary explanation lies in the activation of avoidance behavior to prevent the recurrence of pain sensation [16]. Our analysis of causal predominance among pain sensation, kinesiophobia, and quality of life indicates that pain is the predominant cause both in generating kinesiophobia and in reducing outpatient quality of life.
Our research, even though it is subject to limitations such as the absence of a comparator or control group and the short follow-up window, reveals a positive relationship between pain and kinesiophobia, signifying that higher levels of pain are associated with elevated levels of fear of pain. This relationship, present before treatment, persists and strengthens even after treatment. Therefore, it is reasonable to suppose that after treatment, participants are more sensitive to the persistence of pain, leading them to overreact to pain with higher levels of fear, stress, and anxiety.
The relationships between pain and quality of life are positive, indicating that higher levels of pain are correlated with lower quality of life and increased difficulty during daily activities. It is reasonable to assume that before and after treatment, the persistence of pain leads participants to perceive a high level of difficulty in physical movements. Importantly, after treatment, there was a significant reduction in the crossed-relation coefficient between VAS and KOOS (from −2.179 to −1.264). This reduction is likely due to the positive effect of the treatment on the physical condition and pain perception of participants. These research findings demonstrate that pain management interventions should be prioritized early in rehabilitation to prevent or reduce kinesiophobia. Besides that, our results affirm that the rehabilitative process for knee osteoarthritis exhibits a dynamic multivariate pattern of variables in which biopsychosocial factors play a pivotal role [8,21]. Specifically, rehabilitation demonstrates significant efficacy in alleviating pain levels and kinesiophobia while enhancing overall quality of life.
The CLPM reveals that pain reduction exerts a predominant causal influence on kinesiophobia and life conditions, not only immediately following treatment but also during follow-up. Thus, the efficacy of rehabilitation hinges primarily on its ability to alleviate chronic pain, subsequently leading to decreased psychological anxiety and an enhanced quality of life.
Although the anti-inflammatory effects of quantum molecular resonance (QMR) technology, as already demonstrated in an in vitro model of osteoarthritis-related inflammation [20], may be one of the crucial factors in the management of KOA, on the other hand, the treatment of pain and inflammation alone in patients with KOA is not sufficient to reduce fear of movement. In fact, Selçuk et al. suggest that approaches to increase awareness of fear of movement and physical activity and cognitive behavioral therapy related to fear of movement should be included in the treatment program [34].
Based on our suggestion, it would be beneficial to emphasize that early pain-management interventions should be prioritized in rehabilitation to help prevent or reduce kinesiophobia, ultimately supporting better functional outcomes. Regarding potential confounders, such as the wide age range of participants, acknowledging how these factors might influence the rate or effectiveness of fear reduction is important. Future studies could stratify results by age or other variables to better understand these dynamics. Finally, being transparent about the study’s limitations, like the short follow-up period and the lack of a control group, would indeed strengthen the credibility of the findings. Recognizing these constraints invites further research under more controlled conditions, helping to build a more robust evidence base for managing fear of movement in KOA.
5. Conclusions
In conclusion, outpatients with pain in KOA experience elevated levels of kinesiophobia and diminished quality of life primarily as a result of their pain sensations. A rehabilitative program by physiotherapy with an electro-medical device enhances their psychological and living conditions by alleviating pain. The persistence of pain post-treatment can significantly influence kinesiophobia, as it may signify ongoing leg articulation issues. Effectively reducing pain not only improves physical function but also decreases fear of movement, which can lead to better psychological and social outcomes. The persistent relationship between pain and kinesiophobia, even during follow-up, suggests that alleviating chronic pain is crucial for reducing anxiety, stress, and ultimately enhancing overall quality of life.
Furthermore, our results emphasize the need for an integrated, biopsychosocial approach, combining pain management techniques with strategies like awareness training and cognitive-behavioral therapy. Such approaches can help patients overcome fear of movement, encouraging active participation in therapy and improving functional outcomes.
We hope that our work enables physiotherapists and all medical and psychological professionals and practitioners in the field of musculoskeletal rehabilitation to enhance the efficacy of their treatments through a deeper understanding of the dynamic relationships among various biopsychosocial factors, such as pain, kinesiophobia, and quality of life, that influence healthcare quality.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines13061361/s1. Table S1. Regression coefficients, covariances and variances of model M1 between VAS and TSK scores or between VAS and KOOS scores.
Author Contributions
Conceptualization, T.P and A.P. (Antonia Patruno); methodology, M.T. and M.P.; software, M.T. and A.P. (Alessandro Pozzato); validation, A.P. (Antonia Patruno), T.P., and R.P.; formal analysis, M.T.; investigation, A.C., G.S. and M.Z.; data curation, M.T. and A.P. (Andrea Pantalone); writing—original draft preparation M.T., T.P., and A.P. (Antonia Patruno); writing—review and editing, A.P. (Andrea Pantalone) and A.P. (Antonia Patruno); visualization, all authors; supervision, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by a local Ethical Review Board of University G. D’Annunzio (protocol code 22016, 17 December 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
For privacy and ethical reasons, all data are available upon request to the corresponding author.
Conflicts of Interest
Author Alessandro Pozzato was employed by the company Telea Electronic Engineering. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| KOA | knee osteoarthritis |
| QMR | quantum molecular resonance |
| BPS | biopsychosocial |
| VAS | visual analogue scale |
| KOOS | knee injury and osteoarthritis outcome scale |
| TSK | Tampa scale of kinesiophobia |
| CLPMs | cross-lagged panel models |
References
- Engel, G.L. The need for a new medical model: A challenge for biomedicine. Science 1977, 196, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Adler, R.H. Engel’s biopsychosocial model is still relevant today. J. Psychosom. Res. 2009, 67, 607–611. [Google Scholar] [CrossRef]
- Lehman, B.J.; David, D.M.; Gruber, J.A. Rethinking the biopsychosocial model of health: Understanding health as a dynamic system. Soc. Personal. Psychol. Compass 2017, 11, e12328. [Google Scholar] [CrossRef]
- Appels, A.; Mulder, P.A. Excess fatigue as precursor of myocardial infarction. Eur. Heart J. 1988, 9, 758–764. [Google Scholar] [CrossRef]
- Stern, S.I.; Dhanda, R.; Hazuda, H.P. Hopelessness predicts mortality in older Mexican and European Americans. Psychosom. Med. 2001, 63, 344–351. [Google Scholar] [CrossRef]
- Bolton, D.; Gillett, G. The Biopsychosocial Model of Health and Disease: New Philosophical and Scientific Developments; Springer Nature: Cham, Switzerland, 2019; p. 149. [Google Scholar] [CrossRef]
- Bolton, D. The epistemology of randomized, controlled trials and application in psychiatry. Philos. Psychiatry Psychol. 2008, 15, 159–165. [Google Scholar] [CrossRef]
- van Dijk, H.; Köke, A.J.; Elbers, S.; Mollema, J.; Smeets, R.J.; Wittink, H. Physiotherapists Using the Biopsychosocial Model for Chronic Pain: Barriers and Facilitators—A Scoping Review. Int. J. Environ. Res. Public Health 2023, 20, 1634. [Google Scholar] [CrossRef]
- Moseley, G.L.; Butler, D.S. Fifteen years of explaining pain: The past, present, and future. J. Pain 2015, 16, 807–813. [Google Scholar] [CrossRef]
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; March, L. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef]
- Hunter, D.J.; Schofield, D.; Callander, E. The individual and socioeconomic impact of osteoarthritis. Nat. Rev. Rheumatol. 2014, 10, 437–441. [Google Scholar] [CrossRef]
- Larsson, C.; Hansson, E.E.; Sundquist, K.; Jakobsson, U. Kinesiophobia and its relation to pain characteristics and cognitive affec tive variables in older adults with chronic pain. BMC Geriatr. 2016, 16, 128. [Google Scholar] [CrossRef] [PubMed]
- Dekker, J.; Tola, P.; Aufdemkampe, G.; Winckers, M. Negative affect, pain and disability in osteoarthritis patients: The mediating role of muscle weakness. Behav. Res. Ther. 1993, 31, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.W.; Kraus, R.F. An overview of chronic pain. Hosp. Community Psychiatry 1990, 41, 433–440. [Google Scholar] [CrossRef]
- Lundberg, M.; Larsson, M.E.H.; Ostlund, H.; Styf, J. Kinesiophobia among patients with musculoskeletal pain in primary healthcare. J. Rehabil. Med. 2006, 38, 37–43. [Google Scholar] [CrossRef]
- Vlaeyen, J.W.; Crombez, G.; Linton, S.J. The fear-avoidance model of pain. Pain 2016, 157, 1588–1589. [Google Scholar] [CrossRef]
- Palmas, G.; Palma, D.; Palmas, L. Good Life with Arthritis from Denmark (GLA:D®) as a treatment for kinesiophobia in a patient with knee osteoarthritis. G. Ital. Ortop. Traumatol. 2023, 49, 178–185. [Google Scholar] [CrossRef]
- Unver, B.; Ertekin, Ö.; Karatosun, V. Pain, fear of falling and stair climbing ability in patients with knee osteoarthritis before and after knee replacement: 6 month follow-up study. J. Back Musculoskelet. Rehabil. 2014, 27, 77–84. [Google Scholar] [CrossRef]
- Pells, J.J.; Shelby, R.A.; Keefe, F.J.; Dixon, K.E.; Blumenthal, J.A.; LaCaille, L.; Kraus, V.B. Arthritis self-efficacy and self-efficacy for resisting eating: Relationships to pain, disability, and eating behavior in overweight and obese individuals with osteoarthritic knee pain. Pain 2008, 136, 340–347. [Google Scholar] [CrossRef][Green Version]
- Deveza, L.A.; Hunter, D.J. Pain relief for an osteoarthritic knee in the elderly: A practical guide. Drugs Aging 2016, 33, 11–20. [Google Scholar] [CrossRef]
- Vervullens, S.; Breugelmans, L.; Beckers, L.; Van Kuijk, S.M.; Van Hooff, M.; Winkens, B.; Smeets, R.J. Clinical prediction model for interdisciplinary biopsychosocial rehabilitation in osteoarthritis patients. Eur. J. Phys. Rehabil. Med. 2023, 59, 84. [Google Scholar] [CrossRef]
- Des Jarlais, D.C.; Lyles, C.; Crepaz, N.; TREND Group. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: The TREND statement. Am. J. Public Health 2004, 94, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, T.; Pino, V.; Elsallabi, O.; Gallorini, M.; Pozzato, G.; Pozzato, A.; Lanuti, P.; Reis, V.M.; Pesce, M.; Pantalone, A.; et al. Quantum Molecular Resonance Inhibits NLRP3 Inflammasome/Nitrosative Stress and Promotes M1 to M2 Macrophage Polarization: Potential Therapeutic Effect in Osteoarthritis Model In Vitro. Antioxidants 2023, 12, 1358. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, T.; Tommasi, M.; Pozzato, G.; Pozzato, A.; Pezzi, L.; Zuccarini, M.; Di Lanzo, A.; Palumbo, R.; Porto, D.; Messeri, R.; et al. Management and Rehabilitative Treatment in Osteoarthritis with a Novel Physical Therapy Approach: A Randomized Control Study. Diagnostics 2024, 14, 1200. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thong, I.S.K.; Jensen, M.P.; Mirò, J.; Tan, G. The validity of pain intensity measures: What do the NRS, VAS, VRS, and FPS-R measure? Scand. J. Pain 2018, 18, 99–107. [Google Scholar] [CrossRef]
- Kelly, A.M. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg. Med. J. 2001, 18, 205–207. [Google Scholar] [CrossRef]
- Monticone, M.; Ferrante, S.; Salvaderi, S.; Rocca, B.; Totti, V.; Foti, C.; Roi, G. Development of the Italian version of the knee injury and osteoarthritis outcome score for patients with knee injuries: Cross-cultural adaptation, dimensionality, reliability, and validity. Osteoarthr. Cartil. 2012, 20, 330–335. [Google Scholar] [CrossRef]
- Neblett, R.; Hartzell, M.M.; Mayer, T.G.; Bradford, E.M.; Gatchel, R.J. Establishing clinically meaningful severity levels for the Tampa Scale for Kinesiophobia (TSK-13). Eur. J. Pain 2016, 20, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Monticone, M.; Giorgi, I.; Baiardi, P.; Barbieri, M.; Rocca, B.; Bonezzi, C. Development of the Italian version of the Tampa Scale of Kinesiophobia (TSK-I): Cross-cultural adaptation, factor analysis, reliability, and validity. Spine 2010, 35, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Kearney, M.W. Cross Lagged Panel Analysis. In The SAGE Encyclopedia of Communication Research Methods; Allen, M.R., Ed.; Sage: Thousand Oaks, CA, USA, 2016; ISBN 978-1-48333-8143-5. [Google Scholar]
- JASP, version 0.18.1, Computer Software. JASP Team: Amsterdam, The Netherlands, 2023.
- Penny, K.I.; Purves, A.M.; Smith, B.H.; Chambers, W.A.; Smith, W.C. Relationship between the chronic pain grade and measures of physical, social and psychological well-being. Pain 1999, 79, 275–279. [Google Scholar] [CrossRef]
- Nagyova, I.; Stewart, R.E.; Macejova, Z.; van Dijk, J.P.; van den Heuvel, W.J. The impact of pain on psychological well-being in rheumatoid arthritis: The mediating effects of self-esteem and adjustment to disease. Patient Educ. Couns. 2005, 58, 55–62. [Google Scholar] [CrossRef]
- Selçuk, M.; Karakoyun, A. Is There a Relationship Between Kinesiophobia and Physical Activity Level in Patients with Knee Osteoarthritis? Pain Med. 2020, 21, 3458–3469. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).