Abstract
In recent years, there has been a growing recognition within the medical community that idiopathic pulmonary fibrosis (IPF) cannot be effectively managed through a singular focus on the disease itself. Instead, a dual approach is essential, one that not only aims to prolong survival by targeting the underlying pathological mechanisms of IPF but also addresses the numerous comorbidities that frequently complicate the clinical picture for affected individuals. This narrative review seeks to provide a detailed and comprehensive exploration of the various comorbid conditions associated with IPF, which may include cardiovascular disease (CVD), lung cancer (LC), gastroesophageal reflux disease (GERD), obstructive sleep apnea (OSA), and anxiety/depression, among others. By understanding the interplay between these comorbidities and IPF, healthcare providers can better tailor treatment regimens to meet the holistic needs of patients. Furthermore, this review delves into both current management strategies and emerging therapeutic approaches for these comorbidities, emphasizing the importance of interdisciplinary collaboration in clinical practice. By synthesizing the latest research and clinical insights, this review aims to enhance awareness and understanding of the complexities surrounding IPF management, ultimately guiding clinicians in developing more effective, individualized care plans that address not only the fibrotic lung disease but also the broader spectrum of health challenges faced by patients. Through this comprehensive overview, we hope to contribute to the ongoing dialogue about improving quality of life and survival rates for individuals living with IPF.
1. Introduction
This decline significantly reduces patients’ quality of life, with acute exacerbations of IPF (AE-IPF) frequently interrupting the disease course. It typically exhibits radiological and histological features consistent with usual interstitial pneumonia (UIP) [1]. Clinically, IPF leads to a gradual decline in lung function, accompanied by worsening respiratory symptoms, such as dyspnea on exertion. This decline significantly reduces patients’ quality of life, with acute exacerbations of IPF (AE-IPF) frequently interrupting the disease course. Ultimately, patients may succumb to respiratory failure or associated comorbidities [2]. The etiology of IPF is multifactorial, encompassing genetic, environmental, immunologic, psychological, and social factors. It predominantly affects older adults, with prevalence estimates ranging from 0.33 to 2.51 per 10,000 individuals in Europe and 2.40 to 2.98 per 10,000 individuals in North America [3].
2. Methodological Approach
This narrative review is based on a literature search of PubMed, Embase, and Cochrane Library databases, including articles published up to February 2025. Keywords used included “idiopathic pulmonary fibrosis”, “comorbidities”, “antifibrotic therapy”, “pulmonary hypertension”, “cardiovascular disease”, “lung cancer”, “gastroesophageal reflux disease”, “obstructive sleep apnea”, “anxiety”, “depression”, and specific drug names (pirfenidone and nintedanib). We included systematic reviews, meta-analyses, clinical trials, observational studies, and international clinical practice guidelines relevant to the interplay between IPF, its comorbidities, and management strategies. Articles were selected for their relevance to the epidemiology, pathogenesis, clinical impact, and management of comorbidities in IPF.
Previous guidelines, such as the joint American Thoracic Society (ATS)/European Respiratory Society (ERS)/Japanese Respiratory Society (JRS)/Latin American Thoracic Association (ALAT) guidelines, have addressed the diagnosis and management of IPF [4,5,6]. However, managing the disease remains complex, and prognosis is generally poor. The age-standardized mortality rate for IPF varies significantly, reported to be between 0.5 and 12 per 100,000 persons per year, reflecting regional differences [7].
Current clinical management strategies for IPF include antifibrotic agents (i.e., pirfenidone and nintedanib), anti-inflammatory, and antitussive medications (including oral corticosteroids and opioids) to alleviate cough and enhance quality of life, as well as antacids and proton pump inhibitors (PPIs) to mitigate gastroesophageal reflux. Lung transplantation remains an option, although it is limited to a small subset of patients and is highly dependent on the availability of healthcare resources [1,4,5,6,7]. Without treatment with antifibrotic drugs, IPF can be fatal within 3 to 5 years of diagnosis [8,9,10,11,12,13,14,15].
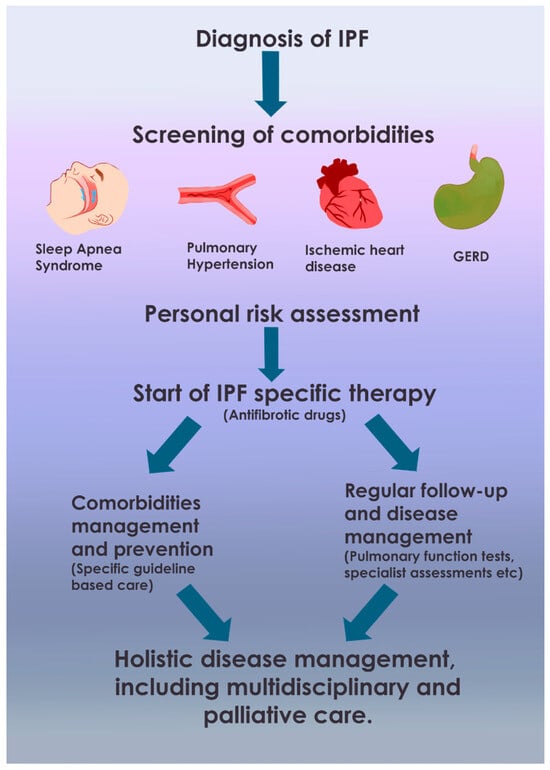
Furthermore, disease management is significantly complicated by comorbidities, which, in conjunction with exacerbations, further diminish patients’ quality of life, potentially reduce survival, and accelerate disease progression. Increasing evidence underscores the importance of early diagnosis and treatment of comorbidities, which are just as critical as managing IPF itself. Comprehensive evaluations and management of existing comorbidities—including pulmonary hypertension (PH), GERD, OSA, and LC—are essential. Regular evaluations every 3–6 months, or more frequently as needed, are recommended to monitor disease progression. Treatment considerations should encompass both pharmacological (nintedanib and pirfenidone) and non-pharmacological (such as oxygen supplementation and pulmonary rehabilitation) therapies. Optimizing strategies to enhance quality of life should include the treatment of comorbidities, promotion of physical activity, attention to emotional well-being, and the palliation of symptoms [16,17,18,19,20,21,22,23,24].
This literature review aims to assess, verify, and delineate the potential reciprocal interactions between IPF therapies and the identified comorbidities, while also summarizing current management strategies for both IPF and these associated conditions. The diagnosis and management of these comorbidities pose significant challenges for pulmonologists specializing in IPF. Addressing these comorbidities may positively influence the quality of life and survival of IPF patients. The focus of this review is primarily on the impact of comorbidities on IPF, its progression, and its management, including therapeutic strategies for both IPF and its associated conditions (Figure 1).
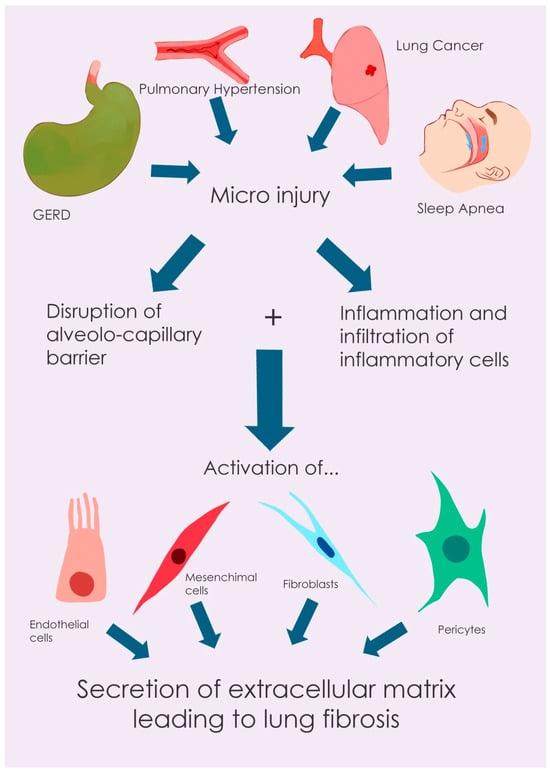
Figure 1.
Illustrative diagram of idiopathic pulmonary fibrosis (IPF) pathogenesis, comorbidity interactions, and therapeutic targets. This figure provides a schematic overview of the main pathogenic mechanisms of idiopathic pulmonary fibrosis (IPF), illustrating how prevalent comorbidities (e.g., GERD, IP, and lung cancer) interact with these processes, and highlighting potential therapeutic targets for both antifibrotic treatments and therapies addressing associated conditions.
Pathogenesis of IPF
The pathogenesis of IPF is characterized by a complex interplay of cellular and molecular events leading to progressive lung fibrosis. The process is initiated by recurrent micro-injuries to the alveolar epithelium, disrupting the alveolar–capillary barrier. This epithelial damage triggers an inflammatory cascade, resulting in the recruitment and infiltration of immune cells, including macrophages. These immune cells release a variety of pro-inflammatory and pro-fibrotic mediators, such as tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-β, monocyte chemoattractant protein-1 (MCP-1/CCL2), macrophage inflammatory protein-1α (MIP-1α/CCL3), and T-helper 2 (Th2) chemokines (CCL17, CCL18, and CCL22). These mediators stimulate the differentiation of various cell types, including fibroblasts, epithelial cells, endothelial cells, fibrocytes, mesenchymal stem cells, and pericytes, into myofibroblasts [25]. Myofibroblasts are the primary effector cells in fibrosis, exhibiting enhanced proliferation, increased extracellular matrix (ECM) production, and contractile properties due to the expression of α-smooth muscle actin (α-SMA) stress fibers. In normal wound healing, myofibroblasts undergo apoptosis after tissue repair. However, in IPF, this process is dysregulated, and myofibroblasts exhibit resistance to apoptosis, leading to their persistent accumulation and excessive ECM deposition [26]. The ECM, under physiological conditions, provides structural support to the lung tissue, maintains mechanical integrity, and contributes to the elastic recoil necessary for proper pulmonary function. Furthermore, it plays a regulatory role in myofibroblast differentiation and activity [27,28]. In IPF, this delicate balance is disrupted, resulting in aberrant lung remodeling characterized by dysregulated ECM deposition, ultimately leading to tissue destruction and functional impairment [28].
3. Identified Comorbidities and Their Management
The following comorbidities have been recognized in association with IPF, reflecting their potential impact on disease progression, patient outcomes, and therapeutic strategies (Figure 2). Their management often requires a multidisciplinary approach, integrating recommendations from international guidelines.
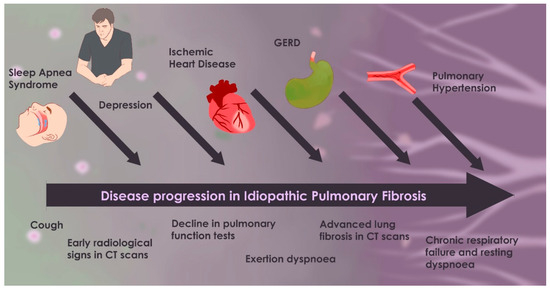
Figure 2.
Timeline of IPF progression with comorbidities.
Figure 2 illustrates the progression of idiopathic pulmonary fibrosis (IPF) from its early stages—characterized by mild symptoms and limited fibrosis—to its advanced phase, which includes severe dyspnea, extensive fibrosis, and respiratory failure. It also highlights the most significant comorbidities (such as GERD, OSA, pulmonary hypertension, ischemic heart disease, lung cancer, and depression). When these comorbidities manifest, they tend to worsen the disease course, contribute to the overall disease burden, and accelerate clinical decline.
In a 2022 review, Podolanczuk et al. summarized the frequent association of comorbidities which may increase symptom burden and impact survival [29,30,31,32,33], concluding that attention to these and other comorbidities has to be part of a comprehensive evaluation and management of patients with IPF. In particular, in the study by Kreuter et al., the median survival of patients with IPF decreased from 66 months for those without comorbidities to 35 months for those with four to seven comorbidities [32]. Prevalent comorbidities and related complications had previously been examined by Cano-Jiménez E. and colleagues in 2018 [34]. The high prevalence of some comorbidities, such as chronic obstructive pulmonary disease (COPD), LC, and CVD, may coexist with IPF partially due to shared risk factors (smoking, older age, and genetic predisposition), while others may arise as a consequence of IPF itself (e.g., AE-IPF or PH).
3.1. Respiratory Comorbidities
Pulmonary Emphysema (Combined Pulmonary Fibrosis and Emphysema)
Emphysema and/or COPD have been reported in 6–67% of patients with IPF, and ~30% of patients with IPF have emphysematous changes on imaging [35,36]. The coexistence of these two patterns is typical of combined pulmonary fibrosis and emphysema (CPFE) [37], which is common in male smokers, with the upper lobes being predominantly affected by emphysema and the lower lobes typically fibrotic. It is not clear whether CPFE is associated with a higher or lower mortality risk than IPF alone, but it is certainly higher than in emphysema alone [38].
Patients with CPFE commonly demonstrate paradoxically preserved lung volumes, despite the severity of dyspnea [33], and/or a combination of obstructive and restrictive ventilatory defects, accompanied by substantial diffusion impairment (measured as diffusing capacity of the lungs for carbon monoxide, DLCO) on pulmonary function tests [39]. Prognostically, DLCO values and the extent of emphysema on high-resolution computed tomography (HRCT) are significant. Given a consistent level of fibrosis, emphysema appears to exert an additive negative effect on outcomes [37,38,40].
CPFE is frequently complicated by LC and PH (reported in 15–30% of CPFE patients). Both LC and PH are predictors of mortality in CPFE, along with age and DLCO values [35,41,42,43].
Regarding treatment, pivotal trials for both Pirfenidone (ASCEND) and Nintedanib (INPULSIS) included patients with emphysema, and both antifibrotic drugs seem to have a similar effect in CPFE as in IPF alone [44,45]. In accordance with the ATS/ERS/JRS/ALAT guidelines and research statements [6,39], management should include antifibrotic treatment, bronchodilator therapy (e.g., LABA-LAMA or LABA-LAMA-ICS), smoking cessation, oxygen supplementation for desaturation, pulmonary rehabilitation, and vaccinations [36,37,38,39,46].
3.2. Cardiovascular Disorders
3.2.1. Pulmonary Hypertension
Pulmonary hypertension (PH), defined hemodynamically as a mean pulmonary arterial pressure (mPAP) > 20 mmHg at rest confirmed by right heart catheterization (RHC), complicates several ILDs, including IPF, contributing to increased disease burden and poor prognosis [47]. Patients with WHO Group 3 PH (PH due to lung diseases and/or hypoxia) related to pulmonary fibrosis have a worse prognosis compared with other types of PH [48]. The COMPERA registry reported estimated 5-year survival rates of 14% in patients with PH-ILD compared to 51.8% in patients with idiopathic pulmonary arterial hypertension (PAH) [49]. Any degree of PH severity impacts survival, with mortality increasing further with severe PH (Pulmonary Vascular Resistance, PVR >5 Wood Units, especially >8 Wood Units) [50,51].
The prevalence of PH in IPF patients ranges from the range of 8–15% at diagnosis to the range of 35–44% prior to lung transplantation evaluation, and up to 86% during lung transplantation [52]. Comorbidities like emphysema, OSA, cardiac diastolic dysfunction, or pulmonary thromboembolism can exacerbate PH in IPF [46,53,54,55]. Two main phenotypes of PH in IPF exist: PH developing secondary to extensive fibrosis, and a smaller subset (10%) with severe PH despite mild-to-moderate fibrosis [56].
Mechanisms for PH development in ILD include fibrotic vascular ablation, alveolar–septal remodeling, chronic inflammation, aberrant angiogenesis, and potentially genetic factors (e.g., Bone Morphogenetic Protein Receptor type 2 (BMPR2) and TGF-β pathway) [56,57,58,59,60]. These lead to impaired gas diffusion, ventilation/perfusion (V/Q) mismatch, and eventually right heart failure [57,61]. Impaired right ventricular (RV) function can occur even without overt PH [62].
Clinical manifestations are often non-specific. Brain natriuretic peptide (BNP) and N-terminal pro-BNP (NT-proBNP) can indicate RV overload [58]. PH screening is indicated when hypoxemia and DLCO are disproportionately low, or with severe exercise desaturation. The 6-minute walk test (6MWT) distance is often reduced [63]. Cardiopulmonary exercise testing (CPET) can help identify PH, showing reduced peak oxygen consumption (VO2) and increased minute ventilation/carbon dioxide production (VE/VCO2) slope [64,65].
Imaging clues include a main pulmonary artery to ascending aorta diameter ratio >1 on chest CT, pulmonary artery enlargement, or RV hypertrophy [66,67,68]. Transthoracic echocardiography (TTE) is the primary non-invasive screening tool, estimating RV systolic pressure (RVSP) [67], though its specificity for PH-IPF can be low [69,70,71]. An echocardiographic score can improve identification of severe PH [72]. RHC remains the gold standard for diagnosis [68,72,73,74]. The Ford Score can aid PH screening in ILD [75]. Exclusion of left heart disease is crucial, as post-capillary PH can occur in ~20% of PH-ILD patients [76]. Currently, specific therapy for PH-IPF is limited. The 2022 ESC/ERS guidelines give a Class IIb recommendation for inhaled treprostinil based on the INCREASE study, which showed improved 6MWD and other secondary endpoints in PH-ILD patients (28% IPF) [74,77]. Post hoc analyses suggested potential antifibrotic properties and benefits in less severe hemodynamics [78,79]. TETON studies are ongoing. Parenteral treprostinil showed some benefit in a small study [80]. Inhaled nitric oxide (iNO) showed mixed results in iNO-PF and REBUILD trials [81,82,83]. Sildenafil showed some benefit in secondary endpoints in the STEP-IPF trial but not primary [84,85,86], and combination with nintedanib (INSTAGE) or pirfenidone (SP-IPF) failed to improve PH outcomes [84,85,86,87,88,89]. Other vasodilators, like riociguat and ambrisentan, were either harmful or ineffective [90,91]. Current ESC/ERS guidelines recommend oxygen therapy for hypoxia, treatment of underlying ILD, and referral for lung transplantation for PH in IPF [68,74]. Pulmonary rehabilitation is also important [74].
3.2.2. Ischemic Heart Disease
Patients with IPF have an elevated risk of CVD, with cardiac issues being the second leading cause of death (~10%) [92,93,94,95,96,97]. Coronary artery disease (CAD) is common, with some studies reporting prevalence up to 68% [95]. Shared pathophysiological mechanisms include endothelial damage, chronic inflammation, and fibrosis [95,98,99,100]. Intermittent hypoxemia can also contribute to atherogenesis [101].
Pulmonary fibrosis has been associated with increased CAD incidence compared to non-fibrotic lung diseases [102]. Both IPF and CAD involve excessive fibrosis [103]. Hypoxia can exacerbate angina, and IPF patients may receive suboptimal cardiovascular preventative care [104,105,106,107]. Low forced expiratory volume in 1 s (FEV1) is a risk factor for CAD, and this association appears stronger for IPF-related FEV1 reduction than for COPD-related reduction [107,108,109,110]. Some studies found higher CAD prevalence in IPF lung-transplant candidates compared to emphysema patients, despite lower smoking rates in the IPF group [111,112,113], though other studies in Asian populations showed lower CAD prevalence, possibly due to ethnic differences [98,113,114,115,116]. Hubbard et al. found increased risk of acute coronary syndrome and angina in IPF patients [93]. Nathan et al. [95] found CAD in 65.8% of IPF patients versus 46.1% in COPD patients, with worse outcomes for IPF patients with significant CAD.
Screening for CAD in IPF is important. HRCT scans, routinely used for IPF monitoring, can also assess coronary artery calcium (CAC), a marker for CAD. Bray et al. found the highest CAC scores to be in IPF, followed by COPD and non-smokers [110]. Nathan et al. showed that HRCT had good sensitivity (81%) and specificity (85%) for detecting significant CAD via calcification assessment, suggesting its utility as a screening tool [117].
3.2.3. Arrhythmias
Arrhythmias (AA) occur in 6–19% of IPF cases [30]. Atrial fibrillation (AF) is particularly common, especially in lung-transplant candidates [2]. Contributing factors include hypoxia, altered pulmonary hemodynamics, CAD, and chronic inflammation [2,95,102]. Biomarkers like C-reactive protein and B-type natriuretic peptide may predict AF risk [118]. Hypoxia increases sympathetic activity, and PH can alter hemodynamics around pulmonary veins, predisposing to AF [103].
The most common arrhythmias are AF and Atrial Flutter (AFL). Orrego et al. found IPF to be a risk factor for post-operative arrhythmias after lung transplantation (25.4% incidence; AF, 17.8%) [119]. Shibata et al. found that decreased FEV1% and FVC% were independent risk factors for AF development in IPF and COPD patients [120]. Azadani et al. identified IPF as an independent predictor of AFL post-lung transplant (13% incidence) [121]. Nielsen et al. also found IPF to be a significant predictor for post-operative AF after lung transplantation [122].
3.3. Gastrointestinal Comorbidities
Gastroesophageal Reflux Disease
Gastroesophageal reflux disease (GERD) is highly prevalent in IPF, potentially affecting 60–80% of patients, often silently [123,124,125,126]. Esophageal pH monitoring is the gold standard for diagnosis [127,128]. In IPF patients with GERD, reflux is often supine, and acid clearance may be slowed despite preserved esophageal function. Severe GERD can lead to microaspiration of gastric fluid, potentially contributing to alveolar damage, IPF progression, and AE-IPF [129]. Elevated pepsin in bronchoalveolar lavage (BAL) fluid during AE-IPF supports this [129]. GERD may be more frequent in asymmetric pulmonary fibrosis [130].
The proposed mechanism involves microaspiration of gastric contents (acid and pepsin), causing subclinical lung injury and fibroproliferation [53,54,124,131,132,133,134,135,136,137]. Detection of gastric molecules in BALF supports this [124,126,135].
The effect of antacid therapy (e.g., PPIs) on IPF progression is controversial. Some observational studies suggested benefits (slower lung function decline, improved survival, and fewer AEs) [137], but post hoc analyses of antifibrotic trials (pirfenidone and nintedanib) showed no significant benefit of antacid therapy over placebo on IPF progression [6,20,124,138,139,140,141]. Current ATS/ERS/JRS/ALAT guidelines conditionally recommend antacid therapy only for IPF patients with symptomatic GERD [6,39,124,131,142]. Anti-reflux surgery is not routinely recommended due to controversial effectiveness but may be considered for selected patients [141]. GERD is a common side effect of pirfenidone [143].
3.4. Sleep-Related Breathing Disorders
Obstructive Sleep Apnea
Obstructive sleep apnea (OSA) is surprisingly common in IPF, with prevalence estimates ranging from 59% to 88% in various studies [144,145], contrary to earlier beliefs that increased ventilatory drive in IPF might be protective. IPF patients often exhibit abnormal sleep macroarchitecture (reduced slow-wave and rapid eye movement (REM) sleep, increased stage 1 sleep, frequent awakenings, and reduced sleep efficiency) and microarchitecture (more microarousals), impacting quality of life [146].
The Apnea–Hypopnea Index (AHI) has been found to correlate positively with body mass index (BMI) and negatively with FEV1 and FVC [146]. Reduced lung volumes in IPF (decreased total lung capacity, TLC) may decrease upper airway stability, particularly during REM sleep, when functional residual capacity is further reduced, facilitating airway collapse [145]. This is because lower lung volumes reduce the caudal traction on the upper airway, making it more susceptible to collapse. The pathogenic link is complex: OSA might develop due to IPF-related lung restriction, or OSA-related chronic nocturnal intermittent hypoxia could promote GERD or increase oxidative lung stress, potentially exacerbating fibrosis [146,147,148,149]. Animal models show that intermittent hypoxia can worsen bleomycin-induced lung fibrosis [150].
IPF patients often have an altered breathing pattern (rapid, shallow breathing) that persists during sleep [148]. This pattern, driven by increased lung stiffness and vagal receptor activity, is not alleviated during sleep. Nocturnal hypoxemia (SpO2 < 90%) is common, especially during REM sleep, due to alveolar hypoventilation, V/Q mismatch, and reduced diffusion capacity [148]. This sleep-related desaturation can be more severe than exercise-induced desaturation and is associated with worse survival and potentially contributes to PH development via mechanisms like increased endothelin-1 [149,151,152].
Early detection of sleep-related breathing disorders (SRBDs) via nocturnal respiratory polygraphy is crucial [146]. Management includes oxygen therapy for REM-related SBD and continuous positive airway pressure (CPAP) for OSA. While long-term oxygen for established respiratory failure in IPF has not shown survival benefits, treating nocturnal hypoxemia might prevent PH development. CPAP for IPF-OSA has been associated with improved quality of life, sleep, daily function, and potentially survival, especially with good adherence [145,146].
Regarding antifibrotic drugs, nintedanib has been linked to improved anxiety/depression and, thus, potentially sleep quality [78], while pirfenidone’s prescribing information notes insomnia in ~10% of cases [44,153,154].
3.5. Oncological Comorbidities
Lung Cancer
IPF patients have an approximately fivefold increased risk of developing lung cancer (LC), affecting 3–22% of cases [154,155,156,157,158,159]. Shared risk factors (e.g., smoking) and pro-carcinogenic biological pathways contribute [35,154,155]. The risk is even higher in CPFE (up to 12%) and in individuals with interstitial lung abnormalities (ILAs) [155,156,157,158,159,160]. Annual HRCT is recommended for monitoring IPF progression and early LC detection [160].
Pathogenic links include persistent TGF-β activity, which influences cell growth, metastasis, and cancer progression, with pulmonary fibrosis providing a supportive microenvironment [161,162,163,164,165]. Increased fibroblast foci in IPF are linked to more aggressive cancer [161,162,163,164,165]. Programmed cell death-ligand 1 (PD-L1) expression is increased in both IPF and LC. Genetic and epigenetic alterations (somatic mutations, DNA methylation, telomere dysfunction, p53 mutations, FHIT alterations, abnormal microRNA (miRNA) expression, reduced Connexin 43 (CX43) and gap junction intercellular communication (GJIC), Thy-1 promoter hypermethylation, and reactive oxygen species (ROS) overproduction) are implicated in both conditions [155,161,166,167].
Squamous cell carcinoma (SCC) is often reported as more prevalent than adenocarcinoma (AC) in IPF-associated LC, frequently arising in peripheral, fibrotic areas, particularly honeycomb regions [154,168,169,170,171]. Survival is poor, often driven by malignancy [164,170,171,172].
Treatment is challenging. Surgical resection and radiation therapy may be limited by extensive fibrosis and risk of post-operative complications, like AE-IPF (5–15% incidence; ~50% short-term mortality) [170,171,172,173,174]. Tissue-sparing surgery, careful fluid management, and low-tidal-volume ventilation are strategies to improve outcomes [171,174]. Peri-operative pirfenidone may reduce post-operative exacerbation risk [175]. Proton therapy is an emerging radiation option [176]. Many antineoplastic agents, including PD-L1 inhibitors, carry a risk of drug-induced ILD or worsening fibrosis [177,178,179,180]. Carboplatin-based regimens appear relatively safer [180,181,182,183,184,185,186].
Antifibrotic drugs show some promise. Nintedanib, initially approved for non-small-cell lung cancer (NSCLC) with docetaxel [183,187,188,189,190,191,192,193], targets Vascular Endothelial Growth Factor Receptor (VEGFR), Platelet-Derived Growth Factor Receptor (PDGFR), and Fibroblast Growth Factor Receptor (FGFR), showing antitumor effects in preclinical models [194,195]. Pirfenidone may reverse epithelial–mesenchymal transition and induce apoptosis in cancer-associated fibroblasts [163,165,170,181,182]. Observational studies suggest that antifibrotic therapy (pirfenidone or nintedanib) may be associated with lower LC incidence and LC-related mortality in IPF patients [184,185,186,187,188,189,190,191,192,193]. Some case reports suggest that nintedanib monotherapy might inhibit tumor progression in NSCLC with IPF [195,196,197,198,199,200,201,202,203,204]. Combination therapy (e.g., atezolizumab + pirfenidone) is under investigation [193,204,205,206]. However, IPF patients are often excluded from LC trials, thus limiting data [186,187,188,189].
3.6. Neuropsychiatric Comorbidities
Anxiety and Depression
Psychiatric comorbidities, particularly anxiety and depression, are highly prevalent in patients with IPF, significantly impacting their quality of life, symptom burden, and overall prognosis [24,206]. Studies indicate that depression (estimated prevalence, ~20–50%) and anxiety (estimated prevalence, ~30–60%) are more common in IPF patients compared to the general population and even some other chronic respiratory diseases [24,30,206]. These conditions are often underdiagnosed and undertreated.
The chronic, progressive nature of IPF; and debilitating symptoms like dyspnea and chronic cough, fatigue, social isolation, and uncertainty about the future contribute to psychological distress. Anxiety and depression can exacerbate physical symptoms, creating a vicious cycle: increased anxiety can worsen the perception of dyspnea, leading to activity avoidance, deconditioning, and further functional decline [206].
Furthermore, psychiatric comorbidities can negatively affect treatment adherence (to antifibrotics, oxygen, and pulmonary rehabilitation), engagement in self-management behaviors, and overall coping abilities, ultimately worsening disease outcomes and increasing healthcare utilization [206].
Management requires a holistic, multidisciplinary approach. Screening for anxiety and depression should be a routine part of IPF care. Non-pharmacological interventions are foundational and include pulmonary rehabilitation (which often incorporates psychosocial support and exercise that can improve mood), cognitive behavioral therapy (CBT), mindfulness-based stress reduction, and patient support groups [207]. Pharmacological treatment with antidepressants (e.g., selective serotonin reuptake inhibitors (SSRIs)) or anxiolytics may be considered, carefully weighing potential benefits against side effects and drug interactions, especially in an older population with polypharmacy [207]. Early palliative care interventions can also address psychosocial and spiritual needs, improving quality of life for both patients and caregivers [21,22].
3.7. Acute Exacerbations of IPF
IPF is a progressive disease with variable rates of progression [1]. Acute exacerbations of IPF (AE-IPF) are episodes of acute, clinically significant respiratory deterioration characterized by new widespread alveolar abnormality on CT, not fully explained by cardiac failure or fluid overload, typically developing over less than one month [208]. AE-IPF account for approximately 40% of IPF-related deaths [96,209], with an incidence of 4–20% per year [210] and high associated morbidity and mortality (often >50%). Antifibrotic therapies (pirfenidone and nintedanib) may reduce AE-IPF incidence [208,211,212,213,214].
The etiology of AE-IPF is often unknown but may involve occult triggers, like viral infections, an altered respiratory microbiome (e.g., Staphylococcus and Streptococcus), GERD with microaspiration, or procedural stress (e.g., surgery and BAL) [208,215,216,217,218,219,220,221,222,223]. Risk factors for AE-IPF include advanced functional impairment (low FVC, DLCO, PaO2, and 6MWT distance), poor baseline oxygenation, younger age, CAD, and high BMI [224]. Chronic comorbidities like PH and CVD are also implicated [209,210,224,225,226,227,228,229,230].
The impact of specific chronic comorbidities on AE-IPF outcomes is an area of active research. A study by Baig and Yoo [230] using a large inpatient database found that chronic kidney disease (CKD) was associated with significantly increased in-hospital mortality among AE-IPF patients, while diabetes was associated with a reduced risk of death. Older age was correlated with higher mortality. The link between CKD and worse AE-IPF outcomes may relate to systemic impacts of AE-IPF exacerbating pre-existing kidney injury [231,232]. The potential protective effect of diabetes is less clear, though some studies suggest lower acute respiratory distress syndrome (ARDS) incidence in diabetics [233,234,235], but the link to ARDS mortality is not established [236,237].
Management of AE-IPF is largely supportive. Corticosteroids are weakly recommended based on low-quality evidence [4]. Mechanical ventilation carries high mortality and is often a bridge to transplant in selected cases [4,209]. Immunomodulatory agents, sometimes combined with antifibrotics, have been explored in small studies, but large-scale controlled data are lacking [219,220,221,222,223,224,225,226,227]. Antifibrotic drugs have shown efficacy in reducing IPF exacerbation frequency, including post-surgically [238,239]. Real-world studies are crucial for understanding antifibrotic effects on comorbidities, as pivotal trials often exclude patients with significant comorbidities [240,241,242,243,244,245,246,247,248,249,250,251]. Both pirfenidone and nintedanib have demonstrated benefits in slowing FVC decline and reducing AE incidence [240,244,245].
4. Conclusions and Future Perspectives
Management of IPF extends far beyond targeting pulmonary fibrosis alone; it requires a comprehensive strategy that actively identifies and addresses its numerous and impactful comorbidities (Table 1).

Table 1.
Comprehensive summary of common comorbidities associated with IPF.
Conditions such as PH, IHD, GERD, OSA, LC, anxiety, and depression are not mere bystanders but can significantly influence IPF progression, symptom burden, quality of life, and survival. Antifibrotic therapies form the cornerstone of IPF management by slowing disease progression, but their optimal use and effectiveness can be modulated by the presence and management of these coexisting conditions (Table 2).

Table 2.
Potential drug–drug interactions between antifibrotic agents and medications for comorbidities.
This review underscores the critical need for a multidisciplinary, patient-centered approach. Pulmonologists, cardiologists, gastroenterologists, oncologists, sleep specialists, mental health professionals, palliative care teams, and specialist nurses must collaborate to develop integrated care plans. Routine screening for common comorbidities at diagnosis and during follow-up is essential, guided by international clinical guidelines.
5. Future Directions
Despite advancements, significant gaps remain in our understanding and management of comorbidities in IPF. Continuous research is imperative. Future research should focus on the following:
Large-scale, multicenter prospective cohort studies: To better delineate the prevalence, incidence, natural history, and prognostic impact of various comorbidities in diverse IPF populations, and to identify risk factors for their development.
Mechanistic studies: To unravel the shared and distinct pathophysiological pathways linking IPF and its comorbidities (e.g., systemic inflammation, shared genetic predispositions, and impact of hypoxia). This could identify novel therapeutic targets.
Development and validation of personalized therapeutic approaches: Tailoring treatment strategies based on individual patient profiles, including their specific comorbidity burden, genetic markers, and biomarker signatures. This includes investigating the efficacy and safety of antifibrotic agents in IPF patients with specific comorbidities and exploring optimal combination therapies.
Clinical trials for comorbidity management in IPF: Dedicated trials are needed to evaluate the impact of treating specific comorbidities (e.g., aggressive GERD management, CPAP for OSA, and targeted PH therapies) on IPF-related outcomes, including lung function decline, exacerbation rates, and survival.
Implementation and evaluation of multidisciplinary care models: Assessing the effectiveness and cost-effectiveness of integrated, multidisciplinary care pathways in improving patient outcomes, quality of life, and healthcare resource utilization.
Patient-Reported Outcomes (PROs): Greater emphasis on PROs in clinical trials and real-world studies to capture the holistic impact of IPF and its comorbidities, and the benefits of integrated management, from the patient’s perspective.
The analysis of drug–drug interactions between antifibrotic agents and medications used to treat comorbidities in IPF is not within the primary scope of this study; however, recognizing and understanding these interactions may enhance treatment safety and efficacy. Table 2 summarizes the current knowledge on this topic.
In conclusion, effectively managing the complex interplay between IPF and its comorbidities is a cornerstone of modern IPF care. By embracing a holistic, evidence-based, and collaborative approach that prioritizes early detection, guideline-adherent management of comorbidities, and shared decision-making, clinicians can strive to improve not only survival but also the quality of life for individuals living with this devastating disease. The journey requires ongoing research and a commitment to translating new knowledge into better, more personalized patient care.
Author Contributions
Conceptualization, A.S., M.C. (Maria Chianese), M.M., and B.R.; methodology, A.R., A.D.N., D.A., A.G., M.C. (Maria Chernovsky), and L.M.; writing—original draft preparation, R.C., M.P., F.S., P.C., P.G., G.B., and M.H.; writing—review and editing, A.S., M.C. (Maria Chianese), M.M., and B.R.; supervision, B.R. and M.C. (Marco Confalonieri). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest. Each author declares that he or she has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangement, etc.) that might pose a conflict of interest in connection with the submitted article.
References
- Lederer, D.J.; Martinez, F.J. Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2018, 378, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Verma, I.; Shah, V.; Agarwal, A.; Sikachi, R.R. Cardiac manifestations of idiopathic pulmonary fibrosis. Intractable Rare Dis. Res. 2016, 5, 70–75. [Google Scholar] [CrossRef]
- Maher, T.M.; Bendstrup, E.; Dron, L.; Langley, J.; Smith, G.; Khalid, J.M.; Patel, H.; Kreuter, M. Global incidence and prevalence of idiopathic pulmonary fibrosis. Respir. Res. 2021, 22, 197. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.-F.; Flaherty, K.R.; Lasky, J.A.; et al. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic Pulmonary Fibrosis: Evidence-based Guidelines for Diagnosis and Management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Rochwerg, B.; Zhang, Y.; Cuello-Garcia, C.; Azuma, A.; Behr, J.; Brozek, J.L.; Collard, H.R.; Cunningham, W.; Homma, S.; et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2015, 192, e3–e19. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef]
- Olson, A.L.; Swigris, J.J.; Lezotte, D.C.; Norris, J.M.; Wilson, C.G.; Brown, K.K. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am. J. Respir. Crit. Care Med. 2007, 176, 277–284. [Google Scholar] [CrossRef]
- Baumgartner, K.B.; Samet, J.M.; Stidley, C.A.; Colby, T.V.; Waldron, J.A. Cigarette smoking: A risk factor for idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 1997, 155, 242–248. [Google Scholar] [CrossRef]
- King, T.E.; Albera, C.; Bradford, W.Z.; Costabel, U.; du Bois, R.M.; Leff, J.A.; Nathan, S.D.; Sahn, S.A.; Valeyre, D.; Noble, P.W. All-cause mortality rate in patients with idiopathic pulmonary fibrosis. Implications for the design and execution of clinical trials. Am. J. Respir. Crit. Care Med. 2014, 189, 825–831. [Google Scholar] [CrossRef]
- Nathan, S.D.; Shlobin, O.A.; Weir, N.; Ahmad, S.; Kaldjob, J.M.; Battle, E.; Sheridan, M.J.; du Bois, R.M. Long-term Course and Prognosis of Idiopathic Pulmonary Fibrosis in the New Millennium. Chest 2011, 140, 221–229. [Google Scholar] [CrossRef]
- Richeldi, L.; Collard, H.R.; Jones, M.G. Idiopathic pulmonary fibrosis. Lancet 2017, 389, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Somogyi, V.; Chaudhuri, N.; Torrisi, S.E.; Kahn, N.; Maher, T.M. The therapy of idiopathic pulmonary fibrosis: What is next? Eur. Respir. Rev. 2019, 28, 190021. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Slutsky, A.S. Idiopathic Pulmonary Fibrosis—New Insights. N. Engl. J. Med. 2007, 356, 1370–1372. [Google Scholar] [CrossRef] [PubMed]
- Wuyts, W.A.; Lauwers, E.; Dupont, L.J. The pathogenesis of pulmonary fibrosis: A moving target. Eur. Respir. J. 2013, 41, 1207–1218. [Google Scholar] [CrossRef]
- Zheng, Q.; Cox, I.A.; Campbell, J.A.; Xia, Q.; Otahal, P.; de Graaff, B.; Corte, T.J.; Teoh, A.K.; Walters, E.H.; Palmer, A.J. Mortality and survival in idiopathic pulmonary fibrosis: A systematic review and meta-analysis. ER. Open Res. 2022, 8, 00591–02021. [Google Scholar] [CrossRef]
- Cottin, V.; Selman, M.; Inoue, Y.; Wong, A.W.; Corte, T.J.; Flaherty, K.R.; Han, M.K.; Jacob, J.; Johannson, K.A.; Kitaichi, M.; et al. Syndrome of Combined Pulmonary Fibrosis and Emphysema: An Official ATS/ERS/JRS/ALAT Research Statement. Am. J. Respir. Crit. Care Med. 2022, 206, e7–e41. [Google Scholar] [CrossRef]
- De Boer, K.; Lee, J.S. Under-recognised co-morbidities in idiopathic pulmonary fibrosis: A review. Respirology 2016, 21, 995–1004. [Google Scholar] [CrossRef]
- Fulton, B.G.; Ryerson, C.J. Managing comorbidities in idiopathic pulmonary fibrosis. Int. J. Gen. Med. 2015, 8, 309–318. [Google Scholar]
- King, C.S.; Nathan, S.D. Idiopathic pulmonary fibrosis: Effects and optimal management of comorbidities. Lancet Respir. Med. 2017, 5, 72–84. [Google Scholar] [CrossRef]
- Kreuter, M.; Spagnolo, P.; Wuyts, W.; Renzoni, E.; Koschel, D.; Bonella, F.; Maher, T.M.; Kolb, M.; Weycker, D.; Kirchgässler, K.U.; et al. Antacid Therapy and Disease Progression in Patients with Idiopathic Pulmonary Fibrosis Who Received Pirfenidone. Respiration 2017, 93, 415–423. [Google Scholar] [CrossRef]
- Lindell, K.O.; Nouraie, M.; Klesen, M.J.; Klein, S.; Gibson, K.F.; Kass, D.J.; Rosenzweig, M.Q. Randomised clinical trial of an early palliative care intervention (SUPPORT) for patients with idiopathic pulmonary fibrosis (IPF) and their caregivers: Protocol and key design considerations. BMJ Open Respir. Res. 2018, 5, e000272. [Google Scholar] [CrossRef] [PubMed]
- Lindell, K.O.; Raghu, G. Palliative care for patients with pulmonary fibrosis: Symptom relief is essential. Eur. Respir. J. 2018, 52, 1802086. [Google Scholar] [CrossRef]
- Oldham, J.M.; Collard, H.R. Comorbid Conditions in Idiopathic Pulmonary Fibrosis: Recognition and Management. Front. Med. 2017, 4, 123. [Google Scholar] [CrossRef] [PubMed]
- Carter, P.; Lagan, J.; Fortune, C.; Bhatt, D.L.; Vestbo, J.; Niven, R.; Chaudhuri, N.; Schelbert, E.B.; Potluri, R.; Miller, C.A. Association of cardiovascular disease with respiratory disease. J. Am. Coll. Cardiol. 2019, 73, 2166–2177. [Google Scholar] [CrossRef]
- Di Gregorio, J.; Pérez-Barroso, V.; López-Novoa, J.M.; Nieto, M.A.; Portillo-Salido, E. The Epithelial-to-Mesenchymal Transition as a Possible Therapeutic Target in Fibrotic Disorders. Front. Cell. Dev. Biol. 2020, 8, 607483. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J.; Horowitz, J.C. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proc. Am. Thorac. Soc. 2006, 3, 350–356. [Google Scholar] [CrossRef]
- Burgstaller, G.; Kulasekaran, S.; Königshoff, M.; Eickelberg, O. The instructive extracellular matrix of the lung: Basic composition and alterations in chronic lung disease. Eur. Respir. J. 2017, 50, 1601805. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, Y.; Zhang, L.; Li, Y.; Zhang, Y. The extracellular matrix and mechanotransduction in pulmonary fibrosis. Int. J. Biochem. Cell Biol. 2020, 126, 105802. [Google Scholar] [CrossRef]
- Podolanczuk, A.; Pirożyński, J.; Białas, A.J.; Brzecka, M. Comorbidities in idiopathic pulmonary fibrosis. Adv. Clin. Exp. Med. 2022, 31, 1187–1194. [Google Scholar]
- Raghu, G.; Amatto, V.C.; Behr, J.; Stowasser, S. Comorbidities in idiopathic pulmonary fibrosis patients: A systematic literature review. Eur. Respir. J. 2015, 46, 1113–1130. [Google Scholar] [CrossRef]
- Torrisi, S.E.; Ley, B.; Kreuter, M.; Wijsenbeek, M.; Vittinghoff, E.; Collard, H.R.; Vancheri, C. The added value of comorbidities in predicting survival in idiopathic pulmonary fibrosis: A multicentre observational study. Eur. Respir. J. 2019, 53, 1801587. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, M.; Ehlers-Tenenbaum, S.; Palmowski, K.; Bruhwyler, J.; Oltmanns, U.; Muley, T.; Heussel, C.P.; Warth, A.; Kolb, M.; Herth, F.J. Impact of Comorbidities on Mortality in Patients with Idiopathic Pulmonary Fibrosis. PLoS ONE 2016, 11, e0151425. [Google Scholar] [CrossRef] [PubMed]
- Cottin, V.; Nunes, H.; Brillet, P.-Y.; Delaval, P.; Devouassoux, G.; Tillie-Leblond, I.; Israel-Biet, D.; Court-Fortune, I.; Valeyre, D.; Cordier, J.-F. Combined pulmonary fibrosis and emphysema: A distinct underrecognised entity. Eur. Respir. J. 2005, 26, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Cano-Jiménez, E.; Hernández González, F.; Peloche, G.B. Comorbidities and Complications in Idiopathic Pulmonary Fibrosis. Med. Sci. 2018, 6, 71. [Google Scholar] [CrossRef]
- Choi, J.S.; Lee, S.H.; Leem, A.Y.; Song, J.H.; Chung, K.S.; Jung, J.Y.; Kang, Y.A.; Park, M.S.; Kim, Y.S.; Chang, J.; et al. Prognostic impact of the ratio of the main pulmonary artery to that of the aorta on chest computed tomography in patients with idiopathic pulmonary fibrosis. BMC Pulm. Med. 2019, 19, 81. [Google Scholar] [CrossRef]
- Silva, D.R.; Gazzana, M.B.; Barreto, S.S.M.; Knorst, M.M. Fibrose pulmonar idiopática simultânea a enfisema em pacientes tabagistas (Simultaneous idiopathic pulmonary fibrosis and emphysema in smokers). J. Bras. Pneumol. 2008, 34, 779–786. (In Portuguese) [Google Scholar] [CrossRef]
- Mejía, M.; Carrillo, G.; Rojas-Serrano, J.; Estrada, A.; Suárez, T.; Alonso, D.; Barrientos, E.; Gaxiola, M.; Navarro, C.; Selman, M. Idiopathic Pulmonary Fibrosis and Emphysema. Chest 2009, 135, 10–15. [Google Scholar] [CrossRef]
- Kurashima, K.; Takayanagi, N.; Tsuchiya, N.; Kanauchi, T.; Ueda, M.; Hoshi, T.; Miyahara, Y.; Sugita, Y. The effect of emphysema on lung function and survival in patients with idiopathic pulmonary fibrosis. Respirology 2010, 15, 843–848. [Google Scholar] [CrossRef]
- Cottin, V.; Azuma, A.; Raghu, G.; Stansen, W.; Stowasser, S.; Schlenker-Herceg, R.; Kolb, M. Therapeutic effects of nintedanib are not influenced by emphysema in the INPULSIS trials. Eur. Respir. J. 2019, 53, 1801655. [Google Scholar] [CrossRef]
- Wells, A.U.; Desai, S.R.; Rubens, M.B.; Goh, N.S.; Cramer, D.; Nicholson, A.G.; Colby, T.V.; Du Bois, R.M.; Hansell, D.M. Idiopathic Pulmonary Fibrosis: A Composite Physiologic Index Derived from Disease Extent Observed by Computed Tomography. Am. J. Respir. Crit. Care. Med. 2003, 167, 962–969. [Google Scholar] [CrossRef]
- Cottin, V.; Le Pavec, J.; Prévot, G.; Mal, H.; Humbert, M.; Simonneau, G.; Cordier, J.F. Pulmonary hypertension in patients with combined pulmonary fibrosis and emphysema syndrome. Eur. Respir. J. 2010, 35, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Platenburg, M.G.J.P.; Van Der Vis, J.J.; Grutters, J.C.; Van Moorsel, C.H.M. Decreased Survival and Lung Function in Progressive Pulmonary Fibrosis. Medicina 2023, 59, 296. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Noble, P.W.; Albera, C.; Bradford, W.Z.; Costabel, U.; Glassberg, M.K.; Kardatzke, D.; King, T.E., Jr.; Lancaster, L.; Sahn, S.A.; Szwarcberg, J.; et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): Two randomised trials. Lancet 2011, 377, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; Du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and Safety of Nintedanib in Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef]
- Cottin, V.; Crestani, B.; Cadranel, J.; Cordier, J.F.; Marchand-Adam, S.; Prévot, G.; Wallaert, B.; Bergot, E.; Camus, P.; Dalphin, J.C.; et al. French practical guidelines for the diagnosis and management of idiopathic pulmonary fibrosis–2017 update. Full-length version. Rev. Mal. Respir. 2017, 34, 900–968. (In French) [Google Scholar] [CrossRef]
- Fiorentù, G.; Bernardinello, N.; Giulianelli, G.; Cocconcelli, E.; Balestro, E.; Spagnolo, P. Pulmonary Hypertension Associated with Interstitial Lung Disease (PH-ILD): Back to the Future. Adv. Ther. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Dhont, S.; Zwaenepoel, B.; Vandecasteele, E.; Brusselle, G.; De Pauw, M. Pulmonary hypertension in interstitial lung disease: An area of unmet clinical need. ERJ Open Res. 2022, 8, 00272–02022. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Behr, J.; Held, M.; Grünig, E.; Vizza, C.D.; Vonk-Noordegraaf, A.; Lange, T.J.; Claussen, M.; Grohé, C.; Klose, H.; et al. Pulmonary Hypertension in Patients with Chronic Fibrosing Idiopathic Interstitial Pneumonias. PLoS ONE 2015, 10, e0141911. [Google Scholar] [CrossRef]
- Olsson, K.M.; Hoeper, M.M.; Pausch, C.; Grünig, E.; Huscher, D.; Pittrow, D.; Rosenkranz, S.; Gall, H. Pulmonary vascular resistance predicts mortality in patients with pulmonary hypertension associated with interstitial lung disease: Results from the COMPERA registry. Eur. Respir. J. 2021, 58, 2101483. [Google Scholar] [CrossRef]
- Piccari, L.; Wort, S.J.; Meloni, F.; Rizzo, M.; Price, L.C.; Martino, L.; Salvaterra, E.; Scelsi, L.; López Meseguer, M.; Blanco, I.; et al. The Effect of Borderline Pulmonary Hypertension on Survival in Chronic Lung Disease. Respiration 2022, 101, 717–727. [Google Scholar] [CrossRef]
- Lettieri, C.J.; Nathan, S.D.; Barnett, S.D.; Ahmad, S.; Shorr, A.F. Prevalence and Outcomes of Pulmonary Arterial Hypertension in Advanced Idiopathic Pulmonary Fibrosis. Chest 2006, 129, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Hobson, A.R.; Shang, Z.M.; Pei, Y.X.; Gao, Y.; Wang, J.X.; Huang, W.N. The prevalence of gastro-esophageal reflux disease and esophageal dysmotility in Chinese patients with idiopathic pulmonary fibrosis. BMC Gastroenterol. 2015, 15, 26. [Google Scholar] [CrossRef] [PubMed]
- Gavini, S.; Finn, R.T.; Lo, W.K.; Goldberg, H.J.; Burakoff, R.; Feldman, N.; Chan, W.W. Idiopathic pulmonary fibrosis is associated with increased impedance measures of reflux compared to non-fibrotic disease among pre-lung transplant patients. Neurogastroenterol. Motil. 2015, 27, 1326–1332. [Google Scholar] [CrossRef]
- Liao, Y.; Wu, B. Analysis of clinical features and risk factors of pulmonary hypertension associated with interstitial lung disease. Biomed. Rep. 2025, 22, 58. [Google Scholar] [CrossRef]
- Collum, S.D.; Klinger, J.R.; Collard, H.R.; Nathan, S.D. Pulmonary Hypertension Associated with Idiopathic Pulmonary Fibrosis: Current and Future Perspectives. Can. Respir. J. 2017, 2017, 1–12. [Google Scholar] [CrossRef]
- Harari, S.; Elia, D.; Humbert, M. Pulmonary Hypertension in Parenchymal Lung Diseases. Chest 2018, 153, 217–223. [Google Scholar] [CrossRef]
- Olsson, K.M.; Corte, T.J.; Kamp, J.C.; Montani, D.; Nathan, S.D.; Neubert, L.; Price, L.C.; Kiely, D.G. Pulmonary hypertension associated with lung disease: New insights into pathomechanisms, diagnosis, and management. Lancet Respir. Med. 2023, 11, 820–835. [Google Scholar] [CrossRef] [PubMed]
- Mondoni, M.; Rinaldo, R.; Ryerson, C.J.; Albrici, C.; Baccelli, A.; Tirelli, C.; Marchetti, F.; Cefalo, J.; Nalesso, G.; Ferranti, G.; et al. Vascular involvement in idiopathic pulmonary fibrosis. ERJ Open Res. 2024, 10, 00550–02024. [Google Scholar] [CrossRef]
- Panagiotou, M.; Church, A.C.; Johnson, M.K.; Peacock, A.J. Pulmonary vascular and cardiac impairment in interstitial lung disease. Eur. Respir. Rev. 2017, 26, 160053. [Google Scholar] [CrossRef]
- Glasser, S.; Noga, O.; Elkayam, U.; Vainshelboim, B.; Fox, B.; Kramer, M.R. Impact of pulmonary hypertension on gas exchange and exercise capacity in patients with pulmonary fibrosis. Respir. Med. 2009, 103, 1634–1641. [Google Scholar] [CrossRef]
- D’Andrea, A.; Stanziola, A.; Di Palma, E.; Martino, M.; D’Alto, M.; Dellegrottaglie, S.; Cocchia, R.; Riegler, L.; Betancourt Cordido, M.V.; Lanza, M.; et al. Right Ventricular Structure and Function in Idiopathic Pulmonary Fibrosis with or without Pulmonary Hypertension. Echocardiography 2016, 33, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Minai, O.A.; Santacruz, J.F.; Alster, J.M.; Budev, M.M.; McCarthy, K. Impact of pulmonary hemodynamics on 6-min walk test in idiopathic pulmonary fibrosis. Respir. Med. 2012, 106, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Boutou, A.K.; Pitsiou, G.G.; Argentou, M.I.; Stanopoulos, I. Exercise capacity in idiopathic pulmonary fibrosis: The effect of pulmonary hypertension. Respir. Med. 2011, 105, 1233–1239. [Google Scholar] [CrossRef]
- Nathan, S.D.; Shlobin, O.A.; Ahmad, S.; Urbanek, S.; Barnett, S.D. Pulmonary hypertension and pulmonary function testing in idiopathic pulmonary fibrosis. Chest 2007, 131, 657–663. [Google Scholar] [CrossRef]
- Alkukhun, L.; Wang, X.F.; Ahmed, M.K.; Baumgartner, M.; Budev, M.M.; Dweik, R.A.; Tonelli, A.R. Non-invasive screening for pulmonary hypertension in idiopathic pulmonary fibrosis. Respir. Med. 2016, 117, 65–72. [Google Scholar] [CrossRef]
- Nathan, S.D.; Shlobin, O.A.; Barnett, S.D.; Saggar, R.; Belperio, J.A.; Ross, D.J.; Ahmad, S.; Libre, E.; Lynch, J.P., 3rd; Zisman, D.A. Right ventricular systolic pressure by echocardiography as a predictor of pulmonary hypertension in idiopathic pulmonary fibrosis. Respir. Med. 2008, 102, 1305–1310. [Google Scholar] [CrossRef]
- Kimura, M.; Taniguchi, H.; Kondoh, Y.; Kimura, T.; Kataoka, K.; Nishiyama, O.; Aso, H.; Sakamoto, K.; Hasegawa, Y. Pulmonary hypertension as a prognostic indicator at the initial evaluation in idiopathic pulmonary fibrosis. Respiration 2013, 85, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Nathan, S.D.; Behr, J.; Brown, K.K.; Egan, J.J.; Kawut, S.M.; Flaherty, K.R.; Martinez, F.J.; Wells, A.U.; Shao, L.; et al. Pulmonary hypertension in idiopathic pulmonary fibrosis with mild-to-moderate restriction. Eur. Respir. J. 2015, 46, 1370–1377. [Google Scholar] [CrossRef]
- Shorr, A.F.; Wainright, J.L.; Cors, C.S.; Lettieri, C.J.; Nathan, S.D. Pulmonary hypertension in patients with pulmonary fibrosis awaiting lung transplant. Eur. Respir. J. 2007, 30, 715–721. [Google Scholar] [CrossRef]
- Zisman, D.A.; Schwarz, M.; Anstrom, K.J.; Collard, H.R.; Flaherty, K.R.; Hunninghake, G.W. A Controlled Trial of Sildenafil in Advanced Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2010, 363, 620–628. [Google Scholar]
- Bax, S.; Bredy, C.; Kempny, A.; Dimopoulos, K.; Devaraj, A.; Walsh, S.; Jacob, J.; Nair, A.; Kokosi, M.; Keir, G. A stepwise composite echocardiographic score predicts severe pulmonary hypertension in patients with interstitial lung disease. ERJ Open Res. 2018, 4, 00124–02017. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Nathan, S.D.; Chandel, A.; Wang, Y.; Xu, J.; Shao, L.; Watkins, T.R.; Diviney, J.; King, C.S.; Han, L. Derivation and validation of a noninvasive prediction tool to identify pulmonary hypertension in patients with IPF: Evolution of the model FORD. J. Heart Lung Transplant. 2024, 43, 547–553. [Google Scholar] [CrossRef]
- Pradhan, A.; Tyagi, R.; Sharma, P.; Bajpai, J.; Kant, S. Shifting Paradigms in the Management of Pulmonary Hypertension. Eur. Cardiol. 2024, 19, e25. [Google Scholar] [CrossRef]
- Waxman, A.; Restrepo-Jaramillo, R.; Thenappan, T.; Ravichandran, A.; Engel, P.; Bajwa, A.; Allen, R.; Feldman, J.; Argula, R.; Smith, P.; et al. Inhaled Treprostinil in Pulmonary Hypertension Due to Interstitial Lung Disease. N. Engl. J. Med. 2021, 384, 325–334. [Google Scholar] [CrossRef]
- Nathan, S.D.; Waxman, A.; Rajagopal, S.; Case, A.; Johri, S.; DuBrock, H.; De La Zerda, D.J.; Sahay, S.; King, C.; Melendres-Groves, L.; et al. Inhaled treprostinil and forced vital capacity in patients with interstitial lung disease and associated pulmonary hypertension: A post-hoc analysis of the INCREASE study. Lancet Respir Med. 2021, 9, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Ruaro, B.; Baratella, E.; Caforio, G.; Confalonieri, P.; Wade, B.; Marrocchio, C.; Geri, P.; Pozzan, R.; Andrisano, A.G.; Cova, M.A.; et al. Chronic Thromboembolic Pulmonary Hypertension: An Update. Diagnostics 2022, 12, 235. [Google Scholar] [CrossRef]
- Saggar, R.; Khanna, D.; Vaidya, A.; Derhovanessian, A.; Maranian, P.; Duffy, E.; Belperio, J.A.; Weigt, S.S.; Dua, S.; Shapiro, S.S.; et al. Changes in right heart haemodynamics and echocardiographic function in an advanced phenotype of pulmonary hypertension and right heart dysfunction associated with pulmonary fibrosis. Thorax 2014, 69, 123–129. [Google Scholar] [CrossRef]
- Nathan, S.D.; Flaherty, K.R.; Glassberg, M.K.; Raghu, G.; Swigris, J.; Alvarez, R.; Ettinger, N.; Loyd, J.; Fernandes, P.; Gillies, H.; et al. A randomized, double-blind, placebo-controlled study of pulsed, inhaled nitric oxide in subjects at risk of pulmonary hypertension associated with pulmonary fibrosis. Chest 2020, 158, 637–645. [Google Scholar] [CrossRef]
- Nathan, S.D.; Rajicic, N.; Dudenhofer, R.; Hussain, R.; Argula, R.; Bandyopadhyay, D.; Luckhardt, T.; Muehlemann, N.; Flaherty, K.R.; Glassberg, M.K.; et al. Inhaled Nitric Oxide in Fibrotic Lung Disease: A Randomized, Double-Blind, Placebo-controlled Trial. Ann. Am. Thorac. Soc. 2024, 21, 1661–1669. [Google Scholar] [CrossRef]
- Nathan, S.D.; Stinchon MRJr Atcheson, S.; Simone, L.; Nelson, M. Shining a spotlight on pulmonary hypertension associated with interstitial lung disease care: The latest advances in diagnosis and treatment. J. Manag. Care Spec. Pharm. 2025, 31, S2–S17. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.; Kolb, M.; Song, J.W.; Luppi, F.; Schinzel, B.; Stowasser, S.; Quaresma, M.; Martinez, F.J. Nintedanib and Sildenafil in Patients with Idiopathic Pulmonary Fibrosis and Right Heart Dysfunction. A Prespecified Subgroup Analysis of a Double-Blind Randomized Clinical Trial (INSTAGE). Am. J. Respir. Crit. Care Med. 2019, 200, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.; Nathan, S.D.; Wuyts, W.A.; Mogulkoc Bishop, N.; Bouros, D.E.; Antoniou, K.; Guiot, J.; Kramer, M.R.; Kirchgaessler, K.U.; Bengus, M.; et al. Efficacy and safety of sildenafil added to pirfenidone in patients with advanced idiopathic pulmonary fibrosis and risk of pulmonary hypertension: A double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2021, 9, 85–95. [Google Scholar] [CrossRef]
- Han, M.K.; Bach, D.S.; Hagan, P.G.; Yow, E.; Flaherty, K.R.; Toews, G.B.; Anstrom, K.J.; Martinez, F.J.; IPFnet Investigators. Sildenafil Preserves Exercise Capacity in Patients With Idiopathic Pulmonary Fibrosis and Right-sided Ventricular Dysfunction. Chest 2013, 143, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E.; Corte, T.; Chambers, D.C.; Palmer, A.J.; Ekström, M.P.; Glaspole, I.; Goh, N.S.L.; Hepworth, G.; Khor, Y.H.; Hoffman, M.; et al. Ambulatory oxygen for treatment of exertional hypoxaemia in pulmonary fibrosis (PFOX trial): A randomised controlled trial. BMJ Open 2020, 10, e040798. [Google Scholar] [CrossRef]
- Kolb, M.; Raghu, G.; Wells, A.U.; Behr, J.; Richeldi, L.; Schinzel, B.; Quaresma, M.; Stowasser, S.; Martinez, F.J.; INSTAGE Investigators. Nintedanib plus sildenafil in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2018, 379, 1722–1731. [Google Scholar] [CrossRef]
- Whitty, J.A.; Rankin, J.; Visca, D.; Tsipouri, V.; Mori, L.; Spencer, L.; Adamali, H.; Maher, T.M.; Hopkinson, N.S.; Birring, S.S.; et al. Cost-effectiveness of ambulatory oxygen in improving quality of life in fibrotic lung disease: Preliminary evidence from the AmbOx Trial. Eur. Respir. J. 2020, 55, 1901157. [Google Scholar] [CrossRef]
- Nathan, S.D.; Behr, J.; Collard, H.R.; Cottin, V.; Hoeper, M.M.; Martinez, F.J.; Corte, T.J.; Keogh, A.M.; Leuchte, H.; Mogulkoc, N.; et al. Riociguat for idiopathic interstitial pneumonia-associated pulmonary hypertension (RISE-IIP): A randomised, placebo-controlled phase 2b study. Lancet Respir. Med. 2019, 7, 780–790. [Google Scholar] [CrossRef]
- Raghu, G.; Behr, J.; Brown, K.K.; Egan, J.J.; Kawut, S.M.; Flaherty, K.R.; Martinez, F.J.; Nathan, S.D.; Wells, A.U.; Collard, H.R.; et al. Treatment of idiopathic pulmonary fibrosis with ambrisentan: A parallel, randomized trial. Ann. Intern. Med. 2013, 158, 641–649. [Google Scholar] [CrossRef]
- Clarson, L.E.; Bajpai, R.; Whittle, R.; Belcher, J.; Abdul Sultan, A.; Kwok, C.S.; Welsh, V.; Mamas, M.; Mallen, C.D. Interstitial lung disease is a risk factor for ischaemic heart disease and myocardial infarction. Heart 2020, 106, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, R.B.; Smith, C.; Le Jeune, I.; Gribbin, J.; Fogarty, A.W. The association between idiopathic pulmonary fibrosis and vascular disease: A population-based study. Am. J. Respir. Crit. Care Med. 2008, 178, 1257–1261. [Google Scholar] [CrossRef]
- Kim, W.Y.; Mok, Y.; Kim, G.W.; Baek, S.J.; Yun, Y.D.; Jee, S.H.; Kim, D.S. Association between idiopathic pulmonary fibrosis and coronary artery disease: A case-control study and cohort analysis. Sarcoidosis. Vasc. Diffuse Lung Dis. 2015, 31, 289–296. [Google Scholar] [PubMed]
- Nathan, S.D.; Basavaraj, A.; Reichner, C.; Shlobin, O.A.; Ahmad, S.; Kiernan, J.; Burton, N.; Barnett, S.D. Prevalence and impact of coronary artery disease in idiopathic pulmonary fibrosis. Respir. Med. 2010, 104, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Panos, R.J.; Mortenson, R.L.; Niccoli, S.A.; King, T.E., Jr. Clinical deterioration in patients with idiopathic pulmonary fibrosis: Causes and assessment. Am. J. Med. 1990, 88, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Tomassetti, S.; Gurioli, C.; Ryu, J.H.; Decker, P.A.; Ravaglia, C.; Tantalocco, P.; Buccioli, M.; Piciucchi, S.; Sverzellati, N.; Dubini, A.; et al. The impact of lung cancer on survival of idiopathic pulmonary fibrosis. Chest 2015, 147, 157–164. [Google Scholar] [CrossRef]
- Kato, S.; Kitamura, H.; Hayakawa, K.; Fukui, K.; Tabata, E.; Otoshi, R.; Iwasawa, T.; Utsunomiya, D.; Kimura, K.; Tamura, K.; et al. Coronary artery disease and heart failure in patients with idiopathic pulmonary fibrosis. Heart. Vessels 2021, 36, 1151–1158. [Google Scholar] [CrossRef]
- Ruaro, B.; Pizzorni, C.; Paolino, S.; Smith, V.; Ghio, M.; Casabella, A.; Alessandri, E.; Patané, M.; Sulli, A.; Cutolo, M. Correlations between nailfold microvascular damage and skin involvement in systemic sclerosis patients. Microvasc. Res. 2019, 125, 103874. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis: An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Hayashi, M.; Fujimoto, K.; Urushibata, K.; Uchikawa, S.; Imamura, H.; Kubo, K. Nocturnal oxygen desaturation correlates with the severity of coronary atherosclerosis in coronary artery disease. Chest 2003, 124, 936–941. [Google Scholar] [CrossRef][Green Version]
- Kizer, J.R.; Zisman, D.A.; Blumenthal, N.P.; Kotloff, R.M.; Kimmel, S.E.; Strieter, R.M.; Arcasoy, S.M.; Ferrari, V.A.; Hansen-Flaschen, J. Association between pulmonary fibrosis and coronary artery disease. Arch. Intern. Med. 2004, 164, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Patane, S.; Marte, F.; Sturiale, M.; Dattilo, G.; Luzza, F. Atrial flutter, ventricular tachycardia and changing axis deviation associated with scleroderma. Int. J. Cardiol. 2011, 153, e25–e28. [Google Scholar] [CrossRef]
- Dalleywater, W.; Powell, H.A.; Hubbard, R.B.; Navaratnam, V.; Fogarty, A.W. Risk factors for cardiovascular disease in people with idiopathic pulmonary fibrosis: A population-based study. Chest 2015, 147, 150–156. [Google Scholar] [CrossRef]
- Bhatt, S.P.; Kazerooni, E.A.; Newell, J.D., Jr.; Hokanson, J.E.; Budoff, M.J.; Dass, C.A.; Martinez, C.H.; Bodduluri, S.; Jacobson, F.L.; Yen, A.; et al. Visual Estimate of Coronary Artery Calcium Predicts Cardiovascular Disease in COPD. Chest 2018, 154, 579–587. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart disease and stroke statistics-2018 update: A report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef]
- Duong, M.; Islam, S.; Rangarajan, S.; Leong, D.; Kurmi, O.; Teo, K.; Killian, K.; Dagenais, G.; Lear, S.; Wielgosz, A.; et al. Mortality and cardiovascular and respiratory morbidity in individuals with impaired FEV1 (PURE): An international, community-based cohort study. Lancet Glob Health 2019, 7, e613–e623. [Google Scholar] [CrossRef] [PubMed]
- Hole, D.J.; Watt, G.C.; Davey-Smith, G.; Hart, C.L.; Gillis, C.R.; Hawthorne, V.M. Impaired lung function and mortality risk in men and women: Findings from the Renfrew and Paisley prospective population study. BMJ 1996, 313, 711–715; discussion 715–716. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.G.; Stockley, R.A.; Harper, L.; Hussell, T.; Sapey, E. Pulmonary infection in Wegener granulomatosis and idiopathic pulmonary fibrosis. Thorax 2009, 64, 692–697. [Google Scholar] [CrossRef]
- Bray, K.; Bodduluri, S.; Kim, Y.I.; Sthanam, V.; Nath, H.; Bhatt, S.P. Idiopathic pulmonary fibrosis is more strongly associated with coronary artery disease than chronic obstructive pulmonary disease. Respir. Med. 2023, 211, 107195. [Google Scholar] [CrossRef]
- Izbicki, G.; Ben-Dor, I.; Shitrit, D.; Bendayan, D.; Aldrich, T.K.; Kornowski, R.; Kramer, M.R. The prevalence of coronary artery disease in end-stage pulmonary disease: Is pulmonary fibrosis a risk factor? Respir. Med. 2009, 103, 1346–1349. [Google Scholar] [CrossRef]
- Le Jeune, I.; Gribbin, J.; West, J.; Smith, C.; Cullinan, P.; Hubbard, R. The incidence of cancer in patients with idiopathic pulmonary fibrosis and sarcoidosis in the UK. Respir. Med. 2007, 101, 2534–2540. [Google Scholar] [CrossRef] [PubMed]
- Bild, D.E.; Detrano, R.; Peterson, D.; Guerci, A.; Liu, K.; Shahar, E.; Ouyang, P.; Jackson, S.; Saad, M.F. Ethnic differences in coronary calcification: The multi-ethnic study of atherosclerosis (MESA). Circulation 2005, 111, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Manolio, T.A.; Arnold, A.M.; Post, W.; Bertoni, A.G.; Schreiner, P.J.; Sacco, R.L.; Saad, M.F.; Detrano, R.L.; Szklo, M. Ethnic differences in the relationship of carotid atherosclerosis to coronary calcification: The multi-ethnic study of atherosclerosis. Atherosclerosis 2008, 197, 132–138. [Google Scholar] [CrossRef]
- Nasir, K.; Shaw, L.J.; Liu, S.T.; Weinstein, S.R.; Mosler, T.R.; Flores, P.R.; Flores, F.R.; Raggi, P.; Berman, D.S.; Blumenthal, R.S.; et al. Ethnic differences in the prognostic value of coronary artery calcification for all-cause mortality. J. Am. Coll. Cardiol. 2007, 50, 953–960. [Google Scholar] [CrossRef]
- Natsuizaka, M.; Chiba, H.; Kuronuma, K.; Otsuka, M.; Kudo, K.; Mori, M.; Bando, M.; Sugiyama, Y.; Takahashi, H. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am. J. Respir. Crit. Care. Med. 2014, 190, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Nathan, S.D.; Weir, N.; Shlobin, O.A.; Urban, B.A.; Curry, C.A.; Basavaraj, A.; Ahmad, S.; Kiernan, J.; Sheridan, M.J.; Earls, J.P. The value of computed tomography scanning for the detection of coronary artery disease in patients with idiopathic pulmonary fibrosis. Respirology 2011, 16, 481–486. [Google Scholar] [CrossRef]
- Krummen, D.E.; Daniels, L.B. Biomarkers in arrhythmias, sudden death, and device therapy. In Cardiac Biomarkers: Case Studies and Clinical Correlations; Maisel, A.S., Jaffe, A.S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 329–343. [Google Scholar]
- Orrego, C.M.; Cordero-Reyes, A.M.; Estep, J.D.; Seethamraju, H.; Scheinin, S.; Loebe, M.; Torre-Amione, G. Atrial arrhythmias after lung transplant: Underlying mechanisms, risk factors, and prognosis. J. Heart Lung Transplant. 2014, 33, 734–740. [Google Scholar] [CrossRef]
- Shibata, Y.; Watanabe, T.; Osaka, D.; Tanahashi, N. Impairment of pulmonary function is an independent risk factor for atrial fibrillation: The Takahata study. Int. J. Med. Sci. 2011, 8, 514–522. [Google Scholar] [CrossRef]
- Azadani, P.N.; Kumar, U.N.; Yang, Y.; Scheinman, M.M.; Hoopes, C.W.; Marcus, G.M.; Rifkin, C.; Olgin, J.E.; Lee, B.K. Frequency of atrial flutter after adult lung transplantation. Am. J. Cardiol. 2011, 107, 922–926. [Google Scholar] [CrossRef]
- Nielsen, T.D.; Bahnson, T.; Davis, R.D.; Palmer, S.M. Atrial fibrillation after pulmonary transplant. Chest 2004, 126, 496–500. [Google Scholar] [CrossRef]
- Bandeira, C.D.; Rubin, A.S.; Cardoso, P.F.; Moreira, J.d.S.; Machado, M.d.M. Prevalência da doença do refluxo gastroesofágico em pacientes com fibrose pulmonar idiopática (Prevalence of gastroesophageal reflux disease in patients with idiopathic pulmonary fibrosis). J. Bras. Pneumol. 2009, 35, 1182–1189. (In Portuguese) [Google Scholar] [CrossRef]
- Raghu, G.; Meyer, K.C. Silent gastro-oesophageal reflux and microaspiration in IPF: Mounting evidence for anti-reflux therapy? Eur. Respir. J. 2012, 39, 242–245. [Google Scholar] [CrossRef]
- Raghu, G.; Freudenberger, T.D.; Yang, P.; Curtis, J.R.; Spada, C.; Hayes, J.; Sillery, J.K.; Pope, C.E., 2nd; Pellegrini, C.A. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur. Respir. J. 2006, 27, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Savarino, E.; Zentilin, P.; Savarino, V. Gastro-oesophageal reflux and gastric aspiration in idiopathic pulmonary fibrosis patients. Eur. Respir. J. 2013, 42, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Madan, K.; Ali, T.; Singh, P.; Ahuja, V.; Sharma, M.P. Impact of 24-h esophageal pH monitoring on the diagnosis of gastroesophageal reflux disease: Defining the gold standard. J. Gastroenterol. Hepatol. 2005, 20, 30–37. [Google Scholar] [CrossRef]
- Rosen, R.; Lord, C.; Nurko, S. The Sensitivity of Multichannel Intraluminal Impedance and the pH Probe in the Evaluation of Gastroesophageal Reflux in Children. Clin. Gastroenterol. Hepatol. 2006, 4, 167–172. [Google Scholar] [CrossRef]
- Lee, J.S.; Song, J.W.; Wolters, P.J.; Elicker, B.M.; King, T.E., Jr.; Kim, D.S.; Collard, H.R. Bronchoalveolar lavage pepsin in acute exacerbation of idiopathic pulmonary fibrosis. Eur. Respir. J. 2012, 39, 352–358. [Google Scholar] [CrossRef]
- Tcherakian, C.; Cottin, V.; Brillet, P.Y.; Freynet, O.; Naggara, N.; Carton, Z.; Cordier, J.F.; Brauner, M.; Valeyre, D.; Nunes, H. Progression of idiopathic pulmonary fibrosis: Lessons from asymmetrical disease. Thorax 2011, 66, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Ruaro, B.; Pozzan, R.; Confalonieri, P.; Tavano, S.; Hughes, M.; Matucci Cerinic, M.; Baratella, E.; Zanatta, E.; Lerda, S.; Geri, P.; et al. Gastroesophageal Reflux Disease in Idiopathic Pulmonary Fibrosis: Viewer or Actor? To Treat or Not to Treat? Pharmaceuticals 2022, 15, 1033. [Google Scholar] [CrossRef]
- Ghebremariam, Y.T.; Cooke, J.P.; Gerhart, W.; Griego, C.; Brower, J.B.; Doyle-Eisele, M.; Moeller, B.C.; Zhou, Q.; Ho, L.; de Andrade, J.; et al. Pleiotropic effect of the proton pump inhibitor esomeprazole leading to suppression of lung inflammation and fibrosis. J. Transl. Med. 2015, 13, 249. [Google Scholar] [CrossRef]
- Hoppo, T.; Jarido, V.; Pennathur, A.; Morrell, M.; Crespo, M.; Shigemura, N.; Bermudez, C.; Hunter, J.G.; Toyoda, Y.; Pilewski, J.; et al. Antireflux Surgery Preserves Lung Function in Patients With Gastroesophageal Reflux Disease and End-stage Lung Disease Before and After Lung Transplantation. Arch. Surg. 2011, 146, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Collard, H.R.; Raghu, G.; Sweet, M.P.; Hays, S.R.; Campos, G.M.; Golden, J.A.; King, T.E., Jr. Does Chronic Microaspiration Cause Idiopathic Pulmonary Fibrosis? Am. J. Med. 2010, 123, 304–311. [Google Scholar] [CrossRef]
- Lozo Vukovac, E.; Lozo, M.; Mise, K.; Gudelj, I.; Puljiz, Ž.; Jurcev-Savicevic, A.; Bradaric, A.; Kokeza, J.; Mise, J. Bronchoalveolar pH and inflammatory biomarkers in newly diagnosed IPF and GERD patients: A case-control study. Med. Sci. Monit. 2014, 20, 255–261. [Google Scholar]
- Soares, R.V.; Forsythe, A.; Hogarth, K.; Sweiss, N.J.; Noth, I.; Patti, M.G. Interstitial lung disease and gastroesophageal reflux disease: Key role of esophageal function tests in the diagnosis and treatment. Arq. Gastroenterol. 2011, 48, 91–97. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, J.S.; Ryu, J.H.; Elicker, B.M.; Lydell, C.P.; Jones, K.D.; Wolters, P.J.; King, T.E., Jr.; Collard, H.R. Gastroesophageal Reflux Therapy Is Associated with Longer Survival in Patients with Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2011, 184, 1390–1394. [Google Scholar] [CrossRef] [PubMed]
- Fidler, L.; Sitzer, N.; Shapera, S.; Shah, P.S. Treatment of Gastroesophageal Reflux in Patients With Idiopathic Pulmonary Fibrosis. Chest 2018, 153, 1405–1415. [Google Scholar] [CrossRef]
- Johannson, K.A.; Strâmbu, I.; Ravaglia, C.; Grutters, J.C.; Valenzuela, C.; Mogulkoc, N.; Luppi, F.; Richeldi, L.; Wells, A.U.; Vancheri, C.; et al. Antacid therapy in idiopathic pulmonary fibrosis: More questions than answers? Lancet Respir. Med. 2017, 5, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Collard, H.R.; Anstrom, K.J.; Martinez, F.J.; Noth, I.; Roberts, R.S.; Yow, E.; Raghu, G. Anti-acid treatment and disease progression in idiopathic pulmonary fibrosis: An analysis of data from three randomised controlled trials. Lancet Respir. Med. 2013, 1, 369–376. [Google Scholar] [CrossRef]
- Raghu, G.; Morrow, E.; Collins, B.F.; Ho, L.A.; Hinojosa, M.W.; Hayes, J.M.; Spada, C.A.; Oelschlager, B.; Li, C.; Yow, E.; et al. Laparoscopic anti-reflux surgery for idiopathic pulmonary fibrosis at a single centre. Eur. Respir. J. 2016, 48, 826–832. [Google Scholar] [CrossRef]
- Glass, D.S.; Chapman, S.J.; Singanayagam, A. Idiopathic pulmonary fibrosis: Current and future treatment. Clin. Respir. J. 2022, 16, 84–96. [Google Scholar] [CrossRef]
- Valeyre, D.; Albera, C.; Bradford, W.Z.; Costabel, U.; King, T.E., Jr.; Leff, J.A.; Noble, P.W.; Sahn, S.A.; du Bois, R.M. Comprehensive assessment of the long-term safety of pirfenidone in patients with idiopathic pulmonary fibrosis. Respirology 2014, 19, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, L.H.; Mason, W.R.; Parnell, J.A.; Rice, T.W.; Loyd, J.E.; Milstone, A.P.; Collard, H.R.; Malow, B.A. Obstructive Sleep Apnea Is Common in Idiopathic Pulmonary Fibrosis. Chest 2009, 136, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Mermigkis, C.; Stagaki, E.; Tryfon, S.; Schiza, S.; Amfilochiou, A.; Polychronopoulos, V.; Panagou, P.; Galanis, N.; Kallianos, A.; Mermigkis, D.; et al. How common is sleep-disordered breathing in patients with idiopathic pulmonary fibrosis? Sleep Breath 2010, 14, 387–390. [Google Scholar] [CrossRef]
- Mermigkis, C.; Stagaki, E.; Amfilochiou, A.; Polychronopoulos, V.; Korkonikitas, P.; Mermigkis, D.; Bregou, M.; Kouris, N.; Bouros, D. Sleep quality and associated daytime consequences in patients with idiopathic pulmonary fibrosis. Med. Princ. Pract. 2009, 18, 10–15. [Google Scholar] [CrossRef]
- Pillai, M.; Olson, A.L.; Huie, T.J.; Solomon, J.J.; Fernandez-Perez, E.R.; Brown, K.K.; Hanna, P.; Lee-Chiong, T.; Swigris, J.J. Obstructive sleep apnea does not promote esophageal reflux in fibrosing interstitial lung disease. Respir. Med. 2012, 106, 1033–1039. [Google Scholar] [CrossRef]
- Perez-Padilla, R.; West, P.; Lertzman, M.; Kryger, M.H. Breathing during sleep in patients with interstitial lung disease. Am. Rev. Respir. Dis. 1985, 132, 224–229. [Google Scholar] [PubMed]
- Kolilekas, L.; Manali, E.; Vlami, K.A.; Lyberopoulos, P.; Triantafillidou, C.; Kagouridis, K.; Baou, K.; Gyftopoulos, S.; Vougas, K.N.; Karakatsani, A.; et al. Sleep oxygen desaturation predicts survival in idiopathic pulmonary fibrosis. J. Clin. Sleep Med. 2013, 9, 593–601. [Google Scholar] [CrossRef]
- Haine, L.; Bravais, J.; Yegen, C.H.; Bernaudin, J.F.; Marchant, D.; Planès, C.; Voituron, N.; Boncoeur, E. Sleep Apnea in Idiopathic Pulmonary Fibrosis: A Molecular Investigation in an Experimental Model of Fibrosis and Intermittent Hypoxia. Life 2021, 11, 973. [Google Scholar] [CrossRef]
- Corte, T.J.; Wort, S.J.; Talbot, S.; Macdonald, P.M.; Hansel, D.M.; Polkey, M.; Renzoni, E.; Maher, T.M.; Nicholson, A.G.; Wells, A.U.; et al. Elevated nocturnal desaturation index predicts mortality in interstitial lung disease. Sarcoidosis. Vasc. Diffuse Lung Dis. 2012, 29, 41–50. [Google Scholar]
- Trakada, G.; Nikolaou, E.; Pouli, A.; Tsiamita, M.; Spiropoulos, K. Endothelin-1 levels in interstitial lung disease patients during sleep. Sleep Breath 2003, 7, 111–118. [Google Scholar] [CrossRef]
- Kim, E.S.; Keating, G.M. Pirfenidone: A review of its use in idiopathic pulmonary fibrosis. Drugs 2015, 75, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, K.M.; Tomassetti, S.; Tsitoura, E.; Vancheri, C. Idiopathic pulmonary fibrosis and lung cancer: A clinical and pathogenesis update. Curr. Opin. Pulm. Med. 2015, 21, 626–633. [Google Scholar] [CrossRef]
- Ballester, B.; Milara, J.; Cortijo, J. Idiopathic Pulmonary Fibrosis and Lung Cancer: Mechanisms and Molecular Targets. Int. J. Mol. Sci. 2019, 20, 593. [Google Scholar] [CrossRef] [PubMed]
- Bouros, D.; Hatzakis, K.; Labrakis, H.; Zeibecoglou, K. Association of malignancy with diseases causing interstitial pulmonary changes. Chest 2002, 121, 1278–1289. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.W.; Dobelle, M.; Padilla, M.; Agovino, M.; Wisnivesky, J.P.; Hashim, D.; Boffetta, P. Idiopathic Pulmonary Fibrosis and Lung Cancer. A Systematic Review and Meta-analysis. Ann. Am. Thorac. Soc. 2019, 16, 1041–1051. [Google Scholar] [CrossRef]
- Choi, W.I.; Park, S.H.; Park, B.J.; Lee, C.W. Interstitial Lung Disease and Lung Cancer Development: A 5-Year Nationwide Population-Based Study. Cancer Res. Treat. 2018, 50, 374–381. [Google Scholar] [CrossRef]
- JafariNezhad, A.; YektaKooshali, M.H. Lung cancer in idiopathic pulmonary fibrosis: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0202360. [Google Scholar] [CrossRef]
- Karampitsakos, T.; Rapti, A.; Ntolios, P.; Drevelegas, K.; Syrigos, K.N. Lung cancer in patients with idiopathic pulmonary fibrosis. Pulm. Pharmacol. Ther. 2017, 45, 1–10. [Google Scholar] [CrossRef]
- Kinoshita, T.; Goto, T. Molecular Mechanisms of Pulmonary Fibrogenesis and Its Progression to Lung Cancer: A Review. Int. J. Mol. Sci. 2019, 20, 1461. [Google Scholar] [CrossRef]
- Saito, A.; Horie, M.; Micke, P.; Nagase, T. The Role of TGF-β Signaling in Lung Cancer Associated with Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2018, 19, 3611. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Tzouvelekis, A.; Antoniou, K.M.; Vancheri, C. Patients with IPF and lung cancer: Diagnosis and management. Lancet Respir. Med. 2018, 6, 86–88. [Google Scholar] [CrossRef]
- Vancheri, C. Common pathways in idiopathic pulmonary fibrosis and cancer. Eur. Respir. Rev. 2013, 22, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Bergers, G. Tumors vs. Chronic Wounds: An Immune Cell’s Perspective. Front. Immunol. 2019, 10, 2178. [Google Scholar] [CrossRef]
- Ortiz-Zapater, E.; Signes-Costa, J.; Montero, P.; Roger, I. Lung Fibrosis and Fibrosis in the Lungs: Is It All about Myofibroblasts? Biomedicines 2022, 10, 1423. [Google Scholar] [CrossRef]
- Park, J.; Kim, D.S.; Shim, T.S.; Lim, C.M.; Koh, Y.; Lee, S.D.; Kim, W.S.; Kim, W.D.; Lee, J.S.; Song, K.S. Lung cancer in patients with idiopathic pulmonary fibrosis. Eur. Respir. J. 2001, 17, 1216–1219. [Google Scholar] [CrossRef]
- Caliò, A.; Lever, V.; Rossi, A.; Gilioli, E.; Brunelli, M.; Dubini, A.; Tomassetti, S.; Piciucchi, S.; Nottegar, A.; Rossi, G.; et al. Increased frequency of bronchiolar histotypes in lung carcinomas associated with idiopathic pulmonary fibrosis. Histopathology 2017, 71, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Higami, T.; Ohori, S.; Koyanagi, T.; Nakashima, S.; Mawatari, T. Is lung cancer resection indicated in patients with idiopathic pulmonary fibrosis? J. Thorac. Cardiovasc. Surg. 2008, 136, 1357–1363, 1363.e1–2. [Google Scholar] [CrossRef]
- Kato, E.; Takayanagi, N.; Takaku, Y.; Kagiyama, N.; Kanauchi, T.; Ishiguro, T.; Sugita, Y. Incidence and predictive factors of lung cancer in patients with idiopathic pulmonary fibrosis. ERJ Open Res. 2018, 4, 00111–02016. [Google Scholar] [CrossRef]
- Kim, H.C.; Lee, S.; Song, J.W. Impact of idiopathic pulmonary fibrosis on clinical outcomes of lung cancer patients. Sci. Rep. 2021, 11, 8312. [Google Scholar] [CrossRef]
- Watanabe, A.; Kawaharada, N.; Higami, T. Postoperative Acute Exacerbation of IPF after Lung Resection for Primary Lung Cancer. Pulm. Med. 2011, 2011, 960316. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Kawabata, Y.; Koyama, N.; Ikeya, T.; Hoshi, E.; Takayanagi, N.; Koyama, S. A clinicopathological study of surgically resected lung cancer in patients with usual interstitial pneumonia. Respir. Med. 2017, 129, 158–163. [Google Scholar] [CrossRef]
- Iwata, T.; Yoshida, S.; Nagato, K.; Nakajima, T.; Suzuki, H.; Tagawa, T.; Mizobuchi, T.; Ota, S.; Nakatani, Y.; Yoshino, I.; et al. Experience with perioperative pirfenidone for lung cancer surgery in patients with idiopathic pulmonary fibrosis. Surg. Today 2015, 45, 1263–1270. [Google Scholar] [CrossRef]
- Kim, H.; Pyo, H.; Noh, J.M.; Lee, W.; Park, B.; Park, H.Y.; Yoo, H. Preliminary result of definitive radiotherapy in patients with non-small cell lung cancer who have underlying idiopathic pulmonary fibrosis: Comparison between X-ray and proton therapy. Radiat. Oncol. 2019, 14, 19. [Google Scholar] [CrossRef]
- Goodman, C.D.; Nijman, S.F.M.; Senan, S.; Nossent, E.J.; Ryerson, C.J.; Dhaliwal, I.; Qu, X.M.; Laba, J.; Rodrigues, G.B.; Palma, D.A.; et al. A Primer on Interstitial Lung Disease and Thoracic Radiation. J. Thorac. Oncol. 2020, 15, 902–913. [Google Scholar] [CrossRef]
- Johkoh, T.; Lee, K.S.; Nishino, M.; Travis, W.D.; Ryu, J.H.; Lee, H.Y.; Ryerson, C.J.; Franquet, T.; Bankier, A.A.; Brown, K.K.; et al. Chest CT Diagnosis and Clinical Management of Drug-related Pneumonitis in Patients Receiving Molecular Targeting Agents and Immune Checkpoint Inhibitors: A Position Paper from the Fleischner Society. Radiology 2021, 298, 550–566. [Google Scholar] [CrossRef] [PubMed]
- Kenmotsu, H.; Naito, T.; Kimura, M.; Ono, A.; Shukuya, T.; Nakamura, Y.; Tsuya, A.; Kaira, K.; Murakami, H.; Takahashi, T.; et al. The risk of cytotoxic chemotherapy-related exacerbation of interstitial lung disease with lung cancer. J. Thorac. Oncol. 2011, 6, 1242–1246. [Google Scholar] [CrossRef]
- Minegishi, Y.; Sudoh, J.; Kuribayasi, H.; Mizutani, H.; Seike, M.; Azuma, A.; Yoshimura, A.; Kudoh, S.; Gemma, A. The safety and efficacy of weekly paclitaxel in combination with carboplatin for advanced non-small cell lung cancer with idiopathic interstitial pneumonias. Lung. Cancer 2011, 71, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, A.; Funaki, S.; Fukui, E.; Kimura, K.; Kanou, T.; Ose, N.; Minami, M.; Shintani, Y. Effects of pirfenidone targeting the tumor microenvironment and tumor-stroma interaction as a novel treatment for non-small cell lung cancer. Sci. Rep. 2020, 10, 10900. [Google Scholar] [CrossRef]
- Mediavilla-Varela, M.; Boateng, K.; Noyes, D.; Antonia, S.J. The anti-fibrotic agent pirfenidone synergizes with cisplatin in killing tumor cells and cancer-associated fibroblasts. BMC Cancer 2016, 16, 176. [Google Scholar] [CrossRef]
- Reck, M.; Kaiser, R.; Mellemgaard, A.; Douillard, J.Y.; Orlov, S.; Krzakowski, M.; von Pawel, J.; Gottfried, M.; Bondarenko, I.; Liao, M.; et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): A phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014, 15, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Saito, T.; Tanaka, T.; Takoi, H.; Yatagai, Y.; Inomata, M.; Nei, T.; Saito, Y.; Gemma, A.; Azuma, A. Reduced incidence of lung cancer in patients with idiopathic pulmonary fibrosis treated with pirfenidone. Respir. Investig. 2018, 56, 72–79. [Google Scholar] [CrossRef]
- Naoi, H.; Suzuki, Y.; Mori, K.; Aono, Y.; Kono, M.; Hasegawa, H.; Yokomura, K.; Inoue, Y.; Hozumi, H.; Karayama, M.; et al. Impact of anti-fibrotic therapy on lung cancer development in idiopathic pulmonary fibrosis. Thorax 2022, 77, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Asahina, H.; Oizumi, S.; Takamura, K.; Harada, T.; Harada, M.; Yokouchi, H.; Kanazawa, K.; Fujita, Y.; Kojima, T.; Sugaya, F.; et al. A prospective phase II study of carboplatin and nab-paclitaxel in patients with advanced non-small cell lung cancer and concomitant interstitial lung disease (HOT1302). Lung Cancer 2019, 138, 65–71. [Google Scholar]
- Kenmotsu, H.; Yoh, K.; Mori, K.; Ono, A.; Baba, T.; Fujiwara, Y.; Yamaguchi, O.; Ko, R.; Okamoto, H.; Yamamoto, N.; et al. Phase II study of nab-paclitaxel + carboplatin for patients with non-small-cell lung cancer and interstitial lung disease. Cancer Sci. 2019, 110, 3738–3745. [Google Scholar] [CrossRef]
- Otsubo, K.; Kishimoto, J.; Kenmotsu, H.; Minegishi, Y.; Ichihara, E.; Shiraki, A.; Kato, T.; Atagi, S.; Horinouchi, H.; Ando, M.; et al. Treatment Rationale and Design for J-SONIC: A Randomized Study of Carboplatin Plus Nab-paclitaxel With or Without Nintedanib for Advanced Non-Small-cell Lung Cancer With Idiopathic Pulmonary Fibrosis. Clin. Lung Cancer 2018, 19, e5–e9. [Google Scholar] [CrossRef]
- Sekine, A.; Satoh, H.; Baba, T.; Ikeda, S.; Okuda, R.; Shinohara, T.; Komatsu, S.; Hagiwara, E.; Iwasawa, T.; Ogura, T.; et al. Safety and efficacy of S-1 in combination with carboplatin in non-small cell lung cancer patients with interstitial lung disease: A pilot study. Cancer Chemother. Pharmacol. 2016, 77, 1245–1252. [Google Scholar] [CrossRef]
- Kijlertsuphasri, S.; Petnak, T.; Moua, T. Late Breaking Abstract-Antifibrotic Therapy and Risk of Lung Cancer in Patients with Idiopathic Pulmonary Fibrosis. Eur. Respir. J. 2024, 64, OA4561. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Kim, H.; Bae, Y.; Song, J.W. Pirfenidone and risk of lung cancer development in IPF: A nationwide population-based study. Eur. Respir. J. 2024, 65, 2401484. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Wu, H.; Liu, Z.; Sun, R.; Li, Y.; Si, Y.; Nie, Y.; Qiao, Y.; Qian, X.; et al. N-Phenyl-2-Pyridone-Derived Endoperoxide Suppressing both Lung Cancer and Idiopathic Pulmonary Fibrosis Progression by Three-Pronged Action. Angew. Chem. Int. Ed. Engl. 2024, 63, e202408473. [Google Scholar] [CrossRef]
- Huang, C.H.; Wick, J.; Komiya, T. Preliminary results of atezolizumab and pirfenidone in non-small cell lung cancer. J. Clin. Oncol. 2024, 42, e15197. [Google Scholar] [CrossRef]
- Hilberg, F.; Roth, G.J.; Krssak, M.; Kautschitsch, S.; Sommergruber, W.; Tontsch-Grunt, U.; Garin-Chesa, P.; Bader, G.; Zoephel, A.; Quant, J.; et al. BIBF 1120: Triple angiokinase inhibitor with sustained receptor blockade and good anti-tumor efficacy. Cancer Res. 2008, 68, 4774–4782. [Google Scholar] [CrossRef] [PubMed]
- Wollin, L.; Maillet, I.; Quesniaux, V.; Holweg, A.; Ryffel, B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J. Pharmacol. Exp. Ther. 2014, 349, 209–220. [Google Scholar] [CrossRef]
- Peng, M.; Deng, J.; Li, X. Clinical advances and challenges in targeting FGF/FGFR signaling in lung cancer. Mol. Cancer 2024, 23, 256. [Google Scholar] [CrossRef]
- Novello, S.; Kaiser, R.; Mellemgaard, A.; Douillard, J.Y.; Orlov, S.; Krzakowski, M.; von Pawel, J.; Gottfried, M.; Bondarenko, I.; Liao, M.; et al. Analysis of patient-reported outcomes from the LUME-Lung 1 trial: A randomised, double-blind, placebo-controlled, Phase III study of second-line nintedanib in patients with advanced non-small cell lung cancer. Eur. J. Cancer 2015, 51, 317–326. [Google Scholar] [CrossRef]
- Reck, M.; Syrigos, K.; Miliauskas, S.; Zöchbauer-Müller, S.; Fischer, J.R.; Buchner, H.; Kitzing, T.; Kaiser, R.; Radonjic, D.; Kerr, K. Non-interventional LUME-BioNIS study of nintedanib plus docetaxel after chemotherapy in adenocarcinoma non-small cell lung cancer: A subgroup analysis in patients with prior immunotherapy. Lung Cancer 2020, 148, 159–165. [Google Scholar] [CrossRef]
- Hanna, N.H.; Kaiser, R.; Sullivan, R.N.; Aren, O.R.; Ahn, M.J.; Tiangco, B.; Voccia, I.; Pawel, J.V.; Kovcin, V.; Agulnik, J.; et al. Nintedanib plus pemetrexed versus placebo plus pemetrexed in patients with relapsed or refractory, advanced non-small cell lung cancer (LUME-Lung 2): A randomized, double-blind, phase III trial. Lung Cancer 2016, 102, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.A.; Obasaju, C.; Gandara, D.; Hirsch, F.R.; Bonomi, P.; Bunn, P.A., Jr.; Kim, E.S.; Langer, C.J.; Natale, R.B.; Novello, S.; et al. Current and Emergent Therapy Options for Advanced Squamous Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 165–183. [Google Scholar] [CrossRef]
- Forster, M.; Hackshaw, A.; De Pas, T.; Cobo, M.; Garrido, P.; Summers, Y.; Dingemans, A.C.; Flynn, M.; Schnell, D.; von Wangenheim, U.; et al. A phase I study of nintedanib combined with cisplatin/gemcitabine as first-line therapy for advanced squamous non-small cell lung cancer (LUME-Lung 3). Lung Cancer 2018, 120, 27–33. [Google Scholar] [CrossRef]
- Kai, Y.; Ishihara, K.; Fujiwara, T.; Ohshimo, S.; Yamashita, M. Remarkable response of non-small cell lung cancer to nintedanib treatment in a patient with idiopathic pulmonary fibrosis. Thorac. Cancer 2021, 12, 1457–1460. [Google Scholar] [CrossRef]
- Fukunaga, K.; Yokoe, S.; Kawashima, S.; Uchida, Y.; Nakagawa, H.; Nakano, Y. Nintedanib prevented fibrosis progression and lung cancer growth in idiopathic pulmonary fibrosis. Respirol. Case Rep. 2018, 6, e00363. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, T.; Tanaka, H.; Tabe, C.; Tsuchiya, J.; Ishioka, Y.; Itoga, M.; Taima, K.; Takanashi, S.; Tasaka, S. Effect of nintedanib on non-small cell lung cancer in a patient with idiopathic pulmonary fibrosis: A case report and literature review. Thorac. Cancer 2020, 11, 1720–1723. [Google Scholar] [CrossRef] [PubMed]
- Kato, R.; Haratani, K.; Hayashi, H.; Sakai, K.; Sakai, H.; Kawakami, H.; Tanaka, K.; Takeda, M.; Yonesaka, K.; Nishio, K.; et al. Nintedanib promotes antitumour immunity and shows antitumour activity in combination with PD-1 blockade in mice: Potential role of cancer-associated fibroblasts. Br. J. Cancer 2021, 124, 914–924. [Google Scholar] [CrossRef]
- Kahlmann, V.; Moor, C.C.; Wijsenbeek, M.S. Managing Fatigue in Patients With Interstitial Lung Disease. Chest 2020, 158, 2026–2033. [Google Scholar] [CrossRef] [PubMed]
- Moor, C.C.; Wijsenbeek, M.S.; Balestro, E.; Biondini, D.; Bondue, B.; Cottin, V.; Flewett, R.; Galvin, L.; Jones, S.; Molina-Molina, M.; et al. Gaps in care of patients living with pulmonary fibrosis: A joint patient and expert statement on the results of a Europe-wide survey. ERJ Open Res. 2019, 5, 00124–02019. [Google Scholar] [CrossRef]
- Collard, H.R.; Ryerson, C.J.; Corte, T.J.; Jenkins, G.; Kondoh, Y.; Lederer, D.J.; Lee, J.S.; Maher, T.M.; Wells, A.U.; Antoniou, K.M.; et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am. J. Respir. Crit. Care Med. 2016, 194, 265–275. [Google Scholar] [CrossRef]
- Judge, E.P.; Fabre, A.; Adamali, H.I.; Egan, J.J. Acute exacerbations and pulmonary hypertension in advanced idiopathic pulmonary fibrosis. Eur. Respir. J. 2012, 40, 93–100. [Google Scholar] [CrossRef]
- Song, J.W.; Hong, S.B.; Lim, C.M.; Koh, Y.; Kim, D.S. Acute exacerbation of idiopathic pulmonary fibrosis: Incidence, risk factors and outcome. Eur. Respir. J. 2011, 37, 356–363. [Google Scholar] [CrossRef]
- Isshiki, T.; Sakamoto, S.; Yamasaki, A.; Shimizu, H.; Miyoshi, S.; Nakamura, Y.; Homma, S.; Kishi, K. Incidence of acute exacerbation of idiopathic pulmonary fibrosis in patients receiving antifibrotic agents: Real-world experience. Respir. Med. 2021, 187, 106551. [Google Scholar] [CrossRef]
- Man, R.K.; Gogikar, A.; Nanda, A.; Janga, L.S.N.; Sambe, H.G.; Yasir, M.; Ramphall, S. A Comparison of the Effectiveness of Nintedanib and Pirfenidone in Treating Idiopathic Pulmonary Fibrosis: A Systematic Review. Cureus 2024, 16, e54268. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, M.; Gu, H.; Liu, M.; Zhao, Y.; Shi, Y.; Wu, S.; Jiang, C.; Ye, X.; Zhu, H.; et al. Animal models of acute exacerbation of pulmonary fibrosis. Respir. Res. 2023, 24, 296. [Google Scholar] [CrossRef] [PubMed]
- de Hemptinne, Q.; Remmelink, M.; Brimioulle, S.; Salmon, I.; Vincent, J.L. ARDS: A clinicopathological confrontation. Chest 2009, 135, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Vannella, K.M.; Moore, B.B. Viruses as co-factors for the initiation or exacerbation of lung fibrosis. Fibrogenesi. Tissue Repair 2008, 1, 2. [Google Scholar] [CrossRef]
- Kakugawa, T.; Sakamoto, N.; Sato, S.; Yura, H.; Harada, T.; Nakashima, S.; Hara, A.; Oda, K.; Ishimoto, H.; Yatera, K.; et al. Risk factors for an acute exacerbation of idiopathic pulmonary fibrosis. Respir. Res. 2016, 17, 79. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, J.; Zhou, X. Lung microbiome: New insights into the pathogenesis of respiratory diseases. Signal Transduct. Target Ther. 2024, 9, 19. [Google Scholar] [CrossRef]
- Knippenberg, S.; Ueberberg, B.; Maus, R.; Bohling, J.; Ding, N.; Tort Tarres, M.; Hoymann, H.G.; Jonigk, D.; Izykowski, N.; Paton, J.C.; et al. Streptococcus pneumoniae triggers progression of pulmonary fibrosis through pneumolysin. Thorax 2015, 70, 636–646. [Google Scholar] [CrossRef]
- Han, M.K.; Zhou, Y.; Murray, S.; Tayob, N.; Noth, I.; Lama, V.N.; Moore, B.B.; White, E.S.; Flaherty, K.R.; Huffnagle, G.B.; et al. Lung microbiome and disease progression in idiopathic pulmonary fibrosis: An analysis of the COMET study. Lancet Respir. Med. 2014, 2, 548–556. [Google Scholar] [CrossRef]
- Molyneaux, P.L.; Cox, M.J.; Willis-Owen, S.A.; Mallia, P.; Russell, K.E.; Russell, A.M.; Murphy, E.; Johnston, S.L.; Schwartz, D.A.; Wells, A.U.; et al. The role of bacteria in the pathogenesis and progression of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2014, 190, 906–913. [Google Scholar] [CrossRef]
- O’Dwyer, D.N.; Ashley, S.L.; Gurczynski, S.J.; Xia, Y.; Wilke, C.; Falkowski, N.R.; Norman, K.C.; Arnold, K.B.; Huffnagle, G.B.; Salisbury, M.L.; et al. Lung microbiota contribute to pulmonary inflammation and disease progression in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2019, 199, 1127–1138. [Google Scholar] [CrossRef]
- Oda, K.; Ishimoto, H.; Yamada, S.; Kushima, H.; Ishii, H.; Imanaga, T.; Harada, T.; Ishimatsu, Y.; Matsumoto, N.; Naito, K.; et al. Autopsy analyses in acute exacerbation of idiopathic pulmonary fibrosis. Respir. Res. 2014, 15, 109. [Google Scholar] [CrossRef]
- Kim, D.S.; Park, J.H.; Park, B.K.; Lee, J.S.; Nicholson, A.G.; Colby, T. Acute exacerbation of idiopathic pulmonary fibrosis: Frequency and clinical features. Eur. Respir. J. 2006, 27, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, Y.; Taniguchi, H.; Katsuta, T.; Kataoka, K.; Kimura, T.; Nishiyama, O.; Sakamoto, K.; Johkoh, T.; Nishimura, M.; Ono, K.; et al. Risk factors of acute exacerbation of idiopathic pulmonary fibrosis. Sarcoidosis. Vasc. Diffuse Lung Dis. 2010, 27, 103–110. [Google Scholar] [PubMed]
- Kishaba, T.; Tamaki, H.; Shimaoka, Y.; Fukuyama, H.; Yamashiro, S. Staging of acute exacerbation in patients with idiopathic pulmonary fibrosis. Lung 2014, 192, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Simon-Blancal, V.; Freynet, O.; Nunes, H.; Cohen-Aubart, F.; Bouvry, D.; Brillet, P.Y.; Denis, D.; Cohen, Y.; Vincent, F.; Valeyre, D.; et al. Acute exacerbation of idiopathic pulmonary fibrosis: Outcome and prognostic factors. Respiration 2012, 83, 28–35. [Google Scholar] [CrossRef]
- van den Bosch, L.; Luppi, F.; Ferrara, G.; Mura, M. Immunomodulatory treatment of interstitial lung disease. Ther. Adv. Respir. Dis. 2022, 16, 17534666221117002. [Google Scholar] [CrossRef]
- Kreuter, M.; Bendstrup, E.; Russell, A.M.; Bajwah, S.; Lindell, K.; Adir, Y.; Brown, C.E.; Calligaro, G.; Cassidy, N.; Corte, T.J.; et al. Palliative care in interstitial lung disease: Living well. Lancet Respir. Med. 2017, 5, 968–980. [Google Scholar] [CrossRef]
- Moua, T.; Westerly, B.D.; Dulohery, M.M.; Daniels, C.E.; Ryu, J.H.; Lim, K.G. Patients With Fibrotic Interstitial Lung Disease Hospitalized for Acute Respiratory Worsening: A Large Cohort Analysis. Chest 2016, 149, 1205–1214. [Google Scholar] [CrossRef]
- Baig, S.H.; Yoo, E.J. The Impact of Chronic Comorbidities on Outcomes in Acute Exacerbations of Idiopathic Pulmonary Fibrosis. Life 2024, 14, 156. [Google Scholar] [CrossRef]
- Emura, I.; Usuda, H. Acute exacerbation of IPF has systemic consequences with multiple organ injury, with SRA+ and TNF-α+ cells in the systemic circulation playing central roles in multiple organ injury. BMC Pulm. Med. 2016, 16, 138. [Google Scholar] [CrossRef]
- Ikezoe, K.; Handa, T.; Tanizawa, K.; Yokoi, H.; Kubo, T.; Aihara, K.; Sokai, A.; Nakatsuka, Y.; Hashimoto, S.; Uemasu, K.; et al. Chronic Kidney Disease Predicts Survival in Patients with Idiopathic Pulmonary Fibrosis. Respiration 2017, 94, 346–352. [Google Scholar] [CrossRef]
- Ji, M.; Chen, M.; Hong, X.; Chen, T.; Zhang, N. The effect of diabetes on the risk and mortality of acute lung injury/acute respiratory distress syndrome: A meta-analysis. Medicine 2019, 98, e15095. [Google Scholar] [CrossRef] [PubMed]
- Moss, M.; Guidot, D.M.; Steinberg, K.P.; Duhon, G.F.; Treece, P.; Wolken, R.; Hudson, L.D.; Parsons, P.E. Diabetic patients have a decreased incidence of acute respiratory distress syndrome. Crit. Care Med. 2000, 28, 2187–2192. [Google Scholar] [CrossRef] [PubMed]
- Jeganathan, N.; Miot, C.; Sathananthan, A.; Sathananthan, M. The association of pulmonary fibrosis with diabetes mellitus. ERJ Open Res. 2020, 6, 00237–02020. [Google Scholar] [CrossRef]
- Singla, A.; Turner, P.; Pendurthi, M.K.; Agrawal, V.; Modrykamien, A. Effect of type II diabetes mellitus on outcomes in patients with acute respiratory distress syndrome. J. Crit. Care 2014, 29, 66–69. [Google Scholar] [CrossRef]
- Yu, S.; Christiani, D.C.; Thompson, B.T.; Bajwa, E.K.; Gong, M.N. Role of diabetes in the development of acute respiratory distress syndrome. Crit. Care Med. 2013, 41, 2720–2732. [Google Scholar] [CrossRef]
- Gori, L.; Bongiolatti, S.; Sorano, A.; Rosi, E.; Amendola, M.; Bonti, V.; Ferrari, K.; Ginanni, R.; Salvicchi, A.; Viggiano, D.; et al. IPF and lung cancer. Retrospective analysis of antifibrotic drugs and thoracic surgery. Eur. Respir. J. 2024, 64, PA515. [Google Scholar]
- Urushiyama, H.; Kondo, K.; Ebata, R.; Kuroda, H.; Taniguchi, T.; Nakajima, J. Preoperative use of pirfenidone and reduced risk of postoperative severe respiratory complications in patients with idiopathic pulmonary fibrosis: Propensity score-matched analysis using a nationwide database in Japan. Respirology 2021, 26, 590–596. [Google Scholar] [CrossRef]
- Ruaro, B.; Gandin, I.; Pozzan, R.; Tavano, S.; Bozzi, C.; Hughes, M.; Kodric, M.; Cifaldi, R.; Lerda, S.; Confalonieri, M.; et al. Nintedanib in Idiopathic Pulmonary Fibrosis: Tolerability and Safety in a Real Life Experience in a Single Centre in Patients also Treated with Oral Anticoagulant Therapy. Pharmaceuticals 2023, 16, 307. [Google Scholar] [CrossRef]
- Reccardini, N.; Chernovsky, M.; Salton, F.; Confalonieri, P.; Mondini, L.; Barbieri, M.; Romallo, A.; Maggisano, M.; Torregiani, C.; Geri, P.; et al. Pirfenidone in Idiopathic Pulmonary Fibrosis: Real-World Observation on Efficacy and Safety, Focus on Patients Under-going Antithrombotic and Anticoagulant. Pharmaceuticals 2024, 17, 930. [Google Scholar] [CrossRef]
- Hughes, M.; Bruni, C.; Cuomo, G.; Delle Sedie, A.; Gargani, L.; Gutierrez, M.; Lepri, G.; Ruaro, B.; Santiago, T.; Suliman, Y.; et al. The role of ultrasound in systemic sclerosis: On the cutting edge to foster clinical and research advancement. J. Scleroderma Relat. Disord. 2021, 6, 123–132. [Google Scholar] [CrossRef]
- Ruaro, B.; Salotti, A.; Reccardini, N.; Kette, S.; Da Re, B.; Nicolosi, S.; Zuccon, U.; Confalonieri, M.; Mondini, L.; Pozzan, R.; et al. Functional Progression after Dose Suspension or Discon-tinuation of Nintedanib in Idiopathic Pulmonary Fibrosis: A Real-Life Multicentre Study. Pharmaceuticals 2024, 17, 119. [Google Scholar] [CrossRef] [PubMed]
- Nathan, S.D.; Albera, C.; Bradford, W.Z.; Costabel, U.; Glaspole, I.; Glassberg, M.K.; Kardatzke, D.R.; Daigl, M.; Kirchgaessler, K.U.; Lancaster, L.H.; et al. Effect of pirfenidone on mortality: Pooled analyses and meta-analyses of clinical trials in idiopathic pulmonary fibrosis. Lancet. Respir. Med. 2017, 5, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Maggisano, M.; Mondini, L.; Chernovsky, M.; Confalonieri, P.; Salton, F.; Reccardini, N.; Kodric, M.; Geri, P.; Confalonieri, M.; Hughes, M.; et al. Safety of Nintedanib in a Patient with Chronic Pulmonary Fibrosis and Kidney Disease. Pharmaceuticals 2024, 17, 1147. [Google Scholar] [CrossRef]
- Kolonics-Farkas, A.M.; Šterclová, M.; Mogulkoc, N.; Kus, J.; Hájková, M.; Müller, V.; Jovanovic, D.; Tekavec-Trkanjec, J.; Littnerová, S.; Hejduk, K.; et al. Anticoagulant Use and Bleeding Risk in Central European Patients with Idiopathic Pulmonary Fibrosis (IPF) Treated with Antifibrotic Therapy: Real-World Data from EMPIRE. Drug. Saf. 2020, 43, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Sulli, A.; Ruaro, B.; Smith, V.; Pizzorni, C.; Zampogna, G.; Gallo, M.; Cutolo, M. Progression of nailfold microvascular damage and antinuclear antibody pattern in systemic sclerosis. J. Rheumatol. 2013, 40, 634–639. [Google Scholar] [CrossRef]
- Rubino, C.M.; Bhavnani, S.M.; Ambrose, P.G.; Forrest, A.; Loutit, J.S. Effect of food and antacids on the pharmacokinetics of pirfenidone in older healthy adults. Pulm. Pharmacol. Ther. 2009, 22, 279–285. [Google Scholar] [CrossRef]
- Costabel, U.; Behr, J.; Crestani, B.; Stansen, W.; Schlenker-Herceg, R.; Stowasser, S.; Raghu, G. Anti-acid therapy in idiopathic pulmonary fibrosis: Insights from the INPULSIS® trials. Respir. Res. 2018, 19, 167. [Google Scholar] [CrossRef]
- Sulli, A.; Ruaro, B.; Cutolo, M. Evaluation of blood perfusion by laser speckle contrast analysis in different areas of hands and face in patients with systemic sclerosis. Ann Rheum Dis. 2014, 73, 2059–2061. [Google Scholar] [CrossRef]
- Kreuter, M.; Costabel, U.; Richeldi, L.; Cottin, V.; Wijsenbeek, M.; Bonella, F.; Bendstrup, E.; Maher, T.M.; Wachtlin, D.; Stowasser, S.; et al. Statin Therapy and Outcomes in Trials of Nintedanib in Idiopathic Pulmonary Fibrosis. Respiration 2018, 95, 317–326. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).