PARP Inhibitors in Ovarian Cancer: Resistance Mechanisms, Clinical Evidence, and Evolving Strategies
Abstract
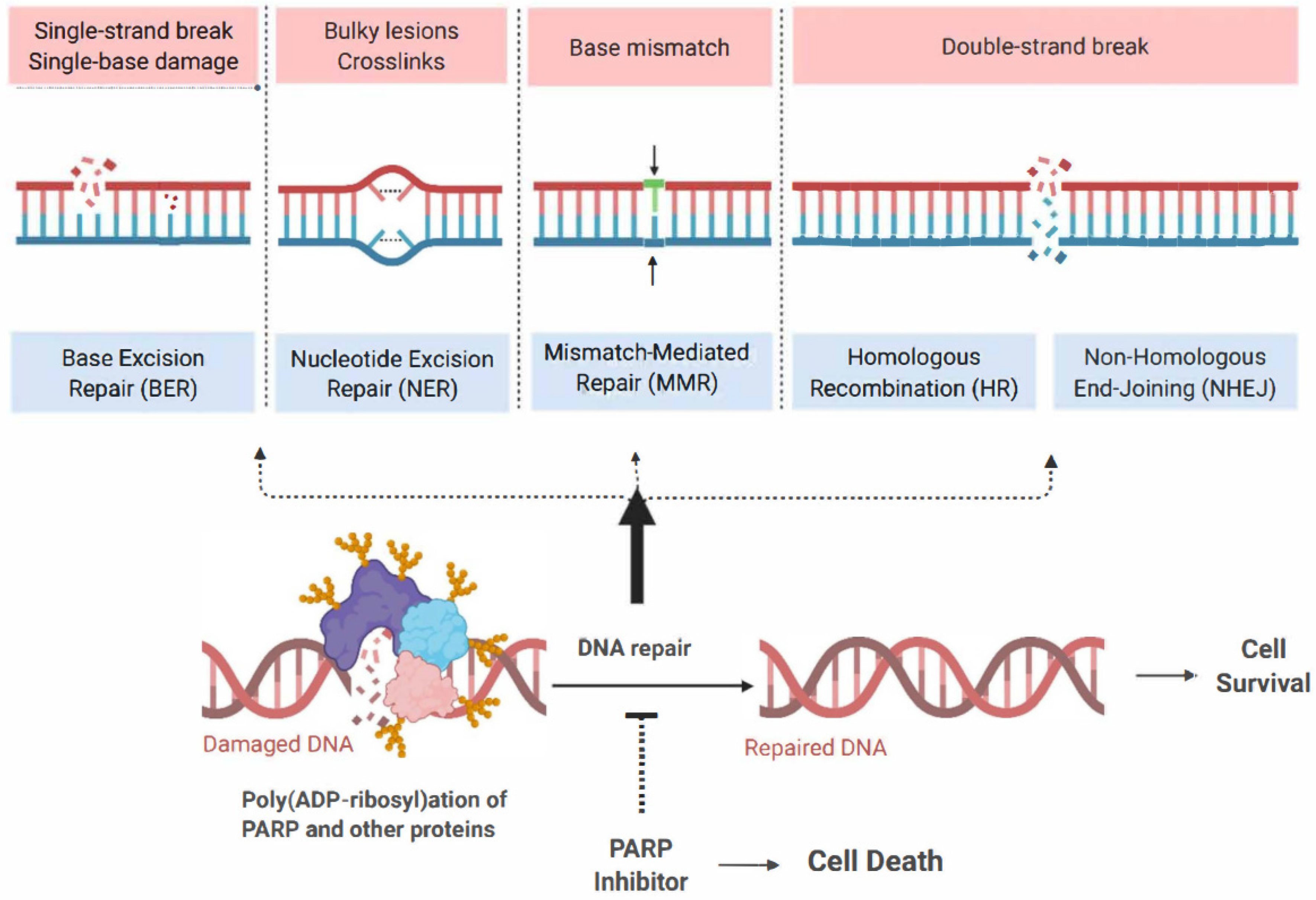
1. Introduction
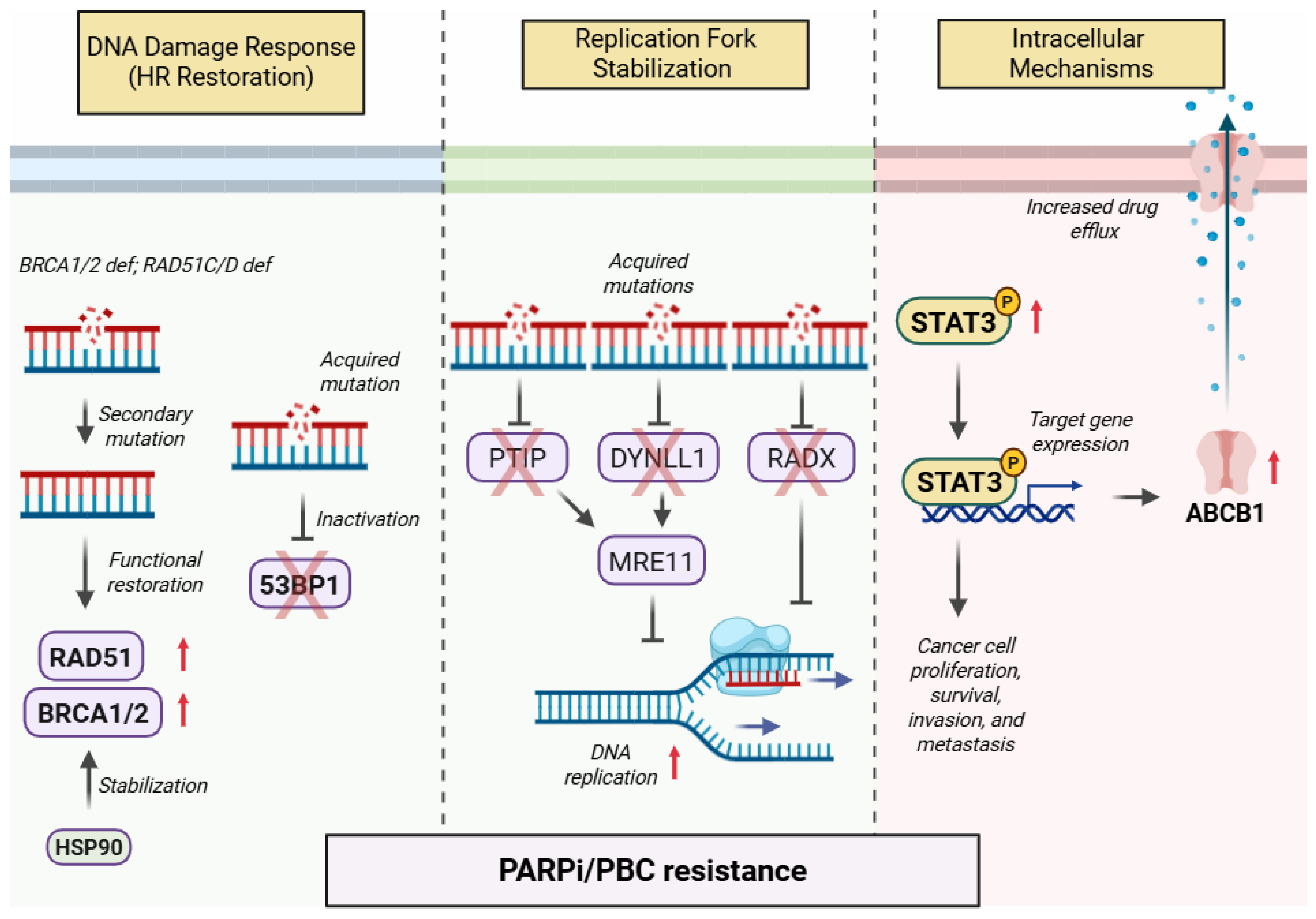
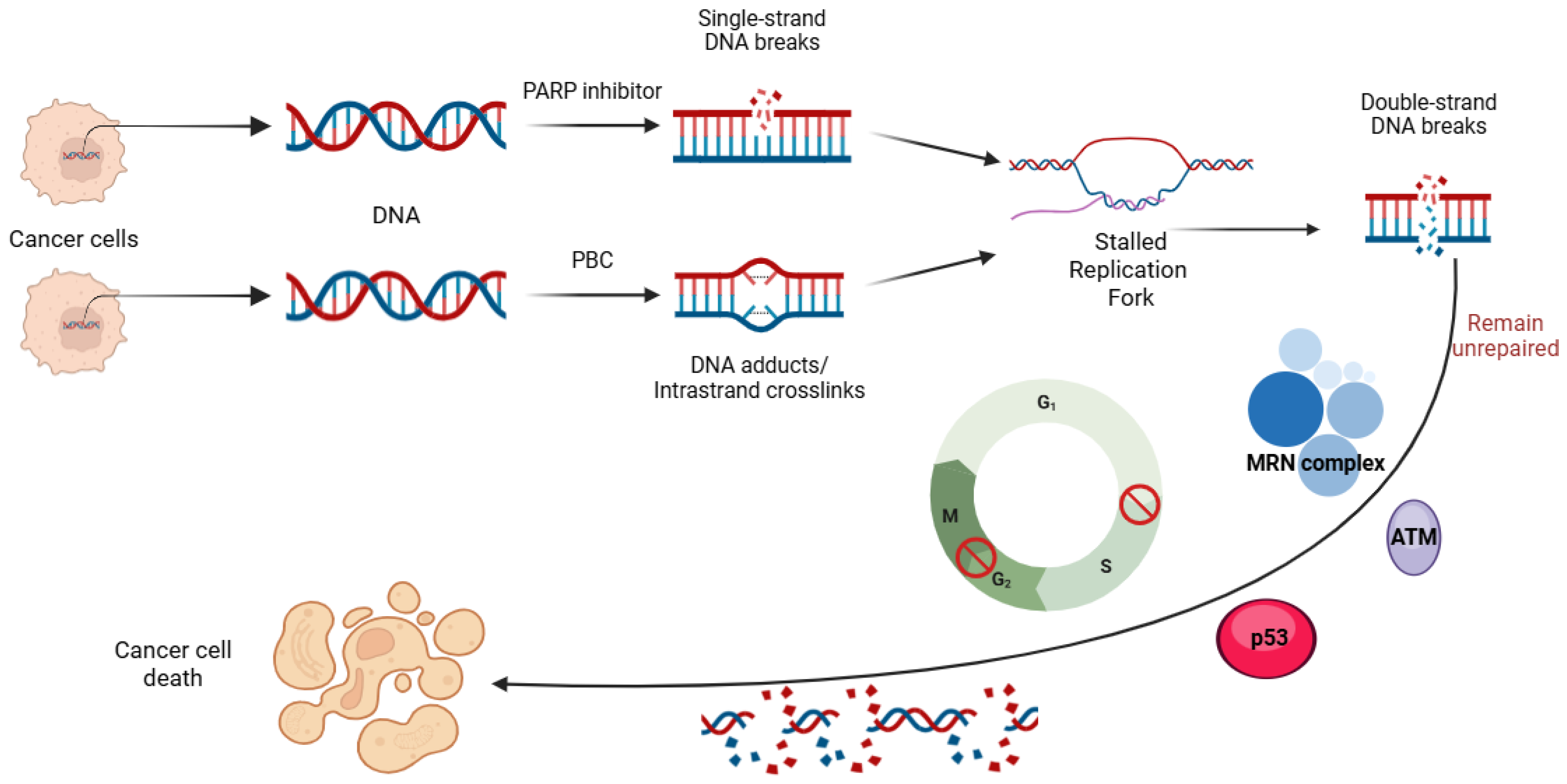
2. Shared Mechanisms of Resistance to PARP Inhibitors and Platinum-Based Chemotherapy
2.1. DNA Damage Repair Response
2.2. Replication Fork Stabilization
2.3. Intracellular Mechanisms
3. Placebo-Controlled Trials Evaluating the Role of PARP Inhibitors in Ovarian Cancer
3.1. Frontline Treatment
3.2. Frontline Maintenance
3.3. Recurrent Treatment
3.4. Recurrent Maintenance
| Setting | Drug | Study | Inclusion Criteria | BRCA/HR Status | Methods | Primary Endpoint | Pertinent Secondary Endpoints |
|---|---|---|---|---|---|---|---|
| Frontline Maintenance | Niraparib | PRIMA [27,61] | High-grade epithelial histology Stage III or IV Primary CRS or Interval CRS Complete or partial response after PBC (bevacizumab not permitted) | gBRCAm gBRCAwt/HRd gBRCAwt/HRp | Phase 3 2:1 ratio to receive niraparib 300 mg or placebo once daily for up to 36 months. | Median PFS: ITT Population: niraparib: 13.8 months placebo: 8.2 months HR, 0.62; 95% CI, 0.50 to 0.76 HRd Population: niraparib: 21.9 months placebo 10.4 months HR, 0.43; 95% CI, 0.31 to 0.59 HRp Population: niraparib: 8.1 months placebo: 5.4 months HR, 0.68; 95% CI, 0.49 to 0.94 | 24-Month OS ITT Population: niraparib: 84% placebo: 77% HR, 0.70; 95% CI, 0.44 to 1.11 HRd Population: niraparib: 91% placebo: 85% HR, 0.61; 95% CI, 0.27 to 1.39 HRp Population: niraparib: 81% placebo: 59% HR, 0.51; 95% CI, 0.27 to 0.97 |
| Rucaparib | ATHENA-MONO/ GOG-3020/ ENGOT-ov45 [62] | High-grade epithelial histology Stage III or IV Primary CRS or Interval CRS Complete or partial response after platinum–taxane chemotherapy (bevacizumab permitted during the chemotherapy phase) | gBRCAm gBRCAwt/HRd gBRCAwt/HRp | Phase 3 4:1 ratio to receive rucaparib 600 mg or placebo twice a day for up to 24 months. | Median PFS: ITT Population: rucaparib: 20.2 months placebo 9.2 months HR, 0.52; 95% CI, 0.40 to 0.68 HRd Population: rucaparib: 28.7 months placebo: 11.3 months HR, 0.47; 95% CI, 0.31 to 0.72 HRp Population: rucaparib: 12.1 months placebo: 9.1 months HR, 0.65; 95% CI, 0.45 to 0.95 | OS results have not yet been reported. | |
| Olaparib | SOLO-1 [26,63] | High-grade epithelial histology Stage III or IV Primary CRS or Interval CRS Complete or partial response after platinum–taxane chemotherapy (bevacizumab not permitted) | gBRCAm sBRCAm | Phase 3 2:1 ratio to receive olaparib 300 mg or placebo twice daily for up to 24 months. | Median PFS: ITT Population: olaparib: 56.0 months placebo: 13.8 months HR 0.33; 95% CI 0.25–0.43 | Median OS: ITT Population: olaparib: Not reached placebo: 75.2 months HR, 0.55; 95% CI, 0.40 to 0.76 | |
| Olaparib + bevacizumab | PAOLA-1 [28,64] | High-grade epithelial histology Stage III or IV Primary CRS or Interval CRS Complete or partial response after platinum–taxane chemotherapy plus bevacizumab | gBRCAm gBRCAwt/HRd gBRCAwt/HRp | Phase 3 2:1 ratio to receive olaparib 300 mg or placebo twice daily for up to 24 months. Bevacizumab 15 mg/kg every three weeks was initiated with chemotherapy and continued as maintenance therapy for up to 15 months. | Median PFS: ITT Population: olaparib: 22.1 months placebo: 13.8 months HR, 0.59; 95% CI 0.49–0.72 HRd Population: olaparib: 37.2 months placebo: 17.7 months HR, 0.33; 95% CI 0.25–0.45 HRp Population: olaparib: 16.6 months placebo: 16.2 months HR, 1.00; 95% CI 0.75–1.35 | Median OS: ITT Population: olaparib: 56.5 months placebo: 51.6 months HR, 0.92; 95% CI 0.76–1.12 HRd Population: olaparib 75.2 months placebo: 57.3 months HR, 0.62; 95% CI 0.45–0.85 HRp Population: olaparib: 36.8 months placebo: 40.4 months HR,1.19; 95% CI 0.88–1.63 | |
| Recurrent Maintenance | Niraparib | ENGOT-OV16/NOVA [23,73,74] | PSROC High-grade epithelial histology A total of ≥ 2 prior lines of platinum-based chemotherapy +/− bevacizumab | gBRCAm gBRCAwt/HRd gBRCAwt/HRp | Phase 3 2:1 ratio to receive niraparib 300 mg or placebo daily until disease progression. | Median PFS: gBRCAm Population: niraparib: 21 months placebo: 5.5 months HR, 0.27; 95% CI 0.17–0.41 gBRCAwt/HRd Population: niraparib: 12.9 months placebo: 3.8 months HR, 0.38; 95% CI 0.24–0.59 gBRCAwt/HRp Population: niraparib: 7.4 months placebo: 4.2 months HR, 0.45; 95% CI 0.34–0.61 | Median OS: gBRCAm Population: niraparib: 40.9 months placebo: 38.1 months HR, 0.85; 95% CI 0.61–1.20 gBRCAwt/HRd Population: niraparib: 35.6 months placebo: 41.4 months HR, 1.29; 95% CI 0.85–1.95 OS HRp Population: niraparib: 27.9 months placebo: 27.9 months HR, 0.93; 95% CI 0.61–1.41 |
| Niraparib | NORA [75] | PSROC High-grade epithelial histology A total of 2 prior lines of platinum-based chemotherapy +/− bevacizumab | gBRCAm gBRCAwt | Phase 3 2:1 to receive niraparib 300 mg or placebo daily until disease progression. | Median PFS: ITT Population: niraparib: 18.3 months placebo: 5.4 months HR, 0.32; 95% CI 0.23–0.45 gBRCAm Population: niraparib: Not reached placebo: 5.5 months HR, 0.22; 95% CI 0.23–0.39 gBRCAwt Population: niraparib: 11.1 months placebo: 3.9 months HR, 0.40; 95% CI 0.26–0.61 | Median OS: ITT Population: niraparib: 46.3 months placebo: 43.4 months HR, 0.83; 95% CI 0.56–1.21 gBRCAm Population: niraparib: Not reached placebo: 47.6 months HR, 0. 77; 95% CI 0. 40–1.47 gBRCAwt Population: niraparib: 43.1 months placebo: 38.4 months HR, 0.86; 95% CI 0.53–1.39 | |
| Rucaparib | ARIEL-3 [76,77] | PSROC High-grade epithelial histology A total of ≥ 2 prior lines of platinum-based chemotherapy +/− bevacizumab | gBRCAm gBRCAwt/HRd gBRCAwt/HRp | Phase 3 2:1 ratio to receive rucaparib 600 mg or placebo twice daily until disease progression. | Median PFS: ITT Population: rucaparib: 10.8 months placebo: 5.4 months HR, 0.36; 95% CI 0.30–0.45 HRd Population: rucaparib: 9.7 months placebo: 5.4 months HR, 0.44; 95% CI 0.29–0.66 HRp Population: rucaparib: 6.7 months placebo: 5.4 months HR, 0.58; 95% CI 0.40–0.85 | Median OS: ITT Population: rucaparib: 36 months placebo: 43.2 months HR, 0.995; 95% CI 0.81–1.22 HRd Population: rucaparib: 40.5 months placebo: 47.8 months HR, 1.01; 95% CI 0.77–1.32 HRp Population: rucaparib: 28.6 months placebo 32.6 months HR, 1.15; 95% CI 0.78–1.70 | |
| Olaparib | Study-19 [80,81] | PSROC High-grade serous histology A total of ≥ 2 prior lines of platinum-based chemotherapy | gBRCAm gBRCAwt | Phase 2 1:1 ratio to receive olaparib 400 mg or placebo twice daily until disease progression. | Median PFS: ITT Population: olaparib: 8.4 months placebo: 4.8 months HR, 0.35; 95% CI 0.25–0.49 gBRCAm Population: olaparib: 11.2 months placebo: 4.3 months HR, 0.18; 95% CI 0.10–0.31 gBRCAwt population: olaparib: 7.4 months placebo: 5.5 months HR, 0.54; 95% CI 0.34–0.85 | Median OS: ITT Population: olaparib: 29.8 months placebo: 27.8 months HR, 0.73; 95% CI 0.55–0.96 gBRCAm Population: olaparib: 34.9 months placebo: 30.2 months HR, 0.62; 95% CI 0.41–0.94 gBRCAwt population: olaparib: 24.5 months placebo: 26.6 months HR, 0.83; 95% CI 0.55–1.24 | |
| Olaparib | SOLO2/ENGOT-Ov21 [78,79] | PSROC High-grade epithelial histology A total of ≥ 2 prior lines of platinum-based chemotherapy | gBRCAm sBRCAm | Phase 3 2:1 ratio to receive olaparib 300 mg or placebo twice daily until disease progression. | Median PFS: ITT Population: olaparib: 19.1 months placebo: 5.5 months HR 0.30; 95% CI 0.22–0.41 | Median OS: ITT Population: olaparib: 51.7 months placebo: 38.8 months HR 0.30; 95% CI 0.22–0.41 | |
| Recurrent treatment | Rucaparib | ARIEL-4 [68,69] | PSROC High-grade epithelial histology A total of ≥ 2 prior lines of chemotherapy (at least one line of PBC), no prior PARP exposure | gBRCAm sBRCAm | Phase 3 2:1 ratio to receive rucaparib 600 mg twice daily or chemotherapy until disease progression. | Median PFS: ITT Population: rucaparib: 7.4 months placebo: 5.7 months HR 0.67; 95% CI 0.52–0.86 | Median OS: ITT Population: rucaparib: 19.4 months placebo: 25.4 months HR 1.31; 95% CI 1.00–1.73 |
| Olaparib | SOLO-3 [70,71] | PSROC High-grade epithelial histology A total of ≥ 2 prior lines of chemotherapy (at least one line of PBC), no prior PARP exposure | gBRCAm | Phase 3 2:1 ratio to receive rucaparib 600 mg twice daily or chemotherapy until disease progression. | Median PFS: ITT Population: 13.2 months in the olaparib group vs. 8.5 months in the placebo group (HR 0.62; 95% CI 0.43–0.91). | Median OS: ITT Population: 34.9 months in the olaparib group vs. 32.9 months in the placebo group (HR 1.07; 95% CI 0.76–1.49). |
4. Does PARP Inhibitor Maintenance Therapy Influence Outcomes of Platinum-Based Chemotherapy Retreatment in Recurrent Disease?
5. Is Retreatment with PARP Inhibitors a Viable Strategy After Prior Maintenance Failure?
6. Challenges and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef]
- Sargent, R.G.; Brenneman, M.A.; Wilson, J.H. Repair of site-specific double-strand breaks in a mammalian chromosome by homologous and illegitimate recombination. Mol. Cell. Biol. 1997, 17, 267–277. [Google Scholar] [CrossRef]
- Arnaudeau, C.; Lundin, C.; Helleday, T. DNA double-strand breaks associated with replication forks are predominantly repaired by homologous recombination involving an exchange mechanism in mammalian cells. J. Mol. Biol. 2001, 307, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Dianov, G.L. Targeting base excision repair to improve cancer therapies. Mol. Aspects Med. 2007, 28, 345–374. [Google Scholar] [CrossRef][Green Version]
- Fisher, A.E.O.; Hochegger, H.; Takeda, S.; Caldecott, K.W. Poly(ADP-Ribose) Polymerase 1 Accelerates Single-Strand Break Repair in Concert with Poly(ADP-Ribose) Glycohydrolase. Mol. Cell. Biol. 2007, 27, 5597–5605. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.; Burgess, J.T.; O’byrne, K.; Richard, D.J.; Bolderson, E. PARP Inhibitors: Clinical Relevance, Mechanisms of Action and Tumor Resistance. Front. Cell Dev. Biol. 2020, 8, 564601. [Google Scholar] [CrossRef]
- Wang, M.; Wu, W.; Wu, W.; Rosidi, B.; Zhang, L.; Wang, H.; Iliakis, G. PARP-1 and Ku compete for repair of DNA double strand breaks by distinct NHEJ pathways. Nucleic Acids Res. 2006, 34, 6170–6182. [Google Scholar] [CrossRef] [PubMed]
- Haince, J.-F.; McDonald, D.; Rodrigue, A.; Déry, U.; Masson, J.-Y.; Hendzel, M.J.; Poirier, G.G. PARP1-dependent Kinetics of Recruitment of MRE11 and NBS1 Proteins to Multiple DNA Damage Sites. J. Biol. Chem. 2008, 283, 1197–1208. [Google Scholar] [CrossRef]
- Sugimura, K.; Takebayashi, S.-I.; Taguchi, H.; Takeda, S.; Okumura, K. PARP-1 ensures regulation of replication fork progression by homologous recombination on damaged DNA. J. Cell Biol. 2008, 183, 1203–1212. [Google Scholar] [CrossRef]
- Bryant, H.E.; Petermann, E.; Schultz, N.; Jemth, A.-S.; Loseva, O.; Issaeva, N.; Johansson, F.; Fernandez, S.; McGlynn, P.; Helleday, T. PARP is activated at stalled forks to mediate Mre11-dependent replication restart and recombination. EMBO J. 2009, 28, 2601–2615. [Google Scholar] [CrossRef]
- Boehler, C.; Gauthier, L.R.; Mortusewicz, O.; Biard, D.S.; Saliou, J.-M.; Bresson, A.; Sanglier-Cianferani, S.; Smith, S.; Schreiber, V.; Boussin, F.; et al. Poly(ADP-ribose) polymerase 3 (PARP3), a newcomer in cellular response to DNA damage and mitotic progression. Proc. Natl. Acad. Sci. USA 2011, 108, 2783–2788. [Google Scholar] [CrossRef] [PubMed]
- Rulten, S.L.; Fisher, A.E.; Robert, I.; Zuma, M.C.; Rouleau, M.; Ju, L.; Poirier, G.; Reina-San-Martin, B.; Caldecott, K.W. PARP-3 and APLF Function Together to Accelerate Nonhomologous End-Joining. Mol. Cell 2011, 41, 33–45. [Google Scholar] [CrossRef]
- Pines, A.; Vrouwe, M.G.; Marteijn, J.A.; Typas, D.; Luijsterburg, M.S.; Cansoy, M.; Hensbergen, P.; Deelder, A.; de Groot, A.; Matsumoto, S.; et al. PARP1 promotes nucleotide excision repair through DDB2 stabilization and recruitment of ALC1. J. Cell Biol. 2012, 199, 235–249. [Google Scholar] [CrossRef]
- Fenton, A.L.; Shirodkar, P.; Macrae, C.J.; Meng, L.; Koch, C.A. The PARP3- and ATM-dependent phosphorylation of APLF facilitates DNA double-strand break repair. Nucleic Acids Res. 2013, 41, 4080–4092. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.; Boehler, C.; Barbat, J.G.; Bonnet, M.-E.; Illuzzi, G.; Ronde, P.; Gauthier, L.R.; Magroun, N.; Rajendran, A.; Lopez, B.S.; et al. PARP3 affects the relative contribution of homologous recombination and nonhomologous end-joining pathways. Nucleic Acids Res. 2014, 42, 5616–5632. [Google Scholar] [CrossRef]
- Bolton, K.L.; Chenevix-Trench, G.; Goh, C.; Sadetzki, S.; Ramus, S.J.; Karlan, B.Y.; Lambrechts, D.; Despierre, E.; Barrowdale, D.; McGuffog, L.; et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA 2012, 307, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-Z.; Jia, H.; Xiao, Q.; Li, R.-Z.; Wang, X.-S.; Yin, H.-Y.; Zhou, X. Efficacy and Prognostic Factors for PARP Inhibitors in Patients With Ovarian Cancer. Front. Oncol. 2020, 10, 958. [Google Scholar] [CrossRef]
- Frey, M.K.; Pothuri, B. Homologous recombination deficiency (HRD) testing in ovarian cancer clinical practice: A review of the literature. Gynecol. Oncol. Res. Pract. 2017, 4, 4. [Google Scholar] [CrossRef]
- Pennington, K.P.; Walsh, T.; Harrell, M.I.; Lee, M.K.; Pennil, C.C.; Rendi, M.H.; Thornton, A.; Norquist, B.M.; Casadei, S.; Nord, A.S.; et al. Germline and Somatic Mutations in Homologous Recombination Genes Predict Platinum Response and Survival in Ovarian, Fallopian Tube, and Peritoneal Carcinomas. Clin. Cancer Res. 2014, 20, 764–775. [Google Scholar] [CrossRef]
- Takaya, H.; Nakai, H.; Takamatsu, S.; Mandai, M.; Matsumura, N. Homologous recombination deficiency status-based classification of high-grade serous ovarian carcinoma. Sci. Rep. 2020, 10, 2757. [Google Scholar] [CrossRef]
- Takaya, H.; Nakai, H.; Sakai, K.; Nishio, K.; Murakami, K.; Mandai, M.; Matsumura, N. Intratumor heterogeneity and homologous recombination deficiency of high-grade serous ovarian cancer are associated with prognosis and molecular subtype and change in treatment course. Gynecol. Oncol. 2020, 156, 415–422. [Google Scholar] [CrossRef]
- Poveda, A.; Floquet, A.; Ledermann, J.A.; Asher, R.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Pignata, S.; Friedlander, M.; et al. Final overall survival (OS) results from SOLO2/ENGOT-ov21: A phase III trial assessing maintenance olaparib in patients (pts) with platinum-sensitive, relapsed ovarian cancer and a BRCA mutation. J. Clin. Oncol. 2020, 38, 6002. [Google Scholar] [CrossRef]
- Mirza, M.R.; Monk, B.J.; Herrstedt, J.; Oza, A.M.; Mahner, S.; Redondo, A.; Fabbro, M.; Ledermann, J.A.; Lorusso, D.; Vergote, I.; et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N. Engl. J. Med. 2016, 375, 2154–2164. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.; Scambia, G.; et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 1949–1961. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Moore, K.N.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1721–1731. [Google Scholar] [CrossRef]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef]
- Dias, M.P.; Moser, S.C.; Ganesan, S.; Jonkers, J. Understanding and overcoming resistance to PARP inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 2021, 18, 773–791. [Google Scholar] [CrossRef]
- McMullen, M.; Karakasis, K.; Madariaga, A.; Oza, A.M. Overcoming Platinum and PARP-Inhibitor Resistance in Ovarian Cancer. Cancers 2020, 12, 1607. [Google Scholar] [CrossRef]
- Hongo, A.; Seki, S.; Akiyama, K.; Kudo, T. A comparison of in vitro platinum-DNA adduct formation between carboplatin and cisplatin. Int. J. Biochem. 1994, 26, 1009–1016. [Google Scholar] [PubMed]
- Damia, G.; Broggini, M. Platinum Resistance in Ovarian Cancer: Role of DNA Repair. Cancers 2019, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Dobbelstein, M.; Sørensen, C.S. Exploiting replicative stress to treat cancer. Nat. Rev. Drug Discov. 2015, 14, 405–423. [Google Scholar] [CrossRef]
- Davar, D.; Beumer, J.H.; Hamieh, L.; Tawbi, H. Role of PARP Inhibitors in Cancer Biology and Therapy. Curr. Med. Chem. 2012, 19, 3907–3921. [Google Scholar] [CrossRef] [PubMed]
- Jubin, T.; Kadam, A.; Jariwala, M.; Bhatt, S.; Sutariya, S.; Gani, A.; Gautam, S.; Begum, R. The PARP family: Insights into functional aspects of poly (ADP-ribose) polymerase-1 in cell growth and survival. Cell Prolif. 2016, 49, 421–437. [Google Scholar] [CrossRef]
- Sakai, W.; Swisher, E.M.; Karlan, B.Y.; Agarwal, M.K.; Higgins, J.; Friedman, C.; Villegas, E.; Jacquemont, C.; Farrugia, D.J.; Couch, F.J.; et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature 2008, 451, 1116–1120. [Google Scholar] [CrossRef]
- Edwards, S.L.; Brough, R.; Lord, C.J.; Natrajan, R.; Vatcheva, R.; Levine, D.A.; Boyd, J.; Reis-Filho, J.S.; Ashworth, A. Resistance to therapy caused by intragenic deletion in BRCA2. Nature 2008, 451, 1111–1115. [Google Scholar] [CrossRef]
- Ganesan, S. Tumor Suppressor Tolerance: Reversion Mutations in BRCA1 and BRCA2 and Resistance to PARP Inhibitors and Platinum. JCO Precis. Oncol. 2018, 2, 1–4. [Google Scholar] [CrossRef]
- Banda, K.; Swisher, E.M.; Wu, D.; Pritchard, C.C.; Gadi, V.K. Somatic reversion of germline BRCA2 mutation confers resistance to PARP inhibitor therapy. JCO Precis. Oncol. 2018, 2, 1–6. [Google Scholar] [CrossRef]
- Carneiro, B.A.; Collier, K.A.; Nagy, R.J.; Pamarthy, S.; Sagar, V.; Fairclough, S.; Odegaard, J.; Lanman, R.B.; Costa, R.; Taxter, T.; et al. Acquired resistance to the PARP inhibitor olaparib in BRCA2-associated prostate cancer due to biallelic BRCA2 reversion mutations restoring both germline and somatic loss of function mutations. JCO Precis. Oncol. 2018, 2, 1–8. [Google Scholar] [CrossRef]
- Cheng, H.H.; Salipante, S.J.; Nelson, P.S.; Montgomery, B.; Pritchard, C.C. Polyclonal BRCA2 Reversion Mutations Detected in Circulating Tumor DNA After Platinum Chemotherapy in a Patient With Metastatic Prostate Cancer. JCO Precis. Oncol. 2018, 2, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kondrashova, O.; Nguyen, M.; Shield-Artin, K.; Tinker, A.V.; Teng, N.N.H.; Harrell, M.I.; Kuiper, M.J.; Ho, G.Y.; Barker, H.; Jasin, M.; et al. Secondary Somatic Mutations Restoring RAD51C and RAD51D Associated with Acquired Resistance to the PARP Inhibitor Rucaparib in High-Grade Ovarian Carcinoma. Cancer Discov. 2017, 7, 984–998. [Google Scholar] [CrossRef]
- Bouwman, P.; Aly, A.; Escandell, J.M.; Pieterse, M.; Bartkova, J.; van der Gulden, H.; Hiddingh, S.; Thanasoula, M.; Kulkarni, A.; Yang, Q.; et al. 53BP1 loss rescues BRCA1 deficiency and is associated with triple-negative and BRCA-mutated breast cancers. Nat. Struct. Mol. Biol. 2010, 17, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.; Johnson, S.F.; Yao, W.; Li, Y.C.; Choi, Y.E.; Bernhardy, A.J.; Wang, Y.; Capelletti, M.; Sarosiek, K.A.; Moreau, L.A.; et al. Stabilization of mutant BRCA1 protein confers PARP inhibitor and platinum resistance. Proc. Natl. Acad. Sci. USA 2013, 110, 17041–17046. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.R.; Callen, E.; Ding, X.; Gogola, E.; Duarte, A.A.; Lee, J.-E.; Wong, N.; Lafarga, V.; Calvo, J.A.; Panzarino, N.J.; et al. Replication fork stability confers chemoresistance in BRCA-deficient cells. Nature 2016, 535, 382–387, Erratum in 2016, 539, 456. [Google Scholar] [CrossRef]
- He, Y.J.; Meghani, K.; Caron, M.-C.; Yang, C.; Ronato, D.A.; Bian, J.; Sharma, A.; Moore, J.; Niraj, J.; Detappe, A.; et al. DYNLL1 binds to MRE11 to limit DNA end resection in BRCA1-deficient cells. Nature 2018, 563, 522–526. [Google Scholar] [CrossRef]
- Dungrawala, H.; Bhat, K.P.; Le Meur, R.; Chazin, W.J.; Ding, X.; Sharan, S.K.; Wessel, S.R.; Sathe, A.A.; Zhao, R.; Cortez, D. RADX Promotes Genome Stability and Modulates Chemosensitivity by Regulating RAD51 at Replication Forks. Mol. Cell 2017, 67, 374–386.e5. [Google Scholar] [CrossRef]
- Jaspers, J.E.; Sol, W.; Kersbergen, A.; Schlicker, A.; Guyader, C.; Xu, G.; Wessels, L.; Borst, P.; Jonkers, J.; Rottenberg, S. BRCA2-Deficient Sarcomatoid Mammary Tumors Exhibit Multidrug Resistance. Cancer Res. 2015, 75, 732–741. [Google Scholar] [CrossRef]
- Kim, H.; Xu, H.; George, E.; Hallberg, D.; Kumar, S.; Jagannathan, V.; Medvedev, S.; Kinose, Y.; Devins, K.; Verma, P.; et al. Combining PARP with ATR inhibition overcomes PARP inhibitor and platinum resistance in ovarian cancer models. Nat. Commun. 2020, 11, 3726. [Google Scholar] [CrossRef]
- Ji, T.; Gong, D.; Han, Z.; Wei, X.; Yan, Y.; Ye, F.; Ding, W.; Wang, J.; Xia, X.; Li, F.; et al. Abrogation of constitutive Stat3 activity circumvents cisplatin resistant ovarian cancer. Cancer Lett. 2013, 341, 231–239. [Google Scholar] [CrossRef]
- Liang, F.; Ren, C.; Wang, J.; Wang, S.; Yang, L.; Han, X.; Chen, Y.; Tong, G.; Yang, G. The crosstalk between STAT3 and p53/RAS signaling controls cancer cell metastasis and cisplatin resistance via the Slug/MAPK/PI3K/AKT-mediated regulation of EMT and autophagy. Oncogenesis 2019, 8, 59. [Google Scholar] [CrossRef]
- Yue, P.; Zhang, X.; Paladino, D.; Sengupta, B.; Ahmad, S.; Holloway, R.W.; Ingersoll, S.B.; Turkson, J. Hyperactive EGF receptor, Jaks and Stat3 signaling promote enhanced colony-forming ability, motility and migration of cisplatin-resistant ovarian cancer cells. Oncogene 2011, 31, 2309–2322. [Google Scholar] [CrossRef] [PubMed]
- Martincuks, A.; Song, J.; Kohut, A.; Zhang, C.; Li, Y.-J.; Zhao, Q.; Mak, E.; Rodriguez-Rodriguez, L.; Yu, H.; Cristea, M. PARP Inhibition Activates STAT3 in Both Tumor and Immune Cells Underlying Therapy Resistance and Immunosuppression In Ovarian Cancer. Front. Oncol. 2021, 11, 724104. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, N.; Yang, H.; Shin, Y.K. Homologous Recombination Deficiency in Ovarian, Breast, Colorectal, Pancreatic, Non-Small Cell Lung and Prostate Cancers, and the Mechanisms of Resistance to PARP Inhibitors. Front. Oncol. 2022, 12, 880643. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhao, H.; Liu, H.; Wang, W.; Dong, H.; Zhao, C. RNA methylation, homologous recombination repair and therapeutic resistance. Biomed. Pharmacother. 2023, 166, 115409. [Google Scholar] [CrossRef]
- Feng, G.; Yuan, Y.; Li, Z.; Wang, L.; Zhang, B.; Luo, J.; Ji, J.; Kong, D. Replication fork stalling elicits chromatin compaction for the stability of stalling replication forks. Proc. Natl. Acad. Sci. USA 2019, 116, 14563–14572. [Google Scholar] [CrossRef]
- Adhikari, S.; Bhattacharya, A.; Adhikary, S.; Singh, V.; Gadad, S.S.; Roy, S.; Das, C. The paradigm of drug resistance in cancer: An epigenetic perspective. Biosci. Rep. 2022, 42. [Google Scholar] [CrossRef]
- Sadida, H.Q.; Abdulla, A.; Marzooqi, S.A.; Hashem, S.; Macha, M.A.; Akil, A.S.A.; Bhat, A.A. Epigenetic modifications: Key players in cancer heterogeneity and drug resistance. Transl. Oncol. 2024, 39, 101821. [Google Scholar] [CrossRef]
- Oza, A.M.; Cibula, D.; Benzaquen, A.O.; Poole, C.; Mathijssen, R.H.; Sonke, G.; Colombo, N.; Špaček, J.; Vuylsteke, P.; Hirte, H.; et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: A randomised phase 2 trial. Lancet Oncol. 2015, 16, 87–97. [Google Scholar] [CrossRef]
- Coleman, R.L.; Fleming, G.F.; Brady, M.F.; Swisher, E.M.; Steffensen, K.D.; Friedlander, M.; Okamoto, A.; Moore, K.N.; Efrat Ben-Baruch, N.; Werner, T.L.; et al. Veliparib with First-Line Chemotherapy and as Maintenance Therapy in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2403–2415. [Google Scholar] [CrossRef]
- Han, S.N.; Monk, B.J.; Gonzalez-Martin, A. Time to first subsequent therapy (TFST) and progression-free survival 2 (PFS2) from the phase 3 randomized, double-blind PRIMA/ENGOT-OV26/GOG-3012 study in patients with newly diagnosed ovarian cancer. Gynecol. Oncol. 2020, 159, 18–19. [Google Scholar] [CrossRef]
- Monk, B.J.; Parkinson, C.; Lim, M.C.; O’Malley, D.M.; Oaknin, A.; Wilson, M.K.; Coleman, R.L.; Lorusso, D.; Bessette, P.; Ghamande, S.; et al. A Randomized, Phase III Trial to Evaluate Rucaparib Monotherapy as Maintenance Treatment in Patients With Newly Diagnosed Ovarian Cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45). J. Clin. Oncol. 2022, 40, 3952–3964. [Google Scholar] [CrossRef] [PubMed]
- DiSilvestro, P.; Banerjee, S.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; et al. Overall Survival With Maintenance Olaparib at a 7-Year Follow-Up in Patients With Newly Diagnosed Advanced Ovarian Cancer and a BRCA Mutation: The SOLO1/GOG 3004 Trial. J. Clin. Oncol. 2022, 41, 609–617. [Google Scholar] [CrossRef]
- Ray-Coquard, I.L.; Leary, A.; Pignata, S.; Cropet, C.; Gonzalez Martin, A.J.; Bogner, G.; Yoshida, H.; Vergote, I.B.; Colombo, N.; Maenpaa, J.; et al. Final overall survival (OS) results from the phase III PAOLA-1/ENGOT-ov25 trial evaluating maintenance olaparib (ola) plus bevacizumab (bev) in patients (pts) with newly diagnosed advanced ovarian cancer (AOC). In Annals of Oncology; European Society of Medical Oncology Congress: Paris, France, 2022. [Google Scholar]
- Ray-Coquard, I.; Leary, A.; Pignata, S.; Cropet, C.; González-Martín, A.; Marth, C.; Nagao, S.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus bevacizumab first-line maintenance in ovarian cancer: Final overall survival results from the PAOLA-1/ENGOT-ov25 trial. Ann. Oncol. 2023, 34, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.J.; Barretina-Ginesta, M.P.; Pothuri, B.; Vergote, I.; Graybill, W.; Mirza, M.R.; McCormick, C.C.; Lorusso, D.; Moore, R.G.; Freyer, G.; et al. Niraparib first-line maintenance therapy in patients with newly diagnosed advanced ovarian cancer: Final overall survival results from the PRIMA/ENGOT-OV26/GOG-3012 trial. Ann. Oncol. 2024, 35, 981–992. [Google Scholar] [CrossRef]
- O’Malley, D.M.; Monk, B.J.; Lim, M.C.; Pradera, J.F.; Buscema, J.; Wilson, M.K.; De Vivo, R.; Herzog, T.J.; Zagouri, F.; Oza, A.M.; et al. Final safety results from ATHENA–MONO (GOG-3020/ENGOT-ov45), a randomized, placebo-controlled, double-blind, phase 3 trial evaluating rucaparib monotherapy as maintenance treatment in patients with newly diagnosed ovarian cancer. J. Clin. Oncol. 2024, 42 (Suppl. S16), 5554. [Google Scholar] [CrossRef]
- Kristeleit, R.; Lisyanskaya, A.; Fedenko, A.; Dvorkin, M.; de Melo, A.C.; Shparyk, Y.; Rakhmatullina, I.; Bondarenko, I.; Colombo, N.; Svintsitskiy, V.; et al. Rucaparib versus standard-of-care chemotherapy in patients with relapsed ovarian cancer and a deleterious BRCA1 or BRCA2 mutation (ARIEL4): An international, open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 465–478. [Google Scholar] [CrossRef]
- Oza, A.; Lisyanskaya, A.; Fedenko, A.; de Melo, A.; Shparik, Y.; Bondarenko, I.; Colombo, N.; Lorusso, D.; Cibula, D.; Póka, R.; et al. 518O Overall survival results from ARIEL4: A phase III study assessing rucaparib vs chemotherapy in patients with advanced, relapsed ovarian carcinoma and a deleterious BRCA1/2 mutation. Ann. Oncol. 2022, 33, S780. [Google Scholar] [CrossRef]
- Penson, R.; Valencia, R.V.; Colombo, N.; Leath, C.; Bidzinski, M.; Kim, J.-W.; Nam, J.-H.; Madry, R.; Hernández, C.; Mora, P.; et al. Final overall survival results from SOLO3: Phase III trial assessing olaparib monotherapy versus non-platinum chemotherapy in heavily pretreated patients with germline BRCA1—And/or BRCA2-mutated platinum-sensitive relapsed ovarian cancer (026). Gynecol. Oncol. 2022, 166, S19–S20. [Google Scholar] [CrossRef]
- Scambia, G.; Villalobos Valencia, R.; Colombo, N.; Cibula, D.; Leath, C.A.; Bidziński, M.; Kim, J.W.; Nam, J.H.; Madry, R.; Hernández, C.; et al. Olaparib as Treatment Versus Nonplatinum Chemotherapy in Patients with Platinum-Sensitive Relapsed Ovarian Cancer: Phase III SOLO3 Study Final Overall Survival Results. J. Clin. Oncol. 2025, 43, 1408–1416. [Google Scholar] [CrossRef]
- Moore, K.N.; Secord, A.A.; Geller, M.A.; Miller, D.S.; Cloven, N.; Fleming, G.F.; Wahner Hendrickson, A.E.; Azodi, M.; DiSilvestro, P.; Oza, A.M.; et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): A multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 636–648. [Google Scholar] [CrossRef]
- Mirza, M.R.; Lindahl, G.; Mahner, S.; Redondo, A.; Fabbro, M.; Rimel, B.J.; Herrstedt, J.; Oza, A.M.; Canzler, U.; Berek, J.S.; et al. Ad hoc Analysis of the Phase III ENGOT-OV16/NOVA Study: Niraparib Efficacy in Germline BRCA Wild-type Recurrent Ovarian Cancer with Homologous Recombination Repair Defects. Cancer Res. Commun. 2022, 2, 1462. [Google Scholar] [CrossRef]
- Luik, S. Dear Health Care Provider Letter (Niraparib): GSK. 2022. Available online: https://www.zejulahcp.com/content/dam/cf-pharma/hcp-zejulahcp-v2/en_US/pdf/ZEJULA%20(niraparib)%20Dear%20HCP%20Letter%20November%202022.pdf (accessed on 20 January 2025).
- Wu, X.; Zhu, J.; Yin, R.; Yang, J.; Liu, J.; Wang, J.; Wu, L.; Liu, Z.; Gao, Y.; Wang, D.; et al. Niraparib maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer using an individualized starting dose (NORA): A randomized, double-blind, placebo-controlled phase III trial. Ann. Oncol. 2021, 32, 512–521. [Google Scholar] [CrossRef]
- Ledermann, J.A.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.R.; Scambia, G.; et al. Rucaparib for patients with platinum-sensitive, recurrent ovarian carcinoma (ARIEL3): Post-progression outcomes and updated safety results from a randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 710–722. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.R.; Scambia, G.; et al. 2022-RA-249-ESGO Overall survival results from ariel3: A phase 3 randomised, double-blind study of rucaparib vs placebo following response to platinum-based chemotherapy for recurrent ovarian carcinoma. Int. J. Gynecol. Cancer 2022, 32 (Suppl. S2), A226. [Google Scholar]
- Pujade-Lauraine, E.; Ledermann, J.A.; Selle, F.; Gebski, V.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Poveda, A.; Pignata, S.; et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Poveda, A.; Floquet, A.; Ledermann, J.A.; Asher, R.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Pignata, S.; Friedlander, M.; et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A final analysis of a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2021, 22, 620–631. [Google Scholar] [CrossRef]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.L.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: A preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014, 15, 852–861. [Google Scholar] [CrossRef]
- Ledermann, J.A.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Overall survival in patients with platinum-sensitive recurrent serous ovarian cancer receiving olaparib maintenance monotherapy: An updated analysis from a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Oncol. 2016, 17, 1579–1589. [Google Scholar] [CrossRef]
- Parmar, M.K.; Ledermann, J.A.; Colombo, N.; du Bois, A.; Delaloye, J.F.; Kristensen, G.B.; Wheeler, S.; Swart, A.M.; Qian, W.; Torri, V.; et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: The ICON4/AGO-OVAR-2.2 trial. Lancet 2003, 361, 2099–2106. [Google Scholar]
- Pignata, S.; Lorusso, D.; Joly, F.; Gallo, C.; Colombo, N.; Sessa, C.; Bamias, A.; Salutari, V.; Selle, F.; Frezzini, S.; et al. Carboplatin-based doublet plus bevacizumab beyond progression versus carboplatin-based doublet alone in patients with platinum-sensitive ovarian cancer: A randomised, phase 3 trial. Lancet Oncol. 2021, 22, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Cecere, S.C.; Giannone, G.; Salutari, V.; Arenare, L.; Lorusso, D.; Ronzino, G.; Lauria, R.; Cormio, G.; Carella, C.; Scollo, P.; et al. Olaparib as maintenance therapy in patients with BRCA 1–2 mutated recurrent platinum sensitive ovarian cancer: Real world data and post progression outcome. Gynecol. Oncol. 2020, 156, 38–44. [Google Scholar] [CrossRef]
- Markman, M.; Markman, J.; Webster, K.; Zanotti, K.; Kulp, B.; Peterson, G.; Belinson, J. Duration of Response to Second-Line, Platinum-Based Chemotherapy for Ovarian Cancer: Implications for Patient Management and Clinical Trial Design. J. Clin. Oncol. 2004, 22, 3120–3125. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, K.; Gao, B.; Mapagu, C.; Fereday, S.; Emmanuel, C.; Alsop, K.; Traficante, N.; Harnett, P.R.; Bowtell, D.D.; Defazio, A. Response rates to second-line platinum-based therapy in ovarian cancer patients challenge the clinical definition of platinum resistance. Gynecol. Oncol. 2018, 150, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Frenel, J.; Kim, J.; Aryal, N.; Asher, R.; Berton, D.; Vidal, L.; Pautier, P.; Ledermann, J.; Penson, R.; Oza, A.; et al. Efficacy of subsequent chemotherapy for patients with BRCA1/2-mutated recurrent epithelial ovarian cancer progressing on olaparib versus placebo maintenance: Post-hoc analyses of the SOLO2/ENGOT Ov-21 trial. Ann. Oncol. 2022, 33, 1021–1028. [Google Scholar] [CrossRef]
- Dugan, K.; Kashi, P.K.; Gough, E.; Wallam, S.; Levinson, K.; Wethington, S.; Gaillard, S. Effectiveness of Platinum-Based Chemotherapy after Progression on Poly ADP Ribose Polymerase (PARP) Inhibitor in Epithelial Ovarian Cancer (042). Gynecol. Oncol. 2022, 166, S29–S30. [Google Scholar] [CrossRef]
- Xu-Vuillard, A.; Guerin-Charbonnel, C.; Bocquet, F.; Cheeseman, S.; Kubelac, P.; Zenatri, M.; Hall, G.; Achimas-Cadariu, P.; Hanvic, B.; Fenton, H.; et al. Efficacy of chemotherapy after progression during or following PARPi exposure in ovarian cancer. ESMO Open 2024, 9, 103694. [Google Scholar] [CrossRef]
- Rose, P.G.; Yao, M.; Chambers, L.M.; Mahdi, H.; DeBernardo, R.; Michener, C.M.; AlHilli, M.; Ricci, S.; Vargas, R. PARP inhibitors decrease response to subsequent platinum-based chemotherapy in patients with BRCA mutated ovarian cancer. Anti-Cancer Drugs 2021, 32, 1086–1092. [Google Scholar] [CrossRef]
- Park, J.; Kim, S.I.; Jeong, S.Y.; Kim, Y.; Bookman, M.A.; Kim, J.W.; Kim, B.G.; Lee, J.Y. Second-line olaparib maintenance therapy is associated with poor response to subsequent chemotherapy in BRCA1/2-mutated epithelial ovarian cancer: A multicentre retrospective study. Gynecol. Oncol. 2022, 165, 97–104. [Google Scholar] [CrossRef]
- Baert, T.; Ataseven, B.; Bommert, M.; Concin, N.; Frindte, J.; Schneider, S.; Harter, P.; du Bois, A.; Heitz, F. 828P Expected versus observed response to platinum-based chemotherapy after poly (ADP-ribose) polymerase inhibitor treatment for relapsed ovarian cancer. Ann. Oncol. 2020, 31, S624. [Google Scholar] [CrossRef]
- Salarich, A.P.; García, I.T.; Burdalo, B.P.; Gil-Martin, M.; Piulats, J.; Planas, C.F.; Ginesta, M.B.; Diez, A.F.; Rincon, L.N.; Badia, A.P.; et al. 824P Real-world-data (RWD) on platinum (Pt) outcomes after PARP inhibitors (PARPi) progression in high grade serous ovarian cancer (HGSOC) patients (p). Ann. Oncol. 2020, 31, S622. [Google Scholar] [CrossRef]
- Gadducci, A.; Cosio, S.; Landoni, F.; Lissoni, A.A.; Zola, P.; Laudani, M.E.; Ardizzoia, A.; Gambino, A.; Sartori, E. Response to Chemotherapy and Clinical Outcome of Patients With Recurrent Epithelial Ovarian Cancer After PARP Inhibitor Maintenance Treatment: A Multicenter Retrospective Italian Study. Anticancer. Res. 2022, 42, 2017–2022. [Google Scholar] [CrossRef] [PubMed]
- Romeo, M.; Gil-Martín, M.; Gaba, L.; Teruel, I.; Taus, Á.; Fina, C.; Masvidal, M.; Murata, P.; Fernández-Plana, J.; Martínez, A.; et al. Multicenter Real-World Data of Subsequent Chemotherapy after Progression to PARP Inhibitors in a Maintenance Relapse Setting. Cancers 2022, 14, 4414. [Google Scholar] [CrossRef]
- Rimel, B.; Secord, A.; Geller, M.; Miller, D.; Cloven, N.; Fleming, G.; Hendrickson, A.W.; Azodi, M.; DiSilvestro, P.; Oza, A.; et al. Safety and Efficacy Results of Retreatment With a PARP Inhibitor Monotherapy in Late-Line Recurrent Ovarian Cancer: Results From a Subset of the QUADRA Trial. Gynecol. Oncol. 2020, 156, e4–e5. [Google Scholar] [CrossRef]
- Yubero, A.; Estévez, P.; Barquín, A.; Sánchez, L.; Santaballa, A.; Pajares, B.; Reche, P.; Salvador, C.; Manso, L.; Márquez, R.; et al. Rucaparib for PARP inhibitor-pretreated ovarian cancer: A GEICO retrospective subgroup analysis from the Spanish Rucaparib Access Program. Gynecol. Oncol. Rep. 2023, 48, 101211. [Google Scholar] [CrossRef] [PubMed]
- Essel, K.; Behbakht, K.; Lai, T.; Hand, L.; Evans, E.; Dvorak, J.; Ding, K.; Konecny, G.; Moore, K. PARPi after PARPi in epithelial ovarian cancer. Gynecol. Oncol. Rep. 2021, 35, 100699. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Selle, F.; Scambia, G.; Asselain, B.; Marmé, F.; Lindemann, K.; Colombo, N.; Mądry, R.; Glasspool, R.; Vergote, I.; et al. Maintenance olaparib rechallenge in patients with platinum-sensitive relapsed ovarian cancer previously treated with a PARP inhibitor (OReO/ENGOT-ov38): A phase IIIb trial. Ann. Oncol. 2023, 34, 1152–1164. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Guo, B.; Li, X.; Tuersun, R.; Cao, Y.; Li, J.; Liu, J.; Li, S.; Liu, T.; et al. Efficacy of PARPi re-maintenance therapy for recurrent ovarian cancer. Front. Oncol. 2025, 14, 1512339. [Google Scholar] [CrossRef]
- Bonadio, R.C.; Estevez-Diz, M.D.P. Perspectives on PARP Inhibitor Combinations for Ovarian Cancer. Front. Oncol. 2021, 11, 754524. [Google Scholar] [CrossRef]
- Trillsch, F.; Okamoto, A.; Kim, J.W.; Reuss, A.; Rubio Pérez, M.J.; Vardar, M.A.; Salutari, V.; Frenel, J.-S.; Kärkkäinen, H.; Colombo, N.; et al. 43O Durvalumab (D) + carboplatin/paclitaxel (CP) + bevacizumab (B) followed by D, B + olaparib (O) maintenance (mtx) for newly diagnosed advanced ovarian cancer (AOC) without a tumour BRCA1/BRCA2 mutation (non-tBRCAm): Updated results from DUO-O. ESMO Open 2024, 9, 103550. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, D.; Wang, J.; Wang, W.; Li, Q.; Tang, J.; Huang, Y.; An, R.; Duan, W.; Wang, L.; et al. 766P A phase II trial of fuzuloparib in combination with apatinib vs. fuzuloparib alone for recurrent ovarian cancer (OC). Ann. Oncol. 2024, 35, S579–S580. [Google Scholar] [CrossRef]
- Kim, Y.N.; Park, B.; Joung, J.-G.; Kim, J.-W.; Kim, B.-G.; Kim, S.W.; Kim, H.S.; Choi, C.H.; Lim, M.C.; Ngoi, N.Y.; et al. Triplet maintenance (olaparib, pembrolizumab, and bevacizumab) in BRCA non-mutated patients with platinum-sensitive recurrent ovarian cancer: A multi-center, single-arm phase II study (OPEB-01). J. Clin. Oncol. 2023, 41 (Suppl. S16), 5584. [Google Scholar] [CrossRef]
- Yap, T.A.; Im, S.-A.; Schram, A.M.; Sharp, A.; Balmana, J.; Baird, R.D.; Brown, J.S.; Schwaederle, M.; Pilling, E.A.; Moorthy, G.; et al. Abstract CT007: PETRA: First in class, first in human trial of the next generation PARP1-selective inhibitor AZD5305 in patients (pts) with BRCA1/2, PALB2 or RAD51C/D mutations. Cancer Res. 2022, 82 (Suppl. S12), CT007. [Google Scholar] [CrossRef]
- Simpkins, F.; Nasioudis, D.; Wethington, S.L.; Martin, L.P.; Tanyi, J.L.; Latif, N.A.; Torigian, D.A.; Omran, D.K.; Rodriguez, D.; Smith, S.; et al. Combination ATR and PARP Inhibitor (CAPRI): A phase 2 study of ceralasertib plus olaparib in patients with recurrent, platinum-sensitive epithelial ovarian cancer (cohort A). J. Clin. Oncol. 2024, 42, 5510. [Google Scholar] [CrossRef]
- Westin, S.N.; Coleman, R.L.; Fellman, B.M.; Yuan, Y.; Sood, A.K.; Soliman, P.T.; Wright, A.A.; Horowitz, N.S.; Campos, S.M.; Konstantinopoulos, P.A.; et al. EFFORT: EFFicacy Of adavosertib in parp ResisTance: A randomized two-arm non-comparative phase II study of adavosertib with or without olaparib in women with PARP-resistant ovarian cancer. J. Clin. Oncol. 2021, 39, 5505. [Google Scholar] [CrossRef]
- Nicum, S.; Ledermann, J.; Mileshkin, L.; Jayson, G.; Gourley, C.; Michael, A.; Lord, R.; Mackay, H.; Hall, M.; Tookman, L.; et al. LBA33 ICON9: International phase III randomized study to evaluate the efficacy of maintenance therapy with olaparib and cediranib or olaparib alone in patients with relapsed platinum-sensitive ovarian cancer following a response to platinum-based chemotherapy. Ann. Oncol. 2024, 35, S1225–S1226. [Google Scholar] [CrossRef]
- Lee, J.-M.; Brady, M.F.; Miller, A.; Moore, R.G.; MacKay, H.; McNally, L.; Lea, J.; Street, D.; Lheureux, S.; McDonald, M.E.; et al. Cediranib and Olaparib Combination Compared With Cediranib or Olaparib Alone, or Chemotherapy in Platinum-Resistant or Primary Platinum-Refractory Ovarian Cancer: NRG-GY005. J. Clin. Oncol. 2024, 42, 4305–4316. [Google Scholar] [CrossRef]
- González-Martín, A.; Rubio, M.J.; Heitz, F.; Christensen, R.D.; Colombo, N.; Van Gorp, T.; Romeo, M.; Ray-Coquard, I.; Gaba, L.; Leary, A.; et al. Atezolizumab Combined With Platinum and Maintenance Niraparib for Recurrent Ovarian Cancer With a Platinum-Free Interval >6 Months: ENGOT-OV41/GEICO 69-O/ANITA Phase III Trial. J. Clin. Oncol. 2024, 42, 4294–4304. [Google Scholar] [CrossRef]
- Lee, J.-M.; Miller, A.; Rose, P.; AlHilli, M.; Washington, C.; John, V.; Shah, C.; Matsuo, K.; Siedel, J.; Miller, D.; et al. 746MO Randomized phase II trial of durvalumab in combination with olaparib and cediranib (DOC) compared to olaparib and cediranib (OC) or durvalumab and cediranib (DC) or standard of care chemotherapy (SOC) in platinum-resistant ovarian cancer with prior bevacizumab (NRG-GY023). Ann. Oncol. 2023, 34, S511. [Google Scholar] [CrossRef]
| First Author | Country | Study Design | Number of Participants | Types of Ovarian Cancer | PARPi Administered | Findings |
|---|---|---|---|---|---|---|
| Cecere et al. [84] | Italy | Retrospective study | 234 | BRCA-mutated recurrent platinum-sensitive | Olaparib | Post-progression response to PBC in terms of ORR of 22.2% in those who had a platinum-free intervals of more than 12 months. |
| Frenel et al. [87] | International | Posthoc analysis on an RCT | 96 | BRCA-mutated recurrent platinum-sensitive | Olaparib | Significantly longer time to a second progression after post-progression PBC initiation in those who have received a placebo compared to those who received olaparib. |
| Rose et al. [90] | USA | Retrospective study | 115 | BRCA-mutated recurrent platinum-sensitive | Various PARPis (niraparib, olaparib, rucaparib, and veliparib) | Following each of the second and third courses of PBC, patients who had not been exposed to PARPi had significantly longer PFS than those with no prior exposure. |
| Park et al. [91] | Republic of Korea | Retrospective study | 197 | BRCA-mutated recurrent platinum-sensitive | Olaparib | Post-progression chemotherapy, either PBC or non-PBC, was associated with a shorter PFS in those who received prior olaparib maintenance therapy. |
| Baert et al. [92,93] | Germany | Retrospective study | 92 | Recurrent | Olaparib and niraparib | Prior PARPi treatment negatively reduced the effectiveness of later PBC, evidenced by a higher progression rate in the PARPi group (40% vs. 9% in the control group, p = 0.003). |
| Plaja Salarich et al. [93] | Spain | Retrospective study | 54 | Recurrent | PARPi | An overall ORR of 33.3% was observed for subsequent PBC, increasing to 42.9% in patients with a platinum-free interval of over 12 months. |
| Gadducci et al. [94] | Italy | Retrospective study | 103 | Recurrent platinum-sensitive | Various PARPis (olaparib, niraparib, and rucaparib) | Subsequent chemotherapy, PBC and non-PBC combined, was associated with an ORR of 41.9% in those who had platinum-free interval of more than 12 months. |
| Romeo et al. [95] | Spain | Retrospective study | 74 | Platinum-sensitive recurrent | Various PARPis (olaparib, niraparib, and rucaparib) | Subsequent PBC after PARPi was associated with an ORR of 41.9% and a median PFS and OS of 6.6 and 20.6 months, respectively. |
| Dugan et al. [88] | USA | Retrospective study | 40 | Platinum-sensitive recurrent | Various PARPis (olaparib, niraparib, and rucaparib) | Median PFS after starting PBC was 219 days (IQR 125–307), significantly longer in those on PARPis for over 18 months. |
| Xu-Vuillard et al. [89] | International | Retrospective study | 291 | Recurrent | PARPis | Patients with a platinum-free interval over six months had better PFS with PBC than non-PBC, though not statistically significant (HR = 0.68, 95% CI of 0.46–1.01). |
| Nakao et al. [88] | Japan | Retrospective study | 10 | Recurrent | Olaparib and niraparib | Post-progression response to PBC showed an ORR of 70%, with all patients having a platinum-free interval of more than six months. |
| NCT Number | Study Title | Phase | Location | Primary Endpoint | Targeted Additional Mechanism |
|---|---|---|---|---|---|
| NCT04729387 | Alpelisib Plus Olaparib in Platinum-resistant/Refractory, High-grade Serous Ovarian Cancer, with no Germline BRCA Mutation Detected | 3 | Australia, Austria, Belgium, Brazil, Canada, China, Czechia, Denmark, Finland, France, Germany, Italy, the Republic of Korea, Malaysia, Mexico, the Netherlands, Portugal, Russia, Singapore, Slovakia, Spain, Taiwan, Turkey, the United Kingdom, and the United States of America | Progression-free survival | The phosphoinositide 3-kinase pathway inhibition |
| NCT04679064 | Trial on Niraparib-TSR-042 (Dostarlimab) vs. Physician’s Choice Chemotherapy in Recurrent, Ovarian, Fallopian Tube, or Primary Peritoneal Cancer Patients Not Candidate for Platinum Retreatment | 3 | Italy | Overall survival | Programmed cell death protein 1 blockade |
| NCT03740165 | Study of Chemotherapy with Pembrolizumab (MK-3475) Followed by Maintenance with Olaparib (MK-7339) for the First-Line Treatment of Women with BRCA Non-mutated Advanced Epithelial Ovarian Cancer (EOC) (MK-7339-001/KEYLYNK-001/ENGOT-ov43/GOG-3036) | 3 | Australia, Belgium, Brazil, Canada, Chile, Colombia, Czechia, France, Germany, Hungary, Israel, Italy, Japan, the Republic of Korea, Poland, Russia, South Africa, Spain, Taiwan, Turkey, Ukraine, and the United States of America | Progression-free survival | Programmed cell death protein 1 blockade |
| NCT04734665 | Niraparib and Bevacizumab Maintenance Therapy in Platinum-sensitive Recurrent Ovarian Cancer Patients Previously Treated with a PARP Inhibitor | 2 | Republic of Korea | 6-month progression-free survival rate | Vascular endothelial growth factor A inhibition |
| NCT04669002 | EP0057 in Combination with Olaparib in Advanced Ovarian Cancer | 2 | Hungary, the United Kingdom, and the United States of America | Objective response rate | Topoisomerase I inhibition |
| NCT04566952 | Anlotinib Combined with Dose-reduced Olaparib in Patients with Platinum-Sensitive Recurrent Ovarian Cancer | 2 | China | Progression-free survival, adverse events | Inhibition of vascular endothelial growth factor receptors, platelet-derived growth factor receptors, fibroblast growth factor receptors, c-Kit, and rearranged during transfection |
| NCT04556071 | Efficacy and Safety of Niraparib Combined with Bevacizumab in Platinum Refractory/Resistant Recurrent Ovarian Cancer | 2 | China | Objective response rate | Vascular endothelial growth factor A inhibition |
| NCT05158062 | Pembrolizumab and Bevacizumab with Chemotherapy Followed by Pembrolizumab, Bevacizumab and Olaparib in Recurrent Ovarian Cancer | 2 | Japan | Two-year progression-free survival rate | Vascular endothelial growth factor A inhibition and programmed cell death protein 1 |
| NCT03574779 | A Study to Evaluate the Efficacy and Safety of Novel Treatment Combinations in Participants with Ovarian Cancer (OPAL) | 2 | Canada, Spain, Turkey, and the United States of America | Objective response rate | Vascular endothelial growth factor A inhibition |
| NCT02953457 | Olaparib, Durvalumab, and Tremelimumab in Treating Patients with Recurrent or Refractory Ovarian, Fallopian Tube or Primary Peritoneal Cancer with BRCA1 or BRCA2 Mutation | 2 | United States of America | Dose-limiting toxicities, 3- and 6-month progression-free survival | Cytotoxic T-lymphocyte–associated protein 4 blockade and programmed death ligand 1 blockade |
| NCT04034927 | Testing the Addition of an Immunotherapy Drug, Tremelimumab, to the PARP Inhibition Drug, Olaparib, for Recurrent Ovarian, Fallopian Tube or Peritoneal Cancer | 2 | United States of America | Progression-free survival, dose-limiting toxicities | Cytotoxic T-lymphocyte–associated Protein 4 blockade |
| NCT02484404 | Phase I/II Study of the Anti-Programmed Death Ligand-1 Durvalumab Antibody (MEDI4736) in Combination with Olaparib and/or Cediranib for Advanced Solid Tumors and Advanced or Recurrent Ovarian, Triple Negative Breast, Lung, Prostate and Colorectal Cancers | 1–2 | United States of America | Maximum tolerated dose, objective response rate | Programmed death ligand 1 and vascular endothelial growth factor receptor 1, receptor 2, and receptor 3 inhibition |
| NCT02571725 | PARP-inhibition and CTLA-4 Blockade in BRCA-deficient Ovarian Cancer | 1–2 | United States of America | Maximum tolerated dose, objective response rate | Cytotoxic T-lymphocyte–associated protein 4 blockade |
| NCT03462212 | Carboplatin-Paclitaxel-Bevacizumab vs. Carbo-Pacli-Beva-Rucaparib vs. Carbo-Pacli-Ruca, Selected According to HRD Status, in Patients with Advanced Ovarian, Primary Peritoneal and Fallopian Tube Cancer, Preceded by a Phase I Dose Escalation Study on Ruca-Beva Combination | 1–2 | Italy | Maximum tolerated dose, progression-free survival | Vascular endothelial growth factor A inhibition |
| NCT04703920 | Talazoparib in Combination with Belinostat for Metastatic Breast Cancer, Metastatic Castration-Resistant Prostate Cancer, and Metastatic Ovarian Cancer | 1 | United States of America | Dose-limiting toxicities | Histone deacetylase inhibition |
| NCT03162627 | Selumetinib and Olaparib in Solid Tumors | 1 | United States of America | Maximum tolerated dose | The mitogen-activated protein kinase kinase 1 and 2 pathway inhibition |
| NCT04673448 | Niraparib and TSR-042 for the Treatment of BRCA-Mutated Unresectable or Metastatic Breast, Pancreas, Ovary, Fallopian Tube, or Primary Peritoneal Cancer | 1 | United States of America | Best objective response | Programmed cell death protein 1 blockade |
| NCT03586661 | Niraparib and Copanlisib in Treating Patients with Recurrent Endometrial, Ovarian, Primary Peritoneal, or Fallopian Tube Cancer | 1 | United States of America | Maximum tolerated dose | The phosphoinositide 3-kinase pathway inhibition |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apelian, S.; Martincuks, A.; Whittum, M.; Yasukawa, M.; Nguy, L.; Mathyk, B.; Andikyan, V.; Anderson, M.L.; Rutherford, T.; Cristea, M.; et al. PARP Inhibitors in Ovarian Cancer: Resistance Mechanisms, Clinical Evidence, and Evolving Strategies. Biomedicines 2025, 13, 1126. https://doi.org/10.3390/biomedicines13051126
Apelian S, Martincuks A, Whittum M, Yasukawa M, Nguy L, Mathyk B, Andikyan V, Anderson ML, Rutherford T, Cristea M, et al. PARP Inhibitors in Ovarian Cancer: Resistance Mechanisms, Clinical Evidence, and Evolving Strategies. Biomedicines. 2025; 13(5):1126. https://doi.org/10.3390/biomedicines13051126
Chicago/Turabian StyleApelian, Shant, Antons Martincuks, Michelle Whittum, Maya Yasukawa, Lindsey Nguy, Begum Mathyk, Vaagn Andikyan, Matthew L. Anderson, Thomas Rutherford, Mihaela Cristea, and et al. 2025. "PARP Inhibitors in Ovarian Cancer: Resistance Mechanisms, Clinical Evidence, and Evolving Strategies" Biomedicines 13, no. 5: 1126. https://doi.org/10.3390/biomedicines13051126
APA StyleApelian, S., Martincuks, A., Whittum, M., Yasukawa, M., Nguy, L., Mathyk, B., Andikyan, V., Anderson, M. L., Rutherford, T., Cristea, M., Stewart, D., & Kohut, A. (2025). PARP Inhibitors in Ovarian Cancer: Resistance Mechanisms, Clinical Evidence, and Evolving Strategies. Biomedicines, 13(5), 1126. https://doi.org/10.3390/biomedicines13051126





