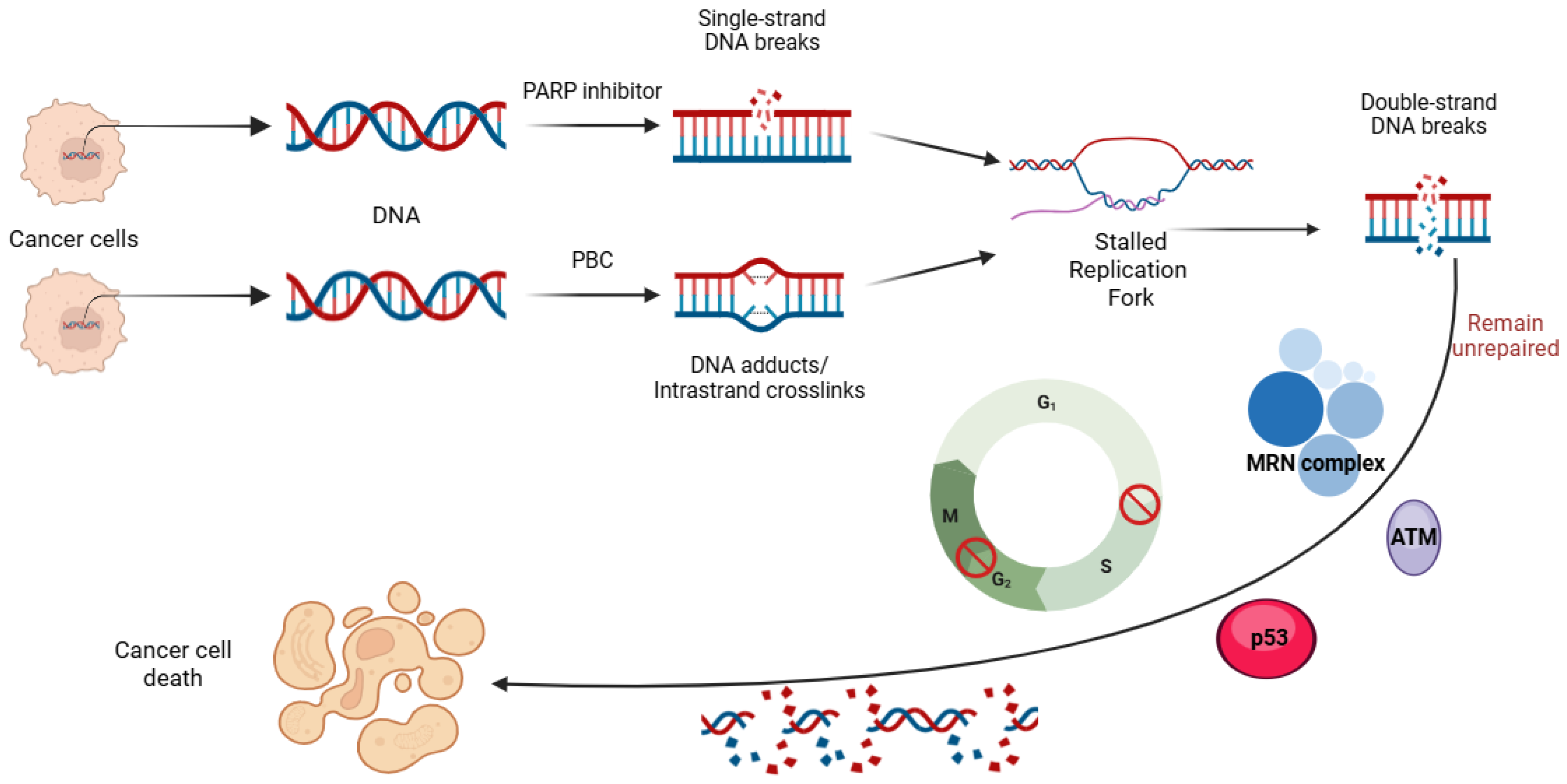
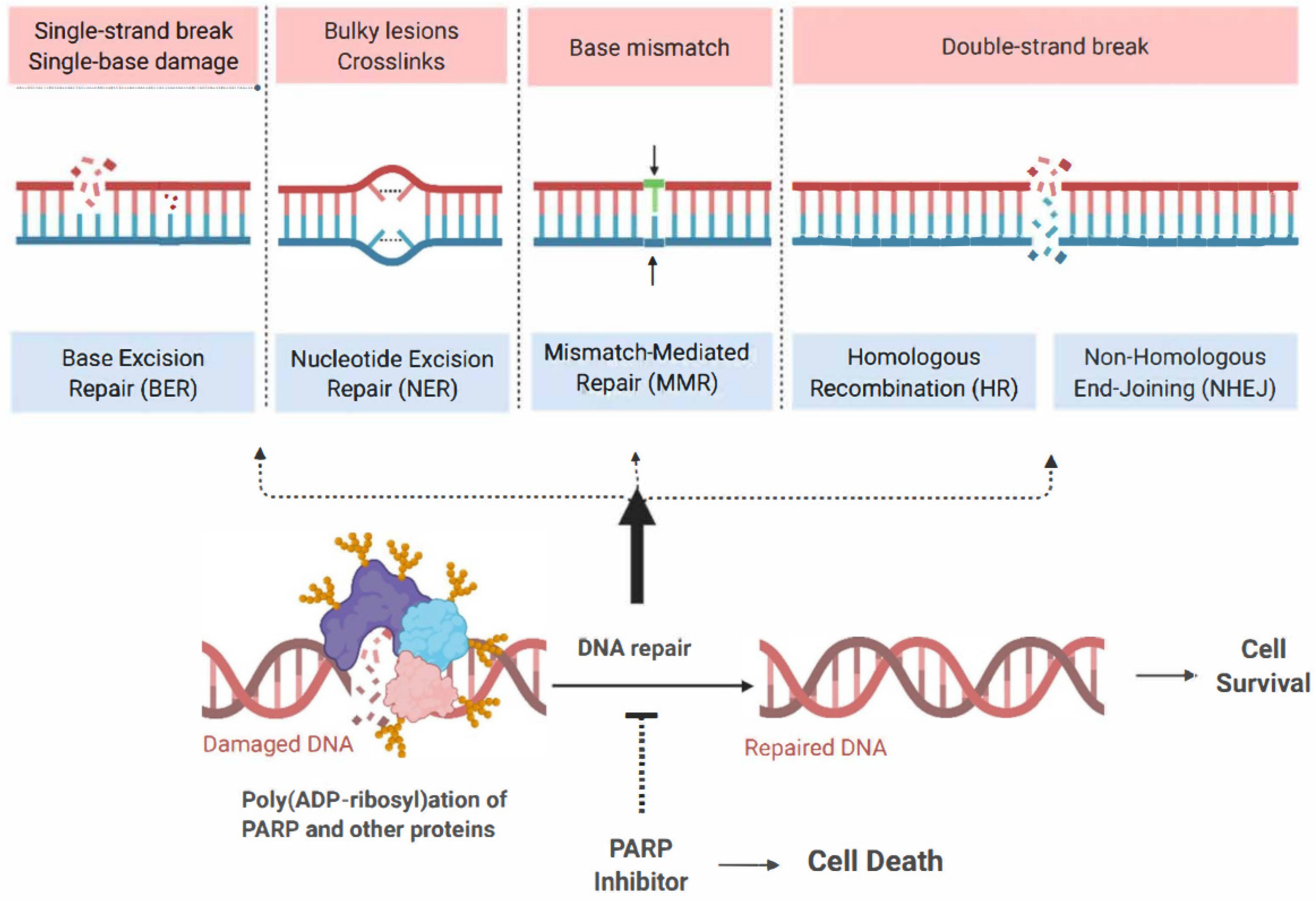
Abstract
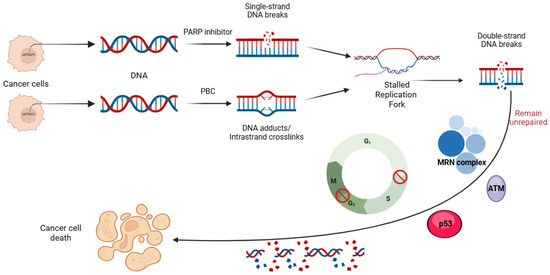
The introduction of poly (ADP-ribose) polymerase inhibitors (PARPi) into the management of ovarian cancer has transformed the treatment landscape for patients affected by this malignancy. However, as the use of PARPi expands into both frontline maintenance and recurrence settings, the emergence of drug resistance has become a significant clinical challenge in the treatment of these patients. Although platinum-based chemotherapy (PBC) and PARPi act through different mechanisms—PBC causes DNA damage while PARPi blocks its repair—both depend on the integrity of DNA damage repair (DDR) pathways, leading to overlapping mechanisms of resistance. Here, we review the key resistance mechanisms shared by PARPi and PBC, and then we discuss their clinical implications in the management of patients with ovarian cancer. We also examine clinical rationale supporting the hypothesis that prior PARPi exposure may reduce the efficacy of subsequent PBC in patients experiencing a disease recurrence. Furthermore, we review preliminary clinical data assessing the potential role of PARPi retreatment in patients who have previously progressed on PARPis.
1. Introduction
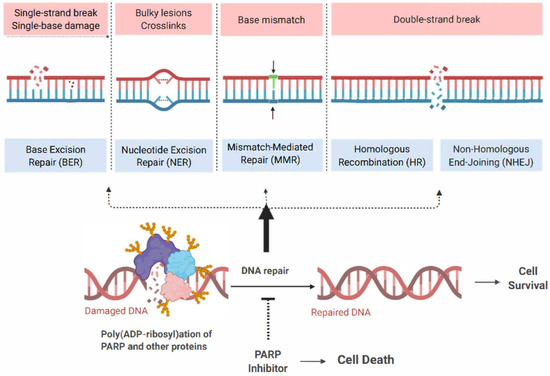
The management of ovarian cancer has undergone considerable innovation over recent years due to the successful integration of poly (ADP- ribose) polymerase (PARP) inhibitors (PARPis) into contemporary therapeutic strategies. Prior to this, the pharmacological standard of care for ovarian cancer primarily involved platinum-based chemotherapy (PBC). PARPis are targeted agents that exploit the dependence of rapidly dividing cells on proficient deoxyribonucleic acid (DNA) repair mechanisms for survival and proliferation. Given the exponential growth of these cells, they rely on an array of DNA repair mechanisms that enable them to maintain a continuous state of cellular division [1]. Critical DNA repair mechanisms include single-strand and double-strand break (SSB and DSB) DNA recognition and repair, nonhomologous end joining (NHEJ), homologous recombination (HR), base excision repair (BER), and nucleotide excision repair (NER) [1,2,3,4,5]. The PARP enzyme family plays a vital role in these DNA repair mechanisms, making them attractive targets in developing cancer therapeutics [5,6,7,8,9,10,11,12,13,14,15].
Early studies on PARPis highlighted the therapeutic potential of these agents in ovarian cancer, particularly due to the high prevalence of germline mutations in critical DNA repair genes such as BRCA1 and BRCA2 [16]. Subsequent studies showed similar benefits in patients with somatic BRCA mutations or BRCA wild-type tumors exhibiting homologous recombination deficiency (HRD), thereby expanding the population of patients with ovarian cancer likely to respond to PARPi [17,18,19,20,21]. These findings support the use of BRCA and HR status as biomarkers predictive of response to PARPi [18].
The initial FDA approvals of PARPis, including olaparib, niraparib, and rucaparib, for ovarian cancer were granted based on phase III clinical trials demonstrating significant efficacy in maintaining remission in recurrent platinum-sensitive ovarian cancer [22,23,24]. The most significant benefit was observed in patients with germline BRCA mutations [22,23,24]. Given the success of these agents in the recurrent setting, PARPis were subsequently evaluated in earlier-stage disease through additional phase III trials, which showed improved survival outcomes. These findings led to FDA approvals for the use of PARPi in the frontline maintenance setting, including olaparib for patients with BRCA-mutated platinum-responsive disease, olaparib plus bevacizumab for patients with HRD-positive, platinum-responsive disease, and niraparib for all patients with platinum-responsive disease, regardless of biomarker status [25,26,27,28].
The successful integration of PARPis in the management of high-grade serous ovarian cancer has, therefore, provided significant clinical benefits and reshaped the therapeutic landscape for these patients. However, it has also introduced new challenges in managing patients with disease recurrence after the use of PARPis. In this review, we explored the complexities of systemic therapy following relapse and provided an overview of the ongoing efforts to address these emerging challenges.
3. Placebo-Controlled Trials Evaluating the Role of PARP Inhibitors in Ovarian Cancer
Table 1 summarizes clinical trials evaluating the efficacy of PARPis (olaparib, niraparib, and rucaparib) in the maintenance and treatment of recurrent ovarian cancers. It details study settings, inclusion criteria, BRCA and HR statuses, and their endpoints, including the primary and secondary ones.
3.1. Frontline Treatment
PARPis have not yet been broadly adopted as concurrent agents with frontline PBC, primarily due to overlapping toxicities [59]. The most notable study in this setting is the VELIA trial, which investigated veliparib in combination with chemotherapy and as maintenance therapy [60]. A statistically significant improvement in median progression-free survival (PFS) was observed across all biomarker-defined subgroups, including patients with BRCA mutations (34.7 vs. 22.0 months; HR 0.44, 95% CI of 0.28 to 0.68), HRD-positive tumors (31.9 vs. 20.5 months; HR 0.57, 95% CI of 0.43 to 0.76), and the intention-to-treat population (23.5 vs. 17.3 months; HR 0.68, 95% CI of 0.56 to 0.83). Importantly, the benefit was observed only when veliparib was continued into maintenance; no survival advantage was seen with veliparib use during the induction phase alone. Therefore, the use of PARPis with frontline chemotherapy is limited by toxicity and minimal added benefit, with their primary value lying in their continuation as maintenance therapy rather than as a standalone frontline treatment.
3.2. Frontline Maintenance
The most consistent and significant clinical impact of PARPis has been observed in the frontline maintenance setting, particularly in those with germline BRCA1/2, somatic BRCA1/2, or germline BRCA1/2 wild-type HR-deficient biomarker statuses. Several phase III trials have shown clinical benefits with niraparib (PRIMA/ENGOT-OV26/GOG-3012), olaparib (SOLO1/GOG3004 and PAOLA-1/ENGOT-ov25), and rucaparib (ATHENA-MONO/GOG-3020/ENGOT-ov45) following initial response to PBC [26,27,28,61,62,63,64]. These findings were further confirmed by mature OS results from the PRIMA and PAOLA-1 trials, which indicated significantly longer median OS among patients with homologous recombination-deficient (HRD) ovarian cancer who received PARPi [65,66]. However, the 7-year follow-up data from the SOLO1/GOG-3004 trial showed no significant difference in OS between patients with BRCA-mutated ovarian cancer who received frontline maintenance olaparib and those who received a placebo [63]. Additionally, final safety results from the ATHENA-MONO trial confirmed a manageable safety profile for rucaparib, though final efficacy results are still pending [67]. Patients with BRCA1/2 wild-type, HR-proficient biomarker statuses experienced less benefit from PARPis, with only niraparib and rucaparib demonstrating statistically significant survival advantages [27,61].
3.3. Recurrent Treatment
PARPis have also been tested as active treatment agents in the setting of platinum-sensitive recurrent ovarian cancer. Key trials include SOLO-3 and ARIEL-4, both limited to BRCA-mutated populations [68,69,70,71,72]. SOLO-3 demonstrated a median PFS of 13.2 months with olaparib vs. 8.5 months with chemotherapy (HR 0.62, 95% CI of 0.43 to 0.91), though the OS difference (34.9 vs. 32.9 months) was not statistically significant. ARIEL-4, which compared rucaparib with chemotherapy in patients with BRCA-mutated recurrent disease, showed a modest PFS benefit (7.4 vs. 5.7 months; HR 0.67, 95% CI of 0.52 to 0.86) [68,69]. However, it raised concern due to a non-statistically significant trend toward worse OS in the rucaparib group (19.4 vs. 25.4 months; HR 1.31, 95% CI of 1.00 to 1.73) [68,69].
Therefore, although PARPis offer an alternative to chemotherapy in recurrent BRCA-mutated disease by improving PFS, inconsistent OS results suggest a need for careful patient selection and further study.
3.4. Recurrent Maintenance
PARPis have shown consistent PFS benefit in the maintenance setting following a response to platinum-based therapy for recurrent disease, particularly in patients with positive biomarker statuses. Trials include NOVA (niraparib), NORA (niraparib, Asian cohort), ARIEL-3 (rucaparib), and SOLO2 (olaparib) [23,73,74,75,76,77,78,79]. However, in this setting, no PARPi has yet demonstrated a significant OS benefit in the gBRCAwt/HRp population [23,73,74,76,77]. The only PARPi to show an OS benefit in the recurrent maintenance setting within a phase III trial was olaparib in the SOLO2 trial [78,79], which included only patients with germline or somatic BRCA or BRCA mutation biomarker statuses. In SOLO2, olaparib significantly improved both PFS (19.1 vs. 5.5 months; HR 0.30, 95% CI of 0.22 to 0.41) and OS (51.7 vs. 38.8 months; HR 0.30, 95% CI of 0.22 to 0.41) in BRCA-mutated patients. OS results in HR-proficient populations were less favorable, with some trials reporting non-significant or negative trends (e.g., ARIEL-3: OS HR for HRp population = 1.15, 95% CI of 0.78 to 1.70) [23,73,74,76,77].

Table 1.
Summary of clinical trials evaluating PARP inhibitors in patients with ovarian cancer.
Table 1.
Summary of clinical trials evaluating PARP inhibitors in patients with ovarian cancer.
| Setting | Drug | Study | Inclusion Criteria | BRCA/HR Status | Methods | Primary Endpoint | Pertinent Secondary Endpoints |
|---|---|---|---|---|---|---|---|
| Frontline Maintenance | Niraparib | PRIMA [27,61] | High-grade epithelial histology Stage III or IV Primary CRS or Interval CRS Complete or partial response after PBC (bevacizumab not permitted) | gBRCAm gBRCAwt/HRd gBRCAwt/HRp | Phase 3 2:1 ratio to receive niraparib 300 mg or placebo once daily for up to 36 months. | Median PFS: ITT Population: niraparib: 13.8 months placebo: 8.2 months HR, 0.62; 95% CI, 0.50 to 0.76 HRd Population: niraparib: 21.9 months placebo 10.4 months HR, 0.43; 95% CI, 0.31 to 0.59 HRp Population: niraparib: 8.1 months placebo: 5.4 months HR, 0.68; 95% CI, 0.49 to 0.94 | 24-Month OS ITT Population: niraparib: 84% placebo: 77% HR, 0.70; 95% CI, 0.44 to 1.11 HRd Population: niraparib: 91% placebo: 85% HR, 0.61; 95% CI, 0.27 to 1.39 HRp Population: niraparib: 81% placebo: 59% HR, 0.51; 95% CI, 0.27 to 0.97 |
| Rucaparib | ATHENA-MONO/ GOG-3020/ ENGOT-ov45 [62] | High-grade epithelial histology Stage III or IV Primary CRS or Interval CRS Complete or partial response after platinum–taxane chemotherapy (bevacizumab permitted during the chemotherapy phase) | gBRCAm gBRCAwt/HRd gBRCAwt/HRp | Phase 3 4:1 ratio to receive rucaparib 600 mg or placebo twice a day for up to 24 months. | Median PFS: ITT Population: rucaparib: 20.2 months placebo 9.2 months HR, 0.52; 95% CI, 0.40 to 0.68 HRd Population: rucaparib: 28.7 months placebo: 11.3 months HR, 0.47; 95% CI, 0.31 to 0.72 HRp Population: rucaparib: 12.1 months placebo: 9.1 months HR, 0.65; 95% CI, 0.45 to 0.95 | OS results have not yet been reported. | |
| Olaparib | SOLO-1 [26,63] | High-grade epithelial histology Stage III or IV Primary CRS or Interval CRS Complete or partial response after platinum–taxane chemotherapy (bevacizumab not permitted) | gBRCAm sBRCAm | Phase 3 2:1 ratio to receive olaparib 300 mg or placebo twice daily for up to 24 months. | Median PFS: ITT Population: olaparib: 56.0 months placebo: 13.8 months HR 0.33; 95% CI 0.25–0.43 | Median OS: ITT Population: olaparib: Not reached placebo: 75.2 months HR, 0.55; 95% CI, 0.40 to 0.76 | |
| Olaparib + bevacizumab | PAOLA-1 [28,64] | High-grade epithelial histology Stage III or IV Primary CRS or Interval CRS Complete or partial response after platinum–taxane chemotherapy plus bevacizumab | gBRCAm gBRCAwt/HRd gBRCAwt/HRp | Phase 3 2:1 ratio to receive olaparib 300 mg or placebo twice daily for up to 24 months. Bevacizumab 15 mg/kg every three weeks was initiated with chemotherapy and continued as maintenance therapy for up to 15 months. | Median PFS: ITT Population: olaparib: 22.1 months placebo: 13.8 months HR, 0.59; 95% CI 0.49–0.72 HRd Population: olaparib: 37.2 months placebo: 17.7 months HR, 0.33; 95% CI 0.25–0.45 HRp Population: olaparib: 16.6 months placebo: 16.2 months HR, 1.00; 95% CI 0.75–1.35 | Median OS: ITT Population: olaparib: 56.5 months placebo: 51.6 months HR, 0.92; 95% CI 0.76–1.12 HRd Population: olaparib 75.2 months placebo: 57.3 months HR, 0.62; 95% CI 0.45–0.85 HRp Population: olaparib: 36.8 months placebo: 40.4 months HR,1.19; 95% CI 0.88–1.63 | |
| Recurrent Maintenance | Niraparib | ENGOT-OV16/NOVA [23,73,74] | PSROC High-grade epithelial histology A total of ≥ 2 prior lines of platinum-based chemotherapy +/− bevacizumab | gBRCAm gBRCAwt/HRd gBRCAwt/HRp | Phase 3 2:1 ratio to receive niraparib 300 mg or placebo daily until disease progression. | Median PFS: gBRCAm Population: niraparib: 21 months placebo: 5.5 months HR, 0.27; 95% CI 0.17–0.41 gBRCAwt/HRd Population: niraparib: 12.9 months placebo: 3.8 months HR, 0.38; 95% CI 0.24–0.59 gBRCAwt/HRp Population: niraparib: 7.4 months placebo: 4.2 months HR, 0.45; 95% CI 0.34–0.61 | Median OS: gBRCAm Population: niraparib: 40.9 months placebo: 38.1 months HR, 0.85; 95% CI 0.61–1.20 gBRCAwt/HRd Population: niraparib: 35.6 months placebo: 41.4 months HR, 1.29; 95% CI 0.85–1.95 OS HRp Population: niraparib: 27.9 months placebo: 27.9 months HR, 0.93; 95% CI 0.61–1.41 |
| Niraparib | NORA [75] | PSROC High-grade epithelial histology A total of 2 prior lines of platinum-based chemotherapy +/− bevacizumab | gBRCAm gBRCAwt | Phase 3 2:1 to receive niraparib 300 mg or placebo daily until disease progression. | Median PFS: ITT Population: niraparib: 18.3 months placebo: 5.4 months HR, 0.32; 95% CI 0.23–0.45 gBRCAm Population: niraparib: Not reached placebo: 5.5 months HR, 0.22; 95% CI 0.23–0.39 gBRCAwt Population: niraparib: 11.1 months placebo: 3.9 months HR, 0.40; 95% CI 0.26–0.61 | Median OS: ITT Population: niraparib: 46.3 months placebo: 43.4 months HR, 0.83; 95% CI 0.56–1.21 gBRCAm Population: niraparib: Not reached placebo: 47.6 months HR, 0. 77; 95% CI 0. 40–1.47 gBRCAwt Population: niraparib: 43.1 months placebo: 38.4 months HR, 0.86; 95% CI 0.53–1.39 | |
| Rucaparib | ARIEL-3 [76,77] | PSROC High-grade epithelial histology A total of ≥ 2 prior lines of platinum-based chemotherapy +/− bevacizumab | gBRCAm gBRCAwt/HRd gBRCAwt/HRp | Phase 3 2:1 ratio to receive rucaparib 600 mg or placebo twice daily until disease progression. | Median PFS: ITT Population: rucaparib: 10.8 months placebo: 5.4 months HR, 0.36; 95% CI 0.30–0.45 HRd Population: rucaparib: 9.7 months placebo: 5.4 months HR, 0.44; 95% CI 0.29–0.66 HRp Population: rucaparib: 6.7 months placebo: 5.4 months HR, 0.58; 95% CI 0.40–0.85 | Median OS: ITT Population: rucaparib: 36 months placebo: 43.2 months HR, 0.995; 95% CI 0.81–1.22 HRd Population: rucaparib: 40.5 months placebo: 47.8 months HR, 1.01; 95% CI 0.77–1.32 HRp Population: rucaparib: 28.6 months placebo 32.6 months HR, 1.15; 95% CI 0.78–1.70 | |
| Olaparib | Study-19 [80,81] | PSROC High-grade serous histology A total of ≥ 2 prior lines of platinum-based chemotherapy | gBRCAm gBRCAwt | Phase 2 1:1 ratio to receive olaparib 400 mg or placebo twice daily until disease progression. | Median PFS: ITT Population: olaparib: 8.4 months placebo: 4.8 months HR, 0.35; 95% CI 0.25–0.49 gBRCAm Population: olaparib: 11.2 months placebo: 4.3 months HR, 0.18; 95% CI 0.10–0.31 gBRCAwt population: olaparib: 7.4 months placebo: 5.5 months HR, 0.54; 95% CI 0.34–0.85 | Median OS: ITT Population: olaparib: 29.8 months placebo: 27.8 months HR, 0.73; 95% CI 0.55–0.96 gBRCAm Population: olaparib: 34.9 months placebo: 30.2 months HR, 0.62; 95% CI 0.41–0.94 gBRCAwt population: olaparib: 24.5 months placebo: 26.6 months HR, 0.83; 95% CI 0.55–1.24 | |
| Olaparib | SOLO2/ENGOT-Ov21 [78,79] | PSROC High-grade epithelial histology A total of ≥ 2 prior lines of platinum-based chemotherapy | gBRCAm sBRCAm | Phase 3 2:1 ratio to receive olaparib 300 mg or placebo twice daily until disease progression. | Median PFS: ITT Population: olaparib: 19.1 months placebo: 5.5 months HR 0.30; 95% CI 0.22–0.41 | Median OS: ITT Population: olaparib: 51.7 months placebo: 38.8 months HR 0.30; 95% CI 0.22–0.41 | |
| Recurrent treatment | Rucaparib | ARIEL-4 [68,69] | PSROC High-grade epithelial histology A total of ≥ 2 prior lines of chemotherapy (at least one line of PBC), no prior PARP exposure | gBRCAm sBRCAm | Phase 3 2:1 ratio to receive rucaparib 600 mg twice daily or chemotherapy until disease progression. | Median PFS: ITT Population: rucaparib: 7.4 months placebo: 5.7 months HR 0.67; 95% CI 0.52–0.86 | Median OS: ITT Population: rucaparib: 19.4 months placebo: 25.4 months HR 1.31; 95% CI 1.00–1.73 |
| Olaparib | SOLO-3 [70,71] | PSROC High-grade epithelial histology A total of ≥ 2 prior lines of chemotherapy (at least one line of PBC), no prior PARP exposure | gBRCAm | Phase 3 2:1 ratio to receive rucaparib 600 mg twice daily or chemotherapy until disease progression. | Median PFS: ITT Population: 13.2 months in the olaparib group vs. 8.5 months in the placebo group (HR 0.62; 95% CI 0.43–0.91). | Median OS: ITT Population: 34.9 months in the olaparib group vs. 32.9 months in the placebo group (HR 1.07; 95% CI 0.76–1.49). |
Bolded text indicates the different settings in which PARPis were used in the trials; underlined italic text highlights the trial endpoints being assessed; italicized text without underlining denotes the patient populations evaluated in the trials.
4. Does PARP Inhibitor Maintenance Therapy Influence Outcomes of Platinum-Based Chemotherapy Retreatment in Recurrent Disease?
As survival data from PARPi maintenance trials continue to mature, conflicting results have emerged regarding the potential benefits on OS. Some studies indicate no OS benefit following PARPi maintenance therapy, suggesting that the long-term effectiveness of these drugs may be limited. The optimal approach to managing patients who experience disease progression while undergoing PARPi treatment remains uncertain, particularly for those who progress while actively receiving a PARPi treatment. Historically, many patients with recurrent ovarian cancer have benefitted from retreatment with agents used in the first-line setting, such as carboplatin, paclitaxel, and bevacizumab [82,83]. However, one of the main concerns after the introduction of PARPis into the management of ovarian cancer is the potential for cross-resistance between PARPis and PBC, which may reduce the efficacy of subsequent treatment options in these patients. Emerging clinical data from several studies have started to explore this issue, providing evidence that progression on PARPi treatment may be associated with a poor prognosis upon retreatment with platinum-based therapies.
A multicenter study on patients with BRCA-mutated ovarian cancer was among the first to highlight the reduced efficacy of PBC following frontline PARPi use [84]. While the study demonstrated that olaparib could be both effective and safe as a maintenance therapy for this patient group, it also revealed a low response rate to subsequent treatments in those who progressed after maintenance. The objective response rate (ORR) was only 22.2% in those with platinum-free intervals of more than 12 months, which is approximately one-third to one-half of the rates reported in previous studies on patients who had not received PARPi treatment [84,85,86]. This finding was also noted in a secondary analysis of the SOLO2/ENGOT Ov-21 trial [87]. In this study, olaparib was administered to patients with platinum-sensitive, relapsed BRCA-mutated ovarian cancer who had undergone at least two prior lines of chemotherapy. As previously mentioned, the results demonstrated a significant improvement in PFS and OS with an acceptable safety profile. This secondary analysis included only patients who received chemotherapy—either PBC or non-PBC—after disease progression following treatment with olaparib or placebo. The findings revealed that the time to second progression was significantly longer in patients who had received a placebo in the SOLO2/ENGOT Ov-21 trial compared to those who had received olaparib, specifically among those who underwent post-progression PBC (HR = 2.89, 95% CI of 1.73–4.82). However, this difference was not observed in patients who received non-PBC (HR = 1.58, 95% CI of 0.86–2.90), raising questions about the role of prior PARPi exposure in the effectiveness of PBC.
Further evidence from retrospective studies has provided additional insight into this issue. In a retrospective chart review study, the outcomes of patients who received PBC after progressing on PARPi treatment were evaluated [88]. This study demonstrated that the median PFS after initiation of PBC was 219 days (interquartile range of 125–307), with the median being significantly longer in patients who had been on PARPis for more than 18 months compared to those who had been on them for 12 to 18 months and those who had received them for less than 12 months. Additionally, a retrospective international study conducted on 291 patients with ovarian cancer who were treated between 2003 and 2021 assessed the response to chemotherapy after progression on PARPi [89]. This study found that among patients with a platinum-free interval of more than six months, those who received PBC did not have worse outcomes compared to those who received non-PBC. The PBC group showed numerically better PFS, although the adjusted HR (0.68, 95% CI of 0.46–1.01) was not statistically significant (p = 0.0547). Another retrospective study observed an ORR of 70% in response to PBC among 10 patients who had progressed on PARPi [88].
A recently published post hoc analysis of the PAOLA-1/ENGOT-ov25 trial provided additional insights into the effectiveness of subsequent treatments in patients with recurrent ovarian cancer following PARPi therapy. This analysis specifically examined the efficacy of chemotherapy after olaparib maintenance, stratifying patients based on whether disease progression occurred during or after olaparib maintenance. Multivariate analysis revealed that the timing of progression was significantly associated with the duration between the first and second subsequent treatments, both in the overall cohort and in the subgroup of patients who received PBC and PARPi retreatment after progression. A detailed summary of studies evaluating the impact of prior PARPi treatment on the efficacy of PBC retreatment after progression is provided in Table 2.

Table 2.
Studies evaluating the effects of PARP inhibitor maintenance therapy on sensitivity to subsequent lines of platinum-based chemotherapy.
These findings collectively do not support the hypothesis that prior exposure to PARPis may negatively influence the response to subsequent PBC. However, the variability in outcomes across different studies underscores the complexity of this relationship and the need for further investigation. Different studies have assessed this issue using varied methodologies and reported different aspects of this issue. Some studies focused on the impact of prior PARPi exposure by evaluating objective response rates (ORR) to PBC, while others examined PFS or OS as key endpoints. Additionally, the studies differed in their inclusion criteria, with some exclusively analyzing BRCA-mutated populations, while others included patients with both BRCA-mutated and wild-type tumors. Differences in the duration of PARPi exposure, timing of disease progression, number of prior lines of retreatment, and platinum-free intervals further complicate direct comparisons across studies. Additionally, the type of platinum regimen used post-PARPi progression varies between studies, which may influence the observed outcomes. Some analyses suggest a detrimental effect of prior PARPi treatment on PBC efficacy. In contrast, others indicate that specific subgroups, particularly those with longer platinum-free intervals, may still benefit from PBC retreatment. Given these discrepancies, more prospective, well-controlled studies are necessary to clarify the impact of prior PARPi exposure on PBC retreatment outcomes.
5. Is Retreatment with PARP Inhibitors a Viable Strategy After Prior Maintenance Failure?
Similar to the challenges and uncertainties surrounding the effectiveness of PBC retreatment following progression on PARPi, the potential clinical benefit of PARPi retreatment after prior progression on PARPi maintenance remains an open question.
Early investigations into PARPi retreatment have yielded mixed results. A secondary analysis of the QUADRA trial, conducted by Rimel et al., specifically focused on patients with prior PARPi exposure [96]. The QUADRA trial was a multicenter, single-arm study conducted across Canada and the United States, evaluating the efficacy and safety of niraparib monotherapy in patients with ovarian cancer who had undergone three or more prior chemotherapy regimens [72]. Among the more than 450 patients enrolled, 37 had previously received a PARPi, with 33 discontinuing it due to disease progression. Within this subgroup, 35 patients had evaluable outcomes, revealing that only 3% achieved a confirmed partial response, 34% had stable disease for at least seven weeks, and a clinical benefit rate of 20% at 16 weeks. Similarly, a post hoc analysis of the Rucaparib Access Programme examined a subset of ovarian cancer patients with prior PARPi exposure, with a median of five prior treatment lines before receiving rucaparib [97]. The study found a median PFS of only 2.5 months (95% CI: 1.0–4.4). Among seven patients with accessible response data, only one achieved stable disease, while six experienced disease progression, reinforcing concerns about limited efficacy in heavily pretreated patients. Essel et al. also conducted a multicenter retrospective analysis of 22 patients with recurrent ovarian cancer who underwent PARPi retreatment [98]. No complete responses were observed in this cohort, but three patients had partial responses, and 13 achieved stable disease. Notably, all three patients with partial responses harbored BRCA mutations. These findings suggest that while a small subset of patients may still derive some benefit from PARPi retreatment, overall response rates remain low.
The first and only prospective study specifically designed to address the concept of PARPi retreatment was the OReO/ENGOT Ov-38 trial [99]. This trial was a randomized, placebo-controlled, double-blinded study to assess the effectiveness and safety of maintenance olaparib on both BRCA-mutated and non-BRCA-mutated patient cohorts with recurrent ovarian cancer who have previously received PARPi treatment and remained platinum-sensitive to their most recent PBC. The study demonstrated that olaparib retreatment was associated with a 43% lower hazard of disease progression or death among patients with BRCA mutations (HR = 0.57, 95% CI of 0.37–0.87) and a 57% lower hazard among those without BRCA mutations (HR = 0.57, 95% CI of 0.37–0.87) with no additional safety concerns reported. An unexpected observation was the comparable or slightly greater benefit seen in the non-BRCA-mutated cohort. This contrasts with previous studies in the PARPi-naïve setting, where BRCA-mutated tumors have consistently demonstrated greater sensitivity to PARPi therapy [23,24,80].
A retrospective study involving 201 patients with recurrent ovarian cancer was conducted to evaluate the efficacy and safety of PARPi retreatment following prior failure and to compare its outcomes with chemotherapy alone [100]. The study demonstrated that patients who underwent PARPi retreatment had a significantly lower progression rate (66.7% vs. 84.0%) and a significantly longer median PFS (10.8 vs. 5.0 months) than those who received chemotherapy alone. Furthermore, the efficacy of PARPi retreatment was notably higher in patients who had achieved a complete response to their most recent therapy compared to those with a partial response. Additionally, the researchers conducted molecular analyses, which revealed that specific mutations were associated with significantly longer PFS following PARPi re-treatment, suggesting molecular profiling may enhance patient selection and improve the clinical utility of PARPi rechallenge in recurrent ovarian cancer.
6. Challenges and Future Directions
Resistance to PARPis remains a significant hurdle in the treatment of ovarian cancer, prompting extensive research into novel therapeutic targets and combination strategies. Several molecular pathways have been identified as contributors to PARPi resistance, and clinical trials are actively evaluating targeted treatments to counteract these mechanisms [101]. Among the promising agents being tested in combination with PARPi are phosphatidylinositol-3-kinase (PI3K) inhibitors, including alpelisib (EPIK-O/ENGOT-ov61 trial, NCT04729387) and copanlisib (NCT03586661); MEK1/2 inhibitors such as selumetinib (NCT03162627); and ATR inhibitors like ceralasertib. Tyrosine kinase inhibitors (TKIs) under investigation include bevacizumab (KGOG 3056/NIRVANA-R trial, NCT04734665; MITO25 trial, NCT03462212) and anlotinib (NCT04566952). Immune checkpoint inhibitors are also being explored in combination with PARPi, including pembrolizumab (MK-7339-001/KEYLYNK-001/ENGOT-ov43/GOG-3036 trial, NCT03740165), dostarlimab (NItCHE/MITO trial, NCT04679064), durvalumab (NCT02953457), tremelimumab (NCT04034927), and TSR-042 (anti-PD-1, NCT04673448). Other investigational therapies combined with PARPi include the histone deacetylase (HDAC) inhibitor belinostat (NCT04703920) and the nanoparticle-drug conjugate (NDC) EP0057, a cyclodextrin-based polymer backbone linked to camptothecin (EP0057-201 trial, NCT04669002). Additionally, various combination regimens are being tested, such as cediranib with durvalumab (NCT02484404), pembrolizumab with bevacizumab (SaINT-ov02 trial, NCT05158062), and dostarlimab with bevacizumab (OPAL trial, NCT03574779). These trials aim to enhance the efficacy of PARPi by targeting key resistance mechanisms. An overview of some of these important ongoing trials with their primary endpoint is provided in Table 3.

Table 3.
Ongoing clinical trials evaluating combination strategies with PARP inhibitors in ovarian cancer.
Encouraging data from some of these trials suggest that certain combination strategies may provide significant benefits. The DUO-O trial, which investigated the addition of bevacizumab, durvalumab, and olaparib to PBC in frontline maintenance therapy, has shown a notable improvement in PFS compared to bevacizumab and PBC in the interim analysis [102]. However, a critical limitation of this study is the absence of a control arm including a PARP inhibitor, reflecting the rapid evolution of treatment paradigms and the challenges in designing trials that remain relevant amid changing standards of care. Other recently published studies have also reported promising outcomes. The NCT04517357 trial demonstrated the superiority of apatinib combined with fuzuloparib over fuzuloparib alone, with a higher ORR [103]. Similarly, pembrolizumab in combination with olaparib (NCT04417192) exhibited an ORR of 70%, while the OPEB-01 trial (NCT04361370), evaluating the triplet regimen of olaparib, pembrolizumab, and bevacizumab, reported an impressive PFS rate of 88.6% at six months [104]. Additionally, findings from the PETRA trial assessed the first-in-class PARP1-selective inhibitor saruparib (AZD5305) in patients with advanced solid tumors harboring BRCA1/2, PALB2, or RAD51C/D mutations [105]. This phase I study demonstrated promising anticancer activity with a favorable safety profile. Several other combination strategies have also shown potential. The CAPRI trial (NCT03462342), a phase 2 study evaluating the ATR inhibitor ceralasertib with olaparib, found the combination to be both well-tolerated and effective [106]. Meanwhile, the EFFORT trial (NCT03579316) investigated the Wee1 inhibitor adavosertib alone and in combination with olaparib in patients with PARPi-resistant ovarian cancer, demonstrating notable efficacy in this challenging patient population [107].
Conversely, not all trials have produced favorable results. The ICON-9 trial (NCT03278717) found that cediranib combined with olaparib did not confer superior efficacy over olaparib alone in terms of PFS and OS in patients with relapsed ovarian cancer following response to PBC [108]. Similarly, the NRG-GY005 trial (NCT02502266), which compared cediranib and olaparib combinations with olaparib or cediranib alone or with standard-of-care chemotherapy, failed to demonstrate a PFS benefit over each of these arms [109]. Furthermore, the ENGOT-OV41/GEICO 69-O/ANITA trial (NCT03598270), which explored the addition of atezolizumab to chemotherapy and maintenance niraparib in recurrent ovarian cancer, did not yield significant improvements in PFS or ORR [110]. Additionally, a trial investigating durvalumab combined with olaparib and/or cediranib found that none of the experimental arms outperformed standard-of-care treatment in terms of PFS [111]. These results underscore the complexity of overcoming drug resistance and highlight the necessity of more refined therapeutic strategies.
A critical challenge in the current adoption of these agents in the management of ovarian cancer is the lack of optimal patient selection criteria. While BRCA mutations and HRD status are key biomarkers for predicting PARPi response, they are not always reliable indicators of long-term efficacy. Many patients without BRCA mutations or HRD positivity still exhibit responses to PARPi, whereas some BRCA-mutated or HRD-positive cases fail to derive sustained benefit. This highlights the need for more precise genomic and molecular signatures to refine patient stratification and guide treatment decisions. Ongoing research explores alternative predictive biomarkers, such as alterations in RAD51 foci formation, replication stress markers, and transcriptomic profiles, to enhance the ability to tailor PARPi-based therapies to individual patients. The toxicity and tolerability of PARPi-based combination regimens present another major hurdle. While these combinations have shown promise in improving efficacy, they often exacerbate treatment-related adverse effects, including myelosuppression (anemia, neutropenia, thrombocytopenia), gastrointestinal toxicity (nausea, vomiting, diarrhea), and fatigue. These toxicities can limit the duration of therapy and reduce patients’ quality of life, necessitating careful dose optimization, supportive care strategies, and proactive management of side effects to maximize therapeutic benefits without compromising tolerability.
Furthermore, the optimal sequencing and integration of PARPi with other treatment modalities remain unresolved. The interplay between PARPi, chemotherapy, immunotherapy, and other targeted agents is complex, and their sequential or concurrent administration can influence both efficacy and resistance mechanisms. For instance, while combining PARPi with immune checkpoint inhibitors holds potential due to PARPi-induced genomic instability and enhanced neoantigen exposure, the timing and dosing of this combination remain areas of active investigation. Similarly, integrating anti-angiogenic agents, ATR inhibitors, and MEK inhibitors with PARPi requires careful consideration of pharmacokinetics, toxicity profiles, and potential antagonistic interactions. Compounding these challenges is that current clinical trial findings on these combination strategies remain conflicting, as mentioned above.
A major barrier to defining optimal treatment strategies is the significant heterogeneity in studies evaluating PARPi-based management. Differences in study designs, treatment arms, primary and secondary endpoints, and patient population characteristics create challenges in drawing consistent conclusions across trials. Additionally, the retrospective nature of several studies, small sample sizes, and variations in prior treatment exposures further complicate the interpretation of results and the establishment of standardized treatment guidelines. To address these challenges, future research should focus on prospective, randomized trials with standardized inclusion criteria, comprehensive biomarker analyses, and long-term follow-up data. Additionally, real-world evidence and large-scale registries may provide valuable insights into the effectiveness and safety of PARPi-based combinations outside controlled clinical settings. As our understanding of resistance mechanisms, patient selection strategies, and treatment sequencing evolves, these efforts will be crucial in optimizing the use of PARPi in ovarian cancer management.
7. Conclusions
In conclusion, while PARPi therapy has revolutionized the treatment of ovarian cancer, significant challenges remain. Drug resistance, patient selection, toxicity management, therapeutic sequencing, and heterogeneity in the current body of evidence all contribute to the complexity of optimizing PARPi use. Continued research and innovative clinical trial designs are essential to address these limitations and develop more effective, personalized strategies for patients. The future of ovarian cancer treatment will likely depend on a multifaceted approach that integrates genomic profiling, novel drug combinations, and adaptive treatment strategies to enhance long-term outcomes.
Author Contributions
Conceptualization and investigation, S.A., A.M., M.W., M.Y., L.N., B.M., V.A., M.L.A., T.R., M.C., D.S. and A.K.; literature review, S.A., M.W., M.Y., V.A. and A.K.; writing—original draft preparation, S.A., M.W., M.Y., D.S., V.A. and A.K.; writing—review and editing, A.M., L.N., B.M., M.L.A., T.R., M.C. and A.K.; and supervision, A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
Author Mihaela Cristea was employed by the company Regeneron Pharmaceuticals. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef]
- Sargent, R.G.; Brenneman, M.A.; Wilson, J.H. Repair of site-specific double-strand breaks in a mammalian chromosome by homologous and illegitimate recombination. Mol. Cell. Biol. 1997, 17, 267–277. [Google Scholar] [CrossRef]
- Arnaudeau, C.; Lundin, C.; Helleday, T. DNA double-strand breaks associated with replication forks are predominantly repaired by homologous recombination involving an exchange mechanism in mammalian cells. J. Mol. Biol. 2001, 307, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Dianov, G.L. Targeting base excision repair to improve cancer therapies. Mol. Aspects Med. 2007, 28, 345–374. [Google Scholar] [CrossRef][Green Version]
- Fisher, A.E.O.; Hochegger, H.; Takeda, S.; Caldecott, K.W. Poly(ADP-Ribose) Polymerase 1 Accelerates Single-Strand Break Repair in Concert with Poly(ADP-Ribose) Glycohydrolase. Mol. Cell. Biol. 2007, 27, 5597–5605. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.; Burgess, J.T.; O’byrne, K.; Richard, D.J.; Bolderson, E. PARP Inhibitors: Clinical Relevance, Mechanisms of Action and Tumor Resistance. Front. Cell Dev. Biol. 2020, 8, 564601. [Google Scholar] [CrossRef]
- Wang, M.; Wu, W.; Wu, W.; Rosidi, B.; Zhang, L.; Wang, H.; Iliakis, G. PARP-1 and Ku compete for repair of DNA double strand breaks by distinct NHEJ pathways. Nucleic Acids Res. 2006, 34, 6170–6182. [Google Scholar] [CrossRef] [PubMed]
- Haince, J.-F.; McDonald, D.; Rodrigue, A.; Déry, U.; Masson, J.-Y.; Hendzel, M.J.; Poirier, G.G. PARP1-dependent Kinetics of Recruitment of MRE11 and NBS1 Proteins to Multiple DNA Damage Sites. J. Biol. Chem. 2008, 283, 1197–1208. [Google Scholar] [CrossRef]
- Sugimura, K.; Takebayashi, S.-I.; Taguchi, H.; Takeda, S.; Okumura, K. PARP-1 ensures regulation of replication fork progression by homologous recombination on damaged DNA. J. Cell Biol. 2008, 183, 1203–1212. [Google Scholar] [CrossRef]
- Bryant, H.E.; Petermann, E.; Schultz, N.; Jemth, A.-S.; Loseva, O.; Issaeva, N.; Johansson, F.; Fernandez, S.; McGlynn, P.; Helleday, T. PARP is activated at stalled forks to mediate Mre11-dependent replication restart and recombination. EMBO J. 2009, 28, 2601–2615. [Google Scholar] [CrossRef]
- Boehler, C.; Gauthier, L.R.; Mortusewicz, O.; Biard, D.S.; Saliou, J.-M.; Bresson, A.; Sanglier-Cianferani, S.; Smith, S.; Schreiber, V.; Boussin, F.; et al. Poly(ADP-ribose) polymerase 3 (PARP3), a newcomer in cellular response to DNA damage and mitotic progression. Proc. Natl. Acad. Sci. USA 2011, 108, 2783–2788. [Google Scholar] [CrossRef] [PubMed]
- Rulten, S.L.; Fisher, A.E.; Robert, I.; Zuma, M.C.; Rouleau, M.; Ju, L.; Poirier, G.; Reina-San-Martin, B.; Caldecott, K.W. PARP-3 and APLF Function Together to Accelerate Nonhomologous End-Joining. Mol. Cell 2011, 41, 33–45. [Google Scholar] [CrossRef]
- Pines, A.; Vrouwe, M.G.; Marteijn, J.A.; Typas, D.; Luijsterburg, M.S.; Cansoy, M.; Hensbergen, P.; Deelder, A.; de Groot, A.; Matsumoto, S.; et al. PARP1 promotes nucleotide excision repair through DDB2 stabilization and recruitment of ALC1. J. Cell Biol. 2012, 199, 235–249. [Google Scholar] [CrossRef]
- Fenton, A.L.; Shirodkar, P.; Macrae, C.J.; Meng, L.; Koch, C.A. The PARP3- and ATM-dependent phosphorylation of APLF facilitates DNA double-strand break repair. Nucleic Acids Res. 2013, 41, 4080–4092. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.; Boehler, C.; Barbat, J.G.; Bonnet, M.-E.; Illuzzi, G.; Ronde, P.; Gauthier, L.R.; Magroun, N.; Rajendran, A.; Lopez, B.S.; et al. PARP3 affects the relative contribution of homologous recombination and nonhomologous end-joining pathways. Nucleic Acids Res. 2014, 42, 5616–5632. [Google Scholar] [CrossRef]
- Bolton, K.L.; Chenevix-Trench, G.; Goh, C.; Sadetzki, S.; Ramus, S.J.; Karlan, B.Y.; Lambrechts, D.; Despierre, E.; Barrowdale, D.; McGuffog, L.; et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA 2012, 307, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-Z.; Jia, H.; Xiao, Q.; Li, R.-Z.; Wang, X.-S.; Yin, H.-Y.; Zhou, X. Efficacy and Prognostic Factors for PARP Inhibitors in Patients With Ovarian Cancer. Front. Oncol. 2020, 10, 958. [Google Scholar] [CrossRef]
- Frey, M.K.; Pothuri, B. Homologous recombination deficiency (HRD) testing in ovarian cancer clinical practice: A review of the literature. Gynecol. Oncol. Res. Pract. 2017, 4, 4. [Google Scholar] [CrossRef]
- Pennington, K.P.; Walsh, T.; Harrell, M.I.; Lee, M.K.; Pennil, C.C.; Rendi, M.H.; Thornton, A.; Norquist, B.M.; Casadei, S.; Nord, A.S.; et al. Germline and Somatic Mutations in Homologous Recombination Genes Predict Platinum Response and Survival in Ovarian, Fallopian Tube, and Peritoneal Carcinomas. Clin. Cancer Res. 2014, 20, 764–775. [Google Scholar] [CrossRef]
- Takaya, H.; Nakai, H.; Takamatsu, S.; Mandai, M.; Matsumura, N. Homologous recombination deficiency status-based classification of high-grade serous ovarian carcinoma. Sci. Rep. 2020, 10, 2757. [Google Scholar] [CrossRef]
- Takaya, H.; Nakai, H.; Sakai, K.; Nishio, K.; Murakami, K.; Mandai, M.; Matsumura, N. Intratumor heterogeneity and homologous recombination deficiency of high-grade serous ovarian cancer are associated with prognosis and molecular subtype and change in treatment course. Gynecol. Oncol. 2020, 156, 415–422. [Google Scholar] [CrossRef]
- Poveda, A.; Floquet, A.; Ledermann, J.A.; Asher, R.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Pignata, S.; Friedlander, M.; et al. Final overall survival (OS) results from SOLO2/ENGOT-ov21: A phase III trial assessing maintenance olaparib in patients (pts) with platinum-sensitive, relapsed ovarian cancer and a BRCA mutation. J. Clin. Oncol. 2020, 38, 6002. [Google Scholar] [CrossRef]
- Mirza, M.R.; Monk, B.J.; Herrstedt, J.; Oza, A.M.; Mahner, S.; Redondo, A.; Fabbro, M.; Ledermann, J.A.; Lorusso, D.; Vergote, I.; et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N. Engl. J. Med. 2016, 375, 2154–2164. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.; Scambia, G.; et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 1949–1961. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Moore, K.N.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1721–1731. [Google Scholar] [CrossRef]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef]
- Dias, M.P.; Moser, S.C.; Ganesan, S.; Jonkers, J. Understanding and overcoming resistance to PARP inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 2021, 18, 773–791. [Google Scholar] [CrossRef]
- McMullen, M.; Karakasis, K.; Madariaga, A.; Oza, A.M. Overcoming Platinum and PARP-Inhibitor Resistance in Ovarian Cancer. Cancers 2020, 12, 1607. [Google Scholar] [CrossRef]
- Hongo, A.; Seki, S.; Akiyama, K.; Kudo, T. A comparison of in vitro platinum-DNA adduct formation between carboplatin and cisplatin. Int. J. Biochem. 1994, 26, 1009–1016. [Google Scholar] [PubMed]
- Damia, G.; Broggini, M. Platinum Resistance in Ovarian Cancer: Role of DNA Repair. Cancers 2019, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Dobbelstein, M.; Sørensen, C.S. Exploiting replicative stress to treat cancer. Nat. Rev. Drug Discov. 2015, 14, 405–423. [Google Scholar] [CrossRef]
- Davar, D.; Beumer, J.H.; Hamieh, L.; Tawbi, H. Role of PARP Inhibitors in Cancer Biology and Therapy. Curr. Med. Chem. 2012, 19, 3907–3921. [Google Scholar] [CrossRef] [PubMed]
- Jubin, T.; Kadam, A.; Jariwala, M.; Bhatt, S.; Sutariya, S.; Gani, A.; Gautam, S.; Begum, R. The PARP family: Insights into functional aspects of poly (ADP-ribose) polymerase-1 in cell growth and survival. Cell Prolif. 2016, 49, 421–437. [Google Scholar] [CrossRef]
- Sakai, W.; Swisher, E.M.; Karlan, B.Y.; Agarwal, M.K.; Higgins, J.; Friedman, C.; Villegas, E.; Jacquemont, C.; Farrugia, D.J.; Couch, F.J.; et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature 2008, 451, 1116–1120. [Google Scholar] [CrossRef]
- Edwards, S.L.; Brough, R.; Lord, C.J.; Natrajan, R.; Vatcheva, R.; Levine, D.A.; Boyd, J.; Reis-Filho, J.S.; Ashworth, A. Resistance to therapy caused by intragenic deletion in BRCA2. Nature 2008, 451, 1111–1115. [Google Scholar] [CrossRef]
- Ganesan, S. Tumor Suppressor Tolerance: Reversion Mutations in BRCA1 and BRCA2 and Resistance to PARP Inhibitors and Platinum. JCO Precis. Oncol. 2018, 2, 1–4. [Google Scholar] [CrossRef]
- Banda, K.; Swisher, E.M.; Wu, D.; Pritchard, C.C.; Gadi, V.K. Somatic reversion of germline BRCA2 mutation confers resistance to PARP inhibitor therapy. JCO Precis. Oncol. 2018, 2, 1–6. [Google Scholar] [CrossRef]
- Carneiro, B.A.; Collier, K.A.; Nagy, R.J.; Pamarthy, S.; Sagar, V.; Fairclough, S.; Odegaard, J.; Lanman, R.B.; Costa, R.; Taxter, T.; et al. Acquired resistance to the PARP inhibitor olaparib in BRCA2-associated prostate cancer due to biallelic BRCA2 reversion mutations restoring both germline and somatic loss of function mutations. JCO Precis. Oncol. 2018, 2, 1–8. [Google Scholar] [CrossRef]
- Cheng, H.H.; Salipante, S.J.; Nelson, P.S.; Montgomery, B.; Pritchard, C.C. Polyclonal BRCA2 Reversion Mutations Detected in Circulating Tumor DNA After Platinum Chemotherapy in a Patient With Metastatic Prostate Cancer. JCO Precis. Oncol. 2018, 2, 1–5. [Google Scholar] [CrossRef] [PubMed]
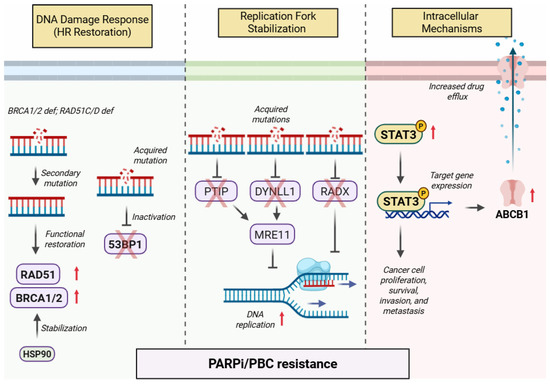
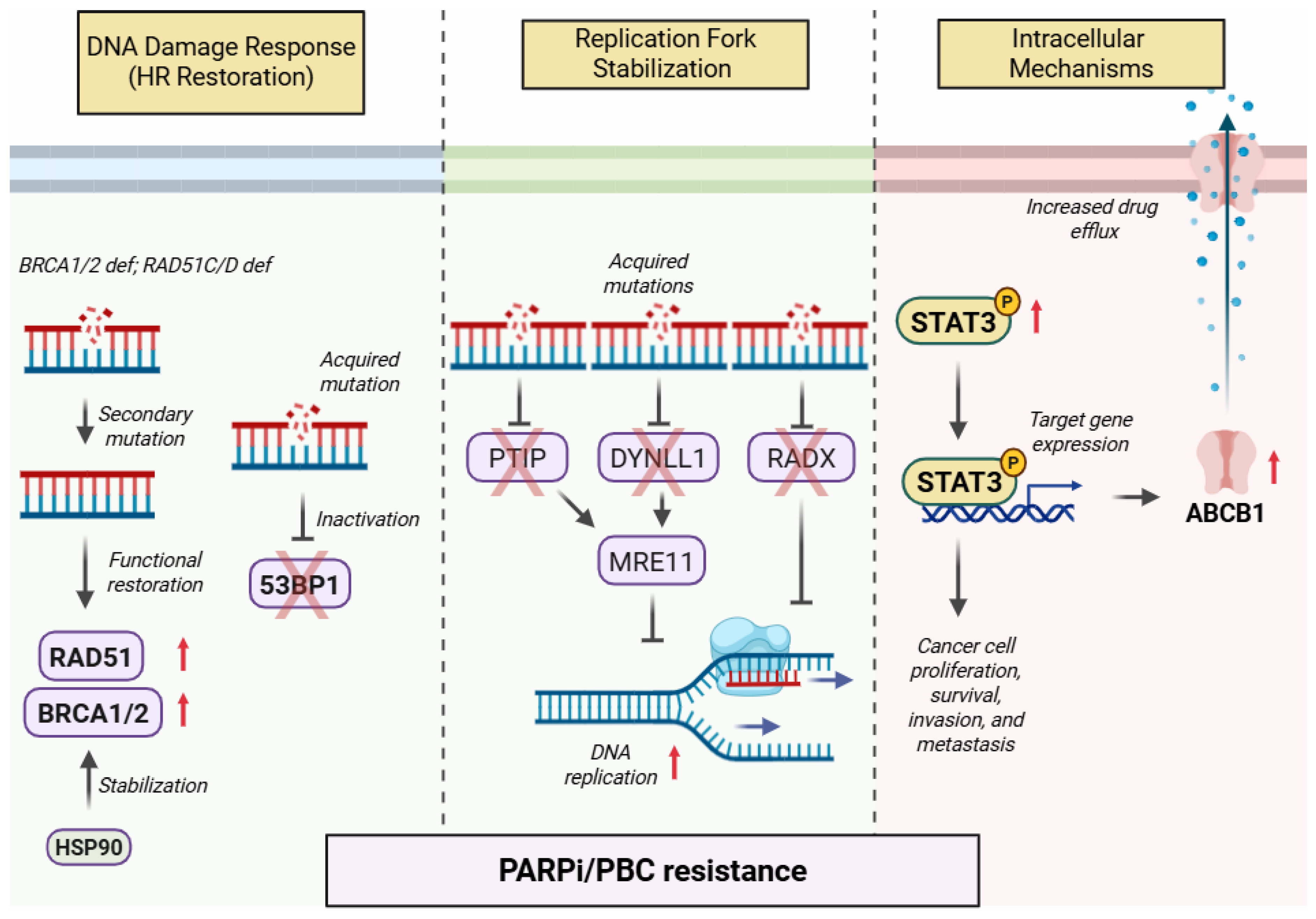
- Kondrashova, O.; Nguyen, M.; Shield-Artin, K.; Tinker, A.V.; Teng, N.N.H.; Harrell, M.I.; Kuiper, M.J.; Ho, G.Y.; Barker, H.; Jasin, M.; et al. Secondary Somatic Mutations Restoring RAD51C and RAD51D Associated with Acquired Resistance to the PARP Inhibitor Rucaparib in High-Grade Ovarian Carcinoma. Cancer Discov. 2017, 7, 984–998. [Google Scholar] [CrossRef]
- Bouwman, P.; Aly, A.; Escandell, J.M.; Pieterse, M.; Bartkova, J.; van der Gulden, H.; Hiddingh, S.; Thanasoula, M.; Kulkarni, A.; Yang, Q.; et al. 53BP1 loss rescues BRCA1 deficiency and is associated with triple-negative and BRCA-mutated breast cancers. Nat. Struct. Mol. Biol. 2010, 17, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.; Johnson, S.F.; Yao, W.; Li, Y.C.; Choi, Y.E.; Bernhardy, A.J.; Wang, Y.; Capelletti, M.; Sarosiek, K.A.; Moreau, L.A.; et al. Stabilization of mutant BRCA1 protein confers PARP inhibitor and platinum resistance. Proc. Natl. Acad. Sci. USA 2013, 110, 17041–17046. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.R.; Callen, E.; Ding, X.; Gogola, E.; Duarte, A.A.; Lee, J.-E.; Wong, N.; Lafarga, V.; Calvo, J.A.; Panzarino, N.J.; et al. Replication fork stability confers chemoresistance in BRCA-deficient cells. Nature 2016, 535, 382–387, Erratum in 2016, 539, 456. [Google Scholar] [CrossRef]
- He, Y.J.; Meghani, K.; Caron, M.-C.; Yang, C.; Ronato, D.A.; Bian, J.; Sharma, A.; Moore, J.; Niraj, J.; Detappe, A.; et al. DYNLL1 binds to MRE11 to limit DNA end resection in BRCA1-deficient cells. Nature 2018, 563, 522–526. [Google Scholar] [CrossRef]
- Dungrawala, H.; Bhat, K.P.; Le Meur, R.; Chazin, W.J.; Ding, X.; Sharan, S.K.; Wessel, S.R.; Sathe, A.A.; Zhao, R.; Cortez, D. RADX Promotes Genome Stability and Modulates Chemosensitivity by Regulating RAD51 at Replication Forks. Mol. Cell 2017, 67, 374–386.e5. [Google Scholar] [CrossRef]
- Jaspers, J.E.; Sol, W.; Kersbergen, A.; Schlicker, A.; Guyader, C.; Xu, G.; Wessels, L.; Borst, P.; Jonkers, J.; Rottenberg, S. BRCA2-Deficient Sarcomatoid Mammary Tumors Exhibit Multidrug Resistance. Cancer Res. 2015, 75, 732–741. [Google Scholar] [CrossRef]
- Kim, H.; Xu, H.; George, E.; Hallberg, D.; Kumar, S.; Jagannathan, V.; Medvedev, S.; Kinose, Y.; Devins, K.; Verma, P.; et al. Combining PARP with ATR inhibition overcomes PARP inhibitor and platinum resistance in ovarian cancer models. Nat. Commun. 2020, 11, 3726. [Google Scholar] [CrossRef]
- Ji, T.; Gong, D.; Han, Z.; Wei, X.; Yan, Y.; Ye, F.; Ding, W.; Wang, J.; Xia, X.; Li, F.; et al. Abrogation of constitutive Stat3 activity circumvents cisplatin resistant ovarian cancer. Cancer Lett. 2013, 341, 231–239. [Google Scholar] [CrossRef]
- Liang, F.; Ren, C.; Wang, J.; Wang, S.; Yang, L.; Han, X.; Chen, Y.; Tong, G.; Yang, G. The crosstalk between STAT3 and p53/RAS signaling controls cancer cell metastasis and cisplatin resistance via the Slug/MAPK/PI3K/AKT-mediated regulation of EMT and autophagy. Oncogenesis 2019, 8, 59. [Google Scholar] [CrossRef]
- Yue, P.; Zhang, X.; Paladino, D.; Sengupta, B.; Ahmad, S.; Holloway, R.W.; Ingersoll, S.B.; Turkson, J. Hyperactive EGF receptor, Jaks and Stat3 signaling promote enhanced colony-forming ability, motility and migration of cisplatin-resistant ovarian cancer cells. Oncogene 2011, 31, 2309–2322. [Google Scholar] [CrossRef] [PubMed]
- Martincuks, A.; Song, J.; Kohut, A.; Zhang, C.; Li, Y.-J.; Zhao, Q.; Mak, E.; Rodriguez-Rodriguez, L.; Yu, H.; Cristea, M. PARP Inhibition Activates STAT3 in Both Tumor and Immune Cells Underlying Therapy Resistance and Immunosuppression In Ovarian Cancer. Front. Oncol. 2021, 11, 724104. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, N.; Yang, H.; Shin, Y.K. Homologous Recombination Deficiency in Ovarian, Breast, Colorectal, Pancreatic, Non-Small Cell Lung and Prostate Cancers, and the Mechanisms of Resistance to PARP Inhibitors. Front. Oncol. 2022, 12, 880643. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhao, H.; Liu, H.; Wang, W.; Dong, H.; Zhao, C. RNA methylation, homologous recombination repair and therapeutic resistance. Biomed. Pharmacother. 2023, 166, 115409. [Google Scholar] [CrossRef]
- Feng, G.; Yuan, Y.; Li, Z.; Wang, L.; Zhang, B.; Luo, J.; Ji, J.; Kong, D. Replication fork stalling elicits chromatin compaction for the stability of stalling replication forks. Proc. Natl. Acad. Sci. USA 2019, 116, 14563–14572. [Google Scholar] [CrossRef]
- Adhikari, S.; Bhattacharya, A.; Adhikary, S.; Singh, V.; Gadad, S.S.; Roy, S.; Das, C. The paradigm of drug resistance in cancer: An epigenetic perspective. Biosci. Rep. 2022, 42. [Google Scholar] [CrossRef]
- Sadida, H.Q.; Abdulla, A.; Marzooqi, S.A.; Hashem, S.; Macha, M.A.; Akil, A.S.A.; Bhat, A.A. Epigenetic modifications: Key players in cancer heterogeneity and drug resistance. Transl. Oncol. 2024, 39, 101821. [Google Scholar] [CrossRef]
- Oza, A.M.; Cibula, D.; Benzaquen, A.O.; Poole, C.; Mathijssen, R.H.; Sonke, G.; Colombo, N.; Špaček, J.; Vuylsteke, P.; Hirte, H.; et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: A randomised phase 2 trial. Lancet Oncol. 2015, 16, 87–97. [Google Scholar] [CrossRef]
- Coleman, R.L.; Fleming, G.F.; Brady, M.F.; Swisher, E.M.; Steffensen, K.D.; Friedlander, M.; Okamoto, A.; Moore, K.N.; Efrat Ben-Baruch, N.; Werner, T.L.; et al. Veliparib with First-Line Chemotherapy and as Maintenance Therapy in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2403–2415. [Google Scholar] [CrossRef]
- Han, S.N.; Monk, B.J.; Gonzalez-Martin, A. Time to first subsequent therapy (TFST) and progression-free survival 2 (PFS2) from the phase 3 randomized, double-blind PRIMA/ENGOT-OV26/GOG-3012 study in patients with newly diagnosed ovarian cancer. Gynecol. Oncol. 2020, 159, 18–19. [Google Scholar] [CrossRef]
- Monk, B.J.; Parkinson, C.; Lim, M.C.; O’Malley, D.M.; Oaknin, A.; Wilson, M.K.; Coleman, R.L.; Lorusso, D.; Bessette, P.; Ghamande, S.; et al. A Randomized, Phase III Trial to Evaluate Rucaparib Monotherapy as Maintenance Treatment in Patients With Newly Diagnosed Ovarian Cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45). J. Clin. Oncol. 2022, 40, 3952–3964. [Google Scholar] [CrossRef] [PubMed]
- DiSilvestro, P.; Banerjee, S.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; et al. Overall Survival With Maintenance Olaparib at a 7-Year Follow-Up in Patients With Newly Diagnosed Advanced Ovarian Cancer and a BRCA Mutation: The SOLO1/GOG 3004 Trial. J. Clin. Oncol. 2022, 41, 609–617. [Google Scholar] [CrossRef]
- Ray-Coquard, I.L.; Leary, A.; Pignata, S.; Cropet, C.; Gonzalez Martin, A.J.; Bogner, G.; Yoshida, H.; Vergote, I.B.; Colombo, N.; Maenpaa, J.; et al. Final overall survival (OS) results from the phase III PAOLA-1/ENGOT-ov25 trial evaluating maintenance olaparib (ola) plus bevacizumab (bev) in patients (pts) with newly diagnosed advanced ovarian cancer (AOC). In Annals of Oncology; European Society of Medical Oncology Congress: Paris, France, 2022. [Google Scholar]
- Ray-Coquard, I.; Leary, A.; Pignata, S.; Cropet, C.; González-Martín, A.; Marth, C.; Nagao, S.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus bevacizumab first-line maintenance in ovarian cancer: Final overall survival results from the PAOLA-1/ENGOT-ov25 trial. Ann. Oncol. 2023, 34, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.J.; Barretina-Ginesta, M.P.; Pothuri, B.; Vergote, I.; Graybill, W.; Mirza, M.R.; McCormick, C.C.; Lorusso, D.; Moore, R.G.; Freyer, G.; et al. Niraparib first-line maintenance therapy in patients with newly diagnosed advanced ovarian cancer: Final overall survival results from the PRIMA/ENGOT-OV26/GOG-3012 trial. Ann. Oncol. 2024, 35, 981–992. [Google Scholar] [CrossRef]
- O’Malley, D.M.; Monk, B.J.; Lim, M.C.; Pradera, J.F.; Buscema, J.; Wilson, M.K.; De Vivo, R.; Herzog, T.J.; Zagouri, F.; Oza, A.M.; et al. Final safety results from ATHENA–MONO (GOG-3020/ENGOT-ov45), a randomized, placebo-controlled, double-blind, phase 3 trial evaluating rucaparib monotherapy as maintenance treatment in patients with newly diagnosed ovarian cancer. J. Clin. Oncol. 2024, 42 (Suppl. S16), 5554. [Google Scholar] [CrossRef]
- Kristeleit, R.; Lisyanskaya, A.; Fedenko, A.; Dvorkin, M.; de Melo, A.C.; Shparyk, Y.; Rakhmatullina, I.; Bondarenko, I.; Colombo, N.; Svintsitskiy, V.; et al. Rucaparib versus standard-of-care chemotherapy in patients with relapsed ovarian cancer and a deleterious BRCA1 or BRCA2 mutation (ARIEL4): An international, open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 465–478. [Google Scholar] [CrossRef]
- Oza, A.; Lisyanskaya, A.; Fedenko, A.; de Melo, A.; Shparik, Y.; Bondarenko, I.; Colombo, N.; Lorusso, D.; Cibula, D.; Póka, R.; et al. 518O Overall survival results from ARIEL4: A phase III study assessing rucaparib vs chemotherapy in patients with advanced, relapsed ovarian carcinoma and a deleterious BRCA1/2 mutation. Ann. Oncol. 2022, 33, S780. [Google Scholar] [CrossRef]
- Penson, R.; Valencia, R.V.; Colombo, N.; Leath, C.; Bidzinski, M.; Kim, J.-W.; Nam, J.-H.; Madry, R.; Hernández, C.; Mora, P.; et al. Final overall survival results from SOLO3: Phase III trial assessing olaparib monotherapy versus non-platinum chemotherapy in heavily pretreated patients with germline BRCA1—And/or BRCA2-mutated platinum-sensitive relapsed ovarian cancer (026). Gynecol. Oncol. 2022, 166, S19–S20. [Google Scholar] [CrossRef]
- Scambia, G.; Villalobos Valencia, R.; Colombo, N.; Cibula, D.; Leath, C.A.; Bidziński, M.; Kim, J.W.; Nam, J.H.; Madry, R.; Hernández, C.; et al. Olaparib as Treatment Versus Nonplatinum Chemotherapy in Patients with Platinum-Sensitive Relapsed Ovarian Cancer: Phase III SOLO3 Study Final Overall Survival Results. J. Clin. Oncol. 2025, 43, 1408–1416. [Google Scholar] [CrossRef]
- Moore, K.N.; Secord, A.A.; Geller, M.A.; Miller, D.S.; Cloven, N.; Fleming, G.F.; Wahner Hendrickson, A.E.; Azodi, M.; DiSilvestro, P.; Oza, A.M.; et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): A multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 636–648. [Google Scholar] [CrossRef]
- Mirza, M.R.; Lindahl, G.; Mahner, S.; Redondo, A.; Fabbro, M.; Rimel, B.J.; Herrstedt, J.; Oza, A.M.; Canzler, U.; Berek, J.S.; et al. Ad hoc Analysis of the Phase III ENGOT-OV16/NOVA Study: Niraparib Efficacy in Germline BRCA Wild-type Recurrent Ovarian Cancer with Homologous Recombination Repair Defects. Cancer Res. Commun. 2022, 2, 1462. [Google Scholar] [CrossRef]
- Luik, S. Dear Health Care Provider Letter (Niraparib): GSK. 2022. Available online: https://www.zejulahcp.com/content/dam/cf-pharma/hcp-zejulahcp-v2/en_US/pdf/ZEJULA%20(niraparib)%20Dear%20HCP%20Letter%20November%202022.pdf (accessed on 20 January 2025).
- Wu, X.; Zhu, J.; Yin, R.; Yang, J.; Liu, J.; Wang, J.; Wu, L.; Liu, Z.; Gao, Y.; Wang, D.; et al. Niraparib maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer using an individualized starting dose (NORA): A randomized, double-blind, placebo-controlled phase III trial. Ann. Oncol. 2021, 32, 512–521. [Google Scholar] [CrossRef]
- Ledermann, J.A.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.R.; Scambia, G.; et al. Rucaparib for patients with platinum-sensitive, recurrent ovarian carcinoma (ARIEL3): Post-progression outcomes and updated safety results from a randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 710–722. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.R.; Scambia, G.; et al. 2022-RA-249-ESGO Overall survival results from ariel3: A phase 3 randomised, double-blind study of rucaparib vs placebo following response to platinum-based chemotherapy for recurrent ovarian carcinoma. Int. J. Gynecol. Cancer 2022, 32 (Suppl. S2), A226. [Google Scholar]
- Pujade-Lauraine, E.; Ledermann, J.A.; Selle, F.; Gebski, V.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Poveda, A.; Pignata, S.; et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Poveda, A.; Floquet, A.; Ledermann, J.A.; Asher, R.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Pignata, S.; Friedlander, M.; et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A final analysis of a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2021, 22, 620–631. [Google Scholar] [CrossRef]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.L.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: A preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014, 15, 852–861. [Google Scholar] [CrossRef]
- Ledermann, J.A.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Overall survival in patients with platinum-sensitive recurrent serous ovarian cancer receiving olaparib maintenance monotherapy: An updated analysis from a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Oncol. 2016, 17, 1579–1589. [Google Scholar] [CrossRef]
- Parmar, M.K.; Ledermann, J.A.; Colombo, N.; du Bois, A.; Delaloye, J.F.; Kristensen, G.B.; Wheeler, S.; Swart, A.M.; Qian, W.; Torri, V.; et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: The ICON4/AGO-OVAR-2.2 trial. Lancet 2003, 361, 2099–2106. [Google Scholar]
- Pignata, S.; Lorusso, D.; Joly, F.; Gallo, C.; Colombo, N.; Sessa, C.; Bamias, A.; Salutari, V.; Selle, F.; Frezzini, S.; et al. Carboplatin-based doublet plus bevacizumab beyond progression versus carboplatin-based doublet alone in patients with platinum-sensitive ovarian cancer: A randomised, phase 3 trial. Lancet Oncol. 2021, 22, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Cecere, S.C.; Giannone, G.; Salutari, V.; Arenare, L.; Lorusso, D.; Ronzino, G.; Lauria, R.; Cormio, G.; Carella, C.; Scollo, P.; et al. Olaparib as maintenance therapy in patients with BRCA 1–2 mutated recurrent platinum sensitive ovarian cancer: Real world data and post progression outcome. Gynecol. Oncol. 2020, 156, 38–44. [Google Scholar] [CrossRef]
- Markman, M.; Markman, J.; Webster, K.; Zanotti, K.; Kulp, B.; Peterson, G.; Belinson, J. Duration of Response to Second-Line, Platinum-Based Chemotherapy for Ovarian Cancer: Implications for Patient Management and Clinical Trial Design. J. Clin. Oncol. 2004, 22, 3120–3125. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, K.; Gao, B.; Mapagu, C.; Fereday, S.; Emmanuel, C.; Alsop, K.; Traficante, N.; Harnett, P.R.; Bowtell, D.D.; Defazio, A. Response rates to second-line platinum-based therapy in ovarian cancer patients challenge the clinical definition of platinum resistance. Gynecol. Oncol. 2018, 150, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Frenel, J.; Kim, J.; Aryal, N.; Asher, R.; Berton, D.; Vidal, L.; Pautier, P.; Ledermann, J.; Penson, R.; Oza, A.; et al. Efficacy of subsequent chemotherapy for patients with BRCA1/2-mutated recurrent epithelial ovarian cancer progressing on olaparib versus placebo maintenance: Post-hoc analyses of the SOLO2/ENGOT Ov-21 trial. Ann. Oncol. 2022, 33, 1021–1028. [Google Scholar] [CrossRef]
- Dugan, K.; Kashi, P.K.; Gough, E.; Wallam, S.; Levinson, K.; Wethington, S.; Gaillard, S. Effectiveness of Platinum-Based Chemotherapy after Progression on Poly ADP Ribose Polymerase (PARP) Inhibitor in Epithelial Ovarian Cancer (042). Gynecol. Oncol. 2022, 166, S29–S30. [Google Scholar] [CrossRef]
- Xu-Vuillard, A.; Guerin-Charbonnel, C.; Bocquet, F.; Cheeseman, S.; Kubelac, P.; Zenatri, M.; Hall, G.; Achimas-Cadariu, P.; Hanvic, B.; Fenton, H.; et al. Efficacy of chemotherapy after progression during or following PARPi exposure in ovarian cancer. ESMO Open 2024, 9, 103694. [Google Scholar] [CrossRef]
- Rose, P.G.; Yao, M.; Chambers, L.M.; Mahdi, H.; DeBernardo, R.; Michener, C.M.; AlHilli, M.; Ricci, S.; Vargas, R. PARP inhibitors decrease response to subsequent platinum-based chemotherapy in patients with BRCA mutated ovarian cancer. Anti-Cancer Drugs 2021, 32, 1086–1092. [Google Scholar] [CrossRef]
- Park, J.; Kim, S.I.; Jeong, S.Y.; Kim, Y.; Bookman, M.A.; Kim, J.W.; Kim, B.G.; Lee, J.Y. Second-line olaparib maintenance therapy is associated with poor response to subsequent chemotherapy in BRCA1/2-mutated epithelial ovarian cancer: A multicentre retrospective study. Gynecol. Oncol. 2022, 165, 97–104. [Google Scholar] [CrossRef]
- Baert, T.; Ataseven, B.; Bommert, M.; Concin, N.; Frindte, J.; Schneider, S.; Harter, P.; du Bois, A.; Heitz, F. 828P Expected versus observed response to platinum-based chemotherapy after poly (ADP-ribose) polymerase inhibitor treatment for relapsed ovarian cancer. Ann. Oncol. 2020, 31, S624. [Google Scholar] [CrossRef]
- Salarich, A.P.; García, I.T.; Burdalo, B.P.; Gil-Martin, M.; Piulats, J.; Planas, C.F.; Ginesta, M.B.; Diez, A.F.; Rincon, L.N.; Badia, A.P.; et al. 824P Real-world-data (RWD) on platinum (Pt) outcomes after PARP inhibitors (PARPi) progression in high grade serous ovarian cancer (HGSOC) patients (p). Ann. Oncol. 2020, 31, S622. [Google Scholar] [CrossRef]
- Gadducci, A.; Cosio, S.; Landoni, F.; Lissoni, A.A.; Zola, P.; Laudani, M.E.; Ardizzoia, A.; Gambino, A.; Sartori, E. Response to Chemotherapy and Clinical Outcome of Patients With Recurrent Epithelial Ovarian Cancer After PARP Inhibitor Maintenance Treatment: A Multicenter Retrospective Italian Study. Anticancer. Res. 2022, 42, 2017–2022. [Google Scholar] [CrossRef] [PubMed]
- Romeo, M.; Gil-Martín, M.; Gaba, L.; Teruel, I.; Taus, Á.; Fina, C.; Masvidal, M.; Murata, P.; Fernández-Plana, J.; Martínez, A.; et al. Multicenter Real-World Data of Subsequent Chemotherapy after Progression to PARP Inhibitors in a Maintenance Relapse Setting. Cancers 2022, 14, 4414. [Google Scholar] [CrossRef]
- Rimel, B.; Secord, A.; Geller, M.; Miller, D.; Cloven, N.; Fleming, G.; Hendrickson, A.W.; Azodi, M.; DiSilvestro, P.; Oza, A.; et al. Safety and Efficacy Results of Retreatment With a PARP Inhibitor Monotherapy in Late-Line Recurrent Ovarian Cancer: Results From a Subset of the QUADRA Trial. Gynecol. Oncol. 2020, 156, e4–e5. [Google Scholar] [CrossRef]
- Yubero, A.; Estévez, P.; Barquín, A.; Sánchez, L.; Santaballa, A.; Pajares, B.; Reche, P.; Salvador, C.; Manso, L.; Márquez, R.; et al. Rucaparib for PARP inhibitor-pretreated ovarian cancer: A GEICO retrospective subgroup analysis from the Spanish Rucaparib Access Program. Gynecol. Oncol. Rep. 2023, 48, 101211. [Google Scholar] [CrossRef] [PubMed]
- Essel, K.; Behbakht, K.; Lai, T.; Hand, L.; Evans, E.; Dvorak, J.; Ding, K.; Konecny, G.; Moore, K. PARPi after PARPi in epithelial ovarian cancer. Gynecol. Oncol. Rep. 2021, 35, 100699. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Selle, F.; Scambia, G.; Asselain, B.; Marmé, F.; Lindemann, K.; Colombo, N.; Mądry, R.; Glasspool, R.; Vergote, I.; et al. Maintenance olaparib rechallenge in patients with platinum-sensitive relapsed ovarian cancer previously treated with a PARP inhibitor (OReO/ENGOT-ov38): A phase IIIb trial. Ann. Oncol. 2023, 34, 1152–1164. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Guo, B.; Li, X.; Tuersun, R.; Cao, Y.; Li, J.; Liu, J.; Li, S.; Liu, T.; et al. Efficacy of PARPi re-maintenance therapy for recurrent ovarian cancer. Front. Oncol. 2025, 14, 1512339. [Google Scholar] [CrossRef]
- Bonadio, R.C.; Estevez-Diz, M.D.P. Perspectives on PARP Inhibitor Combinations for Ovarian Cancer. Front. Oncol. 2021, 11, 754524. [Google Scholar] [CrossRef]
- Trillsch, F.; Okamoto, A.; Kim, J.W.; Reuss, A.; Rubio Pérez, M.J.; Vardar, M.A.; Salutari, V.; Frenel, J.-S.; Kärkkäinen, H.; Colombo, N.; et al. 43O Durvalumab (D) + carboplatin/paclitaxel (CP) + bevacizumab (B) followed by D, B + olaparib (O) maintenance (mtx) for newly diagnosed advanced ovarian cancer (AOC) without a tumour BRCA1/BRCA2 mutation (non-tBRCAm): Updated results from DUO-O. ESMO Open 2024, 9, 103550. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, D.; Wang, J.; Wang, W.; Li, Q.; Tang, J.; Huang, Y.; An, R.; Duan, W.; Wang, L.; et al. 766P A phase II trial of fuzuloparib in combination with apatinib vs. fuzuloparib alone for recurrent ovarian cancer (OC). Ann. Oncol. 2024, 35, S579–S580. [Google Scholar] [CrossRef]
- Kim, Y.N.; Park, B.; Joung, J.-G.; Kim, J.-W.; Kim, B.-G.; Kim, S.W.; Kim, H.S.; Choi, C.H.; Lim, M.C.; Ngoi, N.Y.; et al. Triplet maintenance (olaparib, pembrolizumab, and bevacizumab) in BRCA non-mutated patients with platinum-sensitive recurrent ovarian cancer: A multi-center, single-arm phase II study (OPEB-01). J. Clin. Oncol. 2023, 41 (Suppl. S16), 5584. [Google Scholar] [CrossRef]
- Yap, T.A.; Im, S.-A.; Schram, A.M.; Sharp, A.; Balmana, J.; Baird, R.D.; Brown, J.S.; Schwaederle, M.; Pilling, E.A.; Moorthy, G.; et al. Abstract CT007: PETRA: First in class, first in human trial of the next generation PARP1-selective inhibitor AZD5305 in patients (pts) with BRCA1/2, PALB2 or RAD51C/D mutations. Cancer Res. 2022, 82 (Suppl. S12), CT007. [Google Scholar] [CrossRef]
- Simpkins, F.; Nasioudis, D.; Wethington, S.L.; Martin, L.P.; Tanyi, J.L.; Latif, N.A.; Torigian, D.A.; Omran, D.K.; Rodriguez, D.; Smith, S.; et al. Combination ATR and PARP Inhibitor (CAPRI): A phase 2 study of ceralasertib plus olaparib in patients with recurrent, platinum-sensitive epithelial ovarian cancer (cohort A). J. Clin. Oncol. 2024, 42, 5510. [Google Scholar] [CrossRef]
- Westin, S.N.; Coleman, R.L.; Fellman, B.M.; Yuan, Y.; Sood, A.K.; Soliman, P.T.; Wright, A.A.; Horowitz, N.S.; Campos, S.M.; Konstantinopoulos, P.A.; et al. EFFORT: EFFicacy Of adavosertib in parp ResisTance: A randomized two-arm non-comparative phase II study of adavosertib with or without olaparib in women with PARP-resistant ovarian cancer. J. Clin. Oncol. 2021, 39, 5505. [Google Scholar] [CrossRef]
- Nicum, S.; Ledermann, J.; Mileshkin, L.; Jayson, G.; Gourley, C.; Michael, A.; Lord, R.; Mackay, H.; Hall, M.; Tookman, L.; et al. LBA33 ICON9: International phase III randomized study to evaluate the efficacy of maintenance therapy with olaparib and cediranib or olaparib alone in patients with relapsed platinum-sensitive ovarian cancer following a response to platinum-based chemotherapy. Ann. Oncol. 2024, 35, S1225–S1226. [Google Scholar] [CrossRef]
- Lee, J.-M.; Brady, M.F.; Miller, A.; Moore, R.G.; MacKay, H.; McNally, L.; Lea, J.; Street, D.; Lheureux, S.; McDonald, M.E.; et al. Cediranib and Olaparib Combination Compared With Cediranib or Olaparib Alone, or Chemotherapy in Platinum-Resistant or Primary Platinum-Refractory Ovarian Cancer: NRG-GY005. J. Clin. Oncol. 2024, 42, 4305–4316. [Google Scholar] [CrossRef]
- González-Martín, A.; Rubio, M.J.; Heitz, F.; Christensen, R.D.; Colombo, N.; Van Gorp, T.; Romeo, M.; Ray-Coquard, I.; Gaba, L.; Leary, A.; et al. Atezolizumab Combined With Platinum and Maintenance Niraparib for Recurrent Ovarian Cancer With a Platinum-Free Interval >6 Months: ENGOT-OV41/GEICO 69-O/ANITA Phase III Trial. J. Clin. Oncol. 2024, 42, 4294–4304. [Google Scholar] [CrossRef]
- Lee, J.-M.; Miller, A.; Rose, P.; AlHilli, M.; Washington, C.; John, V.; Shah, C.; Matsuo, K.; Siedel, J.; Miller, D.; et al. 746MO Randomized phase II trial of durvalumab in combination with olaparib and cediranib (DOC) compared to olaparib and cediranib (OC) or durvalumab and cediranib (DC) or standard of care chemotherapy (SOC) in platinum-resistant ovarian cancer with prior bevacizumab (NRG-GY023). Ann. Oncol. 2023, 34, S511. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).