Ultrastructural and Molecular Analysis of Vascular Smooth Muscle Cells During the Switch from a Physiological to a Pathological Phenotype
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Maintenance
2.2. Sample Processing for Transmission Electron Microscopy (TEM)
2.3. Cellular Calcification Induction
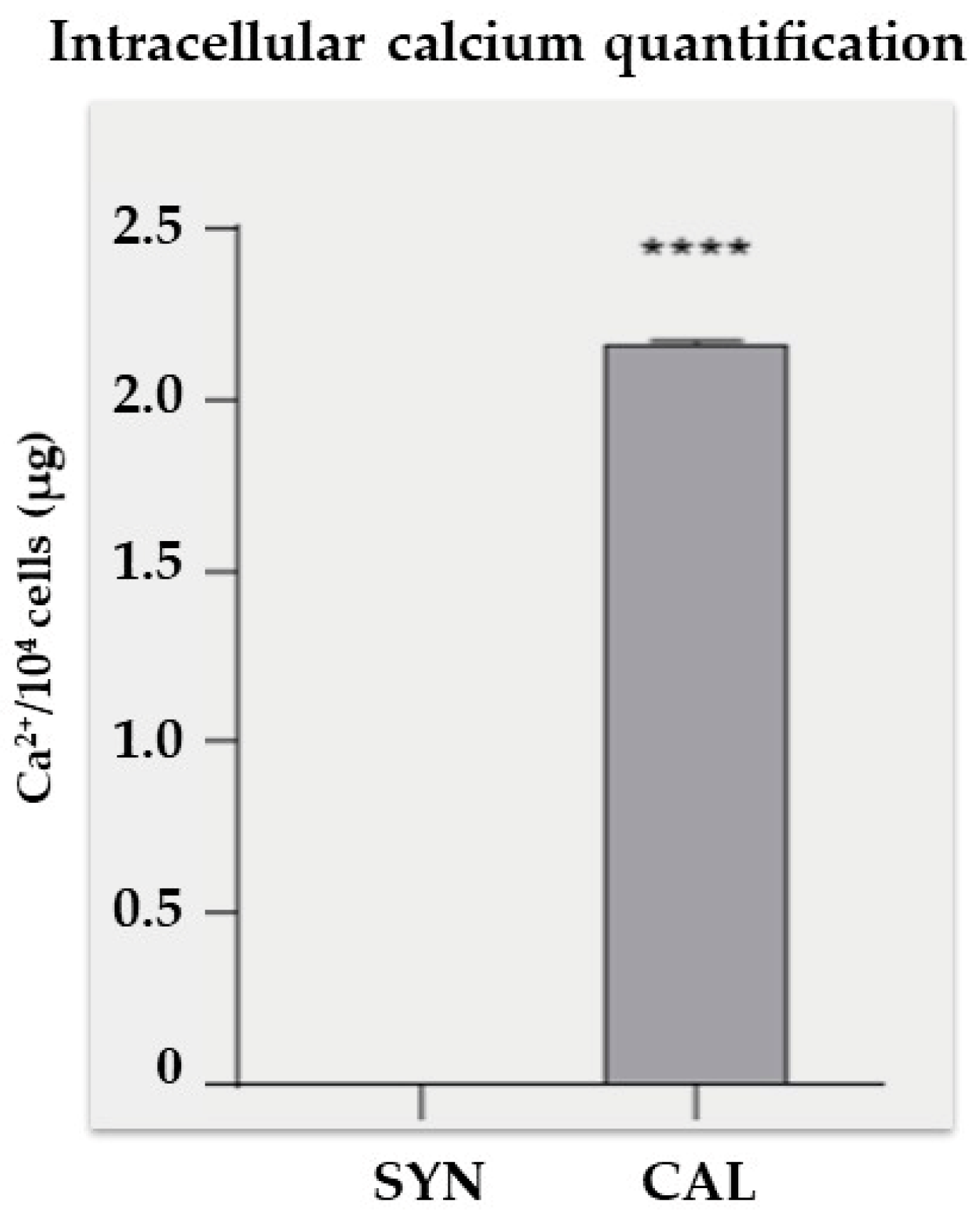
2.4. Cell Lysis and Intracellular Calcium Quantification
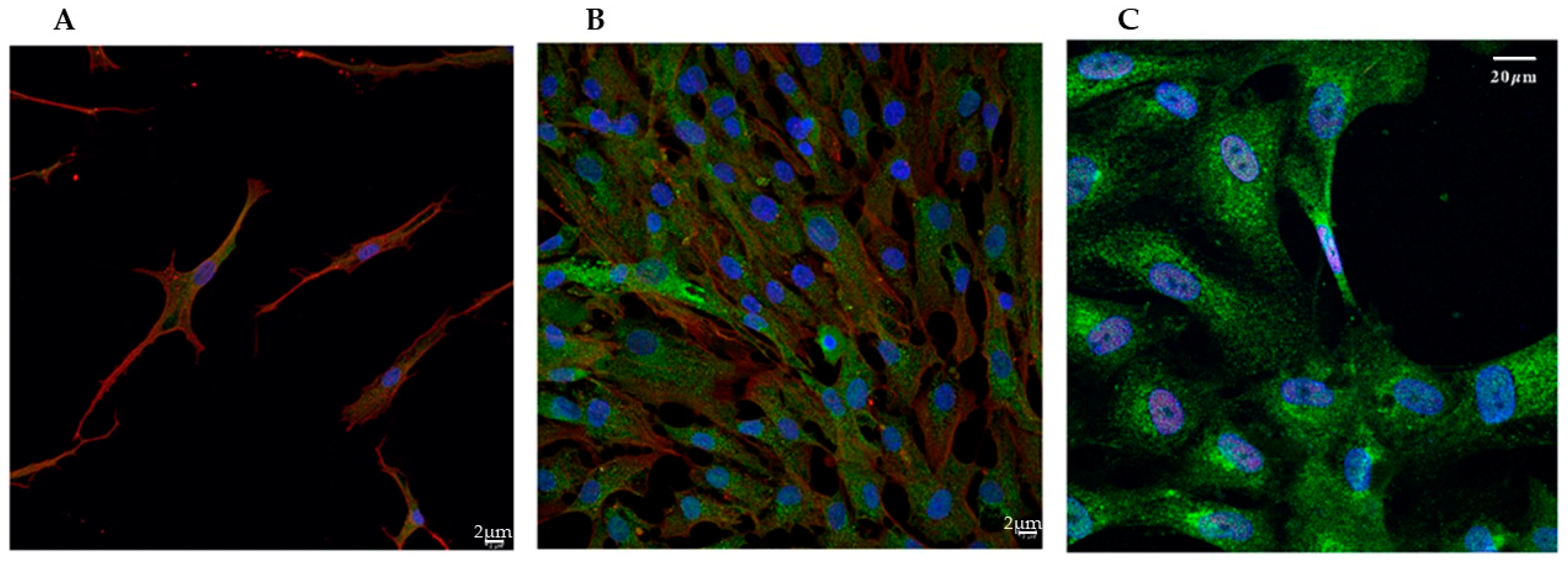
2.5. Immunofluorescence Analysis
2.6. Total RNA Extraction, Quantification, and Quality Assessment, cDNA Synthesis
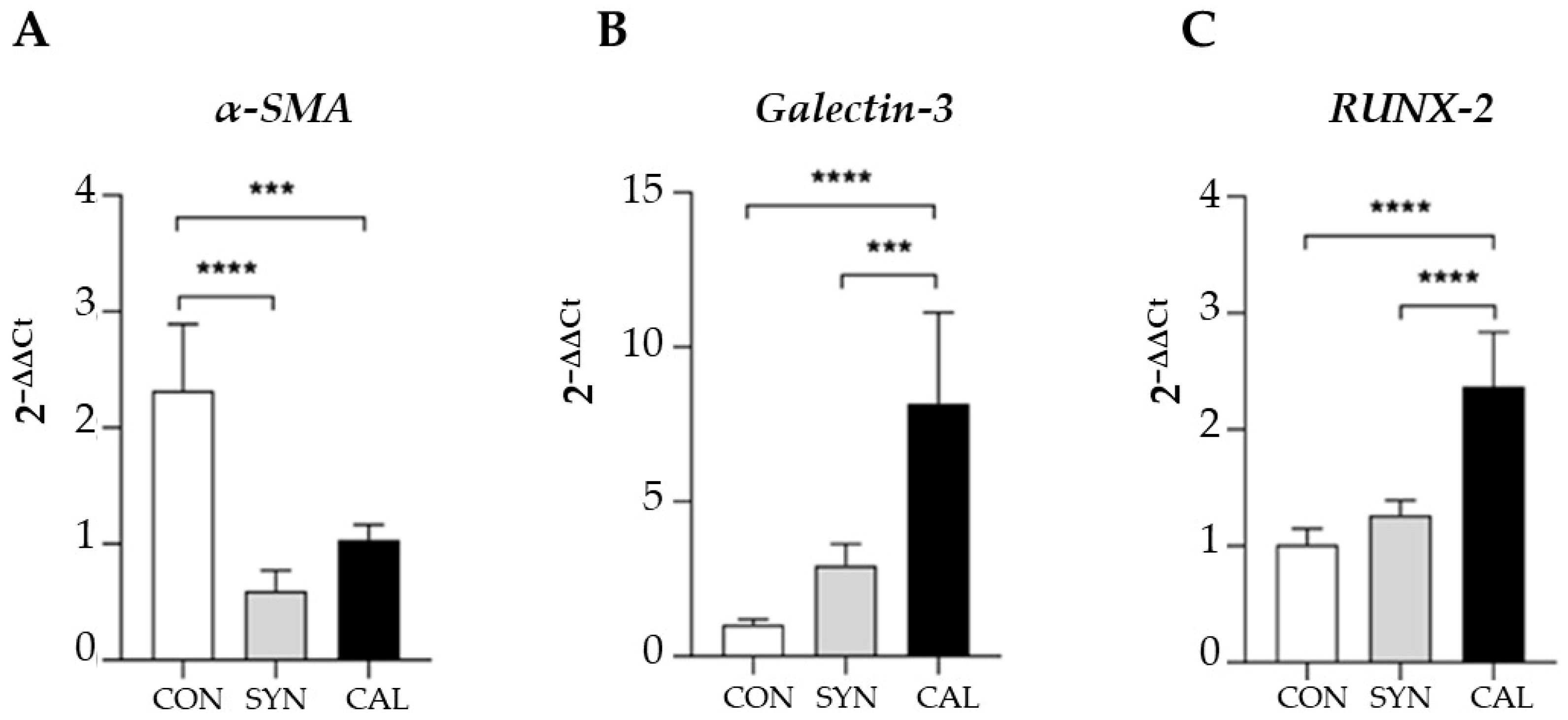
2.7. Real-Time qPCR Analysis
2.8. Statistical Analysis
3. Results and Discussion
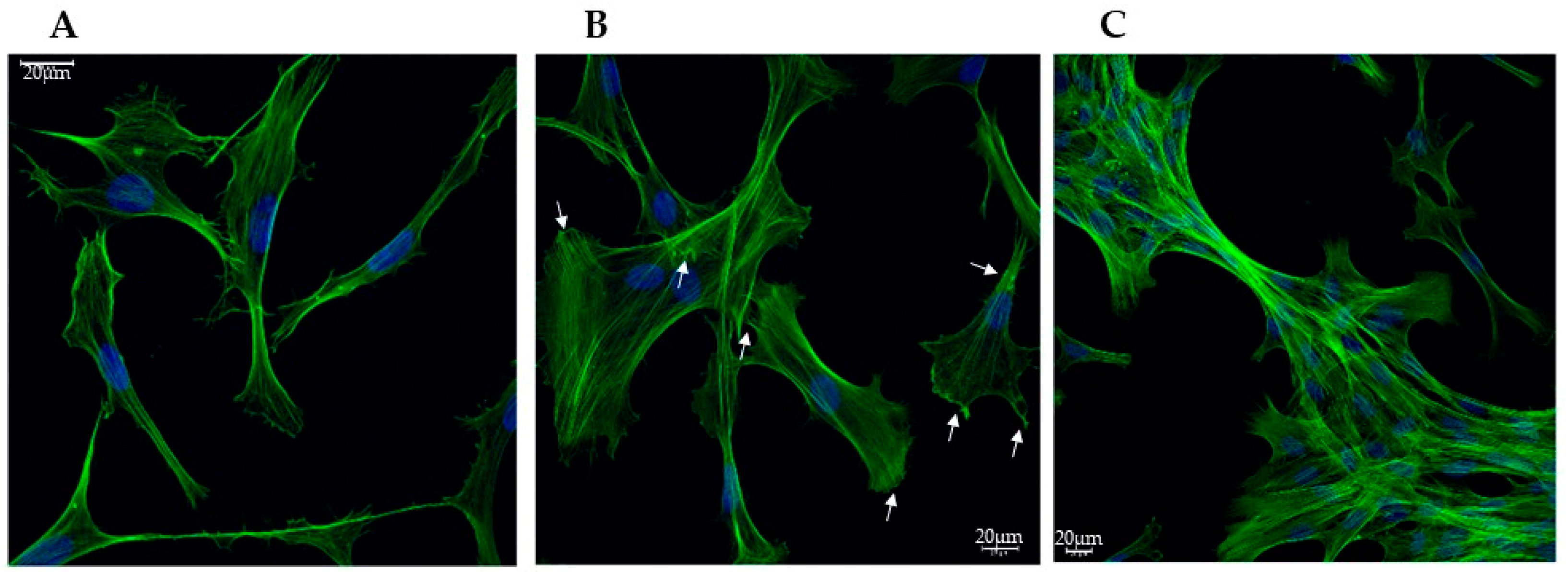
3.1. Confocal Imaging Reveals Morphological Changes During VSMCs Phenotypic Switch
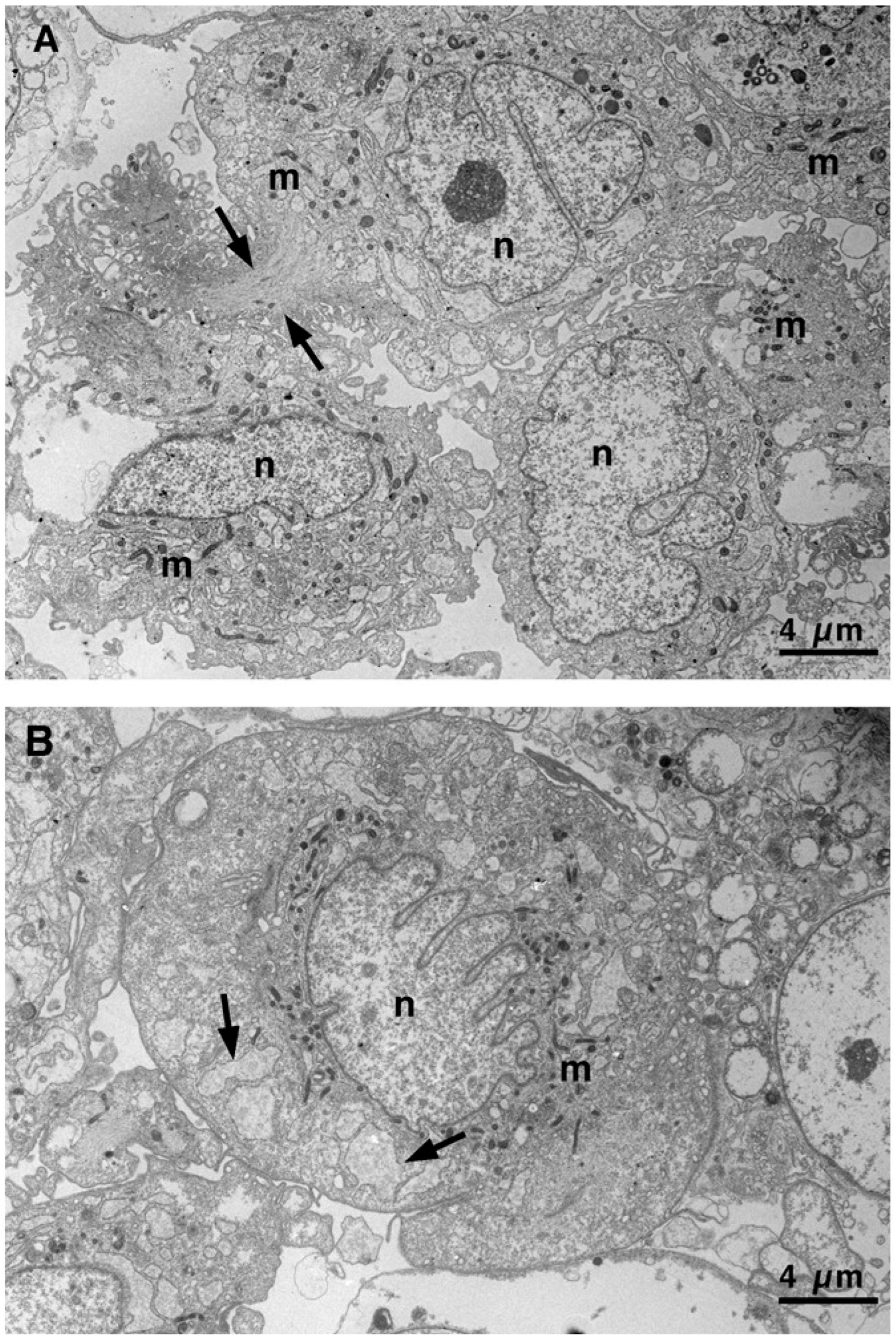
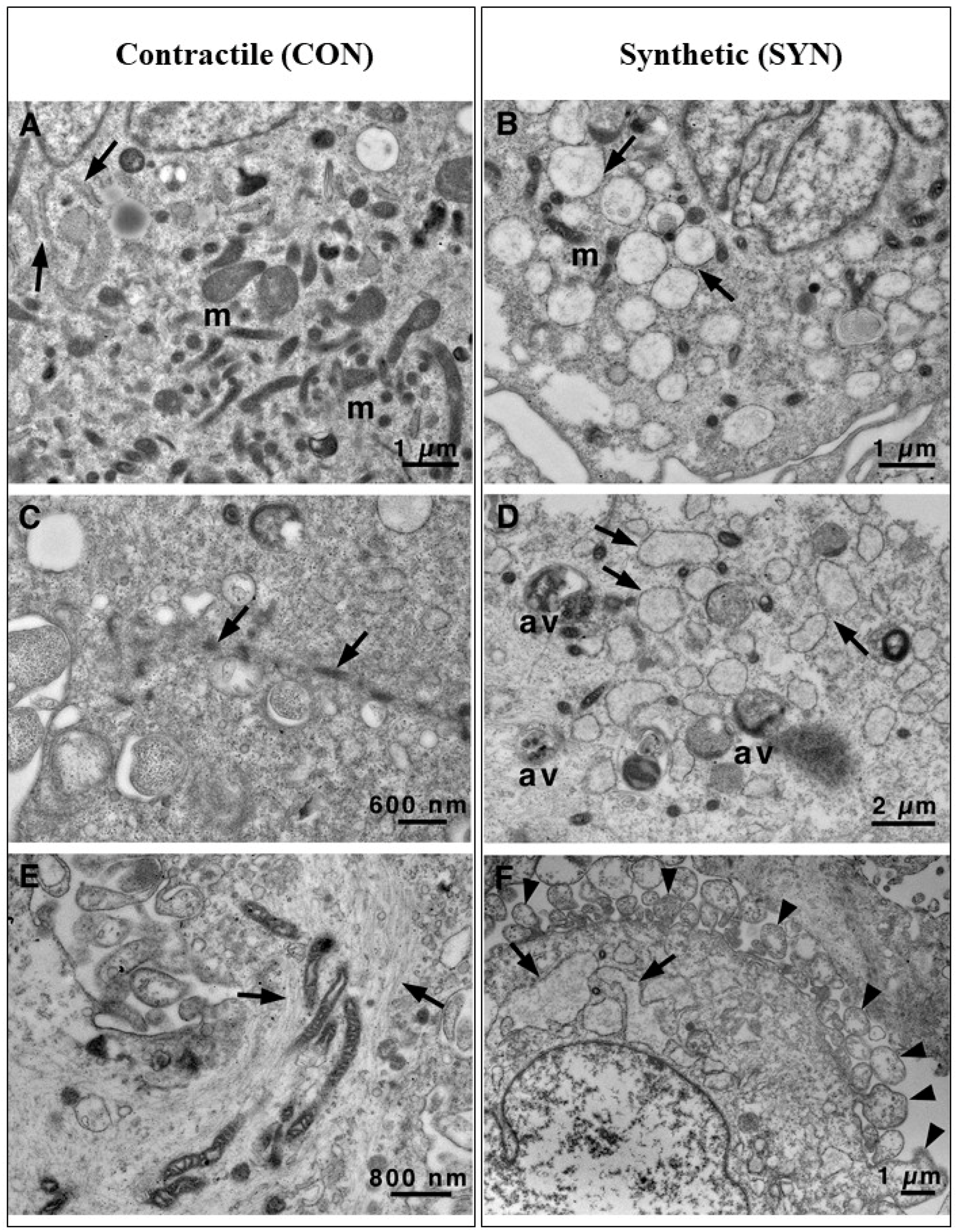
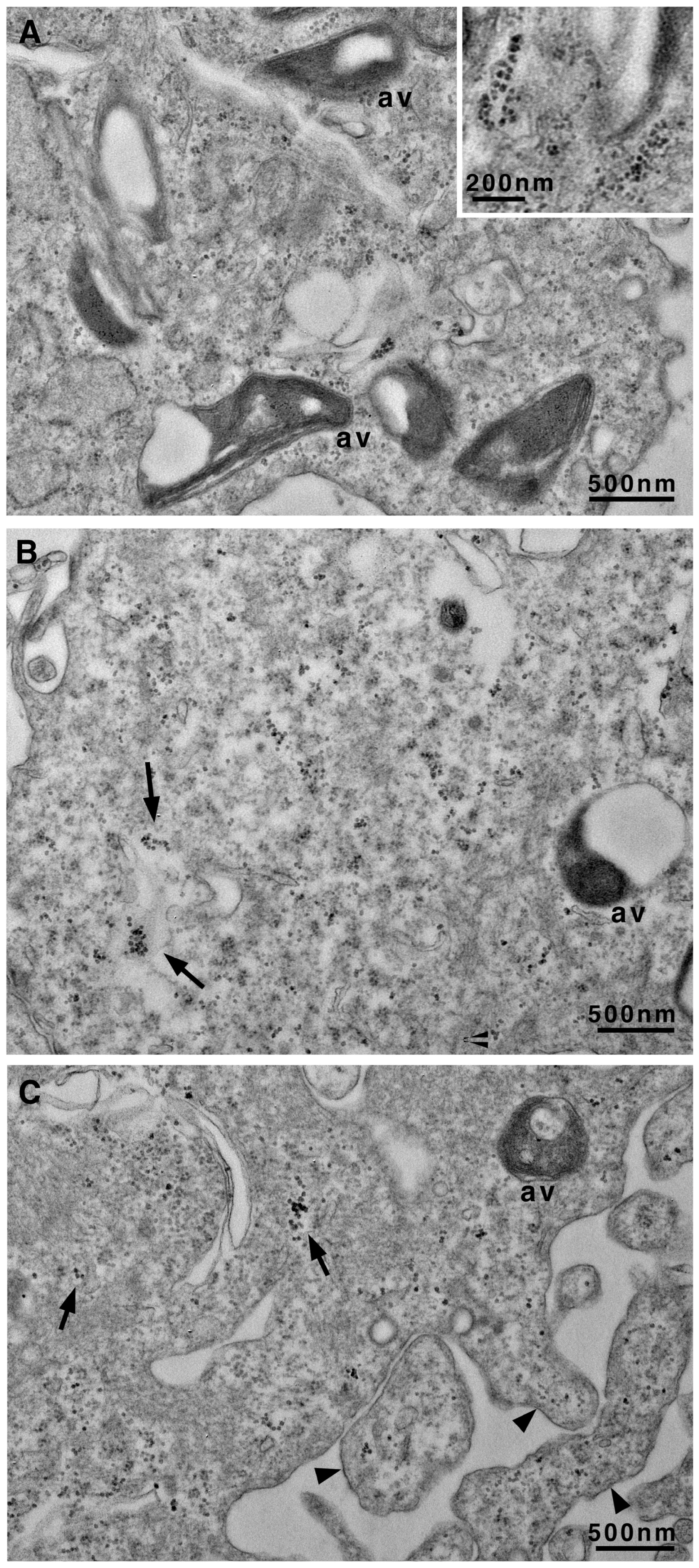
3.2. Transmission Electron Microscopy Images Allow Us to Look Deeply Inside Vascular Smooth Cells
4. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-SMA | Alpha-Smooth Muscle Actin |
| BSA | Bovine Serum Albumin |
| CAL | Calcified |
| CON | Contractile |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FBS | Fetal Bovine Serum |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
| HCASMCs | Human Coronary Artery Smooth Muscle Cells |
| LSCM | Scanning Confocal Microscopy |
| PBS | Phosphate-Buffered Saline |
| RER | Rough Endoplasmic Reticulum |
| RUNX-2 | Runt-Related Transcription Factor 2 |
| SYN | Synthetic |
| TEM | Transmission Electron Microscopy |
| VSMCs | Vascular Smooth Muscle Cells |
References
- Libby, P. Inflammation in Atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis—An Inflammatory Disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.; Mieremet, A.; De Vries, C.J.M.; Micha, D.; De Waard, V. Six Shades of Vascular Smooth Muscle Cells Illuminated by KLF4 (Krüppel-Like Factor 4). Arter. Thromb. Vasc. Biol. 2021, 41, 2693–2707. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Xuan, X.; Hu, J.; Zhang, R.; Jin, H.; Dong, H. How Vascular Smooth Muscle Cell Phenotype Switching Contributes to Vascular Disease. Cell Commun. Signal 2022, 20, 180. [Google Scholar] [CrossRef]
- Chen, R.; McVey, D.G.; Shen, D.; Huang, X.; Ye, S. Phenotypic Switching of Vascular Smooth Muscle Cells in Atherosclerosis. J. Am. Heart Assoc. 2023, 12, e031121. [Google Scholar] [CrossRef]
- Gomez, D.; Owens, G.K. Smooth Muscle Cell Phenotypic Switching in Atherosclerosis. Cardiovasc. Res. 2012, 95, 156–164. [Google Scholar] [CrossRef]
- Petsophonsakul, P.; Furmanik, M.; Forsythe, R.; Dweck, M.; Schurink, G.W.; Natour, E.; Reutelingsperger, C.; Jacobs, M.; Mees, B.; Schurgers, L. Role of Vascular Smooth Muscle Cell Phenotypic Switching and Calcification in Aortic Aneurysm Formation. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1351–1368. [Google Scholar] [CrossRef]
- Zhang, F.; Guo, X.; Xia, Y.; Mao, L. An Update on the Phenotypic Switching of Vascular Smooth Muscle Cells in the Pathogenesis of Atherosclerosis. Cell. Mol. Life Sci. 2022, 79, 6. [Google Scholar] [CrossRef]
- Bochaton-Piallat, M.-L.; Ropraz, P.; Gabbiani, F.; Gabbiani, G. Phenotypic Heterogeneity of Rat Arterial Smooth Muscle Cell Clones: Implications for the Development of Experimental Intimal Thickening. Arter. Thromb. Vasc. Biol. 1996, 16, 815–820. [Google Scholar] [CrossRef]
- Worth, N.F.; Rolfe, B.E.; Song, J.; Campbell, G.R. Vascular Smooth Muscle Cell Phenotypic Modulation in Culture Is Associated with Reorganisation of Contractile and Cytoskeletal Proteins. Cell Motil. Cytoskelet. 2001, 49, 130–145. [Google Scholar] [CrossRef]
- Reutelingsperger, C.; Schurgers, L. Coronary Artery Calcification. JACC Cardiovasc. Imaging 2018, 11, 1324–1326. [Google Scholar] [CrossRef] [PubMed]
- Shioi, A.; Ikari, Y. Plaque Calcification During Atherosclerosis Progression and Regression. J. Atheroscler. Thromb. 2018, 25, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Bobryshev, Y.V. Transdifferentiation of Smooth Muscle Cells into Chondrocytes in Atherosclerotic Arteries In Situ: Implications for Diffuse Intimal Calcification. J. Pathol. 2005, 205, 641–650. [Google Scholar] [CrossRef]
- Durham, A.L.; Speer, M.Y.; Scatena, M.; Giachelli, C.M.; Shanahan, C.M. Role of Smooth Muscle Cells in Vascular Calcification: Implications in Atherosclerosis and Arterial Stiffness. Cardiovasc. Res. 2018, 114, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Tyson, K.L.; Reynolds, J.L.; McNair, R.; Zhang, Q.; Weissberg, P.L.; Shanahan, C.M. Osteo/Chondrocytic Transcription Factors and Their Target Genes Exhibit Distinct Patterns of Expression in Human Arterial Calcification. Arter. Thromb. Vasc. Biol. 2003, 23, 489–494. [Google Scholar] [CrossRef]
- Shanahan, C.M.; Crouthamel, M.H.; Kapustin, A.; Giachelli, C.M. Arterial Calcification in Chronic Kidney Disease: Key Roles for Calcium and Phosphate. Circ. Res. 2011, 109, 697–711. [Google Scholar] [CrossRef]
- Ceccherini, E.; Gisone, I.; Persiani, E.; Ippolito, C.; Falleni, A.; Cecchettini, A.; Vozzi, F. Novel in Vitro Evidence on the Beneficial Effect of Quercetin Treatment in Vascular Calcification. Front. Pharmacol. 2024, 15, 1330374. [Google Scholar] [CrossRef]
- Pugliese, G.; Iacobini, C.; Fantauzzi, C.B.; Menini, S. The Dark and Bright Side of Atherosclerotic Calcification. Atherosclerosis 2015, 238, 220–230. [Google Scholar] [CrossRef]
- Tian, L.; Chen, K.; Cao, J.; Han, Z.; Wang, Y.; Gao, L.; Fan, Y.; Wang, C. Galectin-3 Induces the Phenotype Transformation of Human Vascular Smooth Muscle Cells via the Canonical Wnt Signaling. Mol. Med. Rep. 2017, 15, 3840–3846. [Google Scholar] [CrossRef]
- Alencar, G.F.; Owsiany, K.M.; Karnewar, S.; Sukhavasi, K.; Mocci, G.; Nguyen, A.T.; Williams, C.M.; Shamsuzzaman, S.; Mokry, M.; Henderson, C.A.; et al. Stem Cell Pluripotency Genes Klf4 and Oct4 Regulate Complex SMC Phenotypic Changes Critical in Late-Stage Atherosclerotic Lesion Pathogenesis. Circulation 2020, 142, 2045–2059. [Google Scholar] [CrossRef]
- Giusti, I.; Bianchi, S.; Nottola, S.A.; Macchiarelli, G.; Dolo, V. Clinical Electron Microscopy in the Study of Human Ovarian Tissues. Euromediterr. Biomed. J. 2019, 14, 145–151. [Google Scholar] [CrossRef]
- Franken, L.E.; Grünewald, K.; Boekema, E.J.; Stuart, M.C.A. A Technical Introduction to Transmission Electron Microscopy for Soft-Matter: Imaging, Possibilities, Choices, and Technical Developments. Small 2020, 16, 1906198. [Google Scholar] [CrossRef] [PubMed]
- Torge, D.; Bernardi, S.; Ciciarelli, G.; Macchiarelli, G.; Bianchi, S. Dedicated Protocol for Ultrastructural Analysis of Farmed Rainbow Trout (Oncorhynchus mykiss) Tissues with Red Mark Syndrome: The Skin—Part One. MPs 2024, 7, 37. [Google Scholar] [CrossRef]
- Ferrucci, M.; Lenzi, P.; Lazzeri, G.; Busceti, C.L.; Frati, A.; Puglisi-Allegra, S.; Fornai, F. Combined Light and Electron Microscopy (CLEM) to Quantify Methamphetamine-Induced Alpha-Synuclein-Related Pathology. J. Neural Transm. 2024, 131, 335–358. [Google Scholar] [CrossRef] [PubMed]
- Falleni, A.; Moscato, S.; Fulvio, G.; Polizzi, E.; Bernardeschi, M.; Bianchi, F.; Donati, V.; Cabiati, M.; Ippolito, C.; Del Ry, S.; et al. Connexin Expression in Human Minor Salivary Glands: An Immunohistochemical Microscopy Study. Molecules 2022, 27, 5926. [Google Scholar] [CrossRef]
- Poliseno, L.; Cecchettini, A.; Mariani, L.; Evangelista, M.; Ricci, F.; Giorgi, F.; Citti, L.; Rainaldi, G. Resting Smooth Muscle Cells as a Model for Studying Vascular Cell Activation. Tissue Cell 2006, 38, 111–120. [Google Scholar] [CrossRef]
- Persiani, E.; Ceccherini, E.; Gisone, I.; Cecchettini, A.; Vozzi, F. Protocol to Generate an in Vitro Model to Study Vascular Calcification Using Human Endothelial and Smooth Muscle Cells. STAR Protoc. 2023, 4, 102328. [Google Scholar] [CrossRef]
- Ceccherini, E.; Persiani, E.; Cabiati, M.; Guiducci, L.; Del Ry, S.; Gisone, I.; Falleni, A.; Cecchettini, A.; Vozzi, F. A Dynamic Cellular Model as an Emerging Platform to Reproduce the Complexity of Human Vascular Calcification In Vitro. Int. J. Mol. Sci. 2024, 25, 7427. [Google Scholar] [CrossRef]
- Ni, D.; Mo, Z.; Yi, G. Recent Insights into Atherosclerotic Plaque Cell Autophagy. Exp. Biol. Med. 2021, 246, 2553–2558. [Google Scholar] [CrossRef]
- Zheng, Y.; Tian, C.; Meng, Y.; Qin, Y.; Du, Y.; Du, J.; Li, H. Osteopontin Stimulates Autophagy via Integrin/CD44 and P38 MAPK Signaling Pathways in Vascular Smooth Muscle Cells. J. Cell. Physiol. 2012, 227, 127–135. [Google Scholar] [CrossRef]
- García-Miguel, M.; Riquelme, J.A.; Norambuena-Soto, I.; Morales, P.E.; Sanhueza-Olivares, F.; Nuñez-Soto, C.; Mondaca-Ruff, D.; Cancino-Arenas, N.; San Martín, A.; Chiong, M. Autophagy Mediates Tumor Necrosis Factor-α-Induced Phenotype Switching in Vascular Smooth Muscle A7r5 Cell Line. PLoS ONE 2018, 13, e0197210. [Google Scholar] [CrossRef] [PubMed]
- Rattazzi, M.; Bennett, B.J.; Bea, F.; Kirk, E.A.; Ricks, J.L.; Speer, M.; Schwartz, S.M.; Giachelli, C.M.; Rosenfeld, M.E. Calcification of Advanced Atherosclerotic Lesions in the Innominate Arteries of ApoE-Deficient Mice: Potential Role of Chondrocyte-Like Cells. Arter. Thromb. Vasc. Biol. 2005, 25, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, I.-K.; Jeon, J.-H. Vascular Calcification-New Insights Into Its Mechanism. Int. J. Mol. Sci. 2020, 21, 2685. [Google Scholar] [CrossRef]
- Speer, M.Y.; Yang, H.-Y.; Brabb, T.; Leaf, E.; Look, A.; Lin, W.-L.; Frutkin, A.; Dichek, D.; Giachelli, C.M. Smooth Muscle Cells Give Rise to Osteochondrogenic Precursors and Chondrocytes in Calcifying Arteries. Circ. Res. 2009, 104, 733–741. [Google Scholar] [CrossRef]
- Perisic Matic, L.; Rykaczewska, U.; Razuvaev, A.; Sabater-Lleal, M.; Lengquist, M.; Miller, C.L.; Ericsson, I.; Röhl, S.; Kronqvist, M.; Aldi, S.; et al. Phenotypic Modulation of Smooth Muscle Cells in Atherosclerosis Is Associated with Downregulation of LMOD1, SYNPO2, PDLIM7, PLN, and SYNM. Arter. Thromb. Vasc. Biol. 2016, 36, 1947–1961. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons From Sudden Coronary Death: A Comprehensive Morphological Classification Scheme for Atherosclerotic Lesions. Arter. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef]
- Ceccherini, E.; Cecchettini, A.; Gisone, I.; Persiani, E.; Morales, M.A.; Vozzi, F. Vascular Calcification: In Vitro Models under the Magnifying Glass. Biomedicines 2022, 10, 2491. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Millican, R.; Lynd, T.; Gangasani, M.; Malhotra, S.; Sherwood, J.; Hwang, P.T.; Cho, Y.; Brott, B.C.; et al. Recent Progress in in Vitro Models for Atherosclerosis Studies. Front. Cardiovasc. Med. 2022, 8, 790529. [Google Scholar] [CrossRef]
- Reed, E.; Fellows, A.; Lu, R.; Rienks, M.; Schmidt, L.; Yin, X.; Duregotti, E.; Brandt, M.; Krasemann, S.; Hartmann, K.; et al. Extracellular Matrix Profiling and Disease Modelling in Engineered Vascular Smooth Muscle Cell Tissues. Matrix Biol. Plus 2022, 16, 100122. [Google Scholar] [CrossRef]
| Gene | Primer Sequence (5′–3′) |
|---|---|
| GAPDH | FWD: ACATCGCTCAGACACCATGG REV: GACGGTGCCATGGAATTTGC |
| RUNX2 | FWD: GATTCTTAACCAACCAGCCTTACC REV: AGTGATGTCATTCTGCTCCTCTAA |
| α-SMA | FWD: AGAGTTACGAGTTGCCTGATG REV: GATGAAGGATGGCTGGAACA |
| Galectin-3 | FWD: GCCACTGATTGTGCCTTATT REV: CCGTGCCCAGAATTGTTAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Persiani, E.; Ceccherini, E.; Falleni, A.; Gisone, I.; Ippolito, C.; Mattii, L.; Cecchettini, A.; Vozzi, F. Ultrastructural and Molecular Analysis of Vascular Smooth Muscle Cells During the Switch from a Physiological to a Pathological Phenotype. Biomedicines 2025, 13, 1127. https://doi.org/10.3390/biomedicines13051127
Persiani E, Ceccherini E, Falleni A, Gisone I, Ippolito C, Mattii L, Cecchettini A, Vozzi F. Ultrastructural and Molecular Analysis of Vascular Smooth Muscle Cells During the Switch from a Physiological to a Pathological Phenotype. Biomedicines. 2025; 13(5):1127. https://doi.org/10.3390/biomedicines13051127
Chicago/Turabian StylePersiani, Elisa, Elisa Ceccherini, Alessandra Falleni, Ilaria Gisone, Chiara Ippolito, Letizia Mattii, Antonella Cecchettini, and Federico Vozzi. 2025. "Ultrastructural and Molecular Analysis of Vascular Smooth Muscle Cells During the Switch from a Physiological to a Pathological Phenotype" Biomedicines 13, no. 5: 1127. https://doi.org/10.3390/biomedicines13051127
APA StylePersiani, E., Ceccherini, E., Falleni, A., Gisone, I., Ippolito, C., Mattii, L., Cecchettini, A., & Vozzi, F. (2025). Ultrastructural and Molecular Analysis of Vascular Smooth Muscle Cells During the Switch from a Physiological to a Pathological Phenotype. Biomedicines, 13(5), 1127. https://doi.org/10.3390/biomedicines13051127






