Abstract
Background: The clinical profiles of MNDs are dominated by inexorable motor decline, but subclinical proprioceptive, nociceptive and somatosensory deficits may also exacerbate mobility, dexterity, and bulbar function. While extra-motor pathology and frontotemporal involvement are widely recognised in motor neuron diseases (MNDs), reports of sensory involvement are conflicting. The potential contribution of sensory deficits to clinical disability is not firmly established and the spectrum of sensory manifestations is poorly characterised. Methods: A systematic review was conducted to examine the clinical, neuroimaging, electrophysiology and neuropathology evidence for sensory dysfunction in MND phenotypes. Results: In ALS, paraesthesia, pain, proprioceptive deficits and taste alterations are sporadically reported and there is also compelling electrophysiological, histological and imaging evidence of sensory network alterations. Gait impairment, impaired dexterity, and poor balance in ALS are likely to be multifactorial, with extrapyramidal, cerebellar, proprioceptive and vestibular deficits at play. Human imaging studies and animal models also confirm dorsal column-medial lemniscus pathway involvement as part of the disease process. Sensory symptoms are relatively common in spinal and bulbar muscular atrophy (SBMA) and Hereditary Spastic Paraplegia (HSP), but are inconsistently reported in primary lateral sclerosis (PLS) and in post-poliomyelitis syndrome (PPS). Conclusions: Establishing the prevalence and nature of sensory dysfunction across the spectrum of MNDs has a dual clinical and academic relevance. From a clinical perspective, subtle sensory deficits are likely to impact the disability profile and care needs of patients with MND. From an academic standpoint, sensory networks may be ideally suited to evaluate propagation patterns and the involvement of subcortical grey matter structures. Our review suggests that sensory dysfunction is an important albeit under-recognised facet of MND.
1. Introduction
1.1. The Clinical Spectrum of Motor Neuron Disease
Motor neuron diseases (MNDs) encompass a clinically and pathologically heterogeneous group of neurodegenerative disorders with distinct clinical, neuroimaging and biomarker profiles. Clinical phenotypes in MNDs are classically discussed along the spectrum of upper motor neuron (UMN) to lower motor neuron (LMN) dysfunction predominance including primary lateral sclerosis (PLS), hereditary spastic paraplegia (HSP), amyotrophic lateral sclerosis (ALS), spinal and bulbar muscular atrophy (SBMA or Kennedy’s disease) [1,2,3,4,5,6,7,8]. Another dimension of disease heterogeneity is the varying degree of frontotemporal dysfunction or comorbid frontotemporal dementia (FTD) in MNDs and phenotypes such as ALS-FTD, PLS-FTD, ALS with cognitive impairment (ALSci), and ALS with behavioural impairment (ALSbi) are also often distinguished [9,10]. Many neurologists would also regard HSP as a motor neuron disease [11,12,13], and low-incidence entities, such as monomelic ALS variants, O’Sullivan McLeod syndrome, and post-poliomyelitis syndrome are also often considered as MND subtypes [14,15,16,17,18,19,20,21,22,23].
1.2. Disease Heterogeneity and Extra-Motor Manifestations
Another facet of heterogeneity in MND is the relatively distinct clinical phenotypes associated with specific genetic variants which can often be linked to unique biomarker signatures [24,25,26,27,28,29]. Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease characterised by the concomitant degeneration of both the upper and lower motor neuron systems [30,31]. The core neuroimaging signature of ALS includes motor cortex, brainstem, corticospinal tract, and spinal cord degeneration, with subcortical grey matter degeneration also being observed [32]. It is increasingly recognised as a multi-system disorder, with extra-motor involvement being observed to be part of the disease process [31,33]. Pathology of sensory neurons in the dorsal root ganglia and sensory neuropathy are not widely acknowledged as part of the ALS syndrome [34], and there is a prevailing notion that ALS spares sensory networks [35], despite evidence of somatosensory disturbance that has been observed in ALS patients clinically [36,37,38,39], in electrophysiology [36,40,41,42,43], neuroimaging [41,44,45,46,47] and neuropathology [36,48,49,50,51,52] for several decades [31]. Postcentral neocortex involvement is regarded as a hallmark of “Stage 3” and thalamic involvement is regarded as an indicator of “Stage 2” of the Brettschneider--Braak pathological staging system proposed based on TDP-43 burden patterns [52,53]. From a clinical perspective, numbness [31], pain [54] and paraesthesia [37,55,56,57] are among some the most common sensory changes described by patients. While frank visual and auditory deficits are rarely observed, subtle changes in smell [58,59,60] and taste [61,62] are also occasionally reported. Proprioceptive deficits are also observed [63] and may have implications with regard to gait and dexterity. Additionally, sensory dysfunction may contribute to impaired cough reflex [64] and swallowing [65,66]. However, the findings of sensory pathology in ALS are conflicting with negative findings being reported in several studies [67,68,69,70,71,72,73]. Sensory disturbance is also observed in other motor neuron disease variants such as primary lateral sclerosis (PLS) [74,75,76]. The comprehensive characterisation of somatosensory pathophysiology in ALS is crucial for our academic understanding of disease heterogeneity, and findings of sensory pathology may have practical implications for rehabilitation efforts and the monitoring of disease progression. Furthermore, sensory dysfunction may have a significant impact on patients’ quality of life. The objective of this review is to investigate findings of sensory pathology in ALS and other MND phenotypes.
2. Methods
A formal literature search was performed on PubMed using the core search terms “amyotrophic lateral sclerosis”, “motor neuron disease”, “primary lateral sclerosis”, “spinal and bulbar muscular atrophy”, “hereditary spastic paraplegia”, “kennedy’s disease”, and “post-polio syndrome” individually combined with each of the following keywords: “sensory”, “somatosensory”, “sensory cortex”, “postcentral gyrus”, “dorsal column”, “thalamus”, “proprioception”, “thermoception”, “nociception”, “bulbar sensation”, “taste”, “enjoyment of food”, “facial sensation”, “paraesthesia”, “electrophysiology”, “neurophysiology”, “magnetic resonance imaging”, “PET”, “spinal imaging”, “biopsy”, and “animal models”. Only original research papers specifically assessing somatosensory function were systematically reviewed. Review papers, meta-analyses, conference abstracts, opinion pieces and editorials were excluded. Where relevant, references of original research papers have were also reviewed. The review was conducted between August 2023 and October 2023 and only papers published in English were reviewed. Based on the above criteria, a total of 305 original research papers were selected and reviewed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 recommendations (Figure 1).
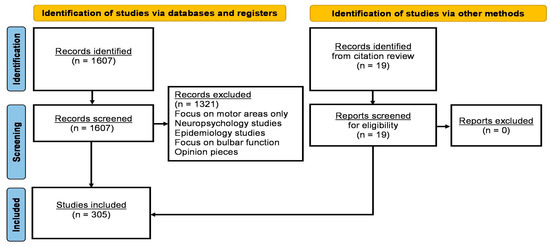
Figure 1.
PRISMA Flow Chart.
3. Results
3.1. Clinical Observations
3.1.1. Pain and Paraesthesia
Somatosensory symptoms and signs are well documented in ALS. According to one study, the most common symptom reported was numbness, followed by neuropathic pain, tingling, and reduced temperature sensation [31]. Sensory disturbance is more common in familial ALS than in sporadic ALS [30,77]. One study showed that 20% of familial ALS (fALS) cases manifest atypical features such as pain, paraesthesia or urgency micturition [55]. Pain is commonly reported in patients with ALS, with a study reporting a prevalence of 66% [54]. Pain was most commonly located in the neck and shoulders [54]. Neuropathic pain was reported in 9% of patients [54]. Pain intensity is not typically correlated with disease duration or physical disability [54]. Paraesthesia is often reported by patients [37,56,57], but it is also regarded as a potential side effect of Riluzole [78,79,80]. A small proportion of patients demonstrated sensory alterations on the quantitative sensory testing (QST) battery [49]. There are conflicting reports of impaired thermal sensory pathways. One study reports thermal threshold abnormalities [81], while another which investigated contact heat-evoked potentials in ALS patients proposed that the nociceptive pathway is not affected, suggesting that small fibres are spared in ALS [69].
3.1.2. Gait and Balance Impairment
Gait impairment is a well-recognised facet of ALS. Despite a multitude of targeted studies [82,83,84], the substrates of impaired postural control and gait abnormalities are relatively challenging to untangle as proprioceptive, extrapyramidal, cerebellar and vestibular components are likely to contribute to these deficits [85,86,87,88,89]. Twenty-five percent of patients assessed in one study had proprioceptive impairments [63]. Nonetheless, abnormal postural reactions have been linked to impaired balance, and it has been proposed that impaired axial control leads to postural abnormalities [90] and impaired gait [91,92]. Extrapyramidal involvement is also suspected to contribute to stiffness and balance impairment in ALS [86,93]. Vestibular deficits have been consistently reported in ALS [88,93,94,95] and evaluated both from a diagnostic and management perspective. These, although they are under-recognised, are thought to increase the risk of falls [93]. Gait impairment and poor balance in ALS are likely to be multifactorial with extrapyramidal, cerebellar, proprioceptive and vestibular deficits at play; therefore, the assessment of the individual contribution of these deficits is very challenging [82,83,84,96].
3.1.3. Gustatory, Olfactory, Pharyngeal and Laryngeal Manifestations
Other sensory manifestations such as a reduction in taste [62,97] have been reported, which may not only have negative quality of life implications, but the loss of enjoyment of eating may lead to reduced caloric intake. While taste and smell impairments [61] were reported by some studies, others detected no marked changes in olfaction and gustation [98], highlighting the need to assess these deficits prospectively in larger studies. It is noteworthy that several studies identified no abnormal findings in the sensory system [68,98,99], adding to the inconsistency in the literature and underlining the need for comprehensive studies to elucidate the degree and substrate of somatosensory dysfunction in MNDs. One study identified that 43.8% of patients with ALS reported taste alterations [100]. Changes in taste perception may have profound negative consequences on quality of life [62]. There is multimodal evidence of laryngeal sensory impairment in ALS. Laryngeal adduction reflex (LAR) abnormalities have been observed in as many as 20% of patients in some studies [101]. Laryngeal sensory changes are also commonly observed in the fibre-optic endoscopic evaluation of swallowing with sensory testing (FEEST) [65,66]. Thirty-three percent of patients with ALS had sensory deficits of the larynx in one study [65], while another detected deficits in as many as 54.5% of patients [66]. Sensory deficits are more commonly observed in bulbar-onset ALS patients [65] and laryngeal and pharyngeal sensory deficits may contribute to dysphagia [102,103,104]. Olfactory impairment has also been observed [58,59,60]. One study showed that changes in respiratory function correlate with deficits in olfaction [60]. Cutaneous sensory and autonomic denervation has been reported in both ALS and PLS, but the pathophysiological mechanisms behind these changes are not well characterised [50]. While the discussion and review of autonomic dysfunction in MNDs is beyond the scope of this paper, autonomic dysfunction has been consistently reported in both sporadic and familial forms of ALS [105,106,107,108,109,110,111,112]) (Table 1).

Table 1.
Clinical evidence for somatosensory impairment in ALS.
3.1.4. Insights from Electrophysiology Studies
Clinical reports of sensory involvement are increasingly complemented by neurophysiology studies (Table 2). The incidence of sensory nerve conduction abnormalities in ALS varies considerably from study to study [121,122] from as low as 14.7% [123] to as high as 66.7% [121]. While a study showed that 22.7% of patients with ALS had sensory abnormalities in at least one nerve [42], another study of 154 patients found that abnormal sensory nerve conduction is only detected in a minority of ALS patients [124]. It has been consistently shown that patients with ALS have a slower sensory conduction velocity [125,126] and it has been suggested that sensory involvement is more common in C9orf72 hexanucleotide repeat expansion carriers. Electrophysiological evidence of a sensory neuropathy was observed in 38% of C9orf72 positive patients compared to 21% of C9orf72-negative ALS patients [127]. Sensory deficits are also commonly seen in SOD1 patients [110], and in general, sensory disturbance is more commonly observed in familial ALS patients [109,118]. A comprehensive review of electrodiagnostic tests in ALS confirmed sensory signs in 32% of patients, and in 27% of patients, sural SNAPs were abnormal [36]. Reduced conduction velocity [43] and abnormal sensory nerve action potentials (SNAPs) are commonly observed [43,124,128], and one study showed that SNAP amplitude deteriorated with disease progression, although it remained within the normal range [39]. SNAP has been proposed as a prognostic indicator, as one study showed a superior prognosis in those with lower median nerve SNAP amplitudes, but only in patients younger than 57 years old [129]. It has also been suggested that compound muscle action potential (CMAP) and SNAP amplitudes of the median nerve are independent prognostic factors of sporadic ALS [129]. Abnormal somatosensory evoked potentials (SEPs) were previously used to exclude a diagnosis of ALS [130]; however, abnormal SEPs are commonly observed in both ALS [40,131,132,133,134,135,136] and PLS [135]. Abnormal median and tibial nerve SEPs have been reported in ALS [134]. A study showed sensory action potential amplitude (SAPa) reductions in 22% of patients, affecting the median, ulnar and sural nerves [137]. Most studies are consistent in confirming that sensory nerve conduction measures are more likely to capture pathological change than conventional sensory measures [121]. One study identified that 60% of patients had abnormal findings on sensory testing, but suggested that the changes may be non-progressive [126]. Despite of a plethora of studies reporting abnormal sensory measures in ALS, there are a handful of studies emphasising the absence of sensory findings in ALS [70,73,138,139,140]. This apparent inconsistency is probably best resolved by large, prospective multi-centre studies applying a methodologically standardised protocol. From a biomarker perspective, sensory cortex hyperexcitability was linked to shorter survival [132]. Several studies seem to indicate that abnormal SEPs and SNAPs often do not manifest in clinical signs of complaints, suggesting that sensory abnormalities may often remain subclinical [39,141]. A-beta or A-delta sensory fibres, and in some cases both, are shown to be impaired in ALS [133]. There is a relative scarcity of longitudinal neurophysiology studies focusing on sensory involvement.

Table 2.
Neurophysiology evidence of sensory impairment in MNDs.
3.1.5. Histopathology and Animal Model Data
pTDP-43 pathology has been consistently detected in the somatosensory cortex [162,163,164,165] as well as the thalamus [52,53]. Anatomical pTDP-43 burden patterns were used in the development of the Brettschneider--Braak pathological staging system in ALS [52,53], which has since been extensively validated by neuroimaging studies [166,167,168,169,170]. Postcentral neocortex involvement is regarded as a hallmark of “Stage 3” [52,53]. Studies also capture the pathology of dorsal root axons and dorsal root ganglion (DRG) cell bodies [171]. Thalamic pathology in ALS also has been extensively studied. Thalamic involvement is regarded as an indicator of “Stage 2” of the pTDP-43 staging system [52,53]. Post-mortem studies have consistently commented on both global thalamic degeneration in ALS [172] as well as the predilection to specific nuclei [173,174]. A study investigating post-mortem brains using 7T MRI as well as histopathology describes iron deposition in the thalamus [175]. Considerable thalamic dipeptide protein repeat (DPR) [176] and moderate p62 [177] burden were identified in individuals carrying the C9orf72 hexanucleotide repeat expansions. An interesting pathology report of seven patients with ALS who were in a locked-in state described considerable somatosensory, auditory, and gustatory pathway involvement with the relative preservation of visual and olfactory pathways [178]. Loss of neurons in the dorsal root ganglia as well as degeneration of posterior columns can be detected both ante and post mortem [73,179]. Contrary to brain and spinal cord reports, peripheral nervous system findings are somewhat inconsistent. Intra-epidermal nerve fibre density has been found to be normal in some studies [114] and reduced in others [48,51]. Both large-calibre and small-calibre sensory fibres are thought to be affected in ALS [36] and the sural nerve has been consistently shown to be affected [180,181,182]. Inflammatory cell infiltrates [183], reduced myelin thickness [184], and axonal loss [182] have all been observed in the sural nerve. Pathological change was detected in 91% of patients who underwent sural nerve biopsy, and large-calibre myelinated fibres may be particularly vulnerable [36]. A reduction in myelinated fibres was also observed in the peroneal nerve [56,185]. Small-fibre neuropathy is also thought to be a feature of ALS. It was demonstrated by a study [51] showing a significant epidermal small fibre density reduction in the distal calf. Laryngeal dysfunction is a cardinal feature of ALS [104] and the sensory components of laryngeal dysfunction have been specifically investigated in dedicated multimodal studies [65,101]. Aberrant or absent intraepidermal fibres were noted on laryngeal biopsies [65]. While murine models of ALS are not universally regarded as representative of the complex pathobiology of human ALS, TDP43 animal models have also consistently shown sensory pathology [186,187]. SOD1 animal models revealed pathology in central sensory regions [188,189,190], DRG neurons [30,33], DRG axons [191] and sensory neurons [192,193]. Wallerian degeneration of sensory nerves is readily observed in SOD1 mouse models [194]. A diffusion tensor imaging (DTI) study of an SOD1 animal model also confirmed sensory involvement in the symptomatic disease phase [188]. Other animal studies, however, revealed that sensory white matter fibres were preserved [72,195] and similarly, sensory deficits were not observed by other studies [72,195,196]. Histological data pertaining to somatosensory pathology are summarised in Table 3.

Table 3.
Histopathological evidence of somatosensory involvement.
3.1.6. Neuroimaging
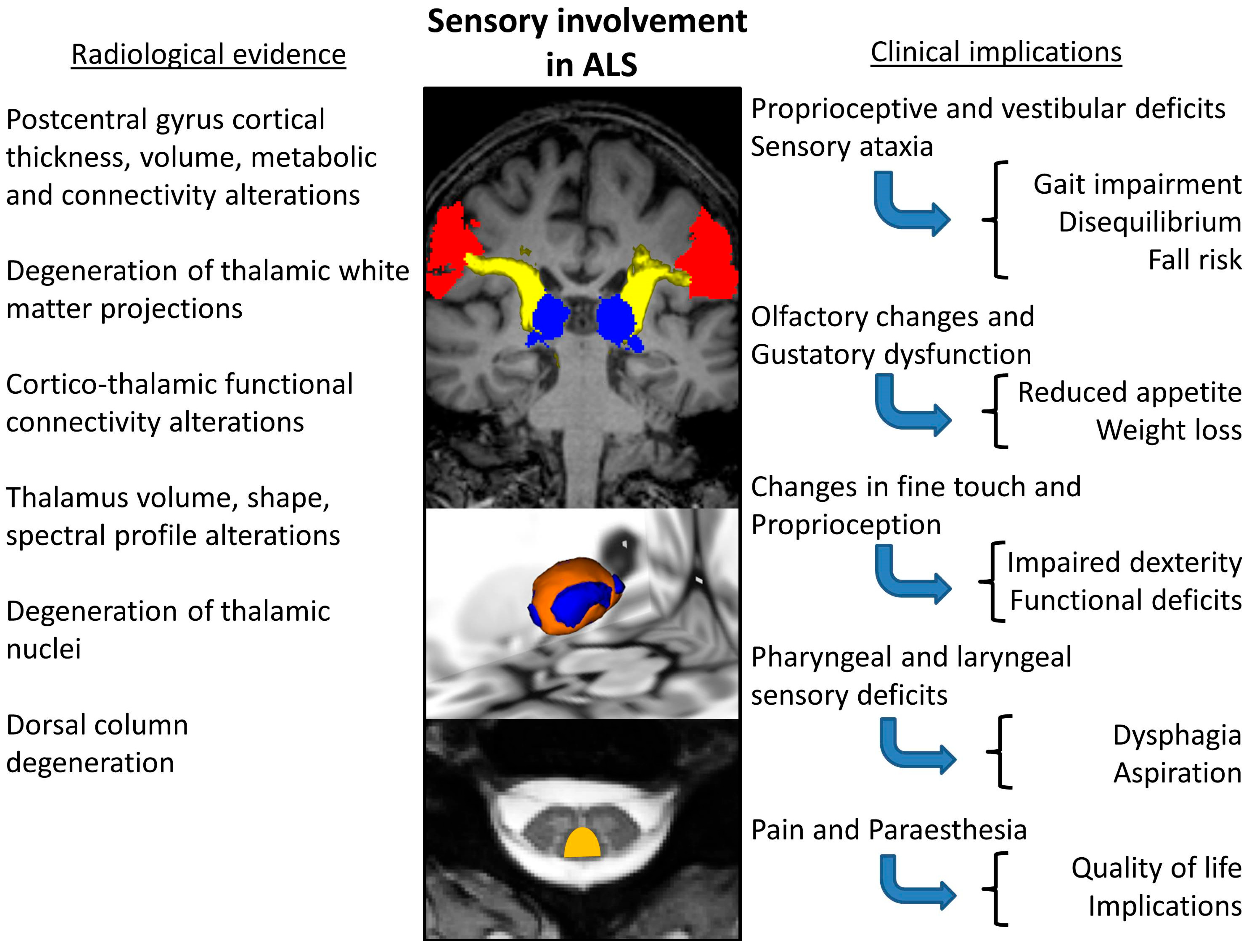
Neuroimaging offers a wealth of evidence for somatosensory involvement in ALS (Table 4.). With the advent of spinal imaging in ALS [204,205], dorsal column degeneration [41,179,206] has been consistently demonstrated by neuroimaging studies [41,179,206]. One study reported a significant correlation between abnormal DTI measures of sensory fibres and N9 amplitude [41]. Combining spinal imaging and neurophysiology has shown sub-clinical deficits of the sensory system in up to 85% of ALS patients [41]. Dorsal column changes are observed soon after symptom onset; therefore, it is possible that sensory involvement is grossly underestimated as an early feature of ALS [41,179]. One study investigated sensory pathway dysfunction in patients with ALS, using a combination of diffusion tensor imaging (DTI), magnetization transfer and atrophy index, demonstrating considerable dorsal column pathology within a year of symptom onset [179]. Thalamic pathology has been extensively studied in the literature of ALS [207,208] and a multitude of segmentation techniques have been implemented to demonstrate focal thalamic involvement affecting specific nuclei projecting to specific cortical [32,47,207,208,209] and limbic regions [209,210]. Thalamic atrophy is particularly significant in ALS-FTD [10,174,209,211,212,213]. Thalamic regions mediating somatosensory circuits are involved in both C9orf72-negative and -positive ALS patients [47]. Thalamus pathology [209,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229] has not only been demonstrated on structural [230,231,232] imaging, but also using diffusion tensor imaging (DTI) [233], functional MRI (fMRI) [46], PET [217,220], and magnetic resonance spectroscopy (MRS) [234,235]. Structural imaging studies are not only consistent in demonstrating postcentral gyrus atrophy [44,45,47,230,231,236,237,238], but insular, superior temporal, transverse temporal, supramarginal, and lateral occipital cortical thickness reductions have also been reported, which are thought to be more marked in hexanucleotide repeat expansion carriers in C9orf72 [47]. In addition to cortical thickness analyses, functional [239,240], morphometric [241], susceptibility [242], spectroscopy [243] and diffusion studies [244] have all demonstrated parietal and occipital pathology in ALS. Functional imaging consistently reveals changes in the somatosensory cortex [245]. Reduced right regional coherence in the postcentral gyrus was seen to correlate with high disease severity, while increased regional coherence in the left postcentral gyrus was associated with longer disease duration [236]. The reduced fractional amplitude of low-frequency fluctuations (fALFF) was also observed in the right postcentral gyrus [238]. A reduced Na/Cr resonance intensity ratio has been demonstrated on spectroscopy in the postcentral gyrus [235]. The quantitative analyses of the integrity of white matter tracts involved in somatosensory processing revealed medial lemniscus posterior thalamic radiation diffusivity alterations in patients with ALS [47]. In summary, neuroimaging offers ample evidence of the degeneration of key cortical, subcortical, thalamic, spinal and white matter components of somatosensory networks in ALS. Consensus imaging findings pertaining to sensory dysfunction in ALS as summarised in Figure 2.

Table 4.
Neuroimaging studies of ALS, highlighting somatosensory involvement.
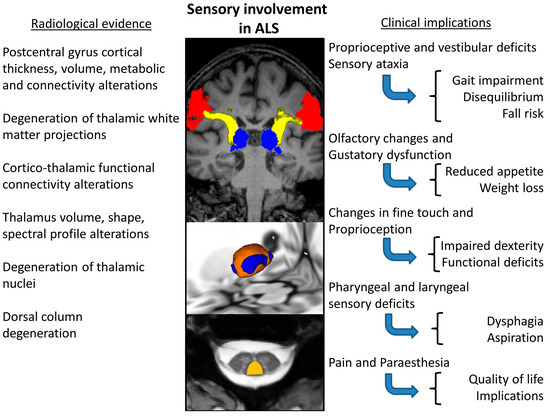
Figure 2.
Consensus of imaging findings pertaining to sensory dysfunction in ALS and a summary of putative clinical ramifications.
3.2. Other Motor Neuron Diseases
Reports of sensory alterations in primary lateral sclerosis (PLS) are inconsistent, and most studies focus on precentral gyrus, corticospinal tract and brainstem degeneration [271,272,273]. More recent studies, however, have demonstrated considerable extra-motor and thalamic pathologies in PLS [274,275]. Increased functional connectivity within the sensorimotor network was observed in patients with a faster progression and greater disability [74]. Thalamic volume reductions and shape deformations have been captured by most PLS studies evaluating this structure [274,276,277]. While the cortical thickness of primary sensory cortex is not reduced in PLS [275,278], some postcentral gyrus grey matter density reductions may be observed on morphometric analyses [275]. While Kennedy’s disease or spinal and bulbar muscular atrophy (SBMA) is primarily regarded as a lower motor neuron disease [1,5], widespread frontal and parietal degeneration has been highlighted by some imaging studies [10,279,280]. Imaging studies of SBMA have also consistently captured cerebellar [279,281], brainstem [279,282] and limbic [279,281] pathologies highlighting that the central nervous system is involved. In SBMA, reduced [75,76,283,284,285,286,287,288,289] or absent [290] SNAP has been consistently observed and SNAP does not appear to be correlated to CAG repeat size [291]. Axonal degeneration and the loss of myelinated nerve fibres were observed in the sural nerve [292]. Altered visual and auditory EPs [293], decreased somatosensory EPs [293] and prolonged somatosensory-evoked responses [294] were also observed. Post-polio syndrome (PPS) is a rare entity that affects poliomyelitis survivors decades after their initial infection. It typically presents after a long period of neurological stability and may manifest in myalgia, limb fatigability, new-onset muscle weakness, muscle bulk loss, and often as generalised fatigue [20,295]. While some post mortem studies have suggested a predilection to thalamic and hypothalamic involvement following poliomyelitis [296] and widespread cerebral involvement [297,298,299,300], other studies emphasise the absence of CNS involvement [301]. Abnormal SEPs have been observed in ageing polio survivors [302], but imaging studies of patients with post-polio syndrome are inconsistent. While early imaging studies have emphasised a considerable white matter hyperintensity burden in the reticular formation and medial lemniscus [296], subsequent quantitative imaging studies have not identified significant postcentral gyrus, thalamic or white matter alterations [21,303]. Hereditary spastic paraplegia (HSP) is a genetically and clinically heterogeneous group of neurodegenerative disorders typically divided into ‘pure’ and ‘complicated’ forms. In addition to spastic paraparesis, complicated HSP (cHSP), may be associated with cerebellar, extrapyramidal, optic nerve, cognitive impairment, and sensory manifestations. Imaging studies have described genotype-specific patterns of cerebral atrophy often including somatosensory regions [12]. Postcentral cortical thinning has been specifically highlighted in SPG4 [304] and SPG8 [305]. Morphometric [306,307,308,309], PET [310,311,312], SPECT [313] and spectroscopy [314] studies have consistently detected thalamic pathology in various HSP genotypes. Diffusion tensor imaging has identified changes in white matter integrity in thalamic radiations [315].
4. Discussion
Somatosensory involvement is an overlooked aspect of motor neuron diseases despite its likely contribution to bulbar dysfunction, impaired dexterity and gait impairment [82,83,84]. While subtle sensory symptoms are often reported by patients, in the face of relentless motor decline, targeted clinical or neurophysiological examination is seldom performed to specifically assess sensory dysfunction in routine clinical care. Clinical care is centred on the most pressing clinical aspects of ALS, such as respiratory and bulbar involvement and the maintenance of motor independence. Nonetheless, raising awareness of sensory involvement and the integration of clinical, imaging and neurophysiological evidence with regard to sensory involvement is crucial both from a clinical and an academic perspective.
From a clinical standpoint, the detection of proprioceptive deficits, addressing pain and the consideration of the sensory aspects of bulbar dysfunction, gait impairment and changes in dexterity have considerable practical relevance (Figure 2). It is also conceivable that in ALS, deficits in sensorimotor integration may contribute to impaired dexterity. ALS patients exhibit poor performance on the nine-hole peg test (NHPT) which has a moderate negative correlation with ALSFRS-R handwriting scores [316]. Impaired dexterity in ALS is multifactorial, and a combination of UMN, LMN, extrapyramidal, cerebellar and sensory components are likely at play. These deficits have a significant impact on independence, affecting writing, typing, driving, and getting dressed, among other essential daily tasks. Gait impairment in ALS is also thought to be multifactorial, encompassing extrapyramidal [84], cerebellar [87], postural [90] and vestibular [93] components in addition to primary motor system degeneration. The exact degree to which sensory afferent dysfunction contributes to gait impairment in ALS is very challenging to assess clinically due to extensive lower motor neuron and pyramidal involvement. Despite the compelling evidence of considerable sensory dysfunction in ALS, current rehabilitation strategies in ALS focus nearly exclusively on motor dysfunction and spasticity. The recognition of proprioceptive and vestibular deficits may guide fall prevention strategies [93]. The underpinnings of impaired dexterity are seldom evaluated comprehensively [316,317] and often are merely attributed to upper and lower motor neuron dysfunction overlooking proprioceptive, cerebellar and extrapyramidal components. Patients with ALS need to interact confidently with communication aids, take their medications, put on their NIV masks, and rely increasingly on their phones, tablets and other electronic devices; therefore, dexterity is a crucial aspect of their condition and the maintenance of function is the mainstay of occupational therapy [318,319]. Sensory dysfunction is also likely to contribute to bulbar dysfunction. Pharyngeal sensory deficits are thought to be more common in bulbar-onset ALS as evidenced by the endoscopic evaluation of swallowing with sensory testing [65,66]. ALS patients also report increased sensitivity to an upper airway irritant [64]. Evidence from Parkinson’s disease suggests that somatosensory deficits can also contribute to dysarthria [320]. Progressive dysphagia and dysarthria are often solely attributed to muscle weakness, overlooking the potential contribution of sensory impairment. Weight-loss and decreased oral intake are hallmarks of bulbar-onset ALS and are typically attributed to progressive dysphagia, metabolic and endocrine changes and apathy. Subtle changes in taste, impaired olfaction and the resulting reduced enjoyment of food are less commonly considered [58,59,60,61,62,321]. While some degree of limb paraesthesia is often experienced in ALS, clinicians rarely ask about sensory symptoms in established ALS cases.
Genotype-associated sensory profiles are difficult to establish based on the available literature. The majority of ALS studies are not stratified for genetic status, do not provide comprehensive screening for genetic variants, or only screen for a panel of the most common genetic variants associated with the disease (SOD1, C9orf72, TARDBP, FUS, etc.). There is therefore a paucity of evidence to link sensory-predominant manifestations to specific genotypes. One of the best characterised ALS cohorts is patients carting the GGGGCC hexanucleotide repeat expansion in C9orf72, a genotype that is typically linked to extensive extra-motor, subcortical, thalamic and cerebellar manifestations [25,27,322] and pre-symptomatic thalamic changes [28,323]. It is noteworthy, however, that extensive subcortical and frontotemporal change is not unique to the C9orf72 genotype [324]. Existing epidemiology studies of ALS focus on genotype-associated survival profiles, neuropsychological traits, longitudinal trajectories and the rate of decline [325], but sensory aspects are often overlooked. Accordingly, future studies that are clinical or radiological should meticulously screen their patients for ALS-associated genetic variants and specifically examine whether sensory manifestations are more common in a certain genotype.
From an academic standpoint, considerable research gaps persist and there is a notable shortage of recent papers evaluating sensory and somatosensory dysfunction in ALS and other MNDs. Despite the practical clinical implications of sensory deficits in ALS, many of the original studies we identified are a few years old. There is a sense that instead of pursuing classical clinico-radiological descriptive studies, there may be a trend in pursuing more “topical” facets of ALS research more recently, such as antisense oligonucleotide (ASO) development, machine learning (ML), artificial intelligence (AI) application, cluster analyses, presymptomatic studies, and assistive and wearable technologies [326,327,328,329,330,331,332]. As outlined in this review, there is ample clinical, imaging, histopathology and neurophysiology evidence that the somatosensory system is not spared in ALS. Despite the considerable amount of the literature, however, the majority of reports are from cross-sectional, single-timepoint studies. It is therefore challenging to determine whether sensory pathology is an early or late feature of the disease. There are sporadic reports of sensory involvement preceding motor pathology [38,333] and there are reports of thalamic pathology preceding cortical involvement in presymptomatic mutation carriers [28,334]. However, there is a notable scarcity of multi-timepoint, longitudinal studies evaluating progressive changes [335,336,337,338] and there is a particular paucity of studies specifically examining the evolution of somatosensory dysfunction. Few studies assess sensory dysfunction comprehensively using multiple complementary methods. Certain sensory domains such as visual, auditory, olfactory and gustatory deficits are particularly commonly overlooked [58,59,60,61,62]. Abnormal visual evoked potentials [339,340], retinal changes on optical coherence tomography (OCT) [341,342] and changes in auditory evoked potentials [40,340] have been consistently demonstrated in ALS. Among a wide range of neuropsychological domains affected in ALS [210,343,344,345], impaired visuospatial ability has been consistently reported in a proportion of patients [110,346]. Pathology in the lateral posterior nucleus of the thalamus, which plays a role in visual saliency and visually guided behaviours with afferents from the superior colliculus, primary visual, auditory and somatosensory cortices and efferents to the parietal association cortex, has also been observed [207]. Visuospatial dysfunction may negatively impact basic daily activities such as ambulation, driving and reading. Subclinical auditory deficits may also have considerable ramifications. Impaired taste and smell may have unrecognised quality-of-life implications as well as impacts on patients’ appetites [62]. While the discussion of therapeutic advances is beyond the scope of this paper, our review highlights the considerable clinical, radiological and genetic heterogeneity of motor neuron diseases, and supports the notion that instead of pursuing the development of a single disease-modifying drug that is effective in all phenotypes, a precision medicine strategy is required which would work in specific genotypes and phenotypes. The transition in drug development from generic “neuroprotective” approaches to precision therapies is well demonstrated by recent antisense oligonucleotide trials [347,348,349].
5. Conclusions
ALS should no longer be considered a condition that exclusive involves motor and frontotemporal regions. Sensory alterations are likely to contribute to some of the core symptoms of ALS, including bulbar dysfunction, gait impairment, and impaired dexterity. Despite the wealth of clinical, neurophysiology, imaging, and histopathology data, sensory deficits remain glaringly under-evaluated in ALS and other motor neuron diseases. The recognition and routine assessment of the sensory system is crucial for precision clinical evaluation, disease monitoring and the understanding of disease biology.
Funding
This project was sponsored by the Health Research Board Ireland (JPND-Cofund-2-2019-1 & HRB EIA-2017-019) and the EU Joint Programme—Neurodegenerative Disease Research (JPND). Professor Bede is also supported by the Spastic Paraplegia Foundation (SPF), the Irish Institute of Clinical Neuroscience (IICN), the Research Motor Neurone (RMN) foundation, and the Science Foundation Ireland (SFI SP20/SP/8953).
Conflicts of Interest
The authors declare no conflict of interest.
Glossary
AI—artificial intelligence; ALFF—amplitude of low-frequency fluctuations; ALS—amyotrophic lateral sclerosis; ALSbi—ALS with behavioural impairment; ALSci—ALS with cognitive impairment; ALSFRS-R—Amyotrophic Lateral Sclerosis Functional Rating Scale; ALS-FTD—amyotrophic lateral sclerosis with frontotemporal degeneration; ASO—antisense oligonucleotide; ATF3—AMP-dependent transcription factor; BAEP—brainstem auditory evoked potential; bvFTD—behavioural variant frontotemporal dementia; C9orf72—chromosome 9 open reading frame 72; CMAP—compound muscle action potential; CPM—conditioned pain modulation; CSAP—compound sensory action potential; CST—corticospinal tract; CV—conduction velocity; DC—degree centrality; DRG—dorsal root ganglion; DTI—diffusion tensor imaging; EMG—electromyography; ENG—electronystagmography; Eps—evoked potentials; FA—fractional anisotropy; fALFF—fractional amplitude of low-frequency fluctuations; fALS—familial ALS; FD—fractal dimensionality; FDA—Food and Drug Administration; FDG-PET—fluorodeoxyglucose (FDG)-positron emission tomography; FEESST—fibre-optic endoscopic evaluation of swallowing with sensory testing; fMRI—functional MRI; FOSMN—facial onset sensory and motor neuronopathy; FTD—frontotemporal degeneration; FTLD—frontotemporal lobar degeneration; GM—grey matter; HSP—hereditary spastic paraplegia; IENFD—intraepidermal nerve fibre density; ihMT—inhomogeneous magnetization transfer; LEP—laser evoked potentials; MCV—motor conduction velocity; MD—mean diffusivity; ML—machine learning; MND—motor neuron disease; MRI—magnetic resonance imaging; MRS—magnetic resonance spectroscopy; MUNE—motor unit number estimation; Na/Cr—N-acetyl to creatine resonance intensity ratios; NCS—nerve conduction study; NCV—nerve conduction velocity; NHPT—nine-hole peg test; NMS—non-motor symptoms; OCT—optical coherence tomography; OEP—olfactory-evoked response; PERK—PKR-like endoplasmic Reticulum Kinase; PLS—primary lateral sclerosis; PMA—progressive muscular atrophy; PPS—post-poliomyelitis syndrome; PRISMA—Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PSVEP—pattern-shift visual evoked response; QoL—quality of life; QST—quantitative sensory testing; RD—radial diffusivity; ROI—region of interest; rsfMRI—resting state functional MRI; SAPAs—sensory action potential amplitudes; SBMA—spinal and bulbar muscular atrophy (Kennedy’s disease); SCNV—sensory nerve conduction velocity; SCV—sensory conduction velocity; SEP—somatosensory evoked potentials; SGCs—satellite glial cells; SMN—superior medial sensory-motor network; SNAP—sensory nerve action potential; SNAPA—sensory nerve action potential amplitude; SOD1—superoxide dismutase 1; SSCV—spinal cord conduction velocity; SSEP—somatosensory evoked potentials; TDP-43—TAR DNA-binding protein 43; UPSIT—University of Pennsylvania Smell Identification Test; UtC—urge to cough; VBM—voxel-based morphometry; VEP—visual evoked potential; WM—white matter.
References
- Querin, G.; Bede, P.; Marchand-Pauvert, V.; Pradat, P.F. Biomarkers of Spinal and Bulbar Muscle Atrophy (SBMA): A Comprehensive Review. Front. Neurol. 2018, 9, 844. [Google Scholar] [CrossRef] [PubMed]
- Prudlo, J.; Bißbort, C.; Glass, A.; Grossmann, A.; Hauenstein, K.; Benecke, R.; Teipel, S.J. White matter pathology in ALS and lower motor neuron ALS variants: A diffusion tensor imaging study using tract-based spatial statistics. J. Neurol. 2012, 259, 1848–1859. [Google Scholar] [CrossRef] [PubMed]
- de Vries, B.S.; Rustemeijer, L.M.M.; Bakker, L.A.; Schröder, C.D.; Veldink, J.H.; van den Berg, L.H.; Nijboer, T.C.W.; van Es, M.A. Cognitive and behavioural changes in PLS and PMA:challenging the concept of restricted phenotypes. J. Neurol. Neurosurg. Psychiatry 2019, 90, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Lebouteux, M.V.; Franques, J.; Guillevin, R.; Delmont, E.; Lenglet, T.; Bede, P.; Desnuelle, C.; Pouget, J.; Pascal-Mousselard, H.; Pradat, P.F. Revisiting the spectrum of lower motor neuron diseases with snake eyes appearance on magnetic resonance imaging. Eur. J. Neurol. 2014, 21, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Pradat, P.F.; Bernard, E.; Corcia, P.; Couratier, P.; Jublanc, C.; Querin, G.; Morelot Panzini, C.; Salachas, F.; Vial, C.; Wahbi, K.; et al. The French national protocol for Kennedy’s disease (SBMA): Consensus diagnostic and management recommendations. Orphanet J. Rare Dis. 2020, 15, 90. [Google Scholar] [CrossRef] [PubMed]
- Finegan, E.; Li Hi Shing, S.; Siah, W.F.; Chipika, R.H.; Chang, K.M.; McKenna, M.C.; Doherty, M.A.; Hengeveld, J.C.; Vajda, A.; Donaghy, C.; et al. Evolving diagnostic criteria in primary lateral sclerosis: The clinical and radiological basis of “probable PLS”. J. Neurol. Sci. 2020, 417, 117052. [Google Scholar] [CrossRef]
- Hardiman, O.; van den Berg, L.H.; Kiernan, M.C. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2011, 7, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Ince, P.G.; Evans, J.; Knopp, M.; Forster, G.; Hamdalla, H.H.; Wharton, S.B.; Shaw, P.J. Corticospinal tract degeneration in the progressive muscular atrophy variant of ALS. Neurology 2003, 60, 1252–1258. [Google Scholar] [CrossRef]
- Strong, M.J.; Abrahams, S.; Goldstein, L.H.; Woolley, S.; McLaughlin, P.; Snowden, J.; Mioshi, E.; Roberts-South, A.; Benatar, M.; HortobaGyi, T.; et al. Amyotrophic lateral sclerosis—frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotroph. Lateral Scler. Front. Degener. 2017, 18, 153–174. [Google Scholar] [CrossRef]
- McKenna, M.C.; Corcia, P.; Couratier, P.; Siah, W.F.; Pradat, P.F.; Bede, P. Frontotemporal Pathology in Motor Neuron Disease Phenotypes: Insights From Neuroimaging. Front. Neurol. 2021, 12, 723450. [Google Scholar] [CrossRef] [PubMed]
- Rezende, T.J.; de Albuquerque, M.; Lamas, G.M.; Martinez, A.R.; Campos, B.M.; Casseb, R.F.; Silva, C.B.; Branco, L.M.; D’Abreu, A.; Lopes-Cendes, I.; et al. Multimodal MRI-based study in patients with SPG4 mutations. PLoS ONE 2015, 10, e0117666. [Google Scholar] [CrossRef] [PubMed]
- Mulkerrin, G.; França, M.C., Jr.; Lope, J.; Tan, E.L.; Bede, P. Neuroimaging in hereditary spastic paraplegias: From qualitative cues to precision biomarkers. Expert Rev. Mol. Diagn. 2022, 22, 745–760. [Google Scholar] [CrossRef]
- Fink, J.K. The hereditary spastic paraplegias. Handb. Clin. Neurol. 2023, 196, 59–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wen, H.; Chen, S.; Wang, H.; Zheng, Y.; Chen, R.; Li, J.; Jiang, K.; Xiang, H.; Zhu, M.; et al. Benign monomelic amyotrophy of lower limb in a cohort of chinese patients. Brain Behav. 2021, 11, e02073. [Google Scholar] [CrossRef] [PubMed]
- Moglia, C.; Calvo, A.; Brunetti, M.; Chiò, A.; Grassano, M. Broadening the clinical spectrum of FUS mutations: A case with monomelic amyotrophy with a late progression to amyotrophic lateral sclerosis. Neurol. Sci. 2021, 42, 1207–1209. [Google Scholar] [CrossRef] [PubMed]
- Matamala, J.M.; Geevasinga, N.; Huynh, W.; Dharmadasa, T.; Howells, J.; Simon, N.G.; Menon, P.; Vucic, S.; Kiernan, M.C. Cortical function and corticomotoneuronal adaptation in monomelic amyotrophy. Clin. Neurophysiol. 2017, 128, 1488–1495. [Google Scholar] [CrossRef]
- Bede, P.; Walsh, R.; Fagan, A.J.; Hardiman, O. “Sand-watch” spinal cord: A case of inferior cervical spinal cord atrophy. J. Neurol. 2014, 261, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Bede, P.; Bokde, A.L.; Byrne, S.C.; Elamin, M.; Walsh, R.J.; Hardiman, O. Waterskier’s Hirayama syndrome. J. Neurol. 2011, 258, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, K.; Tomonaga, M.; Kitano, K.; Yamada, T.; Kojima, S.; Arai, K. Focal cervical poliopathy causing juvenile muscular atrophy of distal upper extremity: A pathological study. J. Neurol. Neurosurg. Psychiatry 1987, 50, 285–290. [Google Scholar] [CrossRef]
- Li Hi Shing, S.; Chipika, R.H.; Finegan, E.; Murray, D.; Hardiman, O.; Bede, P. Post-polio Syndrome: More Than Just a Lower Motor Neuron Disease. Front. Neurol. 2019, 10, 773. [Google Scholar] [CrossRef]
- Li Hi Shing, S.; Lope, J.; McKenna, M.C.; Chipika, R.H.; Hardiman, O.; Bede, P. Increased cerebral integrity metrics in poliomyelitis survivors: Putative adaptation to longstanding lower motor neuron degeneration. J. Neurol. Sci. 2021, 424, 117361. [Google Scholar] [CrossRef]
- Pinto, W.; Nunes, P.P.; Lima, E.T.I.; Assis, A.C.D.; Naylor, F.G.M.; Chieia, M.A.T.; Souza, P.V.S.; Oliveira, A.S.B. O’Sullivan-McLeod syndrome: Unmasking a rare atypical motor neuron disease. Rev. Neurol. 2019, 175, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Ghadiri-Sani, M.; Huda, S.; Larner, A.J. O’Sullivan-McLeod syndrome: Clinical features, neuroradiology and nosology. Br. J. Hosp. Med. 2014, 75, 712–713. [Google Scholar] [CrossRef]
- Walhout, R.; Schmidt, R.; Westeneng, H.J.; Verstraete, E.; Seelen, M.; van Rheenen, W.; de Reus, M.A.; van Es, M.A.; Hendrikse, J.; Veldink, J.H.; et al. Brain morphologic changes in asymptomatic C9orf72 repeat expansion carriers. Neurology 2015, 85, 1780–1788. [Google Scholar] [CrossRef] [PubMed]
- Li Hi Shing, S.; McKenna, M.C.; Siah, W.F.; Chipika, R.H.; Hardiman, O.; Bede, P. The imaging signature of C9orf72 hexanucleotide repeat expansions: Implications for clinical trials and therapy development. Brain Imaging Behav. 2021, 15, 2693–2719. [Google Scholar] [CrossRef] [PubMed]
- Lulé, D.E.; Müller, H.P.; Finsel, J.; Weydt, P.; Knehr, A.; Winroth, I.; Andersen, P.; Weishaupt, J.; Uttner, I.; Kassubek, J.; et al. Deficits in verbal fluency in presymptomatic C9orf72 mutation gene carriers-a developmental disorder. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Cistaro, A.; Pagani, M.; Montuschi, A.; Calvo, A.; Moglia, C.; Canosa, A.; Restagno, G.; Brunetti, M.; Traynor, B.J.; Nobili, F.; et al. The metabolic signature of C9ORF72-related ALS: FDG PET comparison with nonmutated patients. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Bede, P.; Lulé, D.; Müller, H.P.; Tan, E.L.; Dorst, J.; Ludolph, A.C.; Kassubek, J. Presymptomatic grey matter alterations in ALS kindreds: A computational neuroimaging study of asymptomatic C9orf72 and SOD1 mutation carriers. J. Neurol. 2023, 270, 4235–4247. [Google Scholar] [CrossRef]
- Turner, M.R.; Hammers, A.; Al-Chalabi, A.; Shaw, C.E.; Andersen, P.M.; Brooks, D.J.; Leigh, P.N. Distinct cerebral lesions in sporadic and ’D90A’ SOD1 ALS: Studies with [11C]flumazenil PET. Brain 2005, 128, 1323–1329. [Google Scholar] [CrossRef]
- Sábado, J.; Casanovas, A.; Tarabal, O.; Hereu, M.; Piedrafita, L.; Calderó, J.; Esquerda, J.E. Accumulation of misfolded SOD1 in dorsal root ganglion degenerating proprioceptive sensory neurons of transgenic mice with amyotrophic lateral sclerosis. BioMed Res. Int. 2014, 2014, 852163. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.Q.; Wei, Q.; Wu, Z.Y. Sensory nerve disturbance in amyotrophic lateral sclerosis. Life Sci. 2018, 203, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Bede, P.; Omer, T.; Finegan, E.; Chipika, R.H.; Iyer, P.M.; Doherty, M.A.; Vajda, A.; Pender, N.; McLaughlin, R.L.; Hutchinson, S.; et al. Connectivity-based characterisation of subcortical grey matter pathology in frontotemporal dementia and ALS: A multimodal neuroimaging study. Brain Imaging Behav. 2018, 12, 1696–1707. [Google Scholar] [CrossRef] [PubMed]
- Sassone, J.; Taiana, M.; Lombardi, R.; Porretta-Serapiglia, C.; Freschi, M.; Bonanno, S.; Marcuzzo, S.; Caravello, F.; Bendotti, C.; Lauria, G. ALS mouse model SOD1G93A displays early pathology of sensory small fibers associated to accumulation of a neurotoxic splice variant of peripherin. Hum. Mol. Genet. 2016, 25, 1588–1599. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, B.; Robberecht, W. The phenotypic variability of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2014, 10, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Bede, P.; Iyer, P.M.; Schuster, C.; Elamin, M.; McLaughlin, R.L.; Kenna, K.; Hardiman, O. The selective anatomical vulnerability of ALS: ’disease-defining’ and ’disease-defying’ brain regions. Amyotroph. Lateral Scler. Front. Degener. 2016, 17, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Hammad, M.; Silva, A.; Glass, J.; Sladky, J.T.; Benatar, M. Clinical, electrophysiologic, and pathologic evidence for sensory abnormalities in ALS. Neurology 2007, 69, 2236–2242. [Google Scholar] [CrossRef] [PubMed]
- Gubbay, S.S.; Kahana, E.; Zilber, N.; Cooper, G.; Pintov, S.; Leibowitz, Y. Amyotrophic lateral sclerosis. A study of its presentation and prognosis. J. Neurol. 1985, 232, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, J.D.; Dean, A.F.; Shaw, C.E.; Al-Chalabi, A.; Mills, K.R.; Leigh, P.N. Amyotrophic lateral sclerosis with sensory neuropathy: Part of a multisystem disorder? J. Neurol. Neurosurg. Psychiatry 2007, 78, 750–753. [Google Scholar] [CrossRef] [PubMed]
- Gregory, R.; Mills, K.; Donaghy, M. Progressive sensory nerve dysfunction in amyotrophic lateral sclerosis: A prospective clinical and neurophysiological study. J. Neurol. 1993, 240, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Radtke, R.A.; Erwin, A.; Erwin, C.W. Abnormal sensory evoked potentials in amyotrophic lateral sclerosis. Neurology 1986, 36, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, C.; Sangari, S.; El Mendili, M.M.; Benali, H.; Marchand-Pauvert, V.; Pradat, P.F. Electrophysiological and spinal imaging evidences for sensory dysfunction in amyotrophic lateral sclerosis. BMJ Open 2015, 5, e007659. [Google Scholar] [CrossRef] [PubMed]
- Pugdahl, K.; Fuglsang-Frederiksen, A.; de Carvalho, M.; Johnsen, B.; Fawcett, P.R.; Labarre-Vila, A.; Liguori, R.; Nix, W.A.; Schofield, I.S. Generalised sensory system abnormalities in amyotrophic lateral sclerosis: A European multicentre study. J. Neurol. Neurosurg. Psychiatry 2007, 78, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Pugdahl, K.; Fuglsang-Frederiksen, A.; Johnsen, B.; de Carvalho, M.; Fawcett, P.R.; Labarre-Vila, A.; Liguori, R.; Nix, W.A.; Schofield, I.S. A prospective multicentre study on sural nerve action potentials in ALS. Clin. Neurophysiol. 2008, 119, 1106–1110. [Google Scholar] [CrossRef]
- Zhou, C.; Hu, X.; Hu, J.; Liang, M.; Yin, X.; Chen, L.; Zhang, J.; Wang, J. Altered Brain Network in Amyotrophic Lateral Sclerosis: A Resting Graph Theory-Based Network Study at Voxel-Wise Level. Front. Neurosci. 2016, 10, 204. [Google Scholar] [CrossRef]
- Devine, M.S.; Pannek, K.; Coulthard, A.; McCombe, P.A.; Rose, S.E.; Henderson, R.D. Exposing asymmetric gray matter vulnerability in amyotrophic lateral sclerosis. NeuroImage Clin. 2015, 7, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Lule, D.; Diekmann, V.; Muller, H.P.; Kassubek, J.; Ludolph, A.C.; Birbaumer, N. Neuroimaging of multimodal sensory stimulation in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2010, 81, 899–906. [Google Scholar] [CrossRef]
- Chipika, R.H.; Mulkerrin, G.; Murad, A.; Lope, J.; Hardiman, O.; Bede, P. Alterations in somatosensory, visual and auditory pathways in amyotrophic lateral sclerosis: An under-recognised facet of ALS. J. Integr. Neurosci. 2022, 21, 88. [Google Scholar] [CrossRef]
- Dalla Bella, E.; Lombardi, R.; Porretta-Serapiglia, C.; Ciano, C.; Gellera, C.; Pensato, V.; Cazzato, D.; Lauria, G. Amyotrophic lateral sclerosis causes small fiber pathology. Eur. J. Neurol. 2016, 23, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Isak, B.; Pugdahl, K.; Karlsson, P.; Tankisi, H.; Finnerup, N.B.; Furtula, J.; Johnsen, B.; Sunde, N.; Jakobsen, J.; Fuglsang-Frederiksen, A. Quantitative sensory testing and structural assessment of sensory nerve fibres in amyotrophic lateral sclerosis. J. Neurol. Sci. 2017, 373, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Nolano, M.; Provitera, V.; Manganelli, F.; Iodice, R.; Caporaso, G.; Stancanelli, A.; Marinou, K.; Lanzillo, B.; Santoro, L.; Mora, G. Non-motor involvement in amyotrophic lateral sclerosis: New insight from nerve and vessel analysis in skin biopsy. Neuropathol. Appl. Neurobiol. 2017, 43, 119–132. [Google Scholar] [CrossRef]
- Weis, J.; Katona, I.; Muller-Newen, G.; Sommer, C.; Necula, G.; Hendrich, C.; Ludolph, A.C.; Sperfeld, A.D. Small-fiber neuropathy in patients with ALS. Neurology 2011, 76, 2024–2029. [Google Scholar] [CrossRef]
- Braak, H.; Brettschneider, J.; Ludolph, A.C.; Lee, V.M.; Trojanowski, J.Q.; Del Tredici, K. Amyotrophic lateral sclerosis--a model of corticofugal axonal spread. Nat. Rev. Neurol. 2013, 9, 708–714. [Google Scholar] [CrossRef]
- Brettschneider, J.; Del Tredici, K.; Toledo, J.B.; Robinson, J.L.; Irwin, D.J.; Grossman, M.; Suh, E.; Van Deerlin, V.M.; Wood, E.M.; Baek, Y.; et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann. Neurol. 2013, 74, 20–38. [Google Scholar] [CrossRef]
- Moisset, X.; Cornut-Chauvinc, C.; Clavelou, P.; Pereira, B.; Dallel, R.; Guy, N. Is there pain with neuropathic characteristics in patients with amyotrophic lateral sclerosis? A cross-sectional study. Palliat. Med. 2016, 30, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Camu, W.; Khoris, J.; Moulard, B.; Salachas, F.; Briolotti, V.; Rouleau, G.A.; Meininger, V. Genetics of familial ALS and consequences for diagnosis. French ALS Research Group. J. Neurol. Sci. 1999, 165 (Suppl. S1), S21–S26. [Google Scholar] [CrossRef] [PubMed]
- Dyck, P.J.; Stevens, J.C.; Mulder, D.W.; Espinosa, R.E. Frequency of nerve fiber degeneration of peripheral motor and sensory neurons in amyotrophic lateral sclerosis. Morphometry of deep and superficial peroneal nerves. Neurology 1975, 25, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Khoris, J.; Moulard, B.; Briolotti, V.; Hayer, M.; Durieux, A.; Clavelou, P.; Malafosse, A.; Rouleau, G.A.; Camu, W. Coexistence of dominant and recessive familial amyotrophic lateral sclerosis with the D90A Cu, Zn superoxide dismutase mutation within the same country. Eur. J. Neurol. 2000, 7, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Elian, M. Olfactory impairment in motor neuron disease: A pilot study. J. Neurol. Neurosurg. Psychiatry 1991, 54, 927–928. [Google Scholar] [CrossRef][Green Version]
- Hawkes, C.H.; Shephard, B.C.; Geddes, J.F.; Body, G.D.; Martin, J.E. Olfactory disorder in motor neuron disease. Exp. Neurol. 1998, 150, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Gunther, R.; Schrempf, W.; Hahner, A.; Hummel, T.; Wolz, M.; Storch, A.; Hermann, A. Impairment in Respiratory Function Contributes to Olfactory Impairment in Amyotrophic Lateral Sclerosis. Front. Neurol. 2018, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Gunther, R.; Richter, N.; Sauerbier, A.; Chaudhuri, K.R.; Martinez-Martin, P.; Storch, A.; Hermann, A. Non-Motor Symptoms in Patients Suffering from Motor Neuron Diseases. Front. Neurol. 2016, 7, 117. [Google Scholar] [CrossRef] [PubMed]
- Tarlarini, C.; Greco, L.C.; Lizio, A.; Gerardi, F.; Sansone, V.A.; Lunetta, C. Taste changes in amyotrophic lateral sclerosis and effects on quality of life. Neurol. Sci. 2019, 40, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Simmatis, L.; Atallah, G.; Scott, S.H.; Taylor, S. The feasibility of using robotic technology to quantify sensory, motor, and cognitive impairments associated with ALS. Amyotroph. Lateral Scler. Front. Degener. 2019, 20, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Tabor-Gray, L.; Vasilopoulos, T.; Wheeler-Hegland, K.; Wymer, J.; Plowman, E.K. Reflexive Airway Sensorimotor Responses in Individuals with Amyotrophic Lateral Sclerosis. Dysphagia 2021, 36, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Ruoppolo, G.; Onesti, E.; Gori, M.C.; Schettino, I.; Frasca, V.; Biasiotta, A.; Giordano, C.; Ceccanti, M.; Cambieri, C.; Greco, A.; et al. Laryngeal Sensitivity in Patients with Amyotrophic Lateral Sclerosis. Front. Neurol. 2016, 7, 212. [Google Scholar] [CrossRef]
- Amin, M.R.; Harris, D.; Cassel, S.G.; Grimes, E.; Heiman-Patterson, T. Sensory testing in the assessment of laryngeal sensation in patients with amyotrophic lateral sclerosis. Ann. Otol. Rhinol. Laryngol. 2006, 115, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Santos-Bento, M.; de Carvalho, M.; Evangelista, T.; Sales Luís, M.L. Sympathetic sudomotor function and amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2001, 2, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Deepika, J.; Manvir, B.; Sumit, S.; Vinay, G.; Trilochan, S.; Garima, S.; Padma, M.V.; Madhuri, B. Quantitative thermal sensory testing in patients with amyotrophic lateral sclerosis using reaction time exclusive method of levels (MLE). Electromyogr. Clin. Neurophysiol. 2006, 46, 145–148. [Google Scholar] [PubMed]
- Xu, Y.S.; Zhang, J.; Zheng, J.Y.; Zhang, S.; Kang, D.X.; Fan, D.S. Fully intact contact heat evoked potentials in patients with amyotrophic lateral sclerosis. Muscle Nerve 2009, 39, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Argyriou, A.A.; Polychronopoulos, P.; Talelli, P.; Chroni, E. F wave study in amyotrophic lateral sclerosis: Assessment of balance between upper and lower motor neuron involvement. Clin. Neurophysiol. 2006, 117, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Pouget, J. Electroneuromyographic criteria of amyotrophic lateral sclerosis. Rev. Neurol. 2006, 162, 4S34–4S42. [Google Scholar] [PubMed]
- Underwood, C.K.; Kurniawan, N.D.; Butler, T.J.; Cowin, G.J.; Wallace, R.H. Non-invasive diffusion tensor imaging detects white matter degeneration in the spinal cord of a mouse model of amyotrophic lateral sclerosis. Neuroimage 2011, 55, 455–461. [Google Scholar] [CrossRef]
- Rabin, B.A.; Griffin, J.W.; Crain, B.J.; Scavina, M.; Chance, P.F.; Cornblath, D.R. Autosomal dominant juvenile amyotrophic lateral sclerosis. Brain 1999, 122, 1539–1550. [Google Scholar] [CrossRef]
- Agosta, F.; Canu, E.; Inuggi, A.; Chio, A.; Riva, N.; Silani, V.; Calvo, A.; Messina, S.; Falini, A.; Comi, G.; et al. Resting state functional connectivity alterations in primary lateral sclerosis. Neurobiol. Aging 2014, 35, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Anagnostou, E.; Zachou, A.; Breza, M.; Kladi, A.; Karadima, G.; Koutsis, G. Disentangling balance impairments in spinal and bulbar muscular atrophy. Neurosci. Lett. 2019, 705, 94–98. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Q.; Lin, L.; Wang, D.; Zheng, H.; Guan, Y. Comparison of clinical and physiological characteristics between Kennedy disease and amyotrophic lateral sclerosis. Nan Fang Yi Ke Da Xue Xue Bao 2014, 34, 1688–1692. [Google Scholar] [PubMed]
- Li, T.M.; Alberman, E.; Swash, M. Comparison of sporadic and familial disease amongst 580 cases of motor neuron disease. J. Neurol. Neurosurg. Psychiatry 1988, 51, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Siniscalchi, A. Tolerability of riluzole: A review of the literature. Clin. Ter. 2004, 155, 25–28. [Google Scholar] [PubMed]
- Wagner, M.L.; Landis, B.E. Riluzole: A new agent for amyotrophic lateral sclerosis. Ann. Pharmacother. 1997, 31, 738–744. [Google Scholar] [CrossRef]
- Bryson, H.M.; Fulton, B.; Benfield, P. Riluzole. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in amyotrophic lateral sclerosis. Drugs 1996, 52, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Jamal, G.A.; Weir, A.I.; Hansen, S.; Ballantyne, J.P. Sensory involvement in motor neuron disease: Further evidence from automated thermal threshold determination. J. Neurol. Neurosurg. Psychiatry 1985, 48, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Abidi, M.; de Marco, G.; Grami, F.; Termoz, N.; Couillandre, A.; Querin, G.; Bede, P.; Pradat, P.F. Neural Correlates of Motor Imagery of Gait in Amyotrophic Lateral Sclerosis. J. Magn. Reson. Imaging 2021, 53, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Abidi, M.; Pradat, P.F.; Termoz, N.; Couillandre, A.; Bede, P.; de Marco, G. Motor imagery in amyotrophic lateral Sclerosis: An fMRI study of postural control. NeuroImage Clinical 2022, 35, 103051. [Google Scholar] [CrossRef]
- Feron, M.; Couillandre, A.; Mseddi, E.; Termoz, N.; Abidi, M.; Bardinet, E.; Delgadillo, D.; Lenglet, T.; Querin, G.; Welter, M.L.; et al. Extrapyramidal deficits in ALS: A combined biomechanical and neuroimaging study. J. Neurol. 2018, 265, 2125–2136. [Google Scholar] [CrossRef] [PubMed]
- Chipika, R.H.; Mulkerrin, G.; Pradat, P.F.; Murad, A.; Ango, F.; Raoul, C.; Bede, P. Cerebellar pathology in motor neuron disease: Neuroplasticity and neurodegeneration. Neural Regen. Res. 2022, 17, 2335–2341. [Google Scholar] [CrossRef]
- Pradat, P.F.; Bruneteau, G.; Munerati, E.; Salachas, F.; Le Forestier, N.; Lacomblez, L.; Lenglet, T.; Meininger, V. Extrapyramidal stiffness in patients with amyotrophic lateral sclerosis. Mov. Disord. 2009, 24, 2143–2148. [Google Scholar] [CrossRef] [PubMed]
- Bede, P.; Chipika, R.H.; Christidi, F.; Hengeveld, J.C.; Karavasilis, E.; Argyropoulos, G.D.; Lope, J.; Li Hi Shing, S.; Velonakis, G.; Dupuis, L.; et al. Genotype-associated cerebellar profiles in ALS: Focal cerebellar pathology and cerebro-cerebellar connectivity alterations. J. Neurol. Neurosurg. Psychiatry 2021, 92, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Nakamagoe, K.; Yamada, S.; Kawakami, R.; Miyake, Z.; Tozaka, N.; Okune, S.; Takeda, H.; Koganezawa, T.; Tamaoka, A. Vestibular dysfunction as cortical damage with amyotrophic lateral sclerosis. J. Neurol. Sci. 2019, 397, 4–8. [Google Scholar] [CrossRef]
- Tahedl, M.; Tan, E.L.; Kleinerova, J.; Delaney, S.; Hengeveld, J.C.; Doherty, M.A.; McLaughlin, R.L.; Pradat, P.F.; Raoul, C.; Ango, F.; et al. Progressive Cerebrocerebellar Uncoupling in Sporadic and Genetic Forms of Amyotrophic Lateral Sclerosis. Neurology 2024, 103, e209623. [Google Scholar] [CrossRef] [PubMed]
- Krieg, I.; Dalin, D.; Heimbach, B.; Wiesmeier, I.K.; Maurer, C. Abnormal trunk control determines postural abnormalities in Amyotrophic Lateral Sclerosis. NeuroRehabilitation 2019, 44, 599–608. [Google Scholar] [CrossRef]
- Radovanovic, S.; Milicev, M.; Peric, S.; Basta, I.; Kostic, V.; Stevic, Z. Gait in amyotrophic lateral sclerosis: Is gait pattern differently affected in spinal and bulbar onset of the disease during dual task walking? Amyotroph. Lateral Scler. Front. Degener. 2014, 15, 488–493. [Google Scholar] [CrossRef]
- Liao, F.; Wang, J.; He, P. Multi-resolution entropy analysis of gait symmetry in neurological degenerative diseases and amyotrophic lateral sclerosis. Med. Eng. Phys. 2008, 30, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Sanjak, M.; Hirsch, M.A.; Bravver, E.K.; Bockenek, W.L.; Norton, H.J.; Brooks, B.R. Vestibular deficits leading to disequilibrium and falls in ambulatory amyotrophic lateral sclerosis. Arch. Phys. Med. Rehabil. 2014, 95, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Lukomski, M.; Klimek, A. Electronystagmographic examination in patients with amyotrophic lateral sclerosis (ALS). Neurol. I Neurochir. Pol. 1993, 27, 493–498. [Google Scholar]
- Liu, X.; Zhang, S.; Huang, X.; Zhang, Y.; Fan, D. Vestibular evoked myogenic potentials and their clinical utility in patients with amyotrophic lateral sclerosis. Clin. Neurophysiol. 2019, 130, 647–654. [Google Scholar] [CrossRef]
- Bede, P.; Elamin, M.; Byrne, S.; McLaughlin, R.L.; Kenna, K.; Vajda, A.; Fagan, A.; Bradley, D.G.; Hardiman, O. Patterns of cerebral and cerebellar white matter degeneration in ALS. J. Neurol. Neurosurg. Psychiatry 2015, 86, 468–470. [Google Scholar] [CrossRef]
- Pelletier, C.; Abou-Zeid, E.; Bartoshuk, L.; Rudnicki, S. Is Taste Altered in Patients with ALS? Chemosens. Percept. 2013, 6, 101–107. [Google Scholar] [CrossRef]
- Lang, C.J.; Schwandner, K.; Hecht, M. Do patients with motor neuron disease suffer from disorders of taste or smell? Amyotroph. Lateral Scler. 2011, 12, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Mogyoros, I.; Kiernan, M.C.; Burke, D.; Bostock, H. Ischemic resistance of cutaneous afferents and motor axons in patients with amyotrophic lateral sclerosis. Muscle Nerve 1998, 21, 1692–1700. [Google Scholar] [CrossRef]
- Jesus, P.; Massoulard, A.; Marin, B.; Nicol, M.; Laplagne, O.; Baptiste, A.; Gindre-Poulvelarie, L.; Couratier, P.; Fraysse, J.L.; Desport, J.C. First assessment at home of amyotrophic lateral sclerosis (ALS) patients by a nutrition network in the French region of Limousin. Amyotroph. Lateral Scler. 2012, 13, 538–543. [Google Scholar] [CrossRef]
- Ruoppolo, G.; Schettino, I.; Frasca, V.; Giacomelli, E.; Prosperini, L.; Cambieri, C.; Roma, R.; Greco, A.; Mancini, P.; De Vincentiis, M.; et al. Dysphagia in amyotrophic lateral sclerosis: Prevalence and clinical findings. Acta Neurol. Scand. 2013, 128, 397–401. [Google Scholar] [CrossRef]
- Steele, C.M.; Miller, A.J. Sensory input pathways and mechanisms in swallowing: A review. Dysphagia 2010, 25, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Santoso, L.F.; Kim, D.Y.; Paydarfar, D. Sensory dysphagia: A case series and proposed classification of an under recognized swallowing disorder. Head Neck 2019, 41, E71–E78. [Google Scholar] [CrossRef] [PubMed]
- Yunusova, Y.; Plowman, E.K.; Green, J.R.; Barnett, C.; Bede, P. Clinical Measures of Bulbar Dysfunction in ALS. Front. Neurol. 2019, 10, 106. [Google Scholar] [CrossRef]
- Shimizu, T.; Kawata, A.; Kato, S.; Hayashi, M.; Takamoto, K.; Hayashi, H.; Hirai, S.; Yamaguchi, S.; Komori, T.; Oda, M. Autonomic failure in ALS with a novel SOD1 gene mutation. Neurology 2000, 54, 1534–1537. [Google Scholar] [CrossRef]
- Pavlovic, S.; Stevic, Z.; Milovanovic, B.; Milicic, B.; Rakocevic-Stojanovic, V.; Lavrnic, D.; Apostolski, S. Impairment of cardiac autonomic control in patients with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2010, 11, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Shemisa, K.; Kaelber, D.; Parikh, S.A.; Mackall, J.A. Autonomic etiology of heart block in amyotrophic lateral sclerosis: A case report. J. Med. Case Rep. 2014, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Kawata, A.; Kato, S.; Hayashi, H.; Hirai, S. Prominent sensory and autonomic disturbances in familial amyotrophic lateral sclerosis with a Gly93Ser mutation in the SOD1 gene. J. Neurol. Sci. 1997, 153, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Hineno, A.; Nakamura, A.; Shimojima, Y.; Yoshida, K.; Oyanagai, K.; Ikeda, S. Distinctive clinicopathological features of 2 large families with amyotrophic lateral sclerosis having L106V mutation in SOD1 gene. J. Neurol. Sci. 2012, 319, 63–74. [Google Scholar] [CrossRef]
- Marjanović, I.V.; Selak-Djokić, B.; Perić, S.; Janković, M.; Arsenijević, V.; Basta, I.; Lavrnić, D.; Stefanova, E.; Stević, Z. Comparison of the clinical and cognitive features of genetically positive ALS patients from the largest tertiary center in Serbia. J. Neurol. 2017, 264, 1091–1098. [Google Scholar] [CrossRef]
- Beck, M.; Giess, R.; Magnus, T.; Puls, I.; Reiners, K.; Toyka, K.V.; Naumann, M. Progressive sudomotor dysfunction in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2002, 73, 68–70. [Google Scholar] [CrossRef]
- Dalla Vecchia, L.; De Maria, B.; Marinou, K.; Sideri, R.; Lucini, A.; Porta, A.; Mora, G. Cardiovascular neural regulation is impaired in amyotrophic lateral sclerosis patients. A study by spectral and complexity analysis of cardiovascular oscillations. Physiol. Meas. 2015, 36, 659–670. [Google Scholar] [CrossRef]
- Lopes, L.C.G.; Galhardoni, R.; Silva, V.; Jorge, F.M.H.; Yeng, L.T.; Callegaro, D.; Chadi, G.; Teixeira, M.J.; Ciampi de Andrade, D. Beyond weakness: Characterization of pain, sensory profile and conditioned pain modulation in patients with motor neuron disease: A controlled study. Eur. J. Pain 2018, 22, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Truini, A.; Biasiotta, A.; Onesti, E.; Di Stefano, G.; Ceccanti, M.; La Cesa, S.; Pepe, A.; Giordano, C.; Cruccu, G.; Inghilleri, M. Small-fibre neuropathy related to bulbar and spinal-onset in patients with ALS. J. Neurol. 2015, 262, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.; Grisan, E.; Scarpa, F.; Fazio, R.; Comola, M.; Quattrini, A.; Comi, G.; Rama, P.; Riva, N. Corneal confocal microscopy reveals trigeminal small sensory fiber neuropathy in amyotrophic lateral sclerosis. Front. Aging Neurosci. 2014, 6, 278. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, J.D.; Martin, L.J.; Kuncl, R.W. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. New Engl. J. Med. 1992, 326, 1464–1468. [Google Scholar] [CrossRef]
- Wirguin, I.; Brenner, T.; Argov, Z.; Steiner, I. Multifocal motor nerve conduction abnormalities in amyotrophic lateral sclerosis. J. Neurol. Sci. 1992, 112, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Ben Hamida, M.; Hentati, F. Charcot’s disease and juvenile amyotrophic lateral sclerosis. Rev. Neurol. 1984, 140, 202–206. [Google Scholar] [PubMed]
- Jokelainen, M. Amyotrophic lateral sclerosis in Finland. II: Clinical characteristics. Acta Neurol. Scand. 1977, 56, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Alter, M.; Schaumann, B. Hereditary Amyotrophic Lateral Sclerosis. A report of two families. Eur. Neurol. 1976, 14, 250–265. [Google Scholar] [CrossRef]
- Isak, B.; Tankisi, H.; Johnsen, B.; Pugdahl, K.; Torvin MØLler, A.; Finnerup, N.B.; Christensen, P.B.; Fuglsang-Frederiksen, A. Involvement of distal sensory nerves in amyotrophic lateral sclerosis. Muscle Nerve 2016, 54, 1086–1092. [Google Scholar] [CrossRef]
- Kulkantrakorn, K.; Suksasunee, D. Clinical, electrodiagnostic, and outcome correlation in ALS patients in Thailand. J. Clin. Neurosci. 2017, 43, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, X.; Ding, X.; Song, M.; Sui, K. Analysis of clinical and electrophysiological characteristics of 150 patients with amyotrophic lateral sclerosis in China. Neurol. Sci. 2019, 40, 363–369. [Google Scholar] [CrossRef]
- Ren, Y.T.; Cui, F.; Yang, F.; Chen, Z.H.; Ling, L.; Huang, X.S. An analysis of characteristics of nerve conduction in 154 cases of amyotrophic lateral sclerosis. Zhonghua Nei Ke Za Zhi 2016, 55, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Shefner, J.M.; Tyler, H.R.; Krarup, C. Abnormalities in the sensory action potential in patients with amyotrophic lateral sclerosis. Muscle Nerve 1991, 14, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Theys, P.A.; Peeters, E.; Robberecht, W. Evolution of motor and sensory deficits in amyotrophic lateral sclerosis estimated by neurophysiological techniques. J. Neurol. 1999, 246, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Pegat, A.; Bouhour, F.; Mouzat, K.; Vial, C.; Pegat, B.; Leblanc, P.; Broussolle, E.; Millecamps, S.; Lumbroso, S.; Bernard, E. Electrophysiological Characterization of C9ORF72-Associated Amyotrophic Lateral Sclerosis: A Retrospective Study. Eur. Neurol. 2019, 82, 106–112. [Google Scholar] [CrossRef]
- Behnia, M.; Kelly, J.J. Role of electromyography in amyotrophic lateral sclerosis. Muscle Nerve 1991, 14, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Imai, E.; Nakamura, T.; Atsuta, N.; Nakatochi, M.; Suzuki, M.; Harada, Y.; Nakamura, R.; Hayashi, N.; Sobue, G.; Katsuno, M. A nerve conduction study predicts the prognosis of sporadic amyotrophic lateral sclerosis. J. Neurol. 2020, 267, 2524–2532. [Google Scholar] [CrossRef]
- Dasheiff, R.M.; Drake, M.E.; Brendle, A.; Erwin, C.W. Abnormal somatosensory evoked potentials in amyotrophic lateral sclerosis. Electroencephalogr. Clin. Neurophysiol. 1985, 60, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Sangari, S.; Giron, A.; Marrelec, G.; Pradat, P.F.; Marchand-Pauvert, V. Abnormal cortical brain integration of somatosensory afferents in ALS. Clin. Neurophysiol. 2018, 129, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Bokuda, K.; Kimura, H.; Kamiyama, T.; Nakayama, Y.; Kawata, A.; Isozaki, E.; Ugawa, Y. Sensory cortex hyperexcitability predicts short survival in amyotrophic lateral sclerosis. Neurology 2018, 90, e1578–e1587. [Google Scholar] [CrossRef] [PubMed]
- Isak, B.; Tankisi, H.; Johnsen, B.; Pugdahl, K.; Finnerup, N.B.; Fuglsang-Frederiksen, A. Laser and somatosensory evoked potentials in amyotrophic lateral sclerosis. Clin. Neurophysiol. 2016, 127, 3322–3328. [Google Scholar] [CrossRef]
- Subramaniam, J.S.; Yiannikas, C. Multimodality evoked potentials in motor neuron disease. Arch. Neurol. 1990, 47, 989–994. [Google Scholar] [CrossRef]
- Georgesco, M.; Salerno, A.; Carlander, B.; Léger, J.J.; Camu, W.; Billiard, M.; Cadilhac, J. Somatosensory evoked potentials in amyotrophic lateral sclerosis and primary lateral sclerosis. Rev. Neurol. 1994, 150, 292–298. [Google Scholar]
- Constantinovici, A. Abnormal somatosensory evoked potentials in amyotrophic lateral sclerosis. Rom. J. Neurol. Psychiatry 1993, 31, 273–278. [Google Scholar] [PubMed]
- Mondelli, M.; Rossi, A.; Passero, S.; Guazzi, G.C. Involvement of peripheral sensory fibers in amyotrophic lateral sclerosis: Electrophysiological study of 64 cases. Muscle Nerve 1993, 16, 166–172. [Google Scholar] [CrossRef]
- Koszewicz, M.; Bilińska, M.; Podemski, R. Electrophysiological estimation of the peripheral nerves conduction parameters and the autonomic nervous system function in the course of amyotrophic lateral sclerosis. Neurol. I Neurochir. Pol. 2005, 39, 351–357. [Google Scholar]
- de Carvalho, M.; Swash, M. Nerve conduction studies in amyotrophic lateral sclerosis. Muscle Nerve 2000, 23, 344–352. [Google Scholar] [CrossRef]
- Berardelli, A.; Inghilleri, M.; Formisano, R.; Accornero, N.; Manfredi, M. Stimulation of motor tracts in motor neuron disease. J. Neurol. Neurosurg. Psychiatry 1987, 50, 732–737. [Google Scholar] [CrossRef]
- Swash, M. Sensorimotor integration is problematic in amyotrophic lateral sclerosis. Clin. Neurophysiol. 2018, 129, 849–850. [Google Scholar] [CrossRef] [PubMed]
- Harada, Y.; Nakamura, T.; Suzuki, M.; Ueda, M.; Hirayama, M.; Katsuno, M. Impaired pain processing and its association with attention disturbance in patients with amyotrophic lateral sclerosis. Neurol. Sci. 2021, 42, 3327–3335. [Google Scholar] [CrossRef]
- Norioka, R.; Shimizu, T.; Bokuda, K.; Morishima, R.; Kawazoe, T.; Kimura, H.; Asano, Y.; Nakayama, Y.; Takahashi, K. Enlarged high frequency oscillations of the median nerve somatosensory evoked potential and survival in amyotrophic lateral sclerosis. Clin. Neurophysiol. 2021, 132, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Nardone, R.; Golaszewski, S.; Thomschewski, A.; Sebastianelli, L.; Versace, V.; Brigo, F.; Orioli, A.; Saltuari, L.; Höller, Y.; Trinka, E. Disinhibition of sensory cortex in patients with amyotrophic lateral sclerosis. Neurosci. Lett. 2020, 722, 134860. [Google Scholar] [CrossRef] [PubMed]
- Höffken, O.; Schmelz, A.; Lenz, M.; Gruhn, K.; Grehl, T.; Tegenthoff, M.; Sczesny-Kaiser, M. Excitability in somatosensory cortex correlates with motoric impairment in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2019, 20, 192–198. [Google Scholar] [CrossRef]
- Matamala, J.M.; Howells, J.; Dharmadasa, T.; Huynh, W.; Park, S.B.; Burke, D.; Kiernan, M.C. Excitability of sensory axons in amyotrophic lateral sclerosis. Clin. Neurophysiol. 2018, 129, 1472–1478. [Google Scholar] [CrossRef]
- Jin, X.; Jiang, J.Y.; Lu, F.Z.; Xia, X.L.; Wang, L.X.; Zheng, C.J. Electrophysiological differences between Hirayama disease, amyotrophic lateral sclerosis and cervical spondylotic amyotrophy. BMC Musculoskelet. Disord. 2014, 15, 349. [Google Scholar] [CrossRef]
- Simone, I.L.; Tortelli, R.; Samarelli, V.; D’Errico, E.; Sardaro, M.; Difruscolo, O.; Calabrese, R.; Francesco Vde, V.; Livrea, P.; de Tommaso, M. Laser evoked potentials in amyotrophic lateral sclerosis. J. Neurol. Sci. 2010, 288, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Hamada, M.; Hanajima, R.; Terao, Y.; Sato, F.; Okano, T.; Yuasa, K.; Furubayashi, T.; Okabe, S.; Arai, N.; Ugawa, Y. Median nerve somatosensory evoked potentials and their high-frequency oscillations in amyotrophic lateral sclerosis. Clin. Neurophysiol. 2007, 118, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Ogata, K.; Tobimatsu, S.; Furuya, H.; Kira, J. Sporadic amyotrophic lateral sclerosis showing abnormal somatosensory evoked potentials: A report of three cases. Fukuoka Igaku Zasshi 2001, 92, 242–250. [Google Scholar]
- Matsumoto, A.; Kawashima, A.; Doi, S.; Moriwaka, F.; Tashiro, K. The spinal somatosensory evoked potentials in amyotrophic lateral sclerosis in relation to the spinal cord conduction velocities. No Shinkei 1999, 51, 41–47. [Google Scholar]
- Schulte-Mattler, W.J.; Jakob, M.; Zierz, S. Focal sensory nerve abnormalities in patients with amyotrophic lateral sclerosis. J. Neurol. Sci. 1999, 162, 189–193. [Google Scholar] [CrossRef]
- Emeryk-Szajewska, B.; Kostera-Pruszczyk, A.; Rowińska-Marcińska, K.; Karwańska, A. Median nerve electrophysiological assessment in amyotrophic lateral sclerosis. Neurol. I Neurochir. Pol. 1998, 32, 39–49. [Google Scholar]
- Mogyoros, I.; Kiernan, M.C.; Burke, D.; Bostock, H. Strength-duration properties of sensory and motor axons in amyotrophic lateral sclerosis. Brain 1998, 121, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Georgesco, M.; Salerno, A.; Camu, W. Somatosensory evoked potentials elicited by stimulation of lower-limb nerves in amyotrophic lateral sclerosis. Electroencephalogr. Clin. Neurophysiol. 1997, 104, 333–342. [Google Scholar] [CrossRef]
- Zanette, G.; Tinazzi, M.; Polo, A.; Rizzuto, N. Motor neuron disease with pyramidal tract dysfunction involves the cortical generators of the early somatosensory evoked potential to tibial nerve stimulation. Neurology 1996, 47, 932–938. [Google Scholar] [CrossRef]
- Palma, V.; Guadagnino, M.; Brescia Morra, V.; Nolfe, G. Multimodality evoked potentials in sporadic amyotrophic lateral sclerosis: A statistical approach. Electromyogr. Clin. Neurophysiol. 1993, 33, 167–171. [Google Scholar] [PubMed]
- Gao, X.X.; Tang, X. Relation between the clinical manifestations and electromyographic findings in motor neurone disease. Zhonghua Shen Jing Jing Shen Ke Za Zhi 1991, 24, 98–100. [Google Scholar]
- Zanette, G.; Polo, A.; Gasperini, M.; Bertolasi, L.; De Grandis, D. Far-field and cortical somatosensory evoked potentials in motor neuron disease. Muscle Nerve 1990, 13, 47–55. [Google Scholar] [CrossRef]
- Facco, E.; Micaglio, G.; Liviero, M.C.; Ceccato, M.B.; Toffoletto, F.; Martinuzzi, A.; Angelini, C. Sensory-motor conduction time in amyotrophic lateral sclerosis. Riv. Di Neurol. 1989, 59, 108–112. [Google Scholar]
- Cosi, V.; Poloni, M.; Mazzini, L.; Callieco, R. Somatosensory evoked potentials in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 1984, 47, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Fatima, M.; Tan, R.; Halliday, G.M.; Kril, J.J. Spread of pathology in amyotrophic lateral sclerosis: Assessment of phosphorylated TDP-43 along axonal pathways. Acta Neuropathol. Commun. 2015, 3, 47. [Google Scholar] [CrossRef] [PubMed]
- Verde, F.; Del Tredici, K.; Braak, H.; Ludolph, A. The multisystem degeneration amyotrophic lateral sclerosis—neuropathological staging and clinical translation. Arch. Ital. De Biol. 2017, 155, 118–130. [Google Scholar] [CrossRef]
- Geser, F.; Brandmeir, N.J.; Kwong, L.K.; Martinez-Lage, M.; Elman, L.; McCluskey, L.; Xie, S.X.; Lee, V.M.; Trojanowski, J.Q. Evidence of multisystem disorder in whole-brain map of pathological TDP-43 in amyotrophic lateral sclerosis. Arch. Neurol. 2008, 65, 636–641. [Google Scholar] [CrossRef]
- Geser, F.; Martinez-Lage, M.; Robinson, J.; Uryu, K.; Neumann, M.; Brandmeir, N.J.; Xie, S.X.; Kwong, L.K.; Elman, L.; McCluskey, L.; et al. Clinical and pathological continuum of multisystem TDP-43 proteinopathies. Arch. Neurol. 2009, 66, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Behler, A.; Müller, H.P.; Del Tredici, K.; Braak, H.; Ludolph, A.C.; Lulé, D.; Kassubek, J. Multimodal in vivo staging in amyotrophic lateral sclerosis using artificial intelligence. Ann. Clin. Transl. Neurol. 2022, 9, 1069–1079. [Google Scholar] [CrossRef]
- Baldaranov, D.; Khomenko, A.; Kobor, I.; Bogdahn, U.; Gorges, M.; Kassubek, J.; Muller, H.P. Longitudinal Diffusion Tensor Imaging-Based Assessment of Tract Alterations: An Application to Amyotrophic Lateral Sclerosis. Front. Hum. Neurosci. 2017, 11, 567. [Google Scholar] [CrossRef] [PubMed]
- Kassubek, J.; Muller, H.P.; Del Tredici, K.; Brettschneider, J.; Pinkhardt, E.H.; Lule, D.; Bohm, S.; Braak, H.; Ludolph, A.C. Diffusion tensor imaging analysis of sequential spreading of disease in amyotrophic lateral sclerosis confirms patterns of TDP-43 pathology. Brain 2014, 137, 1733–1740. [Google Scholar] [CrossRef] [PubMed]
- Kassubek, J.; Müller, H.P.; Del Tredici, K.; Lulé, D.; Gorges, M.; Braak, H.; Ludolph, A.C. Imaging the pathoanatomy of amyotrophic lateral sclerosis in vivo: Targeting a propagation-based biological marker. J. Neurol. Neurosurg. Psychiatry 2018, 89, 374–381. [Google Scholar] [CrossRef]
- Müller, H.P.; Del Tredici, K.; Lulé, D.; Müller, K.; Weishaupt, J.H.; Ludolph, A.C.; Kassubek, J. In vivo histopathological staging in C9orf72-associated ALS: A tract of interest DTI study. NeuroImage Clin. 2020, 27, 102298. [Google Scholar] [CrossRef]
- Kawamura, Y.; Dyck, P.J.; Shimono, M.; Okazaki, H.; Tateishi, J.; Doi, H. Morphometric comparison of the vulnerability of peripheral motor and sensory neurons in amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 1981, 40, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.M.; South, P.W. The topographic distribution of brain atrophy in frontal lobe dementia. Acta Neuropathol. 1993, 85, 334–340. [Google Scholar] [CrossRef]
- Mackenzie, I.R.A.; Neumann, M.; Baborie, A.; Sampathu, D.M.; Du Plessis, D.; Jaros, E.; Perry, R.H.; Trojanowski, J.Q.; Mann, D.M.A.; Lee, V.M.Y. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011, 122, 111–113. [Google Scholar] [CrossRef]
- McKenna, M.C.; Lope, J.; Bede, P.; Tan, E.L. Thalamic pathology in frontotemporal dementia: Predilection for specific nuclei, phenotype-specific signatures, clinical correlates, and practical relevance. Brain Behav. 2023, 13, e2881. [Google Scholar] [CrossRef]
- De Reuck, J.; Devos, D.; Moreau, C.; Auger, F.; Durieux, N.; Deramecourt, V.; Pasquier, F.; Maurage, C.A.; Cordonnier, C.; Leys, D.; et al. Topographic distribution of brain iron deposition and small cerebrovascular lesions in amyotrophic lateral sclerosis and in frontotemporal lobar degeneration: A post-mortem 7.0-tesla magnetic resonance imaging study with neuropathological correlates. Acta Neurol. Belg. 2017, 117, 873–878. [Google Scholar] [CrossRef]
- Davidson, Y.; Robinson, A.C.; Liu, X.; Wu, D.; Troakes, C.; Rollinson, S.; Masuda-Suzukake, M.; Suzuki, G.; Nonaka, T.; Shi, J.; et al. Neurodegeneration in frontotemporal lobar degeneration and motor neurone disease associated with expansions in C9orf72 is linked to TDP-43 pathology and not associated with aggregated forms of dipeptide repeat proteins. Neuropathol. Appl. Neurobiol. 2016, 42, 242–254. [Google Scholar] [CrossRef]
- Troakes, C.; Maekawa, S.; Wijesekera, L.; Rogelj, B.; Siklos, L.; Bell, C.; Smith, B.; Newhouse, S.; Vance, C.; Johnson, L.; et al. An MND/ALS phenotype associated with C9orf72 repeat expansion: Abundant p62-positive, TDP-43-negative inclusions in cerebral cortex, hippocampus and cerebellum but without associated cognitive decline. Neuropathology 2012, 32, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Oyanagi, K.; Mochizuki, Y.; Nakayama, Y.; Hayashi, K.; Shimizu, T.; Nagao, M.; Hashimoto, T.; Yamazaki, M.; Matsubara, S.; Komori, T. Marked preservation of the visual and olfactory pathways in ALS patients in a totally locked-in state. Clin. Neuropathol. 2015, 34, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Adad, J.; El Mendili, M.M.; Morizot-Koutlidis, R.; Lehericy, S.; Meininger, V.; Blancho, S.; Rossignol, S.; Benali, H.; Pradat, P.F. Involvement of spinal sensory pathway in ALS and specificity of cord atrophy to lower motor neuron degeneration. Amyotroph. Lateral Scler. Front. Degener. 2013, 14, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Bradley, W.G.; Good, P.; Rasool, C.G.; Adelman, L.S. Morphometric and biochemical studies of peripheral nerves in amyotrophic lateral sclerosis. Ann. Neurol. 1983, 14, 267–277. [Google Scholar] [CrossRef]
- Heads, T.; Pollock, M.; Robertson, A.; Sutherland, W.H.; Allpress, S. Sensory nerve pathology in amyotrophic lateral sclerosis. Acta Neuropathol. 1991, 82, 316–320. [Google Scholar] [CrossRef]
- Luigetti, M.; Conte, A.; Del Grande, A.; Bisogni, G.; Romano, A.; Sabatelli, M. Sural nerve pathology in ALS patients: A single-centre experience. Neurol. Sci. 2012, 33, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Devigili, G.; Uçeyler, N.; Beck, M.; Reiners, K.; Stoll, G.; Toyka, K.V.; Sommer, C. Vasculitis-like neuropathy in amyotrophic lateral sclerosis unresponsive to treatment. Acta Neuropathol. 2011, 122, 343–352. [Google Scholar] [CrossRef]
- Sawa, N.; Kataoka, H.; Sugie, K.; Kawahara, M.; Horikawa, H.; Kusunoki, S.; Ueno, S. Clinical analysis and outcomes of amyotrophic lateral sclerosis with demyelinating polyneuropathy. Amyotroph. Lateral Scler. 2012, 13, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Ben Hamida, M.; Letaief, F.; Hentati, F.; Ben Hamida, C. Morphometric study of the sensory nerve in classical (or Charcot disease) and juvenile amyotrophic lateral sclerosis. J. Neurol. Sci. 1987, 78, 313–329. [Google Scholar] [CrossRef]
- Vaughan, S.K.; Sutherland, N.M.; Zhang, S.; Hatzipetros, T.; Vieira, F.; Valdez, G. The ALS-inducing factors, TDP43(A315T) and SOD1(G93A), directly affect and sensitize sensory neurons to stress. Sci. Rep. 2018, 8, 16582. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, S.K.; Kemp, Z.; Hatzipetros, T.; Vieira, F.; Valdez, G. Degeneration of proprioceptive sensory nerve endings in mice harboring amyotrophic lateral sclerosis-causing mutations. J. Comp. Neurol. 2015, 523, 2477–2494. [Google Scholar] [CrossRef] [PubMed]
- Marcuzzo, S.; Bonanno, S.; Figini, M.; Scotti, A.; Zucca, I.; Minati, L.; Riva, N.; Domi, T.; Fossaghi, A.; Quattrini, A.; et al. A longitudinal DTI and histological study of the spinal cord reveals early pathological alterations in G93A-SOD1 mouse model of amyotrophic lateral sclerosis. Exp. Neurol. 2017, 293, 43–52. [Google Scholar] [CrossRef]
- Filali, M.; Lalonde, R.; Rivest, S. Sensorimotor and cognitive functions in a SOD1(G37R) transgenic mouse model of amyotrophic lateral sclerosis. Behav. Brain Res. 2011, 225, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, A.; Murray, E.A. Functional interaction of medial mediodorsal thalamic nucleus but not nucleus accumbens with amygdala and orbital prefrontal cortex is essential for adaptive response selection after reinforcer devaluation. J. Neurosci. 2010, 30, 661–669. [Google Scholar] [CrossRef]
- Fischer, L.R.; Culver, D.G.; Davis, A.A.; Tennant, P.; Wang, M.; Coleman, M.; Asress, S.; Adalbert, R.; Alexander, G.M.; Glass, J.D. The WldS gene modestly prolongs survival in the SOD1G93A fALS mouse. Neurobiol. Dis. 2005, 19, 293–300. [Google Scholar] [CrossRef]
- Guo, Y.S.; Wu, D.X.; Wu, H.R.; Wu, S.Y.; Yang, C.; Li, B.; Bu, H.; Zhang, Y.S.; Li, C.Y. Sensory involvement in the SOD1-G93A mouse model of amyotrophic lateral sclerosis. Exp. Mol. Med. 2009, 41, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.A.; Herrando-Grabulosa, M.; Vilches, J.J.; Navarro, X. Involvement of sensory innervation in the skin of SOD1(G93A) ALS mice. J. Peripher. Nerv. Syst. JPNS 2016, 21, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Schuster, J.E.; Fu, R.; Siddique, T.; Heckman, C.J. Progressive changes in synaptic inputs to motoneurons in adult sacral spinal cord of a mouse model of amyotrophic lateral sclerosis. J. Neurosci. 2009, 29, 15031–15038. [Google Scholar] [CrossRef]
- Cowin, G.J.; Butler, T.J.; Kurniawan, N.D.; Watson, C.; Wallace, R.H. Magnetic resonance microimaging of the spinal cord in the SOD1 mouse model of amyotrophic lateral sclerosis detects motor nerve root degeneration. Neuroimage 2011, 58, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Bernard-Marissal, N.; Médard, J.J.; Azzedine, H.; Chrast, R. Dysfunction in endoplasmic reticulum-mitochondria crosstalk underlies SIGMAR1 loss of function mediated motor neuron degeneration. Brain 2015, 138, 875–890. [Google Scholar] [CrossRef]
- Mehta, A.R.; Gregory, J.M.; Dando, O.; Carter, R.N.; Burr, K.; Nanda, J.; Story, D.; McDade, K.; Smith, C.; Morton, N.M.; et al. Mitochondrial bioenergetic deficits in C9orf72 amyotrophic lateral sclerosis motor neurons cause dysfunctional axonal homeostasis. Acta Neuropathol. 2021, 141, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Shankar, L.; Shankar, S.K.; Santosh, V.; Taly, A.B.; Nagaraja, D.; Devi, G.; Satishchandra, P.; Swamy, H.S.; Das, S.; Nagraj, D.; et al. Light and ultrastructural pathology of spinal cords in sporadic forms of amyotrophic lateral sclerosis from South Asia. Neurol. India 1995, 43, 83–90. [Google Scholar] [PubMed]
- Bączyk, M.; Alami, N.O.; Delestrée, N.; Martinot, C.; Tang, L.; Commisso, B.; Bayer, D.; Doisne, N.; Frankel, W.; Manuel, M.; et al. Synaptic restoration by cAMP/PKA drives activity-dependent neuroprotection to motoneurons in ALS. J. Exp. Med. 2020, 217, e20191734. [Google Scholar] [CrossRef] [PubMed]
- Peng, A.Y.T.; Agrawal, I.; Ho, W.Y.; Yen, Y.C.; Pinter, A.J.; Liu, J.; Phua, Q.X.C.; Koh, K.B.; Chang, J.C.; Sanford, E.; et al. Loss of TDP-43 in astrocytes leads to motor deficits by triggering A1-like reactive phenotype and triglial dysfunction. Proc. Natl. Acad. Sci. USA 2020, 117, 29101–29112. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Soto, M.; Riancho, J.; Tapia, O.; Lafarga, M.; Berciano, M.T. Satellite Glial Cells of the Dorsal Root Ganglion: A New “Guest/Physiopathological Target” in ALS. Front. Aging Neurosci. 2020, 12, 595751. [Google Scholar] [CrossRef] [PubMed]
- Weerasekera, A.; Crabbé, M.; Tomé, S.O.; Gsell, W.; Sima, D.; Casteels, C.; Dresselaers, T.; Deroose, C.; Van Huffel, S.; Rudolf Thal, D.; et al. Non-invasive characterization of amyotrophic lateral sclerosis in a hTDP-43(A315T) mouse model: A PET-MR study. Neuroimage Clin. 2020, 27, 102327. [Google Scholar] [CrossRef]
- Seki, S.; Yamamoto, T.; Quinn, K.; Spigelman, I.; Pantazis, A.; Olcese, R.; Wiedau-Pazos, M.; Chandler, S.H.; Venugopal, S. Circuit-Specific Early Impairment of Proprioceptive Sensory Neurons in the SOD1(G93A) Mouse Model for ALS. J. Neurosci. 2019, 39, 8798–8815. [Google Scholar] [CrossRef] [PubMed]
- Bede, P.; Bokde, A.L.; Byrne, S.; Elamin, M.; Fagan, A.J.; Hardiman, O. Spinal cord markers in ALS: Diagnostic and biomarker considerations. Amyotroph. Lateral Scler. 2012, 13, 407–415. [Google Scholar] [CrossRef] [PubMed]
- El Mendili, M.M.; Querin, G.; Bede, P.; Pradat, P.F. Spinal Cord Imaging in Amyotrophic Lateral Sclerosis: Historical Concepts-Novel Techniques. Front. Neurol. 2019, 10, 350. [Google Scholar] [CrossRef]
- Rasoanandrianina, H.; Grapperon, A.M.; Taso, M.; Girard, O.M.; Duhamel, G.; Guye, M.; Ranjeva, J.P.; Attarian, S.; Verschueren, A.; Callot, V. Region-specific impairment of the cervical spinal cord (SC) in amyotrophic lateral sclerosis: A preliminary study using SC templates and quantitative MRI (diffusion tensor imaging/inhomogeneous magnetization transfer). NMR Biomed. 2017, 30, e3801. [Google Scholar] [CrossRef]
- Chipika, R.H.; Finegan, E.; Li Hi Shing, S.; McKenna, M.C.; Christidi, F.; Chang, K.M.; Doherty, M.A.; Hengeveld, J.C.; Vajda, A.; Pender, N.; et al. “Switchboard” malfunction in motor neuron diseases: Selective pathology of thalamic nuclei in amyotrophic lateral sclerosis and primary lateral sclerosis. NeuroImage Clin. 2020, 27, 102300. [Google Scholar] [CrossRef] [PubMed]
- Chipika, R.H.; Siah, W.F.; Shing, S.L.H.; Finegan, E.; McKenna, M.C.; Christidi, F.; Chang, K.M.; Karavasilis, E.; Vajda, A.; Hengeveld, J.C.; et al. MRI data confirm the selective involvement of thalamic and amygdalar nuclei in amyotrophic lateral sclerosis and primary lateral sclerosis. Data Brief 2020, 32, 106246. [Google Scholar] [CrossRef] [PubMed]
- Machts, J.; Loewe, K.; Kaufmann, J.; Jakubiczka, S.; Abdulla, S.; Petri, S.; Dengler, R.; Heinze, H.J.; Vielhaber, S.; Schoenfeld, M.A.; et al. Basal ganglia pathology in ALS is associated with neuropsychological deficits. Neurology 2015, 85, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Christidi, F.; Kleinerova, J.; Tan, E.L.; Delaney, S.; Tacheva, A.; Hengeveld, J.C.; Doherty, M.A.; McLaughlin, R.L.; Hardiman, O.; Siah, W.F.; et al. Limbic Network and Papez Circuit Involvement in ALS: Imaging and Clinical Profiles in GGGGCC Hexanucleotide Carriers in C9orf72 and C9orf72-Negative Patients. Biology 2024, 13, 504. [Google Scholar] [CrossRef] [PubMed]
- Bocchetta, M.; Gordon, E.; Cardoso, M.J.; Modat, M.; Ourselin, S.; Warren, J.D.; Rohrer, J.D. Thalamic atrophy in frontotemporal dementia—Not just a C9orf72 problem. Neuroimage Clin. 2018, 18, 675–681. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.C.; Li Hi Shing, S.; Murad, A.; Lope, J.; Hardiman, O.; Hutchinson, S.; Bede, P. Focal thalamus pathology in frontotemporal dementia: Phenotype-associated thalamic profiles. J. Neurol. Sci. 2022, 436, 120221. [Google Scholar] [CrossRef]
- Bocchetta, M.; Iglesias, J.E.; Neason, M.; Cash, D.M.; Warren, J.D.; Rohrer, J.D. Thalamic nuclei in frontotemporal dementia: Mediodorsal nucleus involvement is universal but pulvinar atrophy is unique to C9orf72. Hum. Brain Mapp. 2020, 41, 1006–1016. [Google Scholar] [CrossRef]
- Tu, S.; Menke, R.A.L.; Talbot, K.; Kiernan, M.C.; Turner, M.R. Regional thalamic MRI as a marker of widespread cortical pathology and progressive frontotemporal involvement in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Menke, R.A.L.; Proudfoot, M.; Talbot, K.; Turner, M.R. The two-year progression of structural and functional cerebral MRI in amyotrophic lateral sclerosis. NeuroImage Clin. 2018, 17, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, H.; Li, C.; Yao, J.C.; Hu, J.; Wang, J.; Hu, Q.; Zhang, Y.; Zhang, J. Abnormal cortical-basal ganglia network in amyotrophic lateral sclerosis: A voxel-wise network efficiency analysis. Behav. Brain Res. 2017, 333, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Buhour, M.S.; Doidy, F.; Mondou, A.; Pélerin, A.; Carluer, L.; Eustache, F.; Viader, F.; Desgranges, B. Voxel-based mapping of grey matter volume and glucose metabolism profiles in amyotrophic lateral sclerosis. EJNMMI Res. 2017, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Q.; Ji, B.; Zhou, C.Y.; Li, L.C.; Li, Z.H.; Hu, X.P.; Hu, J. Differential Impairment of Thalamocortical Structural Connectivity in Amyotrophic Lateral Sclerosis. CNS Neurosci. Ther. 2017, 23, 155–161. [Google Scholar] [CrossRef]
- Masuda, M.; Senda, J.; Watanabe, H.; Epifanio, B.; Tanaka, Y.; Imai, K.; Riku, Y.; Li, Y.; Nakamura, R.; Ito, M.; et al. Involvement of the caudate nucleus head and its networks in sporadic amyotrophic lateral sclerosis-frontotemporal dementia continuum. Amyotroph. Lateral Scler. Front. Degener. 2016, 17, 571–579. [Google Scholar] [CrossRef]
- Van Laere, K.; Vanhee, A.; Verschueren, J.; De Coster, L.; Driesen, A.; Dupont, P.; Robberecht, W.; Van Damme, P. Value of 18fluorodeoxyglucose-positron-emission tomography in amyotrophic lateral sclerosis: A prospective study. JAMA Neurol. 2014, 71, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, G.; Nicoletti, G.; Cherubini, A.; Trotta, M.; Tallarico, T.; Chiriaco, C.; Nistico, R.; Salvino, D.; Bono, F.; Valentino, P.; et al. Diffusion tensor MRI changes in gray structures of the frontal-subcortical circuits in amyotrophic lateral sclerosis. Neurol. Sci. 2014, 35, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Aoki, S.; Iwata, N.K.; Abe, O.; Mori, H.; Ohtomo, K. Magnetic resonance imaging in patients of amyotrophic lateral sclerosis with and without dementia. Brain Nerve 2009, 61, 1259–1268. [Google Scholar]
- Li, S.; Chen, Q.; Yu, B.; Xue, K.; Luo, C.; Xu, Y.; Gong, Q.; He, C.; Zhou, D.; He, L.; et al. Structural and functional changes mapped in the brains of amyotrophic lateral sclerosis patients with/without dysphagia: A pilot study. Amyotroph. Lateral Scler. 2009, 10, 280–287. [Google Scholar] [CrossRef]
- Thivard, L.; Pradat, P.F.; Lehericy, S.; Lacomblez, L.; Dormont, D.; Chiras, J.; Benali, H.; Meininger, V. Diffusion tensor imaging and voxel based morphometry study in amyotrophic lateral sclerosis: Relationships with motor disability. J. Neurol. Neurosurg. Psychiatry 2007, 78, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.R.; Cagnin, A.; Turkheimer, F.E.; Miller, C.C.; Shaw, C.E.; Brooks, D.J.; Leigh, P.N.; Banati, R.B. Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: An [11C](R)-PK11195 positron emission tomography study. Neurobiol. Dis. 2004, 15, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Sach, M.; Winkler, G.; Glauche, V.; Liepert, J.; Heimbach, B.; Koch, M.A.; Buchel, C.; Weiller, C. Diffusion tensor MRI of early upper motor neuron involvement in amyotrophic lateral sclerosis. Brain 2004, 127, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.R.; Sheriff, S.; Maudsley, A.; Govind, V. Diffusion tensor imaging of basal ganglia and thalamus in amyotrophic lateral sclerosis. J. Neuroimaging 2013, 23, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.L.; Lomen-Hoerth, C.; Murphy, J.; Henry, R.G.; Kramer, J.H.; Miller, B.L.; Gorno-Tempini, M.L. A voxel-based morphometry study of patterns of brain atrophy in ALS and ALS/FTLD. Neurology 2005, 65, 75–80. [Google Scholar] [CrossRef]
- Ahmed, R.M.; Bocchetta, M.; Todd, E.G.; Tse, N.Y.; Devenney, E.M.; Tu, S.; Caga, J.; Hodges, J.R.; Halliday, G.M.; Irish, M.; et al. Tackling clinical heterogeneity across the amyotrophic lateral sclerosis-frontotemporal dementia spectrum using a transdiagnostic approach. Brain Commun. 2021, 3, fcab257. [Google Scholar] [CrossRef] [PubMed]
- Cosottini, M.; Pesaresi, I.; Piazza, S.; Diciotti, S.; Cecchi, P.; Fabbri, S.; Carlesi, C.; Mascalchi, M.; Siciliano, G. Structural and functional evaluation of cortical motor areas in Amyotrophic Lateral Sclerosis. Exp. Neurol. 2012, 234, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Grosskreutz, J.; Kaufmann, J.; Fradrich, J.; Dengler, R.; Heinze, H.J.; Peschel, T. Widespread sensorimotor and frontal cortical atrophy in Amyotrophic Lateral Sclerosis. BMC Neurol. 2006, 6, 17. [Google Scholar] [CrossRef]
- Barry, R.L.; Babu, S.; Anteraper, S.A.; Triantafyllou, C.; Keil, B.; Rowe, O.E.; Rangaprakash, D.; Paganoni, S.; Lawson, R.; Dheel, C.; et al. Ultra-high field (7T) functional magnetic resonance imaging in amyotrophic lateral sclerosis: A pilot study. NeuroImage Clinical 2021, 30, 102648. [Google Scholar] [CrossRef] [PubMed]
- Bede, P.; Elamin, M.; Byrne, S.; McLaughlin, R.L.; Kenna, K.; Vajda, A.; Pender, N.; Bradley, D.G.; Hardiman, O. Basal ganglia involvement in amyotrophic lateral sclerosis. Neurology 2013, 81, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.; Tai, P.; Genge, A.; Arnold, D.L. Rapid improvement in cortical neuronal integrity in amyotrophic lateral sclerosis detected by proton magnetic resonance spectroscopic imaging. J. Neurol. 2006, 253, 1060–1063. [Google Scholar] [CrossRef] [PubMed]
- Pioro, E.P.; Antel, J.P.; Cashman, N.R.; Arnold, D.L. Detection of cortical neuron loss in motor neuron disease by proton magnetic resonance spectroscopic imaging in vivo. Neurology 1994, 44, 1933–1938. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Xu, R.; Dowd, E.; Zang, Y.; Gong, H.; Wang, Z. Alterations in regional functional coherence within the sensory-motor network in amyotrophic lateral sclerosis. Neurosci. Lett. 2014, 558, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Pannek, K.; Bell, C.; Baumann, F.; Hutchinson, N.; Coulthard, A.; McCombe, P.; Henderson, R. Direct evidence of intra- and interhemispheric corticomotor network degeneration in amyotrophic lateral sclerosis: An automated MRI structural connectivity study. NeuroImage 2012, 59, 2661–2669. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Chen, Q.; Huang, R.; Chen, X.; Chen, K.; Huang, X.; Tang, H.; Gong, Q.; Shang, H.F. Patterns of spontaneous brain activity in amyotrophic lateral sclerosis: A resting-state FMRI study. PLoS ONE 2012, 7, e45470. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, S.; Goldstein, L.H.; Simmons, A.; Brammer, M.; Williams, S.C.; Giampietro, V.; Leigh, P.N. Word retrieval in amyotrophic lateral sclerosis: A functional magnetic resonance imaging study. Brain 2004, 127, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, M.; Bede, P.; Turner, M.R. Imaging Cerebral Activity in Amyotrophic Lateral Sclerosis. Front. Neurol. 2018, 9, 1148. [Google Scholar] [CrossRef]
- Bede, P.; Bokde, A.; Elamin, M.; Byrne, S.; McLaughlin, R.L.; Jordan, N.; Hampel, H.; Gallagher, L.; Lynch, C.; Fagan, A.J.; et al. Grey matter correlates of clinical variables in amyotrophic lateral sclerosis (ALS): A neuroimaging study of ALS motor phenotype heterogeneity and cortical focality. J. Neurol. Neurosurg. Psychiatry 2013, 84, 766–773. [Google Scholar] [CrossRef]
- Prell, T.; Hartung, V.; Tietz, F.; Penzlin, S.; Ilse, B.; Schweser, F.; Deistung, A.; Bokemeyer, M.; Reichenbach, J.R.; Witte, O.W.; et al. Susceptibility-weighted imaging provides insight into white matter damage in amyotrophic lateral sclerosis. PLoS ONE 2015, 10, e0131114. [Google Scholar] [CrossRef] [PubMed]
- Verma, G.; Woo, J.H.; Chawla, S.; Wang, S.; Sheriff, S.; Elman, L.B.; McCluskey, L.F.; Grossman, M.; Melhem, E.R.; Maudsley, A.A.; et al. Whole-Brain Analysis of Amyotrophic Lateral Sclerosis by Using Echo-Planar Spectroscopic Imaging. Radiology 2013, 267, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Meoded, A.; Kwan, J.Y.; Peters, T.L.; Huey, E.D.; Danielian, L.E.; Wiggs, E.; Morrissette, A.; Wu, T.; Russell, J.W.; Bayat, E.; et al. Imaging findings associated with cognitive performance in primary lateral sclerosis and amyotrophic lateral sclerosis. Dement. Geriatr. Cogn. Dis. Extra 2013, 3, 233–250. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zhang, Y.; Wang, Y.; Zhang, Y.; Hu, J.; Wang, J.; Zhang, J.; Jiang, T. Disrupted effective connectivity of the sensorimotor network in amyotrophic lateral sclerosis. J. Neurol. 2016, 263, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Huang, N.X.; Zou, T.X.; Chen, H.J. Brain Cortical Complexity Alteration in Amyotrophic Lateral Sclerosis: A Preliminary Fractal Dimensionality Study. BioMed Res. Int. 2020, 2020, 1521679. [Google Scholar] [CrossRef]
- Bueno, A.P.A.; Pinaya, W.H.L.; Moura, L.M.; Bertoux, M.; Radakovic, R.; Kiernan, M.C.; Teixeira, A.L.; de Souza, L.C.; Hornberger, M.; Sato, J.R. Structural and functional papez circuit integrity in amyotrophic lateral sclerosis. Brain Imaging Behav. 2018, 12, 1622–1630. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Liu, M.Q.; Ma, L. Gray Matter Volume Changes over the Whole Brain in the Bulbar- and Spinal-onset Amyotrophic Lateral Sclerosis: A Voxel-based Morphometry Study. Chin. Med. Sci. J. 2018, 33, 20–28. [Google Scholar] [CrossRef]
- Kim, H.J.; de Leon, M.; Wang, X.; Kim, H.Y.; Lee, Y.J.; Kim, Y.H.; Kim, S.H. Relationship between Clinical Parameters and Brain Structure in Sporadic Amyotrophic Lateral Sclerosis Patients According to Onset Type: A Voxel-Based Morphometric Study. PLoS ONE 2017, 12, e0168424. [Google Scholar] [CrossRef] [PubMed]
- de Albuquerque, M.; Anjos, L.G.; Maia Tavares de Andrade, H.; de Oliveira, M.S.; Castellano, G.; Junqueira Ribeiro de Rezende, T.; Nucci, A.; Franca Junior, M.C. MRI Texture Analysis Reveals Deep Gray Nuclei Damage in Amyotrophic Lateral Sclerosis. J. Neuroimaging 2016, 26, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.J.; McMillan, C.T.; Brettschneider, J.; Libon, D.J.; Powers, J.; Rascovsky, K.; Toledo, J.B.; Boller, A.; Bekisz, J.; Chandrasekaran, K.; et al. Cognitive decline and reduced survival in C9orf72 expansion frontotemporal degeneration and amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2013, 84, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Mioshi, E.; Lillo, P.; Yew, B.; Hsieh, S.; Savage, S.; Hodges, J.R.; Kiernan, M.C.; Hornberger, M. Cortical atrophy in ALS is critically associated with neuropsychiatric and cognitive changes. Neurology 2013, 80, 1117–1123. [Google Scholar] [CrossRef]
- Thorns, J.; Jansma, H.; Peschel, T.; Grosskreutz, J.; Mohammadi, B.; Dengler, R.; Münte, T.F. Extent of cortical involvement in amyotrophic lateral sclerosis--an analysis based on cortical thickness. BMC Neurol. 2013, 13, 148. [Google Scholar] [CrossRef]
- Mohammadi, B.; Kollewe, K.; Samii, A.; Krampfl, K.; Dengler, R.; Munte, T.F. Decreased brain activation to tongue movements in amyotrophic lateral sclerosis with bulbar involvement but not Kennedy syndrome. J. Neurol. 2009, 256, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Hayashi, H.; Yagishita, A. Involvement of the frontotemporal lobe and limbic system in amyotrophic lateral sclerosis: As assessed by serial computed tomography and magnetic resonance imaging. J. Neurol. Sci. 1993, 116, 52–58. [Google Scholar] [CrossRef]
- Rajagopalan, V.; Pioro, E.P. Corticospinal Tract and Related Grey Matter Morphometric Shape Analysis in ALS Phenotypes: A Fractal Dimension Study. Brain Sci. 2021, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, V.; Pioro, E.P. Differential involvement of corticospinal tract (CST) fibers in UMN-predominant ALS patients with or without CST hyperintensity: A diffusion tensor tractography study. Neuroimage Clin. 2017, 14, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Sheelakumari, R.; Madhusoodanan, M.; Radhakrishnan, A.; Ranjith, G.; Thomas, B. A Potential Biomarker in Amyotrophic Lateral Sclerosis: Can Assessment of Brain Iron Deposition with SWI and Corticospinal Tract Degeneration with DTI Help? AJNR Am. J. Neuroradiol. 2016, 37, 252–258. [Google Scholar] [CrossRef]
- Trojsi, F.; Caiazzo, G.; Corbo, D.; Piccirillo, G.; Cristillo, V.; Femiano, C.; Ferrantino, T.; Cirillo, M.; Monsurro, M.R.; Esposito, F.; et al. Microstructural changes across different clinical milestones of disease in amyotrophic lateral sclerosis. PLoS ONE 2015, 10, e0119045. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Oh, J.S.; Sung, J.J.; Lee, K.W.; Song, I.C.; Hong, Y.H. Diffusion tensor tractography analysis of the corpus callosum fibers in amyotrophic lateral sclerosis. J. Clin. Neurol. 2014, 10, 249–256. [Google Scholar] [CrossRef]
- Agosta, F.; Valsasina, P.; Absinta, M.; Riva, N.; Sala, S.; Prelle, A.; Copetti, M.; Comola, M.; Comi, G.; Filippi, M. Sensorimotor functional connectivity changes in amyotrophic lateral sclerosis. Cereb. Cortex 2011, 21, 2291–2298. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Lin, J.H.; Cai, L.M.; Shi, J.Y.; Zhang, X.H.; Zou, Z.Y.; Chen, H.J. Abnormal Stability of Dynamic Functional Architecture in Amyotrophic Lateral Sclerosis: A Preliminary Resting-State fMRI Study. Front. Neurol. 2021, 12, 744688. [Google Scholar] [CrossRef]
- Qiu, T.; Zhang, Y.; Tang, X.; Liu, X.; Wang, Y.; Zhou, C.; Luo, C.; Zhang, J. Precentral degeneration and cerebellar compensation in amyotrophic lateral sclerosis: A multimodal MRI analysis. Hum. Brain Mapp. 2019, 40, 3464–3474. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, B.; Hu, J.; Zhou, C.; Li, L.; Li, Z.; Huang, X.; Hu, X. Aberrant interhemispheric homotopic functional and structural connectivity in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2017, 88, 369–370. [Google Scholar] [CrossRef]
- Poujois, A.; Schneider, F.C.; Faillenot, I.; Camdessanche, J.P.; Vandenberghe, N.; Thomas-Anterion, C.; Antoine, J.C. Brain plasticity in the motor network is correlated with disease progression in amyotrophic lateral sclerosis. Hum. Brain Mapp. 2013, 34, 2391–2401. [Google Scholar] [CrossRef]
- Mohammadi, B.; Kollewe, K.; Samii, A.; Dengler, R.; Munte, T.F. Functional neuroimaging at different disease stages reveals distinct phases of neuroplastic changes in amyotrophic lateral sclerosis. Hum. Brain Mapp. 2011, 32, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Pisharady, P.K.; Eberly, L.E.; Cheong, I.; Manousakis, G.; Guliani, G.; Clark, H.B.; Bathe, M.; Walk, D.; Lenglet, C. Tract-specific analysis improves sensitivity of spinal cord diffusion MRI to cross-sectional and longitudinal changes in amyotrophic lateral sclerosis. Commun. Biol. 2020, 3, 370. [Google Scholar] [CrossRef] [PubMed]
- Olney, N.T.; Bischof, A.; Rosen, H.; Caverzasi, E.; Stern, W.A.; Lomen-Hoerth, C.; Miller, B.L.; Henry, R.G.; Papinutto, N. Measurement of spinal cord atrophy using phase sensitive inversion recovery (PSIR) imaging in motor neuron disease. PLoS ONE 2018, 13, e0208255. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Chen, W.; Wang, Z.; Xu, Y.; Luo, J.; Lin, H.; Sun, G. Detecting neuronal dysfunction of hand motor cortex in ALS: A MRSI study. Somatosens. Mot. Res. 2017, 34, 15–20. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Y.; Liu, L.; Ma, L.; Huang, X.; Lou, X.; Wang, Y.; Wu, N.; Liu, T.; Guo, X. Preliminary study on cervical spinal cord in patients with amyotrophic lateral sclerosis using MR diffusion tensor imaging. Acad. Radiol. 2014, 21, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Finegan, E.; Chipika, R.H.; Li Hi Shing, S.; Doherty, M.A.; Hengeveld, J.C.; Vajda, A.; Donaghy, C.; McLaughlin, R.L.; Pender, N.; Hardiman, O.; et al. The clinical and radiological profile of primary lateral sclerosis: A population-based study. J. Neurol. 2019, 266, 2718–2733. [Google Scholar] [CrossRef] [PubMed]
- Bede, P.; Chipika, R.H.; Finegan, E.; Li Hi Shing, S.; Doherty, M.A.; Hengeveld, J.C.; Vajda, A.; Hutchinson, S.; Donaghy, C.; McLaughlin, R.L.; et al. Brainstem pathology in amyotrophic lateral sclerosis and primary lateral sclerosis: A longitudinal neuroimaging study. NeuroImage Clinical 2019, 24, 102054. [Google Scholar] [CrossRef]
- Pioro, E.P.; Turner, M.R.; Bede, P. Neuroimaging in primary lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2020, 21, 18–27. [Google Scholar] [CrossRef]
- Finegan, E.; Li Hi Shing, S.; Chipika, R.H.; Doherty, M.A.; Hengeveld, J.C.; Vajda, A.; Donaghy, C.; Pender, N.; McLaughlin, R.L.; Hardiman, O.; et al. Widespread subcortical grey matter degeneration in primary lateral sclerosis: A multimodal imaging study with genetic profiling. NeuroImage Clinical 2019, 24, 102089. [Google Scholar] [CrossRef]
- Finegan, E.; Shing, S.L.H.; Chipika, R.H.; Chang, K.M.; McKenna, M.C.; Doherty, M.A.; Hengeveld, J.C.; Vajda, A.; Pender, N.; Donaghy, C.; et al. Extra-motor cerebral changes and manifestations in primary lateral sclerosis. Brain Imaging Behav. 2021, 15, 2283–2296. [Google Scholar] [CrossRef]
- Finegan, E.; Hi Shing, S.L.; Chipika, R.H.; McKenna, M.C.; Doherty, M.A.; Hengeveld, J.C.; Vajda, A.; Donaghy, C.; McLaughlin, R.L.; Hutchinson, S.; et al. Thalamic, hippocampal and basal ganglia pathology in primary lateral sclerosis and amyotrophic lateral sclerosis: Evidence from quantitative imaging data. Data Brief 2020, 29, 105115. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, M.C.; Laluz, V.; Rowe, A.; Findlater, K.; Lee, D.H.; Kennedy, K.; Kramer, J.H.; Strong, M.J. Brain atrophy in primary lateral sclerosis. Neurology 2009, 72, 1236–1241. [Google Scholar] [CrossRef]
- Butman, J.A.; Floeter, M.K. Decreased thickness of primary motor cortex in primary lateral sclerosis. AJNR Am. J. Neuroradiol. 2007, 28, 87–91. [Google Scholar] [PubMed]
- Kassubek, J.; Juengling, F.D.; Sperfeld, A.D. Widespread white matter changes in Kennedy disease: A voxel based morphometry study. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1209–1212. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lai, T.H.; Liu, R.S.; Yang, B.H.; Wang, P.S.; Lin, K.P.; Lee, Y.C.; Soong, B.W. Cerebral involvement in spinal and bulbar muscular atrophy (Kennedy’s disease): A pilot study of PET. J. Neurol. Sci. 2013, 335, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Unrath, A.; Muller, H.P.; Riecker, A.; Ludolph, A.C.; Sperfeld, A.D.; Kassubek, J. Whole brain-based analysis of regional white matter tract alterations in rare motor neuron diseases by diffusion tensor imaging. Hum. Brain Mapp. 2010, 31, 1727–1740. [Google Scholar] [CrossRef] [PubMed]
- Pieper, C.C.; Konrad, C.; Sommer, J.; Teismann, I.; Schiffbauer, H. Structural changes of central white matter tracts in Kennedy’s disease—a diffusion tensor imaging and voxel-based morphometry study. Acta Neurol. Scand. 2013, 127, 323–328. [Google Scholar] [CrossRef]
- Ni, W.; Chen, S.; Qiao, K.; Wang, N.; Wu, Z.Y. Genotype-phenotype correlation in Chinese patients with spinal and bulbar muscular atrophy. PLoS ONE 2015, 10, e0122279. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.E.; Freeman, B.K.; Auh, S.; Kokkinis, A.D.; La Pean, A.; Chen, C.; Lehky, T.J.; Shrader, J.A.; Levy, E.W.; Harris-Love, M.; et al. Clinical features of spinal and bulbar muscular atrophy. Brain 2009, 132, 3242–3251. [Google Scholar] [CrossRef]
- Hama, T.; Hirayama, M.; Hara, T.; Nakamura, T.; Atsuta, N.; Banno, H.; Suzuki, K.; Katsuno, M.; Tanaka, F.; Sobue, G. Discrimination of spinal and bulbar muscular atrophy from amyotrophic lateral sclerosis using sensory nerve action potentials. Muscle Nerve 2012, 45, 169–174. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, S.; Fan, D. Upper motor neuron involvement in Kennedy disease evaluated by triple stimulation technique. Zhonghua Yi Xue Za Zhi 2015, 95, 1522–1525. [Google Scholar] [PubMed]
- Ferrante, M.A.; Wilbourn, A.J. The characteristic electrodiagnostic features of Kennedy’s disease. Muscle Nerve 1997, 20, 323–329. [Google Scholar] [CrossRef]
- Xu, Y.S.; Zhang, J.; Lu, M.; Zheng, J.Y.; Zhang, S.; Kang, D.X.; Fan, D.S. Test of sensory nerve in patients with Kennedy disease. Zhonghua Yi Xue Za Zhi 2008, 88, 2771–2774. [Google Scholar] [PubMed]
- Fu, S.C.; Kuo, H.C.; Chu, C.C.; Wu, Y.R.; Ro, L.S.; Liu, C.S.; Huang, C.C. Long-term follow-up of spinal and bulbar muscular atrophy in Taiwan. J. Formos. Med. Assoc. 2013, 112, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, A.; Sugeno, N.; Tateyama, M.; Nishiyama, S.; Kato, M.; Aoki, M. Postural leg tremor in X-linked spinal and bulbar muscular atrophy. J. Clin. Neurosci. 2014, 21, 799–802. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lim, Y.M.; Lee, E.J.; Oh, Y.J.; Kim, K.K. Correlation between the CAG repeat size and electrophysiological findings in patients with spinal and bulbar muscular atrophy. Muscle Nerve 2018, 57, 683–686. [Google Scholar] [CrossRef]
- Meng, L.; Liu, J.; Liu, X.; Wang, Z.; Yuan, Y.; Zhang, W. Pathological features of muscles and peripheral nerves of Kennedy’s disease: A report of 12 cases. Zhonghua Yi Xue Za Zhi 2015, 95, 1681–1685. [Google Scholar]
- Lai, T.H.; Soong, B.W.; Chen, J.T.; Chen, Y.Y.; Lai, K.L.; Wu, Z.A.; Liao, K.K. Multimodal evoked potentials of Kennedy’s disease. Can. J. Neurol. Sci. 2007, 34, 328–332. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suntrup, S.; Kristina Teismann, I.; Steinstraeter, O.; Bernd Ringelstein, E.; Pantev, C.; Dziewas, R. Decreased cortical somatosensory finger representation in X-linked recessive bulbospinal neuronopathy (Kennedy disease): A magnetoencephalographic study. J. Neuroimaging 2010, 20, 16–21. [Google Scholar] [CrossRef]
- Li Hi Shing, S.; Lope, J.; Chipika, R.H.; Hardiman, O.; Bede, P. Extra-motor manifestations in post-polio syndrome (PPS): Fatigue, cognitive symptoms and radiological features. Neurol. Sci. 2021, 42, 4569–4581. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.L.; Cohen, J.M.; Galski, T.; Frick, N.M. The neuroanatomy of post-polio fatigue. Arch. Phys. Med. Rehabil. 1994, 75, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Bodian, D. Histopathologic basis of clinical findings in poliomyelitis. Am. J. Med. 1949, 6, 563–578. [Google Scholar] [CrossRef]
- Barnhart, M.; Rhines, R.; McCarter, J.C.; Magoun, H.W. Distribution of lesions of the brain stem in poliomyelitis. Arch. Neurol. Psychiatry 1948, 59, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Matzke, H.A.; Baker, A.B. Poliomyelitis. IV. A study of the midbrain. AMA Arch. Neurol. Psychiatry 1951, 65, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Luhan, J.A. Epidemic poliomyelitis; some pathologic observations on human material. Arch. Pathol. 1946, 42, 245–260. [Google Scholar]
- Miller, D.C. Post-polio syndrome spinal cord pathology. Case report with immunopathology. Ann. N. Y Acad. Sci. 1995, 753, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Prokhorenko, O.A.; Vasconcelos, O.M.; Lupu, V.D.; Campbell, W.W.; Jabbari, B. Sensory physiology assessed by evoked potentials in survivors of poliomyelitis. Muscle Nerve 2008, 38, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Li Hi Shing, S.; Lope, J.; Chipika, R.H.; Hardiman, O.; Bede, P. Imaging data indicate cerebral reorganisation in poliomyelitis survivors: Possible compensation for longstanding lower motor neuron pathology. Data Brief 2021, 38, 107316. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Z.; Zheng, H.H.; Ma, Q.L.; Wang, C.; Fan, L.P.; Wu, H.M.; Wang, D.N.; Zhang, J.X.; Zhan, Y.H. Cortical Damage Associated With Cognitive and Motor Impairment in Hereditary Spastic Paraplegia: Evidence of a Novel SPAST Mutation. Front. Neurol. 2020, 11, 399. [Google Scholar] [CrossRef] [PubMed]
- Servelhere, K.R.; Rezende, T.J.R.; de Lima, F.D.; de Brito, M.R.; de França Nunes, R.F.; Casseb, R.F.; Pedroso, J.L.; Barsottini, O.G.P.; Cendes, F.; França, M.C., Jr. Brain Damage and Gene Expression Across Hereditary Spastic Paraplegia Subtypes. Mov. Disord. 2021, 36, 1644–1653. [Google Scholar] [CrossRef] [PubMed]
- Montanaro, D.; Vavla, M.; Frijia, F.; Aghakhanyan, G.; Baratto, A.; Coi, A.; Stefan, C.; Girardi, G.; Paparella, G.; De Cori, S.; et al. Multimodal MRI Longitudinal Assessment of White and Gray Matter in Different SPG Types of Hereditary Spastic Paraparesis. Front. Neurosci. 2020, 14, 325. [Google Scholar] [CrossRef] [PubMed]
- Faber, I.; Martinez, A.R.M.; de Rezende, T.J.R.; Martins, C.R., Jr.; Martins, M.P.; Lourenço, C.M.; Marques, W., Jr.; Montecchiani, C.; Orlacchio, A.; Pedroso, J.L.; et al. SPG11 mutations cause widespread white matter and basal ganglia abnormalities, but restricted cortical damage. Neuroimage Clin. 2018, 19, 848–857. [Google Scholar] [CrossRef]
- Navas-Sánchez, F.J.; Fernández-Pena, A.; Martín de Blas, D.; Alemán-Gómez, Y.; Marcos-Vidal, L.; Guzmán-de-Villoria, J.A.; Fernández-García, P.; Romero, J.; Catalina, I.; Lillo, L.; et al. Thalamic atrophy in patients with pure hereditary spastic paraplegia type 4. J. Neurol. 2021, 268, 2429–2440. [Google Scholar] [CrossRef] [PubMed]
- Lindig, T.; Bender, B.; Hauser, T.K.; Mang, S.; Schweikardt, D.; Klose, U.; Karle, K.N.; Schüle, R.; Schöls, L.; Rattay, T.W. Gray and white matter alterations in hereditary spastic paraplegia type SPG4 and clinical correlations. J. Neurol. 2015, 262, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Samaranch, L.; Riverol, M.; Masdeu, J.C.; Lorenzo, E.; Vidal-Taboada, J.M.; Irigoyen, J.; Pastor, M.A.; de Castro, P.; Pastor, P. SPG11 compound mutations in spastic paraparesis with thin corpus callosum. Neurology 2008, 71, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Hehr, U.; Bauer, P.; Winner, B.; Schule, R.; Olmez, A.; Koehler, W.; Uyanik, G.; Engel, A.; Lenz, D.; Seibel, A.; et al. Long-term course and mutational spectrum of spatacsin-linked spastic paraplegia. Ann. Neurol. 2007, 62, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Orlén, H.; Melberg, A.; Raininko, R.; Kumlien, E.; Entesarian, M.; Söderberg, P.; Påhlman, M.; Darin, N.; Kyllerman, M.; Holmberg, E.; et al. SPG11 mutations cause Kjellin syndrome, a hereditary spastic paraplegia with thin corpus callosum and central retinal degeneration. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009, 150b, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Okubo, S.; Ueda, M.; Kamiya, T.; Mizumura, S.; Terashi, A.; Katayama, Y. Neurological and neuroradiological progression in hereditary spastic paraplegia with a thin corpus callosum. Acta Neurol. Scand. 2000, 102, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Schuurs-Hoeijmakers, J.H.; Geraghty, M.T.; Kamsteeg, E.J.; Ben-Salem, S.; de Bot, S.T.; Nijhof, B.; van de, V., II; van der Graaf, M.; Nobau, A.C.; Otte-Höller, I.; et al. Mutations in DDHD2, encoding an intracellular phospholipase A(1), cause a recessive form of complex hereditary spastic paraplegia. Am. J. Hum. Genet. 2012, 91, 1073–1081. [Google Scholar] [CrossRef]
- Aghakhanyan, G.; Martinuzzi, A.; Frijia, F.; Vavla, M.; Hlavata, H.; Baratto, A.; Martino, N.; Paparella, G.; Montanaro, D. Brain white matter involvement in hereditary spastic paraplegias: Analysis with multiple diffusion tensor indices. AJNR Am. J. Neuroradiol. 2014, 35, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Czell, D.; Neuwirth, C.; Weber, M.; Sartoretti-Schefer, S.; Gutzeit, A.; Reischauer, C. Nine Hole Peg Test and Transcranial Magnetic Stimulation: Useful to Evaluate Dexterity of the Hand and Disease Progression in Amyotrophic Lateral Sclerosis. Neurol. Res. Int. 2019, 2019, 7397491. [Google Scholar] [CrossRef] [PubMed]
- Hübner, J.; Hübner, I.; Kroczka, S. Correlation of electrophysiological parameters of peripheral nerves and manual dexterity in patients with amyotrophic lateral sclerosis. Wiad. Lek. 2018, 71, 807–814. [Google Scholar] [PubMed]
- Yamakawa, I.; Yamada, A.; Sonoda, Y.; Wakita, K.; Nishioka, T.; Harada, Y.; Ogawa, N.; Kitamura, A.; Sanada, M.; Tani, T.; et al. Occupational therapy using a robotic-assisted glove ameliorates finger dexterity and modulates functional connectivity in amyotrophic lateral sclerosis. J. Clin. Neurosci. 2023, 107, 144–149. [Google Scholar] [CrossRef]
- Manero, A.C.; McLinden, S.L.; Sparkman, J.; Oskarsson, B. Evaluating surface EMG control of motorized wheelchairs for amyotrophic lateral sclerosis patients. J. Neuroeng. Rehabil. 2022, 19, 88. [Google Scholar] [CrossRef] [PubMed]
- Hammer, M.J.; Barlow, S.M. Laryngeal somatosensory deficits in Parkinson’s disease: Implications for speech respiratory and phonatory control. Exp. Brain Res. 2010, 201, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Viguera, C.; Wang, J.; Mosmiller, E.; Cerezo, A.; Maragakis, N.J. Olfactory dysfunction in amyotrophic lateral sclerosis. Ann. Clin. Transl. Neurol. 2018, 5, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Chipika, R.H.; Christidi, F.; Finegan, E.; Li Hi Shing, S.; McKenna, M.C.; Chang, K.M.; Karavasilis, E.; Doherty, M.A.; Hengeveld, J.C.; Vajda, A.; et al. Amygdala pathology in amyotrophic lateral sclerosis and primary lateral sclerosis. J. Neurol. Sci. 2020, 417, 117039. [Google Scholar] [CrossRef]
- Wen, J.; Zhang, H.; Alexander, D.C.; Durrleman, S.; Routier, A.; Rinaldi, D.; Houot, M.; Couratier, P.; Hannequin, D.; Pasquier, F.; et al. Neurite density is reduced in the presymptomatic phase of C9orf72 disease. J. Neurol. Neurosurg. Psychiatry 2019, 90, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Westeneng, H.J.; Walhout, R.; Straathof, M.; Schmidt, R.; Hendrikse, J.; Veldink, J.H.; van den Heuvel, M.P.; van den Berg, L.H. Widespread structural brain involvement in ALS is not limited to the C9orf72 repeat expansion. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1354–1360. [Google Scholar] [CrossRef]
- Chio, A.; Logroscino, G.; Traynor, B.J.; Collins, J.; Simeone, J.C.; Goldstein, L.A.; White, L.A. Global epidemiology of amyotrophic lateral sclerosis: A systematic review of the published literature. Neuroepidemiology 2013, 41, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Bede, P.; Murad, A.; Hardiman, O. Pathological neural networks and artificial neural networks in ALS: Diagnostic classification based on pathognomonic neuroimaging features. J. Neurol. 2021, 269, 2440–2452. [Google Scholar] [CrossRef] [PubMed]
- Bede, P.; Murad, A.; Lope, J.; Hardiman, O.; Chang, K.M. Clusters of anatomical disease-burden patterns in ALS: A data-driven approach confirms radiological subtypes. J. Neurol. 2022, 269, 4404–4413. [Google Scholar] [CrossRef]
- Bede, P.; Murad, A.; Lope, J.; Li Hi Shing, S.; Finegan, E.; Chipika, R.H.; Hardiman, O.; Chang, K.M. Phenotypic categorisation of individual subjects with motor neuron disease based on radiological disease burden patterns: A machine-learning approach. J. Neurol. Sci. 2021, 432, 120079. [Google Scholar] [CrossRef]
- Dukic, S.; McMackin, R.; Costello, E.; Metzger, M.; Buxo, T.; Fasano, A.; Chipika, R.; Pinto-Grau, M.; Schuster, C.; Hammond, M.; et al. Resting-state EEG reveals four subphenotypes of amyotrophic lateral sclerosis. Brain 2022, 145, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Querin, G.; Bede, P.; El Mendili, M.M.; Li, M.; Pelegrini-Issac, M.; Rinaldi, D.; Catala, M.; Saracino, D.; Salachas, F.; Camuzat, A.; et al. Presymptomatic spinal cord pathology in c9orf72 mutation carriers: A longitudinal neuroimaging study. Ann. Neurol. 2019, 86, 158–167. [Google Scholar] [CrossRef]
- Querin, G.; El Mendili, M.M.; Bede, P.; Delphine, S.; Lenglet, T.; Marchand-Pauvert, V.; Pradat, P.F. Multimodal spinal cord MRI offers accurate diagnostic classification in ALS. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1220–1221. [Google Scholar] [CrossRef]
- Johnson, S.A.; Karas, M.; Burke, K.M.; Straczkiewicz, M.; Scheier, Z.A.; Clark, A.P.; Iwasaki, S.; Lahav, A.; Iyer, A.S.; Onnela, J.P.; et al. Wearable device and smartphone data quantify ALS progression and may provide novel outcome measures. NPJ Digit. Med. 2023, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, A.; Warita, H.; Takahashi, T.; Suzuki, N.; Nishiyama, S.; Tano, O.; Akiyama, T.; Watanabe, Y.; Takahashi, K.; Kuroda, H.; et al. Prominent sensory involvement in a case of familial amyotrophic lateral sclerosis carrying the L8V SOD1 mutation. Clin. Neurol. Neurosurg. 2016, 150, 194–196. [Google Scholar] [CrossRef]
- Chipika, R.H.; Siah, W.F.; McKenna, M.C.; Li Hi Shing, S.; Hardiman, O.; Bede, P. The presymptomatic phase of amyotrophic lateral sclerosis: Are we merely scratching the surface? J. Neurol. 2021, 268, 4607–4629. [Google Scholar] [CrossRef] [PubMed]
- Tahedl, M.; Li Hi Shing, S.; Finegan, E.; Chipika, R.H.; Lope, J.; Hardiman, O.; Bede, P. Propagation patterns in motor neuron diseases: Individual and phenotype-associated disease-burden trajectories across the UMN-LMN spectrum of MNDs. Neurobiol. Aging 2021, 109, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Tahedl, M.; Tan, E.L.; Chipika, R.H.; Hengeveld, J.C.; Vajda, A.; Doherty, M.A.; McLaughlin, R.L.; Siah, W.F.; Hardiman, O.; Bede, P. Brainstem-cortex disconnection in amyotrophic lateral sclerosis: Bulbar impairment, genotype associations, asymptomatic changes and biomarker opportunities. J. Neurol. 2023, 270, 3511–3526. [Google Scholar] [CrossRef]
- Tahedl, M.; Chipika, R.H.; Lope, J.; Li Hi Shing, S.; Hardiman, O.; Bede, P. Cortical progression patterns in individual ALS patients across multiple timepoints: A mosaic-based approach for clinical use. J. Neurol. 2021, 268, 1913–1926. [Google Scholar] [CrossRef] [PubMed]
- Chipika, R.H.; Finegan, E.; Li Hi Shing, S.; Hardiman, O.; Bede, P. Tracking a Fast-Moving Disease: Longitudinal Markers, Monitoring, and Clinical Trial Endpoints in ALS. Front. Neurol. 2019, 10, 229. [Google Scholar] [CrossRef]
- Münte, T.F.; Tröger, M.C.; Nusser, I.; Wieringa, B.M.; Johannes, S.; Matzke, M.; Dengler, R. Alteration of early components of the visual evoked potential in amyotrophic lateral sclerosis. J. Neurol. 1998, 245, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Matheson, J.K.; Harrington, H.J.; Hallett, M. Abnormalities of multimodality evoked potentials in amyotrophic lateral sclerosis. Arch. Neurol. 1986, 43, 338–340. [Google Scholar] [CrossRef]
- Rojas, P.; de Hoz, R.; Ramírez, A.I.; Ferreras, A.; Salobrar-Garcia, E.; Muñoz-Blanco, J.L.; Urcelay-Segura, J.L.; Salazar, J.J.; Ramírez, J.M. Changes in Retinal OCT and Their Correlations with Neurological Disability in Early ALS Patients, a Follow-Up Study. Brain sciences 2019, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Hübers, A.; Müller, H.P.; Dreyhaupt, J.; Böhm, K.; Lauda, F.; Tumani, H.; Kassubek, J.; Ludolph, A.C.; Pinkhardt, E.H. Retinal involvement in amyotrophic lateral sclerosis: A study with optical coherence tomography and diffusion tensor imaging. J. Neural. Transm. 2016, 123, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Burke, T.; Elamin, M.; Bede, P.; Pinto-Grau, M.; Lonergan, K.; Hardiman, O.; Pender, N. Discordant performance on the ’Reading the Mind in the Eyes’ Test, based on disease onset in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2016, 17, 467–472. [Google Scholar] [CrossRef]
- Christidi, F.; Karavasilis, E.; Velonakis, G.; Ferentinos, P.; Rentzos, M.; Kelekis, N.; Evdokimidis, I.; Bede, P. The Clinical and Radiological Spectrum of Hippocampal Pathology in Amyotrophic Lateral Sclerosis. Front. Neurol. 2018, 9, 523. [Google Scholar] [CrossRef]
- Finegan, E.; Chipika, R.H.; Li Hi Shing, S.; Hardiman, O.; Bede, P. Pathological Crying and Laughing in Motor Neuron Disease: Pathobiology, Screening, Intervention. Front. Neurol. 2019, 10, 260. [Google Scholar] [CrossRef]
- Abrahams, S.; Newton, J.; Niven, E.; Foley, J.; Bak, T.H. Screening for cognition and behaviour changes in ALS. Amyotroph. Lateral Scler. Front. Degener. 2014, 15, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.; Cudkowicz, M.; Shaw, P.J.; Andersen, P.M.; Atassi, N.; Bucelli, R.C.; Genge, A.; Glass, J.; Ladha, S.; Ludolph, A.L.; et al. Phase 1-2 Trial of Antisense Oligonucleotide Tofersen for SOD1 ALS. New Engl. J. Med. 2020, 383, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.J.; Zhang, P.W.; Pham, J.T.; Haeusler, A.R.; Mistry, N.A.; Vidensky, S.; Daley, E.L.; Poth, E.M.; Hoover, B.; Fines, D.M.; et al. RNA toxicity from the ALS/FTD C9ORF72 expansion is mitigated by antisense intervention. Neuron 2013, 80, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Ly, C.V.; Miller, T.M. Emerging antisense oligonucleotide and viral therapies for amyotrophic lateral sclerosis. Curr. Opin. Neurol. 2018, 31, 648–654. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).