Abstract
Advanced cutaneous squamous cell carcinoma (aCSCC) comprises locally advanced and metastatic disease not amenable to curative surgery or radiotherapy and is associated with substantial morbidity, mortality, and healthcare costs. This narrative review summarizes current knowledge on the epidemiology, biology, clinical presentation, and staging of aCSCC and critically appraises therapeutic strategies with a focus on programmed death 1 (PD-1) blockade. Immune checkpoint inhibitors now represent the main systemic treatment for advanced cSCC, with clinical trials and observational studies reporting response rates around 45–60%, sustained benefit in a subset of patients, and a manageable yet clinically relevant profile of immune-related toxicities. However, outcomes remain heterogeneous, particularly in elderly, comorbid, and immunosuppressed patients. We therefore review established and emerging prognostic determinants spanning clinical, anatomical, histopathological, metabolic, inflammatory, and on-treatment domains. Priorities for biomarker-enriched studies and harmonized real-world registries to enable more refined risk stratification and genuinely personalized, multidisciplinary management of aCSCC are also outlined.
1. Introduction
Cutaneous squamous cell carcinoma (cSCC) is one of the most prevalent malignancies affecting the skin, ranking second only to basal cell carcinoma among nonmelanoma skin cancers [1,2]. Its global incidence has been steadily increasing over recent decades, with an estimated annual rise of up to 50–200%, making it a significant and growing public health concern [2,3]. While the majority of cSCCs are amenable to surgical excision and have a favorable prognosis, with five-year survival rates exceeding 90%, a subset of tumors exhibits aggressive behaviour, marked by a higher likelihood of local invasion, recurrence, and metastasis [4,5]. Though metastasis is relatively rare compared to other malignancies, it remains clinically relevant due to its association with poor outcomes and its potential to affect lymph nodes and distant organs. The clinical spectrum of cSCC varies widely, ranging from small, well-differentiated, indolent tumors to rapidly growing, poorly differentiated lesions capable of deep tissue destruction involving cartilage or bone [6]. In detail, advanced cutaneous squamous cell carcinoma (aCSCC) comprises locally advanced (laCSCC) and metastatic cutaneous squamous cell carcinoma (mCSCC) not amenable to curative surgery or curative radiotherapy, or both [7]. Risk stratification is therefore essential and must account for clinical presentation, anatomical site, histological features, and patient-related factors. In parallel with the rising incidence of cSCC, the field of dermatology has witnessed significant technological and therapeutic advances aimed at improving patient care. Diagnostic capabilities have been enhanced by the increasing integration of non-invasive imaging modalities, such as dermoscopy, reflectance confocal microscopy (RCM), and line-field confocal optical coherence tomography (LC-OCT), which allow for real-time, in vivo assessment of suspicious lesions and help refine clinical decision-making [8,9]. On the therapeutic front, the treatment paradigm for cSCC has expanded beyond conventional surgery and radiotherapy, particularly for cases that are locally advanced, recurrent, or metastatic. The introduction of immune checkpoint inhibitors has marked a transformative shift in the management of advanced cSCC, offering durable responses in a subset of patients with limited therapeutic options. Among these, cemiplimab, an anti-PD-1 monoclonal antibody, has become the first systemic immunotherapy approved for patients with unresectable or metastatic cSCC, demonstrating favorable efficacy and safety profiles in both clinical trials and real-world settings [10,11].
This narrative review aims to provide a practice-oriented synthesis of advanced cutaneous squamous cell carcinoma, from epidemiology, biology, and staging to current multimodal treatment, with particular focus on PD-1 blockade and cemiplimab. We summarize and contextualize evidence from clinical trials and real-world cohorts on effectiveness and safety, including immune-related adverse events, within contemporary multidisciplinary care. At the same time, we acknowledge the persisting unmet needs and knowledge gaps, such as incomplete prognostic stratification, limited data in vulnerable subgroups, and heterogeneous real-world outcomes, of which only some can be addressed given the current literature. In parallel, we discuss available and emerging prognostic factors across clinical, pathological, metabolic, inflammatory, and on-treatment domains, with specific attention to special populations, and highlight key priorities for future biomarker-enriched studies and harmonized registries to support more personalized, risk-adapted management.
2. Materials and Methods
A comprehensive literature search was conducted independently in each database (PubMed/MEDLINE and Scopus) to identify publications relevant to aCSCC, with complementary searches in Embase, Web of Science, and the Cochrane Library to enhance coverage. Trial registries (ClinicalTrials.gov and World Health Organization—International Clinical Trials Registry Platform) and major conference abstract repositories (American Society of Clinical Oncology, European Society for Medical Oncology, American Academy of Dermatology) were screened for recent and ongoing studies not yet indexed in bibliographic databases. No language or publication date restrictions were applied. The search covered database inception through November 2025. The search strategy combined controlled vocabulary (e.g., MeSH/Emtree) and free-text terms, adapted to each database, using Boolean operators and truncation where appropriate. The core query paired disease terms with treatment, outcomes, and prognostic constructs. Representative terms included, in various combinations: “cutaneous squamous cell carcinoma” OR “cSCC” OR “skin squamous cell carcinoma” AND “advanced” OR “locally advanced” OR “unresectable” OR “metastatic” AND “immunotherapy” OR “immune checkpoint inhibitor” OR “anti–PD-1” OR “cemiplimab” OR “pembrolizumab” OR “nivolumab” OR “checkpoint blockade” OR “EGFR inhibitor” OR “cetuximab” OR “chemotherapy” OR “platinum” OR “radiotherapy” OR “neoadjuvant” OR “adjuvant” OR “multimodal” AND “real-world” OR “observational” OR “registry” OR “cohort” OR “retrospective” OR “prospective” AND “prognostic” OR “risk factor” OR “staging” OR “AJCC” OR “Brigham and Women’s (BWH)” OR “perineural invasion” OR “tumor thickness” OR “depth of invasion” OR “immunosuppressed” OR “solid organ transplant” AND “overall survival” OR “progression-free survival” OR “objective response rate” OR “duration of response” OR “disease control” OR “toxicity” OR “immune-related adverse events” OR “quality of life”. Reference lists of all eligible articles and relevant reviews were manually screened to identify additional studies. Regulatory assessment reports (e.g., European Medicines Agency/Food and Drug Administration labels and reviews) were consulted to contextualize indications and pivotal evidence. Given the narrative scope of this review, no formal meta-analysis was planned. Nevertheless, we applied a structured approach to study selection and appraisal. After duplicate removal, titles and abstracts were screened for relevance, followed by full-text assessment. Preference was given to peer-reviewed original research, including randomized and non-randomized clinical trials, prospective and retrospective cohorts, registries, and large case series reporting predefined clinical outcomes. Studies focusing on non-cutaneous squamous cell carcinomas (e.g., mucosal head-and-neck, anogenital) were excluded. Single-patient case reports were generally excluded unless they provided unique insights pertinent to advanced disease management. The study selection process is summarized in the PRISMA flow diagram (Figure 1).
Figure 1.
PRISMA flow diagram of study selection. Flow of records through identification, screening, eligibility assessment, and final inclusion of 64 studies in the narrative review on advanced cutaneous squamous cell carcinoma.
To guide selection in a reproducible manner while preserving the breadth expected of a narrative synthesis, we used a PICO framework: (i) Population: Adults with advanced cSCC, defined as unresectable locally advanced or metastatic disease; special populations (immunosuppressed, solid-organ transplant recipients) were included when reported; (ii) Intervention/Exposure: Systemic therapies (anti–PD-1 agents such as cemiplimab, pembrolizumab; EGFR inhibitors; cytotoxic chemotherapy), and multimodal strategies integrating radiotherapy and/or surgery; exposures also included prognostic factors and staging systems relevant to advanced disease; (iii) Comparator: Alternative systemic regimens, best supportive care, historical or real-world comparators, or no comparator in single-arm studies; (iv) Outcomes: Treatment effectiveness (ORR, CR, DOR, DCR, PFS, OS), safety (overall and grade ≥ 3 AEs, immune-related AEs), patient-reported outcomes, and prognostic performance (effect sizes, discrimination/calibration).
3. Results
Advanced cutaneous squamous cell carcinoma (aCSCC) is broadly classified into locally advanced (laCSCC) and metastatic (mCSCC) disease, the latter encompassing both locoregional and distant metastatic spread [12]. According to the current European guidelines, laCSCC is defined as non-metastatic cSCC that is not amenable to curative surgery or radiotherapy due to factors such as multiple recurrences, extensive tumour size, bone erosion, deep invasion beyond the subcutaneous tissue into muscle or along major nerves, or anatomical locations where curative resection would result in unacceptable functional impairment, morbidity, or disfigurement. Conversely, mCSCC refers to tumours with regional lymph node involvement and/or distant metastases.
3.1. Epidemiology
cSCC represents the second most frequent cutaneous malignancy after basal cell carcinoma (BCC), with a rapidly increasing incidence worldwide [1]. Epidemiological data for advanced cSCC are limited due to inconsistent reporting and the lack of a uniform definition [12]. The frequency of metastasis in unselected cSCC cohorts is estimated at 3–5% for nodal spread and <1% for distant metastasis [13]. A large retrospective study in the United States reported local recurrence in 4.6% of patients, nodal metastasis in 3.7%, distant metastasis in 0.4%, and disease-specific death in 2.1% [14]. A UK study of over 76,000 patients (National Cancer and Analysis Registration Service—NCRAS, 2013–2015) documented a cumulative incidence of metastatic cSCC of 2.1%, with rates of 2.4% in men and 1.1% in women [15]. Data from Germany (1997–2011) indicated that 2.2% of cases were regional (direct invasion or lymph node involvement) and 0.3% were distant metastases, while in Norway (1963–2011) 2.1% of cSCCs were classified as advanced [16,17]. Importantly, the majority of metastatic events occur early: 85% within the first 2 years and over 90% within 3 years of the primary tumor diagnosis [15]. Survival outcomes are significantly worse for advanced cSCC. In the UK NCRAS dataset, the 3-year survival for patients with mcSCC was 46% in men and 29% in women, compared with 65% and 68% for non-metastatic cases [15]. German cancer registry data reported a relative 5-year survival of 94% for all cSCC cases, but only 58.3% for those with regional metastasis [16]. Norwegian data showed 5-year relative survival rates of 82% in men and 88% in women with localized cSCC, dropping to 51% and 64%, respectively, in advanced disease [17]. The proportion of advanced cSCC among selected populations is illustrated in Figure 2. Distant metastases confer the worst prognosis, with studies showing an eightfold increased risk of death compared to nodal metastasis; 89% of patients with distant spread die within 5 years [18]. Immunosuppressed patients, particularly solid organ transplant recipients (SOTRs), represent a high-risk group for advanced cSCC. The incidence of cSCC in SOTRs is 65–100 times higher than in the general population [19,20]. A meta-analysis reported a pooled metastatic risk of 7.3% in SOTRs compared with 3.1% in immunocompetent patients [21]. In a retrospective cohort of 51 SOTRs with advanced cSCC, 88% had metastatic disease, and the 5-year disease-specific survival was only 30.5% [22]. Aggressive disease tends to present at a younger age in this group, with median diagnosis around 62 years. The time between primary tumor detection and metastasis is often short, averaging 1.4 years [22,23].
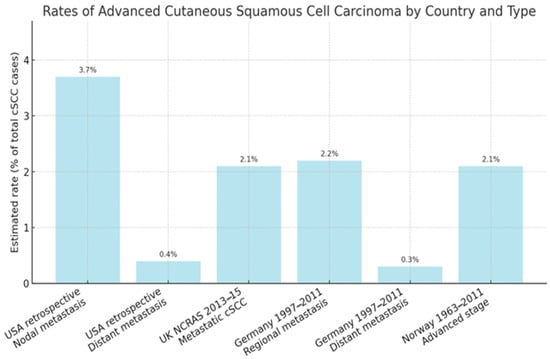
Figure 2.
Estimated rates of advanced cSCC across different population-based studies. “Any advanced stage” includes both locally advanced and metastatic disease, where not otherwise specified. Data are derived from national cancer registry studies conducted in the United Kingdom (2013–2015), Germany (1997–2011), Norway (1963–2011), and epidemiological estimates from the United States (2012) [13,14,15,16,17].
3.2. Pathogenesis and Evolution to Advanced cSCC
3.2.1. Initiating Drivers and Early Carcinogenesis
cSCC arises through the convergence of environmental injury, impaired immune surveillance, and inherited or acquired genomic alterations that together drive a multistep carcinogenic process from actinic keratosis to invasive disease (Figure 3) [24,25,26,27].
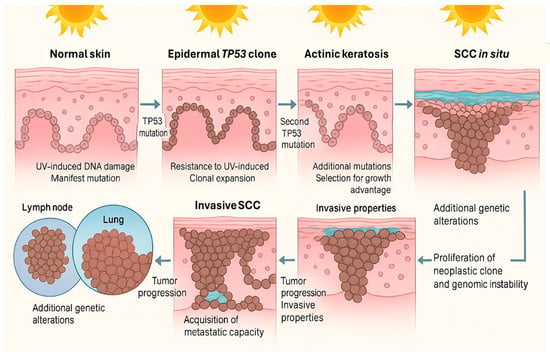
Figure 3.
Multistep progression of cSCC. Chronic UV exposure leads to DNA damage and mutations in keratinocytes, including alterations in the TP53 gene, allowing for clonal expansion and resistance to apoptosis. Accumulation of additional mutations promotes actinic keratosis and progression to squamous cell carcinoma in situ. Further genetic changes confer invasive and metastatic properties, enabling tumor spread to regional lymph nodes and distant organs. Image created using GNU Image Manipulation Program v.2.10.
Chronic ultraviolet radiation (UVR) is the dominant initiator: UVB and UVA generate cyclobutane pyrimidine dimers and 6–4 photoproducts with characteristic C>T transitions at dipyrimidine sites, seeding early driver mutations, most notably in TP53, that permit clonal survival under continued UV stress [25,28]. Risk scales with cumulative exposure (outdoor work, high-insolation latitudes), distinguishing cSCC from BCC’s stronger link to intermittent exposure and sunburns [28,29,30]. Artificial or therapeutic sources add to this burden: indoor tanning at young ages, PUVA in psoriasis (dose-dependent), and ionizing radiation confer substantial additional risk [31]. Chemical carcinogens (arsenic, polycyclic aromatic hydrocarbons, nitrosamines) further damage DNA via adduct formation and hinder repair [32,33]. Immunosuppression is a powerful amplifier: solid organ transplant recipients experience 65–250-fold higher incidence, reflecting reduced immune surveillance, UV synergy, and drug-specific carcinogenicity (e.g., azathioprine, cyclosporine) [34,35]. Elevated risks also occur in hematologic malignancies (CLL, NHL) and advanced HIV infection, where anal SCC is notably enriched, owing to combined cellular and humoral immune defects [36,37]. Beta-HPVs (types 5, 8, 38) likely act as co-carcinogens in sun-exposed skin by E6/E7-mediated interference with p53 and Rb, especially early in tumorigenesis and in immunosuppressed or genetically predisposed hosts (e.g., epidermodysplasia verruciformis) [38,39]. Chronic inflammation, scarring, and non-healing wounds create a pro-tumor microenvironment rich in reactive oxygen species and cytokines (Marjolin ulcers) [40]. Dysregulated TGF-β signaling and an imbalanced IL-18/IL-37 axis further supports immune evasion and aggressive behavior, including in oral SCC [41,42]. Certain drugs modulate risk: BRAF inhibitors can trigger eruptive keratoacanthomas/cSCC via paradoxical MAPK activation in RAS-mutant keratinocytes, an effect mitigated by MEK co-inhibition, while long-term voriconazole and vismodegib have also been linked to increased cSCC incidence [43,44]. Lifestyle factors modestly contribute (smoking, alcohol) and may interact with exposure patterns from occupational vs. recreational activity [45]. Inherited cancer-prone states, such as nucleotide excision repair defects (xeroderma pigmentosum), pigmentation disorders (oculocutaneous albinism), viral susceptibility syndromes (EV), and chronic-wounding genodermatoses (recessive dystrophic epidermolysis bullosa), underscore the primacy of DNA repair, photoprotection, and cutaneous barrier integrity [46,47,48]. Beyond rare syndromes, GWAS highlights common, low-to-moderate effect alleles in pigmentation (MC1R, TYR, IRF4, SLC45A2, HERC2), immunity (HLA-DQA1, FOXP1), and keratinocyte regulation (TP63, BNC2, DEF8) that shape baseline susceptibility [49,50]. Molecularly, early TP53 inactivation, CDKN2A silencing, and frequent truncating mutations in NOTCH1/2 disrupt apoptosis and differentiation; context-dependent RAS activation (notably with BRAF inhibition), KNSTRN mutations, MYC amplification, copy-number losses (3p, 9p; FHIT, PTPRD), and epigenetic dysregulation collectively propel progression within a UV-damaged “field” of mutated clones [51,52,53]. Tumor–stroma crosstalk (angiogenic signaling such as VEGF), recruitment of immunosuppressive cells, MHC-I downregulation, immunosuppressive cytokines (IL-10, TGF-β), and PD-L1 expression consolidate immune escape [54,55]. The main pathogenetic underlying advanced cutaneous squamous cell carcinoma are summarized in Table 1.

Table 1.
Main pathogenetic and progression factors in cutaneous squamous cell carcinoma (cSCC).
3.2.2. Invasion and Dissemination Mechanisms
The progression from cSCC in situ to an advanced and metastatic phenotype is a consequence of stepwise genetic and epigenetic alterations that drive changes in tumor cell behavior and interaction with the surrounding microenvironment. In the transition from in situ to invasive disease, the loss of cell–cell adhesion molecules, such as desmocollin-3 (DSC3), desmoplakin (DSP), and junction plakoglobin (JUP), plays a central role in enabling keratinocytes to breach the basement membrane [56]. This functional impairment, which correlates with somatic mutations and reduced protein expression, facilitates dermal infiltration by disrupting desmosomal integrity and intercellular cohesion. As tumor cells migrate deeper into the dermis, they acquire additional alterations, including mutations in genes such as PTEN, NOTCH1, and CDKN2A, that contribute to proliferative advantage, immune evasion, and matrix degradation [57]. Notably, whole-exome sequencing has revealed a distinct mutational signature in the deeper invasive front, enriched in pathways regulating cell junction organization and epithelial–mesenchymal transition (EMT), suggesting a spatial evolution of tumor clones under selective pressure [58]. Local invasion sets the stage for perineural and lymphovascular involvement. Perineural spread, associated with mutations in adhesion and axon guidance genes such as NTNG2 and RELN, enables tumor extension along nerve sheaths, often without overt mass formation [56,59]. Simultaneously, entry into the lymphatic system is facilitated by tumor-induced lymphangiogenesis and upregulation of VEGF-C/D, promoting access to regional lymph nodes [60]. Clonal selection of subpopulations with increased motility and immune-modulatory capabilities, such as PD-L1 overexpression, further supports immune escape and dissemination [61]. CD274 (gene encoding PD-L1) point mutations are uncommon and are not considered major driver events. Ultimately, hematogenous metastasis occurs in the context of profound genomic instability. Copy number alterations, gain-of-function mutations in driver oncogenes (e.g., EGFR), and loss of tumor suppressors like TP53 and FAT1 contribute to the acquisition of traits necessary for distant colonization [62,63]. These include survival in circulation, endothelial adhesion, and growth in foreign microenvironments. Thus, advanced cSCC represents the culmination of a dynamic evolutionary process shaped by both intrinsic tumor biology and host–tumor interactions.
3.3. Clinical Features, Diagnostic Work-Up, Histology, and Staging
Clinically, aCSCC may present as an enlarging, indurated plaque or nodule with hyperkeratosis, ulceration, or exophytic growth and often ill-defined margins, with rapid enlargement, friability, hemorrhagic crusting, and pain more common in poorly differentiated lesions [1,6]. High-risk anatomical sites (non-glabrous lip, ear, periocular and perinasal regions) are prone to aggressive local behavior and recurrence owing to complex anatomy [64]. Tumors arising in scars or chronic wounds (Marjolin ulcers) show heightened risk of nodal involvement and, in some series, synchronous distant spread [65]. Squamous cell carcinoma of the lip, frequently on a background of actinic cheilitis, behaves more aggressively with a greater likelihood of perineural invasion and regional spread [66]. Perineural invasion (PNI) is an adverse feature: neuropathic pain, paresthesia, hypoesthesia, or weakness along a named nerve distribution should raise suspicion and prompt targeted imaging and multidisciplinary planning [67]. When metastatic, patients may present with firm, sometimes fixed lymphadenopathy (e.g., parotid/cervical for head and neck primaries; axillary/inguinal for extremity primaries) or with organ-specific symptoms. Lung is the most frequent distant site, followed by bone and other viscera [12]. Host immunosuppression further tilts the course toward advanced disease [64]. aCSCC accounts for disproportionate morbidity, healthcare utilization, and mortality relative to overall cSCC burden [12,67,68]. Major prognostic determinants for metastatic spread in invasive cSCC are summarized in Table 2.

Table 2.
Major risk factors for metastasis in invasive cSCC, including clinical, anatomical, and histopathologic parameters associated with aggressive behavior.
3.3.1. Histopathology
Advanced tumors span the differentiation spectrum: well-differentiated aCSCC shows infiltrative nests and lobules of atypical squamous cells with intercellular bridges and keratin pearls; progressive invasion manifests as irregular tongues extending into deep dermis, subcutis, and sometimes muscle or bone. Poorly differentiated tumors exhibit reduced keratinization, marked pleomorphism, brisk mitotic activity, and necrosis. immunohistochemistry may be required to exclude mimics [69,70]. Adverse variants, such as desmoplastic, acantholytic, clear cell, pseudovascular, mucinous, pigmented, and tumors arising in scars or chronic ulcers, are enriched among advanced presentations; desmoplastic SCC, in particular, carries a high local recurrence risk and frequent PNI [71]. Keratoacanthoma-like lesions with pronounced atypia are managed as SCC given potential for invasion/metastasis [71]. Pathology reports in aCSCC should quantify tumor thickness (with >6 mm especially adverse), level of invasion (beyond subcutaneous fat), margin status, degree of differentiation, lymphovascular invasion, and granular PNI metrics (nerve caliber ≥ 0.1 mm, involvement of named nerves, proximal extent), as these parameters drive staging, adjuvant therapy, and follow-up [69,70,71].
3.3.2. Staging and Risk Stratification
Staging underpins prognosis and treatment selection. For cSCC of the head and neck, AJCC 8th edition TNM is used; for sites outside head and neck, AJCC 7th remains a common reference for clinical staging (institution-dependent) [72,73]. Because conventional TNM incompletely captures metastatic risk for cutaneous primaries, the Brigham and Women’s Hospital (BWH) system complements staging by tallying high-risk features—e.g., diameter ≥ 2 cm, poor/undifferentiated histology, PNI (including caliber-based criteria or named nerves), deep invasion (>6 mm or beyond fat), high-risk location (ear or non-glabrous lip), and minor bone erosion—stratifying tumors from T1 (0 features) to T3 (≥4 features) [74]. BWH T2b–T3 tumors have the highest rates of nodal metastasis and recurrence and often define the cohort considered “advanced” at presentation or during follow-up [12,74]. A complementary role in staging is played by imaging techniques. Given that the overall risk of metastasis in cSCC is relatively low, routine radiologic screening is not recommended. However, computed tomography (CT), magnetic resonance imaging (MRI), ultrasound, and positron emission tomography/computed tomography (PET/CT) can be employed in high-risk tumors (AJCC T3–T4 or BWH T2b–T3) to evaluate bone involvement, deep soft tissue invasion, perineural spread, or the presence of nodal and distant metastases [75]. CT has emerged as the most frequently used modality for nodal and osseous assessment, whereas MRI is superior for the evaluation of perineural spread and deep structures; ultrasound with fine-needle aspiration is mainly applied for the assessment of superficial lymph nodes, while PET/CT, despite its higher sensitivity for distant metastatic disease, is limited by high costs and relatively modest impact on patient management. In Europe, in particular, ultrasound is often the first-line modality for the evaluation of regional lymph nodes in high-risk cases [75]. Finally, sentinel lymph node biopsy (SLNB) has been proposed as a tool for the early detection of occult micrometastases in selected patients with high-risk cSCC. In high-risk cSCC, early sentinel lymph node biopsy follows standard melanoma-derived protocols, with peritumoral radiocolloid injection and preoperative lymphoscintigraphy to map the draining basin, followed by radioguided (±blue dye) excision of the sentinel node(s) for serial sectioning and immunohistochemical assessment. Retrospective studies and meta-analyses have demonstrated that the procedure is technically feasible, with low false-negative rates and generally mild complications [76]. Reported positivity rates range between 11% and 24%, especially in tumors classified as BWH T2b or higher, suggesting a potential prognostic benefit in selected subgroups.
3.4. Therapeutic Strategies in Advanced cSCC
Management of aCSCC relies on multidisciplinary decision-making that weighs oncologic control against function, cosmesis, and patient comorbidities, within a guideline framework still constrained by limited randomized evidence [77,78]. North American (American Academy of Dermatology, AAD) and European (European Association of Dermato-Oncology, EADO; European Dermatology Forum, EDF; European Society for Radiotherapy and Oncology, ESTRO; European Union of Medical Specialists, UEMS; European Academy of Dermatology and Venereology, EADV; European Organisation for Research and Treatment of Cancer, EORTC) recommendation converge on a risk-adapted pathway. The therapeutic backbone shifts to systemic treatment and/or radiotherapy, with surgery reserved for selected, non-curative intents.
3.4.1. Surgery in Advanced cSCC
In aCSCC, the surgery is reserved for selected, non-curative intents [77,78]. Operative interventions are considered salvage or adjunctive: (i) conversion/salvage resections after response to systemic therapy or RT when an R0 becomes achievable with acceptable morbidity; (ii) debulking/palliation to control pain, bleeding, infection, malodor, or to facilitate wound care; and (iii) metastasectomy or therapeutic nodal dissection in rigorously selected oligometastatic or regionally confined disease, ideally after multidisciplinary review [77,78,79].
3.4.2. Radiotherapy and Management of Regional Nodal Disease
RT is the principal alternative for inoperable laCSCC or when surgery would incur unacceptable morbidity, and it is a key adjunct after surgery in selected scenarios [79,80]. It is particularly useful for tumors located on the lip, eyelid, nose, ear, or scalp, or in elderly patients with significant comorbidities. A meta-analysis of 14 observational studies including 1018 tumors showed a pooled recurrence rate of 6.4% after radiotherapy, higher than surgery but still within acceptable limits for selected patients [81]. Fractionation is tailored to fitness (standard 60–70 Gy vs. hypofractionation in frail patients) with awareness of late effects [82]. Postoperative RT is indicated when residual disease (R1/R2) cannot be cleared and is commonly advised for extranodal extension or extensive perineural invasion; after R0 resection, its role remains debated and is generally reserved for very high-risk biology (e.g., BWH T2b/T3), with emphasis on locoregional control rather than proven survival benefit [78]. Regional nodal disease should be surgically addressed when operability allows, with the extent of dissection individualized by basin and burden; parotid metastasis often warrants superficial parotidectomy with selective neck dissection, as RT alone underperforms for survival [77,83]. Elective dissection in clinically node-negative patients is not supported [78]. Sentinel lymph node biopsy reports low false-negative rates and double-digit positivity in BWH T2b/T3 tumors but lacks demonstrated survival benefit and remains investigational [77]. In unresectable nodal disease, definitive chemoradiation is an option; for systemic dissemination, systemic therapy becomes central. Among local palliative options, electrochemotherapy (ECT) offers high objective and complete response rates for unresectable cutaneous or in-transit lesions with acceptable toxicity, making it valuable for symptom control and field reduction in frail patients, though durability beyond short follow-up remains uncertain [84]. This technique can complement systemic therapy or defer extensive surgery/RT in selected cases. Notably, both radiotherapy and several cytotoxic regimens can dynamically up-regulate PD-L1 on tumor and immune cells through DNA-damage- and cGAS–STING/IFN-driven pathways, providing a biological rationale for combined chemo-/radio-immunotherapy with PD-1/PD-L1 inhibitors [85,86]. In this setting, RT may act as an in situ vaccine by inducing immunogenic cell death, enhancing antigen presentation, and increasing trafficking and activation of tumor-specific T cells, whereas checkpoint blockade prevents PD-1/PD-L1-mediated adaptive resistance and sustains systemic antitumor immunity [85,86,87,88]. Preclinical data and early clinical series, including patients with locally advanced cSCC treated with concurrent cemiplimab and RT, suggest that such combinations can improve local control and occasionally induce abscopal responses, although optimal dose, fractionation, and sequencing remain to be defined in prospective trials [85].
3.4.3. Non-Immunotherapy Systemic Options
Cytotoxic chemotherapy—historically cisplatin ± 5-fluorouracil—produces short-lived responses and significant toxicity, particularly in the elderly aCSCC population, and is now largely a palliative fallback when immunotherapy is contraindicated or has failed [87]. EGFR-directed therapy (cetuximab; panitumumab) achieves disease control in a subset, with median survivals typically around a year in small series, and may serve as a bridge or radiosensitizer in non-immunotherapy candidates; dermatologic toxicities are frequent and require proactive management [88,89]. EGFR tyrosine kinase inhibitors have modest activity and limited durability [77].
3.4.4. Translational and Emerging Therapies
Translational strategies aim to deepen or rescue responses to PD-1 blockade. Oncolytic immunotherapy (e.g., RP1) seeks to convert immunologically “cold” tumors to “hot,” with phase III (CERPASS) and phase II programs exploring combinations with PD-1 inhibitors; early signals in PD-1–refractory cohorts are encouraging but not yet definitive for practice change [77]. Additional targeted or immune-modulating agents (e.g., PLK1 inhibition with rigosertib in RDEB-associated aCSCC) illustrate the heterogeneity of advanced disease and the need for tailored trials in genetically or clinically defined subsets [78].
3.5. Immune Checkpoint Inhibitors in Advanced cSCC
Immune checkpoint inhibitors (ICIs) have revolutionized the therapeutic landscape of several solid tumors, and their role in advanced cSCC has rapidly gained attention. The strong immunogenicity of cSCC, its high tumor mutational burden, and frequent PD-L1 expression provide a strong biological rationale for PD-1 blockade. In this context, cemiplimab has emerged as the first and currently only approved PD-1 inhibitor in Europe for patients with unresectable locally advanced or metastatic cSCC. The following sections will explore the mechanistic basis of PD-1/PD-L1 inhibition, summarize the clinical trial and real-world evidence, and examine safety considerations with a focus on immune-related adverse events.
3.5.1. Mechanism of Action
PD-1, a CD28-family inhibitory receptor on activated T cells, dampens TCR/CD28 signaling upon binding PD-L1/PD-L2 via SHP-1/2 recruitment to its ITSM motif, dephosphorylating CD3ζ, ZAP-70, and PI3K and suppressing Akt/mTOR/MAPK pathways [90,91]. PD-1 is also expressed on CD4+ T cells, regulatory T cells, B cells, and subsets of NK/NKT cells, where it contributes to broader immunoregulatory effects within the tumor microenvironment [90]. In tumors, chronic antigen exposure drives PD-1–high “exhausted” TILs with reduced proliferation, cytokines (IL-2, IFN-γ, TNF-α), and cytotoxicity [90,91]. cSCC, UV-driven and highly mutagenic, is immunogenic yet exploits adaptive resistance: IFN-γ–induced PD-L1 upregulation on tumor and myeloid cells attenuates antitumor T-cell activity, reinforced by Tregs, IL-10/TGF-β, M2 macrophages, and impaired type I IFN signaling [90,91,92,93]. The markedly higher cSCC incidence in immunosuppressed hosts (e.g., transplant recipients) underscores the role of immune surveillance. Anti-PD-1 antibodies (cemiplimab, pembrolizumab, nivolumab) block PD-1–ligand interactions, releasing the inhibitory brake and restoring antigen-specific T-cell function [94,95]. Mechanistically, PD-1 blockade reinvigorates exhausted CD8+/CD4+ T cells, enhancing proliferation, IFN-γ/TNF-α production, and perforin/granzyme-mediated cytolysis; additional effects include improved NK activity, revitalized tumor-draining node responses, and modulation of myeloid cells, with a possible, but generally outweighed, expansion of PD-1+ Tregs (Figure 4) [96].
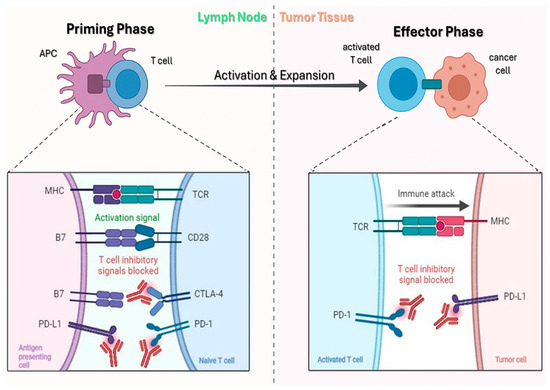
Figure 4.
Mechanism of action of ICIs. Left: during the priming phase, APCs activate naïve T cells through MHC–TCR interaction, while inhibitory signals are blocked by anti–PD-1, anti–PD-L1, and anti–CTLA-4. Right: in the effector phase, activated T cells infiltrate tumor tissue, recognize tumor antigens, and trigger an immune attack, with inhibitory pathways blocked by anti–PD-1 and anti–PD-L1. Adapted from Khan et al. [96].
The high tumor mutational burden and UV-signature neoantigens in cSCC likely underpin the robust clinical responses observed in advanced disease [90,93]. Baseline PD-L1 expression and pre-existing TILs correlate with higher response rates, reflecting the need for a pre-activated immune microenvironment for effective checkpoint blockade [92,93].
3.5.2. Clinical Trials and Regulatory Approvals
cSCC is of great interest for treatment with ICIs. The high tumor mutational burden (TMB), defined as the number of nonsynonymous mutations per coding region, has been correlated to the response rate of PD1 antibody treatment [97]. In fact, high TMB increases the probability of generating neoantigens that can be recognized by tumor-infiltrating T cells, thereby enhancing the likelihood of a pre-existing antitumor immune response that can be unmasked by PD-1 blockade. In addition, immunohistochemical studies demonstrate relatively high expression of PD-L1 in cSCC, expressed not only on tumor cells but also on tumor-associated macrophages and other stromal elements. Increased PD-L1 expression has been shown to correlate with better tumor response to PD-1 antibody treatment, reinforcing the biological rationale for checkpoint blockade in this disease [98]. The pivotal clinical evidence comes from the EMPOWER-CSCC-1 trial, a phase II, non-randomized, multicohort study evaluating cemiplimab, a fully human IgG4 monoclonal antibody against PD-1 [99]. In the first cohort, 59 adult patients with metastatic disease and 78 with locally advanced tumors ineligible for curative surgery or radiotherapy received cemiplimab intravenously at 3 mg/kg every two weeks for up to 96 weeks. A separate cohort of 56 adult patients with metastatic cSCC was treated with a flat fixed dose of 350 mg intravenously every three weeks for up to one year. Of the total enrolled population, 33.7% had previously received systemic anticancer therapy. The primary endpoint was the overall response rate (ORR) as determined by independent central review using RECIST 1.1 criteria for radiographic assessment and modified WHO criteria for photographic evaluation [98]. Results presented at ESMO 2022, with a median follow-up of 15.3 months, reported an ORR of 50.8% in the 3 mg/kg group, which included 20.3% complete responses (CR) and 30.5% partial responses (PR) [74,100]. In the cohort with locally advanced disease, the ORR was 44.9%, with 12.8% CR and 32.1% PR. In the fixed-dose cohort, the ORR was 46.4%, comprising 19.6% CR and 26.8% PR. When all three groups were pooled (n = 193), the ORR was 47.2%, including 17.1% CR and 30.1% PR. The median duration of response reached 41.3 months, progression-free survival (PFS) was 22.1 months, and the median overall survival (OS) had not been reached at data cut-off. Treatment discontinuation due to adverse events occurred in 10.4% of patients. The most frequently observed toxicities were fatigue, diarrhea, nausea, and pruritus, consistent with the expected class profile. No treatment-related deaths were reported. Based on these data, cemiplimab was approved by the FDA in September 2018 and by the EMA in July 2019 for the treatment of patients with locally advanced or metastatic cSCC who are not candidates for curative surgery or radiotherapy [100]. A confirmatory cohort (group 6 of EMPOWER-CSCC-1) tested the regulatory-approved flat dosing regimen of cemiplimab at 350 mg every three weeks. At ESMO 2022, results from 167 patients (59.9% metastatic, 40.4% locally advanced) were presented, with a median treatment exposure of 35.7 weeks [101]. The median follow-up was 8.7 months. The overall response rate was 45.1%, including 5.5% complete responses. Median progression-free survival was 14.7 months, while the medians for both OS and duration of response had not yet been reached. Treatment discontinuation due to adverse events was reported in 13.9% of patients, with fatigue (26.1%), diarrhea (21.2%), and pruritus (21.2%) being the most common. No treatment-related deaths were observed in this cohort. Clinical trials of cemiplimab in advanced cutaneous squamous cell carcinoma are summarized in Table 3.

Table 3.
Clinical trials exploring cemiplimab in advanced cSCC.
Pembrolizumab has also been evaluated in advanced cSCC, most notably in the KEYNOTE-629 trial [102]. This phase II study enrolled 105 patients with locally advanced or metastatic cSCC, the majority of whom had received prior systemic chemotherapy. The ORR was 34.3%, with 3.8% complete responses. Median PFS was 6.9 months, and median OS had not yet been reached, though 60.3% of patients were alive at 12 months. The discontinuation rate due to adverse events was 12.1%, and toxicity was comparable to that reported in cemiplimab studies. Subsequent analyses confirmed durable antitumor activity without unexpected safety concerns [103]. Pembrolizumab received FDA approval in June 2020 for locally advanced or metastatic cSCC but remains unapproved by EMA, leaving cemiplimab as the sole approved PD-1 inhibitor for advanced cSCC in Europe. Indirect comparative analyses have provided further evidence for the superior efficacy of cemiplimab. A systematic review by Keeping and colleagues evaluated 11 studies, including the cemiplimab EMPOWER-CSCC-1 trial (n = 193), seven trials of EGFR inhibitors, two pembrolizumab studies, and one platinum-based chemotherapy trial [104]. Cemiplimab demonstrated improved OS compared with EGFR inhibitors, with HRs ranging from 0.07 to 0.47, and PFS benefits with HRs between 0.30 and 0.67. Compared with pembrolizumab, cemiplimab conferred superior OS (HR range: 0.17–0.52) and PFS (HR range: 0.49–0.55 in KEYNOTE-629), though not in all datasets, as no significant PFS difference was observed in the Maubec et al. pembrolizumab trial [105]. Against platinum-based chemotherapy, cemiplimab showed a marked survival benefit (HR for OS: 0.19, 95% CI: 0.10–0.39), although the PFS advantage was not statistically significant (HR: 0.66, 95% CI: 0.38–1.16). A broader systematic review and comparative analysis by Petzold et al. included 22 studies evaluating chemotherapy, EGFR inhibitors, immune checkpoint inhibitors, and combination regimens [106]. Immunotherapy demonstrated the most favorable survival outcomes, with median PFS of 9.9 months (range 8.1–19.9) compared to 3 months for chemotherapy, 4.9 months for EGFR inhibitors, and 9.1 months for non-immunotherapy combination regimens. Median OS with checkpoint blockade was not reached (95% CI: 31.5 months–not reached), whereas it was 12.6 months for chemotherapy and 12.7 months for EGFR inhibition. At 26 months, survival with checkpoint inhibitors was 70.8% (95% CI: 61.5–81.5), compared with 17.1% (95% CI: 9.5–30.8) for chemotherapy and 37.9% (95% CI: 29.5–48.8) for combination regimens without immunotherapy.
Beyond advanced disease, the potential of cemiplimab in earlier treatment settings has been evaluated. The REGN-ONC-1901 neoadjuvant trial enrolled 79 patients with stage II–IV cSCC (median age 73 years) who received four cycles of cemiplimab 350 mg every three weeks prior to surgery [106]. Pathological complete response (pCR) was achieved in 56% of patients, with an additional 12.7% achieving major pathological response (1–10% viable tumor cells). By contrast, radiological ORR was 68.4%, including only 6.3% complete responses, underscoring the importance of pathological endpoints. PD-L1 expression ≥1% correlated with higher pCR rates, though this did not reach statistical significance. Four deaths occurred, three unrelated to study drug, and one discontinuation was reported. In the adjuvant setting, the REGN-1788 (C-POST) phase III trial is randomizing high-risk cSCC patients to cemiplimab (350 mg every three weeks for 12 weeks, followed by 700 mg every six weeks for 36 weeks) versus placebo after surgery and postoperative radiation [78]. The primary endpoint is disease-free survival. In parallel, the KEYNOTE-630 trial is assessing pembrolizumab (400 mg every six weeks for one year) versus placebo under similar conditions [78]. Alternative delivery strategies are also being tested. An intralesional phase I study (NCT03889912) administered cemiplimab directly into cutaneous lesions at doses ranging from 5 to 44 mg per lesion over 12 weeks prior to surgery [78,107]. Among 17 patients (median age 76 years, 76% with head and neck primaries), 76.5% achieved a pathological complete response. Pruritus (23.5%) and fatigue (17.6%) were the most common adverse events. One patient discontinued at the highest dose level, and no treatment-related deaths occurred. Combination approaches are under investigation as well. The I-TACKLE trial (NCT03666325) studied pembrolizumab monotherapy followed by the addition of cetuximab in patients with progression. Pembrolizumab alone produced an ORR of 44% (12 CR, seven PR) [108]. Among those who relapsed after initial response, two patients treated with pembrolizumab plus cetuximab achieved further partial responses. Among patients with no initial response to pembrolizumab, 38% responded after the addition of cetuximab. Parallel trials are ongoing to assess PD-1/PD-L1 inhibitors in combination with EGFR inhibitors, such as the ongoing study of avelumab with or without cetuximab (NCT03944941) [78].
3.5.3. Real-World Evidence of Cemiplimab in Advanced cSCC
Beyond clinical trials, several real-world studies have provided valuable insights into the safety and efficacy of cemiplimab in patients with advanced cSCC, a population often older and more comorbid than those typically enrolled in pivotal trials. The CASE trial (NCT03836105), a prospective observational study, reported interim data on 196 patients with a median age of 76 years [109]. Most patients presented with locally advanced disease (63.3%), while 36.7% had metastatic cSCC; 42.9% had received prior radiotherapy, 75% prior surgery, and 45.4% prior systemic therapy. The overall response rate (ORR) was 37.4%, with 9.8% complete responses, and the disease control rate (DCR) reached 54.6%. Only 2.6% of patients discontinued treatment due to serious adverse events, and one death was attributed to treatment-related pneumonitis. In Europe, additional real-world cohorts have strengthened these findings. The CEMISKIN study, a large international multicentre non-interventional project conducted across Germany, Austria, and Switzerland, aims to include approximately 400 patients (200 prospective, 200 retrospective) to assess efficacy and tolerability of cemiplimab outside clinical trials [78,110]. Preliminary results highlight the feasibility of long-term data collection and confirm its integration into routine oncology practice. Similarly, the CAREPI study group in France analyzed 245 patients (mean age 77 years, 73% male), of whom 35% had locally advanced and 65% metastatic cSCC [111]. Nearly half had received prior systemic therapy, and 24% were immunocompromised. The best overall response rate was 50.4% (21% CR, 29% PR). With a median follow-up of 12.6 months, median progression-free survival (PFS) was 7.9 months, while median overall survival (OS) and duration of response were not reached. One-year OS was 73% in patients with ECOG < 2 versus 36% in those with ECOG ≥ 2, highlighting performance status as a critical prognostic factor. Severe treatment-related adverse events occurred in 9% of patients, including one fatal toxic epidermal necrolysis, while immunosuppression did not significantly impair efficacy. Data from the Netherlands have further validated these outcomes. In a retrospective multicentre cohort of 65 patients (median age 76, range 30–93), cemiplimab achieved an ORR of 52%, with 22% complete responses [112]. Median PFS was 10.9 months and median OS 26.1 months after a median follow-up of 21.5 months. Grade 3–4 adverse events occurred in 22% of patients, yet treatment was generally well tolerated, even among elderly patients with severe comorbidities. Complementary prospective data from the DRUG Access Protocol (151 patients treated with cemiplimab prior to national reimbursement) confirmed consistent efficacy: clinical benefit rate was 54.3%, ORR 35.1%, and median OS 24.2 months, with manageable safety [113]. Kidney transplant rejection emerged as the most notable treatment-related complication, affecting 9.5% of patients. Finally, a Hungarian single-centre retrospective analysis including 25 patients (median age 78 years) reported an ORR of 52% (3 CR, 10 PR) and a DCR of 76%, with similar efficacy observed in immunocompromised patients (ORR 60%, DCR 80%) [114]. Treatment discontinuation occurred in 24% due to adverse events, and 36% experienced grade ≥3 toxicities. Despite this, cemiplimab demonstrated clinically meaningful activity in a population with advanced age, multiple comorbidities, and frequent immunosuppression.
3.5.4. Safety Profile and Immune-Related Adverse Events
Cemiplimab, as an anti–PD-1 antibody, shares with other ICIs the potential to induce a wide spectrum of immune-related adverse events (irAEs) [115]. PD-1 blockade enhances T-cell proliferation and effector function by preventing PD-1 ligation with PD-L1/PD-L2, but this same mechanism may precipitate autoreactive immune activity against healthy tissues. Breakdown of immune self-tolerance is mediated by clonal expansion of autoreactive T cells, diminished regulatory T-cell function, enhanced B-cell activation with autoantibody production, and proinflammatory cytokine release, which collectively underlie the onset of irAEs [116]. Organs dependent on peripheral tolerance, such as the skin, gastrointestinal tract, endocrine glands, and lungs, are most frequently affected. Clinical data from pivotal phase II trials highlight the incidence and severity of such events. In the open-label study of Migden et al., grade 3–4 treatment-emergent adverse events occurred in 44% of patients, with hypertension (8%) and pneumonia (5%) being most common [117]. Importantly, grade ≥ 3 immune-related events attributable to cemiplimab were reported in 10% of cases [117]. These included pneumonitis, hepatitis with marked transaminase elevation, proctitis, encephalitis, and autoimmune hepatitis, reflecting the multisystemic autoimmune profile of checkpoint blockade. Treatment discontinuation due to adverse events occurred in 8% of patients, and deaths were rarely but occasionally attributed to treatment, such as fatal pneumonia or aspiration pneumonia temporally related to therapy. Population-level real-world analyses have further refined the spectrum of adverse effects. In a retrospective global cohort of 714 cemiplimab-treated patients compared with almost 295,000 controls, significant excess risks were observed for several irAEs [118]. The strongest associations were seen for hypothyroidism (OR 8.06), rash or skin eruption (OR 7.02), elevated alkaline phosphatase (OR 6.54), hyponatremia (OR 5.26), diarrhea (OR 3.43), noninfectious colitis (OR 2.69), and pneumonia (OR 2.08). Notably, endocrine toxicities, especially thyroid dysfunction, were among the most frequent, consistent with other PD-1 inhibitors. Severe pulmonary complications were particularly clinically relevant: pneumonia and pleural effusion not only occurred more frequently but were also associated with a significant reduction in overall survival, with hazard ratios of 2.37 and 1.52, respectively [118].
These data emphasize the dual contribution of autoimmune and infectious etiologies in pulmonary irAEs, underscoring the importance of careful differential diagnosis. Cutaneous toxicities, including maculopapular rash and pruritus, are among the earliest and most common irAEs and were observed in up to 27% of patients in clinical trials [117]. Although generally mild (grade 1–2). Gastrointestinal adverse events, such as diarrhea and colitis, occurred in 7–8% of patients in real-world series and can progress to life-threatening immune-mediated colitis if unrecognized [118]. Hepatotoxicity, though less frequent, remains clinically significant, with elevations in AST/ALT reported in 20% of treated patients and rare cases of autoimmune hepatitis requiring discontinuation. Endocrine toxicities are dominated by thyroiditis leading to hypothyroidism, but rare cases of hypophysitis, adrenal insufficiency, and new-onset diabetes mellitus have also been described, consistent with PD-1–mediated disruption of glandular self-tolerance. Neurological and cardiovascular irAEs are rare but severe. Encephalitis, reported in phase II trials, as well as myocarditis and myositis, have been documented sporadically, carrying high mortality rates [117]. Renal involvement is uncommon but clinically relevant, with elevated creatinine and acute interstitial nephritis reported more frequently in cemiplimab-exposed patients, with renal failure occurring in approximately 20% of real-world cases, though often multifactorial [118]. Electrolyte disturbances such as hyponatremia and hypercalcemia are also more prevalent in cemiplimab recipients, suggesting systemic immune dysregulation.
Overall, the safety profile of cemiplimab in advanced cSCC mirrors that of other PD-1 inhibitors but is characterized by a predominance of cutaneous, endocrine, hepatic, and pulmonary irAEs. The majority are manageable with treatment interruption, corticosteroids, or immunosuppression, yet a subset of events, particularly pneumonitis, myocarditis, and severe hepatitis, carry substantial morbidity and mortality. Early recognition, patient selection, and vigilant monitoring, especially in elderly and comorbid populations typical of advanced cSCC, are therefore critical to optimizing outcomes.
4. Discussion
aCSCC remains a disease of high clinical and societal burden with notable health-system costs. In an analysis of healthcare resource utilisation within the Italian National Health Service, the mean annual cost per patient in 2015 was estimated at €5654 for non-melanoma skin cancers overall, rising to €10,281 for unresectable or advanced cSCC [67]. For decades, systemic options were largely palliative, offering transient control with substantial toxicity. The advent of PD-1 blockade has altered this landscape, introducing the possibility of durable benefit in a meaningful subset of patients. Yet, the key aspect for everyday practice is who benefits most, how durable that benefit is outside trials, and how to integrate systemic therapy with radiotherapy and selective surgery and these issues are far from settled.
In line with contemporary international guidelines, PD-1 blockade with cemiplimab, and, where available, pembrolizumab, is endorsed as the preferred first-line systemic option for most patients with unresectable or metastatic cSCC, given its favorable efficacy–toxicity profile and the potential for durable disease control [7,10,19,99]. Nonetheless, NCCN, ESMO and national societies (e.g., AIOM) explicitly acknowledge a role for alternative systemic strategies in selected scenarios, including EGFR inhibitors such as cetuximab and platinum-based chemotherapy, either as single agents or in combination regimens, particularly in patients with formal contraindications to immunotherapy (e.g., active autoimmune disease, uncontrolled interstitial lung disease), in those who progress on or shortly after PD-1 blockade, and in solid-organ transplant recipients where the risk of allograft rejection is deemed prohibitive [7,15]. Moreover, these agents may be integrated with radiotherapy and/or surgery in a multidisciplinary approach, for instance in the setting of bulky, borderline-resectable tumours or when rapid cytoreduction is required. Within this framework, cemiplimab should be regarded as the current backbone of systemic therapy for aCSCC rather than the sole available option, and its use needs to be contextualised within guideline-based, patient-tailored treatment algorithms.
Across pivotal trials and real-world cohorts, PD-1 blockade with cemiplimab has transformed the prognosis of advanced cSCC, with objective response rates (ORRs) in the range of ~45–60%, complete responses in 10–30% of patients, and median progression-free survival (PFS) that generally exceeds 8–12 months, alongside a tail of durable responders [110,111,112,113,114]. Nonetheless, outcomes remain highly heterogeneous, underscoring the need to understand host- and tumour-related prognostic factors to refine patient selection, counselling, and monitoring strategies. Some determinants have now been consistently validated, whereas others are emerging from broader immuno-oncology literature but remain insufficiently explored specifically in advanced cSCC treated with cemiplimab. Among clinicodemographic variables, performance status has emerged as one of the most powerful and reproducible prognostic determinants. In large real-world cohorts, patients with ECOG ≥ 1–2 systematically show lower ORR, shorter PFS, and reduced overall survival (OS), whereas those with good performance status achieve outcomes comparable to, or in some series approaching, those reported in EMPOWER-CSCC-1 [99]. Disease extent at baseline is another robust driver of prognosis. Pooled analyses of EMPOWER-CSCC-1 and multiple registries confirm that patients with locally advanced but non-metastatic disease derive the greatest relative benefit from cemiplimab, while nodal disease and, particularly, distant metastases are associated with lower response rates and shorter disease control, even though many metastatic cases still obtain meaningful benefit [99,110,111,112,113,114]. Immunosuppression status also carries substantial prognostic weight: real-world cohorts consistently report inferior PFS and OS in patients with solid-organ transplants, hematologic malignancies, chronic corticosteroid therapy, or other immunocompromising conditions, mirroring the historically more aggressive clinical course and poorer outcomes of cSCC in this setting [10,114]. By contrast, age and sex appear to exert limited influence on the probability of benefit. EMPOWER-CSCC-1 did not show meaningful differences in ORR or duration of response when stratified by age or gender, a finding that has been echoed in several elderly and ultra-octogenarian real-world series, supporting the use of cemiplimab irrespective of chronological age provided that functional status is acceptable [99,110,111,112,113,114]. Anatomical site is a more subtle variable. Cemiplimab series with adequate stratification suggest that head-and-neck primaries, which account for the majority of advanced cSCC, often display numerically higher response rates and more favorable survival than tumours on the trunk or extremities. In a large Italian multicenter cohort, head-and-neck localisation and normal baseline haemoglobin were significantly associated with better response, whereas genital primaries, prior chemotherapy, recent systemic antibiotics and chronic corticosteroid use correlated with poorer outcomes [10]. More recently, a multi-institutional UK study found that a head-and-neck primary site independently predicted improved PFS and OS, while concomitant immunosuppression portended worse PFS [119]. These observations are biologically plausible: chronic ultraviolet exposure on sun-exposed skin drives a very high tumour mutational burden and abundant neoantigens, and head-and-neck cSCC often exhibits increased PD-L1 expression and an inflamed tumour microenvironment, which may enhance susceptibility to PD-1 blockade [110,111,112,113,114]. At the same time, tumours on the trunk or extremities frequently arise in the context of chronic ulcers, lymphedema, diabetes or prior radiation, all of which can compromise local immune surveillance. Histopathology adds an additional layer of complexity. Surgical series and risk-stratification guidelines have long recognized that certain histologic variants, such as desmoplastic, spindle-cell, sarcomatoid, keratoacanthoma-like, and other poorly differentiated forms, are associated with higher rates of perineural invasion, local recurrence and disease-specific mortality compared with conventional cSCC [71,120,121]. These variants tend to display epithelial–mesenchymal transition, dense desmoplastic stroma and an immune-excluded phenotype, features that conceptually may reduce sensitivity to PD-1 blockade. However, most cemiplimab trials and registries have not systematically analysed outcomes by histologic subtype, and available data on variant histotypes under immunotherapy remain sparse and largely exploratory. Similarly, classical “high-risk” pathological features such as depth of invasion, lymphovascular invasion and perineural invasion, that are strong predictors of nodal spread and mortality in surgical cohorts, have not emerged as a consistent or strong prognostic signal in the limited cemiplimab datasets where they were captured, raising the possibility that checkpoint inhibition may partially mitigate their adverse impact. This highlights an important knowledge gap and supports systematic histopathologic stratification, including variant subtypes and quantitative measures of thickness and perineural involvement, in future immunotherapy studies. Beyond tumour burden and histology, host metabolic and inflammatory status is emerging as a potentially relevant, yet under-studied, prognostic domain in advanced cSCC. A growing body of pan-cancer evidence supports the so-called “obesity paradox”, whereby overweight or mildly obese patients (BMI ≥ 25 kg/m2) treated with ICIs often experience improved PFS and OS compared with normal-weight individuals, particularly in melanoma, NSCLC and head-and-neck cancers [122,123]. Proposed mechanisms include the immune-endocrine role of adipose tissue, leptin-driven T-cell activation coupled with PD-1 overexpression and functional exhaustion, and adiposity-associated changes in myeloid and lymphoid compartments that create a checkpoint-dependent state in which PD-1 blockade may yield a disproportionate functional gain [124,125]. However, BMI and other lifestyle-related variables (physical activity, smoking and alcohol intake, diet, UV exposure patterns) have been scarcely examined as prognostic modifiers in cemiplimab-treated cSCC, despite the high prevalence of elderly, comorbid and metabolically fragile patients in this population. In parallel, easily available serum biomarkers of systemic inflammation and nutritional status, albumin, neutrophil-to-lymphocyte ratio (NLR), and lactate dehydrogenase (LDH), have repeatedly been linked to ICI outcomes in other tumour types. Meta-analyses show that hypoalbuminemia is consistently associated with poorer survival in patients receiving ICIs, likely integrating systemic inflammation, cancer-associated cachexia and altered monoclonal antibody pharmacokinetics [126,127]. Elevated or rising NLR and dynamic increases in LDH during immunotherapy similarly correlate with treatment resistance and early progression in melanoma, NSCLC and head-and-neck carcinoma, whereas baseline values are less consistently predictive [128,129]. Despite their practicality and biological plausibility, these markers have only sporadically been incorporated into cSCC registries, and no validated threshold or composite score currently guides clinical practice in this setting.
Finally, the development of irAEs appears to be a promising “on-treatment” prognostic signal. Across multiple tumour types and ICI regimens, the occurrence of (mostly low-grade) irAEs, particularly cutaneous and endocrine, has been associated with higher response rates and prolonged survival, whereas severe toxicities requiring prolonged high-dose steroids may attenuate this benefit [129,130]. Evidence in cSCC specifically is still limited and partly conflicting, yet the biological rationale, e.g., shared T-cell clonality and cytokine profiles driving both antitumour and autoimmune responses, potential microbiome-mediated effects, and pharmacokinetic differences in non-cachectic patients, is compelling and justifies prospective collection and time-dependent analysis of irAEs as candidate surrogate markers of benefit with cemiplimab. Taken together, the available literature suggests a layered prognostic architecture in advanced cSCC treated with PD-1 blockade. “Core” determinants such as performance status, disease stage, immunosuppression, and (probably) anatomical site are now reasonably well characterized and should form the backbone of any risk stratification framework. In contrast, several potentially informative domains remain underexplored in this disease: histologic variants and quantitative pathologic features, lifestyle and metabolic factors (including BMI and related comorbidities), and inexpensive inflammatory or nutritional biomarkers (albumin, NLR, LDH and related indices), together with the timing, pattern and severity of irAEs.
Beyond traditional clinico-pathologic factors, emerging AI- and imaging-based approaches may further refine risk stratification in cSCC. Deep learning models applied to digital pathology and cellular morphometry have shown improved prediction of metastatic risk compared with conventional staging, suggesting a potential role in identifying patients who might benefit from intensified surveillance or systemic therapy [131,132]. Similarly, baseline [18F]FDG PET/CT radiomics signatures combined with AI are being explored to predict immunotherapy response and non-invasively approximate tumor grade in advanced cSCC. In parallel, hyperspectral imaging coupled with machine learning has demonstrated encouraging performance for non-invasive characterization and margin assessment of non-melanoma skin cancers, including SCC, and could in principle be integrated into pre-operative planning or longitudinal monitoring of high-risk lesions [133]. However, these tools remain at an early, exploratory stage, and robust prospective validation in dedicated aCSCC cohorts is still lacking.
In this context, future prospective, biomarker-rich studies and large, AI-applications, and harmonized real-world registries should be specifically designed to evaluate these factors in multivariable models, integrating static baseline characteristics with dynamic on-treatment markers within a truly multidisciplinary framework. This is particularly relevant in dermatology, where clinical practice is increasingly shifting towards stratified, team-based care and a greater focus on special populations (such as immunosuppressed, very elderly, highly comorbid or metabolically fragile patients), who may display distinct risk–benefit profiles under PD-1 blockade [134,135]. Robust prognostic tools capable of both identifying these patients upfront and anticipating their trajectory of response during therapy would ultimately allow clinicians to tailor treatment intensity, monitoring and supportive measures to the individual context, thereby refining risk–benefit assessment and personalizing management in advanced cSCC treated with cemiplimab.
5. Conclusions
Based on pivotal trials and accumulating real-world evidence, PD-1 blockade with cemiplimab now represents the standard first-line systemic option for unresectable locally advanced or metastatic cutaneous squamous cell carcinoma and should be considered early for all eligible patients without major contraindications to immunotherapy. Prognosis is not determined by stage alone, but by the interplay of tumor-related (tumor burden, head and neck localization, perineural invasion, metastatic pattern), host-related (age, ECOG performance status, immunosuppression, comorbidities) and inflammatory or nutritional markers (e.g., LDH, NLR, albumin, BMI). These domains should be systematically assessed at baseline to guide treatment goals, set realistic expectations and support shared decision-making, particularly in frail elderly and immunosuppressed patients. Our synthesis supports viewing cemiplimab as the backbone of care in aCSCC, to be integrated with surgery and radiotherapy within a multidisciplinary pathway. In clinical practice, we recommend early referral to dedicated skin cancer boards, adoption of structured follow-up schedules and routine collection of simple blood-based biomarkers and imaging at predefined time-points to detect primary resistance and to identify long-term responders who may be candidates for treatment de-escalation or discontinuation. Future research should prioritize the prospective validation of composite prognostic scores and biomarker-driven studies testing intensified or combined approaches in biologically high-risk subgroups, thereby consolidating cemiplimab at the center of personalized, risk-adapted management algorithms in aCSCC.
Author Contributions
Conceptualization, A.D.G., G.P. and L.F.; methodology, A.D.G., F.T. and C.C.; software, A.D.G. and F.T.; validation, A.D.G., F.T., G.P. and L.F.; formal analysis, A.D.G. and F.R.; investigation, A.D.G., F.T., C.C., F.R., G.D.L., F.M., R.M., A.P. and S.P.N.; resources, C.C., F.R., G.D.L., S.P.N., G.P. and L.F.; data curation, F.T., F.R., G.D.L. and A.P.; writing—original draft preparation, A.D.G. and F.T.; writing—review and editing, C.C., F.R., G.P. and L.F.; visualization, A.D.G., F.T. and R.M.; supervision, G.P., L.F., C.C. and S.P.N.; project administration, A.D.G., F.T. and L.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| cSCC | Cutaneous squamous cell carcinoma |
| aCSCC | Advanced cutaneous squamous cell carcinoma |
| laCSCC | Locally advanced cutaneous squamous cell carcinoma |
| mCSCC | Metastatic cutaneous squamous cell carcinoma |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed cell death ligand 1 |
| ICI | Immune checkpoint inhibitor |
| RT | Radiotherapy |
| EGFR | Epidermal growth factor receptor |
| ORR | Overall response rate |
| PFS | Progression-free survival |
| OS | Overall survival |
| irAE | Immune-related adverse event |
| ECOG | Eastern Cooperative Oncology Group |
| AJCC | American Joint Committee on Cancer |
References
- Que, S.K.T.; Zwald, F.O.; Schmults, C.D. Cutaneous squamous cell carcinoma: Incidence, risk factors, diagnosis, and staging. J. Am. Acad. Dermatol. 2018, 78, 237–247. [Google Scholar] [CrossRef]
- Caudill, J.; Thomas, J.E.; Burkhart, C.G. The risk of metastases from squamous cell carcinoma of the skin. Int. J. Dermatol. 2023, 62, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, C.J.; Eggermont, A.M.M. Shifting landscape in skin cancer incidence: The rising tide of cutaneous squamous cell carcinoma and potential implications for prevention. Br. J. Dermatol. 2024, 190, 460–461. [Google Scholar] [CrossRef]
- Skulsky, S.L.; O’Sullivan, B.; McArdle, O.; Leader, M.; Roche, M.; Conlon, P.J.; O’Neill, J.P. Review of high-risk features of cutaneous squamous cell carcinoma and discrepancies between the American Joint Committee on Cancer and NCCN Clinical Practice Guidelines In Oncology. Head. Neck 2017, 39, 578–594. [Google Scholar] [CrossRef] [PubMed]
- Work Group; Invited Reviewers; Kim, J.Y.S.; Kozlow, J.H.; Mittal, B.; Moyer, J.; Olenecki, T.; Rodgers, P. Guidelines of care for the management of cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 2018, 78, 560–578. [Google Scholar]
- Jiang, R.; Fritz, M.; Que, S.K.T. Cutaneous Squamous Cell Carcinoma: An Updated Review. Cancers 2024, 16, 1800. [Google Scholar] [CrossRef] [PubMed]
- Stratigos, A.J.; Garbe, C.; Dessinioti, C.; Lebbe, C.; Bataille, V.; Bastholt, L.; Dreno, B.; Fargnoli, M.C.; Forsea, A.M.; Frenard, C.; et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: Part 1. epidemiology, diagnostics and prevention. Eur. J. Cancer 2020, 128, 60–82. [Google Scholar] [CrossRef]
- Garcia, C.N.; Wies, C.; Hauser, K.; Brinker, T.J. Noninvasive Technologies for the Diagnosis of Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. JID Innov. 2024, 4, 100303. [Google Scholar] [CrossRef]
- Azimi, A.; Jabbour, S.; Patrick, E.; Fernandez-Penas, P. Non-invasive diagnosis of early cutaneous squamous cell carcinoma. Exp. Dermatol. 2023, 32, 1946–1959. [Google Scholar] [CrossRef]
- Baggi, A.; Quaglino, P.; Rubatto, M.; Depenni, R.; Guida, M.; Ascierto, P.A.; Trojaniello, C.; Queirolo, P.; Saponara, M.; Peris, K.; et al. Corrigendum to “Real world data of cemiplimab in locally advanced and metastatic cutaneous squamous cell carcinoma” [Eur J Canc 157 (2021) 250-258]. Eur. J. Cancer 2022, 166, 309–310. [Google Scholar] [CrossRef]
- Ge, W.; Wu, N.; Chen, C.I.; Inocencio, T.J.; LaFontaine, P.R.; Seebach, F.; Fury, M.; Harnett, J.; Ruiz, E.S. Real-World Treatment Patterns and Outcomes of Cemiplimab in Patients with Advanced Cutaneous Squamous Cell Carcinoma Treated in US Oncology Practices. Cancer Manag. Res. 2024, 16, 841–854. [Google Scholar] [CrossRef]
- Dessinioti, C.; Pitoulias, M.; Stratigos, A.J. Epidemiology of advanced cutaneous squamous cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 39–50. [Google Scholar] [CrossRef]
- Nelson, T.G.; Ashton, R.E. Low incidence of metastasis and recurrence from cutaneous squamous cell carcinoma found in a UK population: Do we need to adjust our thinking on this rare but potentially fatal event? J. Surg. Oncol. 2017, 116, 783–788. [Google Scholar] [CrossRef]
- Schmults, C.D.; Karia, P.S.; Carter, J.B.; Han, J.; Qureshi, A.A. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: A 10-year, single-institution cohort study. JAMA Dermatol. 2013, 149, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Venables, Z.C.; Nijsten, T.; Wong, K.F.; Autier, P.; Broggio, J.; Deas, A.; Harwood, C.A.; Hollestein, L.M.; Langan, S.M.; Morgan, E.; et al. Epidemiology of basal and cutaneous squamous cell carcinoma in the U.K. 2013-15, a cohort study. Br. J. Dermatol. 2019, 181, 474–482. [Google Scholar] [CrossRef]
- Eisemann, N.; Jansen, L.; Castro, F.A.; Chen, T.; Eberle, A.; Nennecke, A.; Zeissig, S.R.; Brenner, H.; Katalinic, A.; GEKID Cancer Survival Working Group. Survival with nonmelanoma skin cancer in Germany. Br. J. Dermatol. 2016, 174, 778–785. [Google Scholar] [CrossRef]
- Robsahm, T.E.; Helsing, P.; Veierød, M.B. Cutaneous squamous cell carcinoma in Norway 1963–2011, increasing incidence and stable mortality. Cancer Med. 2015, 4, 472–480. [Google Scholar] [CrossRef]
- Brunner, M.; Veness, M.J.; Ch’ng, S.; Elliott, M.; Clark, J.R. Distant metastases from cutaneous squamous cell carcinoma—Analysis of AJCC stage IV. Head. Neck 2013, 35, 72–75. [Google Scholar] [CrossRef]
- O’Reilly Zwald, F.; Brown, M. Skin cancer in solid organ transplant recipients: Advances in therapy and management: Part I. Epidemiology of skin cancer in solid organ transplant recipients. J. Am. Acad. Dermatol. 2011, 65, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Euvrard, S.; Kanitakis, J.; Claudy, A. Skin cancers after organ transplantation. N. Engl. J. Med. 2003, 348, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Genders, R.E.; Weijns, M.E.; Dekkers, O.M.; Plasmeijer, E.I. Metastasis of cutaneous squamous cell carcinoma in organ transplant recipients and the immunocompetent population: Is there a difference? a systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 828–841. [Google Scholar] [CrossRef]
- Lanz, J.; Bouwes Bavinck, J.N.; Westhuis, M.; Quint, K.D.; Harwood, C.A.; Nasir, S.; Van-de-Velde, V.; Proby, C.M.; Ferrándiz, C.; Genders, R.E.; et al. Aggressive Squamous Cell Carcinoma in Organ Transplant Recipients. JAMA Dermatol. 2019, 155, 66–71. [Google Scholar] [CrossRef]
- Martinez, J.C.; Otley, C.C.; Stasko, T.; Euvrard, S.; Brown, C.; Schanbacher, C.F.; Weaver, A.L.; Transplant-Skin Cancer Collaborative. Defining the clinical course of metastatic skin cancer in organ transplant recipients: A multicenter collaborative study. Arch. Dermatol. 2003, 139, 301–306. [Google Scholar] [CrossRef]
- Fania, L.; Didona, D.; Di Pietro, F.R.; Verkhovskaia, S.; Morese, R.; Paolino, G.; Donati, M.; Ricci, F.; Coco, V.; Ricci, F.; et al. Cutaneous Squamous Cell Carcinoma: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2021, 9, 171. [Google Scholar] [CrossRef]
- Paulo, M.S.; Symanzik, C.; Ádam, B.; Gobba, F.; Kezic, S.; van der Molen, H.F.; Peters, C.E.; Rocholl, M.; Tenkate, T.; John, S.M.; et al. Risk of cutaneous squamous cell carcinoma due to occupational exposure to solar ultraviolet radiation: Protocol for a systematic review and meta-analysis. PLoS ONE 2023, 18, e0282664. [Google Scholar] [CrossRef]
- Fernandez Figueras, M.T. From actinic keratosis to squamous cell carcinoma: Pathophysiology revisited. J. Eur. Acad. Dermatol. Venereol. 2017, 31 (Suppl. 2), 5–7. [Google Scholar] [CrossRef] [PubMed]
- Willenbrink, T.J.; Ruiz, E.S.; Cornejo, C.M.; Schmults, C.D.; Arron, S.T.; Jambusaria-Pahlajani, A. Field cancerization: Definition, epidemiology, risk factors, and outcomes. J. Am. Acad. Dermatol. 2020, 83, 709–717. [Google Scholar] [CrossRef]
- Al-Sadek, T.; Yusuf, N. Ultraviolet Radiation Biological and Medical Implications. Curr. Issues Mol. Biol. 2024, 46, 1924–1942. [Google Scholar] [CrossRef]
- Liu, C.; Liu, X.; Cao, P.; Li, X.; Xin, H.; Zhu, S. Global, regional, national prevalence, mortality, and disability-adjusted life-years of cutaneous squamous cell carcinoma and trend analysis from 1990 to 2021 and prediction to 2045. Front. Oncol. 2025, 15, 1523169. [Google Scholar] [CrossRef] [PubMed]
- Lergenmuller, S.; Rueegg, C.S.; Perrier, F.; Robsahm, T.E.; Green, A.C.; Lund, E.; Ghiasvand, R.; Veierød, M.B. Lifetime Sunburn Trajectories and Associated Risks of Cutaneous Melanoma and Squamous Cell Carcinoma Among a Cohort of Norwegian Women. JAMA Dermatol. 2022, 158, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Dessinioti, C.; Stratigos, A.J. An Epidemiological Update on Indoor Tanning and the Risk of Skin Cancers. Curr. Oncol. 2022, 29, 8886–8903. [Google Scholar] [CrossRef] [PubMed]
- Yuspa, S.H. Cutaneous chemical carcinogenesis. J. Am. Acad. Dermatol. 1986, 15 Pt 1, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, A.; Chakraborty, S.; Sil, A. Arsenical Squamous Cell Carcinoma. Am. J. Med. Sci. 2021, 361, e45. [Google Scholar] [CrossRef]
- Plasmeijer, E.I.; Sachse, M.M.; Gebhardt, C.; Geusau, A.; Bouwes Bavinck, J.N. Cutaneous squamous cell carcinoma (cSCC) and immunosurveillance—The impact of immunosuppression on frequency of cSCC. J. Eur. Acad. Dermatol. Venereol. 2019, 33 (Suppl. 8), 33–37. [Google Scholar] [CrossRef]
- Khaddour, K.; Murakami, N.; Ruiz, E.S.; Silk, A.W. Cutaneous Squamous Cell Carcinoma in Patients with Solid-Organ-Transplant-Associated Immunosuppression. Cancers 2024, 16, 3083. [Google Scholar] [CrossRef]
- Eggermont, C.J.; Hollatz, A.; Wakkee, M.; Louwman, M.; Dinmohamed, A.G.; Posthuma, E.F.M.; Nijsten, T.; Hollestein, L. Skin cancer risk in more than 200 000 patients with haematological malignancies over 30 years: A nationwide population-based study in the Netherlands. Br. J. Dermatol. 2025, 192, 1029–1037. [Google Scholar] [CrossRef]
- Zhang, E.R.; Pfeiffer, R.M.; Austin, A.; Clarke, M.A.; Hayes, J.; Horner, M.J.; Monterosso, A.; Pawlish, K.S.; Engels, E.A.; Shiels, M.S. Impact of HIV on Anal Squamous Cell Carcinoma Rates in the United States, 2001–2015. J. Natl. Cancer Inst. 2022, 114, 1246–1252. [Google Scholar] [CrossRef]
- Sandoval-Clavijo, A.; Martí-Martí, I.; Ferrándiz-Pulido, C.; Verdaguer-Faja, J.; Jaka, A.; Toll, A. Human Papillomavirus-Related Cutaneous Squamous Cell Carcinoma. Cancers 2025, 17, 897. [Google Scholar] [CrossRef]
- Qian, G.; Wang, D.; Magliocca, K.R.; Hu, Z.; Nannapaneni, S.; Kim, S.; Chen, Z.; Sun, S.Y.; Shin, D.M.; Saba, N.F.; et al. Human papillomavirus oncoprotein E6 upregulates c-Met through p53 downregulation. Eur. J. Cancer 2016, 65, 21–32. [Google Scholar] [CrossRef]
- Kuraitis, D.; Murina, A. Squamous Cell Carcinoma Arising in Chronic Inflammatory Dermatoses. Cutis 2024, 113, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Zhao, X.; Zhu, N.; Zhao, M.; Hu, Q.; Ni, Y. The balance of serum IL-18/IL-37 levels is disrupted during the development of oral squamous cell carcinoma. Surg. Oncol. 2020, 32, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Cohen, D.N.; Rady, P.L.; Tyring, S.K. BRAF inhibitor-associated cutaneous squamous cell carcinoma: New mechanistic insight, emerging evidence for viral involvement and perspectives on clinical management. Br. J. Dermatol. 2017, 177, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Shi, W.; Song, Y.; Han, J. Voriconazole exposure and risk of cutaneous squamous cell carcinoma among lung or hematopoietic cell transplant patients: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2019, 80, 500–507.e10. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.V.; Chang, J.; Li, S.; Henry, A.S.; Wood, D.J.; Chang, A.L. Increased Risk of Cutaneous Squamous Cell Carcinoma After Vismodegib Therapy for Basal Cell Carcinoma. JAMA Dermatol. 2016, 152, 527–532. [Google Scholar] [CrossRef]
- Sawada, Y.; Nakamura, M. Daily Lifestyle and Cutaneous Malignancies. Int. J. Mol. Sci. 2021, 22, 5227. [Google Scholar] [CrossRef]
- Lee, C.H.; Wu, D.C.; Lee, J.M.; Wu, I.C.; Goan, Y.G.; Kao, E.L.; Huang, H.L.; Chan, T.F.; Chou, S.H.; Chou, Y.P.; et al. Carcinogenetic impact of alcohol intake on squamous cell carcinoma risk of the oesophagus in relation to tobacco smoking. Eur. J. Cancer 2007, 43, 1188–1199. [Google Scholar] [CrossRef]
- Leung, A.K.; Barankin, B.; Lam, J.M.; Leong, K.F.; Hon, K.L. Xeroderma pigmentosum: An updated review. Drugs Context 2022, 11, 2022-2-5. [Google Scholar] [CrossRef]
- Kassang, P.; Akakpo, S.A.; Teclessou, J.N.; Gnosike, P.; Lauressergues, E.; Przybylski, C.; Matel, L.; Mahamadou, G.; Mouhari-Toure, A.; Kombate, K.; et al. Skin Cancers in People with Albinism: An Overview and Review of Literature. Int. J. Dermatol. 2025, 64, 1986–1994. [Google Scholar] [CrossRef]
- Chahal, H.S.; Lin, Y.; Ransohoff, K.J.; Hinds, D.A.; Wu, W.; Dai, H.J.; Qureshi, A.A.; Li, W.Q.; Kraft, P.; Tang, J.Y.; et al. Genome-wide association study identifies novel susceptibility loci for cutaneous squamous cell carcinoma. Nat. Commun. 2016, 7, 12048. [Google Scholar] [CrossRef]
- Shete, S.; Liu, H.; Wang, J.; Yu, R.; Sturgis, E.M.; Li, G.; Dahlstrom, K.R.; Liu, Z.; Amos, C.I.; Wei, Q. A Genome-Wide Association Study Identifies Two Novel Susceptible Regions for Squamous Cell Carcinoma of the Head and Neck. Cancer Res. 2020, 80, 2451–2460. [Google Scholar] [CrossRef]
- Huang, A.; Nguyen, J.K.; Austin, E.; Mamalis, A.; Jagdeo, J. Updates on Treatment Approaches for Cutaneous Field Cancerization. Curr. Dermatol. Rep. 2019, 8, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Viros, A.; Milagre, C.; Trunzer, K.; Bollag, G.; Spleiss, O.; Reis-Filho, J.S.; Kong, X.; Koya, R.C.; Flaherty, K.T.; et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N. Engl. J. Med. 2012, 366, 207–215. [Google Scholar] [CrossRef]
- Kitrell, B.; Crew, A.; Wysong, A.; Sutton, A. Refining the Classification of Field Cancerization. Dermatol. Surg. 2023, 49, 228–230. [Google Scholar] [CrossRef]
- Hosseini, T.M.; Park, S.J.; Guo, T. The Mutational and Microenvironmental Landscape of Cutaneous Squamous Cell Carcinoma: A Review. Cancers 2024, 16, 2904. [Google Scholar] [CrossRef]
- Moy, J.D.; Moskovitz, J.M.; Ferris, R.L. Biological mechanisms of immune escape and implications for immunotherapy in head and neck squamous cell carcinoma. Eur. J. Cancer 2017, 76, 152–166. [Google Scholar] [CrossRef]
- Hwang, H.W.; Shin, H.T.; An, H.Y.; Byun, J.W. Genomic progression for local invasion of cutaneous squamous cell carcinoma from the superficial to the deep portion. Int. J. Cancer 2024, 155, 532–544. [Google Scholar] [CrossRef]
- Chang, D.; Shain, A.H. The landscape of driver mutations in cutaneous squamous cell carcinoma. NPJ Genom. Med. 2021, 6, 61. [Google Scholar] [CrossRef]
- Li, H.D.; Cuevas, I.; Zhang, M.; Lu, C.; Alam, M.M.; Fu, Y.X.; You, M.J.; Akbay, E.A.; Zhang, H.; Castrillon, D.H. Polymerase-mediated ultramutagenesis in mice produces diverse cancers with high mutational load. J. Clin. Investig. 2018, 128, 4179–4191. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Yu, M.; Lin, J.; Liu, B.; Xing, H.; Yang, J.; Sun, D.; Chen, F.; Jiang, M.; Tang, C.; et al. The pan-cancer landscape of netrin family reveals potential oncogenic biomarkers. Sci. Rep. 2020, 10, 5224. [Google Scholar] [CrossRef]
- García-Pérez, O.; Melgar-Vilaplana, L.; Sifaoui, I.; Śmietańska, A.; Córdoba-Lanús, E.; Fernández-de-Misa, R. VEGFC Gene Expression Is Associated with Tumor Progression and Disease-Free Survival in Cutaneous Squamous Cell Carcinoma. Int. J. Mol. Sci. 2023, 25, 379. [Google Scholar] [CrossRef] [PubMed]
- Cañueto, J.; Cardeñoso, E.; García, J.L.; Santos-Briz, Á.; Castellanos-Martín, A.; Fernández-López, E.; Blanco Gómez, A.; Pérez-Losada, J.; Román-Curto, C. Epidermal growth factor receptor expression is associated with poor outcome in cutaneous squamous cell carcinoma. Br. J. Dermatol. 2017, 176, 1279–1287. [Google Scholar] [CrossRef]
- Mishra, A.K.; Kadoishi, T.; Wang, X.; Driver, E.; Chen, Z.; Wang, X.J.; Wang, J.H. Squamous cell carcinomas escape immune surveillance via inducing chronic activation and exhaustion of CD8+ T Cells co-expressing PD-1 and LAG-3 inhibitory receptors. Oncotarget 2016, 7, 81341–81356. [Google Scholar] [CrossRef]
- Kim, S.I.; Woo, S.R.; Noh, J.K.; Lee, M.K.; Lee, Y.C.; Lee, J.W.; Ko, S.G.; Eun, Y.G. Clinical significance of FAT1 gene mutation and mRNA expression in patients with head and neck squamous cell carcinoma. Mol. Oncol. 2022, 16, 1661–1679. [Google Scholar] [CrossRef]
- Yan, W.; Wistuba, I.I.; Emmert-Buck, M.R.; Erickson, H.S. Squamous Cell Carcinoma—Similarities and Differences among Anatomical Sites. Am. J. Cancer Res. 2011, 1, 275–300. [Google Scholar]
- Enoch, S.; Miller, D.R.; Price, P.E.; Harding, K.G. Early diagnosis is vital in the management of squamous cell carcinomas associated with chronic non healing ulcers: A case series and review of the literature. Int. Wound J. 2004, 1, 165–175. [Google Scholar] [CrossRef]
- Bota, J.P.; Lyons, A.B.; Carroll, B.T. Squamous Cell Carcinoma of the Lip-A Review of Squamous Cell Carcinogenesis of the Mucosal and Cutaneous Junction. Dermatol. Surg. 2017, 43, 494–506. [Google Scholar] [CrossRef]
- Ronconi, G.; Piccinni, C.; Dondi, L.; Calabria, S.; Pedrini, A.; Esposito, I.; Ascierto, P.A.; Naldi, L.; Martini, N. Identification of cases and estimate of direct costs of unresectable and advanced cutaneous squamous cell carcinoma: Real-world data from a large Italian database. Br. J. Dermatol. 2020, 183, 172–174. [Google Scholar] [CrossRef]
- Hillen, U.; Leiter, U.; Haase, S.; Kaufmann, R.; Becker, J.; Gutzmer, R.; Terheyden, P.; Krause-Bergmann, A.; Schulze, H.J.; Hassel, J.; et al. Advanced cutaneous squamous cell carcinoma: Aretrospective analysis of patient profiles treatment patterns-Results of a non-interventional study of the DeCOG. Eur. J. Cancer 2018, 96, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, L.; Kanitakis, J. Histological classification of cutaneous squamous cell carcinomas with different severity. J. Eur. Acad. Dermatol. Venereol. 2019, 33 (Suppl. 8), 11–15. [Google Scholar] [CrossRef] [PubMed]
- Cocuz, I.G.; Popelea, M.C.; Niculescu, R.; Manea, A.; Sabău, A.H.; Tinca, A.C.; Szoke, A.R.; Budin, C.E.; Stoian, A.; Morariu, S.H.; et al. Pathophysiology, Histopathology, and Differential Diagnostics of Basal Cell Carcinoma and Cutaneous Squamous Cell Carcinoma-An Update from the Pathologist’s Point of View. Int. J. Mol. Sci. 2024, 25, 2220. [Google Scholar] [CrossRef] [PubMed]
- Yanofsky, V.R.; Mercer, S.E.; Phelps, R.G. Histopathological variants of cutaneous squamous cell carcinoma: A review. J. Skin. Cancer 2011, 2011, 210813. [Google Scholar] [CrossRef]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Lydiatt, W.M.; Patel, S.G.; O’Sullivan, B.; Brandwein, M.S.; Ridge, J.A.; Migliacci, J.C.; Loomis, A.M.; Shah, J.P. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 122–137. [Google Scholar] [CrossRef]
- Farasat, S.; Yu, S.S.; Neel, V.A.; Nehal, K.S.; Lardaro, T.; Mihm, M.C.; Byrd, D.R.; Balch, C.M.; Califano, J.A.; Chuang, A.Y.; et al. A new American Joint Committee on Cancer staging system for cutaneous squamous cell carcinoma: Creation and rationale for inclusion of tumor (T) characteristics. J. Am. Acad. Dermatol. 2011, 64, 1051–1059. [Google Scholar] [CrossRef]
- Bander, T.S.; Nehal, K.S.; Lee, E.H. Cutaneous Squamous Cell Carcinoma: Updates in Staging and Management. Dermatol. Clin. 2019, 37, 241–251. [Google Scholar] [CrossRef]
- Costantino, A.; Canali, L.; Festa, B.M.; Spriano, G.; Mercante, G.; De Virgilio, A. Sentinel lymph node biopsy in high-risk cutaneous squamous cell carcinoma of the head and neck: Systematic review and meta-analysis. Head. Neck 2022, 44, 2288–2300. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Couselo, E.; Cañueto, J.; Jerviz Guía, V.; López López, A.M.; Bermejo Segú, J.O.; García Castaño, A.; Puig Sardá, S.; Sanmartín Jiménez, O.; Soria Rivas, A.; Gratal, P.; et al. Recommendations for the management of cutaneous squamous cell carcinoma: A systematic multidisciplinary Delphi consensus approach. Clin. Transl. Oncol. 2025, 27, 3058–3072. [Google Scholar] [CrossRef] [PubMed]
- Stratigos, A.J.; Garbe, C.; Dessinioti, C.; Lebbe, C.; van Akkooi, A.; Bataille, V.; Bastholt, L.; Dreno, B.; Dummer, R.; Fargnoli, M.C.; et al. European consensus-based interdisciplinary guideline for invasive cutaneous squamous cell carcinoma: Part 2. Treatment-Update 2023. Eur. J. Cancer 2023, 193, 113252. [Google Scholar] [CrossRef]
- Moreno-Ramírez, D.; Silva-Clavería, F.; Fernández-Orland, A.; Eiris, N.; Ruiz de Casas, A.; Férrandiz, L. Surgery for Cutaneous Squamous Cell Carcinoma and its Limits in Advanced Disease. Dermatol. Pract. Concept. 2021, 11 (Suppl. 2), e2021167S. [Google Scholar] [CrossRef]
- Soydemir, G.P.; Kandaz, M.; Melikoğlu, M. Erratum: The results of radiotherapy for squamous cell carcinomas of the skin. Dermatol. Ther. 2023, 2023, 9836545. [Google Scholar] [CrossRef]
- Lansbury, L.; Bath-Hextall, F.; Perkins, W.; Stanton, W.; Leonardi-Bee, J. Interventions for non-metastatic squamous cell carcinoma of the skin: Systematic review and pooled analysis of observational studies. BMJ 2013, 347, f6153. [Google Scholar] [CrossRef] [PubMed]
- Muto, P.; Pastore, F. Radiotherapy in the Adjuvant and Advanced Setting of CSCC. Dermatol. Pract. Concept. 2021, 11 (Suppl. 2), e2021168S. [Google Scholar] [CrossRef]
- Meyer, M.F.; Wolber, P.; Arolt, C.; Wessel, M.; Quaas, A.; Lang, S.; Klussmann, J.P.; Semrau, R.; Beutner, D. Survival after parotid gland metastases of cutaneous squamous cell carcinoma of the head and neck. Oral. Maxillofac. Surg. 2021, 25, 383–388. [Google Scholar] [CrossRef]
- Gehl, J.; Sersa, G.; Matthiessen, L.W.; Muir, T.; Soden, D.; Occhini, A.; Quaglino, P.; Curatolo, P.; Campana, L.G.; Kunte, C.; et al. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol. 2018, 57, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.H.; Lei, Z.; Yang, H.N.; Tang, Z.; Yang, M.Q.; Wang, Y.; Sui, J.D.; Wu, Y.Z. Radiation-induced PD-L1 expression in tumor and its microenvironment facilitates cancer-immune escape: A narrative review. Ann. Transl. Med. 2022, 10, 1406. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, H.; Zhang, X.; Zhang, Z.; Liu, Z.; Mao, R.; Fan, Y.; Sun, R.; Chen, M. Chemotherapy modulates PD-L1 expression and its combination with immune checkpoint blockade requires precision. Crit. Rev. Oncol. Hematol. 2025, 214, 104908. [Google Scholar] [CrossRef]
- Trodello, C.; Pepper, J.P.; Wong, M.; Wysong, A. Cisplatin and Cetuximab Treatment for Metastatic Cutaneous Squamous Cell Carcinoma: A Systematic Review. Dermatol. Surg. 2017, 43, 40–49. [Google Scholar] [CrossRef]
- Hourbeigt, K.; Ehret, M.; Visseaux, L.; Durlach, A.; Petit, A.; Sanchez, J.; Grange-Prunier, A.; Barbe, C.; Servagi-Vernat, S.; Grange, F. Efficacy and safety of panitumumab alone or in association with radiotherapy in unresectable cutaneous squamous cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2789–2794. [Google Scholar] [CrossRef]
- Samstein, R.M.; Ho, A.L.; Lee, N.Y.; Barker, C.A. Locally advanced and unresectable cutaneous squamous cell carcinoma: Outcomes of concurrent cetuximab and radiotherapy. J. Skin. Cancer 2014, 2014, 284582. [Google Scholar] [CrossRef]
- Wright, Q.; Gonzalez Cruz, J.L.; Wells, J.W.; Leggatt, G.R. PD-1 and beyond to Activate T Cells in Cutaneous Squamous Cell Cancers: The Case for 4-1BB and VISTA Antibodies in Combination Therapy. Cancers 2021, 13, 3310. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lee, L.F.; Fisher, T.S.; Jessen, B.; Elliott, M.; Evering, W.; Logronio, K.; Tu, G.H.; Tsaparikos, K.; Li, X.; et al. Combination of 4-1BB agonist and PD-1 antagonist promotes antitumor effector/memory CD8 T cells in a poorly immunogenic tumor model. Cancer Immunol. Res. 2015, 3, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Bartkowiak, T.; Curran, M.A. 4-1BB Agonists: Multi-Potent Potentiators of Tumor Immunity. Front. Oncol. 2015, 5, 117. [Google Scholar] [CrossRef]
- Chester, C.; Ambulkar, S.; Kohrt, H.E. 4-1BB agonism: Adding the accelerator to cancer immunotherapy. Cancer Immunol. Immunother. 2016, 65, 1243–1248. [Google Scholar] [CrossRef]
- Azzimonti, B.; Zavattaro, E.; Provasi, M.; Vidali, M.; Conca, A.; Catalano, E.; Rimondini, L.; Colombo, E.; Valente, G. Intense Foxp3+ CD25+ regulatory T-cell infiltration is associated with high-grade cutaneous squamous cell carcinoma and counterbalanced by CD8+/Foxp3+ CD25+ ratio. Br. J. Dermatol. 2015, 172, 64–73. [Google Scholar] [CrossRef]
- Dougan, M.; Luoma, A.M.; Dougan, S.K.; Wucherpfennig, K.W. Understanding and treating the inflammatory adverse events of cancer immunotherapy. Cell 2021, 184, 1575–1588. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.; Qahwaji, R.M.; Alfaifi, M.S.; Mobashir, M. Nivolumab and Ipilimumab Acting as Tormentors of Advanced Tumors by Unleashing Immune Cells and Associated Collateral Damage. Pharmaceutics 2024, 16, 732. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef]
- Stevenson, M.L.; Wang, C.Q.; Abikhair, M.; Roudiani, N.; Felsen, D.; Krueger, J.G.; Pavlick, A.C.; Carucci, J.A. Expression of Programmed Cell Death Ligand in Cutaneous Squamous Cell Carcinoma and Treatment of Locally Advanced Disease with Pembrolizumab. JAMA Dermatol. 2017, 153, 299–303. [Google Scholar] [CrossRef]
- Migden, M.R.; Rischin, D.; Schmults, C.D.; Guminski, A.; Hauschild, A.; Lewis, K.D.; Chung, C.H.; Hernandez-Aya, L.; Lim, A.M.; Chang, A.L.S.; et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 341–351. [Google Scholar] [CrossRef]
- Rischin, D.; Migden, M.R.; Lim, A.M.; Schmults, C.D.; Khushalani, N.I.; Hughes, B.G.M.; Schadendorf, D.; Dunn, L.A.; Hernandez-Aya, L.; Chang, A.L.S.; et al. Phase 2 study of cemiplimab in patients with metastatic cutaneous squamous cell carcinoma: Primary analysis of fixed-dosing, long-term outcome of weight-based dosing. J. Immunother. Cancer 2020, 8, e000775. [Google Scholar] [CrossRef]
- Hughes, B.G.M.; Grob, J.J.; Bowyer, S.E.; Day, F.L.; Ladwa, R.; Stein, B.; Muñoz Couselo, E.; Basset-Seguin, N.; Guminski, A.; Mortier, L.; et al. Phase II confirmatory study of cemiplimab (350mg IVQ3W) in patients with locally advanced or metastatic cutaneous squamous cell carcinoma (CSCC): Study 1540 Group. Annals Oncol. 2022, 33, S921. [Google Scholar] [CrossRef]
- Grob, J.J.; Gonzalez, R.; Basset-Seguin, N.; Vornicova, O.; Schachter, J.; Joshi, A.; Meyer, N.; Grange, F.; Piulats, J.M.; Bauman, J.R.; et al. Pembrolizumab Monotherapy for Recurrent or Metastatic Cutaneous Squamous Cell Carcinoma: A Single-Arm Phase II Trial (KEYNOTE-629). J. Clin. Oncol. 2020, 38, 2916–2925. [Google Scholar] [CrossRef]
- Hughes, B.G.M.; Munoz-Couselo, E.; Mortier, L.; Bratland, Å.; Gutzmer, R.; Roshdy, O.; González Mendoza, R.; Schachter, J.; Arance, A.; Grange, F.; et al. Corrigendum to ‘Pembrolizumab for locally advanced and recurrent/metastatic cutaneous squamous cell carcinoma (KEYNOTE-629 study): An open-label, nonrandomized, multicenter, phase II trial: [Annals of Oncology Volume 32, Issue 10, October 2021, Pages 1276-1285]. Ann Oncol. 2022, 33, 853. [Google Scholar] [PubMed]
- Jarkowski A3rd Hare, R.; Loud, P.; Skitzki, J.J.; Kane JM3rd May, K.S.; Zeitouni, N.C.; Nestico, J.; Vona, K.L.; Groman, A.; Khushalani, N.I. Systemic Therapy in Advanced Cutaneous Squamous Cell Carcinoma (CSCC): The Roswell Park Experience and a Review of the Literature. Am. J. Clin. Oncol. 2016, 39, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Maubec, E.; Boubaya, M.; Petrow, P.; Beylot-Barry, M.; Basset-Seguin, N.; Deschamps, L.; Grob, J.J.; Dréno, B.; Scheer-Senyarich, I.; Bloch-Queyrat, C.; et al. Phase II Study of Pembrolizumab As First-Line, Single-Drug Therapy for Patients with Unresectable Cutaneous Squamous Cell Carcinomas. J. Clin. Oncol. 2020, 38, 3051–3061. [Google Scholar] [CrossRef] [PubMed]
- Petzold, A.; Steeb, T.; Wessely, A.; Schatton, T.; Berking, C.; Heppt, M.V. Comparative efficacy analysis identifies immune checkpoint blockade as a new survival benchmark in advanced cutaneous squamous cell carcinoma. Eur. J. Cancer 2022, 170, 42–53. [Google Scholar] [CrossRef]
- Gross, N.D.; Miller, D.M.; Khushalani, N.I.; Divi, V.; Ruiz, E.S.; Lipson, E.J.; Meier, F.; Su, Y.B.; Swiecicki, P.L.; Atlas, J.; et al. Neoadjuvant Cemiplimab for Stage II to IV Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2022, 387, 1557–1568. [Google Scholar] [CrossRef]
- Intralesional Cemiplimab for Adult Patients with Cutaneous Squamous Cell Carcinoma or Basal Cell Carcinoma. ClinicalTrials.gov Identifier: NCT03889912. Regeneron Pharmaceuticals. Available online: https://clinicaltrials.gov/study/NCT03889912 (accessed on 17 November 2025).
- Alberti, A.; Bergamini, C.; Resteghini, C.; Locati, L.D.; Alfieri, S.; Cavalieri, S.; Colombo, E.; Gurizzan, C.; Lorini, L.; Tovazzi, V.; et al. Immunotherapy followed by cetuximab in locally advanced/metastatic (LA/M) cutaneous squamous cell carcinomas (cSCC): The I-TACKLE trial. J. Clin. Oncol. 2022, 40, 9820. [Google Scholar]
- Migden, M.R.; Chandra, S.; Rabinowits, G.; Chen, C.I.; Desai, J.; Seluzhytsky, A.; Sasane, M.; Campanelli, B.; Chen, Z.; Freeman, M.L.; et al. CASE (CemiplimAb-rwlc Survivorship and Epidemiology) study in advanced cutaneous squamous cell carcinoma. Future Oncol. 2020, 16, 11–19. [Google Scholar] [CrossRef]
- Hober, C.; Fredeau, L.; Pham-Ledard, A.; Boubaya, M.; Herms, F.; Celerier, P.; Aubin, F.; Beneton, N.; Dinulescu, M.; Jannic, A.; et al. Cemiplimab for Locally Advanced and Metastatic Cutaneous Squamous-Cell Carcinomas: Real-Life Experience from the French CAREPI Study Group. Cancers 2021, 13, 3547. [Google Scholar] [CrossRef]
- Rohaana, M.W.; Duizerb, M.L.; Devriesec, L.A.; Meerveld-Egginka, A.; Brandtsa, W.F.; van Thienena, J.V.; Wilgenhofa, S.; van Herpenb, C.M.L.; Haanena, J.B.A.G. Real-world data on tolerability and clinical response of cemiplimab in patients with advanced cutaneous squamous cell carcinoma: A retrospective, multicentre cohort study from the Netherlands. EJC Skin. Cancer 2023, 1, 100007. [Google Scholar] [CrossRef]
- Verkerk, K.; Geurts, B.S.; Zeverijn, L.J.; van der Noort, V.; Verheul, H.M.W.; Haanen, J.B.A.G.; van der Veldt, A.A.M.; Eskens, F.A.L.M.; Aarts, M.J.B.; van Herpen, C.M.L.; et al. Cemiplimab in locally advanced or metastatic cutaneous squamous cell carcinoma: Prospective real-world data from the DRUG Access Protocol. Lancet Reg. Health Eur. 2024, 39, 100875. [Google Scholar] [CrossRef] [PubMed]
- Kuzmanovszki, D.; Kiss, N.; Tóth, B.; Tóth, V.; Szakonyi, J.; Lőrincz, K.; Hársing, J.; Kuroli, E.; Imrédi, E.; Kerner, T.; et al. Real-World Experience with Cemiplimab Treatment for Advanced Cutaneous Squamous Cell Carcinoma-A Retrospective Single-Center Study. J. Clin. Med. 2023, 12, 5966. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Wu, L.; Han, L.; Zheng, X.; Tong, R.; Li, L.; Bai, L.; Bian, Y. Immune-related adverse events of immune checkpoint inhibitors: A review. Front. Immunol. 2023, 14, 1167975. [Google Scholar] [CrossRef]
- Liao, D.; Wang, M.; Liao, Y.; Li, J.; Niu, T. A Review of Efficacy and Safety of Checkpoint Inhibitor for the Treatment of Acute Myeloid Leukemia. Front. Pharmacol. 2019, 10, 609. [Google Scholar] [CrossRef]
- Migden, M.R.; Khushalani, N.I.; Chang, A.L.S.; Lewis, K.D.; Schmults, C.D.; Hernandez-Aya, L.; Meier, F.; Schadendorf, D.; Guminski, A.; Hauschild, A.; et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: Results from an open-label, phase 2, single-arm trial. Lancet Oncol. 2020, 21, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, D.; Lauck, K.C.; Tolkachjov, S.N. Adverse events in cemiplimab therapy for locally advanced or metastatic cutaneous squamous cell carcinoma: A global propensity-matched retrospective cohort study. J. Am. Acad. Dermatol. 2024, 91, 990–993. [Google Scholar] [CrossRef]
- Haigh, J.E.; Rack, S.; Yan, R.; Babu, S.; Donnelly, O.; Walter, H.; Faust, G.; Bhagani, S.; Isola, P.; Metcalf, R. Evaluation of Clinical Parameters Associated with Response and Resistance to Cemiplimab in Locally Advanced and Metastatic Cutaneous Squamous Cell Carcinoma: A Multi-Institutional Retrospective Cohort Study. Curr. Oncol. 2025, 32, 168. [Google Scholar] [CrossRef]
- Brancaccio, G.; Briatico, G.; Pellegrini, C.; Rocco, T.; Moscarella, E.; Fargnoli, M.C. Risk Factors and Diagnosis of Advanced Cutaneous Squamous Cell Carcinoma. Dermatol. Pract. Concept. 2021, 11 (Suppl. 2), e2021166S. [Google Scholar] [CrossRef]
- Hyeraci, M.; Didona, D.; Abeni, D.; Magri, F.; Ricci, F.; Bertagnin, C.; Loregian, A.; Di Lella, G.; Di Guardo, A.; Panebianco, A.; et al. New Insights into Pathogenesis and Management of Keratoacanthoma: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 10040. [Google Scholar] [CrossRef]
- Cortellini, A.; Bersanelli, M.; Buti, S.; Cannita, K.; Santini, D.; Perrone, F.; Giusti, R.; Tiseo, M.; Michiara, M.; Di Marino, P.; et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: When overweight becomes favorable. J. Immunother. Cancer 2019, 7, 57. [Google Scholar] [CrossRef]
- McQuade, J.L.; Daniel, C.R.; Hess, K.R.; Mak, C.; Wang, D.Y.; Rai, R.R.; Park, J.J.; Haydu, L.E.; Spencer, C.; Wongchenko, M.; et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: A retrospective, multicohort analysis. Lancet Oncol. 2018, 19, 310–322. [Google Scholar] [CrossRef]
- Hahn, A.W.; Venkatesh, N.; Msaouel, P.; McQuade, J.L. The Influence of Obesity on Outcomes with Immune Checkpoint Blockade: Clinical Evidence and Potential Biological Mechanisms. Cells 2023, 12, 2551. [Google Scholar] [CrossRef]
- Guven, D.C.; Sahin, T.K.; Erul, E.; Rizzo, A.; Ricci, A.D.; Aksoy, S.; Yalcin, S. The association between albumin levels and survival in patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Front. Mol. Biosci. 2022, 9, 1039121. [Google Scholar] [CrossRef]
- Zheng, M. Serum albumin: A pharmacokinetic marker for optimizing treatment outcome of immune checkpoint blockade. J. Immunother. Cancer 2022, 10, e005670. [Google Scholar] [CrossRef]
- Stares, M.; Ding, T.E.; Stratton, C.; Thomson, F.; Baxter, M.; Cagney, H.; Cumming, K.; Swan, A.; Ross, F.; Barrie, C.; et al. Biomarkers of systemic inflammation predict survival with first-line immune checkpoint inhibitors in non-small-cell lung cancer. ESMO Open. 2022, 7, 100445. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, Y.; Oya, R.; Takemoto, N.; Inohara, H. Neutrophil-to-lymphocyte ratio as a prognostic marker for head and neck squamous cell carcinoma treated with immune checkpoint inhibitors: Meta-analysis. Head. Neck 2022, 44, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Buder-Bakhaya, K.; Hassel, J.C. Biomarkers for Clinical Benefit of Immune Checkpoint Inhibitor Treatment-A Review From the Melanoma Perspective and Beyond. Front. Immunol. 2018, 9, 1474. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, V.; Gallo, F.; Alberti, A.; Paloschi, D.; Ferrari Bravo, W.; Esposito, A.; Cosentini, D.; Grisanti, S.; Pedersini, R.; Petrelli, F.; et al. Immune-related adverse events as potential surrogates of immune checkpoint inhibitors’ efficacy: A systematic review and meta-analysis of randomized studies. ESMO Open. 2023, 8, 100787. [Google Scholar] [CrossRef]
- Bastacky, M.L.; Wang, H.; Fortman, D.; Rahman, Z.; Mascara, G.P.; Brenner, T.; Najjar, Y.G.; Luke, J.J.; Kirkwood, J.M.; Zarour, H.M.; et al. Immune-Related Adverse Events in PD-1 Treated Melanoma and Impact Upon Anti-Tumor Efficacy: A Real World Analysis. Front. Oncol. 2021, 11, 749064. [Google Scholar] [CrossRef]
- Pan, C.; Schoppe, O.; Parra-Damas, A.; Cai, R.; Todorov, M.I.; Gondi, G.; von Neubeck, B.; Böğürcü-Seidel, N.; Seidel, S.; Sleiman, K.; et al. Deep Learning Reveals Cancer Metastasis and Therapeutic Antibody Targeting in the Entire Body. Cell 2019, 179, 1661–1676.e19. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.L.; Mukundan, A.; Karmakar, R.; Avala, P.; Chang, W.Y.; Wang, H.C. Hyperspectral Imaging for Enhanced Skin Cancer Classification Using Machine Learning. Bioengineering 2025, 12, 755. [Google Scholar] [CrossRef] [PubMed]
- Tolino, E.; Skroza, N.; Di Guardo, A.; Proietti, I.; Bernardini, N.; Potenza, C. Managing Atopic Dermatitis with Dupilumab in Special Populations: A Case Series Study. Clin. Cosmet. Investig. Dermatol. 2025, 18, 2859–2868. [Google Scholar] [CrossRef]
- Shan, Q.; Lu, H. Immune Checkpoint Inhibitors in Special Populations. Technol. Cancer Res. Treat. 2021, 20, 15330338211036526. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).