Cardioprotective Signaling: Outline and Future Directions
Abstract
1. Introduction
2. Inducers of Cardioprotection
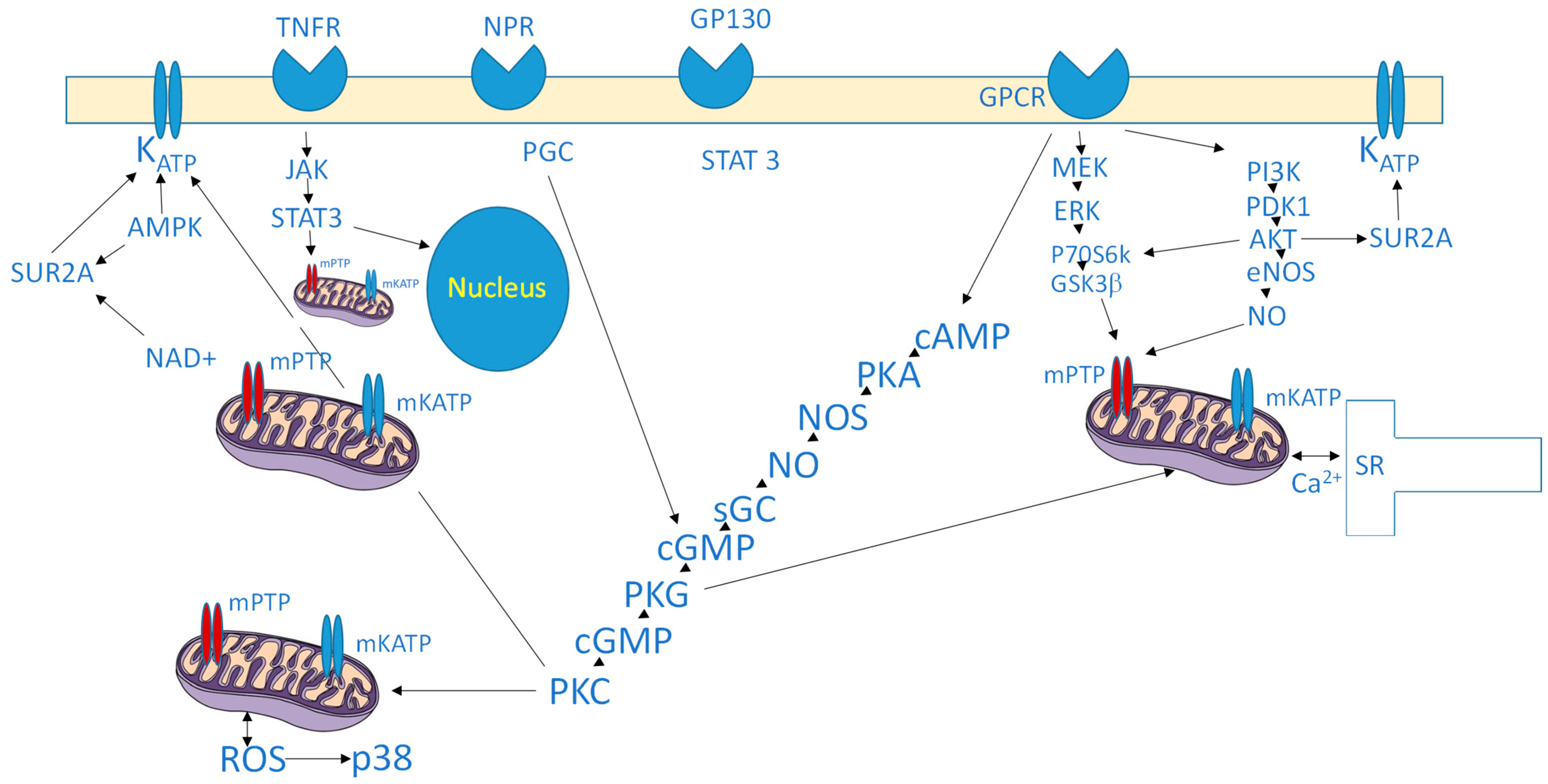
3. Intracellular Signaling of Cardioprotection
3.1. Protein Kinases
3.2. Reperfusion Injury Salvage Kinase (RISK) Pathway
3.3. Survivor Activating Factor Enhancement (SAFE) Pathway
3.4. Transcriptional Regulators and Noncoding RNAs
3.5. Mitochondrial Modulators
3.6. Stress-Responsive Proteins and Enzymes
3.7. Sirtuins and Metabolic Regulators
4. End-Effectors of Cardioprotection
4.1. Mitochondria as Central End-Effectors
4.2. Sarcolemmal KATP Channels and SUR2A
4.3. Hexokinase 2 (HK2)
4.4. Protein Nitrosation
4.5. Sarcoplasmic Reticulum and Calcium Handling
4.6. Cytoskeletal and Ionic Homeostasis
4.7. Autophagy
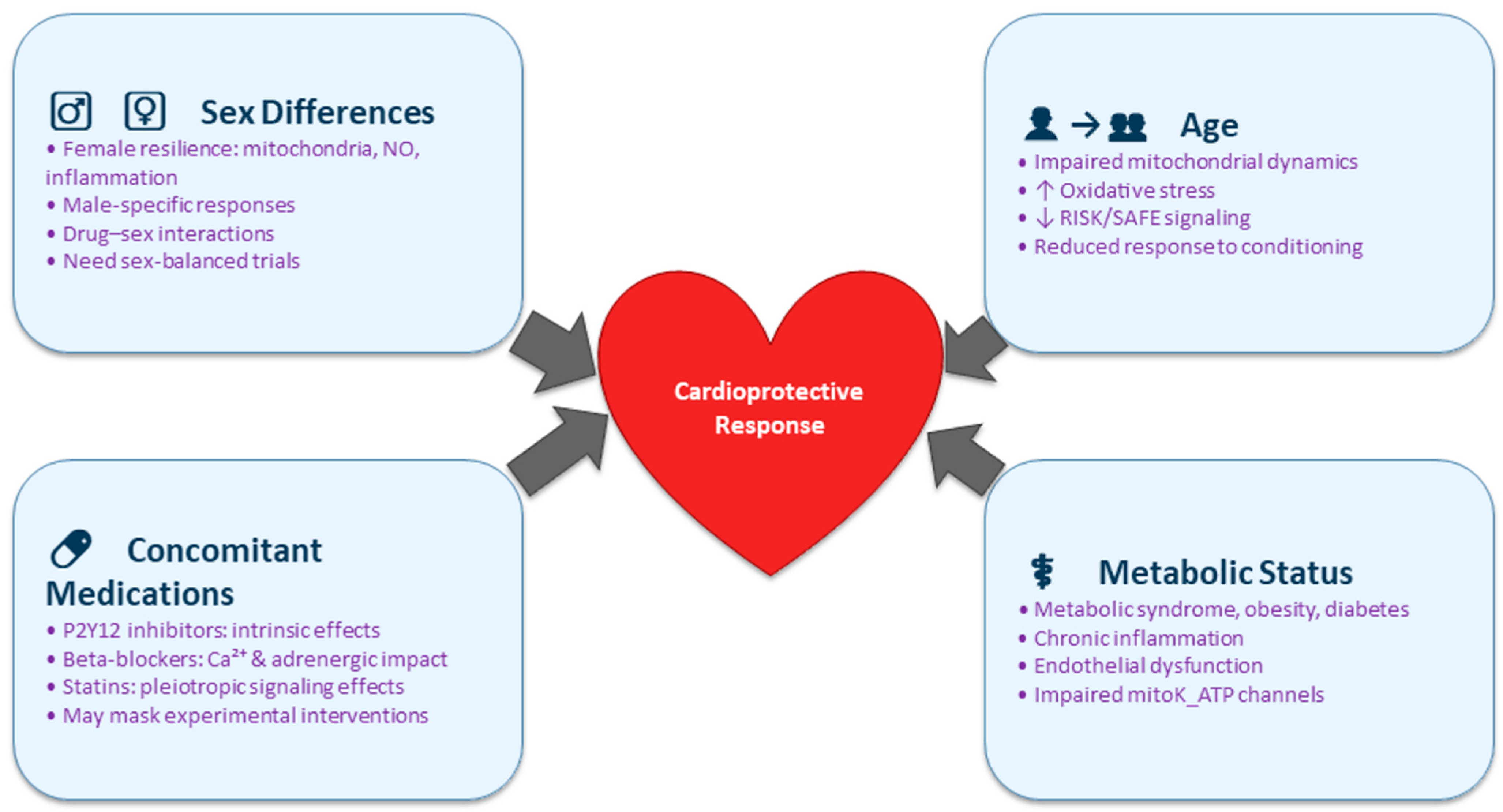
5. Clinical Implications and Patient Stratification
6. Future Directions
- Mechanism-aligned personalized cardioprotection: Instead of broad personalization, future work should define which mechanistic defects dominate in specific patient groups. For example, diabetic patients may require AMPK-sensitizing or mKATP-targeting strategies; elderly patients may benefit from interventions that restore autophagy or augment SAFE signaling; patients on beta-blockers may need conditioning strategies independent of adrenergic pathways.
- Comorbidity-informed combination therapies: Combining pharmacological agents with ischemic conditioning or hypothermia should be tailored to patient-specific defects—e.g., pairing NO-donors with endothelial-dysfunction phenotypes; combining antioxidants with high-ROS aging phenotypes; or integrating SGLT2 inhibitors into conditioning paradigms for diabetics.
- Novel therapeutic targets matched to patient segments: Investigation into microRNAs, exosomes, mitochondrial dynamics regulators, or epigenetic modifiers should identify which targets compensate for pathway impairments unique to metabolic, inflammatory, or aging-associated states.
- Integration with tissue repair and regeneration: Because the capacity for repair varies across phenotypes (e.g., impaired neovascularization in diabetics, reduced progenitor activity in aging), the synergy between cardioprotective strategies and regenerative therapies should be evaluated in comorbidity-specific models.
- Mechanistically stratified clinical trial design: Future trials should incorporate prespecified strata based on mechanistic vulnerabilities—such as diabetes status, age-related mitochondrial dysfunction, or background medications known to activate overlapping pathways. Enrichment strategies (e.g., enrolling patients with clear evidence of residual RISK/SAFE pathway responsiveness), mechanistic biomarkers (e.g., NO-pathway activation, AMPK phosphorylation, mitochondrial function indices), and long-term endpoints (heart failure progression, arrhythmias, mortality) will be essential. Additionally, trials should control for drug–mechanism interactions by harmonizing background therapy or performing drug-stratified analyses.
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALDH2 | Aldehyde Dehydrogenase 2 |
| AMPK | AMP-Activated Protein Kinase |
| ATP | Adenosine Triphosphate |
| A1, A2 | Adenosine Receptor Subtypes 1 and 2 |
| cGMP | Cyclic Guanosine Monophosphate |
| CO | Carbon Monoxide |
| Cx43 | Connexin 43 |
| ERK1/2 | Extracellular Signal-Regulated Kinases 1 and 2 |
| GSK3β | Glycogen Synthase Kinase-3 Beta |
| HIF1α or HIF-1α | Hypoxia-Inducible Factor-1 Alpha |
| HK2 | Hexokinase 2 |
| HO-1 | Heme Oxygenase-1 |
| HSP/HSPs | Heat Shock Protein(s) |
| IPC/IPostC | Ischemic Preconditioning/Ischemic Postconditioning |
| I/R | Ischemia–Reperfusion |
| iNOS | Inducible Nitric Oxide Synthase |
| IPC | Ischemic Preconditioning |
| KATP | ATP-Sensitive Potassium (Channel) |
| MAPK | Mitogen-Activated Protein Kinase |
| MEK1 | MAPK/ERK Kinase 1 |
| miRNA(s) | MicroRNA(s) |
| mitoKATP | Mitochondrial ATP-Sensitive Potassium Channel |
| mPTP | Mitochondrial Permeability Transition Pore |
| miRNAs | MicroRNAs (noncoding regulatory RNAs) |
| NAD+ | Nicotinamide Adenine Dinucleotide (oxidized form) |
| NO | Nitric Oxide |
| NO–cGMP | Nitric Oxide–Cyclic GMP Signaling Pathway |
| PI3K | Phosphoinositide 3-Kinase |
| PKA | Protein Kinase A |
| PKC | Protein Kinase C |
| PKCε | Protein Kinase C Epsilon Isoform |
| PKG | Protein Kinase G |
| RISK | Reperfusion Injury Salvage Kinase (pathway) |
| ROS | Reactive Oxygen Species |
| SAFE | Survivor Activating Factor Enhancement (pathway) |
| SIRT1 | Sirtuin 1 |
| SOD | Superoxide Dismutase |
| SR | Sarcoplasmic Reticulum |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| SUR2A | Sulfonylurea Receptor 2A (subunit of KATP channels) |
| TNF-α | Tumor Necrosis Factor Alpha |
| VEGF | Vascular Endothelial Growth Factor |
References
- Pagliaro, P.; Alloatti, G.; Penna, C. Cardioprotection Reloaded: Reflections on 40 Years of Research. Antioxidants 2025, 14, 889. [Google Scholar] [CrossRef]
- Jovanović, A. Cardioprotective signalling: Past, present and future. Eur. J. Pharmacol. 2018, 833, 314–319. [Google Scholar] [CrossRef]
- Murry, C.E.; Jennings, R.B.; Reimer, K.A. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 1986, 74, 1124–1136. [Google Scholar] [CrossRef]
- Paulino, E.T. Development of cardioprotective class based on pathophysiology of myocardial infarction: A comprehensive review. Curr. Probl. Cardiol. 2024, 49, 102480. [Google Scholar] [CrossRef] [PubMed]
- de Miranda, D.C.; de Oliveira Faria, G.; Hermidorff, M.M.; Dos Santos Silva, F.C.; de Assis, L.V.M.; Isoldi, M.C. Pre- and Post-Conditioning of the heart: An overview of cardioprotective signaling pathways. Curr. Vasc. Pharmacol. 2021, 19, 499–524. [Google Scholar] [CrossRef] [PubMed]
- Mohammed Abdul, K.S.; Jovanović, S.; Du, Q.; Sukhodub, A.; Jovanović, A. A link between ATP and SUR2A: A novel mechanism explaining cardioprotection at high altitude. Int. J. Cardiol. 2015, 189, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Mohammed Abdul, K.S.; Jovanović, S.; Du, Q.; Sukhodub, A.; Jovanović, A. Mild hypoxia in vivo regulates cardioprotective SUR2A: A role for Akt and LDH. Biochim. Biophys. Acta-Mol. Basis Dis. 2015, 1852, 709–719. [Google Scholar] [CrossRef]
- Mohammed Abdul, K.S.; Jovanović, S.; Du, Q.; Sukhodub, A.; Jovanović, A. Upregulation of cardioprotective SUR2A by sub-hypoxic drop in oxygen. Biochim. Biophys. Acta-Mol. Cell Res. 2014, 1843, 2424–2431. [Google Scholar] [CrossRef]
- Mohammed Abdul, K.S.; Jovanović, S.; Jovanović, A. Exposure to 15% oxygen in vivo up-regulates cardioprotective SUR2A without affecting ERK1/2 and Akt: A crucial role for AMPK. J. Cell Mol. Med. 2017, 21, 1342–1350. [Google Scholar] [CrossRef]
- Ramani, S.; Park, S. HSP27 role in cardioprotection by modulating chemotherapeutic doxorubicin-induced cell death. J. Mol. Med. 2021, 99, 771–784. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, H.; Zhai, C.; Jing, L.; Tian, H. Cardioprotective Strategies After Ischemia-Reperfusion Injury. Am. J. Cardiovasc. Drugs 2024, 24, 5–18. [Google Scholar] [CrossRef]
- Kumar, K.; Singh, N.; Yadav, H.N.; Maslov, L.; Jaggi, A.S. Endless journey of adenosine signaling in cardioprotective mechanism of conditioning techniques: Clinical evidence. Curr. Cardiol. Rev. 2023, 19, 56–71. [Google Scholar] [CrossRef]
- Gil-Cabrerizo, P.; Scacchetti, I.; Garbayo, E.; Blanco-Prieto, M.J. Cardiac tissue engineering for myocardial infarction treatment. Eur. J. Pharm Sci. 2023, 185, 106439. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. The cardioprotective role of nitrate-rich vegetables. Foods 2024, 13, 691. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Abdul, K.S.; Faiz, N.; Jovanović, A.; Tan, W. Isosteviol protects H9c2 cells against hypoxia-reoxygenation by activating ERK1/2. Cardiovasc. Hematol. Disord. Drug Targets 2021, 21, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Tappia, P.S.; Shah, A.K.; Ramjiawan, B.; Dhalla, N.S. Modification of ischemia/reperfusion-induced alterations in subcellular organelles by ischemic preconditioning. Int. J. Mol. Sci. 2022, 23, 3425. [Google Scholar] [CrossRef]
- Wojtovich, A.P.; Nadtochiy, S.M.; Brookes, P.S.; Nehrke, K. Ischemic preconditioning: The role of mitochondria and aging. Exp. Gerontol. 2012, 47, 1–7. [Google Scholar] [CrossRef]
- Pedriali, G.; Ramaccini, D.; Bouhamida, E.; Wieckowski, M.R.; Giorgi, C.; Tremoli, E.; Pinton, P. Perspectives on mitochondrial relevance in cardiac ischemia/reperfusion injury. Front. Cell. Dev. Biol. 2022, 10, 1082095. [Google Scholar] [CrossRef]
- Skyschally, A.; Kleinbongard, P.; Lieder, H.; Gedik, N.; Stoian, L.; Amanakis, G.; Elbers, E.; Heusch, G. Humoral transfer and intramyocardial signal transduction of protection by remote ischemic preconditioning in pigs, rats, and mice. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H159–H172. [Google Scholar] [CrossRef]
- Tsiafoutis, I.; Zografos, T.; Karelas, D.; Varelas, P.; Manousopoulos, K.; Nenekidis, I.; Koutouzis, M.; Lagadinos, P.; Koudounis, P.; Agelaki, M.; et al. Ticagrelor potentiates cardioprotection by remote ischemic preconditioning: The ticagrelor in remote ischemic preconditioning (TRIP) randomized clinical trial. Hellenic J. Cardiol. 2024. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Kharbanda, R.K.; Møller, U.K.; Ramlall, M.; Aarøe, J.; Butler, R.; Bulluck, H.; Clayton, T.; Dana, A.; Dodd, M.; et al. CONDI-2/ERIC-PPCI Investigators. Effect of remote ischaemic conditioning on clinical outcomes in patients with acute myocardial infarction (CONDI-2/ERIC-PPCI): A single-blind randomised controlled trial. Lancet 2019, 394, 1415–1424. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, P.; Zhong, J.; Cheng, Y.; Chen, H.; He, Y.; Chen, C. HIF-1α in myocardial ischemia-reperfusion injury (Review). Mol. Med. Rep. 2021, 23, 352. [Google Scholar] [CrossRef] [PubMed]
- Škrlec, I.; Kolomeichuk, S.N. Hypoxia-inducible factor-1α in myocardial infarction. World J. Cardiol. 2024, 16, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Giacca, M. Fulfilling the promise of RNA therapies for cardiac repair and regeneration. Stem Cells Transl. Med. 2023, 12, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Hadebe, N.; Cour, M.; Lecour, S. The SAFE pathway for cardioprotection: Is this a promising target? Basic Res. Cardiol. 2018, 113, 9. [Google Scholar] [CrossRef]
- Upadhyay, J.; Nandave, M.; Thajudeen, K.Y.; Rashid, S.; Ansari, M.N. Molecular mechanisms and therapeutic targeting of heat shock Proteins (HSPs) in cardiovascular disorders. Front. Biosci. 2025, 30, 27324. [Google Scholar] [CrossRef]
- Totzeck, M.; Hendgen-Cotta, U.B.; Rassaf, T. Nitrite-Nitric Oxide Signaling and Cardioprotection. Adv. Exp. Med. Biol. 2017, 982, 335–346. [Google Scholar] [CrossRef]
- Mao, C.; Zhao, J.; Cheng, N.; Xu, Z.; Ma, H.; Song, Y.; Sun, X. S-Nitrosylation in cardiovascular disorders: The state of the art. Biomolecules 2025, 15, 1073. [Google Scholar] [CrossRef]
- Lotz, C.; Herrmann, J.; Notz, Q.; Meybohm, P.; Kehl, F. Mitochondria and Pharmacologic Cardiac Conditioning-At the Heart of Ischemic Injury. Int. J. Mol. Sci. 2021, 22, 3224. [Google Scholar] [CrossRef]
- Singh, H. Mitochondrial ion channels in cardiac function. Am. J. Physiol. Cell. Physiol. 2021, 321, C812–C825. [Google Scholar] [CrossRef]
- Sukhodub, A.; Du, Q.; Jovanović, S.; Jovanović, A. Nicotinamide-rich diet protects the heart against ischaemia-reperfusion in mice: A crucial role for cardiac SUR2A. Pharmacol. Res. 2010, 61, 564–570. [Google Scholar] [CrossRef]
- Kabłak-Ziembicka, A.; Badacz, R.; Okarski, M.; Wawak, M.; Przewłocki, T.; Podolec, J. Cardiac microRNAs: Diagnostic and therapeutic potential. Arch. Med. Sci. 2023, 19, 1360–1381. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhu, C.; Wang, W.; Li, M.; Ma, C.; Gao, B. SIRT1 is a regulator of autophagy: Implications for the progression and treatment of myocardial ischemia-reperfusion. Pharmacol. Res. 2024, 199, 106957. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shi, D.; Guo, M. The roles of PKC-δ and PKC-ε in myocardial ischemia/reperfusion injury. Pharmacol. Res. 2021, 170, 105716. [Google Scholar] [CrossRef] [PubMed]
- Boengler, K.; Eickelmann, C.; Kleinbongard, P. Mitochondrial kinase signaling for cardioprotection. Int. J. Mol. Sci. 2024, 25, 4491. [Google Scholar] [CrossRef]
- Yadav, M.; Kumari, P.; Yadav, V.; Kumar, S. Pharmacological preconditioning with phosphodiestrase inhibitor: An answer to stem cell survival against ischemic injury through JAK/STAT signaling. Heart Fail. Rev. 2020, 25, 355–366. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Y.; Hu, Z.; Yang, S.; Li, L.; Xiong, C.; Gao, Y.; Sun, W.; Zhang, Y. Protein Kinase C Family: Structures, biological functions, diseases, and pharmaceutical interventions. MedComm 2025, 6, e70474. [Google Scholar] [CrossRef]
- Sukhodub, A.; Jovanović, S.; Du, Q.; Budas, G.; Clelland, A.K.; Shen, M.; Sakamoto, K.; Tian, R.; Jovanović, A. AMP-activated protein kinase mediates preconditioning in cardiomyocytes by regulating activity and trafficking of sarcolemmal ATP-sensitive K(+) channels. J. Cell. Physiol. 2007, 210, 224–236. [Google Scholar] [CrossRef]
- Kandula, N.; Kumar, S.; Mandlem, V.K.K.; Siddabathuni, A.; Singh, S.; Kosuru, R. Role of AMPK in myocardial ischemia-reperfusion injury-induced cell death in the presence and absence of diabetes. Oxid. Med. Cell. Longev. 2022, 2022, 7346699. [Google Scholar] [CrossRef]
- Efentakis, P.; Andreadou, I.; Iliodromitis, K.E.; Triposkiadis, F.; Ferdinandy, P.; Schulz, R.; Iliodromitis, E.K. Myocardial protection and current cancer therapy: Two opposite targets with inevitable cost. Int. J. Mol. Sci. 2022, 23, 14121. [Google Scholar] [CrossRef]
- Gallo, S.; Vitacolonna, A.; Bonzano, A.; Comoglio, P.; Crepaldi, T. ERK: A key Player in the pathophysiology of cardiac hypertrophy. Int. J. Mol. Sci. 2019, 20, 2164. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Sandner, P.; Krieg, T. cGMP at the centre of attention: Emerging strategies for activating the cardioprotective PKG pathway. Basic Res. Cardiol. 2018, 113, 24. [Google Scholar] [CrossRef] [PubMed]
- Rakoubian, A.; Khinchin, J.; Yarbro, J.; Kobayashi, S.; Liang, Q. Isoform-specific roles of AMP-activated protein kinase in cardiac physiology and pathophysiology. Front. Cardiovasc. Med. 2025, 12, 1638515. [Google Scholar] [CrossRef] [PubMed]
- Yellon, D.M.; Beikoghli Kalkhoran, S.; Davidson, S.M. The RISK pathway leading to mitochondria and cardioprotection: How everything started. Basic Res. Cardiol. 2023, 118, 22. [Google Scholar] [CrossRef]
- Vainio, L.; Taponen, S.; Kinnunen, S.M.; Halmetoja, E.; Szabo, Z.; Alakoski, T.; Ulvila, J.; Junttila, J.; Lakkisto, P.; Magga, J.; et al. GSK3β Serine 389 Phosphorylation Modulates Cardiomyocyte Hypertrophy and Ischemic Injury. Int. J. Mol. Sci. 2021, 22, 13586. [Google Scholar] [CrossRef]
- Alhassan, H.H.; Janiyani, K.; Surti, M.; Adnan, M.; Patel, M. The dual role of glycogen synthase kinase-3 beta (GSK3β) in neurodegenerative pathologies: Interplay between autophagy and disease progression. Front. Pharmacol. 2025, 16, 1693805. [Google Scholar] [CrossRef]
- Kleinbongard, P. Perspective: Mitochondrial STAT3 in cardioprotection. Basic Res. Cardiol. 2023, 118, 32. [Google Scholar] [CrossRef]
- Xu, L.; Yang, M.; Wei, A.; Wei, Z.; Qin, Y.; Wang, K.; Li, B.; Chen, K.; Liu, C.; Li, C.; et al. Aerobic exercise-induced HIF-1α upregulation in heart failure: Exploring potential impacts on MCT1 and MPC1 regulation. Mol. Med. 2024, 30, 83. [Google Scholar] [CrossRef]
- Rusiecka, O.M.; Montgomery, J.; Morel, S.; Batista-Almeida, D.; Van Campenhout, R.; Vinken, M.; Girao, H.; Kwak, B.R. Canonical and non-canonical roles of connexin43 in cardioprotection. Biomolecules 2020, 10, 1225. [Google Scholar] [CrossRef]
- Boengler, K.; Leybaert, L.; Ruiz-Meana, M.; Schulz, R. Connexin 43 in mitochondria: What do we really know about its function? Front. Physiol. 2022, 13, 928934. [Google Scholar] [CrossRef]
- Tsantoulas, A.; Tsiambas, E.; Spyropoulou, D.; Adamopoulou, M.; Mastronikoli, S.; Roukas, D.; Vylliotis, A.; Kafkas, N.; Fotiades, P.; Agrogiannis, G.; et al. Clinical impact of connexin 43 deregulation on myocardial infraction. Maedica 2024, 19, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Yang, Z.; Ma, H.; Zhang, H. Mitochondrial aldehyde dehydrogenase in myocardial ischemic and ischemia-reperfusion injury. Adv. Exp. Med. Biol. 2019, 1193, 107–120. [Google Scholar] [CrossRef]
- Lamb, R.J.; Griffiths, K.; Lip, G.Y.H.; Sorokin, V.; Frenneaux, M.P.; Feelisch, M.; Madhani, M. ALDH2 polymorphism and myocardial infarction: From alcohol metabolism to redox regulation. Pharmacol. Ther. 2024, 259, 108666. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Cardioprotective effects of sirtuin-1 and its downstream effectors: Potential role in mediating the heart failure benefits of SGLT2 (Sodium-Glucose Cotransporter 2) inhibitors. Circ. Heart Fail. 2020, 13, e007197. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Barnes, K.; Gibson, S.; Fillmore, N. Dual-edged role of SIRT1 in energy metabolism and cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 2024, 327, H1162–H1173. [Google Scholar] [CrossRef]
- Ding, Y.N.; Wang, H.Y.; Chen, X.F.; Tang, X.; Chen, H.Z. Roles of Sirtuins in Cardiovascular diseases: Mechanisms and therapeutics. Circ. Res. 2025, 136, 524–550. [Google Scholar] [CrossRef]
- Sagris, M.; Apostolos, A.; Theofilis, P.; Ktenopoulos, N.; Katsaros, O.; Tsalamandris, S.; Tsioufis, K.; Toutouzas, K.; Tousoulis, D. Myocardial ischemia-reperfusion injury: Unraveling pathophysiology, clinical manifestations, and emerging prevention strategies. Biomedicines 2024, 12, 802. [Google Scholar] [CrossRef]
- Heusch, G. Cardioprotection and its translation: A need for new paradigms? Or for new pragmatism? An opinionated retro- and perspective. J. Cardiovasc. Pharmacol. Ther. 2023, 28, 10742484231179613. [Google Scholar] [CrossRef]
- Skyschally, A.; Kleinbongard, P.; Neuhäuser, M.; Heusch, G. “Expression of concern”: Publication bias for positive preclinical cardioprotection studies. Basic Res. Cardiol. 2024, 119, 397–402. [Google Scholar] [CrossRef]
- Arrell, D.K.; Park, S.; Yamada, S.; Alekseev, A.E.; Garmany, A.; Jeon, R.; Vuckovic, I.; Lindor, J.Z.; Terzic, A. KATP channel dependent heart multiome atlas. Sci. Rep. 2022, 12, 7314. [Google Scholar] [CrossRef]
- Palácio, P.B.; de Freitas Soares, G.C.; Lima, G.M.B.; Cunha, P.L.O.; Varela, A.L.N.; Facundo, H.T. Competitive interaction between ATP and GTP regulates mitochondrial ATP-sensitive potassium channels. Chem. Biol. Interact. 2023, 381, 110560. [Google Scholar] [CrossRef]
- Mahdi, H.; Jovanović, A. SUR2A as a base for cardioprotective therapeutic strategies. Mol. Biol. Rep. 2022, 49, 6717–6723. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zou, Y.; Song, C.; Cao, K.; Cai, K.; Wu, Y.; Zhang, Z.; Geng, D.; Sun, W.; Ouyang, N.; et al. The role of glycolytic metabolic pathways in cardiovascular disease and potential therapeutic approaches. Basic Res. Cardiol. 2023, 118, 48. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.V.; Javadov, S.; Margreiter, R.; Grimm, M.; Hagenbuchner, J.; Ausserlechner, M.J. The role of mitochondria in the mechanisms of cardiac ischemia-reperfusion injury. Antioxidants 2019, 8, 454. [Google Scholar] [CrossRef] [PubMed]
- Cung, T.T.; Morel, O.; Cayla, G.; Rioufol, G.; Garcia-Dorado, D.; Angoulvant, D.; Bonnefoy-Cudraz, E.; Guérin, P.; Elbaz, M.; Delarche, N.; et al. Cyclosporine Before PCI in Patients with Acute Myocardial Infarction. N. Engl. J. Med. 2015, 373, 1021–1031. [Google Scholar] [CrossRef]
- Hefler, J.; Marfil-Garza, B.A.; Campbell, S.; Freed, D.H.; Shapiro, A.M.J. Preclinical systematic review & meta-analysis of cyclosporine for the treatment of myocardial ischemia-reperfusion injury. Ann. Transl. Med. 2022, 10, 954. [Google Scholar] [CrossRef]
- Medzikovic, L.; Azem, T.; Sun, W.; Rejali, P.; Esdin, L.; Rahman, S.; Dehghanitafti, A.; Aryan, L.; Eghbali, M. Sex differences in therapies against myocardial ischemia-reperfusion injury: From basic science to clinical perspectives. Cells 2023, 12, 2077. [Google Scholar] [CrossRef]
- Murphy, E.; Steenbergen, C. Gender-based differences in mechanisms of protection in myocardial ischemia-reperfusion injury. Cardiovasc. Res. 2007, 75, 478–486. [Google Scholar] [CrossRef]
- Pagliaro, P.; Penna, C. Redox signalling and cardioprotection: Translatability and mechanism. Br. J. Pharmacol. 2015, 172, 1974–1995. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Gong, N. Endoplasmic reticulum stress signaling modulates ischemia/reperfusion injury in the aged heart by regulating mitochondrial maintenance. Mol. Med. 2024, 30, 107. [Google Scholar] [CrossRef]
- Oskuye, Z.Z.; Mehri, K.; Mokhtari, B.; Bafadam, S.; Nemati, S.; Badalzadeh, R. Cardioprotective effect of antioxidant combination therapy: A highlight on MitoQ plus alpha-lipoic acid beneficial impact on myocardial ischemia-reperfusion injury in aged rats. Heliyon 2024, 10, e28158. [Google Scholar] [CrossRef]
- Whittington, H.J.; Babu, G.G.; Mocanu, M.M.; Yellon, D.M.; Hausenloy, D.J. The diabetic heart: Too sweet for its own good? Cardiol. Res. Pract. 2012, 2012, 845698. [Google Scholar] [CrossRef]
- Fancher, I.S.; Dick, G.M.; Hollander, J.M. Diabetes mellitus reduces the function and expression of ATP-dependent K+ channels in cardiac mitochondria. Life Sci. 2013, 92, 664–668. [Google Scholar] [CrossRef]
- Hjortbak, M.V.; Hjort, J.; Povlsen, J.A.; Jensen, R.V.; Støttrup, N.B.; Laursen, M.R.; Jespersen, N.R.; Løfgren, B.; Bøtker, H.E. Influence of diabetes mellitus duration on the efficacy of ischemic preconditioning in a Zucker diabetic fatty rat model. PLoS ONE 2018, 13, e0192981. [Google Scholar] [CrossRef]
- Penna, C.; Aragno, M.; Cento, A.S.; Femminò, S.; Russo, I.; Bello, F.D.; Chiazza, F.; Collotta, D.; Alves, G.F.; Bertinaria, M.; et al. Ticagrelor conditioning effects are not additive to cardioprotection induced by direct NLRP3 inflammasome inhibition: Role of RISK, NLRP3, and redox cascades. Oxid. Med. Cell Longev. 2020, 2020, 9219825. [Google Scholar] [CrossRef] [PubMed]
- D’Amario, D.; Galli, M.; Restivo, A.; Canonico, F.; Vergallo, R.; Migliaro, S.; Trani, C.; Burzotta, F.; Aurigemma, C.; Laborante, R.; et al. Ticagrelor enhances the cardioprotective effects of ischemic preconditioning in stable patients undergoing percutaneous coronary intervention: The TAPER-S randomized study. Eur. Heart J. Cardiovasc. Pharmacother. 2024, 10, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Liu, Y.; Yao, Y.; Zhou, S.; Fang, N.; Wang, W.; Li, L. β-blockers and volatile anesthetics may attenuate cardioprotection by remote preconditioning in adult cardiac surgery: A meta-analysis of 15 randomized trials. J. Cardiothorac. Vasc. Anesth. 2013, 27, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Chiari, P.; Fellahi, J.L. Myocardial protection in cardiac surgery: A comprehensive review of current therapies and future cardioprotective strategies. Front. Med. 2024, 11, 1424188. [Google Scholar] [CrossRef]
- Francis, R.; Chong, J.; Ramlall, M.; Bucciarelli-Ducci, C.; Clayton, T.; Dodd, M.; Engstrøm, T.; Evans, R.; Ferreira, V.M.; Fontana, M.; et al. Effect of remote ischaemic conditioning on infarct size and remodelling in ST-segment elevation myocardial infarction patients: The CONDI-2/ERIC-PPCI CMR substudy. Basic Res. Cardiol. 2021, 116, 59. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.; Li, G. Preclinical multi-target strategies for myocardial ischemia-reperfusion injury. Front. Cardiovasc. Med. 2022, 9, 967115. [Google Scholar] [CrossRef]
- Penna, C.; Comità, S.; Tullio, F.; Alloatti, G.; Pagliaro, P. Challenges facing the clinical translation of cardioprotection: 35 years after the discovery of ischemic preconditioning. Vascul. Pharmacol. 2022, 144, 106995. [Google Scholar] [CrossRef]
| Category | Inducer/Example | Mechanism(s) of Action (Proposed) |
|---|---|---|
| Ischemic Procedures | Ischemic preconditioning (brief ischemia–reperfusion before sustained ischemia) | Activates protective signaling cascades; reduces infarct size; improves post-ischemic recovery [3,5,16,17] |
| Ischemic postconditioning (brief ischemia–reperfusion after sustained ischemia) | Limits reperfusion injury; reduces oxidative stress and apoptotic signaling [5,11,18] | |
| Remote ischemic preconditioning (transient ischemia at distant organ/tissue) | Release of humoral factors and neural signaling; systemic activation of cardioprotective pathways [19,20,21] | |
| Remote ischemic postconditioning | Similar to preconditioning; triggers systemic protective signals after prolonged ischemia [19,20,21] | |
| Environmental Stressors | Hypoxia/intermittent hypoxia | Induction of hypoxia-inducible factors (HIFs); metabolic adaptation; angiogenesis [6,7,8,9,22,23] |
| Altered oxygen tension | Modulation of mitochondrial function; activation of stress-response pathways [6,7,8,9,23,24] | |
| Hypothermia | Reduces metabolic demand; inhibits apoptosis and inflammation; preserves ATP [18,25] | |
| Hyperthermia | Heat shock protein (HSP) induction; cytoprotective protein expression [10,26] | |
| Pharmacological Agents | Adenosine | Activates adenosine receptors (A1, A2); reduces calcium overload; improves coronary flow [2,12] |
| Nitric oxide (NO) | Vasodilation; reduces platelet aggregation; modulates mitochondrial respiration [14,27,28] | |
| Isosteviol | Antioxidant and anti-apoptotic effects; modulation of mitochondrial KATP channels [15,29,30] | |
| Nicotinamide | Enhances NAD+ metabolism; supports mitochondrial function; reduces oxidative stress [31,32,33] | |
| Growth factors (e.g., insulin-like growth factor-1, vascular endothelial growth factor) | Promotes cell survival pathways; stimulates angiogenesis and repair mechanisms [4,13,34] | |
| Other Biological Mediators | Heat shock proteins (HSPs) | Act as molecular chaperones; prevent protein misfolding; inhibit apoptosis [10,26] |
| Cytokines and chemokines (e.g., TNF-α at low doses, interleukins) | Can activate pro-survival signaling cascades under controlled conditions [25,35,36] | |
| Endogenous opioids | Modulate receptor-mediated signaling; reduce excitotoxicity and calcium overload [4,25,35] |
| Category | Molecule/Factor | Proposed Mechanism(s) of Action |
|---|---|---|
| Kinases | PKC (especially PKCε) | Isoform-specific activation; phosphorylation of mitochondrial/ion channel targets; modulation of apoptosis [5,11,34,37] |
| PKA | Regulation of calcium handling and contractility; potential role in preconditioning [5,11] | |
| AMPK | Energy sensor; regulates trafficking/opening of KATP channels; supports metabolic adaptation to ischemia [9,38,39,43] | |
| p38 MAPK | Stress kinase; implicated in both protective and deleterious responses depending on context [4,11] | |
| ERK1/2 | Part of RISK pathway; promotes cell survival during reperfusion [11,15,41,44] | |
| PKG | Downstream of NO–cGMP signaling; inhibits mPTP opening; vasodilatory effects [14,27,42] | |
| PI3K–Akt (RISK) | Enhances survival signaling; inhibits pro-death pathways; prevents mPTP opening [11,31,44] | |
| GSK3β | Downstream effector of RISK; role in cardioprotection remains debated [35,40,45] | |
| Transcription Factors & Cytokine Pathways | STAT3 (SAFE pathway) | Activated by cytokines (e.g., TNF-α); regulates transcription of protective genes; modulates mitochondrial respiration [11,25,36,47] |
| HIF-1α | Induces glycolytic shift, angiogenesis, antioxidant enzymes; interacts with noncoding RNAs [6,7,8,22,23,48] | |
| Noncoding RNAs | MicroRNAs (miRNAs) | Post-transcriptional regulation of cardioprotective proteins; fine-tuning of kinase and mitochondrial signaling [4,13,24,32] |
| Mitochondrial Modulators | Connexin 43 (Cx43) | Phosphorylation and mitochondrial translocation; regulates KATP channels and mitochondrial respiration [19,49,50,51,57] |
| Aldehyde dehydrogenase 2 (ALDH2) | Detoxifies reactive aldehydes; activated via PKCε; contributes to isoflurane and remote conditioning [5,52,53,58] | |
| Enzymatic Defenses/Stress Proteins | iNOS (inducible nitric oxide synthase) | Generates protective NO signaling; modulates mitochondrial function [11,22,27,28] |
| SOD (superoxide dismutase) | Detoxifies ROS; preserves redox balance [23,25,32,46] | |
| Aldose reductase | Maintains osmotic and redox homeostasis under ischemia [23,25] | |
| Heme oxygenase-1 (HO-1) | Generates antioxidant molecules (bilirubin, CO); cytoprotective role in ischemia [23,25,32,34] | |
| Metabolic Regulators | Sirtuins (especially SIRT1) | NAD+-dependent deacetylases; promote autophagy, mitochondrial biogenesis, and metabolic resilience [33,54,55,56,59] |
| Category | Effector/Component | Proposed Mechanism(s) of Action |
|---|---|---|
| Mitochondria | Mitochondrial permeability transition pore (mPTP) | Inhibition or delayed opening prevents mitochondrial swelling, cytochrome c release, and cell death [17,18,29,64,65,66] |
| Mitochondrial KATP channels | Mild depolarization; reduced calcium overload; modulation of ROS signaling; preserved ATP production [29,30,57,60,61] | |
| Hexokinase 2 (HK2) | Binds mitochondria; stabilizes mitochondrial membranes; prevents cytochrome c release; reduces ROS [5,11,16,29] | |
| Ion Channels | Sarcolemmal KATP channels (SUR2A subunit) | Modulate membrane potential and ionic flux; SUR2A expression enhances cardioprotection [6,7,8,9,31,38,62] |
| Metabolic Enzymes | ALDH2 (aldehyde dehydrogenase 2) | Detoxifies reactive aldehydes; reduces oxidative stress during ischemia–reperfusion [52,53,57,58] |
| Post-translational Modifications | Protein S-nitrosation | Modulates activity of mitochondrial/cytosolic proteins; attenuates ROS generation during reperfusion [11,22,27,38] |
| Calcium Handling | Sarcoplasmic reticulum (SR) | Modulation of calcium reuptake/release; phospholamban phosphorylation; prevention of calcium overload [16,23,24,57,58] |
| Structural Elements | Cytoskeleton, cell volume, ionic balance, pH stability | Preserve membrane integrity; prevent hypercontracture and cell swelling; stabilize intracellular pH [5,25,49,57,58] |
| Autophagy | Autophagy–lysosomal system | Removal of damaged organelles and toxic proteins (protective during ischemia); excessive activation may trigger autophagic cell death during reperfusion [11,23,32,33,57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovanović, A. Cardioprotective Signaling: Outline and Future Directions. Biomedicines 2025, 13, 2973. https://doi.org/10.3390/biomedicines13122973
Jovanović A. Cardioprotective Signaling: Outline and Future Directions. Biomedicines. 2025; 13(12):2973. https://doi.org/10.3390/biomedicines13122973
Chicago/Turabian StyleJovanović, Aleksandar. 2025. "Cardioprotective Signaling: Outline and Future Directions" Biomedicines 13, no. 12: 2973. https://doi.org/10.3390/biomedicines13122973
APA StyleJovanović, A. (2025). Cardioprotective Signaling: Outline and Future Directions. Biomedicines, 13(12), 2973. https://doi.org/10.3390/biomedicines13122973






