Effects of Different Forms of Selenium in Human Umbilical Cord Mesenchymal Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of hUC-MSCs
2.2. Cell Culture and Selenium Compound Treatment
2.3. Cell Counting Kit-8 Assays
2.4. Flow Cytometry
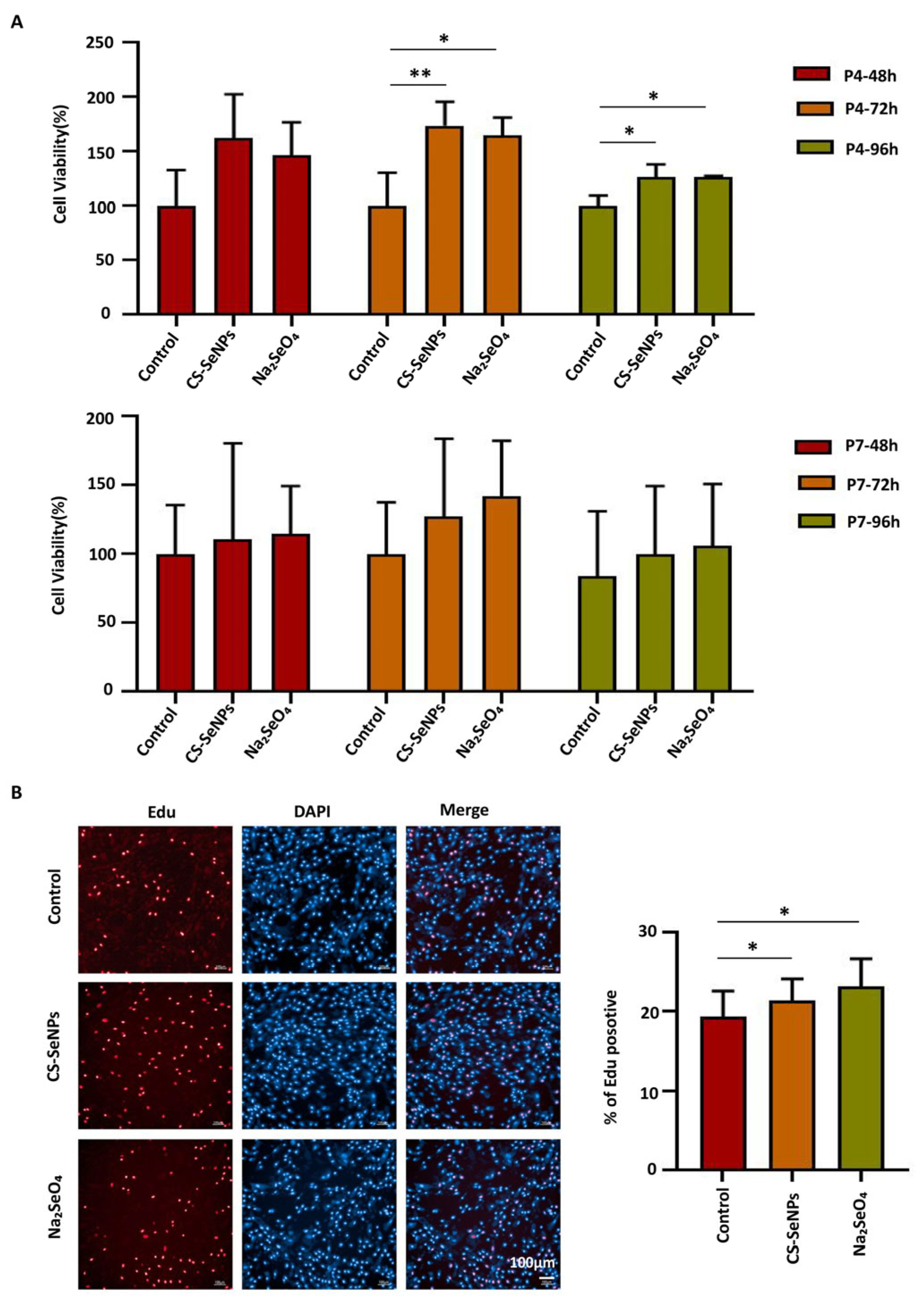
2.5. EdU Assays
2.6. ROS Analysis
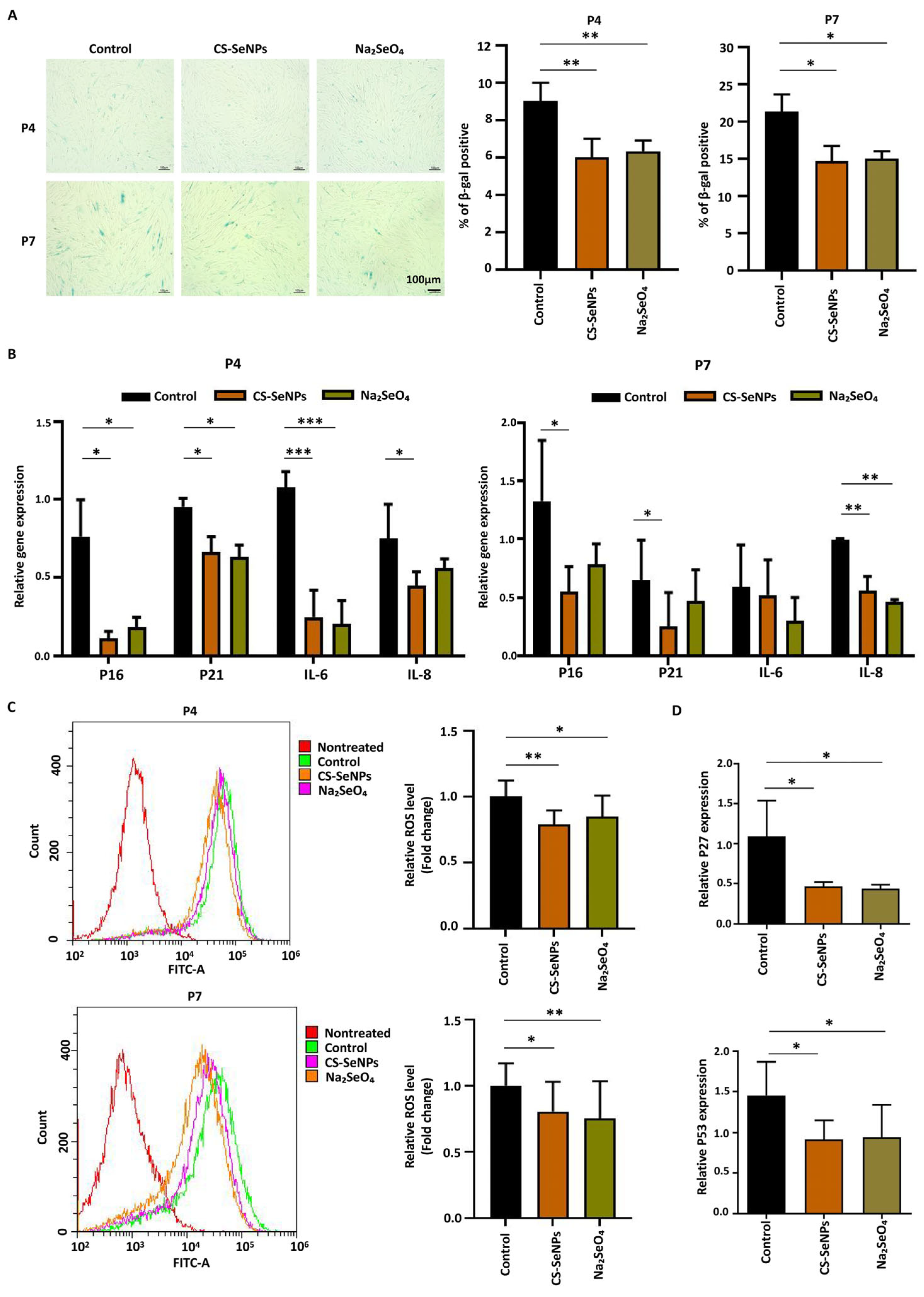
2.7. Quantitative Real-Time PCR Assay (qPCR)
2.8. MSC Adipogenic Differentiation and Osteogenic Differentiation Analysis
2.9. Senescence-Associated β-Galactosidase (SA-β-Gal) Staining Assay
2.10. Statistical Analysis
3. Results
3.1. Choice of Selenium Compounds for Improving the Culture of hUC-MSCs
3.2. Basic Characterization of hUC-MSCs
3.3. Effects of Selenium Compounds on the Proliferation Capacity of hUC-MSCs
3.4. Effects of CS-SeNPs and Na2SeO4 on the Cell Senescence and Oxidative Stress of hUC-MSCs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chehelgerdi, M.; Behdarvand Dehkordi, F.; Chehelgerdi, M.; Kabiri, H.; Salehian-Dehkordi, H.; Abdolvand, M.; Salmanizadeh, S.; Rashidi, M.; Niazmand, A.; Ahmadi, S.; et al. Exploring the promising potential of induced pluripotent stem cells in cancer research and therapy. Mol. Cancer 2023, 22, 189. [Google Scholar] [CrossRef]
- Yadav, P.; Singh, S.K.; Rajput, S.; Allawadhi, P.; Khurana, A.; Weiskirchen, R.; Navik, U. Therapeutic potential of stem cells in regeneration of liver in chronic liver diseases: Current perspectives and future challenges. Pharmacol. Ther. 2024, 253, 108563. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ou, Q.; Shi, S.; Shao, C. Immunomodulatory properties of mesenchymal stem cells/dental stem cells and their therapeutic applications. Cell. Mol. Immunol. 2023, 20, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Ni, B.; Liu, Q.; He, F.; Li, L.; Zhong, X.; Zheng, X.; Lu, J.; Chen, X.; Lin, H.; et al. Human umbilical cord-derived mesenchymal stem cells ameliorate experimental colitis by normalizing the gut microbiota. Stem Cell Res. Ther. 2022, 13, 475. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Ni, B.; Wang, L.; Shan, J.; Pan, L.; He, Y.; Lv, G.; Lin, H.; Chen, W.; Zhang, Q. CCR2-overexpressing mesenchymal stem cells targeting damaged liver enhance recovery of acute liver failure. Stem Cell Res. Ther. 2022, 13, 55. [Google Scholar] [CrossRef]
- Li, C.; Sun, Y.; Xu, W.; Chang, F.; Wang, Y.; Ding, J. Mesenchymal Stem Cells-Involved Strategies for Rheumatoid Arthritis Therapy. Adv. Sci. 2024, 11, e2305116. [Google Scholar] [CrossRef]
- Levy, O.; Kuai, R.; Siren, E.M.J.; Bhere, D.; Milton, Y.; Nissar, N.; De Biasio, M.; Heinelt, M.; Reeve, B.; Abdi, R.; et al. Shattering barriers toward clinically meaningful MSC therapies. Sci. Adv. 2020, 6, eaba6884. [Google Scholar] [CrossRef]
- Attar, A.; Mirhosseini, S.; Mathur, A.; Dowlut, S.; Monabati, A.; Kasaei, M.; Abtahi, F.; Kiwan, Y.; Vosough, M.; Azarpira, N. Prevention of acute myocardial infarction induced heart failure by intracoronary infusion of mesenchymal stem cells: Phase 3 randomised clinical trial (PREVENT-TAHA8). BMJ 2025, 391, e083382. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, Z.; Mei, S.; Wang, Z.; Xu, Z.; Yao, W.; Liu, L.; Yuan, M.; Pan, Y.; Zhu, K.; et al. Dose-escalation studies of mesenchymal stromal cell therapy for decompensated liver cirrhosis: Phase Ia/Ib results and immune modulation insights. Signal Transduct. Target. Ther. 2025, 10, 238. [Google Scholar] [CrossRef]
- Zhao, L.; Ni, B.; Li, J.; Liu, R.; Zhang, Q.; Zheng, Z.; Yang, W.; Yu, W.; Bi, L. Evaluation of the impact of customized serum-free culture medium on the production of clinical-grade human umbilical cord mesenchymal stem cells: Insights for future clinical applications. Stem Cell Res. Ther. 2024, 15, 327. [Google Scholar] [CrossRef]
- Ivanisova, D.; Bohac, M.; Culenova, M.; Smolinska, V.; Danisovic, L. Mesenchymal-Stromal-Cell-Conditioned Media and Their Implication for Osteochondral Regeneration. Int. J. Mol. Sci. 2023, 24, 9054. [Google Scholar] [CrossRef] [PubMed]
- Tee, C.; Roxby, D.; Othman, R.; Denslin, V.; Bhat, S.; Yang, Z.; Han, J.; Tucker-Kellogg, L.; Boyer, L. Metabolic modulation to improve MSC expansion and therapeutic potential for articular cartilage repair. Stem Cell Res. Ther. 2024, 15, 308. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, B.; Huang, Y.; Li, J.; Cao, D.; Chen, Z.; Li, J.; Ran, B.; Yang, J.; Wang, R.; et al. Selenium intake and multiple health-related outcomes: An umbrella review of meta-analyses. Front. Nutr. 2023, 10, 1263853. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhan, S.; Han, F.; Liu, Y.; Wu, H.; Huang, Z. The Possible Mechanism of Physiological Adaptation to the Low-Se Diet and Its Health Risk in the Traditional Endemic Areas of Keshan Diseases. Biol. Trace Elem. Res. 2022, 200, 2069–2083. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Duntas, L.H.; Rayman, M.P. The role of selenium in type-2 diabetes mellitus and its metabolic comorbidities. Redox Biol. 2022, 50, 102236. [Google Scholar] [CrossRef]
- Li, L.; Zhang, W.; Cao, H.; Fang, L.; Wang, W.; Li, C.; He, Q.; Jiao, J.; Zheng, R. Nanozymes in Alzheimer’s disease diagnostics and therapy. Biomater. Sci. 2024, 12, 4519–4545. [Google Scholar] [CrossRef]
- Noguchi, N. Ebselen, a useful tool for understanding cellular redox biology and a promising drug candidate for use in human diseases. Arch. Biochem. Biophys. 2016, 595, 109–112. [Google Scholar] [CrossRef]
- Fatima, S.; Alfrayh, R.; Alrashed, M.; Alsobaie, S.; Ahmad, R.; Mahmood, A. Selenium Nanoparticles by Moderating Oxidative Stress Promote Differentiation of Mesenchymal Stem Cells to Osteoblasts. Int. J. Nanomed. 2021, 16, 331–343. [Google Scholar] [CrossRef]
- Abozaid, O.A.R.; Rashed, L.A.; El-Sonbaty, S.M.; Abu-Elftouh, A.I.; Ahmed, E.S.A. Mesenchymal Stem Cells and Selenium Nanoparticles Synergize with Low Dose of Gamma Radiation to Suppress Mammary Gland Carcinogenesis via Regulation of Tumor Microenvironment. Biol. Trace Elem. Res. 2023, 201, 338–352. [Google Scholar] [CrossRef]
- Shao, C.; Yu, Z.; Luo, T.; Zhou, B.; Song, Q.; Li, Z.; Yu, X.; Jiang, S.; Zhou, Y.; Dong, W.; et al. Chitosan-Coated Selenium Nanoparticles Attenuate PRRSV Replication and ROS/JNK-Mediated Apoptosis in vitro. Int. J. Nanomed. 2022, 17, 3043–3054. [Google Scholar] [CrossRef]
- Soleimani Asl, S.; Amiri, I.; Samzadeh-Kermani, A.; Abbasalipourkabir, R.; Gholamigeravand, B.; Shahidi, S. Chitosan-coated Selenium nanoparticles enhance the efficiency of stem cells in the neuroprotection of streptozotocin-induced neurotoxicity in male rats. Int. J. Biochem. Cell Biol. 2021, 141, 106089. [Google Scholar] [CrossRef]
- Ming, P.; Wei, Y.; Zhu, Y.; Li, K.; Zhu, W.; Qiu, J. Dual-stabilized selenium nanoparticles with chitosan and SS31 peptide: Resolving instability for enhancing ROS elimination, suppressing inflammation, and combating bacterial infections. Colloids Surf. B Biointerfaces 2025, 253, 114749. [Google Scholar] [CrossRef]
- Yang, X.; Fu, Y.; Zhang, J.; Liu, J.; Liu, X.; Peng, Y.; Kyin, S.; Zhang, M.; Zhou, D. Preparation, characterization, and antioxidant and antiapoptotic activities of biosynthesized nano-selenium by yak-derived Bacillus cereus and chitosan-encapsulated chemically synthesized nano-selenium. Int. J. Biol. Macromol. 2023, 242, 124708. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xu, L.; He, L.; Zhao, J.; Zhang, Z.; Chen, Q.; Chen, T. Selenium nanoparticles regulates selenoprotein to boost cytokine-induced killer cells-based cancer immunotherapy. Nanotoday 2020, 35, 100975. [Google Scholar] [CrossRef]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, F.; Khoshmirsafa, M.; Safari, E.; Asgari, M.; Alemrajabi, M.; Nojehdehi, S.; Khorrami, S. Vitamin E and selenium improve mesenchymal stem cell conditioned media immunomodulatory effects. Stem Cell Investig. 2021, 8, 9. [Google Scholar] [CrossRef]
- Gu, W.; Zhao, F.; Huang, W.; Zhu, M.; Huang, H.; Yin, H.; Chen, T. Selenium nanoparticles activate selenoproteins to mitigate septic lung injury through miR-20b-mediated RORγt/STAT3/Th17 axis inhibition and enhanced mitochondrial transfer in BMSCs. J. Nanobiotechnol. 2025, 23, 226. [Google Scholar] [CrossRef]
- Ma, S.; Xue, R.; Zhu, H.; Han, Y.; Ji, X.; Zhang, C.; Wei, N.; Xu, J.; Li, F. Selenomethionine preconditioned mesenchymal stem cells derived extracellular vesicles exert enhanced therapeutic efficacy in intervertebral disc degeneration. Int. Immunopharmacol. 2024, 132, 112028. [Google Scholar] [CrossRef]
- Li, Y.; Liu, T.; Zheng, R.; Lai, J.; Su, J.; Li, J.; Zhu, B.; Chen, T. Translational selenium nanoparticles boost GPx1 activation to reverse HAdV-14 virus-induced oxidative damage. Bioact. Mater. 2024, 38, 276–291. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.H.; Yoon, B.S.; Jun, E.K.; Lee, G.; Kim, I.Y.; You, S. Additive effect of bFGF and selenium on expansion and paracrine action of human amniotic fluid-derived mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 293. [Google Scholar] [CrossRef]
- Mönch, D.; Reinders, M.E.J.; Dahlke, M.H.; Hoogduijn, M.J. How to Make Sense out of 75,000 Mesenchymal Stromal Cell Publications? Cells 2022, 11, 1419. [Google Scholar] [CrossRef]
- Al-Azab, M.; Idiiatullina, E.; Safi, M.; Hezam, K. Enhancers of mesenchymal stem cell stemness and therapeutic potency. Biomed. Pharmacother. 2023, 162, 114356. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, T.; Wang, R.; Shi, P.; Pan, B.; Pang, X. Insulin-Transferrin-Selenium as a Novel Serum-free Media Supplement for the Culture of Human Amnion Mesenchymal Stem Cells. Ann. Clin. Lab. Sci. 2019, 49, 63–71. [Google Scholar] [PubMed]
- Valadbeygi, A.; Naji, T.; Pirnia, A.; Gholami, M. Supplementation freeze-thawed media with selenium protect adipose-derived mesenchymal stem cells from freeze-thawed induced injury. Cryobiology 2016, 73, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh Anvar, L.; Hosseini-Asl, S.; Mohammadzadeh-Vardin, M.; Sagha, M. The Telomerase Activity of Selenium-Induced Human Umbilical Cord Mesenchymal Stem Cells Is Associated with Different Levels of c-Myc and p53 Expression. DNA Cell Biol. 2017, 36, 34–41. [Google Scholar] [CrossRef]
- Suzuki, M.; Endo, M.; Shinohara, F.; Echigo, S.; Rikiishi, H. Differential apoptotic response of human cancer cells to organoselenium compounds. Cancer Chemother. Pharmacol. 2010, 66, 475–484. [Google Scholar] [CrossRef]
- Sinha, R.; Said, T.; Medina, D. Organic and inorganic selenium compounds inhibit mouse mammary cell growth in vitro by different cellular pathways. Cancer Lett. 1996, 107, 277–284. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, Q.; Liu, Q.; Zhang, Y.; Gao, D.; Lu, Q.; Liu, J.; Zheng, W.; Huang, Z. Effect of selenium compounds on ROS and NO production and NOS activity in human umbilical cord mesenchymal stem cells. Chin. J. Pathophysiol. 2010, 26, 2363–2367. [Google Scholar]
- Khabibullaeva, N.; Khaitbaev, A.; Mansurov, D.; Khakimov, Z.; Rakhmanov, A.; Vidović, E.; Benassi, E. Bioactive composite based on chitosan obtained from the exoskeleton of dead Apis mellifera and selenium nanoparticles for accelerated skin wound healing: Synthesis, characterisation and in vivo assesment. Int. J. Biol. Macromol. 2025, 331, 148334. [Google Scholar] [CrossRef]
- Gong, H.; Bai, Y.; Rahoi, D.; Paulson, R.; Prabhu, K. The Impact of Sodium Selenite and Seleno-L-Methionine on Stress Erythropoiesis in a Murine Model of Hemolytic Anemia. J. Nutr. 2025, 155, 540–548. [Google Scholar]
- Chen, F.; Xu, X.; Wu, F.; Wang, F. Effect of selenium compounds on in vitro proliferation of alcohol metabolite damaged mesenchymal stem cells. Chongqing Med. 2023, 21, 3227–3231. [Google Scholar]
- Wang, S.; Wang, Z.; Su, H.; Chen, F.; Ma, M.; Yu, W.; Ye, G.; Cen, S.; Mi, R.; Wu, X.; et al. Effects of long-term culture on the biological characteristics and RNA profiles of human bone-marrow-derived mesenchymal stem cells. Mol. Ther. Nucleic Acids. 2021, 3, 557–574. [Google Scholar] [CrossRef] [PubMed]
- Atashi, F.; Modarressi, A.; Pepper, M. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: A review. Stem Cells Dev. 2015, 24, 1150–1563. [Google Scholar] [CrossRef]
- Li, Q.; Gao, Z.; Chen, Y.; Guan, M. The role of mitochondria in osteogenic, adipogenic and chondrogenic differentiation of mesenchymal stem cells. Protein Cell 2017, 8, 439–445. [Google Scholar] [CrossRef]
- Shen, Y.; Hong, Y.; Huang, X.; Chen, J.; Li, Z.; Qiu, J.; Liang, X.; Mai, C.; Li, W.; Li, X.; et al. ALDH2 regulates mesenchymal stem cell senescence via modulation of mitochondrial homeostasis. Free Radic. Biol. Med. 2024, 223, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, J.; Nam, S.; Kim, B.; Kim, G.; Kim, W.; Choi, Y. Selenium improves stem cell potency by stimulating the proliferation and active migration of 3T3-L1 preadipocytes. Int. J. Oncol. 2014, 44, 336–342. [Google Scholar] [CrossRef]
- Zheng, R.; Chen, D.; Su, J.; Lai, J.; Wang, C.; Chen, H.; Ning, Z.; Liu, X.; Tian, X.; Li, Y.; et al. Inhibition of HAdV-14 induced apoptosis by selenocystine through ROS-mediated PARP and p53 signaling pathways. J. Trace Elem. Med. Biol. 2023, 79, 127213. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, L.; Chen, Y.; Wang, T.; Xing, H. L-selenomethionine inhibits small intestinal ferroptosis caused by ammonia exposure through regulating ROS-mediated iron metabolism. Ecotoxicol. Environ. Saf. 2025, 289, 117477. [Google Scholar] [CrossRef]


| Gene | Forward Primers (5′-3′) | Reverse Primers (5′-3′) |
|---|---|---|
| P27 | AACGTGCGAGTGTCTAACGG | CCCTCTAGGGGTTTGTGATTCT |
| P53 | GAGGTTGGCTCTGACTGTACC | TCCGTCCCAGTAGATTACCAC |
| P16 | CGTACCCCGATTCAGGTG | ACCAGCGTGTCCAGGAAG |
| P21 | GGCAGACCAGCCTGACAGAT | TTCAGGGTTTTCTCTTGCAGAAG |
| IL-6 | AGACAAAGCCAGAGTCCTTC | TTCTGTGACTCCAGCTTATC |
| IL-8 | GAGAGTGATTGAGAGTGGACCAC | CACAACCCTCTGCACCCAGTTT |
| β-ACTIN | TGAAGATCAAGATCATTGCTCCTC | AACTAAGTCATAGTCCGCCTAGAAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, B.; Li, C.; Lin, H.; Chen, W.; Xu, R.; Li, H.; Chen, X.; Lu, J.; Yang, F. Effects of Different Forms of Selenium in Human Umbilical Cord Mesenchymal Stem Cells. Biomedicines 2025, 13, 2861. https://doi.org/10.3390/biomedicines13122861
Ni B, Li C, Lin H, Chen W, Xu R, Li H, Chen X, Lu J, Yang F. Effects of Different Forms of Selenium in Human Umbilical Cord Mesenchymal Stem Cells. Biomedicines. 2025; 13(12):2861. https://doi.org/10.3390/biomedicines13122861
Chicago/Turabian StyleNi, Beibei, Cuiping Li, Huizhu Lin, Wenjie Chen, Ruixuan Xu, Huali Li, Xiaoyan Chen, Jianxi Lu, and Fan Yang. 2025. "Effects of Different Forms of Selenium in Human Umbilical Cord Mesenchymal Stem Cells" Biomedicines 13, no. 12: 2861. https://doi.org/10.3390/biomedicines13122861
APA StyleNi, B., Li, C., Lin, H., Chen, W., Xu, R., Li, H., Chen, X., Lu, J., & Yang, F. (2025). Effects of Different Forms of Selenium in Human Umbilical Cord Mesenchymal Stem Cells. Biomedicines, 13(12), 2861. https://doi.org/10.3390/biomedicines13122861






