Simulated Microgravity Causes Delayed Platelet Activation and Downregulates Acid-Sensing Ion Channel 1/2 Protein Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Platelets from Human Volunteers
2.2. Simulated Microgravity
2.3. BCA Protein Assay
2.4. Membrane Fluidity Assay
2.5. SDS-PAGE, Western Blotting, and Densitometric Analysis
2.6. Immunofluorescence Microscopy
2.7. Thromboelastography
2.8. Statistical Analysis
3. Results
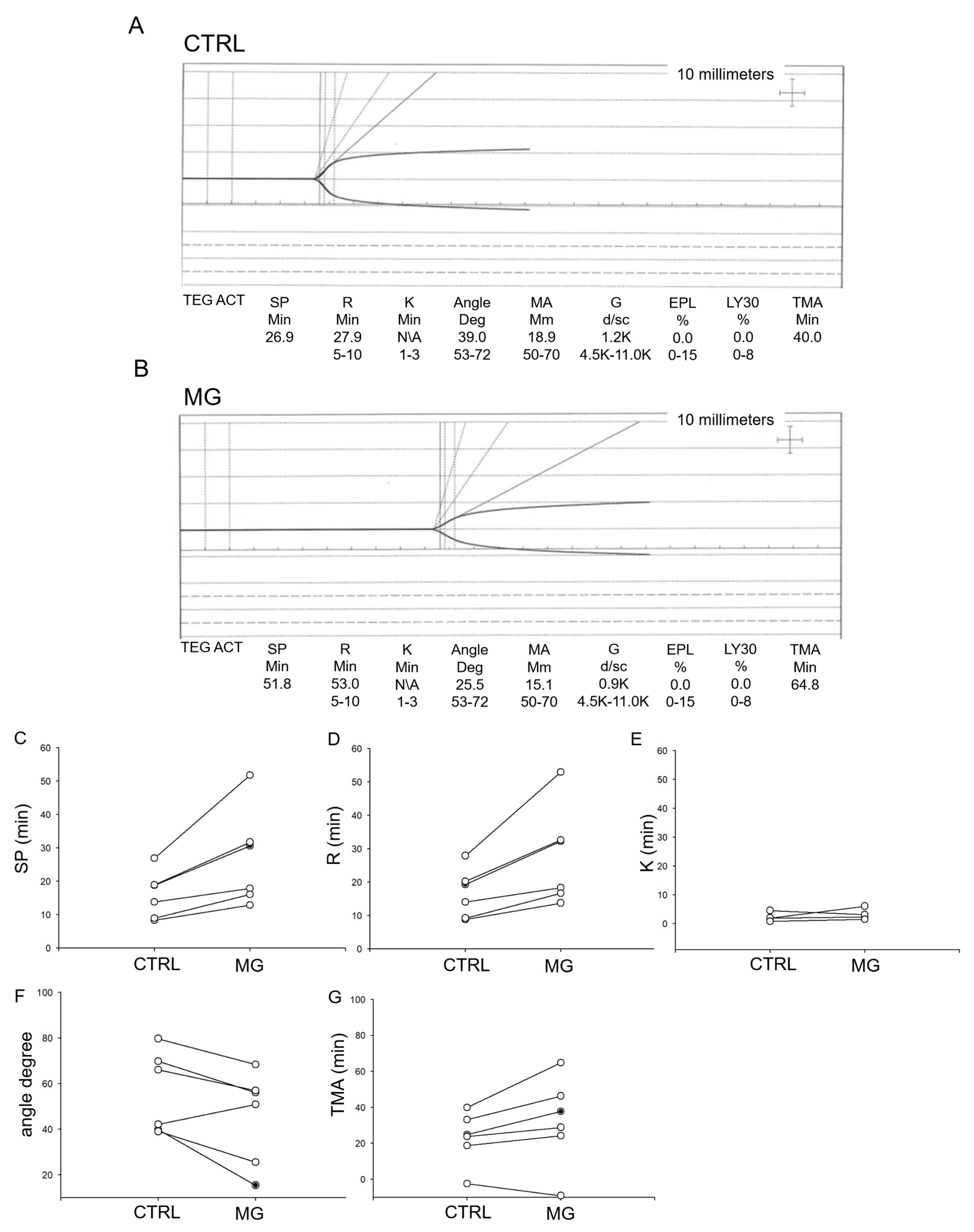
3.1. Simulated Microgravity Causes a Delay in the Activation of Platelets from Human Donors
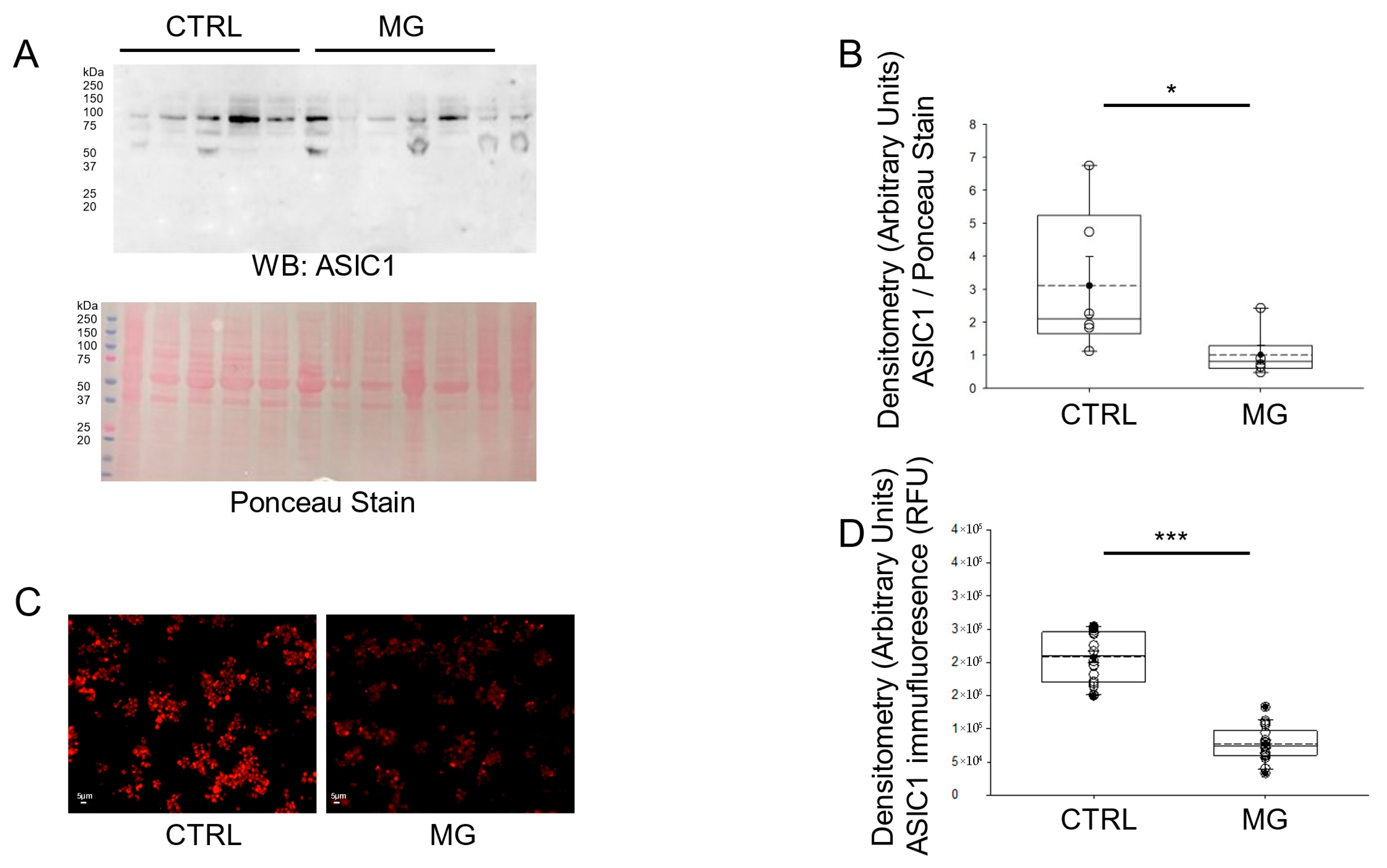
3.2. ASIC1 Protein Expression Is Decreased in Human Platelets Subject to Simulated Microgravity Conditions
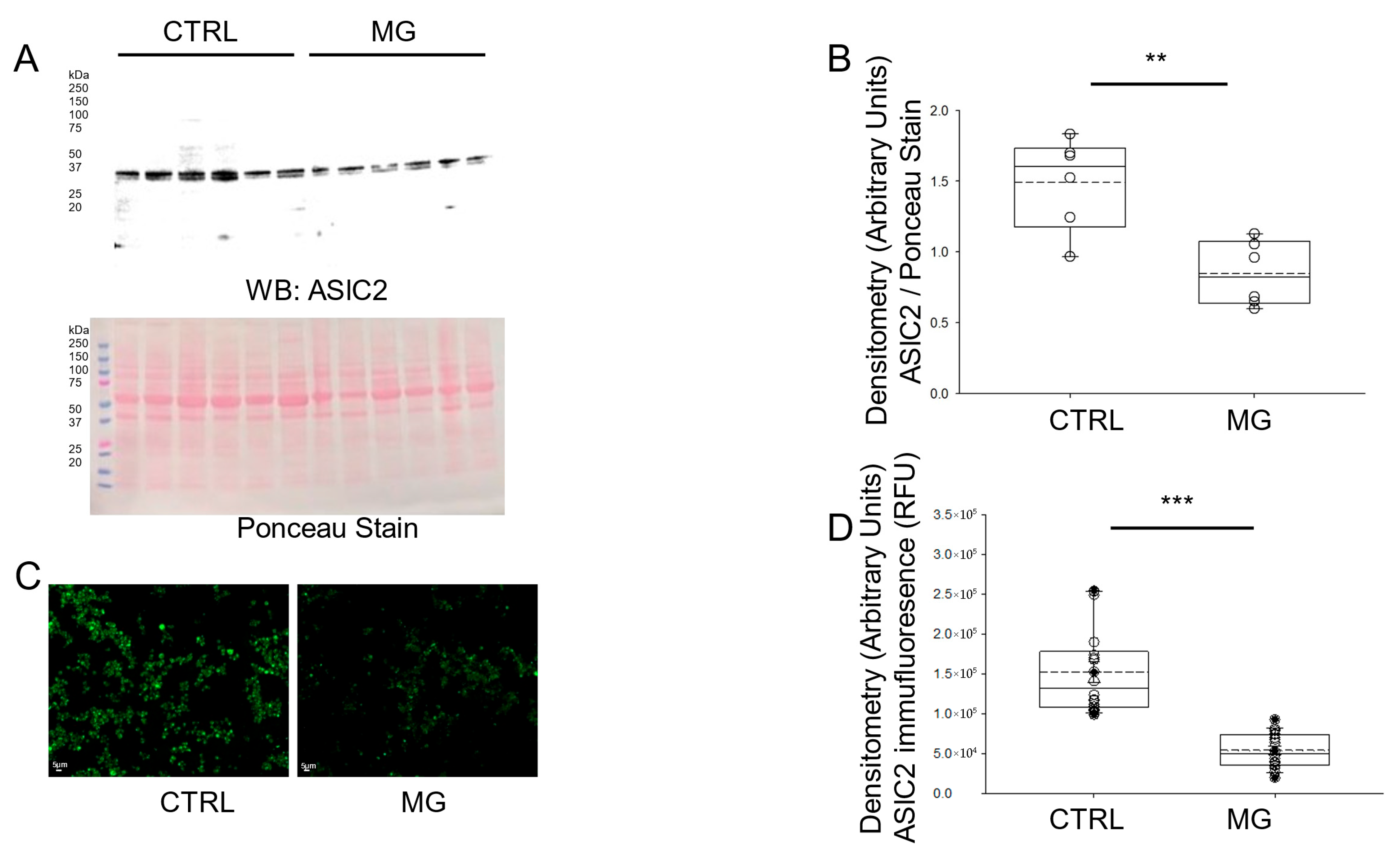
3.3. ASIC2 Protein Expression Is Decreased in Human Platelets Subject to Simulated Microgravity Conditions
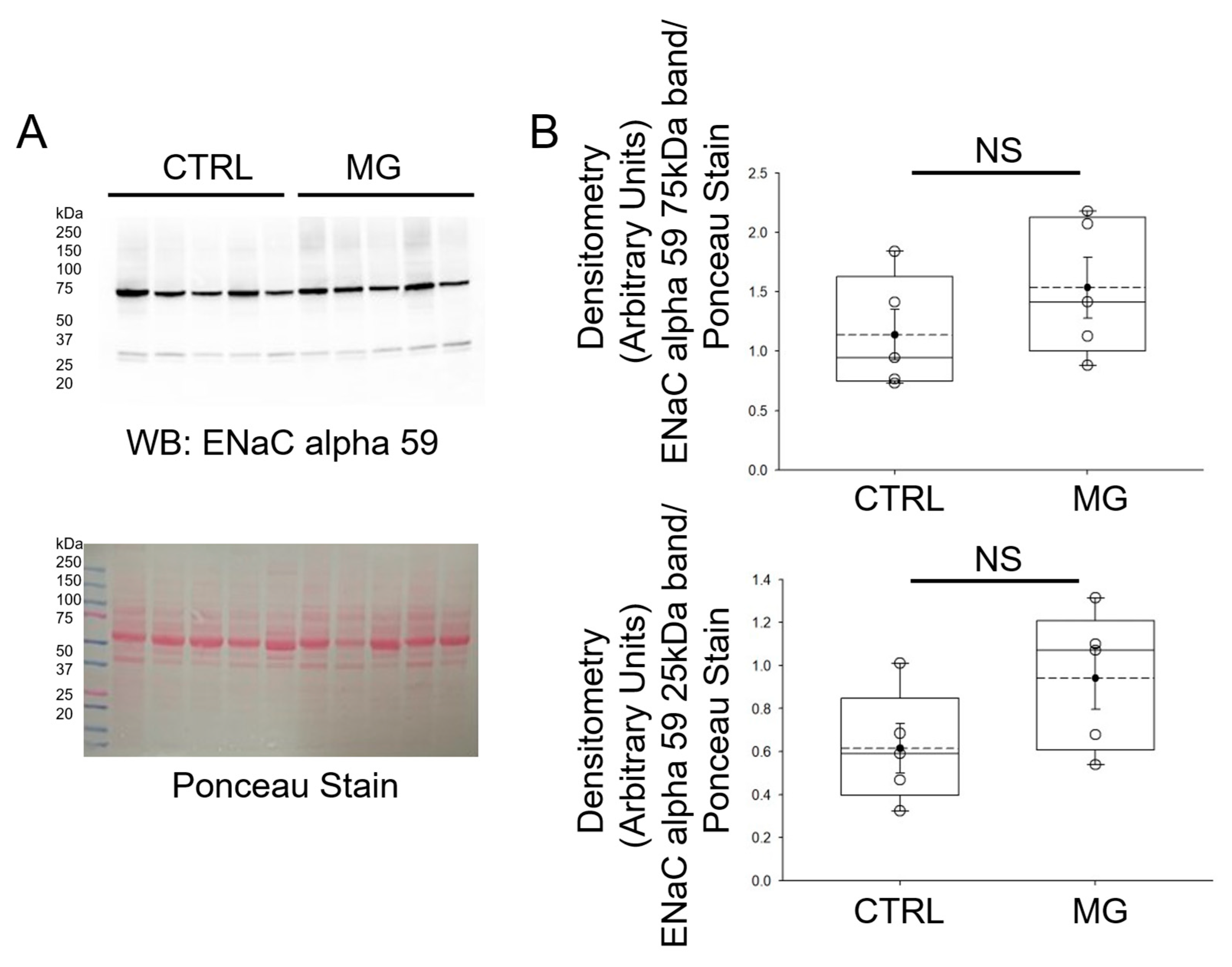
3.4. ENaC Alpha Subunit Protein Expression Is Comparable in Human Platelets Subject to Simulated Microgravity and Normal Gravity Conditions
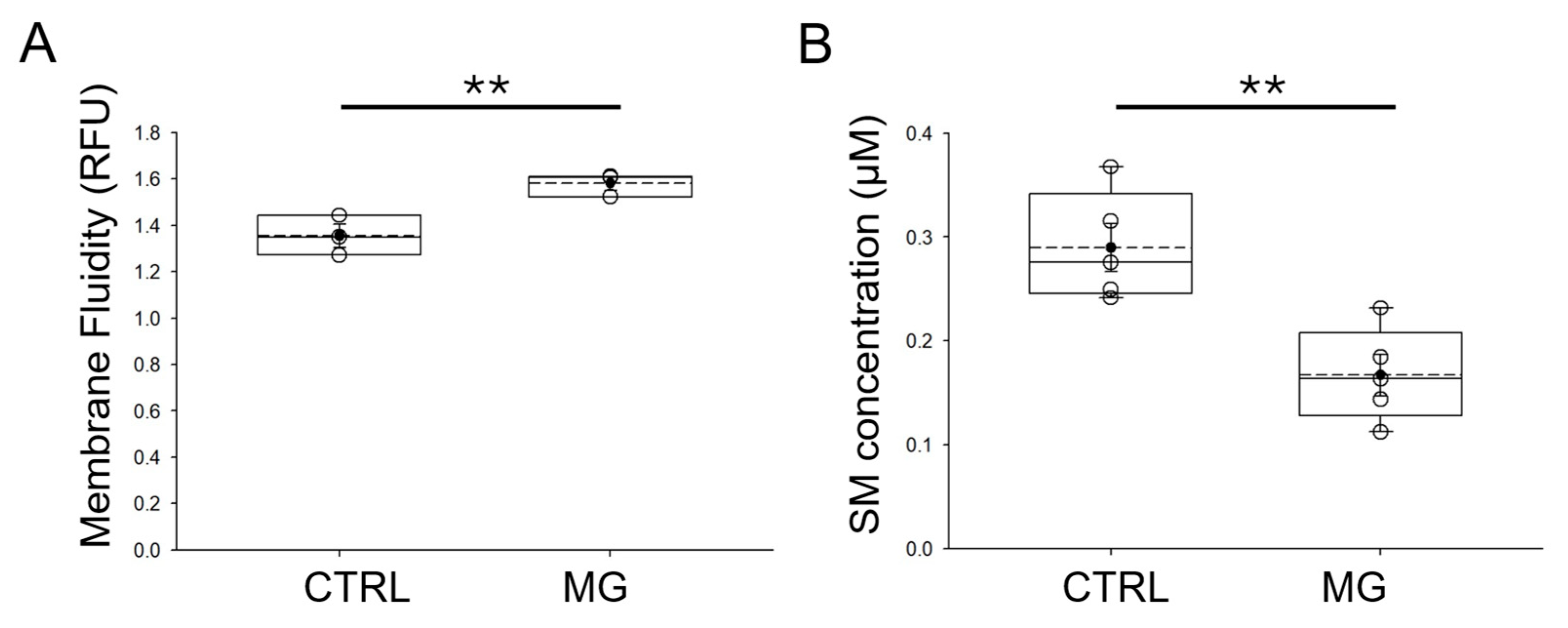
3.5. Membrane Fluidity Is Increased in Human Platelets Subject to Simulated Microgravity Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASIC | Acid-sensing ion channels |
| BCA | bicinchoninic acid |
| CI | Clotting index |
| CTRL | control |
| ENaC | Epithelial sodium channel |
| MG | microgravity |
| PRP | Platelet-rich plasma |
| SDHB | succinate dehydrogenase subunit B |
| SM | sphingomyelin |
| TEG | Thromboelastograph |
| TRP | Transient receptor potential |
References
- Lopez Garzon, N.A.; Pinzon-Fernandez, M.V.; Saavedra, T.J.; Nati-Castillo, H.A.; Arias-Intriago, M.; Salazar-Santoliva, C.; Izquierdo-Condoy, J.S. Microgravity and Cellular Biology: Insights into Cellular Responses and Implications for Human Health. Int. J. Mol. Sci. 2025, 26, 3058. [Google Scholar] [CrossRef]
- Etulain, J. Platelets in wound healing and regenerative medicine. Platelets 2018, 29, 556–568. [Google Scholar] [CrossRef]
- Brill, A.; Dashevsky, O.; Rivo, J.; Gozal, Y.; Varon, D. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc. Res. 2005, 67, 30–38. [Google Scholar] [CrossRef]
- Kisucka, J.; Butterfield, C.E.; Duda, D.G.; Eichenberger, S.C.; Saffaripour, S.; Ware, J.; Ruggeri, Z.M.; Jain, R.K.; Folkman, J.; Wagner, D.D. Platelets and platelet adhesion support angiogenesis while preventing excessive hemorrhage. Proc. Natl. Acad. Sci. USA 2006, 103, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, L.H.; Duchez, A.C.; Cloutier, N.; Soulet, D.; Martin, N.; Bollinger, J.; Pare, A.; Rousseau, M.; Naika, G.S.; Levesque, T.; et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood 2014, 124, 2173–2183, Erratum in Blood 2015, 125, 890. https://doi.org/10.1182/blood-2014-12-615419. [Google Scholar] [CrossRef] [PubMed]
- Le, V.B.; Schneider, J.G.; Boergeling, Y.; Berri, F.; Ducatez, M.; Guerin, J.L.; Adrian, I.; Errazuriz-Cerda, E.; Frasquilho, S.; Antunes, L.; et al. Platelet activation and aggregation promote lung inflammation and influenza virus pathogenesis. Am. J. Respir. Crit. Care Med. 2015, 191, 804–819. [Google Scholar] [CrossRef] [PubMed]
- Sierko, E.; Wojtukiewicz, M.Z. Inhibition of platelet function: Does it offer a chance of better cancer progression control? Semin. Thromb. Hemost. 2007, 33, 712–721. [Google Scholar] [CrossRef]
- Zmigrodzka, M.; Witkowska-Pilaszewicz, O.; Winnicka, A. Platelets Extracellular Vesicles as Regulators of Cancer Progression-An Updated Perspective. Int. J. Mol. Sci. 2020, 21, 5195. [Google Scholar] [CrossRef]
- Brownlow, S.L.; Sage, S.O. Transient receptor potential protein subunit assembly and membrane distribution in human platelets. Thromb. Haemost. 2005, 94, 839–845. [Google Scholar] [CrossRef]
- Liu, D.; Maier, A.; Scholze, A.; Rauch, U.; Boltzen, U.; Zhao, Z.; Zhu, Z.; Tepel, M. High glucose enhances transient receptor potential channel canonical type 6-dependent calcium influx in human platelets via phosphatidylinositol 3-kinase-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 746–751. [Google Scholar] [CrossRef]
- Schmidt, E.M.; Munzer, P.; Borst, O.; Kraemer, B.F.; Schmid, E.; Urban, B.; Lindemann, S.; Ruth, P.; Gawaz, M.; Lang, F. Ion channels in the regulation of platelet migration. Biochem. Biophys. Res. Commun. 2011, 415, 54–60. [Google Scholar] [CrossRef]
- Taylor, K.A.; Wright, J.R.; Vial, C.; Evans, R.J.; Mahaut-Smith, M.P. Amplification of human platelet activation by surface pannexin-1 channels. J. Thromb. Haemost. 2014, 12, 987–998. [Google Scholar] [CrossRef]
- Cerecedo, D.; Martinez-Vieyra, I.; Alonso-Rangel, L.; Benitez-Cardoza, C.; Ortega, A. Epithelial sodium channel modulates platelet collagen activation. Eur. J. Cell Biol. 2014, 93, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Cerecedo, D.; Martinez-Vieyra, I.; Sosa-Peinado, A.; Cornejo-Garrido, J.; Ordaz-Pichardo, C.; Benitez-Cardoza, C. Alterations in plasma membrane promote overexpression and increase of sodium influx through epithelial sodium channel in hypertensive platelets. Biochim. Biophys. Acta 2016, 1858, 1891–1903. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Y.; Zhou, H.; Cai, M.; Liu, J.; Gao, S.; Yang, J.; Tong, L.; Wang, J.; Zhou, S.; et al. Simulated microgravity reduces intracellular-free calcium concentration by inhibiting calcium channels in primary mouse osteoblasts. J. Cell Biochem. 2019, 120, 4009–4020. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, L.; Bai, Y.G.; Song, J.B.; Cheng, J.H.; Ma, H.Z.; Ma, J.; Xie, M.J. miR-137 and its target T-type CaV3.1 channel modulate dedifferentiation and proliferation of cerebrovascular smooth muscle cells in simulated microgravity rats by regulating calcineurin/NFAT pathway. Cell Prolif. 2020, 53, e12774. [Google Scholar] [CrossRef]
- Fu, Z.J.; Xie, M.J.; Zhang, L.F.; Cheng, H.W.; Ma, J. Differential activation of potassium channels in cerebral and hindquarter arteries of rats during simulated microgravity. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H1505–H1515. [Google Scholar] [CrossRef]
- Franco-Obregon, A.; Cambria, E.; Greutert, H.; Wernas, T.; Hitzl, W.; Egli, M.; Sekiguchi, M.; Boos, N.; Hausmann, O.; Ferguson, S.J.; et al. TRPC6 in simulated microgravity of intervertebral disc cells. Eur. Spine J. 2018, 27, 2621–2630. [Google Scholar] [CrossRef] [PubMed]
- Kamkin, A.G.; Mitrokhin, V.M.; Kamkina, O.V.; Kazansky, V.E.; Rodina, A.S.; Zolotareva, A.D.; Zolotarev, V.I.; Sutyagin, P.V.; Mladenov, M.I.; Shenkman, B.S.; et al. Simulated Microgravity Changes the Number of Mechanically Gated and Mechanosensitive Ion Channels Genes Transcripts in Rat Ventricular Cardiomyocytes. Dokl. Biochem. Biophys. 2023, 512, 251–255. [Google Scholar] [CrossRef]
- Hammond, T.G.; Hammond, J.M. Optimized suspension culture: The rotating-wall vessel. Am. J. Physiol.-Ren. Physiol. 2001, 281, F12–F25. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.M.; Yoshida, M.C.; Candelario, T.L.; Hughes-Fulford, M. Spaceflight and simulated microgravity cause a significant reduction of key gene expression in early T-cell activation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R480–R488. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Wuest, S.L.; Gantenbein, B.; Ille, F.; Egli, M. Electrophysiological experiments in microgravity: Lessons learned and future challenges. NPJ Microgravity 2018, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Jeggle, P.; Smith, E.S.; Stewart, A.P.; Haerteis, S.; Korbmacher, C.; Edwardson, J.M. Atomic force microscopy imaging reveals the formation of ASIC/ENaC cross-clade ion channels. Biochem. Biophys. Res. Commun. 2015, 464, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, N.; Lee, W.; Clark, E.; Bartoszewski, R.; McNicholas, C.M.; Latham, C.B.; Bebok, Z.; Parpura, V.; Fuller, C.M.; Palmer, C.A.; et al. Interaction of ASIC1 and ENaC subunits in human glioma cells and rat astrocytes. Am. J. Physiol. Cell Physiol. 2011, 300, C1246–C1259. [Google Scholar] [CrossRef]
- Klipp, R.C.; Bankston, J.R. Structural determinants of acid-sensing ion channel potentiation by single chain lipids. J. Gen. Physiol. 2022, 154, e202213156. [Google Scholar] [CrossRef]
- Ma, H.P.; Saxena, S.; Warnock, D.G. Anionic phospholipids regulate native and expressed epithelial sodium channel (ENaC). J. Biol. Chem. 2002, 277, 7641–7644. [Google Scholar] [CrossRef]
- Yue, G.; Malik, B.; Yue, G.; Eaton, D.C. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates epithelial sodium channel activity in A6 cells. J. Biol. Chem. 2002, 277, 11965–11969. [Google Scholar] [CrossRef]
- Wright, J.R.; Mahaut-Smith, M.P. Why do platelets express K+ channels? Platelets 2021, 32, 872–879. [Google Scholar] [CrossRef]
- Mahaut-Smith, M.P. Chloride channels in human platelets: Evidence for activation by internal calcium. J. Membr. Biol. 1990, 118, 69–75. [Google Scholar] [CrossRef]
- Clifford, E.E.; Parker, K.; Humphreys, B.D.; Kertesy, S.B.; Dubyak, G.R. The P2X1 receptor, an adenosine triphosphate-gated cation channel, is expressed in human platelets but not in human blood leukocytes. Blood 1998, 91, 3172–3181. [Google Scholar] [CrossRef]
- Grimm, D.; Wehland, M.; Corydon, T.J.; Richter, P.; Prasad, B.; Bauer, J.; Egli, M.; Kopp, S.; Lebert, M.; Kruger, M. The effects of microgravity on differentiation and cell growth in stem cells and cancer stem cells. Stem Cells Transl. Med. 2020, 9, 882–894. [Google Scholar] [CrossRef]
- Ahn, C.B.; Lee, J.H.; Han, D.G.; Kang, H.W.; Lee, S.H.; Lee, J.I.; Son, K.H.; Lee, J.W. Simulated microgravity with floating environment promotes migration of non-small cell lung cancers. Sci. Rep. 2019, 9, 14553. [Google Scholar] [CrossRef]
- Hicks, J.; Olson, M.; Mitchell, C.; Juran, C.M.; Paul, A.M. The Impact of Microgravity on Immunological States. Immunohorizons 2023, 7, 670–682. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cubano, L.A.; Lewis, M.L. Fas/APO-1 protein is increased in spaceflown lymphocytes (Jurkat). Exp. Gerontol. 2000, 35, 389–400. [Google Scholar] [CrossRef]
- Battista, N.; Meloni, M.A.; Bari, M.; Mastrangelo, N.; Galleri, G.; Rapino, C.; Dainese, E.; Agro, A.F.; Pippia, P.; Maccarrone, M. 5-Lipoxygenase-dependent apoptosis of human lymphocytes in the International Space Station: Data from the ROALD experiment. FASEB J. 2012, 26, 1791–1798. [Google Scholar] [CrossRef]
- Maccarrone, M.; Battista, N.; Meloni, M.; Bari, M.; Galleri, G.; Pippia, P.; Cogoli, A.; Finazzi-Agro, A. Creating conditions similar to those that occur during exposure of cells to microgravity induces apoptosis in human lymphocytes by 5-lipoxygenase-mediated mitochondrial uncoupling and cytochrome c release. J. Leukoc. Biol. 2003, 73, 472–481. [Google Scholar] [CrossRef]
- Kumari, R.; Singh, K.P.; Dumond, J.W., Jr. Simulated microgravity decreases DNA repair capacity and induces DNA damage in human lymphocytes. J. Cell Biochem. 2009, 107, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Najrana, T.; Sanchez-Esteban, J. Mechanotransduction as an Adaptation to Gravity. Front. Pediatr. 2016, 4, 140. [Google Scholar] [CrossRef] [PubMed]
- Kohn, F.P.M.; Ritzmann, R. Gravity and neuronal adaptation, in vitro and in vivo-from neuronal cells up to neuromuscular responses: A first model. Eur. Biophys. J. 2018, 47, 97–107. [Google Scholar] [CrossRef]
- Pedrera, L.; Fanani, M.L.; Ros, U.; Lanio, M.E.; Maggio, B.; Alvarez, C. Sticholysin I-membrane interaction: An interplay between the presence of sphingomyelin and membrane fluidity. Biochim. Biophys. Acta 2014, 1838, 1752–1759. [Google Scholar] [CrossRef]
- Srivastava, K.; Dash, D. Altered membrane fluidity and signal transduction in the platelets from patients of thrombotic stroke. Mol. Cell Biochem. 2001, 224, 143–149. [Google Scholar] [CrossRef]
- Schootemeijer, A.; Gorter, G.; Tertoolen, L.G.; De Laat, S.W.; Akkerman, J.W. Relation between membrane fluidity and signal transduction in the human megakaryoblastic cell line MEG-01. Biochim. Biophys. Acta 1995, 1236, 128–134. [Google Scholar] [CrossRef]
- Kohn, F.; Hauslage, J.; Hanke, W. Membrane Fluidity Changes, A Basic Mechanism of Interaction of Gravity with Cells? Microgravity Sci. Technol. 2017, 29, 337–342. [Google Scholar] [CrossRef]
- Kohn, F.P.M.; Hauslage, J. The gravity dependence of pharmacodynamics: The integration of lidocaine into membranes in microgravity. NPJ Microgravity 2019, 5, 5. [Google Scholar] [CrossRef]
- Ulbrich, C.; Wehland, M.; Pietsch, J.; Aleshcheva, G.; Wise, P.; van Loon, J.; Magnusson, N.; Infanger, M.; Grosse, J.; Eilles, C.; et al. The impact of simulated and real microgravity on bone cells and mesenchymal stem cells. BioMed Res. Int. 2014, 2014, 928507. [Google Scholar] [CrossRef]
- Mansour, J.; Berwanger, C.; Jung, M.; Eichinger, L.; Fabry, B.; Clemen, C.S. Clinorotation inhibits myotube formation by fluid motion, not by simulated microgravity. Eur. J. Cell Biol. 2023, 102, 151330. [Google Scholar] [CrossRef]
- Wuest, S.L.; Richard, S.; Kopp, S.; Grimm, D.; Egli, M. Simulated microgravity: Critical review on the use of random positioning machines for mammalian cell culture. BioMed Res. Int. 2015, 2015, 971474. [Google Scholar] [CrossRef] [PubMed]
- Herranz, R.; Anken, R.; Boonstra, J.; Braun, M.; Christianen, P.C.; de Geest, M.; Hauslage, J.; Hilbig, R.; Hill, R.J.; Lebert, M.; et al. Ground-based facilities for simulation of microgravity: Organism-specific recommendations for their use, and recommended terminology. Astrobiology 2013, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bala, N.; Yu, L.; Liu, L.P.; Shelton, L.; Xu, Y.; Ghayee, H.K.; Alli, A.A. Metabolic Characterization and Glyceraldehyde-3-Phosphate Dehydrogenase-Dependent Regulation of Epithelial Sodium Channels in hPheo1 Wild-type and SDHB Knockdown Cells. Endocrinology 2023, 164, bqad026. [Google Scholar] [CrossRef] [PubMed]
- Ware, A.W.; Harris, J.J.; Slatter, T.L.; Cunliffe, H.E.; McDonald, F.J. The epithelial sodium channel has a role in breast cancer cell proliferation. Breast Cancer Res. Treat. 2021, 187, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Nakamura, A.; Shimizu, T. Simulated microgravity accelerates aging of human skeletal muscle myoblasts at the single cell level. Biochem. Biophys. Res. Commun. 2021, 578, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, J.; Bai, S.; Wang, G.; Mu, L.; Sun, B.; Wang, D.; Kong, Q.; Liu, Y.; Yao, X.; et al. Simulated microgravity pro-motes cellular senescence via oxidant stress in rat PC12 cells. Neurochem. Int. 2009, 55, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, Z.; Rafay, R.H.; Bala, N.; Dogan, Y.E.; Alli, A.A. The Heart in Space: Effects of Microgravity on Different Cell Types and Their Functions in the Cardiovascular System. Biomedicines 2025, 13, 2336. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Primary Antibody | Company | Catalog Number |
|---|---|---|
| ASIC 1 | Invitrogen, Waltham, MA, USA | pa587945 |
| ASIC 2 | Stress Marq, Victoria, BC, Canada | s271-44 |
| ENaC alpha 59 | James A. Haley Veterans’ Hospital, Tampa, FL, USA | PMID: 22791334 |
| Parameter | Definition of Parameter |
|---|---|
| Clotting index (CI) | coagulation index for the specimen and is a function of the R time, K time and the MA. A hypercoagulable state is defined as CI greater than +3.0 and coagulopathy as CI less than −3.0. |
| Plot upslope angle | Overall speed of the clot formation |
| Maximum amplitude (MA) | measured in millimeters and is the greatest vertical amplitude of the split lines of the trace. It reflects clot strength and is a function both of platelet activity (about 80–85% of the MA) and fibrinogen activity (about 10–20% of the MA). |
| tMA | Time needed to reach maximum MA after start of the clot formation |
| G | Overall total clot strength or shear elastic modulus strength resulting from all coagulation interactions and is calculated from maximum amplitude (MA). Measured in dynes/cm2 |
| K time | time between the trace reaching 2 mm and going up to 20 mm. The “alpha angle” is a slope drawn from the slope of the tracing from the R line to the K value. Both “K” and “alpha” are measurements of the kinetics of clot formation and are dependent on the fibrinogen activity of the specimen and to a certain degree, platelet function. |
| R time | the latency time from placing blood in the cup until the clot starts to form (taken as reaching a tracing amplitude of 2 mm). It is determined by the factors of the intrinsic and common coagulation pathways. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bala, N.; Yu, L.; Harris, N.S.; Mukhtar, F.; Alli, A.A. Simulated Microgravity Causes Delayed Platelet Activation and Downregulates Acid-Sensing Ion Channel 1/2 Protein Expression. Biomedicines 2025, 13, 2860. https://doi.org/10.3390/biomedicines13122860
Bala N, Yu L, Harris NS, Mukhtar F, Alli AA. Simulated Microgravity Causes Delayed Platelet Activation and Downregulates Acid-Sensing Ion Channel 1/2 Protein Expression. Biomedicines. 2025; 13(12):2860. https://doi.org/10.3390/biomedicines13122860
Chicago/Turabian StyleBala, Niharika, Ling Yu, Neil S. Harris, Faisal Mukhtar, and Abdel A. Alli. 2025. "Simulated Microgravity Causes Delayed Platelet Activation and Downregulates Acid-Sensing Ion Channel 1/2 Protein Expression" Biomedicines 13, no. 12: 2860. https://doi.org/10.3390/biomedicines13122860
APA StyleBala, N., Yu, L., Harris, N. S., Mukhtar, F., & Alli, A. A. (2025). Simulated Microgravity Causes Delayed Platelet Activation and Downregulates Acid-Sensing Ion Channel 1/2 Protein Expression. Biomedicines, 13(12), 2860. https://doi.org/10.3390/biomedicines13122860







