Abstract
Agarwood from Aquilaria agallocha, known as chim-hyuang in Korea, is widely distributed throughout Southeast Asia and has traditionally been used to treat asthma, pain, and gastrointestinal disorders. As part of our ongoing efforts to identify bioactive metabolites from natural sources, a phytochemical investigation of the EtOAc fraction of A. agallocha extract led to the isolation and identification of four compounds, N-trans-feruloyltyramine (1), (3R,5R)-octahydrocurcumin (2), 1,7-bis(4-hydroxyphenyl)heptane (3), and trans-caffeoyltyramine (4), via HPLC purification and LC/MS-based analysis. Structural elucidation of the isolated compounds was achieved using NMR spectroscopy, LC/MS, and high-resolution electrospray ionization mass spectrometry (HR-ESIMS). The absolute configuration of compound 2 was further confirmed by optical rotation and electronic circular dichroism (ECD) analyses. All isolated compounds (1–4) were evaluated for their inhibitory activity against tau protein aggregation. Notably, compound 2 exhibited a 43.7% reduction in tau aggregation at 20 μM, without cytotoxicity at the same concentration. These findings indicate that phytochemicals from A. agallocha, particularly the diarylheptanoid compound 2, hold promise as natural lead candidates for the development of therapeutic agents targeting tau protein aggregation.
1. Introduction
The plant genus Aquilaria, belonging to the family Thymelaeaceae, comprises evergreen tropical trees renowned for producing aromatic resins and essential oils. These species are predominantly distributed across Southeast Asia, including Bangladesh, Indonesia, India, and Malaysia [1]. Among them, Aquilaria agallocha is particularly valued for its resinous heartwood, which yields a highly fragrant resin known as chen-xiang in China and chim-hyuang in Korea. This resin is formed as a defensive response to mechanical injury or biotic stress, such as fungal infection or insect attack [2]. For centuries, agarwood has been widely used in traditional medicine throughout Asia. In China, powdered agarwood has been prescribed for the treatment of asthma, pain, and gastrointestinal disorders, whereas in Tibetan medicine, it has been applied to manage psychological and emotional conditions [3,4].
In recent decades, pharmacological studies have confirmed that extracts of A. agallocha exhibit diverse biological activities, including cytotoxicity against lung cancer cells, hepatoprotective effects, anti-inflammatory activity via suppression of nitric oxide (NO) production, and antioxidant and antibacterial properties against Bacillus subtilis [5,6,7]. Phytochemical investigations have led to the identification of a wide range of secondary metabolites from the agarwood of A. agallocha, including sesquiterpenes, triterpenes, 2-(2-phenylethyl)chromone derivatives—the major chemical constituents of agarwood—and various phenolic compounds [8]. Several of these abundant metabolites have demonstrated notable bioactivities in experimental models, such as anti-inflammatory effects mediated by NO inhibition and the upregulation of brain-derived neurotrophic factor (BDNF) expression [9,10].
Neurodegenerative disorders such as Alzheimer’s disease and related tauopathies are characterized by abnormal protein aggregation, with tau aggregation recognized as a central pathogenic event [11]. Although synthetic inhibitors of tau aggregation have been explored, their development has been hampered by limitations related to safety, bioavailability, and therapeutic efficacy, thereby increasing interest in natural products as alternative sources of structurally diverse and biologically safe compounds [12,13]. Given that phytochemicals from A. agallocha have been reported to modulate neuronal pathways, including enhancement of BDNF signaling [9,10] and activation of anti-inflammatory and antioxidant pathways [14,15], this species represents a promising reservoir for the discovery of novel agents targeting tau pathology. Moreover, diarylheptanoids—including octahydrocurcumin analogs—have been reported to modulate oxidative stress [16,17] and endoplasmic reticulum (ER) stress-related pathways in neuronal cells [18], both of which are closely associated with tau oligomerization. These mechanistic properties further support their potential relevance in tau-targeted research.
Despite extensive research on the chemical constituents of A. agallocha, most studies to date have focused on well-known and abundant components, particularly sesquiterpenes and 2-(2-phenylethyl)chromone derivatives. In contrast, minor or less-characterized metabolites—such as diarylheptanoids and phenylpropanoid amides—remain largely unexplored, and their potential neuroprotective or tau modulatory effects have not been investigated. This knowledge gap highlights an opportunity to identify new bioactive constituents with therapeutic relevance.
As part of our ongoing efforts to discover new bioactive natural compounds [19,20,21,22,23,24], we conducted a phytochemical investigation of the EtOAc fraction of A. agallocha, leading to the isolation of four compounds: N-trans-feruloyltyramine (1), (3R,5R)-octahydrocurcumin (2), 1,7-bis(4-hydroxyphenyl)heptane (3), and trans-caffeoyltyramine (4), through successive purification using HPLC. The structures of compounds 1–4 were elucidated by NMR spectroscopy and high-resolution electrospray ionization mass spectrometry (HR-ESIMS), and the absolute configuration of compound 2 was confirmed by specific optical rotation and electronic circular dichroism (ECD) calculations. In this study, we further evaluated these underexplored constituents as potential therapeutic candidates for neurodegenerative disease, with particular emphasis on the tau aggregation inhibitory activity of the diarylheptanoid (3R,5R)-octahydrocurcumin.
2. Materials and Methods
2.1. General Experimental Procedures
The equipment and devices used in the analyzes and experiments are listed in Table 1.

Table 1.
Equipment used for analyses.
2.2. Plant Material
The resinous wood of A. agallocha was collected in Indonesia in June 2022. The plant material was authenticated by Prof. K. H. Kim, one of the authors of this study. A voucher specimen (CH-2022) has been deposited in the herbarium of the School of Pharmacy, Sungkyunkwan University, Suwon, Republic of Korea (Figure 1).

Figure 1.
Photographs of A. agallocha agarwood.
2.3. Extraction and Isolation
The powdered resinous wood of A. agallocha (70.0 g) was extracted with 700 mL of 80% methanol at room temperature for 12 h. This procedure was repeated three times. The combined extracts were filtered and concentrated under reduced pressure to yield 24.7 g of crude extract. The crude extract was suspended in 700 mL of distilled water and successively partitioned three times with n-hexane, dichloromethane (CH2Cl2), ethyl acetate (EtOAc), and n-butanol (n-BuOH), affording 144.0 mg, 1.2 g, 12.5 g, and 10.1 g of the respective fractions. The EtOAc fraction (12.5 g) was subjected to normal-phase silica gel open-column chromatography and eluted using a gradient of CH2Cl2/MeOH (50:1 → 30:1 → 10:1 → 1:1 → 100% MeOH), yielding four fractions (CE1–CE4). Fraction CE2 (758 mg) was further separated by preparative HPLC on a Hector C18 column (250 × 21.2 mm, 5 μm; flow rate: 5 mL/min) with a gradient of 73–100% aqueous MeOH, giving six subfractions (CE21–CE26). Subfraction CE21 (119 mg) was purified by preparative HPLC on a Hector C18 column under the same gradient conditions to afford five subfractions (CE211–CE215). Subfraction CE212 (80 mg) was purified by semi-preparative HPLC on a Phenomenex Luna C18 column (250 × 10 mm, 10 μm; flow rate: 2 mL/min) using isocratic 50% MeOH to yield compounds 1 (6.9 mg, tR 24 min) and 2 (16.9 mg, tR 33 min). Subfraction CE25 (147 mg) was chromatographed on a Sephadex LH-20 column eluted with 80% MeOH to provide seven subfractions (CE251–CE257). Subfraction CE256 (20 mg) was purified by semi-preparative HPLC on a Phenomenex Luna C18 column using 75% aqueous MeOH to yield compound 3 (4.2 mg, tR 41 min). Fraction CE3 (350 mg) was subjected to preparative HPLC on a Hector C18 column using a gradient of 55–80% aqueous MeOH to obtain four subfractions (CE31–CE34). Subfraction CE31 (56 mg) was further purified by semi-preparative HPLC on a Phenomenex Luna C18 column with a gradient of 35–60% aqueous MeOH to afford compound 4 (4.0 mg, tR 30 min).
2.4. UHPLC–QTOF–MS/MS Analysis and GNPS Molecular Networking
Four solvent-partitioned fractions of the A. agallocha extract were subjected to MS/MS analysis using an Agilent 1290 Infinity II UHPLC system coupled to a G6545B quadrupole time-of-flight (Q-TOF) mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) equipped with an electrospray ionization (ESI) source. Each sample was dissolved in HPLC-grade methanol (1 mg/mL), filtered, and 200 μL of each solution was transferred to an HPLC autosampler vial. Chromatographic separation was carried out on an Acquity® UPLC BEH C18 column (150 mm × 2.1 mm, 1.7 μm). The gradient system was optimized according to the polarity of each fraction. For the n-hexane and dichloromethane fractions, the mobile phase gradient was programmed as follows: 50–100% MeCN (0–14 min), 100% MeCN (14–17 min), 100–50% MeCN (17–17.1 min), and 50% MeCN (17.1–20 min). For the EtOAc and n-BuOH fractions, the following gradient was applied: 10–100% MeCN (0–14 min), 100% MeCN (14–17 min), 100–10% MeCN (17–17.1 min), and 10% MeCN (17.1–20 min). The flow rate was 0.3 mL/min, and the injection volume was 5 μL. MS/MS data were acquired in positive ion mode under the following ESI conditions: sheath gas temperature, 350 °C; sheath gas flow, 11 L/min; drying gas temperature, 320 °C; drying gas flow, 8 L/min; nebulizer pressure, 35 psig; nozzle voltage, 1000 V; fragmentor voltage, 175 V; skimmer voltage, 65 V; octopole RF, 750 V. The mass range was set to m/z 50–1200, with fixed collision energies of 10, 20, and 40 V, a maximum of five precursors per cycle, and the isotope model set to “common organic molecules.” Raw data files (.d format) were converted to .mzML using MSConvert (version 3.0), and the resulting .mzML files were uploaded to the GNPS platform via WinSCP for molecular networking analysis. Molecular networking was performed following the GNPS workflow described previously [25], and the resulting networks were visualized and analyzed using Cytoscape software (version 3.10.3).
2.5. 3D Visualization of LC/MS Data
LC/MS analyses were performed on an Agilent 1200 Series HPLC system equipped with a diode array detector and a 6130 Series electrospray ionization (ESI) mass spectrometer. Chromatographic separation was carried out on a Kinetex C18 column (100 × 2.1 mm, 5 μm, 100 Å; Phenomenex, Torrance, CA, USA) at a flow rate of 0.3 mL/min. The mobile phase gradient was programmed from 10% MeOH–H2O to 100% MeOH over 52 min as follows: 10–100% MeOH (0–30 min), 100% MeOH (30–41 min), 100–10% MeOH–H2O (41–42 min), and 10% MeOH–H2O (42–52 min). LC/MS data were processed using MZmine 4.2.0, and three-dimensional chromatographic visualizations were generated. The data processing parameters were as follows: mass range, m/z 100–1000; retention time resolution, 500; m/z resolution, 500; MS level, 1; ionization mode, positive; and spectrum type, centroid.
2.6. Computational Analysis of Electronic Circular Dichroism (ECD) Spectra
Electronic circular dichroism (ECD) calculations for conformers 2a and 2b were performed using time-dependent density functional theory (TD-DFT) at the B3LYP/6-31+G(d,p) level of theory, following previously described literature [26]. The theoretical ECD spectra were generated by summing the individual electronic transitions, each represented by a Gaussian function with a bandwidth (σ) of 0.20 eV at 1/e of its maximum intensity [26]. The excitation energies (ΔEi) and rotatory strengths (Ri) obtained from the TD-DFT calculations were Boltzmann-weighted according to the relative populations of the conformers to produce the final computed spectra. The resulting ECD curves were visualized and plotted using SigmaPlot 14.0 [27].
2.7. Tau-BiFC Cell Culture
HEK293 tau–BiFC cells were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, 100 μg/mL streptomycin, and 100 μg/mL Geneticin (G418). Cells were cultured at 37 °C in a humidified incubator containing 5% CO2.
2.8. Compound Treatment and Image Analysis
HEK293 tau–BiFC cells were seeded into μ-clear 384-well plates. The following day, cells were treated with thapsigargin (TG, 0.05 μM) to induce tau oligomerization, followed by treatment with compounds 1–4 at final concentrations of 1.25, 5, and 20 μM. After 48 h, cell nuclei were counterstained with Hoechst (0.3 μg/mL).
Fluorescence images of BiFC (λex = 460–490 nm; λem = 500–550 nm) and Hoechst (λex = 355–385 nm; λem = 430–500 nm) were automatically acquired using an Operetta® CLS high-content imaging system (PerkinElmer, Shelton, CT, USA) equipped with a built-in sCMOS camera and an 8-channel LED light source. All images were collected using the same 20× water immersion objective (30% LED power, 20 ms exposure time) to ensure identical magnification and image settings across all samples. BiFC fluorescence intensity and total nuclei counts were quantified using Harmony 4.9 software (PerkinElmer). Integrated tau–BiFC intensity was calculated as the product of cell area, mean fluorescence intensity, and number of cells. Data are presented as mean ± standard deviation (SD), and graphs were generated using Prism 10 (GraphPad).
3. Results and Discussion
3.1. LC-MS/MS-Based Molecular Networking Analysis
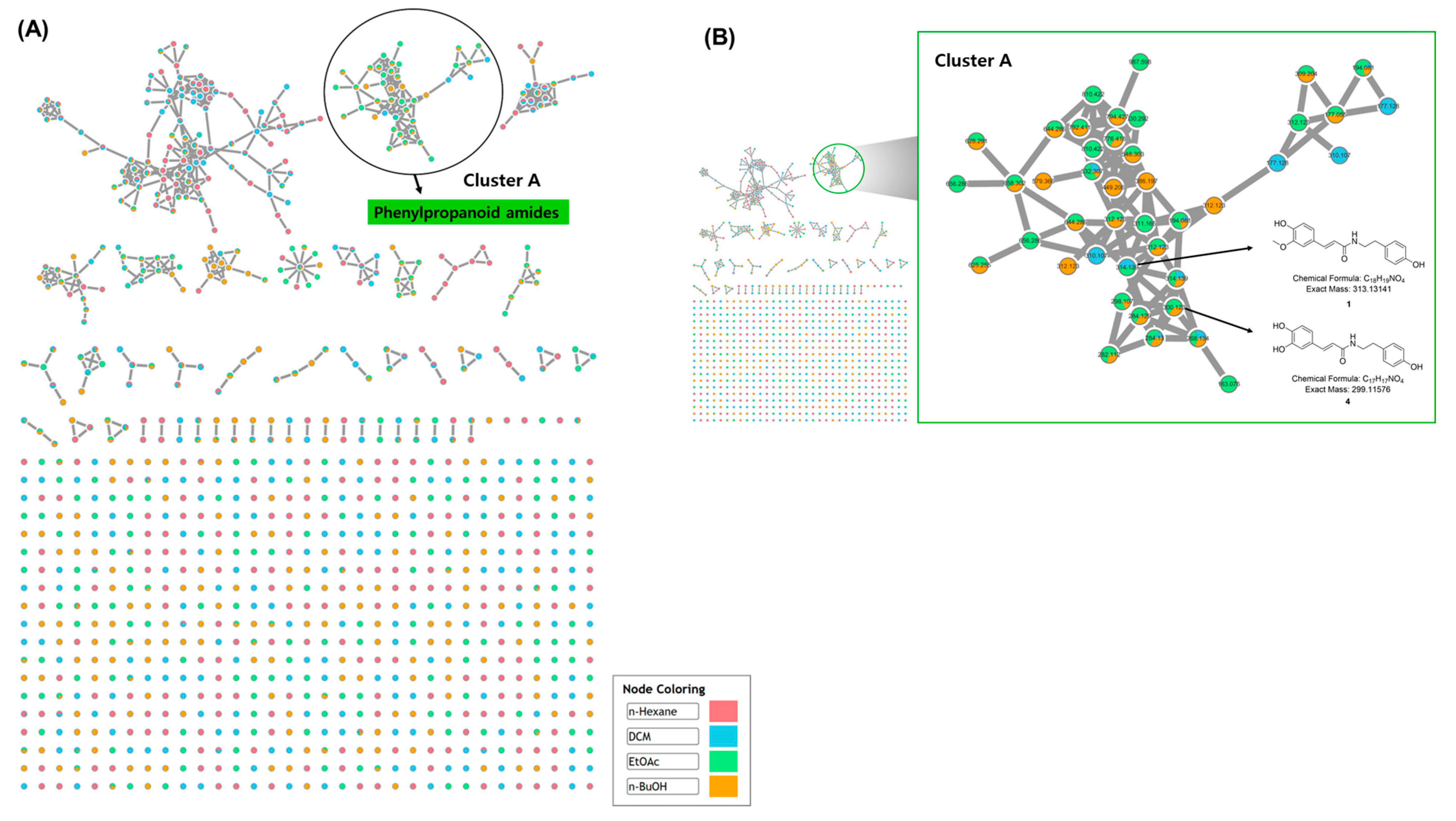
The powdered resinous wood of A. agallocha was extracted with 80% methanol and subsequently partitioned into four fractions using n-hexane, dichloromethane (DCM), EtOAc, and n-BuOH. To investigate the metabolite profile of the A. agallocha extract, four solvent-partitioned fractions were analyzed by ultra-high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (UHPLC–QTOF–MS), and MS/MS spectra were acquired for each fraction. The obtained datasets were subsequently analyzed using the GNPS web platform (https://gnps.ucsd.edu, accessed on 1–5 October 2025). As a result, molecular networking generated a total of 46 clusters, which were automatically grouped based on structural similarities and chemical classes (Figure 2A). Among these, phenylpropanoid amide-type metabolites were identified in Cluster A through GNPS spectral library matching. Within this cluster, the precursor ion at m/z 314.139 [M + H]+ was putatively annotated as feruloyltyramine, and several structurally related nodes within the m/z 260–320 range were also detected (Figure 2B). GNPS analysis further revealed that phenylpropanoid amide derivatives were predominantly present in the EtOAc fraction, with corresponding nodes clearly represented in Cluster A. Several studies have reported that phenylpropanoid amides exhibit diverse pharmacological activities, including anti-inflammatory, antioxidant, antimicrobial, and anticancer effects [28]. In particular, clovamide isolated from Theobroma cacao L. has shown strong anti-inflammatory activity by suppressing the production of superoxide anions and reducing the synthesis of TNF-α and IL-6 in human monocytes [29]. Another group of phenylpropanoid amides, the avenanthramides derived from Avena sativa (oat), demonstrate potent anti-inflammatory and antioxidant effects through free radical scavenging activity and inhibition of NF-κB signaling [30]. Although phenylpropanoid amides are valuable natural compounds with diverse biological activities, studies on their occurrence and bioactivities in A. agallocha remain limited. Based on these findings, the EtOAc fraction was selected for subsequent isolation of secondary metabolites, particularly those that have been rarely characterized in A. agallocha.

Figure 2.
(A) GNPS molecular networking analysis of MS/MS data obtained from four solvent-partitioned fractions of the A. agallocha extract in positive ion mode. Each node in the network is represented as a pie chart, with pink, blue, green, and orange sectors indicating the relative MS intensities of features detected in the four respective fractions. (B) Enlarged view of Cluster A, highlighting nodes annotated as phenylpropanoid amide–type metabolites.
3.2. Isolation and Structural Elucidation of Compounds 1–4 from the EtOAc Fraction
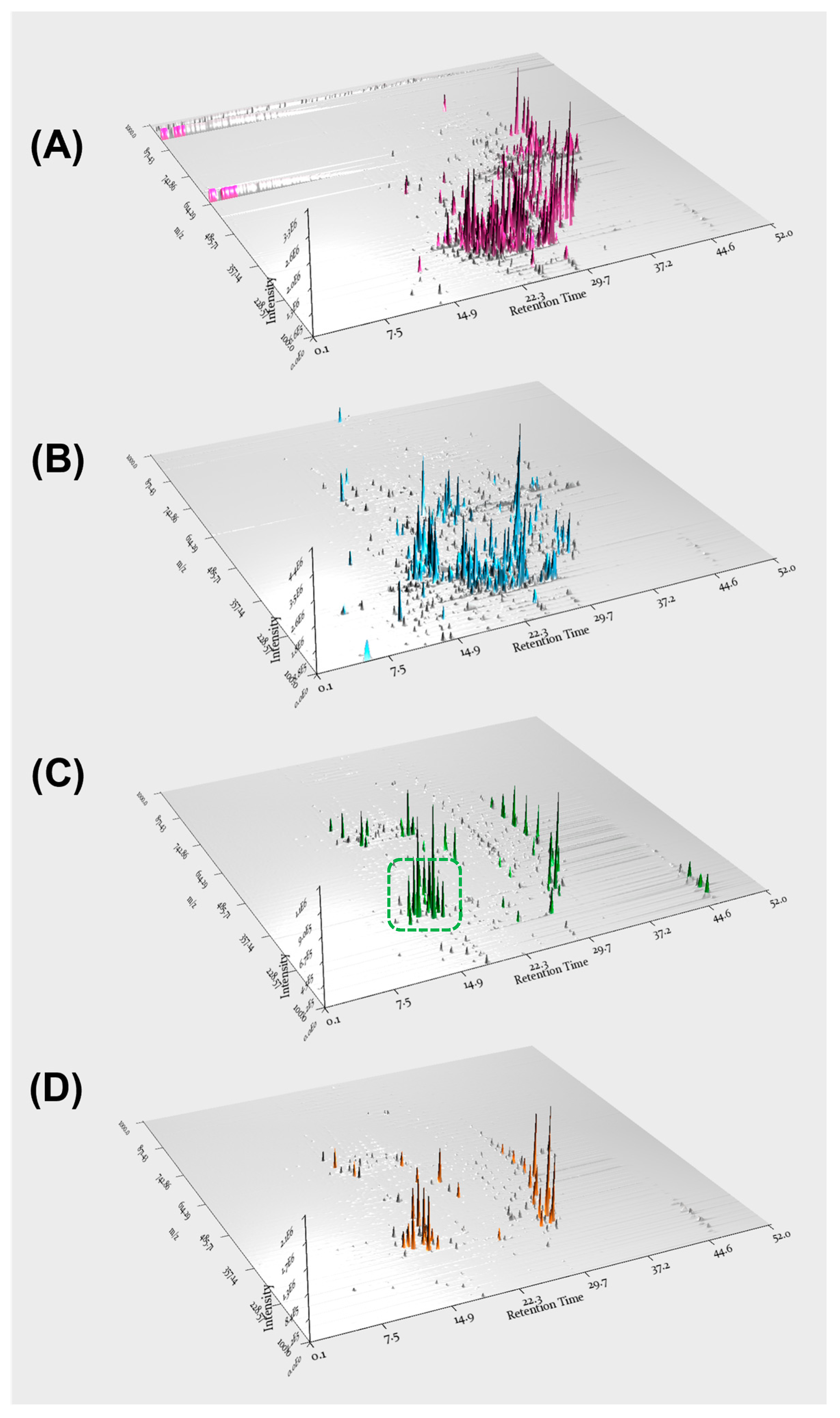
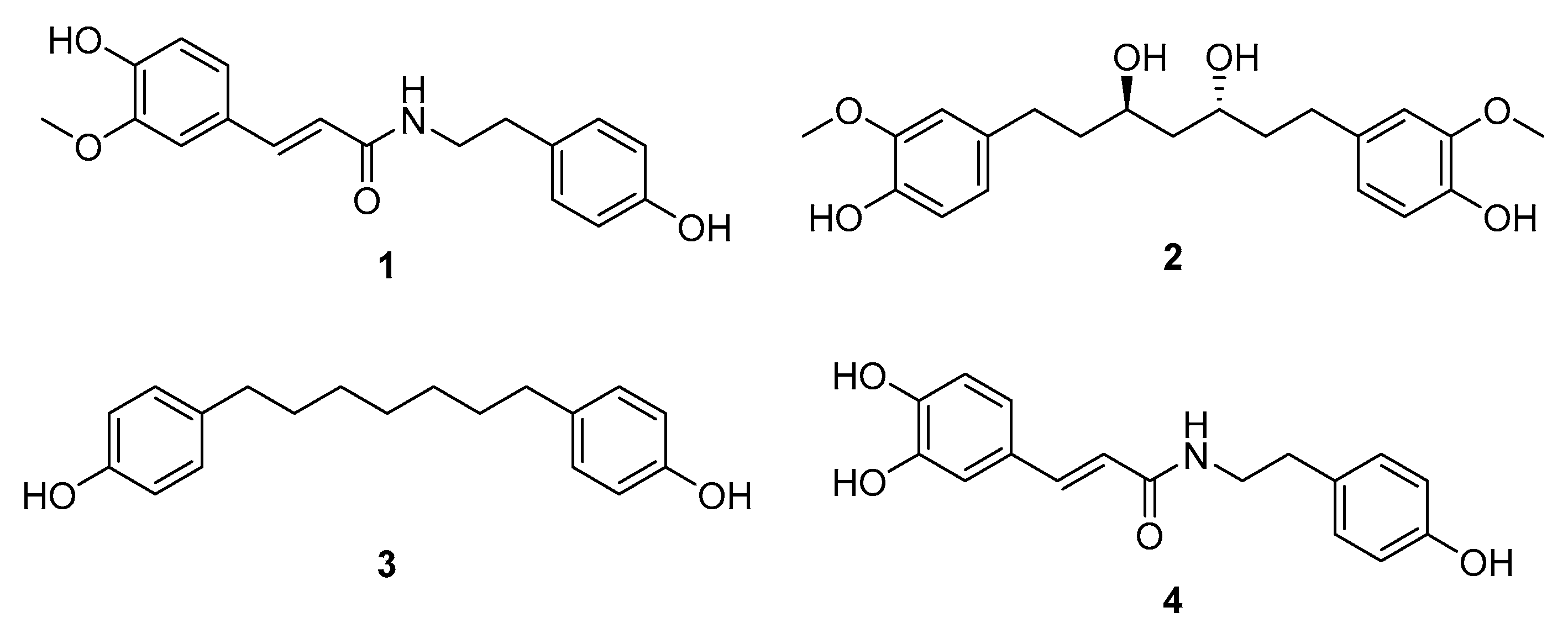
Based on molecular networking analysis as well as LC/MS analysis, the EtOAc fraction was found to contain key constituents of A. agallocha, including phenylpropanoid amides and diarylheptanoids. Furthermore, LC/MS data from the four fractions were processed using MZmine 4.2.0 in positive ion mode, and 3D plot visualizations were generated (Figure 3). These plots demonstrated the presence of diverse secondary metabolites in each fraction and confirmed that the EtOAc fraction was enriched in phenylpropanoid amide–type metabolites, characterized by a distinct m/z range of 280–320. In addition, features corresponding to diarylheptanoids were observed as major constituents of this fraction, appearing predominantly within the m/z 290–400 range. The EtOAc fraction was therefore subjected to repeated chromatographic separation, including open-column chromatography, preparative HPLC, and semi-preparative HPLC, guided by LC/MS profiling to isolate phenylpropanoid amides and diarylheptanoids. As a result, four compounds were successfully isolated; two phenylpropanoid amides (1 and 4) and two diarylheptanoids (2 and 3). Based on comparison of their NMR spectroscopic data with previously reported values, the structures of the isolated compounds were identified as N-trans-feruloyltyramine (1) [31], (3R,5R)-octahydrocurcumin (2) [32], 1,7-bis(4-hydroxyphenyl)heptane (3) [33], and trans-caffeoyltyramine (4) [34] (Figure 4).

Figure 3.
3D plot visualizations of the soluble fractions obtained from the A. agallocha extract: (A) n-hexane, (B) CH2Cl2, (C) EtOAc, and (D) n-BuOH. The green dotted box in panel (C) highlights the region corresponding to phenylpropanoid amides, showing a characteristic m/z range of approximately 290–400.

Figure 4.
Chemical structures of compounds 1–4.
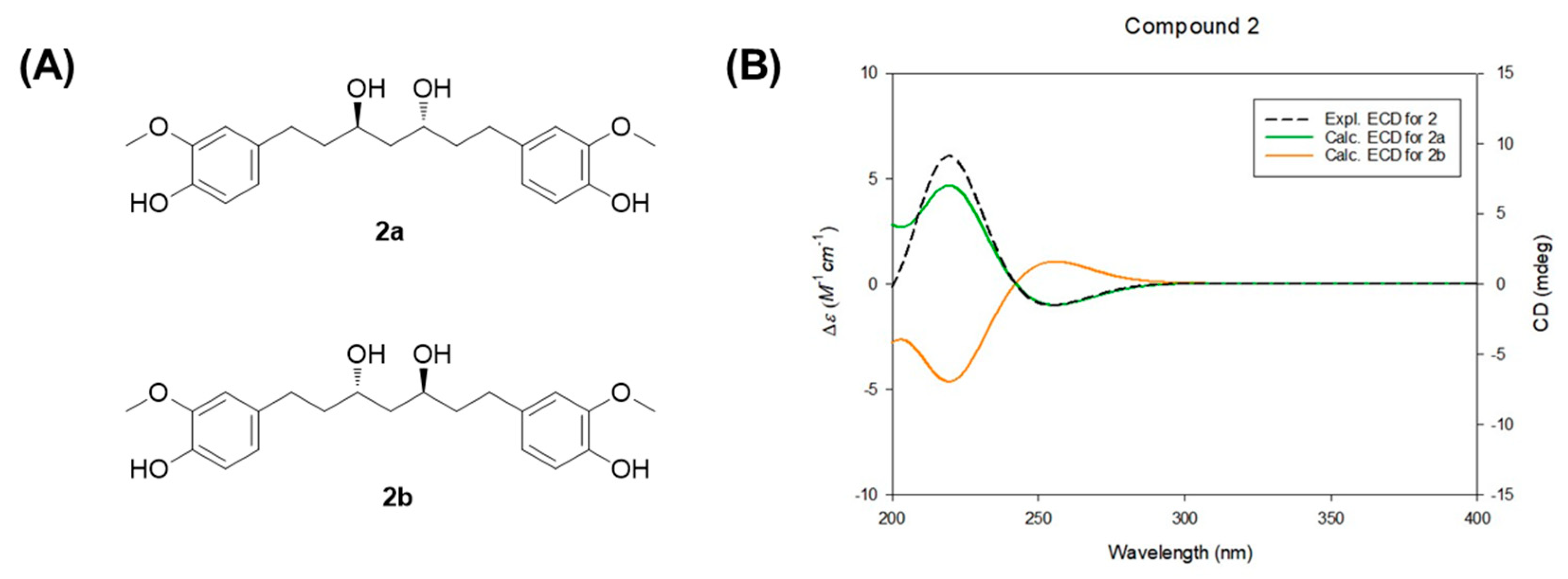
The determination of absolute configuration in diarylheptanoids with some hydroxy groups is often challenging. In this work, the absolute configuration of compound 2 was confirmed through comparison of its NMR data and optical rotation with previously reported values. In earlier 1H NMR data for meso-type octahydrocurcumin, the methylene protons at C-4 appeared as distinct triplets at δH 1.52 (1H, t, J = 14.0 Hz) and 1.62 (1H, t, J = 14.0 Hz) [35]. However, in (3R,5R)-octahydrocurcumin and (3S,5S)-octahydrocurcumin, these protons were observed as triplets at δH 1.54 (2H, t-like) and 1.58 (2H, t), respectively [32,35]. In the spectrum of compound 2, the C-4 methylene protons were also detected at δH 1.55 (2H, m), indicating that compound 2 possesses a configuration different from that of meso-octahydrocurcumin. In addition to the 1H NMR evidence, the specific rotation value of compound 2, +8.4 (c 0.08, MeOH), further supported its assignment as (3R,5R)-octahydrocurcumin. This is consistent with previous findings that the (3S,5S)-isomer exhibits an opposite sign of optical rotation [35]. To more rigorously establish the absolute configuration, electronic circular dichroism (ECD) calculations were performed for the two enantiomers (2a and 2b). The experimental ECD spectrum of compound 2 showed strong agreement with the calculated spectrum of isomer 2a (3R,5R), corroborating its absolute configuration and aligning with previously reported data [36] (Figure 5).

Figure 5.
(A) Two enantiomers of compound 2 for ECD calculations. (B) Experimental and calculated ECD spectra of compound 2.
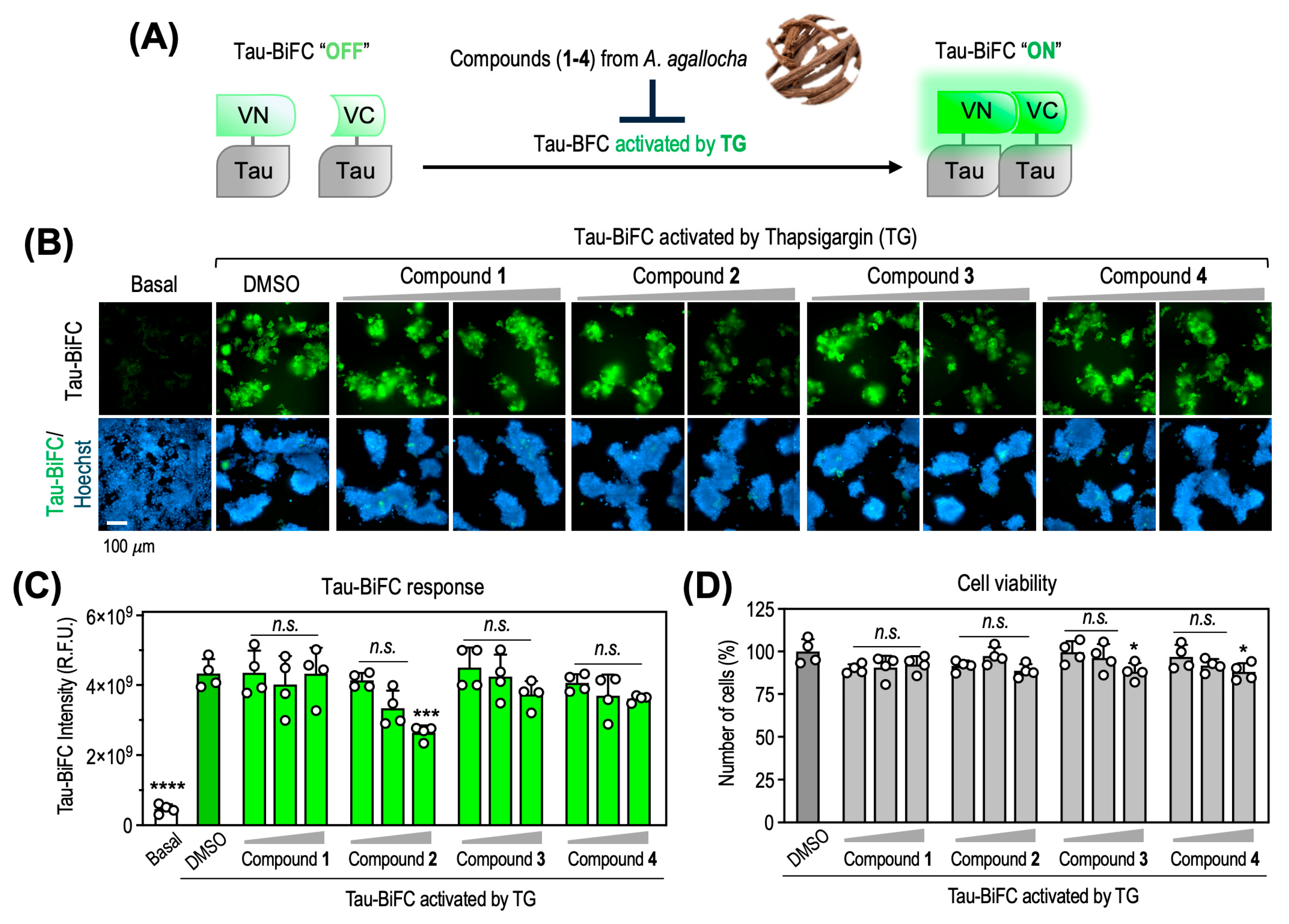
3.3. Inhibition of TG-Induced Tau Oligomerization by Compound 2 in Tau-BiFC Cells
To evaluate the biological effects of the isolated compounds on tau pathology, we employed a tau–BiFC cellular model [37]. This system enables real-time monitoring of tau oligomerization based on reconstitution of a split fluorescent protein upon tau–tau interactions. Tau–BiFC cells were treated with increasing concentrations of each compound (1–4; 1.25, 5, and 20 μM) in the presence of thapsigargin (TG), an inducer of endoplasmic reticulum stress [38]. TG markedly enhanced tau oligomerization, inducing a 9.9 ± 2.9-fold increase in BiFC fluorescence relative to basal levels at 48 h (Figure 6A,B). Among the tested compounds, compound 2 significantly reduced TG-induced tau oligomerization at 20 μM, resulting in a 43.7 ± 5.2% decrease (p < 0.001 vs. TG-treated control). At lower concentrations (1.25 and 5 μM), a dose-dependent reduction in BiFC fluorescence was observed; however, these decreases did not reach statistical significance (Figure 6C). In contrast, compounds 1, 3, and 4 did not exhibit notable inhibitory effects under the same conditions. To exclude the possibility that the reduced BiFC signal was attributable to cytotoxicity, cell viability was assessed in parallel by quantifying Hoechst-stained nuclei. Compound 2 showed no cytotoxicity up to 20 μM, confirming that the attenuation of tau oligomerization was not due to reduced cell survival (Figure 6D). To contextualize the biological significance of these findings, it is important to note that although the inhibitory effect of compound 2 (43.7% at 20 μM) is lower than that of classical tau aggregation inhibitors such as methylene blue, methylene blue exhibits marked cytotoxicity at comparable concentrations [39], whereas compound 2 did not affect cell viability up to 20 μM. Thus, the relevance of compound 2 should be interpreted in the context of both efficacy and safety.

Figure 6.
Effects of compound 2 on TG-induced tau oligomerization and cell viability in tau-BiFC cells. (A) Schematic diagram of the tau-BiFC system. Full-length tau is fused to split Venus fragments (VN173 and VC155), which reconstitute fluorescence upon tau oligomerization under stress conditions. For the tau-BiFC assay, cells were treated with each compound in the presence of TG. (B) Representative BiFC fluorescence images of tau-BiFC cells treated with compounds 1–4 (1.25, 5 and 20 μM) in the presence of TG (0.05 μM). Nuclei were counterstained with Hoechst (blue). Scale bar, 100 μm. (C) Quantification of tau-BiFC fluorescence intensity. (D) Cell viability determined by Hoechst-stained nuclei counting. Data were analyzed by one-way ANOVA followed by Dunnett’s multiple comparisons test; * p < 0.05, *** p < 0.001, **** p < 0.0001 vs. TG-treated control (DMSO).
Diarylheptanoids, including octahydrocurcumin analogs, are known to modulate ER stress and ROS generation—key drivers of TG-induced tau oligomerization—suggesting that compound 2 may attenuate stress-associated pathways rather than directly inhibiting tau–tau interactions. Although BiFC fluorescence is generally considered irreversible once the Venus fragments reconstitute, overall signal intensity can decline when new tau–tau interactions are suppressed or when existing complexes undergo cellular clearance. Nevertheless, because the tau-BiFC assay primarily reports early oligomerization events and cannot fully distinguish direct from indirect mechanisms, its mechanistic resolution is limited. Collectively, these considerations indicate that compound 2 confers moderate but meaningful inhibition of stress-induced tau oligomerization within a favorable cytotoxicity profile.
4. Conclusions
In summary, a phytochemical investigation of the EtOAc fraction of A. agallocha extract, guided by LC/MS-based analysis, led to the isolation and structural elucidation of four compounds: N-trans-feruloyltyramine (1), (3R,5R)-octahydrocurcumin (2), 1,7-bis(4-hydroxyphenyl)heptane (3), and trans-caffeoyltyramine (4). The therapeutic potential of these constituents against neurodegenerative diseases, such as Alzheimer’s disease, was assessed through evaluation of their inhibitory effects on tau protein aggregation. Among them, compound 2, a diarylheptanoid, significantly suppressed tau oligomerization at 20 μM without exhibiting cytotoxicity in the tau–BiFC cell model. These findings highlight the potential of A. agallocha phytochemicals—particularly diarylheptanoids—as promising modulators of tau aggregation and underscore the pharmaceutical relevance of its secondary metabolites. This study further emphasizes the importance of exploring diverse and underinvestigated constituents of A. agallocha for the discovery of novel neuroprotective agents.
Author Contributions
Conceptualization, J.-C.K. and K.H.K.; methodology, Y.R.C., J.K., B.K., D.M.K., Y.K.K. and S.L.; formal analysis, D.M.K., Y.K.K. and S.L.; writing—original draft preparation, Y.R.C., Y.K.K. and K.H.K.; writing—review and editing, Y.R.C., J.K. and K.H.K.; supervision, K.H.K.; funding acquisition, K.H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT; grant numbers RS-2019-NR040057 and RS-2021-NR059240) and the Korea Environment Industry & Technology Institute (KEITI) through a project to make multi-ministerial national biological research resources more advanced funded by the Korea Ministry of Environment (MOE; 2021003420003). This work was supported by the KIST Institutional Program (No. 2E33681 and 2E33711). This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2024-00440614).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, W.; Chen, H.-Q.; Wang, H.; Mei, W.-L.; Dai, H.-F. Natural products in agarwood and Aquilaria plants: Chemistry, biological activities and biosynthesis. Nat. Prod. Rep. 2021, 38, 528–565. [Google Scholar] [CrossRef]
- Chhipa, H.; Chowdhary, K.; Kaushik, N. Artificial production of agarwood oil in Aquilaria sp. by fungi: A review. Phytochem. Rev. 2017, 16, 835–860. [Google Scholar] [CrossRef]
- Barden, A.; Anak, N.A.; Mulliken, T.; Song, M. Heart of the Matter: Agarwood Use and Trade and CITES Implementation for Aquilaria malaccensis; Traffic International: Cambridge, UK, 2000; pp. 17–18. [Google Scholar]
- Liu, Y.-Y.; Wei, J.-H.; Gao, Z.-H.; Zhang, Z.; Lyu, J.-C. A review of quality assessment and grading for agarwood. Chin. Herb. Med. 2017, 9, 22–30. [Google Scholar] [CrossRef]
- Dash, M.; Patra, J.K.; Panda, P.P. Phytochemical and antimicrobial screening of extracts of Aquilaria agallocha Roxb. Afr. J. Biotechnol. 2008, 7, 3531–3534. [Google Scholar]
- Aggarwal, G.; Sharma, M.; Singh, R.; Sharma, U. Ethnopharmacologically important highly subsidized Indian medicinal plants: Systematic review on their traditional uses, phytochemistry, pharmacology, quality control, conservation status and future prospective. J. Ethnopharmacol. 2024, 320, 117385. [Google Scholar]
- Dalkılıç, S.; Dalkılıç, L.K.; İsbenov, E.; Uygur, L.; Taşdemir, C. Investigation of Cytotoxic, Antioxidant, Apoptotic/Necrotic Activity of Aquilaria agallocha Root Extract and Determination of Gene Expression Levels in HepG2, MCF-7 Cancer Cell Lines. Life 2025, 15, 651. [Google Scholar] [CrossRef]
- Chen, H.Q.; Wei, J.H.; Yang, J.S.; Zhang, Z.; Yang, Y.; Gao, Z.H.; Sui, C.; Gong, B. Chemical constituents of agarwood originating from the endemic genus Aquilaria plants. Chem. Biodivers. 2012, 9, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Song, L.; Li, C.; Yang, Y.; Zhang, S.; Xu, H.; Wang, Z.; Han, Z.; Yang, L. Eudesmane-type and agarospirane-type sesquiterpenes from agarwood of Aquilaria agallocha. Phytochem. 2021, 192, 112920. [Google Scholar] [CrossRef]
- Ueda, J.-Y.; Imamura, L.; Tezuka, Y.; Tran, Q.L.; Tsuda, M.; Kadota, S. New sesquiterpene from Vietnamese agarwood and its induction effect on brain-derived neurotrophic factor mRNA expression in vitro. Bioorg. Med. Chem. 2006, 14, 3571–3574. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Zhang, Z.; Ye, K. Tau in neurodegenerative diseases: Molecular mechanisms, biomarkers, and therapeutic strategies. Transl. Neurodegener. 2024, 13, 40. [Google Scholar] [CrossRef]
- Li, C.; Götz, J. Tau-based therapies in neurodegeneration: Opportunities and challenges. Nat. Rev. Drug Discov. 2017, 16, 863–883. [Google Scholar] [CrossRef]
- Basurto-Islas, G.; Diaz, M.C.; Ocampo, L.M.Z.; Martínez-Herrera, M.; López-Camacho, P.Y. Natural products against tau hyperphosphorylation-induced aggregates: Potential therapies for Alzheimer’s disease. Archiv. Pharm. 2025, 358, e2400721. [Google Scholar] [CrossRef] [PubMed]
- Nahar, J.; Boopathi, V.; Rupa, E.J.; Awais, M.; Valappil, A.K.; Morshed, M.N.; Murugesan, M.; Akter, R.; Yang, D.U.; Mathiyalagan, R. Protective effects of Aquilaria agallocha and Aquilaria malaccensis edible plant extracts against lung cancer, inflammation, and oxidative stress—In silico and in vitro study. Appl. Sci. 2023, 13, 6321. [Google Scholar] [CrossRef]
- Durairajan, S.S.; Selvarasu, K.; Bera, M.R.; Rajaram, K.; Iyaswamy, A.; Li, M. Alzheimer’s disease and other tauopathies: Exploring efficacy of medicinal plant-derived compounds in alleviating tau-mediated neurodegeneration. Curr. Mol. Pharmacol. 2022, 15, 361–379. [Google Scholar] [CrossRef]
- Jitsanong, T.; Khanobdee, K.; Piyachaturawat, P.; Wongprasert, K. Diarylheptanoid 7-(3, 4 dihydroxyphenyl)-5-hydroxy-1-phenyl-(1E)-1-heptene from Curcuma comosa Roxb. protects retinal pigment epithelial cells against oxidative stress-induced cell death. Toxicol. In Vitro 2011, 25, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Vattanarongkup, J.; Piyachaturawat, P.; Tuchinda, P.; Sanvarinda, P.; Sanvarinda, Y.; Jantaratnotai, N. Protective effects of a diarylheptanoid from Curcuma comosa against hydrogen peroxide-induced astroglial cell death. Planta Med. 2016, 82, 1456–1462. [Google Scholar] [CrossRef]
- Zhu, H.; Fan, Y.; Sun, H.; Chen, L.; Man, X. Curcumin inhibits endoplasmic reticulum stress induced by cerebral ischemia-reperfusion injury in rats. Exp. Ther. Med. 2017, 14, 4047–4052. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jang, M.; Ryoo, R.; Roh, J.; Ko, S.-K.; Kim, K.H. New autophagy-modulating lanostane-type triterpenoids from a hallucinogenic poisonous mushroom Gymnopilus orientispectabilis. Arch. Pharm. Res. 2024, 47, 272–287. [Google Scholar] [CrossRef]
- Lee, D.E.; Park, K.H.; Hong, J.-H.; Kim, S.H.; Park, K.-M.; Kim, K.H. Anti-osteoporosis effects of triterpenoids from the fruit of sea buckthorn (Hippophae rhamnoides) through the promotion of osteoblast differentiation in mesenchymal stem cells, C3H10T1/2. Arch. Pharm. Res. 2023, 46, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Gil, T.-Y.; Kim, H.-J.; Kim, H.-M.; Sim, H.-Y.; Choi, W.; Lee, B.S.; Kim, K.H.; An, H.-J. Aster glehni ethanol extract inhibits inflammatory responses regulating skin barrier molecules in human keratinocytes. Nat. Prod. Sci. 2024, 30, 262–267. [Google Scholar] [CrossRef]
- Lee, J.-E.; Jeong, S.Y.; Li, Z.; Kim, H.-Y.; Kim, H.-W.; Yoo, M.J.; Jang, H.J.; Kim, D.-K.; Cho, N.; Yoo, H.M. Development of a screening platform to discover natural products active against SARS-CoV-2 infection using lung organoid models. Biomater. Res. 2023, 27, 18. [Google Scholar] [CrossRef]
- You, C.-L.; Lee, S.-J.; Lee, J.; Vuong, T.A.; Lee, H.-Y.; Jeong, S.Y.; Alishir, A.; Walker, A.S.; Bae, G.-U.; Kim, K.H. Inonotus obliquus upregulates muscle regeneration and augments function through muscle oxidative metabolism. Int. J. Biol. Sci. 2023, 19, 4898. [Google Scholar] [CrossRef]
- Cho, C.H.; Chae, S.H.; Kim, S.H.; Kim, K.H. Phenolic compounds isolated from Juncus decipiens and their effects on osteoblast differentiation in the mouse mesenchymal stem cell line C3H10T1/2. Nat. Prod. Sci. 2024, 30, 135–142. [Google Scholar] [CrossRef]
- Lee, S.R.; Schalk, F.; Schwitalla, J.W.; Guo, H.; Yu, J.S.; Song, M.; Jung, W.H.; De Beer, Z.W.; Beemelmanns, C.; Kim, K.H. GNPS-Guided Discovery of Madurastatin Siderophores from the Termite-Associated Actinomadura sp. RB99. Chem. Eur. J. 2022, 28, e202200612. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, C.S.; Yu, J.S.; Kang, H.; Yoo, M.J.; Youn, U.J.; Ryoo, R.; Bae, H.Y.; Kim, K.H. Ergopyrone, a styrylpyrone-fused steroid with a hexacyclic 6/5/6/6/6/5 skeleton from a mushroom Gymnopilus orientispectabilis. Org. Lett. 2021, 23, 3315–3319. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Alishir, A.; Zhang, S.; Zhang, Y.; Choi, S.; Pang, C.; Bae, H.Y.; Jung, W.H.; Kim, K.H. Identification of Obscurolide-Type Metabolites and Antifungal Metabolites from the Termite-Associated Streptomyces neopeptinius BYF101. J. Nat. Prod. 2023, 86, 1891–1900. [Google Scholar] [CrossRef]
- Roumani, M.; Duval, R.E.; Ropars, A.; Risler, A.; Robin, C.; Larbat, R. Phenolamides: Plant specialized metabolites with a wide range of promising pharmacological and health-promoting interests. Biomed. Pharmacother. 2020, 131, 110762. [Google Scholar] [CrossRef]
- Zeng, H.; Locatelli, M.; Bardelli, C.; Amoruso, A.; Coisson, J.D.; Travaglia, F.; Arlorio, M.; Brunelleschi, S. Anti-inflammatory properties of clovamide and Theobroma cacao phenolic extracts in human monocytes: Evaluation of respiratory burst, cytokine release, NF-κB activation, and PPARγ modulation. J. Agric. Food Chem. 2011, 59, 5342–5350. [Google Scholar] [CrossRef]
- Wang, C.; Eskiw, C.H. Cytoprotective effects of Avenathramide C against oxidative and inflammatory stress in normal human dermal fibroblasts. Sci. Rep. 2019, 9, 2932. [Google Scholar] [CrossRef]
- Kim, H.R.; Min, H.-Y.; Jeong, Y.H.; Lee, S.K.; Lee, N.S.; Seo, E.-K. Cytotoxic constituents from the whole plant of Corydalis pallida. Arch. Pharm. Res. 2005, 28, 1224–1227. [Google Scholar] [CrossRef] [PubMed]
- Yokosuka, A.; Mimaki, Y.; Sakagami, H.; Sashida, Y. New diarylheptanoids and diarylheptanoid glucosides from the rhizomes of Tacca chantrieri and their cytotoxic activity. J. Nat. Prod. 2002, 65, 283–289. [Google Scholar] [CrossRef]
- Kawasaki, I.; Matsuda, K.; Kaneko, T. Preparation of 1, 7-bis (p-hydroxyphenyl) heptane. Bull. Chem. Soc. Jpn. 1971, 44, 1986–1987. [Google Scholar] [CrossRef]
- Chen, T.; He, J.; Zhang, J.; Li, X.; Zhang, H.; Hao, J.; Li, L. The isolation and identification of two compounds with predominant radical scavenging activity in hempseed (seed of Cannabis sativa L.). Food chem. 2012, 134, 1030–1037. [Google Scholar] [CrossRef]
- Kikuzaki, H.; Usuguchi, J.; Nakatani, N. Constitutents of Zingiberaceae I. Diarylheptanoids from the rhizomes of ginger (Zingiber officinale Roscoe). Chem. Pharm. Bull. 1991, 39, 120–122. [Google Scholar] [CrossRef]
- Puente, A.R.; Nagabhushanam, K.; Ganjihal, S.; Majeed, M.; Polavarapu, P.L. Chiroptical spectroscopic studies for the absolute configuration determination of hexahydrocurcumin and octahydrocurcumin. Chirality 2022, 34, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Tak, H.; Haque, M.M.; Kim, M.J.; Lee, J.H.; Baik, J.-H.; Kim, Y.; Kim, D.J.; Grailhe, R.; Kim, Y.K. Bimolecular fluorescence complementation; lighting-up tau-tau interaction in living cells. PLoS ONE 2013, 8, e81682. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.-Q.; Yang, Y.; Song, J.; Jiang, Q.; Liu, Z.-C.; Wang, Q.; Zhu, L.-Q.; Wang, J.-Z.; Tian, Q. LiCl attenuates thapsigargin-induced tau hyperphosphorylation by inhibiting GSK-3β in vivo and in vitro. J. Alzheimer’s Dis. 2010, 21, 1107–1117. [Google Scholar] [CrossRef]
- Lim, S.; Shin, S.; Sung, Y.; Lee, H.E.; Kim, K.H.; Song, J.Y.; Lee, G.-H.; Aziz, H.; Lukianenko, N.; Kang, D.M. Levosimendan inhibits disulfide tau oligomerization and ameliorates tau pathology in TauP301L-BiFC mice. Exp. Mol. Med. 2023, 55, 612–627. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).