Abstract
Background/Objectives: Dry eye disease (DED) is an ocular surface disease with unstable tear film hemeostasis that could influence the corneal biomechanics. The study aimed to elucidate the impact of dry eye severity on corneal biomechanics. Methods: This is a prospective cohort study that enrolled 72 participants with or without dry eye severity. All subjects received dry eye and corneal biomechanic assessment. Dry eye patients were divided into non-DED (>6 s) and DED (<6 s) groups based on the average non-invasive keratograph tear break-up time to compare their performance in corneal biomechanics. We further analyzed the correlation between the corneal biomechanic parameters and dry eye indexes for these patients. Results: In this study, 38 non-DED patients and 34 DED patients were enrolled for analysis. The two groups showed significant differences in first applanation (A1) deflection area (p = 0.002), A1 delta arc length (p = 0.024), second applanation (A2) deformation amplitude (p = 0.024), and whole eye movement [mm] (p = 0.021). Moreover, both A1 deflection area and A1 delta arc length revealed significantly correlated with tear meniscus height in DED patients. Conclusions: DED and its severity can affect corneal biomechanics. Tear volume on the ocular surface could be one of the important factors to influence corneal biomechanics.
1. Introduction
Dry eye disease (DED) is a global issue related to tear film instability, ocular surface inflammation, and neurosensory dysfunction [1,2]. It can be marked by complex changes in the tear film and ocular surface, which further cause ocular discomfort and visual issues [2]. Mainstay treatments for DED include, but are not limited to, instilling artificial tears, improving eyelid hygiene, and making lifestyle and environmental modifications [3,4]. With its chronic nature and the need for ongoing management and treatment, DED may lead to long-term cost barriers worldwide and generate a substantial economic burden [5].
In the past decade, only few studies proposed that the disease entity of DED is linked to alterations in corneal biomechanics [6,7,8,9]. The possible mechanisms linking DED to changes in corneal surface characteristics may be attributed to insufficient maintenance of ocular epithelial surface due to decreased lubrication effects of tears [10]. This leads to chronic inflammation and disruption of meibomian glands, driven by the cytokine effects of T helper type 1 (Th1)-derived interferon (IFN)-γ and T helper type 17 (Th17)-derived interleukin (IL)-17 [11,12]. The Th17 pathway may specifically induce the expression of Matrix metalloproteinase (MMP)-3 and MMP-9, leading to corneal epithelial barrier injury and further worsening the severity of DED [12,13]. Previous limited clinical studies have reported that DED patients may have more compliant cornea, which could be related to changes in corneal biomechanics [8,9,10].
Common techniques for evaluating corneal biomechanics are based on the viscoelastic characteristics of the cornea [14]. These techniques, including applanation tonometry, Brillouin scattering, and elastography, are able to apply a well-defined stimulus to generate a subsequent visualized tissue response for quantification and analysis [15]. However, only the applanation tonometry-based device is currently available for clinical use. Corneal Visualization Scheimpflug Technology (Corvis ST; Oculus, Wetzlar, Germany) is a representative applanation tonometry device which provides state-of-the-art corneal biomechanic measurements. Corvis ST can obtain information on corneal biomechanics by applying a noncontact air puff with fixed force to the ocular surface and recording the time-series changes in corneal shape with an ultrahigh-speed Scheimpflug camera [16].
The impact of DED on corneal biomechanics has not yet been well investigated. Moreover, the relationship between tear film homeostasis and corneal biomechanics has not been clearly elucidated. Although some studies have suggested that tear film physiology, hydration status, and ocular surface microstructures may influence corneal biomechanics [17,18,19,20], clinical data directly linking DED to in vivo biomechanical changes remain limited. Hence, this study aimed to compare the corneal biomechanics of DED patients via a corneal biomechanical analyzer, Corvis ST. Furthermore, we tried to explore the association between tear film homeostatic indexes and corneal biomechanical parameters for DED patients with various severities.
2. Materials and Methods
2.1. Participants
This prospective cohort was conducted from October 2020 to September 2023 at Kaohsiung Chang Gung Memorial Hospital (KCGMH), Taiwan. The study adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of KCGMH. We included patients without and with DED and divided them into two groups: a control group (non-DED) and a DED group. DED patients were identified by the presence of positive dry eye symptoms [Ocular Surface Disease Index (OSDI) > 13] and at least one of the following two conditions: average non-invasive keratograph break-up time (NIKBUT-ave) ≤ 6 s or Oxford staining score (OSS) ≥ 1, in accordance with the refined global definition of DED proposed in the Tear Film and Ocular Surface Society Dry Eye WorkShop II (TFOS DEWS II) in 2017 [21]. All measurements were performed in a controlled environment with temperature 24 °C in the summer and 20 °C in the winter and with 55% relative humidity). Patients were instructed to refrain from instillation of artificial tears or excessive blinking for at least 5 min prior to testing.
Subjects were excluded if they were younger than 20 or older than 80 years, had an active ocular infection, or had ocular diseases such as corneal dystrophy, glaucoma, keratoconus, uveitis, or intraocular tumors. Further exclusion criteria included prior ocular or eyelid surgery within 6 months, contact lens use within 1 month before examination, pregnancy, and systemic conditions known to affect corneal biomechanics such as diabetes mellitus and autoimmune diseases. Patients unable to complete the examinations were also excluded.
All participants first completed the OSDI questionnaire to quantify their subjective ocular symptoms. Subsequently, they underwent an orderly series of ocular examinations, including measurement of the tear meniscus height (TMH), detection of the non-invasive tear break-up time, meibography, and corneal fluorescence staining. Finally, corneal biomechanics were analyzed with the Corvis ST. The right eye was chosen as the examined eye if both eyes met the enrolled criteria, while the left eye was measured when the right eye was not qualified.
2.2. Defining the DED and Non-DED
The enrolled subjects were divided into two groups based on having DED or not, which was defined by the values of average NIKBUT (NIKBUT-ave) [22,23,24]. The subjects with NIKBUT-ave < 6 s were classified into the dry eye group, while those with NIKBUT-ave > 6 s were grouped into the control group, as known as non-DED group.
2.3. Evaluating Dry Eye Symptoms
This OSDI questionnaire is a tool designed to assess the subjective severity of dry eye, which comprises 12 questions, scoring from 0 to 4 based on the symptoms, frequency, and the effect of these symptoms on vision-related functioning, with higher scores indicating more severe impairment [25]. The overall score is calculated with a scale of 0 to 100. Scores were then measured and reported for each individual upon completion.
2.4. Assessing the Tear Film Homeostasis
Keratograph® 5M (K5M; Oculus, GmbH, Wetzlar, Germany) is a video topographer that can assess both the quantity and quality of the tear film automatically and objectively in a non-invasive manner [25]. TMHs were initially measured perpendicular to the central eyelid margin relative to the pupil center. NIKBUT can be measured by K5M between a blink and the defect shown on the rings reflected on the tear surface [26]. NIKBUT-1st, NIKBUT-ave, and NIKBUT tolerability (the tolerable time of the NIKBUT test) were then obtained at least 5 min after the TMH measurement. With a near-infrared illumination with 840 nm diode light source, meibography was performed to directly visualize the morphology of meibomian glands in vivo in a non-invasive manner finally. The severity of the meibomian gland dropout can be graded according to the meibograde, from degree 0 to 4, with each degree of rise representing a 25% meibomian gland dropout [27].
2.5. Examining Corneal Surface Injuries
OSS is a grading score for grading corneal surface damage in dry eye by the Oxford Schema [28]. After applying fluorescent dye to the eye, the stained cornea was examined under absorption filters. Staining ranges from 0 to 5 for the cornea. Moreover, the grade can be added if filaments, pupillary area staining, and confluent patches present while examining.
2.6. Determining Corneal Biomechanics
Corvis ST is a clinically available instrument that records the corneal response to a defined air pulse with high-speed Scheimpflug camera, which can capture over 4300 images per second, allowing direct measurement of intraocular pressure (IOP) independent to corneal thickness (biomechanically corrected IOP, bIOP) during first applanation (A1) as well as central corneal thickness. Physicians can also obtain other properties of corneal biomechanics during the phases of A1, highest concavity (HC), and the second applanation (A2) with this instrument. Moreover, some novel parameters can indicate ocular biomechanic limits (limit indices) and biomechanical correction parameters with physiological significance (integrated parameters) [29,30]. Figure S1 showed the representative Corvis ST evaluation for a non-DED (Figure S1a) and a (Figure S1b) DED patients.
2.7. Statistical Analysis and Power Calculation
The subjects’ characters and their ocular parameters for the control and DED groups were compared and statistically tested by the Fisher’s exact test or the Mann–Whitney U test. The association between the Corvis ST parameters and the tear film homeostatic parameters were analyzed via Spearman’s rank-order correlation. All of the data were analyzed using the SPSS software, version 22 (IBM Corp., Armonk, NY, USA). A priori sample size estimation was performed using G*Power 3.1, based on previously reported differences in NIKBUT-ave between DED and normal populations [18]. With an expected effect size (Cohen’s d) of approximately 1.3, a two-tailed test with α = 0.05 and power = 0.8 indicated that a minimum of 22 subjects (11 per group) would be sufficient.
For the above analyses, a two-tailed p value less than 0.05 was considered statistically significant.
3. Results
3.1. Population Characteristics
A total of 72 patients, including 34 patients with DED and 38 patients without DED, were compared and analyzed. Among the basic characters, there was no significant difference between the two groups (Table 1). For the tear film homeostatic parameters, the DED group had significantly shorter NIKBUT-1st, NIKBUT-ave, and tolerable time of the NIKBUT test than the control group (Table 1).

Table 1.
Demographic data for subjects of non-DED and DED.
3.2. Comparison of Corneal Biomechanics Between the Non-DED and DED Patients
DED patients showed significantly different performance in some biomechanical parameters of A1, A2, and limit indices (Table 2). Among A1 indices, both A1 delta arc length (A1 dArcL) and A1 deflection area (A1 Darea) in the DED group showed significantly greater values (p < 0.05) than those in the control group. Among A2 indices, the DED group showed significantly higher A2 deformation amplitude (A2 DA) than the control group. Among the limit indices, DED patients had significantly greater whole eye movement (WEM) than non-DED patients (Table 2).

Table 2.
Comparison of Corvis ST parameters between the non-DED and DED patients.
3.3. Relationships of Corvis ST Parameters and Dry Eye Parameters
Dry eye parameters and biomechanical indices demonstrated a significant positive association between the A1 Darea and NIKBUT-ave in the overall population (Figure 1a). Furthermore, both the A1 Darea and A1 dArcL were found to be significantly positively and negatively correlated with central TMH (p = 0.012 and 0.015, respectively) (Figure 2a,b). In contrast, no significant correlations were observed between the Corvis indices and dry eye parameters within the control group (Figure 1b and Figure 2c,d). However, in the DED group, both the A1 Darea and A1 dArcL showed significant positive and negative associations with central TMH (p = 0.020 and 0.015, respectively) (Figure 1c and Figure 2e,f).
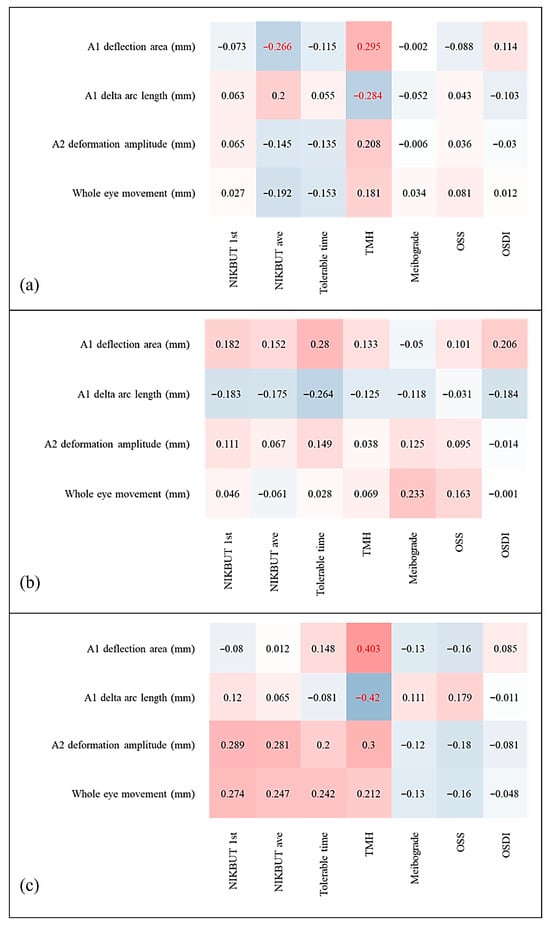
Figure 1.
Correlation matrices between Corvis ST corneal biomechanical parameters and K5M parameters for (a) all patients, (b) non-DED subjects only, and (c) DED subjects only. Red columns show positive correlations, and blue columns show negative correlations; darker shades indicate stronger relationships. Red statistics indicate statistical significance.
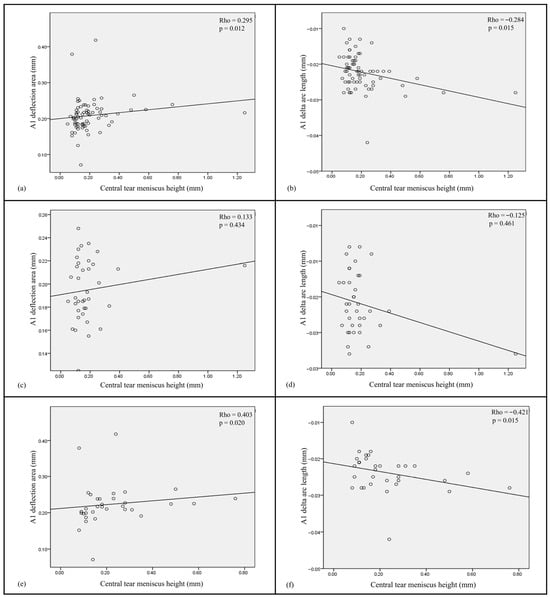
Figure 2.
Scatter plots show correlations between two significant corneal biomechanical parameters (A1 deflection area and A1 delta arc length) and the tear meniscus height for (a,b) all subjects, (c,d) non-DED patients, and (e,f) DED subjects.
4. Discussion
This study explored the impact of DED on corneal biomechanics in DED patients. We found that all participants have distinct presentations in A1, A2, and limit corneal biomechanical indices, including A1 dArcL, A1 Darea, A2 DA, and WEM. Moreover, we examined the association between Corvis indices and dry eye parameters. In all subjects, the result showed positive correlations between A1 Darea and both NIKBUT-ave and central TMH. A1 dArcL was negatively associated with central TMH. In the dry eye group, similar findings were noted as well. However, no such correlations were observed in the control group.
In our study, DED patients had significantly higher A1 Darea, A1 dArcL, A2 DA, and WEM than non-DED patients (Table 2), which implied the former’s corneas were more compliant or had less stiffness. In addition, the severity of DED was found associated with the corneal biomechanics as well, with the more severe the DED was, the more compliant the cornea was (Figure 1c and Figure 2e,f). The A1 Darea is the area of the applanated region at the first applanation, which represents the corneal movement without whole eye motion and is the indicator of corneal elasticity. More compliant corneas are reported to have higher A1 Darea [31,32]. A1 dArcL means the delta arc length at the first applanation. Greater values of A1_dArcL have also been reported in keratoconus, post-laser in situ keratomileusis (LASIK), and post-LASIK keratectasia eyes [33]. DA represents the vertical movement of the central cornea and is related to corneal stiffness and the IOP. The stiffer the cornea is, the smaller the DA. WEM, on the other hand, is the vertical movement of the whole eye, and is affected by scleral and underlying soft tissue’s biomechanical properties.
Compared with previous studies focusing on corneal biomechanics in DED, their results were aligned with ours. Satitpitakul et al. reported that greater DED severity was associated with less stiffness of cornea in patients with either low or normal tear production [8]. They found significant associations between conjunctival staining scores and A2 velocity, between corneal staining scores and A2 length (A2L), and between Schirmer test with A1 time and A2L. Moreover, Long et al. found that dry eye patients had decreased HC time than normal subjects [9]. Furthermore, Yang et al. implied that ocular surface injuries in DED could be made due to increased mean eyelid pressure and increased shear stress, which may affect corneal cell behavior by altering mechanical factors [10].
There has been no previous study dedicated on the correlation between tear film homeostasis properties measured by K5M and the corneal biomechanism analyzed with Corvis ST. According to our analysis, the corneas of the DED patients were more compliant, which could be reflected by the positive association between A1 Darea and NIKBUT-ave as well as between A1 dArcL and NIKBUT-ave. A1 dArcL had a negative association with central TMH, which was partially consistent with the results by Tung et al. [34]. They found that tear volume may not completely reflect the DED severity. Instead, for patients with meibomian gland dysfunction, higher tear volume correlated with worse ocular surface disease due to change in tear osmolarity, tear compositions (e.g., lactoferrin concentration, inflammatory mediators), and delayed tear clearance, which could be related to corneal epithelial damage [34].
Our result showed that DA and WEM also had no significant associations with any of the tear film homeostasis parameters. The complex effects of corneal thickness, IOP, ocular structures, and orbital soft tissue may contribute to this result [35]. Different corneal biomechanical parameters reflect diverse ocular properties, not merely tear film status. DA reflects the maximal displacement of the cornea under load, which may be dominated by the stroma’s bulk elasticity and lamellar microstructure, buffering the more superficial influences of tear film instability. In addition, higher IOP and thicker corneas can lead to lower values of DA [36,37]. WEM may echo the biomechanical status of the sclera, orbital soft tissues, and boundary conditions, making them less sensitive to superficial tear film changes [38]. Thus, it is reasonable to note that not all corneal biomechanical parameters had significant associations with tear film homeostasis parameters.
Beyond alterations in tear volume, the physiology and pathology of the tear film itself are important contributors to corneal biomechanics. The tear film, composed of lipid, aqueous, and mucin layers, maintains ocular surface homeostasis, while its disruption can induce surface irregularities, neovascularization, and inflammation-driven remodeling of the extracellular matrix [17,20,39,40].
Experimental and imaging studies further demonstrate that tear adhesiveness, redistribution, and meniscus dynamics modulate corneal stress distribution [17], and that hydration critically governs lamellar spacing and collagen interactions, with environmental or eyelid factors inducing structural shifts [39]. At the microstructural level, stromal striae arranged in truss-like geometries enhance biomechanical resilience [40,41]. In dry eye disease, chronic tear film instability, epithelial barrier dysfunction, and sustained inflammation may disrupt these hydration-dependent and microarchitectural mechanisms, leading to increased corneal compliance. Additionally, unstable tear film can bias in vivo biomechanical measurements, further complicating interpretation [18,19,42,43]. Collectively, these considerations suggest that the biomechanical alterations observed in DED reflect both genuine structural remodeling and measurement variability mediated by tear film dynamics.
Currently, DED may be clinically diagnosed using various assessment tools, such as various dry eye questionnaires, different ocular surface staining grading systems, invasive or non-invasive tear break-up tests, tear secretion, tear volume, and meibography [44,45]. Among the above measurements, tear break-up time (TBUT) has been proposed to be one of the mostly used modalities since tear film instability is one of the leading pathophysiological features [23,46]. Moreover, NIKBUT can provide similar results to the conventionally invasive means [22,24], and may provide better repeatability and reproducibility in measuring DED due to less influence of reflex tearing during the examination [22,26,47].
In the present study, the classification of non-DED and DED groups were based on NIKBUT-ave threshold of 6 s as the cutoff value, which has been supported in multiple studies as a practical and objective tool for DED diagnosis [22,23,24]. The 2017 TFOS DEWS II report proposed a NIKBUT < 10 s as a general diagnostic criterion for dry eye, with ≤5 s being more specific for clinical disease. But this broad range includes patients with varying severity [44]. Subsequent studies have further illustrated the optimal cutoff duration in stratifying DED and non-DED patients. In a randomized controlled trial by Wang et al., NIKBUT median of 6.3 s was observed in symptomatic DED subjects, suggesting this value represents a clinically meaningful division between symptomatic DED and healthy groups [22]. In addition, for symptomatic DED patients, NIKBUT-ave were found 0.2–2 s longer than TBUT values [23,24], implying that NIKBUT-ave 6 s is one of the reasonable cutoffs for identifying DED patients.
In this study, there were several limitations. First of all, the effects of eyedrops were not analyzed. Most of these subjects were regularly followed up and treated by various lubricants. Despite this, all of the participants were asked to discontinue ointment one day before the examination and stop any eyedrops instillation on the examination day until completion of all tests. Therefore, we deemed that its effects may be relatively subtle in affecting the results. Second, the diagnostic criteria for DED were based on TFOS DEWS II since the patients were recruited before the publication of the latest TFOS DEWS III [48]. Future studies investigating DED patients’ corneal biomechanics based on the latest guideline were needed. Third, we simply use NIKBUT-ave 6 s as cutoff to divide the two groups, which may inevitably neglect the potential impact of other dry eye related parameters. However, TBUT is the most widely used parameter to identify and grade DED and can reflect the severity of DED in most circumstances and correlate to other common parameters [23,46], making it simple yet feasible way to identify these patients clinically. Despite that we had performed power analysis to determine the minimum number of cases required in our study, future studies with a relatively larger sample size are still needed to explore the corneal biomechanics in DED patients.
Our study provides a new clinical perspective on corneal biomechanics in patients with DED. We found that corneas in DED are more compliant, reflecting altered biomechanical properties. These findings have several clinical implications. The severity of DED may be assessed not only by traditional tear film parameters but also by evaluating corneal structural changes, which could be especially useful in the follow-up of patients at risk of corneal melting. Increased corneal compliance in DED may also bias IOP measurements and reduce resistance to mechanical stress, with potential consequences for refractive surgery and keratoplasty outcomes. Moreover, incorporating biomechanical indices with tear film assessments may enhance disease stratification and provide complementary markers for monitoring therapeutic response.
5. Conclusions
In conclusion, corneal biomechanics of DED patients could be different from non-DED subjects. In addition, the more severe the dry eye is, the more compliant the cornea is. Moreover, tear film stability reflected by the tear break-up time may be one of the most crucial factors associated with corneal biomechanics in eyes of DED. For DED patients, especially for those with higher severity, stabilizing the tear film could improve their corneal biomechanics.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biomedicines13102524/s1. Figure S1: Representative Corvis ST evaluation for all participants. a. A non-DED patient. b. A DED patient shows more compliant cornea than a non-DED patient.
Author Contributions
Conceptualization, M.-T.K.; methodology, L.-W.C. and M.-T.K.; validation, R.-W.H., P.-C.F. and H.-J.Y.; formal analysis, L.-W.C.; investigation, L.-W.C. and R.-W.H.; resources, M.-T.K. and R.-W.H.; data curation, H.-J.Y. and P.-C.F.; writing—original draft preparation, L.-W.C.; writing—review and editing, M.-T.K. and H.-J.Y.; visualization, L.-W.C. and M.-T.K.; supervision, M.-T.K., R.-W.H. and I.-H.Y.; project administration, M.-T.K.; funding acquisition, M.-T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Chang Gung Medical Foundation (CMRPG8K1451, CMRPG8M0991) and the Ministry of Science and Technology (NSTC 112-2314-B-182A-019-MY3). The sponsors or funding organizations had no role in the design or conduct of this research.
Institutional Review Board Statement
Institutional review board/ethics committee approval (No. 202001104B0) was obtained from the committee of medical ethics and human experiments of Chang Gung Memorial Hospital on 9 July 2020.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data can be made available by the authors upon reasonable request.
Acknowledgments
We thank Yu-Ting Huang for her assistance in obtaining raw data from K5M and Corvis ST. We acknowledge the use of AI tools for support with writing style and English verification but not for content (OpenAI, GPT 4.1, 2025).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DED | Dry eye disease |
| Corvis ST | Corneal Visualization Scheimpflug Technology |
| K5M | Keratograph® 5M |
| IOP | Intraocular pressure |
| SE | Spherical equivalence |
| OSDI | Ocular surface disease index |
| NIKBUT-1st | The first non-invasive keratograph break-up time |
| NIKBUT-ave | The average non-invasive keratograph break-up time |
| TMH | Tear meniscus height |
| OSS | Oxford staining score |
| A1 | The first applanation |
| A2 | The second applanation |
| DA | Deformation amplitude |
| DA_c | Deflection amplitude |
| DL | Deflection length |
| dArcL | The change of arc length within 7 mm corneal center |
| Darea | Deflection area |
| HC | The highest concavity |
| WEM | Whole eye movement |
| Max | Maximal |
| ICR | Inverse concave radius |
| bIOP | Biomechanically corrected intraocular pressure |
| SSI | Stress strain index |
| Pachy Slope (a.u.) | The slope of corneal thickness (arbitrary unit) |
| DA Ratio 1 mm (2 mm) | DA ratio between cornea apex and paracentral 1 mm (2 mm) |
| ARTh | Ambrosio Relational Thickness horizontal |
| SP-A1 | Stiffness parameter at the first applanation |
| CBI | Corneal (or Corvis) biomechanical index |
References
- Nelson, J.D.; Craig, J.P.; Akpek, E.K.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Clayton, J.A.; Dogru, M.; Dua, H.S.; Foulks, G.N.; et al. TFOS DEWS II Introduction. Ocul. Surf. 2017, 15, 269–275. [Google Scholar] [CrossRef]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-Del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II Management and Therapy Report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Wang, M.T.M.; Vidal-Rohr, M.; Menduni, F.; Dhallu, S.; Ipek, T.; Acar, D.; Recchioni, A.; France, A.; Kingsnorth, A.; et al. Demographic and lifestyle risk factors of dry eye disease subtypes: A cross-sectional study. Ocul. Surf. 2021, 21, 58–63. [Google Scholar] [CrossRef]
- McDonald, M.; Patel, D.A.; Keith, M.S.; Snedecor, S.J. Economic and Humanistic Burden of Dry Eye Disease in Europe, North America, and Asia: A Systematic Literature Review. Ocul. Surf. 2016, 14, 144–167. [Google Scholar] [CrossRef] [PubMed]
- Efraim, Y.; Chen, F.Y.T.; Stashko, C.; Cheong, K.N.; Gaylord, E.; McNamara, N.; Knox, S.M. Alterations in corneal biomechanics underlie early stages of autoimmune-mediated dry eye disease. J. Autoimmun. 2020, 114, 102500. [Google Scholar] [CrossRef]
- Long, Q.; Wang, J.Y.; Xu, D.; Li, Y. Comparison of corneal biomechanics in Sjogren’s syndrome and non-Sjogren’s syndrome dry eyes by Scheimpflug based device. Int. J. Ophthalmol. 2017, 10, 711–716. [Google Scholar] [PubMed]
- Satitpitakul, V.; Taweekitikul, P.; Puangsricharern, V.; Kasetsuwan, N.; Reinprayoon, U.; Kittipibul, T. Alteration of corneal biomechanical properties in patients with dry eye disease. PLoS ONE 2021, 16, e0254442. [Google Scholar] [CrossRef]
- Long, Q.; Wang, J.; Yang, X.; Jin, Y.; Ai, F.; Li, Y. Assessment of Corneal Biomechanical Properties by CorVis ST in Patients with Dry Eye and in Healthy Subjects. J. Ophthalmol. 2015, 2015, 380624. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, J.; Tan, Y.; Wang, Y. Unraveling the mechanobiology of cornea: From bench side to the clinic. Front. Bioeng. Biotechnol. 2022, 10, 953590. [Google Scholar] [CrossRef]
- Reyes, J.L.; Vannan, D.T.; Eksteen, B.; Avelar, I.J.; Rodriguez, T.; Gonzalez, M.I.; Mendoza, A.V. Innate and Adaptive Cell Populations Driving Inflammation in Dry Eye Disease. Mediat. Inflamm. 2018, 2018, 2532314. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Feulner, L.; Djonov, V.; Pavlovic, D.; Volarevic, V. The Molecular Mechanisms Responsible for Tear Hyperosmolarity-Induced Pathological Changes in the Eyes of Dry Eye Disease Patients. Cells 2023, 12, 2755. [Google Scholar] [CrossRef] [PubMed]
- De Paiva, C.S.; Chotikavanich, S.; Pangelinan, S.B.; Pitcher, J.D., 3rd; Fang, B.; Zheng, X.; Ma, P.; Farley, W.J.; Siemasko, K.F.; Niederkorn, J.Y.; et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009, 2, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, Y.; Wei, P.; Jhanji, V. Biomechanics and structure of the cornea: Implications and association with corneal disorders. Surv. Ophthalmol. 2018, 63, 851–861. [Google Scholar] [CrossRef]
- Lan, G.; Twa, M.D.; Song, C.; Feng, J.; Huang, Y.; Xu, J.; Qin, J.; An, L.; Wei, X. In vivo corneal elastography: A topical review of challenges and opportunities. Comput. Struct. Biotechnol. J. 2023, 21, 2664–2687. [Google Scholar] [CrossRef] [PubMed]
- Koprowski, R. Automatic method of analysis and measurement of additional parameters of corneal deformation in the Corvis tonometer. Biomed. Eng. Online 2014, 13, 150. [Google Scholar] [CrossRef]
- Fotovat-Ahmadi, N.; Siddiqui, O.; Ong, J.; Thanitcul, C.; Reinhardt, C.; Cologna, S.M.; Aakalu, V.K. The ocular surface tear film as a biomarker for systemic health. Ocul. Surf. 2025, 37, 283–300. [Google Scholar] [CrossRef]
- Da Silva, F.; Linhares, J.M.M.; Lira, M. The influence of the tear film on the intraocular pressure and the corneal biomechanical properties analyzed with the Ocular Response Analyzer. J. Optom. 2024, 17, 100488. [Google Scholar] [CrossRef]
- Komninou, M.A.; Seiler, T.G.; Enzmann, V. Corneal biomechanics and diagnostics: A review. Int. Ophthalmol. 2024, 44, 132. [Google Scholar] [CrossRef]
- Johnson, M.E.; Murphy, P.J. Changes in the tear film and ocular surface from dry eye syndrome. Prog. Retin. Eye Res. 2004, 23, 449–474. [Google Scholar] [CrossRef]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef]
- Wang, M.T.M.; Craig, J.P. Comparative Evaluation of Clinical Methods of Tear Film Stability Assessment: A Randomized Crossover Trial. JAMA Ophthalmol. 2018, 136, 291–294. [Google Scholar] [CrossRef]
- Vidas Pauk, S.; Petricek, I.; Jukic, T.; Popovic-Suic, S.; Tomic, M.; Kalauz, M.; Jandrokovic, S.; Masnec, S. Noninvasive Tear Film Break-up Time Assessment Using Handheld Lipid Layer Examination Instrument. Acta Clin. Croat. 2019, 58, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Szczesna-Iskander, D.H.; Llorens-Quintana, C. Agreement between invasive and noninvasive measurement of tear film breakup time. Sci. Rep. 2024, 14, 3852. [Google Scholar] [CrossRef]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Qu, J.H.; Zhang, X.Y.; Sun, X.G. Repeatability and Reproducibility of Noninvasive Keratograph 5M Measurements in Patients with Dry Eye Disease. J. Ophthalmol. 2016, 2016, 8013621. [Google Scholar] [CrossRef]
- Pult, H.; Riede-Pult, B. Comparison of subjective grading and objective assessment in meibography. Contact Lens Anterior Eye 2013, 36, 22–27. [Google Scholar] [CrossRef]
- Bron, A.J.; Evans, V.E.; Smith, J.A. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003, 22, 640–650. [Google Scholar] [CrossRef]
- Vinciguerra, R.; Ambrosio, R., Jr.; Elsheikh, A.; Roberts, C.J.; Lopes, B.; Morenghi, E.; Azzolini, C.; Vinciguerra, P. Detection of Keratoconus with a New Biomechanical Index. J. Refract. Surg. 2016, 32, 803–810. [Google Scholar] [CrossRef]
- Lin, F.Y.; Ho, R.W.; Yu, H.J.; Yang, I.H.; Fang, P.C.; Kuo, M.T. Impacts and Correlations on Corneal Biomechanics, Corneal Optical Density and Intraocular Pressure after Cataract Surgery. Diagnostics 2024, 14, 1557. [Google Scholar] [CrossRef]
- Jedzierowska, M.; Koprowski, R. Novel dynamic corneal response parameters in a practice use: A critical review. Biomed. Eng. Online 2019, 18, 17. [Google Scholar] [CrossRef]
- Valbon, B.F.; Ambrosio, R., Jr.; Fontes, B.M.; Luz, A.; Roberts, C.J.; Alves, M.R. Ocular biomechanical metrics by CorVis ST in healthy Brazilian patients. J. Refract. Surg. 2014, 30, 468–473. [Google Scholar] [CrossRef]
- Yang, K.; Xu, L.; Fan, Q.; Gu, Y.; Song, P.; Zhang, B.; Zhao, D.; Pang, C.; Ren, S. Evaluation of new Corvis ST parameters in normal, Post-LASIK, Post-LASIK keratectasia and keratoconus eyes. Sci. Rep. 2020, 10, 5676. [Google Scholar] [CrossRef]
- Tung, C.I.; Perin, A.F.; Gumus, K.; Pflugfelder, S.C. Tear meniscus dimensions in tear dysfunction and their correlation with clinical parameters. Am. J. Ophthalmol. 2014, 157, 301–310.e1. [Google Scholar] [CrossRef]
- Blackburn, B.J.; Jenkins, M.W.; Rollins, A.M.; Dupps, W.J. A Review of Structural and Biomechanical Changes in the Cornea in Aging, Disease, and Photochemical Crosslinking. Front. Bioeng. Biotechnol. 2019, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, Z.S.; Sreenivasaiah, S.; Deshmukh, S.; Mangala, L.; Shroff, S.; Devi, S.; Webers, C.A.; Rao, H.L. Factors affecting corneal deformation amplitude measured by Corvis ST in eyes with open-angle glaucoma. Indian J. Ophthalmol. 2024, 72, 533–537. [Google Scholar] [CrossRef]
- Asaoka, R.; Nakakura, S.; Tabuchi, H.; Murata, H.; Nakao, Y.; Ihara, N.; Rimayanti, U.; Aihara, M.; Kiuchi, Y. The Relationship between Corvis ST Tonometry Measured Corneal Parameters and Intraocular Pressure, Corneal Thickness and Corneal Curvature. PLoS ONE 2015, 10, e0140385. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Kim, E.C.; Kim, M.S.; Yang, S.W. A novel method for quantifying the biomechanical parameters of orbital soft tissue using a corneal dynamic scheimpflug analyser: A retrospective study. BMC Ophthalmol. 2019, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, S.; Sun, H.; He, H.; Shi, Y.; Wu, Y.; Wu, H.; Liu, Z.; Zhuang, J.; Li, W. Lacrimal Gland Microenvironment Changes After Obstruction of Lacrimal Gland Ducts. Investig. Ophthalmol. Vis. Sci. 2022, 63, 14. [Google Scholar] [CrossRef]
- Zhang, M.; Tian, X.; Wu, Y.; Zhang, L.; Zhang, L.; Zheng, X.; Ou, S.; Gu, H. Establishment of A Mouse Model of Aqueous Deficiency Dry Eye. J. Vis. Exp. 2024, 213, e67317. [Google Scholar] [CrossRef]
- Grieve, K.; Ghoubay, D.; Georgeon, C.; Latour, G.; Nahas, A.; Plamann, K.; Crotti, C.; Bocheux, R.; Borderie, M.; Nguyen, T.M.; et al. Stromal striae: A new insight into corneal physiology and mechanics. Sci. Rep. 2017, 7, 13584. [Google Scholar] [CrossRef] [PubMed]
- Dogru, M.; Simsek, C.; Kojima, T.; Aketa, N.; Tsubota, K.; Shimazaki, J. The Impact of Noncontact Tonometry and Icare Rebound Tonometry on Tear Stability and Dry Eye Clinical Practice. J. Clin. Med. 2022, 11, 2819. [Google Scholar] [CrossRef]
- Yousefi, A.; Ma, Y.; Roberts, C.J.; Moroi, S.E.; Reilly, M.A. Hydrodynamic Interaction Between Tear Film and Air Puff from Noncontact Tonometry. Transl. Vis. Sci. Technol. 2022, 11, 2. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef]
- Behrens, A.; Doyle, J.J.; Stern, L.; Chuck, R.S.; McDonnell, P.J.; Azar, D.T.; Dua, H.S.; Hom, M.; Karpecki, P.M.; Laibson, P.R.; et al. Dysfunctional tear syndrome: A Delphi approach to treatment recommendations. Cornea 2006, 25, 900–907. [Google Scholar] [CrossRef]
- Yazdani, M.; Fiskadal, J.; Chen, X.; Utheim, O.A.; Raeder, S.; Vitelli, V.; Utheim, T.P. Tear Film Break-Up Time and Dry Eye Disease Severity in a Large Norwegian Cohort. J. Clin. Med. 2021, 10, 884. [Google Scholar] [CrossRef]
- Alfaro-Juarez, A.; Caro-Magdaleno, M.; Montero-Iruzubieta, J.; Fernandez-Palacin, A.; Munoz-Morales, A.; Castilla-Martino, M.A.; Spinola-Munoz, C.; Rodriguez de la Rua, E. Keratograph 5M As a Useful and Objective Tool for Evaluating the Ocular Surface in Limbal Stem Cell Deficiency. Clin. Ophthalmol. 2019, 13, 2025–2033. [Google Scholar] [CrossRef] [PubMed]
- Wolffsohn, J.S.; Benitez-Del-Castillo, J.M.; Loya-Garcia, D.; Inomata, T.; Iyer, G.; Liang, L.; Pult, H.; Sabater, A.L.; Starr, C.E.; Vehof, J.; et al. TFOS DEWS III: Diagnostic Methodology. Am. J. Ophthalmol. 2025, 279, 387–450. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).