Abstract
Speckle-tracking echocardiography (STE) has become an established, widely available diagnostic method in the past few years, making its value clear in cases of COVID-19 and the further course of the disease, including post-COVID syndrome. Since the beginning of the pandemic, many studies have been published on the use of STE in this condition, enabling, on the one hand, a better understanding of myocardial involvement in COVID-19 and, on the other, a better identification of risk to patients, although some questions remain unanswered in regard to specific pathomechanisms, especially in post-COVID patients. This review takes a closer look at current findings and potential future developments by summarising the extant data on the use of STE, with a focus on left and right ventricular longitudinal strain.
1. Introduction
Speckle-tracking echocardiography (STE), which in daily practice most commonly measures left and right ventricular global longitudinal strain (LV GLS and RV GLS, respectively), is increasingly used to evaluate myocardial function, and this assessment is now considered a valuable tool in the diagnosis and management of all types of cardiomyopathies. Strain, defined as the percentage change in the length of the myocardium, provides a measure of the myocardium’s deformation in response to changes in pressure or volume. Strain values can be derived in each direction (longitudinal, radial, circumferential) and may be assessed from 2D and 3D images as well as in post-processing. STE has proven its value in diverse cardiomyopathies, such as takotsubo cardiomyopathy, myocarditis, cancer therapy related cardiac dysfunction, coronary artery disease and myocardial infarction [1,2,3,4,5]. An example of a reduced LV GLS using 2D-STE is shown in Figure 1, and an example of 3D-STE in a healthy athlete is shown in Figure 2.
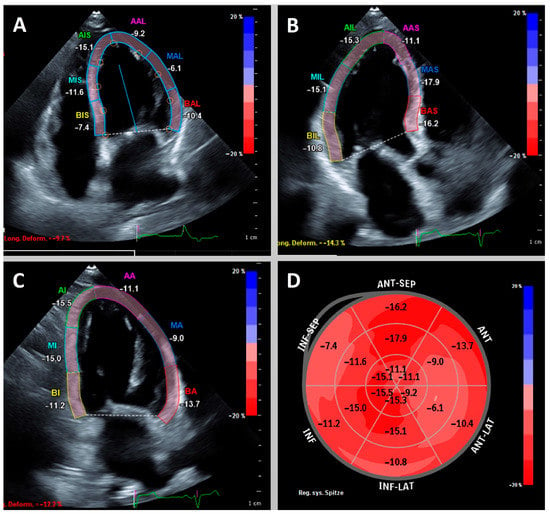
Figure 1.
Example of a patient with reduced left ventricular global longitudinal strain (GLS) of −12.1%. Apical 4-, 3- and 2-chamber view (A–C) with highlighted left ventricular myocardium. Bullseye plot (D) shows globally reduced longitudinal strain with accentuation basally septal and midventricular anterior/anterolateral.
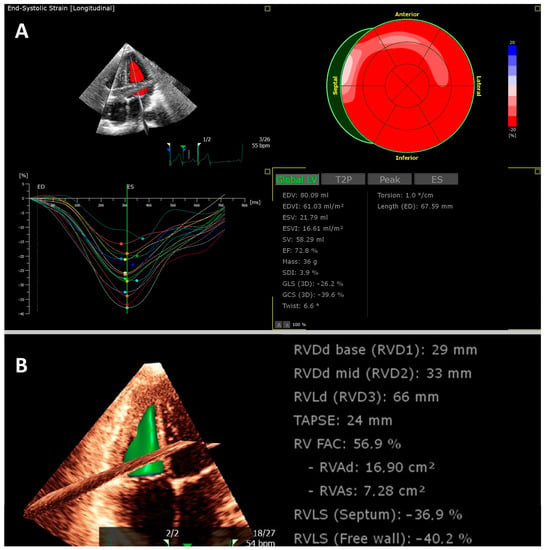
Figure 2.
Example of a young athlete who was examined after COVID-19 before returning to sport. Transthoracic 3D echocardiography revealed normal left ventricular (A) and right ventricular function (B).
Using STE in SARS-CoV-2 virus (COVID-19) patients made sense, as myocardial involvement was commonly found in these infections, especially in critically ill patients. Even in mild forms of myocardial dysfunction, deformation parameters are more accurate than classical volumetric evaluation and ejection fractions and have demonstrated good inter- and intra-observer reliability. Furthermore, the ability to perform echocardiography even at the bedside in a hospital ward gives STE an advantage over other imaging modalities, such as cardiovascular magnetic resonance (CMR) imaging and cardiac computed tomography.
In acute COVID-19, myocardial involvement has been shown to be prognostically significant, with increased mortality in patients with elevated troponin [6]. Various possibilities of myocardial involvement may be conceived, and myocardial infarctions, myocarditis and micro- and macro-embolisms have been detected in autopsy studies [7]. Arrhythmias and stress cardiomyopathies have also been described and affect patient prognosis [8,9]. The pathogenesis is diverse including viral endothelitis, humoral and prothrombotic changes and systemic inflammatory response syndrome [10,11,12]. In particular, the co-expression of angiotensin convertase enzyme 2 (ACE-2) and transmembrane serine protease 2 (TMPRSS2) on the cell surfaces of some tissues as the site of invasion of SARS-CoV-2 appears to have an impact on disease progression and multi-organ involvement [13,14].
Myocardial changes have also been reported in the post-COVID setting. Post-COVID describes a syndrome emerging in the context of COVID-19 that involves persistent symptoms even after three or more months, including fatigue, dyspnoea and chest pain [15,16]. Some studies have identified the presence of heart failure, myocarditis and left ventricular dysfunction following COVID-19, but further research is needed to fully understand the long-term cardiac impact of COVID-19 and the mechanisms underlying these changes. A loss of myocardial function is often mild and observable only in deformation parameters, not in common volumetric assessments, such as the left ventricular ejection fraction.
This review describes the current state of the use of STE in the acute COVID-19 and post-COVID contexts. We illustrate the potential and limitations of the methodology and highlight avenues for further development.
2. Strain in Acute COVID-19
The COVID-19 pandemic demanded a high degree of flexibility from the medical system. Especially in severely ill patients in the alpha- and delta-dominant waves, myocardial involvement was frequently observed with a poor outcome. Hygiene measures were adopted to provide the highest level of safety for medical staffs, but, in many hospitals, this limited the availability of diagnostic measures, e.g., CT and MRI imaging. Consequently, the possibility was soon considered of conducting bedside examinations using STE. An early article by Stöbe et al., which described a comprehensive examination protocol with LV strain in all three orientations in 19 patients during the first wave of the disease, hypothesised that STE could diagnose the myocardial involvement of COVID-19 [17]. A pattern of myocardial dysfunction was seen, with changes predominantly in the inferolateral and anterolateral basal segments. Free wall RV LS was reduced in 4 of the 19 patients and was accompanied by elevation of troponin T and NT-proBNP.
In a small prospective study of only 30 patients with signs of myocardial involvement marked by elevated troponin I, a significantly reduced free wall RV LS was found in nonsurvivors as compared to survivors (−15.6% vs. −24.3%, respectively, p = 0.0018) [18]. Li et al. further investigated the observed impairments in right ventricular strain [19]. In their single-centre study, patients with COVID-19 were examined bedside in their wards on the seventh day (on average) after hospital admission. Patients with known ischaemic and nonischaemic cardiomyopathy were excluded. The final 120 evaluable patients were divided into tertiles according to their free wall RV LS. Patients in the lowest tertile (−10.3% to −20.5%) had acute heart injury, acute respiratory distress syndrome (ARDS) and deep vein thrombosis more often than those in the other tertiles, and the latter finding especially suggests the possibility of thromboembolic events. A cut-off value of worse than −23% in free wall RV LS was associated with a significantly increased mortality. In a receiver operating characteristics analysis, the area under the curve (AUC) of free wall RV LS was 0.87 and thus clearly superior to the fractional area change (FAC) and the tricuspid annular plane systolic excursion (TAPSE) (which yielded AUC 0.72 and AUC 0.67, respectively).
A later study by Bleakley et al. of 90 critically ill patients requiring venovenous extracorporeal membrane oxygenation (ECMO) also examined the parameters of right ventricular function [20]. Classical parameters, such as diameter and FAC, which assess radial rather than longitudinal function, were significantly more likely to diagnose right ventricular dysfunction than free wall RV LS. The authors discuss an altered pattern of right ventricular dysfunction due to the more acute right ventricular afterload increase caused by endotheliitis, microembolism and macroembolism in the pulmonary stromal bed, with different influences on the right ventricular microstructure than those of chronic stress. Whether the specific setting of ECMO patients has an influence remains unclear.
Other studies examining right ventricular strain produced diverse results. For example, free wall RV LS was an independent predictor of mortality in a study of 132 patients by Xie et al., while Park et al. found no association with that outcome in only 48 patients [21,22]. Both studies took place in a similar time frame, so a different SARS-CoV-2 variant could not have significantly influenced the results.
A significant reduction of LV GLS has also been observed in COVID-19 patients. The degree of impairment correlated with NT-proBNP, troponin and inflammatory parameters in several studies [23,24], and an impaired LV GLS was associated with higher mortality. A correlation to the specific SARS-CoV-2 variant was not detectable in the influence on deformation parameters. In a study by Ghantous et al. of 148 patients infected with the omicron variant, no significant differences were found in LV GLS, RV GLS and free wall RV LS in comparison to a matched cohort with the wild-type variant [25], but differences in right ventricular geometry (FAC, RV end-systolic area) and maximal tricuspid regurgitation velocity were found in favour of the omicron-infected patients. In addition, the authors found lower values for the echocardiographically estimated pulmonary vascular resistance index, which they attribute to less lung parenchymal or vascular damage, fewer cytokine storms or combined effects. A study by Bhatia et al. also examined LV GLS in patients with COVID-19 [26]. They found reduced LV GLS values in 91% of patients, with a median value of −13.5% [IQR −15.0%, −10.8%] despite a normal left ventricular ejection fraction (LVEF) with a median of 62% [IQR 56%, 68%]. In this small cohort with follow-up examinations during inpatient stay, there was a trend towards improvement in the LV GLS in patients who could be discharged in contrast to patients with fatal courses.
Strain analysis of the atrial function is also possible, even if most examiners have not yet incorporated it into their daily routine. In a study of left atrial strain in critically ill patients requiring intensive care, left atrial strain was better able to identify patients with diastolic dysfunction than classical echocardiographic parameters [27]. Impaired left atrial strain was correlated with protracted inflammatory states and changes in the differential blood count.
D’Andrea et al. studied 55 patients with COVID-19-associated CMR-diagnosed myocarditis according to the updated Lake Louise criteria [28]. The CMR occurred during the first 14 days after hospital admission. Compared to matched healthy controls, the myocarditis patients saw a reduction in LV GLS, which was correlated to absolute scar burden in terms of late gadolinium enhancement. A follow-up examination showed a functional improvement in the myocarditis patients. Baseline LV GLS, LVEF and the extent of late gadolinium enhancement were independent predictors of LVEF at six months, which leads to the next section. (Table 1 summarises current studies of STE in COVID-19 patients.)

Table 1.
Studies using speckle-tracking echocardiography with a focus on acute COVID-19.
3. Strain in the Diagnostic Work-Up of Post-COVID Syndrome
A strength of STE is that it detects discrete, subclinical functional changes. The published data mostly describe only minor changes that do not reach pathological values in post-COVID patients. However, discrete impairments can be detected in comparison to healthy controls [29,30,31,32]. When interpreting the results of the following studies, it is partly necessary to take a close look at the inclusion criteria. A detailed presentation of these would go beyond the limits of this review due to the heterogeneity and their complexity.
A study by Caiado et al. of 100 post-COVID patients with an examination after 130 ± 70 days found no significant differences in LV GLS compared to healthy subjects [33]. There was a local change in the function of basal segments, however, which is consistent with Stöbe et al.’s descriptions in acute disease as discussed above [17].
Actual epidemiological data are not yet available on the incidence of STE changes in post-COVID patients. Data from the Epidemiology of Long Covid (EPILOC) study will provide further information in the near future. A study (without STE) by Petersen et al. of 443 patients 9.6 months after COVID-19 found reduced LVEF and increased levels of troponin T [34]. With numerical differences of 1.17% and 0.17 ng/L, respectively, the influence on patient treatment is questionable despite their statistical significance. To our knowledge, the largest study to date on STE in post-COVID patients is that of Garcia-Zamora et al. [35], which included 595 patients referred for echocardiography after COVID-19 in 10 hospitals in South America. There was a reduction in LV GLS (defined as >−18.0%) in 5.7% of the patients and a reduction in RV GLS (defined as >−20.0%) in 3.0%.
A study by Young et al. found no significant changes in LV GLS, RV GLS or free wall RV LS among 259 patients on whom STE was performed both before and after COVID-19 [36]. Only 27 patients experienced a worsening of a strain parameter, while 49 patients experienced new or worsening symptoms in the context of COVID-19 (19%). Patients who exhibited the worsening of a strain parameter during COVID-19 were significantly clustered in the group of patients with new symptoms.
Oikonomou and colleagues found a significant reduction in LV GLS in 34 post-COVID patients with STE compared to a healthy cohort (−18.4% vs. −22.0%, respectively, p < 0.001) [30]. LV GLS improved significantly in this COVID cohort up to the six-month follow-up but did not reach the levels seen in healthy subjects. In contrast, Baruch et al.’s study observed no improvement in LV GLS 88 days after infection. In that study, reduced LV GLS values were detected in 33% of patients during their hospitalisation (>−16.1% reduction for men and > −17.3% for women) [37]. At follow-up, this was the case in only 25%, which represents a numerical but not statistically significant improvement. A study by Lassen et al. also found no significant improvement in LV GLS [29]. The researchers studied 91 patients who had to be hospitalised because of COVID-19. They found a reduced LV GLS that did not improve in the follow-up examination 77 days (on average) after the first examination (−17.4 ± 2.9% vs. −17.6 ± 3.3%, respectively, p = 0.6). In contrast, there was a significant improvement in RV GLS (−25.3 ± 5.5% vs. −19.9 ± 5.8%, respectively, p < 0.001), in which the results are consistent with those of Baruch et al. [37].
Relevant long-term symptoms are often found even in mild courses of initial COVID-19. Especially in the case of dyspnoea, the question of myocardial damage thus also arises in oligosymptomatic courses. Indeed, Akkaya et al. found a relevant right ventricular dysfunction in their study of 105 mildly ill patients compared to a healthy control cohort [38]. Both RV GLS (−15.1 ± 3.4% vs. −19.6 ± 5.2%, p < 0.001) and free wall RV LS (−17.2 ± 4.4% vs. −19.6 ± 5.2%, p < 0.001) were significantly reduced in the post-COVID group as compared to controls. This study also found a correlation of the level of inflammatory blood values and D-dimers with right ventricular dysfunction. Therefore, thromboembolic and vasculitic mechanisms are also discussed in this patient population, which seems reasonable, as haematological, inflammatory and rheological changes occur under COVID-19 and often persist for a long time [11,39].
Other studies have found a clear correlation between initial disease severity and right ventricular function in STE during follow-up [32,40]. Hospitalised patients and patients with severe COVID-19 pneumonia were associated with reduced RV strain, and left ventricular function has exhibited similar correlations. For example, in a study by Mahajan et al., LV GLS was significantly worse in patients with severe COVID-19 than in those with moderate or mild disease severity (−15.5 ± 3.1% vs. −18.1 ± 6.9% vs. −21.0 ± 3.4%, p < 0.001) [41]. Overall, 29.9% had reduced LV GLS, which was defined as a value below the mean of a control group studied with the same protocol (−19.2%). This approach potentially overestimates the number of ‘pathological’ findings. The usual adjustment is ±1.96 standard deviations, and other authors obtain threshold values for LV GLS of −16.7% for men and −17.8% for women [42]; this adjustment would significantly reduce the number of abnormal findings in the study of Mahajan et al.
Most investigations find a normal ejection fraction with discrete changes in STE. In fact, the question arises of whether these echocardiographic changes are attributable to the often-high symptom burden or are merely an epiphenomenon. Several studies report correlations between an accumulation of abnormal STE values and patients with increased symptoms [36,43,44,45]. Because this is not consistently detectable in all symptomatic patients with post-COVID, however, a noncausal correlation must be assumed. Discretely elevated inflammatory biomarkers are detectable particularly in patients with a high symptom burden. Relatedly, in other conditions, myocardial dysfunction is often associated with an increase in these markers [46]. In this respect, a reduction of the deformation parameters in post-COVID could be a surrogate parameter of an ongoing disease process. However, other causes of functional abnormalities in patients with a higher symptom burden like detraining must be discussed, as well [47,48].
This assumption may also be supported by another study. In their study of 184 post-COVID patients, Shimoni et al. report a correlation of reduced maximal oxygen uptake in cardiopulmonary exercise testing with reduced LV GLS [31], which suggests a more systemic disease state. Regarding the right ventricle and its good regenerative capacity after acute COVID-19 as mentioned above, pulmonary changes are more likely (e.g., vascular scarification in the case of lung damage, thromboembolism).
In the work-up of patients with persistent symptoms after COVID-19, pre-existing conditions must be considered, as well [49]. These can also be unrecognised so far undetected, such as subclinical coronary artery disease, which also goes in line with a reduced global or regional strain [5]. In a study by Gherbesi et al., this confounder can be excluded [50]. Here, 40 young athletes aged 24.4 ± 8.3 years had worse LV GLS (but not RV GLS) than age- and gender-matched controls. The clinical impact in this asymptomatic cohort with a LV GLS of −22.7 ± 1.6% is unclear. Screening for myocardial involvement in asymptomatic patients is not recommended [49].
Of course, irreversible changes and damage in the context of acute COVID-19 may also be causal for the described changes in STE. For example, post-myocardial scars can lead to a regional restriction of the deformation parameters [28]. In their study of 123 patients, Italia et al. describe a significant reduction in LV GLS in patients with persistently elevated levels of troponin T [51], which suggests persistent and ongoing myocardial damage as the cause of myocardial dysfunction. Studies with cardiovascular magnetic resonance imaging suggest a complex picture with ischaemic and inflammatory signs in noninvasive tissue characterization [52,53]. The cause of such a persistent injury is ultimately unclear, and diverse hypotheses have been proposed, including immunological aberrations, autoimmunity, a change in the patient’s microbiome, metabolic dysregulation and microvascular, endothelial or hormonal dysfunctions [35,54,55,56]. Post-COVID is a multifactorial disease in which microvascular perfusion disturbances and inflammation may be most important denominators for cardiac dysfunction [49]. However, the pathophysiology should be eluded in cardiovascular studies which include parameters of microvascular perfusion and inflammation (Table 2 provides an overview of current data).

Table 2.
Studies using speckle-tracking echocardiography with a focus on post-COVID. Note that the cohorts are very heterogeneous due to the individual inclusion criteria. This could not be presented here in full.
4. Current Limitations of Knowledge and Future Directions
The present review has attempted to give a comprehensive overview of the current data on STE in COVID-19 and post-COVID syndrome. This revealed a great heterogeneity of the studies. Disease severity of COVID-19 and time of the exams were very different, so that presumably no meta-analysis will be possible. Heterogeneity of the cohorts and often a small sample size are most likely the reasons for sometimes contradictory results.
It must also be borne in mind that STE itself has relevant limitations. There is a risk of foreshortening and through-plane motion [57]. There is also a certain dependence of the STE measurement on the vendor and the software used [42,57,58]. Due to a high dependence on image quality, many patients were excluded from the cited studies.
The investigation of myocardial involvement under COVID-19 may be transferable to other diseases. In the future, subclinical myocardial dysfunction may be investigated in the context of other infectious diseases such as influenza, respiratory syncytial virus, Epstein–Barr virus and pneumococcal infections. The development of new and more pathogenic SARS-CoV-2 variants is also still conceivable.
5. Conclusions
STE with its main examination modalities, LV and RV longitudinal strain, is a good diagnostic tool for the detection of myocardial dysfunction, even in the presence of normal ejection fractions. It has shown its prognostic potential in acute COVID-19. Changes in both the right ventricular and left ventricular longitudinal strain were associated with higher mortality and morbidity in hospitalised COVID-19 patients.
In survivors, the deformation parameters are mostly normal, although worse compared to healthy controls. Epidemiological studies are currently not available, so no statement can be made about the actual prevalence of myocardial dysfunction. Despite frequently small and, in part, contradictory studies, it can be said that the RV GLS seems to have a better tendency to recover after infection than the LV GLS. This is most likely due to different pathomechanisms with at least partially reversible changes in the pulmonary stromal bed for right ventricular dysfunction and scarring and persistent microvascular dysfunction as leading causes for left ventricular dysfunction. However, a reduction of the deformation parameters may have various reasons, and a conclusion regarding an exact pathomechanism is not possible in most cases.
Author Contributions
Conceptualization, J.K. (Johannes Kersten) and J.M.S.; writing—original draft preparation, J.K. (Johannes Kersten); writing—review and editing, J.S., A.J., J.K. (Johannes Kirsten), H.P., Y.L., J.M.S.; visualization, J.K. (Johannes Kersten) and J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Doerner, J.; Bunck, A.C.; Michels, G.; Maintz, D.; Baeßler, B. Incremental value of cardiovascular magnetic resonance feature tracking derived atrial and ventricular strain parameters in a comprehensive approach for the diagnosis of acute myocarditis. Eur. J. Radiol. 2018, 104, 120–128. [Google Scholar] [CrossRef]
- Gavara, J.; Rodriguez-Palomares, J.F.; Valente, F.; Monmeneu, J.V.; Lopez-Lereu, M.P.; Bonanad, C.; Ferreira-Gonzalez, I.; Garcia Del Blanco, B.; Rodriguez-Garcia, J.; Mutuberria, M.; et al. Prognostic Value of Strain by Tissue Tracking Cardiac Magnetic Resonance After ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc. Imaging 2018, 11, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Stiermaier, T.; Lange, T.; Chiribiri, A.; Möller, C.; Graf, T.; Villnow, C.; Raaz, U.; Villa, A.; Kowallick, J.T.; Lotz, J.; et al. Left ventricular myocardial deformation in Takotsubo syndrome: A cardiovascular magnetic resonance myocardial feature tracking study. Eur. Radiol. 2018, 28, 5160–5170. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Kokkinidis, D.G.; Kampaktsis, P.N.; Amir, E.A.; Marwick, T.H.; Gupta, D.; Thavendiranathan, P. Assessment of Prognostic Value of Left Ventricular Global Longitudinal Strain for Early Prediction of Chemotherapy-Induced Cardiotoxicity: A Systematic Review and Meta-analysis. JAMA Cardiol. 2019, 4, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Gaibazzi, N.; Bergamaschi, L.; Pizzi, C.; Tuttolomondo, D. Resting global longitudinal strain and stress echocardiography to detect coronary artery disease burden. Eur. Heart J.-Cardiovasc. Imaging 2023, jead046. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury with Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Halushka, M.K.; Vander Heide, R.S. Myocarditis is rare in COVID-19 autopsies: Cardiovascular findings across 277 postmortem examinations. Cardiovasc. Pathol. 2021, 50, 107300. [Google Scholar] [CrossRef]
- Nishiga, M.; Wang, D.W.; Han, Y.; Lewis, D.B.; Wu, J.C. COVID-19 and cardiovascular disease: From basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020, 17, 543–558. [Google Scholar] [CrossRef]
- Timpau, A.-S.; Miftode, R.-S.; Leca, D.; Timpau, R.; Miftode, I.-L.; Petris, A.O.; Costache, I.I.; Mitu, O.; Nicolae, A.; Oancea, A.; et al. A Real Pandora’s Box in Pandemic Times: A Narrative Review on the Acute Cardiac Injury Due to COVID-19. Life 2022, 12, 1085. [Google Scholar] [CrossRef]
- Yamaoka-Tojo, M. Vascular Endothelial Glycocalyx Damage in COVID-19. Int. J. Mol. Sci. 2020, 21, 9712. [Google Scholar] [CrossRef]
- Bizjak, D.A.; John, L.; Matits, L.; Uhl, A.; Schulz, S.V.W.; Schellenberg, J.; Peifer, J.; Bloch, W.; Weiß, M.; Grüner, B.; et al. SARS-CoV-2 Altered Hemorheological and Hematological Parameters during One-Month Observation Period in Critically Ill COVID-19 Patients. Int. J. Mol. Sci. 2022, 23, 15332. [Google Scholar] [CrossRef] [PubMed]
- Vollenberg, R.; Tepasse, P.-R.; Ochs, K.; Floer, M.; Strauss, M.; Rennebaum, F.; Kabar, I.; Rovas, A.; Nowacki, T. Indications of Persistent Glycocalyx Damage in Convalescent COVID-19 Patients: A Prospective Multicenter Study and Hypothesis. Viruses 2021, 13, 2324. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, S.; Cegolon, L.; Khafaei, M.; Gholami, N.; Zhao, S.; Khalesi, N.; Moosavian, H.; Fathi, S.; Izadi, M.; Ghadian, A.; et al. Gastrointestinal cancers, ACE-2/TMPRSS2 expression and susceptibility to COVID-19. Cancer Cell Int. 2021, 21, 431. [Google Scholar] [CrossRef] [PubMed]
- Rahban, M.; Stanek, A.; Hooshmand, A.; Khamineh, Y.; Ahi, S.; Kazim, S.N.; Ahmad, F.; Muronetz, V.; Samy Abousenna, M.; Zolghadri, S.; et al. Infection of Human Cells by SARS-CoV-2 and Molecular Overview of Gastrointestinal, Neurological, and Hepatic Problems in COVID-19 Patients. J. Clin. Med. 2021, 10, 4802. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef]
- Peter, R.S.; Nieters, A.; Kräusslich, H.-G.; Brockmann, S.O.; Göpel, S.; Kindle, G.; Merle, U.; Steinacker, J.M.; Rothenbacher, D.; Kern, W.V. Post-acute sequelae of covid-19 six to 12 months after infection: Population based study. BMJ 2022, 379, e071050. [Google Scholar] [CrossRef]
- Stöbe, S.; Richter, S.; Seige, M.; Stehr, S.; Laufs, U.; Hagendorff, A. Echocardiographic characteristics of patients with SARS-CoV-2 infection. Clin. Res. Cardiol. 2020, 109, 1549–1566. [Google Scholar] [CrossRef]
- Stockenhuber, A.; Vrettos, A.; Androschuck, V.; George, M.; Robertson, C.; Bowers, N.; Clifford, P.; Firoozan, S. A pilot study on right ventricular longitudinal strain as a predictor of outcome in COVID-19 patients with evidence of cardiac involvement. Echocardiography 2021, 38, 222–229. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Zhu, S.; Xie, Y.; Wang, B.; He, L.; Zhang, D.; Zhang, Y.; Yuan, H.; Wu, C.; et al. Prognostic Value of Right Ventricular Longitudinal Strain in Patients With COVID-19. JACC Cardiovasc. Imaging 2020, 13, 2287–2299. [Google Scholar] [CrossRef]
- Bleakley, C.; Singh, S.; Garfield, B.; Morosin, M.; Surkova, E.; Mandalia, M.S.; Dias, B.; Androulakis, E.; Price, L.C.; McCabe, C.; et al. Right ventricular dysfunction in critically ill COVID-19 ARDS. Int. J. Cardiol. 2021, 327, 251–258. [Google Scholar] [CrossRef]
- Park, J.; Kim, Y.; Pereira, J.; Hennessey, K.C.; Faridi, K.F.; McNamara, R.L.; Velazquez, E.J.; Hur, D.J.; Sugeng, L.; Agarwal, V. Understanding the role of left and right ventricular strain assessment in patients hospitalized with COVID-19. Am. Heart J. Plus Cardiol. Res. Pract. 2021, 6, 100018. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, L.; Li, M.; Li, H.; Zhu, S.; Wang, B.; He, L.; Zhang, D.; Zhang, Y.; Yuan, H.; et al. Biventricular Longitudinal Strain Predict Mortality in COVID-19 Patients. Front. Cardiovasc. Med. 2021, 7, 632434. [Google Scholar] [CrossRef] [PubMed]
- Shmueli, H.; Shah, M.; Ebinger, J.E.; Nguyen, L.-C.; Chernomordik, F.; Flint, N.; Botting, P.; Siegel, R.J. Left ventricular global longitudinal strain in identifying subclinical myocardial dysfunction among patients hospitalized with COVID-19. IJC Heart Vasc. 2021, 32, 100719. [Google Scholar] [CrossRef] [PubMed]
- Minhas, A.S.; Gilotra, N.A.; Goerlich, E.; Metkus, T.; Garibaldi, B.T.; Sharma, G.; Bavaro, N.; Phillip, S.; Michos, E.D.; Hays, A.G. Myocardial Work Efficiency, A Novel Measure of Myocardial Dysfunction, Is Reduced in COVID-19 Patients and Associated with In-Hospital Mortality. Front. Cardiovasc. Med. 2021, 8, 461. [Google Scholar] [CrossRef]
- Ghantous, E.; Shetrit, A.; Hochstadt, A.; Banai, A.; Lupu, L.; Levi, E.; Szekely, Y.; Schellekes, N.; Jacoby, T.; Zahler, D.; et al. Cardiologic Manifestations in Omicron-Type Versus Wild-Type COVID-19: A Systematic Echocardiographic Study. JAHA 2023, 12, e027188. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, H.S.; Bui, Q.M.; King, K.; De Maria, A.; Daniels, L.B. Subclinical left ventricular dysfunction in COVID-19. IJC Heart Vasc. 2021, 34, 100770. [Google Scholar] [CrossRef]
- Gonzalez, F.A.; Ângelo-Dias, M.; Martins, C.; Gomes, R.; Bacariza, J.; Fernandes, A.; Borrego, L.M. Left atrial strain is associated with distinct inflammatory and immune profile in patients with COVID-19 pneumonia. Ultrasound J. 2023, 15, 2. [Google Scholar] [CrossRef]
- D’Andrea, A.; Cante, L.; Palermi, S.; Carbone, A.; Ilardi, F.; Sabatella, F.; Crescibene, F.; Di Maio, M.; Giallauria, F.; Messalli, G.; et al. COVID-19 Myocarditis: Prognostic Role of Bedside Speckle-Tracking Echocardiography and Association with Total Scar Burden. Int. J. Environ. Res. Public Health 2022, 19, 5898. [Google Scholar] [CrossRef] [PubMed]
- Lassen, M.C.H.; Skaarup, K.G.; Lind, J.N.; Alhakak, A.S.; Sengeløv, M.; Nielsen, A.B.; Simonsen, J.Ø.; Johansen, N.D.; Davidovski, F.S.; Christensen, J.; et al. Recovery of cardiac function following COVID-19—ECHOVID-19: A prospective longitudinal cohort study. Eur. J. Heart Fail. 2021, 23, 1903–1912. [Google Scholar] [CrossRef]
- Oikonomou, E.; Lampsas, S.; Theofilis, P.; Souvaliotis, N.; Papamikroulis, G.A.; Katsarou, O.; Kalogeras, K.; Pantelidis, P.; Papaioannou, T.G.; Tsatsaragkou, A.; et al. Impaired left ventricular deformation and ventricular-arterial coupling in post-COVID-19: Association with autonomic dysregulation. Heart Vessel. 2023, 38, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Shimoni, O.; Korenfeld, R.; Goland, S.; Meledin, V.; Haberman, D.; George, J.; Shimoni, S. Subclinical Myocardial Dysfunction in Patients Recovered from COVID-19 Disease: Correlation with Exercise Capacity. Biology 2021, 10, 1201. [Google Scholar] [CrossRef] [PubMed]
- Tryfou, E.S.; Kostakou, P.M.; Chasikidis, C.G.; Kostopoulos, V.S.; Serafetinidis, I.I.; Ferdianaki, E.K.; Mihas, C.; Olympios, C.D.; Kouris, N.T. Biventricular myocardial function in Covid-19 recovered patients assessed by speckle tracking echocardiography: A prospective cohort echocardiography study. Int. J. Cardiovasc. Imaging 2021, 38, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Caiado, L.D.C.; Azevedo, N.C.; Azevedo, R.R.C.; Caiado, B.R. Cardiac involvement in patients recovered from COVID-19 identified using left ventricular longitudinal strain. J. Echocardiogr. 2022, 20, 51–56. [Google Scholar] [CrossRef]
- Petersen, E.L.; Goßling, A.; Adam, G.; Aepfelbacher, M.; Behrendt, C.-A.; Cavus, E.; Cheng, B.; Fischer, N.; Gallinat, J.; Kühn, S.; et al. Multi-organ assessment in mainly non-hospitalized individuals after SARS-CoV-2 infection: The Hamburg City Health Study COVID programme. Eur. Heart J. 2022, 43, 1124–1137. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Zamora, S.; Picco, J.M.; Lepori, A.J.; Galello, M.I.; Saad, A.K.; Ayón, M.; Monga-Aguilar, N.; Shehadeh, I.; Manganiello, C.F.; Izaguirre, C.; et al. Abnormal echocardiographic findings after COVID-19 infection: A multicenter registry. Int. J. Cardiovasc. Imaging 2023, 39, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Young, K.A.; Krishna, H.; Jain, V.; Hamza, I.; Scott, C.G.; Pellikka, P.A.; Villarraga, H.R. Serial Left and Right Ventricular Strain Analysis in Patients Recovered from COVID-19. J. Am. Soc. Echocardiogr. 2022, 35, 1055–1063. [Google Scholar] [CrossRef]
- Baruch, G.; Rothschild, E.; Sadon, S.; Szekely, Y.; Lichter, Y.; Kaplan, A.; Taieb, P.; Banai, A.; Hochstadt, A.; Merdler, I.; et al. Evolution of right and left ventricle routine and speckle-tracking echocardiography in patients recovering from coronavirus disease 2019: A longitudinal study. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 1055–1065. [Google Scholar] [CrossRef]
- Akkaya, F.; Yenerçağ, F.N.T.; Kaya, A.; Şener, Y.Z.; Bağcı, A. Long term effects of mild severity COVID-19 on right ventricular functions. Int. J. Cardiovasc. Imaging 2021, 37, 3451–3457. [Google Scholar] [CrossRef]
- García-Abellán, J.; Fernández, M.; Padilla, S.; García, J.A.; Agulló, V.; Lozano, V.; Ena, N.; García-Sánchez, L.; Gutiérrez, F.; Masiá, M. Immunologic phenotype of patients with long-COVID syndrome of 1-year duration. Front. Immunol. 2022, 13, 920627. [Google Scholar] [CrossRef]
- Ozer, P.K.; Govdeli, E.A.; Baykiz, D.; Karaayvaz, E.B.; Medetalibeyoglu, A.; Catma, Y.; Elitok, A.; Cagatay, A.; Umman, B.; Oncul, A.; et al. Impairment of right ventricular longitudinal strain associated with severity of pneumonia in patients recovered from COVID-19. Int. J. Cardiovasc. Imaging 2021, 37, 2387–2397. [Google Scholar] [CrossRef]
- Mahajan, S.; Kunal, S.; Shah, B.; Garg, S.; Palleda, G.M.; Bansal, A.; Batra, V.; Yusuf, J.; Mukhopadhyay, S.; Kumar, S.; et al. Left ventricular global longitudinal strain in COVID-19 recovered patients. Echocardiography 2021, 38, 1722–1730. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Dulgheru, R.; Bernard, A.; Ilardi, F.; Contu, L.; Addetia, K.; Caballero, L.; Akhaladze, N.; Athanassopoulos, G.D.; Barone, D.; et al. Echocardiographic reference ranges for normal left ventricular 2D strain: Results from the EACVI NORRE study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 833–840. [Google Scholar] [CrossRef]
- Luchian, M.-L.; Motoc, A.; Lochy, S.; Magne, J.; Belsack, D.; de Mey, J.; Roosens, B.; van den Bussche, K.; Boeckstaens, S.; Chameleva, H.; et al. Subclinical Myocardial Dysfunction in Patients with Persistent Dyspnea One Year after COVID-19. Diagnostics 2021, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- Baum, P.; Do, L.; Deterding, L.; Lier, J.; Kunis, I.; Saur, D.; Classen, J.; Wirtz, H.; Laufs, U. Cardiac function in relation to functional status and fatigue in patients with post-COVID syndrome. Sci. Rep. 2022, 12, 19575. [Google Scholar] [CrossRef]
- ZeinElabdeen, S.G.; Sherif, A.; Kandil, N.T.; Altabib, A.M.O.; Abdelrashid, M.A. Left atrial longitudinal strain analysis in long COVID-19 syndrome. Int. J. Cardiovasc. Imaging 2023, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulos, A.P.; Georgiopoulou, V.V.; Butler, J. From risk factors to structural heart disease: The role of inflammation. Heart Fail. Clin. 2012, 8, 113–123. [Google Scholar] [CrossRef]
- Schellenberg, J.; Ahathaller, M.; Matits, L.; Kirsten, J.; Kersten, J.; Steinacker, J. Left ventricular global longitudinal strain as a parameter of mild myocardial dysfunction in athletes after COVID-19. Preprint. medRxiv 2023. [Google Scholar] [CrossRef]
- Kersten, J.; Hoyo, L.; Wolf, A.; Hüll, E.; Nunn, S.; Tadic, M.; Scharnbeck, D.; Rottbauer, W.; Buckert, D. Cardiopulmonary Exercise Testing Distinguishes between Post-COVID-19 as a Dysfunctional Syndrome and Organ Pathologies. Int. J. Environ. Res. Public Health 2022, 19, 11421. [Google Scholar] [CrossRef]
- Gluckman, T.J.; Bhave, N.M.; Allen, L.A.; Chung, E.H.; Spatz, E.S.; Ammirati, E.; Baggish, A.L.; Bozkurt, B.; Cornwell, W.K.; Harmon, K.G.; et al. 2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19 in Adults: Myocarditis and Other Myocardial Involvement, Post-Acute Sequelae of SARS-CoV-2 Infection, and Return to Play: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2022, 79, 1717–1756. [Google Scholar] [CrossRef]
- Gherbesi, E.; Bergamaschi, L.; Cusmano, I.; Tien, T.T.; Paolisso, P.; Foà, A.; Pizzi, C.; Barosi, A. The usefulness of speckle tracking echocardiography in identifying subclinical myocardial dysfunction in young adults recovered from mild COVID-19. Echocardiography 2022, 39, 1190–1197. [Google Scholar] [CrossRef]
- Italia, L.; Ingallina, G.; Napolano, A.; Boccellino, A.; Belli, M.; Cannata, F.; Rolando, M.; Ancona, F.; Melillo, F.; Stella, S.; et al. Subclinical myocardial dysfunction in patients recovered from COVID-19. Echocardiography 2021, 38, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.E.; Friedrich, M.G.; Leiner, T.; Elias, M.D.; Ferreira, V.M.; Fenski, M.; Flamm, S.D.; Fogel, M.; Garg, R.; Halushka, M.K.; et al. Cardiovascular Magnetic Resonance for Patients With COVID-19. JACC Cardiovasc. Imaging 2021, 15, 685–699. [Google Scholar] [CrossRef]
- Kotecha, T.; Knight, D.S.; Razvi, Y.; Kumar, K.; Vimalesvaran, K.; Thornton, G.; Patel, R.; Chacko, L.; Brown, J.T.; Coyle, C.; et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur. Heart J. 2021, 42, 1866–1878. [Google Scholar] [CrossRef]
- Haunhorst, S.; Bloch, W.; Wagner, H.; Ellert, C.; Krüger, K.; Vilser, D.C.; Finke, K.; Reuken, P.; Pletz, M.W.; Stallmach, A.; et al. Long COVID: A narrative review of the clinical aftermaths of COVID-19 with a focus on the putative pathophysiology and aspects of physical activity. Oxf. Open Immunol. 2022, 3, iqac006. [Google Scholar] [CrossRef] [PubMed]
- Charfeddine, S.; Ibn Hadj Amor, H.; Jdidi, J.; Torjmen, S.; Kraiem, S.; Hammami, R.; Bahloul, A.; Kallel, N.; Moussa, N.; Touil, I.; et al. Long COVID 19 Syndrome: Is It Related to Microcirculation and Endothelial Dysfunction? Insights From TUN-EndCOV Study. Front. Cardiovasc. Med. 2021, 8, 745758. [Google Scholar] [CrossRef] [PubMed]
- Osiaevi, I.; Schulze, A.; Evers, G.; Harmening, K.; Vink, H.; Kümpers, P.; Mohr, M.; Rovas, A. Persistent capillary rarefication in long COVID syndrome. Angiogenesis 2023, 26, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Amzulescu, M.S.; de Craene, M.; Langet, H.; Pasquet, A.; Vancraeynest, D.; Pouleur, A.C.; Vanoverschelde, J.L.; Gerber, B.L. Myocardial strain imaging: Review of general principles, validation, and sources of discrepancies. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Nicolosi, G.L. The strain and strain rate imaging paradox in echocardiography: Overabundant literature in the last two decades but still uncertain clinical utility in an individual case. Arch. Med. Sci. Atheroscler. Dis. 2020, 5, e297–e305. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).