Abstract
Recently, AAA volume measurement has been proposed as a potentially valuable surveillance method in situations when diameter measurement might fail. Objective: The aim of this systematic review was to analyze the results of previous studies comparing AAA diameter and volume measurements. Methods: A systematic search in PubMed, Cochrane, and EMBASE databases was performed to identify studies investigating the use of diameter and volume measurements in AAA diagnosis and prognosis in English, German, and Russian, published until December 2022. The manuscripts were reviewed by three researchers and scored on the quality of the research using MINORS criteria. Results: After screening 752 manuscripts, 19 studies (n = 1690) were included. The majority (n = 17) of the manuscripts appeared to favor volume. It is, however, important to highlight the heterogeneity of methodologies and lack of standardized protocol for measuring both volume and diameter in the included studies, which hindered the interpretation of the results. Conclusions: The clinical relevance of abdominal aortic aneurysm volume measurement is still unclear, although studies show favorable and promising results for volumetric changes in AAA, especially in follow-up after EVAR.
1. Introduction
To this day, aortic diameter is known to be a key parameter used not only for diagnosing abdominal aortic aneurysms (AAA) but also as a threshold for AAA elective repair and follow-up of already diagnosed aneurysms [1,2]. Although diameter measurements slightly depend on the imaging technology, whether it be ultrasound or computed tomography angiography (CTA), an abdominal aortic diameter larger than 3.0 cm or a diameter that is 1.5 times larger than normal is regarded as aneurysmatic [1]; 5.0 cm and 5.5 cm are considered to be threshold values for elective repair for women and men, respectively, whereas rapid diameter growth (>1 cm/year) requires timely referral to a vascular surgeon [1,3]. Nowadays, more than half of treatment procedures are performed endovascularly, and follow-up is recommended, indicating that even small changes in aortic size can be clinically significant.
Despite the worldwide use of diameter measurement for AAA diagnosis, surveillance, and clinical decision-making, there has been a debate about whether it is the most accurate and reliable method [4]. The accuracy of AAA diameter measurement might be distorted due to poor ability to detect shape changes, tortuosity of the aorta, and high rates of interobserver variability. Even though a larger AAA diameter is traditionally associated with a greater risk of aneurysm rupture, it is estimated that every year up to 2% of small AAAs rupture while some large diameter aneurysms remain stable along the course of a patient‘s life [5,6,7]. Some authors declare that diameter measurement is not able to detect small changes in aneurysm growth, thus, making this method not completely reliable in some clinical scenarios [8].
Recently, AAA volume measurement has been proposed as a potentially valuable surveillance method in situations when diameter measurement might fail. Abdominal aortic volume can be measured using several different techniques, such as three-dimensional reconstruction of computed tomography angiography (3D-CTA) or magnetic resonance angiography (3D-MRA) as well as three-dimensional ultrasound (3D-US), which is an emerging method in the field of AAA volume measurement [8,9]. Measurement of aortic volume is considered to be useful in defining the morphology of the aneurysmal sac in a three-dimensional way; also, it presumably has a higher value for surveillance after endovascular aneurysm repair (EVAR) [10]. Another advantage of AAA volume measurement could be the ability to accurately monitor saccular aneurysms because this type of aneurysm has a weaker relationship between the increased diameter and the risk of rupture [8]. Despite its benefits, volume assessment of AAA is not currently used in daily clinical practice. If performed manually, volume measurement requires skills and specialized software and is time-consuming, which makes it a less attractive method compared to diameter measurement. However, different automatic or semi-automatic segmentation software systems are currently under development in order to provide doctors with fast and accurate AAA volume measurements [11,12].
Notwithstanding the existing evidence that aortic volume measurement provides relevant information about the morphology of AAA, to this day, diameter measurement remains the gold standard for the assessment of this pathology. To the best of our knowledge, no systematic reviews comparing AAA diameter and volume measurements have been reported to date. The aim of this systematic review is to analyze the results of previous studies comparing AAA diameter and volume measurements.
2. Materials and Methods
2.1. Literature Search Strategy
The protocol for the planned systematic review was registered in the international register of systematic reviews (PROSPERO) [13]. Three authors performed an independent literature search to identify studies investigating the use of diameter and volume measurements in AAA diagnosis and prognosis. The PubMed database (https://pubmed.ncbi.nlm.nih.gov, accessed on 30 January 2023) was searched for papers published until 1 December 2022, using the following keywords: “Aortic Aneurysm, Abdominal” (MeSH) AND “Diameter” (MeSH) AND “Volume” (MeSH). Free text words were also used to avoid missing manuscripts that had not yet been given a MeSH label. A total of 370 studies were identified, and 38 studies were deemed eligible after reading the abstracts. The EMBASE database (https://www.embase.com/landing?status=grey, accessed on 30 January 2023) was checked for relevant studies as well, published until 1 December 2022, with the following keywords: “Aortic Aneurysm, Abdominal” (MeSH) AND “Diameter” (MeSH) AND “Volume” (MeSH). A total of 343 studies were found, and 11 were eligible. The Cochrane Database (https://www.cochranelibrary.com, accessed on 30 January 2023) of Systematic Reviews was searched until 1 December 2022 using the following words: “Abdominal aortic aneurysm”, “Volume”, and “Diameter”, with 39 reviews found, 0 eligible. Any disagreement the authors had was resolved after the independent reading of a full text. The literature search strategy, as well as article selection, is outlined in Figure 1, a flow chart of the systematic literature search according to PRISMA guidelines (PRISMA code: CRD4202339635) [14].
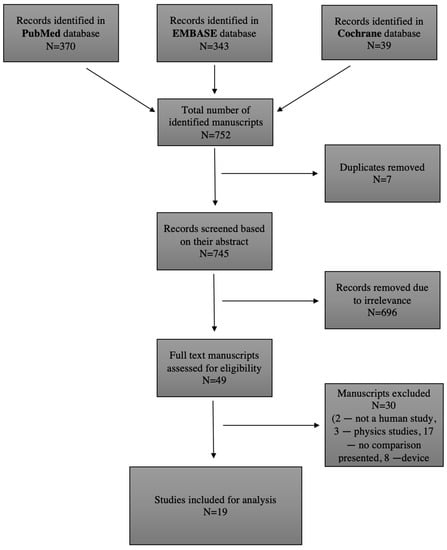
Figure 1.
Literature search strategy and outcomes.
2.2. Inclusion and Exclusion Criteria
All studies included in this systematic review had to meet the selection criteria depicted in Table 1.

Table 1.
Inclusion and exclusion criteria.
2.3. Types of Studies
Human studies.
2.4. Types of Participants
Patients with abdominal aortic aneurysm.
2.5. Types of Outcomes
The outcome measure was defined as the clinical relevance of diameter and volume measurement as well as the comparative usefulness of them in patients with AAA. To assess the relevance of AAA diameter and volume in clinical practice, we specifically reviewed manuscripts that correspond to the following parameters: adequate (>6) methodological index for non-randomized studies (MINORS) quality score, sensitivity, and specificity calculation. The results of sensitivity, specificity, positive predictive value, and negative predictive value calculations were included if provided in the manuscripts reviewed.
2.6. Data Extraction and Critical Appraisal
After identifying relevant titles, all abstracts were screened, and full-text manuscripts were accessed through Vilnius University VPN. A manual cross-reference search of references of included manuscripts was performed to identify other relevant studies. The validity assessment of each manuscript was performed using the MINORS quality score. Non-comparative studies were evaluated on eight quality items, while comparative studies were evaluated on twelve quality items. For each quality item, a score of 0 indicates that it was not reported in the manuscript, 1 indicates that it was reported inadequately, and 2 indicates that it was reported adequately. This adds up to a maximum MINORS score of 16 for non-comparative studies and 24 for comparative studies [15]. In this review, a score of ≤6 was considered poor quality. Quality assessment results of the studies included are demonstrated in Table 2.

Table 2.
Quality assessment according to MINORS criteria.
3. Results
This systematic review analyzed 19 studies (n = 1690) comparing volume and diameter measurements in the diagnosis and prognosis of AAA. Two studies reported no prognostic difference between volume and diameter measurement, while seventeen manuscripts appeared to favor volume. The summary of studies and their outcomes are represented in Table 3 and Table 4, respectively. Studies including the endoleak data are marked with asterisks (*) in both tables.

Table 3.
Summary of studies that found no difference in diagnostic and prognostic values of volume and diameter measurement.

Table 4.
Summary of studies favoring volume measurement.
4. Discussion
To this day, aortic diameter is considered to be a gold standard parameter used in clinical decision-making, while volume measurement is still in its infancy [1]. This systematic review, however, suggests that changes in aortic volume may outweigh the benefits of diameter measurement. On the other hand, it should be emphasized that none of the investigated studies researched the connection between changes in aneurysm volume and mortality. The majority of studies (17 of 19) found volume to be a better tool in characterizing AAAs, while the remaining 2 studies show no significant advantage in using volume measurements against diameter. It has to be noted that most studies favoring volume have a higher or at least equal MINORS score in comparison to studies that did not find a difference between volume and diameter measurements. It is important to highlight the heterogeneity of methodologies and lack of standardized protocol for measuring both volume and diameter in the included studies, which hindered the interpretation of the results.
Six studies included in this systematic review investigated endoleaks after EVAR. Five out of six studies found volume to be a more sensitive measurement in identifying endoleaks after EVAR than diameter. On the other hand, a study conducted by Quan et al. denies the superiority of volume measurement [19]. Quant et al. found that more endoleaks were present in the stable diameter patient group than in the stable volumetric patient group. Nevertheless, according to the authors, 10 patients developed endoleaks without volumetric change. Bargellini et al., on the contrary, found that a change in AAA volume of less than 0.3% at six months follow-up was the strongest independent predictor of endoleak [20]. Results presented by Bargellini et al. were not homogenous as well; a group of patients developed endoleaks without aortic enlargement, while some patients had increased aortic volumes that did not result in endoleaks.
Few of the studies compared the relation of volume and diameter measurements with peak wall stress (PWS) and peak wall rupture index (PWRI) [27,31]. Liljeqvist et al. found that volume growth correlated stronger with PWS and PWRI than diameter. Raghavan et al. found that volume had the strongest correlation with PWS. Such findings put volume measurement forward as being a potentially more feasible parameter for predicting the risk of rupture. The main concern is that this connection is not completely straightforward, as both PWS and PWRI are compound parameters.
Spanos et al. investigated the difference between ruptured and unruptured AAAs [6]. The mentioned study revealed that volumes were significantly different between the groups, while maximum diameter (Dmax) did not have a statistically significant difference. A threshold value of 380 mL was fairly well associated with rupture, accompanied by sensitivity and specificity of 60%. Dmax was not found to be a predictor of rupture.
Although this systematic review included studies comparing aortic diameter and volume measurement, several other potential prognostic markers regarding aneurysm growth and the risk of rupture can be mentioned. Some studies suggest that wall shear stress, wall thickness, and inflammatory markers might be valuable parameters to predict aneurysm rupture [32,33,34,35]. Additionally, hemodynamic features, such as tortuosity or the occlusion of aortic outflow, have been reported to be associated with an increased risk of rupture [36]. Nevertheless, these are complex parameters, and currently, their application in clinical practice is quite limited.
Although the majority of the studies analyzed in this systematic review place AAA volume superior to diameter, we believe that such results cannot be taken as an absolute. These findings might be partially influenced by the methodologies used to calculate diameter and volume. A number of different methods to calculate aortic diameter were used, for example, orthogonal and axial planes, antero–posterior, leading edge to leading edge, inner to inner, and outer to outer measurements [5,9,17,18,19,24,30]. It is important to highlight the lack of a standardized way to measure AAA volume. Most authors measure volume from below the lowest renal artery to the aortic bifurcation [5,6,16,19,20,22,24,25,28,30,31]. Others measure volume in the portion that includes the abdominal aorta, aneurysm, and iliac arteries covered by stent graft [17]. However, a significant portion of AAA volume measurement also involves iliac arteries, which could be measured likewise [21]. Mathematical methods of how centerline is calculated and how a specific target region of interest is chosen also may vary. Furthermore, different methods (CTA, US) are used to measure volume. The aforementioned inconsistencies in the methodologies of included studies produced quite heterogeneous results.
In a study carried out by Ghulam et al., more than one-third of the patients with stable AAA diameters appeared to have a growing AAA volume [9]. More patients from this group compared to those with a stable diameter and volume underwent aortic repair (20% vs. 5%). None of these patients developed a ruptured AAA, and most were treated electively. Only one patient had a symptomatic AAA. All decisions regarding aneurysm repair were made relying on diameter. The results of this study propose volume as a more sensitive tool for evaluating and following AAAs. On the other hand, it raises doubts about whether volume measurement would have any clinical relevance in daily practice. Khan et al. found that volume measurements correlate (r = 0.46) better than diameter with AAA growth [18]. It could be argued whether such a correlation is sufficient to rely completely on this parameter in clinical practice. A few studies included in this systematic review disclose the absence of diameter change with a significant change in volume without having a clear conclusion of such findings [23,28]. Authors note that volume measurement might add important information about AAA expansion and emphasize that more outcome-related studies are needed to assess the clinical significance of volume measurements.
Large-sample-size future studies comparing AAA diameter and volume impact on aneurysm prognosis, growth, and rupture rates are required. Using strictly standardized measurement criteria and having clearly defined expected outcomes could help gather higher quality data and assist in making unbiased decisions regarding the use of diameter and volume measurement of AAAs in daily clinical practice.
5. Conclusions
The clinical relevance of abdominal aortic aneurysm volume measurement is still unclear. Although studies show favorable and promising results for volumetric changes in AAA, especially in follow-up after EVAR, more standardized data are needed to further evaluate the importance of volumetric parameters in AAA. It remains unclear whether volume could replace or be a necessary addition to diameter measurement in assessing the risk of AAA rupture or during the follow-up after EVAR procedures.
Author Contributions
Conceptualization, G.V. and T.B.; methodology, G.V., T.B., V.M., A.R., K.M. and A.S.; software, K.M.; validation, T.B., A.S. and K.M.; formal analysis, G.V., V.M. and A.R.; investigation, G.V., V.M. and A.R.; resources, G.V., V.M. and A.R.; data curation, G.V., V.M. and A.R.; writing—original draft preparation, G.V., V.M. and A.R.; writing—review and editing, K.M., A.S., and T.B.; visualization, A.R., K.M. and A.S.; supervision, G.V. and T.B.; project administration, G.V., T.B., K.M. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This review received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-Iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef] [PubMed]
- Long, A.; Rouet, L.; Lindholt, J.S.; Allaire, E. Measuring the Maximum Diameter of Native Abdominal Aortic Aneurysms: Review and Critical Analysis. Eur. J. Vasc. Endovasc. Surg. 2012, 43, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Chaikof, E.L.; Dalman, R.L.; Eskandari, M.K.; Jackson, B.M.; Lee, W.A.; Mansour, M.A.; Mastracci, T.M.; Mell, M.; Murad, M.H.; Nguyen, L.L.; et al. The Society for Vascular Surgery Practice Guidelines on the Care of Patients with an Abdominal Aortic Aneurysm. J. Vasc. Surg. 2018, 67, 2–77.e2. [Google Scholar] [CrossRef]
- Ricco, J.-B.; Forbes, T.L. Trans-Atlantic Debate: External Diameter for Abdominal Aortic Aneurysm (AAA) Size versus Volume. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2013, 46, 9. [Google Scholar] [CrossRef] [PubMed]
- Kontopodis, N.; Metaxa, E.; Papaharilaou, Y.; Georgakarakos, E.; Tsetis, D.; Ioannou, C.V. Value of Volume Measurements in Evaluating Abdominal Aortic Aneurysms Growth Rate and Need for Surgical Treatment. Eur. J. Radiol. 2014, 83, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Spanos, K.; Nana, P.; Kouvelos, G.; Mpatzalexis, K.; Matsagkas, M.; Giannoukas, A.D. Anatomical Differences Between Intact and Ruptured Large Abdominal Aortic Aneurysms. J. Endovasc. Ther. 2020, 27, 117–123. [Google Scholar] [CrossRef]
- Singh, T.P.; Moxon, J.V.; Gasser, T.C.; Golledge, J. Systematic Review and Meta-Analysis of Peak Wall Stress and Peak Wall Rupture Index in Ruptured and Asymptomatic Intact Abdominal Aortic Aneurysms. J. Am. Heart Assoc. 2021, 10, e019772. [Google Scholar] [CrossRef]
- von Allmen, R.S.; Powell, J.T. Part Two: Against the Motion. External Diameter for AAA Size. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 6–8. [Google Scholar] [CrossRef]
- Ghulam, Q.M.; Bredahl, K.K.; Lönn, L.; Rouet, L.; Sillesen, H.H.; Eiberg, J.P. Follow-up on Small Abdominal Aortic Aneurysms Using Three Dimensional Ultrasound: Volume Versus Diameter. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2017, 54, 439–445. [Google Scholar] [CrossRef]
- van Keulen, J.W.; van Prehn, J.; Prokop, M.; Moll, F.L.; van Herwaarden, J.A. Potential Value of Aneurysm Sac Volume Measurements in Addition to Diameter Measurements after Endovascular Aneurysm Repair. J. Endovasc. Ther. Off. J. Int. Soc. Endovasc. Spec. 2009, 16, 506–513. [Google Scholar] [CrossRef]
- Caradu, C.; Pouncey, A.-L.; Lakhlifi, E.; Brunet, C.; Bérard, X.; Ducasse, E. Fully Automatic Volume Segmentation Using Deep Learning Approaches to Assess Aneurysmal Sac Evolution after Infrarenal Endovascular Aortic Repair. J. Vasc. Surg. 2022, 76, 620–630.E3. [Google Scholar] [CrossRef] [PubMed]
- Adam, C.; Fabre, D.; Mougin, J.; Zins, M.; Azarine, A.; Ardon, R.; d’Assignies, G.; Haulon, S. Pre-Surgical and Post-Surgical Aortic Aneurysm Maximum Diameter Measurement: Full Automation by Artificial Intelligence. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2021, 62, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, J.H. PROSPERO: An International Register of Systematic Review Protocols. Med. Ref. Serv. Q. 2019, 38, 171–180. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological Index for Non-Randomized Studies ( MINORS): Development and Validation of a New Instrument: Methodological Index for Non-Randomized Studies. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Wolf, Y.G.; Tillich, M.; Lee, W.A.; Fogarty, T.J.; Zarins, C.K.; Rubin, G.D. Changes in Aneurysm Volume after Endovascular Repair of Abdominal Aortic Aneurysm. J. Vasc. Surg. 2002, 36, 305–309. [Google Scholar] [CrossRef]
- Skrebunas, A.; Lengvenis, G.; Builyte, I.; Zulpaite, R.; Bliudzius, R.; Baltrunas, T.; Misonis, N.; Marinskis, G. Aortic Sac Enlargement after Endovascular Aneurysm Repair: Volume-Related Changes and the Impact of Intraluminal Thrombus. Pol. J. Radiol. 2019, 84, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Rogers, S.; Carreira, J.; Ghosh, J.; McCollum, C. Aneurysm Geometry Analyzed by the Novel Three-Dimensional Tomographic Ultrasound Relates to Abdominal Aortic Aneurysm Growth. Ann. Vasc. Surg. 2022, 87, 469–477. [Google Scholar] [CrossRef]
- Quan, C.; Oh, Y.K.; Park, S.C.; Won, Y.S.; Yun, S.S.; Suh, Y.J.; Kim, J.Y. Efficacy of Volumetric Analysis of Aorta as Surveillance Tool after EVAR. Asian J. Surg. 2019, 42, 746–754. [Google Scholar] [CrossRef]
- Bargellini, I.; Cioni, R.; Petruzzi, P.; Pratali, A.; Napoli, V.; Vignali, C.; Ferrari, M.; Bartolozzi, C. Endovascular Repair of Abdominal Aortic Aneurysms: Analysis of Aneurysm Volumetric Changes at Mid-Term Follow-Up. Cardiovasc. Intervent. Radiol. 2005, 28, 426–433. [Google Scholar] [CrossRef]
- Fillinger, M. Three-Dimensional Analysis of Enlarging Aneurysms after Endovascular Abdominal Aortic Aneurysm Repair in the Gore Excluder Pivotal Clinical Trial. J. Vasc. Surg. 2006, 43, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Wever, J.J.; Blankensteijn, J.D.; Mali, W.T.M.; Eikelboom, B.C. Maximal Aneurysm Diameter Follow-up Is Inadequate after Endovascular Abdominal Aortic Aneurysm Repair. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2000, 20, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Parr, A.; Jayaratne, C.; Buttner, P.; Golledge, J. Comparison of Volume and Diameter Measurement in Assessing Small Abdominal Aortic Aneurysm Expansion Examined Using Computed Tomographic Angiography. Eur. J. Radiol. 2011, 79, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Schnitzbauer, M.; Güntner, O.; Wohlgemuth, W.A.; Zeman, F.; Haimerl, M.; Stroszczynski, C.; Müller-Wille, R. CT after Endovascular Repair of Abdominal Aortic Aneurysms: Diagnostic Accuracy of Diameter Measurements for the Detection of Aneurysm Sac Enlargement. J. Vasc. Interv. Radiol. 2018, 29, 178–187.e3. [Google Scholar] [CrossRef]
- Olson, S.L.; Panthofer, A.M.; Blackwelder, W.; Terrin, M.L.; Curci, J.A.; Baxter, B.T.; Weaver, F.A.; Matsumura, J.S. Role of Volume in Small Abdominal Aortic Aneurysm Surveillance. J. Vasc. Surg. 2022, 75, 1260–1267.e3. [Google Scholar] [CrossRef]
- Tzirakis, K.; Kontopodis, N.; Metaxa, E.; Ioannou, C.V.; Papaharilaou, Y. Spatial Distribution of Abdominal Aortic Aneurysm Surface Expansion and Correlation With Maximum Diameter and Volume Growth. Ann. Vasc. Surg. 2019, 58, 276–288. [Google Scholar] [CrossRef]
- Lindquist Liljeqvist, M.; Hultgren, R.; Gasser, T.C.; Roy, J. Volume Growth of Abdominal Aortic Aneurysms Correlates with Baseline Volume and Increasing Finite Element Analysis-Derived Rupture Risk. J. Vasc. Surg. 2016, 63, 1434–1442.e3. [Google Scholar] [CrossRef]
- Renapurkar, R.D.; Setser, R.M.; O’Donnell, T.P.; Egger, J.; Lieber, M.L.; Desai, M.Y.; Stillman, A.E.; Schoenhagen, P.; Flamm, S.D. Aortic Volume as an Indicator of Disease Progression in Patients with Untreated Infrarenal Abdominal Aneurysm. Eur. J. Radiol. 2012, 81, e87–e93. [Google Scholar] [CrossRef]
- Franchin, M.; Serafini, M.; Tadiello, M.; Fontana, F.; Rivolta, N.; Venturini, M.; Curti, M.; Bush, R.L.; Dorigo, W.; Piacentino, F.; et al. A Morphovolumetric Analysis of Aneurysm Sac Evolution after Elective Endovascular Abdominal Aortic Repair. J. Vasc. Surg. 2021, 74, 1222–1231.e2. [Google Scholar] [CrossRef]
- Kritpracha, B.; Beebe, H.G.; Comerota, A.J. Aortic Diameter Is an Insensitive Measurement of Early Aneurysm Expansion After Endografting. J. Endovasc. Ther. 2004, 11, 184–190. [Google Scholar] [CrossRef]
- Raghavan, M.L.; Vorp, D.A.; Federle, M.P.; Makaroun, M.S.; Webster, M.W. Wall Stress Distribution on Three-Dimensionally Reconstructed Models of Human Abdominal Aortic Aneurysm. J. Vasc. Surg. 2000, 31, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Meyrignac, O.; Bal, L.; Zadro, C.; Vavasseur, A.; Sewonu, A.; Gaudry, M.; Saint-Lebes, B.; De Masi, M.; Revel-Mouroz, P.; Sommet, A.; et al. Combining Volumetric and Wall Shear Stress Analysis from CT to Assess Risk of Abdominal Aortic Aneurysm Progression. Radiology 2020, 295, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Yuan, D.; Wen, J.; Fan, Y.; Zheng, T. Numerical Identification of the Rupture Locations in Patient-Specific Abdominal Aortic Aneurysmsusing Hemodynamic Parameters. Comput. Methods Biomech. Biomed. Eng. 2018, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.J.; Kuhn, D.C.S.; Lozowy, R.J.; Kulbisky, G.P. Low Wall Shear Stress Predominates at Sites of Abdominal Aortic Aneurysm Rupture. J. Vasc. Surg. 2016, 63, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Teng, B.; Zhou, Z.; Zhao, Y.; Wang, Z. Combined Curvature and Wall Shear Stress Analysis of Abdominal Aortic Aneurysm: An Analysis of Rupture Risk Factors. Cardiovasc. Intervent. Radiol. 2022, 45, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.D.; Chivukula, V.K.; Haller, S.; Vatankhah, N.; Bohannan, C.J.; Moneta, G.L.; Rugonyi, S.; Azarbal, A.F. Aortic Outflow Occlusion Predicts Rupture of Abdominal Aortic Aneurysm. J. Vasc. Surg. 2016, 64, 1623–1628. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).