Contribution of GIP and GLP-1 to the Insulin Response to Oral Administration of Glucose in Female Mice
Abstract
1. Introduction
2. Methods
2.1. Animals
2.2. Experiments
2.3. Analyses
2.4. Statistics
3. Results
3.1. Glucose Controls
3.2. GIP Receptor Antagonism
3.3. GLP-1 Receptor Antagonism
3.4. Combination of GIP and GLP-1 Receptor Antagonism
4. Discussion
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McIntyre, N.; Holdsworth, C.D.; Turner, D.S. New interpretation of oral glucose tolerance. Lancet 1964, 2, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Elrick, H.; Stimmler, L.; Hlad, C.J.; Arai, Y. Plasma insulin responses to oral and intravenous glucose administration. J. Clin. Endocrinol. Metab. 1964, 24, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Creutzfeldt, W. The incretin concept today. Diabetologia 1979, 16, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Homberger, E.; Siegel, E.G.; Allen, R.C.; Eaton, R.P.; Ebert, R.; Creutzfeldt, W. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J. Clin. Endocrinol. Metab. 1986, 63, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Gasbjerg, L.S.; Helsted, M.M.; Hartmann, B.; Jensen, M.H.; Gabe, M.B.N.; Sparre-Ulrich, A.H.; Veedfald, S.; Stensen, S.; Lanng, A.R.; Bergmann, N.C.; et al. Separate and combined gluco-metabolic effects of endogenous glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 in healthy individuals. Diabetes 2019, 68, 906–917. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. The incretin effect in healthy individuals and those with type 2 diabetes: Physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 2016, 4, 525–536. [Google Scholar] [CrossRef]
- Alsalim, W.; Lindgren, O.; Ahrén, B. Glucose-dependent insulinotropic polypeptide and glucagon-like peptie-1 secretion in humans: Characteristics and regulation. J. Diabetes Investig. 2023; in press. [Google Scholar]
- Ahrén, B. Glucose-dependent insulinotropic polypeptide secretion after oral macronutrient ingestion: The human literature revisited and a systematic study in model experiments in mice. J. Diabetes Investig. 2022, 13, 1655–1665. [Google Scholar] [CrossRef]
- Nauck, M.A.; Vardarli, I.; Deacon, C.F.; Holst, J.J.; Meier, J.J. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: What is up, what is down? Diabetologia 2011, 54, 10–18. [Google Scholar] [CrossRef]
- Gasbjerg, L.S.; Helsted, M.M.; Hartmann, B.; Sparre-Ulrich, A.H.; Veedfald, S.; Stensen, S.; Lanng, A.R.; Bergmann, N.C.; Christensen, M.B.; Vilsbøll, T.; et al. GIP and GLP-1 receptor antagonism during a meal in healthy individuals. J. Clin. Endocrinol. Metab. 2020, 105, dgz175. [Google Scholar] [CrossRef]
- Vilsbøll, T.; Krarup, T.; Madsbad, S.; Holst, J.J. Both GLP-1 and GIP are insulinotropic at basal and postprandial glucose levels and contribute nearly equally to the incretin effect of a meal in healthy subjects. Regul. Pept. 2003, 114, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Ahrén, B.; Sörhede Winzell, M.; Pacini, G. The augmenting effect on insulin secretion by oral versus intravenous glucose is exaggerated by high-fat diet in mice. J. Endocrinol. 2008, 197, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Ahlkvist, L.; Vikman, J.; Pacini, G.; Ahrén, B. Synergism by individual macronutrients explains the marked early GLP-1 and islet hormone responses to mixed meal challenge in mice. Regul. Pept. 2012, 178, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Pacini, G.; Ahrén, B. Glucagon-like peptide-1 and glucose-dependent insulinotropic peptide: Effects alone and in combination on insulin secretion and glucose disappearance in mice. Physiol. Rep. 2017, 5, e13280. [Google Scholar] [CrossRef]
- Ahrén, B.; Yamada, Y.; Seino, Y. The incretin effect in female mice with double deletion of GLP-1 and GIP receptors. J. Endocr. Soc. 2020, 4, bvz036. [Google Scholar] [CrossRef]
- Ahrén, B.; Yamada, Y.; Seino, Y. Islet adaptation in GIP receptor knockout mice. Peptides 2020, 125, 170152. [Google Scholar] [CrossRef]
- Ahrén, B.; Yamada, Y.; Seino, Y. The mediation by GLP-1 receptors of glucagon-induced insulin secretion revisited in GLP-1 receptor knockout mice. Peptides 2021, 135, 170434. [Google Scholar] [CrossRef]
- Gasbjerg, L.S.; Christensen, M.B.; Hartmann, B.; Lanng, A.R.; Sparre-Ulrich, A.H.; Gabe, M.B.N.; Dela, F.; Vilsbøll, T.; Holst, J.J.; Rosenkilde, M.M.; et al. GIP(3-30)NH2 is an efficacious GIP receptor antagonist in humans: A randomised, double-blinded, placebo-controlled, crossover study. Diabetologia 2018, 61, 413–423. [Google Scholar] [CrossRef]
- Perry, R.A.; Craig, S.L.; Ng, M.T.; Gault, V.A.; Flatt, P.R.; Irwin, N. Characterization of glucose-dependent insulinotropic polypeptide receptor antagonists in rodent pancreatic beta cells and mice. Clin. Med. Insights Endocrinol. Diabetes 2019, 12, 31548798. [Google Scholar] [CrossRef]
- West, J.A.; Tsakmaki, A.; Ghosh, S.S.; Parkes, D.G.; Grønlund, R.V.; Pedersen, P.J.; Maggs, D.; Rajahopalan, H.; Bewick, G.A. Chronic peptide-based GIP receptor inhibition exhibits glucose metabolic changes in mice when administered either alone or combined with GLP-1 agonism. PLoS ONE 2021, 16, e024239. [Google Scholar] [CrossRef]
- Ovlund, T.; Pacini, G.; Ahrén, B. Impact of incretin hormone receptors on insulin-independent glucose disposal in model experiments in mice. Front. Endocrinol. 2021, 12, 680153. [Google Scholar] [CrossRef] [PubMed]
- Gasbjerg, L.S.; Bari, E.J.; Christensen, M.; Knop, F.K. Exendin (9-39)NH2: Recommendations for clinical use based in a systematic literature review. Diabetes Obes. Metab. 2021, 23, 2419–2436. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Val, T.P.; D´Alessio, D.A. Regulation of islet hormone release and gastric emptying by endogenous glucagon-like peptide 1 after glucose ingestion. J. Clin. Endocrinol. Metab. 2008, 93, 4909–4916. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.M.; Hoselton, A.L.; Krishna, R.; Slentz, C.A.; D´Alessio, D.A. GLP-1 receptor blockade reduces stimulated insulin secretion in fasted subjects with low circulating GLP-1. J. Clin. Endocrinol. Metab. 2022, 107, 2500–2510. [Google Scholar] [CrossRef]
- Sparre-Ulrich, A.H.; Gabe, M.N.; Gasbjerg, L.S.; Christiansen, C.B.; Svendsen, B.; Hartmann, B.; Holst, J.J.; Rosenkilde, M.M. GIP(3-30)NH2 is a potent competitive antagonist of the GIP receptor and effectively inhibits GIP-mediated insulin, glucagon, and somatostatin release. Biochem. Pharmacol. 2017, 131, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.; Stöckmann, F.; Ebert, R.; Creutzfeldt, W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986, 29, 46–52. [Google Scholar] [CrossRef]
- Holst, J.J. The incretin system in healthy humans: The role of GIP and GLP-1. Metabolism 2019, 96, 46–55. [Google Scholar] [CrossRef]
- Miyawaki, K.; Yamada, Y.; Yano, H.; Niwa, H.; Ban, N.; Ihara, Y.; Kubota, A.; Fujimoto, S.; Kajikawa, M.; Kuroe, A.; et al. Glucose intolerance caused by a defect in the entero-insular axis: A study in gastric inhibitory polypeptide receptor knockout mice. Proc. Natl. Acad. Sci. USA 1999, 96, 14843–14847. [Google Scholar] [CrossRef]
- Pamir, N.; Lynn, F.C.; Buchan, A.M.; Ehses, J.; Hinke, S.A.; Pospisilik, J.A.; Miyawaki, K.; Yamada, Y.; Seino, Y.; McIntosh, C.H.S.; et al. Glucose-dependent insulinotropic polypeptide receptor null mice exhibit compensatory changes in the enteroinsular axis. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E931–E939. [Google Scholar] [CrossRef]
- Preitner, F.; Ibberson, M.; Franklin, I.; Binnert, C.; Pende, M.; Gijnovci, A.; Hansotia, T.; Drucker, D.J.; Wollheim, C.; Burcelin, R.; et al. Gluco-incretins control insulin secretion at multiple levels as revealed in mice lacking GLP-1 and GIP receptors. J. Clin. Investig. 2004, 113, 635–645. [Google Scholar] [CrossRef]
- Scrocchi, L.A.; Brown, T.J.; MaClusky, N.; Brubaker, P.L.; Auerbach, A.B.; Joyner, A.L.; Drucker, D.J. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat. Med. 1996, 2, 1254–1258. [Google Scholar] [CrossRef] [PubMed]
- Scrocchi, L.A.; Marshall, B.A.; Cook, S.M.; Brubaker, P.L.; Drucker, D.J. Identification of glucagon-like peptide 1 (GLP-1) actions essential for glucose homeostasis in mice with disruption of GLP-1 receptor signaling. Diabetes 1998, 47, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Ahrén, B.; Yamada, Y.; Seino, Y. The insulin response to oral glucose in GIP and GLP-1 receptor knockout mice: Review of the literature and stepwise glucose dose response studies in female mice. Front. Endocrinol. 2021, 12, 665537. [Google Scholar] [CrossRef] [PubMed]
- Hansotia, T.; Baggio, L.L.; Delmeire, D.; Hinke, S.A.; Yamada, Y.; Tsukiyama, K.; Seino, Y.; Holst, J.J.; Schuit, F.; Drucker, D.J. Double incretin receptor knockout (DIRKO) mice reveal an essential role for the enteroinsular axis in transducing the glucoregulatory actions of DPP-IV inhibitors. Diabetes 2004, 53, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
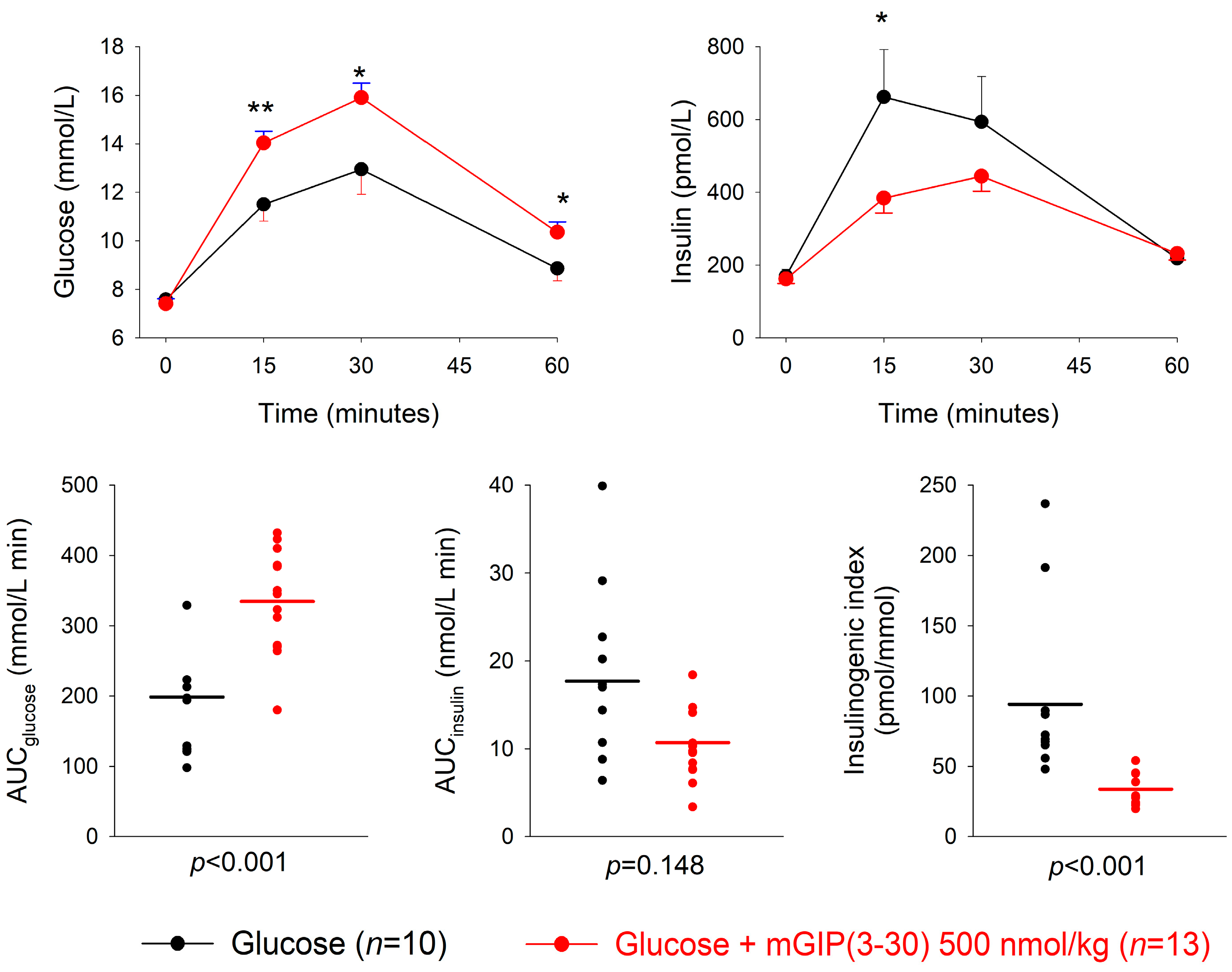
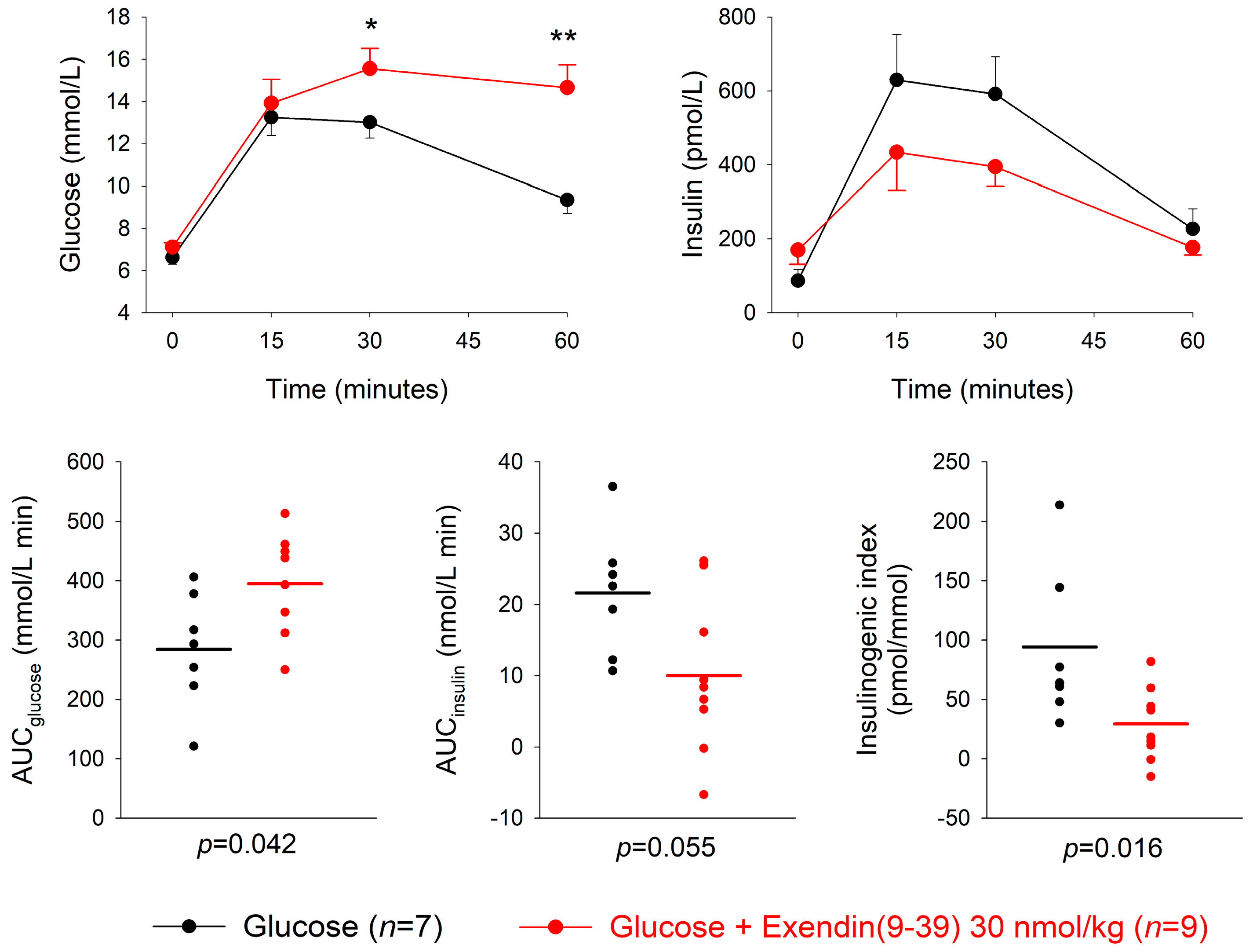
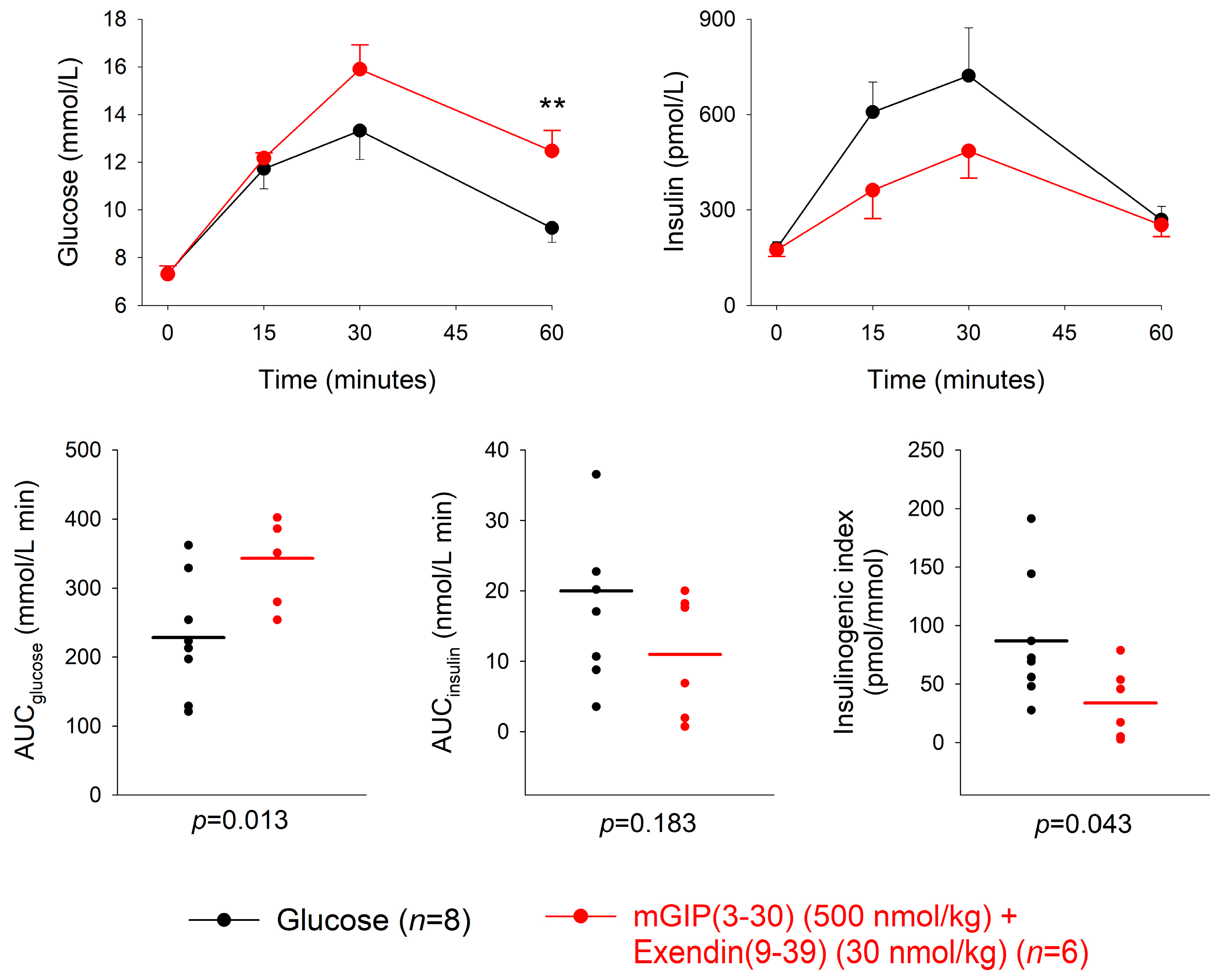
| Dose of Antagonist (nmol/kg) | n | AUCglucose mmol/L min | p | AUCinsulin nmol/L min | p | Insulinogenic Index (pmol/ mmol) | p | |
|---|---|---|---|---|---|---|---|---|
| mGIP(3-30) + glucose | 50 | 14 | 258.5 ± 15.5 | 0.45 | 17.4 ± 1.7 | 0.32 | 71.5 ± 8.6 | 0.076 |
| Glucose alone | 12 | 233.1 ± 30.9 | 20.1 ± 1.9 | 107.2 ± 16.1 | ||||
| mGIP(3-30) + glucose | 500 | 13 | 334.7 ± 20.6 | <0.001 | 10.7 ± 1.1 | 0.148 | 33.8 ± 4.3 | <0.001 |
| Glucose alone | 10 | 198.8 ± 28.1 | 17.7 ± 3.6 | 94.2 ± 21.0 | ||||
| Exendin(9-39) + glucose | 3 | 8 | 272.3 ± 33.9 | 0.80 | 18.0 ± 3.4 | 0.44 | 71.1 ± 12.0 | 0.44 |
| Glucose alone | 8 | 260.5 ± 30.6 | 14.8 ± 3.9 | 53.8 ± 12.8 | ||||
| Exendin(9-39) + glucose | 30 | 9 | 394.6 ± 32.8 | 0.042 | 10.0 ± 3.6 | 0.055 | 29.4 ± 10.2 | 0.016 |
| Glucose | 7 | 284.3 ± 36.6 | 21.6 ± 3.3 | 91.1 ± 24.6 | ||||
| mGIP(3-30) + Exendin(9-39) + glucose | 500 (mGIP) 30 (exendin) | 6 | 343.1 ± 25.2 | 0.013 | 11.0 ± 3.6 | 0.183 | 33.8 ± 12.4 | 0.043 |
| Glucose alone | 8 | 228.4 ± 30.2 | 20.0 ± 4.6 | 86.9 ± 19.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahrén, B. Contribution of GIP and GLP-1 to the Insulin Response to Oral Administration of Glucose in Female Mice. Biomedicines 2023, 11, 591. https://doi.org/10.3390/biomedicines11020591
Ahrén B. Contribution of GIP and GLP-1 to the Insulin Response to Oral Administration of Glucose in Female Mice. Biomedicines. 2023; 11(2):591. https://doi.org/10.3390/biomedicines11020591
Chicago/Turabian StyleAhrén, Bo. 2023. "Contribution of GIP and GLP-1 to the Insulin Response to Oral Administration of Glucose in Female Mice" Biomedicines 11, no. 2: 591. https://doi.org/10.3390/biomedicines11020591
APA StyleAhrén, B. (2023). Contribution of GIP and GLP-1 to the Insulin Response to Oral Administration of Glucose in Female Mice. Biomedicines, 11(2), 591. https://doi.org/10.3390/biomedicines11020591





