Abstract
Bladder cancer is a common disease in men and the elderly. Current treatment paradigms include radical resection of the bladder and lymph nodes or transurethral resection, both supported by chemotherapy and/or radiation. New modalities, such as illumination-based therapies are also being translationally pursued. However, while survival rates have increased due to combined therapies (particularly chemotherapy, radiation, immune checkpoint inhibitors, and surgery), a lack of diagnostic markers leads clinical professionals to rely on frequently invasive and expensive means of monitoring, such as magnetic resonance imaging or bladder cystoscopy. To improve real-time diagnostic capabilities, biomarkers that reflect both the metabolic and metastatic potential of tumor cells are needed. Furthermore, indicators of therapy resistance would allow for rapid changes in treatment to optimize survival outcomes. Fortunately, the presence of nanoscale extracellular vesicles in the blood, urine, and other peripheral fluids allow for proteomic, genomic, and transcriptomic analyses while limiting the invasiveness of frequent sampling. This review provides an overview of the pathogenesis and progression of bladder cancer, standard treatments and outcomes, some novel treatment studies, and the current status of biomarker and therapy development featuring exosome-based analysis and engineering.
1. Introduction
Bladder cancer remains a serious disease with only incremental progress in curative therapies. Men remain far more susceptible to bladder cancer over their lifetimes (hazard ratio 1.08 for men, 0.27 for women) and such malignancies are the 6th most common cancer in European older men (median age 70) [1]. In new bladder cancer patients, roughly 75% start with non-muscle invasive bladder cancer (NMIBC) and 10–50% of these patients will progress to muscle-invasive bladder cancer (MIBC) [1]. As the cancer increases in size, it penetrates the lumen, lamia propria, detrusor muscle, and muscularis propria before invading fatty tissue and spreading to the surrounding organs (Figure 1) [2]. Of note, de novo MIBC occurs in 25% of cases and 90% of these will be urothelial carcinomas (UC) while the remainder consists of squamous cell carcinomas or adenocarcinomas [1]. With over 17,000 deaths and 80,000 cases per year (in the US alone), recurrence rates as high as 30–40%, and the threat of metastasis to the thoracic organs, both progressive or de novo MIBC represent a serious threat to clinical outcomes [1,3]. Incremental gains made over the last 20 years in overall survival (OS) and disease-free survival (DFS) rates of MIBC are the result of coupling radical cystectomy and pelvic lymph node dissection (RC/PLND) surgeries with combinations of chemical, immunological, and radiation therapies (multimodal therapy). Predictive scoring for progression and recurrence do exist; however, the inherently unstable nature of MIBC means that treatment is reactive since reliable biomarkers for tumor progression have not been clinically verified. Thus, clinical research is currently evaluating diverse trimodal therapies to improve quality of life in selected patients even as translational research continues to investigate molecular and other biomarkers for use in predicting MIBC progression and potential treatment resistance.
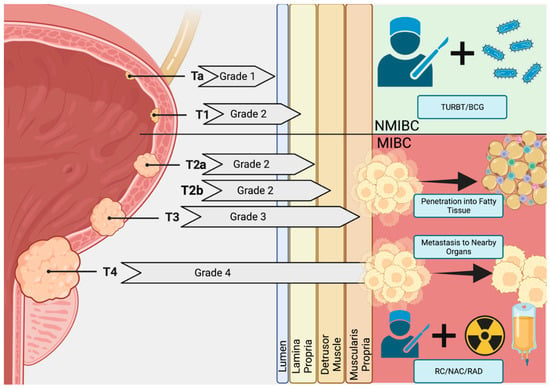
Figure 1.
Bladder Cancer Pathogenesis. Early in situ carcinomas mature into Ta (Grade 1) and T1 (Grade 2) non-muscular invasive bladder cancers (NMIBC) malignancies that begin to penetrate the muscle wall of the bladder. Here, transurethral resection of the bladder (TURBT) and Bacillus Calmette–Guérin (BCG) therapy are often curative. However, a subpopulation is resistant to these therapies and the tumor penetrates the muscle wall and fatty tissue, becoming advanced-stage muscle-invasive bladder cancer (MIBC; Grades 3 and 4). With metastasis to surrounding organs, multimodal therapies featuring radical cystectomy (RC), neoadjuvant chemotherapy (NAC), and radiation (RAD) are used to increase survival by tumor growth and metastasis control. Created at BioRender.com.
2. The Genetic Basis of Muscle-Invasive Bladder Cancer Pathogenesis
NMIBC progresses to MIBC in a subset of cases due to rapid growth and a putative failure of immune surveillance. In addition to the aryl hydrocarbon receptor in NMIBC development due to chemical exposure, carcinogenic chemicals (such as benzene, nitrosamines [fermented foods or fertilizer runoff], or tobacco smoke) may increase the risk of developing bladder cancer through DNA damage to key checkpoint and oncogenes [1,4].
Genetic analyses of MIBC patients point to mutations in FGFR3, PIK3CA, TERT, and p53 for the pathogenesis of NMIBC as well as MIBC progression [3,5]. Additionally, alterations in tumor type are dependent upon these factors, as MIBC seems to be primarily regulated by FGFR3 while NMIBC is more p53 dependent [6]. Heritable factors also play an important role as other genetic studies have found mismatch repair faults (hereditary Lynch syndrome) in MSH2 (and associated genes) to carry higher risk [7]. This was reported in a Swedish study that found MLH1 gene mutations to be present in 40% of affected families, with MSH2 at 36%, MSH6 at 18% and PMS2 at 6% of total cases [7,8]. Lynch syndrome may thus have some influence on bladder cancer pathogenesis due to these oncogene mutations and potential microsatellite instability [8]. However, a study of 164 urothelial carcinoma patients in Japan found a Lynch syndrome prevalence of only 1.8%, similar to a 2017 report in which 6 patients with documented Lynch syndrome were discovered out of 444 total patients (1.3%) while Lynch syndrome-associated neoplasm patients numbered 30/444 (6.7%) [9,10]. Taken together, reports indicate that at least some minor fraction within large populations of UC patients are expected to carry Lynch syndrome (or associated) neoplasms and should be checked for MLH1, MSH2, MSH6, and PMS2 mutations [11].
3. Existing Diagnostic and Imaging Paradigms
With regard to bladder cancer screening and treatment evaluation, imaging has stepped in to fill the diagnostic gap made by a lack of reliable biomarkers. These technologies, while continuously refined for improvements in diagnostic accuracy, remain invasive (cystoscopy) or expensive/troublesome for the patient (magnetic resonance imaging and computed tomography).
3.1. Imaging Paradigms
3.1.1. Magnetic Resonance Imaging and Computed Tomography
Early and accurate detection of NMIBC, progressive MIBC, or de novo MIBC are crucial for treatment planning. Therefore, magnetic resonance imaging (MRI) or computed tomography (CT) have become gold standards for non-invasive imaging and refinement of various imaging parameters and dyes, especially with regard to MRI, are well-reported. As an example, diffusion-weighted MRI, based on the differences in water molecule Gaussian motion, requires careful tuning of B values to enhance discrimination between tumor and normal tissue [12]. Some reports indicate that diffusion kurtosis, which more carefully analyzes water motion in non-Gaussian systems, plus tumor contact length measurements are more accurate than diffusion weighting [13]. Recently, positron Emission/CT (PET/CT), that relies on higher 2F-18-fluoro-2-deoxy-d-glucose (FDG) uptake by more metabolically active tumor cells, has been reported useful for imaging [14]. While frequent urinary flushing can complicate signal retention within the bladder, FDG-CT is useful for metastatic surveillance of the lymph nodes. Newer dye media, such as C-11 acetate and C-11 choline, exploit the retention of radiotracers that cannot be easily excreted via urine and may also facilitate whole-body scanning for metastatic evaluation during initial screening [14].
Even with MRI/CT, diagnostic accuracies from 55–89% are commonly reported and constant evolution, such the development of multiparametric MRI (mpMRI) that does not rely on extensive radiological reading experience, is expected to increase the utility of diagnostic MRI [15]. Unfortunately, the expense and time required to complete imaging sessions is difficult for advanced-stage patients while the data, albeit detailed, is not real-time and is only a snapshot of the physical tumor characteristics. Since prognosis depends on the molecular and metabolic status of the tumor, MRI and PET/CT provides only part of the data needed to plan a complete treatment schedule.
3.1.2. Cystoscopy
A mainstay of initial diagnosis and treatment, cystoscopy is employed in transurethral resection of bladder tumor (TURBT) procedures for suspected NMIBC as the primary imaging pathway to guide the surgery and for biopsy during follow-up visits. An invasive procedure, it also carries the side effects of pain, potential perforation, and distension of the bladder [16]. In MIBC, cystoscopy is useful only for diagnosis but, unfortunately, cystoscopy alone is only 71% accurate [15]. However, a recent trial (BladderPath, ISRCTN 35296862) is currently evaluating mpMRI for replacement of TURBT staging [16] and neutrophil percentage-to-albumin ratio has also been explored for prognostic potential in MIBC patients treated with neoadjuvant chemotherapy (NAC) and RC [17]. Advances in cystoscopy with photosensitizing agents, such as hexyl aminolaevulinic acid, allow for fluorescent highlighting of tumors to improve detection and ensure precision [18].
3.2. Pathology
Histological and histopathological analyses of biopsy specimens remain consistently useful for confirming bladder cancer diagnoses. Paraffin-embedded slicing, fixing, and staining with antibodies specific to driver genes (such as p53 and FGFR3) allow for both aggregate cell counts and morphological confirmation of transformed cells [19]. Morphological and antibody-based classification may be particularly useful in detailing the transformation of NMIBC to MIBC (urothelial to sarcomatoid), especially since PD-L1 expression tends to be higher in MIBC-transformed sarcomatoid cells [20]. However, as with other imaging methods, even the best pathology is a snapshot of a past condition and cannot provide real-time status updates of the molecular and metabolic conditions within a tumor.
4. Treatment Paradigms
4.1. BCG as the First Line
NMIBC is often first treated with Bacillus Calmette-Guerin (BCG), a biotic therapy originally used as a killed tuberculosis vaccine strain, to train the immune system and evasion of this therapy may result in tumor progression [21]. A first-line adjuvant therapy, BCG treatment is effective in about 50% of patients, with the rest either failing to respond, relapsing, or having adverse events [22]. Originally thought to be wholly immune-centered around CD8+ T cells, evidence exists that also demonstrates some direct tumoricidal activity of the bacteria (putatively via oxidative stress or necrotic pathway activation) but increases in PD-L1 expression on tumor cell surfaces might explain BCG evasion [23]. However, for non-responders, valrubicin or pembrolizumab (anti-PD-L1) are the only FDA/European Medicines Agency-approved therapies for NMIBC if BCG is ineffective [24].
4.2. Surgery Types: Radical Cystectomy plus Neo-Adjuvant Therapy
Although radical cystectomy with pelvic lymph node dissection (RC/PLND) is the current gold standard for MIBC, can result in an R0 (complete cure) condition, and has additional benefits in preventing metastasis, it has side effects that can be devastating for quality of life (incontinence, impotence, neurologic damage) and some patients are unsuitable for such surgery. It is the main line of treatment for progressive or de novo MIBC; however, RC alone is also not a definitive treatment, as 5-year survival rates of 40–60% and recurrence in as little as 12 months have been reported [25]. For this reason, RC is usually combined with neo-adjuvant chemotherapy (NAC) to maximize tumor control before and after surgery. Neo-adjuvant cisplatin with RC is the currently recommended multimodal treatment standard for MIBC [26].
While the use of chemotherapy with RC seems obvious, NAC has been reported as underutilized, with less than 20% of patients in the US between 2004–2014 having received it [27]. Meanwhile, a recent meta-study of 35,738 patients in 13 reports found that only 17.2% of patients underwent NAC regimens [28]. Since complete, partial, and downstaged response rates were 16.6%, 14.6%, and 45.0% in that study, NAC may be an important weapon against UC progression [28]. Another meta-study of 8 reports found NAC + RC was superior to RC alone with regard to overall survival (OS; HR 0.79; 95% CI: 0.68–0.92, p = 0.002), bolstering the utility of NAC to provide improved MIBC prognoses [29].
4.3. Trimodal Bladder-Preserving Treatment (TMT)
Trimodal therapy (TMT), consisting of combined chemo- and radiotherapies plus TURBT, has been adopted as an alternative to RC/PLND in selected patients [30]. Such bladder-sparing improves quality of life but requires optimal patient selection with regard to co-morbidities and tumor status (ideally cT2 with no carcinoma in situ) [31]. However, an insufficient number of clinical trials prevents the full clarification of survival and quality-of-life improvements that may be possible with TMT. A recent report simulated 500,000 patients with a 2-D Markov model and, in comparisons between RC and TMT, found that TMT had slightly higher quality of life in elderly patients while RC had better overall survival and life quality in younger patients [30]. Similarly, a US study of 2306 military veterans with MIBC found that TMT was associated with comparable survival to RC with neoadjuvant chemotherapy but only in patients older than 65 years of age [32]. Thus, the utility of TMT may be comparable at best in some patients but worse in older patients. Additionally, a 2018 meta-study of 57 total studies and 30,293 patients found that, while TMT mean 10-year OS was insignificantly lower than RC (30.9% TMT vs. 35.1% RC, p = 0.32), it was chemotherapy response that determined the best survival results with TMT [33]. The concept of RC for long-term survival superiority was also questioned by a 2020 metasudy by Ding et al that found superior OS results for RC after 10 years but only when Charlson comorbidity scores were 0 [34]. Furthermore, within that 10-year timeframe, TMT was comparable, indicating that TMT is a valid therapeutic option for patients who are unsuited for RC or who do not wish to undergo radical resection [34].
These data indicate that, while R0 resection is considered curative, long-term survival also depends on a complete response to chemotherapy and selection of chemoagents by using predictive models for response is therefore a critical component of improving TMT performance. Several proposed and current trials that may finely tune response rate predictive models are currently exploring combinations of immune checkpoint inhibitors and radiation for cisplatin and RC-ineligible patients [35,36]. As such, maintaining a maximum level of tumor control with TMT requires accurate and precise biomarkers to select the proper chemotherapy agents as well as to monitor progress after treatment. The current requirement of frequent CT/MRI or cystoscopy to monitor progress is not clinically feasible and cannot accurately predict resistance to therapy. Outcomes from clinical studies for TMT published after 2010 are summarized in Table 1.

Table 1.
Treatment Outcomes for Trimodal Therapies (TMT) published after 2010.
4.4. Immune Checkpoint Inhibitors
Since atezolizumab was first approved by FDA for metastatic UC in 2016, immune checkpoint inhibitors (ICIs) to PD-1, PD-L1, or CTLA-4, which target angiogenesis and sensitize the immune response to limit both tumor growth and metastasis, are frequently employed as an adjuvant therapy combined with chemotherapy. Anti-PD-L1 therapy on cancer cells prevents binding of PD-1 on T cells to increase apoptosis while CTLA-4 blocks CD80 and CD86 activity, downregulating Treg-mediated immunoregulation and increasing CD8+ cytotoxic activity [44]. In this fashion, antitumor T cell activity is maximized. Both types of drugs are often combined (ICI-ICI) to starve the tumor and increase immune effectiveness since a metastudy of 2 RCTs with 1518 total patients found ICI treatment alone (atezolizumab or nivolumab) was not significantly useful in high-risk muscle-invasive UC [45]. Conversely, a study (CheckMate 032) of combined PD-1 and CTLA-4 inhibitors (nivolumab 1 mg/kg plus ipilimumab 3 mg/kg) found response rate of 38.0% in 92 patients receiving both drugs [46]. Adverse events must also be considered, as a metastudy of 21 reports with 11,454 patients total who received nivolumab, pembrolizumab, atezolizumab, or ipilimumab found a higher incidence of non-fatal adverse events [47]. With the promising results of adjuvant immunotherapy, clinical trials of neoadjunctive immunotherapy have been intensively carried out, and Table 2 summarizes such trials.
Much effort has been made to look for reliable biomarkers for predicting response to ICIs, such as PD-L1 or CD8 expression by immunohistochemistry [48,49,50,51] and/or tumor mutation burden (TMB) [52]. Unfortunately, these biomarkers have not yet to be verified in large clinical trials [51,53].

Table 2.
Outcomes from Select Clinical Studies of neoadjuvant immunotherapy for MIBC.
Table 2.
Outcomes from Select Clinical Studies of neoadjuvant immunotherapy for MIBC.
| Trial | Phase | Regimen | Patients | N | pCR% |
|---|---|---|---|---|---|
| PURE-01 [54] | II | Pembrolizumab | cT ≤ 3bN0 | 114 | 37 |
| ABACUS [55] | II | Atezolizumab | cT2-4N0M0 | 88 | 31 |
| NABUCCO * | Ib | Nivolumab + ipilimumab | cT3-T4aN0M0 /T1-4aN1M0 | 54 | Ipi-high 63 Ipi-low 29 |
| BLASST-1 ** | II | Nivolumab + GC | cT2-4aN ≤ 1M0 | 43 | 49 |
| GU14-188 Cohort 1 *** | II | Pembrolizumab + GC | cT2-4N0M0 | 43 | 44.4 |
| SAKK 06/17 # | II | Durvalumab + GC | cT2-4aN ≤ 1M0 | 53 | 34 |
| RACE IT ## | II | Nivolumab + Radiotherapy | cT3-4N ≤ 1M0 | 33 | 38.7 |
* 2022 ESMO Poster session 18 Abstract 1770P. ** 2020 ASCO-GU. Abstract 439. *** 2020 ASCO. Abstract 5047. # 2022 ASCO. Abstract 4515. ## Annals of Oncology (2022) 33 (suppl_7): S808–S869. GC = gemcitabine + cisplatin chemotherapy.
4.5. FGFR Inhibitors
As mentioned in Section 2, FGFR3 is a common mutation found in patients with MIBC [56]. Erdafitinib, a pan-FGFR tyrosine kinase inhibitor, is the first FDA-approved targeted therapy for mUC with susceptible FGFR2/3 alterations following platinum-containing chemotherapy. A phase II trial of 99 enrolled patients with local advanced and unresectable/metastatic UC with an FGFR3 mutation or FGFR2/3 fusion observed disease progression in all patients following chemotherapy [57]. The confirmed response rate was 40% while an additional 39% of patients were stabilized. For 22 patients with previous immunotherapy, the erdafitinib response rate was 59%. At 24 months median follow-up, the median overall survival was 11.3 months. Adverse events of >grade 3 related to treatment occurred in 46% of patients while 13% had to discontinue erdafitinib due to adverse events [57]. Based on those results, several other FGFR inhibitors are being evaluated, including infigratinib, which has demonstrated promising activity [58].
4.6. Future Treatments Compatible with TMT and RC
Even with an absence of viable biomarkers for prediction and treatment management, development of new modalities for bladder cancer continue to increase specificity by combining drugs with antibody conjugates (antibody-conjugated drugs; ADC). Unlike ICIs, these drugs are manufactured to deliver cytotoxic molecules via antibodies (usually IgG) engineered for strong interaction with specific tumor antigens and very little cross-reactivity [59]. Connecting links between the antibody and payload are usually constructed of disulfide- or protease-dependent bonds in order to prevent premature release of the payload and to exploit the acidic pH and ROS-intensive microenvironments of tumors that are likely to sever the linker [59]. Then, cytotoxic molecules such as auristatins (microtubule destabilizers), maytansinoids, DNA alkylators, or proteotoxins (protein synthesis inhibitors) are connected to the scaffold as effector payloads to slow tumor cell growth and prevent replication. Other antibody-based fusion techniques concurrently being developed to stimulate immunogenic responses (e.g., ALT-803) and deliver current ICIs with higher specificity (e.g., ATOR-1015) have been extensively reviewed by Bogen et al. [60].
With the discovery of fucolsylated glycans as potential biomarkers for pancreatic, MIBC, and other cancers, the potential of lectin-targeted payload delivery to MIBC has become feasible [61,62]. Lectin specificity to these glycans is possible with recombinant technology and future sequencing studies on diverse bladder cancer specimens could allow for a glycan-lectin binding library to be constructed for targeting both non-invasive and invasive bladder cancers [63]. Additionally, these lectins are amenable to conjugation with nontoxic, photoreactive dyes that respond to near-infrared (NIR) light by conformational changes that induce necrotic death in cells [64]. A recent paper by Kuroda et al. has demonstrated the feasibility of this system for pancreatic cancer in a murine model [65]. Since NIR is harmless to human tissue and cystoscopes already have illumination capability, addition of NIR light and lectin-conjugated dye systems to TURBT may be a possibility that removes residual disease and promotes complete response.
5. Scoring Problems and the Search for Biomarkers
A scoring system from the European Organization for Research and Treatment of Cancer (EORTC) has attempted to factor in stage, CIS, tumor grade, size, multifocality, and prior recurrence to create a predictive instrument for progression and recurrence of NMIBC [66]. Other scoring systems take into account responsiveness to BCG, initial TURBT results, CT results, and demographics [66,67]. The vesicle imaging reporting and data system (VI-RADS) scoring method further attempts to integrate MRI imaging to score MIBC and a recent analysis indicated a good sensitivity (0.83) and specificity (0.90) [68,69]. This possibility was previously found to allow discrimination between groups that could and could not optimally benefit from TURBT, as well as agree with interobserver readings in MIBC diagnosis [70,71]. However, these population-based scoring systems only attempt to predict recurrence after treatment based on previous, aggregated patterns and may not be as accurate in predicting individual response to therapy. To rectify this shortcoming, further involvement of neoadjuvant chemotherapy data (nacVI-RADS) has shown some promise in a small group of 10 patients at predicting response, indicating that integration of chemoradiotherapy statistics into existing scoring systems may provide prognostic power [72]. In spite of this progress, more precise benchmarks/biomarkers that do not require invasive repeat TURBT or frequent CT/MRI scanning are needed to measure the response to therapy in as close to real-time as possible.
5.1. Current and Ideal Biomarkers
Typical molecular markers for bladder cancer are BTA, NMP22, and microsatellites (when examining tumor tissue for mismatch repair efficacy) [15]. For MIBC, recent forays into multi-genomic approaches using weighted-gene network analyses have indicated CLK4, DEDD2, ENO1, and STYL1 as genes of interest in addition to Lynch syndrome-associated and checkpoint-associated genes (FGFR3, PIK3CA, TERT, p53, MSH2, MSH6, MLH1, and PMS2) [3,5,8,73]. However, these studies primarily rely on solid tumor tissue analyses after biopsy to check for mutations caused by mismatch repair defects and represent only static snapshots of tumor status. New approaches that check peripheral blood for circulating tumor cells or exosomes, from which genetic analyses can be conducted, may offer more resolution into the progression of MIBC metastasis [74,75]. These approaches are possible with current technology and offer a low-cost, high-throughput lab method adaptable from centrifugal precipitation and microbead protocols used for central nervous system-derived exosomes [76]. Transcriptomic profiling of patients has attempted to overcome the heterogeneous nature of bladder cancer by broad classification into molecular classes [77]. Meanwhile, higher fidelity scanning methods using multi-omics approaches are attempting to categorize metabolic and molecular subtype profiles for MIBC patients to predict non-responders to cisplatin, anti-PD-L1 and other chemotherapies [78].
This section will attempt to detail the most desirable characteristics of biomarkers, particularly the recent discovery of exosomes as a useful clinical tool for cancer profiling and possible adjuvant therapy.
5.2. Liquid Biopsies: The Convenience of Frequent Sampling
The primary characteristic of a useful biomarker is the ability to frequently sample, allowing the tracking of tumor progression with regard to both internal (i.e., microenvironment) and external (i.e., metastatic potential) statuses. This is important as larger amounts of data translate into better prognostic ability for not only individuals but entire populations as aggregate case data can be compared to large control datasets from nested populations (nested case controls) [79]. However, for bladder cancers, cystoscopic biopsy, CT/MRI, and other tissue sampling/scanning methods are time/resource intensive and may create pain and inconvenience for the patients.
In light of the need for accurate, frequent sampling with minimal invasiveness and cost, the concept of liquid biopsies (sampling of circulating tumor cells [CTCs] or exosomes obtained from bodily fluids [e.g., blood, urine]) has seen increased development towards clinically precise separation and analysis methods [80]. Of note is the PredicineBEACON survey, which has already reported the utility of liquid biopsy methods (blood and urine) for individual mutation analysis profiling, demonstrating high precision, accuracy, and sensitivity in rapid evaluation of PIKC3A, FGFR3, and TERT mutations associated with MIBC during neoadjuvant therapy. This was in addition to mutational burden by copy number quantification (2022 ASCO. Abstract 539).
Tumor-derived exosomes, which are nanoscale extracellular vesicles (EVs, 40–150 nm in size) released from cells as metabolic byproducts for communication or waste disposal, can be sampled from blood and are stable both in circulation and collection [81]. In particular, exosomes enriched in nucleic acids or proteins that indicate the genetic or metabolic state of tumors would provide valuable information over time that could reflect the impact of therapies on both tumor cell reproduction, metabolic status, and possible therapy resistance.
The most crucial part of the exosome analysis process is collection and separation, as the blood/urine milieu contains large numbers of mixed solutes, cells, proteins, plasma, and immune components. Of note, several reports have detailed collection methods for urinary exosomes that could be exploited for bladder cancer detection and monitoring, including optimized ultrafiltration with 0.22 µm/10 kDa filters as well as ultracentrifugation, filtration, and protease treatments [82,83]. These low-cost, high-recovery methods use existing technology and are easily adapted for high-throughput clinical lab analysis. As for blood, exosome collection by ultrafiltration, magnetic bead/immunoaffinity capture, and ultracentrifugation have been used to profile diverse other cancers and can easily be applied to bladder cancer with the advantage of capturing CTCs in microcavity or other microfilter systems for culture expansion and profiling [84,85,86]. Combined with solid tumor samples from cystoscopic biopsy and predictive CT/MRI data, exosome/CTC sampling may be a low-cost and rich source of biomarkers to supplement and reinforce conclusions on prognosis and therapy response in bladder cancer patients.
5.3. Amenable to Profiling
The ideal biomarker is also easy to profile with existing technology, especially -omics-based methods. Urinary exosomes, as representative of the bladder milieu, immune interactions, and cellular interactions, are a superior choice [87] (10.3390/pharmaceutics14102027). Starting from an initial 2010 report by Welton et al. in cultured cells, a recent study examined urinary exosomes from patients scheduled for RC and found mass spectrometry to be easily accomplished once a decoy search database and two-peptide matching filters were applied [88,89]. Beads with anti-CD63 were then used to capture free proteins, including CD9, CD63, Rab, heat shock proteins, and CD81 [89]. Thus, proteomic approaches incorporating mass spectrometry could provide high-throughout analysis for clinical applications.
Meanwhile, recent reports have isolated urinary exosomes and found clinically significant DNA amounts amenable to genomic profiling. Lee et al. found that urinary exosomes from 9 patients were similar to cell-free DNA in profiling of mutations, as well as higher correlation between these captured EVs and solid tumor status [90]. A subsequent and similar study by Zhou et al found in 2021 that, after treatment with DNAse I to remove extraluminal nucleic acids, urine and serum exosomes had similar particle trends, markers (flotillin-1 and CD9), and quality [91]. Of importance was their finding that Sanger sequencing was more sensitive than whole-exome sequencing for capturing mutation profiles and that mutation profiles and frequencies (featuring genes such as KLK10, PSCA, PTK2, ETV6, and TBX3) were reproducible and usually located in untranslated regions [91]. Although recent studies have been limited in patient numbers, the potential for commercial development of Sanger-based exosome profiling kits is high in light of this data.
Transcriptomics-based approaches with exosomes are also possible. Discrimination between benign prostate hyperplasia and prostate cancer, for which prostate-specific antigen is insensitive, was achieved through collection and microarray transcriptomic profiling of urinary exosomes to arrive at CDH3 as a definitive biomarker [92]. The impact of RNA on diagnosis was also reinforced by Zheng et al., who detected exosome non-coding RNA for PTENP1 which was secreted by normal cells to target bladder cancer cells and increase apoptosis [93]. Along these lines, RNA analysis could provide valuable insight into transcriptional factors when combined with proteomic data. Thus, transcriptomics, in addition to proteomics and genomics, may serve as a mature platform for exosome and potential CTC analysis and profiling.
5.4. Provides Targeting and Therapeutic Options
Since exosomes are a natural cell-to-cell communication paradigm, they can be readily exploited for delivery of therapeutics. Natively, the endosomal sorting complex (ESCRT 0–3) generates exosomes that are uptaken by diverse mechanisms, including pinocytosis, plasma membrane fusion, or endocytosis [94]. The cargo capacity, although limited by size, can contain nucleic acids, proteins, biometabolites (e.g., sugars, vitamins), and lipids (e.g., cholines, steroids) [94]. It may also be possible to load photosensitive dyes for NIR therapy into these exosomes. Additionally, small chemical molecules, miRNA, or targeted peptides can be loaded and produced en masse for therapies and encased in an exosome package that specifically binds to tumor cells using exosomal surface proteins like LAMP-2B [95,96]. The higher metabolism of tumor cells and more acidic extracellular pH (which itself can be manipulated through drugs or proton pump inhibition) would help facilitate engineered exosome uptake by promoting fusion with the cell membrane [96,97].
Thus, exosomes, coupled with CTCs (if they can be captured efficiently), may provide a singular solution for generating rapid, accurate, and individualized metabolic and genetic profiles of tumors that allow for (1) prediction of therapy response, (2) changes in dosages to compensate for anticipated resistance, (3) screening for metastasis, and (4) delivery of customized drugs or miRNAs to silence key resistance genes, induce apoptosis, or reduce metastatic/growth potential of tumor cells (Figure 2). Although several RNA and natural compound clinical trials are ongoing, no current bladder cancer exosome drug delivery trial has been completed although 3 have been registered (ClinicalTrials.gov NCT04155359, NCT05270174, NCT05559177) [98]. Future studies in large populations with exosome-based screening, plus engineered exosomes to deliver drugs in conjunction with standard-of-care therapy for bladder cancer, will be instructive as to the full potential of these nanoparticles.
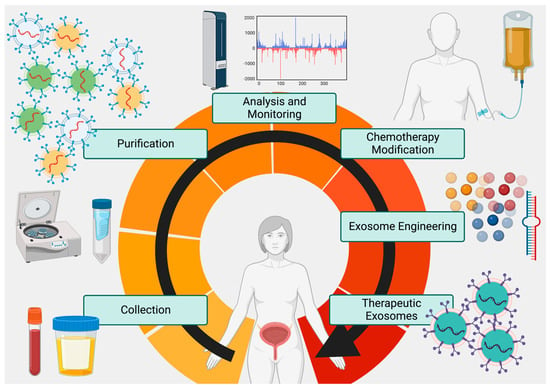
Figure 2.
The Proposed Exosome Cycle. Tumor exosomes can be frequently collected from urine and blood, purified in high volumes, analyzed via -omics technology, and used to evaluate both tumor metabolism and response to therapy. In addition to customizing therapy based on exosome analysis, exosomes engineered to deliver custom payloads to tumor cells to promote chemoradiation susceptibility and apoptosis can be used as a synergistic adjunct to surgery and chemoradiation therapies. Created at BioRender.com.
6. Conclusions
The past 20 years has seen a rise in the overall survival of MIBC due to the advent of multimodal therapy. In particular, combinations of chemotherapy, ICIs, radiation, and surgery (RC/TURBT) have increased both disease-free survival periods as well as overall survival up to 10 years. Additionally, new, immune-based targeting with antibodies and lectin are also being fast-tracked from the lab bench to the bedside. However, the lack of biomarkers to regularly examine the progress of tumors and track their metabolic, genomic, and metastatic conditions have hampered progress in achieving more beneficial outcomes. With the advent of exosome and CTC collection and analysis, frequent sampling for accurate profiling as well as engineered drug delivery are now possibilities to be explored. Successful TMT may thus evolve into quadrimodal therapy (QMT) as TURBT, chemotherapy, radiation, and ICI/ADC combinations are evaluated, profiled, and assisted by tumor exosome analysis and engineered exosome treatment (Figure 2).
Contribution to the Field: While bladder cancer diagnosis and treatment have undergone refinement and incremental improvement over the last 20 years, neo-adjuvant therapy remains underutilized and 10-year survival rates have not demonstrated dramatic gains. New therapies that preserve quality of life, such as bladder-sparing surgeries, are being investigated along with novel therapeutic modalities that could synergistically increase tumor control and prevent metastasis. However, the lack of effective biomarkers renders the real-time evaluation of tumor status impossible and treatment paradigms are reduced to reactive guesswork instead of accurate prognosis. Exosome sampling may provide some relief for the clinician as analysis of these tumor-produced, nanoscale particles can be easily collected, profiled, and used to customize therapy. Additionally, they can be engineered to deliver small molecular cargoes to tumor cells, opening up a new field of therapy. This paper contributes to the field by overviewing current treatments, analyzing new treatments, and exploring the new field of exosomes as both biomarker sources and treatment options.
Author Contributions
Conceptualization, B.J.M., Y.S. and X.Y.; writing—original draft preparation, B.J.M. and Y.S.; writing—review and editing, Y.S. and X.Y.; figures, B.J.M.; tables, Y.H.; project administration, X.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Omorphos, N.P.; Piedad, J.C.P.; Vasdev, N. Guideline of guidelines: Muscle-invasive bladder cancer. Turk. J. Urol. 2021, 47, S71–S78. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.A.; Hurst, C.D. Molecular biology of bladder cancer: New insights into pathogenesis and clinical diversity. Nat. Rev. Cancer 2015, 15, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Matulewicz, R.S.; Steinberg, G.D. Non-muscle-invasive Bladder Cancer: Overview and Contemporary Treatment Landscape of Neoadjuvant Chemoablative Therapies. Rev. Urol. 2020, 22, 43–51. [Google Scholar] [PubMed]
- Yu, J.; Lu, Y.; Muto, S.; Ide, H.; Horie, S. The Dual Function of Aryl Hydrocarbon Receptor in Bladder Carcinogenesis. Anticancer Res. 2020, 40, 1345–1357. [Google Scholar] [CrossRef]
- Sjodahl, G.; Eriksson, P.; Patschan, O.; Marzouka, N.A.; Jakobsson, L.; Bernardo, C.; Lovgren, K.; Chebil, G.; Zwarthoff, E.; Liedberg, F.; et al. Molecular changes during progression from nonmuscle invasive to advanced urothelial carcinoma. Int. J. Cancer 2020, 146, 2636–2647. [Google Scholar] [CrossRef]
- Zhao, M.; He, X.L.; Teng, X.D. Understanding the molecular pathogenesis and prognostics of bladder cancer: An overview. Chin. J. Cancer Res. 2016, 28, 92–98. [Google Scholar] [CrossRef]
- Lagerstedt-Robinson, K.; Rohlin, A.; Aravidis, C.; Melin, B.; Nordling, M.; Stenmark-Askmalm, M.; Lindblom, A.; Nilbert, M. Mismatch repair gene mutation spectrum in the Swedish Lynch syndrome population. Oncol. Rep. 2016, 36, 2823–2835. [Google Scholar] [CrossRef]
- Lindner, A.K.; Schachtner, G.; Tulchiner, G.; Thurnher, M.; Untergasser, G.; Obrist, P.; Pipp, I.; Steinkohl, F.; Horninger, W.; Culig, Z.; et al. Lynch Syndrome: Its Impact on Urothelial Carcinoma. Int. J. Mol. Sci. 2021, 22, 531. [Google Scholar] [CrossRef]
- Ito, T.; Kono, K.; Eguchi, H.; Okazaki, Y.; Yamamoto, G.; Tachikawa, T.; Akagi, K.; Okada, Y.; Kawakami, S.; Morozumi, M.; et al. Prevalence of Lynch syndrome among patients with upper urinary tract carcinoma in a Japanese hospital-based population. Jpn. J. Clin. Oncol. 2020, 50, 80–88. [Google Scholar] [CrossRef]
- Harper, H.L.; McKenney, J.K.; Heald, B.; Stephenson, A.; Campbell, S.C.; Plesec, T.; Magi-Galluzzi, C. Upper tract urothelial carcinomas: Frequency of association with mismatch repair protein loss and lynch syndrome. Mod. Pathol. 2017, 30, 146–156. [Google Scholar] [CrossRef]
- Therkildsen, C.; Eriksson, P.; Hoglund, M.; Jonsson, M.; Sjodahl, G.; Nilbert, M.; Liedberg, F. Molecular subtype classification of urothelial carcinoma in Lynch syndrome. Mol. Oncol. 2018, 12, 1286–1295. [Google Scholar] [CrossRef]
- Yoshida, S.; Koga, F.; Kobayashi, S.; Tanaka, H.; Satoh, S.; Fujii, Y.; Kihara, K. Diffusion-weighted magnetic resonance imaging in management of bladder cancer, particularly with multimodal bladder-sparing strategy. World J. Radiol. 2014, 6, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cao, B.; Liu, K.; Sun, H.; Ding, Y.; Yan, C.; Wu, P.Y.; Dai, C.; Rao, S.; Zeng, M.; et al. Detecting the muscle invasiveness of bladder cancer: An application of diffusion kurtosis imaging and tumor contact length. Eur. J. Radiol. 2022, 151, 110329. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K. Role of PET/CT in muscle-invasive bladder cancer. Transl. Androl. Urol. 2020, 9, 2908–2919. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhu, S.; Cui, X.; Xu, W.; Kong, C.; Zhang, Z.; Qian, W. Identifying non-muscle-invasive and muscle-invasive bladder cancer based on blood serum surface-enhanced Raman spectroscopy. Biomed. Opt. Express 2019, 10, 3533–3544. [Google Scholar] [CrossRef]
- Bryan, R.T.; Liu, W.; Pirrie, S.J.; Amir, R.; Gallagher, J.; Hughes, A.I.; Jefferson, K.P.; Knight, A.; Nanton, V.; Mintz, H.P.; et al. Comparing an Imaging-guided Pathway with the Standard Pathway for Staging Muscle-invasive Bladder Cancer: Preliminary Data from the BladderPath Study. Eur. Urol. 2021, 80, 12–15. [Google Scholar] [CrossRef]
- Ferro, M.; Baba, D.F.; de Cobelli, O.; Musi, G.; Lucarelli, G.; Terracciano, D.; Porreca, A.; Busetto, G.M.; Del Giudice, F.; Soria, F.; et al. Neutrophil percentage-to-albumin ratio predicts mortality in bladder cancer patients treated with neoadjuvant chemotherapy followed by radical cystectomy. Future Sci. OA 2021, 7, FSO709. [Google Scholar] [CrossRef]
- Zainfeld, D.; Daneshmand, S. Transurethral Resection of Bladder Tumors: Improving Quality Through New Techniques and Technologies. Curr. Urol. Rep. 2017, 18, 34. [Google Scholar] [CrossRef]
- Shigeta, K.; Matsumoto, K.; Ogihara, K.; Murakami, T.; Anno, T.; Umeda, K.; Izawa, M.; Baba, Y.; Sanjo, T.; Shojo, K.; et al. The clinicopathological characteristics of muscle-invasive bladder recurrence in upper tract urothelial carcinoma. Cancer Sci. 2021, 112, 1084–1094. [Google Scholar] [CrossRef]
- Genitsch, V.; Kollar, A.; Vandekerkhove, G.; Blarer, J.; Furrer, M.; Annala, M.; Herberts, C.; Pycha, A.; de Jong, J.J.; Liu, Y.; et al. Morphologic and genomic characterization of urothelial to sarcomatoid transition in muscle-invasive bladder cancer. Urol. Oncol. 2019, 37, 826–836. [Google Scholar] [CrossRef]
- Hensley, P.J.; Bree, K.K.; Campbell, M.T.; Alhalabi, O.; Kokorovic, A.; Miest, T.; Nogueras-Gonzalez, G.M.; Gao, J.; Siefker-Radtke, A.O.; Guo, C.C.; et al. Progression of Disease after Bacillus Calmette-Guerin Therapy: Refining Patient Selection for Neoadjuvant Chemotherapy before Radical Cystectomy. J. Urol. 2021, 206, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.; Yoo, S. Role of immunotherapy in Bacillus Calmette-Guerin unresponsive: Non-muscle invasive bladder cancer. Transl. Cancer Res. 2020, 9, 6537–6545. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Gu, X.; Li, Y.; Wu, Q. Mechanisms of BCG in the treatment of bladder cancer-current understanding and the prospect. Biomed. Pharmacother. 2020, 129, 110393. [Google Scholar] [CrossRef] [PubMed]
- Shore, N.D.; Palou Redorta, J.; Robert, G.; Hutson, T.E.; Cesari, R.; Hariharan, S.; Rodriguez Faba, O.; Briganti, A.; Steinberg, G.D. Non-muscle-invasive bladder cancer: An overview of potential new treatment options. Urol. Oncol. 2021, 39, 642–663. [Google Scholar] [CrossRef]
- Ogawa, K.; Shimizu, Y.; Uketa, S.; Utsunomiya, N.; Kanamaru, S. Prognosis of patients with muscle invasive bladder cancer who are intolerable to receive any anti-cancer treatment. Cancer Treat Res. Commun. 2020, 24, 100195. [Google Scholar] [CrossRef]
- Apolo, A.B.; Msaouel, P.; Niglio, S.; Simon, N.; Chandran, E.; Maskens, D.; Perez, G.; Ballman, K.V.; Weinstock, C. Evolving Role of Adjuvant Systemic Therapy for Kidney and Urothelial Cancers. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 311–326. [Google Scholar] [CrossRef]
- Carvalho, F.L.; Zeymo, A.; Egan, J.; Kelly, C.H.; Zheng, C.; Lynch, J.H.; Hwang, J.; Stamatakis, L.; Krasnow, R.E.; Kowalczyk, K.J. Determinants of neoadjuvant chemotherapy use in muscle-invasive bladder cancer. Investig. Clin. Urol. 2020, 61, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tian, J.; Zhang, S.; Yang, E.; Shen, H.; Li, F.; Li, K.; Zhang, T.; Wang, H.; Svatek, R.S.; et al. The utilization status of neoadjuvant chemotherapy in muscle-invasive bladder cancer: A systematic review and meta-analysis. Minerva Urol. Nephrol. 2021, 73, 144–153. [Google Scholar] [CrossRef]
- Kang, D.H.; Cho, K.S.; Moon, Y.J.; Chung, D.Y.; Jung, H.D.; Lee, J.Y. Effect of neoadjuvant chemotherapy on overall survival of patients with T2-4aN0M0 bladder cancer: A systematic review and meta-analysis according to EAU COVID-19 recommendation. PLoS ONE 2022, 17, e0267410. [Google Scholar] [CrossRef]
- Magee, D.; Cheung, D.; Hird, A.; Sridhar, S.S.; Catton, C.; Chung, P.; Berlin, A.; Warde, P.; Zlotta, A.; Fleshner, N.; et al. Trimodal therapy vs. radical cystectomy for muscle-invasive bladder cancer: A Markov microsimulation model. Can. Urol. Assoc. J. 2022, 16, E197–E204. [Google Scholar] [CrossRef]
- Tholomier, C.; Souhami, L.; Kassouf, W. Bladder-sparing protocols in the treatment of muscle-invasive bladder cancer. Transl. Androl. Urol. 2020, 9, 2920–2937. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Cherry, D.R.; Courtney, P.T.; Nalawade, V.; Kotha, N.; Riviere, P.J.; Efstathiou, J.; McKay, R.R.; Karim Kader, A.; Rose, B.S.; et al. Outcomes for Muscle-invasive Bladder Cancer with Radical Cystectomy or Trimodal Therapy in US Veterans. Eur. Urol. Open Sci. 2021, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, O.; Khairul-Asri, M.G.; Schubert, T.; Renninger, M.; Malek, R.; Kubler, H.; Stenzl, A.; Gakis, G. A systematic review and meta-analysis on the oncological long-term outcomes after trimodality therapy and radical cystectomy with or without neoadjuvant chemotherapy for muscle-invasive bladder cancer. Urol. Oncol. 2018, 36, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Fan, N.; Ning, Z.; Ma, D. Trimodal Therapy vs. Radical Cystectomy for Muscle-Invasive Bladder Cancer: A Meta-Analysis. Front. Oncol. 2020, 10, 564779. [Google Scholar] [CrossRef]
- Sekino, Y.; Ishikawa, H.; Kimura, T.; Kojima, T.; Maruo, K.; Azuma, H.; Yoshida, K.; Kageyama, Y.; Ushijima, H.; Tsuzuki, T.; et al. Bladder preservation therapy in combination with atezolizumab and radiation therapy for invasive bladder cancer (BPT-ART)—A study protocol for an open-label, phase II, multicenter study. Contemp. Clin. Trials Commun. 2021, 21, 100724. [Google Scholar] [CrossRef]
- Satkunasivam, R.; Lim, K.; Teh, B.S.; Guzman, J.; Zhang, J.; Farach, A.; Chen, S.H.; Wallis, C.J.; Efstathiou, E.; Esnaola, N.F.; et al. A phase II clinical trial of neoadjuvant sasanlimab and stereotactic body radiation therapy as an in situ vaccine for cisplatin-ineligible MIBC: The RAD VACCINE MIBC trial. Future Oncol. 2022, 18, 2771–2781. [Google Scholar] [CrossRef] [PubMed]
- Lagrange, J.L.; Bascoul-Mollevi, C.; Geoffrois, L.; Beckendorf, V.; Ferrero, J.M.; Joly, F.; Allouache, N.; Bachaud, J.M.; Chevreau, C.; Kramar, A.; et al. Quality of life assessment after concurrent chemoradiation for invasive bladder cancer: Results of a multicenter prospective study (GETUG 97-015). Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 172–178. [Google Scholar] [CrossRef]
- Choudhury, A.; Swindell, R.; Logue, J.P.; Elliott, P.A.; Livsey, J.E.; Wise, M.; Symonds, P.; Wylie, J.P.; Ramani, V.; Sangar, V.; et al. Phase II study of conformal hypofractionated radiotherapy with concurrent gemcitabine in muscle-invasive bladder cancer. J. Clin. Oncol. 2011, 29, 733–738. [Google Scholar] [CrossRef]
- James, N.D.; Hussain, S.A.; Hall, E.; Jenkins, P.; Tremlett, J.; Rawlings, C.; Crundwell, M.; Sizer, B.; Sreenivasan, T.; Hendron, C.; et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N. Engl. J. Med. 2012, 366, 1477–1488. [Google Scholar] [CrossRef]
- Tunio, M.A.; Hashmi, A.; Qayyum, A.; Mohsin, R.; Zaeem, A. Whole-pelvis or bladder-only chemoradiation for lymph node-negative invasive bladder cancer: Single-institution experience. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e457–e462. [Google Scholar] [CrossRef]
- Zapatero, A.; Martin De Vidales, C.; Arellano, R.; Ibanez, Y.; Bocardo, G.; Perez, M.; Rabadan, M.; Garcia Vicente, F.; Cruz Conde, J.A.; Olivier, C. Long-term results of two prospective bladder-sparing trimodality approaches for invasive bladder cancer: Neoadjuvant chemotherapy and concurrent radio-chemotherapy. Urology 2012, 80, 1056–1062. [Google Scholar] [CrossRef]
- Giacalone, N.J.; Shipley, W.U.; Clayman, R.H.; Niemierko, A.; Drumm, M.; Heney, N.M.; Michaelson, M.D.; Lee, R.J.; Saylor, P.J.; Wszolek, M.F.; et al. Long-term Outcomes After Bladder-preserving Tri-modality Therapy for Patients with Muscle-invasive Bladder Cancer: An Updated Analysis of the Massachusetts General Hospital Experience. Eur. Urol. 2017, 71, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, G.S.; Hermanns, T.; Wei, Y.; Bhindi, B.; Satkunasivam, R.; Athanasopoulos, P.; Bostrom, P.J.; Kuk, C.; Li, K.; Templeton, A.J.; et al. Propensity Score Analysis of Radical Cystectomy Versus Bladder-Sparing Trimodal Therapy in the Setting of a Multidisciplinary Bladder Cancer Clinic. J. Clin. Oncol. 2017, 35, 2299–2305. [Google Scholar] [CrossRef]
- Simsek, M.; Tekin, S.B.; Bilici, M. Immunological Agents Used in Cancer Treatment. Eurasian J. Med. 2019, 51, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, F.S.M.; Soares, A.; Souza, V.C.; Sperandio, R.C.; Grande, E.; Santoni, M.; Fay, A.P.; Sasse, A.D. A Systematic Review and Meta-Analysis of the Role of Immune Checkpoint Inhibitors (ICI) as Adjuvant Treatment for Localized High-Risk Muscle-Invasive Urothelial Carcinoma (MIUC). Clin. Genitourin. Cancer 2022, 20, 391–398. [Google Scholar] [CrossRef]
- Sharma, P.; Siefker-Radtke, A.; de Braud, F.; Basso, U.; Calvo, E.; Bono, P.; Morse, M.A.; Ascierto, P.A.; Lopez-Martin, J.; Brossart, P.; et al. Nivolumab Alone and With Ipilimumab in Previously Treated Metastatic Urothelial Carcinoma: CheckMate 032 Nivolumab 1 mg/kg Plus Ipilimumab 3 mg/kg Expansion Cohort Results. J. Clin. Oncol. 2019, 37, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- De Velasco, G.; Je, Y.; Bosse, D.; Awad, M.M.; Ott, P.A.; Moreira, R.B.; Schutz, F.; Bellmunt, J.; Sonpavde, G.P.; Hodi, F.S.; et al. Comprehensive Meta-analysis of Key Immune-Related Adverse Events from CTLA-4 and PD-1/PD-L1 Inhibitors in Cancer Patients. Cancer Immunol. Res. 2017, 5, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; van der Heijden, M.S.; Castellano, D.; Galsky, M.D.; Loriot, Y.; Petrylak, D.P.; Ogawa, O.; Park, S.H.; Lee, J.L.; De Giorgi, U.; et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020, 21, 1574–1588. [Google Scholar] [CrossRef]
- Powles, T.; Csoszi, T.; Ozguroglu, M.; Matsubara, N.; Geczi, L.; Cheng, S.Y.; Fradet, Y.; Oudard, S.; Vulsteke, C.; Morales Barrera, R.; et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 931–945. [Google Scholar] [CrossRef]
- Yoshida, T.; Ohe, C.; Ito, K.; Takada, H.; Saito, R.; Kita, Y.; Sano, T.; Tsuta, K.; Kinoshita, H.; Kitamura, H.; et al. Clinical and molecular correlates of response to immune checkpoint blockade in urothelial carcinoma with liver metastasis. Cancer Immunol. Immunother. 2022, 71, 2815–2828. [Google Scholar] [CrossRef]
- Barone, B.; Calogero, A.; Scafuri, L.; Ferro, M.; Lucarelli, G.; Di Zazzo, E.; Sicignano, E.; Falcone, A.; Romano, L.; De Luca, L.; et al. Immune Checkpoint Inhibitors as a Neoadjuvant/Adjuvant Treatment of Muscle-Invasive Bladder Cancer: A Systematic Review. Cancers 2022, 14, 2545. [Google Scholar] [CrossRef] [PubMed]
- Kandoth, C.; McLellan, M.D.; Vandin, F.; Ye, K.; Niu, B.; Lu, C.; Xie, M.; Zhang, Q.; McMichael, J.F.; Wyczalkowski, M.A.; et al. Mutational landscape and significance across 12 major cancer types. Nature 2013, 502, 333–339. [Google Scholar] [CrossRef]
- Stuhler, V.; Rausch, S.; Maas, J.M.; Stenzl, A.; Bedke, J. Combination of immune checkpoint inhibitors and tyrosine kinase inhibitors for the treatment of renal cell carcinoma. Expert Opin. Biol. Ther. 2021, 21, 1215–1226. [Google Scholar] [CrossRef]
- Necchi, A.; Raggi, D.; Gallina, A.; Madison, R.; Colecchia, M.; Luciano, R.; Montironi, R.; Giannatempo, P.; Fare, E.; Pederzoli, F.; et al. Updated Results of PURE-01 with Preliminary Activity of Neoadjuvant Pembrolizumab in Patients with Muscle-invasive Bladder Carcinoma with Variant Histologies. Eur. Urol. 2020, 77, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Kockx, M.; Rodriguez-Vida, A.; Duran, I.; Crabb, S.J.; Van Der Heijden, M.S.; Szabados, B.; Pous, A.F.; Gravis, G.; Herranz, U.A.; et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat. Med. 2019, 25, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2018, 174, 1033. [Google Scholar] [CrossRef] [PubMed]
- Loriot, Y.; Necchi, A.; Park, S.H.; Garcia-Donas, J.; Huddart, R.; Burgess, E.; Fleming, M.; Rezazadeh, A.; Mellado, B.; Varlamov, S.; et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2019, 381, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; Rosenberg, J.E.; Hoffman-Censits, J.H.; Berger, R.; Quinn, D.I.; Galsky, M.D.; Wolf, J.; Dittrich, C.; Keam, B.; Delord, J.P.; et al. Efficacy of BGJ398, a Fibroblast Growth Factor Receptor 1-3 Inhibitor, in Patients with Previously Treated Advanced Urothelial Carcinoma with FGFR3 Alterations. Cancer Discov. 2018, 8, 812–821. [Google Scholar] [CrossRef]
- Ungaro, A.; Tucci, M.; Audisio, A.; Di Prima, L.; Pisano, C.; Turco, F.; Delcuratolo, M.D.; Di Maio, M.; Scagliotti, G.V.; Buttigliero, C. Antibody-Drug Conjugates in Urothelial Carcinoma: A New Therapeutic Opportunity Moves from Bench to Bedside. Cells 2022, 11, 803. [Google Scholar] [CrossRef]
- Bogen, J.P.; Grzeschik, J.; Jakobsen, J.; Bahre, A.; Hock, B.; Kolmar, H. Treating Bladder Cancer: Engineering of Current and Next Generation Antibody-, Fusion Protein-, mRNA-, Cell- and Viral-Based Therapeutics. Front. Oncol. 2021, 11, 672262. [Google Scholar] [CrossRef]
- Peixoto, A.; Ferreira, D.; Azevedo, R.; Freitas, R.; Fernandes, E.; Relvas-Santos, M.; Gaiteiro, C.; Soares, J.; Cotton, S.; Teixeira, B.; et al. Glycoproteomics identifies HOMER3 as a potentially targetable biomarker triggered by hypoxia and glucose deprivation in bladder cancer. J. Exp. Clin. Cancer Res. 2021, 40, 191. [Google Scholar] [CrossRef] [PubMed]
- Hasehira, K.; Furuta, T.; Shimomura, O.; Asada, M.; Oda, T.; Tateno, H. Quantitative structural analysis of glycans expressed within tumors derived from pancreatic cancer patient-derived xenograft mouse models. Biochem. Biophys. Res. Commun. 2021, 534, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Oinam, L.; Tateno, H. Glycan Profiling by Sequencing to Uncover Multicellular Communication: Launching Glycobiology in Single Cells and Microbiomes. Front. Cell Dev. Biol. 2022, 10, 919168. [Google Scholar] [CrossRef]
- Nakajima, T.; Sano, K.; Mitsunaga, M.; Choyke, P.L.; Kobayashi, H. Real-time monitoring of in vivo acute necrotic cancer cell death induced by near infrared photoimmunotherapy using fluorescence lifetime imaging. Cancer Res. 2012, 72, 4622–4628. [Google Scholar] [CrossRef]
- Kuroda, Y.; Oda, T.; Shimomura, O.; Hashimoto, S.; Akashi, Y.; Miyazaki, Y.; Furuya, K.; Furuta, T.; Nakahashi, H.; Louphrasitthiphol, P.; et al. Lectin-based phototherapy targeting cell surface glycans for pancreatic cancer. Int. J. Cancer 2022, 152, 1425–1437. [Google Scholar] [CrossRef]
- Kim, L.H.C.; Patel, M.I. Transurethral resection of bladder tumour (TURBT). Transl. Androl. Urol. 2020, 9, 3056–3072. [Google Scholar] [CrossRef] [PubMed]
- Prelevic, R.; Stojadinovic, M.M.; Simic, D.; Spasic, A.; Petrovic, N. Scoring system development for prediction of extravesical bladder cancer. Vojnosanit. Pregl. 2014, 71, 851–857. [Google Scholar] [CrossRef]
- Panebianco, V.; Narumi, Y.; Altun, E.; Bochner, B.H.; Efstathiou, J.A.; Hafeez, S.; Huddart, R.; Kennish, S.; Lerner, S.; Montironi, R.; et al. Multiparametric Magnetic Resonance Imaging for Bladder Cancer: Development of VI-RADS (Vesical Imaging-Reporting and Data System). Eur. Urol. 2018, 74, 294–306. [Google Scholar] [CrossRef]
- Woo, S.; Panebianco, V.; Narumi, Y.; Del Giudice, F.; Muglia, V.F.; Takeuchi, M.; Ghafoor, S.; Bochner, B.H.; Goh, A.C.; Hricak, H.; et al. Diagnostic Performance of Vesical Imaging Reporting and Data System for the Prediction of Muscle-invasive Bladder Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Oncol. 2020, 3, 306–315. [Google Scholar] [CrossRef]
- Del Giudice, F.; Leonardo, C.; Simone, G.; Pecoraro, M.; De Berardinis, E.; Cipollari, S.; Flammia, S.; Bicchetti, M.; Busetto, G.M.; Chung, B.I.; et al. Preoperative detection of Vesical Imaging-Reporting and Data System (VI-RADS) score 5 reliably identifies extravesical extension of urothelial carcinoma of the urinary bladder and predicts significant delayed time to cystectomy: Time to reconsider the need for primary deep transurethral resection of bladder tumour in cases of locally advanced disease? BJU Int. 2020, 126, 610–619. [Google Scholar] [CrossRef]
- Ueno, Y.; Tamada, T.; Takeuchi, M.; Sofue, K.; Takahashi, S.; Kamishima, Y.; Urase, Y.; Kido, A.; Hinata, N.; Harada, K.; et al. VI-RADS: Multiinstitutional Multireader Diagnostic Accuracy and Interobserver Agreement Study. AJR Am. J. Roentgenol. 2021, 216, 1257–1266. [Google Scholar] [CrossRef]
- Pecoraro, M.; Del Giudice, F.; Magliocca, F.; Simone, G.; Flammia, S.; Leonardo, C.; Messina, E.; De Berardinis, E.; Cortesi, E.; Panebianco, V. Vesical Imaging-Reporting and Data System (VI-RADS) for assessment of response to systemic therapy for bladder cancer: Preliminary report. Abdom. Radiol. 2022, 47, 763–770. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Zhang, S.; Zhu, Y.; Wang, J. Exploration of Prognostic Biomarkers of Muscle-Invasive Bladder Cancer (MIBC) by Bioinformatics. Evol. Bioinform. Online 2021, 17, 11769343211049270. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Dong, X.; Yang, F.; Xing, N. Comparative Analysis of Differentially Mutated Genes in Non-Muscle and Muscle-Invasive Bladder Cancer in the Chinese Population by Whole Exome Sequencing. Front. Genet. 2022, 13, 831146. [Google Scholar] [CrossRef] [PubMed]
- Matsusaka, S.; Kozuka, M.; Takagi, H.; Ito, H.; Minowa, S.; Hirai, M.; Hatake, K. A novel detection strategy for living circulating tumor cells using 5-aminolevulinic acid. Cancer Lett. 2014, 355, 113–120. [Google Scholar] [CrossRef]
- Hornung, S.; Dutta, S.; Bitan, G. Cns-Derived Blood Exosomes as a Promising Source of Biomarkers: Opportunities and Challenges. Front. Mol. Neurosci. 2020, 13, 38. [Google Scholar] [CrossRef]
- Kamoun, A.; de Reynies, A.; Allory, Y.; Sjodahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Groeneveld, C.S.; Al-Ahmadie, H.; Choi, W.; et al. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. Eur. Urol. 2020, 77, 420–433. [Google Scholar] [CrossRef]
- Mo, Q.; Li, R.; Adeegbe, D.O.; Peng, G.; Chan, K.S. Integrative multi-omics analysis of muscle-invasive bladder cancer identifies prognostic biomarkers for frontline chemotherapy and immunotherapy. Commun. Biol. 2020, 3, 784. [Google Scholar] [CrossRef]
- Graziano, F.; Valsecchi, M.G.; Rebora, P. Sampling strategies to evaluate the prognostic value of a new biomarker on a time-to-event end-point. BMC Med. Res. Methodol. 2021, 21, 93. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Li, Y.; Wang, M.; Gu, J.; Xu, W.; Cai, H.; Fang, X.; Zhang, X. Exosomes as a new frontier of cancer liquid biopsy. Mol. Cancer 2022, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef]
- He, L.; Zhu, D.; Wang, J.; Wu, X. A highly efficient method for isolating urinary exosomes. Int. J. Mol. Med. 2019, 43, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Gheinani, A.H.; Vogeli, M.; Baumgartner, U.; Vassella, E.; Draeger, A.; Burkhard, F.C.; Monastyrskaya, K. Improved isolation strategies to increase the yield and purity of human urinary exosomes for biomarker discovery. Sci. Rep. 2018, 8, 3945. [Google Scholar] [CrossRef]
- Konoshenko, M.; Sagaradze, G.; Orlova, E.; Shtam, T.; Proskura, K.; Kamyshinsky, R.; Yunusova, N.; Alexandrova, A.; Efimenko, A.; Tamkovich, S. Total Blood Exosomes in Breast Cancer: Potential Role in Crucial Steps of Tumorigenesis. Int. J. Mol. Sci. 2020, 21, 7341. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Ofuji, K.; Hiramatsu, K.; Nosaka, T.; Naito, T.; Matsuda, H.; Endo, K.; Higuchi, M.; Ohtani, M.; Nemoto, T.; et al. Circulating tumor cells detected with a microcavity array predict clinical outcome in hepatocellular carcinoma. Cancer Med. 2021, 10, 2300–2309. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, T.; Yanagitani, N.; Suga, K.; Yoshizawa, T.; Nishikawa, S.; Kitazono, S.; Horiike, A.; Shiba, K.; Ishizuka, T.; Nishio, M.; et al. A Novel System to Detect Circulating Tumor Cells Using Two Different Size-selective Microfilters. Anticancer Res. 2020, 40, 5577–5582. [Google Scholar] [CrossRef]
- Walker, J.M.; O’Malley, P.; He, M. Applications of Exosomes in Diagnosing Muscle Invasive Bladder Cancer. Pharmaceutics 2022, 14, 2027. [Google Scholar] [CrossRef] [PubMed]
- Welton, J.L.; Khanna, S.; Giles, P.J.; Brennan, P.; Brewis, I.A.; Staffurth, J.; Mason, M.D.; Clayton, A. Proteomics analysis of bladder cancer exosomes. Mol. Cell Proteom. 2010, 9, 1324–1338. [Google Scholar] [CrossRef]
- Hiltbrunner, S.; Mints, M.; Eldh, M.; Rosenblatt, R.; Holmstrom, B.; Alamdari, F.; Johansson, M.; Veerman, R.E.; Winqvist, O.; Sherif, A.; et al. Urinary Exosomes from Bladder Cancer Patients Show a Residual Cancer Phenotype despite Complete Pathological Downstaging. Sci. Rep. 2020, 10, 5960. [Google Scholar] [CrossRef]
- Lee, D.H.; Yoon, H.; Park, S.; Kim, J.S.; Ahn, Y.H.; Kwon, K.; Lee, D.; Kim, K.H. Urinary Exosomal and cell-free DNA Detects Somatic Mutation and Copy Number Alteration in Urothelial Carcinoma of Bladder. Sci. Rep. 2018, 8, 14707. [Google Scholar] [CrossRef]
- Zhou, X.; Kurywchak, P.; Wolf-Dennen, K.; Che, S.P.Y.; Sulakhe, D.; D’Souza, M.; Xie, B.; Maltsev, N.; Gilliam, T.C.; Wu, C.C.; et al. Unique somatic variants in DNA from urine exosomes of individuals with bladder cancer. Mol. Ther. Methods Clin. Dev. 2021, 22, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Royo, F.; Zuniga-Garcia, P.; Torrano, V.; Loizaga, A.; Sanchez-Mosquera, P.; Ugalde-Olano, A.; Gonzalez, E.; Cortazar, A.R.; Palomo, L.; Fernandez-Ruiz, S.; et al. Transcriptomic profiling of urine extracellular vesicles reveals alterations of CDH3 in prostate cancer. Oncotarget 2016, 7, 6835–6846. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Du, M.; Wang, X.; Xu, W.; Liang, J.; Wang, W.; Lv, Q.; Qin, C.; Chu, H.; Wang, M.; et al. Exosome-transmitted long non-coding RNA PTENP1 suppresses bladder cancer progression. Mol. Cancer 2018, 17, 143. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering exosomes for targeted drug delivery. Theranostics 2021, 11, 3183–3195. [Google Scholar] [CrossRef]
- Jafari, D.; Shajari, S.; Jafari, R.; Mardi, N.; Gomari, H.; Ganji, F.; Forouzandeh Moghadam, M.; Samadikuchaksaraei, A. Designer Exosomes: A New Platform for Biotechnology Therapeutics. BioDrugs 2020, 34, 567–586. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, D.; Ma, X.; Wang, J.; Hou, W.; Zhang, W. Exosomes as drug carriers for cancer therapy and challenges regarding exosome uptake. Biomed. Pharmacother. 2020, 128, 110237. [Google Scholar] [CrossRef]
- Hao, G.; Xu, Z.P.; Li, L. Manipulating extracellular tumour pH: An effective target for cancer therapy. RSC Adv. 2018, 8, 22182–22192. [Google Scholar] [CrossRef]
- Rezaie, J.; Feghhi, M.; Etemadi, T. A review on exosomes application in clinical trials: Perspective, questions, and challenges. Cell Commun. Signal 2022, 20, 145. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).