Abstract
Proline is water soluble amino acid extensively used in drug delivery systems. Compounds of cobalt (Co) transition metal have potent antimicrobial and anticancer activities. However, a drug delivery system combining proline cobalt is not reported yet. For the first time, new hybrid semi-organic single crystals of proline cobalt chloride (PCC) are prepared. The novelty of the article is also that single crystal proline cobalt chloride showed potent antimicrobial and antitumor activity. Doping of PCC by Ag0NPs significantly increased these biological activities. The anisotropic magnetic properties of single crystals can mitigate the cytotoxicity of Ag0NPs on normal cells. Silver nanoparticles (Ag0NPs) improved the crystal habits and physicochemical properties. Ag0NPs showed the best performance, paramagnetic materials n-type semiconductors due to delocalized excess electrons of Ag0NPs incorporated in the crystal lattice interstitially. Crystals have high absorptivity for UV-radiation electromagnetic radiation. Ag0NPs enhanced AC electrical conductivity up to 2.3 × 104 Ω cm−1 due to high electron density. Proline doped crystals are obtained in good purity as triclinic unit cell with having anisotropic magnetism. PCCAg0NPs crystal exhibited: high antimicrobial activities to various bacterial and fungal species, inhibition zone (mm): 21, 25, 24, 26, 30, 28, 12, and 46 for S. aureus, E. faecalis, S. typhi, E. coli, P. aerugino, K. pneumoniae, A. braselienses, and C. albicans, respectively, in comparison to ciprofloxacin antibiotic (23, 0, 26, 26, 25, 0, 0, 0) for the same tested species, respectively; higher cytotoxicity against breast cancer cells (IC50 22.1 μM) than the reference drug cisplatin (IC50 11.7 μM); and lower cytotoxicity to normal healthy lung cells MRC-5, (IC50 145.5 μM) than cisplatin (IC50 30.2 μM). Hence, this crystal is a candidate for chemotherapy of breast cancer.
1. Introduction
Single crystals in literature are described as polar crystals, thermoelectric materials that generate electricity on heating that change intrinsic magnetic moments and permittivity; tiny small sensors in electronic and power generators convert chaotic waste heat into useful electric work [1,2]. They store electrical energy after removal of an applied electric field as charge-reservoirs parallel plate capacitors; piezoelectric materials applied in automobiles electronics, touch screens of laptop and mobile phones; microwave filters, and energy storage systems [3,4]. In electric fields, the electron clouds in atoms polarize. The dielectric constant or refractive index is affected by the frequency of absorbed quantized energy UV-Vis.; and absorb UV-Vis. of sunlight generating electron–hole pairs [5]. The current flow depends on photon energy. Polarization in electric fields is controlled by dielectric constant; and AC conductivity measures macroscopic dielectric properties and polarization [5]. Dielectric materials are used as capacitors, insulators and semiconductors. Ferroelectric dielectric perovskite crystals undergo a phase change from a non-polar para-electric phase to a polar ferro-electric polar phase depending on heating or pressure at Curie temperature, TC providing no thermal decomposition at TC, and are applied as electrostrictive actuators due to strong electronic power generation and saving [6,7].
Single crystals of glycine amino acid have high nonlinear optical (NLO) and ferroelectric properties [8]. Glycine cobalt chloride (GCC) single crystals are used in: infrared detectors [9]; and pervoskite crystals used in conversion waste heat of combustion, exhausted from pipes, automobiles, batteries, furnaces, and chimneys into electricity [10].
No details are reported about doping glycine and proline-single crystals by Ag0NPs [11]. Perovskites metal oxides single crystals have a molecular formula, ABO3 are pillars in electronics and nanotechnology as superconductive electrodes, magnets, insulators, etc.; have cubic or semi-cubic structure; and applied ferroelectric piezoelectric lead-based perovskites. Examples include Pb(ZrxTi1−x)O3 or Pb(Mg0.33Nb0.67)O3-PbTiO3, which are low cost; however, lead is a toxic element [12]. The phases bithmus titanate Bi4Ti3O12 and barium titanate BaTiO3, alkali Li, Na, K niobates NbO3, bismuth-alkali titanates (Na0.5Bi0.5)TiO3, K0.5Bi0.5TiO3 and their solid solutions (Ba, Ca)(Ti, Zr)O3, (Na0.5Bi0.5)TiO3-BaTiO3 are ecofriendly crystals [13,14].
Hybrid semi-organic single crystals (HSOSCs) have ferroelectric and magnetic properties in a single phase and are good alternatives to conventional materials such as BiFeO3, BiMnO3, FeO3, and LaFeO3. Polar semi-organic crystals possess ferroelectricity, antiferroelectricity, piezoelectricity, thermal stability, metallic conductivity, superconductivity, ferromagnetism, anti-ferromagnetism, etc. [15,16].
The use of Ag0NPs as bridging linkers in proline single crystals is not reported. This study aims to prepare, characterize and enhance the quality and physicochemical properties of new single crystals (structure, magnetic properties and NLO activity by Ag0NPs doping). The synergistic effect of dopants CoCl2 and Ag0NPs could affect such properties.
2. Experimental
2.1. Materials and Methods for Preparation of Single Crystals
All chemicals used in this study are all of analytical grades used as received without further purification. Molecular weight and purity of glycine, proline, silver nanoparticles (Ag0NPs) and cobalt chloride hexa-hydrate, CoCl2·6H2O, are collected in Table 1.

Table 1.
Materials used in crystals growth.
Appropriate salts weights are mixed in stoichiometric molar ratio: (glycine or proline)1−x (CoCl2)x (x is dopant weight percent of either CoCl2 in absence and presence of Ag0NPs). Glycine single crystal is grown as a control single crystal.
Single crystals are grown following a slow evaporation method [12], at optimum experimental conditions: temperature 25 °C ± 0.1, pH 5.5 and 100 rpm agitation speed for 2 h. The salts mixture is continuously agitated in deionized water green nontoxic solvent until attaining a homogeneous saturated solution at the same temperature. The saturated solution is filtered and left covered with porous filter paper. Crystal nucleation and growth are allowed through slow water evaporation. Pink colored single crystals: proline cobalt chloride (PCC) in the presence of 0.15 wt.% Ag0NPs are harvested after three weeks of representative visual appearances as shown in Figure 1 in comparison to glycine cobalt chloride (GCC).
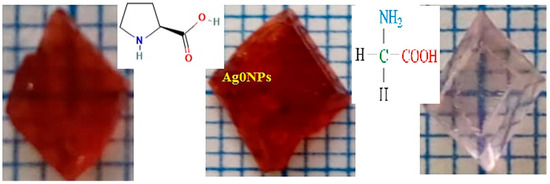
Figure 1.
Visual inspection of cobalt chloride doped crystals: proline, proline-Ag0NPs and glycine, respectively.
High quality crystals have a large size, and perfect octahedral (Oh) geometry with defined edges. Crystals containing Ag0NPs have a more intense pink color, suggesting applications as new colored materials in the second harmonic generation.
2.2. Characterization of Single Crystals
Crystals are characterized using different spectroscopic methods of analysis. Carbon, hydrogen, and nitrogen CHN elemental analysis (EA) is determined using Malvern analytical elemental analyzers. The content of Co(II) ion is determined via several digestion decompositions in aqua-regia to dissolve organic matter. Cobalt residue is dissolved in double distilled water and is determined using a Shimadzu 6650 atomic absorption spectrophotometer.
Fourier transformer infrared (FTIR) spectra are recorded using Bruker Tensor 27FTIR-spectrophotometer at frequency range 400–5000 cm−1, and Nujol mull UV-Vis. electronic spectra are recorded using Lambda 4B Perkin Elmer spectrophotometer at wavelength range 200–900 nm. Molar magnetic susceptibilities and Pascal’s constants are determined using Faraday’s method at 25 °C while calibration spectrophotometer using Hg[Co (SCN)4].
Thermogravimetric analysis, TGA and differential thermal analysis, DTA are determined using Shimadzu DTA/TGA-50, heating rate 10 °C/min, platinum cell under nitrogen, and flow rate 20 mL min−1 [17,18].
Powder X-ray diffraction at 2θ range 5–80° with Cu-Kα X-ray (λ 1.54 Å) radiation source. Density, ρ, is determined by floatation technique in a saturated solution of NaCl, KBr and benzene separately. The number of formula units per unit cell (Z) is calculated by using the equation [17,18]:
where V is volume of unit cell, and N is Avogadro’s number.
X-band electron spin resonance spectra at room temperature using a reflection (JES-RE1X ESR. ESR spectrometer) at 9.43 GHz in cylindrical resonance cavity, 100 kHz modulation, 5 mW electric power and LMR Gauss meter control applied magnetic field.
AC electrical conductivity of single crystal sample is measured using four probes Agilent 4294 A Impedance Bridge applying sine AC signal, 10 amplitude. Thin gold layers (10 nm) are deposited on two opposite sides of the pellet sample by thermal evaporation under vacuum 10−5 mbar using Joule evaporator. Silver wire is glued on each deposit with silver lacquer.
Antimicrobial activity is determined using paper disk diffusion method against Gram-positive bacteria: Staphylococcus aureus, Enterococcus faecalis; Gram-negative bacteria: Escherichia coli; Pseudomonas aeruginosa, Salmonella typhi, Klebsiella pneumoniae); and Fungi: Aspergillus brasiliensis; Candida albicans. Cytotoxic activity this metal complexes on cancer cell lines are screened using MTT assay against MCF-7 (human breast adenocarcinoma), and healthy MRC-5 human lung fibroblasts (control cell lines). Results of in vitro cytotoxic activity are compared with reference standard Cis-platin in terms of IC50.
3. Results and Discussion
The chemical composition and atomic percent are collected in Table 2.

Table 2.
Analytical and physical data of crystals.
High atomic percent C, H, N, and O atoms indicated that proline and glycine amino acids are the mother materials for crystals [19]. Both proline and glycine have a white color. Doping of the crystal lattice of both proline and glycine by CoCl2 produced optically active have high molecular weight (Mw.) single crystals of pink color. Ag0NPs intensified the pink color of PCC and increased Mw. up to 1051.54 g mol−1 forming a self-supramolecular assembled single crystal [20]. CoCl2 incorporated into glycine and proline, forming a crystal with a 1:2 molar ratio. The crystals are stable, non-hygroscopic and soluble in water polar green solvent.
The infrared spectra of the crystals are compared with that of amino acids to deduce the intercalation mode. The charge transfer from ligand (proline or glycine) to Co(II) ion decreased the force constant of bond causing red shift of bond position and enhanced optical activity of crystals. Some blue shift occurs on back donation of the electron from Co(II) ion to the electron donor atom to reinforce the coordinate bond [21]. Assignments of main spectral vibrational band are given in Supplementary Information, Table S1.
Proline bands at 3430, 2983, 2504 and 1312 cm−1 are assigned to υ(OH), υas(CH2), υs(CH2), and υ(C-N), respectively. υ, δ, and γ vibration of NH2+, υC-O of carboxylate COOH group vibrational bands at 3066, 1623, 871, and 1290 cm−1, respectively. Bending vibration bands of CH2 pyrrolidine ring of proline appeared at 1454, 1402, 1367, 1318, 1164, 834, 589 and 538 cm−1, respectively. The absence of a νCOOH band at 1725 cm−1 is due to the deprotonated COOH group in Zwitter ion form. Two bands at 1559 cm−1 and 1357 cm−1 signified asymmetric and symmetric stretching of the deprotonated carboxylate COO− group, respectively [22].
FTIR spectral bands of proline are compared with that of single crystal to declare bonding mode with Co(II) ion. IR spectral bands of two PCC2 crystals showed a νCOOH band at the 1730–1732 cm−1 range (Carbonyl group). The absence υasy COO– is due to protonation on coordination to the Co(II) ion. υNH and δNH are red shift by 101–107 and 17–19 cm−1, respectively, relative to proline. υC-N and γNH changed in shapes and positions, indicating the participation of a N atom in chelation. PCC showed a new band at 437–441 cm−1 (υCo-N). IR spectra explored amino acid is bidentate ligand coordinate Co(II) ion through N, O atoms, see Figure 2.
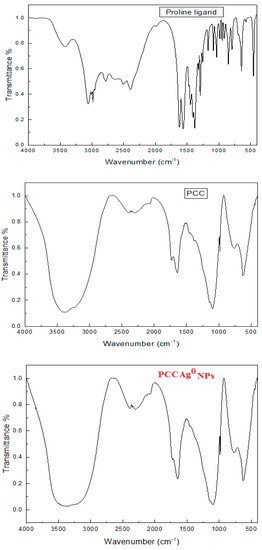
Figure 2.
FTIR spectra of single crystal.
Optical activity confirmed electronic spectral and magnetic properties of crystals investigated by using Nujol mull absorption spectroscopy at room temperature [23,24]. Calculated optical parameters: ligand field splitting and stabilization energy (CFSE), 10 Dq are collected in Table 3. Ligand field parameters for Co(II)-Racah inter-electronic repulsion parameter B′: 595–753 cm−1. The lowering B of free Co(II)ion complexation suggests orbital overlap and electrons delocalization on Co(II)ion. In nephelauxetic ratio, the β less than one indicating partial covalent bond “σ” between Co and amino acid [25]. The parameters of tetragonal distortion in crystals (Ds and Dt) and the crystal field parameter (Dq) are derived from the energy of different electronic transitions.

Table 3.
Electronic absorption spectral data λmax (nm) and effective magnetic values (µeff 298 K) of crystals.
The values of magnetic moment have B.M suggesting high spin distorted tetragonal geometry, (t2g)5(eg)2 configuration and 4A2g ground state. PCC displayed five absorption bands indicating axial distorted Oh symmetry around Co(II) ion [26] due to transitions ν2 4A2g → 4B2g, ν3 4A2g → 4Eg(b) and ν4 4A2g → 4B1g, 4A2g → 4Eg(c) [4T1g (P)] and 4A2g → 4A2g(c) [4T1g (P)]. Bands corresponding to ν1 4A2g → 4Eg(a) were not observed in the spectra [27,28].
Thermal degradation confirmed the molecular structure and thermal stability. TGA and DTA thermograms of crystal are shown in Figure 3, Figure 4 and Figure 5. TGA showed weight loss of tested sample as a function of temperature or time. DTA measures temperature difference (ΔT = TS − TR) between the sample (S) and reference (R) materials at zero heat flow difference (ΔH = HS − HR = 0).
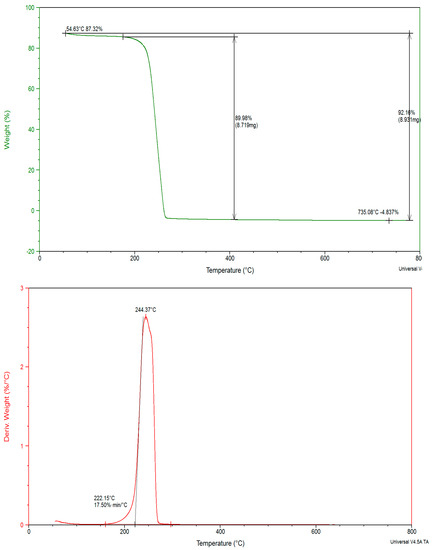
Figure 3.
Thermal gravimetric analysis and differential thermal analysis thermograms of proline.
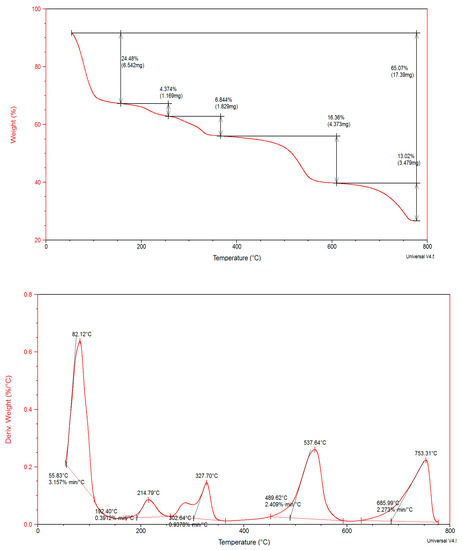
Figure 4.
Thermal gravimetric analysis and differential thermal analysis of PCC.
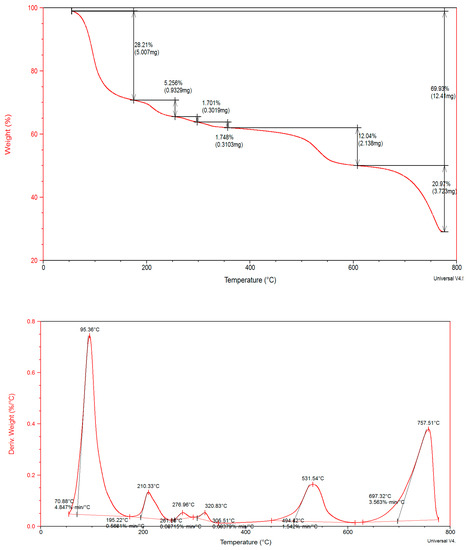
Figure 5.
Thermal gravimetric analysis and differential thermal analysis thermograms of PCCAg0NPs.
TGA and DTA of proline and crystals showed distinguished coordination and stability ranges in peak temperatures and kinetic parameters. Shape index symmetry of peak “S” ratio of slopes of curve tangents at inflection points a/b depends on reaction order, n (1°, 2° order, etc.) and is determined from DTA, see Supplementary Information SI.1 [29].
Applying least square method, the plot (ln ΔT versus 1/T) is represented in Figure S1 and gave straight lines obeying Arrhenius relation. Activation energy Ea of decomposition is calculated [30]. The TGA %wt.loss for decomposition steps is correlated to the proposed chemical formula, see Table 4.

Table 4.
DTA analysis of proline and crystals.
Proline showed 2.14% wt.loss at 199 °C, which caused a weak DTG peak at 66 °C corresponding to dehydration. Thermal degradation from 199–266 °C, 97.86% wt.loss due to complete decomposition is associated with broad DTG peak at 244.37 °C, in a narrow temperature range indicating rapid thermal decomposition. The corresponding Ea kJ/mol−1 and n values for two exothermic steps are 32.42, (1.05) and 12.88, (1.09), respectively [31]. Consecutive thermal degradation followed 1° order kinetic according to Scheme 1.
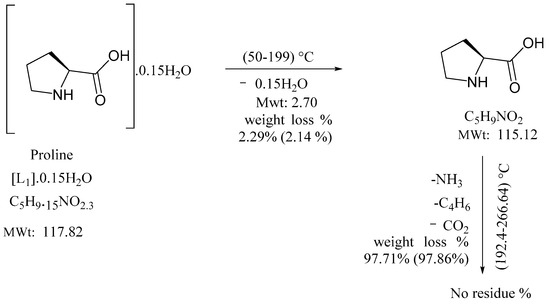
Scheme 1.
Complete thermal degradation of proline.
PCC five degradation stages: 24.48% wt.loss is due to partial dehydration and removal six water molecules give strong DTG peaks, and Tmax 82 °C. Decomposition steps are at temperature ranges 192–302, 302–366 and 366–600 °C. In DTG weak, medium and strong peaks located at 214, 327 and 537 °C, respectively. Thermal decompositions steps suggested elimination: two coordinated H2O molecules, 2OH group and fraction residue 4 C atoms. The final degradation step showed wt.loss 13.02% at 600 °C, DTG peaks at 753 °C is due to release 1.0 mole H2(g) + 2.0 moles NH3 (Ea 122.22 kJ/mol, n 1.41), respectively. The final residue is CoO + 6C. Thermal decomposition pathway is represented in Scheme 2 [32].
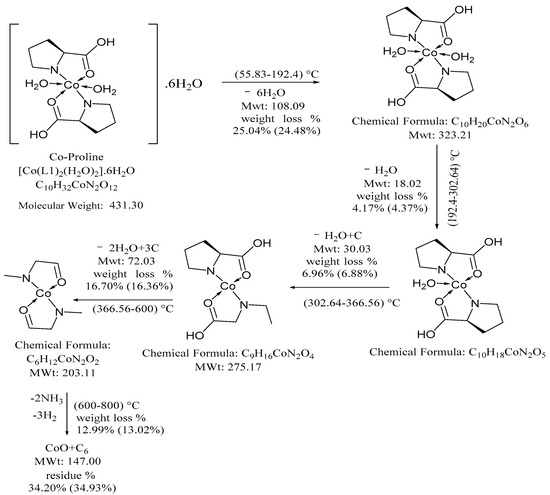
Scheme 2.
Thermal degradation of PCC [Co(L1)2(H2O)2]6H2O complex.
TGA thermograms PCC and PCCAg0NPs showed nearly similar behavior indicating isostructural and isothermally behavior. PCCAg0NPs crystal exhibited five decomposition stages. wt.loss at 70.88–195.4°C is due to removal of lattice-water molecules with sharp strong DTG peaks at 95.38 °C at narrow temperature ranges signifying rapid thermolysis. Anhydrous crystal started decomposition through two overlapped continuous steps at 195.4–261.88 °C and 261.88–305.5 °C giving weak broad diffused DTG peaks at: 210, 276 and 320 °C, respectively, wt.loss 5.24 and 1.70% due to loss 4.0 coordinated H2O molecules. Fourth and fifth thermal decomposition processes Wt.loss 12.04 and 22.66% due to release 2H2O + C7H6 and C13H14 + 2NH3(g), respectively, leaving AgCo2O5 + C residue. These steps showed small different thermal stability and Tmax. DTG peaks showed increasing ΔH due to Ag0NPs interaction with OH− of proline forming a robust self-assembled monolayer on Ag0NPs via strong Ag O covalent bond and Van der Waals interaction. The bond energy Ag-O is 217 kJ mol−1. There is a small difference in ΔH confirmed supramolecular structure [33], see Scheme 3.
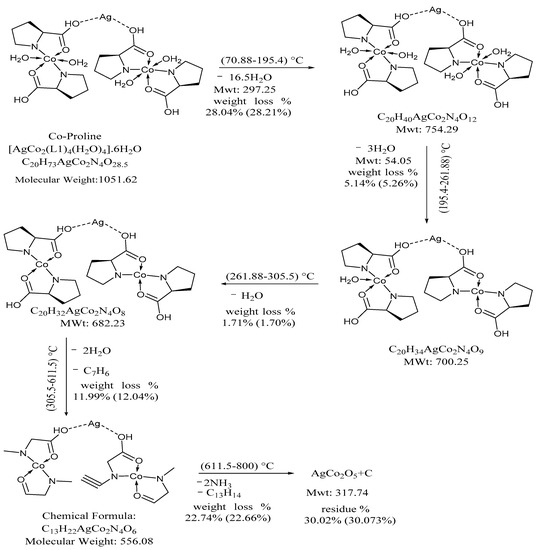
Scheme 3.
Thermal degradation of PCCAg0NPs. Co(II) ion is a covalently linked proline through nitrogen or oxygen atom. Doping proline CoCl2 crystal by Ag0NPs gives thermally stable self-assembled crystals. Ag0NPs is an inorganic linker. Thermal parameters and activation parameters, Ea, ΔS# (J K−1 mol−1), ΔH# (kJ K−1 mol−1) are collected in Table 4.
By profile fitting and indexing the PXRD pattern, the crystal structure is solved using direct methods with simulated annealing implemented in software EXPO2014. The unit-cell parameters for PCC crystals are refined using Pawley/LeBail fit analysis [17,34]. PXRD data of PCCAg0NPs crystals are indexed with N-TREOR, and in Figure 6, the crystal lattice parameters are collected in Table 5 and Table 6 including unit cell parameters and Rietveld refinements Rp, Rwp, and S 2.03 parameters are also described.
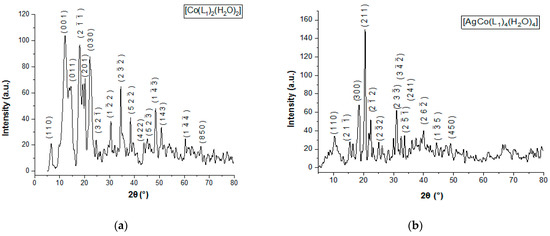
Figure 6.
PXRD pattern: (a) PCC and (b) PCCAg0NPs.

Table 5.
Powder XRD parameters.

Table 6.
PXRD parameters.
Figure 7 showed the refined bond distance in PCC and PCCAg0NPs.
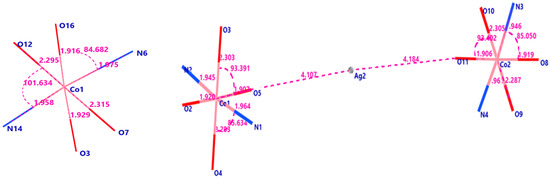
Figure 7.
Bond distances and bond angles around Co(II) in PCC and PCCAg0NPs.
The crystal structure is shown in Figure 8.
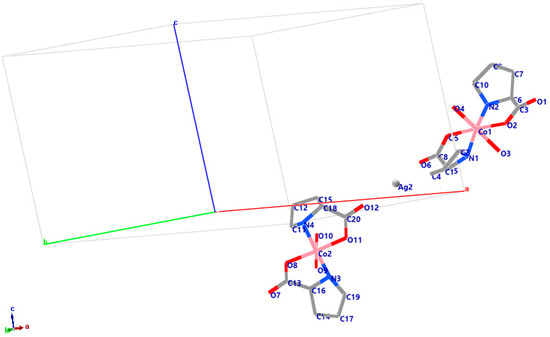
Figure 8.
Crystal structure of PCC, interstitial Ag0NPs.
Figure 9 demonstrated the refined pXRD of PCCAg0NPs.
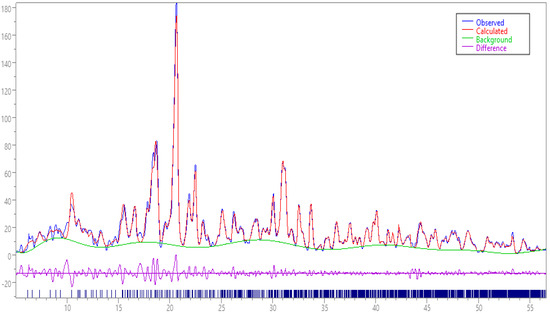
Figure 9.
(Red Color line) Rietveld plot for refinement of pXRD PCCAg0NPs.
Refinement PXRD patterns convert the approximate structure of PCCAg0NPs crystal into an actual structure, see Figure 10.
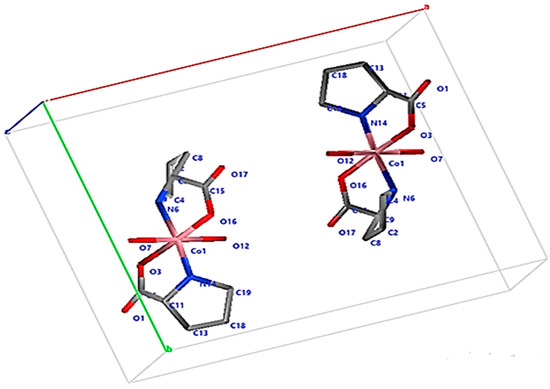
Figure 10.
Crystal structure of PCC.
NH group and O atoms of proline give bidentate chelating, forming a distorted octahedral geometry because imperfect bond angles. The equatorial plane is by O3, O16 of 2 COOH groups and (N6, N14) atoms. Bond angles ranges 84.83 (2)–95.74 (5)°. Axial sites are occupied by O7, O12 of 2 coordinated water with bond angles 78.29 (9)–101.63 (4)°. Geometry around Co(II) ion is distorted elongated Oh with short bonds at equatorial positions at range [1.91 (6)–1.97 (5) Å]. Long bonds formed at axial positions [Co(1)–O(7) is 2.31 (4) Å; Co(1)–O(12) is 2.29 (4) Å]. The axial angle O7–Co1–O6 is 179.09 (4)°. The equatorial angles [O3-Co1-O16; N6-Co1-N14 are 172.34 (2)° and 177.00 (3)°, respectively, are close to linearity. Average Co–O and Co–N bond lengths confirmed Co(II) distorted Oh geometry. Doped Ag0NPs had unchanged crystal geometry around the Co(II) ion type, but changed dimensions of the crystal size by expanding the crystal size [35]. The geometry around the Co(II) ion has the same chelating manner, bond length and bond angle as PCC formed distorted Oh, Ag0NPs bind Co and proline via Van der Waals interaction [36,37]. All bond lengths and bond angle° of PCC are collected in Table S2.
The room temperature polycrystalline X-band ESR spectral patterns Figure 11 showed a typical distorted axial pattern for high-spin Co(II) with a three resonances structure. The perpendicular signal is split into two components due to rhombic distortion. Effective g values are gy 2.15, gx 1.98, and gz 1.89 for PCC and gy 2.12, gx 1.98 and gz 1.88 for PCCAg0NPs. Hyperfine coupling of electron spin with 59Co nucleus (I3/2) produces three equally spaced lines in the gy region with [Ay 35 × 10−4 cm−1] and in the gz region with [Az 45 × 10−4 cm−1] corresponding to |−3/2> → |−1/2>, |+3/2> → |+1/2> and |−1/2> → |−1/2>. Splitting in the gx region is unobserved due to line-width. Ax equals 15 × 10−4 cm−1] [38,39,40].
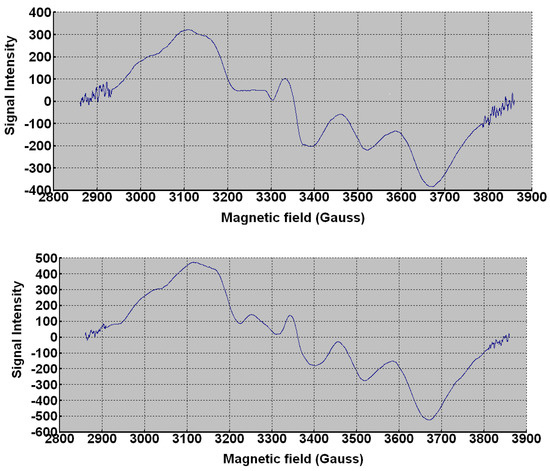
Figure 11.
ESR spectra of PCC and PCCAg0NPs, respectively.
Molecular orbital calculations, performed by qualitative analysis performed by Ab initio calculations following CASSCF method, Gaussian software program suggested distorted Oh symmetry around Co(II) ion. Elongated Co-O bonds with water molecules in comparison to proline favors magnetic anisotropy, (Figure 12) zero field splitting (ZFS) parameters D and E in Griffith Hamiltonian are obtained using five magnetic parameters: gx, gy, gz, D, and η following Equations (7) and (8), Bond length, Å and bond angle° of PCC are collected in Table S2 [41]:
where parameters Dxx, Dyy, and Dzz are principal values of ZFS tensor.
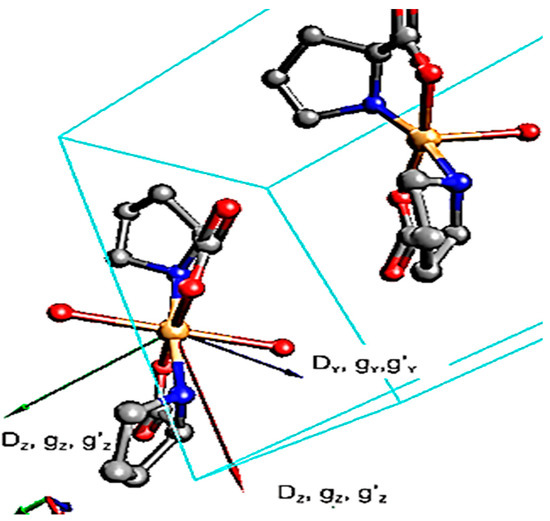
Figure 12.
Orientated tensors D, g, g′ obtained with CASSCF calculations for PCC.
ZFS term causes splitting quartet ground state into two Kramers doublets with energy gap, Δ.
where η = E/D is the rhombicity parameter. Table 7 showed a positive D value and relative high energy. Splitting between two Kramers doublets nevertheless remains larger than 130 cm−1, a value that is within the characteristic range observed for other distorted Oh symmetry. Slow relaxation of single-molecule magnets (SMMs) arises from strong magnetic anisotropy, see Figure 12 and Table 7 and Table 8 [42].

Table 7.
The calculated parameters for anistropic magnetic parameters.

Table 8.
Results of Ab initio calculations.
Excitation energy, Δ, includes spin–orbit coupling effects [43].
Electrochemical behavior of crystals is represented in Figure 13.
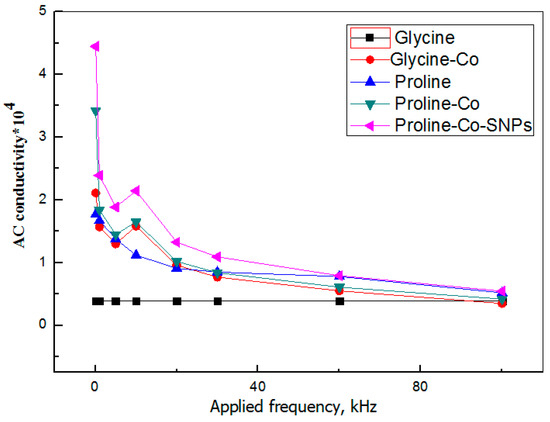
Figure 13.
Variation of AC conductivity of crystals with applied frequency.
The AC conductivity of the sample is decreased with increasing frequency less than 105 Hz due to charges entrapped between grain boundaries and grains. The AC conductivity of crystals is enhanced by SNPs doping. High electric conductivity of samples enabled the applications as new thermoelectric materials for decreasing heat wastes for the 4th generation of solar cells, new high-temperature superconductors and mini-magnets [44].
Proline showed no antimicrobial activity to any tested species. SNPs enhanced antibacterial activity of Co(II)-proline single crystals based on inhibition zones (IZ, mm), Table 9.

Table 9.
Antimicrobial activities of the crystals expressed in inhibition zone.
Proline exhibited no inhibition effect on all tested microorganisms. PCC and PCCAg0NPs exhibited potent antibacterial and antifungal activities. High microbial activity of PCC crystals is due to dopant Ag0NPs. PCC and PCC Ag0NPs showed significant inhibition toward K. pneumoniae, E. faecalis, A. brasiliensis and C. Albicans in comparison to the reference standard ciprofloxacin antibiotic. Doping by Ag0NPs increased chelation complexation between the crystal and DNA of microbes [44].
The concentration response profiles of crystals against MCF-7 and MRC-5 are given in Figure 14a,b and Table 10. PCC and PCCAg0NPs exhibited good antitumor activity against human breast adenocarcinoma (MCF-7), IC50 values < 62.2 μM. PCCAg0NPs exhibited 2-fold more cytotoxicity against MCF-7 (IC50 22.1 μM) than the reference cisplatin, IC50 11.7 μM. Additionally, PCCAg0NPs showed 6-fold more cytotoxicity against MCF-7 than PCC (IC50 62.2 μM).
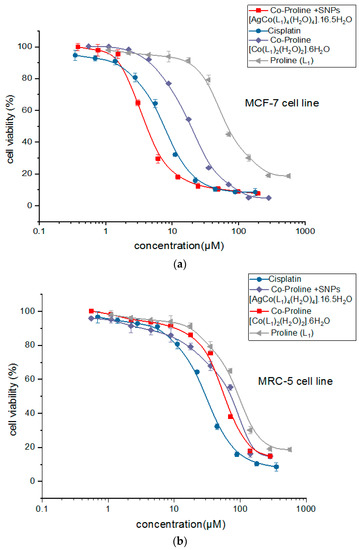
Figure 14.
Concentration-dependent cytotoxicity curves for the tested single crystals for: (a) MCF-7 cell lines, (b) MRC-5 cell line.

Table 10.
IC50 of proline and PCCAg0NPs on breast MCF-7 cell and healthy lung cell lines.
Higher values are observed for IC50 of the tested samples for MRC-5 normal healthy lung cells: cisplatin (30.2) < PCCAg0NPs (145.5) < PCC (255.8). This trend showed that Ag0NPs improved antitumor activity of PCC crystal with low toxicity for normal lung cells.
Same mechanism of action leading antimicrobial and anticancer activity, metal complexes binding DNA of cancer cells.
High cytotoxic activity of PCCAg0NPs is due to Ag0NPs dopant increased DNA complexation. Cytotoxicity against the breast carcinoma cell line MCF-7 followed the order: PCCAg0NPs > PCC > proline.
PCCAg0NPs exhibited over 5-fold less cytotoxicity against MRC-5 (IC50 145.5 μM) than the reference cisplatin antitumor therapeutic drug. High toxicity of PCCAg0NPs against MRC-5 can be mitigated by using PCCAg0NPs in medication as a vial under the applied magnetic field to target tumor cells without affecting normal cells [45].
The dependence of cells viability, human breast adenocarcinoma (MCF-7) and human lung fibroblasts (healthy control) MRC-5, on crystal concentration confirmed that the SNPs crystal showed the best performance in terms of: highest toxicity to cancer cells and less toxicity on the normal lung cell lines approach effect of cisplatin [46,47,48]. All findings obtained in this current study confirmed that silver nanoparticles possess unique physicochemical characteristic-enabled applications in all field technologies [49,50].
4. Conclusions
New single crystals of proline amino acid doped by either cobalt chloride (CoCl2) or CoCl2 + AgNPs are prepared by an innovative low-cost approach (slow evaporation method at room temperature). AgNPs: successfully incorporated into proline single crystals; improved magnetic properties of showed good magnetic properties (µeff. (B.M) for PCC from 4.44 B.M to 4.55 B.M.
Powder XRD diffraction patterns for PCC and PCCAg0NPs are obtained in good purity as a triclinic unit cell. Cobalt chloride created anisotropy magnetism in proline single crystals. PCCAg0NPs exhibited good antitumor activity against human breast adenocarcinoma (MCF-7). PCCAg0NPs crystal showed six-fold more cytotoxicity against breast cancer cell MCF-7, IC50 22.1 μM, than the reference drug cisplatin, IC50 11.7 μM. The mechanism of action of PCCAg0NPs could be binding and complexing DNA of cancer cells.
PCCAg0NPs showed five-fold lower cytotoxicity to normal healthy lung cells MRC-5, (IC50 145.5 μM) than cisplatin (IC50 30.2 μM). The high toxicity of PCCAg0NPs against MCF-7 and its low toxicity against MRC-5 makes it a promising candidate for chemotherapy of breast cancer.
PCCAg0NPs single crystal showed potent antimicrobial activities to various bacterial and fungal species, inhibition zone (mm): 21, 25, 24, 26, 30, 28, 12, and 46 for S. aureus, E. faecalis, S. typhi, E. coli, P. aerugino, K. pneumoniae, A. braselienses, and C. albicans, respectively, in comparison to ciprofloxacin (23, 0, 26, 26, 25, 0, 0, 0) for the same tested species.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biomedicines11020360/s1, Table S1: Assigned vibrational FTIR bands spectra of crystals, Table S2: Bond length, Å and bond angle of PCC.
Author Contributions
Conceptualization, H.A.F.; Methodology, H.A.F. and N.S.E.; Software, M.A.K.; Formal analysis, H.A.F., N.S.E., M.A.K. and R.S.A.; Investigation, A.E.A., M.E.E., H.A.-H.; Data curation, M.A.K. and R.S.A.; Writing-original draft, H.A.F., R.S.A. and N.S.E.; Writing – review & editing, A.E.A., M.E.E., H.A.F., A.E.A. and M.E.E.; Supervision, A.E.A., M.E.E. and H.A.-H.; Funding acquisition, R.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R316), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data in this study will be available on request.
Acknowledgments
Work on this research was carried out at the Chemistry Department, Faculty of Science, Damanhour University, Egypt. The authors would like to thank Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R316), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia for funding this research article. Additionally, we would like to thank J.M. Dessouky, Clinical Pathology Department, Faculty of Medicine, and Alexandria University, Egypt. She helped interpretation antitumor activity and the effectiveness of magnetic single crystals for cancer treatment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- El Batouti, M.; Fetouh, H.A. A facile new modified method for the preparation of a new cerium-doped lanthanium cuperate perovskite energy storage system using nanotechnology. New J. Chem. 2021, 45, 8506–8515. [Google Scholar] [CrossRef]
- Polinger, V.; Bersuker, I.B. Origin of polar nanoregions and relaxor properties of ferroelectrics. Phys. Rev. B 2018, 98, 214102. [Google Scholar] [CrossRef]
- Tulina, N.A. Memristor properties of high temperature superconductors. arXiv 2018, arXiv:1801.09428. [Google Scholar]
- Tulina, N.A.; Ivanov, A.A. Memristive Properties of Oxide-based High-Temperature Superconductors. J. Supercond. Nov. Magn. 2020, 33, 2279–2286. [Google Scholar] [CrossRef]
- Poplavko, I.M. Dielectric Spectroscopy of Electronic Materials; Woodhead Publishing: Cambridge, UK, 2021. [Google Scholar]
- Kour, P.; Pradhan, S.K. Perovskite Ferroelectric. Multifunctional Ferroelectric Materials; Intech Open: London, UK, 2021; p. 31. [Google Scholar]
- Uchino, K. Ferroelectric devices; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Li, J.; Nishikawa, H.; Kougo, J.; Zhou, J.; Dai, S.; Tang, W.; Zhao, X.; Hisai, Y.; Huang, M.; Aya, S. Development of ferroelectric nematic fluids with giant-ε dielectricity and nonlinear optical properties. Sci. Adv. 2021, 7, eabf5047. [Google Scholar] [CrossRef] [PubMed]
- Critelli, R.A.; Sumodjo, P.T.; Bertotti, M.; Torresi, R.M. Influence of glycine on Co electrodeposition: IR spectroscopy and near-surface pH investigations. Electrochim. Acta 2018, 260, 762–771. [Google Scholar] [CrossRef]
- Yuan, G.; Tu, H.; Li, M.; Liu, J.; Zhao, C.; Liao, J.; Yang, Y.; Yang, J.; Liu, N. Glycine derivative-functionalized metal-organic framework (MOF) materials for Co(II) removal from aqueous solution. Appl. Surf. Sci. 2019, 466, 903–910. [Google Scholar] [CrossRef]
- Bera, S.; Guerin, S.; Yuan, H.; O’Donnell, J.; Reynolds, N.P.; Maraba, O.; Ji, W.; Shimon, L.J.W.; Cazade, P.-A.; Tofail, S.A.M.; et al. Molecular engineering of piezoelectricity in collagen-mimicking peptide assemblies. Nat. Commun. 2021, 12, 2634. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhao, G.; Wang, G.; Irvine, J.T. Synthesis and applications of nanoporous perovskite metal oxides. Chem. Sci. 2018, 9, 3623–3637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Z.; Mishra, A.; Yu, M.; Shasti, M.; Tress, W.; Kubicki, D.J.; Avalos, C.E.; Lu, H.; Liu, Y.; et al. Intermediate Phase Enhances Inorganic Perovskite and Metal Oxide Interface for Efficient Photovoltaics. Joule 2019, 4, 222–234. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Lin, H. To Be Higher and Stronger—Metal Oxide Electron Transport Materials for Perovskite Solar Cells. Small 2019, 16, e1902579. [Google Scholar] [CrossRef]
- El Housni, I.; El Mekkaoui, N.; Khalladi, R.; Idrissi, S.; Mtougui, S.; Labrim, H.; Ziti, S.; Bahmad, L. The magnetic properties of the multiferroic transition metal oxide perovskite-type Pb (Fe1/2Nb1/2)O3: Monte Carlo simulations. Ferroelectrics 2020, 568, 191–213. [Google Scholar] [CrossRef]
- Stojanovic, B.D. Magnetic, Ferroelectric, and Multiferroic Metal Oxides; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Mailoud, O.M.; Elsayed, A.H.; El Fetouh, H.A.; ELazm, A.A. Synthesis and characterization of paramagnetic isotropic glycine manganese chloride single crystal with various dopant concentrations. Results Phys. 2019, 12, 925–933. [Google Scholar] [CrossRef]
- Mailoud, O.M.; Elsayed, A.H.; Abo-Elazm, A.; Fetouh, H. Synthesis and study the structure, optical, thermal and dielectric properties of promising Glycine Copper Nitrate (GCN) single crystals. Results Phys. 2018, 10, 512–520. [Google Scholar] [CrossRef]
- Senthil, R.; Vijayaragavan, G.; Ayeshamariam, A.; Kaviyarasu, K. Nonlinear optical properties of single crystal of L-OOMHCL incorporation with Glycine Oxalic Acid (GOA) with high chemical stability for optoelectronic applications. Surf. Interf. 2020, 18, 100417. [Google Scholar] [CrossRef]
- Fetouh, H.; Hefnawy, A.; Attia, A.; Ali, E. Facile and low-cost green synthesis of eco-friendly chitosan-silver nanocomposite as novel and promising corrosion inhibitor for mild steel in chilled water circuits. J. Mol. Liq. 2020, 319, 114355. [Google Scholar] [CrossRef]
- Jin, J.; Wang, G.; Zhou, M. Infrared Spectroscopy and Bonding of the B (NN)3+ and B2(NN)3,4+ Cation Complexes. J. Phys. Chem. A 2021, 125, 6246–6253. [Google Scholar] [CrossRef]
- Beć, K.B.; Huck, C.W. Breakthrough Potential in Near-Infrared Spectroscopy: Spectra Simulation. A Review of Recent Developments. Front. Chem. 2019, 7, 48. [Google Scholar] [CrossRef]
- Perontsis, S.; Dimitriou, A.; Fotiadou, P.; Hatzidimitriou, A.G.; Papadopoulos, A.N.; Psomas, G. Cobalt(II) complexes with the non-steroidal anti-inflammatory drug diclofenac and nitrogen-donor ligands. J. Inorg. Biochem. 2019, 196, 110688. [Google Scholar] [CrossRef] [PubMed]
- El-Gammal, O.A.; Al-Hossainy, A.F.; El-Brashy, S.A. Spectroscopic, DFT, optical band gap, powder X-ray diffraction and bleomycin-dependant DNA studies of Co(II), Ni(II) and Cu(II) complexes derived from macrocyclic Schiff base. J. Mol. Struct. 2018, 1165, 177–195. [Google Scholar] [CrossRef]
- Sathiyaraj, E.; Thirumaran, S. Structural, morphological and optical properties of iron sulfide, cobalt sulfide, copper sulfide, zinc sulfide and copper-iron sulfide nanoparticles synthesized from single source precursors. Chem. Phys. Lett. 2020, 739, 136972. [Google Scholar] [CrossRef]
- Tripathi, S.; Vaidya, S.; Ahmed, N.; Klahn, E.A.; Cao, H.; Spillecke, L.; Koo, C.; Spachmann, S.; Klingeler, R.; Rajaraman, G.; et al. Structure-property correlation in stabilizing axial magnetic anisotropy in oh Co(II) complexes. Cell Rep. Phys. Sci. 2021, 2, 100404. [Google Scholar] [CrossRef]
- Wang, S.; Song, Z.; Kong, Y.; Xia, Z.; Liu, Q. Crystal field splitting of 4fn−15d-levels of Ce3+ and Eu2+ in nitride compounds. J. Lumin. 2018, 194, 461–466. [Google Scholar] [CrossRef]
- Tanda, B.C.; Eksteen, J.J.; Oraby, E.A. Kinetics of chalcocite leaching in oxygenated alkaline glycine solutions. Hydrometalurgy 2018, 178, 264–273. [Google Scholar] [CrossRef]
- Rego, F.; Dias, A.P.S.; Casquilho, M.; Rosa, F.C.; Rodrigues, A. Pyrolysis kinetics of short rotation coppice poplar biomass. Energy 2020, 207, 118191. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.; Zoromba, M.S.; Bassyouni, M.; Zwawi, M.; Alshehri, A.; Al-Hossainy, A. Synthesis and characterization of Co-Al mixed oxide nanoparticles via thermal decomposition route of layered double hydroxide. J. Mol. Struct. 2020, 1206, 127679. [Google Scholar] [CrossRef]
- Naglah, A.M.; Al-Omar, M.A.; Almehizia, A.A.; AlKahtani, H.M.; Bhat, M.A.; Al-Shakliah, N.S.; Belgacem, K.; Majrashi, B.M.; Refat, M.S.; Adam, A.M.A. Synthesis, thermogravimetric, and spectroscopic characterizations of three palladium metal(II) ofloxacin drug and amino acids mixed ligand complexes as advanced antimicrobial materials. J. Mol. Struct. 2020, 1225, 129102. [Google Scholar] [CrossRef]
- Mahmoud, M.; Zaitone, S.; Ammar, A. Binary and ternary Cu(II) complexes of pregabalin with excitatory and inhibitory neurotransmitters and their antiepileptic effect. Mater. Sci. Eng. C 2020, 110, 110650. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Ganji, N.; Daravath, S.; Kanneboina, K.; Rangan, K. Crystal structure, DNA interactions, antioxidant and antitumor activity of thermally stable Cu(II), Ni(II) and Co(III) complexes of an N, O donor Schiff base ligand. Polyhedron 2019, 171, 86–97. [Google Scholar] [CrossRef]
- Korschelt, K.; Schwidetzky, R.; Pfitzner, F.; Strugatchi, J.; Schilling, C.; von der Au, M.; Kirchhoff, K.; Panthöfer, M.; Lieber-wirth, I.; Tahir, M.N.; et al. CeO2−x nanorods with intrinsic urease-like activity. Nanoscale 2018, 10, 13074–13082. [Google Scholar] [CrossRef]
- Matias, J.A.L.; Silva, I.B.; da Silva, A.O.; Oliveira, J.B.; da Silva, D.R.; Morales, M.A. (Bi13Co11)Co2O40–Co3O4 nanocomposites: Approach to different fuels in sol-gel combustion synthesis using the Box-Behnken design. Ceram. Int. 2022, 48, 481–494. [Google Scholar] [CrossRef]
- Mahmoud, M.; Abbas, A.M.; Zaitone, S.; Ammar, A.; Sallam, S. Copper(II) ternary complexes with gabapentin and neurotransmitters as antiepileptic drug. J. Mol. Struct. 2019, 1180, 861–877. [Google Scholar] [CrossRef]
- Bello-Vieda, N.J.; Pastrana, H.F.; Garavito, M.F.; Ávila, A.G.; Celis, A.M.; Muñoz-Castro, A.; Restrepo, S.; Hurtado, J.J. Antibacterial Activities of Azole Complexes Combined with Silver Nanoparticles. Molecules 2018, 23, 361. [Google Scholar] [CrossRef] [PubMed]
- Husain, A.; Kumar, G.; Sood, T.; Walia, S.; Justino, L.L.; Fausto, R.; Kumar, R. Synthesis, structural characterization and DFT analysis of an unusual tryptophan copper(II) complex bound via carboxylate monodentate coordination: Tetraaquabis(l-tryptophan) copper(II) picrate. Inorg. Chim. Acta 2018, 482, 324–332. [Google Scholar] [CrossRef]
- Hoffmann, S.K.; Goslar, J. Anisotropy of the electron spin–lattice relaxation. PO32− radical in glycinium phosphite gly·H3PO3 crystal. J. Magn. Reson. 2018, 294, 93–100. [Google Scholar] [CrossRef]
- Dey, K.K.; Ghosh, M. Investigation of the Structure and Dynamics of Antiviral Drug Adefovir Dipivoxil by Site-Specific Spin–Lattice Relaxation Time Measurements and Chemical Shift Anisotropy Tensor Measurements. ACS Omega 2020, 5, 29373–29381. [Google Scholar] [CrossRef]
- Dobrov, A.; Darvasiová, D.; Zalibera, M.; Bučinský, L.; Puškárová, I.; Rapta, P.; Shova, S.; Dumitrescu, D.; Martins, L.M.; Pombeiro, A.J.; et al. Nickel(II) complexes with redox noninnocent octaazamacrocycles as catalysts in oxidation reactions. Inorg. Chem. 2019, 58, 11133–11145. [Google Scholar] [CrossRef]
- Al-Shehri, B.M.; Shkir, M.; Bawazeer, T.M.; AlFaify, S.; Hamdy, M.S. A rapid microwave synthesis of Ag2S nano-particles and their photocatalytic performance under UV and visible light illumination for water treatment applications. Phys. E Low Dimens. Syst. Nanostruct. 2020, 121, 114060. [Google Scholar] [CrossRef]
- Fetouh, H.; Ismail, A.; Hamid, H.A.; Bashier, M. Synthesis of promising nanocomposites from an antitumer and biologically active heterocyclic compound uploaded by clay and chitosan polymers. Int. J. Biol. Macromol. 2019, 137, 1211–1220. [Google Scholar] [CrossRef]
- Sallam, E.; Aboulnaga, S.; Samy, A.; Beltagy, D.; El Desouky, J.M.; Abdel-Hamid, H.; Fetouh, H. Synthesis, characterization of new heterocyclic compound: Pyrazolyl hydrazino quinoxaline derivative: 3-[5-(hydroxy1methyl)-1-phenylpyrazol-3-yl]-2-[2,4,5-trimethoxybenzylidine] hydrazonyl-quinoxaline of potent antimicrobial, antioxidant, antiviral, and antitumor activity. J. Mol. Struct. 2023, 1271, 133983. [Google Scholar] [CrossRef]
- Annan, N.A.; Butler, I.S.; Titi, H.M.; El-Lazeik, Y.; Jean-Claude, B.J.; Mostafa, S.I. DNA interaction and anticancer evaluation of new zinc(II), ruthenium(II), rhodium(III), palladium(II), silver(I) and platinum(II) complexes based on kojic acid; X-ray crystal structure of [Ag (ka)(PPh3)]·H2O. Inorg. Chim. Acta 2019, 487, 433–447. [Google Scholar] [CrossRef]
- Thanetchaiyakup, A.; Borwornpinyo, S.; Rattanarat, H.; Kanjanasirirat, P.; Jearawuttanakul, K.; Seemakhan, S.; Chuanop-parat, N.; Ngernmeesri, P. Copper-catalyzed synthesis and anticancer activity evaluation of indolo [1,2-a] quinoline derivatives. Tetrahedron Lett. 2021, 82, 153365. [Google Scholar] [CrossRef]
- Lin, J.; Qin, H.; Han, Y.; Li, X.; Zhao, Y.; Zhai, G. CircNRIP1 Modulates the miR-515-5p/IL-25 Axis to Control 5-Fu and Cisplatin Resistance in Nasopharyngeal Carcinoma. Drug Des. Dev. Ther. 2021, 15, 323–330. [Google Scholar] [CrossRef]
- Zharkov, M.N.; Brodovskaya, E.P.; Kulikov, O.A.; Gromova, E.V.; Ageev, V.P.; Atanova, A.V.; Kozyreva, Z.V.; Tishin, A.M.; Pyatakov, A.P.; Pyataev, N.A.; et al. Enhanced cytotoxicity caused by AC magnetic field for polymer microcapsules containing packed magnetic nanoparticles. Colloids Surf. B Biointerfaces 2021, 199, 111548. [Google Scholar] [CrossRef] [PubMed]
- Fetouh, H.A.; Abd-El-Nabey, B.A.; Goher, Y.M.; Karam, M.S. An electrochemical investigation in the anticorrosive properties of silver nanoparticles for the acidic corrosion of aluminium. J. Electrochem. 2018, 24, 89. [Google Scholar]
- Fetouh, H.A.; Abd-Elnaby, H.M.; Alsubaie, M.S.; Sallam, E.R. New experimental low-cost nanoscience technology for formulation of silver nanoparticles-activated carbon composite as a promising antiviral, biocide, and efficient catalyst. J. Exp. Nanosci. 2022, 17, 297–314. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).