Abstract
While a dramatic increase in obesity and related comorbidities is being witnessed, the underlying mechanisms of their spread remain unresolved. Epigenetic and other non-genetic mechanisms tend to be prominent candidates involved in the establishment and transmission of obesity and associated metabolic disorders to offspring. Here, we review recent findings addressing those candidates, in the context of maternal and paternal influences, and discuss the effectiveness of preventive measures.
Keywords:
obesity; HFD; epigenetics; DOHaD; POHaD; non-genetic inheritance; intergenerational inheritance; reversibility 1. Introduction
Obesity is a medical condition that increases the risk of developing type 2 diabetes (T2D), cardiovascular diseases, and several types of cancer, among other pathologies [1]. It has a complex etiology, involving genetics, the environment, and the crosstalk between them. This complexity became more evident when genome-wide association studies (GWAS) began to detect more genetic variants associated with obesity, such as those in fat mass and obesity-associated (FTO) or melanocortin 4 receptor (MC4R) genes, among many others [2,3,4,5,6]. However, genetic variants alone are unable to explain the rapid spread of obesity in the population and the inherited individual susceptibility to metabolic disease development [7,8]. On the other hand, environmental changes in modern society, including poor quality diets, sedentary lifestyles [9,10], and changed working habits [11,12] tend to explain the recent obesity epidemic. Current obesity-related studies have focused on diet-induced weight gain, because the rise in obesity prevalence in recent decades is strongly associated with the composition of modern diets [13,14,15,16,17].
Interestingly, diets not only lead to a self-directed impact; evidence suggests that parental diets can also affect the health of future generations [18,19,20,21]. This is well illustrated in humans by two diet-related conditions: historical famines and modern diet-associated obesity. During the periconceptional period and/or pregnancy, both food-related events have been shown to result in offspring obesity, T2D, and metabolic phenotypes, such as hyperglycemia and cardiovascular diseases [18,22]. Apparently unrelated phenotypes, such as schizophrenia or proliferative diabetic retinopathy in association with famine [19,20], or cognitive impairment in association with obesity [21], have also been observed. Overall, although such dietary challenges differ with the amount of food that is available, food-related events during both the periconceptional period and/or pregnancy made it evident that parental malnutrition has detrimental effects on the metabolic health of offspring.
Considering the duration of direct in utero or postnatal influences, the role of a maternal diet has been largely acknowledged and extensively addressed within the scope of the developmental origins of health and disease (DOHaD), which is a study of the consequences of maternal early-life influences on offspring during pregnancy and lactation. During these periods, diseases can be developmentally programmed and result in long-term adaptive responses and adult phenotypes [23]. Paternal contributions, however, have only recently gained increased attention in this context. Associations between the increase in the prevalence of male obesity and infertility [24], as well as observed metabolic and cardiovascular negative outcomes in the offspring of obese fathers [25], have prompted a refinement in considering the paternal effects on the metabolic health of offspring. In fact, the recently coined concept of paternal origin of health and disease (POHaD) emphasizes the emerging role of the impact of paternal preconception health and paternal inheritance on offspring health, including diet-induced obesity [26].
The parental environmental information that is passed on to the following generation is mainly encrypted in the germ cells, putatively in the germ cell epigenome [27]. First coined by C. H. Waddington in 1942, the epigenome was understood as the interaction between the genotype and the phenotype that allows for cell differentiation and the canalization of the terminally differentiated state [28]. This epigenetic information is transmitted from the mother cell to the daughter cell, a process that is known as epigenetic inheritance. Twenty years later, R. Holliday introduced the idea that the epigenetic modifications could be transmitted through generations, a concept that is now known as inter- or transgenerational epigenetic inheritance, depending on the number of generations affected [29]. Contemporary researchers now define epigenetics as “the study of changes in gene function that are mitotically and/or meiotically heritable and that do not entail a change in DNA sequence” [30].
Epigenetic changes, which are also referred to as epimutations, can be triggered by various environmental stimuli. They are subject to forward–backward dynamics and occur at a more frequent rate than spontaneous genetic mutations, allowing an organism to adapt faster to the environment [31,32]. Epimutations could be transferred from one generation to the next to confer an advantage for evolutionary adaptation and fitness, but they may increase susceptibility to “environmental diseases” (such as obesity and diabetes) when the ancestral environment (e.g., famine) is different from the offspring environment (e.g., feast). This idea has been adopted in the context of obesity and is referred to as metabolic thriftiness [33]. Originally, the concept implied that parental exposure to food shortage can initiate better adaptation to the environment by promoting efficient energy storage, and therefore a higher chance of survival [33]. However, when the environment imposes the opposite situation—excessive nutrition—this feature can result in obesity and associated T2D.
Mechanistically, metabolic thriftiness and the associated evolutionary adaptation were originally attributed to so-called “thrifty genes”, which were supposedly selected during evolution to promote the efficient handling of energy [33]. Because the speed of genetic adaptation and variation cannot explain the current pace of the obesity epidemic [7,8], metabolic thriftiness has lately been attributed to epimutation (the so-called thrifty epigenotype), which—by overcoming slow genetic evolution—could be responsible for the establishment and transmission of the thrifty phenotype across generations [33,34,35].
When referring to the epigenetic inheritance across generations, important distinctions are drawn with respect to specificities of parent–offspring interactions. In fact, maternal environmental exposures that generate epigenetic changes in offspring (F1) do not represent epigenetic inheritance per se. Instead, these epigenetic modifications are the epigenetic response of the embryo/offspring to direct exposure to the maternal environment in the uterus, which is more accurately described as developmental programming. When such responses persist in further generations in the absence of the environmental insult, it is assumed that the original information was stored in the primordial germ cells, persisted throughout development, and led to epigenetic inheritance. These responses can reach one or more generations, a process that is called inter- (F2) or transgenerational (F3 and further) epigenetic inheritance, respectively. On the other hand, as father–offspring interaction and information transfer are indirect (i.e., the father does not directly interact with the offspring during development) and limited in space (i.e., interaction only in the maternal tract) and time (i.e., conception), paternal intergenerational epigenetic inheritance applies to F1, while paternal transgenerational epigenetic inheritance applies to F2 and further generations.
To mimic dietary obesity in humans, diet-induced obesity has been modelled in rodents. Parental obesogenic diets that are rich in fat and/or sugar have confirmed the transmission of metabolic phenotypes inter- and/or transgenerationally in such experimental models [36,37]. Current research addresses how an obesogenic diet influences the parental epigenome, how epimutations are transferred to the next generation, and how these epigenetic changes affect offspring health. Focusing on the effects of parental diet-induced obesity in animal models—as a result of a high-fat diet (HFD) or a high-fat, high-sugar “western diet” (WD)—and in humans, the aim of this review is to provide an overview of the likely underlying molecular mechanisms behind the intergenerational consequence of parental obesity and the current approaches to its reversibility.
2. Maternal Obesity Affects Offspring Health
Mothers have a close interaction with offspring during pregnancy and early life. Research in humans and mice has made us aware that maternal obesity is directly related to poor offspring health [38], not only as a consequence of obesity-triggered pregnancy complications [39], but also as a result of epigenetic changes that are stable throughout generations. Indeed, several studies have shown that maternal obesity or increased fat intake induce transcriptional reprogramming [40], histone modifications [41,42], and DNA methylation changes in offspring [43,44,45,46]. These epigenetic modifications have been reported in human and animal fetal tissues [41], placenta [46], cord blood [43,44], newborns [45], and adults [42].
The interaction between the mother and the offspring can be divided into three susceptibility time windows: (i) before pregnancy (preconception) [44,46,47,48], (ii) during gestation [46], and (iii) during the postnatal period (lactation) [42,49]. Exposures during each of these susceptibility windows affect distinct offspring developmental stages and involve a specific mediator of information transfer: the oocyte during preconception; mainly the placenta during pregnancy; and breast milk postnatally. In addition, there are other less-studied putative mediators, such as vaginal and breast milk microbiota [50,51,52,53,54], the maternal immune system [55], and early postnatal care [56].
2.1. Preconceptional Maternal Obesity: Effects in the Oocyte
Oogenesis in humans starts while the female embryo is in the maternal uterus, continues after puberty, and completes development if fertilization takes place. Throughout its lifetime, all these cells are permanently exposed to maternal environmental conditions. There is growing information about the developmental epigenetic changes that occur during oogenesis and about their inheritance [57]. Although there is limited information regarding epigenetic changes in the event of environmental insults, it is well known that before pregnancy, oocyte quality can be affected by obesity, resulting in poor fertility [58].
It has been suggested that maternal preconceptional feeding with a HFD is sufficient to induce epigenetic changes in oocytes and transmit metabolic phenotypes to offspring. Some studies in mice have addressed such changes globally. For example, oocytes from HFD-fed mothers present a decrease in 5-methylcytosine (5mC) levels and an increase in 5-hydroxymethylcytosine (5hmC) levels [59,60]. The same studies also found an increase of histone H3 lysine 4 trimethylation (H3K4me3) and histone H3 lysine 9 trimethylation (H3K9me3) [60]. Some studies in mice have also demonstrated that a HFD causes epigenetic changes, such as altered methylation of the leptin promoter [61]. Although the specific epigenetic changes present in F1 are unknown, there is evidence from non-HFD-related studies that suggests that the epigenetic changes in the oocyte may be present in offspring. For example, the same alterations in DNA methylation of the insulin-like growth factor 2 (Igf2) gene found in rat oocytes that are exposed to an excess of androgens were also detected in F1 β-cells [62].
There is a potential mechanism to explain how a HFD changes the epigenome in the oocyte, which can be transmitted to the following generation. A HFD reduces the levels of Stella protein in oocytes. This protein is a maternal factor that is essential for early development and has a role in global epigenetic remodeling after fertilization. Stella downregulation in murine oocytes that originate from HFD-fed females is coupled with compromised development and reduced 5mC levels in the maternal pronucleus in the zygote. The latter prevents epigenetic asymmetry in the early embryo. Overexpressing Stella in these oocytes recovers, to a certain extent, 5mC levels in the maternal pronucleus in the zygote and in specific methylated regions [63]. These results suggest, at least in part, that HFD effects could be mediated by Stella protein.
Although maternal intergenerational effects are the most studied in mammals, detailed information on diet-induced epigenome reprogramming in oocytes is limited, most likely by the fact that oocytes are difficult to obtain in big numbers in physiological settings (e.g., without superovulation). New single-cell technologies, however, have already provided detailed DNA methylation and chromatin accessibility in mice and humans during oogenesis [64,65], promising to fill this gap and thereby enabling us to tackle questions raised by the current data: What are the genes on which epigenetic marks act upon? Can the same epigenetic mark in the oocyte be found in the adult offspring tissues? What could be the reason for a discrepancy between oocyte and F1 tissues?
2.2. Maternal Obesity during Pregnancy: Effects in the Placenta
During pregnancy, the placenta acts as the interface between the mother and the fetus. Its roles include fetal nutrient supply, removal of fetal metabolic waste, and hormone production [66]. Obesity during pregnancy can affect placental physiology and structure. Indeed, some studies suggest that mothers with high body mass indices (BMI) display heavier placentas [67], similar to HFD-fed mice [68]. The alterations in a placenta due to obesity can have a negative impact on offspring, as recent studies indicate that a HFD can affect placental function, leading to fetal growth restriction [69,70]. Little is known about how the maternal diet and/or obesity can alter placental function and/or structure. A study of mice showed that a HFD causes hypomethylation, in comparison with a control diet, but this difference was only significant in female placentas [68]. Another study, of humans, showed that maternal obesity can negatively affect the placenta by altering the DNA methylation of genes related to the biological processes of sterol regulatory element-binding protein (SREBP) signaling and phospholipid transport, among other processes [71]. SREBPs are membrane-bound transcription factors that control lipid synthesis and uptake [72]. The authors of that study also found that maternal saturated-fat intake altered the methylation levels of genes that are involved in biological processes related to chromatin remodeling, insulin-like growth factor (IGF) receptor signaling, phosphoinositid-3-kinase (PI3-kinase) signaling, and nitric oxide synthase [71]. Researchers have proposed that it is necessary to determine if the DNA methylation dysregulation affects placental nutrient transport, which could explain the fetal growth restriction [71]. Gabory et al. observed that a HFD downregulated the histone H3 lysine 9 (H3K9) trimethylase, Kmt1a. Interestingly, they did not detect global changes in the levels of H3K9me3 by western blot. The authors suggested that subtle changes may have occurred [73].
2.3. Maternal Obesity Postnatally: Effects in the Breast Milk
Breast milk carries a myriad of nutrients and molecules, such as antibodies [49], microRNA (miRNAs) [74], and antioxidants [75]. It feeds and provides immunological defenses for the growing baby. The maternal diet and obesity can influence the lipid content [76,77], the protein levels [78], and the levels of some miRNAs and other non-coding RNA (ncRNAs) [79] in breast milk.
Early postnatal life exposures can influence the development of a metabolic phenotype, which is believed to be mediated through breast milk [49,80]. The importance of breast milk’s effects has been demonstrated by Gorski et al. [81]. Their study showed that obesity-prone rat pups that were cross-fostered with lean mothers during suckling ameliorated their insulin resistance as adults. On the other hand, obesity-resistant pups that were cross-fostered with obese mothers showed reduced insulin sensitivity and had a diet-induced increase in adiposity. These effects were possibly associated with changes in hypothalamic neuropeptides, such as insulin and leptin receptors [81]. Moreover, in rats, a maternal HFD during suckling can program visceral adiposity and the epigenetic regulation of epididymal white adipose tissue (eWAT). In that study, the male offspring of HFD-fed mothers presented an increment in eWAT that was associated with higher levels of stearoyl-CoA desaturase-1 (Scd1), coupled with a decrease in methylation levels in its promoter [80]. Scd1 is a key enzyme of fatty acid (FA) metabolism that converts saturated FAs to monounsaturated FAs, which are the predominant substrates for triglyceride synthesis [80]. Importantly, the resulting increment in FA content in breast milk prompted the authors to propose them as metabolic mediators.
Overall, these studies show that breast milk from HFD-fed or obese mothers affect offspring health. However, the mechanism is not clear. It is possible that breast milk components could be involved. More research is required to determine whether it is the fat itself, ncRNAs, their combination, or something else that is involved in the mechanism.
2.4. Maternal Effects: What Is Known and What Is Missing
There are three developmental windows of susceptibility for a HFD. This environmental challenge induces epigenetic alterations in the oocyte, the placenta, and, putatively, in breast milk composition, thereby impacting the next generation (Figure 1 and Table 1). However, further studies are required to fully elucidate the mechanistic details of how such epigenetic information is encoded during the different susceptibility time windows and how it is inherited by offspring. It is also important to determine whether one of these windows confers more susceptibility than the others. It is still not clear if epigenetic signals are reversible and how they are maintained during F1 development and adulthood.
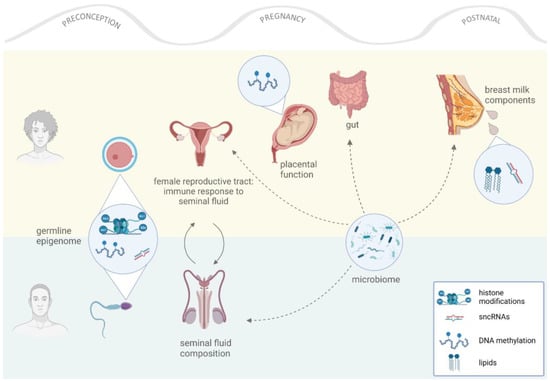
Figure 1.
Effects of parental nutrition in offspring. Both parents contribute to their offspring’s health in different ways. The maternal contribution ranges from conception until postnatal care, while the paternal contribution occurs at conception. Different mediators (for example, germ cells) play roles during different developmental windows, transferring “environmental information” to offspring through epigenetic and/or non-genetic mechanisms.

Table 1.
Putative epigenetic mechanisms that are associated with the intergenerational consequences of maternal obesity.
3. Paternal Obesity Affects the Offspring Health
Unlike mothers, fathers have one short time window within which to pass their “environmental memory” to their offspring: at conception. Originally, this served as one of the reasons for underestimating the paternal contribution to offspring health. Global reprogramming of the sperm epigenome in early embryogenesis and transcriptional silencing during sperm maturation have rendered paternal intergenerational inheritance less feasible in affecting offspring health [82,83,84]. However, seminal studies of various environmental influences strongly suggest the opposite [85,86,87,88]. Paternal exposure to a HFD has been shown to induce a broad range of inter- and transgenerational negative effects, including programming of β-cell dysfunction; dysregulation of hepatic genes; cognitive, metabolic, and reproductive impairments; and female predisposition to breast cancer [87,89].
With advancements in research, several key findings have increased interest in the epigenetic inheritance of environmental effects via male germ cells. On the one hand, although most of the histones are gradually replaced with protamines during mammalian spermatogenesis, up to 8% and 15% of nucleosomes are retained in mice and humans, respectively [90]. Enriched for the genes involved in embryonic development, these escaped regions tend to be a plausible candidate for paternal epigenetic inheritance [91,92]. On the other hand, the incomplete erasure of paternal methylome in early embryogenesis on intergenic and protein-coding regions also applies to genes that are involved in neuronal development [93]. Moreover, imprinted regions escape the first wave of global demethylation; therefore, they remain methylated in the somatic cells of offspring. Despite comprising ~1% of the human genes [94], some portion of environmentally acquired epigenetic marks could be transferred via those parent-of-origin-specific signatures. Thus, differentially methylated regions (DMRs) of paternal origin, within or outside imprinted regions, can evade reprogramming during early embryogenesis. In addition, emerging evidence supports the role of transcription factors as carriers of epigenetic information during spermatogenesis and pre-implantation development, defining the methylation status of paternal DNA [95]. Finally, although transcriptionally inert, mature spermatozoa is rich in small non-coding RNAs (sncRNAs), another putative epifactor that could reflect and pass along environmental influences through generations [96].
Interestingly, in addition to germline effects, seminal fluid components and microbiota have also been implicated in intergenerational inheritance [55,97]. These additional layers provide further support for epidemiological studies that reveal the extent and mechanistic complexity of the paternal contribution to offspring health (Figure 1).
3.1. Paternal Obesity at Conception: Effects in Sperm
The original study of intergenerational non-genetic effects in a HFD rat model addressed whether excessive fat consumption could induce changes in methylation and gene expression levels in pancreatic islets [87]. Whether metabolic effects can also be established in sperm and, therefore, be transmitted to the following generation has been addressed in multiple studies since then (Table 2). As discussed in the following sections, the current research is focused on DNA methylation, histone modifications, and sncRNAs, the latter being the most broadly researched and most likely mediator of paternal effects.

Table 2.
Putative epigenetic mechanisms associated with the intergenerational consequences of paternal obesity.
3.1.1. DNA Methylation
Pioneering rodent studies examining changes in male germ cells revealed that the methylome of spermatozoa is sensitive to a HFD. For example, a chronic HFD caused global hypomethylation in mouse sperm [98] and resulted in DMRs in spermatozoa from F0 as well as F1 rats, with the latter being unexposed to dietary challenge [99]. However, whether these changes are essential and/or sufficient for transmitting metabolic phenotypes through generations remains subject to debate. In fact, although there were some common DMRs detected in the founder and offspring sperm, gene regulation associated with established methylation signatures did not persist in the affected somatic tissues across generations [99]. Such inconsistencies have also been reported in other rodent studies [88,100] and have been, to a certain degree, ascribed to technical bias from methylome experiments [91,101]. Moreover, a study closely examining genome-wide methylation in the sperm suggested that most of the signal originates from genetic or epigenetic variations that are not linked to a HFD [101]. Despite these discrepancies, differential methylation remains one of the broadly addressed readouts for studying the transmission of HFD effects. An interesting hypothesis prevails in the field; it established that modest changes in sperm methylome may modulate HFD-induced changes at early stages of developmental programming, which can manifest as a metabolic dysfunction later in life.
Several human studies have also evidenced DMRs in the sperm of obese individuals versus lean individuals. For example, Donkin et al. identified DMRs in the proximity of genes responsible for central nervous system development and metabolic processes [102]. Consistently, differential methylation within and outside paternally imprinted loci have been recently reported in gametes of overweight and obese men and were further associated with unhealthy diets rich in fat [103,104,105,106].
Although direct testing of the transmission of such changes intergenerationally in humans is ethically limited, paternal obesity has been shown to be associated with offspring methylation status in the cord blood in paternally imprinted genes, indicating a possible transmission of such signatures via sperm [107]. Interestingly, a similar approach has been attempted in a larger cohort undergoing in vitro fertilization or intracytoplasmic sperm injection. That study revealed an association between differential methylation levels in some paternally imprinted regions in the sperm of obese donors and the cord blood of their babies [104].
3.1.2. Histone Modification
Obesity-related impairments during spermatogenesis and its possible mediation via altered histone modifications have been reported by multiple research groups [108,109,110,111]. However, the transmission of altered histone epimutations to the following generation remains unresolved.
HFD-induced differential profiles of histone post-translational modifications have been identified in a few studies. For instance, Terashima et al. revealed a differential occupancy of histone H3 in genes involved in early embryonic development in HFD-fed mouse sperm [108]. More recently, decreased H3K9 dimethylation was also reported in a similar experimental setting [109]. However, the transfer of altered sperm histone signatures to the embryo is challenged by the current understanding of early developmental processes, in which sperm histone marks become heavily reprogrammed [112]. Regardless, findings from transgenic mouse experiments strongly support the critical role of sperm histones in embryonic development and, importantly, reveal the propagation of the resulting phenotype transgenerationally [110]. In fact, germline-specific overexpression of lysine demethylase KDM1A and the resulting reduced H3K4 methylation levels lead to reduced survival and developmental defects spanning multiple generations that originate from heterozygous breeding. Interestingly, a recent follow-up study combined the mentioned transgenic effect with the HFD challenge. The results suggested that altered H3K4 trimethylation levels correspond to embryonic transcription and chromatin profiles in HFD-fed wild-type (WT) mice as well as in transgenic mice, with the latter resulting in a stronger metabolic phenotype transmitted transgenerationally [111].
Overall, although undetected in a human study [102], increasing evidence from experimental models suggests that sperm-derived histones can sense and possibly transfer information on the paternal metabolic state. A precise mechanistic model, however, remains to be elucidated.
3.1.3. sncRNAs
Several rodent studies have detected alterations of sperm-derived sncRNAs levels as a consequence of an obesogenic diet. For example, a HFD and a WD induced differential expression of miRNAs, tRNA-derived fragments (tRFs), and piwi-interacting RNAs (piRNAs); upon their subsequent injection into the normal oocyte or one-cell embryo, a compromised metabolic phenotype resulted in offspring [98,113,114]. Interestingly, some researchers examined individual biotypes (e.g., miRNAs and tRFs) or a specific subtype to define their significance and sufficiency in establishing a metabolic phenotype. For instance, Grandjean et al. focused on the most differentially expressed miR19b (microRNA 19b) in WD mouse sperm. This single miRNA subtype led to partial penetrance of metabolic alterations [114]. In another study, a total tRF pool resulted in efficient mimicking of total sperm RNA-induced phenotypes [113].
Further supported by human studies, sncRNAs appear to sense diet-induced changes in a metabolic environment and to transmit the signature to the following generations [91,102]. The pioneering human study comparing sncRNA expression profiles in obese and normal individuals revealed differential abundance of piRNAs with putative targets involved in neurogenesis. Interestingly, a reanalysis of the sncRNA expression data with a more fine-tuned pipeline revealed that a set of tRFs were also differentially expressed between the two groups, suggesting their role as the carrier of “environmental information” in human spermatozoa [115]. An increasing number of findings that support the contribution of tRFs has followed the discovery of the essential role of RNA modifications. In fact, post-transcriptional methylation of tRFs proved to be critical in transmitting the HFD-induced phenotype intergenerationally [113,116].
sncRNAs have been recently implicated in multigenerational effects resulting from ancestral diet-induced obesity [117]. Exposing male mice to WD for five consecutive generations resulted in metabolic phenotype exacerbating across downstream generations. Interestingly, sncRNAs were differentially expressed in F1 but not in F5. To further investigate the role of sncRNAs in inter- or transgenerational metabolic effects, the total RNAs from F1 and F5 obese mice were microinjected into the naïve zygotes and the resulting effects were compared to those of natural matings. While the metabolic phenotype persisted through the following two generations, it did not aggravate along the generations, as was observed with natural matings. Altogether, these findings indicate the role of sncRNAs in the relatively short-term paternal inheritance of diet-induced obesity and related phenotypes, whereas long-term penetrance of the effects points at a more complex mechanistic level, such as cooperation between epigenetic factors.
Overall, while rapidly advancing, the research requires better tools to identify the origin of sncRNAs and to trace their transmission and effects at the site of action during embryonic or postnatal development. Although microinjection can be a robust tool to ascribe altered phenotypes to sperm head content, it does not enable us to detect when, where, and how the effects are established. Therefore, several key aspects remain to be addressed. On the one hand, due to sncRNA dilution throughout mammalian embryonic cleavages, the information they carry could only be communicated at an early stage of development. Alternatively, sncRNAs transferred from sperm to embryos may activate a self-amplifying mechanism to overcome this caveat and persist throughout development. In turn, it has been shown that the offspring of HFD males exert small RNA profiles in the adipose tissue, similar to the founder sperm [99]. Considering the observed acquisition of small RNAs from epididymis by sperm during its maturation, the overall picture becomes even more complex [118]. Therefore, distinguishing the sperm-born sncRNAs from those obtained through soma-to-germline transition and exploring their trajectory throughout the different stages of offspring development are some primary requirements to eliminate discrepancies that are related to the paternal epigenetic inheritance of diet-induced obesity. As an example, a recently developed tool for the efficient tagging and tracking of sncRNAs in vivo can help address the mentioned aspects in future studies [119].
4. Beyond Epigenome
We have provided an overview of the intergenerational consequences of parental obesity that are induced or mediated bona fide by epigenetic mechanisms. Lately, studies have highlighted an equally important role of parental modifiers of offspring health beyond the epigenome (for a review, see [120,121]). In the following section, we briefly review the known facts about the relevance of the oocyte environment, the seminal fluid, and the parental microbiota composition in the intergenerational transmission of non-genetic information and phenotype programming (Figure 1). This is an emerging part of the epigenetic inheritance field; the obvious question on whether these reported phenomena are really non-epigenetic or, rather, signaling mediators to the parental epigenomes is yet to be answered.
4.1. Oocyte Environment
Oocyte quality is essential for female fertility and offspring health, and the developmental environment of the maturing oocyte is essential for optimal oocyte quality. An obesogenic diet, for example, has been shown to alter the reproductive function and offspring health by changing the biochemical environment of the follicle and inducing endoplasmic reticulum stress, telomere dysfunction [122], mitochondrial dysfunction, oxidative stress, and lipid droplet accumulation [123].
4.2. Seminal Plasma and the Maternal Reproductive Tract Immune Response
The seminal plasma is the fluid that contains the secretions from the male accessory glands (i.e., the seminal vesicle and the prostate and bulbourethral glands), the testes, and the epididymis. Until recently, the seminal plasma has been seen merely as the medium to transport and support the sperm into the maternal reproductive tract. Recent studies—pioneered by the research group of Sarah Robertson et al.—have shown that the composition of the seminal plasma is important in signaling the female tract at conception by priming a tolerogenic response that is essential for embryo implantation, remodeling of the uterine blood vessels, and proper placentation [124,125,126,127]. The depletion of uterine regulatory T-cells (the main mediators of immune tolerance) results in implantation failure, fetal loss, or fetal growth restriction in mice [128,129,130].
Interestingly, the composition of the seminal plasma is affected by lifestyle factors. For example, exposure to an obesogenic diet changes the composition of the seminal plasma toward a pro-inflammatory cytokine repertoire, which alters female immune adaptation to pregnancy and ultimately affects pregnancy and offspring health [55,131,132,133]. Furthermore, we have recently shown that paternal circadian disruption at conception reprograms offspring metabolism by affecting the rhythmicity of corticosterone secretion into the seminal plasma and inducing placental dysfunction and fetal growth restriction [134].
Altogether, these findings suggest that the seminal plasma, although not essential for fertilization and pregnancy establishment, is important for pregnancy outcomes, including embryo implantation, placentation, post-fertilization embryonic development, and fetal growth, and ultimately affects adult offspring health [135].
4.3. Microbiota
Mammalian bodies are colonized by microbes, which respond to the environment mainly by altering the circulating metabolome and, thereby, influencing host physiology, health, and disease trajectories (for a review, see [136]). The most important feature of the microbiota is its ability to transfer phenotypes across organisms, which has recently boosted microbiota transplantation as a therapeutic tool for non-communicable diseases, including diabetes, obesity, and chronic inflammatory disorders (for a review, see [137]).
Of utmost importance for this review, microbiota—and in particular those of maternal origin—are of critical importance in determining offspring health [138]. The alteration of microbiota during pregnancy affects the development and adult phenotypes in mice [138,139,140,141,142] and is one of the main causes of adverse pregnancy outcomes affecting neonatal and infant health in humans [143,144,145,146].
In addition to the indirect effects of the maternal microbiome during pregnancy, there is experimental and clinical evidence showing that the maternal microbiome is present in the breast milk [147] and in the vaginal tract [143]; from these sites, they are directly transferred to the offspring gut [147,148]. Breast milk microbiota composition is modified by the maternal diet and by obesity in mice and humans [50,51,52,53,149] and has been reported as affecting offspring development and health [149]. Likewise, the composition of the vaginal environment, including microbiome, is modified by the maternal diet [150,151] and is critical for offspring metabolic and neurobehavioral phenotypes [152,153,154].
Less is known about the effects of the paternal microbiota. Two studies from the same research group reported the presence of a microbiome in the mouse seminal plasma and its responsiveness to a HFD treatment [97,155]. Although largely unexplored, there is ongoing discussion in the scientific community about the potential role of the seminal microbiome for offspring health, most likely through immune modulation in the female tract at conception [156].
Although the highlighted biological features of the microbiome make it a potential novel modulator of acquired inheritance, the available evidence in mammals is still too uncertain to support conclusions about a role for the microbiota in the parental programming of offspring phenotypes.
5. Reversibility
To what extent can the effects of parental obesity for offspring health be reversed? Having demonstrated the legacy of parental and offspring health in mammals, the epigenetic inheritance field is focusing on the prevention or reversal of this legacy. The vast majority of studies aimed at rescuing offspring health by re-establishing parental health preconceptionally and/or during gestation. The three most often reported approaches for parental obesity (summarized below) are dietary intervention, exercise, and bariatric surgery. In addition, other studies that are not reported below have tested probiotics and dietary supplements [157,158,159].
5.1. Lifestyle Interventions: Diet and Exercise
Lifestyle interventions, including diet, exercise, and their combination, are the most commonly used approaches, in humans and model organisms, to reverse common obesity. The scientific community has applied lifestyle interventions to test the reversibility of intergenerational obesity in several mammalian model organisms.
Studies of mice have shown that the correction of maternal obesity with a diet that is poor in fat may improve the metabolism in offspring who are on a control diet [160,161,162,163,164,165], while worsening their susceptibility to diet-induced obesity when the change of diet is short- or medium-term before pregnancy [161,162,163,166]. Maternal exercise before and during pregnancy has been proven to be efficient in reducing the degree of liver damage in offspring of obese female mice [167], while a combination of diet and exercise improved placental vascularization and prevented macrosomia in offspring of obese mice mothers [168]. These findings have been further supported by independent studies of rats [21] or Japanese macaques [169], showing that correction of maternal obesity before pregnancy partially improves the overall health of offspring when they are raised on a standard diet.
Interestingly, the same lifestyle interventions appear to be effective for the intergenerational consequences of paternal obesity. Studies of mice have the reported beneficial effects of paternal dietary intervention for the metabolic and reproductive health of offspring [170,171]. Likewise, preconceptional exercise, alone or in combination with a dietary intervention, improved metabolic health in obese male mice and positively affected offspring embryonic development [172], adult metabolic health (in particular, body composition and lipid homeostasis) [173], and reproductive health [170,174].
5.2. Bariatric Surgery
In addition to common lifestyle interventions, the more severe forms of obesity are now treated by bariatric surgery, which is becoming increasingly common. Reported evidence based on studies of humans suggests that bariatric surgery is associated with both positive and negative pregnancy and neonatal outcomes [39,175,176]. These findings have been corroborated by studies in diet-induced obesity rats, where ventral sleeve gastrectomy has led to worsened pregnancies and offspring health [177,178,179]. Not many studies have been reported on paternal preconceptional bariatric surgery, but an interesting recent report revealed DMRs in spermatozoa before and after gastric bypass surgery at time points of one week and one year, suggesting the potential of bariatric surgery to modify the sperm epigenome and, therefore, potentially have paternal intergenerational consequences [102].
The evidence on the benefits of weight loss via surgery before pregnancy is still contradictory or unclear. The effects may depend on the initial maternal BMI, the weight reduction, the surgical procedure chosen, the metabolic effects of the surgery, and the age of the offspring when comparing the data [180]. Given that a change in diet long before pregnancy has positive effects in offspring, it is possible that women should wait after surgery before becoming pregnant and establish a healthy diet following the surgical procedure. Although it is recommended that women wait 12 to 18 months after the surgery, there is no clear data supporting the benefit of such a wait [39,175].
5.3. Reversibility: Chances of Success
Contrary to genetic mutations, epigenetic reprogramming is, by definition, reversible. Therefore, modifying one’s lifestyle and restoring parental health before conception or during pregnancy has the potential to mitigate the effects of parental obesity and its consequences on offspring health.
While reporting interesting results, available studies, including the ones mentioned in this review, provide a non-conclusive patchwork of different intervention methods and lengths, exposure windows, dietary combinations, and phenotypic analyses that would need to be harmonized by more robust experimental designs, independent cross-validation studies, and aligned phenotyping pipelines to deliver useful results for the wider scientific and clinical communities. This assumes that parental epigenome reprogramming is secondary to a certain phenotypic manifestation rather than, for example, a specific environmental challenge.
6. Concluding Remarks: When and How Can a Parent Affect Offspring Health?
The central OneGenotype–OnePhenotype dogma was rejected many years ago by the observation of significant phenotypic plasticity in isogenic individuals, mostly due to gene–gene and gene–environment interactions. The discovery of epigenetic inheritance, more than a decade ago, has further highlighted the observation that individual phenotypes are not only determined by one’s own genes and gene–environment interaction, but also by parental effects through epigenetic mechanisms. This hypothesis is particularly fascinating in the case of non-communicable diseases, such as obesity and diabetes, whose pathogenesis is significantly contributed to by gene–environment interactions and, therefore, by lifestyle choices.
Here, we provided an overview of the state-of-the-art view of the role of epigenetic inheritance and parental effects in the rapid worldwide spread of obesity and associated comorbidities. Although compelling evidence supports the relevance of parental metabolic health at conception or during gestation for offspring metabolic homeostasis and susceptibility to metabolic diseases, the field still lacks a comprehensive understanding of the underlying molecular mechanisms. Indeed, both germ-cell and non-germ cell factors have been implicated in such parental effects.
We further touched upon the possibility of reversing parental effects by restoring parental health via lifestyle intervention and bariatric surgery. Although interesting, this set of findings is still far from being conclusive and, as highlighted, needs better and more substantial experimental and clinical evidence.
Where do we stand? We have unearthed an interesting and fascinating realm of knowledge, which surely holds secrets that are fundamental to our understanding of human physiology and pathophysiology, and which is useful for the management and prevention of diseases—such as obesity and diabetes—that are spreading like wildfire, especially among the younger generation. Further effort by the entire scientific community is required. Greater international cooperation, cross-validation, harmonization of protocols, constructive feedback, and exchanges of ideas are suggested ways of transforming epigenetic inheritance and parental programming from fascinating ideas into a useful reality.
Author Contributions
Conceptualization (G.C.-A., L.M., M.G.-V. and R.T.), funding acquisition (R.T.), writing—original draft preparation, review, and editing (G.C.-A., L.M., M.G.-V. and R.T.), Figure 1 and Table 1 and Table 2 preparation (G.C.-A., L.M. and M.G.-V.). All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported by grants of the German Diabetes Research Center (DZD NEXT grant 2019) and the Fritz Thyssen Stiftung (Az. 10.19.2.027MN) to R.T., which fund the position of G.C.-A. We thank the Helmholtz Association and the German Diabetes Research Center for funding the positions of M.G.-V. and R.T.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Acronyms
| 5hmC | 5-hydroxymethylcytosine |
| 5mC | 5-methylcytosine |
| BMI | body mass index |
| DMRs | differentially methylated regions |
| DOHaD | developmental origins of health and disease |
| eWAT | epididymal white adipose tissue |
| FA | fatty acid |
| FTO | fat mass and obesity associated gene |
| GWAS | genome wide association studies |
| H3K4 | histone H3 lysine 4 |
| H3K4me3 | histone H3 lysine 4 trimethylation |
| H3K9 | histone H3 lysine 9 |
| H3K9me3 | histone H3 lysine 9 trimethylation |
| HE | high energy |
| HFD | high-fat diet |
| IGF | insulin-like growth factor |
| Igf2 | insulin-like growth factor 2 |
| KDM1A | lysine demethylase 1A |
| Kmt1a | H3K9 trimethylase |
| MC4R | melanocortin 4 receptor gene |
| MEG3-IG | maternally expressed gene 3 intergenic |
| miR19b | microRNA 19b |
| miRNA | microRNA |
| NA | not applicable |
| ncRNA | non-coding RNA |
| PI3-kinase | phosphoinositid-3-kinase |
| piRNA | piwi-interacting RNA |
| POHaD | paternal origin of health and disease |
| Pparα | peroxisome proliferator-activated receptor alpha |
| Scd1 | stearoyl-CoA desaturase-1 |
| sncRNA | small non-coding RNA |
| SREBP | sterol regulatory element-binding protein |
| T2D | type 2 diabetes |
| tRFs | tRNA-derived fragments |
| WD | Western diet |
| WT | wild-type |
References
- Wang, T.; Jia, W.; Hu, C. Advancement in Genetic Variants Conferring Obesity Susceptibility from Genome-Wide Association Studies. Front. Med. 2015, 9, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, C.H.; Andersen, G. Gene-Environment Interactions and Obesity--Further Aspects of Genomewide Association Studies. Nutr. Burbank Los Angel. Cty. Calif 2009, 25, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Frayling, T.M.; Timpson, N.J.; Weedon, M.N.; Zeggini, E.; Freathy, R.M.; Lindgren, C.M.; Perry, J.R.B.; Elliott, K.S.; Lango, H.; Rayner, N.W.; et al. A Common Variant in the FTO Gene Is Associated with Body Mass Index and Predisposes to Childhood and Adult Obesity. Science 2007, 316, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Dina, C.; Meyre, D.; Gallina, S.; Durand, E.; Körner, A.; Jacobson, P.; Carlsson, L.M.S.; Kiess, W.; Vatin, V.; Lecoeur, C.; et al. Variation in FTO Contributes to Childhood Obesity and Severe Adult Obesity. Nat. Genet. 2007, 39, 724–726. [Google Scholar] [CrossRef]
- Scuteri, A.; Sanna, S.; Chen, W.-M.; Uda, M.; Albai, G.; Strait, J.; Najjar, S.; Nagaraja, R.; Orrú, M.; Usala, G.; et al. Genome-Wide Association Scan Shows Genetic Variants in the FTO Gene Are Associated with Obesity-Related Traits. PLoS Genet. 2007, 3, e115. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.J.F.; Lindgren, C.M.; Li, S.; Wheeler, E.; Zhao, J.H.; Prokopenko, I.; Inouye, M.; Freathy, R.M.; Attwood, A.P.; Beckmann, J.S.; et al. Common Variants near MC4R Are Associated with Fat Mass, Weight and Risk of Obesity. Nat. Genet. 2008, 40, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.J.F.; Yeo, G.S.H. The Genetics of Obesity: From Discovery to Biology. Nat. Rev. Genet. 2022, 23, 120–133. [Google Scholar] [CrossRef]
- Bogardus, C. Missing Heritability and GWAS Utility. Obesity 2009, 17, 209–210. [Google Scholar] [CrossRef]
- Ferrari, G.; Herrera-Cuenca, M.; Zalcman Zimberg, I.; Guajardo, V.; Gómez, G.; Quesada, D.; Rigotti, A.; Yadira Cortés, L.; Yépez García, M.; Pareja, R.G.; et al. A Comparison of Associations Between Self-Reported and Device-Based Sedentary Behavior and Obesity Markers in Adults: A Multi-National Cross-Sectional Study. Assessment 2022, 29, 1441–1457. [Google Scholar] [CrossRef]
- da Costa, N.; Silveira, J.F.d.C.; Schneiders, L.d.B.; Sehn, A.P.; Reuter, É.M.; Hobkirk, J.P.; Carroll, S.; Reuter, C.P. Moderating Role of Physical Fitness in the Association Between TV Time and Adiposity Parameters in Adolescents. Am. J. Health Promot. AJHP 2022, 36, 1104–1111. [Google Scholar] [CrossRef]
- Sugiyama, T.; Hadgraft, N.; Clark, B.K.; Dunstan, D.W.; Owen, N. Sitting at Work & Waist Circumference-A Cross-Sectional Study of Australian Workers. Prev. Med. 2020, 141, 106243. [Google Scholar] [CrossRef] [PubMed]
- Tam, A.C.T.; Steck, V.A.; Janjua, S.; Liu, T.Y.; Murphy, R.A.; Zhang, W.; Conklin, A.I. A Systematic Review of Evidence on Employment Transitions and Weight Change by Gender in Ageing Populations. PLoS ONE 2022, 17, e0273218. [Google Scholar] [CrossRef] [PubMed]
- Boushey, C.; Ard, J.; Bazzano, L.; Heymsfield, S.; Mayer-Davis, E.; Sabaté, J.; Snetselaar, L.; Van Horn, L.; Schneeman, B.; English, L.K.; et al. Dietary Patterns and Growth, Size, Body Composition, and/or Risk of Overweight or Obesity: A Systematic Review; USDA Nutrition Evidence Systematic Reviews: Alexandria, VA, USA, 2020. [Google Scholar]
- De Amicis, R.; Mambrini, S.P.; Pellizzari, M.; Foppiani, A.; Bertoli, S.; Battezzati, A.; Leone, A. Ultra-Processed Foods and Obesity and Adiposity Parameters among Children and Adolescents: A Systematic Review. Eur. J. Nutr. 2022, 61, 2297–2311. [Google Scholar] [CrossRef] [PubMed]
- Neri, D.; Martínez-Steele, E.; Khandpur, N.; Levy, R. Associations Between Ultra-Processed Foods Consumption and Indicators of Adiposity in US Adolescents: Cross-Sectional Analysis of the 2011–2016 National Health and Nutrition Examination Survey. J. Acad. Nutr. Diet. 2022, 122, 1474–1487.e2. [Google Scholar] [CrossRef]
- Bradley, P. Diet Composition and Obesity. Lancet 2012, 379, 1100, author reply 1100–1101. [Google Scholar] [CrossRef]
- Astrup, A.; Brand-Miller, J. Diet Composition and Obesity. Lancet 2012, 379, 1100, author reply 1100–1101. [Google Scholar] [CrossRef]
- Schoonejans, J.M.; Ozanne, S.E. Developmental Programming by Maternal Obesity: Lessons from Animal Models. Diabet. Med. J. Br. Diabet. Assoc. 2021, 38, e14694. [Google Scholar] [CrossRef]
- Li, C.; Lumey, L.H. Exposure to the Chinese Famine of 1959-61 in Early Life and Long-Term Health Conditions: A Systematic Review and Meta-Analysis. Int. J. Epidemiol. 2017, 46, 1157–1170. [Google Scholar] [CrossRef]
- Fedotkina, O.; Luk, A.; Jain, R.; Prasad, R.B.; Shungin, D.; Simó-Servat, O.; Özgümüs, T.; Cherviakova, L.; Khalimon, N.; Svietleisha, T.; et al. Perinatal Famine Is Associated with Excess Risk of Proliferative Retinopathy in Patients with Type 2 Diabetes. Acta Ophthalmol. (Copenh.) 2022, 100, e539–e545. [Google Scholar] [CrossRef]
- Rodriguez, J.S.; Rodríguez-González, G.L.; Reyes-Castro, L.A.; Ibáñez, C.; Ramírez, A.; Chavira, R.; Larrea, F.; Nathanielsz, P.W.; Zambrano, E. Maternal Obesity in the Rat Programs Male Offspring Exploratory, Learning and Motivation Behavior: Prevention by Dietary Intervention Pre-Gestation or in Gestation. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2012, 30, 75–81. [Google Scholar] [CrossRef]
- Vaiserman, A.M. Early-Life Nutritional Programming of Type 2 Diabetes: Experimental and Quasi-Experimental Evidence. Nutrients 2017, 9, E236. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, V.; Bautista, R.J.; Frausto-González, O.; Rodríguez-Peña, N.; Betancourt, E.T.; Bautista, C.J. Developmental Programming in Animal Models: Critical Evidence of Current Environmental Negative Changes. Reprod. Sci. Thousand Oaks Calif 2022, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Lane, M.; Owens, J.A.; Bakos, H.W. Paternal Obesity Negatively Affects Male Fertility and Assisted Reproduction Outcomes: A Systematic Review and Meta-Analysis. Reprod. Biomed. Online 2015, 31, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Eberle, C.; Kirchner, M.F.; Herden, R.; Stichling, S. Paternal Metabolic and Cardiovascular Programming of Their Offspring: A Systematic Scoping Review. PLoS ONE 2020, 15, e0244826. [Google Scholar] [CrossRef]
- Soubry, A. POHaD: Why We Should Study Future Fathers. Environ. Epigenetics 2018, 4, dvy007. [Google Scholar] [CrossRef]
- Huypens, P.; Sass, S.; Wu, M.; Dyckhoff, D.; Tschöp, M.; Theis, F.; Marschall, S.; Hrabě de Angelis, M.; Beckers, J. Epigenetic Germline Inheritance of Diet-Induced Obesity and Insulin Resistance. Nat. Genet. 2016, 48, 497–499. [Google Scholar] [CrossRef]
- Waddington, C.H. The Epigenotype. Int. J. Epidemiol. 2012, 41, 10–13. [Google Scholar] [CrossRef]
- Holliday, R. The Inheritance of Epigenetic Defects. Science 1987, 238, 163–170. [Google Scholar] [CrossRef]
- Russo, V.E.A.; Martienssen, R.A.; Riggs, A.D. Epigenetic Mechanisms of Gene Regulation; Cold Spring Harbor Monograph Series; Cold Spring Harbor Laboratory Press: Plainview, NY, USA, 1996; ISBN 978-0-87969-490-6. [Google Scholar]
- van der Graaf, A.; Wardenaar, R.; Neumann, D.A.; Taudt, A.; Shaw, R.G.; Jansen, R.C.; Schmitz, R.J.; Colomé-Tatché, M.; Johannes, F. Rate, Spectrum, and Evolutionary Dynamics of Spontaneous Epimutations. Proc. Natl. Acad. Sci. USA. 2015, 112, 6676–6681. [Google Scholar] [CrossRef]
- Burggren, W. Epigenetic Inheritance and Its Role in Evolutionary Biology: Re-Evaluation and New Perspectives. Biology 2016, 5, 24. [Google Scholar] [CrossRef]
- Stöger, R. The Thrifty Epigenotype: An Acquired and Heritable Predisposition for Obesity and Diabetes? BioEssays 2008, 30, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.N.; Barker, D.J. Type 2 (Non-Insulin-Dependent) Diabetes Mellitus: The Thrifty Phenotype Hypothesis. Diabetologia 1992, 35, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.H.; Ozanne, S.E. Fetal Programming of Glucose-Insulin Metabolism. Mol. Cell. Endocrinol. 2009, 297, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Hariri, N.; Thibault, L. High-Fat Diet-Induced Obesity in Animal Models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef]
- Musial, B.; Vaughan, O.R.; Fernandez-Twinn, D.S.; Voshol, P.; Ozanne, S.E.; Fowden, A.L.; Sferruzzi-Perri, A.N. A Western-Style Obesogenic Diet Alters Maternal Metabolic Physiology with Consequences for Fetal Nutrient Acquisition in Mice. J. Physiol. 2017, 595, 4875–4892. [Google Scholar] [CrossRef]
- Dahlen, C.R.; Borowicz, P.P.; Ward, A.K.; Caton, J.S.; Czernik, M.; Palazzese, L.; Loi, P.; Reynolds, L.P. Programming of Embryonic Development. Int. J. Mol. Sci. 2021, 22, 11668. [Google Scholar] [CrossRef]
- Spann, R.A.; Grayson, B.E. Curbing Obesity from One Generation to Another: The Effects of Bariatric Surgery on the In Utero Environment and Beyond. Reprod. Sci. Thousand Oaks Calif 2020, 27, 1821–1833. [Google Scholar] [CrossRef]
- Hedegger, K.; Philippou-Massier, J.; Krebs, S.; Blum, H.; Kunzelmann, S.; Förstemann, K.; Gimpfl, M.; Roscher, A.A.; Ensenauer, R.; Wolf, E.; et al. Sex-Specific Programming Effects of Parental Obesity in Pre-Implantation Embryonic Development. Int. J. Obes. 2005 2020, 44, 1185–1190. [Google Scholar] [CrossRef]
- Aagaard-Tillery, K.M.; Grove, K.; Bishop, J.; Ke, X.; Fu, Q.; McKnight, R.; Lane, R.H. Developmental Origins of Disease and Determinants of Chromatin Structure: Maternal Diet Modifies the Primate Fetal Epigenome. J. Mol. Endocrinol. 2008, 41, 91–102. [Google Scholar] [CrossRef]
- Zheng, S.; Li, Q.; Zhang, Y.; Balluff, Z.; Pan, Y.-X. Histone Deacetylase 3 (HDAC3) Participates in the Transcriptional Repression of the P16 (INK4a) Gene in Mammary Gland of the Female Rat Offspring Exposed to an Early-Life High-Fat Diet. Epigenetics 2012, 7, 183–190. [Google Scholar] [CrossRef]
- Sharp, G.C.; Lawlor, D.A.; Richmond, R.C.; Fraser, A.; Simpkin, A.; Suderman, M.; Shihab, H.A.; Lyttleton, O.; McArdle, W.; Ring, S.M.; et al. Maternal Pre-Pregnancy BMI and Gestational Weight Gain, Offspring DNA Methylation and Later Offspring Adiposity: Findings from the Avon Longitudinal Study of Parents and Children. Int. J. Epidemiol. 2015, 44, 1288–1304. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.L.; Jima, D.; Sharp, G.C.; McCullough, L.E.; Park, S.S.; Gowdy, K.M.; Skaar, D.; Cowley, M.; Maguire, R.L.; Fuemmeler, B.; et al. Maternal Pre-Pregnancy Obesity, Offspring Cord Blood DNA Methylation, and Offspring Cardiometabolic Health in Early Childhood: An Epigenome-Wide Association Study. Epigenetics 2019, 14, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Phang, M.; Ross, J.; Raythatha, J.H.; Dissanayake, H.U.; McMullan, R.L.; Kong, Y.; Hyett, J.; Gordon, A.; Molloy, P.; Skilton, M.R. Epigenetic Aging in Newborns: Role of Maternal Diet. Am. J. Clin. Nutr. 2020, 111, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, D.; Ouidir, M.; Workalemahu, T.; Zeng, X.; Tekola-Ayele, F. Placental DNA Methylation Changes Associated with Maternal Prepregnancy BMI and Gestational Weight Gain. Int. J. Obes. 2005 2020, 44, 1406–1416. [Google Scholar] [CrossRef]
- Chang, E.; Hafner, H.; Varghese, M.; Griffin, C.; Clemente, J.; Islam, M.; Carlson, Z.; Zhu, A.; Hak, L.; Abrishami, S.; et al. Programming Effects of Maternal and Gestational Obesity on Offspring Metabolism and Metabolic Inflammation. Sci. Rep. 2019, 9, 16027. [Google Scholar] [CrossRef]
- Lassi, Z.S.; Kedzior, S.G.; Tariq, W.; Jadoon, Y.; Das, J.K.; Bhutta, Z.A. Effects of Preconception Care and Periconception Interventions on Maternal Nutritional Status and Birth Outcomes in Low- and Middle-Income Countries: A Systematic Review. Nutrients 2020, 12, 606. [Google Scholar] [CrossRef]
- Ozkan, H.; Tuzun, F.; Taheri, S.; Korhan, P.; Akokay, P.; Yılmaz, O.; Duman, N.; Özer, E.; Tufan, E.; Kumral, A.; et al. Epigenetic Programming Through Breast Milk and Its Impact on Milk-Siblings Mating. Front. Genet. 2020, 11, 569232. [Google Scholar] [CrossRef]
- Collado, M.C.; Laitinen, K.; Salminen, S.; Isolauri, E. Maternal Weight and Excessive Weight Gain during Pregnancy Modify the Immunomodulatory Potential of Breast Milk. Pediatr. Res. 2012, 72, 77–85. [Google Scholar] [CrossRef]
- Cabrera-Rubio, R.; Collado, M.C.; Laitinen, K.; Salminen, S.; Isolauri, E.; Miras, A.D.; Jackson, R.N.; Jackson, S.N.; Goldstone, A.P.; Olbers, T.; et al. The Human Milk Microbiome Changes over Lactation and Is Shaped by Maternal Weight and Mode of Delivery. Am. J. Clin. Nutr. 2012, 96, 544–551. [Google Scholar] [CrossRef]
- Kumar, H.; du Toit, E.; Kulkarni, A.; Aakko, J.; Linderborg, K.M.; Zhang, Y.; Nicol, M.P.; Isolauri, E.; Yang, B.; Collado, M.C.; et al. Distinct Patterns in Human Milk Microbiota and Fatty Acid Profiles Across Specific Geographic Locations. Front. Microbiol. 2016, 7, 1619. [Google Scholar] [CrossRef]
- Williams, J.E.; Carrothers, J.M.; Lackey, K.A.; Beatty, N.F.; York, M.A.; Brooker, S.L.; Shafii, B.; Price, W.J.; Settles, M.L.; McGuire, M.A.; et al. Human Milk Microbial Community Structure Is Relatively Stable and Related to Variations in Macronutrient and Micronutrient Intakes in Healthy Lactating Women. J. Nutr. 2017, 147, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Newell-Fugate, A.E.; Lenz, K.; Skenandore, C.; Nowak, R.A.; White, B.A.; Braundmeier-Fleming, A. Effects of Coconut Oil on Glycemia, Inflammation, and Urogenital Microbial Parameters in Female Ossabaw Mini-Pigs. PLoS ONE 2017, 12, e0179542. [Google Scholar] [CrossRef] [PubMed]
- Schjenken, J.E.; Moldenhauer, L.M.; Sharkey, D.J.; Chan, H.Y.; Chin, P.Y.; Fullston, T.; McPherson, N.O.; Robertson, S.A. High-Fat Diet Alters Male Seminal Plasma Composition to Impair Female Immune Adaptation for Pregnancy in Mice. Endocrinology 2021, 162, bqab123. [Google Scholar] [CrossRef] [PubMed]
- Dumas, T.C. Adult Health and Early Life Adversity: Behind the Curtains of Maternal Care Research. Front. Physiol. 2022, 13, 804239. [Google Scholar] [CrossRef]
- Stäubli, A.; Peters, A.H. Mechanisms of Maternal Intergenerational Epigenetic Inheritance. Curr. Opin. Genet. Dev. 2021, 67, 151–162. [Google Scholar] [CrossRef]
- Gu, L.; Liu, H.; Gu, X.; Boots, C.; Moley, K.H.; Wang, Q. Metabolic Control of Oocyte Development: Linking Maternal Nutrition and Reproductive Outcomes. Cell. Mol. Life Sci. CMLS 2015, 72, 251–271. [Google Scholar] [CrossRef]
- Hou, Y.-J.; Zhu, C.-C.; Duan, X.; Liu, H.-L.; Wang, Q.; Sun, S.-C. Both Diet and Gene Mutation Induced Obesity Affect Oocyte Quality in Mice. Sci. Rep. 2016, 6, 18858. [Google Scholar] [CrossRef]
- Huang, J.; Ru, G.; Sun, J.; Sun, L.; Li, Z. Elevated RIF1 Participates in the Epigenetic Abnormalities of Zygotes by Regulating Histone Modifications on MuERV-L in Obese Mice. Mol. Med. Camb. Mass 2022, 28, 17. [Google Scholar] [CrossRef]
- Ge, Z.-J.; Luo, S.-M.; Lin, F.; Liang, Q.-X.; Huang, L.; Wei, Y.-C.; Hou, Y.; Han, Z.-M.; Schatten, H.; Sun, Q.-Y. DNA Methylation in Oocytes and Liver of Female Mice and Their Offspring: Effects of High-Fat-Diet-Induced Obesity. Environ. Health Perspect. 2014, 122, 159–164. [Google Scholar] [CrossRef]
- Tian, S.; Lin, X.-H.; Xiong, Y.-M.; Liu, M.-E.; Yu, T.-T.; Lv, M.; Zhao, W.; Xu, G.-F.; Ding, G.-L.; Xu, C.-M.; et al. Prevalence of Prediabetes Risk in Offspring Born to Mothers with Hyperandrogenism. EBioMedicine 2017, 16, 275–283. [Google Scholar] [CrossRef][Green Version]
- Han, L.; Ren, C.; Li, L.; Li, X.; Ge, J.; Wang, H.; Miao, Y.-L.; Guo, X.; Moley, K.H.; Shu, W.; et al. Embryonic Defects Induced by Maternal Obesity in Mice Derive from Stella Insufficiency in Oocytes. Nat. Genet. 2018, 50, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Gu, C.; You, D.; Huang, Z.; Qian, J.; Yang, Q.; Cheng, X.; Zhang, L.; Wang, H.; Wang, P.; et al. Decoding Dynamic Epigenetic Landscapes in Human Oocytes Using Single-Cell Multi-Omics Sequencing. Cell Stem Cell 2021, 28, 1641–1656.e7. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Liu, S.; Wu, Q.; Zhang, L.; Guo, F. Integrative Single-Cell Analysis of Transcriptome, DNA Methylome and Chromatin Accessibility in Mouse Oocytes. Cell Res. 2019, 29, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Higgins, L.; Greenwood, S.L.; Wareing, M.; Sibley, C.P.; Mills, T.A. Obesity and the Placenta: A Consideration of Nutrient Exchange Mechanisms in Relation to Aberrant Fetal Growth. Placenta 2011, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, L.; Franx, A.; Vogelvang, T.E.; Houben, M.L.; van Rijn, B.B.; Nikkels, P.G. Association of Maternal Prepregnancy Body Mass Index With Placental Histopathological Characteristics in Uncomplicated Term Pregnancies. Pediatr. Dev. Pathol. Off. J. Soc. Pediatr. Pathol. Paediatr. Pathol. Soc. 2019, 22, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Gallou-Kabani, C.; Gabory, A.; Tost, J.; Karimi, M.; Mayeur, S.; Lesage, J.; Boudadi, E.; Gross, M.-S.; Taurelle, J.; Vigé, A.; et al. Sex- and Diet-Specific Changes of Imprinted Gene Expression and DNA Methylation in Mouse Placenta under a High-Fat Diet. PLoS ONE 2010, 5, e14398. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, T.; Turnwald, E.-M.; Janoschek, R.; Zentis, P.; Bae-Gartz, I.; Beers, T.; Handwerk, M.; Wohlfarth, M.; Ghilav, M.; Bloch, W.; et al. Maternal High Fat Diet-Induced Obesity Affects Trophoblast Differentiation and Placental Function in Mice. Biol. Reprod. 2020, 103, 1260–1274. [Google Scholar] [CrossRef] [PubMed]
- de Barros Mucci, D.; Kusinski, L.C.; Wilsmore, P.; Loche, E.; Pantaleão, L.C.; Ashmore, T.J.; Blackmore, H.L.; Fernandez-Twinn, D.S.; Carmo, M.d.G.T.d.; Ozanne, S.E. Impact of Maternal Obesity on Placental Transcriptome and Morphology Associated with Fetal Growth Restriction in Mice. Int. J. Obes. 2005 2020, 44, 1087–1096. [Google Scholar] [CrossRef]
- Thakali, K.M.; Zhong, Y.; Cleves, M.; Andres, A.; Shankar, K. Associations between Maternal Body Mass Index and Diet Composition with Placental DNA Methylation at Term. Placenta 2020, 93, 74–82. [Google Scholar] [CrossRef]
- Shimano, H.; Sato, R. SREBP-Regulated Lipid Metabolism: Convergent Physiology—Divergent Pathophysiology. Nat. Rev. Endocrinol. 2017, 13, 710–730. [Google Scholar] [CrossRef]
- Gabory, A.; Ferry, L.; Fajardy, I.; Jouneau, L.; Gothié, J.-D.; Vigé, A.; Fleur, C.; Mayeur, S.; Gallou-Kabani, C.; Gross, M.-S.; et al. Maternal Diets Trigger Sex-Specific Divergent Trajectories of Gene Expression and Epigenetic Systems in Mouse Placenta. PLoS ONE 2012, 7, e47986. [Google Scholar] [CrossRef] [PubMed]
- Alsaweed, M.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Human Milk MiRNAs Primarily Originate from the Mammary Gland Resulting in Unique MiRNA Profiles of Fractionated Milk. Sci. Rep. 2016, 6, 20680. [Google Scholar] [CrossRef] [PubMed]
- Gila-Díaz, A.; Herranz Carrillo, G.; Cañas, S.; Saenz de Pipaón, M.; Martínez-Orgado, J.A.; Rodríguez-Rodríguez, P.; López de Pablo, Á.L.; Martin-Cabrejas, M.A.; Ramiro-Cortijo, D.; Arribas, S.M. Influence of Maternal Age and Gestational Age on Breast Milk Antioxidants During the First Month of Lactation. Nutrients 2020, 12, 2569. [Google Scholar] [CrossRef] [PubMed]
- Burianova, I.; Bronsky, J.; Pavlikova, M.; Janota, J.; Maly, J. Maternal Body Mass Index, Parity and Smoking Are Associated with Human Milk Macronutrient Content after Preterm Delivery. Early Hum. Dev. 2019, 137, 104832. [Google Scholar] [CrossRef]
- Alexandre-Gouabau, M.-C.; David-Sochard, A.; Royer, A.-L.; Parnet, P.; Paillé, V. Moderate High Caloric Maternal Diet Impacts Dam Breast Milk Metabotype and Offspring Lipidome in a Sex-Specific Manner. Int. J. Mol. Sci. 2020, 21, 5428. [Google Scholar] [CrossRef]
- Lowry, D.E.; Paul, H.A.; Reimer, R.A. Impact of Maternal Obesity and Prebiotic Supplementation on Select Maternal Milk MicroRNA Levels and Correlation with Offspring Outcomes. Br. J. Nutr. 2022, 127, 335–343. [Google Scholar] [CrossRef]
- Hicks, S.D.; Confair, A.; Warren, K.; Chandran, D. Levels of Breast Milk MicroRNAs and Other Non-Coding RNAs Are Impacted by Milk Maturity and Maternal Diet. Front. Immunol. 2021, 12, 785217. [Google Scholar] [CrossRef]
- Butruille, L.; Marousez, L.; Pourpe, C.; Oger, F.; Lecoutre, S.; Catheline, D.; Görs, S.; Metges, C.C.; Guinez, C.; Laborie, C.; et al. Maternal High-Fat Diet during Suckling Programs Visceral Adiposity and Epigenetic Regulation of Adipose Tissue Stearoyl-CoA Desaturase-1 in Offspring. Int. J. Obes. 2019, 43, 2381–2393. [Google Scholar] [CrossRef]
- Gorski, J.N.; Dunn-Meynell, A.A.; Hartman, T.G.; Levin, B.E. Postnatal Environment Overrides Genetic and Prenatal Factors Influencing Offspring Obesity and Insulin Resistance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R768–R778. [Google Scholar] [CrossRef]
- Davis, T.L.; Yang, G.J.; McCarrey, J.R.; Bartolomei, M.S. The H19 Methylation Imprint Is Erased and Re-Established Differentially on the Parental Alleles during Male Germ Cell Development. Hum. Mol. Genet. 2000, 9, 2885–2894. [Google Scholar] [CrossRef]
- Reik, W.; Dean, W.; Walter, J. Epigenetic Reprogramming in Mammalian Development. Science 2001, 293, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.W.C.; Kobayashi, T.; Irie, N.; Dietmann, S.; Surani, M.A. Specification and Epigenetic Programming of the Human Germ Line. Nat. Rev. Genet. 2016, 17, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Drake, A.J.; Walker, B.R.; Seckl, J.R. Intergenerational Consequences of Fetal Programming by in Utero Exposure to Glucocorticoids in Rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R34–R38. [Google Scholar] [CrossRef]
- Kaati, G.; Bygren, L.O.; Pembrey, M.; Sjöström, M. Transgenerational Response to Nutrition, Early Life Circumstances and Longevity. Eur. J. Hum. Genet. EJHG 2007, 15, 784–790. [Google Scholar] [CrossRef]
- Ng, S.-F.; Lin, R.C.Y.; Laybutt, D.R.; Barres, R.; Owens, J.A.; Morris, M.J. Chronic High-Fat Diet in Fathers Programs β-Cell Dysfunction in Female Rat Offspring. Nature 2010, 467, 963–966. [Google Scholar] [CrossRef]
- Radford, E.J.; Ito, M.; Shi, H.; Corish, J.A.; Yamazawa, K.; Isganaitis, E.; Seisenberger, S.; Hore, T.A.; Reik, W.; Erkek, S.; et al. In Utero Effects. In Utero Undernourishment Perturbs the Adult Sperm Methylome and Intergenerational Metabolism. Science 2014, 345, 1255903. [Google Scholar] [CrossRef] [PubMed]
- Fontelles, C.C.; da Cruz, R.S.; Gonsiewski, A.K.; Barin, E.; Tekmen, V.; Jin, L.; Cruz, M.I.; Loudig, O.; Warri, A.; de Assis, S. Systemic Alterations Play a Dominant Role in Epigenetic Predisposition to Breast Cancer in Offspring of Obese Fathers and Is Transmitted to a Second Generation. Sci. Rep. 2021, 11, 7317. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Hada, M.; Fukuda, Y.; Inoue, E.; Makino, Y.; Katou, Y.; Shirahige, K.; Okada, Y. Re-Evaluating the Localization of Sperm-Retained Histones Revealed the Modification-Dependent Accumulation in Specific Genome Regions. Cell Rep. 2018, 23, 3920–3932. [Google Scholar] [CrossRef]
- Donkin, I.; Barrès, R. Sperm Epigenetics and Influence of Environmental Factors. Mol. Metab. 2018, 14, 1–11. [Google Scholar] [CrossRef]
- Erkek, S.; Hisano, M.; Liang, C.-Y.; Gill, M.; Murr, R.; Dieker, J.; Schübeler, D.; van der Vlag, J.; Stadler, M.B.; Peters, A.H.F.M. Molecular Determinants of Nucleosome Retention at CpG-Rich Sequences in Mouse Spermatozoa. Nat. Struct. Mol. Biol. 2013, 20, 868–875. [Google Scholar] [CrossRef]
- Tang, W.W.C.; Dietmann, S.; Irie, N.; Leitch, H.G.; Floros, V.I.; Bradshaw, C.R.; Hackett, J.A.; Chinnery, P.F.; Surani, M.A. A Unique Gene Regulatory Network Resets the Human Germline Epigenome for Development. Cell 2015, 161, 1453–1467. [Google Scholar] [CrossRef] [PubMed]
- Anisimov, S.V. A Prevalence of Imprinted Genes within the Total Transcriptomes of Human Tissues and Cells. Mol. Biol. Int. 2012, 2012, 793506. [Google Scholar] [CrossRef] [PubMed]
- Kremsky, I.; Corces, V.G. Protection from DNA Re-Methylation by Transcription Factors in Primordial Germ Cells and Pre-Implantation Embryos Can Explain Trans-Generational Epigenetic Inheritance. Genome Biol. 2020, 21, 118. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, R.J.; Anton, E.; Krawetz, S.A. Isolating MRNA and Small Noncoding RNAs from Human Sperm. Methods Mol. Biol. Clifton NJ 2013, 927, 385–396. [Google Scholar] [CrossRef]
- Javurek, A.B.; Spollen, W.G.; Johnson, S.A.; Bivens, N.J.; Bromert, K.H.; Givan, S.A.; Rosenfeld, C.S. Consumption of a High-Fat Diet Alters the Seminal Fluid and Gut Microbiomes in Male Mice. Reprod. Fertil. Dev. 2017, 29, 1602–1612. [Google Scholar] [CrossRef]
- Fullston, T.; Ohlsson Teague, E.M.C.; Palmer, N.O.; DeBlasio, M.J.; Mitchell, M.; Corbett, M.; Print, C.G.; Owens, J.A.; Lane, M. Paternal Obesity Initiates Metabolic Disturbances in Two Generations of Mice with Incomplete Penetrance to the F2 Generation and Alters the Transcriptional Profile of Testis and Sperm MicroRNA Content. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 4226–4243. [Google Scholar] [CrossRef]
- de Castro Barbosa, T.; Ingerslev, L.R.; Alm, P.S.; Versteyhe, S.; Massart, J.; Rasmussen, M.; Donkin, I.; Sjögren, R.; Mudry, J.M.; Vetterli, L.; et al. High-Fat Diet Reprograms the Epigenome of Rat Spermatozoa and Transgenerationally Affects Metabolism of the Offspring. Mol. Metab. 2015, 5, 184–197. [Google Scholar] [CrossRef]
- Carone, B.R.; Fauquier, L.; Habib, N.; Shea, J.M.; Hart, C.E.; Li, R.; Bock, C.; Li, C.; Gu, H.; Zamore, P.D.; et al. Paternally Induced Transgenerational Environmental Reprogramming of Metabolic Gene Expression in Mammals. Cell 2010, 143, 1084–1096. [Google Scholar] [CrossRef]
- Shea, J.M.; Serra, R.W.; Carone, B.R.; Shulha, H.P.; Kucukural, A.; Ziller, M.J.; Vallaster, M.P.; Gu, H.; Tapper, A.R.; Gardner, P.D.; et al. Genetic and Epigenetic Variation, but Not Diet, Shape the Sperm Methylome. Dev. Cell 2015, 35, 750–758. [Google Scholar] [CrossRef]
- Donkin, I.; Versteyhe, S.; Ingerslev, L.R.; Qian, K.; Mechta, M.; Nordkap, L.; Mortensen, B.; Appel, E.V.R.; Jørgensen, N.; Kristiansen, V.B.; et al. Obesity and Bariatric Surgery Drive Epigenetic Variation of Spermatozoa in Humans. Cell Metab. 2016, 23, 369–378. [Google Scholar] [CrossRef]
- Keyhan, S.; Burke, E.; Schrott, R.; Huang, Z.; Grenier, C.; Price, T.; Raburn, D.; Corcoran, D.L.; Soubry, A.; Hoyo, C.; et al. Male Obesity Impacts DNA Methylation Reprogramming in Sperm. Clin. Epigenetics 2021, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Potabattula, R.; Dittrich, M.; Schorsch, M.; Hahn, T.; Haaf, T.; El Hajj, N. Male Obesity Effects on Sperm and Next-Generation Cord Blood DNA Methylation. PLoS ONE 2019, 14, e0218615. [Google Scholar] [CrossRef] [PubMed]
- Soubry, A.; Guo, L.; Huang, Z.; Hoyo, C.; Romanus, S.; Price, T.; Murphy, S.K. Obesity-Related DNA Methylation at Imprinted Genes in Human Sperm: Results from the TIEGER Study. Clin. Epigenetics 2016, 8, 51. [Google Scholar] [CrossRef]
- Soubry, A.; Murphy, S.K.; Vansant, G.; He, Y.; Price, T.M.; Hoyo, C. Opposing Epigenetic Signatures in Human Sperm by Intake of Fast Food Versus Healthy Food. Front. Endocrinol. 2021, 12, 625204. [Google Scholar] [CrossRef] [PubMed]
- Soubry, A.; Murphy, S.K.; Wang, F.; Huang, Z.; Vidal, A.C.; Fuemmeler, B.F.; Kurtzberg, J.; Murtha, A.; Jirtle, R.L.; Schildkraut, J.M.; et al. Newborns of Obese Parents Have Altered DNA Methylation Patterns at Imprinted Genes. Int. J. Obes. 2005 2015, 39, 650–657. [Google Scholar] [CrossRef]
- Terashima, M.; Barbour, S.; Ren, J.; Yu, W.; Han, Y.; Muegge, K. Effect of High Fat Diet on Paternal Sperm Histone Distribution and Male Offspring Liver Gene Expression. Epigenetics 2015, 10, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Claycombe-Larson, K.G.; Bundy, A.N.; Roemmich, J.N. Paternal High-Fat Diet and Exercise Regulate Sperm MiRNA and Histone Methylation to Modify Placental Inflammation, Nutrient Transporter MRNA Expression and Fetal Weight in a Sex-Dependent Manner. J. Nutr. Biochem. 2020, 81, 108373. [Google Scholar] [CrossRef]
- Siklenka, K.; Erkek, S.; Godmann, M.; Lambrot, R.; McGraw, S.; Lafleur, C.; Cohen, T.; Xia, J.; Suderman, M.; Hallett, M.; et al. Disruption of Histone Methylation in Developing Sperm Impairs Offspring Health Transgenerationally. Science 2015, 350, aab2006. [Google Scholar] [CrossRef]
- Pepin, A.-S.; Lafleur, C.; Lambrot, R.; Dumeaux, V.; Kimmins, S. Sperm Histone H3 Lysine 4 Tri-Methylation Serves as a Metabolic Sensor of Paternal Obesity and Is Associated with the Inheritance of Metabolic Dysfunction. Mol. Metab. 2022, 59, 101463. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, H.; Huang, B.; Li, W.; Xiang, Y.; Peng, X.; Ming, J.; Wu, X.; Zhang, Y.; Xu, Q.; et al. Allelic Reprogramming of the Histone Modification H3K4me3 in Early Mammalian Development. Nature 2016, 537, 553–557. [Google Scholar] [CrossRef]
- Chen, Q.; Yan, M.; Cao, Z.; Li, X.; Zhang, Y.; Shi, J.; Feng, G.; Peng, H.; Zhang, X.; Zhang, Y.; et al. Sperm TsRNAs Contribute to Intergenerational Inheritance of an Acquired Metabolic Disorder. Science 2016, 351, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, V.; Fourré, S.; De Abreu, D.A.F.; Derieppe, M.-A.; Remy, J.-J.; Rassoulzadegan, M. RNA-Mediated Paternal Heredity of Diet-Induced Obesity and Metabolic Disorders. Sci. Rep. 2015, 5, 18193. [Google Scholar] [CrossRef] [PubMed]
- Nätt, D.; Kugelberg, U.; Casas, E.; Nedstrand, E.; Zalavary, S.; Henriksson, P.; Nijm, C.; Jäderquist, J.; Sandborg, J.; Flinke, E.; et al. Human Sperm Displays Rapid Responses to Diet. PLoS Biol. 2019, 17, e3000559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Shi, J.; Tuorto, F.; Li, X.; Liu, Y.; Liebers, R.; Zhang, L.; Qu, Y.; Qian, J.; et al. Dnmt2 Mediates Intergenerational Transmission of Paternally Acquired Metabolic Disorders through Sperm Small Non-Coding RNAs. Nat. Cell Biol. 2018, 20, 535–540. [Google Scholar] [CrossRef]
- Raad, G.; Serra, F.; Martin, L.; Derieppe, M.-A.; Gilleron, J.; Costa, V.L.; Pisani, D.F.; Amri, E.-Z.; Trabucchi, M.; Grandjean, V. Paternal Multigenerational Exposure to an Obesogenic Diet Drives Epigenetic Predisposition to Metabolic Diseases in Mice. eLife 2021, 10, e61736. [Google Scholar] [CrossRef]
- Trigg, N.A.; Eamens, A.L.; Nixon, B. The Contribution of Epididymosomes to the Sperm Small RNA Profile. Reprod. Camb. Engl. 2019, 157, R209–R223. [Google Scholar] [CrossRef]
- Darr, J.; Tomar, A.; Lassi, M.; Gerlini, R.; Berti, L.; Hering, A.; Scheid, F.; Hrabě de Angelis, M.; Witting, M.; Teperino, R. ITAG-RNA Isolates Cell-Specific Transcriptional Responses to Environmental Stimuli and Identifies an RNA-Based Endocrine Axis. Cell Rep. 2020, 30, 3183–3194.e4. [Google Scholar] [CrossRef]
- Tomar, A.; Teperino, R. Genetic Control of Non-Genetic Inheritance in Mammals: State-of-the-Art and Perspectives. Mamm. Genome Off. J. Int. Mamm. Genome Soc. 2020, 31, 146–156. [Google Scholar] [CrossRef]
- Brodie, E.D.; Gregory, B.; Lisch, D.; Riddle, N.C. The Epigenome and Beyond: How Does Non-Genetic Inheritance Change Our View of Evolution? Integr. Comp. Biol. 2022, 61, 2199–2207. [Google Scholar] [CrossRef]
- Ge, J.; Li, C.; Sun, H.; Xin, Y.; Zhu, S.; Liu, Y.; Tang, S.; Han, L.; Huang, Z.; Wang, Q. Telomere Dysfunction in Oocytes and Embryos from Obese Mice. Front. Cell Dev. Biol. 2021, 9, 617225. [Google Scholar] [CrossRef]
- Rao, A.; Satheesh, A.; Nayak, G.; Poojary, P.S.; Kumari, S.; Kalthur, S.G.; Mutalik, S.; Adiga, S.K.; Kalthur, G. High-Fat Diet Leads to Elevated Lipid Accumulation and Endoplasmic Reticulum Stress in Oocytes, Causing Poor Embryo Development. Reprod. Fertil. Dev. 2020, 32, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A. Seminal Plasma and Male Factor Signalling in the Female Reproductive Tract. Cell Tissue Res. 2005, 322, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Aluvihare, V.R.; Kallikourdis, M.; Betz, A.G. Regulatory T Cells Mediate Maternal Tolerance to the Fetus. Nat. Immunol. 2004, 5, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Woidacki, K.; Meyer, N.; Schumacher, A.; Goldschmidt, A.; Maurer, M.; Zenclussen, A.C. Transfer of Regulatory T Cells into Abortion-Prone Mice Promotes the Expansion of Uterine Mast Cells and Normalizes Early Pregnancy Angiogenesis. Sci. Rep. 2015, 5, 13938. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; Care, A.S.; Moldenhauer, L.M. Regulatory T Cells in Embryo Implantation and the Immune Response to Pregnancy. J. Clin. Investig. 2018, 128, 4224–4235. [Google Scholar] [CrossRef] [PubMed]
- Zenclussen, A.C.; Gerlof, K.; Zenclussen, M.L.; Sollwedel, A.; Bertoja, A.Z.; Ritter, T.; Kotsch, K.; Leber, J.; Volk, H.-D. Abnormal T-Cell Reactivity against Paternal Antigens in Spontaneous Abortion: Adoptive Transfer of Pregnancy-Induced CD4+CD25+ T Regulatory Cells Prevents Fetal Rejection in a Murine Abortion Model. Am. J. Pathol. 2005, 166, 811–822. [Google Scholar] [CrossRef]
- Shima, T.; Sasaki, Y.; Itoh, M.; Nakashima, A.; Ishii, N.; Sugamura, K.; Saito, S. Regulatory T Cells Are Necessary for Implantation and Maintenance of Early Pregnancy but Not Late Pregnancy in Allogeneic Mice. J. Reprod. Immunol. 2010, 85, 121–129. [Google Scholar] [CrossRef]
- Chan, H.Y.; Moldenhauer, L.M.; Groome, H.M.; Schjenken, J.E.; Robertson, S.A. Toll-like Receptor-4 Null Mutation Causes Fetal Loss and Fetal Growth Restriction Associated with Impaired Maternal Immune Tolerance in Mice. Sci. Rep. 2021, 11, 16569. [Google Scholar] [CrossRef]
- Leisegang, K.; Bouic, P.J.D.; Menkveld, R.; Henkel, R.R. Obesity Is Associated with Increased Seminal Insulin and Leptin alongside Reduced Fertility Parameters in a Controlled Male Cohort. Reprod. Biol. Endocrinol. RBE 2014, 12, 34. [Google Scholar] [CrossRef]
- Binder, N.K.; Sheedy, J.R.; Hannan, N.J.; Gardner, D.K. Male Obesity Is Associated with Changed Spermatozoa Cox4i1 MRNA Level and Altered Seminal Vesicle Fluid Composition in a Mouse Model. Mol. Hum. Reprod. 2015, 21, 424–434. [Google Scholar] [CrossRef]
- Fan, W.; Xu, Y.; Liu, Y.; Zhang, Z.; Lu, L.; Ding, Z. Obesity or Overweight, a Chronic Inflammatory Status in Male Reproductive System, Leads to Mice and Human Subfertility. Front. Physiol. 2018, 8, 1117. [Google Scholar] [CrossRef] [PubMed]
- Lassi, M.; Tomar, A.; Comas-Armangué, G.; Vogtmann, R.; Dijkstra, D.J.; Corujo, D.; Gerlini, R.; Darr, J.; Scheid, F.; Rozman, J.; et al. Disruption of Paternal Circadian Rhythm Affects Metabolic Health in Male Offspring via Nongerm Cell Factors. Sci. Adv. 2021, 7, eabg6424. [Google Scholar] [CrossRef] [PubMed]
- Morgan, H.L.; Watkins, A.J. The Influence of Seminal Plasma on Offspring Development and Health. Semin. Cell Dev. Biol. 2020, 97, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Hayes, R.B. Environmental Influences on the Human Microbiome and Implications for Noncommunicable Disease. Annu. Rev. Public Health 2021, 42, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Ooijevaar, R.E.; Terveer, E.M.; Verspaget, H.W.; Kuijper, E.J.; Keller, J.J. Clinical Application and Potential of Fecal Microbiota Transplantation. Annu. Rev. Med. 2019, 70, 335–351. [Google Scholar] [CrossRef]
- Kimura, I.; Miyamoto, J.; Ohue-Kitano, R.; Watanabe, K.; Yamada, T.; Onuki, M.; Aoki, R.; Isobe, Y.; Kashihara, D.; Inoue, D.; et al. Maternal Gut Microbiota in Pregnancy Influences Offspring Metabolic Phenotype in Mice. Science 2020, 367, eaaw8429. [Google Scholar] [CrossRef] [PubMed]
- Myles, I.A.; Fontecilla, N.M.; Janelsins, B.M.; Vithayathil, P.J.; Segre, J.A.; Datta, S.K. Parental Dietary Fat Intake Alters Offspring Microbiome and Immunity. J. Immunol. Baltim. Md 1950 2013, 191, 3200–3209. [Google Scholar] [CrossRef]
- Ma, J.; Prince, A.L.; Bader, D.; Hu, M.; Ganu, R.; Baquero, K.; Blundell, P.; Alan Harris, R.; Frias, A.E.; Grove, K.L.; et al. High-Fat Maternal Diet during Pregnancy Persistently Alters the Offspring Microbiome in a Primate Model. Nat. Commun. 2014, 5, 3889. [Google Scholar] [CrossRef]
- Wankhade, U.D.; Zhong, Y.; Kang, P.; Alfaro, M.; Chintapalli, S.V.; Thakali, K.M.; Shankar, K. Enhanced Offspring Predisposition to Steatohepatitis with Maternal High-Fat Diet Is Associated with Epigenetic and Microbiome Alterations. PLoS ONE 2017, 12, e0175675. [Google Scholar] [CrossRef]
- Bhagavata Srinivasan, S.P.; Raipuria, M.; Bahari, H.; Kaakoush, N.O.; Morris, M.J. Impacts of Diet and Exercise on Maternal Gut Microbiota Are Transferred to Offspring. Front. Endocrinol. 2018, 9, 716. [Google Scholar] [CrossRef]
- Feng, T.; Liu, Y. Microorganisms in the Reproductive System and Probiotic’s Regulatory Effects on Reproductive Health. Comput. Struct. Biotechnol. J. 2022, 20, 1541–1553. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.M.; Antony, K.M.; Ma, J.; Prince, A.L.; Showalter, L.; Moller, M.; Aagaard, K.M. The Early Infant Gut Microbiome Varies in Association with a Maternal High-Fat Diet. Genome Med. 2016, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Ponzo, V.; Ferrocino, I.; Zarovska, A.; Amenta, M.B.; Leone, F.; Monzeglio, C.; Rosato, R.; Pellegrini, M.; Gambino, R.; Cassader, M.; et al. The Microbiota Composition of the Offspring of Patients with Gestational Diabetes Mellitus (GDM). PLoS ONE 2019, 14, e0226545. [Google Scholar] [CrossRef] [PubMed]
- Babakobi, M.D.; Reshef, L.; Gihaz, S.; Belgorodsky, B.; Fishman, A.; Bujanover, Y.; Gophna, U. Effect of Maternal Diet and Milk Lipid Composition on the Infant Gut and Maternal Milk Microbiomes. Nutrients 2020, 12, E2539. [Google Scholar] [CrossRef]
- Sindi, A.S.; Geddes, D.T.; Wlodek, M.E.; Muhlhausler, B.S.; Payne, M.S.; Stinson, L.F. Can We Modulate the Breastfed Infant Gut Microbiota through Maternal Diet? FEMS Microbiol. Rev. 2021, 45, fuab011. [Google Scholar] [CrossRef]
- Korpela, K. Impact of Delivery Mode on Infant Gut Microbiota. Ann. Nutr. Metab. 2021, 77, 11–19. [Google Scholar] [CrossRef]
- Treichel, N.S.; Prevoršek, Z.; Mrak, V.; Kostrić, M.; Vestergaard, G.; Foesel, B.; Pfeiffer, S.; Stres, B.; Schöler, A.; Schloter, M. Effect of the Nursing Mother on the Gut Microbiome of the Offspring During Early Mouse Development. Microb. Ecol. 2019, 78, 517–527. [Google Scholar] [CrossRef]
- Sun, H.; Yamada, P.; Paetow, A.; Chan, M.; Arslan, A.; Landberg, R.; Dominguez-Bello, M.G.; Young, B.K. A Randomized Controlled Trial of the Effects of Whole Grains versus Refined Grains Diets on the Microbiome in Pregnancy. Sci. Rep. 2022, 12, 7509. [Google Scholar] [CrossRef]
- Whyte, J.J.; Alexenko, A.P.; Davis, A.M.; Ellersieck, M.R.; Fountain, E.D.; Rosenfeld, C.S. Maternal Diet Composition Alters Serum Steroid and Free Fatty Acid Concentrations and Vaginal PH in Mice. J. Endocrinol. 2007, 192, 75–81. [Google Scholar] [CrossRef]
- Jašarević, E.; Howard, C.D.; Morrison, K.; Misic, A.; Weinkopff, T.; Scott, P.; Hunter, C.; Beiting, D.; Bale, T.L. The Maternal Vaginal Microbiome Partially Mediates the Effects of Prenatal Stress on Offspring Gut and Hypothalamus. Nat. Neurosci. 2018, 21, 1061–1071. [Google Scholar] [CrossRef]
- Jašarević, E.; Hill, E.M.; Kane, P.J.; Rutt, L.; Gyles, T.; Folts, L.; Rock, K.D.; Howard, C.D.; Morrison, K.E.; Ravel, J.; et al. The Composition of Human Vaginal Microbiota Transferred at Birth Affects Offspring Health in a Mouse Model. Nat. Commun. 2021, 12, 6289. [Google Scholar] [CrossRef] [PubMed]
- Pocheron, A.-L.; Le Dréan, G.; Billard, H.; Moyon, T.; Pagniez, A.; Heberden, C.; Le Chatelier, E.; Darmaun, D.; Michel, C.; Parnet, P. Maternal Microbiota Transfer Programs Offspring Eating Behavior. Front. Microbiol. 2021, 12, 672224. [Google Scholar] [CrossRef] [PubMed]
- Javurek, A.B.; Spollen, W.G.; Ali, A.M.M.; Johnson, S.A.; Lubahn, D.B.; Bivens, N.J.; Bromert, K.H.; Ellersieck, M.R.; Givan, S.A.; Rosenfeld, C.S. Discovery of a Novel Seminal Fluid Microbiome and Influence of Estrogen Receptor Alpha Genetic Status. Sci. Rep. 2016, 6, 23027. [Google Scholar] [CrossRef] [PubMed]
- Altmäe, S.; Franasiak, J.M.; Mändar, R. The Seminal Microbiome in Health and Disease. Nat. Rev. Urol. 2019, 16, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kwon, J.; Kim, M.-S.; Park, H.; Ji, Y.; Holzapfel, W.; Hyun, C.-K. Protective Effects of Bacillus Probiotics against High-Fat Diet-Induced Metabolic Disorders in Mice. PLoS ONE 2018, 13, e0210120. [Google Scholar] [CrossRef]
- Li, S.; Tse, I.M.Y.; Li, E.T.S. Maternal Green Tea Extract Supplementation to Rats Fed a High-Fat Diet Ameliorates Insulin Resistance in Adult Male Offspring. J. Nutr. Biochem. 2012, 23, 1655–1660. [Google Scholar] [CrossRef]
- Su, X.; Wang, W.; Fang, C.; Ni, C.; Zhou, J.; Wang, X.; Zhang, L.; Xu, X.; Cao, R.; Lang, H.; et al. Vitamin K2 Alleviates Insulin Resistance in Skeletal Muscle by Improving Mitochondrial Function Via SIRT1 Signaling. Antioxid. Redox Signal. 2021, 34, 99–117. [Google Scholar] [CrossRef]
- Krasnow, S.M.; Nguyen, M.L.T.; Marks, D.L. Increased Maternal Fat Consumption during Pregnancy Alters Body Composition in Neonatal Mice. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1243–E1253. [Google Scholar] [CrossRef]
- Xu, H.; Fu, Q.; Zhou, Y.; Xue, C.; Olson, P.; Lynch, E.C.; Zhang, K.K.; Wu, C.; Murano, P.; Zhang, L.; et al. A Long-Term Maternal Diet Intervention Is Necessary to Avoid the Obesogenic Effect of Maternal High-Fat Diet in the Offspring. J. Nutr. Biochem. 2018, 62, 210–220. [Google Scholar] [CrossRef]
- Summerfield, M.; Zhou, Y.; Zhou, T.; Wu, C.; Alpini, G.; Zhang, K.K.; Xie, L. A Long-Term Maternal Diet Transition from High-Fat Diet to Normal Fat Diet during Pre-Pregnancy Avoids Adipose Tissue Inflammation in next Generation. PLoS ONE 2018, 13, e0209053. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, H.; Xu, H.; Li, J.; Golovko, M.; Cheng, H.; Lynch, E.C.; Liu, L.; McCauley, N.; Kennedy, L.; et al. Maternal Diet Intervention before Pregnancy Primes Offspring Lipid Metabolism in Liver. Lab. Investig. J. Tech. Methods Pathol. 2020, 100, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ding, Z.; Lynch, E.C.; McCauley, N.; Zhou, Y.; Zhang, K.K.; Xie, L. Pregestational Diet Transition to Normal-Fat Diet Avoids the Deterioration of Pancreatic β-Cell Function in Male Offspring Induced by Maternal High-Fat Diet. J. Nutr. Biochem. 2020, 86, 108495. [Google Scholar] [CrossRef] [PubMed]
- Panchenko, P.E.; Lacroix, M.-C.; Jouin, M.; Voisin, S.; Badonnel, K.; Lemaire, M.; Meunier, N.; Safi-Stibler, S.; Persuy, M.-A.; Jouneau, L.; et al. Effect of Maternal Obesity and Preconceptional Weight Loss on Male and Female Offspring Metabolism and Olfactory Performance in Mice. Nutrients 2019, 11, 948. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Olson, P.; Rasmussen, D.; Keith, B.; Williamson, M.; Zhang, K.K.; Xie, L. A Short-Term Transition from a High-Fat Diet to a Normal-Fat Diet before Pregnancy Exacerbates Female Mouse Offspring Obesity. Int. J. Obes. 2016, 40, 564–572. [Google Scholar] [CrossRef]
- Kasper, P.; Breuer, S.; Hoffmann, T.; Vohlen, C.; Janoschek, R.; Schmitz, L.; Appel, S.; Fink, G.; Hünseler, C.; Quaas, A.; et al. Maternal Exercise Mediates Hepatic Metabolic Programming via Activation of AMPK-PGC1α Axis in the Offspring of Obese Mothers. Cells 2021, 10, 1247. [Google Scholar] [CrossRef]
- Son, J.S.; Liu, X.; Tian, Q.; Zhao, L.; Chen, Y.; Hu, Y.; Chae, S.A.; de Avila, J.M.; Zhu, M.-J.; Du, M. Exercise Prevents the Adverse Effects of Maternal Obesity on Placental Vascularization and Fetal Growth. J. Physiol. 2019, 597, 3333–3347. [Google Scholar] [CrossRef]
- Wesolowski, S.R.; Mulligan, C.M.; Janssen, R.C.; Baker, P.R.; Bergman, B.C.; D’Alessandro, A.; Nemkov, T.; Maclean, K.N.; Jiang, H.; Dean, T.A.; et al. Switching Obese Mothers to a Healthy Diet Improves Fetal Hypoxemia, Hepatic Metabolites, and Lipotoxicity in Non-Human Primates. Mol. Metab. 2018, 18, 25–41. [Google Scholar] [CrossRef]
- McPherson, N.O.; Fullston, T.; Bakos, H.W.; Setchell, B.P.; Lane, M. Obese Father’s Metabolic State, Adiposity, and Reproductive Capacity Indicate Son’s Reproductive Health. Fertil. Steril. 2014, 101, 865–873. [Google Scholar] [CrossRef]
- McPherson, N.O.; Owens, J.A.; Fullston, T.; Lane, M. Preconception Diet or Exercise Intervention in Obese Fathers Normalizes Sperm MicroRNA Profile and Metabolic Syndrome in Female Offspring. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E805–E821. [Google Scholar] [CrossRef]
- McPherson, N.O.; Bakos, H.W.; Owens, J.A.; Setchell, B.P.; Lane, M. Improving Metabolic Health in Obese Male Mice via Diet and Exercise Restores Embryo Development and Fetal Growth. PLoS ONE 2013, 8, e71459. [Google Scholar] [CrossRef]
- McPherson, N.O.; Lane, M.; Sandeman, L.; Owens, J.A.; Fullston, T. An Exercise-Only Intervention in Obese Fathers Restores Glucose and Insulin Regulation in Conjunction with the Rescue of Pancreatic Islet Cell Morphology and MicroRNA Expression in Male Offspring. Nutrients 2017, 9, E122. [Google Scholar] [CrossRef] [PubMed]
- Palmer, N.O.; Bakos, H.W.; Owens, J.A.; Setchell, B.P.; Lane, M. Diet and Exercise in an Obese Mouse Fed a High-Fat Diet Improve Metabolic Health and Reverse Perturbed Sperm Function. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E768–E780. [Google Scholar] [CrossRef] [PubMed]
- Maggard, M.A.; Yermilov, I.; Li, Z.; Maglione, M.; Newberry, S.; Suttorp, M.; Hilton, L.; Santry, H.P.; Morton, J.M.; Livingston, E.H.; et al. Pregnancy and Fertility Following Bariatric Surgery: A Systematic Review. JAMA 2008, 300, 2286–2296. [Google Scholar] [CrossRef] [PubMed]
- Alwan, N.A.; Grove, G.; Taylor, E.; Ziauddeen, N. Maternal Weight Change between Successive Pregnancies: An Opportunity for Lifecourse Obesity Prevention. Proc. Nutr. Soc. 2020, 79, 272–282. [Google Scholar] [CrossRef]
- Grayson, B.E.; Schneider, K.M.; Woods, S.C.; Seeley, R.J. Improved Rodent Maternal Metabolism but Reduced Intrauterine Growth after Vertical Sleeve Gastrectomy. Sci. Transl. Med. 2013, 5, 199ra112. [Google Scholar] [CrossRef]
- Spann, R.A.; Taylor, E.B.; Welch, B.A.; Grayson, B.E. Altered Immune System in Offspring of Rat Maternal Vertical Sleeve Gastrectomy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R852–R863. [Google Scholar] [CrossRef]
- Spann, R.A.; Lawson, W.J.; Bidwell, G.L.; Zamarripa, C.A.; Maranon, R.O.; Bandyopadhyay, S.; Taylor, E.R.; Reckelhoff, J.F.; Garrett, M.R.; Grayson, B.E. Rodent Vertical Sleeve Gastrectomy Alters Maternal Immune Health and Fetoplacental Development. Clin. Sci. Lond. Engl. 1979 2018, 132, 295–312. [Google Scholar] [CrossRef]
- Willmer, M.; Berglind, D.; Sørensen, T.I.A.; Näslund, E.; Tynelius, P.; Rasmussen, F. Surgically Induced Interpregnancy Weight Loss and Prevalence of Overweight and Obesity in Offspring. PLoS ONE 2013, 8, e82247. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).