Abstract
In this paper, a novel NL-Fe3+ ensemble was designed as a fluorescent chemosensor for highly selective detection of pyrophosphate (PPi) in DMSO/H2O (2:8/v:v, pH = 7.2) solution and living cells. NL showed a strong affinity for Fe3+ and was accompanied by obvious fluorescence quenching. Upon the addition of PPi to the generated NL-Fe3+ ensemble, the fluorescence and absorption spectra were recovered completely. Spectroscopic investigation showed that the interference provoked by common anions such as adenosine-triphosphate (ATP), adenosine diphosphate (ADP), and phosphates (Pi) can be ignored. The detection limit of NL-Fe3+ to PPi was calculated to be 1.45 × 10−8 M. Intracellular imaging showed that NL-Fe3+ has good membrane permeability and could be used for the detection of PPi in living cells. A B3LYP/6-31G(d,p) basis set was used to optimize NL and NL-Fe3+ complex.
1. Introduction
Phosphates and their derivatives play an important role in the process of building the most fundamental molecules in living organisms. As we know, DNA and RNA make up the main macromolecules necessary for all known life. Phosphates are also a major ingredient of lipid membranes and are involved in many biological processes, which include skeletal development and integrity, cell sensing, and regulation of protein synthesis [1,2]. The change of PPi concentration in biological environments can be used as a diagnostic indicator for various clinical conditions. For example, crystal deposition of calcium pyrophosphate dehydration (CPPD) may result in abnormally high levels of PPi in synovial fluid [3,4], the level of intracellular PPi can provide important information about cellular processes for cancer diagnosis [5]. The fluctuations in PPi concentration in physiological fluids such as synovial fluid and urine can also be used to identify series of diseases such as chondrocalcification and so on [6]. Therefore, the identification of PPi has become a very important research hotspot.
Among various methods for detecting anions, anion-selective fluorescent probes have attracted more and more attention because of their high sensitivity and easy-to-operate features [7,8]. Numerous probes for detecting anions have been devised and are based on hydrogen bonding, anion-π interactions, and metal-anion chelation [9,10,11]. Of these, metal complex chemosensors have been reported for the detection of anions because the coordination flexibility of metal centers can provide a good way for organizing anion binding groups. These complexes are usually not fluorescent due to metal-ion-induced fluorescence quenching. By virtue of the high affinity between metal and anion, the metal ion is detached from the complex in the presence of anion, switching on fluorescence for quantitative detection [12,13,14,15]. Over the past few years, many excellent fluorescent chemosensors for PPi have been reported. However, there are relatively few chemosensors for detecting PPi in aqueous media and biological systems reported to date [16,17,18], which are also not free from disadvantages such as low sensitivity, slow response time, interference from ATP, ADP, and so on [19]. Recently, based on the displacement strategy, we reported a Fe3+-based chemosensor for detecting PPi in DMSO/H2O (3:7/v:v, pH = 7.2) solution [20], which showed good selectivity and sensitivity to PPi, and the interference caused by other common anions such as ATP, ADP, and Pi is low or not relevant. However, there is still a demand to develop homogeneous PPi sensing systems that are selective over di- and trinucleoside phosphates.
In this work, considering the flexible coordination way of heteroatoms-metal ion and the excellent fluorescence properties of coumarin, a novel coumarin-based fluorescent ligand, NL, was synthesized and characterized (Scheme 1). After the complexation of NL with Fe3+, the NL-Fe3+ complex showed no fluorescence owing to the paramagnetic quenching effect of Fe3+. In the presence of PPi, the resultant NL-Fe3+ complex is switched on upon addition of PPi due to the high affinity of Fe3+ to PPi. The detection limit of NL-Fe3+ to PPi was determined to be 1.45 × 10−8 M. This “OFF-ON” fluorescence identification can provide a convenient approach for the detection of PPi in biological samples (Scheme 2).
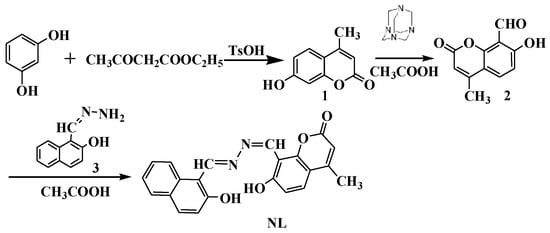
Scheme 1.
Synthesis of fluorescence chemosensor NL.
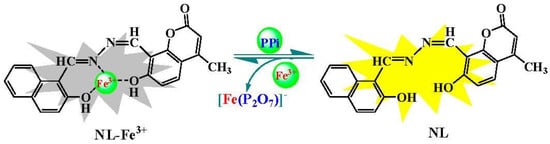
Scheme 2.
Proposed response mechanism of NL-Fe3+ towards pyrophosphate (PPi).
2. Materials and Methods
2.1. Apparatus and Reagents
UV-Vis and fluorescence spectra were measured on a Lambda-900 spectrometer and Perkin-Elmer LS-55 fluorescence spectrophotometer (Perkin-Elmer, Waltham, MA, USA). 1H NMR and 13C NMR (internal standard TMS) were recorded on a Bruker 500 and 125 MHz spectrometers (Karlsruhe, Germany), respectively. Mass spectrometry was performed with a Bruker Solari X70 high-resolution mass spectrometer (Bruker, Karlsruhe, Germany). The elemental analysis was tested using a Perkin-Elmer 240 microanalyzer (PerkinElmer, Waltham, MA, USA).
All chemical reagents were purchased from Aladdin Reagents Company (Shanghai, China). The solvents used for synthesis and spectroscopic studies were analytic grade and spectroscopic grade reagent. Water was double distilled and all metal salts except ferric chloride and zinc chloride are nitrates in the experiments.
2.2. Synthesis of Compound NL
The synthetic route of probe NL is shown in Scheme 1, 4-methyl-7-hydroxy coumarin 1, 7-hydroxy-4-formyl coumarin 2, and naphthalene hydrazine 3 were prepared according to reported procedures [21,22]. A mixture of intermediates 2 (3 mmol) and 3 (3 mmol) were dissolved in 25 mL of acetic acid and refluxed until complete reaction of raw material. An orange precipitate appeared in the process of reaction; the filtered crude was purified by column chromatography to obtain a yellow solid NL (yield: 56%). 1H NMR (500 MHz, DMSO-d6) δ(ppm): 12.56 (s, 1H), 12.51 (s, 1H), 9.97 (d, J = 10.4 Hz, 1H), 9.33 (s, 1H), 8.64 (d, J = 8.2 Hz, 1H), 8.02 (d, J = 8.5 Hz, 1H), 7.89 (d, J = 8.2 Hz, 1H), 7.77 (d, J = 8.8 Hz, 1H), 7.64–7.57 (m, 1H), 7.42 (t, J = 7.3 Hz, 1H), 7.24 (d, J = 8.4 Hz, 1H), 7.00 (d, J = 8.6 Hz, 1H), 6.26 (s, 1H), 2.39 (s, 3H). 13C NMR (125 MHz, DMSO-d6) δ(ppm): 162.8, 161.9, 160.2, 158.9, 157.3, 153.6, 135.1, 132.2, 129.7, 128.7, 128.0, 127.7, 123.7, 121.9, 118.7, 118.6, 113.3, 111.8, 110.7, 108.2, 105.2, 18.1. FT-MS, m/z: 373.11879 (M+H+); Anal. Calcd for C22H16N2O4: C, 70.96; H, 4.33; N, 7.52. Found: C, 70.91; H, 4.35; N, 7.56.
2.3. Synthesis of NL-Fe3+ Complex
Ligand NL (0.5 mmol) and ferric chloride (0.6 mmol) were dissolved in acetonitrile (20 mL). The resulting suspension was heated under reflux for 5 h until a green precipitate formed. It was filtered off while hot, washed with Et2O, and dried in vacuo. The solid obtained was filtered and recrystallized from ethanol to afford a green solid. FT-IR (cm−1): 3433 (OH), 2919 (CH), 1545 (C=N), 845 (C–Cl), 542 (Fe–N), 457 (Fe–O). FT-MS, m/z: 498.94274; Anal. Calcd for C22H16Cl2FeN2O4: C, 52.96; H, 3.23; N, 5.61; Cl, 14.21. Found: C, 52.91; H, 3.24; N, 5.63; Cl, 14.24. Conductivity:109 S·cm−1·mole−1.
2.4. Computational Details
Density functional theory (DFT) method was used to optimize the geometry and electronic structures of NL and NL-Fe3+ at the B3LYP/6-311+G (d,p) level by using Gaussian 09 software [23].
2.5. Calculation of Association Constant
The association constant of NL-Fe3+ was calculated using the Benesi–Hildebrand (B–H) plot (Equation (1)) [24].
where F0 and F are the fluorescence intensity of NL and the emission intensity in the presence of analyte with different concentrations, respectively; Fmin is the minimum emission intensity value obtained by fluorescence titration; and Ka is the association constant and was determined by the slope of the linear plot.
1/(F0 − F) = 1/{Ka (F0 − Fmin) C} + 1/(F0 − Fmin)
2.6. Cytotoxicity Assays
Hela cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) in an atmosphere of 5% CO2 and 95% air at 37 °C for 24 h, and then 0–30 μM NL dissolved in DMSO/H2O solution (2:8/v:v) was added. Then, the labeled cells were cultured in the same environment for 48 h. Finally, 20 mL of 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrabromazole (MTT) was added and cultured for 6 h.
3. Results and Discussion
3.1. Synthesis and Spectroscopic Characterization of the NL-Fe3+
NL and NL-Fe3+ complex were characterized by 1H NMR, 13C NMR, FT-MS, IR, and elemental analysis (Figures S1–S6, Supplementary materials). The stability of NL was studied in DMSO/H2O (2:8/v:v, pH = 7.2) solution. As shown in Figure S9, the fluorescence of NL (10 μM) did not change significantly within 24 h, which showed that NL was stable under experimental conditions. Then, 1.0 equivalent of FeCl3 was added to NL (10 μM) solution to prepare the stock solution of NL-Fe3+ for detecting PPi. The complexation behavior of NL with Fe3+ was investigated by a fluorescence titration experiment. The ligand NL has a strong emission at 557 nm when the excitation wavelength is 410 nm, the fluorescence intensity of NL was gradually quenched with the increase of Fe3+ concentration (0–2 equiv.) (Figure 1A), and the emission color of the solution changed from yellow to colorless under the ultraviolet lamp (Figure 1A inset), indicating a complete formation of NL-Fe3+ complex [25]. A remarkable fluorescence quenching (quenching efficiency at 557 nm, (I0 − I)/I0 × 100% = 86 %) is observed in the presence of 10 μM Fe3+ (Figure S10). However, NL had no obvious fluorescence response upon the addition of other metal ions such as Cr3+, Cu2+, Ag+, Co2+, K+, Hg2+, Pb2+, Ba2+, Ni2+, Mg2+, Cd2+, Ca2+, Zn2+, Al3+, and Na+ (Figure 1B).
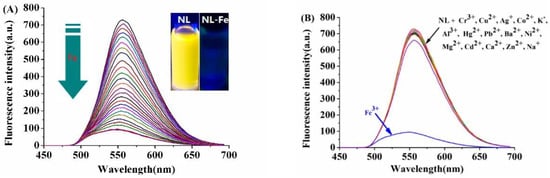
Figure 1.
(A) Fluorescence titration of NL (10 μM) in the presence of different amounts of Fe3+ (0–20 μM) in DMSO/H2O (2:8/v:v, 20 mM HEPES, pH = 7.2) solution. Inset: Changes in fluorescence color of NL (10 μM) upon the addition of Fe3+ (10 μM). (B) Fluorescence responses of NL (10 μM) to different cations (20 μM): Cr3+, Cu2+, Ag+, Co2+, K+, Hg2+, Pb2+, Fe3+, Ba2+, Ni2+, Mg2+, Cd2+, Ca2+, Zn2+, Al3+, and Na+.
In addition, potential interferences were investigated by adding Fe3+ to the mixture of NL and multiple coexisting cations. As shown in the Figure S11, the quenching effect induced by Fe3+ was not interfered with by other competitive cations, indicating that NL has high specificity for Fe3+ binding. The complexation stoichiometry of NL-Fe3+ was determined by Job plot; the results showed that NL coordinates to Fe3+ to form a complex in 1:1 stoichiometry (Figure 2A) [26]. According to Benesi–Hildebrand equation, the intensity (1/(F0−F)) of NL changed linearly with 1/[Fe3+] (R2 = 0.9949), and the association constant of NL with Fe3+ was calculated to be 2.29 × 105 M–1(Figure 2B).
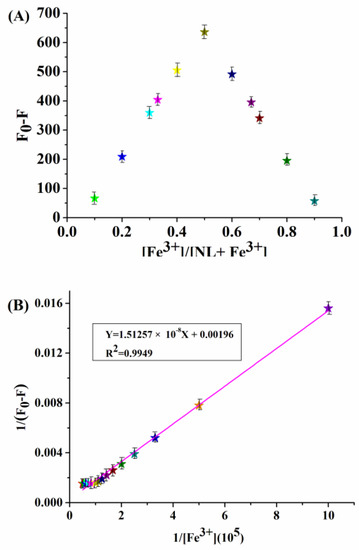
Figure 2.
(A) The Job’s plot of the reaction between NL and Fe3+ (total concentration was kept at 20 μM). (B) The Benesi-Hildebrand plot of NL (10 μM) with Fe3+.
The specific recognition of NL for Fe3+ was then studied by UV-Vis absorption spectroscopy. As shown in Figure 3A, the ligand NL showed a maximum absorption at 412 nm in DMSO/H2O (2:8/v:v, pH = 7.2) solution, the UV-Vis absorption of NL increased gradually when Fe3+ (0–20 μM) was added to NL (10 μM). The addition of other cations has a negligible effect on the UV spectrum of NL (Figure 3B). The association constant of NL with Fe3+ by UV-Vis spectroscopy was calculated to be 2.16 × 105 M–1(R2 = 0.9952) (Figure S12). A Job plot by UV-Vis spectroscopy showed that NL coordinates to Fe3+ to form a complex in 1:1 stoichiometry (Figure S13).
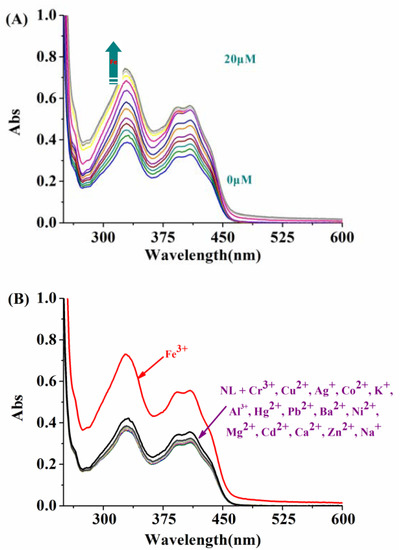
Figure 3.
(A) UV-Vis titration spectra of NL (10 μM) in the presence of different amounts of Fe3+ (0–20 μM) in DMSO/H2O (2:8/v:v, 20 mM HEPES, pH = 7.2) solution. (B) Fluorescence responses of NL (10 μM) to different cations (20 μM): Cr3+, Cu2+, Ag+, Co2+, K+, Hg2+, Pb2+, Fe3+, Ba2+, Ni2+, Mg2+, Cd2+, Ca2+, Zn2+, Al3+, and Na+.
3.2. Fluorescence Responses of the NL-Fe3+ towards Anions
Due to the coordination property of Fe3+-PPi, NL-Fe3+ has the potential to be used as a fluorescent chemosensor for PPi recognition by Fe3+ displacement approach [27]. The NL-Fe3+ complex was then synthesized in situ by adding Fe3+ (10 μM) into NL (10 μM) in DMSO/H2O (2:8/v:v, 20 mM HEPES, pH = 7.2) solution. Fluorescence responses of NL-Fe3+ to various anions were investigated (Figure 4A). It was found that NL-Fe3+ showed an “off-on” fluorescence response toward PPi (40 μM) selectively, the changes of the emission color of the NL-Fe3+ chemosensor from colorless to yellow could be observed by the naked eye under UV lamp, while other anions and polyphosphates such as F–, Cl–, Br–, Ac–, HSO4–, SCN–, HCO3–, CO32–, S2–, NO2–, SO42–, AMP, ADP, ATP, and phosphate (Pi) had no significant effect on the fluorescence intensity of NL-Fe3+. This may be attributed to the strong complexation between PPi and Fe3+, which releases NL from the NL-Fe3+ and restores the fluorescence in the presence of PPi. Therefore, NL-Fe3+ can be used as a selective sensor to detect PPi in aqueous solution.
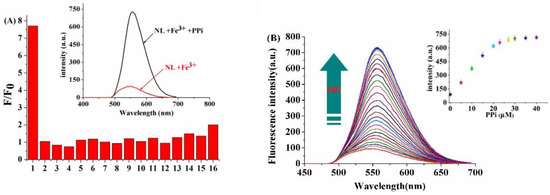
Figure 4.
(A) Fluorescence responses of NL-Fe3+ (10 μM) towards various anionic analytes (40 μM): (1) PPi (2) NO2–, (3) S2–, (4) F–, (5) SCN–, (6) Ac–, (7) HCO3–, (8) HSO4–, (9) CO32–, (10) Cl–, (11) Br–, (12) SO42–, (13) AMP, (14) ADP, (15) ATP, (16) Pi. (B) Fluorescence spectra of NL-Fe3+ (10 μM) for different amounts of PPi (0–40 μM) in DMSO/H2O (2:8/v:v, 20 mM HEPES, pH = 7.2) solution. Inset: Relationship between the fluorescence intensity of NL-Fe3+ and concentration of PPi.
One of the important indicators of sensor performance is its high selectivity toward the analyte over other competitive species. The selectivity of NL-Fe3+ toward PPi in the presence of different anions and polyphosphates was investigated. As shown in Figure S14, upon the addition of 4 equiv. of PPi to the solution of NL-Fe3+ containing other anions and polyphosphates, the competitive anions and polyphosphate species did not lead to any significant interference, which indicates the high selectivity of NL-Fe3+ to PPi under the interference of competitive anions.
To further investigate the recognition performance of NL-Fe3+ toward PPi, fluorescence titration of NL-Fe3+ with different concentrations of PPi was performed in HEPES buffer solution. As shown in Figure 4B, upon addition of increasing concentrations of PPi to NL-Fe3+, the fluorescence intensity of NL-Fe3+ increased gradually and reached the maximum when 3 equiv. of PPi were added, which is the same as the fluorescence intensity of NL (Figure S15), showing the full release of Fe3+ by PPi. The detection limit of NL-Fe3+ for PPi was calculated to be 1.45 × 10−8 M (R2 = 0.9978) (Figure S16), indicating that NL-Fe3+ can identify PPi sensitively in buffer solution, and the decomplexation constant of NL-Fe3+ toward PPi was calculated to be 6.41 × 1010 M–1 (R2 = 0.9946) (Figure S17).
Regeneration is also an important factor to evaluate the performance of a fluorescent chemosensor. The fluorescence spectra of NL-Fe3+ (10 μM) were observed by adding PPi (30 μM) and Fe3+(10 μM) alternately in DMSO/H2O (2:8/v:v, 20 mM HEPES, pH = 7.2) solution, as shown in Figure 5. The reversible fluorescence transformation of NL-Fe3+ could be repeated more than 5 times, suggesting that NL can be used as a reversible fluorescent chemosensor for detecting PPi.
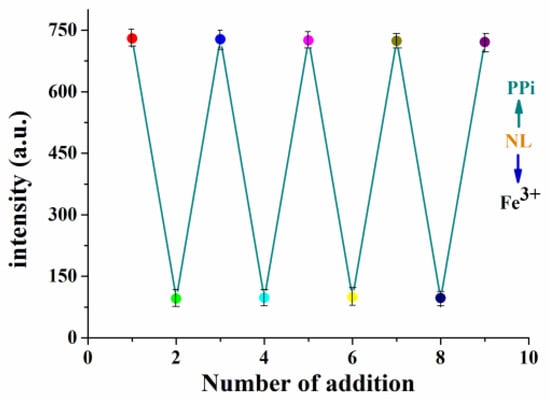
Figure 5.
Fluorescence spectral changes of NL (10 μM) upon the alternate addition of Fe3+ (10 μM) and PPi (30 μM) in HEPES buffer.
The fluorescence response time of NL-Fe3+ to PPi was further investigated. As shown in Figure S18, significant fluorescence enhancement was observed within 60 s, indicating that NL-Fe3+ could be used as a fluorescent probe for real-time detection of PPi.
3.3. Ultraviolet Spectrum Responses of the NL-Fe3+ towards Anions
The recognition response of NL-Fe3+ ensemble towards PPi was studied by UV-Vis absorption spectroscopy. As shown in Figure 6A, with the increase of PPi (0–40 μM), the intensity of NL-Fe3+ decreased gradually and was completely consistent with that of free chemosensor NL, which indicates that the sensor NL is recyclable. In addition, the addition of other anions and polyphosphates such as F–, Cl–, Br–, Ac–, HSO4–, SCN–, HCO3–, CO32–, S2–, NO2–, SO42–, AMP, ADP, ATP, and phosphate (Pi) had no effect on the absorption spectrum of the NL-Fe3+ ensemble (Figure 6B), suggesting the high selectivity of NL-Fe3+ towards PPi, and the decomplexation constant of NL-Fe3+ toward PPi by UV-Vis titration was calculated to be 6.23 × 1010 M–1 (R2 = 0.9918) (Figure S19).
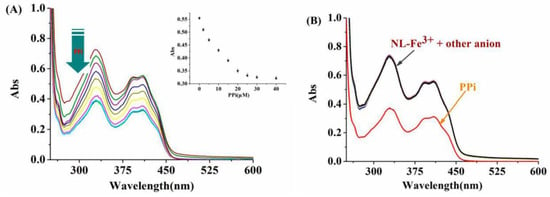
Figure 6.
(A) UV-Vis spectrum of NL-Fe3+ (10 μM) toward different amounts of PPi (0–40 μM) in DMSO/H2O (2:8/v:v, 20 mM HEPES, pH = 7.2) solution. (B) UV-Vis spectrum of NL-Fe3+ (10 μM) towards various anionic analytes (40 μM): F–, Cl–, Br–, Ac–, HSO4–, SCN–, HCO3–, CO32–, S2–, NO2–, SO42–, AMP, ADP, ATP, PPi, and Pi.
3.4. Effects of pH
The effects of pH value on the fluorescence of NL and NL-Fe3+ were studied. As shown in Figure S20, the fluorescence of NL-Fe3+ is almost completely quenched at pH 5–10 in DMSO/H2O (2:8/v:v, 20 mM HEPES) solution, the strong fluorescence was observed when pyrophosphate was added to the NL-Fe3+ complex, indicating that NL-Fe3+ complex is suitable for the recognition of PPi under physiological conditions.
3.5. The Plausible Mechanism of NL towards Fe3+ and PPi
The displacement strategy for PPi detection was further validated by the results of MS spectroscopy studies. MS spectra of NL showed a molecular-ion peak [NL+H+]+ at m/z 373.11879 (Figure S3). After adding Fe3+ into the NL solution, the peak generated at m/z 498.94286 was attributed to [NL+Fe3++2Cl−]+ (Figure S7). This evidence supported the formation of the NL-Fe3+ ensemble convincingly. Further addition of PPi to the generated NL-Fe3+, the MS generated a molecular-ion peak at m/z 373.11883 again (Figure S8), which proves that PPi promotes the release of Fe3+ from NL-Fe3+ complex and results in the restore of ligand NL.
Therefore, the reasonable complexion mode of NL and Fe3+ was proposed according to the above analysis results (Scheme 2). The results showed that Fe3+ chelates with the N atom of imino, O atom of hydroxy in naphthol as well as O atom of hydroxy in coumarin. The chelation pattern was confirmed by DFT calculations at the level of B3LYP using 6-31G (d,p) basis set for NL and LANL2DZ basis set for NL-Fe3+ complex using Gaussian 09 program. The configurations of NL and NL-Fe3+ complex and their corresponding energy levels of HOMO and LUMO molecular orbitals were depicted (Figure 7), the HOMO was distributed on the naphthalene ring of NL, and LUMO was distributed on the whole molecule of NL. Meanwhile, the π electrons in HOMO orbitals of NL-Fe3+ complex were distributed on the coumarin. The LUMO was distributed on the complexation part between the heteroatom and the iron in NL-Fe3+. The HOMO-LUMO energy gaps of NL and NL-Fe3+ were 0.11 eV and 0.03 eV, showing that the combination of Fe3+ and NL reduces the HOMO-LUMO energy gap of NL-Fe3+ and makes the system more stable.
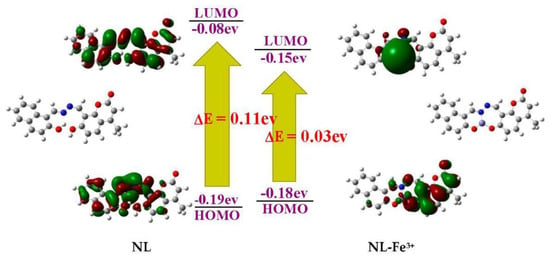
Figure 7.
HOMO and LUMO energies of NL and NL-Fe3 + complexes.
3.6. Fluorescence Imaging of Intracellular PPi
The reduction activity of methyl thiazolyl tetrazolium (MTT[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra-zoliumbromide]) assay was used to determine the cytotoxicity of NL in Hep G2 cells. As shown in Figure S21, when NL (2–16 μM) were incubated with Hep G2 cells at 37 °C for 24 h, no significant change in the proliferation of Hep G2 cells was observed, and the cell viability remained above 90% after 24 h incubation with NL, which indicated that NL exhibited low cytotoxicity and had good biocompatibility, and could be used for fluorescence imaging of living cells. To investigate the cytotoxicity of NL-Fe3+ towards Hep G2 cells, the cells were incubated with NL-Fe3+ (10 μM) for 48 h. The Hep G2 cells were used as the control group. As shown in Figure S22, the survival of Hep G2 cells incubated with NL-Fe3+(10 μM) was still above 92% after 48 h.
Due to its high sensitivity and biocompatibility, the potential application of biological imaging of PPi recognition in live Hep G2 cell was investigated. The cells were incubated with the DMSO/H2O (2:8/v:v, pH = 7.2) solution of NL (10 μM) in a CO2 incubator for 30 min at 37 °C. The cells were washed 3 times and treated with Fe3+(10 μM) for 20 min, then were incubated with PPi (30 μM) for another 20 min. As expected, Hep G2 cells cultured with NL showed strong yellow fluorescence (Figure 8A), while the fluorescence of cells treated with Fe3+ was quenched (Figure 8B), suggesting the formation of NL-Fe3+ complex in the cell. Then, the strong yellow fluorescence of cells was observed after further incubation with PPi (Figure 8C), which indicates that PPi could lead to the recovery of fluorescence by penetrating the living cell membrane and interacting with NL-Fe3+. This indicated that the NL-Fe3+ complex can be used to detect PPi in biological systems.
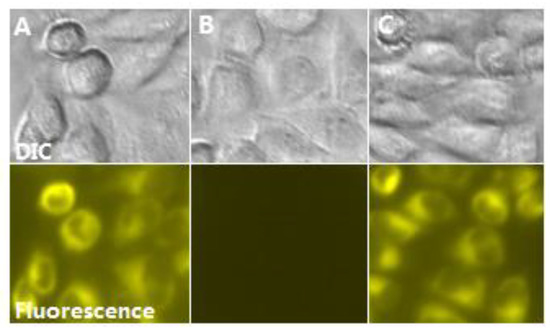
Figure 8.
Confocal bright-field (top) and fluorescence (bottom) imaging of Fe3+ and PPi in living Hep G2 cells. (A) Cells stained with NL (10 μM) in a CO2 incubator for 30 min at 37 °C; (B) cells treated with Fe3+ (10 μM) for 20 min, (C) cells then incubated with PPi (30 μM) for another 20 min.
4. Conclusions
In conclusion, a novel coumarin-Fe3+ complex was investigated as fluorescent chemosensor for PPi in DMSO/H2O (2:8/v:v, pH = 7.2) solution and biological samples. The chelation of Fe3+ with ligand NL results in a strong fluorescence quenching. Then, the NL-Fe3+ complex can be used for PPi recognition by fluorescence “off-on” signaling based on displacement process. The fluorescence ‘‘on-off-on’’circle can be repeated more than five times when PPi and Fe3+ are added alternately, indicating that NL-Fe3+ is a recyclable chemosensor for detecting PPi. Confocal microscopy showed that NL-Fe3+ could be utilized for the biological imaging of PPi in Hep G2 cells. In view of the above experimental results, we believe that the designed metal complex provided a flexible strategy for the identification of PPi in biological samples.
Supplementary Materials
The following are available online at https://www.mdpi.com/2227-9040/9/3/48/s1, Figure S1: 1H NMR of NL, Figure S2: 13C NMR of NL, Figure S3: FT-MS of NL, Figure S4: FT-MS of NL-Fe3+ complex, Figure S5: IR of NL-Fe3+ complex, Figure S6: UV-Vis of NL-Fe3+ complex, Figure S7: FT-MS by adding Fe3+ into NL, Figure S8: FT-MS of NL-Fe3+ + PPi, Figure S9: Fluorescence spectra of NL at different times, Figure S10: Fluorescence intensities of NL as a function of Fe3+ concentration, Figure S11: Fluorescence response of NL to Fe3+ in the presence of other common metal ions, Figure S12: The Benesi–Hildebrand of NL with Fe3+ by UV-Vis spectroscopy, Figure S13: The Job’s plot of the reaction between NL and Fe3+ by UV-Vis spectroscopy, Figure S14: Fluorescence response of NL-Fe3+ in the presence of various analytes, Figure S15: Fluorescence spectra of NL, sequential upon addition of Fe3+ and PPi, Figure S16: The linear responses of NL-Fe3+ versus the concentration of PPi, Figure S17: The decomplexation constant of NL-Fe3+ toward PPi by fluorescence titration, Figure S18: The fluorescence response time of NL-Fe3+ in the presence of PPi, Figure S19: The decomplexation constant of NL-Fe3+ toward PPi by UV-Vis spectroscopy, Figure S20: Fluorescence intensity of NL-Fe3+ in the absence and presence of PPi at various pH values, Figure S21: Cell viability values assessed using an MTT proliferation test versus incubation concentrations of NL, Figure S22: MTT assay of Hep G2 cells treated with NL-Fe3+.
Author Contributions
Conceptualization and methodology, W.W. and Y.G.; investigation, H.Z., B.Z., H.L., and Q.L.; writing—original draft preparation, W.W. and Y.G.; writing—review and editing, Y.G.; funding acquisition, B.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (21506106) and the Key Laboratory Project of Liaoning Province (2008S127).
Acknowledgments
We thank Yanan Lei at the School of Chemical and Environmental Engineering, Shanghai Institute of Technology for their help in the cell imaging experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lee, G.J.; Marks, J. Intestinal phosphate transport: A therapeutic target in chronic kidney disease and beyond. Pediatr. Nephrol. 2015, 30, 363–371. [Google Scholar] [CrossRef]
- Hansen, N.M.; Felix, R.; Bisaz, S.; Fleisch, H. Aggregation of hydroxyapatite crystals. Biochim. Biophys. Acta 1976, 451, 549–559. [Google Scholar] [CrossRef]
- Florence, W.L.T. Genetics and mechanisms of crystal deposition in calcium pyrophosphate deposition disease. Curr. Rheumatol. Rep. 2012, 14, 155–160. [Google Scholar]
- Costello, J.C.; Rosenthal, A.K.; Kurup, I.V.; Masuda, I.; Medhora, M.; Ryan, L.M. Parallel regulation of extracellular ATP and inorganic pyrophosphate: Roles of growth factors, transduction modulators, and ANK. Connect. Tissue Res. 2011, 52, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Ghosh, P.; Gharami, S.; Kumar Mondal, T.; Murmu, N. A novel coumarin based molecular switch for the sequential detection of Al3+ and F−: Application in lung cancer live imagine and construction of logic gate. Sens. Actuators B Chem. 2017, 242, 338–346. [Google Scholar] [CrossRef]
- Rosenthal, A.K.; Ryan, L.M. Calcium pyrophosphate deposition disease. N. Engl. J. Med. 2016, 374, 2575–2584. [Google Scholar] [CrossRef]
- Yang, X.F.; Xie, L.J.; Ning, R.; Gong, X.Q.; Liu, Z.; Li, Y.X.; Zheng, L.Y.; Zhang, G.G.; Gao, B.; Cui, Y.; et al. A diketopyrrolopyrrole-based near-infrared sensor for selective recognition of fluoride ions. Sens. Actuators B Chem. 2015, 210, 784–794. [Google Scholar] [CrossRef]
- Yasuhiro, S.; Rikako, N.; Shunsuke, T.; Chiharu, Y.; Takayuki, H. A naphthalimide-sulfonylhydrazine conjugate as a fluorescent chemodosimeter for hypochlorite. Chemosensors 2020, 8, 123–135. [Google Scholar]
- Miao, R.; Zheng, Q.Y.; Chen, C.F.; Huang, Z.T. A novel calix [4] arene fluorescent receptor for selective recognition of acetate anion. Tetrahedron Lett. 2005, 46, 2155–2158. [Google Scholar] [CrossRef]
- Kumar, R.; Srivastava, A. Anion binding-induced white light emission using a water-tolerant fluorescent molecular tweezer. Chem. Eur. J. 2016, 22, 3224–3229. [Google Scholar] [CrossRef]
- Hu, Z.Q.; Wang, X.M.; Feng, Y.C.; Ding, L.; Li, M.; Lin, C.S. A novel colorimetric and fluorescent chemosensor for acetate ions in aqueous media based on a rhodamine 6G-phenylurea conjugate in the presence of Fe(III) ions. Chem. Commun. 2011, 47, 1622–1624. [Google Scholar] [CrossRef]
- Bhalla, V.; Vij, V.; Kumar, M.; Sharma, P.R.; Kaur, T. Recognition of adenosine monophosphate and H2PO4− using zinc ensemble of new hexaphenylbenzene derivative: Potential bioprobe and multichannel keypad system. Org. Lett. 2012, 14, 1012–1015. [Google Scholar] [CrossRef]
- Nadella, S.; Sahoo, J.; Subramanian, P.S.; Sahu, A.; Mishra, S.; Albrecht, M. Sensing of phosphates by using luminescent EuIII and TbIII complexes: Application to the microalgal cell Chlorella vulgaris. Chem. Eur. J. 2014, 20, 6047–6053. [Google Scholar] [CrossRef]
- Ganjali, M.R.; Hosseini, M.; Memari, Z.; Faridbod, F.; Norouzi, P.; Goldooz, H.; Badiei, A. Selective recognition of monohydrogen phosphate by fluorescence enhancement of a new cerium complex. Anal. Chim. Acta 2011, 708, 107–110. [Google Scholar] [CrossRef]
- Zhang, M.; Lu, W.; Zhou, J.; Du, G.; Jiang, L.; Ling, J.; Shen, Z. A simple and effective fluorescent chemosensor for the cascade recognition of Zn2+ and H2PO4− ions in protic media. Tetrahedron 2014, 70, 1011–1015. [Google Scholar] [CrossRef]
- Rao, A.S.; Singha, S.; Choi, W.; Ahn, K.H. Studies on acedan-based mononuclear zinc complexes toward selective fluorescent probes for pyrophosphate. Org. Biomol. Chem. 2012, 10, 8410–8417. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.Y.; Li, K.; Zhang, W.; Liu, Y.H.; Huang, Z.; Yu, X.Q. Cd(II)-terpyridine-based complex as a ratiometric fluorescent probe for pyrophosphate detection in solution and as an imaging agent in living cells. Dalton Trans. 2015, 44, 1358–1365. [Google Scholar] [CrossRef] [PubMed]
- Anbu, S.; Kamalraj, S.; Paul, A.; Jayabaskaran, C.; Pombeiro, A.J.L. The phenanthroimidazole-based dizinc (II) complex as a fluorescent probe for the pyrophosphate ion as generated in polymerase chain reactions and pyrosequencing. Dalton Trans. 2015, 44, 3930–3933. [Google Scholar] [PubMed]
- Roy, B.; Rao, A.S.; Ahn, K.H. Mononuclear Zn(II) and Cu(II) complexes of a hydroxynaphthalene-derived dipicolylamine: Fluorescent sensing behaviours toward pyrophosphate ions. Org. Biomol. Chem. 2011, 9, 7774–7779. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, J.; Liu, Q.L.; Gao, Y.; Liu, H.M.; Zhao, B. A highly selective coumarin-based chemosensor for the sequential detection of Fe3+ and pyrophosphate and its application in living cell imaging. Tetrahedron Lett. 2018, 59, 1860–1865. [Google Scholar] [CrossRef]
- Bender, D.R.; Kanne, D.; Frazier, J.D.; Rapoport, H. Synthesis and Derivitization of 8-Acetylpsoralens. Acetyl Migrations during Claisen Rearrangement. J. Org. Chem. 1983, 48, 2709–2719. [Google Scholar]
- Wang, W.; Wu, M.; Liu, H.M.; Liu, Q.L.; Gao, Y. A novel on-off-on fluorescent chemosensor for relay detection of Fe3+ and PPi in aqueous solution and living cells. Tetrahedron Lett. 2019, 60, 1631–1635. [Google Scholar] [CrossRef]
- Benesi, H.A.; Hildebrand, J.H. A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- Beneto, A.J.; Thiagarajan, V.; Siva, A. A tunable ratiometric pH sensor based on phenanthro [9, 10–d] imidazole covalently linked with vinylpyridine. RSC Adv. 2015, 83, 67849–67852. [Google Scholar] [CrossRef]
- Yan, L.Q.; Ma, Y.; Cui, M.F.; Qi, Z.J. A novel coumarin-based fluorescence chemosensor containing L-histidine for aluminium(III) ions in aqueous solution. Anal. Methods 2015, 7, 6133–6138. [Google Scholar] [CrossRef]
- Job, P. Formation and Stability of Inorganic Complexes in Solution. Ann. Chim. 1928, 9, 113–203. [Google Scholar]
- Zhang, R.; Song, B.; Dai, Z.; Ye, Z.; Xiao, Y.; Liu, Y.; Yuan, J. Highly sensitive and selective phosphorescent chemosensors for hypochlorous acid based on ruthenium(II) complexes. Biosens. Bioelectron. 2013, 50, 1–7. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).