Abstract
Wastewaters are considered one of the main sources of pollution in the aquatic environment as release a large number of contaminants every day. Emerging contaminants such as pharmaceuticals have special interest due to the high levels of consumption by the global population, their bioactive properties and because actual directives do not include the monitoring of pharmaceuticals. Moreover, it is well-known that pharmaceuticals can be degraded to metabolites or transformation products (TPs), which could be more toxic than the parental compound. In this study, we have developed an analytical method based on solid-phase extraction (SPE) and liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) to determine 76 highly consumed pharmaceuticals, including metabolites and TPs in wastewater effluents. In the 10 wastewaters analysed, the mean concentrations were in the µg L−1 levels, being mycophenolic acid, levodopa, ibuprofen, 4-aminoantypirine, losartan, amylmetacresol, amoxicillin, fluticasone, tramadol, budesonide, chlorpheniramine and diclofenac the pharmaceuticals with the highest concentrations. This study provides a comprehensive optimization on the MS conditions to determine pharmaceutical compounds and their metabolites and provides a spectral characterization to be used for the identification of these compounds in water.
1. Introduction
In the last years, pharmaceuticals and personal care products (PPCPs), classified as Emerging Organic Contaminants (EOC), have affected the environment integrity because of their pseudo-persistent properties and because they are continuously discharged to the environment as they are non-regulated contaminants [1]. PPCPs of different families are detected in treated or incompletely treated wastewater and in receiving waters in a recurrent way [2]. Sewage waters collected across the urban sewage system contain PPCPs that have been excreted by the population and are directed to wastewater treatment plants (WWTPs), which are generally not equipped to remove these pollutants. For this reason, those contaminants remain biologically active for long periods and have been found in effluents of WWTPs and in surface waters [3,4,5,6]. Actual Directives do not include the monitoring of PPCPs, although the European Commission aims at implementing a Strategic Approach to Pharmaceuticals in the Environment to counteract the negative effects of pharmaceuticals on the environment, covering their whole lifecycle from design and production to disposal (http://www.waterjpi.eu/resources/newsletter/copy_of_2019/newsletter-december-2020/pharmaceuticals-in-the-environment-aquaticpollutants-expected-to-contribute-to-the-eu-strategic-approach-to-reduce-their-adverse-effects, accessed on 22 June 2021).
Pharmaceuticals have been investigated in the aquatic environment throughout the last 20 years [7,8,9]. However, less attention has been paid to the analysis of metabolites or transformation products (TPs) [10,11,12]. The chemical structure of Active Pharmaceutical Ingredients (APIs) can be changed by biotransformation, biodegradation and non-biotic transformation, resulting in a change in their physicochemical and pharmaceutical properties. Metabolites are defined as compounds resulting from structural changes of pharmaceuticals within the human body, as biochemical processes. Moreover, metabolites are formed by enzymes and bacterial activity [13]. The biotransformation of APIs is the introduction of a functional group such as hydroxy, carboxyl, amine or sulfhydryl which increase hydrophilicity and then is followed by the conjugation with polar molecules like glucuronic acid, acetate esters, carboxamides or sulphate [14]. On the other side, TPs are molecules formed under environmental conditions after the excretion of parent compounds and their metabolites through processes of hydrolysis, light, oxidation or biotic process. In addition, compounds resulting from chemical reactions in technical facilities like sewage and drinking water treatment plants are considered TPs.
The advances in the sensibility of the analytical techniques have played an important role in the detection of EOCs at very low concentrations in water [2]. Different instrumental techniques are used for the identification and determination of pharmaceuticals in the environment. However, the most used analytical technique to identify PPCPs is LC-MS/MS with electrospray ionization (ESI), due to the high selectivity and sensitivity [15].
Thus, the aim of this study was to develop an analytical method for the simultaneous determination of 76 pharmaceuticals including related metabolites and a TP using solid-phase extraction (SPE) and liquid chromatography coupled to mass spectrometry (LC-MS/MS). Moreover, the presence of these pharmaceuticals in effluents of WWTP was determined as a means to prove the applicability of the developed methodology.
2. Experimental
2.1. Chemicals and Reagents
Table S1 displays the 76 pharmaceuticals, metabolites and a TP studied classified following their Anatomical Therapeutic Chemical Classification System (ATC) code. All pharmaceutical standards of 98–99% of purity were purchased from Sigma-Aldrich (St. Louis, MO, USA). Moreover, ibuprofen-d3, diclofenac-acetophenyl ring-13C6, atenolol-d7, lidocaine-diethyl-d10, acetaminophen-methyl-d3, acetylsalicylic acid-methyl-d3, propranolol-d7, carbamazepine-13C6, gabapentin-13C3 and L-dopa-phenyl-d3 were also acquired from Sigma and were used as internal standard mixture (ISM). HPLC grade methanol (MeOH) and acetonitrile (ACN) were supplied by VWR Chemicals Prolabo (Leuven, Belgium). Ammonium hydroxide (NH4OH) and ammonium formate (NH4COOH) were supplied by Sigma-Aldrich (St. Louis, MO, USA). Formic acid (HCOOH) and hydrochloric acid 37% (HCl) were supplied by Fisher Scientific Chemical (Bridgewater, MA, USA) and Panreac AppliChem (Darmstadt, Germany), respectively. Finally, ultra-pure Milli-Q water was obtained through a Millipore purification system (Millipore, Bedford, MA, USA). Stock standard solutions were prepared at a concentration of 1000 ng µL−1 in methanol and working solutions were prepared at 10 and 100 ng µL−1 in 90% of Milli-Q® water and 10% of methanol. Table S2 shows the main physicochemical properties of all target compounds.
2.2. Wastewaters: Sampling, Pretreatment and Extraction
Ten effluent wastewaters (WW1–WW10) were sampled on different days in April 2021 in a WWTP located in a small coastal village close to Barcelona (41°14′20″ N 1°46′23″ E), Catalonia, Spain. The sampling consisted of twenty-four-hour composite samples collected using an automated position sample collector. Wastewater samples were kept in the fridge until analysis, to prevent the possible degradation of pharmaceuticals. Once in the laboratory, wastewaters were filtered using 0.22 µm nylon filters to remove the solid particulate phase.
The method was optimized using wastewater spiked at 4 µg L−1 of a mix of all target compounds and the ISM at 0.25 µg L−1, which were used as extraction and analytical control. Then, solid-phase extraction (SPE) Oasis HLB cartridges (200 mg, 6 cc, Waters, Mildford, MA, USA) were used to preconcentrate target analytes. Two different pHs (2 and 7, adjusted with HCl 37% with a pH meter SensionTM + PH3, HACH®, Colorado, CO, USA) and three different elution conditions were tested to concentrate 50 mL of water. The cartridges were conditioned with 6 mL of MeOH and 6 mL of Milli-Q® water, and the sample was loaded at flow 1 mL min−1. Once preconcentrated, cartridges were dried over 15–20 min at room temperature and then eluted with different conditions. Test A consisted of the elution of 2 × 6 mL MeOH; Test B consisted of the elution with 6 mL MeOH and 6 mL MeOH + 0.1% formic acid; and Test C consisted of the elution of 4 mL MeOH, 4 mL MeOH + 0.1% formic acid and 4 mL MeOH + 0.1% NH4OH. At pH 7, only the elution Test A and Test C were tested. After the elution, the extracts were evaporated to almost dryness under a current of N2, and then, transferred to a chromatographic vial with 1 mL MeOH as washing solvent. Finally, samples were evaporated with a ReactiVap® until dryness and reconstituted with 200 µL of a 10:90 (v/v) MeOH:Milli-Q® water solution.
2.3. LC-MS/MS Analysis
Pharmaceuticals were measured using a liquid chromatography connected to a triple quadrupole mass spectrometer (Xevo TQS, Acquity H-Class, Waters, Milford, CT, USA) (LC-MS/MS). For the chromatographic separation, a CORTECS T3 column (100 mm × 2.1 mm, particle size 1.6 µm, Waters, Milford, CT, USA) was used. Mobile phase composition consisted of binary mixtures with 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B), following a previously published study [16]. Gradient elution started at 95% A and 5% B, increasing to 60% B in 7 min, to 50% B in 12 min and to 100% of B in 3 min, held for 3 min and returned to initial conditions in 2 min, with a holding time of 5 min. Flow rates of 300, 350 and 400 µL min−1 were tested in order to improve the resolution and selectivity of target compounds and 300 µL min−1 was the best option. In all cases, 5 µL were injected. Most of the compounds were measured under positive electrospray ionization (ESI+), except acetylsalicylic acid, furosemide and hydrochlorothiazide that were detected in negative electrospray ionization (ESI−). Cone voltage (C.V.) was optimized from 1 to 90 V to obtain the precursor ion for each target compound using flow injection analysis (FIA). Moreover, in order to obtain the two intense fragment ions, the collision energy (C.E.) was optimized from 1 to 40 eV. Following the acquisition by selected reaction monitoring (SRM), two transitions from the precursor ion to the product ion were used in order to identify each compound. The optimal parameters are displayed in Table 1 for pharmaceuticals, metabolites, the TP and internal standards. The desolvation temperature was set at 350 °C whereas the desolvation gas flow and the cone gas flow were optimized at 900 L h−1 and 150 L h−1, respectively. The system and data management were processed using MassLynx v4.1 software package.

Table 1.
LC-MS/MS optimized parameters for selected pharmaceuticals (ordered by ATC code), metabolites (*), transformation product (**), and their internal standards (^). C.V.: cone voltage (V); C.E.: collision energy (eV); q1: product ion for quantification; q2: product ion for confirmation.
2.4. Quality Assurance
Calibration was performed over a concentration range from 0.01 to 1 ng µL−1 using six calibration points in MeOH:Milli-Q® water 10:90 (v/v) and ISM at 0.25 µg L−1. Recoveries of pharmaceuticals were estimated using wastewater samples spiked at 4 µg L−1 with the mixture of pharmaceuticals and the ISM. Ten pharmaceutical internal standards were used as extraction and analytical control and external calibration which offered a better response for some pharmaceuticals was used. The instrumental detection limit (IDL) is the minimum amount of analyte required to produce a signal that is statistically distinguishable from the background noise level within a specified confidence level. IDL was determined using the lowest concentration of a standard solution that generated an S/N ratio equal to 3. On the other hand, method detection limit (MDL) was calculated from the injection of spiked wastewater samples at 4 µg L−1 using the minimum concentration of analyte providing an S/N ratio of 3 for the MDL and a ratio of 10 for the LOQ. Moreover, the precision of the method was determined by the estimation of the intra-day assay, expressed as the percentage relative standard deviation (%RSD) of replicate measurements. The variation was assessed by five consecutive injections of 0.1 ng µL−1 standard solution. Finally, matrix effect (ME) was assessed in order to evaluate the degree of signal suppression or enhancement. The ME was calculated by dividing the areas of each pharmaceutical in a solution in wastewater following Equation (1). Values of 100% indicate that there is no matrix effect. However, values higher than 100% means ion enhancement whereas values lower than 100% indicate ion suppression.
where A is the peak area of each analyte from spiked wastewater samples; B is the peak area of each analyte from non-spiked wastewater; and C is the peak area of each analyte in the standard solution. Table 2 displays the quality parameters studied.

Table 2.
Quality parameters obtained for 76 compounds ordered following de ATC code for the pharmaceuticals, metabolites (*) and transformation product (**).
3. Results and Discussion
3.1. Optimization of the Ionization Parameters
Optimization of the ionization conditions was performed using LC-MS/MS in ESI+ and ESI−. For the large screening of PPCPs, the pH of the mobile phase affects the ionization, where acidic pH obtains molecular ions that are charged positively ([M + H]+) and basic pH leads to negative ions ([M − H]−). Both modes can work together, as some molecules may be ionized in positive and negative mode simultaneously, obtaining both mass spectrums, one with higher intensity than the other. Table 1 summarizes the precursor ions and the two most intense fragments, including the optimum C.V. and C.E. voltages for each target compound. C.V. was the major parameter influencing the intensity of signal ions and it was adjusted from 6 to 96 V to achieve the best response for the molecular ion. Then, the molecular ion was fragmented to produce intense product ions, using the C.E which was optimized from 5 to 46 eV. The two most abundant fragments were monitored for each compound, one for quantification purposes and the second for confirmation. Mass spectral characterization for target compounds is included in the Supplementary information, classified by ATC code for selected pharmaceuticals, including their metabolites, and the internal standards. Table S3 shows the MRM transition of the compounds studied. The section “Mass spectral characterization” has been added in the supplementary information describing the fragmentation pattern of each compound.
3.2. Optimization of the Chromatographic Conditions
In order to achieve the optimum resolution and identification of target compounds, the mobile phase composition and gradient conditions were tested using a CORTECS T3 column (Waters, Mildford, OH, USA) using a standard solution at a concentration of 1 ng µL−1. In all the experiments, the mobile phase composition was formed by 0.1% formic acid in water (A) and 0.1% formic acid in ACN (B). Firstly, the composition of the standards was 100% of methanol but results showed that part of the compounds were not retained in the stationary phase, and presented wide peaks. In addition, for some compounds, two or three peaks were formed which means low interaction between the stationary phase and the mobile phase with the compounds. Moreover, for acetylsalicylic acid and hydrochlorothiazide analysed in ESI−, no-signal was observed. Secondly, different proportions of formic acid (0.1%, 0.5% and 1.0%) were added to the standard made in methanol in order to increase the affinity between the stationary phase and the analytes, and the ionization of target compounds. Although the shape of the chromatographic peaks was improved, most of the compounds were not well-retained presenting retention times <1 min. Finally, the last tests were performed changing the proportion of MeOH (10%) and Milli-Q® water (90%), and also, adding 0.1% formic acid at the aqueous phase of standards, providing better results in terms of resolution when high proportions of aqueous solvent were used. However, the addition of formic acid did not represent any improvements in the chromatographic resolution. For this reason, a composition of 10% MeOH and 90% Milli-Q® water was employed, solving the peak splitting, too.
Once the standard solution conditions were optimized, the gradient of the mobile phase composition was tested, following a previously published paper about the presence of pharmaceuticals in wastewaters from senior residences [16]. As indicated before, the mobile phase composition consisted of 0.1% formic acid in water (A) and 0.1% formic acid in ACN (B), testing different gradients. Firstly, Test 1 started at 100% A and increased to 70% B in 12 min, and to 100% of B in 3 min, holding for 5 min with a total run time of 27 min, using a flow of 0.3 mL min−1. Moreover, in this gradient, small variations were performed with the proportion of the mobile phases at 12 min being 70%, 50%, 40%, and 30% of solvent B. On the other hand, Test 2 started at 100% A and increased to 30% B in 7 min, holding 1 min, and increasing to 70% of B in 7 min, holding 1 min, and finally at 100% of B in 4 min with a total run time of 27 min using a flow rate of 0.3 mL min−1. The initial high proportion of the aqueous phase allowed the elution of most water-soluble analytes, whereas low and continuously increasing of the organic mobile phase offer lowest retention time for compounds very soluble in water such as levodopa or paracetamol. Thus, Test 2, reported better results in terms of efficiency and resolution. On the other hand, Test 3 started at 100% A and decreased to 70% A in 7 min, followed by 50% of solvent B in 5 min and to 100% of B in 4 min with a total run time of 28 min using a flow of 0.3 mL min−1. Based on it, some variations were carried out to obtain a good separation and peak resolution. Firstly, different proportions of the mobile phases (95%, 90%, 80%, 70% and 60% of solvent A) were used in minute 7 to adjust the best gradient increase of the organic phase. Secondly, the flow rate of the chromatographic analysis was optimised at 300, 350 and 400 µL min−1, with the same gradient as Test 3. Finally, the best results were obtained with Test 4, starting at 95% A and 5% B, increasing to 40% B in 7 min, followed by 50% of solvent B in 5 min and to 100% of B in 3 min with a total run time of 25 min using a flow of 300 µL min−1, producing better efficiency, resolution and sensitivity for most of pharmaceuticals and metabolites. However, the structural isomers acetylsalicylic acid/salicylic acid, omeprazole/esomeprazole, citalopram/escitalopram and 1-hydroxy ibuprofen/2-hydroxy ibuprofen could not be chromatographically separated due to the same MS transitions. The ion chromatogram of a mixed solution at 1 ng µL−1 containing all the target analytes and using the CORTECS T3 column is shown in Figure 1.


Figure 1.
LC-MS/MS separation of the 76 pharmaceuticals, metabolites and transformation product (TP) with CORTECS T3 column. The compounds acetylsalicylic acid/salicylic acid, omeprazole/esomeprazole, citalopram/escitalopram and 1-hydroxy ibuprofen/2-hydroxy ibuprofen coeluted at the same retention times due to the same MS transitions.
3.3. Optimization of Extraction Procedure and Quality Parameters
Table 2 shows the linearity studied that was in the range of 0.01–1 ng µL−1 for all selected compounds, but different linear responses were obtained, depending on the sensitivity for each analyte: 65 compounds were linear from 0.01 to 1 ng µL−1 with good correlation (R2 ≥ 0.99); pantoprazole, erythromycin, clarithromycin, donepezil and chlorpheniramine were linear in the range of 0.01 to 0.5 ng µL−1; and finally, omeprazole/esomeprazole were linear in the range of 0.01 to 0.1 ng µL−1. The IDLs ranged from 0.02 (propranolol) to 5.87 pg (amylmetacresol), the MDL ranged from 0.4 (levetiracetam) to 40 ng L−1 (dichlorobenzyl alcohol) and the LOQ ranged between 1 to 135 ng L−1. Moreover, the intra-day precision (repeatability) of the method ranged between 1 to 18% (N = 3).
For SPE, different extraction procedures were tested at pH 2 and pH 7 using wastewater. Target compounds present different physicochemical properties linked with their solubility in aqueous samples, so developing two extraction procedures will increase the possibilities to retain most of them. As commented before, at pH 2, three different elution methods were tested to evaluate the efficiency of the elution solvents with Oasis HLB cartridges (200 mg, 6cc, Waters, Mildford, MA, USA); and at pH 7, two different elution methods were tested with the same cartridges. The high complexity of the type of water analysed (wastewater) affected considerably the results. Results showed that Test C allowed achieving the best efficiency as elution solvent at both pH 2 and pH 7, where 76 compounds were recovered in the range of 26% (4-aminoantipyrine) to 160% (hydrochlorothiazide) (Table 2). The matrix effect has significant relevance in LC-MS/MS analysis as signal intensity may be considerably changed affecting the recovery rates of pharmaceuticals in complex wastewater samples. For some target compounds such as acetylsalicylic acid/salicylic acid, amiodarone, verapamil, ezetimibe, dutasteride, N4-acetylsulfamethoxazole, ciprofloxacin, 1-hydroxyibuprofen/2-hydroxyibuprofen, paracetamol, gabapentin, clomethiazole, fluticasone and tiotropium, the presence of matrix compound in the wastewater results in a strong signal suppression presenting values lower than 60%. However, valsartan and diclofenac had signal enhancement, presenting matrix effect values over 120% (see Table 2).
3.4. Presence of Pharmaceutical Residues and Metabolites in Wastewater Samples
To validate the extraction method, 10 wastewater samples were analyzed by SPE and Table 3 displays the levels of the 76 pharmaceuticals and metabolites studied. All compounds were detected in wastewaters except acetylsalicylic acid/salicylic acid, hydrochlorothiazide, furosemide, cyproterone and rasagiline. Figure 2 shows a boxplot in logarithmic scale (in µg L−1) of the pharmaceuticals detected at the highest concentrations in the wastewaters sampled. Mycophenolic acid, an immunosuppressant medication used to prevent rejection following organ transplantation and to treat autoimmune conditions such as Crohn’s disease and lupus, was detected at levels between 1.3 and 23.2 µg L−1 (Table 3 and Figure 2). Recently, Santos et al. developed and validated a single analytical methodology to identify and quantify seven cytostatics in waters and reported levels of mycophenolic acid in effluent samples from a WWTP equipped with a UV disinfection process located in Porto between 0.4 and 0.8 µg L−1 [17]. The authors concluded that the UV disinfection step could contribute to increasing the removal/degradation of mycophenolic acid and other pharmaceuticals in wastewater. Another pharmaceutical with high concentrations was levodopa (L-DOPA), which was detected in wastewaters at levels between <LOQ and 8.8 µg L−1 (see Table 3 and Figure 2). Levodopa is a drug used in the clinical treatment of Parkinson’s disease and dopamine-responsive dystonia. Another pharmaceutical detected with high concentrations was ibuprofen, a nonsteroidal anti-inflammatory drug (NSAID) class widely used for treating pain, fever, and inflammation. Ibuprofen was detected in the 10 wastewater samples at levels between 1.1 and 7.3 µg L−1 (Table 3). A recent review indicates the widespread presence of ibuprofen in wastewaters at µg L−1 [18]. In 2015, Caballo et al. reported levels of ibuprofen between 0.63 and 0.68 µg L−1 in 3 different WWTPs in Córdoba (South of Spain) [19]. Recently, Whang et al. published levels of the enantiomeric fraction of ibuprofen of 0.32 µg L−1 in a wastewater treatment plant located in Yuhong District of Shenyang, China [20]. On the other hand, 4-aminoantipyrine, also known as ampyrone, is a metabolite of aminopyrine with analgesic, anti-inflammatory, and antipyretic properties. 4-aminoantipyrine ranged from 1.9 to 7.4 µg L−1 (Table 3 and Figure 2). To the best of the author’s knowledge, this is the first time that 4-aminoantypirine has been detected in wastewater. Losartan, a medication mainly used to treat high blood pressure and amylmetacresol, with mean levels of 1.82 and 1.81 µg L−1, respectively (Table 3) were also detected. Other pharmaceuticals with mean values over 1 µg L−1 were amoxicillin, fluticasone, tramadol, budesonide, chlorpheniramine and diclofenac.

Table 3.
Levels of 76 pharmaceuticals, metabolites (*) and TP (**) in effluents of wastewater (μg L−1). WW: wastewater.
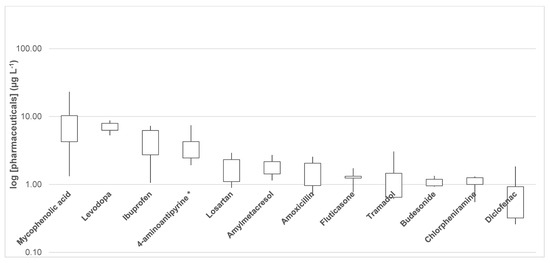
Figure 2.
Boxplot in logarithmic scale (in µg L−1) of eleven pharmaceuticals with highest concentrations in the 10 wastewater samples. * 4-aminoantipyrine is a metabolite of aminopyrine.
The novelty of the present study is the monitoring of pharmaceutical metabolites such as salicylic acid, enalaprilat, N4-acetylsulfamethoxazole, N-(2,6-dichlorophenyl)-2-indoline, 1-hydroxy ibuprofen, 2-hydroxy ibuprofen, 4-aminoantipyrine and carbamazepine 10,11 epoxide. Figure 3 displays the levels of the target metabolites in the wastewater samples. In environmental monitoring, the importance of identifying pharmaceutical metabolites is often disregarded, where biotransformation is assumed to be compared with bioremoval, despite the fact that metabolites can be more harmful than the parent compound [21]. In the present study, the metabolites monitored have been detected at levels of µg L−1, being 4-aminoantipyrine detected at the highest concentrations. On the other hand, carbamazepine 10,11 epoxide, the active metabolite of carbamazepine had levels between 0.50 and 0.81 µg L−1 (Table 3), being the second metabolite with the highest concentrations. In this case, carbamazepine 10,11 epoxide had mean levels (0.63 µg L−1), higher than its parental compound carbamazepine (0.31 µg L−1). Leclercq et al. studied the presence of carbamazepine, oxcarbazepine, and seven of their metabolites (carbamazepine-10,11-epoxide, 10-hydroxy-10,11-dihydrocarbamazepine, 10,11-dihydro-10,11-trans-dihydroxycarbamazepine, 2-hydroxycarbamazepine, iminostilbene, acridine, and acridone) at three different treatment plants in France. Authors reported levels of carbamazepine-10,11-epoxide between 0.008 and 0.029 µg L−1, concluding that metabolites were released in surface water associated with the parent compounds, indicating that aquatic ecosystems are exposed to these molecules [22].
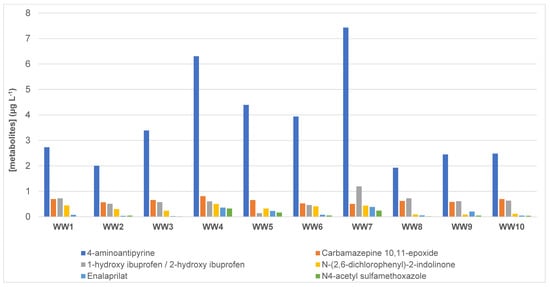
Figure 3.
Concentrations (in µg L−1) of the target metabolites and N-(2.6-dichlorophenyl)-2-indolinone (TP of diclofenac) in wastewater samples. WW: wastewater.
1-hydroxy ibuprofen and 2-hydroxy ibuprofen are the main metabolites of ibuprofen and were in the range of 0.14 to 1.2 µg L−1 (Table 3 and Figure 3). Ferrando-Climent et al. monitored ibuprofen and ibuprofen metabolites in sewage and natural water samples from Girona (Catalonia, Spain), where they were found at higher levels than expected: the maximum concentration in effluent wastewater samples were 1.9, 1.4, 10.7, 5.9 µg L−1 for ibuprofen, 1-hydroxy ibuprofen, ibuprofen carboxylic acid and 2-hydroxyibuprofen, respectively [12].
N-(2,6-dichlorophenyl)-2-indolinone is the main transformation product (TP) produced by the degradation of diclofenac when exposed to UV light [23] and was detected between 0.086 and 0.50 µg L−1 (Table 3 and Figure 3). To the best of the author’s knowledge, this is the first time that this TP is monitored in effluents of wastewater. On the other hand, enalaprilat is the active metabolite of the orally available pro-drug, enalapril. It is an antihypertensive agent used for the management of hypertension when oral therapy is not practical. Enalaprilat was detected at levels between 0.026 and 0.39 µg L−1. In 2014, Kostich et al. measured concentrations of 56 active pharmaceutical ingredients (APIs) in effluent samples from 50 large wastewater treatment plants across the US. The authors reported limits of enalaprilat around 0.009 µg L−1 and predicted environmental concentrations (PECs) of 0.369 µg L−1 [24,25]. Finally, N4-acetylsulfamethoxazole, which is a metabolite of the sulfonamide bacteriostatic antibiotic sulfamethoxazole excreted in urine and can be used as a probe for the molecular percentage enrichment of liver extramitochondrial acetyl-CoA. In the present study, N4-acetylsulfamethoxazole has been detected in the range of <LOQ to 0.32 µg L−1 (Table 3).
4. Conclusions
A comprehensive optimization of a solid phase extraction method followed by LC-MS/MS was performed for the unequivocal identification of 76 pharmaceuticals, metabolites and N-(2,6-dichlorophenyl)-2-indolinone as the main TP of diclofenac in wastewater effluents. The analytical performance of the LC-MS/MS method provides high selectivity and sensitivity and enhanced identification capabilities. In the 10 wastewater samples analyzed, only five drugs gave results below the limit of quantification. Mycophenolic acid, a cytostatic drug, was the pharmaceutical detected at the highest concentrations with levels between 1.3 and 23.2 µg L−1, followed by levodopa and ibuprofen with levels between 1.1 and 8.8 µg L−1. Moreover, pharmaceutical metabolites such as enalaprilat, N4-acetylsulfamethoxazole, 1-hydroxiibuprofen, 2-hydroxiibuprofen, 4-aminoantipyrine and carbamazepine 10,11 epoxide and N-(2,6-dichlorophenyl)-2-indoline as TP have been detected in effluents of wastewater at levels of µg L−1. Thus, the presence of pharmaceuticals and related metabolites in wastewater represents a high impact source of pollution of the aquatic environment.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/chemosensors9100273/s1, Table S1: Anatomical Therapeutic Chemical classification system (ATC) of target pharmaceuticals, Table S2: Physicochemical properties of the 76 pharmaceuticals, metabolites (*) and transformation product (**) studied. Mw: molecular weight. Values have been reported from EPI Suite, Table S3: MS/MS fragmentation of the pharmaceuticals ordered following de ATC code for the pharmaceuticals; and including their metabolites, transformation product and internal standard (*).
Author Contributions
S.E.: Investigation, Methodology; N.R.: Investigation, Methodology; G.G.: Investigation; S.L.: Writing—review and editing; C.G.-C.: Conceptualization, Resources, Supervision, Funding acquisition, Writing—Review and Editing, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universitat Ramon Llull (project 2021-URL-Proj-024; C.G.-C.) and the Spanish Government (PID2020-113371RA-C22; C.G-C and PID2019-105732GB-C21, S.L.).
Acknowledgments
Authors acknowledge the personnel of the WWTP studied for their kind support in the sampling of wastewaters.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Daughton, C.G. Non-regulated water contaminants: Emerging research. Environ. Impact Assess. Rev. 2004, 24, 711–732. [Google Scholar] [CrossRef]
- Richardson, S.D.; Ternes, T.A. Water analysis: Emerging contaminants and current issues. Anal. Chem. 2011, 83, 4616–4648. [Google Scholar] [CrossRef]
- Gurke, R.; Rossmann, J.; Schubert, S.; Sandmann, T.; Rößler, M.; Oertel, R.; Fauler, J. Development of a SPE-HPLC-MS/MS method for the determination of most prescribed pharmaceuticals and related metabolites in urban sewage samples. J. Chromatogr. B 2015, 990, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Babié, S.; Pavlovic, D.M.; Asperger, D.; Perisa, M.; Zrneié, M.; Horvat, A.J.M.; Kastelan-Macan, M. Determination of multi-class pharmaceuticals in wastewater by liquid chromatography–tandem mass spectrometry (LC-MS–MS). Anal. Bioanal. Chem. 2010, 1185–1194. [Google Scholar] [CrossRef]
- Paíga, P.; Santos, L.H.M.L.M.; Delerue-Matos, C. Development of a multi-residue method for the determination of human and veterinary pharmaceuticals and some of their metabolites in aqueous environmental matrices by SPE-UHPLC–MS/MS. J. Pharm. Biomed. Anal. 2017, 135, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Nödler, K.; Licha, T.; Bester, K.; Sauter, M. Development of a multi-residue analytical method, based on liquid chromatography–tandem mass spectrometry, for the simultaneous determination of 46 micro-contaminants in aqueous samples. J. Chromatogr. A 2010, 1217, 6511–6521. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, V.K.; Terry, K.A.; Toito, J. Determination of sulfonamide antibiotics in wastewater: A comparison of solid phase microextraction and solid phase extraction methods. J. Chromatogr. A 2006, 1131, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pedrouzo, M.; Borrull, F.; Marcé, R.M.; Pocurull, E. Simultaneous determination of macrolides, sulfonamides, and other pharmaceuticals in water samples by solid-phase extraction and LC-(ESI) MS. J. Sep. Sci. 2008, 31, 2182–2188. [Google Scholar] [CrossRef]
- Gracia-lor, E.; Sancho, J.V.; Hernández, F. Multi-class determination of around 50 pharmaceuticals, including 26 antibiotics, in environmental and wastewater samples by ultra-high performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 2264–2275. [Google Scholar] [CrossRef]
- Gracia-Lor, E.; Ibáñez, M.; Zamora, T.; Sancho, J.V.; Hernández, F. Investigation of pharmaceutical metabolites in environmental waters by LC-MS/MS. Environ. Sci. Pollut. Res. 2014, 21, 5496–5510. [Google Scholar] [CrossRef] [Green Version]
- Miao, X.S.; Yang, J.J.; Metcalfe, C.D. Carbamazepine and its metabolites in wastewater and in biosolids in a municipal wastewater treatment plant. Environ. Sci. Technol. 2005, 39, 7469–7475. [Google Scholar] [CrossRef] [PubMed]
- Ferrando-Climent, L.; Collado, N.; Buttiglieri, G.; Gros, M.; Rodriguez-Roda, I.; Rodriguez-Mozaz, S.; Barceló, D. Comprehensive study of ibuprofen and its metabolites in activated sludge batch experiments and aquatic environment. Sci. Total Environ. 2012, 438, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. The presence of pharmaceuticals in the environment due to human use-present knowledge and future challenges. J. Environ. Manag. 2009, 90, 2354–2366. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Drewek, A.; Klimaszyk, P. Pharmaceutical pollution of aquatic environment: An emerging and enormous challenge. Limnol. Rev. 2017, 17, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.L.; Zhou, J.L. Simultaneous determination of various pharmaceutical compounds in water by solid-phase extraction-liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2007, 1154, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Canela, C.; Sala-Comorera, T.; Pueyo, V.; Barata, C.; Lacorte, S. Analysis of 44 pharmaceuticals consumed by elderly using liquid chromatography coupled to tandem mass spectrometry. J. Pharm. Biomed. Anal. 2019, 168, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.S.F.; Franquet-Griell, H.; Alves, A.; Lacorte, S. Development of an analytical methodology for the analysis of priority cytostatics in water. Sci. Total Environ. 2018, 645, 1264–1272. [Google Scholar] [CrossRef]
- Petrie, B.; Camacho-Muñoz, D. Analysis, Fate and Toxicity of Chiral Non-Steroidal Anti-Inflammatory Drugs in Wastewaters and the Environment: A Review; Springer: Berlin/Heidelberg, Germany, 2021; Volume 19, ISBN 0123456789. [Google Scholar]
- Caballo, C.; Sicilia, M.D.; Rubio, S. Enantioselective determination of representative profens in wastewater by a single-step sample treatment and chiral liquid chromatography-tandem mass spectrometry. Talanta 2014, 134, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, P.; Zhu, B.; Jiang, Z.; Guo, X. Magnetic solid-phase extraction based on Fe3O4/graphene nanocomposites for enantioselective determination of representative profens in the environmental water samples and molecular docking study on adsorption mechanism of graphene. J. Pharm. Biomed. Anal. 2018, 156, 88–96. [Google Scholar] [CrossRef]
- Nguyen, P.Y.; Carvalho, G.; Reis, M.A.M.; Oehmen, A. A review of the biotransformations of priority pharmaceuticals in biological wastewater treatment processes. Water Res. 2021, 188, 116446. [Google Scholar] [CrossRef]
- Leclercq, M.; Mathieu, O.; Gomez, E.; Casellas, C.; Fenet, H.; Hillaire-Buys, D. Presence and fate of carbamazepine, oxcarbazepine, and seven of their metabolites at wastewater treatment plants. Arch. Environ. Contam. Toxicol. 2009, 56, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Ameri, A.; Shakibaie, M.; Pournamdari, M.; Ameri, A.; Foroutanfar, A. Journal of Photochemistry & Photobiology A: Chemistry Degradation of diclofenac sodium using UV/biogenic selenium nanoparticles/H2O2: Optimization of process parameters. J. Photochem. Photobiol. A Chem. 2020, 392, 112382. [Google Scholar] [CrossRef]
- Kostich, M.S.; Batt, A.L.; Lazorchak, J.M. Concentrations of prioritized pharmaceuticals in effluents from 50 large wastewater treatment plants in the US and implications for risk estimation. Environ. Pollut. 2014, 184, 354–359. [Google Scholar] [CrossRef]
- Kostich, M.S.; Lazorchak, J.M. Risks to aquatic organisms posed by human pharmaceutical use. Sci. Total Environ. 2008, 389, 329–339. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).