Modified Diamide and Phosphine Oxide Extracting Compounds as Membrane Components for Cross-Sensitive Chemical Sensors
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
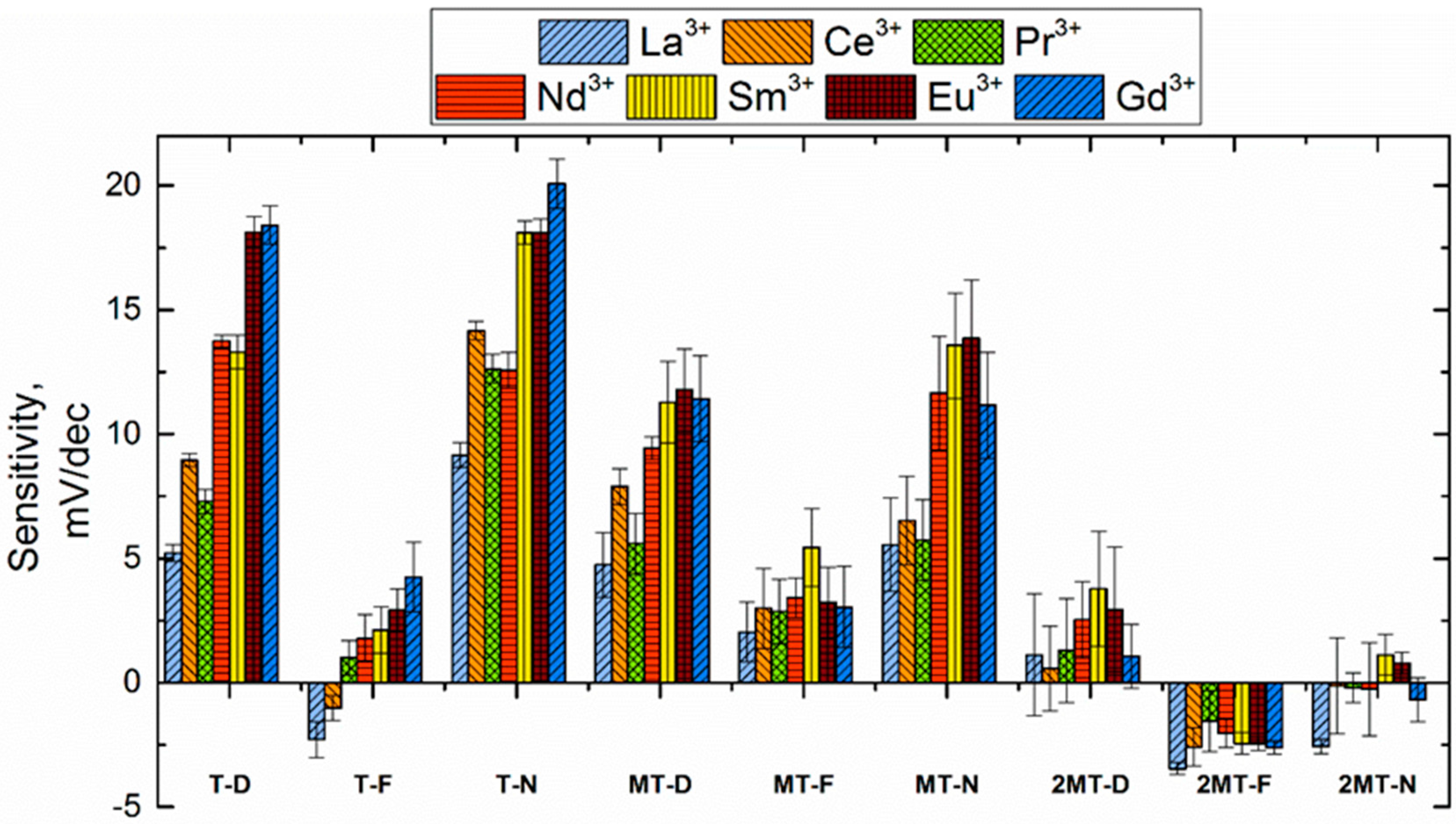
3.1. Sensitivity Towards Single-Charged Ions
3.2. Sensitivity Towards Double-Charged Ions
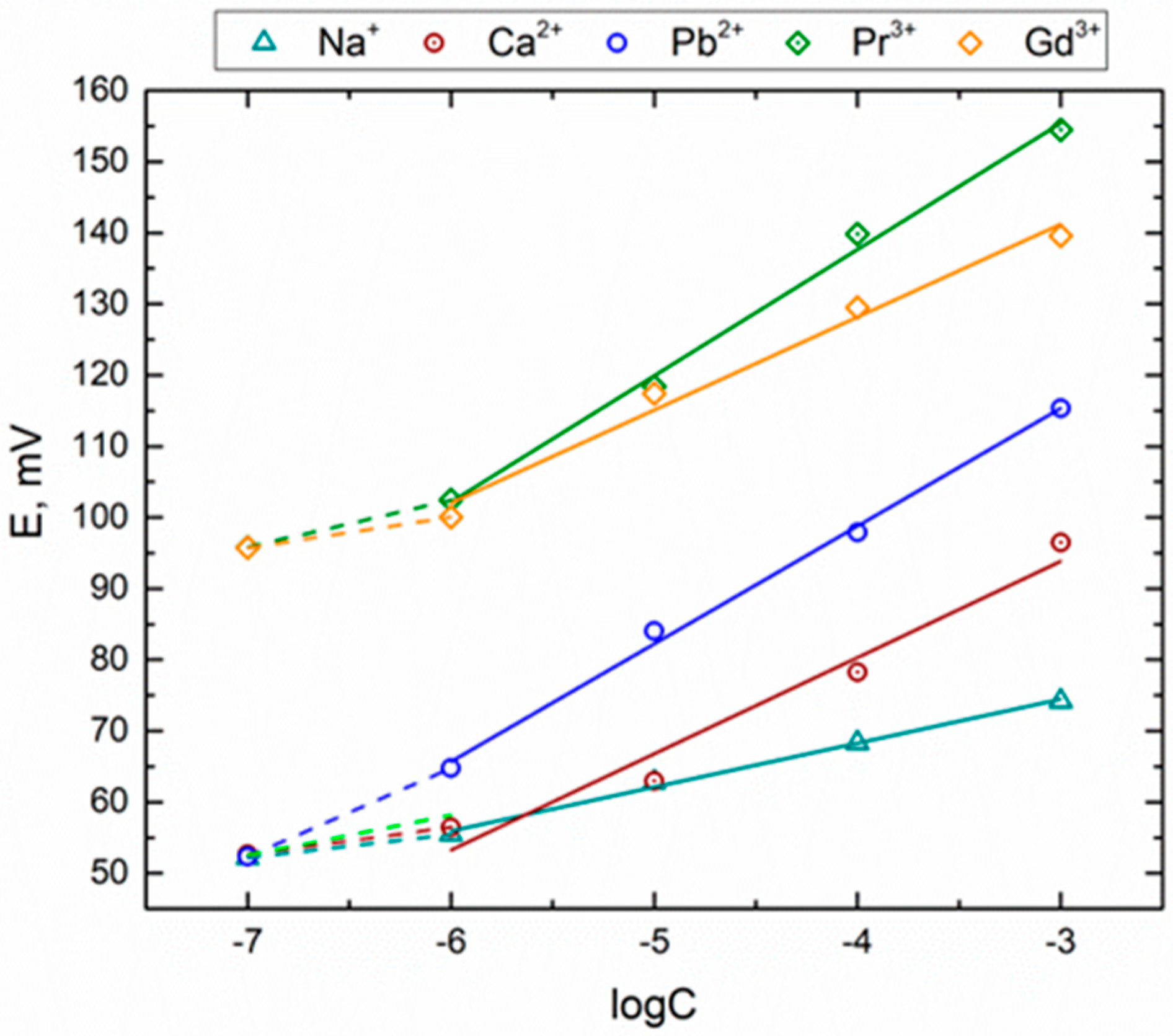
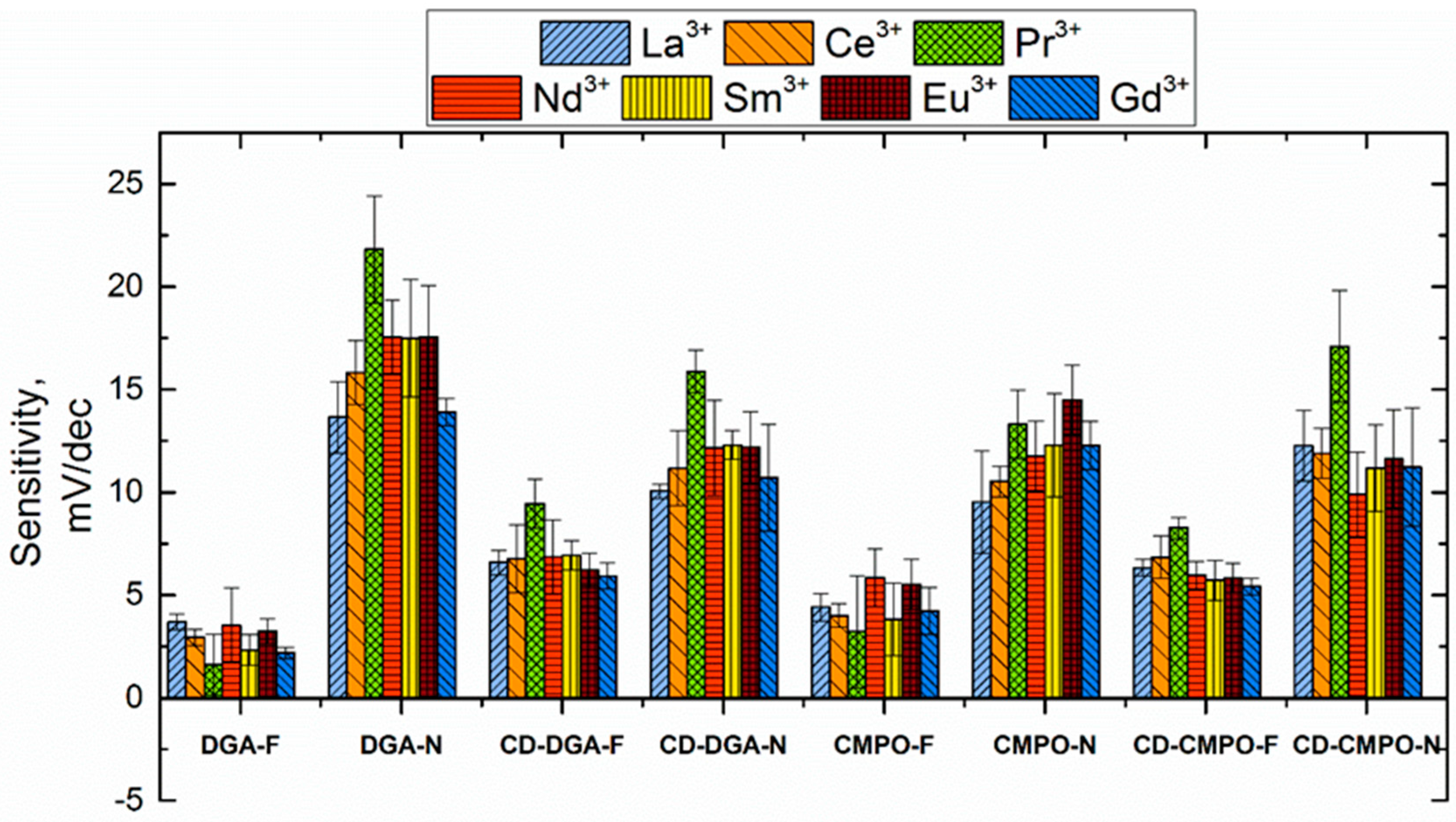
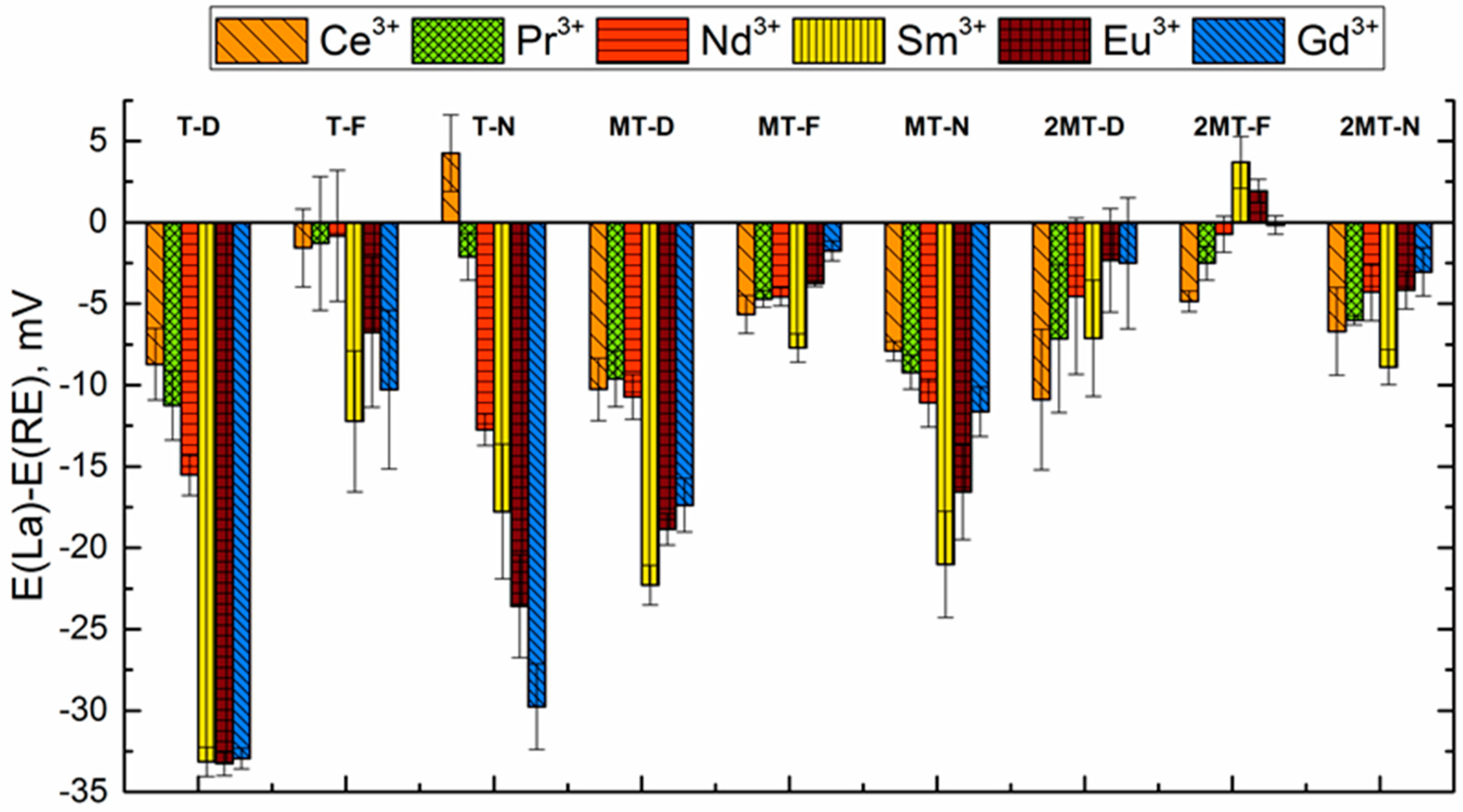
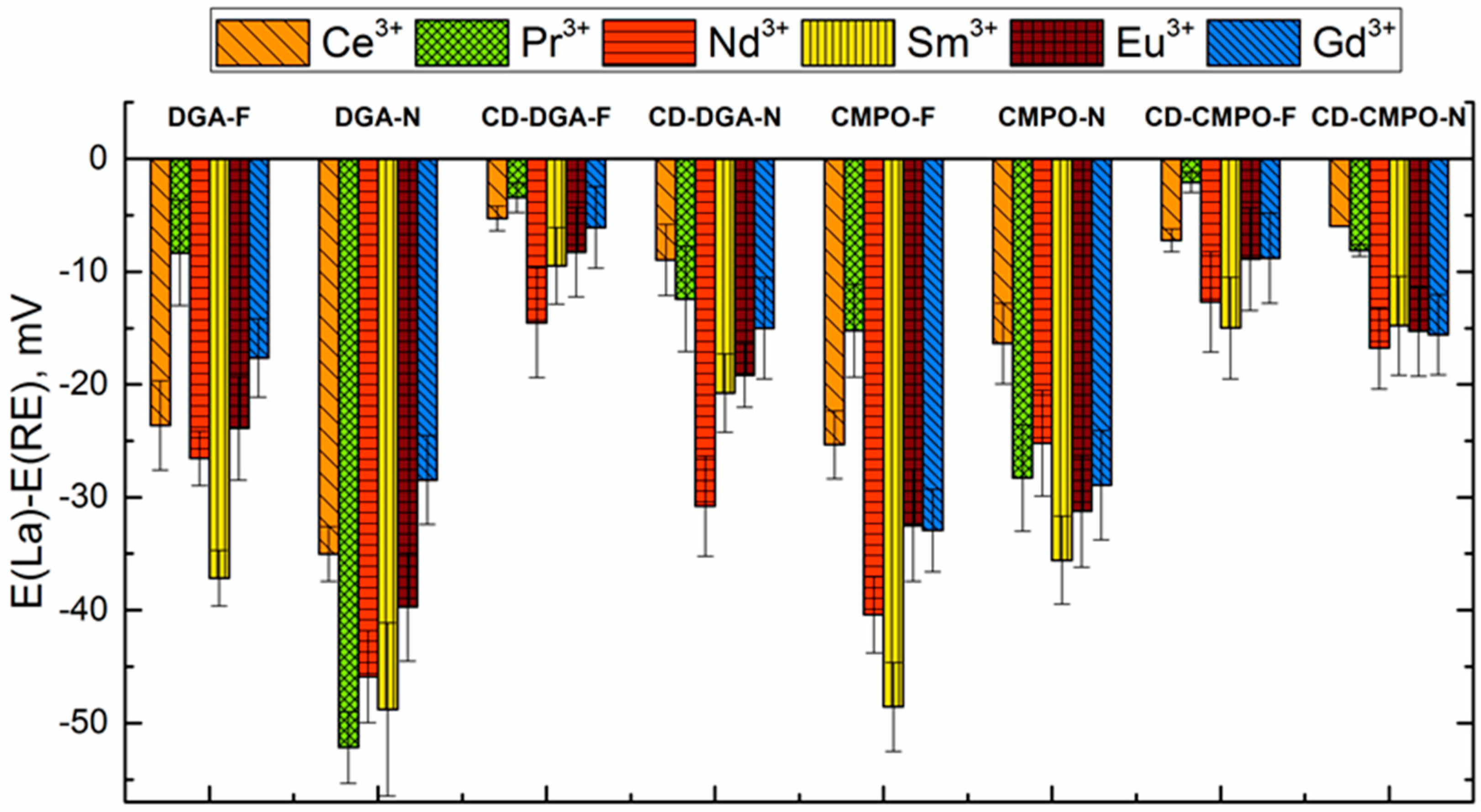
3.3. Sensitivity and Selectivity towards Triple-Charged Ions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vlasov, Y.; Legin, A.; Rudnitskaya, A.; Di Natale, C.; D’Amico, A. Nonspecific sensor arrays (“electronic tongue”) for chemical analysis of liquids: (IUPAC technical report). Pure Appl. Chem. 2005, 77, 1965–1983. [Google Scholar] [CrossRef]
- Del Valle, M. Electronic tongues employing electrochemical sensors. Electroanalysis 2010, 22, 1539–1555. [Google Scholar] [CrossRef]
- Smyth, H.; Cozzolino, D. Instrumental methods (Spectroscopy, Electronic Nose, and Tongue) as tools to predict taste and aroma in beverages: Advantages and limitations. Chem. Rev. 2013, 113, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Śliwińska, M.; Wiśniewska, P.; Dymerski, T.; Namieśnik, J.; Wardencki, W. Food analysis using artificial senses. J. Agric. Food Chem. 2014, 62, 1423–1448. [Google Scholar] [CrossRef] [PubMed]
- Nash, K.L.; Lumetta, G.J.; Nash, K.L.; Braley, J.C.; Alena, P.; Arm, S.; Phillips, C.; Bryan, S.A.; Levitskaia, T.G.; Casella, A.J.; et al. Advanced Separation Techniques for Nuclear Fuel Reprocessing and Radioactive Waste Treatment; Nash, K.L., Lumetta, G.J., Eds.; Woodhead Publishing: Cambridge, UK, 2011; p. 512. [Google Scholar] [CrossRef]
- Croudace, I.W.; Russell, B.C.; Warwick, P.W. Plasma source mass spectrometry for radioactive waste characterisation in support of nuclear decommissioning: A review. J. Anal. At. Spectrom. 2017, 32, 494–526. [Google Scholar] [CrossRef]
- Ganjali, M.R.; Gupta, V.K.; Faridbod, F.; Norouzi, P. Chapter 5-Electrochemical Determination of Lanthanides Series. In Lanthanides Series Determination by Various Analytical Methods; Ganjali, M.R., Gupta, V.K., Faridbod, F., Norouzi, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 91–208. [Google Scholar] [CrossRef]
- Kirsanov, D.; Khaydukova, M.; Tkachenko, L.; Legin, A.; Babain, V. Potentiometric Sensor Array for Analysis of Complex Rare Earth Mixtures. Electroanalysis 2012, 24, 121–130. [Google Scholar] [CrossRef]
- Kirsanov, D.; Babain, V.; Legin, A. Developing sensing materials for multisensor systems on the basis of extraction data. In Multisensor Systems for Chemical Analysis: Materials and Sensors; Lvova, L., Kirsanov, D., Di Natale, C., Legin, A., Eds.; Pan Stanford Publishing: Singapore, 2013; pp. 1–40. [Google Scholar] [CrossRef]
- Alyapyshev, M.Y.; Babain, V.A.; Boyko, V.I.; Eliseev, I.I.; Kirsanov, D.O.; Klimchuk, O.V.; Legin, A.V.; Mikhailina, E.S.; Rodik, R.V.; Smirnov, I.V. Calixarenes functionalized with phosphine oxide and diamide functions as extractants and ionofores for rare-earth metals. J. Incl. Phenom. Macrocycl. Chem. 2010, 67, 117–126. [Google Scholar] [CrossRef]
- Bereczki, R.; Csokai, V.; Grün, A.; Bitter, I.; Tóth, K. Crown bridged thiacalix[4]arenes as cesium-selective ionophores in solvent polymeric membrane electrodes. Anal. Chim. Acta 2006, 569, 42–49. [Google Scholar] [CrossRef]
- Sasaki, Y.; Sugo, Y.; Suzuki, S.; Tachimori, S. The novel extractants diglycolamides for the extraction of lanthanides and actinides in HNO3-n-dodecane system. Solvent Extr. Ion Exch. 2001, 19, 91–103. [Google Scholar] [CrossRef]
- Ansari, S.A.; Pathak, P.N.; Manchanda, V.K.; Husain, M.; Prasad, A.K.; Parmar, V.S. N,N,N′,N′-Tetraoctyl Diglycolamide (TODGA): A Promising Extractant for Actinide-Partitioning from High-Level Waste (HLW). Solvent Extr. Ion Exch. 2005, 23, 463–479. [Google Scholar] [CrossRef]
- Horwitz, P.E.; Kalina, D.G. The extraction of Am(III) from nitric acid by octyl(phenyl)-N,N-diisobutylcarbamoylmethylphosphine oxide-tri-n-butyl phosphate mixtures. Solvent Extr. Ion Exch. (USA) 1984, 2, 179–200. [Google Scholar] [CrossRef]
- Mathur, J.N.; Murali, M.S.; Natarajan, P.R.; Badheka, L.P.; Banerji, A.; Ramanujam, A.; Dhami, P.S.; Gopalakrishnan, V.; Dhumwad, R.K.; Rao, M.K. Partitioning of actinides from high-level waste streams of purex process using mixtures of CMPO and TBP in dodecane. Waste Manag. 1993, 13, 317–325. [Google Scholar] [CrossRef]
- Legin, A.V.; Kirsanov, D.O.; Babain, V.A.; Borovoy, A.V.; Herbst, R.S. Cross-sensitive rare-earth metal sensors based on bidentate neutral organophosphorus compounds and chlorinated cobalt dicarbollide. Anal. Chim. Acta 2006, 572, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Legin, A.V.; Babain, V.A.; Kirsanov, D.O.; Mednova, O.V. Cross-sensitive rare earth metal ion sensors based on extraction systems. Sens. Actuators B Chem. 2008, 131, 29–36. [Google Scholar] [CrossRef]
- Mahanty, B.; Satpati, A.K.; Mohapatra, P.K. Development of a potentiometric sensor for europium(III) based on N,N,N′,N′-tetraoctyldiglycolamide (TODGA) as the ionophore. J. Electroanal. Chem. 2018, 808, 340–347. [Google Scholar] [CrossRef]
- Iqbal, M.; Huskens, J.; Verboom, W.; Sypula, M.; Modolo, G. Synthesis and Am/Eu extraction of novel TODGA derivatives. Supramol. Chem. 2010, 22, 827–837. [Google Scholar] [CrossRef]
- Wilden, A.; Modolo, G.; Lange, S.; Sadowski, F.; Beele, B.B.; Skerencak-Frech, A.; Panak, P.J.; Iqbal, M.; Verboom, W.; Geist, A.; et al. Modified Diglycolamides for the an(III) + Ln(III) Co-separation: Evaluation by Solvent Extraction and Time-Resolved Laser Fluorescence Spectroscopy. Solvent Extr. Ion Exch. 2014, 32, 119–137. [Google Scholar] [CrossRef]
- Rais, J.; Grüner, B. Extraction with metal bis(dicarbollide) anions: Metal bis(dicarbollide) extractants and their applications in separation chemistry. In Ion Exchange and Solvent Extraction; Marcus, Y., SenGupta, A.K., Dekker, M., Eds.; Taylor & Francis Inc.: Bosa Raton, FL, USA, 2004; pp. 243–334. [Google Scholar]
- Paulenova, A.; Alyapyshev, M.Y.; Babain, V.A.; Herbst, R.S.; Law, J.D. Extraction of Lanthanoids with Diamides of Dipcolinic Acid from Nitric Acid Solutions. II. Synergistic Effect of Ethyl-Tolyl Derivates and Dicarbollide Cobalt. Solvent Extr. Ion Exch. 2013, 31, 184–197. [Google Scholar] [CrossRef]
- Grüner, B.; Plešek, J.; Báča, J.; Císařová, I.; Dozol, J.F.; Rouquette, H.; Viňas, C.; Selucký, P.; Rais, J. Cobalt bis(dicarbollide) ions with covalently bonded CMPO groups as selective extraction agents for lanthanide and actinide cations from highly acidic nuclear waste solutions. New J. Chem. 2002, 26, 1519–1527. [Google Scholar] [CrossRef]
- Selucký, P.; Rais, J.; Lučaníková, M.; Grüner, B.; Kvíčalová, M.; Fejfarová, K.; Císařová, I. Lanthanide and actinide extractions with anionic ligands based on cobalt bis(dicarbollide) ions with covalently bonded CMPO functions. Radiochim. Acta 2008, 96, 273–284. [Google Scholar] [CrossRef]
- Grüner, B.; Kvíčalová, M.; Selucký, P.; Lučaníková, M. Anionic alkyl diglycoldiamides with covalently bonded cobalt bis(dicarbollide) (1-) ions for lanthanide and actinide extractions. J. Organomet. Chem. 2010, 695, 1261–1264. [Google Scholar] [CrossRef]
- Bubeníková, M.; Selucký, P.; Rais, J.; Grüner, B.; Švec, P. Studies on Am(III) separation from simulated high-level waste using cobalt bis(dicarbollide) (1-) ion derivative covalently bound to N,N-di-n-octyl diglycol diamide as extractant and DTPA as stripping agent. J. Radioanal. Nucl. Chem. 2012, 293, 403–408. [Google Scholar] [CrossRef]
- Vaňura, P.; Makrlík, E.; Selucký, P. Extraction of trivalent europium and americium by the synergistic mixture of bis-1,2-dicarbollylcobaltate and N,N,N′,N′-tetraoctyl diglycolamide in highly polar 3-nitro-α,α,α-trifluorotoluene solvent. J. Radioanal. Nucl. Chem. 2018, 317, 443–449. [Google Scholar] [CrossRef]
- Fu, B.; Bakker, E.; Yun, J.H.; Yang, V.C.; Meyerhoff, M.E. Response Mechanism of Polymer Membrane-Based Potentiometric Polyion Sensors. Anal. Chem. 1994, 66, 2250–2259. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.; Bühlmann, P.; Pretsch, E. Carrier-based ion-selective electrodes and bulk optodes. 1. General characteristics. Chem. Rev. 1997, 97, 3083–3132. [Google Scholar] [CrossRef]
- Stauthamer, W.P.R.V.; Engbersen, J.F.J.; Verboom, W.; Reinhoudt, D.N. Influence of plasticizer on the selectivity of nitrate-sensitive CHEMFETs. Sens. Actuators B Chem. 1994, 17, 197–201. [Google Scholar] [CrossRef][Green Version]
- Eugster, R.; Rosatzin, T.; Rusterholz, B.; Aebersold, B.; Pedrazza, U.; Rüegg, D.; Schmid, A.; Spichiger, U.E.; Simon, W. Plasticizers for liquid polymeric membranes of ion-selective chemical sensors. Anal. Chim. Acta 1994, 289, 1–13. [Google Scholar] [CrossRef]
- Arada Pérez, M.D.L.A.; Marín, L.P.; Quintana, J.C.; Yazdani-Pedram, M. Influence of different plasticizers on the response of chemical sensors based on polymeric membranes for nitrate ion determination. Sens. Actuators B Chem. 2003, 89, 262–268. [Google Scholar] [CrossRef]
- Bakker, E.; Pretsch, E.; Bühlmann, P. Selectivity of potentiometric ion sensors. Anal. Chem. 2000, 72, 1127–1133. [Google Scholar] [CrossRef]
- Lee, M.H.; Yoo, C.L.; Lee, J.S.; Cho, I.S.; Kim, B.H.; Cha, G.S.; Nam, H. Tweezer-Type Neutral Carrier-Based Calcium-Selective Membrane Electrode with Drastically Reduced Anionic Interference. Anal. Chem. 2002, 74, 2603–2607. [Google Scholar] [CrossRef]
- Bedlechowicz-Śliwakowska, I.; Lingenfelter, P.; Sokalski, T.; Lewenstam, A.; Maj-Zurawska, M. Ion-selective electrode for measuring low Ca2+ concentrations in the presence of high K+, Na+ and Mg2+ background. Anal. Bioanal. Chem. 2006, 385, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Bühlmann, P.; Pretsch, E.; Bakker, E. Carrier-based ion-selective electrodes and bulk optodes. 2. Ionophores for potentiometric and optical sensors. Chem. Rev. 1998, 98, 1593–1687. [Google Scholar] [CrossRef] [PubMed]
- Makrlík, E.; Vaňura, P.; Selucký, P. Extraction of calcium and strontium into phenyltrifluoromethyl sulfone by using synergistic mixture of hydrogen dicarbollylcobaltate and “classical” CMPO. Acta Chim. Slov. 2010, 57, 485–490. [Google Scholar] [PubMed]
- Zhu, Z.X.; Sasaki, Y.; Suzuki, H.; Suzuki, S.; Kimura, T. Cumulative study on solvent extraction of elements by N,N,N′,N′-tetraoctyl-3-oxapentanediamide (TODGA) from nitric acid into n-dodecane. Anal. Chim. Acta 2004, 527, 163–168. [Google Scholar] [CrossRef]
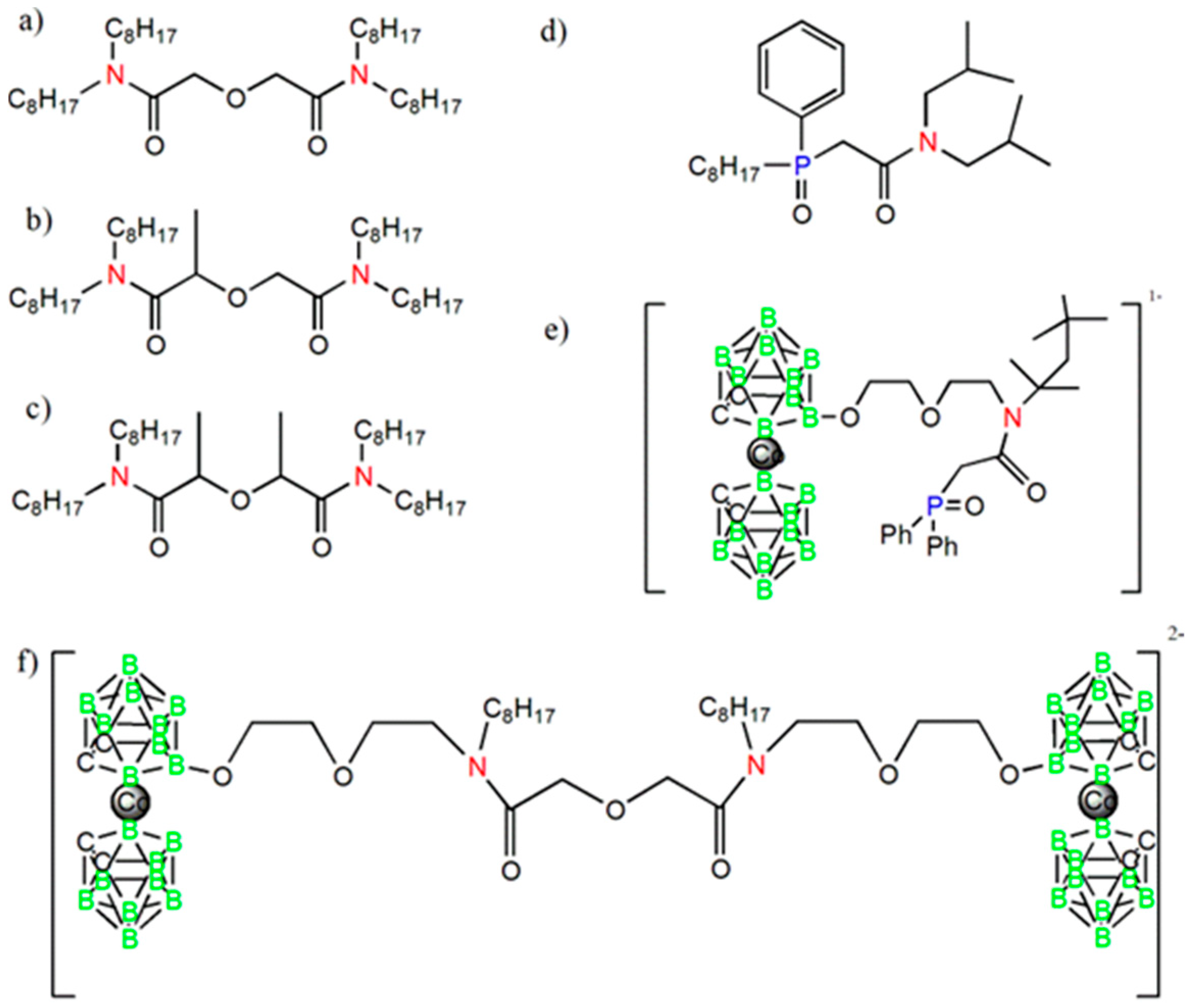
| Group Number | Sensor Number | Sensor Encoding | Components | wt % |
|---|---|---|---|---|
| I | 1 | T-D | TODGA | 2.9 |
| CCD | 0.5 | |||
| DOS | 64.3 | |||
| 2 | T-F | TODGA | 2.9 | |
| CCD | 0.5 | |||
| 2F2N | 64.3 | |||
| 3 | T-N | TODGA | 1.9 | |
| CCD | 0.5 | |||
| NPOE | 64.4 | |||
| 4 | MT-D | Me-TODGA | 3.0 | |
| CCD | 0.5 | |||
| DOS | 64.2 | |||
| 5 | MT-F | Me-TODGA | 3.0 | |
| CCD | 0.5 | |||
| 2F2N | 64.2 | |||
| 6 | MT-N | Me-TODGA | 3.0 | |
| CCD | 0.5 | |||
| NPOE | 64.2 | |||
| 7 | 2MT-D | 2Me-TODGA | 3.0 | |
| CCD | 0.5 | |||
| DOS | 64.2 | |||
| 8 | 2MT-F | 2Me-TODGA | 3.0 | |
| CCD | 0.5 | |||
| 2F2N | 64.2 | |||
| 9 | 2MT-N | 2Me-TODGA | 3.0 | |
| CCD | 0.5 | |||
| NPOE | 64.2 | |||
| II | 10 | DGA-F | TODGA | 1.5 |
| CCD | 3.3 | |||
| 2F2N | 63.5 | |||
| 11 | DGA-N | TODGA | 1.5 | |
| CCD | 3.3 | |||
| NPOE | 63.5 | |||
| 12 | CD-DGA-F | CD–DGA | 6.1 | |
| 2F2N | 62.6 | |||
| 13 | CD-DGA-N | CD–DGA | 6.1 | |
| NPOE | 62.6 | |||
| III | 14 | CMPO-F | CMPO | 1.0 |
| CCD | 3.3 | |||
| 2F2N | 63.8 | |||
| 15 | CMPO-N | CMPO | 1.0 | |
| CCD | 3.3 | |||
| NPOE | 63.8 | |||
| 16 | CD–CMPO-F | CD–CMPO | 4.0 | |
| 2F2N | 64.0 | |||
| 17 | CD–CMPO-N | CD–CMPO | 4.0 | |
| NPOE | 64.0 |
| Group Number | Sensor Number | Membrane | Sensitivity towards Ions in mV/dec | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| K+ | Na+ | Mg2+ | Ca2+ | Cu2+ | Zn2+ | Cd2+ | Pb2+ | |||
| I | 1 | T-D | 1 | 0 | 0 | 29 | 0 | 0 | 7 | 23 |
| 2 | T-F | 2 | 2 | 1 | 28 | 4 | 2 | 9 | 20 | |
| 3 | T-N | 1 | 11 | 3 | 28 | 5 | 1 | 9 | 26 | |
| 4 | MT-D | 1 | 2 | 0 | 28 | 6 | 8 | 7 | 27 | |
| 5 | MT-F | 1 | 1 | 0 | 25 | 1 | 4 | 7 | 29 | |
| 6 | MT-N | 1 | 1 | 0 | 27 | 10 | 6 | 11 | 26 | |
| 7 | 2MT-D | 1 | 1 | 0 | 25 | 12 | 12 | 12 | 27 | |
| 8 | 2MT-F | 1 | 0 | 1 | 26 | 12 | 12 | 12 | 24 | |
| 9 | 2MT-N | 2 | 2 | 0 | 27 | 11 | 7 | 11 | 23 | |
| II | 10 | DGA-F | 18 | 4 | 3 | 6 | 14 | 5 | 5 | 15 |
| 11 | DGA-N | 17 | 3 | 5 | 16 | 13 | 23 | 17 | 23 | |
| 12 | CD-DGA-F | 29 | 7 | 9 | 10 | 7 | 5 | 5 | 17 | |
| 13 | CD-DGA-N | 31 | 7 | 10 | 12 | 9 | 7 | 10 | 31 | |
| III | 14 | CMPO-F | 18 | 5 | 3 | 5 | 10 | 4 | 4 | 12 |
| 15 | CMPO-N | 20 | 4 | 5 | 10 | 17 | 7 | 5 | 25 | |
| 16 | CD–CMPO-F | 25 | 5 | 6 | 9 | 10 | 5 | 6 | 16 | |
| 17 | CD–CMPO-N | 28 | 6 | 9 | 15 | 13 | 7 | 9 | 32 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaydukova, M.; Militsyn, D.; Karnaukh, M.; Grüner, B.; Selucký, P.; Babain, V.; Wilden, A.; Kirsanov, D.; Legin, A. Modified Diamide and Phosphine Oxide Extracting Compounds as Membrane Components for Cross-Sensitive Chemical Sensors. Chemosensors 2019, 7, 41. https://doi.org/10.3390/chemosensors7030041
Khaydukova M, Militsyn D, Karnaukh M, Grüner B, Selucký P, Babain V, Wilden A, Kirsanov D, Legin A. Modified Diamide and Phosphine Oxide Extracting Compounds as Membrane Components for Cross-Sensitive Chemical Sensors. Chemosensors. 2019; 7(3):41. https://doi.org/10.3390/chemosensors7030041
Chicago/Turabian StyleKhaydukova, Maria, Danila Militsyn, Mikhail Karnaukh, Bohumir Grüner, Pavel Selucký, Vasily Babain, Andreas Wilden, Dmitry Kirsanov, and Andrey Legin. 2019. "Modified Diamide and Phosphine Oxide Extracting Compounds as Membrane Components for Cross-Sensitive Chemical Sensors" Chemosensors 7, no. 3: 41. https://doi.org/10.3390/chemosensors7030041
APA StyleKhaydukova, M., Militsyn, D., Karnaukh, M., Grüner, B., Selucký, P., Babain, V., Wilden, A., Kirsanov, D., & Legin, A. (2019). Modified Diamide and Phosphine Oxide Extracting Compounds as Membrane Components for Cross-Sensitive Chemical Sensors. Chemosensors, 7(3), 41. https://doi.org/10.3390/chemosensors7030041







