Abstract
This research is devoted to the development and study of novel cross-sensitive sensors based on modified extracting ligands. According to the previous results of liquid extraction studies, the chemical modification of membrane active components would change the analytical characteristics of a sensor comprising them. The sensing elements of the studied sensors consisted of various derivatives of N,N,N′,N′-tetraoctyldiamide of diglycolic acid (TODGA) and di-phenyl-N,N-di-i-sobutylcarbamoylmethylen phoshine oxide (CMPO) used as neutral carriers, CCD (chlorinated cobalt dicarbollide) as a lipophilic additive, different plasticizers, and poly(vinyl chloride) (PVC) as a polymer. TODGA-based sensors demonstrated a stable and reproducible response towards rare earth cations in acidic media (pH = 2). Changing the concentrations and ratio of neutral carriers and the lipophilic additive, it is possible to modify the sensitivity and selectivity of the sensors towards the same target ions. Bonded ligands, such as cobalt dicarbollide covalently attached to TODGA and CMPO, exhibited lower selectivity and sensitivity to rare earth cations. A possibility to vary the cross-sensitivity patterns of the sensors in a wide range might be of great interest for the development of multisensor systems allowing the simultaneous determination of several analytes in multicomponent solutions.
1. Introduction
Potentiometric sensors are attractive analytical tools giving an opportunity to analyze liquids in a simple, fast, and non-destructive way with high sensitivity and reasonable accuracy. Furthermore, ion-selective electrodes (ISEs), being the most popular potentiometric sensors, are recommended for pH determination by several international standards (e.g., ISO 10523:2008(en)). Nevertheless, frequently observed non-Nernstian behavior and insufficient selectivity complicate the wider implementation of ISEs into analytical practice. Thus, a majority of existing sensors exhibit, to a different extent, a property of cross-sensitivity, being sensitive and selective not only to a target analyte, but also to other components. When chemically and physically similar analytes are simultaneously present in a sample solution (e.g., rare earth elements), it is hardly possible to develop a highly selective sensor for each individual component. A multisensor approach allows turning this problem of ISEs into an advantage [1]. According to this method, an array of cross-sensitive and low-selective sensors demonstrating different sensitivity patterns to the components of a complex mixture is used instead of a single sensor. The application of multivariate data processing techniques to interpret a response of such multisensor arrays helps to determine and quantify several analytes at the same time. Obviously, composition and quantity of cross-sensitive sensors in a multisensor set should be adapted for a particular analytical task.
Recently, various multisensor systems have been successfully applied in different areas [2,3,4]. This approach could be particularly useful to analyze rare earth elements (REEs) in complex media. Simultaneous determination of multiple REEs is of great interest for industrial analytical control of the spent nuclear fuel (SNF) recycling process [5]. Currently, such high-precision spectroscopic techniques based on inductively coupled plasma (ICP) spectrometry such as ICP-MS (mass spectrometry), ICP-AAS (atomic absorption spectroscopy), and ICP-AES (atomic emission spectroscopy) are applied for REE quantification in different SNF reprocessing steps [6]. Despite the remarkable analytical characteristics of these methods, their direct implementation into a processing line is impossible since they do not allow the continuous sampling and real-time analysis of target compounds. Moreover, these offline spectroscopic techniques require high-cost equipment, time-consuming preparation of radioactive probe, and trained staff. In contrast, chemical sensors are cost-effective and suitable for remote in-line measurements. Due to this fact, a multitude of individual ISEs have been suggested for the determination of REE cations [7]. However, most of the proposed sensors work properly only in a pH range of 3 to 8, which is far from real industrial conditions. The selectivity of most ISEs is not enough to perform reliable measurements in complex mixtures. At the same time, multisensor systems can be successfully used for REE determination in their mixtures, as was shown in our previous work [8].
A wide range of chemical molecules acting as liquid extracting agents can be used as ionophores in a polymeric matrix of potentiometric sensors due to the similar selective ion capture mechanisms [9,10,11]. For example, N,N,N′,N′-tetraoctyldiamide of diglycolic acid (TODGA) [12,13] and octylphenyl-N,N-di-isobutylcarbamoylmethylen phosphine oxide (CMPO) [14,15] were proposed for effective lanthanide and actinide cation extraction from highly acidic media. These ligands were applied as sensitive components of potentiometric membranes [16,17,18]; it was shown that their sensing properties were similar to the extractive ones. Since the properties of some extracting agents allow their application as ionophores, it could be reasonable to study the properties of their derivatives. However, it is worth noting that the extracting behavior of a given molecule does not always result in the outstanding performance of a sensor with this component.
The extraction properties of TODGA modified by the addition of one or two methyl groups to the central carbon atoms have been recently studied [19,20]. Both derivatives demonstrated a low extraction ability towards all lanthanides, but for each derivative the distribution ratio varied in a different way from light to heavy REEs [20]. The sensors based on these molecules exhibited alternative patterns of sensitivity and selectivity in comparison with TODGA. New ligands are stable in acidic medium, which makes them attractive as potential active substances for chemical sensors.
Another interesting concept of a sensor’s development is based on the so-called pre-organization. Different individual compounds are chemically bounded into a single molecule to achieve a synergistic effect previously known for a mixture of these compounds. Chlorinated cobalt dicarbollide (CCD), used in many extraction systems for high-level radioactive waste processing, was suggested as an extracting agent for cesium [21]. It was shown that CCD can effectively cross the phase boundary and its combination with a neutral ligand promotes extraction both through solvation and cation-exchange mechanisms [22]. Therefore, cobalt dicarbollide (CD) ions were covalently bonded with neutral ligands, and the extracting properties of such pre-organized molecules were studied [23,24,25,26]. It was shown that compounds with CD covalently bonded with diglycol amides are more efficient for the extraction of Eu (III) and Am (III) than the mixture of TODGA and CCD [25]. Similar extracting ability improvement was observed for the covalent combination of CD and CMPO molecules [24]. The application of neutral ligands covalently bonded with a cation-exchanger can be interesting for the development of cations’ sensors, especially due to the fact that CCD was previously used in polymeric membranes as a cation-exchanger and can be a good alternative for conventional tetraphenylborate derivatives [17].
A significant synergistic effect was observed earlier for the mixtures of CCD and carbamoyl phosphine oxides. In such mixtures, the distribution coefficients of Am(III) and Eu(III) differ from the individual molecules’ characteristics by several orders of magnitude [21]. A similar effect was reported for CCD and diglycolic acid diamides [27].
In this study, we applied several modified ligands originally developed for liquid extraction as active components of chemical sensors. A number of sensors based on TODGA and its derivatives and on compounds with a CD group covalently bounded to neutral ligands (TODGA and CMPO) were prepared and their sensing properties were studied. The main purpose of this work was to expand the number of cross-sensitive sensors with variable sensitivity and selectivity patterns toward lanthanides. This would be highly useful for developing multisensor systems finely adapted for sophisticated analytical tasks, e.g., radioactive waste reprocessing analysis.
2. Materials and Methods
High-molecular weight poly(vinylchloride) (PVC), dioctyl sebacate (DOS), 2-fluorophenyl-2′-nitrophenyl ether (2F2N), and o-nitrophenyloctyl ether (NPOE) were purchased in Fluka (Switzerland). N,N,N′,N′-tetraoctyldiamide of diglycolic acid (TODGA) and the structurally modified derivatives (Me-TODGA and 2Me-TODGA) were synthesized according to [19,20]. Diglycolic acid (DGA) and CMPO bonded with CD (CD-DGA and CD-CMPO) were provided by Katchem (Kralupy nad Vltavou, Czech Republic) and synthesized according to [23,25]. Ligand structures are shown in Figure 1. Cesium salt of chlorinated cobalt dicarbollide (CCD) was provided by Katchem (Kralupy nad Vltavou, Czech Republic).
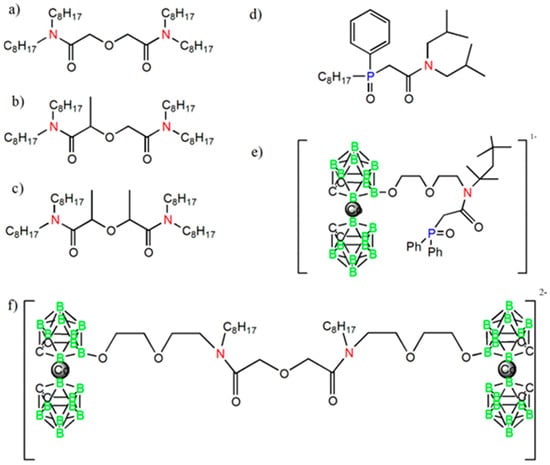
Figure 1.
Ligand structures: (a) N,N,N′,N′-tetraoctyldiamide of diglycolic acid (TODGA); (b) methylated TODGA (Me-TODGA); (c) dimethylated TODGA (2Me-TODGA); (d) octylphenyl-N,N-di-isobutylcarbamoylmethylen phoshine oxide (CMPO); (e) CMPO-cobalt bis(dicarbollides) (CD-CMPO); (f) TODGA-di-cobalt bis(dicarbollides) (CD-DGA).
Polymeric sensor membranes were prepared according to the standard procedure [8]. Corresponding quantities of membrane components were weighted, transferred to a glass beaker, and dissolved in freshly distilled tetrahydrofuran under mixing for 20 min. After the complete dissolution of all components, the mixture was transferred into a Teflon beaker with a flat bottom and was left for 24 h for solvent evaporation. The final thickness of the parent membranes was about 0.5 mm. Discs of 8 mm in diameter were chopped out from the parent membranes. Each disc was glued onto a sensor body (PVC tube). The sensors were dried in ambient conditions for 48 h. Later, they were immersed into and filled in with a 0.01 M NaCl solution for 24 h before the measurements. At least three replicated sensors were prepared for each membrane composition. Sensor membrane compositions are given in the Table 1. Typically, there is an excess of the neutral ligand over the ion exchanger in the plasticized membrane (the corresponding ratio is about 5:1), but in the case of covalently bonded combinations of CD and ligands, the ratio was 1:3 (in the case of TODGA) and 1:2 (for CMPO) due to the molecular structure of the resulting compound. Therefore, additional membranes with a molar ratio of CCD and neutral ligands equal to that of pre-organized molecules were prepared (DGA-N, DGA-F, CMPO-N, and CMPO-F).

Table 1.
Compositions of the sensor membranes. All membranes contained 31–32 wt % of poly(vinyl chloride) (PVC).
The response mechanism of potentiometric sensors with plasticized polymeric membranes is well known and extensively described in numerous comprehensive reviews [28,29]. By default, the membrane matrix (PVC + plasticizer) is considered as neutral; however, many plasticizers may significantly affect the sensor’s sensitivity and lifetime [30,31,32]. Several polymeric plasticizers with different dielectric constants—2F2N, NPOE, and DOS (εr = 50.0 [30], 24, and 3.9 [31], respectively)—were used in this study.
Potentiometric measurements were performed in the following galvanic cell: Cu | Ag | AgCl, KClsat | sample solution | sensor membrane | 0.01 M NaCl | AgCl | Ag | Cu.
Sensor sensitivities were studied in aqueous solutions of alkaline, alkaline earth, transition, and rare earth metal ions (K, Na, Mg, Ca, Cu, Zn, Cd, Pb, La, Ce, Pr, Nd, Sm, Eu, Gd) in the concentration range of 10−7–10−3 M. Measurements of REE ions were performed at pH 2 (addition of nitric acid) to ensure the presence of triple-charged cations. The pH values were controlled by a glass pH electrode (ZIP Gomel, Belarus). REE solutions were prepared by dissolution of the metal oxides (99.9%) in nitric acid. Other calibration solutions were prepared by dissolution of the relevant salts in the water. Oxides, salts, and other chemicals used for the preparation of the calibration solutions were purchased from Vekton (St. Petersburg, Russia). Bidistilled water was used in the experiments. Sensor potentials were measured continuously every 6 s against a Ag/AgCl reference electrode (Izmeritelnaya Tekhnika LLC, Moscow, Russia) with 0.1 mV precision using a high-impedance multichannel digital mV-meter HAN-32 (Sensor Systems, LLC, St. Petersburg, Russia). The measurement time for each sample was 3 min, and the last 5 readings of the sensor during this period were averaged for data processing. Sensitivity values were calculated as a slope of the linear part of a calibration curve in the concentration range of 10−5–10−3 M. Biionic potentials (BIP) used for the selectivity estimation were calculated according to [17,33] as the difference between the electromotive force (EMF) measured in a 10−3 M La3+ aqueous solution and in a solution with the same concentration of another REE cation, divided by the slope value. Thermodynamically strict application of selectivity evaluation methods requires theoretical (Nernstian) sensitivity towards both the target and the interfering ion. When this condition is not fulfilled, a simplified evaluation procedure can be used—the calculation of the difference in EMF values in solutions of two ions.
3. Results and Discussion
3.1. Sensitivity Towards Single-Charged Ions
The sensitivity of novel sensors was studied in solutions of single-, double-, and triple-charged cations. The measurements were repeated three times in each solution on different days of the experiment; typical standard deviations of potential values were in the range of 2–5 mV. Typical dependence between the logarithm of the ionic concentration and the sensor potential is demonstrated in Figure 2. The calculated sensor sensitivity values are presented in Table 2. The dynamic response of certain sensors is shown in Figure S1 (Supplementary Materials).
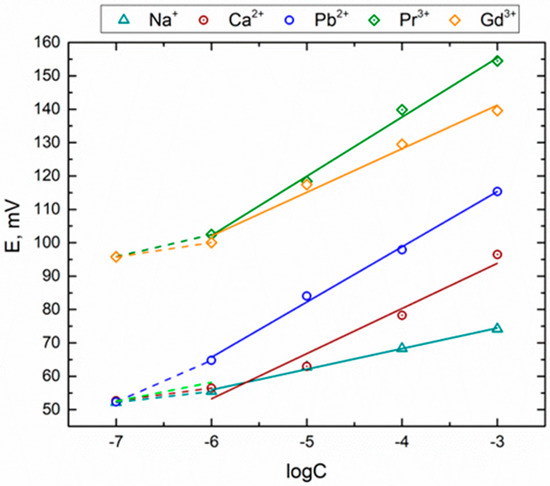
Figure 2.
Typical calibration curves shown for the CD-CMPO-N sensor.

Table 2.
Sensitivity values of the sensors in the solutions of single- and double-charged cations. Standard deviations of the slope values were in the range of 1–3 mV/dec.
The sensors from group I (Table 1) did not show any significant response in alkaline cation solutions. The sensors with CD bonded to neutral ligands (CD-DGA-F, CD-DGA-N, CD-CMPO-F, and CD-CMPO-N) demonstrated some sensitivity towards K+ (up to 31 ± 1 mV/dec), while the mixtures of CCD and ligands (DGA-F, DGA-N, CMPO-F, and CMPO-N) were characterized by lower sensitivity to K+ (less than 20 ± 1 mV/dec), regardless of the plasticizer. The growth of sensitivity to single-charged cations for the sensors with pre-organized molecules is possibly caused by the additional complexation of K+ by oxygen atoms of the polyethyleneglycol-type part of the molecules like CD-DGA and CD-CMPO.
3.2. Sensitivity Towards Double-Charged Ions
In alkaline earth metal ion solutions, the sensors from group II (Table 1) demonstrated a good sensitivity towards Ca2+ cations, with a slope of at least 25 mV/dec. The observed sensitivity can be explained by the influence of a diglycol diamide TODGA backbone, since the ligands with similar chemical structures were used as Ca2+ ionophores [34,35,36]. The sensitivity to Ca2+ decreases down to 6–16 mV/dec with a reduction of the amount of TODGA in membranes (DGA-F and DGA-N, respectively). Sensors 14–17 did not exhibit the same properties towards Ca2+ cations (the slope did not exceed 15 mV/dec); it was previously found that carbamoyl phosphine oxides demonstrated a very low extraction ability to calcium [37].
It is important to note that we studied sensing membranes with different concentrations and ratios of the ligands and CCD. While the concentrations of these components were typical for ISEs for group I, the sensors from groups II and III (DGA-F, DGA-N, CMPO-F, and CMPO-N) had membranes with equimolar quantities of ligand and CCD in order to compare them with the sensors based on bonded ligands.
The highest sensitivity to transition metal ions was observed in Pb2+ solutions. The slope values varied depending on the ionophore and plasticizer. The ionophores with covalently bonded CD demonstrated a higher response to Pb2+ compared to those with equimolar mixtures of the ligand and CCD; the same results were obtained for different plasticizers. In most cases, the sensitivity of the sensors to Zn, Cd, and Cu ions did not exceed 15 mV/dec.
3.3. Sensitivity and Selectivity towards Triple-Charged Ions
The sensitivity of TODGA-based sensors generally correlates with the atomic number of REEs, which agrees with the trends observed in liquid–liquid extraction for this ligand [12,38]. The sensitivity of the sensors was higher when TODGA was used as an ionophore rather than its methylated derivatives (Figure 3). The sensors based on di-methylated TODGA exhibited no significant sensitivity to REE ions. The sensitivity reduction upon incorporation of the methyl group in the DGA structure can be related to the decrease of the molecule flexibility and corresponding steric hindrance for the efficient interaction between the receptor and cations. This is consistent with the decrease in extraction efficiency of modified DGA [19,20].
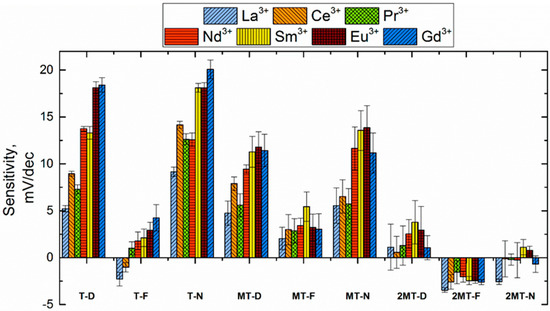
Figure 3.
Sensitivity values measured in rare earth element (REE) solutions for the sensors based on TODGA and its methylated derivatives.
The sensitivity of all TODGA- (and CMPO)-based sensors notably increased when the 2F2N plasticizer was replaced by DOS and then by NPOE in the membrane composition (Figure 3 and Figure 4). This untypical influence of plasticizers on sensing properties will be a subject of further research.
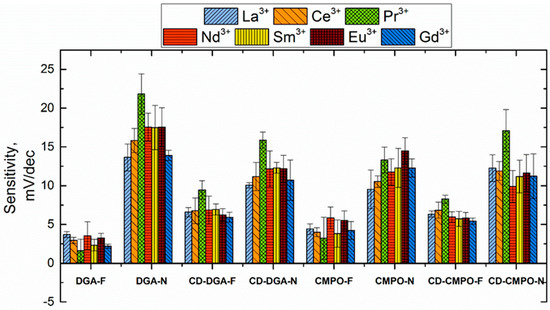
Figure 4.
Sensitivity values measured in REE solutions for sensors with ligands covalently bonded to the CD and for sensors with equimolar mixtures of the ligand and CCD.
The sensitivity to some REE ions (like La3+, Ce3+, Pr3+, Nd3+) and Zn2+ slightly increased with the growth of CCD concentration in the sensing membranes from 0.5 % (group I) to 3.3 % (DGA-F and DGA-N sensors). A similar effect was previously reported for sensors with an analogous composition [17]. Sensitivity dependence on component concentration was also observed for CMPO [16]. However, it is important to note that the increase in CCD content resulted in a change in the neutral carrier and lipophilic additive ratio, and the role of these factors should be additionally studied. It was previously shown that extraction efficiency is higher for DGA bonded with CD than for the mixture of TODGA and CCD [25]. However, the response slope was lower for the sensors with CD-DGA (CD-DGA-F and CD-DGA-N) for NPOE membranes than for the sensors based on the mixtures of ligand, cation exchanger, and the same plasticizer. In the case of 2F2N-plasticized membranes, the covalent attachment of a ligand to a cation exchanger resulted in some enhancement of the sensitivity, though it was still too small for analytical tasks. A similar behavior was observed for the sensors based on modified TODGA-calixarenes [10]; TODGA attachment to the large calixarene rim equalized the selectivity differences in extraction and sensing mechanisms of these ligands. Such effects were less pronounced for mixed and bonded versions of CMPO-based sensors (Figure 4); a slight sensitivity increase can be also noticed for 2F2N-plasticized membranes.
The selectivity of the sensors was estimated by biionic potentials calculated as the difference between sensor potentials measured in a La3+ 10−3 M solution and in a solution with the same concentration of a target REE ion. It has to be mentioned that these sensitivity values can be used only for qualitative comparison of the sensors. The more negative is the difference of the potentials, the more selective is a particular sensor to the corresponding cation in the presence of La3+. The apparent selectivity of T-D and T-N sensors (Figure 5) slightly increases with the growth of an REE ion atomic mass. In the case of methylated TODGA (M* sensors), the selectivity patterns are different. The most negative BIP difference was registered for Sm3+.
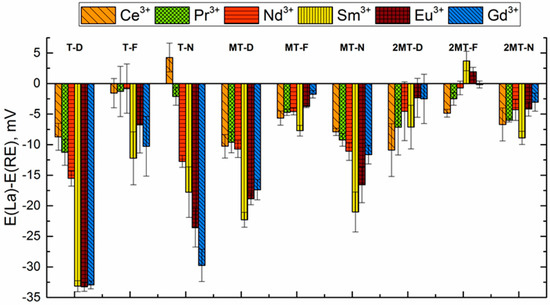
Figure 5.
Biionic potentials in solutions of REEs for the sensors based on TODGA and its methylated derivatives.
Remarkable changes in selectivity patterns were observed for the sensors with bonded ligands (Figure 6). For both ligands (DGA and CMPO) and both plasticizers (2F2N and NPOE), the selectivity of bonded ligands to different REEs significantly decreased in comparison with mixed ionophores.
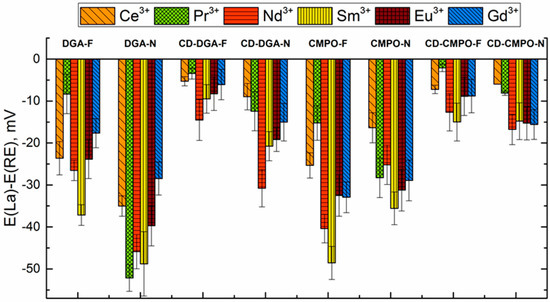
Figure 6.
Biionic potentials in solutions of REEs for sensors with ligands covalently bonded to the CD and for sensors with equimolar mixtures of the ligand and CCD.
4. Conclusions
The sensing properties of novel ion sensors based on different modified extracting agents have been studied. It was shown that the sensitivity and selectivity of a sensor can be changed and adapted for a particular application by modifying the structure of the ligand it comprises.
NPOE-based sensors demonstrated the best sensing characteristics and reproducible behavior in most cases.
The sensors based on TODGA and its methylated derivatives demonstrated good sensitivity to Ca2+ and Pb2+ ions, with response slopes close to theoretical values.
While the TODGA-based sensors demonstrated cross-sensitivity to a wide range of lanthanides, the increase of the methyl group number in the TODGA backbone did not result in any improvement of the sensing properties. Two additional methyl groups significantly further hindered the response to REE ions.
The covalent attachment of the cation exchanger (CD) to DGA resulted in the decreased sensitivity of such sensors towards REEs compared to the sensors based on the mixtures of these components. The selectivity of the membranes with bonded ionophores also became lower in comparison with mixed ionophores.
For CMPO, the sensitivity to REE ions was comparable for the mixed and bonded ligands, while the selectivity to REE became significantly lower for the bonded ligands.
It can be concluded that the sensors with bonded ligands are suitable for multisensor systems for simultaneous determination of several REE cations. The will be the next step of our research.
Supplementary Materials
The following are available online at https://www.mdpi.com/2227-9040/7/3/41/s1, Figure S1: Dynamic response of the sensors DGA-N, CD-DGA-N, CMPO-N, CD-CMPO-N in presence of the praseodymium cations.
Author Contributions
Conceptualization, V.B., D.K. and A.L.; methodology, D.M. and M.K. (Mikhail Karnaukh); formal analysis, M.K. (Maria Khaydukova); investigation, D.M., M.K. (Mikhail Karnaukh), and P.S.; resources, B.G, P.S. and D.K.; data curation, M.K. (Maria Khaydukova); writing—original draft preparation, A.L.; writing—review and editing, V.B., D.K., and A.W.; visualization, M.K. (Maria Khaydukova); supervision, V.B., A.W., D.K., and A.L.; project administration, D.K.; funding acquisition, D.K.
Funding
This research was funded by the Russian Science Foundation, grant number 18-19-00151.
Acknowledgments
M.K. (Maria Khaydukova) would like to thank Ekaterina Oleneva for the final text revision.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vlasov, Y.; Legin, A.; Rudnitskaya, A.; Di Natale, C.; D’Amico, A. Nonspecific sensor arrays (“electronic tongue”) for chemical analysis of liquids: (IUPAC technical report). Pure Appl. Chem. 2005, 77, 1965–1983. [Google Scholar] [CrossRef]
- Del Valle, M. Electronic tongues employing electrochemical sensors. Electroanalysis 2010, 22, 1539–1555. [Google Scholar] [CrossRef]
- Smyth, H.; Cozzolino, D. Instrumental methods (Spectroscopy, Electronic Nose, and Tongue) as tools to predict taste and aroma in beverages: Advantages and limitations. Chem. Rev. 2013, 113, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Śliwińska, M.; Wiśniewska, P.; Dymerski, T.; Namieśnik, J.; Wardencki, W. Food analysis using artificial senses. J. Agric. Food Chem. 2014, 62, 1423–1448. [Google Scholar] [CrossRef] [PubMed]
- Nash, K.L.; Lumetta, G.J.; Nash, K.L.; Braley, J.C.; Alena, P.; Arm, S.; Phillips, C.; Bryan, S.A.; Levitskaia, T.G.; Casella, A.J.; et al. Advanced Separation Techniques for Nuclear Fuel Reprocessing and Radioactive Waste Treatment; Nash, K.L., Lumetta, G.J., Eds.; Woodhead Publishing: Cambridge, UK, 2011; p. 512. [Google Scholar] [CrossRef]
- Croudace, I.W.; Russell, B.C.; Warwick, P.W. Plasma source mass spectrometry for radioactive waste characterisation in support of nuclear decommissioning: A review. J. Anal. At. Spectrom. 2017, 32, 494–526. [Google Scholar] [CrossRef]
- Ganjali, M.R.; Gupta, V.K.; Faridbod, F.; Norouzi, P. Chapter 5-Electrochemical Determination of Lanthanides Series. In Lanthanides Series Determination by Various Analytical Methods; Ganjali, M.R., Gupta, V.K., Faridbod, F., Norouzi, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 91–208. [Google Scholar] [CrossRef]
- Kirsanov, D.; Khaydukova, M.; Tkachenko, L.; Legin, A.; Babain, V. Potentiometric Sensor Array for Analysis of Complex Rare Earth Mixtures. Electroanalysis 2012, 24, 121–130. [Google Scholar] [CrossRef]
- Kirsanov, D.; Babain, V.; Legin, A. Developing sensing materials for multisensor systems on the basis of extraction data. In Multisensor Systems for Chemical Analysis: Materials and Sensors; Lvova, L., Kirsanov, D., Di Natale, C., Legin, A., Eds.; Pan Stanford Publishing: Singapore, 2013; pp. 1–40. [Google Scholar] [CrossRef]
- Alyapyshev, M.Y.; Babain, V.A.; Boyko, V.I.; Eliseev, I.I.; Kirsanov, D.O.; Klimchuk, O.V.; Legin, A.V.; Mikhailina, E.S.; Rodik, R.V.; Smirnov, I.V. Calixarenes functionalized with phosphine oxide and diamide functions as extractants and ionofores for rare-earth metals. J. Incl. Phenom. Macrocycl. Chem. 2010, 67, 117–126. [Google Scholar] [CrossRef]
- Bereczki, R.; Csokai, V.; Grün, A.; Bitter, I.; Tóth, K. Crown bridged thiacalix[4]arenes as cesium-selective ionophores in solvent polymeric membrane electrodes. Anal. Chim. Acta 2006, 569, 42–49. [Google Scholar] [CrossRef]
- Sasaki, Y.; Sugo, Y.; Suzuki, S.; Tachimori, S. The novel extractants diglycolamides for the extraction of lanthanides and actinides in HNO3-n-dodecane system. Solvent Extr. Ion Exch. 2001, 19, 91–103. [Google Scholar] [CrossRef]
- Ansari, S.A.; Pathak, P.N.; Manchanda, V.K.; Husain, M.; Prasad, A.K.; Parmar, V.S. N,N,N′,N′-Tetraoctyl Diglycolamide (TODGA): A Promising Extractant for Actinide-Partitioning from High-Level Waste (HLW). Solvent Extr. Ion Exch. 2005, 23, 463–479. [Google Scholar] [CrossRef]
- Horwitz, P.E.; Kalina, D.G. The extraction of Am(III) from nitric acid by octyl(phenyl)-N,N-diisobutylcarbamoylmethylphosphine oxide-tri-n-butyl phosphate mixtures. Solvent Extr. Ion Exch. (USA) 1984, 2, 179–200. [Google Scholar] [CrossRef]
- Mathur, J.N.; Murali, M.S.; Natarajan, P.R.; Badheka, L.P.; Banerji, A.; Ramanujam, A.; Dhami, P.S.; Gopalakrishnan, V.; Dhumwad, R.K.; Rao, M.K. Partitioning of actinides from high-level waste streams of purex process using mixtures of CMPO and TBP in dodecane. Waste Manag. 1993, 13, 317–325. [Google Scholar] [CrossRef]
- Legin, A.V.; Kirsanov, D.O.; Babain, V.A.; Borovoy, A.V.; Herbst, R.S. Cross-sensitive rare-earth metal sensors based on bidentate neutral organophosphorus compounds and chlorinated cobalt dicarbollide. Anal. Chim. Acta 2006, 572, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Legin, A.V.; Babain, V.A.; Kirsanov, D.O.; Mednova, O.V. Cross-sensitive rare earth metal ion sensors based on extraction systems. Sens. Actuators B Chem. 2008, 131, 29–36. [Google Scholar] [CrossRef]
- Mahanty, B.; Satpati, A.K.; Mohapatra, P.K. Development of a potentiometric sensor for europium(III) based on N,N,N′,N′-tetraoctyldiglycolamide (TODGA) as the ionophore. J. Electroanal. Chem. 2018, 808, 340–347. [Google Scholar] [CrossRef]
- Iqbal, M.; Huskens, J.; Verboom, W.; Sypula, M.; Modolo, G. Synthesis and Am/Eu extraction of novel TODGA derivatives. Supramol. Chem. 2010, 22, 827–837. [Google Scholar] [CrossRef]
- Wilden, A.; Modolo, G.; Lange, S.; Sadowski, F.; Beele, B.B.; Skerencak-Frech, A.; Panak, P.J.; Iqbal, M.; Verboom, W.; Geist, A.; et al. Modified Diglycolamides for the an(III) + Ln(III) Co-separation: Evaluation by Solvent Extraction and Time-Resolved Laser Fluorescence Spectroscopy. Solvent Extr. Ion Exch. 2014, 32, 119–137. [Google Scholar] [CrossRef]
- Rais, J.; Grüner, B. Extraction with metal bis(dicarbollide) anions: Metal bis(dicarbollide) extractants and their applications in separation chemistry. In Ion Exchange and Solvent Extraction; Marcus, Y., SenGupta, A.K., Dekker, M., Eds.; Taylor & Francis Inc.: Bosa Raton, FL, USA, 2004; pp. 243–334. [Google Scholar]
- Paulenova, A.; Alyapyshev, M.Y.; Babain, V.A.; Herbst, R.S.; Law, J.D. Extraction of Lanthanoids with Diamides of Dipcolinic Acid from Nitric Acid Solutions. II. Synergistic Effect of Ethyl-Tolyl Derivates and Dicarbollide Cobalt. Solvent Extr. Ion Exch. 2013, 31, 184–197. [Google Scholar] [CrossRef]
- Grüner, B.; Plešek, J.; Báča, J.; Císařová, I.; Dozol, J.F.; Rouquette, H.; Viňas, C.; Selucký, P.; Rais, J. Cobalt bis(dicarbollide) ions with covalently bonded CMPO groups as selective extraction agents for lanthanide and actinide cations from highly acidic nuclear waste solutions. New J. Chem. 2002, 26, 1519–1527. [Google Scholar] [CrossRef]
- Selucký, P.; Rais, J.; Lučaníková, M.; Grüner, B.; Kvíčalová, M.; Fejfarová, K.; Císařová, I. Lanthanide and actinide extractions with anionic ligands based on cobalt bis(dicarbollide) ions with covalently bonded CMPO functions. Radiochim. Acta 2008, 96, 273–284. [Google Scholar] [CrossRef]
- Grüner, B.; Kvíčalová, M.; Selucký, P.; Lučaníková, M. Anionic alkyl diglycoldiamides with covalently bonded cobalt bis(dicarbollide) (1-) ions for lanthanide and actinide extractions. J. Organomet. Chem. 2010, 695, 1261–1264. [Google Scholar] [CrossRef]
- Bubeníková, M.; Selucký, P.; Rais, J.; Grüner, B.; Švec, P. Studies on Am(III) separation from simulated high-level waste using cobalt bis(dicarbollide) (1-) ion derivative covalently bound to N,N-di-n-octyl diglycol diamide as extractant and DTPA as stripping agent. J. Radioanal. Nucl. Chem. 2012, 293, 403–408. [Google Scholar] [CrossRef]
- Vaňura, P.; Makrlík, E.; Selucký, P. Extraction of trivalent europium and americium by the synergistic mixture of bis-1,2-dicarbollylcobaltate and N,N,N′,N′-tetraoctyl diglycolamide in highly polar 3-nitro-α,α,α-trifluorotoluene solvent. J. Radioanal. Nucl. Chem. 2018, 317, 443–449. [Google Scholar] [CrossRef]
- Fu, B.; Bakker, E.; Yun, J.H.; Yang, V.C.; Meyerhoff, M.E. Response Mechanism of Polymer Membrane-Based Potentiometric Polyion Sensors. Anal. Chem. 1994, 66, 2250–2259. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.; Bühlmann, P.; Pretsch, E. Carrier-based ion-selective electrodes and bulk optodes. 1. General characteristics. Chem. Rev. 1997, 97, 3083–3132. [Google Scholar] [CrossRef]
- Stauthamer, W.P.R.V.; Engbersen, J.F.J.; Verboom, W.; Reinhoudt, D.N. Influence of plasticizer on the selectivity of nitrate-sensitive CHEMFETs. Sens. Actuators B Chem. 1994, 17, 197–201. [Google Scholar] [CrossRef][Green Version]
- Eugster, R.; Rosatzin, T.; Rusterholz, B.; Aebersold, B.; Pedrazza, U.; Rüegg, D.; Schmid, A.; Spichiger, U.E.; Simon, W. Plasticizers for liquid polymeric membranes of ion-selective chemical sensors. Anal. Chim. Acta 1994, 289, 1–13. [Google Scholar] [CrossRef]
- Arada Pérez, M.D.L.A.; Marín, L.P.; Quintana, J.C.; Yazdani-Pedram, M. Influence of different plasticizers on the response of chemical sensors based on polymeric membranes for nitrate ion determination. Sens. Actuators B Chem. 2003, 89, 262–268. [Google Scholar] [CrossRef]
- Bakker, E.; Pretsch, E.; Bühlmann, P. Selectivity of potentiometric ion sensors. Anal. Chem. 2000, 72, 1127–1133. [Google Scholar] [CrossRef]
- Lee, M.H.; Yoo, C.L.; Lee, J.S.; Cho, I.S.; Kim, B.H.; Cha, G.S.; Nam, H. Tweezer-Type Neutral Carrier-Based Calcium-Selective Membrane Electrode with Drastically Reduced Anionic Interference. Anal. Chem. 2002, 74, 2603–2607. [Google Scholar] [CrossRef]
- Bedlechowicz-Śliwakowska, I.; Lingenfelter, P.; Sokalski, T.; Lewenstam, A.; Maj-Zurawska, M. Ion-selective electrode for measuring low Ca2+ concentrations in the presence of high K+, Na+ and Mg2+ background. Anal. Bioanal. Chem. 2006, 385, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Bühlmann, P.; Pretsch, E.; Bakker, E. Carrier-based ion-selective electrodes and bulk optodes. 2. Ionophores for potentiometric and optical sensors. Chem. Rev. 1998, 98, 1593–1687. [Google Scholar] [CrossRef] [PubMed]
- Makrlík, E.; Vaňura, P.; Selucký, P. Extraction of calcium and strontium into phenyltrifluoromethyl sulfone by using synergistic mixture of hydrogen dicarbollylcobaltate and “classical” CMPO. Acta Chim. Slov. 2010, 57, 485–490. [Google Scholar] [PubMed]
- Zhu, Z.X.; Sasaki, Y.; Suzuki, H.; Suzuki, S.; Kimura, T. Cumulative study on solvent extraction of elements by N,N,N′,N′-tetraoctyl-3-oxapentanediamide (TODGA) from nitric acid into n-dodecane. Anal. Chim. Acta 2004, 527, 163–168. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).